Open Access Article
Open Access ArticleSynthesis and structural analysis of a ZIF-9/GO composite for green catalytic C–C bond formation and N-formylation: a spectroscopic study†
Lalita Kumari ,
Rupali Mittal
,
Rupali Mittal ,
Mehak Khurana and
Satish Kumar Awasthi
,
Mehak Khurana and
Satish Kumar Awasthi *
*
Chemical Biology Laboratory, Department of Chemistry, University of Delhi, Delhi-110007, India. E-mail: satishpna@gmail.com
First published on 9th June 2025
Abstract
We report a new synthetic approach for a ZIF-9/GO composite material at room temperature and its comprehensive characterization using a variety of techniques, including PXRD, FT-IR, Raman, UV-vis, DRS, TGA, SEM, FE-SEM, EDX, TEM, ICP-MS, XPS, and BET. The material exhibited a moderate surface area and good thermal stability, as indicated by BET and TGA results. DRS revealed a band gap of 3.4 eV, which indicates slight semiconductor nature of the material. The crystalline nature of the ZIF-9/GO composite was confirmed by PXRD. The ZIF-9/GO composite exhibited excellent catalytic efficiency as a heterogeneous catalyst in Knoevenagel condensation and N-formylation, achieving yields of up to 98% under green reaction conditions with a wide range of substrate tolerance. The E-factor (0.10) and atom economy (91.7%) values were very close to ideal green chemistry parameters. In situ and UV-vis studies revealed reaction intermediates. The practical utility of this protocol was demonstrated by the gram scale synthesis of the value-added compounds 2-imino-2H-1-benzopyran-3-carbonitrile and paracetamol. Single-crystal XRD analysis of three of the compounds confirmed their structural integrity. The catalyst was easily separated via filtration and reused for up to six catalytic cycles without significant loss of activity, which is a crucial component of green synthesis, and hot-filtration experiments confirmed its heterogeneity. The probable mechanisms for both reactions are also well presented.
Introduction
Over the years, heterogeneous catalysis coalesced with sustainable approaches has become as a vital methodology for producing a wide range of chemicals, playing a crucial role in promoting environmentally friendly chemical processes. Compared with homogeneous catalysts, heterogeneous catalysts offer advantages such as ease of product separation, efficient catalyst recycling, and reduced chemical waste generation.1,2 Furthermore, to address concerns pertaining to the recovery and recyclability of catalytic systems, it is possible to immobilize them on a solid insoluble support. This approach offers several advantages, including improved stability, selectivity, and catalyst activity.3Metal–organic frameworks (MOFs) are crystalline porous materials composed via self-assembly of metal atoms and organic ligands through coordination bonds possessing properties such as a large surface area, controllable pore structures, multiple functionalities, high chemical and thermal resistance, and organic-inorganic hybrid nature4–6 and have been used for many applications such as heterogeneous catalysis,7,8 energy storage,9,10 sensors,11,12 drug delivery13 and gas separation.14 One subtype of MOFs is zeolitic imidazolate frameworks (ZIFs), which possess a permanent porous structure and exhibit remarkable stability under thermal and chemical conditions. These properties, such as their high specific area and chemically versatile frameworks, make them highly suitable for use in catalytic applications. Therefore, ZIFs have been widely used for this purpose.15 Among various ZIFs, ZIF-9 possesses high chemical and thermal stability and includes a network of corner-sharing tetrahedral CoN4, where the most stable N-donor ligand is present between the Co(II) bond and the benzimidazolate anion.16,17
Materials composed of graphene oxide (GO) have demonstrated intriguing physical, optical, and mechanical characteristics. Additionally, they have been successfully used as support materials for organic transformations owing to their substantial specific surface area, high surface-to-volume ratio, thermal stability, and chemical stability.18–21 GO is a single-layered substance, an oxidized derivative of graphene, and because of its intriguing characteristics, including hydrophilicity, high biocompatibility, and cost-effectiveness, graphene oxide is a desirable candidate as a support material.22
The combination of ZIF-9 and GO creates a unique class of composites with improved functionality and a large surface area in addition to the inherent properties of both the starting materials. The surface oxygenated groups and defects on GO sheets provide nucleation sites for Co2+ ions for the growth of the ZIF-9 framework through coordination bonds.23 These oxygenated groups and defects stabilizing and anchoring actions on GO may be able to stop ZIF-9 from aggregating and leaking.24 The use of MOFs or ZIFs with various organic ligands and metals as heterogeneous catalysts for various organic transformations is one of the most diverse fields of research, and from a view of sustainability has attracted considerable attention.25,26
The Knoevenagel condensation reaction is the widely used reaction for producing α, β-unsaturated compounds, substituted alkenes and carbon–carbon bonds,25,27 which are valuable precursors for the synthesis of fine chemicals in the pharmaceuticals industry28,29 such as nitrendipine, entacapone, lumefantrine and other pharmacological products of calcium channel blockers and antihypertensive, anticancer, antitumor, antimalarial, and antiviral drugs (Fig. 1).30,31 Knoevenagel condensation can be catalyzed by organic bases,32 MOFs,33,34 zeolites,35 solid-supported materials,36,37 alkali metal hydroxide and metal oxides,38–41 and MOFs.34,42 Interestingly, ZIFs, one of the most promising heterogeneous catalysts, are used in the Knoevenagel condensation reaction.
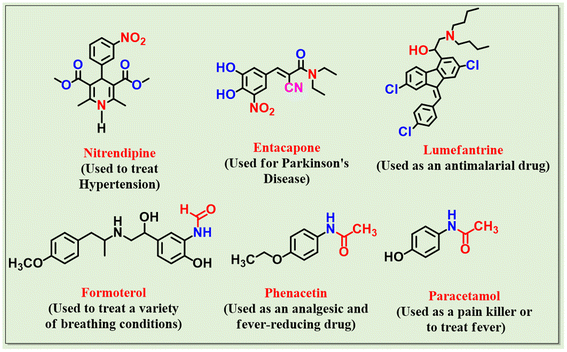 | ||
| Fig. 1 Knoevenagel condensation-derived (A–C) and amide bond containing (D–F) commercially available therapeutic drugs. | ||
In addition, the N-formylation of amines is an important reaction. In addition to being useful reagents in Vilsmeier formylation,43 formamides are important intermediates in the synthesis of pharmaceutically valuable compounds like oxazolidinones,44 and cancer chemotherapeutic agents.45 Additionally, formamides have been widely used as Lewis base catalysts in reactions like the synthesis of acid chlorides, hydrosilyation, allylation of carbonyl derivatives,46,47 isocyanides,48 and as important amino-protecting groups in peptide synthesis.49 Chloral,50 formic acid-DCC,51 formic acid esters,52 (chlorodimethoxytriazine) CDMT,53 ammonium formate,54 formic acid in sodium formate, and in polyethylene glycol55,56 are few reported examples of formylating agents.57 Formic acid (HCOOH), one of many formylating agents, has recently attracted much attention due to its affordability and availability from renewable biomass-derived sources. One of the most recent developments in N-formylation reactions is the catalytic reaction using HCOOH and several different catalysts, such as acidic catalysts (ZnCl2, AlCl3, NiCl2, etc.), transition metal/metal oxide catalysts (ZnO, NiO, etc.), and inorganic solid oxides (alumina, silica gel, zeolite), oaryloxide-N-heterocyclic carbene ruthenium(II) ([Ru–NHC] complex), molybdate sulfuric acid, sulfated tungstate catalyst, indium nano–cerium oxide, K3PO4, heteropoly anion-based ionic liquids (HPAILS), and many others.58–60 However, these approaches possess several drawbacks, including being expensive and toxic, susceptible to moisture, thermal instability of the reagent/catalyst involved, lack of generality, long reaction time and the need for solvent, anhydrous conditions and elevated temperature.61,62 According to statistical calculations, amide bonds are present in almost one-fourth of all pharmaceutical medications, including paracetamol, formoterol and phenacetin (Fig. 1).63
Thus, in this study, we report ZIF-9/GO as an effective heterogeneous catalyst with high reusability for low-temperature liquid-phase Knoevenagel condensation reaction using malononitrile or ethyl cyanoacetate as the active methylene group and N-formylation of amine using formic acid for the synthesis of benzylidene malononitrile or ethyl-3-cyanoacrylate and formamide derivatives, respectively. The catalytic system works well under greener reaction conditions and demonstrates high substrate applicability, scalability, easy recoverability, and great recyclability.
Experimental section
Catalyst synthesis
Results and discussion
In this study, a ZIF-9/GO composite material was synthesized by integrating ZIF-9 and GO materials to achieve their inherent properties as a single material. After successful synthesis, the ZIF-9/GO composite material was subjected to several sophisticated characterizations to gain insight into its structure, composition, and morphology. An illustration of the synthesis procedure of the ZIF-9/GO composite material is provided in Scheme 1.Evaluation of crystallinity, surface and structural properties, morphology, thermal stability and composition of ZIF-9/GO material
Fig. 2a shows the FT-IR spectra of ZIF-9, GO and ZIF-9/GO. The FT-IR spectrum of ZIF-9 shows peaks at 3020 and 2965 cm−1 belonging to aromatic C–H stretching vibrations. The peaks at 1738, 1608 and 1465, 1365, 1235,1277 and 1118 and 745 cm−1 correspond to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching, C
N stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching, C–N stretching, C–C stretching, C–H bending, and C
C stretching, C–N stretching, C–C stretching, C–H bending, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C–H bending, respectively, in the benzimidazole ring, while the peak at 464 cm−1 could be correspond to Co–N stretching.16,66–68 For GO, four characteristic peaks at 3300, 1720, 1615 and 1035 cm−1 correspond to the stretching modes of O–H, C
C/C–H bending, respectively, in the benzimidazole ring, while the peak at 464 cm−1 could be correspond to Co–N stretching.16,66–68 For GO, four characteristic peaks at 3300, 1720, 1615 and 1035 cm−1 correspond to the stretching modes of O–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (carbonyl or carboxylic acid group), C
O (carbonyl or carboxylic acid group), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (aromatic structure) and C–O (epoxy groups), respectively.18,22 Some of the GO peaks vanished/reduced in intensity because they were converted to reduced GO during the process. In the FT-IR spectra of ZIF-9/GO, there was a slight shift in some peaks for ZIF-9/GO after the integration of both materials. These attributed peaks of ZIF-9 and GO were noted in the FT-IR spectrum of the composite material ZIF-9/GO, demonstrating that the interaction of the benzimidazole ligand with the cobalt(II) was unaffected by GO immobilization.
C (aromatic structure) and C–O (epoxy groups), respectively.18,22 Some of the GO peaks vanished/reduced in intensity because they were converted to reduced GO during the process. In the FT-IR spectra of ZIF-9/GO, there was a slight shift in some peaks for ZIF-9/GO after the integration of both materials. These attributed peaks of ZIF-9 and GO were noted in the FT-IR spectrum of the composite material ZIF-9/GO, demonstrating that the interaction of the benzimidazole ligand with the cobalt(II) was unaffected by GO immobilization.
Raman spectroscopy is the most significant characterization tool for carbon materials. To understand the crystalline structure and the aberrations, the Raman spectra of GO and ZIF-9/GO were obtained, as shown in Fig. 2b. In the Raman spectrum of GO, two characteristic peaks of carbon materials corresponding to the D band (vibration of sp3 carbon atoms of disorder in graphitic hexagonal lattice) and G band (vibration of sp2 carbon atoms in graphitic hexagonal lattice) at 1356 and 1599 cm−1, respectively, were observed.18,22,69 For the ZIF-9/GO composite material, the peaks were observed at 1357 and 1601 cm−1. In composite materials comprising carbon components, the disorder in the structure can be predicted by determining the intensity ratio of the D band and G band (ID/IG). In the case of GO and ZIF-9/GO, the ID/IG ratios were 0.82 and 0.91, respectively. The higher ID/IG ratio value in the case of ZIF-9/GO in comparison to pristine GO is related to the increase in the disorder of the GO structure after the immobilization of ZIF-9 MOF on its surface.15,18,22
Brunauer–Emmett–Teller (BET) surface analysis was performed to obtain insights into the textural and surface characteristics of GO, ZIF-9 and ZIF-9/GO via nitrogen adsorption–desorption experiment and presented in Fig. 2c. The BET surface analysis of GO and ZIF-9 revealed surface areas of 14.31 and 18.11 m2 g−1 (Fig. S4†). The pore radius of GO and ZIF-9 were 15.32 and 17.06 Å respectively. The pore volumes of GO and ZIF-9 were 0.032 and 0.019 cc/g, respectively (Fig. S4†). The nitrogen adsorption–desorption isotherm of the ZIF-9/GO composite material displays a type IV isotherm with a hysteresis loop. ZIF-9/GO was found to have a BET surface area of 29.18 m2 g−1. Using the Barrett–Joyner–Halenda (BJH) method, the pore size and pore volume were 8.04 nm and 0.0507 cm3 g−1, respectively. The hysteresis loop in nitrogen adsorption–desorption isotherm and the pore size indicate the mesoporous nature of the ZIF-9/GO composite material.
The crystallinity and phase purity of the synthesized materials were studied using their powder XRD patterns, which are presented in Fig. 2d. The XRD patterns of the ZIF-9 and GO samples were in good agreement with the previous literature. The sharp peaks around 2θ = 9.1°, 16.51°, 17.40°, 19.87°, 21.55°, 23.03°, 27.47°, 31.51°, 33.39°, 35.27°, and 37.73° represent the crystalline nature of the ZIF-9 material.70 For GO, a broad diffraction peak at 2θ = 11.2° (d = 0.79 nm) indicated the presence of (001) plane of the hexagonal system in addition to a minor peak at 2θ = 42.5° (d = 0.21 nm) related to (100) plane related to the hexagonal structure of carbon. The XRD diffraction pattern of ZIF-9/GO composite material displays peaks corresponding to both ZIF-9 and GO, indicating the successful immobilization of ZIF-9 onto the surface of GO.
To evaluate the morphological characteristics of the synthesized samples, SEM and TEM techniques were used. The obtained SEM images for ZIF-9, GO and ZIF-9/GO establish the formation of rhombic dodecahedron-shaped particles, characteristic sheet-like structures, and sheets embedded with rhombic dodecahedron particles, respectively (Fig. 3a–c). Fig. S1† shows the SEM-EDX spectra of ZIF-9/GO, which indicates that elements C, O, N, and Co were found in the sample. The TEM images in Fig. 3d and e show the rhombic dodecahedron and sheet-type morphology of ZIF-9 and GO, respectively. The TEM image of ZIF-9/GO shows the successful growth of ZIF-9 rhombic dodecahedron particles on the sheet-like structure of graphene oxide (Fig. 3f).
 | ||
| Fig. 3 SEM images of (a) ZIF-9, (b) GO, and (c) ZIF-9/GO, and TEM images of (d) ZIF-9, (e) GO, and (f) ZIF-9/GO. | ||
Furthermore, to understand the spatial distribution of elements in ZIF-9/GO, FESEM-EDS elemental mapping was performed, as shown in Fig. S3a–S3f†. The images show the uniform dispersion of C, N, O, and Co elements in the ZIF-9/GO composite material.
The thermogravimetric analysis results of ZIF-9, GO and ZIF-9/GO are shown in Fig. S2a.† The ZIF-9 thermogram shows two weight loss stages. The initial minor weight loss of 5% from 100–50 °C was due to the elimination of solvents such as DMF and H2O present in the Co-ZIF-9 framework. The second sharp weight loss of ∼20% in the range of 560–680 °C was observed along with a weight loss of a total of 46% until 900 °C, indicating the decomposition of the zeolitic imidazolate framework.17,66,70,71 For GO, a cumulative weight loss of 58% was observed across the three phases. First, at approximately 110 °C, a weight loss of 12% occurred because of the evaporation of adsorbed water molecules. Then, in the temperature range of 160–220 °C, a rapid weight loss of 22% occurred due to the disintegration of groups containing oxygen. Finally, in the temperature range of 220–900 °C, the carbon skeleton of GO undergoes pyrolysis, resulting in a weight loss of 28%.18 The TGA curve of ZIF-9/GO shows a first weight loss step of 10% up to150 °C because of moisture loss, a second weight loss of 12% up to 212 °C due to the loss of organic solvent, a third weight loss of 16% from 215–400 °C due to degradation of organic moieties, and finally the fourth weight loss of almost 42%, in the temperature range 400–700 °C is observed due to the pyrolysis of the carbon backbone of GO. At last, 20% of the initial weight of ZIF-9/GO remained, indicating the high thermal stability of the material across a broad temperature range. In addition, the cobalt metal content calculated by Inductively Coupled Plasma Mass Spectroscopy (ICP-MS) was approximately 16.52%, which was similar to the TGA result.
X-ray photoelectron spectroscopy (XPS) measurements provide information about the elemental composition, chemical environment of the elements, and oxidation states of the metals contained in the ZIF-9/GO sample. The survey spectrum of ZIF-9/GO obtained using XPS is shown in Fig. S2b,† which confirms the existence of O, C, N, and Co elements in the sample, as seen in the elemental mapping and EDS characterizations. The high-resolution spectrum of the O 1s state can be deconvoluted into two bands at 530 eV and 531 eV, as shown in Fig. 4a. The peak at 530 eV is caused by the existence of O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O or C–OH groups remaining in the sample, while the peak at 531 eV is indicative of the C–O–C of the epoxy group or the C
O or C–OH groups remaining in the sample, while the peak at 531 eV is indicative of the C–O–C of the epoxy group or the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of the carboxyl group in the GO sheet.72 Three peaks can be distinguished in the high-resolution C 1s XPS spectra (Fig. 4b). The peak at 283.4 eV corresponds to the occurrence of adventitious hydrocarbons (C–C/C
O bond of the carboxyl group in the GO sheet.72 Three peaks can be distinguished in the high-resolution C 1s XPS spectra (Fig. 4b). The peak at 283.4 eV corresponds to the occurrence of adventitious hydrocarbons (C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) on the sample's surface. The presence of C–N
C) on the sample's surface. The presence of C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in ZIF-9 and O–C
C bonds in ZIF-9 and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds from the COOH group of GO, respectively, can be ascribed to the peaks at 285.3 and 286.9 eV. However, in contrast to those of GO, other functional groups (C–C/C–H, C–O, and O–C–O) disappear, which is explained by the chemical interaction between GO and ZIF-9.27The deconvoluted N 1s spectrum shows two bands at 397.4 and 399 eV, which is explained by the existence of C–NH/C
O bonds from the COOH group of GO, respectively, can be ascribed to the peaks at 285.3 and 286.9 eV. However, in contrast to those of GO, other functional groups (C–C/C–H, C–O, and O–C–O) disappear, which is explained by the chemical interaction between GO and ZIF-9.27The deconvoluted N 1s spectrum shows two bands at 397.4 and 399 eV, which is explained by the existence of C–NH/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds and Co–N bonds, respectively (Fig. 4c). The Co2p XPS spectrum shown in Fig. 4d can be deconvoluted into four peaks. The peaks at 780.50 eV and 795.54 eV can be assigned to the spin–orbit splitting doublets Co2p3/2 and Co2p1/2 in their Co2+ state, respectively, while the peaks at 784.7 eV and 801 eV represent the corresponding satellite that arises from the Co2+ ion as well.3,73,74
N bonds and Co–N bonds, respectively (Fig. 4c). The Co2p XPS spectrum shown in Fig. 4d can be deconvoluted into four peaks. The peaks at 780.50 eV and 795.54 eV can be assigned to the spin–orbit splitting doublets Co2p3/2 and Co2p1/2 in their Co2+ state, respectively, while the peaks at 784.7 eV and 801 eV represent the corresponding satellite that arises from the Co2+ ion as well.3,73,74
 | ||
| Fig. 4 High-resolution deconvoluted XPS spectra of O 1s (a), C 1s (b), N 1s (c) and Co2p (d). (e) Diffuse reflectance UV-vis spectra and (f) corresponding Tauc plot of ZIF-9/GO. | ||
Using the UV-vis absorption spectra, the coordination of ZIF-9 and GO was examined. The UV-vis absorption spectra of GO, ZIF-9, and ZIF-9/GO dispersed in ethanol are shown in Fig. S5.† The GO absorption peaks at 233 or 243 nm and the shoulder peak at approximately 288 nm can be attributed to the π → π transition of the aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and n → π transition of the C
C bond and n → π transition of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, respectively. These peak placements are in good agreement with the findings of numerous other investigations.22,75 The pristine ZIF-9 absorption spectrum includes a broad absorption band in a range of 400–500 nm and three absorption bands at 243, 272 and 278 nm, corresponding to π → π* transition,76 while the UV-vis absorption spectra of ZIF-9/GO composites exhibit a little shift in the absorption bands at approximately 332 nm relative to the pristine ZIF-9 absorption spectrum. This shift in the absorption band may be caused by the charge or energy transfer interaction between the polyaromatic scaffold in GO and ZIF-9, suggesting the possibility of a coordination connection between the two.24
O bond, respectively. These peak placements are in good agreement with the findings of numerous other investigations.22,75 The pristine ZIF-9 absorption spectrum includes a broad absorption band in a range of 400–500 nm and three absorption bands at 243, 272 and 278 nm, corresponding to π → π* transition,76 while the UV-vis absorption spectra of ZIF-9/GO composites exhibit a little shift in the absorption bands at approximately 332 nm relative to the pristine ZIF-9 absorption spectrum. This shift in the absorption band may be caused by the charge or energy transfer interaction between the polyaromatic scaffold in GO and ZIF-9, suggesting the possibility of a coordination connection between the two.24
The UV-vis diffuse reflectance spectra (DRS), as shown in Fig. 4e, were examined for the coordination geometry and band gap estimation in the ZIF-9/GO composite material. As shown in Fig. 4e, the detection of the absorption band in the range of 480–620 nm with two maxima at 435 and 585 nm can be attributed to tetrahedrally coordinated Co2+ ions.17,73 The absorption band at 255 nm with a shoulder peak at 305 nm is due to π–π* and n–π* transitions in graphene oxide. The band gap was calculated using Tauc's relation (Fig. 4f) by plotting the graph between (αhν) and energy (hν)n, where, α is the absorption coefficient, h is Plank's constant, n is the number for direct or indirect allowed transition, and hν is the energy of the photon, found to be 3.42 eV for direct allowed transition, which shows slight semiconductor nature of the material.77 The increased band gap energy may be due to the presence of insulating graphene oxide in the material.
Catalytic activity studies of ZIF-9/GO in the Knoevenagel condensation reaction
To demonstrate the merits of the ZIF-9/GO composite as a catalytic material in organic synthesis, we applied it to the Knoevenagel condensation reaction of diversified aldehyde substrates with malononitrile and ethyl cyanoacetate. Initial optimization studies were conducted by reacting 4-nitrobenzaldehyde 1c and malononitrile 2 as model substrates. First, the reaction was performed in polar solvents such as ethanol and water without any catalyst, which, after 6 and 12 hours of reaction, resulted in poor yield (30–50%) of the desired product, respectively (Table 1, entries 1 and 2). The catalytic activity of ZIF-9/GO (5 mg) was examined in the absence of a solvent and in the presence of a variety of solvents such as toluene, water, and ethanol at various temperatures (Table 1, entries 3–7). From this optimization, the highest yield of 4-nitrobenzylidine malononitrile 3c was obtained using ethanol as solvent at room temperature (Table 1, entry 7). Furthermore, the amount of ZIF-9/GO (5–20 mg) was optimized in ethanol solvent at room temperature (Table 1, entries 8–10). There was no increment in the product yield upon increasing the amount of catalyst. It was determined that using ZIF-9/GO (10 mg) as a catalyst in ethanol (2 mL) produced the optimal reaction conditions at room temperature (Table 1, entry 8). Under the optimized conditions, the product4-nitrobenzylidene malononitrile was isolated in 98% yield after 10 min of reaction. The gram scale synthesis of 2-imino-2H-1-benzopyran-3-carbonitrile 7 was also carried out with excellent yield, as shown in Scheme S1.†| Entry | Catalyst amount (mg) | Solvent | Time (h) | Temp. (°C) | Yield b(%) |
|---|---|---|---|---|---|
| a Reaction conditions: aromatic aldehyde (1 mmol), malononitrile (2 mmol), solvent (2 mL), and catalyst (mg).b Isolated yields. | |||||
| 1 | No catalyst | Ethanol | 6 | 60 | 50 |
| 2 | No catalyst | Water | 6 | 80 | 30 |
| 3 | 5 | Solvent-free | 4 | 80 | 70 |
| 4 | 5 | Toluene | 4 | 80 | 10 |
| 5 | 5 | Water | 4 | 80 | 50 |
| 6 | 5 | Ethanol | 2 | 60 | 80 |
| 7 | 5 | Ethanol | 2 | RT | 80 |
| 8 | 10 | Ethanol | 0.10 | RT | 98 |
| 9 | 15 | Ethanol | 0.10 | RT | 98 |
| 10 | 20 | Ethanol | 0.10 | RT | 96 |
Substrate scope of the ZIF-9/GO-catalyzed Knoevenagel condensation reaction using benzaldehyde and malononitrile
The substrate scope of the ZIF-9/GO catalysed the Knoevenagel condensation reaction was assessed following the optimisation of reaction conditions. The substrate scope findings are summarised in Table 2. The benzylidene malononitrile derivatives were produced in good to excellent yields (85–98%).High yields of condensation products were obtained from aromatic aldehydes with electron-withdrawing groups at the ortho/para position than from those substituted with electron-donating groups. The substrate scope was extended to aldehydes with heterocyclic rings, which also produced the condensation product in excellent yields (Table 2, entries 3b, 3q and 3t). Among all heterocycles, the pyridine-substituted aldehyde produced the highest yield (Table 2, entry 3t). The gram scale synthesis of compound 2-imino-2H-1-benzopyran-3-carbonitrile was performed, and the obtained yield was 94% (Table 2, entry 7), as shown in the ESI.† All products were characterized by 1H NMR and 13C NMR (Fig. S9–S30 and S40†).
Substrate scope of the Knoevenagel condensation reaction using benzaldehydes and ethyl cyanoacetate
To extend the scope of the Knoevenagel condensation reaction, the reaction of aldehydes with ethyl cyanoacetate acting as an active methylene compound was also performed under similar circumstances for the reaction (Table 3). However, the reaction of ethyl cyanoacetate 4 with 4-nitrobenzaldehyde 1c required an elevated temperature (60 °C). The reaction proceeded smoothly, giving an excellent yield of the Knoevenagel condensation product (E)-ethyl 2-cyano-3-(4-nitrophenyl) acrylate 5c in a slightly longer period than the benzylidene malononitrile derivatives (Table 2).Table 3 summarizes the substrate scope results of the Knoevenagel condensation reaction between aldehydes and ethyl cyanoacetate derivatives, displaying good to excellent yields (85–96%). Higher yields of condensation products were obtained when aromatic aldehydes were substituted at the ortho/para position with groups that donated electrons compared to those that withdrew electrons. Aldehydes with heterocyclic rings also produced condensation products in excellent yields (Table 3, entries 5a and 5j). The high yield of (E)-ethyl 2-cyano-3-(pyridin-4-yl) acrylate (Table 3, entry 5j) is due to the high reactivity of pyridine towards electrophilic substitution reaction. All products were characterized by 1H NMR and 13C NMR (Fig. S31–S40†).
Green chemistry parameters
The parameters of green chemistry for the conversion of 4-nitrobenzaldehyde 1c and 4-fluorobenzaldehyde 1d to (4-nitrobenzylidine) malononitrile 3c and (E)-ethyl 2-cyano-3-(4- fluorophenyl) acrylate 5d were also calculated (Table S1 and S2†). The results of the calculations shown as radar maps in Fig. S6a and S6b† show how closely the values computed using this methodology align with the optimal values of the parameters related to green chemistry. Our green chemistry metrics values show better results than the previously reported ones as shown in Table 4.Single-crystal X-ray analysis of compound 3q and 5i
The molecular structure of compounds 2-(thiophen-2-ylmethylene) malononitrile 3q and (E)-ethyl 2-cyano-3-(4-cyanophenyl) acrylate 5i was confirmed by single-crystal X-ray analysis, which is one of the most thorough methods for determining the chemical structure of compounds. X-ray-grade crystals were formed through the slow solution evaporation growth method in ethanol. The crystal structures (CCDC No. 2236687 and 2233222, respectively) of compounds 3q and 5i are shown in Table S3.† They crystallized in a monoclinic cell (P21/c space group; Z value = 4) and a triclinic cell (P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group; Z value = 2). The ESI† contains additional single-crystal X-ray crystallographic data for compounds 3q and 5i (Table S3†).
space group; Z value = 2). The ESI† contains additional single-crystal X-ray crystallographic data for compounds 3q and 5i (Table S3†).
Probable reaction pathway for the Knoevenagel condensation reaction
The possible mechanism for the Knoevenagel condensation reaction between benzaldehyde and the active methylene compound (malononitrile) is illustrated in Fig. 5a. First, the aldehyde group's carbonyl oxygen makes an electrostatic bond with the exposed Co(II) site (Lewis's acid) of the ZIF-9/GO catalyst, turning the carbonyl carbon atom into the electrophilic carbon center (Intermediate I). To produce a carbanion intermediate (II), an uncoordinated benzimidazole (Lewis's base) site adsorbed on ZIF-9/GO acts as a substrate to encourage the deprotonation of methylene. The formed carbanions attack the electrophilic carbonyl carbon. After protonation and dehydration, the addition intermediate (III) can be rapidly transformed into benzylidene malononitrile following the release of the used catalyst.5,29,39,80The UV-visible spectra for the reaction progress from 4-nitrobenzaldehyde (4-NBA) to 4-nitrobenzylidine malononitrile using malononitrile (MN) in the presence of ZIF-9/GO are shown in Fig. 5b. The absorbance peak at 268 nm for 4-nitrobenzaldehyde disappeared and a new absorbance peak at 305 nm for 4-nitrobenzylidine malononitrile appeared, which confirmed the formation of the pure desired product. The absorbance peaks at 272 and 280 nm for the intermediate can be attributed to the malononitrile present in the reaction mixture. All 4-nitrobenzaldehyde was converted into 4-nitrobenzylidine malononitrile within 10 min.
In situ IR study of Knoevenagel condensation
3D in situ time-dependent IR spectra of the Knoevenagel condensation reaction were collected to investigate the reaction progress using 4-bromo benzaldehyde and malononitrile as substrates. As shown in Fig. 6, the bands observed in a frequency range of 1550–1750 cm−1 were found for the parent benzaldehyde group (Fig. 6a). | ||
| Fig. 6 3D spectra of in situ time-dependent infra-red for the Knoevenagel condensation reaction. Zoomed in situ IR spectra of the absorption bands of the (a) reactant and (b) product. | ||
Meanwhile, the bands observed after some time in the frequency range of 1350–1550 cm−1 were found to be for alkene and nitrile groups present in the product, and the intensity of these bands increased linearly and reached a maximum in 10 min (Fig. 6b). A strong band at 1720 cm−1 and a medium band at 1596 cm−1 were assigned to the stretching frequency of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of the aromatic group, respectively, and a weak band at 1390 cm−1 assigned to C–H bending of aldehyde, which diminished completely within 10 min, respectively.81 The other bands at 1520 and 1410 cm−1 were assigned to νC
C of the aromatic group, respectively, and a weak band at 1390 cm−1 assigned to C–H bending of aldehyde, which diminished completely within 10 min, respectively.81 The other bands at 1520 and 1410 cm−1 were assigned to νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of the aromatic ring and νC
C of the aromatic ring and νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of the alkene. The medium absorption bands at 1150 and 1080 cm−1 were attributed to the C–O bond present in the intermediate, which disappeared after reaction completion.
C of the alkene. The medium absorption bands at 1150 and 1080 cm−1 were attributed to the C–O bond present in the intermediate, which disappeared after reaction completion.
Catalytic activity of ZIF-9/GO towards N-formylation and of amines
The versatility of the catalytic behavior of ZIF-9/GO was examined for the N-formylation of amines with formic acid (9) under various reaction conditions (Table 5). Aniline 8r (1 mmol) and formic acid (3.0 mmol) were used as model substrates for the optimization of reaction parameters, including catalyst quantity, solvent effect, and temperature. Thin-layer chromatography (TLC) was used to carefully monitor the reaction completion.| Entry | Catalyst amount (mg) | Solvent | Time (h) | Temp. (°C) | Yield b(%) |
|---|---|---|---|---|---|
| a Reaction conditions: aniline (1 mmol), formic acid (3 mmol), solvent (2 mL), and catalyst (mg).b Isolated yields. | |||||
| 1 | No catalyst | Ethanol | 120 | 60 | N.R. |
| 2 | No catalyst | Water | 150 | 80 | N.R. |
| 3 | 5 | Ethanol | 90 | 60 | 50 |
| 4 | 5 | Toluene | 120 | 80 | 10 |
| 5 | 5 | Water | 120 | 80 | 30 |
| 6 | 5 | SF | 60 | 60 | 60 |
| 7 | 5 | SF | 45 | RT | 70 |
| 8 | 10 | SF | 5 | RT | 98 |
| 9 | 15 | SF | 5 | RT | 98 |
| 10 | 20 | SF | 10 | RT | 98 |
We began our research by examining the role of the synthesized ZIF-9/GO catalyst in the synthesis of a substituted formamide to optimize the reaction conditions. Without the aid of a catalyst, 1 mmol of aniline 8r and 3 mmol of formic acid 9 were used as substrates to achieve the above reaction. There was no product formed when the reaction was conducted without a catalyst, demonstrating the need for a catalyst to produce the required formamide product. The selected reaction of aniline and formic acid was then conducted by varying the amount of synthesized ZIF-9/GO catalyst to examine the impact of catalyst quantity on the reaction. When the catalyst loading was increased from 5 mg to 20 mg, a striking increase in the yield with a decrease in time for the synthesis of the formamide 10r product was observed, which can be explained by the increase in the number of catalytically active sites. When the amount of catalyst was further increased, no discernible drop in the reaction time or increment in the yield of the desired product was noticed. To achieve the highest conversion rate and for subsequent trials, 10 mg of catalyst was fixed.
Furthermore, the formation of the product was significantly influenced by the solvent used. By modifying the polarity, solubility, and dispersion of the catalyst, solvents have the power to change the reaction environment. Hence, to examine the effect of solvent in formamide synthesis, we optimized the reaction with different types of solvents, including water, ethanol, and toluene, as well as solvent-free conditions. No such increase in the product's % yield was noticed. However, the above experiments show that a polar solvent such as ethanol somehow increases the yield of the desired product compared to a non-polar solvent such as toluene. The highest yield of acetanilide was obtained under solvent-free conditions. Despite this, the reaction proceeded smoothly when water was used as the solvent, but a longer time was required to complete the reaction.
Substrate scope of the synthesis of formamide derivatives using ZIF-9/GO as a catalyst
The synthesized ZIF-9/GO catalyst was employed to produce different substituted formamide derivatives by condensation between different aliphatic, aromatic, and heterocyclic amines substituted with electron-withdrawing or electron-donating groups and formic acid under the optimized reaction conditions. Aniline 8a–8w (1.0 mmol), formic acid 9 (3.0 mmol), and catalyst (10 mg) were placed in an RB and allowed to react for a predetermined amount of time at room temperature without the presence of any solvent. From various substituted aromatic amines and formic acids, a wide variety of useful substituted formamide derivatives can be produced in good to excellent yields. The electronic environment of the substituted amines affects the yield of the desired product and the time taken for the completion of the reaction. Aromatic amines containing ortho- or para-substituted electron-donating groups, such as CH3 and OCH3, produced better product yields in slightly less time as compared to meta-substituted amines and amines with electron-withdrawing groups. The formylation reaction of secondary, aliphatic or heterocyclic amines required slightly more time than aromatic primary amines (Table 6, entries 10l, 10m, and 10t) under optimized conditions to produce a good yield of the desired product. Aliphatic and alicyclic amines (Table 6, entry 10u and 10v) like morpholine and piperidine were also investigated for the formylation reaction and proved to be effective substrates producing the corresponding products in good to excellent yields. The chemoselectivity of the reaction was also investigated in the formylation of amines in bifunctional compounds (Table 6, entries 10n and 10t). The gram-scale synthesis of compound 10w (Paracetamol) was carried out with excellent yield (Table 6, entry 10w), with the reaction shown in the ESI.† All compounds were characterized by 1H-NMR, 13C-NMR (Fig. S43–S65†) and HRMS analysis.Single-crystal X-ray analysis of compound 10o
Single-crystal X-ray analysis of compound N-(2,5-difluorophenyl) formamide 10o was used to confirm the validity of the present method for the synthesis of formamide derivatives. X-ray-grade crystals were formed through slow solution evaporation growth in ethanol. The crystal structure (CCDC No. 2341900) of compound 10o is presented in Table S3.† It crystallized in a monoclinic cell, Pn space group, Z value of 2. The ESI† contains additional single-crystal X-ray crystallographic data for compound 10o (Table S3†).Recyclability and post-catalytic characterization of the ZIF-9/GO sample
The recyclability of the ZIF-9/GO catalyst was also tested in the Knoevenagel and N-formylation reactions under optimized conditions. After completion of the reaction, the catalyst was extracted from the reaction mixture by filtration. The separated catalyst was rinsed with ethanol and acetone three to four times to remove any organic substances deposited on its surface, followed by drying in an air oven and finally using for further catalytic run under the optimized reaction conditions. The ZIF-9/GO catalyst was reused for six successive runs without a considerable decrease in its catalytic efficiency for both reactions, as shown in Fig. S7a.†The reused ZIF-9/GO was again characterized by SEM, XRD, and FT-IR spectroscopy (Fig. S7b and S7d†) to investigate its stability, morphology, and phase, which showed similar characteristics to those of the original ZIF-9/GO composite material. The observed changes in the XRD peaks of fresh and reused ZIF-9/GO may be due to the presence of reactant or product molecules that may remain adsorbed in the recovered catalyst.
Hot filtration test of the catalyst
Additionally, the “hot filtration method” was used for the synthesis of 3e as the substrate to study the heterogeneity of ZIF-9/GO. The catalyst was separated from the reaction mixture via filtration after 4 minutes, and a 40% conversion of the substrate was observed. Subsequently, the filtrate (without catalyst) was maintained under similar conditions for further reaction. Even after 1 hour, no additional conversion of 3e was observed (Fig. 7). According to this investigation, the filtrate contained almost no soluble catalytically active species, demonstrating the applicability and heterogeneity of the catalyst.Probable mechanism for N-formylation of amines using ZIF-9/GO
The exact mechanism of how formylation processes occur using the catalyst remains unclear. However, a putative mechanism in Fig. S8† has been proposed, indicating that the carbonyl group of formic acid is activated by the acidic cobalt ion of the ZIF-9/GO catalyst through a coordination bond, which makes it more electrophilic. The reactive nucleophilic nitrogen atom of the amine group attacks the activated electrophilic carbonyl group to yield the corresponding formylated products.82,83Comparison of catalytic activity of ZIF-9/GO composite material with those reported in previous literature for the Knoevenagel condensation reaction and N-formylation of amine
The importance of this study is shown by comparing the results with previously published work (Tables S4 and S5†). From the comparison table, the present composite material ZIF-9/GO exhibited excellent catalytic activity for the Knoevenagel condensation reaction and synthesis of formamide derivatives with benefits such as easy preparation of the catalyst, mild reaction conditions, high yield of the product, and good recyclability at low catalyst loading.Conclusions
In conclusion, we report a new synthetic approach for the ZIF-9/GO composite using a straightforward and economical process that combines the conductive and dispersive properties of graphene oxide with the large surface area and stability of ZIF-9. Physiochemical, spectroscopic, and microscopic characterizations confirmed the composition, good crystallinity, thermal stability, structural features, and surface properties of the ZIF-9/GO composite material. ZIF-9/GO was used as a heterogeneous catalyst in Knoevenagel condensation and N-formylation of amines, delivering excellent yields with wide substrate scopes. In situ IR study validated the proposed protocol for Knoevenagel condensation. The plausible mechanisms of both reactions were explained in detail. The salient features of this study are outstanding catalytic performance and reusability, easy catalyst recovery, green reaction conditions, low catalyst loading, gram-scale applicability and tolerance to various sensitive substrates in both reactions, highlighting the durability and commercial potential of ZIF-9/GO as a reusable, environmentally benign heterogeneous catalyst.Data availability
The data supporting the findings of this work are available in the article and its accompanying ESI.† (ESI†) includes Materials and methods,; characterization techniques; SEM-EDX of ZIF-9/GO; TGA analysis and XPS-survey spectrum of ZIF-9/GO; calculation of green chemistry metrics for 3c and 5d; FESEM and elemental mapping of ZIF-9/GO; N2 adsorption–desorption isotherm of GO and ZIF-9; single X-ray crystallographic parameters for 3q, 5i and 10o; UV-visible spectra; radar plot displays green-chemistry metrics of 3c and 5d; post-catalytic characterisation; gram-scale synthesis of compound 7; probable mechanism for both the reactions; comparison table; 1H and 13C NMR chemical shift values; 1H and 13C NMR spectra; FT-IR of 7; and mass spectra of some compounds.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
L. K. is appreciative of the senior research fellowship she received from the CSIR in New Delhi, India. S. K. A is thankful to the Institute of Eminence (IoE/Ref.No./IoE/2023–24/12/FRP), University of Delhi, Delhi, India, for financial support. We gratefully acknowledge the University Science Instrumentation Centre (USIC), University of Delhi, Delhi, for analytical data. We are thankful to Sophisticated Analytical Instrument Facility (SAIF)-AIIMS, New Delhi, and IIT-Roorkee, India, for TEM and XPS facility, respectively.References
- R. Mittal and S. K. Awasthi, ACS Sustain. Chem. Eng., 2022, 10, 1702–1713 CrossRef CAS.
- F. Monehzadeh and Z. Rafiee, Appl. Organomet. Chem., 2020, 34, e5631 CrossRef CAS.
- R. Mittal and S. K. Awasthi, ChemCatChem, 2021, 13, 4799–4813 CrossRef CAS.
- J. Cong, F. Lei, T. Zhao, H. Liu, J. Wang, M. Lu, Y. Li, H. Xu and J. Gao, J. Solid State Chem., 2017, 256, 10–13 CrossRef CAS.
- E. Zare and Z. Rafiee, Appl. Organomet. Chem., 2020, 34, e5516 CrossRef CAS.
- A. Das and A. K. Das, New J. Chem., 2023, 47, 5347–5355 RSC.
- D. Chen, S. Chen, Y. Jiang, S. Xie, H. Quan, L. Hua, X. Luo and L. Guo, RSC Adv., 2017, 7, 49024–49030 RSC.
- Z. Hasan, J. W. Jun and S. H. Jhung, Chem. Eng. J., 2015, 278, 265–271 CrossRef CAS.
- C. Zhang and K. Huang, Chem. Commun., 2017, 53, 10564–10567 RSC.
- J. Linnemann, L. Taudien, M. Klose and L. Giebeler, J. Mater. Chem. A, 2017, 5, 18420–18428 RSC.
- M. Chhatwal, R. Mittal, R. D. Gupta and S. K. Awasthi, J. Mater. Chem. C, 2018, 6, 12142–12158 RSC.
- S. G. Surya, S. S. Nagarkar, S. K. Ghosh, P. Sonar and V. R. Rao, Sens. Actuators, B, 2016, 223, 114–122 CrossRef CAS.
- H. Furukawa, N. Ko, Y. B. Go, N. Aratani, S. B. Choi, E. Choi, A. Ö. Yazaydin, R. Q. Snurr, M. O'Keeffe and J. Kim, Science, 2010, 329, 424–428 CrossRef CAS PubMed.
- J. Duan, M. Higuchi, J. Zheng, S.-i. Noro, I.-Y. Chang, K. Hyeon-Deuk, S. Mathew, S. Kusaka, E. Sivaniah and R. Matsuda, J. Am. Chem. Soc., 2017, 139, 11576–11583 CrossRef CAS PubMed.
- R. Mittal and S. K. Awasthi, ACS Appl. Nano Mater., 2022, 5, 7831–7840 CrossRef CAS.
- Q. Li and H. Kim, Fuel Process. Technol., 2012, 100, 43–48 CrossRef CAS.
- J. Zakzeski, A. Debczak, P. C. Bruijnincx and B. M. Weckhuysen, Appl. Catal., A, 2011, 394, 79–85 CrossRef CAS.
- R. Mittal, A. Mishra and S. K. Awasthi, Synthesis, 2020, 52, 591–601 CrossRef CAS.
- H. J. Shin, K. K. Kim, A. Benayad, S. M. Yoon, H. K. Park, I. S. Jung, M. H. Jin, H. K. Jeong, J. M. Kim and J. Y. Choi, Adv. Funct. Mater., 2009, 19, 1987–1992 CrossRef CAS.
- N. R. Wilson, P. A. Pandey, R. Beanland, R. J. Young, I. A. Kinloch, L. Gong, Z. Liu, K. Suenaga, J. P. Rourke and S. J. York, ACS Nano, 2009, 3, 2547–2556 CrossRef CAS PubMed.
- L. Gao, J. R. Guest and N. P. Guisinger, Nano Lett., 2010, 10, 3512–3516 CrossRef CAS PubMed.
- R. Mittal, A. Kumar and S. K. Awasthi, RSC Adv., 2021, 11, 11166–11176 RSC.
- Q. Yang, R. Lu, S. Ren, C. Chen, Z. Chen and X. Yang, Chem. Eng. J., 2018, 348, 202–211 CrossRef CAS.
- H. Wang, Y. Wang, A. Jia, C. Wang, L. Wu, Y. Yang and Y. Wang, Catal. Sci. Technol., 2017, 7, 5572–5584 RSC.
- A. Das, D. Chavda, M. Manna and A. K. Das, New J. Chem., 2024, 48, 18249–18260 RSC.
- A. K. Das, S. Nandy and S. Bhar, Appl. Organomet. Chem., 2021, 35, e6282 CrossRef CAS.
- R. Tokala, D. Bora and N. Shankaraiah, ChemMedChem, 2022, 17, e202100736 CrossRef CAS PubMed.
- A. V. Narsaiah and K. Nagaiah, Synth. Commun., 2003, 33, 3825–3832 CrossRef CAS.
- H. Tian, S. Liu, Z. Zhang, T. Dang, Y. Lu and S. Liu, ACS Sustain. Chem. Eng., 2021, 9, 4660–4667 CrossRef CAS.
- Y. Yang, D. Wang, P. Jiang, W. Gao, R. Cong and T. Yang, Mol. Catal., 2020, 490, 110914 CrossRef CAS.
- K. Jain, S. Chaudhuri, K. Pal and K. Das, New J. Chem., 2019, 43, 1299–1304 RSC.
- D. B. Jackson, D. J. Macquarrie and J. H. Clark, Spec. Publ.-R. Soc. Chem., 2001, 266, 203–213 CAS.
- R. Cortese and D. Duca, Phys. Chem. Chem. Phys., 2011, 13, 15995–16004 RSC.
- U. P. Tran, K. K. Le and N. T. Phan, ACS Catal., 2011, 1, 120–127 CrossRef CAS.
- M. Opanasenko, A. Dhakshinamoorthy, M. Shamzhy, P. Nachtigall, M. Horáček, H. Garcia and J. Čejka, Catal. Sci. Technol., 2013, 3, 500–507 RSC.
- V. Calvino-Casilda, R. Martin-Aranda, A. Lopez-Peinado, I. Sobczak and M. Ziolek, Catal. Today, 2009, 142, 278–282 CrossRef CAS.
- J. Roggenbuck and M. Tiemann, J. Am. Chem. Soc., 2005, 127, 1096–1097 CrossRef CAS PubMed.
- H. Dabbagh and B. H. Davis, J. Mol. Catal., 1988, 48, 117–122 CrossRef CAS.
- H. Teterycz, R. Klimkiewicz and B. Licznerski, Appl. Catal., A, 2001, 214, 243–249 CrossRef CAS.
- V. N. Panchenko, M. M. Matrosova, J. Jeon, J. W. Jun, M. N. Timofeeva and S. H. Jhung, J. Catal., 2014, 316, 251–259 CrossRef CAS.
- N. Liu, Q. Shang, K. Gao, Q. Cheng and Z. Pan, New J. Chem., 2020, 44, 6384–6393 RSC.
- Z. S. Rozveh, S. P. Aledavoud, S. M. Hosseini, M. Karimi and V. Safarifard, J. Solid State Chem., 2023, 327, 124270 CrossRef.
- I. M. Downie, M. J. Earle, H. Heaney and K. F. Shuhaibar, Tetrahedron, 1993, 49, 4015–4034 CrossRef CAS.
- B. B. Lohray, S. Baskaran, B. S. Rao, B. Y. Reddy and I. N. Rao, Tetrahedron Lett., 1999, 40, 4855–4856 CrossRef CAS.
- G. Pettit, M. Kalnins, T. Liu, E. Thomas and K. Parent, J. Org. Chem., 1961, 26, 2563–2566 CrossRef CAS.
- S. Kobayashi and K. Nishio, J. Org. Chem., 1994, 59, 6620–6628 CrossRef CAS.
- S. Kobayashi, M. Yasuda and I. Hachiya, Chem. Lett., 1996, 25, 407–408 CrossRef.
- M.-A. Bonin, D. Giguere and R. Roy, Tetrahedron, 2007, 63, 4912–4917 CrossRef CAS.
- J. Martinez and J. Laur, Synthesis, 1982, 1982, 979–981 CrossRef.
- F. Blicke and C.-J. Lu, J. Am. Chem. Soc., 1952, 74, 3933–3934 CrossRef.
- M. Waki and J. Meienhofer, J. Org. Chem., 1977, 42, 2019–2020 CrossRef CAS PubMed.
- H. Yale, J. Org. Chem., 1971, 36, 3238–3240 CrossRef CAS.
- L. LD, Synlett, 2004, 2570 Search PubMed.
- P. G. Reddy, G. K. Kumar and S. Baskaran, Tetrahedron Lett., 2000, 41, 9149–9151 CrossRef.
- G. Brahmachari and S. Laskar, Tetrahedron Lett., 2010, 51, 2319–2322 CrossRef CAS.
- B. Das, M. Krishnaiah, P. Balasubramanyam, B. Veeranjaneyulu and D. N. Kumar, Tetrahedron Lett., 2008, 49, 2225–2227 CrossRef CAS.
- B. Krishnakumar and M. Swaminathan, J. Mol. Catal. A: Chem., 2011, 334, 98–102 CrossRef CAS.
- M. Nasrollahzadeh, N. Motahharifar, M. Sajjadi, A. M. Aghbolagh, M. Shokouhimehr and R. S. Varma, Green Chem., 2019, 21, 5144–5167 RSC.
- M. N. Timofeeva, V. N. Panchenko, I. A. Lukoyanov and S. H. Jhung, React. Kinet. Mech. Catal., 2021, 133, 355–369 CrossRef CAS.
- R. Tandon, S. Patil, N. Tandon and P. Kumar, Lett. Org. Chem., 2022, 19, 616–626 CrossRef CAS.
- M. Tajbakhsh, H. Alinezhad, M. Nasrollahzadeh and T. A. Kamali, J. Colloid Interface Sci., 2016, 471, 37–47 CrossRef CAS PubMed.
- M. I. Ansari, M. K. Hussain, N. Yadav, P. K. Gupta and K. Hajela, Tetrahedron Lett., 2012, 53, 2063–2065 CrossRef CAS.
- J. K. Yadav, P. Yadav, S. K. Awasthi and A. Agarwal, RSC Adv., 2020, 10, 41229–41236 RSC.
- W. S. Hummers Jr and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef.
- J. H. Lee, J. Jaworski and J. H. Jung, Nanoscale, 2013, 5, 8533–8540 RSC.
- J. Liu, C. Liu and A. Huang, Int. J. Hydrogen Energy, 2020, 45, 703–711 CrossRef CAS.
- S.-F. Zhang, H. Li, C. Hou, L.-N. Liu, Y. Wang, M.-K. Zhao and C. Liang, Cellulose, 2020, 27, 3287–3300 CrossRef CAS.
- G. Xiao, W. Chen, Y. Cai, S. Zhang, D. Wang and D. Cai, Catalysts, 2022, 12, 688 CrossRef CAS.
- Y. Kim and S. Shanmugam, ACS Appl. Mater. Interfaces, 2013, 5, 12197–12204 CrossRef CAS PubMed.
- L. T. Nguyen, K. K. Le, H. X. Truong and N. T. Phan, Catal. Sci. Technol., 2012, 2, 521–528 RSC.
- Z. Öztürk, J. P. Hofmann, M. Lutz, M. Mazaj, N. Z. Logar and B. M. Weckhuysen, Eur. J. Inorg. Chem., 2015, 2015, 1625–1630 CrossRef.
- R. Al-Gaashani, A. Najjar, Y. Zakaria, S. Mansour and M. Atieh, Ceram. Int., 2019, 45, 14439–14448 CrossRef CAS.
- S. Yan, S. Ouyang, H. Xu, M. Zhao, X. Zhang and J. Ye, J. Mater. Chem. A, 2016, 4, 15126–15133 RSC.
- Y. Yang, F. Li, W. Li, W. Gao, H. Wen, J. Li, Y. Hu, Y. Luo and R. Li, Int. J. Hydrogen Energy, 2017, 42, 6665–6673 CrossRef CAS.
- C. Zhang, L. Ren, X. Wang and T. Liu, J. Phys. Chem. C., 2010, 114, 11435–11440 CrossRef CAS.
- M. Jahan, Z. Liu and K. P. Loh, Adv. Funct. Mater., 2013, 23, 5363–5372 CrossRef CAS.
- N. Sharma, S. Tomar, M. Shkir, R. K. Choubey and A. Singh, Mater. Today: Proc., 2021, 36, 730–735 CAS.
- K. Kumari, P. Choudhary, D. Sharma and V. Krishnan, Ind. Eng. Chem. Res., 2022, 62, 158–168 Search PubMed.
- A. Mishra, P. Yadav and S. K. Awasthi, ACS Org. Inorg. Au, 2023, 3, 254–265 CrossRef CAS PubMed.
- E. Rahmati and Z. Rafiee, J. Porous Mater., 2021, 28, 19–27 Search PubMed.
- J. P. Li, E. M. Kennedy, A. A. Adesina and M. Stockenhuber, J. Catal., 2019, 369, 157–167 Search PubMed.
- M. Seddighi, F. Shirini and O. Goli-Jolodar, C. R. Chim., 2016, 19, 1003–1010 CrossRef CAS.
- R. Chutia and B. Chetia, New J. Chem., 2018, 42, 15200–15206 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: The supporting information includes chemicals and materials; instrumentations; SEM-EDS spectra of ZIF-9/GO; TGA analysis; green chemistry metrics of compound 3c and 5d; FESEM and elemental mapping images; BET analysis of ZIF-9 and GO; single-crystal X-ray crystallographic data of compounds 3q, 5i and 10o; UV-vis spectra; post-catalytic characterization; gram-scale synthesis of compounds 7 and 10w; probable reaction pathway for N-formylation; tables comparing this study with other reported literature; and 1H NMR and 13C NMR spectra. CCDC 2236687 2233222 2341900. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ra02165k |
| This journal is © The Royal Society of Chemistry 2025 |