 Open Access Article
Open Access ArticleAdvancements in preceramic inorganic polymers for environmental applications: properties, synthesis, and potential uses
Mohammad Mehdi Salehi†
a,
Maryam Mohammadi†
b,
Ali Reza Akbarzadeh
 c,
Mohsen Babamoradi
b,
Ali Maleki
c,
Mohsen Babamoradi
b,
Ali Maleki
 *a and
Ehsan Nazarzadeh Zare
*a and
Ehsan Nazarzadeh Zare
 *d
*d
aCatalysts and Organic Synthesis Research Laboratory, Department of Chemistry, Iran University of Science and Technology, Tehran 16846-13114, Iran. E-mail: maleki@iust.ac.ir; Fax: +98-21-73021584; Tel: +98-21-73228313
bDepartment of Physics, Iran University of Science and Technology, Tehran 16846-13114, Iran
cDepartment of Chemistry, Iran University of Science and Technology, Tehran, Iran
dSchool of Chemistry, Damghan University, Damghan, 36716-45667, Iran. E-mail: ehsan.nazarzadehzare@gmail.com; e.nazarzadeh@du.ac.ir
First published on 18th July 2025
Abstract
Preceramic inorganic polymers (PCIPs) have garnered attention due to their unique properties and potential applications in environmental contexts. They are highly resistant to heat, making them suitable for high-temperature processes like pollution control, waste management, and water purification. Additionally, PCIPs possess strong mechanical properties, including high strength and stiffness, which enable them to be used in harsh conditions. Their excellent chemical resistance also makes them useful in corrosive environments and as protective coatings for metals. This review explores different methods for synthesizing PCIPs, including sol–gel processing, polymerization, and pyrolysis. Each method has its benefits and challenges, depending on the desired characteristics and intended applications. PCIPs can be utilized in various environmental solutions, including catalytic converters and air filters for pollution control, stabilizing hazardous waste, and treating wastewater. They also have potential applications in coatings, composites, and sensors, demonstrating their versatility across various industries. This review offers a comprehensive discussion of PCIPs, concentrating on their properties, production methods, and environmental applications. It also examines future opportunities for utilizing PCIPs in sustainable environmental solutions, emphasizing their crucial role in addressing environmental challenges.
1. Introduction
The interface between polymers and ceramics is critical in materials science. Ceramics can be conceptualized as polymer systems with extensive crosslinking, where the three-dimensional structure imparts strength, rigidity, and thermal resistance.1,2 While often overlooked, certain ceramics are entirely organic, specifically those based on carbon.3 Notable examples include melamine–formaldehyde resins, phenol–formaldehyde materials, and carbon fibres.4–6 Inorganic ceramics, particularly those composed of elements such as silicon (Si), aluminium (Al), or boron (B) in conjunction with oxygen (O), carbon (C), or nitrogen (N), are widely recognized.7 Within the realm of inorganic ceramics, two primary categories can be identified: oxide ceramics and non-oxide materials. Oxide ceramics often feature silicate structures characterized by their relatively low melting points. Conversely, non-oxide ceramics, including silicon carbide (SiC), silicon nitride (Si3N4), aluminum nitride (AIN), and boron nitride (BN), represent some of the highest melting point materials known.8–11 Non-oxide ceramics typically possess such high melting points that they present challenges in shaping and fabricating through conventional melt- or powder-fusion methods, which are often used for oxide materials. More than thirty years ago, polymer-derived ceramics (PDCs) were developed by introducing PCIPs, which serve as precursors for manufacturing predominantly silicon-based advanced ceramics.12 The concept of employing molecular precursors to fabricate ceramic materials was initially proposed by Ainger and Herbert in 1960.13 In the early 1970s, Verbeek introduced the pyrolysis of organosilicon polymers to produce ceramic materials suitable for high-temperature applications.14 This technique was specifically designed to produce Si3N4/SiC ceramic fibers. The research undertaken by Yajima et al. in 1975 on producing SiC fibres from polycarbosilane marked a pivotal advancement in polymer pyrolysis, explicitly targeting the generation of PDCs. Following this basic study, polycarbosilane-derived inorganic polymers (PCIPs), particularly organosilicon polymers, have gained recognition as a highly effective approach for fabricating advanced ceramic materials.15 It is essential to note that organosilicon polymers are referred to by various terms, including silicon-based polymers, silicon-based preceramic polymers, and silicone resins.16 The transformation of polymers into ceramics has facilitated remarkable advancements in ceramic science and technology. This method has produced ceramic fibers, coatings, and ceramics that remain stable even at very high temperatures, up to 2000 °C. They also resist breaking down, crystallizing, separating into different phases, and deforming under stress.17 PCIPs, particularly those formulated as organo-silicon compounds comprising a silicon atom backbone along with C, O, N, B, and hydrogen (H), serve as highly effective means of producing advanced ceramics. Silicon-based polymers containing N, C, and B are used to create different types of non-oxide ceramics, including silicon carbide (SiC), silicon carbonitride (SiCN), and silicon boron carbonitride (SiBCN).18,19 Ceramic materials have excellent physical, chemical, thermal, mechanical, electrical, magnetic, and optical properties. These properties stem from their strong atomic bonds, which can be either ionic or covalent.20 These properties make ceramics useful in various industries, including automotive, defense, construction, energy, healthcare, consumer products, and sensor technology.21 Ceramic materials are grouped into two main types: traditional ceramics and advanced (or technical) ceramics. Traditional ceramics are crafted from natural materials, such as clay and sand. On the other hand, advanced ceramics are generally created through intricate manufacturing processes. Notable examples are SiC, Si3N4, BN, aluminium oxide (Al2O3), zirconium oxide (ZrO2), and various composites.22 Furthermore, recent advancements in the analysis of their nanoscale structure have significantly enhanced the understanding of the distinctive and beneficial properties of polycrystalline diamond composites, particularly regarding their exceptionally high creep resistance, chemical durability, and semiconducting characteristics.23 From a processing perspective, PCIPs have served as reactive binders in the fabrication of technical ceramics. The design of these materials has been optimized to enable the formation of structured pores at the mesoscale level. Their effectiveness in bonding advanced ceramic components has been assessed, and they can be converted into either bulk or macroporous forms. Consequently, researchers have significantly expanded the possible uses of PDCs.14 Due to their distinctive characteristics and applications, PCIPs hold considerable promise for addressing environmental issues. These organic polymers can transform ceramic materials via regulated pyrolysis techniques, creating inorganic, ceramic-like structures. This conversion opens avenues for utilizing preceramic polymers in various environmental applications, such as pollution remediation, water purification, and sustainable energy solutions.2,12In environmental remediation, preceramic polymers serve as a foundation for creating complex ceramic materials designed with specific characteristics that aim to capture and eliminate pollutants from air, water, and soil. These engineered materials can be optimized for elevated surface areas, enhanced chemical reactivity, and superior adsorption capabilities, effectively addressing environmental contaminants, including heavy metals, organic pollutants, and industrial waste.24 Additionally, preceramic polymers contribute to technologies by facilitating the production of ceramic components that can withstand high temperatures, which are essential for sensor applications.25,26
These polymers, for example, are applicable in fabricating ceramic membranes used for gas separation and purification, as well as solid oxide fuel cells and thermal insulation materials designed for energy-efficient applications.27–29 The study of preceramic polymers within environmental frameworks involves examining their synthesis, processing techniques, and the characteristics of the resulting ceramic materials relevant to ecological applications. Researchers are focused on advancing novel polymer chemistries and processing methodologies to enhance the efficacy of ceramic materials in environmental remediation and sustainable energy solutions.
In summary, preceramic polymers present significant potential for enhancing environmental sustainability by providing novel approaches to pollution management, water treatment, and clean energy generation. Their distinctive capacity to be transformed into functional ceramic materials underscores their ability to address pressing environmental issues and promote the development of environmentally friendly technologies. Recent studies have focused on applying ceramics to develop novel approaches for environmental remediation. Ceramics play a crucial role in various environmental applications, including detecting, observing, and measuring pollutants, as well as preventing, managing, and remedying their effects.30 Preceramic polymer materials, characterized by their unique properties and versatile applications, have emerged as crucial components in environmental reform. This review has examined the studies on PDCs, their synthesis, and their diverse ecological applications. Readers are expected to develop a deeper understanding and appreciation of this field, which they can subsequently apply in their research endeavours.
2. Preceramic polymers
Preceramic polymers, including those containing Si, Al, and B, can lead to a diverse array of compositions in PDCs. Prominent examples of these preceramic polymers typically feature a silicon backbone composed of C, O, N, B, and H elements. Notable types include polysilazanes, polysiloxanes, and polycarbosilanes.31–33Fig. 1a–d illustrates the prevalent silicon-based polymers. Through pyrolysis, silicon-based preceramic polymers can be transformed into various ceramic materials, including silicon oxide (SiO2), SiC, silicon oxycarbide (SiOC), and SiCN. In contrast to traditional ceramic powders, preceramic polymers provide significantly greater flexibility in fabricating ceramic components characterized by intricate geometric configurations and forms. This technology facilitates the production of a diverse array of high-performance non-oxide ceramics that cannot be produced using traditional ceramic manufacturing techniques. Modifying the chemistry of preceramic polymers, along with the size and morphology of nanofillers, can facilitate the application of nanocomposites (NCs) in intricate shapes and coatings, leading to enhanced thermal and mechanical properties. This versatility is achieved through a range of manufacturing techniques, including casting, moulding, and additive manufacturing (AM).2,34,35
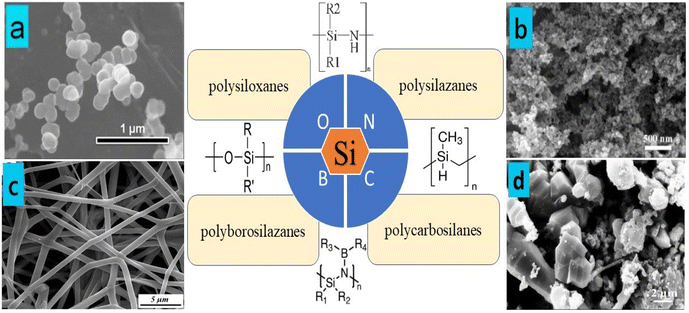 | ||
| Fig. 1 Common silicon-based polymers: (a) formation of spherical silicon-based nanostructures from polysiloxane using the CVD process. Figure (a) was adapted by permission from: Surface and Coatings Technology, 2024, 484, 130804.36 (b) SEM images of SiCN aerogels. Figure (b) was adapted by permission from: Journal of Advanced Ceramics, 2021, 10, 1140–1151.37 (c) SEM image of SiBCN nanofibers after pyrolysis at 1000 °C. Figure (c) was adapted by permission from: Ceramics International, 2021, 47(8), 10958–10964.38 (d) SEM images showing the structure of β-SiC powders. Figure (d) was adapted by permission from: Ceramics International, 2021, 47(12), 17502–17509.39 | ||
PDC technology presents considerable benefits over traditional powder-based ceramic processing and shaping methods, as follows: (1) it can operate entirely as a liquid-based process, thereby circumventing the issues linked to the use of ceramic powders, (2) it provides enhanced formability through the use of soluble and crosslinkable precursors, (3) it facilitates the flexible adjustment of microstructures and compositions at the molecular scale, leading to the development of new capabilities, and (4) the transformation from polymer to ceramic occurs at comparatively lower temperatures (800–1200 °C) than those required in conventional ceramic processing. These benefits have led to PCPs being regarded as exceptionally suitable materials for AM processes, and the integration of AM with PDC technologies has paved the way for potential solutions to the challenges encountered in the synthesis and production of traditional ceramics.12
Preceramic polymers exhibit a variety of configurations and microstructures that can influence the microstructure, composition, porosity, and characteristics of the resulting ceramics. Notable silicon-based preceramic polymers, including polysilazanes, polysiloxanes, polycarbosilanes, and polyborosilazanes, are utilized in the production of ceramic materials (Fig. 1a–d).36–39
2.1 Si-based preceramic polymers
A general formula has been established for an organosilicon polymer that serves as a precursor in ceramic synthesis. This formula is defined by two essential parameters that affect the alteration and formulation of the preceramic composition at the molecular scale. The first parameter is the group (X) present in the polymer core structure, while the second comprises the substituents R1 and R2 bonded to silicon. Changes in the (X) group lead to the development of various categories of silicon-based polymers. These materials comprise multiple types of silicon-based polymers, characterized by the element or group (X) present in their structure. When X is Si, they are referred to as poly(organosilanes). If X is a methylene group (CH2), they are known as poly(organo-carbosilanes). When X is O, they are referred to as poly(organosiloxanes). If X is N and H, they are called poly(organosilazanes). Finally, when X is a carbodiimide group ([N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N]), they are known as poly(organosilylcarbodiimides).14
N]), they are known as poly(organosilylcarbodiimides).14
SiC finds applications in the production of semiconductors, the generation of nuclear energy, and various other industries. Likewise, SiOC, a non-crystalline ceramic produced from polymer precursors, presents a range of applications in both medical and environmental fields.40,41 Developing composite particles comprising SiC and SiOC represents a promising approach to enhancing material performance. The production of SiOC/SiC ceramics using the polymer-derived ceramics (PDC) method depends on the heat treatment (pyrolysis) of organo-modified polysiloxanes –[Si(R2)–O]n– or polysilanes containing oxygen-based functional groups –[Si(OR3)]n–.42,43 Silicon, recognized as an excellent precursor for silicon carbide (SiC), is further enhanced by the promising capabilities of polysiloxane as a polymer precursor for silicon oxycarbide (SiOC).
Guo et al. utilized organosilane slurry residue (OSR), an organic–inorganic hybrid due to its silicon and polysiloxane components, to synthesize SiOC/SiC. OSR is a byproduct that is a liquid–solid mixture generated within the organosilane manufacturing industries, often regarded as waste. Integrating such waste materials into the synthesis of Stabler NCs presents economic and environmental benefits. The researchers concentrated on the pyrolysis of OSR as a method for synthesizing SiC/SiOC nanocomposites. They aimed to gain an in-depth understanding of the transformation mechanisms, phase transitions, and structural modifications that occur throughout the pyrolysis process.44
| Methods | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Sol–gel processes | • Fine control over polymer architecture | • It may exhibit a prolonged duration owing to the incremental characteristics of hydrolysis and condensation, particularly when creating structures with significant crosslinking | 48 |
| • Yield high-purity products | • Excessive water or uncontrolled moisture levels can lead to undesirable side reactions, which may cause the polymer network to become overly | ||
| • Non-toxic alkoxysilanes, mild conditions such as relatively low temperatures and pressures, and reduced hazardous byproducts | • Post-synthesis treatment: frequently, the produced material necessitates additional curing or ageing processes to attain the intended mechanical characteristics | ||
| Anionic ring-opening polymerization | • This technique yields polysiloxanes characterized by elevated molecular weights, resulting in enhanced mechanical properties, including improved elasticity and toughness | • The anionic polymerization process exhibits a significant sensitivity to impurities, such as water and oxygen, which can potentially halt the polymerization reaction prematurely | 49 |
| • This method enables the precise regulation of polymer chain length and architecture, yielding polymers with | • Demands the use of pure functional monomers—resulting in increased costs and complexity. The high expense of reagents and the necessity for purity require a specialized environment | ||
| • Functionalized monomers facilitate modifications after the polymerization process | |||
| Grignard reaction | • The method facilitates the incorporation of diverse organic functional groups, such as alkyl and aryl, into the polymer structure, thereby allowing for precise modification of the polymer's chemical and physical properties | • Grignard reagents exhibit a high degree of sensitivity to moisture, which can lead to undesired side reactions and reduced yields | 50 |
| • The procedure exhibits significant reactivity, frequently resulting in the swift synthesis of the intended organosilicon compound | • The reaction frequently produces byproducts, including magnesium salts, necessitating meticulous removal and disposal procedures | ||
| Hydrosilylation | • It typically takes place under mild conditions characterized by moderate temperature and pressure. This approach effectively reduces the likelihood of side reactions and the degradation of sensitive functional groups | • Platinum catalysts are costly, resulting in increased total expenses associated with polymer synthesis, particularly in large-scale industrial applications | 51 |
| • The process of selective bond formation exhibits a high degree of specificity, facilitating the establishment of particular Si–C linkages. This characteristic is advantageous for the synthesis of tailor-made organosilicon polymers | • Hydrosilylation reactions may be adversely affected by specific impurities, such as sulfur, phosphorus, or amines, which can introduce complications in the synthesis process if not meticulously managed | ||
| • This process produces minimal byproducts, rendering it an effective and environmentally friendly synthesis pathway | |||
| Condensation polymerization | • The condensation polymerization of chlorosilanes exhibits high efficiency and can be readily scaled for industrial manufacturing | • The reaction produces hydrochloric acid (HCl) as a byproduct, necessitating meticulous management and disposal to prevent corrosion and mitigate environmental risks | 52 |
| • Selecting various chlorosilanes, such as those substituted with methyl, phenyl, or vinyl groups, allows for the customization of the resultant polymer's characteristics, including its flexibility and thermal resistance | • Controlling the extent of crosslinking in polymers can be challenging, particularly in systems with high reactivity, which may result in brittle materials | ||
| Polycondensation of silanols | • The procedure is uncomplicated, typically necessitating gentle conditions and yielding the intended polymer without the formation of intricate byproducts | • The condensation reaction may proceed sluggishly, particularly in systems characterized by low reactivity, requiring prolonged reaction durations | 53 |
| • The reaction yields water as the sole byproduct, thereby contributing to the high purity of the resulting polymers | • Attaining an exact level of crosslinking can pose challenges, resulting in variability in the mechanical properties of the final polymer |
To date, polycarbosilane has found extensive applications in the fields of electrical and photoconductive materials, photoresists, and nonlinear optical substances, in addition to serving as preceramic precursors for the production of SiC fibers, powders, whiskers, composites, and nanomaterials. To explore the relationships and distinctions between polycarbosilane and polysilane in the context of SiC ceramics fabrication, Shukla et al. conducted a comparative analysis of the thermal properties of various polysilanes and polycarbosilanes. Their research indicated that poly-dimethyl-silane (PDMS) and poly-dimethyl-methyl-phenyl-silane (PDMMPS) are suitable for the synthesis of polycarbosilane through the Kumada rearrangement process.59 Poly-dimethyl-methyl-silane (PDMMS) has the potential to generate SiC directly, bypassing the need for polycarbosilane synthesis, owing to its significant crosslinking capacity during thermal processing. In contrast, for polycarbosilanes, an increase in molecular weight is correlated with a higher carbon yield, which plays a crucial role in determining the quality of SiC ceramics.
Because of its exceptional thermal stability (≤400 °C), resistance to oxidation, and corrosion protection, polysilazane is used as a heat exchanger barrier and as a coating to protect steel surfaces from environmental deterioration and oxidation.62 Polysiloxane can be synthesized via ammonolysis reactions between chlorosilanes and ammonia or through ammonolysis processes.63 Another practical approach for synthesizing polysilazane is the ring-opening polymerization of cyclic polysilanane.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and the R groups can be hydrogen, phenyl, ethyl, methyl, and others. These compounds are used as starting materials to produce SiCN-based ceramics.69 Polysilylcarbodiimides can be synthesized through a reaction using pyridine as a catalyst, with chlorosilanes and bis(trimethylsilylcarbodiimide); however, they are sensitive to moisture.70 In a recent study, researchers identified hyperbranched polycarbosiloxanes as distinct ceramic precursors of SiCO, which improve ceramic yields.71 Organosilicon polymers containing boron, such as polyborosilane, polyborosilazane, polyborosiloxane, and their derivatives, are frequently precursors in producing borosilicon ceramics. In contrast to conventional organosilicon polymers, incorporating metal elements (such as zirconium and iron) into the main chain or side chains of preceramic polymers facilitates the creation of cermet.72
N, and the R groups can be hydrogen, phenyl, ethyl, methyl, and others. These compounds are used as starting materials to produce SiCN-based ceramics.69 Polysilylcarbodiimides can be synthesized through a reaction using pyridine as a catalyst, with chlorosilanes and bis(trimethylsilylcarbodiimide); however, they are sensitive to moisture.70 In a recent study, researchers identified hyperbranched polycarbosiloxanes as distinct ceramic precursors of SiCO, which improve ceramic yields.71 Organosilicon polymers containing boron, such as polyborosilane, polyborosilazane, polyborosiloxane, and their derivatives, are frequently precursors in producing borosilicon ceramics. In contrast to conventional organosilicon polymers, incorporating metal elements (such as zirconium and iron) into the main chain or side chains of preceramic polymers facilitates the creation of cermet.72Many studies have demonstrated progress in developing affordable and straightforward methods for producing functional organosilicon polymers with high ceramic yields. These studies also explored how the structure of the polymers affects the properties of the ceramics made from them. It is essential to note that the final properties of the ceramics depend significantly on the subsequent processes, such as shaping, crosslinking, and sintering.
3. Properties of preceramic inorganic polymers
Polymers of both organic and inorganic composition differ primarily in their backbone composition; organic polymers contain C atoms, while inorganic polymers lack carbon atoms in their structure and incorporate Si, phosphorus, and N instead. The structural forms of organic polymers are typically more straightforward than those of inorganic polymers, which tend to be more intricate and highly branched.73 It is also possible for hybrid polymers to contain both organic and inorganic segments within the same polymer backbone. Other characteristics of polymers made from mineral materials include their unique properties, which are not found in organic materials, such as flexibility at low temperatures, conductivity, and flame resistance. Unlike silicate minerals, which are extensively crosslinked, inorganic polymers are typically one-dimensional. This section will elucidate several of these characteristics.743.1 Thermal stability
Preceramic polymers demonstrate essential thermal stability for high-temperature applications and ceramic conversion, maintaining structural integrity during pyrolysis. This property enables their use in aerospace components, thermal protection systems, and high-temperature coatings where decomposition resistance is critical.56 Several factors affect the thermal stability of preceramic inorganic polymers, including:(I) Chemical composition: the type of inorganic elements and their bonding in the polymer play a key role. Ceramic precursors with more robust, thermally stable bonds exhibit better thermal stability.75
(II) Polymer structure: the configuration of atoms within the polymer chain significantly influences its thermal properties. Typically, polymers with well-defined and organized structures exhibit better thermal stability than those with disordered arrangements.
(III) Crosslinking density: increased crosslinking within the polymer framework can improve thermal stability. Crosslinked networks exhibit a reduced susceptibility to degradation when subjected to high temperatures.
(IV) Presence of volatile components: certain preceramic polymers may possess volatile constituents that can be emitted at elevated temperatures, thereby influencing the overall stability of the material.
(V) Pyrolysis conditions: the thermal stability of the preceramic polymer can be influenced by the specific conditions of pyrolysis or heat treatment, including temperature, duration, and atmospheric environment.
Researchers and materials scientists investigate and enhance these variables to create preceramic inorganic polymers that exhibit superior thermal stability. The objective is to formulate materials capable of withstanding the necessary processing temperatures throughout the conversion to ceramics while preserving the desired characteristics.76,77 The thermal characteristics of PDCs play a crucial role in their utilization in high-temperature applications, such as heat-protective coatings and insulating materials. The main thermal factors related to PDCs include thermal conductivity (κ), electrical conductivity (σ), coefficient of thermal expansion (CTE), specific heat capacity (Cp), thermal diffusion rate (α), and thermal shock resistance (TSR). Among these, thermal conductivity is the most critical factor, and this discussion will focus mainly on it.78
The transition of phonons primarily governs the κ of ceramic substrates characterized by robust covalent bonds. The microstructural features of these materials, including impurities, crystallinity, grain boundaries, microcracks, and micropores, have a significant impact on phonon scattering, thereby influencing the thermal properties of the materials. Subsequently, we will discuss how the microstructure of PDCs affects their thermal efficiency. Both crystallinity and impurities significantly influence the thermal characteristics of a PDC. The impurities can be categorized into two components: the first pertains to the phase produced by PDCs within the heat treatment process, primarily referring to free carbon.
In contrast, the second involves intentionally adding dopants to modify thermal characteristics. Free carbon has varied effects on PDCs depending on the system in which it appears. In the case of the PDCs–SiOC composite, the presence of free carbon markedly enhances the κ of SiOC. For instance, incorporating cost-effective, readily available carbon-based fillers such as lamp black (LB), graphite (GR), carbon black (CB), and activated carbon (AC) into a SiOC system was successfully achieved through abrasion milling, then spark plasma sintering, resulting in dense, bulk composites without crack. The carbon-enriched SiOC systems (C–SiOC) exhibited improved κ (Fig. 2a). The SiOC performed 63% better when GR flakes were integrated than when standard SiOCs were used. Furthermore, CB did not exhibit a 46% increase in κ due to phonon and electron transfer efficiency, as it created a permeating network of highly interconnected and tortuous carbon domains within a graphene-like structure. The amorphous SiOC exhibits a notably low intrinsic κ, measured at ∼1.2 W (m K)−1 at ambient temperature. Consequently, free carbon significantly enhances the κ within the PDCs–SiOC system.79
 | ||
| Fig. 2 (a) κ of incorporating GR, LB, CB, and AC into a SiOC matrix named SGR, SLB, SCB, and SCA. Figure (a) was adapted by permission from: Journal of Alloys and Compounds, 2021, 889, 161698.79 (b) An analysis of the κ reduction at 300 K for the extrinsic N- and B-doped case, focusing on the contributions of ph–el and ph–def scattering mechanisms, both independently and in combination, across a range of defect concentrations from 1016 to 1021 cm−3. (c) The temperature dependence of κ within the range of 200 to 700 K for both nitrogen-doped and boron-doped cases is characterized by defect concentrations of 1019 and 1020 cm−3. This analysis encompasses ph–def and ph–el scattering mechanisms, considered separately and in conjunction. (d) The ph–def scattering rates for neutral B0C, neutral N0C, and charged N+1C defects at a defect concentration (Cdef) of 1020 cm−3, alongside the associated ph–el scattering rates at an electron concentration of 7 × 1018 cm−3. Additionally, the three-ph scattering rates at a temperature of 300 K in 4H–SiC are included. Figures (b)–(d) were adapted by permission from: Materials Today Physics, 2024, 41, 101346.80 Presents the κ quantities at room temperature for the following materials: (e) SiOC and (f) SiCN(O) ceramic foams. The triangles denote the open cell structures, classified as P-type, while the black circles represent the ceramic foams' closed cell configurations, identified as M-type. (For a detailed understanding of the colour references in this figure legend, readers are encouraged to consult the online version of this article). Figures (e) and (f) were adapted by permission from: Ceramics International, 2020, 46, 5594–5601.81 SEM images depicting mean fibre diameter, corresponding histograms, and optical images of (g) D1800_PAN_1000, (h) HTTS_PAN_1000, (i) PAN_D1800_1000, and (j) PAN_HTTS_1000, (k) TGA curves of C-rich SiCN(O) and carbon nonwovens for assessing oxidation resistance (heating rate: 5 K min−1; atmosphere: synthetic air). Figures (g)–(k) were adapted by permission from: Journal of the European Ceramic Society, 2024, 44(9), 5308–5318.82 | ||
Defects, including micropores, microcracks, grain boundaries, impurities, and variations in crystallinity, significantly influence the thermal characteristics of polycrystalline diamond composites. The effect of these defects on thermal characteristics primarily occurs through their impact on phonon scattering. The presence of small pores, cracks, and grain boundaries can increase the scattering of phonons, which reduces thermal conductivity. Pang et al. calculated the κ of heavily N-doped and B-doped cubic SiC by considering how phonons scatter with electrons and defects. Their analysis revealed that in the case of N-doping, both types of scattering contribute to reducing thermal conductivity. Specifically, phonon–electron (ph–el) scattering primarily contributes to the decrease in κ when defect concentrations are below approximately 1020 cm−3 at room temperature. However, phonon–defect ph–def scattering becomes the prevailing mechanism as the doping concentration exceeds this threshold.
In contrast, for B-doping, ph–def scattering mainly reduces κ across all the temperatures and doping levels tested (Fig. 2b). The study also looked at how κ changes with temperature for two different defect concentrations, Cdef = 1019 and 1020 cm−3, as shown in Fig. 2c. For N-doping at a concentration of 1019 cm−3, the effect of ph–def scattering on reducing κ is small, especially at temperatures higher than room temperature, like at 300 K. This pattern is true for both individual and combined scattering effects in N- and B-doped 3C–SiC. Fig. 2d shows the intrinsic rates of three-phonon, ph–el, and ph–def scattering at room temperature with a concentration of Cdef = 1020 cm−3.
For B-doping, the ionization energy of B defects is about 0.65 eV, which is much higher. This higher energy reduces the ph–el scattering rates, so they can be ignored because the carrier concentration is very low. The focus should be on ph–def scattering from neutral defects, as their scattering rates are similar to three-phonon scattering rates. On the other hand, the ionization energy of the N-defect is much lower, around 0.07 eV. This makes both ph–el and ph–def scattering essential to consider in the low-frequency range, where phonons mainly affect κ, the scattering rates from neutral defects and ph–el interactions are similar to those of three-phonon processes. Therefore, both ph–el and ph–def scattering are expected to reduce significantly κ in highly N-doped 4H–SiC, unlike in B-doped materials.
Because ph–def scattering is not influenced by temperature (T) and intrinsic anharmonic scattering escalates linearly with T, the relative reduction κ diminishes at elevated temperatures. This phenomenon is particularly observable in the case of B dopant. Specifically, ph–def scattering, particularly when B is present, exhibits minimal temperature dependence; consequently, the decline in κ remains consistent even at high temperatures.
In contrast, the increase in ph–el scattering with T is significant in the case of N dopant (NC), resulting in a more rapid decrease in κ compared to BC. This results in a lower κ above 400 K for NC with a concentration of 1020 cm−3. These findings underscore the importance of considering the ph–el scattering mechanism alongside other phonon scattering processes when assessing the thermal conductivity of doped semiconductors, such as the development of thermal insulators and thermal shields in extreme conditions.80
Santhosh et al. created foam materials from polymers, specifically SiCN(O) and SiOC, and tested their thermal properties. Their results showed that, within the temperature range of 30–850 °C, the linear thermal expansion coefficients for the SiCN(O) and SiOC foams were 1.72 × 10−6 K−1 and 1.93 × 10−6 K−1, respectively. Additionally, both types of foams had very low thermal conductivity at room temperature, with SiCN(O) ranging from 0.03 to 0.2 W (m K)−1 and SiOC from 0.03 to 0.6 W (m K)−1. These results highlight the critical role of the foam's structure on its thermal properties.81 To understand how the structure affects thermal conductivity, especially when comparing open-cell and closed-cell foams, the researchers presented data on the relative density of these materials, showing apparent differences between the two types of cells (Fig. 2e and f). The findings in Fig. 2e and f show that P-type and open-cell polyurethane (PU) foams generally have a lower relative density than M-type PU foams. In particular, PU foams with semi-closed cells can accommodate more preceramic polymers, resulting in denser materials after undergoing thermal decomposition.
Nevertheless, despite this significant distinction between the two foam types, the relationship between κ and relative density remains consistent, with all data points for each chemical composition aligning along the same primary trend line. Balestrat et al. synthesized amorphous Si–B–C powder using B-modified polycarbosilane and modified its thermal characteristics. Their research indicated that the porosity of the sintered sample diminished considerably as the boron content increased, subsequently enhancing the κ of the material. Polycarbosilanes with 0.7% boron have demonstrated that incorporating boron into SiC powders enables the formation of dense ceramics at temperatures as low as 1750 °C. These ceramics have a Vickers hardness between 9.6 and 17.3 GPa and a thermal conductivity ranging from 17.7 to 45.1 W (m K)−1.83
3.2 Mechanical properties
The industrial production of SiC-based ceramic fibers is achieved by transforming polycarbosilane, utilizing a method pioneered by Yajima and colleagues in the 1970s and 1980s.15 SiC-based ceramic fibers have undergone significant improvements over time. The first generation had too much carbon, high oxygen levels, and an unclear structure. The third generation, however, has a nearly balanced composition and a crystal-like structure. Polycarbosilane is the primary component of Nicalon fibre, which is produced using Nippon Carbon's Yajima process. During the curing of the first generation of Nicalon fibres, oxygen is introduced into the system at temperatures of up to 12 wt%. Si(O)C fibres were more suitable for describing these fibres. High-temperature stability and creep were found to be adversely affected by the presence of oxygen. When oxygen is present in SiOC, it decomposes above 1200 °C, resulting in weight loss, the growth of SiC crystals, and a loss of strength. The strength and elastic modulus of these fibres were found to reach 3 and 200 GPa at room temperature. Curing processes utilizing ion irradiation were developed to reduce oxygen content and enhance high-temperature stability.84–86 Recent advancements in chemical composition have enabled the development of SiC fibres with properties similar to those of bulk SiC. These fibres are powerful at temperatures up to 1400 °C, an elastic modulus of 400 GPa.87 In 1976, Yajima and his group attempted to enhance the thermal stability of these fibres by incorporating filler elements such as Al and Ti into the material system. To improve thermal stability, titanium alkoxides were used to modify polycarbosilane, resulting in Si–Ti–C–O fibers with high-temperature resistance.88 Al-modified SiC fibres have been developed based on a similar concept. A unique feature of the SiAlCO fibres is that they maintain their mechanical integrity at temperatures as high as 1900 °C and display a tensile strength of 2.5 GPa and an elastic modulus of 300 GPa.89Consequently, traditional methods make it challenging to achieve dense PDCs. PDCs cannot be further used due to these issues, which limit their integrity and mechanical strength. Liquid polymer precursor compositions can be adjusted to achieve ceramics with an integrated functional structure, in contrast to traditional ceramic preparation methods. Thus, PDCs must be strengthened mechanically. Research has explored methods to increase PDC density, including optimizing processes, incorporating passive or active fillers, applying pressure, or repeating the impregnation-pyrolysis process. For instance, Hanniet et al. used direct photolithography of photocurable B-containing polyceramic polymers with molecular design to create dense, crack-free SiBCN microparticles (20–200 μm) with a Young's modulus of 60 GPa.92
Additionally, using the pyrolysis approach of polymer crosslinked polysiloxane, Sorarù et al. created SiOC glasses in thin, dense, and crack-free samples last year. In this study, the amount of free carbon in the final SiOC materials ranged from 18% to 60%. The nanoindentation technique was used to measure the mechanical properties of SiOC glasses, and both Young's modulus and hardness decreased with increasing free carbon content, following a simple law of the mixture model.95 For fracture toughness (KIC), SiCN and SiOC PDCs produced through liquid and powder processing methods are evaluated. KIC values range from 0.56 to 3 MPa m1/2, with higher values observed in powder-fabricated PDCs due to R-curve behaviour influenced by the specific processing technique.96,97 KIC values for liquid-produced PDCs are typically lower, with a particular measurement of 0.70 MPa m1/2 for a SiOC synthesized from a sol–gel precursor. Full-density SiCN derived from liquid precursors has KIC values ranging from 0.56 to 1.3 MPa m1/2.93,97 Through the integration of multi-phase design, doping, fiber reinforcement, and process control, dense SiC-based PDCs can be engineered to attain elevated levels of fracture toughness, mechanical strength, and thermal resilience, essential attributes for rigorous applications in the aerospace, nuclear, and energy industries.98 The incorporation of fillers represents a well-recognized method for enhancing the characteristics of PDCs. For example, Yamamura et al. successfully synthesized Ti-doped Si–O–C, achieving thermal stability beyond 1200 °C. In a similar vein, Al was investigated as a filler material, with the resulting Si–Al–C–O fibers demonstrating exceptional thermo-mechanical stability at temperatures exceeding 1900 °C in an inert environment and 1000 °C in air.89 This indicates that the inclusion of Ti and Al dopants contributes to improvements in both mechanical properties and high-temperature resistance. To further enhance the KIC of PDCs, an attempt was made to incorporate nanofillers into the material. For example, the introduction of 2 mass% CNT resulted in a 60% increase in KIC. This improvement was linked to the toughening mechanisms of fibre bridging and fibre pullout (CNTs function as crack deflectors and toughening agents, thereby significantly improving KIC).99
The processing parameters, including thermolysis temperature, significantly influence the outcomes. The Vickers hardness exhibited an increase from 7.9 to 12.8 GPa as the thermolysis temperature rose to 1400 °C; however, it subsequently declined at 1550 °C due to the effects of crystallisation and porosity. Thus, optimizing the pyrolysis temperature is essential for enhancing densification while reducing microstructural defects. The integration of chemical modifications (such as dopants including Ti, Al, and B), physical reinforcements (like CNTs and fibers), and optimization of processing techniques (for instance, SPS, thermolysis control, and additive manufacturing) provides a thorough approach to improving fracture toughness and mechanical strength in SiC-based PDCs while maintaining high-temperature stability.87
3.3 Chemical resistance
Kovalčíková et al. studied how SiC ceramics react to oxidation at temperatures between 1350 and 1450 °C for up to 204 hours. They examined how various processing methods impacted this reaction. The SiC ceramics were produced using a fast-hot-pressing method with granular SiC powder, without the addition of any oxide sintering aids. Compared to traditional techniques, this novel technology enables a reduction in sintering temperature of approximately 200 °C. The SiC produced without additives exhibited superior oxidation resistance compared to SiC that underwent hot pressing with liquid-phase sintering, demonstrating a roughly three orders of magnitude lower parabolic oxidation rate. The newly developed SiC ceramics have impressive properties, including a Vickers hardness greater than 27.4 GPa, a fracture toughness of 3.42 MPa m1/2, and excellent oxidation resistance, measured at 4.91 × 10−5 mg2 (cm4 h)−1 at 1450 °C.103 Recent research has yielded new findings regarding the thermodynamic stability of SiC(O) and SCN(O) ceramics, which are produced by heating various polymer precursors (polycarbosilane, polysilazane) at 1200 °C. While the polysilazanes have similar structures, they differ in their crosslinking temperatures. High-resolution X-ray photoelectron spectroscopy reveals significant differences in the microstructure of each material. The enthalpies of formation  for SiC(O) (from polycarbosilane), SCN(O) (from polysilazane), and SCN(O) (from polysilazane) are measured at −20 ± 4.63, −78.55 ± 2.32, and −85.09 ± 2.18 kJ (mol)−1, respectively. Among these, the PDC derived from polysilazane exhibits the highest level of thermodynamic stability. The findings indicate that incorporating N into the microstructure of PDCs enhances thermodynamic stabilization. The thermodynamic analysis reveals a greater thermodynamic impetus for forming SiCN(O) microstructures as the proportion of SiNxC4−x mixed bonds increases while the presence of silica diminishes. Overall, the enthalpies of formation demonstrate that SiNxC4−x mixed bonds exert a more pronounced stabilizing influence than SiOxC4−x mixed bonds. The findings imply that a decrease in silicon and oxygen concentration is correlated with a systematic stabilizing of SiCN(O) complexes. Furthermore, the destabilization of PDCs associated with elevated silicon levels may plateau at higher concentrations.104
for SiC(O) (from polycarbosilane), SCN(O) (from polysilazane), and SCN(O) (from polysilazane) are measured at −20 ± 4.63, −78.55 ± 2.32, and −85.09 ± 2.18 kJ (mol)−1, respectively. Among these, the PDC derived from polysilazane exhibits the highest level of thermodynamic stability. The findings indicate that incorporating N into the microstructure of PDCs enhances thermodynamic stabilization. The thermodynamic analysis reveals a greater thermodynamic impetus for forming SiCN(O) microstructures as the proportion of SiNxC4−x mixed bonds increases while the presence of silica diminishes. Overall, the enthalpies of formation demonstrate that SiNxC4−x mixed bonds exert a more pronounced stabilizing influence than SiOxC4−x mixed bonds. The findings imply that a decrease in silicon and oxygen concentration is correlated with a systematic stabilizing of SiCN(O) complexes. Furthermore, the destabilization of PDCs associated with elevated silicon levels may plateau at higher concentrations.104
Compared to their crystalline forms, like Si3N4, SiC, and graphite, carbon-rich SiCN ceramics made from polysilylcarbodiimides (which do not have mixed bonds) show slightly positive or nearly zero heat changes when tested for oxidative dissolution in a molten oxide solvent.105 The findings indicate that the enthalpy of formation for PDCs is significantly influenced by the existence of mixed bonding within their atomic framework. Additionally, research has explored the effects of incorporating supplementary elements into SiCN PDCs on their oxidation characteristics.
In the study by Ramlow et al., polyacrylonitrile (PAN) solutions were mixed with different amounts of oligosilazane (Durazane 1800) or polysilazane (HTTS made from Durazane 1800) and electrospun to create C-rich SiCN(O) nonwovens. By adjusting the viscosity of the solutions, they created fine fibers with diameters ranging from 0.32 μm to 0.62 μm. The fibers were then heated to 1000 °C in a N2 environment, transforming them into amorphous C-rich ceramic nonwovens with various Si–C–N–O phases dispersed throughout the fibers. The diameter of the fibres following pyrolysis at 1000 °C was unexpectedly found to range from 0.26 to 0.63 inches, likely attributable to the fusion of fibres during the process (Fig. 2a–d). Using oligosilazane with PAN resulted in increased oxygen contamination, which enhanced ceramic yields through supplementary crosslinking reactions.
Additionally, all samples exhibited a higher initial oxidation temperature than pure carbon, approximately 500 °C. Materials with a higher content of PAN demonstrated minimal impact on the protective capabilities of the matrix. This is attributed to the limited formation of ceramic phases, which were insufficient to establish a dense passive layer necessary for safeguarding the substantial quantity of free carbon present. Consequently, the HTTS_PAN_1000 sample, characterized by a high polysilazane content, exhibited the most effective oxidation resistance at temperatures of up to 600 °C (Fig. 2k). The polysilazane helps create a strong protective layer that shields the free carbon areas. As a result, the improved resistance to oxidation of the nonwoven HTTS_PAN_1000 is primarily due to the specific chemical composition and molecular structure of the polymers, which facilitate a uniform distribution of free carbon within the fiber matrix. The study demonstrates that carbon-rich SiCN(O) nonwovens possess significant potential for use as catalyst supports in high-temperature, harsh environments, as well as for lightweight materials due to their low density. It also highlights the advantages of PDC technology, showing that the properties of the resulting ceramics can be easily adjusted by modifying the composition or chemical structure of the polymers.82
The role of boron seems to be complex. The initially observed low oxidation rates for Si–B–C–N ceramics may have been underestimated for several reasons, including the small ratio of oxide to ceramic volume, the thick flow of borosilicate, and the evaporation of B2O3.106,107 Studies have shown Kp (or αKp) values similar to those of SiC and Si3N4 at a temperature of 1500 °C. When aluminum is added to Si–C–N–(O), it causes the oxidation behavior to change, especially at temperatures above 1000 °C. This results in a quicker decrease in oxidation rates, eventually reaching a low point. At 1400 °C, the oxidation rate becomes stable. It follows a parabolic pattern for over 20 hours, with rate constants approximately ten times lower than those found in Si–C–N samples without aluminum. Aluminum influences the structural evolution of SiAlCN ceramics by stabilizing the SiCN3 structure at high temperatures and thus delays the crystallization of SiAlCN ceramics. Aluminum also enhances the phase transformation of α-Si3N4 to β-Si3N4 during the crystallization of polymer-derived SiAlCN ceramics. Also, the oxidation rate of SiAlCNs is more than one order of magnitude lower than that of SiCN without aluminum and CVD-derived SiC and Si3N4. The reduced oxidation kinetics of SiAlCNs is due to the unique structures of the oxide precipitates formed by SiAlCNs, in which the aluminum atoms are located in 6-membered Si–O rings, preventing the penetration of oxygen. These aluminum-doped ceramics exhibit remarkable resistance to water vapor-related corrosion at high temperatures. Compared to other silicon-based non-oxide ceramics, SiAlCNs have the lowest corrosion rate. Additionally, SiAlCNs have smooth surfaces and retain high strength after heat treatment. This unique property is attributed to the formation of a protective Si–Al–O layer, in which a small amount of aluminum significantly reduces the activity of silica.108
4. Synthesis of preceramic inorganic polymers
PDCs are ceramics made from polymeric materials, unlike those produced through conventional powder processing methods. Thermosetting polymers are preferred as precursors due to their high molecular weight, ability to prevent uncontrolled polymerization, adequate solubility, functional groups for subsequent reactions, and precise molecular architecture for synthesising stoichiometric ceramic materials like SiC or Si3N4. Over the past five decades, various Si-based preceramic polymers with diverse molecular architectures have been developed, with organochlorosilanes being the primary precursors. Examples include polycarbosilanes, polysiloxanes, and polysilazanes, which are produced through reactions with sodium or potassium, water, and ammonia.110 Making ceramic materials from organosilicon polymer precursors usually involves three main steps: (1) choosing or making an organosilicon polymer with the right composition, structure, and controlled molecular weight, (2) shaping the polymer precursor and then crosslinking it, either through heat or a catalyst, at temperatures between 100 and 300 °C to form an organic/inorganic composite, and (3) heating the crosslinked composite in a controlled atmosphere at temperatures between 500 and 1500 °C to turn it into the final ceramic, which can be either amorphous or crystalline.111Synthesizing preceramic polymers entails the polymerization of monomers that can subsequently be transformed into ceramic materials via thermal treatment. A prevalent approach for synthesizing preceramic polymers involves the use of polysiloxanes. This synthesis typically consists of the reaction of silane monomers in conjunction with a catalyst or initiator, resulting in either linear or cross-linked polymers. Additionally, sol–gel techniques may be employed to create preceramic polymers with tailored compositions and structures. The preceramic polymers produced can then undergo thermal treatment, resulting in ceramic materials characterized by high-temperature stability and other advantageous properties. We will now elucidate several of these methods.112
4.1 Sol–gel process
The sol–gel method, which involves the hydrolysis and condensation of hybrid silicon alkoxides, can be used to produce crosslinked polysiloxanes, also known as silicon resins, as illustrated in Fig. 3a. This method was first used by early researchers who founded the field of SiOC glasses. The precursors used in this process are organically modified silicon alkoxides, which have the general formula RxSi(OR′)4−x. After the gelation phase, these precursors form silicon resins, RxSiO(4−x)/2. R′ typically represents a CH3 or C2H5 group, while R stands for an alkyl, allyl, or aryl group. By co-hydrolyzing different hybrid silicon alkoxides, the sol–gel method enables precise control over the composition of the original silicon resin.112 As a result, precursors for silicon-containing organic compounds (SiOCs) that are stoichiometric, excess-C, or excess-Si have been created.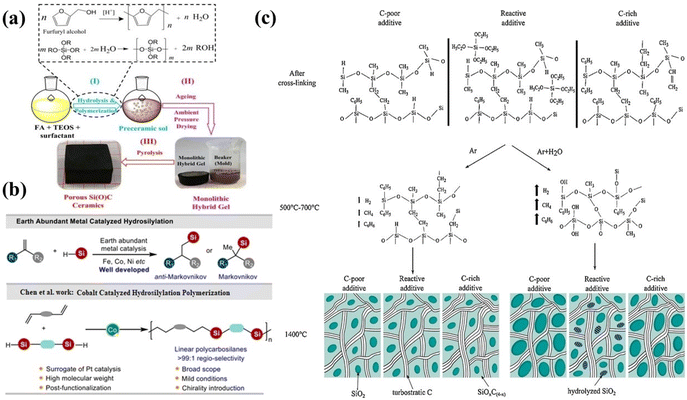 | ||
| Fig. 3 (a) Schematic representation for synthesising porous SiC-based materials utilizing the sol–gel method. Figure (a) was adapted by permission from: Materials, 2022, 16(1), 220.113 (b) Earth-abundant metal-catalyzed hydrosilylation. Figure (b) was adapted by permission from: Macromolecules, 2024, 57(16), 8146–8153.57 (c) Depiction demonstrating the influence of additives and the pyrolysis environment on the microstructure of SiOC. Figure (c) was adapted by permission from: Journal of the European Ceramic Society, 2017, 37(15), 4547–4557.114 | ||
This approach also allows for the uniform incorporation of supplementary elements, such as Al, Ti, or B, into the preceramic network using their respective metal alkoxides.115 However, the sol–gel process has some limitations, such as poor control over viscosity, which limits the use of shaping methods like extrusion and injection molding. Additionally, the considerable shrinkage during the drying process makes it more challenging to produce samples without cracks. Many researchers are studying the relationship between processing, microstructure, and properties of macroporous SiC materials.116,117 The main challenges that limit the widespread use of polymer-derived porous SiC materials are the high cost of preceramic polymers, the use of hazardous materials, and the lengthy time required for the synthesis process.118,119 Sol–gel methods that use affordable carbon-based monomers and silicon alkoxides, followed by pyrolysis, have been widely studied as alternative approaches. However, shrinkage and cracking during the sol–gel process make it difficult to produce bulk SiC materials. To prevent the collapse of bulk gels, expensive drying methods such as supercritical fluid drying and freeze drying, along with long solvent exchange processes, are often used.113,120,121
Li et al. created organic–inorganic gels that can be turned into SiC hierarchical porous materials by heating them. They employed a simple and cost-effective method involving sodium dodecyl sulfate (SDS) to facilitate sol–gel drying at room temperature (Fig. 3a). The presence of SDS facilitates the coarsening of colloidal particles within the hybrid cell, enabling the formation of three-dimensional solid networks characterized by larger pore sizes. This structural enhancement is advantageous as it mitigates the capillary pressure that typically leads to cracking. The hybrid gels transformed β-SiC upon heating to 1400 °C. The porosity and size of the pores in the macroporous SiC monoliths can be adjusted by changing the amount of SDS used in the hybrid cells. The specific surface area of the SiC monoliths can reach up to 171.5 m2 (g)−1. The compressive strength and Young's modulus of the SiC composites were found to be 7.0 ± 0.8 MPa and 407 MPa, respectively.113
4.2 Polymerization methods
Over the past century, methods for producing polymeric organosilicon have undergone significant improvements and expansion. Since silica is found naturally, the monomers for polymeric organosilicon are created through synthetic processes. Silanes have the general formula R4−nSiXn, where X represents reactive groups like Cl, –OR, –OOCR, and –NR2. These groups are essential for starting the polymerization of organosilicon compounds. There are a few ways to make these silanes: (i) by reacting an organic compound directly with silicon at high temperatures; (ii) by chlorinating silicon and then using organic groups and organometallic reagents, such as organolithium compounds, Grignard reagents, and organic zinc compounds; or (iii) by turning silicon into silyl hydrides, which then react with multiple bonds in a hydrosilylation reaction.122 It's essential to note that hydrosilylation is a key reaction in organosilicon chemistry and has become a primary method for combining organic groups with silicon structures.123 Polymerization is necessary for the synthesis of preceramic inorganic polymers. Following pyrolysis, these polymers can produce inorganic ceramics, including SiC, Si3N4, and SiOC. For example, polycarbosilanes are distinctive precursors for synthesising SiC ceramics and fibres. Traditional manufacturing processes often involve severe reaction conditions and utilize low-molecular-weight compounds, whereas catalytic hydrosilylation polymerization generally relies on noble metal catalysts. Chen et al. have documented a cobalt-catalyzed hydrosilylation polymerization process involving dienes and bis-silanes. This approach presents a viable alternative for producing linear polycarbosilanes (Fig. 3b).574.3 Pyrolysis techniques
Pyrolysis is a thermal degradation process that occurs in the absence of oxygen or an inert environment. This method has been widely employed in various organosilicon polymers, including silicones, polysilanes, and polysiloxanes.124,125 The resulting materials are utilized in multiple applications, including electronics, coatings, and sealants, owing to their exceptional thermal stability, flexibility, and durability against environmental factors. The PDCs transform a ceramic material via a range of thermal techniques. These techniques encompass spark plasma sintering,126 chemical vapour deposition,127 rapid thermal annealing,128 laser pyrolysis,129 microwave heating,130 and the predominant method employed, pyrolysis125 conducted in an Ar or N2 atmosphere. PDCs represent a highly adaptable category of materials produced through the pyrolysis of silicon-containing polymers. For example, SiOC materials, in particular, are noteworthy due to their distinctive capability to achieve near-net shapes at lower temperatures compared to conventional ceramics. These SiOC systems find diverse applications, including coatings, microwave-absorbing devices, and components designed for high-temperature environments.131 Among these applications, SiOC-based systems have been the focus of extensive research. The precursor materials typically comprise a Si–O backbone with side groups such as –H, –CH3, –C2H3, and –C6H5. At the same time, the pyrolyzed material forms an amorphous SiOC structure with different carbon areas. The transition from polymer to ceramic is a crucial process in which Si–H and Si–C bonds are broken, hydrogen is removed from phenyl rings and –CH3 groups, and new radical species and phases are formed. The various stages that emerge within the system are primarily influenced by the pyrolysis temperature and the choice of precursor(s).Additionally, during the decomposition of the polymer, several cyclic species can form, including cyclic SiO species, while some side groups may remain unaffected. Si–Si bond formation may also occur within these cyclic intermediates125 at approximately 1000 °C; SiOC (or SiO4−xCx, where 0 ≤ x ≤ 4) demonstrates an amorphous ceramic network architecture characterized by silicon atoms that are tetra-coordinated in conjunction with carbon and oxygen atoms.132 Erb et al. prepared bulk SiOC materials from a polysiloxane-based system by adding various organic substances and utilizing different pyrolysis atmospheres. They also treated the materials with HF etching (Fig. 3c). The additives changed the material's structure by affecting the formation of SiO2 nanodomains. SiOC ceramics made in an Ar + H2O pyrolysis atmosphere had less SiC and more SiO2 compared to those made in an Ar atmosphere. Adding water vapor during pyrolysis significantly increased the specific surface area. When 10% tetraethyl orthosilicate (TEOS) was added during pyrolysis in an Ar + H2O atmosphere, the surface area reached 1953.94 m2 g−1, compared to 880.09 m2 g−1 for the polysiloxane base treated in Ar. This study examines the principles behind phase composition and development, providing a novel approach to creating materials with exceptionally high surface areas. Using specific pyrolysis atmospheres and organic additives to enhance surface area is a promising method for producing highly porous SiOC ceramics.114
5. Potential uses of preceramic inorganic polymers
Different materials can be used for cleaning up the environment, offering various methods to achieve this goal. Given the intricate nature of these materials, their propensity for rapid evaporation, and their restricted reactivity, it is essential to address the containment and breakdown of environmental contaminants. Consequently, contemporary studies have focused on utilizing ceramics to develop innovative strategies for ecological restoration. Inorganic preceramics play a crucial role in various environmental applications, including the detection, monitoring, and quantification of pollutants, as well as their involvement in the prevention, management, and remediation of these contaminants. Numerous ceramic materials are currently under examination for their potential as catalysts in pollution prevention, control, and remediation efforts. Inorganic preceramic materials have emerged as essential components in environmental remediation, attributed to their unique characteristics and versatile applications. This review section examines the body of research concerning ceramics and their diverse ecological applications. Readers are expected to develop a deeper understanding and appreciation of this field through this exploration, which may inform their research endeavours.5.1 Coatings and composites
It has been reported that preceramic polymers can produce low-dimensional products, particularly coatings.133 These polymers can be applied to various substrates through various deposition methods, which may utilize liquid or vapour phases. Preceramic polymers can be enhanced by incorporating various fillers to modify specific properties and facilitate the formation of thicker layers without cracking. When fillers are incorporated into a matrix, several technological challenges arise, particularly when achieving the desired particle fragmentation and uniformity levels. As a result, there are constraints on achieving the requisite layer thickness. This can lead to uncontrolled cracks and pores, which diminish the adhesion between the layer and the underlying surface.134 It is recommended to make ceramic thin films using unfilled polymer precursors. For instance, the meticulous preparation of the polysiloxane network enables SiOC materials to demonstrate advantageous characteristics, including excellent thermal stability, abrasion resistance, and reduced surface energy.Furthermore, this process can yield dense layers that protect coatings in challenging environments, such as those exposed to corrosive gases with moisture at elevated temperatures or radiation.135,136 Research on synthesising polysiloxane films devoid of additive incorporation has encountered difficulties related to the alteration of precursors and the structural stability of the resultant materials. This problem is attributed to the use of unmodified polysiloxanes or small alkoxysilanes with only one Si atom during the sol–gel polymerization process.137,138 While achieving a well-defined precursor structure is possible, this necessitates the use of monomers that possess multiple Si atoms and substantial quantities of solvent to address the diminished solubility in water.139
Malinowska et al. created SiOC layers on stainless steel surfaces using polysiloxane networks without the addition of fillers (Fig. 4a–e). This approach minimizes shrinkage during the pyrolysis process. It improves the adhesion of the SiOC layer to the substrate—the design of the precursor aimed to reduce weight loss during annealing, thereby achieving material shrinkage. The cross-linking of polymers to create precursor coatings was accomplished through a hydrosilylation reaction. Given that this reaction necessitates the presence of asymmetric groups, two distinct SiOC coatings were developed on stainless steel, resulting from the thermal decomposition of the polymer precursors polymethylvinylsiloxane/2,4,6,8-tetramethylcyclotetrasiloxane (V3/D4H) and polymethylhydrosiloxane/1,3,5,7-tetravinyl-1,3,5,7-tetramethylcyclotetrasiloxane (PMHS/D4Vi). Applying SiOC layers on the chosen steel substrate significantly improved its harsh temperature corrosion resistance, with the one coated with pyroV3/D4H exhibiting superior oxidation resistance compared to the one coated with PMHS/D4Vi. Without a protective coating, a clear difference in corrosion resistance is observed between layers made from precursors with vinyl groups in the polymer chain (pyroV3/D4H) and those linked to the cross-linking agent (PMHS/D4Vi). This difference is primarily due to the latter having more microcracks. These microcracks happen because the Si–H bonds in PMHS are blocked, which slows down the material buildup during the spin coating process. Additionally, lower bonding density makes the material more likely to form microcracks.134
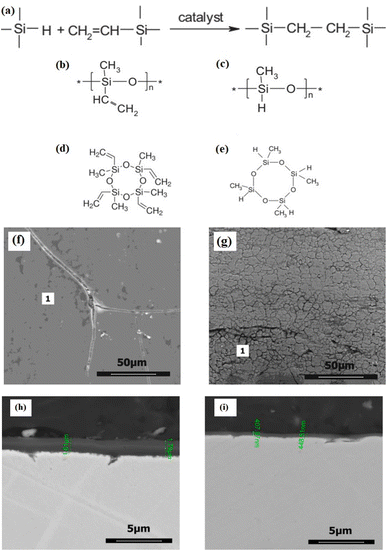 | ||
| Fig. 4 (a) Schematic illustration of the hydrosilylation process, along with the structural representations of the compounds utilized in the research, (b) V3 polymer, (c) PMHS polymer, (d) D4Vi, (e) D4H. SEM images depicting the surface morphology of steel coated with SiOC layers: (f) pyroV3/D4H, (g) pyroPMHS/D4Vi. Also, cross-section SEM images of steel coated with SiOC layers: (h) pyroV3/D4H, (i) pyroPMHS/D4Vi. Figures (a)–(i) were adapted by permission from: Surface and Coatings Technology, 2021, 407, 126760.134 | ||
Fig. 4f and g illustrates notable variations in layer morphology contingent upon the precursor utilized. In contrast to the PMHS/D4Vi layer, the V3/D4H layer exhibits sporadic cracking, characterized by a uniform coating with minimal defects. The occurrence of cracks in the thicker layer (∼1.7 μm) of V3/D4H (Fig. 4h) can be attributed to the coating's thickness, which also influences the thermal evolution of the layer. During the pyrolysis process, the polymer precursor undergoes a mass transformation and experiences an increase in density. This transformation leads to in-layer shrinkage, which subsequently results in the formation of cracks. Bulky layers are particularly susceptible to this phenomenon, thereby imposing a limitation on the achievable layer thickness.
Conversely, the layer formed from the PMHS polymer is significantly thinner, with a maximum thickness of approximately ∼450 nm (as shown in Fig. 4i), indicating a reduced impact of shrinkage on thinner layers. It is essential to highlight that, despite the presence of cracks, the layer maintains a uniform structure and exhibits strong adhesion to the metal substrate.134 Furthermore, the process temperature can be kept at relatively low levels (below 800 °C) to minimize the risk of harm to the underlying substrate. Finding low-temperature and financially feasible methods to shield metal surfaces from oxidation, wear, and corrosion is a top priority. Although the interactions at the interface during processing might enhance adherence to the metal, it is crucial to prevent the formation of brittle phases—coatings created by incorporating silicon or carbon clusters, which may be suitable for optoelectronic applications. Carbon fibres and carbon/carbon composites are coated with oxidation protective layers.140 By incorporating fillers, layers with controlled porosity can be fabricated, making them suitable for use as catalyst supports or in biomedical systems. The application of preceramic layers processed at low temperatures is particularly noteworthy for their ability to coat extensive surfaces, such as train carriages. These layers form transparent, strong coatings that stop inks, permanent markers, spray paints, stickers, dirt, and other contaminants from sticking.
They also help protect against weathering, corrosion, and oxidation. In addition, preceramic polymer films have been successfully used as a bonding agent for ceramic matrix composites and solid ceramic materials.141 In this context, the incorporation of fillers plays a crucial role in reaching desirable characteristics and an appropriate microstructure within the ceramic interlayer. Barroso et al. created a dual-layered thermal barrier coating (TBC) system utilizing polysilazane, comprising both a bond coat and a top coat. The upper layer combines passive fillers like YSZ and active ones like ZrSi2 within an (organic)silazane matrix. The top coat application onto steel substrates was achieved through tape casting, a method noted for its excellent reproducibility and minimal use of suspension. Following the removal of solvents at 110 °C, the coatings and their constituents underwent pyrolysis for one hour at temperatures of 500 °C and 1000 °C, respectively, for the top coats. After this, they were subjected to further investigation. The findings indicated that when subjected to temperatures exceeding 500 °C, ZrSi2 undergoes a controlled oxidation process, resulting in the formation of thermally stable ZrO2 and SiO2 phases. These phases serve as protective barriers, preventing further degradation in oxidative environments up to 1000 °C. Moreover, the volumetric expansion resulting from the oxidation of ZrSi2 effectively offsets the natural shrinkage of polymers during pyrolysis, leading to the development of dense and crack-free coatings.
In contrast, coatings that consist solely of passive fillers, such as YSZ, demonstrate significant cracking and compromised structural integrity due to the absence of reactive compensation mechanisms. The synergistic integration of both active and passive fillers enhances thermal expansion compatibility with metal substrates and mitigates thermal mismatch stresses. These results underscore the essential function of active fillers such as ZrSi2 in optimizing the thermo-structural performance of PDC-based TBC systems for prolonged high-temperature applications.142
Niu et al. created a MoSi2–SiOC–Si3N4 (MSS) coating on SiC-coated C/C composites using a slurry method. The slurry was made by mixing MoSi2 and Si3N4 powders in high hydrogen silicone oil (H–PSO), with tetramethyl tetravinylcyclotetrasiloxane (Vi-D4) as the cross-linking agent and platinum chloride as the catalyst. The coating was heat-treated at 1000 °C for 2 hours in nitrogen gas to form MSS, with an average thickness of 140 μm. They also made a MoSi2–SiOC outer coating using the same slurry method for comparison. The oxidation behavior of the C/C composites with different outer coatings was tested at 1500 °C in air. The results showed that the MSS coating provided better protection against oxidation than the MoSi2–SiOC coating. Additionally, the antioxidant properties of the MSS coatings are strongly influenced by the amount of Si3N4.143
5.2 Removal of environmental pollutants
A lot of research is currently focused on creating new adsorbents and improving their ability to adsorb. Preceramic polymers offer numerous benefits because their molecular structure can be easily modified, and they can be shaped into various forms using plastic-forming methods.144 Materials made from preceramic polymers can be modified to function as effective adsorbents for removing heavy metals such as lead (Pb), mercury (Hg), and arsenic (As) from industrial wastewater.145 These materials' high surface area and chemical versatility make them suitable for capturing toxic ions. Polysiloxanes are recognized as the most cost-effective among all polymeric precursors. Their availability is high and can be processed under normal atmospheric conditions. Amorphous SiOC can be made by heating it to temperatures between 800 and 1400 °C. It remains an undeniable fact that there is a notable lack of scientific literature on the specific applications of porous and high-surface-area PDCs. Recently, materials derived from complex processing methods, including PDCs aerogels146 and composites created from polysiloxane and wood,147 have been evaluated for their efficacy as dye adsorbents.148 Zeydanli et al. produced a SiOC with exceptional permeability and a large surface area achieved through a preceramic polymer blend combined with a catalyst. Following the annealing and thermal decomposition processes, specific samples underwent etching with HF to yield carbon-rich SiOC (C-rich SiOC), which was explicitly identified as sample W–HF.W and W–HF were first tested to assess their ability to remove heavy metals, particularly Cr(III), Pb(II), and Cd(II), as well as cationic dyes such as methylene blue (MB), rhodamine B (RhB), and crystal violet (CV). The tests were done separately and in combinations. HF-treated SiOC samples with high surface area showed a strong ability to adsorb cationic dyes. The higher adsorption of dyes, compared to metal ions, is due to the Si–OH bonds, which are more common in HF-treated SiOC and free carbon. These bonds interact with organic dyes through (a) van der Waals forces between the dyes and the sp2 hybridized carbon, and (b) electrostatic interactions involving Si–OH groups and carbon–oxygen complexes.149 Research conducted over the last thirty years identified Si3N4 as one of the most durable and resilient ceramic materials for structural applications. However, recent investigations have revealed its extraordinary surface biochemistry in the present century. This paper provides a comprehensive overview of the potential applications of Si3N4 in various fields, including disease diagnosis and treatment, personalized healthcare, agricultural practices, food and water safety, and environmental conservation.150 Si3N4 exhibits a notable ability to support metal oxides, thereby preventing agglomeration of these oxides. Si3N4 exhibits many remarkable characteristics, including exceptional oxidation resistance, significant mechanical strength, impressive thermal stability, and low density. In the realm of photocatalytic activities, it serves a vital role as a support material that facilitates the reduction of charge recombination and enhances the efficient transport of charge carriers. Based on this, Sharma et al. have developed a Fe3O4/ZnO/Si3N4 nanocomposite, which may serve as an effective photocatalyst. The photocatalytic performance of the material has been investigated for the degradation of yellow sunset and methyl orange (MO) in aqueous solutions. The findings indicated that the degradation efficiencies exceeded 90% for sunset yellow and 96% for MO. Inhibition experiments revealed that hydroxyl radicals (˙OH) play a crucial role in the degradation mechanism. Furthermore, a reusability assessment conducted over six consecutive cycles demonstrated sustained photocatalytic activity, highlighting its effectiveness even after multiple uses. Additionally, antimicrobial tests performed on E. coli and Pseudomonas strains exhibited a notable inhibition zone.151
Polysilsesquioxane (PSQ) represents a class of organic–inorganic hybrid materials characterized by a backbone of Si–O inorganic bonds complemented by organic groups in the side chains. This unique composition and structural arrangement endow PSQ with remarkable properties. Notably, the organic groups attached to silicon can participate in various chemical reactions, enabling further functionalization. Moreover, PSQ exhibits exceptional resistance to corrosion, impressive thermal stability, and notable chemical reactivity, making it suitable for applications in semiconductor technology, catalysis, adsorption, and other domains. As an adsorbent, PSQ typically demonstrates a high capacity and rapid rate of adsorption.145 Research conducted by Niu et al. involved the synthesis of a thiol-functionalized PSQ designed explicitly for the adsorption of Hg(II) and Mn(II) ions from solutions.152 Additionally, Wang et al. synthesized two unique varieties of fibrous adsorbents through the application of thiol- and amino-functionalized PSQ onto poly(p-phenyleneterephthalamide) fibres and subsequently evaluated their effectiveness in the adsorption of Hg(II).153
Xu et al. created magnetic composites of PSQ/CNTs using the sol–gel method, which involved carboxylic carbon nanotubes (CNTs–COOH), 3-aminopropyl-trimethoxysilane (APTMS), and 3-mercaptopropyl-trimethoxysilane (MPTMS). At the same time, they added magnetite to the composites to improve their ability to separate from water. These composites were used to recover Au(III) from wastewater, and the results showed that the PSQ/CNTs magnetic composites were very effective at adsorbing Au(III).145
5.3 Microwave absorption
The rapid growth of electronic devices and information technology has led to extensive research on materials that can mitigate the harmful effects of interference and radiation caused by electromagnetic waves (EMW).14 In particular, ceramics produced through the PDC method have garnered significant attention in recent research due to their improved capabilities in microwave absorption and electromagnetic field (EMF) shielding. Recent years have witnessed rapid progress in the development of microwave absorption materials, which significantly diminish the strength of incoming electromagnetic waves. This plays a crucial role in implementing stealth technology within the defense and security sectors. Among these, ceramic materials, characterized primarily by their dielectric loss, exhibit distinctive physicochemical properties. The distinctive oxidation resistance and remarkable stability at elevated temperatures of ceramics make them more advantageous than alternative materials for application as microwave absorption substances, especially in modern high-temperature and demanding conditions.154 PDCs represent a method for producing microwave-absorbing ceramics via a conversion process that transforms polymers into ceramics. The unique characteristics of polymers and ceramic materials enable the use of PDCs in fabricating a wide range of microwave-absorbing ceramics. This is achieved through various processing techniques, including fibres, composites, powders, and specialized structures created through 3D printing. SiCN ceramics have attracted considerable attention in Si-based PDCs in recent decades, mainly owing to their enhanced thermal and oxidation resistance relative to SiOC and SiC ceramics.Recent developments in the utilization of ceramics derived from polysilazane for electromagnetic interference (EMI) absorption have led to the creation of various composites, including SiCN,155 SiCN/Fe,156 and SiCN/Ni.157 The electrospinning technique, combined with subsequent thermal processing, has been utilized for the template-free production of PDC fibers exhibiting EMF absorption characteristics.158 Incorporating ferric and nickel compounds into SiCN fibers typically induces magnetic loss,159,160 which may lead to increased weight density, susceptibility to corrosion, processing challenges, and elevated costs. Incorporating carbon into SiCN ceramics can enhance dielectric characteristics, facilitate the production of lighter materials, and bolster resistance in harsher environments. The effectiveness of EMF shielding provided by PDC fibres integrated with carbon has been demonstrated in various studies, including those SiC/Si3N4 fibres that incorporate in situ embedded graphite,161 C/SiC fibres,162 graphite–SiC wires.163 Ramlow et al. investigated the microwave absorption and EMF shielding characteristics of electrospun SiCN/C fibres. A hybrid approach utilizing electrospinning alongside the PDC method was implemented to produce SiCN fibres, which integrated carbon structures formed in situ. This fabrication technique was specifically designed for applications related to microwave absorption and EMF shielding within the Ku band (12.4–18 GHz). For comparative purposes, additional SiCN fibres were synthesized. In this process, polysilazane was used to provide SiCN. At the same time, PAN was added to the solution to improve the electrospinning of the ceramic-filled polymer and serve as a carbon source. The analysis of the samples revealed varying trends in EMF shielding effectiveness, specifically regarding their reflection, transmission, and absorption characteristics. Notably, incorporating carbon into the SiCN matrix resulted in a doubling of EMF absorption compared to SiCN fibres alone. The SiCN/C fibres exhibited a minimum reflection coefficient (S11) of −12 dB, indicating a high shielding efficiency that effectively blocked over 90% of EMF energy.
In contrast, SiCN fibres exhibited an S11 value of −7 dB, translating to roughly 80% attenuation of EMF energy. Computational simulations further elucidated the superior electromagnetic shielding properties of SiCN/C relative to SiCN, attributing this enhancement to increased conduction losses from the presence of free conductive carbon and diminished dipole and surface polarization effects due to defects. The fibres demonstrated resilience against acidic and alkaline conditions, as well as oxidation, at temperatures of up to 600 °C. Additionally, incorporating a carbon source resulted in a 17% reduction in weight for SiCN/C fibres compared to SiCN fibres. This study presents a viable method for producing SiCN/C fibres with EMF absorption characteristics, suitable for application as lightweight materials in challenging environments.164
Another Si-based PDC, quaternary PDC SiBCN, noted for its exceptional high-temperature stability and resistance to oxidation, stands out as the primary material in the realm of high-temperature microwave absorption ceramics documented in the literature. Currently, to address the issue of low dielectric loss associated with PDC–SiBCN, many researchers are enhancing EMW attenuation through the incorporation of high dielectric-loss materials such as carbon-based materials or by employing transition metals like iron and hafnium to catalyze in situ crystallization processes.165,166 For composite materials, filler distribution has consistently been a challenge. Even though transition metals help transform amorphous SiBCN ceramics into crystals, oxidation resulting from phase separation weakens the ceramic's resistance to oxidation. Creating amorphous SiBCN ceramics with microwave-absorbing properties via a single-source precursor exemplifies a novel strategy to overcome the constraints associated with the techniques above. Luo et al. conducted a study in which they developed amorphous SiBCN ceramics for microwave absorption applications. This was achieved using a single-source hyperbranched polyborosilazane containing benzene rings, utilizing a one-pot synthesis approach. Dichlorodiphenylsilane increased carbon incorporation in the pyrolyzed SiBCN ceramics and improved the ordered carbon ratio. Changing the monomer ratio and heating temperature of the ceramic material produced PDCs with strong microwave absorption properties while maintaining their structure. This process resulted in a lowest reflection coefficient (RCmin) of −30.79 dB and a good absorption bandwidth (EABmax) of 4.07 GHz.
Furthermore, the PDC-derived SiBCN ceramics exhibited remarkable thermal stability, showing negligible mass loss in Ar and air atmospheres up to 1400 °C. This innovative and efficient method for preparing PDC–SiBCN materials holds promise for large-scale production. It offers new perspectives for developing microwave absorption materials suitable for extreme conditions.167
5.4 Sensors for environmental monitoring
Sensors play a crucial role in advanced systems that necessitate detection and regulation. The research conducted by Nagaiah et al. investigates the applicability of high-temperature heat flux (HTHF) sensors within gas turbine systems. They have effectively engineered and fabricated these sensors by leveraging recent innovations in a novel ceramic material sourced from PDCs. The PDC–HTHF sensor was developed using SiCN, which was synthesized from an innovative polymer via lithography. This method was proposed as a viable microfabrication technique for sensor production, with conductivity regulated through the careful selection of composition and treatment parameters. SiCN sensors preserve their structural and functional integrity in extremely high-temperature conditions due to a combination of inherent material stability, electrical tunability, and versatile fabrication methods. The amorphous SiCN matrix demonstrates remarkable thermal and oxidation resistance, maintaining stability at temperatures surpassing 1400–1500 °C, even in reactive environments such as combustion gases or turbine flows.Additionally, these ceramics provide adjustable electrical conductivity and a high temperature coefficient of resistance (TCR), with values reaching up to 4000 ppm per °C, facilitating dependable thermal sensing throughout extensive thermal cycling. Structurally, the monolithic, crack-free design achieved via direct pyrolysis of preceramic polymers eliminates interfacial failures that are typical in multilayered or bonded systems. Moreover, compatibility with lithographic and micro-lithographic fabrication techniques enables the precise integration of SiCN sensors onto complex microscale surfaces.
In contrast, traditional heat flux sensors that utilize metal films or wires are constrained by lower thermal thresholds (approximately 1100 °C), susceptibility to oxidation, and mechanical fragility. Consequently, SiCN-based PDC sensors offer a robust and scalable alternative for high-temperature sensing applications in aerospace, energy, and thermal protection systems.168 Additionally, PDC sensors are produced due to their semiconducting properties, particularly their temperature-dependent conductivity. SiC sensors have found extensive application in environments with temperatures not exceeding 500 °C. PDC sensors can function as reliable high-temperature microsensors, utilizing the PDCs' piezoresistive principle or doping, which requires sensing materials to withstand harsh environments at high temperatures without compromising their functionality and structure.169 Developing strain sensors that exhibit high responsiveness across a broad spectrum of temperatures and strains remains a significant challenge, particularly in high-temperature conditions. Yang et al. have introduced a dual-functional sensor featuring a beam concealer structure constructed from polymer-derived SiCN ceramics capable of monitoring temperature and strain. The performance of this integrated sensor was evaluated across a temperature range extending from ambient conditions to 1000 °C, with a comprehensive investigation of the fundamental sensing mechanism. The resulting strain sensor demonstrated an impressive response time of 60 ms within a strain range of 0–3500 με, with a maximum strain rate reaching 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 με S−1.
200 με S−1.
Furthermore, this sensor exhibited remarkable stability and exceptional sensitivity in high-temperature environments. Notably, temperatures can be detected independently up to 1000 °C without interference. With its competitive sensitivity, outstanding stability, rapid response time, and extensive operational range, this innovative integrated sensor is promising for detecting temperature and strain signals in practical applications within extremely high-temperature settings.170 Fig. 5 illustrates the schematic diagrams summarizing synthesis methods and advanced applications. Table 2 represents the potential uses of preceramic inorganic polymers (preparation method, morphology, application, and advantages).
| Preparation method | Nanocomposite | Morphology | Application | Advantages | Ref. |
|---|---|---|---|---|---|
| Pyrolysis | Ni/SiOC(H) nanocomposites | A homogeneous blending of the composites | Removal of acid fuchsin | The OCMC–CS films showed improved adsorption characteristics compared to OCMC–CS–SiC and OCMC–CS–SiC@SiO2 films. At the same time, incorporating SiC and SiC@SiO2 nanoparticles conferred certain structural advantages that reduced the overall adsorption capacity | 171 |
| Oxidation and casting method | OCMC–CS–SiC@SiO2 nanocomposite film | A porous and interconnected network that becomes less porous upon the incorporation of SiC@SiO2 nanoparticles | MB dye adsorption | • The film presents a hopeful and eco-friendly answer for reducing dye contamination in water-based systems | 172 |
| • The presence of SiC or SiC@SiO2 in OCMC–CS led to a reduction in adsorption efficiency, which was unexpected | |||||
| • The decline in adsorption effectiveness may be attributed to the interplay between polymer functional groups and SiC or SiC@SiO2 nanoparticles, reducing accessibility for MB dye adsorption | |||||
| • The decline in adsorption effectiveness is corroborated by the observation that including SiC or SiC@SiO2 in pristine CS polymer enhanced adsorption efficiency, suggesting augmentation of surface functionalities and surface area within CS films | |||||
| Electrospinning followed by heat treatment | SiC-doped Yb2O3 structure | Nanofibers | Photocatalytic hydrogen evolution reactions | • The increased activity level can be credited to the biopolymer's combined impact (cellulose or chitosan) and the inorganic catalyst (Yb2O3/SiC) | 173 |
| • Cellulose and chitosan function as mediators and bio-templates in the photocatalytic water splitting reaction, which augments the photocatalytic activities of bio-templated catalysts | |||||
| Vat-based photopolymerization | Polymer-derived Ni/SiOC materials | Film | CO2 methanation | • A new method for making Ni-modified polymer-derived SiOC using vat-based photopolymerization in 3D printing is introduced | 174 |
| • The SiOC/Ni composites display catalytic properties that are appropriate for the methanation of CO2 | |||||
| • The SiOC/Ni materials that can be printed offer a promising approach to combining metal-modified polymer-derived ceramics with 3D printing, particularly for applications in catalysis | |||||
| Slow dip coating approach | SiOC ceramic composite membranes | Layered structure | Water purification | • The SiOC composite nanofiltration membranes showed strong mechanical strength and remained stable for long periods during cross-flow filtration | 175 |
| • The gradual immersion coating method enhanced the spreading and even distribution of the solution on the surface, resulting in a thick SiOC separation layer | |||||
| • The SiOC-1 composite nanofiltration membrane demonstrated a permeate flow rate of 8.97 L m−2 h−1 bar−1 and a significant dye molecule rejection rate of 95.1% | |||||
| • The potential applications of SiOC ceramic membranes with reduced sintering temperature are numerous and promising for future development | |||||
| Co-electrodeposition process | Si3N4–PbO2 nanocomposite | Multilayer heterojunction – the pyramidal shape of a typical β-PbO2 structure, which steadily decreases as the concentration of Si3N4-doping nanoparticles increases, enhances the crystallinity of lead dioxide | Electrocatalytic degradation of sulfathiazole | • The Si3N4–PbO2 electrode was used as the anode in breaking down sulfathiazole-simulated and natural sulfonamide wastewater through electrocatalysis | 176 |
| • The introduction of 6 g L−1 of Si3N4 resulted in a notable enhancement in the durability of the lead dioxide electrode, enabling it to operate for 94 hours | |||||
| • The electrocatalytic degradation process using the novel electrode is considered environmentally friendly for water treatment | |||||
| Vacuum hot-pressing at 1800 °C | SiCw/Si3N4 composite | The SiC whiskers are uniformly distributed at the Si3N4 grain boundaries and have a long, rod-shaped morphology | Microwave absorption | • SiCw incorporation led to grain refinement, elevated grain aspect ratio (GAR), and diminished average grain size (GAvg) in Si3N4 | 177 |
| • Adding SiCw significantly improved the mechanical properties of the ceramic material | |||||
| • Better microwave absorption was achieved because of the dielectric loss from the SiC phase and good impedance matching | |||||
| • Adding SiCw to the Si3N4 matrix results in improved mechanical properties of the ceramics and an enhanced ability to absorb microwave wavelengths | |||||
| Solvent-thermal method and in situ polymerization | MWCNT/Si3N4/PANI ternary composites | Carbon nanotubes (CNTs) are adhered to and intertwined on the exterior of Si3N4 and PANI clusters | Microwave absorption | • The presence of a conductive network involving MWCNT, Si3N4, and PANI contributes to an increase in conductive loss | 178 |
| • The composites display several loss mechanisms and excellent impedance matching | |||||
• The feeding ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 for MWCNT 40 for MWCNT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Si3N4 Si3N4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PANI resulted in an optimal reflection loss (RL) of −42.57 dB at 8.8 GHz PANI resulted in an optimal reflection loss (RL) of −42.57 dB at 8.8 GHz |
|||||
| • By incorporating Si3N4, the composites can display satisfactory resistance to elevated temperatures | |||||
| One-pot method of single-source precursors | Amorphous SiBCN | Hierarchical structure | Microwave absorption | • By adjustment of carbon content, the microwave absorption properties of amorphous SiBCN ceramics were enhanced | 167 |
| • Up to 1400 °C, the amorphous ceramic materials showed excellent stability | |||||
| • Its hierarchical structure and nano boundaries improve its electromagnetic wave attenuation capabilities | |||||
| • The maximum adequate absorption bandwidth was 4.07 GHz at a thickness of 2.80 mm | |||||
| Polymer infiltration pyrolysis method | Ti3SiC2 modified SiCf/BN/SiBCN composites | The EMI shielding | • The material exhibited a notable combination of impedance matching and electromagnetic loss at 1400 °C oxidation, leading to the optimal electromagnetic interference shielding effectiveness | 179 | |
| Controlled impregnation–pyrolysis cycle method | SiCN(Fe)/Al2O3 composite ceramics | The porous matrix | Electromagnetic wave absorption | • Enhancing iron and carbon can improve magnetic and dielectric losses, thereby enhancing the material's absorption properties | 180 |
| • The sample absorbs approximately 99% of the electromagnetic waves with 3 wt% FeAA at 1000 °C, with a reflectivity of −18 dB at 12 GHz | |||||
| Electrospinning and pyrolysis | SiCN/C fibers | Fibres | Microwave absorption and EMF shielding in Ku-band | • SiCN/C fibres are lightweight ceramic materials that are resistant to corrosion and heat | 164 |
| • The fibres offer superior EMF protection within the Ku-band frequency range of 12.4–18 GHz | |||||
| Ball mill and pyrolysis | SiBCN ceramics | A rough, porous, and randomly distributed microstructure | Temperature sensor | • SiBCN ceramics have a sensitivity of 3.5% per K from room temperature to 980 °C, with B-values reaching up to 3118 K | 181 |
| • The SiBCN sensor exhibits a more rapid rate of temperature response | |||||
| • The SiBCN ceramic temperature sensor is ideal for accurately measuring temperatures in extremely high heat and harsh environments | |||||
| Solvothermal, freeze-casting and pyrolysis | rGO reinforced SiBCN aerogels | SiBCN macroblocks form a necklace-like 3D structure interwoven with rGO nanosheets | Thermal insulation | The rGO/SiBCN aerogels provide excellent thermal insulation, withstand high temperatures, resist ablation, and remain stable in different conditions. They can resist oxidation at 800 °C in still air and maintain their structure at 1200 °C in an argon gas environment | 182 |
| RF magnetron sputtering | Cu/SiC/Si photo-sensor | Thin film | High-temperature devices | • The nanocrystalline thin film demonstrates a high level of efficiency in absorbing light | 183 |
| • The occurrence of traps that lead to extra photocurrents | |||||
| • The photodetector's exceptional responsivity is a result of the combination of various elements |
6. Conclusion and future prospects
PDCs constitute a fascinating category of advanced materials, characterized by their distinctive blend of structural integrity, adjustable functionality, and high-temperature resilience. In the last fifty years, these materials have garnered increasing interest due to their potential applications in extreme environmental and industrial settings. This review has explored the current status and emerging capabilities of PDCs in environmental contexts, focusing on their microstructural properties, the processes of ceramic transformation, and the interactions between dopants and fillers. There has been an increasing focus on combining PDCs with innovative methods, including 3D printing, utilizing ceramics derived from biomass, and investigating the biocompatibility of functionalized PDCs.Despite notable advancements, various limitations continue to obstruct the comprehensive implementation of PDCs. Specifically, a significant portion of existing research remains limited to controlled laboratory environments, lacking adequate long-term performance data in complex, real-world conditions. Obstacles remain in attaining enduring mechanical durability, resistance to oxidation, and functional reliability under cyclic thermal stresses and chemically hostile atmospheres. Likewise, although PDCs have demonstrated potential in MEMS and high-temperature sensor applications, additional miniaturization and system integration are still restricted by present fabrication capabilities and cost-related issues.
To address these challenges, forthcoming studies should adopt a multi-disciplinary strategy. The creation of doped or multi-phase PDC systems featuring delayed crystallization and improved oxidation resistance will be crucial for enhancing durability. Additionally, the progress in scalable, cost-effective preceramic polymer synthesis and solvent-free processing techniques is essential for facilitating high-volume production. Methods such as additive manufacturing, micro-stereolithography, and ink-based printing provide promising avenues for producing intricate sensor designs with reduced waste and enhanced performance. Simultaneously, the integration of PDCs with innovative technologies—including the utilization of bio-derived precursors and the investigation of biocompatibility in functionalized PDCs—offers exciting new possibilities for expanding their range of applications.
As research on PDC progresses, global initiatives are increasingly directed towards customizing these materials for multifunctional applications in extreme conditions. The integration of material innovation, precise fabrication, and functional synergy is set to pave the way for the next generation of high-performance PDCs, solidifying their role as essential facilitators of future technologies in aerospace, energy, electronics, and environmental monitoring.
Abbreviations
| PCIPs | Preceramic inorganic polymers |
| PDCs | Polymer-derived ceramics |
| SiC | Silicon carbide |
| Si3N4 | Silicon nitride |
| BN | Boron nitride |
| Al2O3 | Aluminum oxide |
| ZrO2 | Zirconium oxide |
| AIN | Aluminum nitride |
| SiO2 | Silicon oxide |
| SiOC | Silicon oxycarbide |
| SiCN | Silicon carbonitride |
| AM | Additive manufacturing |
| Mg | Magnesium |
| PDMS | Polydimethylsilane |
| PDMMPS | Polydimethyl-methylphenylsilane |
| PDMMS | Polydimethyl-methylsilane |
| κ | Thermal conductivity |
| CTE | Coefficient of thermal expansion |
| Cp | Specific heat capacity |
| α | Thermal diffusion rate |
| TSR | Thermal shock resistance |
| BNNTs | Boron nitride nanotubes |
| PUMVS | Poly(ureamethylvinyl)-silazane |
| TBC | Thermal barrier coating |
| PHPS | Perhydropolysilazane |
| DCP | Dicumyl peroxide |
| PBDPS | Poly-borodiphenylsiloxane |
| DPDCS | Diphenyldichlorosilane |
| PAlC | Polyaluminocarbosilane |
| PSQ | Polysilsesquioxane |
| OSR | Organosilane slurry residue |
| PEO | Polyethylene oxide |
| KIC | Fracture toughness |
| PAN | Polyacrylonitrile |
| SDS | Sodium dodecyl sulfate |
| V3 | polymethylvinylsiloxane |
| D4H | 2,4,6,8-Tetramethylcyclotetrasiloxane |
| PMHS | Polymethylhydrosiloxane |
| D4Vi | 1,3,5,7-Tetravinyl-1,3,5,7-tetramethylcyclotetrasiloxane |
| EMW | Electromagnetic wave |
| EMF | Electromagnetic field |
| C | Carbon |
| O | Oxygen |
| N | Nitrogen |
| B | Boron |
| H | Hydrogen |
| Al | Aluminium |
| CH2 | Methylene group |
[N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N] N] | Carbodiimide group |
| NCs | Nanocomposite |
| NC | Nitrogen dopant |
| LB | Lamp black |
| GR | Graphite |
| CB | Carbon black |
| AC | Activated carbon |
| Cdef | Defect concentration |
| PU | Polyurethane |
| PEO | Polyethene oxide |
| SiOCs | Silicon carbon oxides |
| HF | Hydrofluoric acid |
| SiOCs | Silicon-containing organic compounds |
| SDS | Sodium dodecyl sulfate |
| TEOS | Tetraethyl orthosilicate |
| PMHS/D4Vi | Polymethylhydrosiloxane/1,3,5,7-tetravinyl-1,3,5,7-tetramethylcyclotetrasiloxane |
| TBC | Thermal barrier coating |
| MSS | MoSi2–SiOC–Si3N4 |
| H–PSO | Hydrogen silicone oil |
| Pb | Lead |
| Hg | Mercury |
| AS | Arsenic |
| C-rich SiOC | C-rich SiOC |
| MB | Methylene blue |
| RhB | Rhodamine B |
| CV | Crystal violet |
| MO | Methyl orange |
| ˙OH | Hydroxyl radicals |
| CNTs–COOH | Carboxylic carbon nanotubes |
| APTMS | 3-Aminopropyl-trimethoxysilane |
| MPTMS | 3-Mercaptopropyl-trimethoxysilane |
| EMI | Electromagnetic interference |
| RCmin | Reflection coefficient |
| CNTs | Carbon nanotubes |
| GAR | Grain aspect ratio |
| GAvg | Average grain size |
| RL | Reflection loss |
Data availability
The data supporting the findings of this review can be obtained from the corresponding author upon request. Due to privacy concerns and other restrictions, the data are not publicly accessible.Author contributions
M. M. S. ideation, search, resources, and writing. M. M. conceptualization, supervision, graphics, and editing. A. R. A. review and editing. M. B. review and editing. A. M. co-supervision, project administration, and editing. E. N. Z. co-supervision, project administration, and editing.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors are thankful for the partial support of the Iran University of Science & Technology (IUST).References
- C. Vakifahmetoglu, D. Zeydanli and P. Colombo, Mater. Sci. Eng., R, 2016, 106, 1–30 CrossRef.
- T. Lacelle, K. L. Sampson, H. Yazdani Sarvestani, A. Rahimizadeh, J. Barroeta Robles, M. Mirkhalaf, M. Rafiee, M. B. Jakubinek, C. Paquet and B. Ashrafi, APL Mater., 2023, 11(7), 070602 CrossRef CAS.
- Q. Wen, Z. Yu and R. Riedel, Prog. Mater. Sci., 2020, 109, 100623 CrossRef CAS.
- K. Zhong, Z. Wang, J. Cui, X. Yu, Z. Yu, Y. Wang, Z. He, Y. Zhao and J. Zhao, Ceram. Int., 2024, 50, 36521–36536 CrossRef CAS.
- A. Dorieh, M. F. Pour, S. G. Movahed, A. Pizzi, P. P. Selakjani, M. V. Kiamahalleh, H. Hatefnia, M. H. Shahavi and R. Aghaei, Prog. Org. Coat., 2022, 165, 106768 CrossRef CAS.
- Y. Arai, R. Inoue, K. Goto and Y. Kogo, Ceram. Int., 2019, 45(12), 14481–14489 CrossRef CAS.
- D. Jia, B. Liang, Z. Yang and Y. Zhou, Prog. Mater. Sci., 2018, 98, 1–67 CrossRef CAS.
- B. Matsoso, W. Hao, Y. Li, V. Vuillet-a-Ciles, V. Garnier, P. Steyer, B. Toury, C. Marichy and C. Journet, J. Phys.: Mater., 2020, 3, 034002 CAS.
- K. Lin, X. Zong, P. Sheng, S. Zhao, Y. Qi, W. Zhang and S. Wu, J. Eur. Ceram. Soc., 2023, 43, 6804–6814 CrossRef CAS.
- A. H. Mir and S. N. Ahmad, Proc. Inst. Mech. Eng., Part L, 2021, 235, 1739–1756 CrossRef CAS.
- R. Ji, Y. Liu, Y. Zhang and F. Wang, Int. J. Refract. Met. Hard Mater., 2011, 29, 117–122 CrossRef CAS.
- R. P. Chaudhary, C. Parameswaran, M. Idrees, A. S. Rasaki, C. Liu, Z. Chen and P. Colombo, Prog. Mater. Sci., 2022, 128, 100969 CrossRef CAS.
- F. W. Ainger and J. M. Herbert, Angew. Chem., Int. Ed., 1959, 71, 653 Search PubMed.
- P. Colombo, G. Mera, R. Riedel and G. D. Soraru, J. Am. Ceram. Soc., 2010, 93, 1805–1837 CrossRef CAS.
- S. Yajima, J. Hayashi, M. Omori and K. Okamura, Nature, 1976, 261, 683–685 CrossRef CAS.
- F. Sarraf, S. V Churakov and F. Clemens, Polymers, 2023, 15, 4360 CrossRef CAS PubMed.
- R. Riedel, A. Kienzle, W. Dressler, L. Ruwisch, J. Bill and F. Aldinger, Nature, 1996, 382(6594), 796–798 CrossRef CAS.
- B. J. Ackley, K. L. Martin, T. S. Key, C. M. Clarkson, J. J. Bowen, N. D. Posey, J. F. Ponder Jr, Z. D. Apostolov, M. K. Cinibulk and T. L. Pruyn, Chem. Rev., 2023, 123, 4188–4236 CrossRef CAS PubMed.
- G. Mera and E. Ionescu, Encyclopedia of Polymer Science and Technology, 2002 Search PubMed.
- D. Eliche-Quesada, L. Pérez-Villarejo and P. J. Sánchez-Soto, Ceramic Materials - Synthesis, Characterization, Applications and Recycling, 2019 Search PubMed.
- R. N. Katz, Materials and Mechanical Design, 2006, p. 433 Search PubMed.
- R. Riedel, E. Ionescu and I. Chen, Ceramics Science and Technology: Volume 1: Structures, 2008, pp. 1–38 Search PubMed.
- V. Khabashesku, V. Filonenko, R. Bagramov, I. Zibrov and A. Anokhin, ACS Appl. Mater. Interfaces, 2021, 13(49), 59560–59566 CrossRef CAS PubMed.
- O. Icin, D. Zeydanlı, M. Biesuz and G. D. Soraru, Open Ceram., 2025, 100803 CrossRef CAS.
- Q. D. Nghiem, S. J. Cho and D.-P. Kim, J. Mater. Chem., 2006, 16, 558–562 RSC.
- S. Zhou, L. Yao, T. Zhao, H. Mei, L. Cheng and L. Zhang, Carbon, 2022, 196, 253–263 CrossRef CAS.
- M. Chen, R. Shang, P. M. Sberna, M. W. J. Luiten-Olieman, L. C. Rietveld and S. G. J. Heijman, Sep. Purif. Technol., 2020, 253, 117496 CrossRef CAS.
- Y. Jüttke, H. Richtera, I. Voigta, R. M. Prasadb, M. S. Bazarjanib, A. Gurlob and R. Riedelb, Chem. Eng., 2013, 32, 1891–1896 Search PubMed.
- A. H. Raza, S. Farhan, A. Ali, A. Sarfraz, M. A. Ahmad, M. Syväjärvi and R. Raza, J. Solid State Electrochem., 2024, 1–9 CAS.
- A. Samadi, L. Gao, L. Kong, Y. Orooji and S. Zhao, Resour., Conserv. Recycl., 2022, 185, 106497 CrossRef CAS.
- Y. Wang, X. Pei, H. Li, X. Xu, L. He, Z. Huang and Q. Huang, Ceram. Int., 2020, 46, 28300–28307 CrossRef CAS.
- K. Takeuchi, T. Suga and E. Higurashi, Sci. Rep., 2024, 14, 1267 CrossRef CAS PubMed.
- J. E. Mark, Acc. Chem. Res., 2004, 37, 946–953 CrossRef CAS PubMed.
- G. D. Sorarù, K. Girardini, M. Narisawa and M. Biesuz, J. Am. Ceram. Soc., 2021, 104, 3097–3104 CrossRef.
- M. Pelanconi, S. Bottacin, P. Colombo and A. Ortona, J. Eur. Ceram. Soc., 2023, 43, 5871–5881 CrossRef CAS.
- K. Sokolowski, S. Blazewicz, M. M. Marzec, A. Bernasik and A. Fraczek-Szczypta, Surf. Coat. Technol., 2024, 484, 130804 CrossRef CAS.
- K. Yuan, D. Han, J. Liang, W. Zhao, M. Li, B. Zhao, W. Liu, H. Lu, H. Wang and H. Xu, J. Adv. Ceram., 2021, 10, 1140–1151 CrossRef CAS.
- Q. Chen, D. Jia, B. Liang, Z. Yang, Y. Zhou, D. Li, R. Riedel, T. Zhang and C. Gao, Ceram. Int., 2021, 47, 10958–10964 CrossRef CAS.
- G. S. Krishnan, T. Ramcharan, R. R. Rao and S. Naveen, Ceram. Int., 2021, 47, 17502–17509 CrossRef.
- M. Arango-Ospina, F. Xie, I. Gonzalo-Juan, R. Riedel, E. Ionescu and A. R. Boccaccini, Appl. Mater. Today, 2020, 18, 100482 CrossRef.
- T. Centofanti, M. d. A. Silva, M. G. Segatelli and C. R. T. Tarley, in Recent Advancements in Polymeric Materials for Electrochemical Energy Storage, Springer, 2023, pp. 449–465 Search PubMed.
- C. Stabler, F. Roth, M. Narisawa, D. Schliephake, M. Heilmaier, S. Lauterbach, H.-J. Kleebe, R. Riedel and E. Ionescu, J. Eur. Ceram. Soc., 2016, 36, 3747–3753 CrossRef CAS.
- A. V Rau, K. Knott and K. Lu, Mater. Chem. Front., 2021, 5, 6530–6545 RSC.
- X. Guo, H. Wang, N. Fu, P. Xing, S. Wang, B. Wu and Y. Zhuang, Int. J. Appl. Ceram. Technol., 2024, 21, 1372–1381 CrossRef CAS.
- E. Ionescu and R. Riedel, Ceramics and composites processing methods, 2012, pp. 235–270 Search PubMed.
- Z. Ren, S. Bin Mujib and G. Singh, Materials, 2021, 14, 614 CrossRef CAS PubMed.
- L. J. Print, J. J. Liggat, S. Moug, H. Seaton and D. C. Apperley, Silicon, 2023, 15, 1355–1379 CrossRef CAS.
- A. C. Pierre, Introduction to sol-gel processing, Springer Nature, 2020 Search PubMed.
- K. A. Yur'evich, D. F. Valer'evich, V. S. Ivanov, A. S. Yegorov and V. V. Men'shikov, Orient. J. Chem., 2018, 34, 612 CrossRef.
- B. Arkles, Chemical Industries, Marcel Dekker, New York, 1996, pp. 667–676 Search PubMed.
- R. P. D'Amelia, J. Mancuso, W. F. Nirode and S. Singh, World J. Org. Chem., 2019, 7, 5–13 CrossRef.
- S. C. Shit and P. Shah, Natl. Acad. Sci. Lett., 2013, 36, 355–365 CrossRef CAS.
- E. V. Egorova, N. G. Vasilenko, N. V. Demchenko, E. A. Tatarinova and A. M. Muzafarov, in Doklady Chemistry, Springer, 2009, vol. 424, pp. 15–18 Search PubMed.
- F. Sarraf, S. V. Churakov and F. Clemens, Polymers, 2023, 15(22), 4360 CrossRef CAS PubMed.
- S. Beppu, Y. Tachibana and K. I. Kasuya, MRS Online Proc. Libr., 2023, 12(4), 536–542 CAS.
- S. Fu, M. Zhu and Y. Zhu, J. Adv. Ceram., 2019, 8, 457–478 CrossRef CAS.
- C. Chen, Z. Luo, H. Zeng, C. Hao, K.-F. Yang, Z. Li, G.-Q. Lai and P. Zhang, Macromolecules, 2024, 57, 8146–8153 CAS.
- M. Gerwig, U. Böhme and M. Friebel, Chem.–Eur. J., 2024, e202400013 CrossRef CAS PubMed.
- S. K. Shukla, R. K. Tiwari, A. Ranjan, A. K. Saxena and G. N. Mathur, Thermochim. Acta, 2004, 424, 209–217 CrossRef CAS.
- C. Vakifahmetoglu, I. Menapace, A. Hirsch, L. Biasetto, R. Hauser, R. Riedel and P. Colombo, Ceram. Int., 2009, 35, 3281–3290 CrossRef CAS.
- C. W. Chen, C. C. Huang, Y. Y. Lin, L. C. Chen and K. H. Chen, Diamond and Related Materials, 2005, 14, 1126–1130 CrossRef CAS.
- J. F. Wendel, N. Matthée, S. Schafföner and G. Motz, J. Am. Ceram. Soc., 2025, e20634 CrossRef.
- A. V Barysheva, G. M. Mochalov and S. S. Suvorov, Russ. J. Appl. Chem., 2021, 94, 1226–1231 CrossRef.
- C. Racles, M. Dascalu, A. Bele and M. Cazacu, Reactive and Functional Polymers Volume One: Biopolymers, Polyesters, Polyurethanes, Resins and Silicones, 2020, pp. 235–291 Search PubMed.
- F. Hosseini, K. J. Fadaee, Z. Salari and M. Ebrahimi, Chem. Methodol., 2023, 7(5), 383–391 CAS.
- J. L. Jayanthi, R. Palavalasa, S. Jampani, V. Miditana and G. Jalem, Chem. Methodol., 2023, 7(7), 569–580 Search PubMed.
- A. Abd Al Zahra and A. K. M. A. Al Sammarraie, Chem. Methodol., 2022, 6(1), 67–73 CAS.
- D. Erb and K. Lu, Microporous Mesoporous Mater., 2018, 266, 75–82 CrossRef CAS.
- S. J. Widgeon, Spectroscopic and calorimetric investigation of short and intermediate-range structures and energetics of amorphous SiCO, SiCN, and SiBCN polymer-derived ceramics, University of California, Davis, 2013 Search PubMed.
- M. Weinmann, Chemical Processing of Ceramics, 2005, p. 439 Search PubMed.
- H. Zhang, L. Xue, J. Li and Q. Ma, Polymers, 2020, 12, 672 CrossRef CAS PubMed.
- S. Widgeon, G. Mera, Y. Gao, S. Sen, A. Navrotsky and R. Riedel, J. Am. Ceram. Soc., 2013, 96, 1651–1659 CrossRef CAS.
- R. D. Archer, Inorganic and organometallic polymers, John Wiley & Sons, 2004 Search PubMed.
- J. Yuan and A. H. E. Müller, Polymer, 2010, 51, 4015–4036 CrossRef CAS.
- S. Mazerat and R. Pailler, Ceram. Int., 2021, 47, 2888–2891 CrossRef CAS.
- H. G. Hackbarth, T. S. Key, B. J. Ackley, G. Opletal, A. Rawal, L. Gallington, Y. Yang, L. Thomsen, M. B. Dickerson and T. L. Pruyn, J. Eur. Ceram. Soc., 2024, 44, 1932–1945 CrossRef CAS.
- Q. Wang, L. Yu, H. Nagasawa, M. Kanezashi and T. Tsuru, Sep. Purif. Technol., 2020, 248, 117067 CrossRef CAS.
- A. Xia, J. Yin, X. Chen, X. Liu and Z. Huang, Crystals, 2020, 10, 824 CrossRef CAS.
- M. A. Mazo, A. C. Caballero and J. Rubio, J. Alloys Compd., 2021, 889, 161698 CrossRef.
- G. Pang, F. Meng, Y. Chen, A. Katre, J. Carrete, B. Dongre, G. K. H. Madsen, N. Mingo and W. Li, Mater. Today Phys., 2024, 41, 101346 CrossRef CAS.
- B. Santhosh, C. Vakifahmetoglu, E. Ionescu, A. Reitz, B. Albert and G. D. Soraru, Ceram. Int., 2020, 46, 5594–5601 CrossRef CAS.
- H. Ramlow, L. F. B. Ribeiro, S. Schafföner, G. Motz and R. A. F. Machado, J. Eur. Ceram. Soc., 2024, 44, 5308–5318 CrossRef CAS.
- M. Balestrat, E. D. Acosta, O. Hanzel, N. Tessier-Doyen, R. Machado, P. Šajgalík, Z. Lenčéš and S. Bernard, J. Eur. Ceram. Soc., 2020, 40, 2604–2612 CrossRef CAS.
- P. Wang, F. Liu, H. Wang, H. Li and Y. Gou, J. Mater. Sci. Technol., 2019, 35(12), 2743–2750 CrossRef CAS.
- A. Xia, J. Yin, X. Chen, X. Liu and Z. Huang, Crystals, 2020, 10(9), 824 CrossRef CAS.
- L. C. Pardini and M. L. Gregori, J. Aerosp. Technol. Manage., 2010, 2(2), 183–194 CrossRef CAS.
- R. Sujith, S. Jothi, A. Zimmermann, F. Aldinger and R. Kumar, Int. Mater. Rev., 2021, 66, 426–449 CrossRef CAS.
- S. Yajima, T. Iwai, T. Yamamura, K. Okamura and Y. Hasegawa, J. Mater. Sci., 1981, 16, 1349–1355 CrossRef CAS.
- T. Ishikawa, Y. Kohtoku, K. Kumagawa, T. Yamamura and T. Nagasawa, Nature, 1998, 391, 773–775 CrossRef CAS.
- J. Liu, Z. Ahmad and J. Chen, Ceram. Int., 2024, 50, 16284–16291 CrossRef CAS.
- M. A. Mazo, D. Soriano and J. Rubio, Ceram. Int., 2023, 49, 12866–12875 CrossRef CAS.
- Q. Hanniet, E. Petit, S. Calas-Etienne, P. Etienne, K. Aissou, C. Gervais, P. Miele, B. Charlot and C. Salameh, Mater. Des., 2022, 223, 111234 CrossRef CAS.
- T. Rouxel, J. Sanglebœuf, J. Guin, V. Keryvin and G. Soraru, J. Am. Ceram. Soc., 2001, 84, 2220–2224 CrossRef CAS.
- S. Walter, G. D. Soraru, H. Brequel and S. Enzo, J. Eur. Ceram. Soc., 2002, 22, 2389–2400 CrossRef CAS.
- G. D. Sorarù, L. Kundanati, B. Santhosh and N. Pugno, J. Am. Ceram. Soc., 2019, 102, 907–913 CrossRef.
- N. Janakiraman, Z. Burghard and F. Aldinger, J. Non-Cryst. Solids, 2009, 355, 2102–2113 CrossRef CAS.
- A. Bauer, M. Christ, A. Zimmermann and F. Aldinger, J. Am. Ceram. Soc., 2001, 84, 2203–2207 CrossRef CAS.
- T. Noda, Handbook of Advanced Ceramics and Composites, 2019, pp. 1–26 Search PubMed.
- Y. Katsuda, P. Gerstel, J. Narayanan, J. Bill and F. Aldinger, J. Eur. Ceram. Soc., 2006, 26, 3399–3405 CrossRef CAS.
- S. Bernard, D. Cornu, P. Miele, M. Weinmann and F. Aldinger, Mechanical Properties and Performance of Engineering Ceramics and Composites: Ceramic Engineering and Science Proceedings, 2005, vol. 26, pp. 35–42 Search PubMed.
- G. Chollon, J. Eur. Ceram. Soc., 2000, 20, 1959–1974 CrossRef CAS.
- G. Chollon, Polymer Derived Ceramics: From Nano-structure to Applications, 2010, pp. 292–308 Search PubMed.
- A. Kovalčíková, J. Sedláček, Z. Lenčéš, R. Bystrický, J. Dusza and P. Šajgalík, J. Eur. Ceram. Soc., 2016, 36, 3783–3793 CrossRef.
- G. J. Leonel, X. Guo, G. Singh, C. Gervais and A. Navrotsky, J. Am. Ceram. Soc., 2023, 106, 5086–5101 CrossRef CAS.
- R. Michelle Morcos, G. Mera, A. Navrotsky, T. Varga, R. Riedel, F. Poli and K. Müller, J. Am. Ceram. Soc., 2008, 91, 3349–3354 CrossRef.
- E. Butchereit and K. G. Nickel, J. Mater. Process. Manuf. Sci., 1998, 15–21 CrossRef CAS.
- K. G. Nickel, E. Butchereit and C. Schumacher, On the Role of Boron in the Oxidation of Si-CNB Ceramics, The Electrochemical Society, 2000, p. 365 Search PubMed.
- Y. Wang, Y. Fan, L. Zhang, W. Zhang and L. An, Scr. Mater., 2006, 55, 295–297 CrossRef CAS.
- G. D. Sorarù, S. Modena, E. Guadagnino, P. Colombo, J. Egan and C. Pantano, J. Am. Ceram. Soc., 2002, 85, 1529–1536 CrossRef.
- R. G. Jones and S. J. Holder, Silicon-Containing Polymers: The Science and Technology of Their Synthesis and Applications, 2000, pp. 353–373 Search PubMed.
- M. Parchovianský, I. Parchovianská, P. Švančárek, D. Medveď, M. Lenz-Leite, G. Motz and D. Galusek, Molecules, 2021, 26, 2388 CrossRef PubMed.
- G. Mera, M. Gallei, S. Bernard and E. Ionescu, Nanomaterials, 2013, 5(2), 468–540 CrossRef.
- F. Li, L. Zhou, J.-X. Liu and G.-J. Zhang, Materials, 2022, 16, 220 CrossRef PubMed.
- D. Erb and K. Lu, J. Eur. Ceram. Soc., 2017, 37, 4547–4557 CrossRef CAS.
- E. Bernardo, L. Fiocco, G. Parcianello, E. Storti and P. Colombo, Materials, 2014, 7(3), 1927–1956 CrossRef CAS PubMed.
- G. Hasegawa, K. Kanamori, K. Nakanishi and T. Hanada, J. Mater. Chem., 2009, 19, 7716–7720 RSC.
- C. Durif, M. Wynn, M. Balestrat, G. Franchin, Y.-W. Kim, A. Leriche, P. Miele, P. Colombo and S. Bernard, J. Eur. Ceram. Soc., 2019, 39, 5114–5122 CrossRef CAS.
- J. Gu, S. H. Lee, H. S. Lee and J. S. Kim, J. Eur. Ceram. Soc., 2021, 41(4), 2297–2305 CrossRef CAS.
- B. J. Ackley, K. L. Martin, T. S. Key, C. M. Clarkson, J. J. Bowen, N. D. Posey, J. F. Ponder Jr, Z. D. Apostolov, M. K. Cinibulk, T. L. Pruyn and M. B. Dickerson, Chem. Rev., 2023, 123(8), 4188–4236 CrossRef CAS PubMed.
- Z. Yu, N. Yang, V. Apostolopoulou-Kalkavoura, B. Qin, Z. Ma, W. Xing, C. Qiao, L. Bergström, M. Antonietti and S. Yu, Angew. Chem., Int. Ed., 2018, 57, 4538–4542 CrossRef CAS PubMed.
- A. Feinle, M. S. Elsaesser and N. Huesing, Chem. Soc. Rev., 2016, 45, 3377–3399 RSC.
- H. Shi, J. Yang, Z. Li and C. He, Silicon Containing Hybrid Copolymers, 2020, pp. 1–21 Search PubMed.
- H. Shi, J. Yang, Z. Li and C. He, Silicon Containing Hybrid Copolymers, 2020, pp. 1–21 Search PubMed.
- P. Zhang, J. Duan, G. Chen, J. Li and W. Wang, Sol. Energy, 2018, 175, 44–53 CrossRef CAS.
- H. Chaney and K. Lu, Chem. Mater., 2023, 35, 3902–3910 CrossRef CAS.
- S. Sun, J. Yuan, W. Guo, X. Duan, D. Jia and H. Lin, J. Am. Ceram. Soc., 2024, 107, 777–784 CrossRef CAS.
- A. Baux, A. Goillot, S. Jacques, C. Heisel, D. Rochais, L. Charpentier, P. David, T. Piquero, T. Chartier and G. Chollon, J. Eur. Ceram. Soc., 2020, 40, 2834–2854 CrossRef CAS.
- Y. Jia, T. D. Ajayi, M. A. Roberts Jr, C.-C. Chung and C. Xu, ACS Appl. Mater. Interfaces, 2020, 12, 46254–46266 CrossRef CAS PubMed.
- Z. Cui, X. Chen, X. Li and G. Sui, Sens. Actuators, A, 2023, 350, 114144 CrossRef CAS.
- Y. J. Joo and K. Y. Cho, Mater. Res. Express, 2021, 8, 035603 CrossRef CAS.
- Y. Jia, M. A. R. Chowdhury, D. Zhang and C. Xu, ACS Appl. Mater. Interfaces, 2019, 11, 45862–45874 CrossRef CAS PubMed.
- B. H. Costa, C. R. T. Tarley, E. S. Ribeiro and M. G. Segatelli, Process. Appl. Ceram., 2023, 17, 118–132 CrossRef CAS.
- A. Francis, R. Detsch and A. R. Boccaccini, Ceram. Int., 2016, 42, 15442–15448 CrossRef CAS.
- A. Nyczyk-Malinowska, W. Niemiec, G. Smoła, R. Gaweł, M. Szuwarzyński and Z. Grzesik, Surf. Coat. Technol., 2021, 407, 126760 CrossRef CAS.
- C. Linck, E. Ionescu, B. Papendorf, D. Galuskova, D. Galusek, P. Ŝajgalík and R. Riedel, Int. J. Mater. Res., 2012, 103, 31–39 CrossRef CAS.
- M. Nastasi, Q. Su, L. Price, J. A. C. Santana, T. Chen, R. Balerio and L. Shao, J. Nucl. Mater., 2015, 461, 200–205 CrossRef CAS.
- P. Colombo, T. E. Paulson and C. G. Pantano, J. Sol-Gel Sci. Technol., 1994, 2, 601–604 CrossRef CAS.
- K. Wang, M. Günthner, G. Motz and R. K. Bordia, J. Eur. Ceram. Soc., 2011, 31, 3011–3020 CrossRef CAS.
- W. Niemiec, P. Szczygieł, P. Jeleń and M. Handke, J. Mol. Struct., 2018, 1164, 217–226 CrossRef CAS.
- J. Bill and D. Heimann, J. Eur. Ceram. Soc., 1996, 16, 1115–1120 CrossRef CAS.
- B. Liu, J. Sun, L. Guo, H. Shi, G. Feng, L. Feldmann, X. Yin, R. Riedel, Q. Fu and H. Li, Mater. Sci. Eng., R, 2025, 163, 100936 CrossRef.
- G. S. Barroso, W. Krenkel and G. Motz, J. Eur. Ceram. Soc., 2015, 35, 3339–3348 CrossRef CAS.
- F. Niu, Y. Wang, I. Abbas, S. Fu and C. Wang, Ceram. Int., 2017, 43, 3238–3245 CrossRef CAS.
- M. C. Bruzzoniti, M. Appendini, B. Onida, M. Castiglioni, M. Del Bubba, L. Vanzetti, P. Jana, G. D. Sorarù and L. Rivoira, Environ. Sci. Pollut. Res., 2018, 25, 10619–10629 CrossRef CAS PubMed.
- T. Xu, R. Qu, Y. Zhang, C. Sun, Y. Wang, X. Kong, X. Geng and C. Ji, Frontiers in Environmental Chemistry, 2021, 2, 706254 CrossRef.
- M. C. Bruzzoniti, M. Appendini, L. Rivoira, B. Onida, M. Del Bubba, P. Jana and G. D. Sorarù, J. Am. Ceram. Soc., 2018, 101, 821–830 CrossRef CAS.
- J. Pan, J. Ren, Y. Xie, X. Wei, Y. Guan, X. Yan, H. Tang and X. Cheng, Res. Chem. Intermed., 2017, 43, 3813–3832 CrossRef CAS.
- J. Pan, W. Shen, Y. Zhao, H. Sun, T. Guo, Y. Cheng, N. Zhao, H. Tang and X. Yan, Colloids Surf., A, 2020, 584, 124041 CrossRef CAS.
- D. Zeydanli, S. Akman and C. Vakifahmetoglu, J. Am. Ceram. Soc., 2018, 101, 2258–2265 CrossRef CAS.
- G. Pezzotti, J. Ceram. Soc. Jpn., 2023, 131, 398–428 CrossRef CAS.
- G. Sharma, A. Kumar, S. Sharma, M. Naushad, P. Dhiman, D.-V. N. Vo and F. J. Stadler, Mater. Lett., 2020, 278, 128359 CrossRef CAS.
- Y. Niu, R. Qu, X. Liu, L. Mu, B. Bu, Y. Sun, H. Chen, Y. Meng, L. Meng and L. Cheng, Mater. Res. Bull., 2014, 52, 134–142 CrossRef CAS.
- Y. Wang, R. Qu, F. Pan, X. Jia, C. Sun, C. Ji, Y. Zhang, K. An and Y. Mu, Chem. Eng. J., 2017, 317, 187–203 CrossRef CAS.
- C. Moysan, R. Riedel, R. Harshe, T. Rouxel and F. Augereau, J. Eur. Ceram. Soc., 2007, 27, 397–403 CrossRef CAS.
- K. Lu, D. Erb and M. Liu, J. Mater. Chem. C, 2016, 4, 1829–1837 RSC.
- Y. Wang, X. Guo, Y. Feng, X. Lin and H. Gong, Ceram. Int., 2017, 43, 15551–15555 CrossRef CAS.
- Y. Liu, Y. Feng, H. Gong, Y. Zhang, X. Lin, B. Xie and J. Mao, J. Alloys Compd., 2018, 749, 620–627 CrossRef CAS.
- Y. Hou, L. Cheng, Y. Zhang, Y. Yang, C. Deng, Z. Yang, Q. Chen, X. Du and L. Zheng, ACS Appl. Mater. Interfaces, 2017, 9, 43072–43080 CrossRef CAS PubMed.
- Y. Feng, X. Guo, J. Lu, J. Liu, G. Wang and H. Gong, Ceram. Int., 2021, 47, 19582–19594 CrossRef CAS.
- X. Guo, J. Lu, J. Liu, C. Liu, Y. Tong, J. Li, H. Sun, H. Peng, S. Wu and Y. Feng, Ceram. Int., 2022, 48, 20495–20505 CrossRef CAS.
- P. Wang, L. Cheng, Y. Zhang and L. Zhang, ACS Appl. Mater. Interfaces, 2017, 9, 28844–28858 CrossRef CAS PubMed.
- P. Wang, L. Cheng and L. Zhang, Carbon, 2017, 125, 207–220 CrossRef CAS.
- P. Wang, L. Cheng, Y. Zhang, W. Yuan, H. Pan and H. Wu, Composites, Part A, 2018, 104, 68–80 CrossRef CAS.
- H. Ramlow, L. L. Silva, C. Marangoni, M. R. Baldan and R. A. F. Machado, Diamond and Related Materials, 2024, 144, 110985 CrossRef CAS.
- W. Zhu, Z. Chen, J. Liang and D. Su, Carbon, 2022, 197, 65–75 CrossRef CAS.
- Y. Song, S. Jin, K. Hu, Y. Du, J. Liang and J. Kong, J. Am. Ceram. Soc., 2021, 104, 5244–5256 CrossRef CAS.
- C. Luo, Y. Wang, X. Hu, Y. Wu, Y. Wang, M. Chao and L. Yan, Mater. Res. Bull., 2024, 174, 112718 CrossRef CAS.
- N. R. Nagaiah, J. S. Kapat, L. An and L. Chow, J. Phys.: Conf. Ser., 2006, 34, 458 CrossRef.
- N. Li, Y. Cao, R. Zhao, Y. Xu and L. An, Sens. Actuators, A, 2017, 263, 174–178 CrossRef CAS.
- M. Yang, C. Ma, Y. Hu, K. Wang, R. Zhao, Y. Liang, D. Han, H. Wang, R. Zhang and G. Shao, Adv. Funct. Mater., 2024, 2400400 CrossRef CAS.
- Z. Yu, S. Li, P. Zhang, Y. Feng and X. Liu, Ceram. Int., 2017, 43, 4520–4526 CrossRef CAS.
- A. Javed, M. Islam, Y. O. Al-Ghamdi, M. Iqbal, M. Aljohani, S. Sohni, S. S. A. Shah and S. A. Khan, Int. J. Biol. Macromol., 2024, 256, 128363 CrossRef CAS PubMed.
- M. T. Genc, A. Sarilmaz, E. Aslan, F. Ozel and I. H. Patir, Mol. Catal., 2024, 556, 113915 CAS.
- J. Essmeister, L. Schachtner, E. Szoldatits, S. Schwarz, A. Lichtenegger, B. Baumann, K. Föttinger and T. Konegger, Open Ceram., 2023, 14, 100350 CrossRef CAS.
- Z. Xu, J. Lu, G. Zhang, R. Liu, W. Zhang and Q. Meng, Ceram. Int., 2024, 50, 23115–23123 CrossRef CAS.
- N. Yu, Y. Wang, H. Cao, R. Si, Z. Xu, X. Hong, X. Mao, K. Shen and J. Wu, Chem. Eng. J., 2024, 490, 151851 CrossRef CAS.
- J. Jing, Y. Zhao, A. Saleam, H. Gong, Y. Zhang, M. Sheng and J. Lu, J. Mater. Sci.: Mater. Electron., 2023, 34, 1384 CrossRef CAS.
- X. Ji, R. Qiao, Z. Xu, J. Liu and H. Yuan, Phys. Status Solidi A, 2024, 221, 2400121 CrossRef CAS.
- Q. Wang, Y. Peng, W. Jiang, H. Xu, W. Hai, Q. Shao and A. Li, Ceram. Int., 2024, 50, 20167–20175 CrossRef CAS.
- X. Lin, H. Gong and Z. Chen, Ceram. Int., 2023, 49, 23851–23863 CrossRef CAS.
- Q. Yan, S. Chen, H. Shi, X. Wang, S. Meng and J. Li, J. Eur. Ceram. Soc., 2023, 43, 7373–7380 CrossRef CAS.
- H. Wang, Z. Chen and D. Su, J. Mater. Sci. Technol., 2024, 179, 145–154 CrossRef CAS.
- A. Arora, P. Chander, S. Mourya, S. Singh, R. Chandra and V. K. Malik, Appl. Surf. Sci., 2024, 665, 160292 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |







