Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Sustainable wet-spun cellulose-Moringa oleifera composite fibres for potential water purification†
Abimbola Oluwatayo Orisawayi *ab,
Prithivi Boyllaa,
Krzysztof K. Koziola and
Sameer S. Rahatekar*a
*ab,
Prithivi Boyllaa,
Krzysztof K. Koziola and
Sameer S. Rahatekar*a
aComposites and Advanced Materials Centre, Faculty of Engineering and Applied Sciences, Cranfield University, College Road, Cranfield, Bedfordshire, MK43 0AL, UK. E-mail: abimbola.orisawayi@cranfield.ac.uk; bimboris_t@yahoo.com; S.S.Rahatekar@cranfield.ac.uk
bDepartment of Mechanical Engineering, School of Engineering and Engineering Technology, Olusegun Agagu University of Science and Technology (OAUSTECH), Okitipupa, Nigeria. E-mail: ao.orisawayi@oaustech.edu.ng
First published on 28th May 2025
Abstract
This study explores a pioneering fabrication of novel cellulose-Moringa oleifera (M. oleifera) composite fibres (CeL-MoFs) and comparable pure regenerated cellulose fibres (CeFs) using the ionic liquid 1-ethyl-3-methylimidazolium diethyl phosphate (EMIM DEP) and the simple traditional wet-spinning process. The composites, CeL-MoFs at 0.5%, 1%, 2%, and 3%, were characterised. Fourier-transform infrared (FTIR) spectroscopy and scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM-EDX) confirmed the successful integration of M. oleifera seed powder (MoP) into the cellulose matrix. The results of preliminary adsorption studies demonstrated high selectivity for copper ions (Cu2+), with no detectable selectivity towards nickel (Ni2+) or cadmium (Cd2+). Thermogravimetric analysis (TGA) and derivative thermogravimetric (DTG) analysis revealed thermal stability variations with increasing MoP content, while atomic force microscopy (AFM) showed surface roughness and fibre defects. Rheological testing validated spinnability, and tensile analysis identified CeL-MoFs (2%) as the optimal composite, balancing mechanical strength and adsorption efficiency. These novel CeL-MoF composites, fabricated using EMIM DEP, are proposed as scalable, eco-friendly materials for selective heavy metal removal. Future work will focus on adsorption kinetics, thermodynamic modelling, and scaling production for industrial water purification applications.
1. Introduction
In recent days, the rapid growth in global industrialisation aimed at improving human life has significantly affected the environment.1,2 Studies have emphasised the widespread environmental effects of environmental pollution, which adversely affect land, air, and water.3,4 Among these, water pollution has emerged as a pressing concern, posing severe risks to human health and contributing to numerous hazardous health-related issues.5,6 Water pollution is majorly caused by pollutant discharge into water bodies and contaminants such as pathogens, heavy metals, dyes, and pigments from industrial or domestic waste, which may either be absorbed by the soil or flow directly into the water system, affecting the concentration of underground and surface water.7,8Among these pollutants, heavy metal ions, such as chromium (Cr), lead (Pb), cadmium (Cd), mercury (Hg), copper (Cu), nickel (Ni), and manganese (Mn), stand out for their toxic and persistent nature, contributing to significant risks to ecosystems and human health.5,9,10 Heavy metals primarily originate from industrial activities such as machinery manufacturing, mineral smelting, electroplating, electronics production, oil refining, and chemical processing.9,11 Therefore, heavy metal ion contamination in water bodies is a critical problem. Developing effective methods for removing these toxic metals remains a prominent focus in environmental research. Several conventional and advanced technologies have been used to address water challenges, including traditional water treatment methods, such as oxidation, electro-precipitation, membrane separation, coagulation–flocculation, evaporation, flotation, and ion exchange, but these methods are often inadequate in addressing efficient water treatment1,12
In heavy metal ion removal, an adsorption method is usually considered one of the effective physical methods preferred for addressing the removal of metal ions from an aqueous solution because of its advantages of multiple adsorbent selection, high-efficiency removal, selective compliance, simple and easy operation, good reversibility and low cost.13 There is a demand for efficient, sustainable materials as an alternative to existing adsorbent materials, sorbent biopolymers and biomaterials.14
Studies on the application of novel chemically modified cellulose for the adsorption of heavy metal ions by Fakhre and Ibrahim.15 revealed that some effective chelating ion-exchange materials are composed of biopolymers and their derivatives, which is attributed to the presence of various functional groups, such as –NH2 and –OH, which readily interact to form bonds with other chemical entities, which may include metal ions from aqueous solution.16,17 These biopolymers may comprise cellulose, alginates, proteins, chitin, and chitin derivatives, such as chitosan, to exhibit outstanding efficiency in reducing metal ion concentrations to considerable concentrations;5,13,18 further reports from these studies stated that cellulose and most of its derivatives can adsorb metals effectively using their hydroxyl groups, which can be replaced by other functional groups.19–22
Modified cellulose can exhibit a 40–80% higher adsorption capacity for heavy metal ions than unmodified cellulose.23,24 Therefore, from the sustainability perspective, biopolymers, such as cellulose, are used in various applications, such as medical devices, construction applications, textiles, pharmaceuticals, aerospace, and automotive, including water treatment.10 Cellulose is the most abundant natural polymer produced by plants and may be biodegradable. It has been discussed for ages as one of the oldest polymeric materials known in the chemical industries; cellulose remains a significant focus and important polymer in research to date, and it has attracted wide interest even in polymer science owing to its excellent biocompatibility, strong and structural forming ability, and environmentally sustainable properties, making it a key material for advanced applications.25,26
Cellulose polymer consists of β(1→4)-polysaccharide with an extensive network of intra- and inter-hydrogen bonds, which enables it to adopt a highly ordered structure.23,27 This is responsible for cellulose having desirable chemical and mechanical properties for healing, bactericide and fungicide, drug delivery, and adsorbents for organic and inorganic pollutants.27 M. oleifera seed powder has been extensively studied owing to its bioactive properties and excellent adsorption capabilities for heavy metals and other contaminants. The seed's high protein content provides functional groups, such as amino acids and carboxyl, enabling interactions with pollutants through various adsorption mechanisms.28–30
M. oleifera seeds also possess antimicrobial properties, making them suitable for water purification applications. However, their direct use in filtration systems is limited by their mechanical instability and tendency to disperse in aqueous environments, as previous studies have shown that it can cause secondary pollution if not properly encapsulated.10 To address these limitations, we propose using M. oleifera seed powder and polymers, such as cellulose, which could provide a synergistic approach. Cellulose, being a mechanically robust and biodegradable polymer, offers a stable structural matrix that can encapsulate and support the active components of M. oleifera.
Previous studies have explored cellulose's potential, including using ionic liquids and M. oleifera separately for water purification.31–33 Cellulose-based materials have been extensively investigated owing to their mechanical strength, chemical stability, and ability to adsorb heavy metals; M. oleifera seed powder is valued for its bioactive properties and superior adsorption capabilities.34,35 However, the direct use of M. oleifera is hindered by mechanical instability and dispersion issues in aqueous environments. Similarly, although biopolymer composites, such as alginate-M. oleifera,10 have been developed to address these limitations, combining cellulose and M. oleifera using green, scalable techniques, such as ionic liquid-based wet spinning, remains unexplored. This gap must be addressed by highlighting the need for innovative, sustainable solutions.
