 Open Access Article
Open Access ArticleMechanistic insights and therapeutic innovations in engineered nanomaterial-driven disruption of biofilm dynamics
Sadeeq Ullah†
 a,
Yong Chen†b,
Chunyan Wua,
Yasir Abbasd,
Yangqing Zhonga,
Xiaohui Chen
a,
Yong Chen†b,
Chunyan Wua,
Yasir Abbasd,
Yangqing Zhonga,
Xiaohui Chen a,
Junyin Tana,
Hefa Cheng
a,
Junyin Tana,
Hefa Cheng
 *c and
Lu Li
*a
*c and
Lu Li
*a
aGuangdong Provincial Key Laboratory of Medical Immunology and Molecular Diagnostics, The First Dongguan Affiliated Hospital, School of Medical Technology, Guangdong Medical University, Dongguan 523808, China. E-mail: lilu2698@gdmu.edu.cn
bDepartment of Medical Laboratory, Affiliated Cancer Hospital of Chengdu Medical College, Chengdu Seventh People's Hospital, Chengdu 610231, China
cMOE Laboratory for Earth Surface Processes, College of Urban and Environmental Sciences, Peking University, Beijing 100871, China. E-mail: hefac@umich.edu
dInterdisciplinary Research Center for Membranes and Water Security, King Fahd University of Petroleum & Minerals, Dhahran, 31261, Saudi Arabia
First published on 7th July 2025
Abstract
Bacteria employ biofilm formation as a survival strategy, characterized by the self-assembly of cells into 3D architectures encapsulated in an extracellular polymeric substance (EPS) that results in reduced antibiotic efficacy, increased tolerance, and emergence of multidrug resistance phenotypes. To overcome this challenge, persistent efforts are directed toward developing cutting-edge approaches and agents that rejuvenate antibiotic efficacy, mitigate biofilm formation, and eradicate biofilm-associated bacterial infections. Within this framework, nanotechnology has emerged as a pivotal tool for developing innovative functional materials with tailored attributes, exhibiting substantial potential in addressing the global health challenge of antibiotic resistance and biofilm-associated infections. This updated review article provides a comprehensive overview, commencing with a thorough analysis of biofilm formation and its implications, followed by a critical evaluation of cutting-edge strategies derived from recent research advancements. Our discussion encompasses novel strategies, including traditional nanomaterials, micro-nanobubbles, multifunctional nanozyme-mimetic platforms, artificial phage-like structures, and sophisticated nano-microrobotic systems. Each strategy is assessed for its potential to effectively target biofilms, enhance antimicrobial penetration, and restore antibiotic susceptibility. We anticipate that this timely review will inform and inspire innovative research directions, focusing on the rational design and application of advanced nanomaterials for targeted biofilm modulation and efficacious treatment, thereby advancing healthcare solutions.
1. Introduction
Bacteria are essential for survival, but when they aggregate into communities, they can cause significant harm and pose a health risk. The rise of antibiotic resistance in bacteria has led to the challenge of persistent infections, with multidrug-resistant (MDR) bacteria representing a global crisis. These resistant strains increase the morbidity and mortality rates among infected people and have a detrimental impact on the clinical results of patients in intensive care units, transplantation, surgery, or cancer treatment.1 Antimicrobial resistance (AMR) is linked to around 4.95 million deaths worldwide and imposes a global economic burden exceeding $300 billion.2,3 Despite a predominant global strategy centered on discovering new antibiotic agents to counteract drug resistance, diminishing returns have been increasingly evident due to perceived poor profitability. Consequently, no new class of antibiotics has obtained regulatory approval since the late 1980s.4 The fundamental scientific hurdles, including challenges with penetration, efflux, and the rapid emergence of resistance, have worsened the deficiencies in antimicrobial pipelines. In addition, the use, sometimes misuse, of antibiotics, coupled with the limited development of new therapeutics in the antibiotic repertoire, worsens this public health threat.5Planktonic (free-floating) bacteria are pivotal in numerous health threats, posing acute risks that are becoming increasingly challenging to address due to escalating rates of acquired antibiotic resistance. This challenge becomes even more severe when bacteria develop biofilms, as biofilms are difficult to penetrate and treat, leading to chronic and recurrent infections.6 Bacteria employ diverse mechanisms of resistance, with some being intrinsic allowing the cell to utilize genes it already owns to withstand antibiotic exposure, while others are acquired involving the acquisition of new genetic material that offers new aptitudes for existence.7 The shift from free-floating to biofilm evolution entails a sequence of changes, including, metabolic physiological, and phenotypic alterations regulated by c-di-GMP (cyclic diguanosine-5′-monophosphate), the secondary messenger. Elevated cellular content of c-di-GMP promotes biofilm development, while decreased levels lead to biofilm dispersal.8,9
1.1 Bacterial biofilm formation and properties
One of the predominant and safeguarded strategies for bacterial growth and survival is the formation of biofilms, which are functional aggregates of bacteria enveloped with an extracellular polymeric substances (EPS) matrix. Biofilms constitute an organized assembly of bacterial cells that can evolve into structured aggregates with qualitatively distinct properties compared to planktonic counterparts of similar size. Biofilms represent one of the most prevalent and thriving lifestyles on Earth. They can consist of populations of the same bacteria or diverse communities comprising multiple species, all coexisting under a protective dome. This dome is formed by a matrix of EPS, containing a blend of sugars, proteins, fats, and DNA molecules. Within biofilms, there are specialized regions, some are involved in nutrient recycling, while others are responsible for producing new EPS components or transmitting messages between different areas of the biofilm.10 The diverse components of EPS contribute to the establishment of biofilm structure, which is an ongoing and dynamic process resulting in a distinct organization that encourages the clustering of cells into microcolonies. Moreover, EPS fragments occupy and define the intercellular space within the biofilm, directly influencing the environment and living settings of the cells while imparting mechanical stability to the biofilm. Of particular importance, extracellular DNA (eDNA) is crucial for maintaining the structural integrity of the EPS matrix and is among the most commonly encountered matrix polymers.11 eDNA also aids in cell adhesion and the early stages of biofilm development, along with DNA damage repair and gene transfer. Furthermore, it plays a role in inactivating cationic antibiotics, thereby contributing to antibiotic resistance.12 It is fascinating that cells known as swimmers can create pores in the matrix as they actively move through it. These pores remain open for a sufficient duration, allowing the diffusion of nutrients into the biofilm. This discovery has led to the concept of a rudimentary circulation system within the biofilm.13 Furthermore, bacterial colonies within biofilms withstand water loss by consistently generating hydrated molecules in the EPS, serving as a hydrogel to retain water.14 Moreover, the formation of a shielding skin by the uppermost EPS layers creates an effective barrier against evaporation. Evidence suggests that, under the influence of specific enzymes, desiccated biofilm samples replace their water content upon exposure to wet conditions.15 Therefore, the biofilm lifestyle is predicted to offer significantly greater protection against desiccation compared to free-living bacterial cells, which do not benefit from the EPS matrix (Fig. 1).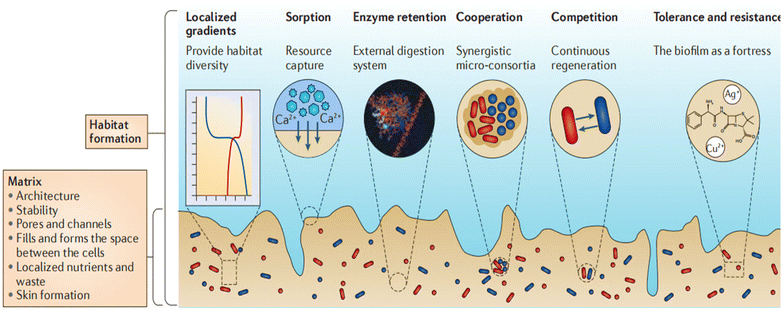 | ||
| Fig. 1 Bacteria within biofilms and the emergence of unique properties. Bacteria secrete a protective envelope that contributes to the stability of the biofilm. The specialized architecture permits the entry of nutrients through pores and channels. The outer layer of the skin serves as a protective barrier against desiccation. Bacteria within biofilms acquire distinct properties, including the ability to capture nutrients, engage in metabolic activities in harsh conditions, and exhibit survival capabilities in stressful environments. Reproduced from ref. 13 with permission from Springer Nature 2016. | ||
1.2 Tolerance to antimicrobial agents
Bacteria have developed various survival strategies to avoid the lethal effects of antibiotics. They tolerate antibiotic exposure either by entering a dormant state when antibiotics are at their highest concentrations such as tolerance or by acquiring active biochemical resistance mechanisms known as resistance.16 As tolerant organisms have a higher probability of surviving, they are more likely to subsequently acquire resistance mutations. Antibiotic tolerance denotes the bacterial population's capacity to temporarily resist high antibiotic concentrations, typically achieved by slowing down vital processes. The assumption of a biofilm lifestyle might contribute to this tolerance, given that a considerable portion of bacteria within biofilms either grows slowly or remains non-growing.17 Therefore, managing biofilm infections demands administering antibiotics at higher concentrations and for extended durations compared to what is required for treating planktonic cells.18 The effectiveness of antibiotics hinges on their ability to reach the intended targets within bacterial cells at adequate concentrations. However, achieving sufficient concentrations against targeted bacteria within biofilms is impeded by the matrix, which can delay the permeation of antibiotics into bacterial cells, as well as by the limited availability of bacterial targets due to the sluggish growth of bacteria in the biofilm.19,20 | ||
| Fig. 2 The mechanisms underlying antimicrobial tolerance in biofilms. The surface-active subpopulations within the biofilm show heightened expression of efflux pumps or β-lactamases, while the metabolically inactive community with slower growth rates displays diminished or minimal expression of antibiotic targets and decreased dynamic uptake of antibiotics. Matrix components bind to positively charged drugs, slowing their penetration into the biofilm. The adaptive stress activation responses and transient resistance, involving the upregulation of resistance genes, reduce the effectiveness of antimicrobials, thereby contributing to the antibiotic tolerance of biofilms. Reproduced from ref. 6 with permission from Springer Nature 2022. | ||
1.3 Tolerance leads to resistance in biofilms
Antibiotic failure often arises from resistance, which encompasses several mechanisms. These include mutations that diminish drug-target binding, heightened efflux pump expression, altered metabolic activity, the barrier created by EPS against antibiotics, horizontal gene transfer, and the presence of diverse populations of species.1,4 Resistance mutations lead to a reduction in the drug's effective concentration, measured by the minimum inhibitory concentration (MIC), indicating the lowest drug concentration required to inhibit visible growth of the microorganism.16 Conversely, tolerance mutations extend the minimum time required to eliminate the population without altering the MIC.17,51 However, bacteria also employ alternative mechanisms to endure antibiotic exposure.52 The emergence of antibiotic resistance in biofilms is supported by various factors, the sustained presence of a sizable population of bacterial cells that withstand antibiotic treatment due to the resilience of slow-growing segments and the existence of persisters, alongside a high mutation rate. The dormant subset of cells within biofilms endures high antibiotic concentrations, displaying considerable tolerance that eventually fosters the emergence of resistant strains. The susceptibility of bacteria to antibiotics is influenced by their cellular metabolic state, and a shift in metabolism is often linked to antibiotic resistance (Fig. 3).53–55 | ||
| Fig. 3 Various mechanisms employed by bacteria within biofilms to withstand the effectiveness of administered antibiotics. Bacteria within biofilms employ four mechanisms to develop resistance: (1) production of EPS, which obstructs antibiotic penetration and the specialized microenvironment (hypoxic and acidic) which degrades antimicrobials, (2) entering dormancy with decreased metabolic activities, (3) possessing horizontal gene transfer abilities, and (4) experiencing enhanced spontaneous mutations alongside the persistence of multispecies populations. Reproduced from ref. 4 with permission from Springer Nature 2023. Colored regions in the figure represent different subpopulations within the biofilm: metabolically active cells (e.g., red or orange) located at the surface, slow-growing or dormant persister cells (e.g., blue or purple) embedded deeper within the matrix, and regions of horizontal gene transfer activity (e.g., green) indicative of genetic exchange. | ||
A recent study demonstrated that fluctuations in intracellular glucose levels can induce resistance development in E. coli subjected to repeated cycles of daily intermittent exposure to ampicillin.56 The conclusion drawn was that the activation of the cAMP/CRP pathway a global transcriptional regulator involving cyclic adenosine monophosphate (cAMP) and the cAMP receptor protein (CRP) triggers resistance when glucose levels gradually decrease below normal, consistent with findings from previous studies.57 Additionally, cAMP/CRP promotes the reduction of ROS, facilitates DNA repair, and upregulates ampC expression, thereby enhancing bacterial survival under stress conditions. Clinical isolates exposed to high drug concentrations often exhibit mutations in multiple genes, and cells that are already tolerant can acquire mutations that confer resistance.58,59 In biofilms, when only a portion of the clonal population adopts a non-growing phenotype, this subset of persisters is responsible for treatment failures.34,60 In contrast to planktonic bacterial populations, which are uniform and homogeneous, the spatially organized and diverse environment within biofilms creates varied niches with localized selective pressures.61,62 This diversity allows for the coexistence and persistence of a wider range of resistant mutants within biofilms.63 While resistance mutations in both biofilms and planktonic cultures may target the same genes, the nature of these mutations and the dynamics of how resistant mutants are generated and sustained differ between the two bacterial growth modes. These distinctions characterize the evolution of resistance in biofilms compared to planktonic cultures. The structural diversity within biofilms and their ability to tolerate antimicrobials may account for the unique evolutionary pathways observed in biofilms.64–66
An accepted mechanism through which bacterial populations in biofilms develop resistance to antibiotics is through an increased capacity for horizontal gene transfer (Fig. 3).67 This process is facilitated by factors such as high population density, heightened genetic competence, and the accumulation of mobile genetic elements.68 Horizontal gene transfer in biofilms commonly occurs through plasmid conjugation, which is significantly more efficient, about 700 times, in biofilms compared to planktonic cells. For instance, research on Staphylococcus aureus (S. aureus) demonstrated that conjugal plasmid transferal was observable in biofilms but not in free-living bacterial cultures. This phenomenon highlights a process specific to biofilms that is not achievable in free-living bacterial populations.69 The dense population and intimate cellular connections within biofilms facilitate the mechanism of horizontal gene transfer, as demonstrated in a study of V. cholerae biofilms. These biofilms employ type VI secretion as a novel mechanism for gene transfer, emphasizing the necessity of close cell proximity for these secretions to occur. It was observed that the type VI system can lyse nearby cells and subsequently acquire the released genetic materials.70 Additionally, it has been reported that the biofilm matrix can aid in binding and stabilizing plasmid DNA, facilitating its subsequent uptake by cells within the biofilm.71 This indicates that bacterial populations within biofilms employ various mechanisms for the transfer of resistant genes, yielding them the capability to withstand the effectiveness of antibiotics and endure in challenging environments.
2. Emerging treatment strategies
Although antibiotics remain the primary approach to inhibit bacterial growth and biofilm formation, the emergence of multidrug-resistant pathogenic strains requires an urgent pursuit of innovative, non-antibiotic-based therapeutic strategies. These novel approaches aim to either restore and synergize antibiotic efficacy or independently eradicate bacterial biofilms by targeting and compromising bacterial physiological and structural barriers, thus overcoming antibiotic resistance and ensuring effective bacterial eradication. Accumulating evidence from up-to-date research underscores the substantial promise of nanotechnology-based strategies in devising innovative nanostructures with diverse compositions and multifaceted functionalities, offering a potent solution to mitigate the global health crisis.72–752.1 Nanotechnology offers hope
Extensive and sustained efforts have been directed toward developing alternative strategies to eradicate biofilms and overcome the antimicrobial resistance of the bacterial strains embedded within them. Nanotechnology stands out as a promising alternative among these efforts. Nanomaterial-based antibiofilm strategies are their interaction with the host immune system, which plays a central role in both biofilm persistence and clearance. Nanomaterials can act as immunomodulatory agents, potentially enhancing or impairing host responses depending on their physicochemical properties. For example, certain nanoparticles have demonstrated adjuvant-like effects, enhancing antigen presentation and immune activation, while others can provoke excessive pro-inflammatory cytokine release (e.g., IL-6, TNF-α), potentially exacerbating tissue damage.76 Conversely, surface-engineered or biodegradable nanomaterials (e.g., PEGylated, zwitterionic, or ROS-scavenging systems) may exhibit anti-inflammatory properties, reducing chronic inflammation that sustains biofilm niches.77 Emerging evidence suggests some nanoplatforms can modulate immune cell behavior for instance, by enhancing macrophage phagocytosis, repolarizing M2 to M1 phenotypes, or improving neutrophil-mediated bacterial clearance, thereby synergizing with antimicrobial effects.78 Therefore, an integrated understanding of nanomaterial immune interactions is essential for optimizing therapeutic efficacy and safety in biofilm-related infections. By crafting nanomaterials with precise sizes, shapes, and desired functionalities, it becomes possible to circumvent the conventional strategies of antibiotic resistance commonly employed by various bacterial strains. Nanomaterials offer antimicrobial strategies that are unfamiliar to bacteria and are not part of their natural defense mechanisms. Recent progress in nanomaterial-based systems presents new prospects for combating MDR planktonic and biofilm infections, serving either as standalone therapeutics or as carriers for antimicrobial agents. The distinctive physicochemical attributes of nanomaterials, including their size, shape, and surface chemistry, impact their therapeutic efficacy.79 Nanomaterials with high surface-to-volume ratios and multivalent interactions can easily penetrate biofilms and are crucial for developing antibacterial agents.80,81 Nanomaterials have been shown to hinder elements of EPS, consequently impeding biofilm formation and causing the demise of the resident cells.82,83 Although nanomaterials exhibit multifaceted antimicrobial mechanisms (e.g., membrane disruption, ROS generation, ion release), the claim that they pose lower risks of resistance development requires caution. Recent studies indicate that bacteria can adapt through several mechanisms, such as efflux pump upregulation,84 membrane remodeling, EPS thickening, or secretion of enzymes that degrade or inactivate nanoparticle coatings. For example, P. aeruginosa has shown increased resistance to AgNPs via biofilm matrix densification and increased expression of oxidative stress response genes.85 Additionally, ion chelation, surface charge masking, and horizontal gene transfer of resistance traits may also occur, especially under sublethal, chronic exposure conditions. Therefore, while nanoparticles may delay resistance development relative to conventional antibiotics, long-term selective pressures and bacterial plasticity require continuous monitoring and design optimization. Therapeutic nanomaterials can be utilized in three main forms: (1) bare nanoparticles, which primarily encompass inorganic, carbon-based, and polymeric nanoparticles, (2) conjugated nanoparticles formed by functionalizing bare nanoparticles with bioactive substances, and (3) carrier materials loaded with specific drugs to efficiently inhibit biofilm formation.86–89 Disrupting biofilms and subsequently destroying the bacteria within them has shown promise in successfully treating infections caused by biofilms.90 Moreover, nanomaterials composed of various metals have demonstrated the ability to disrupt bacterial quorum sensing behavior, representing a notable strategy to inhibit pathogenicity without facilitating the emergence of resistant populations.91,92 A crucial aspect of nanomaterials as nanomedicine is their ability to target multiple sites and activate various mechanisms to kill bacteria. Despite their broad-spectrum antimicrobial activity, AgNPs present several translational challenges. Their cytotoxicity is highly dose-dependent, and they may induce oxidative stress and membrane damage not only in bacteria but also in mammalian cells.93 Furthermore, AgNPs can form protein halos in biological fluids, which may alter their targeting ability and biodistribution.94 A significant limitation is the lack of standardization in particle size, surface chemistry, and synthesis methods, which affects reproducibility and regulatory approval. Scalable, cost-effective production of uniformly stable AgNPs remains nontrivial, and accumulation in tissues raises concerns regarding long-term environmental and systemic toxicity.95 This characteristic reduces the possibility of bacterial resistance. In contrast, traditional antibiotics adhere strictly to specific target points, which bacteria can alter to reduce their effectiveness and develop resistance more easily compared to therapies based on nanomaterials. Furthermore, resistant strains possess enzymes capable of neutralizing administered antibiotics and limiting their binding capacity. The diverse mechanisms of action of nanoparticles allow them to evade deactivation by these enzymes.96,97 Compared to conventional antibiotics that primarily depend on diffusion or membrane protein-facilitated transport entry and are frequently thwarted by efflux pumps or enzymatic inactivation engineered nanomaterials attack bacterial defenses through multiple, simultaneous entry pathways and intracellular effects. For example, zinc oxide and AgNPs physically disrupt bacterial membranes, creating pores that allow direct cytoplasmic access and ion influx, while also generating ROS under light or physiological conditions to damage DNA and proteins.98–100 Furthermore, carbon-based nanomaterials like graphene oxide use their sharp edges to mechanically breach membranes and penetrate biofilm matrices. Once inside, these nanoparticles release antimicrobial payloads, interfere with respiration, inhibit efflux systems, and induce oxidative stress attacking cells from the inside out. This multimodal mechanism enables nanomaterials to bypass classic resistance pathways and achieve robust antimicrobial effects, even against multidrug-resistant strains. Treating biofilms containing dormant cells persisters with antibiotics poses challenges because many antibiotics require active targets to exert lethal effects.101,102 In contrast to many antibiotics that require active cellular processes such as replication, transcription, or cell wall synthesis to exert their bactericidal effects, several nanomaterials reveal non-specific, metabolism-independent mechanisms of action that may extend to persister cells. For instance, AgNPs exert toxicity through a combination of membrane disruption, generation of ROS, and interference with intracellular enzymes and DNA mechanisms that do not strictly require bacterial growth or division.103 Similarly, other nanomaterials like cationic polymers, graphene oxide, and metal oxides cause physical damage to the cell envelope or induce oxidative stress, which can affect even dormant cells.104 However, while these properties may make nanomaterials effective against persisters, it is important to note that they are not immune to resistance development. Bacteria have been shown to adapt by thickening biofilm matrices, upregulating efflux pumps, remodeling membranes, or scavenging ROS.105 For example, P. aeruginosa can develop resistance to silver via enhanced matrix density and oxidative stress responses.106 Therefore, while nanomaterials offer promising avenues for targeting biofilm-associated and dormant infections, their long-term efficacy requires careful optimization and surveillance for emergent resistance.2.2 Conventional metal-based nanomaterials
AgNP stands out as the most extensively investigated antibacterial nanoagent among the array of engineered nanoparticles utilized in antibacterial treatments. This prominence is owed to its broad-spectrum antimicrobial properties and strong efficacy against various bacteria, fungi, and viruses.107–109 The effectiveness of antimicrobial properties is highly influenced by factors such as particle size, surface structure, shape, and charge.110 A thorough comprehension of the mechanisms underlying the action of Ag-based nanomaterials is crucial for the design of selective and efficient nanoagents. AgNPs are believed to act through various mechanisms, including, attaching to the cell membrane and causing disintegration or disruption, resulting in the leakage of cellular components, and internalization into the cell, the Ag ions can deactivate crucial metabolic enzymes and cellular processes, causing DNA damage, and inducing oxidative stress, leading to damage to multiple vital cellular components (Fig. 4).111 As Ag is recognized as a Lewis acid, it typically exhibits a propensity to interact with Lewis bases, such as sulfur and phosphorous-containing biomolecules, which are key constituents of proteins, cell membranes, and DNA bases.112–114 Therefore, initially, AgNPs may gather on the cell wall and membrane, resulting in observable morphological alterations, ultimately leading to destruction. This destruction facilitates the penetration of Ag/AgNPs into the cells, consequently exerting additional lethal effects. Due to their multifaceted mode of action, AgNPs have demonstrated significant potential in combating resistant strains and have been successfully utilized for the eradication of biofilms.115,116 | ||
| Fig. 4 Multiple modes of action of Ag+ from AgNPs. Ag+ kills bacteria by targeting multiple vital targets. Reproduced from ref. 115 with permission from Springer Nature 2021. | ||
Research has indicated that materials capable of influencing multiple mechanisms within biofilms, such as causing physical damage, disrupting quorum sensing, inducing ROS, inhibiting oxidative stress responses, and causing DNA damage, hold promise for effectively eliminating both developing and mature biofilms.
In a detailed investigation, Zhang et al.117 explored how AgNPs affect the biofilm of multidrug-resistant P. aeruginosa. They conducted proteomic analysis to elucidate the impact of AgNPs on protein expression in the bacterial biofilm. The researchers noted that AgNPs can entirely inhibit biofilm formation and can also disrupt pre-existing biofilms, resulting in a significant reduction in biomass in treatments dependent on both dosage and time (Fig. 5a). Scanning electron microscopy (SEM) imaging revealed the detachment and rupture of biofilms following exposure to AgNPs, accompanied by noticeable deformation in bacterial morphology (Fig. 5c). Bacterial motility through flagella and surface attachment via fimbriae are crucial factors in biofilm formation, and inhibiting these characteristics is key to preventing biofilm development. Proteomic analysis showed that exposure to AgNPs led to the downregulation of flagellins, which are essential for flagellar structure and function, as well as fimbrillins involved in fimbria formation, thereby impeding bacterial adhesion and motility (Fig. 5d). Additional research unveiled that exposure to AgNPs can stimulate the generation of ROS and impact both aerobic and anaerobic respiratory pathways. P. aeruginosa can endure hypoxic conditions by employing cytochrome cbb3 type oxidases, and AgNPs-treated bacteria were observed to modify the expression of these proteins. Moreover, AgNPs can disrupt iron homeostasis, further contributing to interference with biofilm development. Proteomic analysis revealed notable decreases in iron storage protein and iron-sulfur cluster protein synthesis in AgNP-exposed biofilms. Conversely, there was an increase in iron uptake receptor proteins and a decrease in proteins responsible for iron processing, indicating an imbalance in iron homeostasis within biofilms, which could elevate ROS induction and result in oxidative damage. Treatment with AgNPs also influenced the quorum sensing (QS) behavior of the cells by increasing the expression of proteins (AntA, AntB) capable of degrading the QS signal precursor (anthranilic acid), thus inhibiting the production of virulence factors and biofilm formation (Fig. 5e). In conclusion, AgNPs demonstrate the capability to inhibit and eradicate biofilms through multiple mechanisms, indicating significant promise for potential clinical applications in treating bacterial infections associated with biofilms.
 | ||
| Fig. 5 (a) Biofilm dispersion upon treatment with AgNPs (b and c) SEM photographs depicting control and AgNPs-treated P. aeruginosa biofilms. (d) Influence of AgNPs on bacterial motility. (e) Impact of AgNPs on quorum sensing, showing the expression levels of antA and antB proteins. (f) The volcano map of differentially expressed proteins. Reproduced from ref. 117 with permission from American Chemical Society 2020. Relative expression levels of biofilm-specific genes in response to (g) AgNPs and (b) Ag+. (h) Relative expression of genes related to exopolysaccharides, motility, efflux transporters, quorum sensing, DNA replication, and outer membrane. (i and j) TEM images of control and AgNPs treated biofilm, respectively. Reproduced from ref. 118 with permission from RSC. | ||
Similarly, another comprehensive study documented the effects of AgNPs and Ag ions on gene regulation related to biofilm formation and bacterial metabolic activities. The study revealed that 1599 transcripts showed differential expression in response to AgNPs, while 2458 transcripts were affected by Ag ions.118 The extracellular polymeric substance is majorly composed of several polysaccharides, which provide a barrier to antimicrobial penetration. Transcriptomic analysis revealed that most genes encoding EPS polysaccharides were downregulated following exposure to AgNPs and Ag+ ions. Additionally, both AgNPs and Ag+ notably influenced adhesion factors and related genes, as well as type IV pili and fimbriae, crucial for recognizing adhesion surfaces. Further analysis revealed that the treatment of biofilms with AgNPs and Ag+ also influenced the QS signaling regulators and other virulence factors. It was concluded that AgNPs and Ag+ inhibit the synthesis of several virulence factors significantly and impact QS-mediated regulation, potentially increasing sensitivity and ultimately leading to the disruption of biofilms (Fig. 5g and h). The AgNPs and Ag ions were also demonstrated to inhibit the genes accountable for the defense mechanism against oxidative stress, as well as the efflux system responsible for expelling antimicrobials. The findings from the transcriptomic analysis were corroborated by TEM investigations (Fig. 5i and j). TEM analysis demonstrated that exposure to both Ag+ and AgNPs led to the detachment of biofilms and a reduction in biomass. Moreover, bacterial cells exhibited deformed and ruptured morphology compared to control cells. The comprehensive molecular-level analysis suggests that both Ag ions and AgNPs are effective agents in inhibiting the growth of biofilms through multiple mechanisms, highlighting their potential significance in eliminating biofilms and related medical conditions.
The weakening of biofilms and subsequent exposure of resident bacteria can be accomplished by inhibiting various elements of the EPS. Among these, targeting functional amyloids presents a novel approach to close biofilm formation. Bacterial amyloids are pivotal in facilitating the deposition of polysaccharides, serving to: (a) boost the integrity of the enclosed community, (b) enhance cell adhesion, motility, intercellular communications, and quorum sensing, and (c) hinder the entry of antimicrobial agents.14,119,120
In a recent study, Huma et al.121 examined the impact of AgNPs and AgNCs coated with branched polyethyleneimine (bPEI) on the anti-amyloid activity by targeting FabC, a key protein component of the extracellular amyloid matrix in P. aeruginosa. When exposed to AgNPs, the formation of FapC fibrils was completely inhibited (Fig. 6a), as indicated by changes in the secondary structure of FapC observed through CD spectroscopy before and after fibrillization (Fig. 6b). These results suggest that AgNPs impeded FapC fibrillization by sequestering monomeric FapC (Fig. 6c). TEM observations also revealed the complete inhibition of FapC fibril formation in the sample treated with FapC + AgNPs. However, AgNCs were found to cluster with FapC monomers and promote the formation of short FapC fibrils (Fig. 6d). The superior effectiveness of AgNPs over AgNCs was attributed to their size disparity; AgNPs are larger, providing ample surface area for favorable interaction with the studied protein, thereby preventing FapC aggregation into fibrils. The comprehensive findings indicate that AgNPs inhibited biofilm formation by preventing FapC amyloidosis without exhibiting bactericidal activity (Fig. 6f). Conversely, AgNCs, owing to their smaller size, inhibited biofilm formation and also conferred bactericidal properties. Likewise, AgNPs coated with citrate and bPEI have exhibited considerable potential in hindering the aggregation of human islet amyloid polypeptide122 (Fig. 6g–l). This indicates that AgNPs could serve as a promising nanomedicine for combating biofilms and addressing other medical conditions linked to fibril formation.123 Gao et al. recently developed a series of AgPd-based nanomaterials that exhibited potent biofilm eradication and bactericidal activity through surface-bound ROS-mediated mechanisms.124 Notably, upon incorporation as a coating additive on polydimethylsiloxane (PDMS), AgPd0.38 exhibited pronounced biofilm inhibition activity, conferring biofilm-resistance on the previously inert surface. It was hypothesized that AgPd0.38 confers the substrate with enhanced anti-protein adsorption, antibacterial, and anti-biofilm properties.
 | ||
| Fig. 6 (a) ThT assay upon mixing FapC with AgNPs or AgNCs. (b) CD spectra and (c) percent secondary structure of FapC monomers, fibrils, and FapC monomers post-incubation with AgNPs or AgNCs. TEM images illustrating (d) FapC + AgNPs and (e) FapC + AgNCs. (f) The impact of AgNPs on inhibiting biofilm formation and bacterial viability. Reproduced from ref. 121 with permission from Wiley 2020. (g and h) ThT assay of IAPP upon exposure to c-AgNPs and bPEI-AgNPs. TEM images of (i) control fibrils, (j) fibrils treated with 500 μg per mL c-AgNPs, and (k and l) fibrils exposed to 100 and 500 μg per mL bPEI-AgNPs. Reproduced from ref. 122 with permission from RSC. | ||
Similarly, to AgNPs, AuNPs have also shown considerable promise in combating bacterial biofilms. The surface modification of AuNPs with antimicrobial peptides has been demonstrated to effectively inhibit biofilm formation, resulting in a 90% reduction in biofilm biomass of Acinetobacter baumannii and P. aeruginosa. Furthermore, the conjugates can also effectively disrupt mature biofilms, leading to an 80% reduction in biofilm viability.125 Extensive research has revealed that gold-based nanomaterials mediate their antibiofilm effects primarily through the production of ROS, which target bacterial cells and biofilms via diverse mechanisms, such as lipid peroxidation, protein denaturation, and biofilm matrix degradation.126–128
2.3 Microbubbles and nanobubbles-based treatment strategies
The utilization of microbubbles (MBs) as a strategy is deemed an innovative method to eliminate biofilms during ultrasound treatment, due to their exceptional acoustic attributes. MBs are spheres measuring micrometers in size, comprising a gas core encased with a stabilizing shell.129,130 When subjected to ultrasound stimulation, these MBs can physically damage the architecture of biofilms and enhance the penetration of loaded antimicrobials into the biofilm.131–133 Taking inspiration from the concept of MBs, Xiu et al.134 engineered Fe3O4-shelled MBs encapsulating piperacillin antibiotic. Upon exposure to ultrasound stimulation, these hybrid MBs physically disrupted biofilms by inducing pore formation, thereby facilitating the penetration of Fe3O4 nanoparticles and Piperacillin into Pseudomonas aeruginosa biofilms. The internalized Fe3O4 particles exhibited peroxidase-like activity, generating ROS, which initiated the chemical breakdown of biofilms and consequent bacterial eradication (Fig. 7a). Additionally, the released piperacillin from the ruptured MBs can effectively inhibits the exposed bacteria, while the Fe3O4 nanoparticles stimulate the immune response to eliminate biofilms (Fig. 7b). An effective strategy to enhance antibiotic efficacy at low doses involves improving their penetration into biofilms by disrupting the biofilm architecture and creating intercellular spaces, allowing for targeted drug internalization. Teirlinck et al.135 introduced an innovative approach, where AuNPs administered to the biofilm are triggered by laser irradiation to produce vapor nanobubbles (VNBs). The AuNPs' photothermal properties enable the absorption of radiation, generating localized heat that rapidly evaporates surrounding water, creating transient VNBs that compromise the biofilm's structural integrity (Fig. 7c). The VNB-induced confined shockwaves create increased intercellular distances, enabling more effective delivery of tobramycin to target cells, including those embedded within dense cell clusters. A key benefit of laser-induced VNB is the efficient thermal-to-mechanical energy conversion within AuNP, which minimizes heat transfer to adjacent healthy tissue, overcoming a major drawback of conventional photothermal therapies. Despite the promising capabilities of microbubble and nanobubble technologies in disrupting biofilm structures, significant challenges persist in effectively targeting deep-seated biofilms, such as those found in chronic wounds, cystic fibrosis lungs, and indwelling medical devices. The EPS matrix in mature biofilms presents a formidable barrier, impeding the diffusion and infiltration of therapeutic agents, particularly larger constructs like microrobots and bubble-generating systems.136 Achieving physiologically relevant concentrations of these agents at infection sites without inducing systemic toxicity or off-target effects remains a critical hurdle. Active targeting strategies, including ligand functionalization and magnetically guided navigation, offer potential solutions but are often limited by biofilm heterogeneity, dynamic fluid environments, and host immune responses. Conversely, passive targeting approaches, relying on size or charge, lack the precision required for effective biofilm penetration.137 Therefore, while advancements in nanotechnology provide innovative avenues for biofilm disruption, a comprehensive understanding of the limitations and challenges associated with both active and passive targeting strategies is essential for the development of effective therapies.138 Post-treatment addition of tobramycin was found to potentiate its effectiveness by up to three orders of magnitude, with the degree of enhancement being organism- and condition-dependent. | ||
| Fig. 7 (a) Illustrates the design and functionality of Pip-loaded microbubbles (MB-Pip). Under ultrasound (US) stimulation, MB-Pip can disrupt biofilm architecture, degrade EPS, and activate macrophages. (b) In the context of P. aeruginosa biofilm-infected lungs, US-triggered rupture of MB-Pip enables physical biofilm disruption. The released Fe3O4 NPs catalytically generate ROS, degrading biofilm EPS and enhancing the antibacterial efficacy of Pip. This process also activates the host immune response, as evidenced by increased production of pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β), ultimately contributing to biofilm elimination. Reproduced from ref. 134 with permission from The American Association for the Advancement of Science 2023. (c) Biofilm disruption via vapor nanobubble (VNB)-mediated mechanisms. Dark field microscopy images depict the impact of a single 561 nm laser pulse (1.69 J cm−2) on biofilm architecture, with the laser-targeted region demarcated by a yellow circle. The induction of VNB is evident, as indicated by red arrowheads. A control biofilm cluster devoid of gold nanoparticles (AuNP) serves as a reference (CTRL). Reproduced from ref. 135 with permission from Springer Nature 2018. | ||
2.4 Nanozymes and biofilms inhibition
Artificial nanomaterials with enzyme-like catalytic properties, referred to as nanozymes, have emerged as a focal point of research in recent years. These nanozymes boast several benefits over natural enzymes, including lower production costs, improved stability, and adjustable activity, making them attractive alternatives for use in biological, industrial, and medical applications.139,140 The adaptable catalytic activity of enzymes modulated through composition, size, and surface modifications is a major advantage, achieving predictable and controllable enzymatic function in vivo remains a formidable challenge.141 In contrast to controlled in vitro buffer systems, biological milieus present dynamic pH fluctuations, ionic strength variations, and abundant ROS scavengers such as glutathione and catalase, which can significantly reduce catalytic turnover.142 Moreover, the rapid adsorption of biomolecules onto nanozyme surfaces a phenomenon known as protein corona formation can mask active sites or alter substrate affinity, leading to unpredictable bioactivity. Additionally, enzyme inhibitors, competitive substrates, and inflammatory microenvironments can further compromise activity. These factors collectively highlight the gap between promising in vitro data and reliable in vivo efficacy, warranting the development of robust predictive models and adaptive nanozyme designs to accommodate physiological variability. Furthermore, the development of drug resistance is hindered due to the multifaceted antibacterial capabilities of these nanomaterials. Notably, their antibacterial efficacy can be precisely modulated by tailoring parameters such as particle size, structural configuration, surface topography, composition, surface charge, and other material properties. Additionally, synergistic enhancements in antibacterial performance can be achieved through the integration of multiple functional mechanisms within a single nanomaterial entity.143–145 The antimicrobial properties of nanozymes have garnered significant attention, as they can produce surface-bound ROS, triggering oxidative damage in pathogens via multiple pathways.146,147 Notably, when applied as surface coatings, these nanozymes exhibit considerable promise in suppressing the formation of bacterial biofilms, thereby mitigating the risk of chronic infections.148 Since ROS can oxidize and impair various cellular constituents crucial for cell function, nanozymes can eradicate drug-resistant bacteria and potentially mitigate the progression of bacterial resistance.149,150 The ROS are widely cited as antimicrobial agents, it is important to distinguish between different types such as hydroxyl radicals (˙OH), superoxide anions (O2−˙), singlet oxygen (1O2), and H2O2.151 These species differ in redox potential, reactivity, diffusion distance, and cellular targets. For instance, ˙OH is extremely reactive but short-lived, damaging DNA and lipids locally, 1O2 is longer-lived and can diffuse to damage proteins and membranes, H2O2 serves more as a signaling molecule and is less directly bactericidal. Moreover, many nanozymes depend on endogenous H2O2 levels to activate peroxidase-like functions, however, H2O2 is often present in very low concentrations in infected tissue typically <10 μM far below optimal catalytic thresholds. Alternatively, some studies use exogenous H2O2, which poses challenges due to instability, diffusion limits, and off-target toxicity, particularly in sensitive tissues. These issues underscore the need for H2O2-independent ROS generators (e.g., Fenton-like catalysts, photoactivated 1O2 producers) or self-supplying nanozymes that co-deliver or catalyze precursor molecules in situ.152 In addition to direct oxidative killing, nanozymes have also shown promise in targeting bacterial communication systems, particularly QS, which plays a pivotal role in initiating and maintaining biofilms. Over the past decade, there has been significant research into designing nanozyme-based nanomaterials to tackle the growing issue of bacterial resistance and the challenges posed by biofilm-related infections.153Targeting the QS mechanism in bacteria is a crucial objective to effectively hinder the formation of biofilms. This mechanism relies on various signaling molecules, with N-acyl homoserine lactones (AHLs) being utilized by Gram-negative bacteria. AHLs have been observed to lose their functionality upon halogenation (in presence of hypohalous acids), leading to quorum quenching (QQ) and thereby impeding biofilm formation further. The naturally occurring vanadium haloperoxidase (V-HPO), produced by marine algae, catalyzes this reaction in the presence of H2O2, making it effective in suppressing biofilm formation through quorum quenching (QQ). Considering the remarkable ability of HPO to obstruct QS and hinder biofilm formation, Zhou and colleagues154 sought to develop a Ce-metal organic framework (Ce-MOF) to mimic HPO for governing surface-adhered biofilms. The synthesized nanozyme successfully replicated HPO's function, catalyzing the generation of HBrO with encouraging antibacterial and quorum quenching (QQ) capabilities (Fig. 8a). Ce-MOF exhibited significant potential in both bactericidal potency and inhibiting surface-adhered biofilm formation (Fig. 8b). Likewise, researchers noted HPO-like characteristics in V2O5 nanowires, capable of catalyzing the production of hypobromous acid (HOBr) and singlet molecular oxygen.155 Paints containing V2O5 nanowires were observed to deter surface biofouling when submerged in seawater for a duration of 60 days (Fig. 8c–f). The generated singlet oxygen exhibited potent antibacterial effects, while the produced HOBr disrupted quorum sensing, effectively inhibiting biofouling.
 | ||
| Fig. 8 (a) Diagram depicting the antibacterial and biofilm formation inhibitory functions of Ce-MOF-808 due to its HPO-like behavior. (b) SEM images showing biofilms formed by E. coli and P. aeruginosa, where 1 and 3 represent control groups, and 2 and 4 depict bacteria treated with 100 μg per mL Ce-MOF-808. Reproduced from ref. 154 with permission from Wiley 2022. (c and d) Photograph depicting a stainless steel plate coated with paint for boat hulls, comparing one without (−V2O5 nw) and one with (+V2O5 nw) V2O5 nanowires. Both plates remain clean after installation. (e) The painted stainless-steel plate without V2O5 nanowires experienced significant natural biofouling. (f) Plates with V2O5 nanowires exhibited no biofouling after 60 days. Reproduced from ref. 155 with permission from Springer Nature 2012. (g) Diagram depicting the synthetic procedure of CeO2@ZrO2. (h) Time-dependent UV-vis spectra showing the bromination of phenol red catalyzed by CeO2@ZrO2 (50 × 10−6 m phenol red, 25 × 10−3 m Br−, 350 × 10−6 m H2O2, 600 minutes, 25 °C). (i) Illustration demonstrating the anti-biofilm mechanism of CeO2@ZrO2 nanozyme in marine environments. Reproduced from ref. 157 with permission from Wiley 2022. (j and k) Schematic representation of the synthetic pathway and antibacterial mechanism of action for V-Fe2O3 nanozyme, respectively. Reproduced from ref. 160 with permission from Wiley 2023. | ||
Halogenation of QS signal molecules, particularly AHL, leads to the loss of their biological activity, prompting ongoing endeavors to develop nanozymes that mimic haloperoxidases (HPO) to impede bacterial communication and biofilm formation. Among these, CeO2-based materials have emerged as efficient candidates with haloperoxidase-mimicking properties. CeO2 nanocrystals (NCs) have been identified as potent quorum quenching (QQ) agents.156 CeO2NCs are known to deactivate QS signals through bromination, rendering brominated AHL ineffective as QS signals. Consequently, bacterial communication is disrupted, preventing the formation of biofilms. In a recent study, Luo et al.157 illustrated that CeO2 coating on zirconia (Fig. 9g) exhibits robust haloperoxidase (HPO) mimicking capabilities (Fig. 8h), effectively catalyzing the production of HOBr by oxidizing bromine in the presence of H2O2. The generated HOBr is recognized for its antibacterial properties and QS inhibition,158,159 contributing to its remarkable antibacterial and anti-biofouling capabilities (Fig. 8i). The pronounced enzymatic activity was credited to the abundant oxygen vacancies and surface acid attributes present in the hybrid material. Ongoing research has focused on enhancing the antibacterial efficacy of engineered materials against methicillin-resistant S. aureus (MRSA) and its associated biofilms. V-Fe2O3 nanozymes have shown potent peroxidase-like activity and ROS generation, resulting in a substantial reduction of MRSA burden in vitro under optimized conditions.160 However, the reported 100% eradication was achieved at relatively high concentrations (e.g., 500 μg mL−1) under static culture conditions, which may not be replicable in complex in vivo settings. Moreover, this evaluation was conducted in monoculture planktonic or early-stage biofilms, rather than established, mature biofilms embedded in host tissue. Therefore, these promising results require careful dose-toxicity validation and biofilm maturity modeling before clinical translation.
 | ||
| Fig. 9 (a) Schematic illustration depicting the mechanisms of Cu-CDs with dual-enzyme mimicking catalytic activity adapted for oral applications. (b) The inhibitory activity of CDs and Cu-CDs against biofilm formation at various concentrations (60, 80, 120, and 240 μg mL−1). Reproduced from. Ref. 161 with permission from American Chemical Society 2022. (c) The diagram illustrates how CoNCDs eliminate biofilms and cause bacterial death. Reproduced from ref. 162 with permission from RSC. (d) A schematic depiction illustrating the self-adaptive mechanism of Cu2MoS4 in exerting antibiofilm and immune modulation effects. Reproduced from ref. 163. (e) The biofilm eradication mechanism is facilitated by CPP nanoflares. (f–h) SEM images depicting biofilms subjected to various treatments: (f) CDs + H2O2, (g) PP + H2O2, (h) CPP + H2O2. Reproduced from ref. 165 with permission from American Chemical Society 2023. | ||
Besides Ce and vanadium-based nanozymes, various other metal-containing materials have shown significant potential as enzyme mimics for effectively inhibiting and eradicating biofilms formed by diverse bacterial strains. For example, copper-doped carbon dots (Cu-CDs) were discovered to possess catalase and peroxidase-like activities, effectively eradicating bacteria and impeding or destroying biofilm formation through mechanisms involving O2 bubbling and ROS mediation.161 This material exhibits a robust affinity for cell wall components like LPS and peptidoglycan, ensuring efficient capture and a concentrated dose at the bacterial cell level, thereby demonstrating remarkable antibacterial efficacy against various bacterial strains. The enzyme-driven Cu-doped carbon dots (Cu-CDs) efficiently eliminate S. mutans biofilms on tooth surfaces and exhibit notable tooth-whitening effects. Furthermore, Cu-CDs display significant efficacy in combatting infections and accelerating wound healing in a rat model of infectious skin wounds (Fig. 9a). Cu-CDs showed considerable efficacy in inhibiting biofilm formation, achieving 97% inhibition at 60 μg mL−1, demonstrating their capability to hinder bacterial adhesion and aggregation (Fig. 9b). Similarly, treatment with Cu-CDs in the presence of H2O2 resulted in over 90% biofilm eradication activity. Likewise, cobalt and nitrogen-doped carbon dots (CoNCDs) were found to exhibit oxidase-like properties and dismantle bacterial biofilms through mechanisms involving ROS mediation without the addition of H2O2.162 The ROS generated by CoNCDs were observed to degrade eDNA, leading to the subsequent eradication of biofilms. Additionally, CoNCDs disrupt bacterial cell membranes, resulting in bacterial death, and have shown promising therapeutic potentials in vivo, particularly in wound curing and acute peritonitis (Fig. 9c). To mitigate the collateral damage to healthy cells resulting from excessive ROS generation during bacterial infection treatment, it is essential to design a material that can dichotomously function as both a ROS-generating agent for bacterial elimination and a ROS-scavenging entity to neutralize the resultant oxidative stress. In this context, hollow Cu2MoS4 nanospheres (H-CMS NSs) have demonstrated significant promise by exhibiting as mimics of catalase and peroxidase. Initially, they initiate ROS generation through catalase-like activity, effectively targeting bacteria and their associated biofilms for destruction. Following biofilm eradication, Cu2MoS4 functions as peroxidase mimics, scavenging the produced ROS inhibiting the polarization of macrophages (M1), and reducing the expression of proinflammatory cytokines (Fig. 9d).163 It has been demonstrated that combining metal with carbon can enhance catalytic activity compared to individual metal and carbon nanomaterials, owing to their efficient interfacial electron transfer.164 Following this principle, Liang and colleagues165 integrated Pt nanoparticles (PtNPs) with carbon dots to create CDs@PtNPs (CPP), which exhibited promising potential in catalyzing H2O2 to produce OH radicals for effective activity against resistant bacteria and biofilm inhibition (Fig. 9e). The CPP nanozyme enables sensitive detection of H2O2, antioxidants, and pathogens. Additionally, the enhanced peroxidase activity of CPP could facilitate rapid and efficient bacterial inactivation and biofilm eradication. Compared to CDs and PtNPs (PP), CPP demonstrated outstanding efficacy in dispersing and destroying biofilms (Fig. 9f–h), highlighting the enhanced peroxidase mimicking properties resulting from the synergistic interaction of CDs and integrated PtNPs in the CPP composite. Similarly, carbon-based nanomaterials have garnered attention for their multifunctional properties in antimicrobial and antibiofilm applications. Materials such as carbon dots (CDs), graphene oxide (GO), and carbon nanotubes (CNTs) exhibit high surface area, tunable surface functionalities, and intrinsic photothermal or redox activity. For instance, copper-doped carbon dots (Cu-CDs) demonstrated peroxidase- and catalase-like activities, enabling ROS generation and biofilm inhibition while also promoting wound healing.166 Similarly, Co- and N-doped carbon dots (CoNCDs) were shown to degrade extracellular DNA and disrupt biofilm architecture without the need for exogenous H2O2.167 Graphene oxide-based composites have also been reported to impair quorum sensing and enhance the delivery of antibacterial agents into biofilm matrices. These findings underscore the emerging potential of carbon nanostructures as effective platforms for nanozyme and non-nanozyme-based biofilm therapies.168
2.5 Combination therapy with nanozymes
Recent research has demonstrated the effectiveness of combination therapy in inhibiting both biofilms and resident bacteria. In a recent investigation, Huang and colleagues169 devised a combination therapy involving ferumoxytol (Fer) and SnF2 (Fig. 10a), aiming to investigate the synergistic effects of this formulation on antibacterial and antibiofilm activities in dental caries. Fer, an FDA-approved iron oxide nanoparticle, is recognized for its ability to eradicate biofilms by catalytically activating H2O2. Combining Fer with SnF2 significantly enhanced the catalytic activity of Fer, thereby increasing its antimicrobial effectiveness. This combination was remarkably successful in preventing dental caries, surpassing the efficacy of either component alone, and completely halting enamel cavitation. The findings indicated that the Fer and SnF2 formulation significantly decreased the biovolume (Fig. 10b) and EPS (Fig. 10c), underscoring the enhanced efficacy of this combined therapy in inhibiting biofilms. Similarly, Zhu et al.170 engineered a hybrid composite nanozyme system (HA@MRuO2-Cip/GOx) consisting of a mesoporous RuO2 core (MRuO2) loaded with the ciprofloxacin (Cip) and glucose oxidase (GOx), as depicted in (Fig. 10d). The developed hybrid composite exhibited notable peroxidase mimicry, leading to the generation of ROS that cleaves eDNA, disrupting biofilms and thereby augmenting the bactericidal effect of released ciprofloxacin from the nanozyme. Nanozymes exhibit greater efficacy under acidic conditions, and the inclusion of GOx in HA@MRuO2-Cip/GOx generates gluconic acid and H2O2, maintaining an acidic pH in the system. Additionally, the produced H2O2 catalyzes the generation of ROS (Fig. 10e). Crucially, in vivo assessments of therapeutic efficacy demonstrated that HA@MRuO2-Cip/GOx can stimulate macrophage-mediated immunity and efficiently relieve MRSA-based lung infections. Hence, the integration of nanocatalytic therapy with targeted antibiotic delivery holds promise for enhancing the treatment of bacterial infections. Likewise, nitric oxide (NO) and singlet oxygen (1O2) are recognized as potent antibacterial agents, and a material capable of releasing both species would likely exhibit synergistic antibacterial activity.171–173 Moreover, nanozymes can catalyze the production of ROS in the presence of H2O2, and the development of a material that can autonomously supply H2O2 while producing ROS and NO would represent a promising strategy for combating multidrug-resistant bacteria. Motivated by the multimodal concept, Li, et al.174 engineered a nanozyme by encapsulating glucose oxidase, a NO donor (BNN6), and chloroperoxidase (CPO) within a metal–organic framework (MOF) carrier, MIL-101(Fe), resulting in a potent antibacterial nanocomposite, designated as MGBC. Mechanistic studies revealed that the nanocomposite initially catalyzes glucose oxidation, generating H2O2 via the integrated glucose oxidase. This H2O2 subsequently triggers the generation of NO and 1O2 through the NO donor and CPO, respectively (Fig. 10f). The CPO-catalyzed reactions enhance ROS production, synergistically augmenting the antibacterial activity of the MGBC nanocomposite. Investigations demonstrated that MGBC exhibits potent efficacy in mitigating MRSA toxicity drug and resistance by substantially downregulating genes involved in QS, virulence, multidrug efflux, and biofilm formation. The collective impact of these genetic modifications underlies the exceptional performance of MGBC in suppressing bacterial proliferation and associated biofilm formation, highlighting its potential as a novel therapeutic agent. Following the preceding research, the synergistic conjunction of L-arginine and NiCo2O4@AlO(OH) nanozyme exhibited a pronounced enhancement of antibacterial potency, with a 1000-fold augmentation in efficacy. A thorough mechanistic elucidation revealed that this combinatory regimen instigates membrane disruption and fosters the intracellular accrual of ROS. Subsequent molecular-level dissection revealed that ROS-mediated damage impedes vital cellular processes, encompassing the tricarboxylic acid cycle and oxidative phosphorylation, thereby destabilizing redox homeostasis and precipitating a disequilibrium between antioxidant and oxidant systems (Fig. 10g).175 This combination therapy exhibited exceptional efficacy in preventing biofilm formation (Fig. 10h), and demonstrated limited efficacy in eliminating established biofilms or persister cells. The observed ineffectiveness against dormant persisters was attributed to their characteristic slow metabolic activity, which renders them less susceptible to combination therapy. In contrast, the therapy effectively targeted rapidly proliferating cells, highlighting a selective efficacy against actively growing microbial populations. | ||
| Fig. 10 (a) Schematic illustration of the synthesis and synergistic enhancement of antimicrobial activity resulting from the combination of ferric (Fer) and tin(II) fluoride (SnF2). (b) Quantitative analysis of the biovolume of bacterial cells, (c) EPS production in biofilms, with and without treatment with Fer + SnF2, revealing the impact of this combination on biofilm formation and structure. Reproduced from ref. 169 with permission from Wiley 2020. (d) Synthetic route for the preparation of HA@MRuO2-Cip/GOx nanostructures. (e) Mechanistic depiction of H2O2 and OH generation by HA@MRuO2-Cip/GOx, showcasing the cooperative catalytic action of MRuO2 and GOx, resulting in the production of ROS, crucial for antimicrobial efficacy. Reproduced from ref. 170 with permission from Wiley 2023. (f) Diagrammatic representation of the MGBC synthesis route, depicting the enzyme cascade catalytic process that facilitates the tandem production of NO and 1O2, demonstrating the material's ability to generate multiple ROS species through a single catalytic pathway. Adopted from ref. 174 with permission from Wiley 2024. (g and h) Schematic representation of the proposed mechanistic framework underlying the biological activity of NiCo2O4@AlO(OH), elucidating the key molecular interactions and cellular processes involved in its mode of action and the anti-biofilm efficacy of NiCo2O4@AlO(OH) (25 μg mL−1), L-arginine (0.4%), and their combined treatment, respectively. Reproduced from ref. 175 with permission from Wiley 2023. | ||
Analogously, a micellar nanocomposite consisting of NO-releasing functional groups, palladium (Pd)-based photocatalytic core (Pd-PC), and tertiary amine (TA) was observed to exhibit potent efficacy in eliminating ciprofloxacin-resistant biofilms.176 Detailed mechanistic studies demonstrated that these micellar nanostructures possess the ability to reorganize in response to the biofilm environment and release NO upon irradiation at a wavelength of 630 nm, facilitating effective biofilm eradication. The integration of TA functionalities was a critical design feature, enabling a bifunctional role as ROS scavengers and proton acceptors. Under alkaline and normoxic conditions typical of biofilm peripheries, deprotonated TA species effectively neutralized 1O2, preventing oxygen exhaustion of the Pd-PC and promoting NO liberation. The observed results demonstrate the material's robust performance under diverse biofilm conditions, underscoring its promise as an effective treatment strategy for combating multidrug-resistant biofilms.
Although current research emphasizes the innovative functionalities and therapeutic potential of advanced nanostructures, there is often insufficient attention given to the practical limitations that hinder clinical translation. The complexity of synthesizing, purifying, and characterizing these multi-component nanosystems particularly those involving precise morphology, multiple functional domains, and responsive behaviors makes large-scale, cost-effective production extremely difficult. Most studies report laboratory-scale fabrication under controlled conditions without addressing whether these methods are compatible with good manufacturing practice standards, reproducibility across batches, or regulatory compliance. Consequently, while the biological efficacy is highlighted, the logistical and economic feasibility for real-world deployment remains underexplored, limiting the pathway toward widespread clinical adoption.177
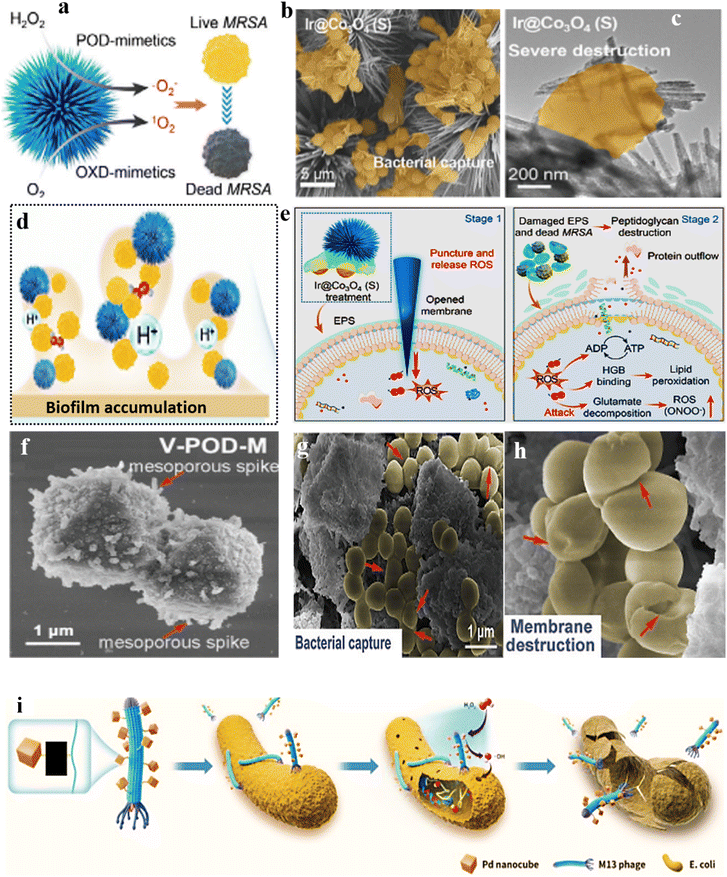 | ||
| Fig. 11 (a) Schematic depiction of ROS generation mechanisms via enzyme mimicry by Ir@Co3O4. (b and c) Antibacterial activity of Ir@Co3O4, demonstrating bacterial capture and elimination. Biofilm accumulation is depicted in (d), and the proposed mechanism of biofilm infiltration and disruption by Ir@Co3O4 (S) particles is illustrated in (e). Adopted from ref. 178 with permission from Wiley 2024. (f) SEM image of V-POD-M, revealing its structural morphology. (g) Bacterial capture efficiency and (h) membrane destruction indicated by red arrows by V-POD-M nanozyme. Adopted from ref. 181 with permission from Wiley 2021. (i) Diagrammatic illustration of the phage@Pd nanoconstruct, showcasing its structural composition and elucidating its antimicrobial mode of action. Reproduced from ref. 182 with permission from Wiley 2023. | ||
Motivated by this strategy, Yuan et al.189 designed and synthesized a pioneering hybrid nanozyme architecture, wherein MnO2 nanozymes were immobilized onto mesoporous polydopamine nanoparticles harboring the photosensitizer indocyanine green (ICG), yielding the MI-MPDA NP construct (Fig. 12a). MI-MPDA NPs can generate oxygen in the infection microenvironment characterized by low pH and high H2O2 levels, thus alleviating biofilm hypoxia. The continual provision of oxygen can augment the production of 1O2 upon exposure to near-infrared radiation (NIR), thereby facilitating efficient biofilm eradication through oxygen-enhanced PDT/PTT (Fig. 12b). Additionally, MI-MPDA decreases the expression of factors involved in inflammatory signaling pathways by scavenging ROS mediated by MnO2, thereby improving the inflammatory condition. MnO2-based nanoplatforms like MI-MPDA can release hypoxia via H2O2-motivated O2 generation, enhancing PDT efficacy. However, in inflamed tissues with elevated ROS and disordered redox balance, uncontrolled O2 release may increase oxidative stress and cause collateral damage. The in vivo kinetics and spatial control of MnO2 catalysis remain poorly studied, highlighting the need for cautious evaluation of redox safety together with antimicrobial activity. Beside its enhanced antibiofilm activity, the irradiated nanozyme also mitigates oxidative stress and the associated inflammation, while triggering a cascade reaction for immunomodulation-based wound healing (Fig. 12c). The integration of photodynamic and photothermal therapies has emerged as a robust treatment strategy for mitigating bacterial infections and associated biofilms. Research has revealed that a novel heterocomposite material, comprising iron atoms embedded in quantum dots (Fe@SAC CQDs) and conjugated with a photothermally active agent (PTA), significantly suppresses experimental bacterial growth and biofilm development, highlighting its potential for wound healing.190 Insights into the mechanistic underpinnings revealed that the iron core in the composite material functions as an enzyme mimic, facilitating ROS production, and the photoactive agent triggers photothermal heat generation. The combined chemodynamic and photothermal effects synergistically contribute to the material's enhanced antibacterial and biofilm-inhibiting efficacy (Fig. 12d). Similarly, a composite material (Cu,N-GQDs@Ru-NO) consisting of Cu,N-doped graphene quantum dots (GQDs) functionalized with a NO-releasing agent (RU-NO) was found to exhibit NADH dehydrogenase-mimicry, catalyzing the photo-oxidation of NADH to NAD+ upon near-infrared (NIR) irradiation.191 Mechanistic investigations revealed that NIR irradiation (808 nm) of the material triggers the oxidation and depletion of NADH, thereby disrupting bacterial redox homeostasis and leading to bacterial cell death. Simultaneously, the NIR light-induced NO release and photothermal effect acted in concert to potentiate bacterial and biofilm eradication, as well as enhance wound healing (Fig. 12e). Cumulative evidence from these and other investigations has unequivocally demonstrated that nanomaterials possessing enzyme-like activity, capable of inducing photothermal and photodynamic effects, serve as effective nanozymes for the eradication of biofilms through multifaceted mechanisms, including the generation of ROS, depletion of reduced glutathione reserves, and the imposition of thermal stress.192,193 For instance, a recent investigation demonstrated that PNMn hydrogel exhibits multifaceted enzymatic activity, comprising peroxidase (POD), oxidase (OXD), and catalase (CAT)-mimicking activities when subjected to NIR-II radiation. This photo-triggered activation instigates the production of a diverse array of ROS, exhibiting pronounced antimicrobial efficacy against bacterial pathogens and biofilm matrices.194 Consistent with this, a plethora of recent scientific studies have reported the development of advanced nanozymes exhibiting photothermal properties, including Cu2O-integrated polydopamine (CuxO@PDA) nanostructures,195 Pt-V2C MXene hybrids,196 lactate oxidase integrated TiO2/γ-Fe3O4 nanocomposites.,197 and Fe-CDs (iron-doped carbon dots) injectable hydrogel.198 These nanozymes have shown considerable promise in efficaciously preventing biofilm growth and enhancing wound healing capacities. Similarly, the strategic creation of vacancy sites in nanozyme materials can enhance the NIR-triggered production of ROS, leading to significantly augmented photothermal and photodynamic antibacterial efficacy and biofilm inhibition capabilities. Notably, a recent study reported the development of a sulfur vacancy-rich Bi2S3−x nanomaterial (Bi2S3−X@PDA) functionalized with catechol-rich polydopamine, which demonstrated significant antibacterial efficacy through NIR-triggered ROS induction.199 Concurrently, the catechol residues conferred antioxidant properties, protecting adjacent cells from oxidative stress-induced damage. The exceptional photocatalytic performance of the fabricated nanozyme was primarily attributed to the enhanced charge separation efficiency under light irradiation, which underscores the crucial role of vacancy sites in facilitating charge carrier dynamics. This highlights the potential of defect engineering as a versatile tool to tailor the electronic properties and charge density of fabricated materials, allowing for the fine-tuning of their photocatalytic activities and maximizing their efficiency. The developed strategy provides a groundbreaking approach, exhibiting a dual-functional mechanism. On one hand, it markedly induces the generation of ROS in infected tissues, effectively eradicating biofilm formations. Conversely, it simultaneously scavenges ROS in adjacent healthy tissues, thereby preventing oxidative stress-mediated inflammation and promoting accelerated wound healing.
 | ||
| Fig. 12 (a) Schematic depiction detailing the preparation of MI-MPDA. (b) The mechanism of NIR-irradiated MI-MPDA for oxygen-potentiated PDT/PTT. (c) NIR-irradiated MI-MPDA for ROS scavenging and immune modulation. Reproduced from ref. 189 with permission from Wiley 2023. (d) Mechanistic Insights into the photothermal catalytic antibacterial activity of Fe@SAC CQDs/PTA nanohybrids. Adopted from ref. 190 with permission from American Chemical Society 2024. (e and f) Schematic illustration of the Cu,N-GQDs@Ru-NO nanozyme and the antibacterial and antibiofilm activities, mediated by the generation of ROS and reactive nitrogen species (RNS), culminating in cellular dysfunction and ultimately, microbial eradication. Targeted antibacterial therapy. Adopted from ref. 191 with permission from Wiley 2023. (g) Schematic illustration of the photo-regulated switching of Bi2S3-X@PDA between antibacterial and antioxidant modalities, enabling the precise control of its biological functions through light activation. Adopted from ref. 199 with permission from Wiley 2024. | ||
2.6 Intratumor biofilm and nanomaterials
The oncogenic potential of Fusobacterium nucleatum (F. nucleatum) is mediated by its capacity to form intratumoral biofilms, which have been implicated in the pathogenesis of diverse malignancies, including colorectal and breast cancer. F. nucleatum exhibits a specific tropism for colorectal cancer (CRC) cells, characterized by the high-affinity binding of its Fap2 adhesin to Gal-GalNAc glycoconjugates on the CRC cell surface, facilitating selective colonization and biofilm formation.200–202 This precise molecular interaction enables F. nucleatum to target and colonize CRC cells with high specificity, leading to the dysregulation of critical host signaling pathways, including those involved in cell proliferation, apoptosis, and immune evasion, thereby contributing to tumorigenesis and cancer progression. The administration of antibiotics to combat intratumoral bacteria poses a significant challenge due to the potential collateral damage to beneficial commensal microbiota, thereby compromising the efficacy of conventional treatment strategies. Consequently, the development of targeted therapeutic approaches has become a pressing need. In this context, the utilization of nanovehicles for site-specific delivery of antibiotics to tumor tissues, enabling precise and selective inhibition of the target bacteria, has emerged as a promising area of research, garnering substantial scientific interest. Building upon this pioneering concept, Chen et al.203 engineered a novel nano vehicle platform by integrating F. nucleatum membrane (FM) fragments with liposomal structures (FM-Lipo), creating a hybrid nanocomplex (Fig. 13a). This innovative vehicle was designed to encapsulate antibiotic (colistin), facilitating targeted delivery to specific sites. The targeted specificity was achieved through the strategic display of Fab2 receptors on the FM component of the nanovehicle, which selectively bind to Gal-GalNAc epitopes on colorectal cancer (CRC) cells, thereby ensuring precise and efficient delivery of the antibiotic payload (Fig. 13b). F. nucleatum exerts a profound influence on the tumor microenvironment,204 inducing an immunosuppressive state characterized by MDSC (myeloid-derived suppressor cells) upregulation and compromised immunotherapeutic efficacy, including reduced responsiveness to ICB (immune checkpoint blockade).205–207 Nonetheless, administration of colistin-LipoFM effectively counteracts the F. nucleatum mediated peritumoral microenvironment, thereby reinstating the therapeutic effectiveness of anti-CTLA4 (cytotoxic T-lymphocyte-associated antigen 4) antibodies (Fig. 13c). Moreover, it was demonstrated that colistin-LipoFM also restores the therapeutic efficacy of anti-PD-1 in MC-38 tumor models infected with F. nucleatum (Fig. 14d), highlighting its potential as an adjunctive therapeutic strategy. Consistent with prior findings, a recent investigation employed the cytoplasmic membrane of F. nucleatum (FM) to encapsulate a liposome-encapsulated antibiotic (colistin-LipoFM), facilitating targeted therapy against F. nucleatum in breast cancer (Fig. 13e).208 The nanovehicle's selectivity was conferred by exploiting the Fab2 receptors present on the FM, which specifically bind to the Gal-GalNAc moieties expressed on the surface of breast tumor cells (Fig. 13f), thereby ensuring precise targeting and minimizing off-target effects. The colistin-LipoFM formulation was observed to exert a profound inhibitory effect on intratumoral F. nucleatum, thereby reinstating chemotherapeutic efficacy in a murine breast tumor. Additionally, LipoFM encapsulating doxycycline was found to disrupt the intratumoral microbiota and impede pulmonary metastasis of breast cancer associated with a polymicrobial infection (Fig. 13g). Therefore, the strategic exploitation of antibiotic-loaded nanovehicles that mimic the molecular signature of F. nucleatum offers a viable approach to mitigate chemoresistance in multiple cancers and may facilitate the rational design of novel, mechanistically informed chemotherapeutic strategies that account for the complex, reciprocal interactions between tumor cells and bacterial colonizers. In another scientific investigation, a cholesterol-modified F. nucleatum membrane liposome formulation (LipoFM-CPG) was demonstrated to effectively and selectively eliminate intratumoral F. nucleatum populations.209 This approach was designed to facilitate the co-delivery of multiple antigenic epitopes and adjuvants, thereby augmenting antigen presentation by dendritic cells and triggering a robust cellular immune response, characterized by the activation and maturation of CD8+ cytotoxic T lymphocytes and B lymphocytes. The cytolytic effector functions of activated T lymphocytes and the targeted humoral immune responses mediated by B cell-derived antibodies collectively exert bactericidal activity against the intratumoral F. nucleatum population, resulting in enhanced chemotherapeutic efficacy and reduced metastatic propensity in F. nucleatum positive CRC. The F. nucleatum is a key contributor to chemoresistance through β-catenin activation and immune suppression,210 emerging evidence indicates that other intratumoral bacteria such as Bacteroides fragilis, E. coli, and Clostridium spp. also play roles in tumor progression and therapeutic resistance via genotoxin production, immune modulation, and drug metabolism.211 Therefore, LipoFM encapsulating doxycycline reduced F. nucleatum and co-resident anaerobes, including B. fragilis and Clostridium spp., as confirmed by 16S rRNA profiling, indicating broader disruption of intratumoral microbiota relevant to chemoresistance. This highlights the capacity of FM-coated liposomes to target polymicrobial tumor microenvironments and improve therapeutic outcomes beyond single-species elimination.212 Taken together, these recent studies suggest that intratumoral colonizing bacteria can be efficiently eliminated through the rational design of liposomal formulations incorporating targeted bacterial mimetic functionalities, enabling selective delivery of nanomedicines and eliciting specific immunological responses to achieve efficacious treatment of infected tissues. Although the Fap2/Gal-GalNAc targeting system offers promising selectivity, its translational scope may be limited by the heterogeneous and context-dependent expression of Gal-GalNAc on tumor surfaces. Gal-GalNAc overexpression is observed mainly in certain adenocarcinomas (e.g., colorectal, pancreatic),184 but its expression can vary with tumor stage, microenvironmental signals, and glycosyltransferase activity, necessitating pre-treatment stratification using glycomic profiling.213 Furthermore, the in vivo stability of F. nucleatum membrane (FM)-coated liposomes is challenged by potential immune tagging and recognition by innate immune components such as complement proteins and pattern recognition receptors (e.g., TLR2, NOD2).214 This may lead to rapid clearance via hepatic and splenic macrophages and enzymatic degradation by circulating phospholipases and proteases.215 Surface engineering strategies such as PEGylation,216 hybrid membrane integration,217 or selective masking of immunogenic epitopes may be required to preserve functionality, prolong circulation time, and minimize immunogenicity under systemic conditions.218 | ||
| Fig. 13 (a) Schematic illustration of F. nucleatum-mimetic antibiotic-loaded liposomal nanovehicles; (b) targeted specificity of the nanomedicine, leveraging specific receptor-ligand interactions; (c and d) selective depletion of tumor-colonizing F. nucleatum populations, resulting in the alleviation of F. nucleatum driven immunosuppressive mechanisms and augmentation of immunotherapy efficacy. Adopted from ref. 203 with permission from Wiley 2023. (e) Preparation of F. nucleatum mimicking nanovehicles involved the heterologous integration of cytoplasmic membrane derived from F. nucleatum with liposomes encapsulating antibiotics. (f) Selective intratumoral bacterial clearance mediated by specific receptor–ligand recognition between the nanovehicle and malignant cells, facilitating targeted antimicrobial therapy. (g) The LipoFM nanovehicle, loaded with antibiotics, exhibited broad-spectrum antimicrobial activity against heterogeneous intratumoral microbiota, effectively attenuating pulmonary metastasis of breast cancer associated with polymicrobial infections, and significantly augmenting the therapeutic efficacy of conventional chemotherapy protocols for breast cancer management. Reproduced from ref. 208 with permission from Elsevier 2024. | ||
 | ||
| Fig. 14 (a) High-resolution STEM imaging of TiO2/Pt microrobots, providing insight into their nanostructural architecture. (b and c) Sequential imaging of TiO2/Pt microrobots in motion, suspended in a solution comprising 0.5 wt% H2O2 and 0.1 wt% surfactant, showcasing their kinetic behavior and locomotion capabilities. Scale bars: 10 μm. (d) In vitro viability assays evaluating the antibiofilm efficacy of TiO2/Pt microrobots at various concentrations, with and without 1 wt% H2O2 supplementation, demonstrating their potential in mitigating dental biofilm formation and promoting oral health. Reproduced from ref. 225 with permission from Elsevier 2020. Kinematic analysis of Fe3O4-MnO2 motor propulsion. (e) Intrinsic dynamics: FMMs exhibit stochastic spinning motion, characteristic of autonomous propulsion. (f and g) Magnetically actuated motion: the application of external magnetic fields enables precise control over FMM trajectories, achieving directed movement along straight or helical paths. The magnetic field vector, denoted by the arrow, dictates motor orientation and navigation. (h) High-resolution SEM imaging of untreated S. aureus biofilm, showcasing its inherent ultrastructure and cellular arrangement. (i) SEM micrographs of S. aureus biofilm following treatment with Fe3O4-MnO2 motors (FMMs) at a concentration of 600 μg mL−1, in combination with 2 wt% H2O2, demonstrating significant biofilm disruption and degradation. Reproduced from ref. 226 with permission from Elsevier 2020. (j) EDX spectroscopic analysis of Ag/B-TiO2, providing elemental mapping and compositional insights. (k–m) Time-lapse imaging of Ag/B-TiO2 under illumination, demonstrating dynamic motion modes. (n) Live/dead fluorescence assay of biofilm under static conditions (no motion). (o) Live/dead fluorescence assay of biofilm under dynamic conditions (motion). (p) Quantitative analysis of biofilm viability, expressing the percentage of dead cells under motion and no-motion states, highlighting the material's antibiofilm efficacy. Adopted from ref. 228 with permission from Wiley 2022. | ||
2.7 Biofilm eradication with micro–nano robotics
Micro- and nanorobotic platforms pioneer an innovative framework for intra-body navigation and biofilm-associated infection management, defined by autonomous navigation, remote control, and reconfigurable design. These small-scale robotic entities synergistically combine antimicrobial surfaces, precision drug delivery systems, and calibrated shear force application, enabling a comprehensive strategy for biofilm elimination, prevention, and inhibition. Stimuli-responsive remote control facilitates precise kinematic manipulation, while advanced imaging techniques provide spatiotemporal localization. Through synergistic induction of mechanical destabilization of the biofilm and targeted eradication of persister (dormant) bacterial subpopulations, these robotic systems significantly enhance the therapeutic efficacy of biofilm elimination strategies, ultimately leading to improved clinical outcomes and augmented patient care.180,219Unlike conventional metallic nanomaterials, which rely on passive biodistribution and bioaccumulation, these robotic materials demonstrate autonomous navigation and targeted delivery attributes, harnessing external energy inputs to generate mechanical motion. This facilitates active trans-barrier migration and precise localization at designated therapeutic sites. The harmonious convergence of autonomous motility, cargo conveyance, and augmented tissue permeability endows these micro/nanomotors (MNM) with diverse functional modalities, highlighting their immense promise for revolutionary biomedical applications, therapies, and diagnostic interventions.220,221 Microscale machines can be engineered to encapsulate a diverse array of antibacterial payloads, including peptides, enzymes, and antibodies, enabling precise transport and localized release at biofilm-impacted areas. This multimodal therapeutic approach enhances selectivity and efficacy by concentrating bioactive agents at the site of infection, optimizing antimicrobial therapy.222,223
Recent advancements have highlighted the potential of externally applied light or magnetic stimuli as viable alternatives to H2O2-based propulsion for nanorobotic systems. This shift is driven by the limitations of H2O2-based approaches, including (1) limited availability of H2O2 in physiological environments and (2). Potential cytotoxicity of H2O2, posing risks to cellular systems and restricting its practical applications.227 A recent investigation successfully integrated silver-decorated black titanium dioxide (Ag/B-TiO2) into a UV-visible light-responsive micro-robotic hybrid, specifically designed for biofilm inhibition on commercial titanium miniplate implants used in facial applications (Fig. 14j).228 Under illumination with 450–500 nm (blue) and 540–550 nm (green) light, the microrobot exhibited diverse motion modes, including autonomous fast rotation and random movement (Fig. 14k–m). Notably, this hybrid material demonstrated exceptional antibiofilm efficacy, attributed to its dynamic motion patterns and the ROS generated by the incorporated silver particles (Fig. 14n–p). The synergistic combination of mechanical disruption and ROS-mediated oxidative stress effectively inhibited biofilm formation, showcasing the potential of this light-controlled microrobot for implant-related infection prevention.
Addressing the limitations of H2O2-dependent nanozymes, as endogenous H2O2 levels in tumors (typically <100 μM) are often insufficient for efficient catalytic activity, and exogenous H2O2 poses toxicity risks.229 Recent designs address this using self-supplying systems incorporating glucose oxidase or H2O2-independent oxidase-mimetic nanozymes (e.g., CoNCDs, V-Fe2O3).230 Similarly, light-driven therapies face limited tissue penetration (∼1–5 mm with visible/NIR-I), though advances in NIR-II photothermal agents and upconversion nanomaterials have improved depth performance.231 However, enhancing catalytic efficiency under physiological conditions remains essential for clinical use.
The Mayorga-Martinez C. C. et al. recently developed a novel magnetic microrobot, designated HNT-Fe3O4@PEI/Amp, through a sequential fabrication process.241 Initially, halloysite nanotubes (HNTs) were functionalized with Fe3O4 nanoparticles to form the HNT-Fe3O4 composite. Subsequently, the HNT-Fe3O4 composite was coated with polyethyleneimine (PEI) to yield HNT-Fe3O4@PEI. Finally, ampicillin (Amp) was conjugated to the magnetic microrobots, resulting in the formation of HNT-Fe3O4@PEI/Amp (Fig. 15a). The fabricated magnetic microrobots demonstrated multimodal locomotion capabilities (Fig. 15b), enabling precise navigation and transportation of therapeutic payloads. Furthermore, these robotic systems demonstrate advanced motion capabilities, including reversible tumbling-to-spinning transitions and adaptive swarm behavior, toggling between vortex and ribbon patterns (Fig. 15c). Importantly, the vortex mode facilitates substantial viability reduction and targeted dismantling of S. aureus biofilms on titanium mesh, underscoring its potential for efficacious biofilm elimination (Fig. 15d and e). The HNTFe3O4@PEI/Amp nanocomposites significantly disrupted biofilm biomass in vitro, the claim of nearly complete eradication was based on short-duration treatment (∼6 h) in monoculture biofilms of S. aureus.241 The model lacked polymicrobial complexity, immune interaction, and dynamic fluidic conditions of real infections. As such, these findings, while compelling, should be interpreted within the scope of preliminary proof-of-concept studies. Future studies should address biofilm maturity, in vivo localization, and long-term biocompatibility for clinical feasibility.
 | ||
| Fig. 15 (a) Schematic illustration of the fabrication process for HNT-Fe3O4@PEI/Amp microrobots. (b) Dynamic motion modalities of a single magnetic Fe3O4-HNT/PEI@Amp microrobot, demonstrating reversible transitions between tumbling and spinning modes (1 Hz) and obstacle avoidance capabilities. (c) The collective multimodal motion of Fe3O4-HNT/PEI@Amp microrobot swarms, exhibiting reversible transformations between ribbon and vortex patterns (frequency: 8 Hz; magnetic field intensity: 5 mT). (d) Comparative analysis of viable bacterial counts in intact biofilms (green) and treated biofilms (red) exposed to Fe3O4-HNT/PEI@Amp microrobots with magnetic actuation, alongside static Fe3O4-HNT/PEI@Amp (yellow) and dynamic Fe3O4-HNT/PEI microrobots without ampicillin (orange). (e) Spectrophotometric analysis (590 nm) of crystal violet absorption from untreated and treated biofilms dissolved in DMSO, indicating biofilm disruption efficacy. Reproduced from ref. 241 with permission from Wiley 2023. (f) Modular microrobot system fabrication scheme, integrating CS and MA modules, with dynamic locking and unlocking mechanism via pH-responsive MA module volume changes. (g) Experimental images showcasing the modular microrobot's capabilities in the BD model: (i) targeted deployment via catheter. (ii and iii) Bifurcation navigation and target acquisition. (iv) On-demand disassembly at the lesion. (v and vi) MA module retrieval, demonstrating modular design and control. Adopted from ref. 242 with permission from The American Association for the Advancement of Science 2023. | ||
The rational design of magnetically and pH-responsive robotic materials with modular architectures is emerging as a crucial strategy to optimize cellular cargo delivery and release kinetics at diverse target locations, thereby improving therapeutic efficacy. In a recent publication in Science Advances,242 the authors employed a modular design approach to develop a hybrid robotic material, comprising a magnetically responsive core (NdFeB), magnetic actuation (MA) module, and cell scaffold (CS) module, functionalized with surface-bound carboxylic groups, thereby endowing the material with dual magnetic and pH-responsive properties (Fig. 15f). In a proof-of-concept study involving cellular delivery to the bile duct (BD), the fabricated microrobot was deployed adjacent to the targeted lesion via catheter-based delivery, followed by magnetically actuated navigation, leveraging a rotating magnetic field to traverse the complex and twisted ductal morphology. On reaching the targeted site characterized by an acidic microenvironment, the microrobot's assembled configuration underwent pH-induced disassembly, triggered by the contraction of the pH-responsive MA module. Subsequent exposure to a low-frequency rotating magnetic field prompted the release of the decomposable cell scaffold (CS) module, enabling targeted cellular therapeutic delivery. The navigation through anatomically complex structures such as the bile duct relies heavily on high-resolution imaging modalities and precise external field control. The loss of guidance due to anatomical obstructions, signal attenuation, or misalignment may result in off-target deposition or device entrapment. To address these limitations, current strategies increasingly incorporate biodegradable materials and real-time, closed-loop control mechanisms to improve navigational reliability and safety in vivo.243 The dissociated magnetic actuation (MA) module was magnetically guided back to the catheter, enabling retrieval and removal, thereby mitigating potential safety risks and minimizing the likelihood of long-term adverse effects (Fig. 15g). This modular design strategy demonstrated herein offers a promising framework for engineering advanced robotic systems, targeting multidrug-resistant bacterial biofilms, and unlocking innovative avenues for next-generation antibiofilm interventions. Consistent with the aforementioned studies, numerous reports corroborate these findings, demonstrating the efficacious potential of magnetic-responsive robotic materials in achieving targeted delivery of therapeutic agents, synergistically combined with the inherent mechanical shear stress generated by these materials, facilitating efficient biofilm disruption and removal.244–247
3. Challenges and outlook
The nanomaterials excel at killing bacteria via non-metabolism-dependent routes, but clear evidence against true persister cells remains limited. Take AgNPs their bactericidal activity stems from oxidative dissolution, releasing Ag+ ions that bind thiol-rich enzymes, disrupt membrane integrity, intercalate DNA, and generate ROS actions that do not require bacterial growth.248 However, AgNPs are effective against metabolically inert cells in vitro, recent studies highlight that there is currently no information available on the use of AgNPs to specifically target persister cells. Besides, resilience can emerge biofilm-forming pathogens like S. aureus and P. aeruginosa can adapt to silver exposure by thickening EPS matrices, upregulating efflux pumps, and activating antioxidative stress responses.249 Other nanomaterials such as graphene oxide, metal-oxide particles, and functionalized polymers cause mechanical membrane damage, disrupt proton gradients, or release photocatalytic ROS, yet their activity against dormant persisters is often shown only under idealized conditions. The nanomaterials offer multi-pronged antimicrobial mechanisms from ion-mediated enzymatic blockade to irreversible physical membrane damage their real-world impact on persister populations and their potential to drive resistance remains underexplored. However, resistance to nanomaterials is not impossible. Bacteria can adapt by upregulating efflux pumps, thickening the EPS matrix, producing stress-response enzymes (e.g., catalase, superoxide dismutase), or altering membrane lipid composition to reduce the uptake of nanoparticles. Studies have shown P. aeruginosa and E. coli can evolve increased tolerance to AgNPs via biofilm densification, oxidative stress gene activation, and ion sequestration, especially under chronic sublethal exposure. Horizontal gene transfer of metal resistance operons has also been observed. Therefore, nanomaterials may delay resistance development, the assumption that they are immune to resistance is scientifically inaccurate. A more accurate perspective is that their multimodal actions raise the evolutionary barrier for resistance, but do not eliminate the risk. This underscores the need for long-term investigation, optimized dosing strategies, and resistance modeling in both in vitro and in vivo systems.4. Conclusion and perspectives
The synergistic co-aggregation of phylogenetically diverse bacterial strains within a self-produced, protective biofilm matrix enhances their collective resilience, facilitating survival under harsh environmental conditions. This adaptive trait enables biofilm-embedded microorganisms to rapidly regrow upon transitioning to favorable conditions. Importantly, biofilm-associated strains have developed sophisticated mechanisms to evade conventional antimicrobial therapies, rendering them refractory to traditional antibiotic-based treatments. This phenomenon presents a significant clinical challenge, as biofilm-mediated antimicrobial resistance necessitates the development of innovative, targeted therapeutic strategies to effectively eradicate these resilient microbial biofilms. Nanotechnology-based interventions exhibit potential for revitalizing antibiotic efficacy against biofilm-encapsulated, antibiotic-resistant bacterial populations by harnessing their intrinsic antibiofilm and antibacterial activities to precisely target and dismantle the biofilm matrix, consequently augmenting bacterial susceptibility to antibiotic therapy. Advanced nanomaterial-based strategies exploit their multifunctional capabilities to: (1) degrade the biofilm matrix, compromising its structural integrity. (2) Achieve deep penetration into the biofilm. (3) Employ dual physical and chemical modalities to eliminate resident microbial populations. This integrated approach effectively disrupts biofilm architecture, facilitating thorough eradication of embedded microorganisms. Stimuli-responsive nano–micro robotic formulations synergistically optimize antimicrobial interactions, mechanical biofilm disruption, and spatially-controlled antimicrobial delivery. Concurrently, enzyme-mimetic nanomaterials leverage biofilm microenvironmental cues to potentiate therapeutic outcomes via ROS-mediated bacteriolysis, PTT-induced biofilm eradication, and PDT-facilitated inactivation. This integrated approach harnesses biofilm dynamics to enhance antimicrobial efficacy, selectively targeting recalcitrant bacterial pathogens and presenting a promising paradigm for mitigating biofilm-associated infections.Although nanomaterials have shown considerable promise, their clinical translation is impeded by substantial knowledge deficits, notably concerning toxicity profiles, clearance pathways, and metabolic disposition. Therefore, a comprehensive pharmacological characterization, integrating detailed pharmacokinetic and pharmacodynamics investigations, is imperative to optimize translational research, ensure nanomaterial safety, and accelerate the development of clinically effective nanotherapeutics. Moreover, existing studies predominantly assess anti-biofilm activities against monoculture bacterial strains, whereas chronic wound infections often involve complex polymicrobial biofilms composed of diverse pathogenic species.74 These polymicrobial biofilms display increased resistance to antimicrobial therapies, highlighting a critical clinical concern. Additionally, to address the complex microbial ecosystem within the host, where pathogens and commensals coexist, nanomaterials must be engineered with targeted ligands to enhance their discriminative capacity and reduce off-target toxicity to surrounding tissues. Although significant hurdles persist, the potential advantages of nanomaterial-based strategies in combating antibiotic resistance are substantial and warrant further investigation. Continued research in this domain is crucial to surmount existing challenges and translating these innovative therapies into clinical applications.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project was supported by grant from the National Natural Science Foundation of China (32300048, 22405063), Guangdong Basic and Applied Basic Research Foundation (2022A1515110158, 2023A1515110122, 2024A1515012577, 2024A1515010677), Construction Project of Nano Technology and Application Engineering Research Center of Guangdong Medical University (4SG24179G), 100 Youth Research Project Funding Program of Guangdong Medical University in 2023 (GDMUD2023001), Dongguan Science and Technology of Social Development Program (20231800936272).References
- J. M. V. Makabenta, et al., Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections, Nat. Rev. Microbiol., 2021, 19, 23–36 CrossRef CAS PubMed.
- C. J. Murray, et al., Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis, Lancet, 2022, 399, 629–655 CrossRef CAS PubMed.
- J. Taylor, et al., Estimating the Economic Costs of Antimicrobial Resistance: Model and Results, 2014 Search PubMed.
- V. Choi, J. L. Rohn, P. Stoodley, D. Carugo and E. Stride, Drug delivery strategies for antibiofilm therapy, Nat. Rev. Microbiol., 2023, 21, 555–572 CrossRef CAS PubMed.
- W. Cassandra, The drug-resistant bacteria that pose the greatest health threats, Nature, 2017, 543, 15 CrossRef PubMed.
- O. Ciofu, C. Moser, P. Ø. Jensen and N. Høiby, Tolerance and resistance of microbial biofilms, Nat. Rev. Microbiol., 2022, 20, 621–635 CrossRef CAS PubMed.
- E. M. Darby, et al., Molecular mechanisms of antibiotic resistance revisited, Nat. Rev. Microbiol., 2023, 21, 280–295 Search PubMed.
- M. Valentini, D. Gonzalez, D. A. Mavridou and A. Filloux, Lifestyle transitions and adaptive pathogenesis of Pseudomonas aeruginosa, Curr. Opin. Microbiol., 2018, 41, 15–20 CrossRef CAS PubMed.
- X. Zhan, et al., A c-di-GMP signaling module controls responses to iron in Pseudomonas aeruginosa, Nat. Commun., 2024, 15, 1860 CrossRef CAS PubMed.
- H.-C. Flemming, et al., The biofilm matrix: multitasking in a shared space, Nat. Rev. Microbiol., 2023, 21, 70–86 CrossRef CAS PubMed.
- L. K. Jennings, et al., Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 11353–11358 CrossRef CAS PubMed.
- H. Panlilio and C. V. Rice, The role of extracellular DNA in the formation, architecture, stability, and treatment of bacterial biofilms, Biotechnol. Bioeng., 2021, 118, 2129–2141 CrossRef CAS PubMed.
- H.-C. Flemming, et al., Biofilms: an emergent form of bacterial life, Nat. Rev. Microbiol., 2016, 14, 563–575 CrossRef CAS PubMed.
- H.-C. Flemming and J. Wingender, The biofilm matrix, Nat. Rev. Microbiol., 2010, 8, 623–633 CrossRef CAS PubMed.
- L. Weaver, J. Webber, A. Hickson, P. Abraham and M. Close, Biofilm resilience to desiccation in groundwater aquifers: a laboratory and field study, Sci. Total Environ., 2015, 514, 281–289 CrossRef CAS PubMed.
- I. Levin-Reisman, et al., Antibiotic tolerance facilitates the evolution of resistance, Science, 2017, 355, 826–830 CrossRef CAS PubMed.
- A. Brauner, O. Fridman, O. Gefen and N. Q. Balaban, Distinguishing between resistance, tolerance and persistence to antibiotic treatment, Nat. Rev. Microbiol., 2016, 14, 320–330 CrossRef CAS PubMed.
- N. Høiby, et al., ESCMID guideline for the diagnosis and treatment of biofilm infections 2014, Clin. Microbiol. Infect., 2015, 21, S1–S25 CrossRef PubMed.
- W. Hengzhuang, H. Wu, O. Ciofu, Z. Song and N. Høiby, Pharmacokinetics/pharmacodynamics of colistin and imipenem on mucoid and nonmucoid Pseudomonas aeruginosa biofilms, Antimicrob. Agents Chemother., 2011, 55, 4469–4474 CrossRef PubMed.
- N. Høiby, et al., Formation of Pseudomonas aeruginosa inhibition zone during tobramycin disk diffusion is due to transition from planktonic to biofilm mode of growth, Int. J. Antimicrob. Agents, 2019, 53, 564–573 CrossRef PubMed.
- M. Fernández-Billón, A. E. Llambías-Cabot, E. Jordana-Lluch, A. Oliver and M. D. Macià, Mechanisms of antibiotic resistance in Pseudomonas aeruginosa biofilms, Biofilm, 2023, 100129 CrossRef PubMed.
- B. Cao, et al., Diffusion retardation by binding of tobramycin in an alginate biofilm model, PLoS One, 2016, 11, e0153616 Search PubMed.
- O. Ciofu and T. Tolker-Nielsen, Tolerance and resistance of Pseudomonas aeruginosa biofilms to antimicrobial agents—how P. aeruginosa can escape antibiotics, Front. Microbiol., 2019, 10, 453354 CrossRef PubMed.
- N. Bagge, et al., Pseudomonas aeruginosa biofilms exposed to imipenem exhibit changes in global gene expression and β-lactamase and alginate production, Antimicrob. Agents Chemother., 2004, 48, 1175–1187 CrossRef CAS PubMed.
- C. Kranjec, et al., Staphylococcal biofilms: Challenges and novel therapeutic perspectives, Antibiotics, 2021, 10, 131 Search PubMed.
- S. M. Mondol, et al., Unveiling a high-risk epidemic clone (ST 357) of ‘Difficult to Treat Extensively Drug-Resistant’(DT-XDR) Pseudomonas aeruginosa from a burn patient in Bangladesh: A resilient beast revealing coexistence of four classes of beta lactamases, J. Global Antimicrob. Resist., 2024, 36, 83–95 CrossRef CAS PubMed.
- C. W. Hall and T.-F. Mah, Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria, FEMS Microbiol. Rev., 2017, 41, 276–301 CrossRef CAS PubMed.
- P. R. Secor, et al., Pf bacteriophage and their impact on Pseudomonas virulence, mammalian immunity, and chronic infections, Front. Immunol., 2020, 11, 511573 Search PubMed.
- J. Jo, A. Price-Whelan and L. E. Dietrich, Gradients and consequences of heterogeneity in biofilms, Nat. Rev. Microbiol., 2022, 20, 593–607 CrossRef CAS PubMed.
- E. M. Waters, S. E. Rowe, J. P. O'Gara and B. P. Conlon, Convergence of Staphylococcus aureus persister and biofilm research: can biofilms be defined as communities of adherent persister cells?, PLoS Pathog., 2016, 12, e1006012 CrossRef PubMed.
- I. Keren, D. Shah, A. Spoering, N. Kaldalu and K. Lewis, Specialized persister cells and the mechanism of multidrug tolerance in Escherichia coli, J. Bacteriol., 2004, 186, 8172–8180 CrossRef CAS PubMed.
- D. Nguyen, et al., Active starvation responses mediate antibiotic tolerance in biofilms and nutrient-limited bacteria, Science, 2011, 334, 982–986 CrossRef CAS PubMed.
- J. M. Stokes, A. J. Lopatkin, M. A. Lobritz and J. J. Collins, Bacterial metabolism and antibiotic efficacy, Cell Metab., 2019, 30, 251–259 CrossRef CAS PubMed.
- J. Yan and B. L. Bassler, Surviving as a community: antibiotic tolerance and persistence in bacterial biofilms, Cell Host Microbe, 2019, 26, 15–21 CrossRef CAS PubMed.
- F. Baquero and B. R. Levin, Proximate and ultimate causes of the bactericidal action of antibiotics, Nat. Rev. Microbiol., 2021, 19, 123–132 CrossRef CAS PubMed.
- N. Q. Balaban, et al., Definitions and guidelines for research on antibiotic persistence, Nat. Rev. Microbiol., 2019, 17, 441–448 CrossRef CAS PubMed.
- S. L. Percival, K. E. Hill, S. Malic, D. W. Thomas and D. W. Williams, Antimicrobial tolerance and the significance of persister cells in recalcitrant chronic wound biofilms, Wound Repair Regen., 2011, 19, 1–9 CrossRef PubMed.
- K. Lewis, Persister cells: molecular mechanisms related to antibiotic tolerance, Antibiotic Resistance, 2012, ch. 8, pp. 121–133, DOI:10.1007/978-3-642-28951-4_8.
- K. Lewis and Y. Shan, Why tolerance invites resistance, Science, 2017, 355, 796 CrossRef CAS PubMed.
- E. Maisonneuve, L. J. Shakespeare, M. G. Jørgensen and K. Gerdes, Bacterial persistence by RNA endonucleases, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 13206–13211 CrossRef CAS PubMed.
- L. R. Mulcahy, J. L. Burns, S. Lory and K. Lewis, Emergence of Pseudomonas aeruginosa strains producing high levels of persister cells in patients with cystic fibrosis, J. Bacteriol., 2010, 192, 6191–6199 CrossRef CAS PubMed.
- J. B. Parsons, et al., In-patient evolution of a high-persister Escherichia coli strain with reduced in vivo antibiotic susceptibility, Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e2314514121 CrossRef CAS PubMed.
- G. Anderson and G. O’toole, Innate and induced resistance mechanisms of bacterial biofilms, Curr. Top. Microbiol. Immunol., 2008, 322, 85–105 CAS.
- P. S. Stewart, et al., Contribution of stress responses to antibiotic tolerance in Pseudomonas aeruginosa biofilms, Antimicrob. Agents Chemother., 2015, 59, 3838–3847 CrossRef CAS PubMed.
- A. Soares, K. Alexandre and M. Etienne, Tolerance and persistence of Pseudomonas aeruginosa in biofilms exposed to antibiotics: Molecular mechanisms, antibiotic strategies and therapeutic perspectives, Front. Microbiol., 2020, 11, 573403 Search PubMed.
- M. A. Kohanski, D. J. Dwyer and J. J. Collins, How antibiotics kill bacteria: from targets to networks, Nat. Rev. Microbiol., 2010, 8, 423–435 CrossRef CAS PubMed.
- J. Blázquez, J. Rodríguez-Beltrán and I. Matic, Antibiotic-induced genetic variation: how it arises and how it can be prevented, Annu. Rev. Microbiol., 2018, 72, 209–230 CrossRef PubMed.
- W. Mi, S. Tang, Y. Jin and N. Shao, Au/Ag bimetallic nanoclusters stabilized by glutathione and lysozyme for ratiometric sensing of H2O2 and hydroxyl radicals, ACS Appl. Nano Mater., 2021, 4, 1586–1595 CrossRef CAS.
- L. A. Bekale, D. Sharma, B. Bacacao, J. Chen and P. L. Santa Maria, Eradication of Bacterial Persister Cells By Leveraging Their Low Metabolic Activity Using Adenosine Triphosphate Coated Gold Nanoclusters, Nano Today, 2023, 51, 101895 CrossRef CAS PubMed.
- C. Zhang, R. Sun and T. Xia, Adaption/resistance to antimicrobial nanoparticles: Will it be a problem?, Nano Today, 2020, 34, 100909 CrossRef CAS.
- B. R. Levin and D. E. Rozen, Non-inherited antibiotic resistance, Nat. Rev. Microbiol., 2006, 4, 556–562 CrossRef CAS PubMed.
- J. M. Blair, M. A. Webber, A. J. Baylay, D. O. Ogbolu and L. J. Piddock, Molecular mechanisms of antibiotic resistance, Nat. Rev. Microbiol., 2015, 13, 42–51 CrossRef CAS PubMed.
- S. Zhang, et al., Reduced redox-dependent mechanism and glucose-mediated reversal in gentamicin-resistant Vibrio alginolyticus, Environ. Microbiol., 2019, 21, 4724–4739 Search PubMed.
- B. Peng, H. Li and X.-X. Peng, Functional metabolomics: from biomarker discovery to metabolome reprogramming, Protein Cell, 2015, 6, 628–637 CrossRef CAS PubMed.
- A. J. Lopatkin, et al., Clinically relevant mutations in core metabolic genes confer antibiotic resistance, Science, 2021, 371, eaba0862 Search PubMed.
- M. Jiang, et al., Ampicillin-controlled glucose metabolism manipulates the transition from tolerance to resistance in bacteria, Sci. Adv., 2023, 9, eade8582 Search PubMed.
- R. Yao, et al., Catabolic regulation analysis of Escherichia coli and its crp, mlc, mgsA, pgi and ptsG mutants, Microb. Cell Factories, 2011, 10, 1–11 CrossRef PubMed.
- O. Fridman, A. Goldberg, I. Ronin, N. Shoresh and N. Q. Balaban, Optimization of lag time underlies antibiotic tolerance in evolved bacterial populations, Nature, 2014, 513, 418–421 Search PubMed.
- E. Trampari, et al., Exposure of Salmonella biofilms to antibiotic concentrations rapidly selects resistance with collateral tradeoffs, npj Biofilms Microbiomes, 2021, 7, 3 Search PubMed.
- N. Q. Balaban, J. Merrin, R. Chait, L. Kowalik and S. Leibler, Bacterial persistence as a phenotypic switch, Science, 2004, 305, 1622–1625 CrossRef CAS PubMed.
- A. H. Melnyk, A. Wong and R. Kassen, The fitness costs of antibiotic resistance mutations, Evol. Appl., 2015, 8, 273–283 CrossRef PubMed.
- P. B. Rainey, A. Buckling, R. Kassen and M. Travisano, The emergence and maintenance of diversity: insights from experimental bacterial populations, Trends Ecol. Evol., 2000, 15, 243–247 CrossRef PubMed.
- K. B. Harris, K. M. Flynn and V. S. Cooper, Polygenic adaptation and clonal interference enable sustained diversity in experimental Pseudomonas aeruginosa populations, Mol. Biol. Evol., 2021, 38, 5359–5375 CrossRef CAS PubMed.
- T. Beaudoin, L. Zhang, A. J. Hinz, C. J. Parr and T.-F. Mah, The biofilm-specific antibiotic resistance gene ndvB is important for expression of ethanol oxidation genes in Pseudomonas aeruginosa biofilms, J. Bacteriol., 2012, 194, 3128–3136 CrossRef CAS PubMed.
- S. Disney-McKeethen, S. Seo, H. Mehta, K. Ghosh and Y. Shamoo, Experimental evolution of Pseudomonas aeruginosa to colistin in spatially confined microdroplets identifies evolutionary trajectories consistent with adaptation in microaerobic lung environments, mBio, 2023, 14, e01506–e01523 Search PubMed.
- S. Yang, et al., Biofilm tolerance, resistance and infections increasing threat of public health, Microb. Cell, 2023, 10, 233 CrossRef CAS PubMed.
- T.-F. Mah, Biofilm-specific antibiotic resistance, Future Microbiol., 2012, 7, 1061–1072 CrossRef CAS PubMed.
- C. A. Fux, J. W. Costerton, P. S. Stewart and P. Stoodley, Survival strategies of infectious biofilms, Trends Microbiol., 2005, 13, 34–40 CrossRef CAS PubMed.
- V. J. Savage, I. Chopra and A. J. O'Neill, Staphylococcus aureus biofilms promote horizontal transfer of antibiotic resistance, Antimicrob. Agents Chemother., 2013, 57, 1968–1970 CrossRef CAS PubMed.
- S. Borgeaud, L. C. Metzger, T. Scrignari and M. Blokesch, The type VI secretion system of Vibrio cholerae fosters horizontal gene transfer, Science, 2015, 347, 63–67 CrossRef CAS PubMed.
- R. T. Merod and S. Wuertz, Extracellular polymeric substance architecture influences natural genetic transformation of Acinetobacter baylyi in biofilms, Appl. Environ. Microbiol., 2014, 80, 7752–7757 CrossRef PubMed.
- L. Kumar, et al., Advances in nanotechnology for biofilm inhibition, ACS Omega, 2023, 8, 21391–21409 CrossRef CAS PubMed.
- A. R. Kirtane, et al., Nanotechnology approaches for global infectious diseases, Nat. Nanotechnol., 2021, 16, 369–384 CrossRef CAS PubMed.
- Q. AlGabbani, Nanotechnology: A promising strategy for the control of parasitic infections, Exp. Parasitol., 2023, 250, 108548 CrossRef CAS PubMed.
- J. Zhang, et al., Nanotechnology-driven strategies to enhance the treatment of drug-resistant bacterial infections, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2024, 16, e1968 CAS.
- K. Las Heras, et al., Modulating the immune system towards a functional chronic wound healing: A biomaterials and Nanomedicine perspective, Adv. Drug Delivery Rev., 2024, 210, 115342 CrossRef CAS PubMed.
- J. Dong, et al., Immunomodulatory biomaterials for implant-associated infections: from conventional to advanced therapeutic strategies, Biomater. Res., 2022, 26, 72 CrossRef PubMed.
- Q. Xu, et al., Targeted hot ion therapy of infected wound by glycol chitosan and polydopamine grafted Cu-SiO2 nanoparticles, Nano Today, 2021, 41, 101330 CrossRef CAS.
- B. Li, et al., Nano–Bio Interactions: Biofilm-Targeted Antibacterial Nanomaterials, Small, 2024, 20, 2306135 CrossRef CAS PubMed.
- E. M. P. Dos Santos, et al., Silver nanoparticles–chitosan composites activity against resistant bacteria: Tolerance and biofilm inhibition, J. Nanopart. Res., 2021, 23, 196 CrossRef CAS PubMed.
- L. Liu, et al., Tannic acid-modified silver nanoparticles for enhancing anti-biofilm activities and modulating biofilm formation, Biomater. Sci., 2020, 8, 4852–4860 RSC.
- Q. Cheng, et al., Effects of Fe2O3 nanoparticles on extracellular polymeric substances and nonylphenol degradation in river sediment, Sci. Total Environ., 2021, 770, 145210 CrossRef CAS PubMed.
- F. A. Al-Wrafy, A. A. Al-Gheethi, S. K. Ponnusamy, E. A. Noman and S. A. Fattah, Nanoparticles approach to eradicate bacterial biofilm-related infections: A critical review, Chemosphere, 2022, 288, 132603 CrossRef CAS PubMed.
- N. Dey, et al., Role of nanomaterials in deactivating multiple drug resistance efflux pumps–A review, Environ. Res., 2022, 204, 111968 CrossRef CAS PubMed.
- T. I. Hasan and A. A. Ahmed, Biogenic Silver Nanoparticles by Pseudomonas aeruginosa Reduce Expression of Biofilm and Quorum Signaling Genes in Multi-drug Resistant Acinetobacter baumannii, Jordan J. Biol. Sci., 2023, 16(4), 621–632 CAS.
- L. Hu, The use of nanoparticles to prevent and eliminate bacterial biofilms, Antimicrobial Research Novel Bioknowledge and Educational Programs, ed. Mendez-Vilas, A., Formatex, Spain, 2017, pp. 344–350 Search PubMed.
- A. Bahrami, R. Delshadi and S. M. Jafari, Active delivery of antimicrobial nanoparticles into microbial cells through surface functionalization strategies, Trends Food Sci. Technol., 2020, 99, 217–228 CrossRef CAS.
- F. Cui, et al., Recent advances in carbon-based nanomaterials for combating bacterial biofilm-associated infections, J. Hazard. Mater., 2022, 431, 128597 CrossRef CAS PubMed.
- K. R. Sims, et al., Enhanced design and formulation of nanoparticles for anti-biofilm drug delivery, Nanoscale, 2019, 11, 219–236 RSC.
- H. Koo, R. N. Allan, R. P. Howlin, P. Stoodley and L. Hall-Stoodley, Targeting microbial biofilms: current and prospective therapeutic strategies, Nat. Rev. Microbiol., 2017, 15, 740–755 CrossRef CAS PubMed.
- F. A. Qais, M. S. Khan and I. Ahmad, Nanoparticles as quorum sensing inhibitor: Prospects and limitations, Biotechnological Applications of Quorum Sensing Inhibitors, 2018, pp. 227–244 Search PubMed.
- S. Hayat, et al., Quorum quenching: role of nanoparticles as signal jammers in Gram-negative bacteria, Future Microbiol., 2019, 14, 61–72 CrossRef CAS PubMed.
- D. Bamal, et al., Silver nanoparticles biosynthesis, characterization, antimicrobial activities, applications, cytotoxicity and safety issues: An updated review, Nanomaterials, 2021, 11, 2086 CrossRef CAS PubMed.
- E. Casals, T. Pfaller, A. Duschl, G. J. Oostingh and V. Puntes, Time evolution of the nanoparticle protein corona, ACS Nano, 2010, 4, 3623–3632 CrossRef CAS PubMed.
- E. Fröhlich, The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles, Int. J. Nanomed., 2012, 7, 5577–5591 CrossRef PubMed.
- R. Y. Pelgrift and A. J. Friedman, Nanotechnology as a therapeutic tool to combat microbial resistance, Adv. Drug Delivery Rev., 2013, 65, 1803–1815 CrossRef CAS PubMed.
- J. A. Lemire, J. J. Harrison and R. J. Turner, Antimicrobial activity of metals: mechanisms, molecular targets and applications, Nat. Rev. Microbiol., 2013, 11, 371–384 CrossRef CAS PubMed.
- Y. Luo, et al., Bacteria-activated macrophage membrane coated ROS-responsive nanoparticle for targeted delivery of antibiotics to infected wounds, J. Nanobiotechnol., 2024, 22, 781 CrossRef CAS PubMed.
- D. Gupta, A. Singh and A. U. Khan, Nanoparticles as efflux pump and biofilm inhibitor to rejuvenate bactericidal effect of conventional antibiotics, Nanoscale Res. Lett., 2017, 12, 1–6 CrossRef CAS PubMed.
- N. Parvin and S. W. Joo, Nanomaterial-Based Strategies to Combat Antibiotic Resistance: Mechanisms and Applications, Antibiotics (Basel), 2025, 14(2), 207 CrossRef CAS PubMed.
- A. Pourmehdiabadi, M. S. Nobakht, B. Hajjam Balajorshari, M. R. Yazdi and K. Amini, Investigating the effects of zinc oxide and titanium dioxide nanoparticles on the formation of biofilm and persister cells in Klebsiella pneumoniae, J. Basic Microbiol., 2023, e2300454 Search PubMed.
- Z. Cao, et al., Gold nanocluster adjuvant enables the eradication of persister cells by antibiotics and abolishes the emergence of resistance, Nanoscale, 2022, 14, 10016–10032 RSC.
- A. Panáček, et al., Bacterial resistance to silver nanoparticles and how to overcome it, Nat. Nanotechnol., 2018, 13, 65–71 CrossRef PubMed.
- V. Borøy, Drug Transport and Delivery Research Group (DTD), UiT Norges arktiske universitet, 2022 Search PubMed.
- N. Blanco-Cabra, J. Alcàcer-Almansa, J. Admella, B. V. Arévalo-Jaimes and E. Torrents, Nanomedicine against biofilm infections: A roadmap of challenges and limitations, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2024, 16, e1944 CAS.
- H. Wang, et al., Nano-sized polystyrene and magnetite collectively promote biofilm stability and resistance due to enhanced oxidative stress response, J. Hazard. Mater., 2024, 476, 134974 CrossRef CAS PubMed.
- Z.-m. Xiu, Q.-b. Zhang, H. L. Puppala, V. L. Colvin and P. J. Alvarez, Negligible particle-specific antibacterial activity of silver nanoparticles, Nano Lett., 2012, 12, 4271–4275 CrossRef CAS PubMed.
- E. Matras, A. Gorczyca, S. W. Przemieniecki and M. Oćwieja, Surface properties-dependent antifungal activity of silver nanoparticles, Sci. Rep., 2022, 12, 18046 CrossRef CAS PubMed.
- S. S. Jeremiah, K. Miyakawa, T. Morita, Y. Yamaoka and A. Ryo, Potent antiviral effect of silver nanoparticles on SARS-CoV-2, Biochem. Biophys. Res. Commun., 2020, 533, 195–200 CrossRef CAS PubMed.
- A. Ivask, et al., Toxicity mechanisms in Escherichia coli vary for silver nanoparticles and differ from ionic silver, ACS Nano, 2014, 8, 374–386 CrossRef CAS PubMed.
- S. Tang and J. Zheng, Antibacterial activity of silver nanoparticles: structural effects, Adv. Healthcare Mater., 2018, 7, 1701503 CrossRef PubMed.
- T. C. Dakal, A. Kumar, R. S. Majumdar and V. Yadav, Mechanistic basis of antimicrobial actions of silver nanoparticles, Front. Microbiol., 2016, 7, 1831 Search PubMed.
- N. Durán, et al., Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity, Nanomed. Nanotechnol. Biol. Med., 2016, 12, 789–799 CrossRef PubMed.
- N. Tripathi and M. K. Goshisht, Recent advances and mechanistic insights into antibacterial activity, antibiofilm activity, and cytotoxicity of silver nanoparticles, ACS Appl. Bio Mater., 2022, 5, 1391–1463 CrossRef CAS PubMed.
- H. Wang, et al., Multi-target mode of action of silver against Staphylococcus aureus endows it with capability to combat antibiotic resistance, Nat. Commun., 2021, 12, 3331 CrossRef CAS PubMed.
- R. Padilla-Hernández, A. Ramos-Jacques & A. Hernandez-Martinez in Silver Nanoparticles for Drug Delivery, Elsevier, vol. 157–164, 2024 Search PubMed.
- Y. Zhang, et al., Quantitative proteomics reveals the mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa biofilms, J. Proteome Res., 2020, 19, 3109–3122 CrossRef CAS PubMed.
- N. Singh, K. M. Paknikar and J. Rajwade, RNA-sequencing reveals a multitude of effects of silver nanoparticles on Pseudomonas aeruginosa biofilms, Environ. Sci.: Nano, 2019, 6, 1812–1828 RSC.
- T. Seviour, et al., Functional amyloids keep quorum-sensing molecules in check, J. Biol. Chem., 2015, 290, 6457–6469 CrossRef CAS PubMed.
- N. Van Gerven, S. E. Van der Verren, D. M. Reiter and H. Remaut, The role of functional amyloids in bacterial virulence, J. Mol. Biol., 2018, 430, 3657–3684 CrossRef CAS PubMed.
- Z. e. Huma, et al., Nanosilver mitigates biofilm formation via FapC amyloidosis inhibition, Small, 2020, 16, 1906674 CrossRef CAS PubMed.
- M. Wang, A. Kakinen, E. H. Pilkington, T. P. Davis and P. C. Ke, Differential effects of silver and iron oxide nanoparticles on IAPP amyloid aggregation, Biomater. Sci., 2017, 5, 485–493 RSC.
- K. A. Conway, J. D. Harper and P. T. Lansbury, Accelerated in vitro fibril formation by a mutant α-synuclein linked to early-onset Parkinson disease, Nat. Med., 1998, 4, 1318–1320 CrossRef CAS PubMed.
- F. Gao, T. Shao, Y. Yu, Y. Xiong and L. Yang, Surface-bound reactive oxygen species generating nanozymes for selective antibacterial action, Nat. Commun., 2021, 12, 745 CrossRef CAS PubMed.
- U. Rajchakit, et al., Size-controlled synthesis of gold nanoparticles tethering antimicrobial peptides with potent broad-spectrum antimicrobial and antibiofilm activities, Mol. Pharm., 2024, 21, 596–608 CrossRef CAS PubMed.
- Y. Luo, H. Zhang, Q. Cui, H. Li and L. Li, Gold nanoclusters-decorated graphitic carbon nitride nanocomposites with antibacterial and anti-biofilm activities, Colloids Surf., A, 2023, 676, 132189 CrossRef CAS.
- Y. Gong, et al., Gold nanoclusters cure implant infections by targeting biofilm, J. Colloid Interface Sci., 2024, 674, 490–499 CrossRef CAS PubMed.
- E. Bhatia and R. Banerjee, Hybrid silver–gold nanoparticles suppress drug resistant polymicrobial biofilm formation and intracellular infection, J. Mater. Chem. B, 2020, 8, 4890–4898 RSC.
- Y. Wang and D. S. Kohane, External triggering and triggered targeting strategies for drug delivery, Nat. Rev. Mater., 2017, 2, 1–14 Search PubMed.
- Y. Gao, et al., Controlled nanoparticle release from stable magnetic microbubble oscillations, NPG Asia Mater., 2016, 8, e260 CrossRef CAS.
- K. R. Lattwein, et al., Sonobactericide: an emerging treatment strategy for bacterial infections, Ultrasound Med. Biol., 2020, 46, 193–215 CrossRef PubMed.
- S. Wang, et al., Accelerating thrombolysis using a precision and clot-penetrating drug delivery strategy by nanoparticle-shelled microbubbles, Sci. Adv., 2020, 6, eaaz8204 CrossRef CAS PubMed.
- Y. Dong, et al., Antibiofilm effect of ultrasound combined with microbubbles against Staphylococcus epidermidis biofilm, Int. J. Med. Microbiol., 2017, 307, 321–328 CrossRef PubMed.
- W. Xiu, et al., Ultrasound-responsive catalytic microbubbles enhance biofilm elimination and immune activation to treat chronic lung infections, Sci. Adv., 2023, 9, eade5446 CrossRef CAS PubMed.
- E. Teirlinck, et al., Laser-induced vapour nanobubbles improve drug diffusion and efficiency in bacterial biofilms, Nat. Commun., 2018, 9, 4518 CrossRef PubMed.
- A. Luo, et al., Mechanism by which micro-nano bubbles impact biofilm growth in drinking water distribution systems, Environ. Sci.: Water Res. Technol., 2025, 11, 754–767 RSC.
- Y. Xie, H. Liu, Z. Teng, J. Ma and G. Liu, Nanomaterial-enabled anti-biofilm strategies: new opportunities for treatment of bacterial infections, Nanoscale, 2025, 17, 5605–5628 RSC.
- S. Park, N. Barak, T. Lotan and G. Yossifon, Biohybrid Microrobots Based on Jellyfish Stinging Capsules and Janus Particles for In Vitro Deep-Tissue Drug Penetration, Small Sci., 2024, 2400551 Search PubMed.
- H. Wei and E. Wang, Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes, Chem. Soc. Rev., 2013, 42, 6060–6093 RSC.
- H. Wang, K. Wan and X. Shi, Recent advances in nanozyme research, Adv. Mater., 2019, 31, 1805368 CrossRef CAS PubMed.
- C. J. Neal, E. Kolanthai, F. Wei, M. Coathup and S. Seal, Surface chemistry of biologically active reducible oxide nanozymes, Adv. Mater., 2024, 36, 2211261 CrossRef CAS PubMed.
- S. Zhang, J. Chen, M.-L. Lian, W.-S. Yang and X. Chen, An engineered, self-propelled nanozyme as reactive oxygen species scavenger, Chem. Eng. J., 2022, 446, 136794 CrossRef CAS.
- Y. Zhang, et al., Emerging nanozyme-based multimodal synergistic therapies in combating bacterial infections, Theranostics, 2022, 12, 5995 CrossRef CAS PubMed.
- Y. Wang, Y. Yang, Y. Shi, H. Song and C. Yu, Antibiotic-free antibacterial strategies enabled by nanomaterials: progress and perspectives, Adv. Mater., 2020, 32, 1904106 CrossRef CAS PubMed.
- F. Cao, et al., Defect-rich adhesive nanozymes as efficient antibiotics for enhanced bacterial inhibition, Angew. Chem., Int. Ed., 2019, 58, 16236–16242 CrossRef CAS PubMed.
- J. Wu, et al., Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II), Chem. Soc. Rev., 2019, 48, 1004–1076 RSC.
- L. Li, et al., ROS-catalytic transition-metal-based enzymatic nanoagents for tumor and bacterial eradication, Adv. Funct. Mater., 2022, 32, 2107530 CrossRef CAS.
- Y. Li, W. Zhu, J. Li and H. Chu, Research progress in nanozyme-based composite materials for fighting against bacteria and biofilms, Colloids Surf., B, 2021, 198, 111465 CrossRef CAS PubMed.
- C. Zhang, X. Wang, J. Du, Z. Gu and Y. Zhao, Reactive oxygen species-regulating strategies based on nanomaterials for disease treatment, Adv. Sci., 2021, 8, 2002797 CrossRef CAS PubMed.
- J. Shan, et al., Cu2MoS4 nanozyme with NIR-II light enhanced catalytic activity for efficient eradication of multidrug-resistant bacteria, Small, 2020, 16, 2001099 CrossRef CAS PubMed.
- H. Sies, et al., Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology, Nat. Rev. Mol. Cell Biol., 2022, 23, 499–515 CrossRef CAS PubMed.
- L. Zhao, et al., H2O2 self-supplied CuFeOx nanosystem as fenton-like reaction agents for endogenous/exogenous responsive synergetic antibacterial therapy, Chem. Eng. J., 2024, 492, 152265 CrossRef CAS.
- A. Zarepour, M. R. Venkateswaran, A. Khosravi, S. Iravani and A. Zarrabi, Bioinspired nanomaterials to combat microbial biofilm and pathogen challenges: A review, ACS Appl. Nano Mater., 2024, 7, 25287–25313 CrossRef CAS.
- Z. Zhou, et al., Cerium-Based Metal–Organic Framework with Intrinsic Haloperoxidase-Like Activity for Antibiofilm Formation, Adv. Funct. Mater., 2022, 32, 2206294 CrossRef CAS.
- F. Natalio, et al., Vanadium pentoxide nanoparticles mimic vanadium haloperoxidases and thwart biofilm formation, Nat. Nanotechnol., 2012, 7, 530–535 CrossRef CAS PubMed.
- E. Pütz, et al., Communication breakdown: Into the molecular mechanism of biofilm inhibition by CeO2 nanocrystal enzyme mimics and how it can Be exploited, ACS Nano, 2022, 16, 16091–16108 CrossRef PubMed.
- Q. Luo, et al., Stabilizing Ultrasmall Ceria-Cluster Nanozyme for Antibacterial and Antibiofouling Applications, Small, 2022, 18, 2107401 CrossRef CAS PubMed.
- D. Su, X. He, J. Zhou, C. Yuan and X. Bai, Facet-dependent haloperoxidase-like activities of CeO2 nanoparticles contribute to their excellent biofilm formation suppression abilities, J. Hazard. Mater., 2024, 465, 133433 CrossRef CAS PubMed.
- M. Hu, et al., Nanozymes in nanofibrous mats with haloperoxidase-like activity to combat biofouling, ACS Appl. Mater. Interfaces, 2018, 10, 44722–44730 CrossRef CAS PubMed.
- H. Huang, et al., Spiky Artificial Peroxidases with V− O− Fe Pair Sites for Combating Antibiotic-Resistant Pathogens, Angew. Chem., 2024, 136, e202310811 CrossRef.
- M. Liu, et al., Copper doped carbon dots for addressing bacterial biofilm formation, wound infection, and tooth staining, ACS Nano, 2022, 16, 9479–9497 CrossRef CAS PubMed.
- W. Dong, et al., Co-, N-doped carbon dot nanozymes based on an untriggered ROS generation approach for anti-biofilm activities and in vivo anti-bacterial treatment, J. Mater. Chem. B, 2024, 12, 1052–1063 RSC.
- K. Yang, et al., Self-adaptive antibiofilm effect and immune regulation by hollow Cu2MoS4 nanospheres for treatment of implant infections, ACS Appl. Mater. Interfaces, 2023, 15, 18720–18733 CrossRef CAS PubMed.
- Y. Dong, et al., Superior peroxidase mimetic activity of carbon dots–Pt nanocomposites relies on synergistic effects, New J. Chem., 2015, 39, 4141–4146 RSC.
- M. Liang, et al., Engineering inorganic nanoflares with elaborate enzymatic specificity and efficiency for versatile biofilm eradication, Small, 2020, 16, 2002348 CrossRef CAS PubMed.
- S.-N. Song, et al., Nanoarchitectonics of bimetallic Cu-/Co-doped nitrogen–carbon nanozyme-functionalized hydrogel with NIR-responsive phototherapy for synergistic mitigation of drug-resistant bacterial infections, ACS Appl. Mater. Interfaces, 2024, 16, 16011–16028 CrossRef CAS PubMed.
- L. Xie, H. Wu, Y. Li, L. Shi and Y. Liu, Recent development of nanozymes for combating bacterial drug resistance: a review, Adv. Healthcare Mater., 2025, 14, 2402659 CrossRef CAS PubMed.
- A. Aljaafari, F. Ahmed and F. M. Husain, Bio-inspired facile synthesis of graphene-based nanocomposites: Elucidation of antimicrobial and biofilm inhibitory potential against foodborne pathogenic bacteria, Coatings, 2020, 10, 1171 CrossRef CAS.
- Y. Huang, et al., Iron oxide nanozymes stabilize stannous fluoride for targeted biofilm killing and synergistic oral disease prevention, Nat. Commun., 2023, 14, 6087 CrossRef CAS PubMed.
- X. Zhu, J. Guo, Y. Yang and J. Liu, Macrophage Polarization Induced by Bacteria-Responsive Antibiotic-Loaded Nanozymes for Multidrug Resistance-Bacterial Infections Management, Small, 2023, 19, 2204928 CrossRef CAS PubMed.
- S. Yu, G. Li, R. Liu, D. Ma and W. Xue, Dendritic Fe3O4@ poly (dopamine)@ PAMAM nanocomposite as controllable NO-releasing material: a synergistic photothermal and NO antibacterial study, Adv. Funct. Mater., 2018, 28, 1707440 CrossRef.
- J. Cao, et al., Bacteria-adhesive nitric oxide-releasing graphene oxide nanoparticles for MRPA-infected wound healing therapy, ACS Appl. Mater. Interfaces, 2022, 14, 50507–50519 CrossRef CAS PubMed.
- L. Zheng, et al., Flexible modulation of cellular activities with cationic photosensitizers: insights of alkyl chain length on reactive oxygen species antimicrobial mechanisms, Adv. Mater., 2023, 35, 2302943 CrossRef CAS PubMed.
- H. Li, K. Luo, W. Liu, S. Yu and W. Xue, Neutrophil-Mimicking Nanozyme with Cascade Catalytic Releasing Nitric Oxide and Signet Oxygen Property for Synergistic Bimodal Therapy of Methicillin-Resistant Staphylococcus Aureus Infections, Small, 2024, 2403527 CrossRef CAS PubMed.
- Z. Zhao, et al., Arginine-Enhanced Antimicrobial Activity of Nanozymes against Gram-Negative Bacteria, Adv. Healthcare Mater., 2024, 13, 2301332 CrossRef CAS PubMed.
- J. Cheng, et al., Biofilm heterogeneity-adaptive photoredox catalysis enables red light-triggered nitric oxide release for combating drug-resistant infections, Nat. Commun., 2023, 14, 7510 CrossRef CAS PubMed.
- Q. Tian, et al., Nanozyme-enabled biomedical diagnosis: advances, trends, and challenges, Adv. Healthcare Mater., 2025, 14, 2401630 CrossRef CAS PubMed.
- S. Xiao, et al., Artificial Phages with Biocatalytic Spikes for Synergistically Eradicating Antibiotic-Resistant Biofilms, Adv. Mater., 2024, 2404411 CrossRef CAS PubMed.
- W. Zhong, et al., Miniature robots for battling bacterial infection, ACS Nano, 2024, 18, 32335–32363 CrossRef CAS PubMed.
- Z. Zhang, et al., Micro-/Nanorobots in antimicrobial applications: recent progress, challenges, and opportunities, Adv. Healthcare Mater., 2022, 11, 2101991 CrossRef CAS PubMed.
- Y. Yang, et al., Bioinspired spiky peroxidase-mimics for localized bacterial capture and synergistic catalytic sterilization, Adv. Mater., 2021, 33, 2005477 CrossRef CAS PubMed.
- L. Jin, et al., Microenvironment-activated Nanozyme-armed bacteriophages efficiently combat bacterial infection, Adv. Mater., 2023, 35, 2301349 CrossRef CAS PubMed.
- J. Ye, et al., Current Status and Future Developments in NIR-II-emitting Organic Small Molecule Fluorophores for Bioimaging and Phototherapy, Small, 2409722 Search PubMed.
- R. Luo, et al., NIR-II upconversion nanomaterials for biomedical applications, Nanoscale, 2025, 17, 2985–3002 RSC.
- Q. Deng, et al., Porphyrin MOF dots–based, function-adaptive nanoplatform for enhanced penetration and photodynamic eradication of bacterial biofilms, Adv. Funct. Mater., 2019, 29, 1903018 CrossRef.
- J. H. Lee, J. S. Ryu, Y. K. Kang, H. Lee and H. J. Chung, Polydopamine sensors of bacterial hypoxia via fluorescence coupling, Adv. Funct. Mater., 2021, 31, 2007993 CrossRef CAS.
- X. Sun, et al., Oxygen self-sufficient nanoplatform for enhanced and selective antibacterial photodynamic therapy against anaerobe-induced periodontal disease, Adv. Funct. Mater., 2021, 31, 2101040 CrossRef CAS.
- Y. Hu, et al., Biofilm microenvironment-responsive nanoparticles for the treatment of bacterial infection, Nano Today, 2022, 46, 101602 CrossRef CAS.
- Z. Yuan, et al., A Photo-Therapeutic Nanocomposite with Bio-Responsive Oxygen Self-Supplying Combats Biofilm Infections and Inflammation from Drug-Resistant Bacteria, Adv. Funct. Mater., 2023, 33, 2302908 CrossRef CAS.
- W. Wang, et al., One-stop integrated nanoagent for bacterial biofilm eradication and wound disinfection, ACS Nano, 2024, 18, 4089–4103 CrossRef CAS PubMed.
- Y. Wang, et al., A Multifunctional Nanozyme with NADH Dehydrogenase-Like Activity and Nitric Oxide Release under Near-Infrared Light Irradiation as an Efficient Therapeutic for Antimicrobial Resistance Infection and Wound Healing, Adv. Healthcare Mater., 2023, 12, 2300568 CrossRef CAS PubMed.
- Q. Xu, et al., A biofilm microenvironment-activated single-atom iron nanozyme with NIR-controllable nanocatalytic activities for synergetic bacteria-infected wound therapy, Adv. Healthcare Mater., 2021, 10, 2101374 CrossRef CAS PubMed.
- H. Song, et al., Iron/Molybdenum Sulfide Nanozyme Cocatalytic Fenton Reaction for Photothermal/Chemodynamic Efficient Wound Healing, Langmuir, 2024, 40(28), 14346–14354 CrossRef CAS PubMed.
- X. Ren, et al., Multi-Enzyme-Based Superabsorbent Hydrogel for Self-Enhanced NIR-II Photothermal-Catalytic Antibacterial Therapy, Adv. Healthcare Mater., 2024, 13, 2303537 CrossRef CAS PubMed.
- L. Luo, et al., A Robust Photothermal-Mediated Nanozyme Engineering with Efficient Synergistic Antibacterial Therapy for Wound Healing, ACS Mater. Lett., 2024, 6, 2487–2496 CrossRef CAS.
- X. He, et al., Platinum Nanoparticles Regulated V2C MXene Nanoplatforms with NIR-II Enhanced Nanozyme Effect for Photothermal and Chemodynamic Anti-Infective Therapy, Adv. Mater., 2024, 2400366 CrossRef CAS PubMed.
- Y. Shi, et al., A small pore black TiO 2/-large pore Fe 3 O 4 cascade nanoreactor for chemodynamic/photothermal synergetic tumour therapy, J. Mater. Chem. B, 2023, 11, 4498–4510 RSC.
- T. Li, et al., Injectable Hydrogel Incorporated with Iron-Doped Carbon Dots Exhibiting Peroxidase-Like Activity for Antibacterial Therapy and Wound Healing, Adv. Therapeut., 2024, 7, 2300368 CrossRef CAS.
- D. Mo, et al., Sulfur Vacancy-Rich Bi2S3-X@ PDA Heterojunctions with Light-Controlled Reactive Oxygen Species Generation and Elimination to Combat Biofilm Infection and Inflammation Caused by Drug-Resistant Bacteria, Adv. Funct. Mater., 2024, 2313569 CrossRef CAS.
- S. Zamani, et al., Enterotoxigenic Bacteroides fragilis: a possible etiological candidate for bacterially-induced colorectal precancerous and cancerous lesions, Front. Cell. Infect. Microbiol., 2020, 9, 449 CrossRef PubMed.
- J. Abed, et al., Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed Gal-GalNAc, Cell Host Microbe, 2016, 20, 215–225 CrossRef CAS PubMed.
- L. Parhi, et al., Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression, Nat. Commun., 2020, 11, 3259 CrossRef CAS PubMed.
- L. Chen, et al., Antibacterial Fusobacterium nucleatum-mimicking nanomedicine to selectively eliminate tumor-colonized bacteria and enhance immunotherapy against colorectal cancer, Adv. Mater., 2023, 35, 2306281 CrossRef CAS PubMed.
- J. L. Galeano Niño, et al., Effect of the intratumoral microbiota on spatial and cellular heterogeneity in cancer, Nature, 2022, 611, 810–817 CrossRef PubMed.
- M. Vétizou, et al., Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota, Science, 2015, 350, 1079–1084 CrossRef PubMed.
- L. F. Mager, et al., Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy, Science, 2020, 369, 1481–1489 CrossRef CAS PubMed.
- S.-S. Jiang, et al., Fusobacterium nucleatum-derived succinic acid induces tumor resistance to immunotherapy in colorectal cancer, Cell Host Microbe, 2023, 31, 781–797 CrossRef CAS PubMed.
- L. Chen, et al., Fusobacterium nucleatum-mimicking nanovehicles to overcome chemoresistance for breast cancer treatment by eliminating tumor-colonizing bacteria, Chem, 2024, 1783–1803 CAS.
- L. Chen, et al., An emerging antibacterial nanovaccine for enhanced chemotherapy by selectively eliminating tumor-colonizing bacteria, Sci. Bull., 2024, 2565–2579 CrossRef CAS PubMed.
- S. Bullman, et al., Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer, Science, 2017, 358, 1443–1448 CrossRef CAS PubMed.
- D. Nejman, et al., The human tumor microbiome is composed of tumor type–specific intracellular bacteria, Science, 2020, 368, 973–980 CrossRef CAS PubMed.
- S. Fan, W. Zhang, L. Zhou, D. Wang and D. Tang, Potential role of the intratumoral microbiota in colorectal cancer immunotherapy, Int. Immunopharmacol., 2024, 137, 112537 CrossRef CAS PubMed.
- S. S. Pinho and C. A. Reis, Glycosylation in cancer: mechanisms and clinical implications, Nat. Rev. Cancer, 2015, 15, 540–555 CrossRef CAS PubMed.
- J. Ma, L. Jiang and G. Liu, Cell membrane-coated nanoparticles for the treatment of bacterial infection, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2022, 14, e1825 CAS.
- A. T. Babakr, Scavenger receptors: different classes and their role in the uptake of oxidized low-density lipoproteins, Biomed. Pharmacol. J., 2024, 17 DOI:10.13005/bpj/2897.
- D. E. Owens III and N. A. Peppas, Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles, Int. J. Pharm., 2006, 307, 93–102 CrossRef PubMed.
- C.-M. J. Hu, et al., Nanoparticle biointerfacing by platelet membrane cloaking, Nature, 2015, 526, 118–121 CrossRef CAS PubMed.
- J. Huang, et al., Nanomedicine-boosting tumor immunogenicity for enhanced immunotherapy, Adv. Funct. Mater., 2021, 31, 2011171 CrossRef CAS.
- H. Zhang, et al., Review of the Applications of Micro/Nanorobots in Biomedicine, ACS Appl. Nano Mater., 2024, 7(15), 17151–17192 CrossRef CAS.
- B. Wang, K. Kostarelos, B. J. Nelson and L. Zhang, Trends in micro-/nanorobotics: materials development, actuation, localization, and system integration for biomedical applications, Adv. Mater., 2021, 33, 2002047 CrossRef CAS PubMed.
- J. Llacer-Wintle, et al., Biodegradable small-scale swimmers for biomedical applications, Adv. Mater., 2021, 33, 2102049 CrossRef CAS PubMed.
- G. Hwang, et al., Catalytic antimicrobial robots for biofilm eradication, Sci. Robot., 2019, 4, eaaw2388 CrossRef PubMed.
- Y. Dong, et al., Endoscope-assisted magnetic helical micromachine delivery for biofilm eradication in tympanostomy tube, Sci. Adv., 2022, 8, eabq8573 CrossRef CAS PubMed.
- C. C. Mayorga-Martinez, L. Zhang and M. Pumera, Chemical multiscale robotics for bacterial biofilm treatment, Chem. Soc. Rev., 2024, 2284–2299 RSC.
- K. Villa, et al., Chemical microrobots as self-propelled microbrushes against dental biofilm, Cell Rep. Phys. Sci., 2020, 1, 100181 CrossRef.
- H. Ji, et al., Precisely controlled and deeply penetrated micro-nano hybrid multifunctional motors with enhanced antibacterial activity against refractory biofilm infections, J. Hazard. Mater., 2022, 436, 129210 CrossRef CAS PubMed.
- W. Liu, et al., Biomedical Micro-/Nanomotors: Design, Imaging, and Disease Treatment, Adv. Funct. Mater., 2023, 33, 2212452 CrossRef CAS.
- M. Ussia, et al., Light-Propelled Nanorobots for Facial Titanium Implants Biofilms Removal, Small, 2022, 18, 2200708 CrossRef CAS PubMed.
- X. Li, et al., Nanozyme-augmented tumor catalytic therapy by self-supplied H2O2 generation, ACS Appl. Bio Mater., 2020, 3, 1769–1778 CrossRef CAS PubMed.
- D. H. Lee and M. Kamruzzaman, Advancements in organic materials-based nanozymes for broader applications, Trends Chem., 2024, 540–555 CrossRef CAS.
- Y. Lu and M. Li, Rational Design of Near-infrared II Plasmonic Optofunctional Materials for Diagnostic and Therapeutic Applications, Adv. Funct. Mater., 2024, 34, 2312753 CrossRef CAS.
- H. Zhou, C. C. Mayorga-Martinez, S. Pané, L. Zhang and M. Pumera, Magnetically driven micro and nanorobots, Chem. Rev., 2021, 121, 4999–5041 CrossRef CAS PubMed.
- G. Gardi, S. Ceron, W. Wang, K. Petersen and M. Sitti, Microrobot collectives with reconfigurable morphologies, behaviors, and functions, Nat. Commun., 2022, 13, 2239 CrossRef CAS PubMed.
- P. I. Baburova, et al., Magnetic soft robot for minimally invasive urethral catheter biofilm eradication, ACS Nano, 2023, 17, 20925–20938 CrossRef PubMed.
- M. Urso, M. Ussia and M. Pumera, Smart micro-and nanorobots for water purification, Nat. Rev. Bioeng., 2023, 1, 236–251 CrossRef CAS PubMed.
- Z.-C. Zhao, Y. Zhou, G. Tan and J. Li, Research progress about the effect and prevention of blue light on eyes, Int. J. Ophthalmol., 2018, 11, 1999 Search PubMed.
- S. Wahl, M. Engelhardt, P. Schaupp, C. Lappe and I. V. Ivanov, The inner clock—Blue light sets the human rhythm, J. Biophotonics, 2019, 12, e201900102 CrossRef CAS PubMed.
- X. Chen, C. Tian, H. Zhang and H. Xie, Biodegradable magnetic hydrogel robot with multimodal locomotion for targeted cargo delivery, ACS Appl. Mater. Interfaces, 2023, 15, 28922–28932 CrossRef CAS PubMed.
- J. Li, et al., Development of a magnetic microrobot for carrying and delivering targeted cells, Sci. Robot., 2018, 3, eaat8829 CrossRef PubMed.
- X. Deng, et al., Magnetic Micro/nanorobots for biological detection and targeted delivery, Biosens. Bioelectron., 2023, 222, 114960 CrossRef CAS PubMed.
- C. C. Mayorga-Martinez, et al., Multimodal-Driven Magnetic Microrobots with Enhanced Bactericidal Activity for Biofilm Eradication and Removal from Titanium Mesh, Adv. Mater., 2023, 35, 2300191 CrossRef CAS PubMed.
- L. Su, et al., Modularized microrobot with lock-and-detachable modules for targeted cell delivery in bile duct, Sci. Adv., 2023, 9, eadj0883 CrossRef CAS PubMed.
- J. Choi, J. Hwang, J. y. Kim and H. Choi, Recent progress in magnetically actuated microrobots for targeted delivery of therapeutic agents, Adv. Healthcare Mater., 2021, 10, 2001596 CrossRef CAS PubMed.
- B. Sun, et al., Magnetic hydrogel micromachines with active release of antibacterial agent for biofilm eradication, Adv. Intell. Syst., 2024, 6, 2300092 CrossRef.
- Z. Huang, et al., A magnetic-guided nano-antibacterial platform for alternating magnetic field controlled vancomycin release in staphylococcus aureus biofilm eradication, Drug Delivery Transl. Res., 2024, 1–16, 1249–1264 Search PubMed.
- Z. Zhu, C. Huang, L. Liu, J. Wang and X. Gou, Magnetically actuated pandanus fruit-like nanorobots for enhanced pH-stimulated drug release and targeted biofilm elimination in wound healing, J. Colloid Interface Sci., 2024, 661, 374–388 CrossRef CAS PubMed.
- M. Ussia, et al., Active Light-Powered Antibiofilm ZnO Micromotors with Chemically Programmable Properties, Adv. Funct. Mater., 2021, 31, 2101178 CrossRef CAS.
- S. Sarkar, et al., Escaping the ESKAPE pathogens: A review on antibiofilm potential of nanoparticles, Microb. Pathog., 2024, 194, 106842 CrossRef CAS PubMed.
- A. S. Rodrigues, et al., Advances in silver nanoparticles: a comprehensive review on their potential as antimicrobial agents and their mechanisms of action elucidated by proteomics, Front. Microbiol., 2024, 15, 1440065 CrossRef PubMed.
Footnote |
| † These authors have contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |



