Open Access Article
Open Access ArticleAn isophorone-derived AIE-active probe for peroxynitrite detection and bioimaging applications†
Raguraman Lalithaa,
Vishnu Halnor Viveka,
Yueh-Hsun Lub,
Shu Pao Wub and
Sivan Velmathi *a
*a
aOrganic and Polymer Synthesis Laboratory, Department of Chemistry, National Institute of Technology, Tiruchirappalli – 620 015, India. E-mail: velmathis@nitt.edu
bDepartment of Applied Chemistry, National Yang Ming Chiao Tung University, Hsinchu 30010, Republic of China
First published on 11th July 2025
Abstract
Compounds exhibiting aggregation-induced emission (AIE) are remarkable due to their characteristic restriction of intramolecular rotation, making them highly versatile for a wide range of applications. Herein, an isophorone based probe (IHP) was developed for the detection of peroxynitrite (ONOO−) using the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N as the reactive group. Photophysical investigations were conducted to explore the aggregation behaviour of IHP in two different solvent systems: DMSO:PBS and DMSO. In DMSO, only the monomeric form of the dye was observed, showing no unusual behaviour. However, in DMSO
N as the reactive group. Photophysical investigations were conducted to explore the aggregation behaviour of IHP in two different solvent systems: DMSO:PBS and DMSO. In DMSO, only the monomeric form of the dye was observed, showing no unusual behaviour. However, in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PBS (1
PBS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), multiple emitting species were detected, indicating the coexistence of dye aggregates and monomers. The fluorescence spectra exhibited wavelength-dependent changes, with the aggregates displaying a red-shift compared to the monomers, indicating J-aggregation. The AIE properties of IHP resulted in red emission, with a fluorescence response towards ONOO− through oxidative cleavage of the C
1), multiple emitting species were detected, indicating the coexistence of dye aggregates and monomers. The fluorescence spectra exhibited wavelength-dependent changes, with the aggregates displaying a red-shift compared to the monomers, indicating J-aggregation. The AIE properties of IHP resulted in red emission, with a fluorescence response towards ONOO− through oxidative cleavage of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond to an aldehyde accompanied by the shift in emission maximum. Under physiological conditions, the probe exhibited good selectivity and high sensitivity to ONOO−, low detection limit of 1.1 μM and rapid recognition within 200 seconds. Furthermore, the probe was effectively used to image exogenous and endogenous peroxynitrite in living cells.
N bond to an aldehyde accompanied by the shift in emission maximum. Under physiological conditions, the probe exhibited good selectivity and high sensitivity to ONOO−, low detection limit of 1.1 μM and rapid recognition within 200 seconds. Furthermore, the probe was effectively used to image exogenous and endogenous peroxynitrite in living cells.
1. Introduction
Nitric oxide (NO) is converted into intracellular reactive nitrogen species (RNS) by electron transfer reactions.1,2 Peroxynitrite (ONOO−), a RNS produced by the reaction of nitric oxide (NO) and superoxide anion radicals (O2−˙).3 This substance is produced in mitochondria and is important for cell signal transduction, stress response and antibacterial activity.4 Elevated levels of ONOO− can damage vital cellular components, and may contribute to the development of various diseases,5–7 including inflammation, circulatory shock, cancer, diabetes etc.8,9 At a pH of 7.4, ONOO− shows higher reactivity towards a wide range of bioactive substances, including proteins and lipids, exhibiting exceptionally high reactivity levels.8,10–12 Besides its inherent oxidation, nucleophilic, and nitration properties, ONOO− has the capability to transform into more potent secondary free radicals, such as nitro radicals , hydroxyl radicals (OH˙), and carbonate radicals (CO3−˙). These secondary radicals subsequently interact with biomolecules, and ultimately cause cell death.13,14 Therefore, it is crucial to establish an accurate approach for ONOO− detection in living cells.
, hydroxyl radicals (OH˙), and carbonate radicals (CO3−˙). These secondary radicals subsequently interact with biomolecules, and ultimately cause cell death.13,14 Therefore, it is crucial to establish an accurate approach for ONOO− detection in living cells.
The use of small organic fluorescent probes has become an effective method for identifying species that are physiologically active within living systems, providing high temporal resolution.12,15–18 Their affordability, along with their notable attributes such as high selectivity, sensitivity, and easy monitoring, has garnered significant attention from numerous researchers.19 Nevertheless, certain drawbacks are linked to these conventional fluorescent sensors. They are highly fluorescent in the molecular state but weakly fluorescent or non-emissive in the aggregated state, leading to aggregation-caused quenching (ACQ), which adversely affects their sensing capabilities.20,21 Moreover, highly polar solvents exert a considerable impact on the fluorescence intensity and stability of certain fluorescent compounds.22 As a result, the design of fluorescent sensors that exhibit remarkable durability in polar solvents and high fluorescence in an aggregated state has gained growing importance. In dye chemistry, self-association, or aggregation, of dyes in solution or at solid–liquid interfaces is a common phenomenon due to the intermolecular van der Waals attractive forces between dye molecules.23–26
In recent years, fluorogens with a unique feature known as aggregation-induced emission (AIE) have become an emergent category of fluorescent sensors. The AIE fluorogens typically exhibit little to no emission when dissolved at the molecular level but exhibit strong emission when they aggregate into larger molecular clusters, operating through a mechanism of restriction of intramolecular motions (RIMs).19,27–29 Primarily, various molecular interactions, such as steric effects, hydrogen bonding inhibit intramolecular rotations favouring a radiative pathway, leading to strong fluorescence emission. This phenomenon enhances the potential for on-site sensing using a AIE fluorophore. Furthermore, the AIE phenomenon offers numerous advantages to AIE based sensors including reduced background noise, a large Stoke's shift, and enhanced signal amplification which is ideal for biosensing and bioimaging.19,27–29 These appealing photophysical properties have prompted researchers to develop numerous AIE-active fluorescent molecules. These molecules are being utilized in numerous applications, such as optoelectronic devices, biosensors, and the detection of explosives and metal ions.30–34
There are few AIE probes reported in the literature for ONOO−˙,35–43 nevertheless, developing AIE-active fluorescent probes for the efficient detection of ONOO− remains essential due to the significant role this analyte plays in both biological and chemical fields. While numerous fluorescent probes have been reported for the detection of ONOO−,44–48 many of these probes suffer from slow response times, which is not suitable for peroxynitrite detection due to the short lifetime of ONOO− under physiological conditions and operate in the short-wavelength window (<600 nm) (Table S1†), thus leading to low signal-to-background ratio and poor accuracy of imaging limiting their biological applications.