To prepare cellulose solutions, ionic liquids (IL) have been widely used because they offer a promising alternative to the commonly used acidic solvents in dissolving cellulose. Ionic liquids (ILs), with melting points below 100 °C, have garnered attention owing to their exceptional properties, including high thermal stability, non-flammability, low vapour pressure, and remarkable polymer solubility.36 Carefully selected ionic liquids with low toxicity and excellent recyclability offer significant environmental and safety advantages over traditional fibre production processes. The ionic liquid used in the study is imidazolium-based, such as 1-ethyl-3-methylimidazolium diethyl phosphate, which was reported in one study as an eco-friendly approach toward downstream processing of bacterial biomass for the extraction of an intracellular potential bioplastic material, polyhydroxyalkanoates, replacing chlorinated organic solvents.37 ILs are frequently used for dissolution with a sustainable and efficient medium for processing materials, preserving their bioactive properties to enable uniform dispersion and enhancing their integration with other materials like cellulose.38
The novelty of this study lies in the pioneering integration of M. oleifera (MoP) with cellulose (CeL) using ionic liquids through wet spinning, a fibre production technique in which a polymer solution is extruded through a spinneret into a coagulation bath (in this case, water was used as a coagulation bath because it is eco-friendly). Wet-spinning is particularly advantageous for processing materials that require precise control over fibre morphology and properties, making it ideal for creating biopolymer composites with enhanced mechanical stability and functional performance. By leveraging cellulose's exceptional mechanical strength and MoP's superior adsorption capabilities, this study aims to develop robust composite fibres tailored for water treatment applications. In addition, we expect that this pioneering CeL-MoF composite would deliver a sustainable and scalable solution for water purification, merging MoP's superior adsorption capabilities with cellulose's mechanical durability. MoP may affect mechanical properties, but it could still be suitable for application in water treatment. This advancement has the potential to be transformative for industrial-scale water treatment and environmental remediation technologies.
In this work, we first developed pure CeF fibres and their composites (CeL-MoF composite fibres) through an ionic liquid-mediated wet-spinning process using the same wet spinning setup as our previous studies.10 Specific characterisation of the composite fibres was performed using FTIR to analyse chemical interactions and bonding, TGA to evaluate thermal stability, and SEM-EDX to study morphological features and elemental composition, with further characterisation using AFM to assess the surface topography and rheology to examine the flow and viscoelastic properties of the spinning solution. In addition, the mechanical properties of the composite fibres were assessed to ensure their robustness and suitability for practical applications. Tensile testing was conducted to determine tensile strength, Young's modulus, and elongation at break, providing insights into the ability of the fibres to withstand mechanical stresses. The composite developed demonstrated structural and functional advantages, such as an eco-friendly, efficient, and sustainable solution, for potential water purification applications.
2. Materials and methods
2.1 Materials
The cellulose used in this study was high-purity cellulose. Pulp sheets were procured from Rayonier, Fernandina Beach (USA), with a degree of polymerization (DP) of 890. The Ionic Liquid (IL) used as a solvent was 1-ethyl-3-methylimidazolium diethyl phosphate (C10H21N2O4P), EMIM DEP, molecular weight (Mw):264.26 g mol−1, ≤ 100% (specifically 99% high purity), product number: 671541, CAS-No.: 848641-69-0, CAS-No.: 848641-69-0, procured from Sigma-Aldrich, UK. M. oleifera seeds used were the ones previously procured through our trusted supplier (Purely Agro Ltd, “DGT”d 2, London, UK).10,34 Other chemicals used for preliminary adsorption studies include copper(II) acetate monohydrate (C4H6CuO4·H2O), cadmium acetate dihydrate (C4H6CdO4·2H2O), and nickel(II) acetate tetrahydrate (C4H6NiO4·2H2O) procured from Sigma-Aldrich, UK. All chemicals were of high purity, and deionized water was supplied by the university laboratory supplier, as reported in previous studies.102.2 Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 90
5, 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 80
10, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, and 70
20, and 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, These ratios were carefully selected to prevent agglomeration, which was observed during preliminary preparations at higher MoP concentrations. The resulting mixtures were stirred for an additional 4 hours. The final mixtures had cellulose concentrations of 5.7 wt%, 5.4 wt%, 4.8 wt%, and 4.2 wt%, respectively, and MoP concentrations of 0.5 wt%, 1.0 wt%, 2.0 wt%, and 3.0 wt%. The control solution consisting of 6 wt% CeL in IL served as a baseline for comparison. Each solution was loaded into a 60 mL syringe. To remove air bubbles and ensure uniformity, the samples were degassed using a vacuum oven. Table 1 presents a comprehensive summary of the prepared samples, serving as a reference point for the subsequent characterisation and evaluation of their properties. These compositions were linked to the preparation of solutions used in the wet spinning process to produce fibres.
30, These ratios were carefully selected to prevent agglomeration, which was observed during preliminary preparations at higher MoP concentrations. The resulting mixtures were stirred for an additional 4 hours. The final mixtures had cellulose concentrations of 5.7 wt%, 5.4 wt%, 4.8 wt%, and 4.2 wt%, respectively, and MoP concentrations of 0.5 wt%, 1.0 wt%, 2.0 wt%, and 3.0 wt%. The control solution consisting of 6 wt% CeL in IL served as a baseline for comparison. Each solution was loaded into a 60 mL syringe. To remove air bubbles and ensure uniformity, the samples were degassed using a vacuum oven. Table 1 presents a comprehensive summary of the prepared samples, serving as a reference point for the subsequent characterisation and evaluation of their properties. These compositions were linked to the preparation of solutions used in the wet spinning process to produce fibres.
| Sample name | CeL (wt%) | MoP (wt%) | IL (wt%) | Description |
|---|---|---|---|---|
| CeL–IL | 6.0 | 0.0 | 94.0 | Control with only cellulose in IL |
| MoP–IL | 0.0 | 10.0 | 90.0 | MoP solution in IL |
| CeL–MoP–IL (0.5%) | 5.7 | 0.5 | 93.8 | 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (w/w) mix of CeL–IL and MoP–IL 5 (w/w) mix of CeL–IL and MoP–IL |
| CeL–MoP–IL (1%) | 5.4 | 1.0 | 93.6 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (w/w) mix of CeL–IL and MoP–IL 10 (w/w) mix of CeL–IL and MoP–IL |
| CeL–MoP–IL (2%) | 4.8 | 2.0 | 93.2 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (w/w) mix of CeL–IL and MoP–IL 20 (w/w) mix of CeL–IL and MoP–IL |
| CeL–MoP–IL (3%) | 4.2 | 3.0 | 92.8 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (w/w) mix of CeL–IL and MoP–IL 30 (w/w) mix of CeL–IL and MoP–IL |
2.3 Characterisations and instrumentations
3. Results and discussion
3.1 Rheology of freshly prepared CeL–IL and CeL–MoP–IL solutions
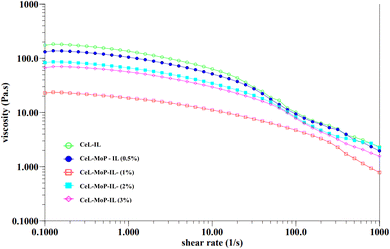
The rheology of a solution, influenced by the choice of solvent, including ionic liquids, plays a crucial role in determining spinnability during the wet spinning process.47,48 This analysis of the rheology of the spinning solutions can also help to understand the processes and control of the MoP introduced into the solutions to enhance the good spinnability of the composites during the wet spinning process. To examine how solution preparation affects fibre spinnability, Fig. 2 presents the rheological properties of cellulose solutions combined with varying concentrations of MoP in spinning dopes. These curves highlight the interactions between pure cellulose solutions and their composites, illustrating how these interactions influence the spinnability of fibres. The addition of 0.5% MoP in the ionic liquid resulted in a marginal viscosity reduction of CeL–MoP–IL (0.5%). A further increment of MoP of 1% resulted in a significant reduction in the viscosity of CeL–MoP–IL (1%); this phenomenon might be linked to contents in the MoP, such as protein, carbohydrates, flavonoids, fatty acids, glucosinolates, oils, and some present minerals.34 Similar trends were observed in the rheological behaviour of cellulose/silk fibroin blend solutions with ionic liquid as solvent. Furthermore, there were intractable increases and reductions in CeL–MoP (2%) and CeL–MoP–IL (3%). Further additions of MoP at 2% and 3% concentrations led to variable increases and decreases in viscosity, indicating shear-thinning behaviour, as observed in previous literature.49–52 This result agrees with previous studies on the rheological properties of solutions of non-woven fabric made from fine regenerated cellulose fibres produced using a wet solution blow spinning method from an ionic-liquid solution and other studies on the structural and property changes in regenerated cellulose fibres caused by the presence of metal ion impurities.48,52 Furthermore, our findings confirm the phenomenon of Newtonian fluid plateau properties of minimal shear rates, resulting in a stable solution structure during the flow process, which helps to facilitate the manufacturing process of polymers, including wet spinning fibres at 65 ± 1 °C used for the wet spinning process when the ionic liquid was used for fabrications.49 These results indicate that MoP incorporation increases spinning solution instability, negatively impacting the mechanical properties of cellulose fibres. However, the successful integration of MoP into the cellulose fibre matrix suggests potential enhancements that could improve the material's suitability for our proposed applications.3.2 Fourier transform infrared (FTIR) analysis of fibres
