Open Access Article
Open Access ArticleHigh-response humidity sensing with graphene oxide/lignosulfonate and laser-induced graphene for respiratory health†
Yanbo Peng,
Yuhong Zhao,
Ying Yuan,
Wei Meng,
Wenhe Jiang and
Xiluan Wang *
*
Beijing Key Laboratory of Lignocellulosic Chemistry, Beijing Forestry University, Beijing, 100083, P. R. China. E-mail: wangxiluan@bjfu.edu.cn
First published on 15th April 2025
Abstract
Most current commercial humidity sensors rely on precious metals and chemicals. In this study, alkali lignin produced in the paper industry was utilized to form a film with hydroxyethyl cellulose to generate laser-induced graphene (LIG) as an electrode material for a sensor by the laser-induction technique. LIG exhibits excellent conductivity, and the experimental results demonstrate that its resistivity can be adjusted by laser power without the necessity for additional conductive materials. A solution comprising a blend of graphene oxide and sodium lignosulfonate was introduced to the LIG surface in a dropwise manner, thereby establishing a sensing surface. This process resulted in the introduction of hydrophilic groups, including carboxyl, phenolic hydroxyl, and sulfonic acid. The integration of these hydrophilic groups enhanced the surface's sensitivity to humidity, thereby facilitating the precise capture of alterations in ambient air humidity. The humidity sensor, which employs alkali lignin and lignin laser-induced graphene as electrodes and graphene oxide (GO) as the humidity-sensitive layer, exhibits an exceptionally high degree of sensitivity to humidity. The response reached 42.74 (RRH/R0) at 80% relative humidity and 133.96 (RRH/R0) at 90% humidity with a sensitivity of 147.73%/% RH. Moreover, the sensor displays an impressively brief recovery period, which remains unaltered even after multiple cycles. Additionally, the humidity sensor exhibits excellent stability for a period of up to 30 days. This study has successfully developed a simple and efficient method for preparing graphene, and has produced a flexible resistive sensor with high sensitivity, repeatability, and stability, thereby opening up new avenues for the high-value utilisation of lignin.
Introduction
Humidity sensors are of great importance in the field of agriculture,1 industrial production and human activities.2,3 Researchers have investigated a multitude of humidity detection mechanisms, including resistors, photoreactors, capacitors, field effect transistors (FETs), and quartz crystal microbalances (QCMs).4–7 Among the existing sensing technologies, resistive sensors are worthy of further investigation due to their straightforward structure, cost-effectiveness and straightforward circuit design. To enhance the sensing performance, a plethora of novel sensing materials have been developed, encompassing carbon materials,8,9 electrospun fibres,10 metal oxides,11,12 semiconductor particles13 and composite nanomaterials.14 Nevertheless, the primary impediments to the industrialisation of sensors are the elevated cost of electrodes, the high cost of raw materials and the intricate nature of the manufacturing process. The large-scale development of low-cost humidity sensing materials represents a significant challenge.Lignin has low intrinsic value and a low utilization rate.15 But most of lignin is burned as fuel, and only a small part of lignin is converted into commercial products.16 However, about 50 million tons of lignin are produced worldwide through industrial and biological refining processes every year.17 As the second largest natural polymer on the earth, lignin is the most important source of aromatic biomass. Its antioxidant, antibacterial, biodegradable, and CO2-neutral properties have garnered increasing attention. With the emergence of environmental pollution and resource crisis, the effective utilization of lignin has attracted increasing attention, because it not only brings economic benefits to the biomass industry, but also helps to reduce the dependence on fossil energy.18,19 Through previous studies, modern lignin extraction technology has been developed and mature, mainly used to prepare high value-added products such as pyrolysis oil,20 phenolic resin, polyurethane, epoxy resin and heavy metal adsorbent.16,21,22 The current research project is focused on the preparation of graphene from woody biomass resources, including wood and paper, using laser etching.23 Nevertheless, the considerable heterogeneity of lignin represents a significant obstacle to the large-scale utilisation of lignin-based materials, particularly those based on graphene. Fortunately, laser etching technology can effectively circumvent the influence of lignin heterogeneity, thereby enabling the efficient preparation of graphene.24
The disposal and recycling of electronic products also present long-term challenges, as traditional electrodes are often composed of expensive and non-recyclable metals, including copper, gold, silver, platinum, nickel, chromium and aluminium.25,26 Research in the field of humidity sensors has seldom addressed the electrode component. Lignin-derived laser-induced graphene (LIG) represents an optimal electrode material, offering a cost-effective and renewable alternative. Furthermore, industrial lignin can be transformed into water-soluble lignosulfonate (LS), which is rich in hydrophilic groups, through sulfonation.27 This material has attracted considerable interest due to its renewability, low cost and potential as a surfactant and water adsorbent. The functionality of resistive humidity sensors depends on changes in ambient humidity signal.28 Chemical resistance materials, as potential materials for wearable sensors, can directly convert external stimuli into electrical signals for detection. It boasts a straightforward structure, simple signal collection, and straightforward manufacturing.10,29,30 Moreover, the sensitivity of such sensors can be markedly enhanced by the introduction of hydrophilic groups, including carboxyl groups, phenolic hydroxyl groups, and sulfonic acid groups. Graphene is a low-dimensional material with superior electrical sensitivity and a large surface-to-volume ratio, which renders it a promising candidate for incorporation into low-dimensional humidity sensing materials.31–34 Graphene oxide (GO), a derivative of graphene, exhibits excellent hydrophilicity owing to the existence of epoxy, hydroxyl, and carboxyl groups on its surface and edges.35,36 This property endows GO with a remarkable capacity for humidity sensing. Further improvements to the humidity sensor's performance may be achieved through modifications to the sensitive layer.37
Expanding the response range of sensor to humidity and reducing their response and recovery times have always been focal points in sensor research. Alrammouz deposited aluminum IDE onto highly porous GO-coated cellulose paper, created by immersing the paper in a GO solution via vacuum thermal evaporation, and subsequently designed a capacitive humidity sensor. While this sensor is capable of reducing resistivity, its sensing humidity range is limited to only 30% to 90%.38 Zhu developed a humidity sensor utilizing a blend of nanocellulose and carbon nanotubes as the moisture-sensitive material. Although this sensor's response humidity range has been expanded to 11–95%, its response/recovery times are quite lengthy, at 330 s/377 s respectively.9 Through in situ polymerization, hydrophilic PANI with varying concentrations was coated onto the surface of nanofibers, subsequently enabling the assembly of a resistive humidity sensor. The sensor's humidity response range can be enhanced from the original 49% to 83%, with a response/recovery time of 33/88 seconds.39 Sulfides such as MoS2 are also widely used in humidity sensors.40 A flexible humidity sensor was developed on textiles using silver conductive ink and carbon conductive ink through screen printing technology. It has preferably linear response in the humidity range of 28.8–91.5%, but its response recovery time at the highest humidity is 20 s and 35 s respectively.1
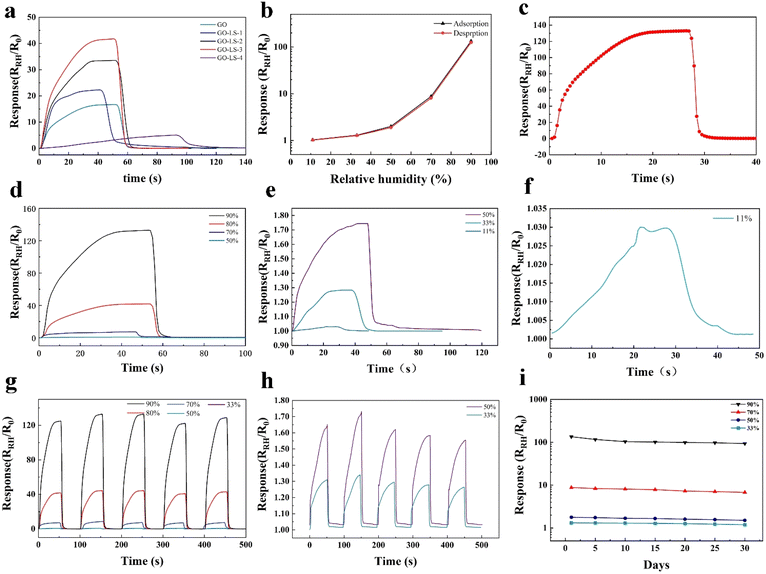
This study presents the design of a lignin-based laser-induced graphene electrode, which incorporates graphene oxide as a humidity-sensitive layer and integrates LS as a moisture-absorbing enhancement material. The experimental outcomes indicate a response of 41.74 (RRH/R0) at 80% relative humidity (RH), approximately tripling that of a pristine graphene oxide film. Furthermore, the peak response observed at 90% RH was 132.96 (RRH/R0), with a sensitivity of 147.73%/% RH.
The field of humidity sensing technologies is experiencing continuous refinement and enhancement, particularly within the domains of industrial Internet of Things and healthcare. Despite the progress made by commercial capacitive polymer and MEMS sensors, which have achieved response times of less than a second and the capability to detect a broad spectrum of humidity levels, these sensors still face technical obstacles when applied to the realm of flexible wearable respiration detection. The primary challenges include the inherent limitations of rigid substrates and the complexity of lithography processes associated with MEMS sensors. Furthermore, the production of electrodes in most commercially accessible sensors relies on the use of precious metals and chemicals. Furthermore, most commercially available sensors are reliant on precious metals and chemicals for electrode fabrication. Finally, medical-grade respiratory monitoring devices currently lack portability. In light of the pressing demand for high-performance, cost-effective, and portable humidity sensors, laser-induced graphene (LIG) humidity sensors that utilize graphene oxide/LS thin films have emerged as a promising solution. These sensors offer several distinct advantages, including their flexibility, wide applicability, and the potential for cost-effective mass production. The integration of the sensing film with flexible LIG finger electrodes endows the sensor with the capacity to undergo folding and bending, rendering it optimal for incorporation into portable devices. The integration of the proposed sensor with a mouthpiece and an embedded microcontroller unit (MCU) facilitates the detection and counting of breaths. Furthermore, the ultra-high sensitivity of the sensor in high humidity environments ensures that the motion state sensing profiles measured can fulfil the requirements of high-frequency dynamic response.
Results and discussion
Synthetic procedures
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds are broken, and the oxygen element is released in the form of gas (Fig. 1b). At the same time, the higher content of AL in the film undergoes C–C bond reorganisation by reaction to form an aromatic backbone. The material is transformed from a sp3 hybrid state to a more structurally ordered sp2 hybrid state.
O bonds are broken, and the oxygen element is released in the form of gas (Fig. 1b). At the same time, the higher content of AL in the film undergoes C–C bond reorganisation by reaction to form an aromatic backbone. The material is transformed from a sp3 hybrid state to a more structurally ordered sp2 hybrid state.
LS exhibits clear hygroscopic characteristics, enabling the adsorption of water molecules from the surrounding air. The combination of LS and GO results in the formation of additional hydrogen bond sites, which facilitates the adsorption of a greater number of water molecules onto the film. This, in turn, enhances the sensitivity and responsiveness of the material to fluctuations in humidity. Furthermore, the sensitivity can be augmented by depositing a GO–LS sensing solution onto a porous LIG electrode. The porous structure of the electrode increases the surface area of the GO that is susceptible to the effects of moisture. The deposition of the GO–LS sensing solution onto the highly porous structure forms rough films with a larger surface area than planar surfaces.
An illustration of the humidity-response mechanism is depicted in Fig. 1d. Under a consistent voltage, as humidity escalates, the electrode's resistance diminishes and the current intensifies, thereby altering the sensing signal across varying humidity conditions. With the escalation of humidity levels, water molecules are progressively adsorbed onto the surface of graphene oxide (GO) and lignosulfonate (LS). The LS molecules, abundant in highly hydrophilic sulfonic acid groups, effectively capture water molecules from the environment via hydrogen bonding and dipolar interactions. The sulfonic acid group facilitates the dissociation of hydrogen ions in the presence of water, forming an ion migration channel that markedly enhances the material's ionic conductivity. Additionally, the natural polymer framework of LS offers adsorption sites for water molecules, and its porous or cross-linked structure expedites the diffusion and penetration of these molecules. The amalgamation of LS and GO yields a humidity sensor characterized by high responsiveness, minimal lag, repeatability, and stability (Fig. 1c). At reduced humidity levels, a limited number of water molecules adhere to the sulfonic acid groups of LS and the oxygen-functional groups of GO, partially dissociating to form localized ion migration pathways, which initiates a decrease in resistance. However, the absence of a continuous water layer on the sensor material's surface impedes the transfer of H2O or H3O+ in the discontinuous regions, resulting in relatively minor impedance variations under low humidity conditions. As humidity continues to rise, a continuous water layer develops on the GO–LS interface, and the porous or cross-linked structure of LS promotes the transfer of H2O or H3O+ ions. The natural macromolecular backbone of LS provides adsorption sites for water molecules, and the Grotthuss mechanism28 describes the ion transport process involving proton transfer between H2O and H3O+ within these water layers. The reaction is represented by the equation: H2O + H3O+ → H3O+ + H2O. This ion-centric process results in a substantial reduction in impedance as relative humidity increases, and the swift ion transfer within the aqueous layer leads to a significant decrease in impedance, thereby enhancing the sensor's sensitivity. Nonetheless, due to the absence of a continuous electron conduction pathway in LS and the swelling effect of LS in high humidity conditions, which can influence the ion transport of water molecules and GO.
Characterisation of graphene electrodes
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond near 284.8 eV, and the C–O and C
C bond near 284.8 eV, and the C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds near 286.5 eV and 288.5 eV, respectively. Among these, the oxygen-containing functional groups in AL include C–O, C
O bonds near 286.5 eV and 288.5 eV, respectively. Among these, the oxygen-containing functional groups in AL include C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and carboxyl groups, while HEC contains a substantial number of hydroxyl groups. Consequently, the C 1s spectra exhibit significant signal peaks corresponding to the C–C, C–O, and C
O, and carboxyl groups, while HEC contains a substantial number of hydroxyl groups. Consequently, the C 1s spectra exhibit significant signal peaks corresponding to the C–C, C–O, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds. Furthermore, it is evident that the intensity of the signal peaks for the C–C bonds of the AL-HEC films remains largely constant after the intensity of laser etching, while the intensities of the signal peaks for the C–O and C
O bonds. Furthermore, it is evident that the intensity of the signal peaks for the C–C bonds of the AL-HEC films remains largely constant after the intensity of laser etching, while the intensities of the signal peaks for the C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds are considerably reduced. The XPS analysis yielded analogous results in the case of varying power etching (Fig. S2a–d†). As illustrated in Table 1, a discernible shift in the quantitative ratios of C
O bonds are considerably reduced. The XPS analysis yielded analogous results in the case of varying power etching (Fig. S2a–d†). As illustrated in Table 1, a discernible shift in the quantitative ratios of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–OH, and C–C components was evident before and after laser treatment of the AL-HEC films. The atomic energy share of C–C exhibited a notable increase from 63.41% to 78.55%, accompanied by a reduction in the binding energies of C–OH and C
O, C–OH, and C–C components was evident before and after laser treatment of the AL-HEC films. The atomic energy share of C–C exhibited a notable increase from 63.41% to 78.55%, accompanied by a reduction in the binding energies of C–OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O to varying extents. It can be posited that the oxygen and carbon atoms experienced dissociation during the laser etching process, resulting in alterations to the LIG functional groups.
O to varying extents. It can be posited that the oxygen and carbon atoms experienced dissociation during the laser etching process, resulting in alterations to the LIG functional groups.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–OH and C–C fractions before and after laser processing of AL-HEC films
O, C–OH and C–C fractions before and after laser processing of AL-HEC films
| Chemical state | AL-HEC films (at %) | LIG-P100 (at %) |
|---|---|---|
| C–C | 63.41% | 78.55% |
| C–OH | 31.07% | 16.41% |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
5.53% | 5.04% |
Humidity sensing test
In order to evaluate the performance of the GO/LS-LIG humidity sensor, we conducted a series of experiments. Initially, as previously described, 10 mL of a 0.5 mg per mL GO solution was added to different masses of LS, with the LS added in a sequential manner. The sequence of additions was 0 mg, 1 mg, 5 mg, 10 mg, and 100 mg, and the resulting samples were labeled as GO–LS-n (n = 0, 1, 2, 3, and 4). In order to provide a quantitative description of the response of the aforementioned humidity sensor, we define response (R) and sensitivity (S) as follows:| R = RRH/R0 | (1) |
| S = (RRH − R0)/(RHX − RH0) | (2) |
| Material | Sensitivity | Humidity range | Response (s)/recovery (s) | LOD | Reference |
|---|---|---|---|---|---|
| Borophene–MoS2 | 155 (ΔI/I0) | 0–97% | 2.5/3.1 | LOD >0.9736% RH | 41 |
| rGO/MoS2 | 24.94 (ΔR/R0) | 5–85% | 6.3/30.8 | LOD >0.0109% RH | 42 |
| GO-based | 37.43% | 11–97% | 94/134 | — | 43 |
| CNTs-paper | 0.998/% RH | 33–98% | 0.5/90 | — | 44 |
| LIG/poly | 1700 kΩ/% RH | 11–97% | 4.2/6.8 | — | 45 |
| LS/GO-LIG | 147.73/% RH | 11–90% | 12/2 | LOD >0.4429% RH | This work |
| Hmax = FFS − FFR | (3) |
Applications in respiratory monitoring
The humidity of human exhaled air is typically extremely high, approaching 100% relative humidity. This phenomenon is attributable to the absorption of moisture by the air as it traverses the lungs and respiratory tract, whereby the exhaled air attains a moisture content approaching saturation. The assembled GO/LS-LIG humidity sensor was placed into a disposable mask, as illustrated in Fig. 6a. The device was connected to an electrochemical workstation, and the response curves of the sensor to the human breathing were tested at a room temperature of 25 °C and an ambient humidity of 60% RH under fast, normal, and deep breathing states (Fig. 6b and c). During the fast breathing phase, the sensor's sensitivity to high humidity gases enabled it to record each breathing cycle with sufficient accuracy, even though it did not reach the maximum response value. The respiratory rate of the human body could then be calculated by determining the number of peaks per minute.We proceeded to integrate the sensor into an embedded microcontroller unit (MCU). Upon the exhalation of gas by the human body, which causes a change in sensor resistance, the MCU (microcontroller unit) receives the analog signals and converts them into digital signals, thereby generating the appropriate commands to control the computer. In conjunction with the respiration cycle resulting from the alteration in resistance, the MCU-converted digital signal also undergoes a transformation. Through the implementation of a programmed “counting signal,” each instance of the digital signal exceeding the “counting signal” results in the MCU's initiation of a data accumulation process, which is then displayed on the screen (for further details, please refer to Video 1†). We also tested the respiratory sensing under various exercise conditions, recording the sensing curves of the test subjects while standing, walking, and running (Fig. 6d–f). When standing, a relatively rounded curve is visible. During walking and running, the peaks and valleys of the sensing curve become sharper. In the case of running, due to the increased breathing rate, there are denser and sharper peaks and valleys, exhibiting a similar sensing curve trend to that observed during previous rapid breathing detection.
In the presence of high humidity gas, the resistance value of GO/LS-LIG undergoes significant changes, demonstrating high sensitivity. It can detect human respiration using only an MCU and provide valuable respiratory information under different motion conditions. This paves the way for advancements in portable respiratory monitoring devices.
Experimental
Materials
The alkaline lignin (L0082) was procured from TCI, while the sodium lignosulfonate was obtained from Macklin. The hydroxyethyl cellulose was sourced from Meryer, and the glycerol was purchased from Aladdin. The polyethylene terephthalate (PET) substrate was acquired from the Oudifu company.Preparation of laser-induced graphene electrodes
A solution of 4.0 g of alkaline lignin (AL) in 50 mL of deionised water was prepared and mixed with 1.0 g of hydroxyethyl cellulose (HEC). Subsequently, 1.5 mL of glycerol should be added. The AL-HEC solution was then poured onto the PET plastic substrate and scraped into a film using a coating machine. The coating speed was set to 60 mm s−1 and the film thickness was set to 0.8 mm. Subsequently, the AL-HEC solution was subjected to a drying process at 60 °C for 30 min, after which the AL-HEC solution had undergone a process of film formation and had been affixed to the PET substrate. A semiconductor laser engraver (D3 laser engraver, DAJA) was selected for the laser etching of the AL-HEC film. The peak laser power of the laser engraver was 7 W, with the applied laser power representing a range of 50–100%. The scanning rate was set at 50–70% of the full scanning speed (100 mm s−1). The LIG prepared at X% laser power is denoted as LIG-PX. The fork finger electrodes were designed to consist of 14 electrodes, with individual electrodes measuring 10.0 mm in length and 1.0 mm in width, and a spacing of 0.5 mm between electrodes. The fork finger electrodes were etched onto the surface of AL-HEC film to form LIG electrodes, and the remaining film was rinsed with deionised water to obtain the fork finger electrodes on the PET substrate, as illustrated in Fig. 1.Humidity sensor preparation
GO was prepared by oxidising natural graphite powder (325 mesh, Qingdao Huatai Lubricant Seal Technology Co., Ltd, Qingdao, China) in accordance with the modified Hummers' method. A GO/LS mixture was prepared by adding varying masses of LS to a 10 mL solution of 0.5 mg per mL GO. Subsequently, 0.3 mL of the prepared solution was pipetted onto the surface of the forked electrode and dried at 60 °C. The solution was then subjected to a drying process at 60 °C. Following this, copper foil was attached to both sides of the forked electrode, thus completing the assembly of the sensor.Characterisation of materials
The square resistance of the LIGs was determined utilising a four-point probe resistivity measurement system (RTS-2, Guangzhou Four Points Technology Co., Ltd, Guangzhou, China). Scanning electron microscope images were obtained using a field emission scanning electron microscope (Regulus 8100, Hitachi, Japan), and transmission electron microscopy observations were conducted using a Hitachi H-7650 B transmission electron microscope operated at 80 kV. Furthermore, high-resolution transmission electron microscopy (HRTEM) imaging was conducted using a FEI Tecnai F20 HRTEM at 200 kV. Raman spectra were obtained using a confocal Raman microscope (Horiba Jobin Yvon, France) with a 532 nm laser and a 50× objective lens, with wave numbers ranging from 500 to 2800 cm−1. X-ray diffraction was conducted using a Rigaku Ultima IV diffractometer (Japan), while XPS measurements were performed with a K-Alpha spectrometer (Thermo Fisher Scientific, USA) with a beam size of 400 μm. The specific surface area was calculated using the BET method with a BUILDER Kubo-X1000 tester, based on nitrogen adsorption–desorption analysis.Humidity sensing measurements
Ambient humidity conditions ranging from 40% to 90% were provided by a constant temperature and humidity chamber (HWS-80, Zhejiang Shangcheng, China). When a relative humidity of less than 40% was desired, we added 50 mL of saturated lithium chloride to one of the sealed solvents and fifty mL of saturated magnesium chloride solution to the other, which was used to create a relative humidity of 11% versus 33%. A 0% RH environment was obtained by using phosphorus pentoxide (P2O5) powder as a desiccant. The RH response of the sensor was achieved by humidifying the sensor in a constant temperature and humidity chamber and then quickly transferring it to a sealed container at 0% RH to remove water molecules. This iterative process of moisture absorption and release is defined as the humidification–dehumidification cycle. The resistance change of the prepared humidity sensor was measured using an electrochemical workstation (CHI 660E, ChenHua).Conclusions
By integrating mass-producible LEG, graphene oxide and lignin-based materials, we have developed a flexible humidity sensor. With an enhanced surface area of 242.83 m2 g−1 and a rich mesoporous structure, LIG provided the structural basis for sensor performance. The hygroscopicity of LS significantly improves the response time of the sensors, enabling a rapid response time of 12 seconds and a recovery time of 2 seconds at 90% relative humidity. Notably, the sensors exhibit precise and linear response signals across various humidity levels, demonstrating their reliability in sensing applications. Furthermore, the sensor demonstrates good repeatability and stability during testing. It can also be embedded into microcontrollers to develop portable humidity response devices, which have good application prospects in the field of smart materials and provide new ideas for the development of sensors for lignin-based materials.Data availability
All data generated or analyzed in this study are included in this manuscript document as well as in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51603012).Notes and references
- M. Tekcin, D. R. T. Hamzaoglu and S. Kursun, Flexible Printed Electron., 2023, 8, 035003 CrossRef CAS.
- J.-W. Han, B. Kim, J. Li and M. Meyyappan, J. Phys. Chem. C, 2012, 116, 22094–22097 CrossRef CAS.
- M. Y. Cho, S. Kim, I. S. Kim, E. S. Kim, Z. J. Wang, N. Y. Kim, S. W. Kim and J. M. Oh, Adv. Funct. Mater., 2020, 30, 1907449 CrossRef CAS.
- Y. Zhao, X.-G. Li, X. Zhou and Y.-N. Zhang, Sens. Actuators, B, 2016, 231, 324–340 CrossRef CAS.
- S. R. Vaughan, N. C. Speller, P. Chhotaray, K. S. McCarter, N. Siraj, R. L. Pérez, Y. Li and I. M. Warner, Talanta, 2018, 188, 423–428 CrossRef CAS PubMed.
- D. Pawar and S. N. Kale, Microchim. Acta, 2019, 186, 1–34 CrossRef PubMed.
- Q. Zheng, J.-h. Lee, X. Shen, X. Chen and J.-K. Kim, Mater. Today, 2020, 36, 158–179 CrossRef CAS.
- P. Zhou, L. Chen, L. Yao, M. Weng and W. Zhang, Nanoscale, 2018, 10, 8422–8427 RSC.
- P. Zhu, Y. Liu, Z. Fang, Y. Kuang, Y. Zhang, C. Peng and G. Chen, Langmuir, 2019, 35, 4834–4842 CrossRef CAS PubMed.
- L. Lu, B. Yang and J. Liu, Chem. Eng. J., 2020, 400, 125928 CrossRef CAS.
- K. Sahoo, B. Mohanty, A. Biswas and J. Nayak, Mater. Sci. Semicond. Process., 2020, 105, 104699 CrossRef CAS.
- C. Huo, H. Chen, L. Chen, S. Yang, P. Cui and J. Song, J. Electron. Mater., 2024, 53, 5238–5245 CrossRef CAS.
- C. Yu, Y. Wu, X. Liu, F. Fu, Y. Gong, Y.-J. Rao and Y. Chen, Sens. Actuators, B, 2017, 244, 107–113 CrossRef CAS.
- S. Hu, S. Wu, C. Li, R. Chen, E. Forsberg and S. He, Sens. Actuators, B, 2020, 305, 127517 CrossRef CAS.
- A. J. Ragauskas, G. T. Beckham, M. J. Biddy, R. Chandra, F. Chen, M. F. Davis, B. H. Davison, R. A. Dixon, P. Gilna and M. Keller, Science, 2014, 344, 1246843 CrossRef.
- A. Duval and M. Lawoko, React. Funct. Polym., 2014, 85, 78–96 CrossRef CAS.
- L. Cao, K. Iris, Y. Liu, X. Ruan, D. C. Tsang, A. J. Hunt, Y. S. Ok, H. Song and S. Zhang, Bioresour. Technol., 2018, 269, 465–475 CrossRef CAS PubMed.
- S. Laurichesse and L. Avérous, Prog. Polym. Sci., 2014, 39, 1266–1290 CrossRef CAS.
- M. Witzler, A. Alzagameem, M. Bergs, B. El Khaldi-Hansen, S. E. Klein, D. Hielscher, B. Kamm, J. Kreyenschmidt, E. Tobiasch and M. Schulze, Molecules, 2018, 23, 1885 CrossRef PubMed.
- J. Becker and C. Wittmann, Biotechnol. Adv., 2019, 37, 107360 CrossRef CAS PubMed.
- J. H. Lora and W. G. Glasser, J. Polym. Environ., 2002, 10, 39–48 CrossRef CAS.
- E. Ten and W. Vermerris, J. Appl. Polym. Sci., 2015, 132, 42069 CrossRef.
- M. F. El-Kady, V. Strong, S. Dubin and R. B. Kaner, Science, 2012, 335, 1326–1330 CrossRef CAS PubMed.
- A. Lamberti, F. Perrucci, M. Caprioli, M. Serrapede, M. Fontana, S. Bianco, S. Ferrero and E. Tresso, Nanotechnology, 2017, 28, 174002 CrossRef PubMed.
- H. Tai, Z. Duan, Y. Wang, S. Wang and Y. Jiang, ACS Appl. Mater. Interfaces, 2020, 12, 31037–31053 CrossRef CAS PubMed.
- P. Zhu, Y. Kuang, Y. Wei, F. Li, H. Ou, F. Jiang and G. Chen, Chem. Eng. J., 2021, 404, 127105 CrossRef CAS PubMed.
- T. Aro and P. Fatehi, ChemSusChem, 2017, 10, 1861–1877 CrossRef CAS PubMed.
- S. Hajian, X. Zhang, P. Khakbaz, S.-M. Tabatabaei, D. Maddipatla, B. B. Narakathu, R. G. Blair and M. Z. Atashbar, IEEE Sens. J., 2020, 20, 7517–7524 CAS.
- L. Duan, D. R. D'hooge and L. Cardon, Prog. Mater. Sci., 2020, 114, 100617 CrossRef.
- J. Meng and Z. Li, Adv. Mater., 2020, 32, 2000130 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D.-E. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183–191 CrossRef CAS PubMed.
- F. Schedin, A. K. Geim, S. V. Morozov, E. W. Hill, P. Blake, M. I. Katsnelson and K. S. Novoselov, Nat. Mater., 2007, 6, 652–655 CrossRef CAS PubMed.
- A. K. Geim, Science, 2009, 324, 1530–1534 CrossRef CAS PubMed.
- K. P. Loh, Q. Bao, G. Eda and M. Chhowalla, Nat. Chem., 2010, 2, 1015–1024 Search PubMed.
- Y. Tu, L. Zhao, J. Sun, Y. Wu, X. Zhou, L. Chen, X. Lei, H. Fang and G. Shi, Chin. Phys. Lett., 2020, 37, 066803 CrossRef CAS.
- G. Zhu, Z. Huang, L. Zhao and Y. Tu, Nanoscale, 2021, 13, 15231–15237 RSC.
- R. Alrammouz, J. Podlecki, A. Vena, R. Garcia, P. Abboud, R. Habchi and B. Sorli, Sens. Actuators, B, 2019, 298, 126892 CrossRef CAS.
- S. Yan, D. Shen, M. A. A. Newton, S. Zhu and B. Xin, Colloids Surf., A, 2024, 695, 134198 CrossRef CAS.
- D. J. Late, Y.-K. Huang, B. Liu, J. Acharya, S. N. Shirodkar, J. Luo, A. Yan, D. Charles, U. V. Waghmare and V. P. Dravid, ACS Nano, 2013, 7, 4879–4891 CrossRef CAS PubMed.
- C. Hou, G. Tai, Y. Liu, Z. Wu, Z. Wu and X. Liang, J. Mater. Chem. A, 2021, 9, 13100–13108 RSC.
- S. Y. Park, Y. H. Kim, S. Y. Lee, W. Sohn, J. E. Lee, D. H. Kim, Y.-S. Shim, K. C. Kwon, K. S. Choi and H. J. Yoo, J. Mater. Chem. A, 2018, 6, 5016–5024 RSC.
- D. Zhang, J. Tong and B. Xia, Sens. Actuators, B, 2014, 197, 66–72 CrossRef CAS.
- A. Liang and X. Chen, J. Mater. Chem. A, 2024, 12, 29081–29091 RSC.
- J. Wang, N. Wang, D. Xu, L. Tang and B. Sheng, Sens. Actuators, B, 2023, 375, 132846 CrossRef CAS.
- J. Li, Y. Lu, Q. Ye, M. Cinke, J. Han and M. Meyyappan, Nano Lett., 2003, 3, 929–933 CrossRef CAS.
- V. Dua, S. P. Surwade, S. Ammu, S. R. Agnihotra, S. Jain, K. E. Roberts, S. Park, R. S. Ruoff and S. K. Manohar, Angew. Chem., 2010, 122, 2200–2203 CrossRef.
- Y. H. Kim, S. J. Kim, Y.-J. Kim, Y.-S. Shim, S. Y. Kim, B. H. Hong and H. W. Jang, ACS Nano, 2015, 9, 10453–10460 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra01765c |
| This journal is © The Royal Society of Chemistry 2025 |