Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Catalytic system-controlled divergent reactions of pyrazolidinones with 3-alkynyl-3-hydroxyisoindolinones to construct diversified nitrogen-containing heterocyclic scaffolds†
Zhenwei Zhang‡
*abc,
Xiaoxue Zhang‡bcd,
Hao Wangbc,
Geyao Xieabc and
Yu Zhou *abcd
*abcd
aSchool of Pharmaceutical Science and Technology, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, Hangzhou 310024, China. E-mail: zhouyu@simm.ac.cn; zhangzhenwei@ucas.ac.cn
bDrug Discovery & Development Center, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai 201203, China
cUniversity of Chinese Academy of Sciences, Beijing 100049, China
dSchool of Chinese Materia Medica, Nanjing University of Chinese Medicine, Nanjing 210023, China
First published on 26th June 2025
Abstract
A catalytic system-controlled divergent reaction was reported to construct three distinct nitrogen-containing heterocycles from readily available starting materials via a precise chemical bond activation and annulation cascade. Notably, this is the first capture of pyrazolidinones and propargyl alcohols to construct tetrahydropyrimidinones via selective N–N bond activation and to generate previously unreported 3-acylindoles. This protocol demonstrates a broad substrate scope, moderate to good yields, and valuable transformations, laying a robust foundation for drug discovery applications.
Introduction
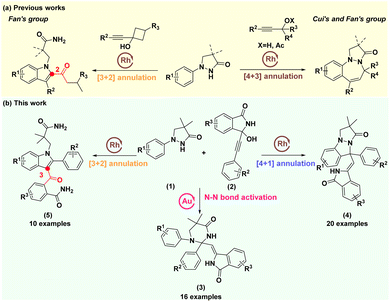
Nitrogen-containing heterocyclic structures are highly valued patterns present in a wide range of natural products, pharmaceuticals, agrochemicals, and organic materials.1–3 Recently, pyrazolidinones with a free NH moiety have been employed as a traceless directing group for constructing diverse privileged nitrogen-containing heterocyclic frameworks, including N-substituted indoles,4–6 pyrazolo[1,2-a]pyrazolones,7–9 N,N-bicyclic pyrazolidinones,10,11 diazepines12–14 and CF3-tethered indazoles.15 The propargylic alcohols have also been successfully involved in constructing diverse nitrogen-containing heterocyclic compounds.16,17 In 2020, Fan's group developed a pioneering Rh(III)-catalyzed redox-neutral coupling of 1-phenylpyrazolidinones with alkynyl cyclobutanols, enabling selective construction of 2-acylindoles and pyrazolo[1,2-a]pyrazolones through [3 + 2] and [4 + 1] annulations, respectively, by adjusting reaction conditions (Scheme 1a, left).18 In 2018, Ji's group reported three examples where Rh(III)-catalyzed [4 + 1] annulation of substituted 1-phenylpyrazolidinones with 1-phenylbut-2-yn-1-ol was used to construct desired pyrazolo[1,2-a]indazolones.19 In 2020, the groups of Cui and Fan independently accomplished the Rh(III)-catalyzed [4 + 3] annulations of pyrazolidinones with propargyl alcohols or propargylic acetates to construct benzodiazepines (Scheme 1a, right).20,21 Despite these elegant works, they mainly focused on C–H bond activation/annulation cascades, with limited exploration of N–N bond activation for heterocyclic framework construction from these substrates.In our continuous efforts to pursue rapid construction of diverse drug-like heterocyclic skeletons via transition-metal catalysed chemical bond activation/annulation cascades,22–27 we sought to develop more distinct and structurally complex nitrogen-containing heterocycles by employing the polycyclic propargyl alcohols. Interestingly, we observed an interesting phenomenon when the propargylic alcohols (2) with a large sterically hindered group were introduced as the coupling partner, 1-phenylpyrazolidinones (1) looked like a generalist that could be precisely and independently assembled into three distinct types of nitrogen-containing heterocyclic skeletons at different reaction sites of itself under different catalytic conditions (Scheme 1b). Of note, except for passing through a C(sp2)–H/N–H activation to generate pyrazolo[1,2-a]pyrazolones (4), we have captured a new N–N bond activation product tetrahydropyrimidinones (3) and an unusual product 3-acylindoles (5). These findings provide a versatile strategy for synthesizing diverse and biologically active nitrogen-containing heterocycles.