Fig. 3 shows the FTIR spectra of pure cellulose (CeL) and the MoP-loaded composite (CeL-MoF 3%) before and after immersion in aqueous solutions containing Cu2+, Cd2+, and Ni2+ ions. The 3% MoP concentration was selected owing to its superior spectral resolution, functional group diversity, and consistent performance. Spectral comparisons demonstrate key shifts and intensity variations associated with metal ion adsorption. FTIR profiles for additional MoP concentrations (0.5%, 1%, and 2%) are presented in figure in the ESI.† | ||
| Fig. 3 FTIR spectra of CeF (a) before adsorption and (b) after adsorption of heavy metals in aqueous solution and CeL-MoF (3%) composite fibres. | ||
From the FT-IR spectra shown in Fig. 3a, the pure cellulose exhibited characteristic peaks at 3377.33 cm−1 (O–H stretching), 2917.41 cm−1 (C–H stretching), and 1025.99 cm−1 (C–O–C stretching), which are consistent with literature values for regenerated cellulose.53–55 The spectral bands of the pure cellulose fibres predominantly occur within the wave number regions of 3750–2800 cm−1 and 1750–600 cm−1. The spectra in these regions correspond to FTIR data previously reported for cellulose fibres derived from various wood pulp derivatives.56 The peaks observed in the wave number range of 3750–3000 cm−1 are attributed to the stretching vibrations of the O–H and C–H bonds typical of polysaccharides. The broad peak at 3378.33 cm−1 is characteristic of hydroxyl group stretching in polysaccharides, while the sharp and intense peak at 2917.41 cm−1 corresponds to the C–H stretching vibration of hydrocarbon (CH2) moieties within the polysaccharide structure.55 Furthermore, sharp peaks in the 1550–1750 cm−1 range are assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of the carbonyl linkages present in the fatty acid constituents of Moringa seed extract.34 These peaks exhibited increased intensity upon the addition of MoP. The peak at 1465.21 cm−1 is attributed to the O–H bending vibration, while the characteristic peak at 1028.40 cm−1 correlates with C–O bond stretching. With the incorporation of MoP, several new absorption peaks emerged, and notable shifts in the existing peaks were also observed. For example, a prominent peak at approximately 1653.25 cm−1 attributed to the C
O stretching vibrations of the carbonyl linkages present in the fatty acid constituents of Moringa seed extract.34 These peaks exhibited increased intensity upon the addition of MoP. The peak at 1465.21 cm−1 is attributed to the O–H bending vibration, while the characteristic peak at 1028.40 cm−1 correlates with C–O bond stretching. With the incorporation of MoP, several new absorption peaks emerged, and notable shifts in the existing peaks were also observed. For example, a prominent peak at approximately 1653.25 cm−1 attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of proteins and other organic compounds present in MoP57,58 became increasingly pronounced with higher MoP concentrations. In addition, a slight shift in the O–H stretching peak was observed, suggesting the formation of hydrogen bonds between the hydroxyl groups of cellulose and the functional moieties in MoP. Additionally, new sharp and intense peaks at 2920.30 cm−1 and 2851.36 cm−1 correspond to the enhanced asymmetric and symmetric C–H stretching vibration of hydrocarbon (CH2 and CH3) moieties within the CeL-MoF composite structure. Similar spectral shifts were reported by Zhang et al.59 In their study, they investigated the incorporation of grape seed extract into regenerated cellulose films. These FTIR spectral modifications collectively suggest that MoP is successfully integrated into the cellulose matrix and that hydrogen bonding interactions occur between the functional groups of MoP and cellulose. This interpretation aligns with the findings of Castro-López et al.60 who observed comparable peak intensity reductions and shifts following the incorporation of M. oleifera seed extract into carboxymethyl cellulose. Specifically, a small signal at 1540.43 cm−1 was assigned to the N–O stretching vibration of amino compounds, while a band at 1745.82 cm−1 was attributed to the C
O stretching vibrations of proteins and other organic compounds present in MoP57,58 became increasingly pronounced with higher MoP concentrations. In addition, a slight shift in the O–H stretching peak was observed, suggesting the formation of hydrogen bonds between the hydroxyl groups of cellulose and the functional moieties in MoP. Additionally, new sharp and intense peaks at 2920.30 cm−1 and 2851.36 cm−1 correspond to the enhanced asymmetric and symmetric C–H stretching vibration of hydrocarbon (CH2 and CH3) moieties within the CeL-MoF composite structure. Similar spectral shifts were reported by Zhang et al.59 In their study, they investigated the incorporation of grape seed extract into regenerated cellulose films. These FTIR spectral modifications collectively suggest that MoP is successfully integrated into the cellulose matrix and that hydrogen bonding interactions occur between the functional groups of MoP and cellulose. This interpretation aligns with the findings of Castro-López et al.60 who observed comparable peak intensity reductions and shifts following the incorporation of M. oleifera seed extract into carboxymethyl cellulose. Specifically, a small signal at 1540.43 cm−1 was assigned to the N–O stretching vibration of amino compounds, while a band at 1745.82 cm−1 was attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of M. oleifera. In the present study, the intensity of the newly formed peaks increased as the MoP content increased, thereby confirming the presence and distribution of MoP within the cellulose fibres.
O stretching of M. oleifera. In the present study, the intensity of the newly formed peaks increased as the MoP content increased, thereby confirming the presence and distribution of MoP within the cellulose fibres.
After the immersion of the fibres into the aqueous heavy metal solution, the adsorption of Cu2+, Cd2+, and Ni2+ ions onto the regenerated composite fibres was assessed through variations in the FTIR spectrum, as shown in Fig. 3b. The characteristic functional groups of cellulose and MoP—namely hydroxyl (−OH), carboxyl (−COOH), amine (−NH2), and glycosidic (C–O–C) linkages—are theoretically known to exhibit vibrational frequency shifts upon interaction with metal ions.61–63
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration, normally observed around 1700–1750 cm−1, is generally expected to shift toward a lower wavenumber upon the formation of metal-carboxylate complexes. This shift demonstrates metal–oxygen interactions that weaken the C
O stretching vibration, normally observed around 1700–1750 cm−1, is generally expected to shift toward a lower wavenumber upon the formation of metal-carboxylate complexes. This shift demonstrates metal–oxygen interactions that weaken the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond. However, in this study, the peak around 1744.37 cm−1 did not shift, implying that carboxylate coordination was absent or significantly hindered.
O bond. However, in this study, the peak around 1744.37 cm−1 did not shift, implying that carboxylate coordination was absent or significantly hindered.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/b_char_e001.gif) O–NR′R′′) groups. The N–H stretching band observed in the region of 3200–3500 cm−1 and the amide I band (C
O–NR′R′′) groups. The N–H stretching band observed in the region of 3200–3500 cm−1 and the amide I band (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–NR′R′′ stretching) near 1647.46 cm−1 are also expected to shift following metal ion adsorption. These changes may be attributed to the coordination between the metal ions and nitrogen atoms in the amino or amide groups of MoP. However, the lack of this shift indicates that the CeL-MoF compound does not provide active amino/amide groups for metal coordination compound formation.
O–NR′R′′ stretching) near 1647.46 cm−1 are also expected to shift following metal ion adsorption. These changes may be attributed to the coordination between the metal ions and nitrogen atoms in the amino or amide groups of MoP. However, the lack of this shift indicates that the CeL-MoF compound does not provide active amino/amide groups for metal coordination compound formation.These spectral shifts collectively provide evidence of metal ion adsorption onto the fibre, where the metal ions form coordination bonds with the oxygen or nitrogen atoms in the functional groups of cellulose and M. oleifera seed powder fibre. This is supported by studies conducted by Acheampong et al.64,65 and Meneghel et al.,66,67 who found that their FTIR spectra analysis revealed the presence of many functional groups that can bind metal ions by removal using ion exchange. The observed spectral shifts, disappearance, and increased intensity of some peaks after sorption experiments indicate an interaction between metal ions and the M. oleifera seed. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, amino groups, and –NH2 were involved in sorption by MoP. The resulting FTIR data suggest that structural modifications in the functional groups due to CeL–MoP interactions may block or limit some active metal binding in the CeL-MoF composites, thus impeding efficient coordination complex formation with heavy metal ions. This phenomenon may cause the observed preferential adsorption of metals such as Cu2+ compared to Cd2+ and Ni2+, potentially indicating a degree of selectivity in the fibre's binding behaviour.
O, amino groups, and –NH2 were involved in sorption by MoP. The resulting FTIR data suggest that structural modifications in the functional groups due to CeL–MoP interactions may block or limit some active metal binding in the CeL-MoF composites, thus impeding efficient coordination complex formation with heavy metal ions. This phenomenon may cause the observed preferential adsorption of metals such as Cu2+ compared to Cd2+ and Ni2+, potentially indicating a degree of selectivity in the fibre's binding behaviour.
3.3 Thermogravimetric analysis of CeF and CeL-MoF
The TGA of CeF and CeL-MoF was conducted, and the combination of the TGA–DTG curve is shown in Fig. 4. As shown in Fig. 4a, the initial temperature of the pure cellulose fibre is approximately 212 °C, while the final decomposition temperature of the CeL reaches a peak of about 342 °C. This is similar to the results obtained in the literature.41 The onset degradation temperature of the composites is similar, with little variation in the degradation of each of those composites in the range of 249–243 °C. However, changes were observed in the endset thermal degradation properties of the composites, as shown in Fig. 4. With CeL-MoF (0.5%) degrading at about 314 °C, CeL-MoF (1%) at 325 °C, and CeL-MoF (2%) and (3%) at approximately 465.6 °C and 322.4 °C, respectively. Similar trends were reported in the characteristics of cellulose isolated from the selected biomass, as described by Zhang et al.41 The thermogravimetric (DTG) curve of the samples also reflects more on the degradation profiles of the fibre samples, with the quantitative summary presented in Table 2. The CeL fibre shows a single sharp peak observed at a temperature of 272.7 °C, which corresponds to the composition stage primarily driven by cellulose breakdown, as reported in the literature.41,68 With little addition of CeL-MoF (0.5%), the shift in peaks to approximately 303.06 °C resulted in traces of MoP in the matrix. However, the further addition of MoP in CeL-MoF composites resulted in a shift in peaks of CeL-MoF (1%), which presented two distinct peaks at 316.08 °C and 394.9 °C, respectively, resulting in a sequential thermal decomposition of the cellulose matrix and MoP, respectively. This may be ascribed to the presence of the major constituents of MoP, such as protein, as the content of the MoP increases to 2%, and the second peak shifts to a higher temperature of 427.55 °C, but the profile on the DTG profile appears to overlap with the thermal profile. The CeL-MoF (3%) reflects complex degradation that presents multiple peaks at 276.04 °C, 350.79 °C, and 377.81 °C. These peaks correspond to the thermal decomposition of both the CeL and the combination of the MoP composites, and their synergistic interactions within the composite, as similar trends were observed in studies conducted by Orisawayi et al.10 when MoP was loaded into a sodium alginate matrix. Conclusively, the overall study of the TGA and the DTG analysis demonstrates that MoP was incorporated into the CeL matrix, which significantly altered the thermal stability and degradation properties of the composites. Conclusively, the deduction from the TGA–DTG studies demonstrated that the degradation of the composites of MoP at higher concentrations occurred in a wide temperature range over time. | ||
| Fig. 4 Combined TGA–DTG curve of (a) CeF and CeL-MoF (0.5%); (b) CeL-MoF at different concentrations. | ||
| Sample | T5% (°C) | T10% (°C) | T50% (°C) | T1peak (°C) | T2peak (°C) | T3peak (°C) |
|---|---|---|---|---|---|---|
| CeL | 111.93 | 219.31 | 275.36 | 272.70 | — | — |
| CeL-MoF (0.5%) | 102.22 | 247.15 | 305.38 | 303.06 | — | — |
| CeL-MoF (1%) | 108.47 | 257.04 | 321.71 | 316.08 | 394.90 | — |
| CeL-MoF (2%) | 91.5 | 240.37 | 318.21 | 313.92 | 427.55 | — |
| CeL-MoF (3%) | 136.52 | 233.65 | 287.96 | 276.04 | 350.79 | 377.81 |
3.4 SEM structure morphology of CeL and CeL–MoP composite fibres
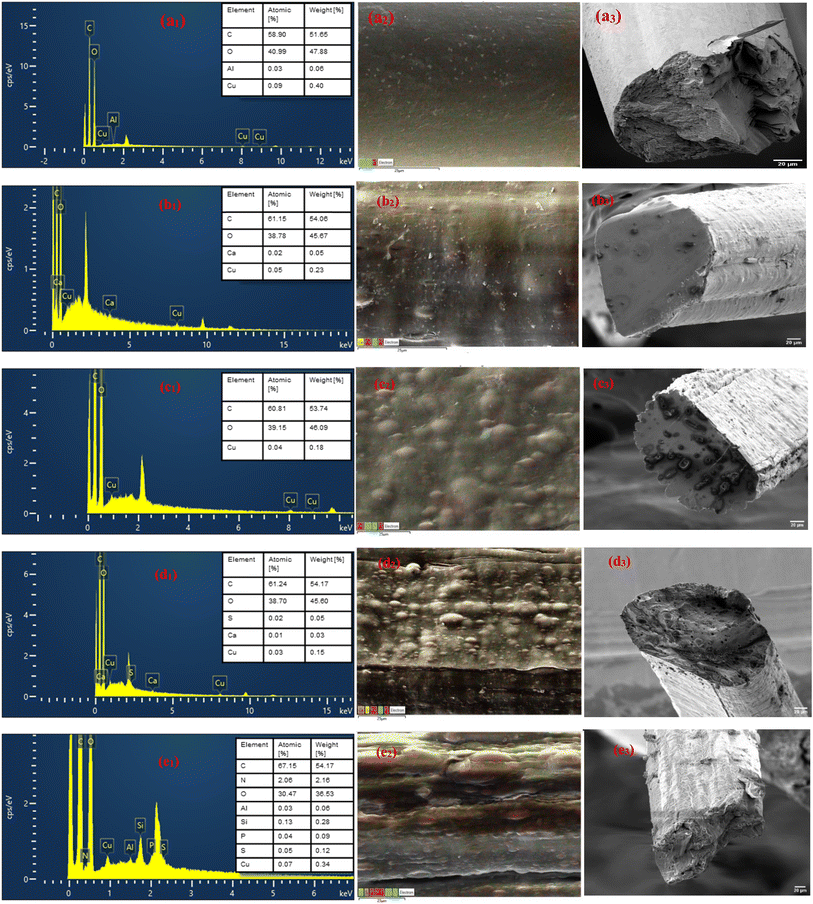
The structural morphology and cross-sectional views of the wet-spun fibre samples are presented in Fig. 5. The images of the pure cellulose fibre (CeF) are shown in Fig. 5a1 and a2. CeF reveals a smooth, uniform surface morphology, consistent with the characteristics of no visible inter-fibre gaps on the surface of pure regenerated cellulose fabricated from IL, as reported in previous studies.51,69 The cross-sectional view of the CeF exhibits a near-circular structure with a smooth and well-defined surface. Internally, the fibres appear homogenous, indicating the high quality of the wet-spinning process.10,69,70 The magnified cross-section in Fig. 5a2 features fracture patterns that do not seem to originate from inherent defects. This demonstrates the excellent structural integrity of the CeF. These characteristics establish CeF as a reliable baseline for further material modifications or the incorporation of additives, such as MoP.Fig. 5b1–e2 shows the surface morphology of CeL-MoF fibres at various MoP concentrations. The CeL-MoF (0.5%) composite in Fig. 5b2 presents a well-dispersed MoP particle distribution within the cellulose matrix. The surface appears slightly irregular compared to the CeF fibres. There were features of few or minimal processing and intrinsic defects observed on the surface of the fibres. These features are consistent with prior studies on low MoP loading in biopolymer matrices, such as alginate, as noted by Orisawayi et al.10
CeL-MoF (1%) composites (Fig. 5c2) exhibit a rougher surface than CeL-MoFs (0.5%), with features resembling the ridges on the surface of the fibres. These features differ from those observed in CeL fibres and the CeL-MoF (0.5%) composite. Protrusions were also observed on the surface during fractures, suggesting modifications caused by MoP incorporation that must have resulted from the factored surfaces. As the concentration of MoP increases to 2%, as illustrated in Fig. 5d2, the CeL-MoF (2%) composite fibres exhibit more pronounced surface irregularities, with visible MoP particles and increased inter-fibre gaps with aggregations. The morphology shows moderate roughness, disrupting the previously smooth structure. The pronounced ridges on the surface seam disappear but are slightly observed.
