 Open Access Article
Open Access ArticleQuassinoids from Malaysian Eurycoma longifolia significantly increased the expression of the melatonin biosynthesis-related enzyme gene (AANAT)†
Chunguang Han a,
Maki Nagataa,
Masako Matsumotoa,
Yhiya Amen
a,
Maki Nagataa,
Masako Matsumotoa,
Yhiya Amen b,
Marwa Elsbaey
b,
Marwa Elsbaey b,
Liwei Menga,
Yutaka Kurokicd and
Kuniyoshi Shimizu*a
b,
Liwei Menga,
Yutaka Kurokicd and
Kuniyoshi Shimizu*a
aDepartment of Agro-Environmental Sciences, Graduate School of Bioresource and Bioenvironmental Sciences, Kyushu University, 819-0395 Fukuoka, Japan. E-mail: shimizu.kuniyoshi.381@m.kyushu-u.ac.jp
bDepartment of Pharmacognosy, Faculty of Pharmacy, Mansoura University, Mansoura 35516, Egypt
cD-LAB, Japan Tobacco Inc., 4-1-1, Toranomon, Minato-ku, Tokyo 105-6927, Japan
dDelightex Pte. Ltd., 230 Victoria Street, #15-01 Bugis Junction Towers, 188024, Singapore
First published on 20th May 2025
Abstract
Eurycoma longifolia Jack, also known as Tongkat Ali, is a native plant in the family of Simaroubaceae that grows in Southeast Asian rain forests. E. longifolia has garnered significant attention due to its profound pharmacological properties (antimalarial, anti-pyretic, antiulcer, cytotoxic and aphrodisiac properties). However, the recent report that the intake of Tongkat Ali supplements improve sleep quality in healthy adults has aroused our interest. Three the most characteristic C20-quassinoids (1–3) were isolated from the roots of Eurycoma longifolia, along with eight known secondary metabolites with other structural types (4–11). Their structures were identified by comprehensive analyses of NMR spectroscopy, and HRMS data. The sleep-promoting activity of three quassinoids based on the melatonin (sleep hormone) biosynthesis-related enzyme gene (AANAT) expression test was also evaluated, and the results revealed that quassinoids (1–3) significantly increased AANAT gene expression compared to the control. These results provide new ideas for the development and utilization of the plant for clinical applications of sleep disorders in the future.
1. Introduction
Eurycoma longifolia Jack (commonly called Tongkat Ali) is a flowering plant in the family Simaroubaceae. It is native to Indochina (Cambodia, Laos, Malaysia, Myanmar, Thailand and Vietnam) and Indonesia (the islands of Borneo and Sumatra).1 The root of E. longifolia has been used in folk medicine in the Southeast Asian region to treat malaria, dysentery, glandular swelling, persistent fever, aches, and sexual insufficiency.2 In modern times it has found common use as supplements, as well as food and drink additives.Insufficient sleep is a pervasive and prominent problem in the modern 24 h society. Medical experts have said that about one-third of the people in the world suffer from sleep disorders. Insufficient sleep is prevalent across various age groups, considered to be a public health epidemic. After decades of research, the case can be confidently made that sleep loss and sleep disorders have profound and widespread effects on human health.3 It leads to increased incidences of cardiovascular morbidity, increased chances of diabetes mellitus, obesity, derailment of cognitive functions, vehicular accidents, and increased accidents at workplaces.2
The latest research indicated the intake of Tongkat Ali supplement containing about 402 mg of Tongkat Ali powder a day enhanced mood state and consequently improve the sleep quality in a healthy population.4 These unique properties make Tongkat Ali (E. longifolia Jack) plant valuable for potential applications, and it is worth further exploring the active substances in E. longifolia. Evaluation of its sleep-promoting activity of these compounds can provide valuable insights into the role of E. longifolia in improving the sleep disorders that is the worldwide public health epidemic.
In this study, we investigated further related constituents from n-hexane-, dichloromethane- and ethyl acetate-soluble extract of the water extract of the roots of E. longifolia, and isolated three quassinoids (1–3), along with several known secondary metabolites (4–11) are displayed in Fig. 1. We herein describe the isolation, structure elucidation of these compounds, and evaluation of the sleep-promoting activity of the most characteristic chemical components (quassinoids) of this plant based on the gene expression test of enzymes related to melatonin (sleep hormone) biosynthesis.
2. Results and discussion
2.1. Chemistry
The HPLC chromatograms of three quassinoids (1–3) were presented in Fig. 2a. Three peaks were observed at 11.57 (purple), 12.89 (red), and 16.29 min (black), which corresponded to eurycomanone (1) and eurycomanol (2) and 14,15β-dihydroxyklaineanone (3), respectively. High-Resolution Mass Spectrum (HRMS) shows the observed [M + H]+ peak at m/z 409.1514 (calcd for C20H25O9, 409.1499) of 1, 411.1647 (calcd for C20H27O9, 411.1655) of 2 and 397.1862 (calcd for C20H29O8, 397.1862) of 3 in Fig. 2b.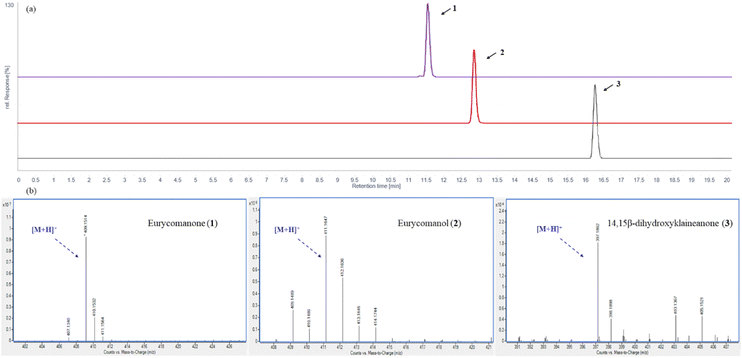 | ||
| Fig. 2 (a) HPLC analysis result of three quassinoids (1–3); (b) HRMS spectra of the three quassinoids (1–3). | ||
Compound (1) was obtained as colorless needle with the molecular formula C20H25O9, which was determined by the ion peaks at m/z 409.1514 [M + H]+ observed by HR-ESIMS. In the 1H and 13C NMR spectra of 1 revealed the presence of two methyl groups (δH 1.62 and 1.77, δC 10.8 and 22.8), two methine (δH 3.26, δC 42.6) and (δH 3.83, δC 48.1), a methylene (δH 2.21 and 2.32, δC 26.1), one oxymethylene (δH 4.01 and 4.55, δC 68.1), and four oxymethines (δH 4.53, 4.81, 5.26, 5.67, δC 84.9, 81.4, 72.2, 76.2), a trisubstituted double bond (δH 6.15, δC 126.4, 162.9), a 1,1-disubstituted double bond (δH 5.65 and 6.12, δC 119.7 and 148.5), a ketone carbonyl (δC 197.9), a ester carbonyl (δC 174.3), two oxygenated quaternary carbon (δC 79.8, 109.9), two quaternary sp3 carbons (δC 46.3, 53.0) (Fig. 3 and Table 1), suggesting a C20-quassinoid skeleton. By the comparison with literature values,5 compound 1 was established as a C20-type quassinoid, eurycomanone (Fig. 3).