Results and discussion
We initially investigated this transformation by employing the 4,4-dimethyl-1-phenylpyrazolidin-3-one (1a, 0.24 mmol) and 3-hydroxy-3-(phenylethynyl)isoindolin-1-one (2a, 0.2 mmol) as model substrates, and the results showed that the desired N–N bond activation product 3aa could be constructed with a 20% yield under [Cp*RhCl2]2 (5 mol%) as the catalyst and 1,2-dichloroethane (DCE) as the solvent at 90 °C for 4 h (Table 1, entry 1). Surprisingly, adding silver acetate and acetic acid into the catalytic system would result in a new [4 + 1] annulation product 4aa with 8% yield (Table 1, entry 2), while the addition of cesium pivalate and acetic acid into the catalytic system simultaneously formed 4aa (28% yield) and a [3 + 2] annulation product 5aa (10% yield) (Table 1, entry 3). Interestingly, the yield of 5aa could be increased to 50% when we used dioxane as the solvent (Table 1, entry 4). Encouraged by these results, we further screened different catalysts, including Rh2(esp)2, CyJohnPhos AuCl, (Ph3P)AuCl, Ph3PAuNTf2, CyJohnPhos AuNTf2 and AuCl(IPr) (Table 1, entries 5–10). The results showed that the AuCl(IPr) offered the best catalytic activity (Table 1, entry 10) for the formation of 3aa, the yield of which could be increased to 54%. Subsequent solvent screening indicated that DCE was the most conducive to this transformation (Table 1, entries 11–13). Next, lowering the reaction temperature to 70 °C could further increase the yield to 65% (Table 1, entry 14), while increasing the reaction temperature to 100 °C resulted in a slight decrease in yield (Table 1, entry 15). In brief, the optimal reaction conditions for 3aa were as follows: 1a (0.24 mmol), 2a (0.2 mmol), and AuCl(IPr) (5 mol%) in DCE at 70 °C for 4 h (condition A). Likewise, the optimal reaction conditions for 4aa and 5aa were also separately investigated (see Tables S1 and S2†). The results displayed that when using 1a (0.24 mmol), 2a (0.2 mmol), [Cp*Rh(CH3CN)3](SbF6)2 (5 mol%), LiOAc (0.2 mmol), BzOH (0.1 mmol) at 100 °C for 8 h (Table 1, entry 16, condition B) and 1a (0.2 mmol), 2a (0.24 mmol), [Cp*RhCl2]2 (5 mol%), CsOPiv (0.2 mmol) and AcOH (0.2 mmol) in diglyme at 80 °C for 2 h (Table 1, entry 17, condition C) as their respective catalytic systems, the products 4aa and 5aa were effectively prepared with 60% and 80%, respectively. Their structures were confirmed by 1H and 13C NMR, MS and X-ray spectra.| Entry | Catalytic system | Solvent | Yield (%) | ||
|---|---|---|---|---|---|
| 3aa | 4aa | 5aa | |||
| a Reaction conditions: 1a (0.24 mmol), 2a (0.2 mmol), catalyst (5.0 mol%) in solvent (4 mL) at 90 °C for 4 h, NMR yield (1,3,5-trimethoxybenzene as the internal standard).b 1a (0.2 mmol), 2a (0.24 mmol), inorganic salts (0.4 mmol), AcOH (0.2 mmol) at 80 °C for 8 h, isolated yield.c 70 °C.d 100 °C.e 1a (0.24 mmol), 2a (0.2 mmol), [Cp*Rh(CH3CN)3](SbF6)2 (5 mol%), LiOAc (0.2 mmol), BzOH (0.1 mmol) at 100 °C for 8 h, isolated yield.f 1a (0.2 mmol), 2a (0.24 mmol), [Cp*RhCl2]2 (5 mol%), CsOPiv (0.2 mmol), AcOH (0.2 mmol) at 80 °C for 2 h, isolated yield. | |||||
| 1 | [Cp*RhCl2]2 | DCE | 20 | — | — |
| 2b | [Cp*RhCl2]2/AgOAc/AcOH | DCE | — | 8 | — |
| 3b | [Cp*RhCl2]2/CsOPiv/AcOH | DCE | — | 28 | 10 |
| 4b | [Cp*RhCl2]2/CsOPiv/AcOH | Dioxane | — | — | 50 |
| 5 | Rh2(esp)2 | DCE | 44 | — | — |
| 6 | CyJohnPhos AuCl | DCE | 31 | — | — |
| 7 | CyJohnPhos AuNTf2 | DCE | 44 | — | — |
| 8 | (Ph3P)AuCl | DCE | 44 | — | — |
| 9 | Ph3PAuNTf2 | DCE | 36 | — | — |
| 10 | AuCl(IPr) | DCE | 54 | — | — |
| 11 | AuCl(IPr) | MeCN | 15 | — | — |
| 12 | AuCl(IPr) | CHCl3 | 41 | — | — |
| 13 | AuCl(IPr) | HFIP | Trace | — | — |
| 14c | AuCl(IPr) | DCE | 65 | — | — |
| 15d | AuCl(IPr) | DCE | 50 | — | — |
| 16e | [Cp*Rh(CH3CN)3](SbF6)2/LiOAc/BzOH | CH3CN | — | 60 | Trace |
| 17f | [Cp*RhCl2]2/CsOPiv/AcOH | Diglyme | — | — | 80 |
With the optimized reaction conditions in hand, we firstly conducted a comprehensive investigation into the scope of pyrazolidinones 1 and coupling partners 2 under the optimal reaction condition to build diversified tetrahydropyrimidinones 3. As shown in Scheme 2, whether introducing halogen atoms (F, Cl and Br) or electron-donating groups (CH3 and OCH3) into 4-position of benzene ring of substrate 1, the reaction efficiencies were maintained at a moderate yield (Scheme 2, 3ba–3fa, 36–64%). However, introducing an F atom into 3-position of phenyl ring resulted in a decrease in yield (3ia, 25%). Subsequently, we investigated the effect of various substituents on the coupling partners 2. Notably, substrates 2 bearing halogen atoms (F, Cl and Br) or electron-donating groups (CH3, OCH3 and (CH2)4CH3) at the 4-position of the phenyl ring afforded corresponding products in moderate to good yields (3ab–3ag, 49–72%). Introducing CH3 and Cl groups into the 3-position of the phenyl ring also gave satisfactory results (3ai–3ak, 69–72%). Replacing the phenyl ring of 2 with the thiophene ring also provided a good yield (3al, 66%).
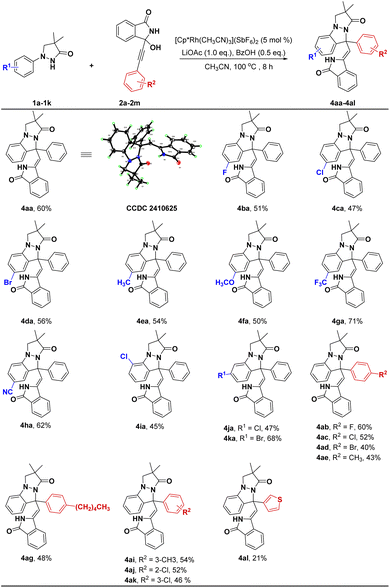
During the reaction condition screening, we observed the pyrazolo[1,2-a]pyrazolone derivative 4aa could be selectively constructed via a C(sp2)–H bond activation/[4 + 1] annulation cascade. As shown in Scheme 3, the reaction worked well with electron-withdrawing and electron-donating groups at the 4-position of the phenyl ring of substrates 1, affording 4ba–4ha with 47–71% yields (Scheme 3). Translocating Cl or Br to the 3-position of the phenyl ring could also successfully generate corresponding products with 45–68% yields (4ia–4ka). Next, various substituted groups at the 4-position of the phenyl ring of substrate 2 were explored, and the results indicated that all substrates could react smoothly with 1a to provide the corresponding products 4ab–4ag in moderate to good yields (40–60%). Likewise, introducing CH3 or Cl into the meta or ortho position of the benzene ring was also satisfactory (4ai–4ak). However, replacing benzene ring of 2 with thiophene ring gave a relatively low yield (4al, 21%).