In the CeL-MoF (3%) composite shown in Fig. 5e2, features of significantly pronounced inter-fibre gaps were observed compared to other composites of CeL-MoF and CeF fibres. The surface displays a high concentration of defects, with noticeable aggregations of MoP at these concentrations disrupting uniformity, which must have affected the mechanical properties. The cross-sectional view reveals severe inter-fibre gaps and considerable heterogeneity, suggesting that excess MoP loading alters the cellulose matrix. This could be attributed to interactions between the bioactive compounds in MoP and the cellulose matrix, which affect the structural integrity of the fibres during the coagulation process.
Previous studies have reported increased surface roughness in fibres due to MoP incorporation into biopolymers. This phenomenon is often linked to interactions between bioactive compounds in MoP and the polymer matrix, which disrupt homogeneity and contribute to surface irregularities, as noted by Orisawayi et al.,10 Coscia et al.,71 and Yang et al.72
The findings of this study are consistent with these observations, showing a significant increase in the MoP content of approximately 8% when incorporated into alginate biopolymers.10 This increase prevents further loading, as it leads to greater morphological disruptions and inter-fibre gaps. Similarly, our findings indicate that during preparation, the agglomeration of MoP is minimized, preventing the further addition of MoP into the CeL matrix.
In conclusion, higher MoP concentrations significantly affect fibre morphology, increasing surface roughness, inter-fibre gaps, and heterogeneity. The 3% MoP sample exhibits the most pronounced changes, with aggregated MoP clusters causing structural disorders. These results highlight the impact of MoP loading on the cellulose matrix, providing valuable insights for optimising composite material properties.
3.5 Atomic force microscopy
Over a few decades, Atomic Force Microscopy (AFM) has been employed as a multipurpose analytical technique and has proven to be extremely useful in characterising complicated topographic images of materials from micrometres to nanometres.73,74 To the best of our knowledge, only few studies have reported the use of AFM to characterise the nature of the microscopic topographic image of wet-spun fibre. The 2-D and 3-D topographic images of the CeL and CeL-MoF composite samples obtained are illustrated in Fig. 6. The roughness parameters, arithmetic mean roughness (Ra), root mean square roughness (Rq), and maximum roughness depth (Rmax) are commonly accepted and frequently used to determine roughness measurement.75 Table 3 presents the quantitative results from the AFM surface roughness properties of the CeF and CeL-MoF fibres. The (Ra) and (Rq) values for pure CeL fibre were 23.7 nm and 29.2 nm, respectively. This is an attribute of CeF, presenting a smooth surface topology, as observed in Fig. 6a. This illustration shows a moderate level of surface roughness structures compared to CeL-MoF composite fibres. These features could be evidence of the complete dissolution of the CeF fragments in IL, which is crucial for excellent fibre formation during the wet spinning process. This finding aligns with studies on the common characteristics of pure cellulose, where a similar topology was observed in fibres produced through dry-jet wet-spinning.76 As depicted in Fig. 6b–e, the incorporation of MoP at different concentrations of 0.5%, 1%, 2% and 3% wt. resulted in increments in Ra and Rq values to 30.6 and 42.2, 75.1 and 92.5, 79.1 and 104, as well as 175 and 207 nm, respectively. This rapid increase in the value of Rmax from 603 nm to about 1181 nm, nearly doubling the value, resulted in a noticeable change, as shown in Fig. 6e. Thus, the surface's topography can be modulated to increase the surface area, as observed in Fig. 6e. Although this change influences the mechanical properties, it strongly indicates the successful distinctive feature attributed to the incorporation of MoP into the CeF matrix composites.| Fibre Code | Ra (nm) | Rq (nm) | Rmax (nm) |
|---|---|---|---|
| CeF | 23.7 | 29.2 | 200 |
| CeL-MoF (0.5%) | 30.6 | 42.2 | 386 |
| CeL-MoF (1%) | 75.1 | 92.5 | 464 |
| CeL-MoF (2%) | 79.1 | 104 | 603 |
| CeL-MoF (3%) | 175 | 206 | 1181 |
3.6 Fibre diameter and mechanical properties of CeF and CeL-MoF
The fibre diameter and the mechanical properties are important in determining the behaviour of wet-spun fibre.75 Fig. 7 shows the CeL and CeL-MoF composite's average diameter properties and the mechanical properties of CeF and CeL-MoF composite fibres with the stress–strain curve, ultimate tensile strength, breaking strain, and Young's modulus.The fibre diameter variations during wet spinning, influenced by factors such as filler dispersion, particle concentration, spinning speed, and drying conditions, significantly affect the mechanical properties, explaining why they change and how this facilitates the incorporation of MoF.70 Fig. 7a illustrates these variations and their impact on CeF and CeL-MoF fibres. Regarding mechanical properties, the control sample, CeF, exhibited the highest values (Fig. 7b–e), with UTS of approximately 199.1 MPa (±4.76), breaking strain of 25.9% (±3.88), and Youngs' Modulus (YM) of 7.66 GPa (±0.57), demonstrating the intrinsic strength and flexibility of pure regenerated CeL fibres developed in this study. In comparison, the mechanical properties of CeL-MoF composites varied with MoP concentration, particularly MoP at 0.5% and 1%, possessing similar trends.
In detail, the introduction of 0.5 MoP reduces the UTS of the CeL-MoF to approximately 83.23 MPa (±8.31), which is significantly lower than that of the CeF. This reflects the initial impact of MoP incorporated on the CeF fibre's mechanical properties, with a breaking strain of 11.07% (±0.65). This impact was observed to reduce the flexibility of pure CeF. The YM also substantially decreased compared to 3.21 GPa (±0.4) compared to that of CeF, which may be due to disruption in its molecular arrangement, leading to a decline in mechanical properties, even at low concentrations of MoP. These results may be related to the results of the study conducted by Ejeta et al.77 on the influence of filler concentrations and processing parameters on the mechanical properties of lignocellulose loaded with bio-fillers. The CeL-MoF (1%) UTS is similar to that of MoP at 0.5% with little improvement to about 82.74 MPa (±5.16) at this concentration, while the breaking strain is 10.63% (±1), showing a further reduction in flexibility at this trend; the YM is approximately 3.99 GPa (±0.6), demonstrating a slight improvement compared to 0.5% MoP. This change is potentially due to improved particle dispersion during solution preparation with IL or interactions between bioactive compounds in MoP, such as proteins, lipids, fatty acids, or antioxidants, which may weaken the cellulose structure.77,78
Interestingly, the fibres of the CeL-MoF (2%) composites show a UTS of approximately 113.42 MPa (±14.09), which is an improvement over the 0.5% and 1% MoP composites, suggesting optimal alignment of MoP particles at this concentration. The result obtained from the breaking strain is about 18.98% (±2.32), which is significantly higher than other composites, indicating improved ductility. The YM of 4.1 GPa (±0.23) is the highest among the composites, but there is approximately a 43.0% reduction in UTS and a 46.5% reduction compared to the CeF control. This improvement can be compared with studies by Coscia et al.79 on the particle dispersion of curcumin actively loaded on regenerated cellulose, thereby improving the mechanical properties of the composites at a certain threshold. Furthermore, CeL-MoF (3%) with a UTS of approximately 87.97 MPa (±4.66), a decrease compared to 2% MoP, can be attributed to particle agglomeration, causing structural defects. This decrease in tensile strength aligns with the SEM and AFM analyses, which reveal increased surface roughness at higher MoP concentrations. The breaking strain at this concentration was 8.86% (±1.84), which is the lowest among the composites, indicating increased brittleness, with a YM of 1.46 GPa (±0.55), which was drastically reduced, highlighting the adverse effects of agglomeration at higher MoP concentrations.
Conclusively, the key finding from the result of the mechanical test of our composites was that the mechanical properties of the composites were lower than those of our CeF owing to varied concentrations of MoP. Our study finally shows that among the composites, CeL-MoF at 2% MoP demonstrated the best mechanical performance with a breaking strain of 18.98%, which was significantly reduced to 8.86%, which is a higher concentration of MoP of 3%. This progressive decrease could be attributed to the earlier stated weakness in the interface bonding caused by the agglomeration of the polysaccharide matrix associated with the loading of MoP particles reported by Orisawayi et al.10 Studies on alginate polysaccharide matrix loaded with MoP at 8% could also be related to agglomeration disrupting the cellulose structure, causing more brittleness at this concentration, possibly associated with easier crack propagation within the structures of previously reported polysaccharide composites.80,81
3.7 SEM-energy dispersive X-ray spectroscopy (EDX) of CeF and CeL-MoF samples
The analyses of the CeF and CeL-MoF samples were performed. The key elements used are the same and agree with our previous studies.10 The spectra from this analysis are presented in Fig. 8, showing the presence of common elements, such as carbon and oxygen, associated with CeL and MoP, respectively.82,83Additional elements such as aluminum, calcium, sulphur, and silicon are characteristic of MoP, as reported in previous studies.10 The immersed fibre spectrum of the CeL-MoF presented an additional peak related to that of MoP content, consistent with the increasing concentration of MoP in the composite fibres. This was observed in the pure samples. This is corroborated by the observations made in previous studies on the use of EDX analysis on M. oleifera seed derivatives, as reported by Acheampong et al.,64 Benettayeb et al.,84 Mateus et al.,85 Belbali et al.,86 and Okoya et al.87 Compared with our FTIR findings, this provides visual and elemental evidence of the successful incorporation of MoP into the cellulose matrix.
The adsorption of heavy metals was also validated using EDX as a qualitative measure, where copper (Cu2+) has shown the most affinity for adsorption through the CeL-MoF composite fibres. Nickel (Ni2+) and cadmium (Cd2+) are not visible on such spectra, indicating that they were present in trace amounts and could not be detected by the sensors. Similar studies conducted by Acheampong et al.,64 on adsorption of Cu(II) using M. oleifera seed powder, inferred that there is an involvement of ion exchange mechanisms in the biosorption process for the removal of copper by M. oleifera seeds by the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group. However, Ni2+ and Cd2+ ion absorption mechanisms could have been hampered by a competitive sorption environment due to the multi-metal presence in the test solution. In addition, the literature suggests88–90 that the sorption affinity of the metal ions depends on the atomic weight, electronegativity, electrode potential, and ionic size. Matouq et al.90 demonstrated, for raw M. oleifera seed powder, that Cu2+ has a high affinity for sorption in a solution with Ni2+, Cr3+, Cu2+, and Zn2+. This aligns with our results, explaining why other heavy metals, such as Ni2+ and Cd2+, were not visible in the spectra. Ni2+ and Cd2+ were not visible in the spectra. This preliminary analysis highlights the capacity of the developed composites to capture heavy metals. The EDX mapping illustrated in Fig. 9 presents the CeL-MoF (2%). The mapping shows that the metal ions after immersion are uniformly distributed and homogeneous within the CeL-MoF matrix, which is consistent with previous studies,10 indicating similar effective and uniform adsorption related to studies conducted by Orisawayi et al., 2024. The deduction from these studies shows that composites can capture heavier metals compared to CeF. Thus, CeL-MoF composites have significant potential for removing heavy metals from water.
O group. However, Ni2+ and Cd2+ ion absorption mechanisms could have been hampered by a competitive sorption environment due to the multi-metal presence in the test solution. In addition, the literature suggests88–90 that the sorption affinity of the metal ions depends on the atomic weight, electronegativity, electrode potential, and ionic size. Matouq et al.90 demonstrated, for raw M. oleifera seed powder, that Cu2+ has a high affinity for sorption in a solution with Ni2+, Cr3+, Cu2+, and Zn2+. This aligns with our results, explaining why other heavy metals, such as Ni2+ and Cd2+, were not visible in the spectra. Ni2+ and Cd2+ were not visible in the spectra. This preliminary analysis highlights the capacity of the developed composites to capture heavy metals. The EDX mapping illustrated in Fig. 9 presents the CeL-MoF (2%). The mapping shows that the metal ions after immersion are uniformly distributed and homogeneous within the CeL-MoF matrix, which is consistent with previous studies,10 indicating similar effective and uniform adsorption related to studies conducted by Orisawayi et al., 2024. The deduction from these studies shows that composites can capture heavier metals compared to CeF. Thus, CeL-MoF composites have significant potential for removing heavy metals from water.
4. Conclusion
This study demonstrates the novel fabrication of CeL-MoF composite potential materials for the selective adsorption of heavy metal ions from an aqueous solution. The M. oleifera seed, identified as a natural biosorbent, affects the mechanical properties of cellulose in the developed composites owing to the incorporation of MoP and affects adsorption performance. FTIR and SEM-EDX analyses confirm the successful integration of MoP into the cellulose matrix. CeL-MoF (2%) exhibits improved mechanical properties and enhanced adsorption efficiency compared to pure CeF. The results from the rheology confirm that the solution is suitable for spinning, and the TGA–DTG results confirm variations in the properties of the developed CeL-MoF through the demonstration of variations in the thermal properties. Notably, the preliminary study confirmed that the composites have more affinity towards the adsorption of copper (Cu2+). The MoP concentration exhibited an optimal balance between the mechanical properties and adsorption performance, highlighting the unique alignment of MoP particles within the matrix. The use of ionic liquids in wet spinning provides a scalable, environmentally friendly fabrication method for dissolving cellulose, which can offer an alternative method to help in fabrication compared to conventional membrane technologies. This work addresses critical gaps in heavy metal adsorption by presenting a sustainable, cost-effective solution that aligns with green chemistry principles. Future work will focus on optimisation of the CeL-MoF composites by exploring more characterisation and evaluating properties such pore size distribution using the BJH (Barrett–Joyner–Halenda) method, DFT (Density Functional Theory), viscoelasticity, fibre-matrix interactions and stimulating the composite to determine properties such as adsorption capacity, sorption kinetics, and thermodynamics using models such as Langmuir, Freundlich, Temkin, and pseudo-second-order, to explore its selectivity, considering additional metals such as Fe, As, and other mutagenic agents from the wastewater. Furthermore, we will consider the scalability of our Cel-MoF fibres for practical water purification applications, ensuring that our research contributes to both scientific knowledge and real-world solutions.Data availability
The data and supporting documents are available from the corresponding author upon reasonable request.Author contributions
Abimbola Oluwatayo Orisawayi: formal analysis, conceptualization, investigation, methodology, funding acquisition, project administration, investigation, methodology, software, visualization, writing the original draft, and writing – review & editing. Prithivi Boylla: software, visualization, writing the original draft, and writing – review & editing. Krzysztof Koziol and Sameer S. Rahatekar: supervision, visualization, writing – review & editing, supervision, and resources.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors acknowledge the Petroleum Technology Development Fund (PTDF) of Nigeria for funding through a PhD Overseas scholarship programme (No. PTDF/ED/OSS/PHD/AOO/1844/2020PHD152). Special thanks to Jim Hurley and Shaun Flippance for their technical and motivational support during the experiments. The authors also acknowledge Diane Johnson, Kris Bramley, and Christine Kimpton for their expertise and training in SEM, EDS, and AFM analyses. Gratitude is extended to Dr David Ayre for his guidance on TGA and Professor Argyrios Zolotas for their internal review; Eva Peláez-Álvarez for rheology and FTIR training. Finally, sincere thanks to the staff of the Composites and Advanced Materials Centre, Cranfield University, for their technical support.References
- A. J. Sayyed, D. V. Pinjari, S. H. Sonawane, B. A. Bhanvase, J. Sheikh and M. Sillanpää, J. Environ. Chem. Eng., 2021, 9, 1–17 CrossRef.
- M. Musa, Y. Gao, P. Rahman, A. Albattat, M. A. S. Ali and S. K. Saha, Clean Technol. Environ. Policy, 2024, 26, 1799–1823 CrossRef.
- G. Venkatraman, N. Giribabu, P. S. Mohan, B. Muttiah, V. K. Govindarajan, M. Alagiri, P. S. Abdul Rahman and S. A. Karsani, Chemosphere, 2024, 351, 1–13 CrossRef PubMed.
- Y. Dinakarkumar, G. Ramakrishnan, K. R. Gujjula, V. Vasu, P. Balamurugan and G. Murali, Environ. Chem. Ecotoxicol., 2024, 6, 293–302 CrossRef CAS.
- P. Zhang, M. Yang, J. Lan, Y. Huang, J. Zhang, S. Huang, Y. Yang and J. Ru, Toxics, 2023, 11, 1–20 Search PubMed.
- T. Dippong, M.-A. Resz, C. Tănăselia and O. Cadar, J. Hazard. Mater., 2024, 476, 1–21 CrossRef PubMed.
- B. S. Rathi, P. S. Kumar and D.-V. N. Vo, Sci. Total Environ., 2021, 797, 1–22 CrossRef PubMed.
- S. Afroze and T. K. Sen, Water, Air, Soil Pollut., 2018, 229, 1–50 CrossRef CAS.
- F. Zhu, Y.-M. Zheng, B.-G. Zhang and Y.-R. Dai, J. Hazard. Mater., 2021, 401, 1–22 Search PubMed.
- A. O. Orisawayi, K. Koziol and S. S. Rahatekar, Carbohydr. Polym. Technol. Appl., 2024, 1–43 Search PubMed.
- A. Celebioglu, F. Topuz, Z. Irem Yildiz and T. Uyar, ACS Omega, 2019, 4(4), 7850–7860 CrossRef CAS PubMed.
- D. W. Hendricks, Water Treatment Unit Processes, CRC Press, Boca Raton, 2018 Search PubMed.
- M. Ince and O. Kaplan Ince, in Biochemical Toxicology – Heavy Metals and Nanomaterials, ed. M. Ince, O. K. Ince and G. Ondrasek, IntechOpen, 2020, pp. 1–19 Search PubMed.
- L. Li, X. Qian and J. Shen, Carbohydr. Polym., 2022, 293, 1–10 Search PubMed.
- N. A. Fakhre and B. M. Ibrahim, J. Hazard. Mater., 2018, 343, 324–331 CrossRef CAS PubMed.
- X. Pei, L. Gan, Z. Tong, H. Gao, S. Meng, W. Zhang, P. Wang and Y. Chen, J. Hazard. Mater., 2021, 406, 1–11 CrossRef PubMed.
- F. Ji, C. Li, B. Tang, J. Xu, G. Lu and P. Liu, Chem. Eng. J., 2012, 209, 325–333 CrossRef CAS.
- A. N. Doyo, R. Kumar and M. A. Barakat, J. Taiwan Inst. Chem. Eng., 2023, 151, 1–18 Search PubMed.
- J. Sánchez, B. Butter and B. L. Rivas, J. Chil. Chem. Soc., 2020, 65, 5004–5010 CrossRef.
- Z. Zia, A. Hartland and M. R. Mucalo, Int. J. Environ. Sci. Technol., 2020, 17, 4389–4406 CrossRef CAS.
- T. M. Mututuvari and C. D. Tran, J. Hazard. Mater., 2014, 264, 449–459 CrossRef CAS PubMed.
- S. F. Hosseini, Z. Nahvi and M. Zandi, Food Hydrocolloids, 2019, 89, 637–648 CrossRef CAS.
- M. H. Rahaman, M. A. Islam, M. M. Islam, M. A. Rahman and S. M. N. Alam, Curr. Res. Green Sustainable Chem., 2021, 4, 1–8 Search PubMed.
- Y.-L. Wu, S. Xu, T. Wang and C.-F. Wang, ACS Appl. Mater. Interfaces, 2018, 10, 32736–32746 CrossRef CAS PubMed.
- S. Kobayashi, J. Sakamoto and S. Kimura, Prog. Polym. Sci., 2001, 26, 1525–1560 CrossRef CAS.
- I. O. Oladele, T. B. Origbemisoye, A. S. Taiwo, S. A. Oyegunna, S. O. Adelani, O. F. Olanrewaju and A. O. Orisawayi, Adv. Mat. Sustain. Manuf., 2024, 1, 1–12 Search PubMed.
- S. Nagaraja, P. B. Anand, K. Mohan Kumar and M. I. Ammarullah, RSC Adv., 2024, 14, 17594–17611 RSC.
- T. G. Kebede, S. Dube and M. M. Nindi, Mater. Res. Express, 2018, 5, 125015 CrossRef.
- T. A. Aderinola, A. M. Alashi, I. D. Nwachukwu, T. N. Fagbemi, V. N. Enujiugha and R. E. Aluko, Food Hydrocolloids, 2020, 101, 1–10 CrossRef.
- F. O. Adetuyi, E. S. Akintimehin, K. O. Karigidi and A. O. Orisawayi, Int. J. Food Sci., 2025, 2025, 1–12 Search PubMed.
- P. P. Mishra, C. Mohanty, N. Das, M. Mishra, A. K. Mohanty, S. Manna and A. K. Behera, Water, Air, Soil Pollut., 2024, 235, 1–22 CrossRef.
- V. C. Obuseng, M. N. Moshoeshoe, F. M. Nareetsile, H. Kwaambwa and I. Maina, Front. Chem., 2022, 10, 1–14 Search PubMed.
- A. Vázquez-Guerrero, R. Cortés-Martínez, R. Alfaro-Cuevas-Villanueva, E. Rivera-Muñoz and R. Huirache-Acuña, Water, 2021, 13, 1–28 CrossRef.
- A. O. Orisawayi, K. Koziol, S. Hao, S. Tiwari and S. S. Rahatekar, RSC Adv., 2024, 14, 8502–8512 RSC.
- U. Ravikumar and J. Mater, J. Mater. Environ. Sci., 2020, 2020, 123–138 Search PubMed.
- I. Kuznik, I. Kruppke and C. Cherif, Polymers, 2022, 14, 1–11 CrossRef PubMed.
- S. Dubey, P. Bharmoria, P. S. Gehlot, V. Agrawal, A. Kumar and S. Mishra, ACS Sustain. Chem. Eng., 2018, 6, 766–773 CrossRef CAS.
- D. Zhao, H. Li, J. Zhang, L. Fu, M. Liu, J. Fu and P. Ren, Carbohydr. Polym., 2012, 87, 1490–1494 CrossRef CAS.
- Z. Zhao, J. Wang, H. Yuan, J. Xu, H. Gao and Y. Nie, ACS Appl. Mater. Interfaces, 2024, 16, 18063–18074 CrossRef CAS PubMed.
- T. Qiao, C. Yang, L. Zhao, Y. Feng, X. Feng, Z. Mao and B. Wang, Int. J. Biol. Macromol., 2024, 277, 1–11 Search PubMed.
- Z. Zhang, M. Zhu and D. Zhang, Appl. Energy, 2018, 220, 87–93 CrossRef CAS.
- A. Hadou, A. Belaadi, I. M. H. Alshaikh and D. Ghernaout, Case Stud. Therm. Eng., 2024, 64, 1–23 Search PubMed.
- H. Lu, J. A. Butler, N. S. Britten, P. D. Venkatraman and S. S. Rahatekar, Nanomaterials, 2021, 11, 1–16 Search PubMed.
- A. M. Sivalingam and A. Pandian, Carbohydr. Polym. Technol. Appl., 2024, 8, 1–13 Search PubMed.
- N. Sabapathi, S. Ramalingam, K. N. Aruljothi, J. Lee and S. Barathi, Plants, 2023, 12, 707–713 CrossRef CAS PubMed.
- E. E. Jaekel, G. R. Torres, M. Antonietti, O. J. Rojas and S. Filonenko, Sci. Rep., 2024, 14, 1–9 CrossRef PubMed.
- N. Nygren, I. Schlapp-Hackl, S. Heimala, H. Sederholm, M. Rissanen and M. Hummel, Carbohydr. Polym., 2024, 346, 1–11 CrossRef PubMed.
- J. Wei, Y. Long, B. Wang, H. Wu, H. Gao and Y. Nie, Int. J. Biol. Macromol., 2024, 255, 1–10 CrossRef PubMed.
- M. J. Lundahl, M. Berta, M. Ago, M. Stading and O. J. Rojas, Eur. Polym. J., 2018, 109, 367–378 CrossRef CAS.
- M. K. Yoo, M. S. Reza, I. M. Kim and K. J. Kim, Fibers Polym., 2015, 16, 1618–1628 CrossRef CAS.
- Y. Ma, B. Nasri-Nasrabadi, X. You, X. Wang, T. J. Rainey and N. Byrne, J. Nat. Fibers, 2021, 18, 2338–2350 CrossRef CAS.
- J. Zhang, H. Kitayama, Y. Gotoh, A. Potthast and T. Rosenau, Carbohydr. Polym., 2019, 226, 1–8 CrossRef PubMed.
- T. Heinze, in Advances in Polymer Science, 2015, pp. 1–52 Search PubMed.
- W. Shang, Z. Sheng, Y. Shen, B. Ai, L. Zheng, J. Yang and Z. Xu, Carbohydr. Polym., 2016, 141, 135–142 CrossRef CAS PubMed.
- Z. Liu, H. Wang, Z. Li, X. Lu, X. Zhang, S. Zhang and K. Zhou, Mater. Chem. Phys., 2011, 128, 220–227 CrossRef CAS.
- V. Hospodarova, E. Singovszka and N. Stevulova, 09, Am. J. Anal. Chem., 2018, 303–310 CrossRef CAS.
- C. S. T. Araújo, V. N. Alves, H. C. Rezende, I. L. S. Almeida, R. M. N. de Assunção, C. R. T. Tarley, M. G. Segatelli and N. M. M. Coelho, Water Sci. Technol., 2010, 62, 2198–2203 CrossRef PubMed.
- A. Alghamdi, K. P. Rajan and S. P. Thomas, Case Stud. Chem. Environ. Eng., 2024, 9, 100635 CrossRef CAS.
- Y. Zhang, J. Li, X. Huang, C. Yang, C. Wu, Z. Yang and D. Li, Int. J. Biol. Macromol., 2023, 232, 123290 CrossRef CAS PubMed.
- C. Castro-López, C. Espinoza-González, R. Ramos-González, V. D. Boone-Villa, M. A. Aguilar-González, G. C. G. Martínez-Ávila, C. N. Aguilar and J. M. Ventura-Sobrevilla, Food Res. Int., 2021, 144, 110291 CrossRef PubMed.
- I. W. Maina, V. Obuseng and F. Nareetsile, J. Chem., 2016, 2016, 1–13 CrossRef.
- D. H. K. Reddy, K. Seshaiah, A. V. R. Reddy, M. M. Rao and M. C. Wang, J. Hazard. Mater., 2010, 174, 831–838 CrossRef CAS PubMed.
- I. Hegazy, M. E. A. Ali, E. H. Zaghlool and R. Elsheikh, Appl. Water Sci., 2021, 11, 95 CrossRef CAS.
- M. A. Acheampong, J. P. C. Pereira, R. J. W. Meulepas and P. N. L. Lens, J. Chem. Technol. Biotechnol., 2011, 86, 1184–1194 CrossRef CAS.
- M. A. Acheampong, E. D. O. Ansa, M. Y. Woode and E. Awuah, Chem. Eng. Commun., 2015, 202, 946–953 CrossRef CAS.
- A. P. Meneghel, A. C. Gonçalves Jr, L. Strey, F. Rubio, D. Schwantes and J. Casarin, Quim. Nova, 2013, 36, 1104–1110 CrossRef CAS.
- A. P. Meneghel, A. C. Gonçalves, F. Rubio, D. C. Dragunski, C. A. Lindino and L. Strey, Water, Air, Soil Pollut., 2013, 224, 1383 CrossRef.
- M. Muddasar, N. Menéndez, Á. Quero, M. A. Nasiri, A. Cantarero, J. García-Cañadas, C. M. Gómez, M. N. Collins and M. Culebras, Adv. Compos. Hybrid Mater., 2024, 7, 1–14 CrossRef.
- Y. J. Lee, S. J. Lee, S. W. Jeong, H. Kim, T. H. Oh and S. G. Lee, Fibers Polym., 2019, 20, 501–511 CrossRef CAS.
- H. D. Jeong, S. G. Kim, G. M. Choi, M. Park, B.-C. Ku and H. S. Lee, Chem. Eng. J., 2021, 412, 1–11 CrossRef.
- M. G. Coscia, J. Bhardwaj, N. Singh, M. G. Santonicola, R. Richardson, V. K. Thakur and S. Rahatekar, Ind. Crops Prod., 2018, 111, 536–543 CrossRef CAS.
- S.-C. Yang, Y. Liao, K. G. Karthikeyan and X. J. Pan, Environ. Pollut., 2021, 286, 1–10 Search PubMed.
- S. A. Khan, M. A. Ahmed, M. M. Baig, M. M. Rehman, Y. Yang, S. G. Lee, J. W. Choi and W. Y. Kim, Chem. Eng. J., 2024, 485, 1–12 Search PubMed.
- H. Zhang, J. Huang, Y. Wang, R. Liu, X. Huai, J. Jiang and C. Anfuso, Opt. Commun., 2018, 406, 3–17 CrossRef CAS.
- Z. Zhang, Y. Kong, J. Gao, X. Han, Z. Lian, J. Liu, W.-J. Wang and X. Yang, Nanoscale, 2024, 16, 6383–6401 Search PubMed.
- T. Nypelö, S. Asaadi, G. Kneidinger, H. Sixta and J. Konnerth, Cellulose, 2018, 25, 5297–5307 CrossRef PubMed.
- L. O. Ejeta, Y. Zheng and Y. Zhou, Mech. Compos. Mater., 2024, 60, 659–670 CrossRef CAS.
- A. B. Falowo, F. E. Mukumbo, E. M. Idamokoro, J. M. Lorenzo, A. J. Afolayan and V. Muchenje, Food Res. Int., 2018, 106, 317–334 Search PubMed.
- M. G. Coscia, J. Bhardwaj, N. Singh, M. G. Santonicola, R. Richardson, V. K. Thakur and S. Rahatekar, Ind. Crops Prod., 2018, 111, 536–543 CrossRef CAS.
- P. Sampath and S. K. V. Santhanam, Polímeros, 2019, 29, 1–7 CrossRef.
- A. Vázquez-Guerrero, R. Cortés-Martínez, R. Alfaro-Cuevas-Villanueva, E. Rivera-Muñoz and R. Huirache-Acuña, Water, 2021, 13, 1–28 CrossRef.
- F. Guan, J. Tao, Q. Yao, Z. Li, Y. Zhang, S. Feng, J. Sun, Q. Yang, X. Song, J. Guo and Y. Liu, Colloids Surf. A Physicochem. Eng. Asp., 2024, 680, 1–12 CrossRef.
- Z. Ajmal, H. Ali, S. Ullah, A. Kumar, M. Abboud, H. Gul, Y. Al-hadeethi, A. S. Alshammari, N. Almuqati, G. A. Ashraf, N. Hassan, A. Qadeer, A. Hayat, M. Ul Haq, I. Hussain and A. Murtaza, Fuel, 2024, 367, 1–42 CrossRef.
- A. Benettayeb and B. Haddou, Int. J. Environ. Anal. Chem., 2023, 103, 6859–6884 CrossRef CAS.
- G. A. P. Mateus, M. P. Paludo, T. R. T. dos Santos, M. F. Silva, L. Nishi, M. R. Fagundes-Klen, R. G. Gomes and R. Bergamasco, J. Environ. Chem. Eng., 2018, 6, 4084–4092 CrossRef CAS.
- A. Belbali, A. Benghalem, K. Gouttal and S. Taleb, Int. J. Environ. Anal. Chem., 2023, 103, 8689–8705 CrossRef CAS.
- A. A. Okoya, O. O. Olaiya, A. B. Akinyele and N. O. Ochor, J. Chem., 2020, 2020, 1–11 CrossRef.
- Y. Sağ, B. Akçael and T. Kutsal, Sep. Sci. Technol., 2002, 37, 279–309 CrossRef.
- B. Mattuschka and G. Straube, J. Chem. Technol. Biotechnol., 1993, 58, 57–63 CrossRef CAS.
- M. Matouq, N. Jildeh, M. Qtaishat, M. Hindiyeh and M. Q. Al Syouf, J. Environ. Chem. Eng., 2015, 3, 775–784 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra02386f |
| This journal is © The Royal Society of Chemistry 2025 |