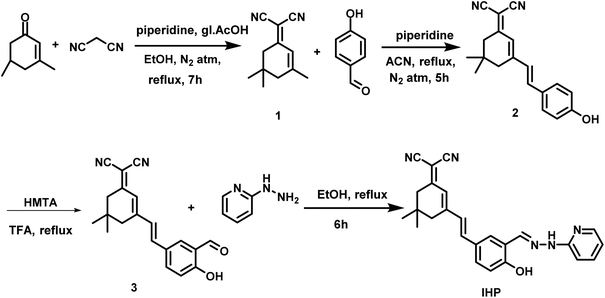
In this work, we developed an isophorone derivative, 2-(3-((E)-4-hydroxy-3-((E)-(2-(pyridin-2-yl)hydrazineylidene)methyl)styryl)-5,5-dimethylcyclohex-2-en-1-ylidene)malononitrile(IHP). The probe has hydrazinyl pyridine moiety which act as electron donor and the dicyanoisophorone moiety as the electron acceptor. This donor–acceptor groups can render the molecule to Intramolecular Charge Transfer (ICT) behaviour. A systematic investigation of IHP's photophysical properties was conducted in different ratios of DMSO (good solvent) and PBS (a bad solvent) to explore its aggregation behaviour and the resulting changes in fluorescence properties. Notably, IHP contains a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N moiety, which serves as a reactive site for oxidative cleavage by ONOO−. The IHP probe exhibits a shift in emission maxima in response to ONOO−, also demonstrated better analytical properties for detecting ONOO−, including rapid response time, high sensitivity, and strong selectivity. Owing to these properties, IHP can function as a sensitive fluorescent sensor for ONOO−, making it valuable for detecting this species in living cells through fluorescence microscopy.
N moiety, which serves as a reactive site for oxidative cleavage by ONOO−. The IHP probe exhibits a shift in emission maxima in response to ONOO−, also demonstrated better analytical properties for detecting ONOO−, including rapid response time, high sensitivity, and strong selectivity. Owing to these properties, IHP can function as a sensitive fluorescent sensor for ONOO−, making it valuable for detecting this species in living cells through fluorescence microscopy.
2. Materials
2.1 Chemicals and instruments
All chemicals were sourced from commercial dealers and used as such. Silica gel with a mesh size of 100–200 was employed for column chromatography. Proton and carbon NMR were recorded on a Bruker AV-500 NMR spectrometer. An Agilent QTOF G6545 spectrometer with a resolution of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 in electrospray ionization (ESI) mode was used to measure HRMS. Absorption spectra were recorded with a Shimadzu UV-2600 spectrophotometer, and emission spectra were obtained using a Shimadzu RF-5301PC fluorescence spectrophotometer. HeLa cells, a human cervical cancer cell line, were obtained from the Culture Collection and Research Center, Food Industry Research and Development Institute (FIRDI) in Hsinchu, Taiwan.
000 in electrospray ionization (ESI) mode was used to measure HRMS. Absorption spectra were recorded with a Shimadzu UV-2600 spectrophotometer, and emission spectra were obtained using a Shimadzu RF-5301PC fluorescence spectrophotometer. HeLa cells, a human cervical cancer cell line, were obtained from the Culture Collection and Research Center, Food Industry Research and Development Institute (FIRDI) in Hsinchu, Taiwan.
2.2 Synthesis of the sensing molecule
The synthesis procedure of compound 1–3 is given in ESI data† and their characteristic data is given in Fig. S1–S6.†1H NMR (500 MHz, DMSO-d6) δ (ppm): 1.02 (s, 6H), 2.55 (s, 2H), 2.60 (s, 2H), 6.78–6.8 (m, 1H), 6.85 (s, 1H), 6.94 (d, J = 8.5 Hz, 1H), 7.14 (d, J = 8. Hz, 1H), 7.27 (d, J = 16 Hz, 1H), 7.30 (d, J = 16 Hz, 1H), 7.59–7.61 (m, 1H), 7.66–7.68 (m, 1H), 7.93 (d, J = 2.05 Hz, 1H), 8.14–8.15 (m, 1H), 8.28 (s, 1H), 10.96 (s, 1H), 11.0 (s, 1H).
13C NMR (125 MHz, DMSO-d6) δ (ppm): 27.4, 31.6, 38.2, 42.3, 75.1, 106.3, 113.2, 114.0, 115.1, 116.6, 121.1, 121.6, 127.0, 127.3, 127.6, 129.0, 137.4, 137.9, 147.8, 156.3, 156.5, 157.4, 170.2 (Fig. S7 and S8†).
HRMS (ESI, m/z): calculated [C25H24N5O]+, 410.1976; found 410.1984 (Fig. S9†).
2.3 Spectral acquisition
1 mM stock solution of IHP in DMSO and 10 mM of analyte solution in water was made. For absorption and emission studies 20 μM of IHP and 100 μM of analytes were added and diluted to 2 mL using DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PBS (10 mM, pH 7.4, 1
PBS (10 mM, pH 7.4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). For consistency, each sensing experiment was carried out three times at room temperature. Excitation wavelength 460 nm was used to record the fluorescence spectra.
1). For consistency, each sensing experiment was carried out three times at room temperature. Excitation wavelength 460 nm was used to record the fluorescence spectra.
2.4 Cytotoxicity assay
Using the conventional MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) assay, the cytotoxicity of IHP towards the experimental cells was assessed. In summary, HeLa cells were cultured for one night in 96-well plates, and then for one day at 37 °C. The cells were exposed to several doses of IHP (0, 5, 10, 15, 20, 25 μM) after which the cells were treated with 50 μL of MTT solution (5 mg mL−1) and incubated at 37 °C for an extra 4 hours. Following the extraction of the leftover MTT, 150 μL of DMSO was introduced into every well to dissolve the formazan crystals formed. The absorbance of each well at 570 nm was measured with a microplate reader. The cells showed 90% cell viability (Fig. S10†) which indicated that IHP is less toxic to the cells and can be used for bioimaging.2.5 Cell imaging
HeLa cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% (v/v) antibiotic solution at 37 °C in incubator containing 5% CO2. For imaging experiments, cells were seeded into 6-well plates and allowed to adhere for 24 hours. The culture medium was then replaced with fresh DMEM, and cells were incubated with the fluorescent probe IHP (10 μM) for 20 minutes. After incubation, the medium was removed, and cells were washed twice with phosphate-buffered saline (PBS) to remove probe.Cells were then treated with peroxynitrite (ONOO−, 100 μM) for 5 minutes. For the ONOO− scavenger group, cells were co-incubated with ONOO− (50 μM) and uric acid (200 μM) for 15 minutes. To stimulate endogenous ONOO− production, lipopolysaccharide (LPS, 1 μg mL−1) and phorbol 12-myristate 13-acetate (PMA, 10 nM) were dissolved in DMEM and applied to the cells for 1 hour.
Following treatment, the medium was removed, and cells were washed twice with PBS. Nuclear staining was performed using 4′,6-diamidino-2-phenylindole (DAPI, 0.02 mg mL−1) for 15 minutes at room temperature, followed by PBS washes to remove excess dye. The cells were fixed with 4% formaldehyde for 15 minutes. The coverslips were then mounted for confocal fluorescence imaging.
3. Results and discussion
3.1 Synthesis and design
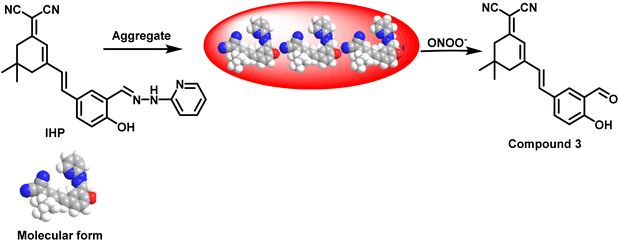
The probe was synthesised by four steps as detailed above in the experimental section and characterised using NMR and HRMS. The NMR characterisation of probe and intermediates and the HRMS data of the probe is given in ESI (Fig. S1 to S9).† The probe IHP was designed for peroxynitrite detection, initially assumed to be non-emissive due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization. Upon oxidative cleavage of the C
N isomerization. Upon oxidative cleavage of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond, it was expected to regenerate compound 3, an Excited State Intramolecular Proton Transfer (ESIPT) emissive molecule.49 However, IHP exhibited AIE behaviour, displaying red emission upon aggregation. Upon reaction with ONOO−, the emission maximum shifted, corresponding to the formation of compound 3.
N bond, it was expected to regenerate compound 3, an Excited State Intramolecular Proton Transfer (ESIPT) emissive molecule.49 However, IHP exhibited AIE behaviour, displaying red emission upon aggregation. Upon reaction with ONOO−, the emission maximum shifted, corresponding to the formation of compound 3.
3.2 Photophysical properties
UV-vis and fluorescence studies were conducted using 10 μM of IHP in different solvents varying from non-polar to polar solvents. The absorbance spectra showed two bands at approximately around 360 nm and 440 nm, and it was almost the same in all solvents, which implies that the absorption profiles are free from the effect of solvent polarity. In the emission spectra, IHP showed solvatochromic behaviour. The molecule IHP has the pyridinyl moiety as the donor group and the dicyanoisophorone moiety as the acceptor group. The lower energy absorption band corresponds to the n to π* transition of the pyridinyl moiety and the high energy absorption band corresponds to ICT transitions from the electron releasing pyridinyl moiety to the electron accepting isophorone moiety and the π to π* transition. There was shift in the emission maximum towards longer wavelength as we moved from non-polar to polar solvents (Fig. S11†). This can be attributed to the ICT effect of the molecule.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerisation of the molecule which makes it non-emissive.
N isomerisation of the molecule which makes it non-emissive.When the PBS buffer ratio was increased to 50% there was a red shift in absorption bands of IHP from 364 nm and 440 nm to 435 nm and 540 nm respectively, and the level off tails observed in the visible region (Fig. S12a†) shows the AIE behaviour of IHP.30 This shift is likely attributed to the aggregation of IHP induced by this solvent mixture. Since aggregation is a process mediated by solvents, the behaviour of molecules during aggregation will be strongly influenced by the inherent hydrophilicity and hydrophobicity of both the solvents and the molecules involved. It's well-known that many organic compounds have the tendency to aggregate in poor solvents, including water.19
In the emission spectra, IHP was non-emissive in DMSO (good solvent), when the PBS (bad solvent) fraction increased to 50%, there was dramatic increase in the emission intensity (at 635 nm) with no shift in emission maxima, this dramatic increase can be attributed to the AIE behaviour of IHP, which was proved by the SEM analysis which shows spherical aggregates in 50% PBS (Fig. 1a). When the PBS fraction was greater than 50%, there was a new peak at 644 nm with reduced intensity which may be due to the formation of aggregates of different morphology31 (Fig. S12b†). The SEM results taken in 90% PBS showed the formation of aggregates of different morphology which had flakelike structure (Fig. 1b). To ascertain that the aggregates contain the IHP molecule rather than components from the PBS buffer, elemental mapping was conducted. The results confirmed the presence of the IHP molecule within the aggregates, as illustrated in Fig. S13.†
 | ||
Fig. 1 (a) SEM image of IHP (10 μM) in DMSO (b) SEM image of IHP (10 μM) in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PBS (1 PBS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, pH 7.4) (c) SEM image of IHP (10 μM) in DMSO 1, v/v, pH 7.4) (c) SEM image of IHP (10 μM) in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PBS (1 PBS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v, pH 7.4). 9, v/v, pH 7.4). | ||
The fluorescence lifetime was measured Fig. S14,† and the lifetime in DMSO (good solvent) was 0.02 ns, because there was minimal aggregation and the molecules are well-dispersed, the fluorescence intensity was low due to the intramolecular rotations leading to the non-radiative decay and hence the fluorescence lifetime is short as the non-radiative pathway dominates.
The fluorescence lifetime in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PBS (1
PBS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), where the AIE effect was prominent was 0.06 ns. The lifetime is longer than in pure DMSO, reflecting the enhanced radiative pathway. The lifetime in 90% PBS is 0.057 ns, where there is slight decrease in the lifetime as compared to the emissive AIE form.
1), where the AIE effect was prominent was 0.06 ns. The lifetime is longer than in pure DMSO, reflecting the enhanced radiative pathway. The lifetime in 90% PBS is 0.057 ns, where there is slight decrease in the lifetime as compared to the emissive AIE form.
When fluorescence quenching is observed upon increasing the PBS fraction beyond 50%, it may result from the formation of large aggregates or agglomerates. These structures can facilitate non-radiative decay pathways, such as π–π stacking interactions or light scattering, leading to a significant decrease in fluorescence lifetime.
Conversely, if the quenching is primarily due to the IFE, where the sample itself absorbs excitation or emission light, the fluorescence lifetime typically remains unchanged, as IFE affects the intensity but not the excited-state deactivation pathways.50–54
Herein, the slight decrease in fluorescence lifetime from 0.06 ns in the DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PBS (1
PBS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture to 0.057 ns in 90% PBS suggests that both aggregation-induced non-radiative decay and IFE contribute to the observed quenching. The minimal change in lifetime indicates that while aggregation introduces some non-radiative decay pathways, IFE plays a significant role in reducing fluorescence intensity without substantially affecting the lifetime.
1) mixture to 0.057 ns in 90% PBS suggests that both aggregation-induced non-radiative decay and IFE contribute to the observed quenching. The minimal change in lifetime indicates that while aggregation introduces some non-radiative decay pathways, IFE plays a significant role in reducing fluorescence intensity without substantially affecting the lifetime.
Therefore, the observed fluorescence quenching at higher PBS concentrations is likely due to a combined effect of agglomerate formation and the inner filter effect.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PBS (10 mM, pH 7.4 1
PBS (10 mM, pH 7.4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is explained by the Kasha's molecular exciton theory.55 This theory put forward that an end-to-end alignment of chromophore transition dipoles, or J-aggregates, is allowed to transition from the ground state to a lower energy exciton state, which causes the bathochromic shift in the UV spectra (Fig. S15a†). In contrast, a face-to-face alignment of dipole moments, or H-aggregation, results in a hypsochromic shift, which signifies a transition to a higher energy exciton state. Thus, the observed shift in absorption of IHP indicates the formation of J-aggregates.56,57 In DMSO solvent, there was no significant fluorescence emission of IHP. However, with increasing PBS fraction up to 50%, a red emission is observed with emission maximum at 635 nm (Fig. S15b†). Restrictions in intramolecular rotation, cause the emission intensity to increase and activate the radiative pathway, with a higher degree of aggregation at 50% PBS. This observation was supported by increase in quantum yield from 0.004 to 0.08 with the increase in PBS fraction from 0 to 50%.
1) is explained by the Kasha's molecular exciton theory.55 This theory put forward that an end-to-end alignment of chromophore transition dipoles, or J-aggregates, is allowed to transition from the ground state to a lower energy exciton state, which causes the bathochromic shift in the UV spectra (Fig. S15a†). In contrast, a face-to-face alignment of dipole moments, or H-aggregation, results in a hypsochromic shift, which signifies a transition to a higher energy exciton state. Thus, the observed shift in absorption of IHP indicates the formation of J-aggregates.56,57 In DMSO solvent, there was no significant fluorescence emission of IHP. However, with increasing PBS fraction up to 50%, a red emission is observed with emission maximum at 635 nm (Fig. S15b†). Restrictions in intramolecular rotation, cause the emission intensity to increase and activate the radiative pathway, with a higher degree of aggregation at 50% PBS. This observation was supported by increase in quantum yield from 0.004 to 0.08 with the increase in PBS fraction from 0 to 50%.In both DMSO and DMSO:PBS solutions, we examined the emission spectra of IHP throughout a range of excitation wavelengths that covered the red and blue margins of the absorption spectra. The emission spectra in DMSO obtained at various excitation wavelengths showed no discernible spectral changes. The emission maximum was at 656 nm even on exciting at various excitation wavelength (Fig. 2a). However, in DMSO:PBS, the difference in the emission spectra was notably low when the monitoring excitations were maintained at the blue edge of the spectrum (Fig. 2b), indicating emissions from monomeric species. Conversely, a bathochromic shift was seen in the emission maxima when the excitation wavelengths were situated at the red edge of the spectrum (Fig. 2c), known as the red-edge effect, which deviates from Kasha's rule. This red shift in emission peak, with a magnitude as large as 39 nm, persisted until a noise-free spectrum could be recorded (Fig. 2d),58 suggesting the presence of multiple emitting species when IHP is dissolved in DMSO:PBS. These multiple species arise from aggregation, with the aggregates predominantly being strongly fluorescent J-aggregates. Since each aggregated species possesses its own fluorescence maxima, a change in the excitation wavelength results in the excitation of a different species and the observation of emission characteristic of that species. A 3D model directly representing the Excitation Emission Matrices (EEM) data in three dimensions, showing excitation wavelength, emission wavelength, and fluorescence intensity represented in Fig. S16† shows strong, red-shifted emissions, narrow peaks and relatively small Stoke's shift also proves the J-type behaviour of the aggregates.
To further validate the aggregation behaviour of IHP and ascertain whether the change in fluorescence was a result of aggregation or the presence of salts in the PBS buffer, emission spectra of IHP were conducted in different ratios of DMSO to H2O. It was found that similar emission pattern was observed as it was in DMSO:PBS. The maximum emission intensity was observed in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Fig. S17†). On conducting the excitation-dependent emission experiments, there was red-edge effect observed even in DMSO:H2O (Fig. 3a) but the magnitude of the red shift was less (29 nm) compared to the red-shift seen in DMSO:PBS (39 nm).
1) (Fig. S17†). On conducting the excitation-dependent emission experiments, there was red-edge effect observed even in DMSO:H2O (Fig. 3a) but the magnitude of the red shift was less (29 nm) compared to the red-shift seen in DMSO:PBS (39 nm).
This led us to contemplate the factors influencing this change, and our focus turned to pH. Consequently, we conducted experiments to record the emission spectra of IHP in DMSO:PBS at various pH levels ranging from 4 to 9 (Fig. 3b). The observations revealed a decrease in the emission of IHP under acidic conditions. This can be attributed to the conversion of the pyridine moiety in IHP to pyridinium ion in acidic pH, rendering it water-soluble.59 Hence, this transformation prevents aggregation and, consequently, diminishes the emission of IHP in acidic environments. As the pH is made alkaline, the emission maximum intensity increases up to pH 10. A dramatic increase in intensity is observed from pH 7 to 8, potentially explaining the lower magnitude of the red-shift emission intensity in water (pH 7) as compared to the PBS buffer (10 mM, pH 7.4). The emission intensity is almost same from pH 8 to pH 10 and based on that we could say that IHP can be useful in physiological conditions. Then IHP was taken in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), where the pH was adjusted to pH 7.4 and the emission spectra was recorded by increasing the excitation wavelength in the red-edge of the spectra and then found that the magnitude of the red-shift (38 nm) was almost equal to the magnitude of the shift in DMSO:PBS (Fig. 3c). All these results proved that IHP forms J-aggregate in DMSO
1), where the pH was adjusted to pH 7.4 and the emission spectra was recorded by increasing the excitation wavelength in the red-edge of the spectra and then found that the magnitude of the red-shift (38 nm) was almost equal to the magnitude of the shift in DMSO:PBS (Fig. 3c). All these results proved that IHP forms J-aggregate in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PBS (10 mM, pH 7.4, 1
PBS (10 mM, pH 7.4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Similar pattern of absorption and emission was observed when IHP was studied in different DMSO
1). Similar pattern of absorption and emission was observed when IHP was studied in different DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratios. This confirms the AIE behaviour of IHP.
H2O ratios. This confirms the AIE behaviour of IHP.
3.3 Sensing studies
With our aim to utilize IHP for ONOO− and considering the alkaline-dependent stability of ONOO−,60 extensive experimentation with various buffer solutions was conducted, ultimately leading to the selection of PBS buffer (10 mM, pH 7.4) for spectral studies. All the sensing studies were done in DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PBS (10 mM, pH 7.4, 1
PBS (10 mM, pH 7.4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) system due to the alkaline stability of ONOO−.
1) system due to the alkaline stability of ONOO−.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PBS buffer in a 1
PBS buffer in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (10 mM, pH = 7.4) in the presence of ONOO− (50 μM). As shown in Fig. S18,† the absorption spectra of IHP showed two distinct bands at 435 nm and 540 nm. Upon the addition of ONOO−, these absorption bands shifted to 460 nm, which was accompanied by a noticeable colour change as shown in the insets of Fig. S18a,† enabling the colorimetric sensing of ONOO−.
1 ratio (10 mM, pH = 7.4) in the presence of ONOO− (50 μM). As shown in Fig. S18,† the absorption spectra of IHP showed two distinct bands at 435 nm and 540 nm. Upon the addition of ONOO−, these absorption bands shifted to 460 nm, which was accompanied by a noticeable colour change as shown in the insets of Fig. S18a,† enabling the colorimetric sensing of ONOO−.Initially, IHP when excited at 460 nm displayed a strong fluorescence emission at 635 nm. However, the addition of ONOO− resulted in the reduction of fluorescence intensity of IHP and a blue shift in the emission maximum to 562 nm with reduced intensity. This change in behaviour was also marked by the change in fluorescence emission from red to orange as depicted in Fig. S18b.† The large Stoke's shift (∼100 nm) observed can prevent it from autofluorescence and thus helps in bioimaging applications.
A plot between concentration of ONOO− against fluorescence intensity at I562/I651 (Fig. 4b) exhibited a linear relationship with linear coefficient (R2 = 0.97). The detection limit was calculated using 3σ/m, where σ is the standard deviation of the blank, obtained by running the blank sample ten times, and m is the slope of the plot obtained. The LOD was found to be 1.1 μM. The above experimental data showed that the probe IHP can detect ONOO− even in its low concentration.
As illustrated in the fluorescence selectivity graph of IHP with various analytes (Fig. S19a†), no changes in the emission of IHP was seen, even on the addition of 100 μM of these similar analytes, except for ONOO− (50 μM). These spectral studies confirm the high selectivity of IHP for ONOO− ions in a DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PBS (1
PBS (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solution.
1) solution.
To further assess the resistance of IHP to interference, the emission at 635 nm was measured in the presence of other active species at higher concentrations along with ONOO− (Fig. S19b†). From the experimental results, it was observed that only a small portion of these substances interfered with the probe's response to ONOO−. Several factors may explain this outcome. Firstly, the probe's selectivity plays a crucial role in minimizing interference, as it is specifically designed to interact with the unique chemical structure of ONOO−. The probe may also exhibit kinetic preference, reacting more rapidly with ONOO− than with other similar analytes, allowing it to produce a detectable signal for ONOO− while remaining unaffected by other substances. Additionally, the chemical properties of ONOO− contribute to the observed specificity. As a short-lived and highly reactive species, ONOO− can interact with the probe before less reactive analytes can do so. Furthermore, the pH dependence of ONOO− stability is a key factor. Since ONOO− is stable only within a specific pH range, probes and assays are often optimized for this condition, reducing interference from other analytes that may be less stable or reactive in the same environment. These factors collectively explain why only a limited number of substances interfered with the probe's response to ONOO−, featuring its potential suitability for detecting ONOO− in complex environments. This robust selectivity and interference resistance highlight IHP's effectiveness as a fluorescent sensor for peroxynitrite in physiological conditions.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization of the hydrazone moiety, is expected to be weakly or non-fluorescent. However, based on its AIE characteristics, IHP exhibits strong red emission. Upon the addition of ONOO−, an oxidative cleavage of the C
N isomerization of the hydrazone moiety, is expected to be weakly or non-fluorescent. However, based on its AIE characteristics, IHP exhibits strong red emission. Upon the addition of ONOO−, an oxidative cleavage of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond occurs, leading to a deprotection reaction that produces compound 3 (Scheme 2). Compound 3 demonstrates ESIPT behaviour and is emissive. The observed shift in emission maxima is attributed to the formation of de-aggregated species and compound 3. Compared to IHP, the emission maximum of compound 3 is blue shifted, validating the transition from red to orange emission. The blue-shifted peak appears less intense, potentially due to insufficient excitation energy at the chosen wavelength or because compound 3 is inherently less emissive compared to the aggregates of IHP under the conditions used for sensing studies.
N bond occurs, leading to a deprotection reaction that produces compound 3 (Scheme 2). Compound 3 demonstrates ESIPT behaviour and is emissive. The observed shift in emission maxima is attributed to the formation of de-aggregated species and compound 3. Compared to IHP, the emission maximum of compound 3 is blue shifted, validating the transition from red to orange emission. The blue-shifted peak appears less intense, potentially due to insufficient excitation energy at the chosen wavelength or because compound 3 is inherently less emissive compared to the aggregates of IHP under the conditions used for sensing studies.
To verify the above mechanism, the reaction between IHP and ONOO− was monitored using 1H NMR. As seen in Fig. S20,† IHP had a sharp singlet at δ = 8.34 ppm, which correspond to the –CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– group. However, on addition of ONOO−, the sharp singlet at δ = 8.34 ppm disappeared and two peaks at δ 10.25 ppm and 11.02 ppm appears which correspond to the –CHO and –OH proton in compound 3 respectively. The mechanism was further confirmed by HRMS in which the mass observed 319.1441 matched with the calculated mass of compound 3 (Fig. S21†).
N– group. However, on addition of ONOO−, the sharp singlet at δ = 8.34 ppm disappeared and two peaks at δ 10.25 ppm and 11.02 ppm appears which correspond to the –CHO and –OH proton in compound 3 respectively. The mechanism was further confirmed by HRMS in which the mass observed 319.1441 matched with the calculated mass of compound 3 (Fig. S21†).
3.3.6.1 Theoretical calculations. To investigate the sensing mechanism and solvent behaviour of IHP, theoretical calculations were conducted using Density Functional Theory (DFT) with the B3LYP/6-31G basis set. Time-Dependent DFT (TDDFT) analysis revealed that in IHP, the electron cloud in the Highest Occupied Molecular Orbital (HOMO) is distributed across the molecule, with a significant concentration on the hydrazinyl pyridine moiety. In contrast, the Lowest Unoccupied Molecular Orbital (LUMO) shows electron density predominantly localized on the electron-deficient dicyanoisophorone moiety (Fig. 5).
Upon the addition of ONOO−, IHP is converted to compound 3, wherein the electron densities in both HOMO and LUMO are uniformly distributed throughout the molecule which supports the ICT behaviour. The HOMO–LUMO energy gap of IHP is smaller compared to compound 3, aligning with the observed blue shift in the absorption and emission spectra of the probe and its complex with ONOO−.
4. Conclusion
A donor–acceptor-based fluorescent probe was designed by integrating 2-hydrazinyl pyridine with a dicyanoisophorone fluorophore to specifically detect peroxynitrite (ONOO−). Leveraging the AIE characteristics, the probe exhibited excitation-dependent fluorescence changes, highlighting the presence of multiple emission species. A red shift in emission further indicated the formation of J-aggregates. The synthesized probe demonstrated several advantageous properties for ONOO− detection, including a pronounced fluorescence response, high selectivity, and strong anti-interference capability against structurally similar analytes. These results were attributed to the incorporation of a strategically designed hydrazone functional group. Additionally, the probe was effective across the physiological pH range and exhibited low cytotoxicity, enabling the visualization of both exogenous and endogenous ONOO− in living cells. A fluorescent probe exploiting the AIE behaviour was developed was developed that offer robust and precise detection of peroxynitrite (ONOO−) in both biological and environmental systems.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Raguraman Lalitha: conceptualization, methodology, investigation, writing – original draft, review and editing. Vivek Vishnu Halnor: investigation, conceptualization, methodology. Yueh-Hsun Lu: investigation, conceptualization, methodology. Shu Pao Wu: visualisation, investigation, resources. Sivan Velmathi: resources, supervision, writing – review and editing, validation.Conflicts of interest
The authors state that “there are no conflicts to declare”.Acknowledgements
The author (R. Lalitha, File Number 09/895(0015)/2020-EMR-I) is grateful to the Council of Scientific and Industrial Research – HRDG for the financial support.References
- J. Sun, X. Cao, W. Lu, Y. Wei, L. Kong, W. Chen, X. Shao and Y. Wang, Theranostics, 2023, 13, 1716–1744 CrossRef PubMed.
- Z. Chen, S. Zhang, X. Li and H. wang Ai, Cell Chem. Biol., 2021, 28, 1542–1553 CrossRef CAS PubMed.
- C. Liu, X. Zhang, Z. Li, Y. Chen, Z. Zhuang, P. Jia, H. Zhu, Y. Yu, B. Zhu and W. Sheng, J. Agric. Food Chem., 2019, 67, 6407–6413 CrossRef CAS PubMed.
- Q. An, S. Su, L. Chai, Y. Wang, X. Wang, X. Li, T. Liang, W. Hu, X. Song and C. Li, Talanta, 2023, 253, 124073 CrossRef CAS.
- X. Sun, K. Lacina, E. C. Ramsamy, S. E. Flower, J. S. Fossey, X. Qian, E. V. Anslyn, S. D. Bull and T. D. James, Chem. Sci., 2015, 6, 2963–2967 RSC.
- Y. Fu, H. Nie, R. Zhang, F. Xin, Y. Tian, J. Jing and X. Zhang, RSC Adv., 2018, 8, 1826–1832 RSC.
- J. Lu, Z. Li, Q. Gao, J. Tan, Z. Sun, L. Chen and J. You, Anal. Chem., 2021, 93, 3426–3435 CrossRef CAS PubMed.
- M. Zhu, H. Zhou, D. Ji, G. Li, F. Wang, D. Song, B. Deng, C. Li and R. Qiao, Dyes Pigments, 2019, 168, 77–83 CrossRef CAS.
- Y. Zhang and D. Ma, Spectrochim. Acta, Part A, 2021, 244, 1–5 Search PubMed.
- Z. Wang, W. Wang, P. Wang, X. Song, Z. Mao and Z. Liu, Anal. Chem., 2021, 93, 3035–3041 CrossRef CAS PubMed.
- Z. Li, J. Lu, Q. Pang and J. You, Analyst, 2021, 146, 5204–5211 RSC.
- C. Sun, W. Du, P. Wang, Y. Wu, B. Wang, J. Wang and W. Xie, Biochem. Biophys. Res. Commun., 2017, 494, 518–525 CrossRef CAS PubMed.
- J. Zhang, Y. Li, J. Zhao and W. Guo, Sens. Actuators, B, 2016, 237, 67–74 CrossRef CAS.
- Z. Liu, S. Mo, Z. Hao and L. Hu, Int. J. Mol. Sci., 2023, 24(16), 12821 CrossRef CAS PubMed.
- S. Xu, K. C. Yan, Z. H. Xu, Y. Wang and T. D. James, Chem. Soc. Rev., 2024, 53, 7590–7631 RSC.
- M. Zhou, Y. Lin, T. Bai, T. Ye, Y. Zeng, L. Li, Z. Qian, L. Guo, H. Liu and J. Wang, Sens. Actuators, B, 2024, 414, 135994 CrossRef CAS.
- Y. Shen, X. Zhang, Y. Zhang, H. Li and Y. Chen, Sens. Actuators, B, 2018, 258, 544–549 CrossRef CAS.
- Y. Shen, X. Zhang, Y. Zhang, Y. Wu, C. Zhang, Y. Chen, J. Jin and H. Li, Sens. Actuators, B, 2018, 255, 42–48 CrossRef CAS.
- Khadija, H. Irshad, S. Rafique, A. M. Khan, S. Nawazish, H. ur Rehman, M. Imran, S. A. Shahzad and U. Farooq, Spectrochim. Acta, Part A, 2023, 290, 122273 CrossRef CAS PubMed.
- R. X. Zhang, P. F. Li, W. J. Zhang, N. Li and N. Zhao, J. Mater. Chem. C, 2016, 4, 10479–10485 RSC.
- G. J. Mao, G. Q. Gao, W. P. Dong, Q. Q. Wang, Y. Y. Wang, Y. Li, L. Su and G. Zhang, Talanta, 2021, 221, 121607 CrossRef CAS PubMed.
- S. Kim, C. W. Ko, T. Lim, S. Yoo, H. J. Ham, S. Y. Kang, S. Kang, S. K. Cho and M. S. Han, Dyes Pigments, 2019, 171, 107762 CrossRef CAS.
- R. F. Khairutdinov and N. Serpone, J. Phys. Chem. B, 1997, 101, 2602–2610 CrossRef CAS.
- J. L. Bricks, Y. L. Slominskii, I. D. Panas and A. P. Demchenko, Methods Appl. Fluoresc., 2017, 6, 012001 CrossRef PubMed.
- P. Mahato, S. Saha, S. Choudhury and A. Das, Chem. Commun., 2011, 47, 11074–11076 RSC.
- F. Xu, T. T. Testoff, L. Wang and X. Zhou, Molecules, 2020, 25, 4478 CrossRef CAS PubMed.
- J. Heo, D. P. Murale, H. Y. Yoon, V. Arun, S. Choi, E. Kim, J. S. Lee and S. Kim, Aggregate, 2022, 3, 1–35 CrossRef.
- S. Hussain, H. Muhammad Junaid, M. Tahir Waseem, W. Rauf, A. Jabbar Shaikh and S. Anjum Shahzad, Spectrochim. Acta, Part A, 2022, 272, 121021 CrossRef CAS PubMed.
- K. Mukherjee, T. I. Chio, H. Gu, A. Banerjee, A. M. Sorrentino, D. L. Sackett and S. L. Bane, ACS Sens., 2017, 2, 128–134 CrossRef CAS PubMed.
- P. Das, A. Kumar, A. Chowdhury and P. S. Mukherjee, ACS Omega, 2018, 3, 13757–13771 CrossRef CAS PubMed.
- Y. Zhou, H. Xu, Q. X. Li, Z. R. Hou, Y. W. Wang and Y. Peng, Org. Biomol. Chem., 2023, 21, 1270–1274 RSC.
- M. Zhang, J. Li, L. Yu, X. Wang and M. Bai, RSC Adv., 2020, 10, 14520–14524 RSC.
- Q. Hu, Q. Huang, K. Liang, Y. Wang, Y. Mao, Q. Yin and H. Wang, Dyes Pigments, 2020, 176, 108229 CrossRef CAS.
- H. Sun, X. X. Tang, R. Zhang, W. H. Sun, B. X. Miao, Y. Zhao and Z. H. Ni, Dyes Pigments, 2020, 174, 108051 CrossRef CAS.
- G. Jiang, C. Li, Q. Lai, X. Liu, Q. Chen, P. Zhang, J. Wang and B. Z. Tang, Sens. Actuators, B, 2021, 329, 129223 CrossRef CAS.
- J. Li, J. Tang, X. Yang, P. Xie, J. Liu, D. Zhang and Y. Ye, Sens. Actuators, B, 2022, 358, 131513 CrossRef CAS.
- Z. Wang, J. Gong, P. Wang, J. Xiong, F. Zhang and Z. Mao, Talanta, 2023, 252, 123811 CrossRef CAS PubMed.
- X. Han, X. Yang, Y. Zhang, Z. Li, W. Cao, D. Zhang and Y. Ye, Sens. Actuators, B, 2020, 321, 128510 CrossRef CAS.
- H. Xie, J. Zhang, C. Chen, F. Sun, H. Liu, X. He, K. W. K. Lam, Z. Li, J. W. Y. Lam, G. Q. Zhang, D. Ding, R. T. K. Kwok and B. Z. Tang, Mater. Chem. Front., 2021, 5, 1830–1835 RSC.
- B. Guo, W. Shu, W. Liu, H. Wang, S. Xing, J. Chen and X. Zhang, Sens. Actuators, B, 2021, 344, 130206 CrossRef CAS.
- M. Li, H. Han, S. Song, S. Shuang and C. Dong, Spectrochim. Acta, Part A, 2021, 261, 120044 CrossRef CAS PubMed.
- Y. Shen, M. Li, M. Yang, Y. Zhang, H. Li and X. Zhang, Spectrochim. Acta, Part A, 2019, 222, 117230 CrossRef CAS PubMed.
- Y. Dong, Y. Yang, Y. Tao, M. Fang, C. Li and W. Zhu, J. Fluoresc., 2024 DOI:10.1007/s10895-024-03961-w.
- X. F. Hou, Y. L. Xue, J. G. Yang, Z. S. Li, Z. H. Xu, W. Li and L. Yuan, Anal. Chem., 2024, 96(44), 17657–17664 CrossRef CAS PubMed.
- X. Hou, Y. Xue, C. Liu, Z. Li and Z. Xu, Spectrochim. Acta, Part A, 2024, 320, 124665 CrossRef CAS PubMed.
- J. Wu, Y. Lin, Y. Yu, Y. Li, T. Ye, H. Zhou, L. Li and J. Wang, Dyes Pigments, 2022, 206, 110597 CrossRef CAS.
- L. Zhong, H. Niu, Y. Lin, T. Ye, S. Zheng, K. Chen, L. Li, L. Guo and J. Wang, Microchem. J., 2024, 206, 111540 CrossRef CAS.
- Y. Shen, X. Zhang, Y. Zhang, H. Li, L. Dai, X. Peng, Z. Peng and Y. Xie, Anal. Chim. Acta, 2018, 1014, 71–76 CrossRef CAS PubMed.
- F. Huo, Y. Zhang, Y. Yue, J. Chao, Y. Zhang and C. Yin, Dyes Pigments, 2017, 143, 270–275 CrossRef CAS.
- J. Harathi and K. Thenmozhi, Mater. Chem. Front., 2020, 4, 1471–1482 RSC.
- A. Jose, A. Tharayil and M. Porel, Polym. Chem., 2023, 14, 3309–3316 RSC.
- Y. Meng, X. Yao, K. Zhong, Y. Li and L. Tang, Eur. J. Org. Chem., 2023, 26(18), e202300022 CrossRef CAS.
- C. Xia and Y. Qian, New J. Chem., 2016, 40, 144–150 RSC.
- T. T. Trang, T. T. H. Pham, N. Van Dang, P. T. Nga, M. Van Linh and X. H. Vu, RSC Adv., 2024, 14, 9538–9546 RSC.
- Y. Kong, R. Wu, X. Wang, G. Qin, F. Wu, C. Wang, M. Chen, N. Wang, Q. Wang and D. Cao, RSC Adv., 2022, 12, 27933–27939 RSC.
- P. Verma and H. Pal, J. Phys. Chem. A, 2014, 118, 6950–6964 CrossRef CAS PubMed.
- N. H. Mudliar, P. M. Dongre and P. K. Singh, Sens. Actuators, B, 2019, 301, 127089 CrossRef CAS.
- A. Samanta, J. Phys. Chem. B, 2006, 110, 13704–13716 CrossRef CAS PubMed.
- V. D. Singh, R. S. Singh, R. P. Paitandi, B. K. Dwivedi, B. Maiti and D. S. Pandey, J. Phys. Chem. C, 2018, 122, 5178–5187 CrossRef CAS.
- T. Hou, K. Zhang, X. Kang, X. Guo, L. Du, X. Chen, L. Yu, J. Yue, H. Ge, Y. Liu, A. M. Asiri, K. A. Alamry, H. Yu and S. Wang, Talanta, 2019, 196, 345–351 CrossRef CAS PubMed.
- L. Yuan, W. Lin, S. Zhao, W. Gao, B. Chen, L. He and S. Zhu, J. Am. Chem. Soc., 2012, 134, 13510–13523 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra02185e |
| This journal is © The Royal Society of Chemistry 2025 |