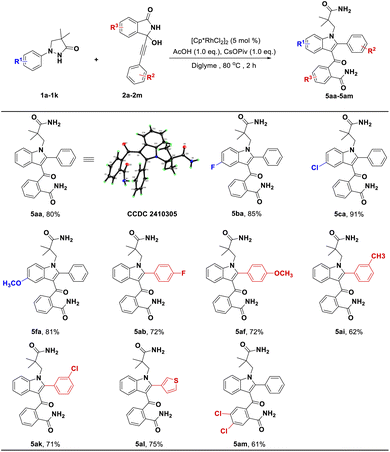
Another discovery was that we successfully obtained previously unreported 3-acylindoles via a C(sp2)–H bond activation and [3 + 2] annulation cascade from pyrazolidinone 1 and its coupling partner 2. As shown in Scheme 4, whether introducing electron-donating or electron-withdrawing groups into pyrazolidinones 1 or 3-alkynyl-3-hydroxyisoindolinones 2 could generate desired products in good yields (5ba–5fa, 81–91%; 5ab–5ak, 62–72%). In particular, in this transformation, replacing the benzene ring of 2 with the thiophene ring could provide a good yield (Scheme 4, 5al, 75%). Additionally, we also attempted to introduce the group into R3 position of substrate 2, the corresponding product 5am was obtained with good yield.
Given the potential application of these intriguing heterocycles, the gram-scale preparations for the products 3aa, 4aa, and 5aa were performed with 61%, 47%, and 67% isolated yields, respectively (Scheme 5a). Then, the synthetic transformations of compounds 4aa and 5aa were also explored. For example, the C–C double bond of 4aa could be selectively reduced using hydrogen gas and Pd/C, generating the desired product 6 with 80% yield (Scheme 5b). Interestingly, we successfully fulfilled a simultaneous transformation of two amide groups in 5aa, in which compound 5aa could be converted into nitrile product 7 with 84% yield in the presence of cyanuric chloride (Scheme 5b). Additionally, treatment of 5aa with triethylsilane and trifluoroacetic acid afforded a ring-closed product 8 (Scheme 5b).
Having established the substrate scope and utility of the product, we then conducted a series of experiments to explore the preliminary mechanism (Scheme 6). Firstly, 95% of ortho H could be deuterated by treating 1a with D2O under standard condition B, 25% of the ortho H could be deuterated by treating 1a with D2O under standard condition C, suggesting the reversibility of the C(aryl)–H bond cleavage during the formation of products 4 and 5 (Scheme 6a). Next, the kinetic isotope effect (KIE) values of 1.70 for 4aa and 1.17 for 5aa indicated that the C–H bond cleavage was likely not the turnover-limiting step (Scheme 6b). Moreover, three competitive experiments within three reaction conditions by introducing different substrates with electron-withdrawing and electron-donating groups were carried out. Treating a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of fluorine/methoxy substituted pyrazolidinone (1b/1f) with 2a under conditions A and C, respectively, showed that there is no apparent difference in the reaction rate between two substrates 1 with electron-withdrawing and electron-donating groups (Scheme 6c). These results indicated that these different groups are well tolerated in these transformations. However, we observed a high ratio (8.0) between 4ga and 4fa when treating a 1
1 mixture of fluorine/methoxy substituted pyrazolidinone (1b/1f) with 2a under conditions A and C, respectively, showed that there is no apparent difference in the reaction rate between two substrates 1 with electron-withdrawing and electron-donating groups (Scheme 6c). These results indicated that these different groups are well tolerated in these transformations. However, we observed a high ratio (8.0) between 4ga and 4fa when treating a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of trifluoromethyl/methoxy substituted pyrazolidinone (1g/1f) with 2a under condition B (Scheme 6c), suggesting that the electron-withdrawing substrates reacted faster in this transformation.
1 mixture of trifluoromethyl/methoxy substituted pyrazolidinone (1g/1f) with 2a under condition B (Scheme 6c), suggesting that the electron-withdrawing substrates reacted faster in this transformation.
Based on the above observations and literature precedents,18,21,24,28–32 three plausible catalytic mechanisms are proposed for N–N bond activations and C–H functionalizations. As shown in Scheme 7a, the propargylic alcohol 2a initially forms the intermediate A by coordinating with the Au(I) catalyst. Upon the entry of the pyrazolone 1a into the system, it undergoes alkyne hydroamination with Au(I) to yield complex B, which further forms the intermediate C after the intramolecular electrophilic addition. Finally, elimination of the Au(I) catalyst and the hydroxy group from C gives the desired product 3aa. The speculated mechanisms of the desired products 4aa and 5aa are shown in Scheme 7b. The NH directing group of 1a assists the activated Rh catalyst with the cleavage of the ortho C(sp2)–H bond, enabling the formation of a five-membered rhodacycle D. Next, the complexation of Rh(III) with the triple bond of 2a gives intermediate E. The following regioselective insertion of the alkynyl unit of 2a into the C–Rh bond of E generates intermediate F, which is most likely driven by additional coordination of the OH group with Rh(III) to overcome the unfavorable steric interactions between the isoindolin-1-one group and the Cp ligand. Further, the cleavage of the C–Rh bond and elimination of water under the assistance of benzoic acid offers the allenyl intermediate G. Finally, G undergoes nucleophilic addition and followed by protodemetalation to form target product 4aa. Specially, using [Cp*RhCl2]2 as the catalyst in diglyme results in the opposite regioselective insertion of the alkyne unit of 2a into the C–Rh bond of E to form intermediate H, probably because the solvent prevents the additional coordination of the OH group of propargylic alcohol with Rh(III) complex. Then protonation of intermediate H leads to the generation of intermediate I, which then undergoes an oxidative addition with N–N bond to form intermediate J. Finally, intermediate J undergoes a reductive elimination and a following N-demethylation/protonation to produce the desired product 5aa.
Conclusions
In summary, we have successfully fulfilled a catalytic system-controlled divergent reaction strategy to construct three types of nitrogen-containing heterocyclic skeletons through chemical bond activation/annulation routes of pyrazolidinones with 3-alkynyl-3-hydroxyisoindolinones. More importantly, this is the first capture of pyrazolidinones and propargyl alcohols to construct tetrahydropyrimidinones and unusual 3-acylindoles. These strategies exhibit a broad range of substrates, moderate to good yields, and valuable transformations, offering structural and biological potentials for further drug discovery.Data availability
The data supporting this article have been included as part of the ESI,† which includes experimental details, characterization data, NMR spectra, and HPLC spectra, along with single-crystal X-ray data for compounds 3aa (CCDC 2410303), 4aa (CCDC 2410625), and 5aa (CCDC 2410305).Conflicts of interest
The authors declare no competing interests.Acknowledgements
We thank the National Natural Science Foundation of China (82173656, 82373709, 82151219), Shandong Laboratory Program (SYS202205), and China Postdoctoral Science Foundation (2023M730821) for financial support.References
- C. Wallwey and S.-M. Li, Ergot alkaloids: structure diversity, biosynthetic gene clusters and functional proof of biosynthetic genes, Nat. Prod. Rep., 2011, 28, 496–510 RSC
.
- C. Yang, F. Song, J. Chen and Y. Huang, Construction of Pyridazine Analogues via Rhodium-mediated C-H Activation, Adv. Synth. Catal., 2017, 359, 3496–3502 CrossRef CAS
.
- W. Zi, Z. Zuo and D. Ma, Intramolecular dearomative oxidative coupling of indoles: a unified strategy for the total synthesis of indoline alkaloids, Acc. Chem. Res., 2015, 48, 702–711 CrossRef CAS PubMed
.
- Z. Zhang, H. Jiang and Y. Huang, Ruthenium-catalyzed redox-neutral C-H activation via N-N cleavage: synthesis of N-substituted indoles, Org. Lett., 2014, 16, 5976–5979 CrossRef CAS PubMed
.
- Z. Yang, Q. Yue, M. Yang, H. Zhang and X. Cui, Ru(II)-Catalyzed Tunable Cascade Reaction via C-H/C-C Bond Cleavage, J. Org. Chem., 2020, 85, 12960–12970 CrossRef CAS PubMed
.
- Z. Fan, H. Lu, W. Li, K. Geng and A. Zhang, Rhodium-catalyzed redox-neutral coupling of phenidones with alkynes, Org. Biomol. Chem., 2017, 15, 5701–5708 RSC
.
- N. Strašek, L. Lavrenčič, A. Oštrek, D. Slapšak, U. Grošelj, M. Klemenčič, H. Brodnik Žugelj, J. Wagger, M. Novinec and J. Svete, Tetrahydro-1H,5H-pyrazolo[1,2-a]pyrazole-1-carboxylates as inhibitors of Plasmodium falciparum dihydroorotate dehydrogenase, Bioorg. Chem., 2019, 89, 102982 CrossRef PubMed
.
- L. Yang, Y. Lv, F. Wang and G. Zhong, Chiral NHC-catalyzed 1,3-dipolar [3 + 2] cycloaddition of azomethine imines with α-chloroaldehydes for the synthesis of bicyclic pyrazolidinones, Org. Biomol. Chem., 2018, 16, 4433–4438 RSC
.
- R. Na, C. Jing, Q. Xu, H. Jiang, X. Wu, J. Shi, J. Zhong, M. Wang, D. Benitez, E. Tkatchouk, W. A. Goddard, H. Guo and O. Kwon, Phosphine-catalyzed annulations of azomethine imines: allene-dependent [3 + 2], [3 + 3], [4 + 3], and [3 + 2 + 3] pathways, J. Am. Chem. Soc., 2011, 133, 13337–13348 CrossRef CAS PubMed
.
- M. P. Clark, S. K. Laughlin, M. J. Laufersweiler, R. G. Bookland, T. A. Brugel, A. Golebiowski, M. P. Sabat, J. A. Townes, J. C. VanRens, J. F. Djung, M. G. Natchus, B. De, L. C. Hsieh, S. C. Xu, R. L. Walter, M. J. Mekel, S. A. Heitmeyer, K. K. Brown, K. Juergens, Y. O. Taiwo and M. J. Janusz, Development of orally bioavailable bicyclic pyrazolones as inhibitors of tumor necrosis factor-alpha production, J. Med. Chem., 2004, 47, 2724–2727 CrossRef CAS PubMed
.
- W. Lewgowd and A. Stanczak, Cinnoline derivatives with biological activity, Arch. Pharm., 2007, 340, 65–80 CrossRef CAS PubMed
.
- V. A. Assimon, Y. Tang, J. D. Vargas, G. J. Lee, Z. Y. Wu, K. Lou, B. Yao, M. K. Menon, A. Pios, K. C. Perez, A. Madriaga, P. K. Buchowiecki, M. Rolfe, L. Shawver, X. Jiao, R. Le Moigne, H. J. Zhou and D. J. Anderson, CB-6644 Is a Selective Inhibitor of the RUVBL1/2 Complex with Anticancer Activity, ACS Chem. Biol., 2019, 14, 236–244 CrossRef CAS PubMed
.
- J. Velcicky, U. Bodendorf, P. Rigollier, R. Epple, D. R. Beisner, D. Guerini, P. Smith, B. Liu, R. Feifel, P. Wipfli, R. Aichholz, P. Couttet, I. Dix, T. Widmer, B. Wen and T. Brandl, Discovery of the First Potent, Selective, and Orally Bioavailable Signal Peptide Peptidase-Like 2a (SPPL2a) Inhibitor Displaying Pronounced Immunomodulatory Effects In Vivo, J. Med. Chem., 2018, 61, 865–880 CrossRef CAS PubMed
.
- F. A. Romero, A. M. Taylor, T. D. Crawford, V. Tsui, A. Côté and S. Magnuson, Disrupting Acetyl-Lysine Recognition: Progress in the Development of Bromodomain Inhibitors, J. Med. Chem., 2016, 59, 1271–1298 CrossRef CAS PubMed
.
- H. Li, M. Shen, B. Li, X. Zhang and X. Fan, Solvent-Dependent Selective Synthesis of CF(3)-Tethered Indazole Derivatives Based on Multiple Bond Activations, Org. Lett., 2023, 25, 720–725 CrossRef CAS PubMed
.
- X.-R. Song, R. Yang and Q. Xiao, Recent Advances in the Synthesis of Heterocyclics via Cascade Cyclization of Propargylic Alcohols, Adv. Synth. Catal., 2021, 363, 852–876 CrossRef CAS
.
- P. Sihag and M. Jeganmohan, Regioselective Synthesis of Isocoumarins via Iridium(III)-Catalyzed Oxidative Cyclization of Aromatic Acids with Propargyl Alcohols, J. Org. Chem., 2019, 84, 2699–2712 CrossRef CAS PubMed
.
- Y. Xu, M. Shen, X. Zhang and X. Fan, Selective Synthesis of Pyrazolo[1,2-a]pyrazolones and 2-Acylindoles via Rh(III)-Catalyzed Tunable Redox-Neutral Coupling of 1-Phenylpyrazolidinones with Alkynyl Cyclobutanols, Org. Lett., 2020, 22, 4697–4702 CrossRef CAS PubMed
.
- X. Wu and H. Ji, Rhodium-Catalyzed [4 + 1] Cyclization via C-H Activation for the Synthesis of Divergent Heterocycles Bearing a Quaternary Carbon, J. Org. Chem., 2018, 83, 4650–4656 CrossRef CAS PubMed
.
- L. Zhang, Y. Xu, X. Zhang, X. Zhang and X. Fan, Synthesis of pyrazolone fused benzodiazepines via Rh(III)-catalyzed [4 + 3] annulation of 1-phenylpyrazolidinones with propargyl alcohols, Org. Chem. Front., 2020, 7, 2284–2290 RSC
.
- T. Li, Z. Yang, Z. Song, R. Chauvin and X. Cui, Rhodium(III)-Catalyzed [4+3] Annulation of N-Aryl-pyrazolidinones and Propargylic Acetates: Access to Benzo[c][1,2]diazepines, Org. Lett., 2020, 22, 4078–4082 CrossRef CAS PubMed
.
- F. Fang, C. Zhang, C. Zhou, Y. Li, Y. Zhou and H. Liu, Rh(III)-Catalyzed C-H Activation of Benzoylacetonitriles and Tandem Cyclization with Diazo Compounds to Substituted Benzo[ de]chromenes, Org. Lett., 2018, 20, 1720–1724 CrossRef CAS PubMed
.
- X. Wu, B. Wang, S. Zhou, Y. Zhou and H. Liu, Ruthenium-Catalyzed Redox-Neutral [4 + 1] Annulation of Benzamides and Propargyl Alcohols via C–H Bond Activation, ACS Catal., 2017, 7, 2494–2499 CrossRef CAS
.
- F. Fang, S. Hu, C. Li, Q. Wang, R. Wang, X. Han, Y. Zhou and H. Liu, Catalytic System-Controlled Divergent Reaction Strategies for the Construction of Diversified Spiropyrazolone Skeletons from Pyrazolidinones and Diazopyrazolones, Angew. Chem., Int. Ed., 2021, 60, 21327–21333 CrossRef CAS PubMed
.
- H. Wang, Y. Li, L. Huang, H. Xu, Y. Jiao, Z. Zhou, Z. Tang, F. Fang, X. Zhang, K. Ding, W. Yi, H. Liu, X. Wu and Y. Zhou, The synthesis of spirocyclopropane skeletons enabled by Rh(III)-catalyzed enantioselective C–H activation/[4 + 2] annulation, Chem Catal., 2023, 3, 100822 CrossRef CAS
.
- J. Gao, K. Luo, X. Wei, H. Wang, H. Liu and Y. Zhou, Rh(III)-Catalyzed Successive C-H Activations of 2-Phenyl-3H-indoles and Cyclization Cascades to Construct Highly Fused Indole Heteropolycycles, Org. Lett., 2023, 25, 3341–3346 CrossRef CAS PubMed
.
- X. Hou, R. Wang, F. Fang, Z. Qu, J. Zhou, T. Yu, D. Wang, H. Liu and Y. Zhou, Rh(III)-Catalyzed C-H Activation/Annulation for the Construction of Quinolizinones and Indolizines, Org. Lett., 2024, 26, 4451–4456 CrossRef CAS PubMed
.
- Y. Yang, N. Li, J. Zhao, Y. Jiang, X. Zhang and X. Fan, Selective Synthesis of 3-(α-Fluorovinyl)indoles and 3-Acylindoles via the Cascade Reactions of 1-Phenylpyrazolidinones with α,α-Difluoromethylene Alkynes, Adv. Synth. Catal., 2021, 363, 3600–3606 CrossRef CAS
.
- Z. Yang, Z. Song, L. Jie, L. Wang and X. Cui, Iridium(III)-catalysed annulation of pyrazolidinones with propiolates: a facile route to pyrazolo[1,2-a] indazoles, Chem. Commun., 2019, 55, 6094–6097 RSC
.
- D. Campeau, D. F. León Rayo, A. Mansour, K. Muratov and F. Gagosz, Gold-Catalyzed Reactions of Specially Activated Alkynes, Allenes, and Alkenes, Chem. Rev., 2021, 121, 8756–8867 CrossRef CAS PubMed
.
- A. S. K. Hashmi, Gold-Catalyzed Organic Reactions, Chem. Rev., 2007, 107, 3180–3211 CrossRef CAS PubMed
.
- S. K. Yadav, B. Ramesh and M. Jeganmohan, Cobalt(III)-Catalyzed Chemo- and Regioselective [4 + 2]-Annulation of Aromatic Sulfoxonium Ylides with 1,3-Diynes, J. Org. Chem., 2022, 87, 4134–4153 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2410303, 2410305 and 2410625. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ra01992c |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |