Open Access Article
Open Access ArticleSimultaneous analysis of multi-class antibiotics in bottled water using large-volume direct-injection LC-MS/MS†
Haijun Wanga,
Qiao Zhanga,
Xiaolin Lia,
Huan Chen b,
Xiaolan Zhuc,
Liming Yangd,
Hongling Yina,
Jing Sun
b,
Xiaolan Zhuc,
Liming Yangd,
Hongling Yina,
Jing Sun a,
Shuhong Fanga and
Hui Zhang
a,
Shuhong Fanga and
Hui Zhang *ae
*ae
aCollege of Resources and Environment, Chengdu University of Information Technology, Chengdu 610225, China. E-mail: zhanghui@cuit.edu.cn
bDepartment of Environmental Engineering and Earth Science, Clemson University, South Carolina 29634, USA
cAgilent Technologies (China) Co., Ltd, 100102, China
dDepartment of Chemical and Biomolecular Engineering, National University of Singapore, 117585, Singapore
eKey Laboratory of Atmospheric Environment Simulation and Pollution Control at Chengdu University of Information Technology of Sichuan Province, Chengdu 610225, China
First published on 20th May 2025
Abstract
A large-volume direct-injection (LVDI) method was developed and validated for the simultaneous analysis of 69 antibiotics in bottled water using ultra-high performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS). Target antibiotics included 23 sulfonamides, 19 quinolones, 12 macrolides, 11 β-lactams, and 4 tetracyclines. Optimization of ion source parameters led to enhanced signal intensities for 55 antibiotics. The 100 μL injection volume was both feasible and preferred, resulting in increased signal intensities while maintaining unchanged peak shapes for the antibiotics. Good absolute recoveries for the 69 antibiotics were obtained with the LVDI method, primarily ranging from 80% to 120%, whereas lower absolute recoveries of macrolides, quinolones, sulfonamides and β-lactams were observed with a solid-phase extraction (SPE) method. The limits of detection (LODs) for antibiotics were generally comparable between the LVDI and SPE methods, with values below 1 ng L−1 for most antibiotics (0.00271–26.6 ng L−1). Analysis of 25 brands of bottled water using the LVDI method revealed the presence of 54 antibiotics from 5 classes, with detection frequencies (DFs) ranging from 4% to 100% and detected concentrations between 0.0453 and 37.4 ng L−1. Multiple antibiotics were detected simultaneously in bottled water, with more than 10 antibiotics identified in each of 9 different brands. Quinolones and sulfonamides were the predominant antibiotics, accounting for over 80% of the total concentration. Only sulfaclozine showed significantly different concentrations between purified drinking water and natural mineral water (22.2 vs. 17.2 ng L−1, p < 0.05).
1. Introduction
Antibiotics are used primarily to treat bacterial infections in humans and animals. Previous data showed that 48% of antibiotics in China were used for human treatment, with per capita usage 5 to 7 times higher than in developed countries such as the United States, Canada, and the United Kingdom, while 52% were used in animal husbandry and aquaculture.1,2 A small amount of antibiotics is used in plant treatment, but their use is much lower than in human medicine and livestock farming.3,4 Commonly used antibiotics include sulfonamides (SAs), quinolones (QNs), macrolides (MLs), lincosamides (LMs), tetracyclines (TCs), β-lactams (β-Ls), aminoglycosides (AGs), and peptides (PTs), which have distinct structures and properties.5,6 The manufacturing and use of antibiotics have led to their widespread occurrence in various environmental compartments, such as wastewater,7–9 surface water,10,11 groundwater,12,13 sediment,14,15 soil,16,17 and biota samples.18 Their presence in the environment poses risks to non-target organisms and contribute to the development of antibiotic-resistant bacteria.19,20Drinking water is a crucial pathway for human exposure to environmental antibiotics, potentially posing health risks. The widespread presence of antibiotics in drinking water sources and various types of drinking water has been reported in previous studies. For instance, Feng et al. detected 8 antibiotics, such as sulfonamides and macrolides, across 9 drinking water sources, with concentrations ranging from 13.9 to 188.1 ng L−1.21 Leung et al. found 6 antibiotics from 4 classes, such as macrolides and sulfonamides, in tap water samples from 13 Chinese cities, with concentrations as high as 104.0 ng L−1.22 Limited studies also revealed the occurrence of antibiotics in bottled water, which is a major type of drinking water nowadays. For instance, Ben et al. detected as many as 45 antibiotics in bottled water, mainly including macrolides, quinolones and tetracyclines.23 Wang et al. found florfenicol in bottled water with concentrations ranging from 0.60 to 1.00 ng L−1.24 Given the ubiquitous presence of various antibiotics in the environment, and especially the potential exposure risks through drinking water, there is a need for a simple, sensitive, and rapid method to analyze multi-class antibiotics in drinking water.
Extraction of antibiotics from aqueous samples is usually required prior to LCMS analysis. This is commonly achieved through methods such as solid-phase extraction (SPE),25 online SPE,26 dispersive liquid–liquid microextraction,27 and ionic liquid membrane microextraction.28 Although SPE is a preferred method for analysis of antibiotics in water, its operation can be relatively complex and time-consuming.29 Furthermore, the simultaneous extraction of multiple classes of antibiotics with diverse physicochemical properties is a challenge when using a single SPE protocol. To extract a wide range of antibiotics by SPE, adjustments to sample conditions (e.g., pH) and the use of different adsorbents may be necessary,30 further complicating the analytical process.
Direct injection (DI) coupled with LC-MS/MS has emerged as a promising technique for the analysis of diverse water contaminants. By eliminating extensive sample preparation steps, DI provides a rapid, cost-effective, and environmentally friendly approach.31,32 This approach has been successfully applied to the analysis of emerging contaminants, such as pharmaceuticals and personal care products (PPCPs),31,33 per- and polyfluoroalkyl substances (PFAS),34,35 and pesticides.36,37 Advancements in mass spectrometry, particularly enhanced sensitivity,33,38 combined with large volume injection,33,39 have made it possible to detect trace-level contaminants in water by DI-LC-MS/MS analysis. However, the application of DI-LC-MS/MS for antibiotic analysis remains relatively limited, and its feasibility for the simultaneous analysis of multi-class antibiotics with different properties has not been well studied. For instance, Bayen et al. used LC-MS/MS with small volume direct injection to screen for only 7 specific antibiotics in surface water and seawater.31 More recently, Edvaldo et al. developed a rapid DI-LC-MS/MS method for determining β-lactam antibiotics in various types of water, such as tap water, surface water and domestic wastewater.40 Simarro-Gimeno et al. evaluated a DI-LC-MS/MS method for detecting 8 antibiotics in different water matrices, including groundwater, surface water and wastewater.41 Compared to wastewater and surface water, the matrix of drinking water is much less complex, making large volume direct injection (LVDI) LC-MS/MS particularly well-suited for the analysis of trace-level antibiotics.
In this study, we aimed to develop and validate an LVDI-LC-MS/MS method for simultaneous analysis of 69 antibiotics from 5 classes in drinking water at trace-level. An optimized SPE method was compared with the LVDI method to assess their respective merits, and especially the feasibility of the LVDI method. The developed LVDI-LC-MS/MS method was applied to the analysis of antibiotics in 25 brands of bottled water from China, Southeast Asia and Europe, including both purified drinking water (PDW) and natural mineral water (NMW), to investigate the occurrence and distribution of these antibiotics.
2. Materials and methods
2.1. Chemicals and reagents
Authentic standards for 58 antibiotics, including 23 sulfonamides, 19 quinolones, 12 macrolides, and 4 tetracyclines, were obtained from ANPEL Laboratory Technologies (Shanghai, China). Authentic standards for 11 β-Lactams were obtained from Alta Scientific (Tianjin, China). Deuterium-labeled internal standards for sulfonamides, quinolones and β-lactams were obtained from Alta Scientific (Tianjin, China), while those for macrolides were obtained from ANPEL Laboratory Technologies (Shanghai, China). Detailed information on the 69 antibiotics and 10 deuterium-labeled internal standards is provided in Table S1.† HPLC grade methanol was purchased from Chron Chemicals (Chengdu, Sichuan, China). Ethylenediaminetetraacetic acid disodium (Na2EDTA) and LC-MS grade formic acid were both purchased from Aladdin Scientific (Shanghai, China). LC-MS grade water was purchased from ANPEL Laboratory Technologies (Shanghai, China). Hydrophilic–lipophilic balance (HLB) cartridges (6 mL/200 mg) were purchased from ANPEL Laboratory Technologies (Shanghai, China).2.2. Sample collection
Bottled water samples were purchased from local supermarkets and online shops, including 20 brands in China and 5 widely consumed imported brands. For each brand, three bottles of water were analyzed. The bottled water samples were categorized into PDW and NMW. Their water sources included 10 provinces and one prefecture-level city in China, as well as locations in Thailand, Malaysia, New Zealand, Norway and France. The detailed information on bottled water samples is provided in Table S2.†2.3. Method development and validation
An LVDI method for analysis of antibiotics in drinking water was developed and validated. In brief, 950 μL of drinking water was transferred into an amber glass vial, followed by the addition of 50 μL of internal standards to achieve a final concentration of 0.02 μg L−1. The sample was then vortexed thoroughly prior to instrumental analysis. The method was validated by assessing recoveries of spiked antibiotics at 0.005, 0.02, 0.1, and 1 μg L−1, respectively. Linearity was evaluated by analyzing antibiotic standards at concentrations ranging from 0.002 to 4 μg L−1.An SPE method was adopted from previous studies and validated for comparison purposes.42–44 In brief, 500 mL of the water sample was acidified to pH 2 by adding hydrochloric acid, followed by the addition of 0.25 g Na2EDTA and internal standards (0.04 μg L−1). An HLB cartridge was conditioned successively with 10 mL of methanol and 10 mL of acidified ultrapure water (pH 2). The well-mixed water sample was loaded at a flow rate of 8–12 mL min−1. After sample loading, the HLB column was rinsed with 10 mL of acidified ultrapure water (pH 2) and dried under vacuum for 5 minutes. The antibiotics was eluted with 9 mL of methanol. The eluate was concentrated to around 1.5 mL using a centrifugal vacuum concentrator at 30 °C, then transferred to an amber glass vial. The eluate was further concentrated to 1 mL under gentle nitrogen gas prior to instrumental analysis. The SPE method was validated by assessing recoveries of spiked antibiotics at 0.02 μg L−1.
2.4. Instrumental analysis
An Agilent 1290 Infinity II LC coupled to a 6495C Triple Quad MS (Agilent, Santa Clara, CA, USA) was used for sample analysis. A ZORBAX Eclipse Plus C18 column (1.8 μm, 2.1 mm × 50 mm, Agilent, Santa Clara, CA, USA) was used to separate the antibiotics at 35 °C. The mobile phase consisted of water (phase A) and methanol (phase B), both containing 0.1% formic acid. The flow rate was 0.3 mL min−1. The gradient program (phase B) was as follows: 5% was held for 4 min, increased to 40% in 5 min, increased to 100% in 7 min, held at 100% for 3 min, then decreased to 5% in 0.5 min, and finally held at 5% for 3.5 min. The total run time was 23 min.An Agilent Jet Stream Electrospray Ionization (AJS ESI) source in positive mode was employed to ionize the antibiotics, with the optimized ion source parameters shown in Table S3.† Dynamic multiple reaction monitoring (dMRM) with delta time of 1 min for each antibiotic was used to acquire data. Detailed information, such as precursor and product ions, collision energies, retention times, is provided in Table S1.†
2.5. Data analysis
Identification and quantification of the antibiotics were performed using Agilent's MassHunter Quantitative Analysis software (version 10.1). Antibiotics in the samples were identified following these criteria: (1) the signal to noise ratio was above 3; (2) the uncertainty of qualifier to quantifier ratio was below 30%, and (3) the retention time shift was within 0.1 min. Blank samples (LC-MS grade water) were analyzed together with water samples to evaluate background contamination. Blank-corrected concentrations were reported when the concentration in a sample exceeded the average concentration plus 3 times the standard deviation in the blank samples. Limits of detection (LODs) and limits of quantification (LOQs) were estimated as the concentrations corresponding to signal-to-noise (S/N) ratios of 3 and 10, respectively. Data analysis was conducted using R (version 4.4.1). The Mann–Whitney U test was applied to compare antibiotic concentrations between PDW and NMW. For this analysis, non-detectable concentrations were replaced with half of the LODs.3. Results and discussion
3.1. Optimization of MS parameters
A standard mixture of the 69 antibiotics was prepared and used to optimize MS parameters without chromatographic separation. The detailed information on MRM optimization is provided in the ESI.† For each antibiotic, 2 MRM transitions (i.e., precursor ion → product ion) with the highest intensities were selected (Table S1†).To further enhance the sensitivity for antibiotic analysis, ion source parameters were optimized using the Agilent MassHunter Optimizer software. The optimization was performed by directly injecting the antibiotic mixture into the LCMS system without chromatographic separation. The default and optimized ion source parameters, including nebulizer pressure, capillary voltage, nozzle voltage, dry gas temperature and flow rate, sheath gas temperature and flow rate, as well as high-pressure and low-pressure funnel radio frequency (RF), are provided in Table S3.† After the ion source optimization, signal intensities increased for 55 antibiotics, but decreased for 14 antibiotics (Fig. 1). In comparison, the signal intensities increased more than twofold for 40 antibiotics, while they decreased less than twofold for 13 antibiotics. Sulfanitran was the only antibiotic that exhibited a drastic signal drop after the optimization. Nevertheless, the optimized parameters were applied in this study due to the significant signal enhancement observed for most antibiotics.
3.2. LVDI method
The effect of injection volume on peak area and peak shape was evaluated by injecting 10, 50, and 100 μL of a standard mixture of antibiotics (0.01 μg L−1 for each antibiotic). To minimize solvent effects, the mixture consisted of methanol and water (v/v, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95), matching the initial composition of the mobile phase. The peak area ratios ranged from 8.26 to 11.7 (10.5 ± 0.703), 4.62 to 5.67 (5.28 ± 0.240), and 1.72 to 2.15 (1.99 ± 0.0846) when comparing injection volumes of 100 to 10 μL, 50 to 10 μL, and 100 to 50 μL, respectively. The results indicated that the peak areas all increased proportionally with the injection volume (Fig. 2a), demonstrating the feasibility of large-volume injection for antibiotic analysis.
95), matching the initial composition of the mobile phase. The peak area ratios ranged from 8.26 to 11.7 (10.5 ± 0.703), 4.62 to 5.67 (5.28 ± 0.240), and 1.72 to 2.15 (1.99 ± 0.0846) when comparing injection volumes of 100 to 10 μL, 50 to 10 μL, and 100 to 50 μL, respectively. The results indicated that the peak areas all increased proportionally with the injection volume (Fig. 2a), demonstrating the feasibility of large-volume injection for antibiotic analysis.
 | ||
| Fig. 2 Comparison of peak area (a) and FWHM (b) ratios for antibiotics analyzed using different injection volumes. | ||
The peak shapes were comparable across the injection volumes, except for the two earliest eluted antibiotics (i.e., sulfacetamide and amoxicillin), as indicated by the full width at half maximum (FWHM) of each peak (Fig. 2b). The FWHM ratios between different injection volumes typically ranged from 0.8 to 1.2. Excluding sulfacetamide and amoxicillin, the FWHM values for the antibiotics at injection volumes of 10, 50, and 100 μL were 0.090 ± 0.012, 0.090 ± 0.013, and 0.091 ± 0.014 minutes, respectively. To maximize the sensitivity for antibiotic analysis, an injection volume of 100 μL was selected.
The recoveries of antibiotics using the LVDI method was assessed by analyzing ultrapure water spiked with antibiotics at concentrations of 0.005, 0.02, 0.1 and 1 μg L−1, respectively. Absolute recoveries (ARs) and relative recoveries (RRs) of the antibiotics were in the range of 80% to 120%, except for tylosin (AR: 126.3%), cefotaxime (AR: 78.2%), and cefazolin (AR: 58.3%, RR: 66.8%) at 0.005 μg L−1, and demeclocycline (AR: 75.0%, RR: 78.5%) at 0.02 μg L−1 (Fig. 3a and Table S4†).
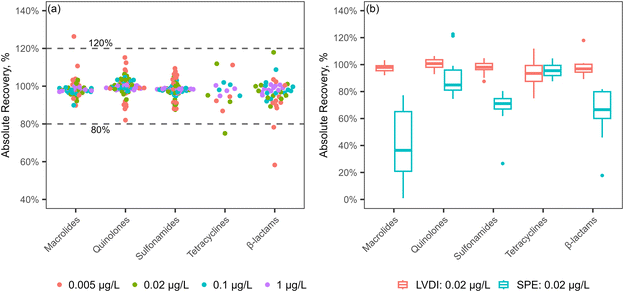 | ||
| Fig. 3 Absolute recoveries of antibiotics (a) by the LVDI method at 0.005, 0.02, 0.1 and 1 μg L−1 and (b) comparison of the LVDI and SPE methods at 0.02 μg L−1. | ||
A series of antibiotic mixtures with concentrations ranging from 0.002 to 4 μg L−1 was used to assess the linear range of the method. The results showed good linear relationships and correlation coefficients (R2 > 0.99) for the antibiotics, with typical linear ranges of 0.002–4 μg L−1 and 0.01–4 μg L−1 (Table S5†). Sulfanitran had narrower linear range (i.e., 0.2–4 μg L−1), due to its high LOD (Table S5†).
3.3. SPE method
SPE using an HLB cartridge was selected for antibiotic extraction due to its ability to retain acidic, basic and neutral compounds.45 This sorbent has been used to extract multiple classes of antibiotics with various physicochemical properties. Sample pH is a key parameter for SPE performance by affecting the dominant form of molecules in a solution, and pH values lower than the pKa values of target analytes are usually preferred.40,46 Following the U.S. EPA Method 1694,44 the water sample in this study was conditioned to pH 2 prior to extraction.Methanol, methanolic ammonia (e.g., 1–5% ammonium hydroxide in methanol) and methanol with 0.1% formic acid have been used as elution solvents in previous studies.47–49 Our data showed that methanolic ammonia (1% ammonium hydroxide) enhanced signal intensities for lactams but reduced those for both tetracyclines and macrolides compared to methanol. In contrast, methanol with 0.1% formic acid resulted in lower signal intensities for both lactams and macrolides (Fig. S1†). Out of the 69 antibiotics, methanol generally eluted more antibiotics and was selected as the elution solvent.
An insufficient elution volume may lead to low recoveries of antibiotics. Our data showed that elution with 9 mL of methanol yielded higher signal intensities for the antibiotics compared to 6 mL. The improvement was especially notable for sulfadimethoxine, virginiamycin M1, cefoperazone, ceftazidime, cefpirome, ceftiofur, ampicillin, amoxicillin, and cefotetan, with enhancement ratios ranging from 1.9 to 10.9 (Fig. S2†). Further increase of the elution volume to 12 mL had negligible effects on signal enhancement. An elution volume of 9 mL was selected to achieve acceptable recoveries of the antibiotics while minimizing the use of organic solvent.
3.4. Comparison between LVDI and SPE methods
Absolute recoveries of spiked antibiotics in ultrapure water at 0.02 μg L−1 were compared between the LVDI and SPE methods (Fig. 3b). The recoveries of tetracyclines were comparable between the two methods, but lower absolute recoveries of macrolides, quinolones, sulfonamides and β-lactams were observed with the SPE method. Notably, most macrolides and oxacillin had ARs below 40%, and penicillin G was not detected with the SPE method (Table S4†). However, good RRs were obtained for most antibiotics with the SPE method, except for some macrolides (<50%) and β-lactams (>130%) (Table S4†). These results confirmed the better performance of the LVDI method over the SPE method for the simultaneous analysis of multiple classes of antibiotics.The LODs for the antibiotics were generally comparable between the LVDI and SPE methods, with most values between 0.01 and 1 ng L−1 (Fig. 4 and S3†). In the LVDI method, the LODs for 63 antibiotics ranged from 0.00417 to 0.977 ng L−1, while higher values were observed for the other 6 antibiotics, ranging from 1.16 to 26.6 ng L−1. In the SPE method, the LODs for 67 antibiotics ranged from 0.00271 to 0.864 ng L−1, but the LOD for penicillin G was not estimated due to its poor recovery (Table S5†). In comparison, the higher LODs for macrolides, quinolones and tetracyclines were observed in the LVDI method, whereas higher LODs for sulfonamides and β-lactams were found in the SPE method (Fig. 4). The two methods in our study provided lower LODs for antibiotics compared to previous studies, which were mainly above 1 ng L−1 (Table S6†). In the SPE method, 500 mL of the water sample was extracted and then concentrated to 1 mL of extract. However, only 1 μL of the extract was injected for LCMS analysis, which is 100 times lower than the injection volume used in the LVDI method. It is noteworthy that the SPE method may achieve lower LODs by increasing the injection volume.
The SPE method comprises a more complex protocol, including extraction, concentration and solution transfer steps, which can introduce significant amounts of matrix components that may interfere with instrumental analysis. In contrast, the LVDI method employs a simpler workflow, involving only sample transfer and the addition of internal standards, greatly minimizing matrix effects. The LVDI method was validated for the analysis of antibiotics in bottled water, which is either purified through advanced technologies such as membrane filtration or sourced from carefully selected locations for drinking purposes. In general, bottled water contains negligible matrix interference in LCMS analysis. However, special attention may be required for natural mineral water, which can contain relatively high levels of minerals due to geological factors. For the simultaneous analysis of multi-class antibiotics in water samples with minimal matrix effects (e.g., bottled water and tap water), the LVDI method is preferred due to its simplicity, time and cost efficiency, and broad analyte coverage. However, the SPE method may still be necessary when analyzing ultra-trace concentrations of antibiotics.
3.5. Application to antibiotic analysis in bottled water
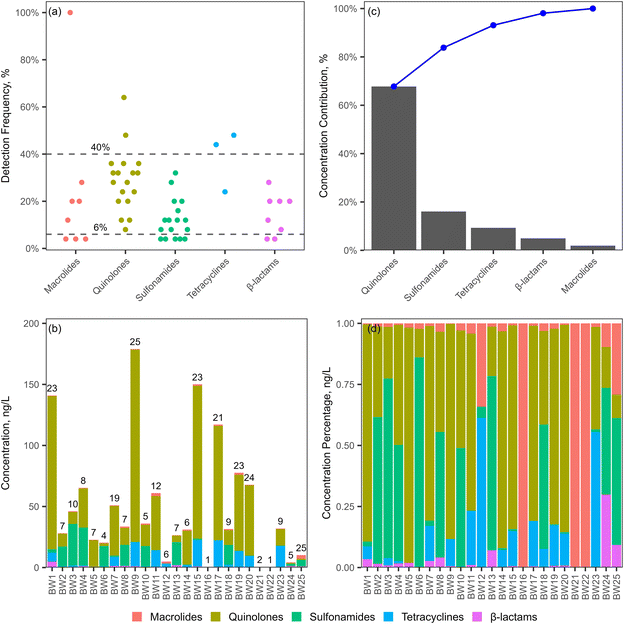
The concentrations of the detected antibiotics ranged from 0.0453 to 37.4 ng L−1, and the average concentrations for 24 antibiotics were above 1 ng L−1 (Fig. 5b and Table S7†). Especially, sulfaclozine, demeclocycline, and 6 quinolones, including moxifloxacin, oxolinic acid, norfloxacin, ciprofloxacin, lomefloxacin, and enoxacin, had concentrations above 10 ng L−1. The antibiotic concentrations were comparable to those in bottled water from the study by Ben et al.,23 although several antibiotics showed relatively higher concentrations in our study. Antibiotic concentrations were relatively lower in bottled water compared to those in tap water, with mean concentrations ranging from 2.8 to 7.0 ng L−1 for macrolides and from 8.0 to 9.4 ng L−1 for sulfonamides in 13 major Chinese cities,22 and from 2 to 270 ng L−1 for quinolones in Guangzhou, China,52 respectively. The total concentrations of antibiotics in each brand of bottled water were in the range of 0.442 to 179 ng L−1, with concentrations higher than 50 ng L−1 in 9 brands (Fig. 6b). Quinolones and sulfonamides were the major antibiotics, accounting for over 80% of the total concentration (Fig. 6c). This was further confirmed by the significant contributions of quinolones and sulfonamides to the total concentration in each brand of bottled water. Quinolones accounted for more than 50% of the total concentration in 10 brands, while sulfonamides exceeded 50% in 7 brands (Fig. 6d).
 | ||
| Fig. 7 Distribution of frequently detected antibiotics (a) and 5 antibiotic classes (b) in natural mineral water and purified drinking water (*p < 0.05). | ||
NMW originates from underground sources that were less affected by human activities, such as springs or bore holes, and is usually free of contamination and characterized by the mineral content.53 PDW usually originates from tap water or groundwater, which is processed to remove impurities, mainly physical, chemical and biological contaminants as well as minerals, to ensure that the purified water is safe for human consumption. The purification process typically involves a combination of techniques such as filtration, absorption, electrodialysis, ion exchange, disinfection and desalination.54,55 The comparable distribution of antibiotics in NMW and PDW suggested that the purification process may not remove specific antibiotics efficiently. For instance, a study by Simazaki et al. revealed that pharmaceuticals were not totally removed at drinking water purification plants utilizing ozonation and granular activated carbon filtration processes.56 Sulfaclozine, a broad-spectrum sulfonamide antibiotic, is primarily used in veterinary medicine, but it is usually poorly absorbed by animals,57,58 which may result in high concentrations in the environment. The significantly higher concentrations of sulfaclozine in PDW suggest that water sources for PDW may be more polluted and the water purification processes may be insufficient in removing sulfaclozine.
4. Conclusions
A LVDI-LC-MS/MS method for simultaneous analysis of 69 antibiotics from 5 classes in bottled water was developed and validated. Compared to injection volumes of 10 and 50 μL, an injection volume of 100 μL led to proportionally increased signal intensities while maintaining the peak shapes for the antibiotics. The LVDI method provided both good absolute and relative recoveries for the 69 antibiotics at various concentrations, which were in the range of 80% to 120% for 65 antibiotics and 58.3% to 126.3% for 4 antibiotics. The SPE method had lower ARs for macrolides, quinolones, sulfonamides and β-lactams, but good RRs for most antibiotics. Both the LVDI and SPE methods achieved low LODs, with most values below 1 ng L−1, but relatively higher LODs for macrolides, quinolones and tetracyclines were observed with the LVDI method. The application of the LVDI method revealed that 54 antibiotics were detected in bottled water, including 18 quinolones, 17 sulfonamides, 8 β-lactams, 8 macrolides and 3 tetracyclines. Our results highlighted that multiple antibiotics usually co-existed in bottled water, with more than 10 antibiotics identified in each of 9 different brands. The DFs ranged from 4% to 100%, with erythromycin detected in all the samples. The detected concentrations ranged from 0.0453 to 37.4 ng L−1, with quinolones and sulfonamides being the dominant antibiotics, comprising over 80% of the total concentration. The distribution of antibiotics in PDW and NMW was comparable, except for sulfaclozine, which had significantly higher concentrations in PDW. Our study provides a simple, sensitive and rapid method for simultaneous analysis of multi-class antibiotics in bottled water, which can also be applied to other types of drinking water, such as tap water and groundwater, aiding future contaminant monitoring and risk assessment of antibiotics.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Haijun Wang: methodology, investigation, formal analysis, visualization, writing – original draft. Qiao Zhang: investigation, data curation. Xiaolin Li: investigation, data curation. Huan Chen: methodology, writing – review & editing. Xiaolan Zhu: methodology, investigation. Liming Yang: conceptualization, writing – review & editing. Hongling Yin: funding acquisition, resources. Jing Sun: resources. Shuhong Fang: resources. Hui Zhang: conceptualization, methodology, formal analysis, visualization, funding acquisition, writing – review & editing.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This study was supported by the Natural Science Foundation of Sichuan Province, China (2023NSFSC0290, 2023NSFSC0287), and the Sichuan Province’s Talent Program (Tianfu Emei Plan Youth Talent Project, 2022).References
- X. He, M. Deng, Q. Wang, Y. Yang, Y. Yang and X. Nie, Aquaculture, 2016, 458, 38–46 CrossRef CAS.
- Q. Q. Zhang, G. G. Ying, C. G. Pan, Y. S. Liu and J. L. Zhao, Environ. Sci. Technol., 2015, 49, 6772–6782 CrossRef CAS PubMed.
- P. S. McManus, V. O. Stockwell, G. W. Sundin and A. L. Jones, Annu. Rev. Phytopathol., 2002, 40, 443–465 CrossRef CAS PubMed.
- K. Liu, X. Yin, D. Zhang, D. Yan, L. Cui, Z. Zhu and L. Wen, Mar. Pollut. Bull., 2018, 129, 859–865 CrossRef CAS.
- D. Li and W. Shi, Chin. J. Catal., 2016, 37, 792–799 CrossRef CAS.
- F. Wong, E. J. Zheng, J. A. Valeri, N. M. Donghia, M. N. Anahtar, S. Omori, A. Li, A. Cubillos-Ruiz, A. Krishnan, W. Jin, A. L. Manson, J. Friedrichs, R. Helbig, B. Hajian, D. K. Fiejtek, F. F. Wagner, H. H. Soutter, A. M. Earl, J. M. Stokes, L. D. Renner and J. J. Collins, Nature, 2024, 626, 177–185 CrossRef CAS.
- S. Cheng, R. Zhang, Q. Liu, S. He, J. Sun and L. Xing, Environ. Sci. Pollut. Res. Int., 2025, 32, 1223–1235 CrossRef CAS.
- R. Feng, K. Mao, H. Zhang, H. Zhu, W. Du, Z. Yang and S. Wang, Mikrochim. Acta, 2024, 192, 19 CrossRef.
- M. Wang, J. Li, Y. Zhou, W. Zhou and S. Huang, PLoS One, 2024, 19, e0310865 CrossRef CAS.
- G. Zhang, S. Lu, Y. Wang, X. Liu, Y. Liu, J. Xu, T. Zhang, Z. Wang and Y. Yang, Environ. Pollut., 2020, 257, 113365 CrossRef CAS.
- S. Akhter, M. A. Bhat, S. Ahmed and W. A. Siddiqui, Environ. Geochem. Health, 2024, 46, 387 CrossRef CAS.
- N. Yashir, Q. Sun, X. Zhang, M. Ma, D. Wang, Y. Feng and X. Song, Sci. Total Environ., 2025, 961, 178373 CrossRef CAS.
- W. Yuan, Y. Liu, R. Liu, L. Li, P. Deng, S. Fu, L. Riaz, J. Lu, G. Li and Z. Yang, Environ. Geochem. Health, 2024, 46, 309 CrossRef CAS.
- P. Sun, Y. Tan, Z. Zhu, T. Yang, S. Thevarajan and L. Zhang, Antibiotics, 2024, 13, 820 CrossRef CAS PubMed.
- J. Zhou, J. Kang, C. Lin, Q. Xu, W. Yang, K. Fan and J. Li, Toxics, 2024, 12, 411 CrossRef CAS PubMed.
- X. Yi, C. Lin, E. J. L. Ong, M. Wang and Z. Zhou, Chemosphere, 2019, 216, 213–223 CrossRef CAS.
- L. Fang, C. Chen, S. Li, P. Ye, Y. Shi, G. Sharma, B. Sarkar, S. M. Shaheen, S. S. Lee, R. Xiao and X. Chen, Ecotoxicol. Environ. Saf., 2023, 262, 115175 CrossRef CAS.
- T. Fayaz, N. Renuka and S. K. Ratha, Chemosphere, 2024, 349, 140822 CrossRef CAS PubMed.
- P. Barathe, K. Kaur, S. Reddy, V. Shriram and V. Kumar, J. Hazard. Mater. Lett., 2024, 5, 100105 CrossRef CAS.
- B. Puri, R. Vaishya and A. Vaish, Med. J. Armed Forces India, 2025, 81, 247–258 CrossRef.
- L. Feng, Y. Cheng, Y. Zhang, Z. Li, Y. Yu, L. Feng, S. Zhang and L. Xu, Environ. Res., 2020, 185, 109386 CrossRef CAS PubMed.
- H. W. Leung, L. Jin, S. Wei, M. M. Tsui, B. Zhou, L. Jiao, P. C. Cheung, Y. K. Chun, M. B. Murphy and P. K. Lam, Environ. Health Perspect., 2013, 121, 839–846 CrossRef.
- Y. Ben, M. Hu, X. Zhang, S. Wu, M. H. Wong, M. Wang, C. B. Andrews and C. Zheng, Water Res., 2020, 175, 115699 CrossRef CAS.
- H. Wang, N. Wang, B. Wang, Q. Zhao, H. Fang, C. Fu, C. Tang, F. Jiang, Y. Zhou, Y. Chen and Q. Jiang, Environ. Sci. Technol., 2016, 50, 2692–2699 CrossRef CAS PubMed.
- I. Ferrer, J. A. Zweigenbaum and E. M. Thurman, J. Chromatogr. A, 2010, 1217, 5674–5686 CrossRef CAS.
- V. R. Panditi, S. R. Batchu and P. R. Gardinali, Anal. Bioanal. Chem., 2013, 405, 5953–5964 CrossRef CAS.
- N. Salgueiro-Gonzalez, E. Concha-Grana, I. Turnes-Carou, S. Muniategui-Lorenzo, P. Lopez-Mahia and D. Prada-Rodriguez, J. Chromatogr. A, 2012, 1223, 1–8 CrossRef CAS.
- Y. Tao, J. F. Liu, X. L. Hu, H. C. Li, T. Wang and G. B. Jiang, J. Chromatogr. A, 2009, 1216, 6259–6266 CrossRef CAS PubMed.
- M. E. I. Badawy, M. A. M. El-Nouby, P. K. Kimani, L. W. Lim and E. I. Rabea, Anal. Sci., 2022, 38, 1457–1487 CrossRef CAS.
- M. N. L. Suseela, M. K. Viswanadh, A. K. Mehata, V. Priya, Vikas, A. Setia, A. K. Malik, P. Gokul, J. Selvin and M. S. Muthu, J. Chromatogr. A, 2023, 1695, 463937 CrossRef CAS.
- S. Bayen, X. Yi, E. Segovia, Z. Zhou and B. C. Kelly, J. Chromatogr. A, 2014, 1338, 38–43 CrossRef CAS.
- C. Hao, P. A. Helm, D. Morse and E. J. Reiner, Chemosphere, 2018, 191, 288–295 CrossRef CAS.
- C. Boix, M. Ibanez, J. V. Sancho, J. Rambla, J. L. Aranda, S. Ballester and F. Hernandez, Talanta, 2015, 131, 719–727 CrossRef CAS.
- M. A. Mottaleb, Q. X. Ding, K. G. Pennell, E. N. Haynes and A. J. Morris, J. Chromatogr. A, 2021, 1653, 462426 CrossRef CAS PubMed.
- S.-H. Liang, M. Chakraborty and J. A. Steimling, J. Chromatogr. Open, 2024, 6, 100188 CrossRef.
- A. K. Brown and A. Farenhorst, Chemosphere, 2024, 349, 140924 CrossRef CAS.
- M. Sargazi, M. Bücking and M. Kaykhaii, Open Chem., 2020, 18, 1339–1348 CAS.
- T. S. Oliveira, M. Murphy, N. Mendola, V. Wong, D. Carlson and L. Waring, Sci. Total Environ., 2015, 518–519, 459–478 CrossRef CAS.
- T. S. Thompson, D. K. Noot, F. Forrest, J. P. van den Heever, J. Kendall and J. Keenliside, Anal. Chim. Acta, 2009, 633, 127–135 CrossRef CAS PubMed.
- E. V. S. Maciel, D. A. Vargas-Medina and F. M. Lancas, Talanta Open, 2023, 7, 100185 CrossRef.
- C. Simarro-Gimeno, B. Garlito, E. Pitarch and F. Hernández, Microchem. J., 2023, 193, 108985 CrossRef CAS.
- R. Mirzaei, M. Yunesian, S. Nasseri, M. Gholami, E. Jalilzadeh, S. Shoeibi, H. S. Bidshahi and A. Mesdaghinia, J. Environ. Health Sci. Eng., 2017, 15, 21 CrossRef.
- L. Tong, P. Li, Y. Wang and K. Zhu, Chemosphere, 2009, 74, 1090–1097 CrossRef CAS PubMed.
- U. S. EPA, Method 1694, 2007, EPA-821-R-808-002.
- O. Opriş, M.-L. Soran, V. Coman, F. Copaciu and D. Ristoiu, Open Chem., 2013, 11, 1343–1351 CrossRef.
- Q. Wu, S. K. Xiao, C. G. Pan, C. Yin, Y. H. Wang and K. F. Yu, Sci. Total Environ., 2022, 806, 150439 CrossRef CAS PubMed.
- Z. Yin, Environ. Sci.: Processes Impacts, 2021, 23, 1088–1100 RSC.
- J. L. Zhou, K. Maskaoui and A. Lufadeju, Anal. Chim. Acta, 2012, 731, 32–39 CrossRef CAS.
- N. Liang, P. Huang, X. Hou, Z. Li, L. Tao and L. Zhao, Anal. Bioanal. Chem., 2016, 408, 1701–1713 CrossRef CAS.
- B. H. Schafhauser, L. A. Kristofco, C. M. R. de Oliveira and B. W. Brooks, Environ. Pollut., 2018, 238, 440–451 CrossRef CAS PubMed.
- Y. Wang, X. Dong, J. Zang, X. Zhao, F. Jiang, L. Jiang, C. Xiong, N. Wang and C. Fu, Water Res., 2023, 236, 119940 CrossRef CAS.
- Yiruhan, Q. J. Wang, C. H. Mo, Y. W. Li, P. Gao, Y. P. Tai, Y. Zhang, Z. L. Ruan and J. W. Xu, Environ. Pollut., 2010, 158, 2350–2358 CrossRef CAS.
- S. Quattrini, B. Pampaloni and M. L. Brandi, Clin. Cases Miner. Bone Metab., 2016, 13, 173–180 Search PubMed.
- N. B. Singh, G. Nagpal and S. Agrawal, Rachna, Environ. Technol. Innovation, 2018, 11, 187–240 CrossRef.
- A. Ahmad and T. Azam, in Bottled and Packaged Water, ed. A. M. Grumezescu and A. M. Holban, Woodhead Publishing, 2019, pp. 83–120 Search PubMed.
- D. Simazaki, R. Kubota, T. Suzuki, M. Akiba, T. Nishimura and S. Kunikane, Water Res., 2015, 76, 187–200 CrossRef CAS.
- L. Ismail, A. Rifai, C. Ferronato, L. Fine, F. Jaber and J.-M. Chovelon, Appl. Catal., B, 2016, 185, 88–99 CrossRef CAS.
- I. Sentepe and G. Eraslan, Food Chem. Toxicol., 2010, 48, 448–451 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra01764e |
| This journal is © The Royal Society of Chemistry 2025 |