| No. | 1b δC, type δH | 2b δC, type δH | 3c δC, type δH | |||
|---|---|---|---|---|---|---|
| a Overlapped signals were reported without designating multiplicity.b Measured in C5D5N, 400 MHz for 1H NMR, 100 MHz for 13C NMR.c Measured in CDCl3, 400 MHz for 1H NMR, 100 MHz for 13C NMR. | ||||||
| 1 | 84.9 CH | 4.53, s | 84.1, CH | 4.07, d (8.2) | 82.6, CH | 4.04, s |
| 2 | 197.9, C | — | 73.1, CH | 4.66, m | 198.2, C | — |
| 3 | 126.4, CH | 6.15, br s | 127.4, CH | 5.82, br s | 124.3, CH | 6.09, br s |
| 4 | 162.9, C | — | 135.4, C | — | 164.5, C | — |
| 5 | 42.6, CH | 3.26, br d (12.8) | 42.0, CH | 2.85, br d (12.4) | 43.6, CH | 2.87, br d (12.8) |
| 6 | 26.1, CH2 | 2.21, ddd (14.8, 12.8, 2.8) | 26.0, CH2 | 1.93, br t (14.4) | 26.1, CH2 | 2.10, m |
| 2.32, dt (14.8, 2.8) | 2.20, br d (14.4) | 2.30, td (3.2, 14.8) | ||||
| 7 | 72.2, CH | 5.26, t (2.8) | 72.3, CH | 5.19, br s | 81.5, CH | 4.63, t (3.2) |
| 8 | 53.0, C | — | 53.1, C | — | 48.2, C | — |
| 9 | 48.1, CH | 3.83, s | 48.3, CH | 3.58, s | 45.0, CH | 2.11, d (3.2) |
| 10 | 46.3, C | — | 42.7, C | — | 43.0, C | — |
| 11 | 109.9, C | — | 110.2, C | — | 73.6, CH | 4.88, br s |
| 12 | 81.4, CH | 4.81, s | 81.5, CH | 4.83, s | 77.0, CH | 3.91, t (2.8) |
| 13 | 148.5, C | — | 149.0, C | — | 35.8, CH | 2.43, dq (2.8, 7.3) |
| 14 | 79.8, C | — | 79.8, C | — | 75.8, C | — |
| 15 | 76.2, CH | 5.67, s | 76.9, CH | 5.55, s | 71.3, CH | 5.33, s |
| 16 | 174.3, C | — | 174.3, C | — | 175.9, C | — |
| 18 | 22.8, CH3 | 1.77, s 1H) | 21.7, CH3 | 1.64, br s | 22.8, CH3 | 1.97, s |
| 19 | 10.8, CH3 | 1.62, s (1H) | 11.3, CH3 | 1.76, s | 12.4, CH3 | 1.18, s |
| 20 | 68.1, CH2 | 4.01, d (8.8) | 68.3, CH2 | 4.07, d (8.2) | 17.4, CH3 | 1.51, s |
| 4.55, d (8.8) | 4.58, d (8.2) | |||||
| 21 | 119.7, CH2 | 5.65, d (1.6) | 119.7, CH2 | 5.66, d (1.6) | 12.5, CH3 | 1.22, d (7.2) |
| 6.12, d (1.6) | 6.13, d (1.6) | |||||
The stereochemistry of compound 1 was proved based on measured optical rotation consistent with literature values.5 [α] = + 37.8 (c 0.3, MeOH); 1H and 13C NMR data are listed in Table 1.
The 1H and 13C NMR spectroscopic data of compounds 2 was closely similar to those of 1 and was identical with eurycomanol by the comparison of the chemical shifts reported in the literature,5,6 as illustrated in Fig. 4a. Eurycomanol (2): colorless needles; [α] = + 83.9 (c 0.04, C5H5N). 1H and 13C NMR data were shown as Table 1.
 | ||
| Fig. 4 (a) Eurycomanol (2): chemical structure and 1H/13C NMR spectra; (b) 14,15β-dihydroxyklaineanone (3): chemical structure and 1H/13C NMR spectra. | ||
Compound (3) was obtained as colorless needle with the molecular formula C20H29O8, which was determined by the ion peaks at m/z 397.1862 [M + H]+ observed by HR-ESIMS. In the 1H NMR spectrum of 3, four methyl groups at δH 1.18, 1.22, 1.51, 1.97, five oxygenated methine protons at δH 3.91, 4.04, 4.63, 4.88 and 5.33, a trisubstituted double bond proton at δH 6.09 were observed, a ketone carbonyl (δC 198.2), a ester carbonyl (δC 175.9), one oxygenated quaternary carbon (δC 75.8), two quaternary sp3 carbons (δC 43.0, 48.2), suggesting the structural similarity of a C20-quassinoid skeleton. By the comparison with literature values,5 compound 3 was established as 14,15β-dihydroxyklaineanone (Fig. 4b). The stereochemistry of compound 3 was proved based on measured optical rotation consistent with literature values.6 [α] = + 53.2 (c 0.5, MeOH); 1H and 13C NMR data are listed in Table 1.
A furanoid lignan, a triterpenoid, a squalene-type derivative, two β-carboline alkaloids and three heterocyclic compounds were determined as (+)-syringaresinol (4),7–10 bourjotinolone A (5),11–13 eurylene (6),14,15 canthin-6-one (7),16–18 9-methoxycanthin-6-one (8),19–21 vanillin (9),22 3,5-dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one (10)23 and 5-(hydroxymethyl)furfural (11)24,25 by comparing the 1H, 13C NMR and HRMS data with those reported in literature.
2.2. Biological activity
In this study, we firstly investigated the influence of E. longifolia extract on melatonin biosynthesis by AANAT, which is a rate-limiting enzyme in melatonin biosynthesis. The expression of AANAT gene was significantly 1.6 times higher than control when E. longifolia extract applied to SH-SY5Y cells. This result indicate that the extract of E. longifolia have a possible to increase melatonin biosynthesis by up-regulating AANAT genes (Fig. 5).
The quassinoids are a fascinating class of highly oxygenated degraded triterpene natural product, and many quassinoids have shown the exciting biological properties, including anti-malarial, anti-inflammatory, antiviral, neuroprotective and antifeeding-particularly potent anti-cancer activity.27,28 In a controlled trial on the effect of Tongkat Ali supplement intake on the sleep quality in Japanese healthy adults, statistical analysis showed that a significant improvement sleep quality was observed only in the Tongkat Ali group (intaking 402 mg of Tongkat Ali power a day) over a 4 week period, compared with the placebo group.4 This finding aligns with the present study, which, for the first time, demonstrates that three C20-type quassinoids isolated from E. longifolia significantly upregulate the expression of the AANAT gene, a rate-limiting enzyme critical for the biosynthesis of melatonin (the sleep hormone), thereby revealing the potential of quassinoids in improving and treating “sleep disorders”. However, based on the number of carbon atoms involving the construction of their basic scaffolds, quassinoids are commonly categorized into six distinct groups: C26, C25, C22, C20, C19, and C18 types.5,27 The C20-type skeleton structure isolated in this study, which is characterized by a tetracyclic ring system often containing a d-ring lactone. Investigating further the structural–activity correlation for six types of quassinoids involved in upregulating AANAT gene activity will serve as a significant driving force for our ongoing exploration of additional quassinoid types from this plant.
Quassinoids are the main components responsible for various biological activities in E. longifolia extract, and we speculate that they have hypnotic effects. We observed that E. longifolia extract and its isolated product significantly increased the expression of AANAT gene compared with the control in SH-SY5Y cells line. Although the preliminary findings are promising, further extensive studies are required, such as using the three-dimensional structure and molecular docking of the target enzyme combined with computer-aided design to identify their binding sites (associated amino acid residues) of quassinoids to target receptor. Additionally, based on increased AANAT gene expression, investigating elevated levels of its acetylation product, N-acetyl-5-hydroxytryptamine (NAS) and ultimately the increased intracellular melatonin content, are necessary to elucidate the mechanism by which it improve the sleep quality in healthy adults.4 These unique activities make E. longifolia a potentially valuable insight for developing natural products or sleep supplements that enhance sleep.
3. Conclusions
In conclusion, three most characteristic C20-type quassinoids (1–3) were successfully isolated from E. longifolia, and their chemical structures and stereochemistry were elucidated by spectroscopic methods. The effects of three components from E. longifolia on the melatonin biosynthesis-related enzyme AANAT were investigated. Eurycomanone, eurycomanol and 14,15β-dihydroxykaineanone significantly increased the expression of the rate-limiting enzyme AANAT gene for melatonin synthesis on SH-SY5Y cell compared to the control. These results strongly indicated that the C20-type quassinoids (1–3) contained in E. longifolia may be expected to promote melatonin biosynthesis by up-regulating the AANAT gene. This study helps to reveal the second metabolites from E. longifolia can yield promising drug candidates for improving and treating “sleep disorders” and to deepen our understanding of the existence of a class of highly oxygenated degraded triterpenoids in E. longifolia. The use of C20-type quassinoids from E. longifolia which have potential sleep-promoting activities will have broad application prospects in functional food and medicine. Future studies will focus on exploring quassinoids with stronger gene up-regulation ability, investigating changes in melatonin content both inside and outside the cell based on its activity, as well as exploring mechanism of action.4. Experimental
4.1. General
Organic solvents were purchased from Wako Pure Chemical Industries (Osaka, Japan). Silica gel (75–120 mesh) and RP-C18 silica (38–63 μm) were purchased from Wako Pure Chemical Industries (Osaka, Japan). Buchi Reveleris® Prep system equipped with NP-silica gel, RP-C18 flash column or Develosil ODS-UG-5 column (5 μm, ∅ 20 × 250 mm, Nomura Chemical, Japan), were used for fractionation and fine purification. Optical rotations were recorded on a P-2200 polarimeter (JASCO, Japan). High-performance liquid chromatography (HPLC) was performed using a high-pressure Agilent 1260 Infinity II LC system equipped with UV and ELSD detector (Agilent Technologies Japan, Ltd). All NMR measurements spectra were obtained on a JNM-ECS 400 (400 MHz for 1H) (JEOL) or DRX-600 spectrometer (600 MHz for 1H) (Bruker Daltonics, USA). The chemical shifts (ppm) were referenced to the solvent peak of chloroform-d1 (δH 7.26/δC 77.0), methanol-d4 (δH 3.31/δC 49.0) or pyridine-d5 (δH 7.22/δC 123.9) as an internal standard. High-resolution electrospray ionization time-of-flight mass spectra (HRESITOFMS) were recorded on a 6545 LC-QTOF/MS system (Agilent Technologies) in the positive ion mode.4.2. Materials
The freeze-dried water extract from dried roots (Radix) of Eurycoma longifolia Jack (Simaroubaceae) was kindly provided by Phytes Biotek Sdn Bhd (Malaysia).4.3. Extraction and isolation
One hundred and five grams of the provided powder was subjected to fractionation with solvents of increasing polarity to give n-hexane-, dichloromethane- and ethyl acetate-soluble fractions. The weights of them were as following: 302 mg, 1.811 g, 1.843 g and 100 g. The n-hexane-soluble fraction was chromatographed on Buchi Reveleris® Prep system equipped with NP-silica gel set with UV-ELSD detector and using a gradient mobile phase of n-hexane/EtOAc (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 → 0
0 → 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100). Fractions eluted with approximately n-hexane/EtOAc (40
100). Fractions eluted with approximately n-hexane/EtOAc (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60) was recovered as a pure compound 8 (3.2 mg) from this run. Fractions [eluted with n-hexane/EtOAc (70
60) was recovered as a pure compound 8 (3.2 mg) from this run. Fractions [eluted with n-hexane/EtOAc (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30)] was chromatographed on Buchi Reveleris® Prep system equipped with a preparative Inertsil ODS-3 column (5 μm, ∅ 20 × 250 mm, GL Sciences Inc., Japan) using H2O/MeOH (60
30)] was chromatographed on Buchi Reveleris® Prep system equipped with a preparative Inertsil ODS-3 column (5 μm, ∅ 20 × 250 mm, GL Sciences Inc., Japan) using H2O/MeOH (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 → 0
40 → 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) to afford compounds 9 (20 mg), 7 (1 mg), 6 (1 mg) and 5 (0.8 mg) eluted with H2O/MeOH (50
100) to afford compounds 9 (20 mg), 7 (1 mg), 6 (1 mg) and 5 (0.8 mg) eluted with H2O/MeOH (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 → 27
50 → 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 73 → 8
73 → 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 92 → 2
92 → 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 98), respectively. The DCM-soluble fraction was chromatographed on Buchi Reveleris® Prep system equipped with RP-C18 silica set with UV-ELSD detector and using a gradient mobile phase of H2O/MeOH (95
98), respectively. The DCM-soluble fraction was chromatographed on Buchi Reveleris® Prep system equipped with RP-C18 silica set with UV-ELSD detector and using a gradient mobile phase of H2O/MeOH (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 → 0
5 → 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) to give 11 fractions (D1–D11). Fractions D1 and D2 were proved to be pure compounds and assigned as 10 (20 mg) and 11 (10.8 mg). Fraction D5 was chromatographed on Buchi Reveleris® Prep system equipped with RP-C18 silica set with UV-ELSD detector and using a gradient mobile phase of H2O/MeOH (95
100) to give 11 fractions (D1–D11). Fractions D1 and D2 were proved to be pure compounds and assigned as 10 (20 mg) and 11 (10.8 mg). Fraction D5 was chromatographed on Buchi Reveleris® Prep system equipped with RP-C18 silica set with UV-ELSD detector and using a gradient mobile phase of H2O/MeOH (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 → 60
5 → 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) to afford compound 4 (4 mg). The EtOAc-soluble fraction was subjected to a siliga flash column (FlashPure ID HP 20 μm particles, 12 g) and eluted with n-hexane/EtOAc (2
40) to afford compound 4 (4 mg). The EtOAc-soluble fraction was subjected to a siliga flash column (FlashPure ID HP 20 μm particles, 12 g) and eluted with n-hexane/EtOAc (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, EtOAc) and EtOAc–MeOH (9
1, EtOAc) and EtOAc–MeOH (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 4
1 → 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → MeOH) to give five fractions (Fr.1–Fr.5). The Fr.3 (77.3 mg) was separated by semi-preparative HPLC (GL Sciences Inc. ∅ 20 × 250 mm, 5 μm particles; UV detector 205 nm, flow rate: 8 mL min−1) with MeCN/H2O gradient (20
1 → MeOH) to give five fractions (Fr.1–Fr.5). The Fr.3 (77.3 mg) was separated by semi-preparative HPLC (GL Sciences Inc. ∅ 20 × 250 mm, 5 μm particles; UV detector 205 nm, flow rate: 8 mL min−1) with MeCN/H2O gradient (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 → 100
80 → 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) as a mobile phase to yield to 3 (21.3 mg, tR = 30 min). The Fr.4 (70 mg) was separated by semi-preparative HPLC (GL Sciences Inc. ∅ 20 × 250 mm, 5 μm particles; UV detector 205 nm, flow rate: 8 mL min−1) with MeCN/H2O gradient (5
0) as a mobile phase to yield to 3 (21.3 mg, tR = 30 min). The Fr.4 (70 mg) was separated by semi-preparative HPLC (GL Sciences Inc. ∅ 20 × 250 mm, 5 μm particles; UV detector 205 nm, flow rate: 8 mL min−1) with MeCN/H2O gradient (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 → 40
95 → 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60) to yield to 1 (5.1 mg, tR = 38 min) and 2 (2.2 mg, tR = 41.6 min).
60) to yield to 1 (5.1 mg, tR = 38 min) and 2 (2.2 mg, tR = 41.6 min).
4.4. Cell culture
Human neuroblastoma SH-SY5Y (ATCC, Manassas, VA, USA) were cultured in Dulbecco's Modified Eagle's medium (high glucose) (Wako, Osaka, Japan). The medium was supplemented with 10% heat-inactivated fetal bovine serum (FBS, Corning, CA, USA) and antibiotics 100 U mL−1 penicillin–streptomycin (Wako, Osaka, Japan). The cells were grown at 37 °C in 5% CO2 humidified incubator. To estimate the expression of AANAT gene by real-time qPCR, SH-SY5Y cells (1.0 × 105 cells per mL) were cultured for 24 hours and then treated with TA or compounds for 24 hours.4.5. RNA extraction and real-time qPCR
Total RNA was prepared from cells using PureLink RNA Mini kit (Invtrogen, MA, USA) following the manufacturer's instructions. cDNA was synthesized from the extracted total RNA by ReverTra Ace qPCR RT Master Mix with gDNA Remover (TOYOBO, Osaka, Japan). Real-Time quantitative PCR was performed by Agilent AriaMX real-time PCR system (Agilent Technologies, CA, USA). Using the THUNDERBIRD SYBR qPCRMix (TOYOBO, Osaka, Japan) for the real-time qPCR reaction. The real-time qPCR reaction conditions were initial denaturation at 95 °C for 60 s cDNA samples were amplified for 40 cycles (95 °C for 15 s and 60 °C for 60 s). Primers used for amplification were TGCCAGTGAGTTTCGCTGCCTC and GTCAGGAAGTGCCGGATCTCAT for AANAT, or GTCTCCTCTGACTTCAACAGCG and ACCACCCTGTTGCTGTAGCCAA for GAPDH. The expression level of the AANAT gene was analyzed using ΔΔCt analysis mode with AriaMx Real-Time PCR software.Abbreviations
| AANAT | Arylalkymine N-acetyltransferase |
| TLC | Thin layer chromatography |
| HPLC | High-performance liquid chromatography |
| NMR | Nuclear magnetic resonance |
| HRMS | High resolution mass spectrometry |
| PCR | Polymerase chain reaction |
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Isolation & Structure elucidation of quassinoids: C. Han, L. Meng. Isolation of other known secondary metabolites: Y. Amen and M. Elsbaey. Writing original draft: C. Han. Evaluation of Activity: M. Nagata and M. Matsumoto. Funding acquisition: Y. Kuroki. Project administration: Kuniyoshi Shimizu.Conflicts of interest
The authors have declared no conflicts of interest.Acknowledgements
The authors would like to thank PHYTES BIOTEK SDN BHD (Malaysia) for providing the powder of the roots of Eurycoma longifolia.References
- Eurycoma longifolia Jack, Plants of the World Online, Royal Botanic Gardens, Kew, retrieved 2019-01-27, https://en.wikipedia.org/wiki/Eurycoma_longifolia Search PubMed.
- R. Bhat and A. A. Karim, Fitoterapia, 2010, 81, 669–679 CrossRef.
- R. C. Harvey and M. A. Bruce, Sleep Disorders and Sleep Deprivation, National Academies Press (US), 2006, https://www.ncbi.nlm.nih.gov/books/NBK19960/ Search PubMed.
- H. Toyama, Y. Kuroki, A. Nishide, K. Shimizu and K. Ohnuki, Jpn. Pharmacol. Ther., 2022, 50(5), 871–876 Search PubMed.
- W.-Q. Yang, W. Tang, X.-J. Huang, J.-G. Song, Y.-Y. Li, Y. Xiong, C.-L. Fan, Z.-L. Wu, Y. Wang and W.-C. Ye, Molecules, 2021, 26, 5939–5950 CrossRef CAS PubMed.
- H. Morita, E. Kishi, K. Takeya, H. Itokawa and O. Tanaka, Chem. Lett., 1990, 749–752 CrossRef CAS.
- W. Monthong, S. Pitchuanchom, N. Nuntasaen and W. Pompimo, Am. J. Appl. Sci., 2011, 8(12), 1268–1271 CrossRef CAS.
- M. Brenes, F. J. Hidalgo, A. Garcia, J. J. Rios, P. Garcia, R. Zamora and A. Garrido, J. Am. Oil Chem. Soc., 2000, 77(7), 715–720 CrossRef CAS.
- G. L. Norman and B. D. Laurence, Plant Polyphenols, ed. R. W. Hemingway and E. E. L. Aks, Plenum Press, New York, 1992 Search PubMed.
- T. Deyama, Chem. Pharm. Bull., 1983, 31(9), 2993–2997 CrossRef CAS.
- S. D. Jolad, J. J. Hoffmann and J. R. Cole, J. Org. Chem., 1980, 45(15), 3132–3135 CrossRef CAS.
- A. M. C. Arriaga, A. C. D. Mesquita, Y. B. M. Pouliquen, R. A. D. Lima, S. H. Cavalcante, M. G. D. Carvalho, J. A. D. Siqueira, L. V. Alegrio and R. Braz-Filho, An. Acad. Bras. Cienc., 2002, 74(3), 415–424 CrossRef CAS PubMed.
- G. J. W. Breen, E. Ritchie, W. T. L. Sidwell and W. C. Taylor, Aust. J. Chem., 1966, 19(3), 455–481 CrossRef CAS.
- H. Itokawa, E. Kishi, H. Morita, K. Takeya and Y. Itaka, Tetrahedron Lett., 1991, 32, 1803–1804 CrossRef CAS.
- H. Itokawa, E. Kishi, H. Morita and K. Takeya, Chem. Pharm. Bull., 1992, 40, 1053–1055 CrossRef CAS.
- L. A. Mitscher, H. D. Showalter, M. T. Shipchandler, R. P. Leu and J. L. Beal, Lloydia, 1972, 35, 177–180 CAS.
- P. Liu, H. Li, R. Luan, G. Huang, Y. Liu, M. Wang, Q. Chao, L. Wang, D. Li and H. Fan, J. Nat. Med., 2019, 73, 124–130 CrossRef CAS PubMed.
- N. Fukamyia, M. Okano, T. Aratani, K. Negoro, Y. M. Lin and K. H. Lee, Planta Med., 1987, 53, 140–143 CrossRef.
- H. Mori, E. Kishi, K. Takeya, H. Itokawa and O. Tanaka, Chem. Lett., 1990, 5, 749 Search PubMed.
- A. M. Giesbrecht, H. E. Gottlieb, O. RGottlieb, M. O. F. Goulart, R. A. DeLama and A. E. G. Santana, Phytochemistry, 1980, 19, 313 CrossRef CAS.
- L. B. S. Kardono, C. K. Angerhofer, S. Tsauri, K. Padmawinata, J. M. Pezzuto and A. D. Kinghorn, J. Nat. Prod., 1991, 54(5), 1360–1367 CrossRef CAS PubMed.
- M. Elsbaey and B. Abdel, Int. J. Pharmacogn, Phytochem. Res., 2017, 9, 1288–1292 Search PubMed.
- Y. Beppu, H. Komura, T. Izumo, Y. Horii, J. Shen, M. Tanida, T. Nakashima, N. Tsuruoka and K. Nagai, J. Agric. Food Chem., 2012, 60, 11044–11049 CrossRef CAS.
- Y.-X. Li, Y. Li, Z.-J. Qian, M.-M. Kim and S.-K. Kim, J. Microbiol. Biotechnol., 2009, 19(11), 1319–1327 CAS.
- C. Zhao, B. Zhang, J. Fan and J. Shao, J. Yangzhou Univ., 2010, 4, 39–41 Search PubMed.
- A. Miranda-Riestra, R. Estrada-Reyes, E. D. Torres-Sanchez, S. Carreño-García, G. G. Ortiz and G. Benítez-King, Molecules, 2022, 27(22), 7742 CrossRef CAS.
- Z. K. Duan, Z. J. Zhang, S. H. Dong, Y. X. Wang, S. J. Song and X. X. Huang, Phytochemistry, 2021, 187, 112769 CrossRef CAS.
- W. Y. Zhao, X. Y. Song, L. Zhao, C. X. Zou, W. Y. Zhou, B. Lin, G. D. Yao, X. X. Huang and S. J. Song, J. Nat. Prod., 2019, 82, 714–723 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra01703c |
| This journal is © The Royal Society of Chemistry 2025 |




