Open Access Article
Open Access ArticleMIL-101-SO3H: functionalized MOF for enhanced barium ion adsorption and environmental remediation
Abouzar Tahkora,
Seyed Dariush Taherzade *bc,
Niloufar Akbarzadeha,
Alireza Rezvania and
Janet Soleimannejad*b
*bc,
Niloufar Akbarzadeha,
Alireza Rezvania and
Janet Soleimannejad*b
aDepartment of Chemistry, University of Sistan and Baluchestan, P. O. Box 98135-674, Zahedan, Iran
bSchool of Chemistry, College of Science, University of Tehran, P. O. Box 14155-6455, Tehran, Iran. E-mail: jsoleimannejad@ut.ac.ir
cCollege of Materials Science and Engineering, Hunan University, 2 Lushan S Rd, Changsha, 410082, PR China. E-mail: d.taherzade@hnu.edu.cn
First published on 28th April 2025
Abstract
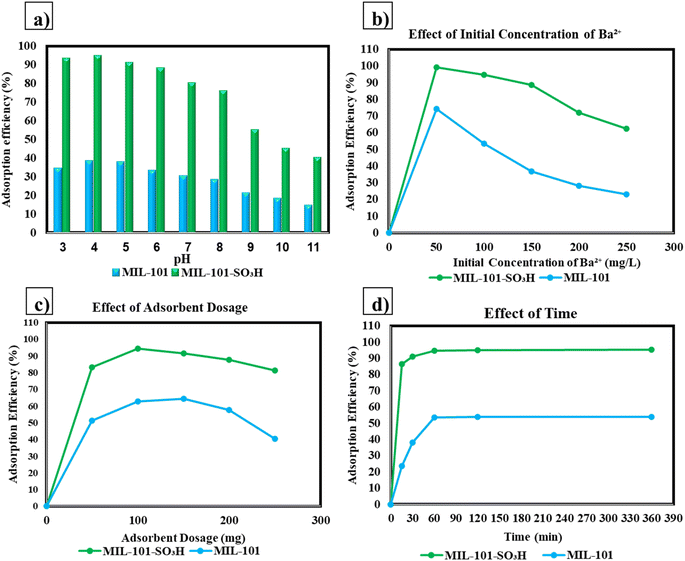
Cationic pollution from barium ions in wastewater poses environmental and health risks including cardiovascular effects and disrupted aquatic ecosystems. This study investigates the use of Metal–Organic Frameworks (MOFs), including both MIL-101(Cr) and its sulfonated derivative MIL-101-SO3H, for efficient removal of barium ions in contaminated water sources. Sulfonation of MIL-101 at the metal center was shown to considerably increase its adsorption capacity compared to unmodified MIL-101 for barium ions, achieving 141.9 mg g−1 for Ba2+ as compared to 54 mg g−1 for MIL-101. The study deeply looked into the effects of the pH, adsorbent dosage, initial Ba2+ concentration, and contact time on adsorption efficiency. Approximately pH = 4 has been determined as the best for Ba2+ adsorption at which the sulfonated MOF gave essentially complete removal efficiencies of 99% of the barium cations. The adsorption correlated with the Langmuir model indicates a homogeneous monolayer adsorption on the surface. MIL-101-SO3H, given the superior characteristics of its adsorptive ability compared with other materials including MIL-101, is tested with ion coexistence and retention trials. These results indicate that MIL-101-SO3H would be an extremely efficient adsorbent for barium ion removal from wastewater that could prove beneficial for a future large-scale application in environmental remediation.
Introduction
Cationic pollution of wastewater with barium ions is one of the biggest environmental concerns due to their toxicity and possible health hazards.1,2 High levels of this metal result in cardiovascular problems, hypertension, and other harmful effects in humans upon ingestion through contaminated water.3 Besides, barium interferes with aquatic life by breaking the ionic balance in water bodies.3 In light of this, the effective removal of barium from wastewater is a key concern for both environmental and human health requirements.4–6 Metal–Organic Frameworks (MOFs) have the potential to change this landscape owing to their incredible porosity,7 adjustable properties,8 and their various applications. Such properties make MOFs very suitable for a plethora of applications including gas storage,9 separation,10 catalysis11 and also wastewater remediation.12,13 Thus, the employment of MOFs is one important green and very efficient method for elimination of barium from wastewater.14,15 The MOF family of materials called Materials of Institute Lavoisier (MIL) is a family of materials with desirable structural stability, porosity and tunability.16,17 Most of these MOFs are made from metals such as aluminum,18 iron,19 and chromium,20 and linkers such as terephthalic acid21 that create a volumetric structure with high porosity. A number of them, such as MIL-53,17 MIL-88,19 MIL-101 (ref. 20) demonstrate diverse structural features and functionality.Among the abovementioned MILs, MIL-101(Cr) is known for its fascinating layer structure and high industrial value.22,23 MIL-101(Cr) has an unusual large cavity system with two different kinds of mesopores, providing high surface area and large volume of pores which enhance its adsorption tendency.24,25 The material showcases outstanding stability to elevated temperatures as well as to many different chemicals, alongside considerable freedom in the envisaged structural plan which can be further modified through changing the organic linkers or the metal centers.26 This makes MIL-101(Cr) very useful in a variety of applications such as gas storage,27 catalysis28 or separation.16 Moreover, the material with large pores and a high surface area allows for incorporation of a range of guest molecules, opening up its application for environmental cleaning29–31 among others in advanced material science.
This article describes MIL-101(Cr) in detail, including its preparation, properties, and modification, as well as its sulfonated form, MIL-101-SO3H, which is designed to improve the functional properties of this framework for different advanced applications. To the best of our knowledge, in most cases, sulfonation is used to modify the organic linkers within MOFs, which is because sulfonate groups bonded to the linkers improve the hydrophilicity and ion-exchange capacity as well as the catalytic activity.32,33 In this work however, we consider an alternative way using sulfonate functional group covalent attachment to metal centers of MIL-101(Cr). This strategy introduces a new approach of improving the functionalities of MOFs by looking at the metal nodes. This can potentially lead to the development of new materials with specific interactions with ions that are required in their applications. Not only the previous reports, but also the current study indicates that the node-functionalization influences the adsorption and desorption efficiencies of the material, especially speaking about bivalent cations, like barium cations.34,35 These studies pave the way for a variety of new applications of MIL-101-SO3H, including environmental and catalytic applications where selective capture and release of ions is likely to be critical. After gaining the knowledge of the design, development, and alteration of these structures, this paper opens the door to the exploration of the applicability of functionalized MOFs across several industries, the environment, and for catalytic purposes.
Experimental section
Materials and instruments
The reactants and solvents were acquired from commercial sources and utilized without additional purification. Fourier transforms infrared (FT-IR) spectra were obtained using a Bruker Tensor II spectrometer, which was coupled with a single reflection ATR system. The catalysts were examined via field emission scanning electron microscopy (FE-SEM) on a KYKY EM3900M instrument with a SE detector. X-ray diffraction (XRD) patterns were obtained using a Bruker Advance D8 diffractometer, operated at 40 kV and 30 mA utilizing Cu-Kα radiation (λ = 1.5406 Å) within the 2θ range of 4–80°. The surface area and pore volume of the samples were determined by nitrogen adsorption/desorption measurements using a Belsorp-mini gas analyzer from MicrotracBel Corp. company at liquid nitrogen temperature. Prior to the adsorption experiments, the samples were degassed under vacuum at 150 °C for 12 h. Thermogravimetric analysis (TGA) was performed utilizing a TGA/DTA (STA 504) instrument from Bahr Company, where the samples were heated from 50 to 700 °C in air at a constant rate of 20 °C min−1. For X-ray Photoelectron Spectroscopy (XPS) analysis, a SPECS UHV analysis system (Germany) was used to acquire the spectra of the samples.Synthesis of MIL-101(Cr)
Synthesis of the MIL-101(Cr) was carried out as previously described in the literature,36 with some modifications. In this case, 3 mmol (0.5 g) of terephthalic acid ligand were mixed with 3 mmol (1.2 g) of chromium(III) nitrate nonahydrate in a 25 mL autoclave. Then 0.6 mL of 5 M hydrofluoric acid was added to the mixture as a catalyst. This solution was topped up with 15 mL of distilled water, put into the autoclave, and maintained at a temperature of 220 °C for 8 hours. Temperature program was implemented to allow the increase in the room temperature to 220 °C over 4 hours. After 8 hours, the autoclave was allowed to cool to ambient temperature for 24 hours, then it was removed from the oven. The resulting green precipitate of MIL-101(Cr) was collected via centrifugation and subsequently refluxed in DMF for 3 hours at 60 °C, followed by reflux in ethanol for 2.5 hours at 70 °C. The final precipitate was then dried in an oven at 70 °C following the filtration process.Synthesis of MIL-101(Cr)-SO3H
The MIL-101(Cr)-SO3H was prepared by using the procedure described in earlier reports.37 The synthesis started with 2 mmol cysteamine hydrochloride and 2 mmol NaOH dissolved in 20 mL of hot absolute ethanol, followed by stirring of the solution to displace hydrochloride. The forming NaCl was separated by centrifugation, and the supernatant was combined with 1 g of dehydrated MIL-101(Cr) in 20 mL ethanol in a round bottom flask, which was then refluxed for 16 hours. The mixture was subsequently filtered and washed twice with deionized water and ethanol, followed by drying at 80 °C for twelve hours. Then, the thiol groups of MIL-101(Cr)-SO3H were oxidized with hydrogen peroxide and sulfonic acid groups were introduced. In a typical experiment, 0.5 g of MIL-101(Cr)-SO3H was dispersed in a water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol (1
ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture with stirring, and treated with 10 mL of H2O2 (1% weight) solution for 24 hours at room temperature. The product was collected by filtration, washed with ethanol and deionized water, and then stirred in 0.1 M H2SO4 for four hours. Finally, MIL-101(Cr)-SO3H was thoroughly rinsed with deionized water and ethanol and finally placed in a vacuum at 120 °C for drying.
1) mixture with stirring, and treated with 10 mL of H2O2 (1% weight) solution for 24 hours at room temperature. The product was collected by filtration, washed with ethanol and deionized water, and then stirred in 0.1 M H2SO4 for four hours. Finally, MIL-101(Cr)-SO3H was thoroughly rinsed with deionized water and ethanol and finally placed in a vacuum at 120 °C for drying.
Adsorption experiment
In this study, batch experiments were conducted by adding 10 mL of aqueous solution containing specific concentrations of barium to a specific amount of MOFs. The impact of varying initial barium concentrations from 50 ppm (mg L−1) to 200 ppm on adsorption capacity and removal efficiency was evaluated. Equilibrium studies were performed at different time intervals, with a maximum time of 360 min to determine the equilibrium contact time and capacity. To determine the optimal amount of MOF for maximum removal of barium from water, different amounts ranging from 10 and 50 mg were tested. The dependency of barium removal efficiency on pH was studied by conducting experiments at different pH values ranging from 3 to 11. To achieve the desired pH, acid or base was added drop by drop after measuring the initial pH of the solution. All experiments were conducted in triplicate, and the average values are reported.It should be noted that in this study, nonradioactive Ba2+ was utilized instead of radioactive 133Ba2+ in all experiments. To perform the adsorption experiments, 10 mg of the adsorbent was added to a 10 mL Ba2+ solution and mixed using a constant temperature shaker. The resulting solution was then filtered to obtain a clear filtrate which was analyzed for its concentration using an ICP-OES spectrometer. The amount of Ba2+ adsorbed was determined using the following equation:
 | (1) |
Adsorption kinetics study
The kinetics of Ba2+ adsorption were further assessed. The experimental data were analyzed according to the pseudo-first-order and pseudo-second-order models. The linear forms of these equations are given by eqn (2) (pseudo-first-order) and eqn (3) (pseudo-second-order).| ln(Qe − Qt) = ln Qe − k1t | (2) |
 | (3) |
The pseudo-first-order and pseudo-second-order models yielded the adsorption capacity Qt of Ba2+ at time t, whereby k1 is the first-order rate constant and k2 is the second-order rate constant.
Adsorption mechanism study
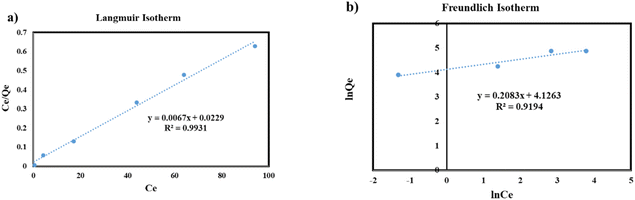
The Freundlich and Langmuir isotherm models were employed to determine the maximum adsorption capacity and sorption energy of barium on adsorbents. The resulting linear mathematical expressions for these models are given below.
 | (4) |
 | (5) |
Eqn (4) and (5) were utilized to describe the barium adsorption capacity on the adsorbent. Eqn (4) corresponds to the Freundlich isotherm model and gives the relationship between Ce, Qe, and the Freundlich empirical constants, KF and n. KF signifies the sorption capacity of the adsorbent while n represents the intensity of sorption. The Langmuir isotherm model was expressed by eqn (5) which relates the maximum adsorption capacity, Xm (mg g−1), and the Langmuir equilibrium constant, K, to Ce.
Selectivity test
To assess how much MIL-101-SO3H can selectively adsorb Ba2+ ions in a multi cation environment, competitive adsorption studies were performed. These studies focused on how these competing co ions, Cr2+, Ni2+, Co2+, and Zn2+, would affect the adsorption of Ba2+. The interfering ions were all obtained by the dissolution of their respective nitrate salts in deionized water at 10 mg L−1 concentrations. Ba2+ was prepared separately at 10 mg L−1 concentration. All solutions were adjusted to the desired pH range of 6 to 7 by dilute HCl or NaOH. For each competitive adsorption study, 10 mg of MIL-101-SO3H was dispersed in 10 mL Ba2+ solution with those of the coexisting metal ions to agitate at 200 rpm for 360 minutes at room temperature (25 ± 2 °C) in constant temperature shaker. Then, the suspensions were filtered through a 0.45 micrometer membrane filter to obtain the clear filtrates. The amounts of Ba2+ and the interfering metal ions present in the filtrate were quantified using ICP-OES. The effectiveness of MIL-101-SO3H in removing Ba2+ in the presence of competing ions was then determined and compared. The selectivity coefficient was determined to assess the affinity of MIL-101-SO3H toward Ba2+ relative to the interfering cations. The data obtained were analyzed for the contribution of sulfonic acid (–SO3H) functional groups toward the selectivity of adsorption of Ba2+.Results and discussion
Characterization
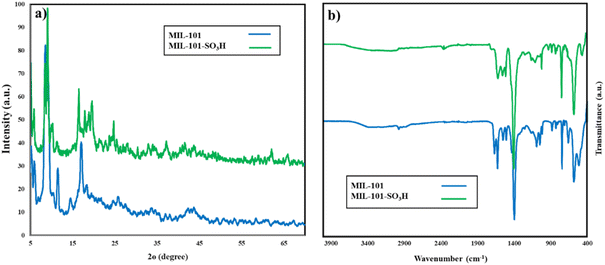
The XRD pattern of MIL-101-SO3H shows roughly the same diffraction pattern compared to pristine MIL-101, inferring that the sulfonation process does not remarkably affect the overall crystalline structure of the material. The XRD pattern of MIL-101(Cr)-SO3H still shows some of the characteristic peaks of MIL-101. However, there might be a slight displacement or broadening of these peaks, which will be indicative that the introduction of sulfonate groups is likely to have a minor effect on interplanar spacing or the lattice parameters. The changes in the X-ray diffraction pattern are minor and are consistent with sulfonate groups that are directly tied to the Cr unsaturated sites in the framework and only slightly change local structure without disrupting the overall crystallographic framework.38,39
The FT-IR spectrum of MIL-101-SO3H shows distinct changes, which already confirm that the MIL-101 framework underwent sulfonation. Prominent changes were noted within the wavenumber range of 1000–1200 cm−1, wherein several strong peaks emerged around 1170 cm−1. These peaks are used to identify the asymmetric and symmetric stretching vibrations of the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds found in the sulfonic acid group (SO3H). The presence of these peaks confirms the successful incorporation of sulfonate groups onto the MIL-101 structure. Additionally, the peak around 1120 cm−1 attributed to the S–O stretching vibrations further supports the presence of sulfonate groups. These spectral modifications demonstrate the successful functionalization of MIL-101 with SO3H groups, resulting in the formation of MIL-101-SO3H with enhanced acidic properties.
O bonds found in the sulfonic acid group (SO3H). The presence of these peaks confirms the successful incorporation of sulfonate groups onto the MIL-101 structure. Additionally, the peak around 1120 cm−1 attributed to the S–O stretching vibrations further supports the presence of sulfonate groups. These spectral modifications demonstrate the successful functionalization of MIL-101 with SO3H groups, resulting in the formation of MIL-101-SO3H with enhanced acidic properties.
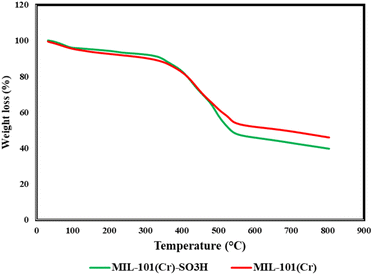
The collected characterization data (XRD patterns, FT-IR spectra, and TGA profiles) for MIL-101(Cr) show excellent agreement with previously reported results, confirming the successful synthesis of the parent framework.26,36,41 Similarly, the modified MIL-101-SO3H material demonstrates all expected functional group vibrations in FT-IR, while maintaining the parent framework's thermal stability in TGA.37 These collective results verify both the preservation of the MIL-101 structure during functionalization and the successful incorporation of sulfonic acid groups, consistent with literature reports.
Adsorption of Ba2+
Table 1 compares the adsorption capacities of various materials towards Ba2+ ions, demonstrating the exceptional performance of MIL-101-SO3H. Adsorption capacities vary widely, from the low capacity of activated carbon (3.1 × 10−6 mg g−1) to the impressive 295.52 mg g−1 for Ti3C2Tx MXene. MIL-101-SO3H stands out with a capacity of 141.9 mg g−1, significantly surpassing materials like titanium nanotubes (109.6 mg g−1) and the unmodified MIL-101 (54.0 mg g−1). This enhanced performance is attributed to the functionalized –SO3H groups on MIL-101-SO3H, which introduce additional active sites for barium ion adsorption, resulting in improved efficiency. Notably, MIL-101-SO3H outperforms materials such as expanded perlite (2.486 mg g−1) and zero-valent iron (22.56 mg g−1), highlighting its potential for environmental applications like water treatment. Although certain materials, such as Ti3C2Tx MXene (295.52 mg g−1) and [CH3CH2NH3]3In2S5 (211.73 mg g−1), exhibit higher adsorption capacities, MIL-101-SO3H offers a competitive performance while maintaining structural stability, ease of synthesis, and cost-effectiveness. These factors make MIL-101-SO3H a highly promising candidate for large-scale, cost-effective Ba2+ removal in industrial and environmental applications.
| No. | Material | Adsorption capacity | Ref. |
|---|---|---|---|
| 1 | Activated carbon | 3.1 × 10−6 mg g−1 | 42 |
| 2 | Titanate nanotube | 109.6 mg g−1 | 43 |
| 3 | MOF-808-SO4 | 131.1 mg g−1 | 1 |
| 4 | Expanded perlite | 2.486 mg g−1 | 44 |
| 5 | Zero-valent iron | 22.56 mg g−1 | 45 |
| 6 | Niobate | 13.7 mg g−1 | 46 |
| 7 | Ca-exchanged clinoptilolite | 71.885 mg g−1 | 47 |
| 8 | Titanium silicate | 144 mg g−1 | 48 |
| 9 | [CH3CH2NH3]6In8S15 | 211.73 mg g−1 | 49 |
| 10 | TiY2O5@g-C3N4 | 295.52 mg g−1 | 50 |
| 11 | K3.4(CH3NH3)0.45(NH4)0.15Zn2Sn3Se10·3.4H2O | 108.94 mg g−1 | 51 |
| 12 | Ti3C2Tx MXene | 180 mg g−1 | 52 |
| 13 | MIL-101(Cr) | 54.0 mg g−1 | This work |
| 14 | MIL-101-SO3H | 141.9 mg g−1 | This work |
Previous studies have reported an adsorption capacity of 70.5 mg g−1 for MIL-101-SO3H when the sulfonated functional group was located on the ligand.1 In contrast, our results show a significantly higher adsorption capacity of 141.9 mg g−1 when the sulfonation is introduced at the metal center of MIL-101. This enhancement in adsorption capacity can be attributed to several factors. First, the sulfonation of the metal center increases the number of accessible active sites for barium ion binding, improving the overall adsorption efficiency. The sulfonic acid groups at the metal centers likely interact more strongly with Ba2+ ions due to electrostatic and coordination interactions, leading to higher binding strength compared to the sulfonation at the ligand. Additionally, the proximity of the functional group to the metal center enhances the structural integrity and stability of the framework, preventing leaching of the functional groups during adsorption. These combined effects contribute to the superior adsorption performance of MIL-101-SO3H when the sulfonation is positioned on the metal center.
| Desorption efficiency of Ba2+ after 2 hours | ||
|---|---|---|
| Initial concentration | MIL-101 | MIL-101-SO3H |
| 50 ppm | 28.7% | 75.4% |
| 100 ppm | 22.7% | 81.2% |
| 150 ppm | 14.7% | 89.6% |
The high desorption efficiency of Ba2+ in water (75.4–89.6%, Table 2) arises from: competitive ion exchange and concentration-driven diffusion.
(1) Competitive ion exchange: water's weak acidity (pH ∼7) provides H+ ions that displace Ba2+ from sulfonate sites (–SO3−) via reversible ion exchange (Fig. 10). This is consistent with studies on sulfonated MOFs, where H+/Ba2+ competition governs regeneration efficiency.1
(2) Concentration-driven diffusion: at low Ba2+ concentrations (50 ppm), the steep chemical potential gradient between the MOF and water maximizes desorption. At higher concentrations (150 ppm), site saturation weakens Ba2+ binding, enhancing release despite a reduced gradient.14,53
While acidic eluents (e.g., 0.1 M HCl) could fully regenerate –SO3H groups,33 water offers a greener alternative that preserves MIL-101-SO3H's crystallinity (XRD, Fig. 9a) and porosity (BET, Fig. 11), critical for reuse.54
Furthermore, as illustrated in Fig. 9b, the FT-IR spectra show significant insights into the Ba2+ adsorption effect on both MIL-101 and MIL-101-SO3H. For MIL-101-SO3H, the presence of sulfonic acid (–SO3H) functional groups is confirmed by the characteristic peaks near 1040–1150 cm−1, corresponding to S–O and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
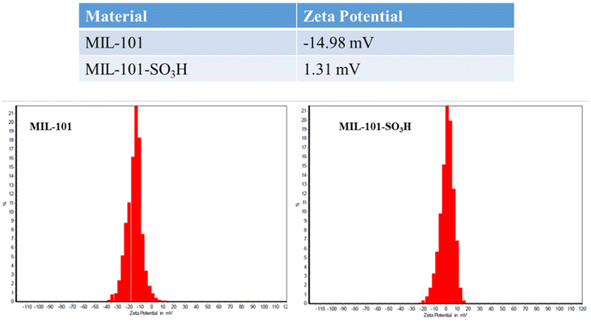
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibrations. After Ba2+ adsorption, these peaks exhibit a slight broadening, suggesting strong interaction between the Ba2+ cations and the –SO3H groups. This interaction likely involves ion exchange or electrostatic attraction. In MIL-101 without –SO3H functionalization, the absence of such pronounced shifts in the FT-IR spectrum indicates weaker or less specific adsorption of Ba2+, aligning with its lower adsorption efficiency. Additionally, any changes in the broader O–H stretching region (∼3200–3600 cm−1) suggest a role of hydroxyl groups in binding Ba2+ ions. Overall, the sulfonic acid functionalization in MIL-101-SO3H enhances Ba2+ interaction, as evident from the spectral changes.
O vibrations. After Ba2+ adsorption, these peaks exhibit a slight broadening, suggesting strong interaction between the Ba2+ cations and the –SO3H groups. This interaction likely involves ion exchange or electrostatic attraction. In MIL-101 without –SO3H functionalization, the absence of such pronounced shifts in the FT-IR spectrum indicates weaker or less specific adsorption of Ba2+, aligning with its lower adsorption efficiency. Additionally, any changes in the broader O–H stretching region (∼3200–3600 cm−1) suggest a role of hydroxyl groups in binding Ba2+ ions. Overall, the sulfonic acid functionalization in MIL-101-SO3H enhances Ba2+ interaction, as evident from the spectral changes.
 | ||
| Fig. 12 XPS spectra of pristine MIL-101 (a), MIL-101 after Ba2+ adsorption (b), pristine MIL-101-SO3H (c), and MIL-101-SO3H after Ba2+ adsorption (d). | ||
The XPS spectrum of MIL-101 after adsorption of Ba2+ shows some noticeable changes compared to the pristine MIL-101 sample. The overall intensity of the peaks has increased, particularly the primary peaks around 400 eV, 600 eV, and 1200 eV, which suggests an alteration in the electronic environment of the material. After Ba2+ adsorption, new peaks corresponding to Ba 3d may appear around the 780–800 eV region, which indicates successful adsorption of Ba2+ ions onto the MIL-101 structure. This shift reflects the binding interaction between Ba2+ ions and the functional groups present in MIL-101. Moreover, peaks related to the oxygen species (often around 531 eV for O 1s) and carbonyl groups (around 285 eV for C 1s) may exhibit shifts or intensity changes. This is due to the interaction of Ba2+ with oxygen-containing groups, which enhances binding affinity. The increase in electron counts across various peaks suggests that Ba2+ adsorption may have introduced additional electronic density, consistent with the coordination of Ba2+ with the oxygen or sulfonate.
The XPS analysis of pristine MIL-101-SO3H also shows a peak at C 1s region (approx. ∼285 eV). Deconvolution of this peak could show components related to aromatic carbon, oxygen-bonded carbon, and possibly sulfur-containing groups. The peak at O 1s region (∼532 eV) represents oxygen contributions from hydroxyl groups, sulfonic acid groups (–SO3H), and the carboxyl groups in the framework structure. S 2p region (∼168–170 eV) confirms the presence of sulfonic acid groups (–SO3H) in the modified MIL-101. The binding energy aligns with the sulfur atom in a highly oxidized state (as in SO3).
The XPS spectrum of MIL-101-SO3H after Ba2+ adsorption shows several significant features when compared to its pristine counterpart. The characteristic binding energy for the sulfur atoms in the –SO3H groups may experience a slight shift or intensity variation. This indicates interactions between the –SO3H groups and the adsorbed Ba2+ ions, likely through ion-exchange mechanisms or electrostatic attraction. A clear indication of Ba2+ adsorption is the appearance of new peaks in the Ba 3d region (around 780–795 eV), representing the presence of barium on the material's surface. The oxygen (O 1s) and sulfur (S 2p) peaks may shift slightly due to changes in the electronic environment after interaction with Ba2+. This highlights the role of –SO3H functional groups in binding barium ions. Moreover, peaks corresponding to framework metals (e.g., Cr in MIL-101) might exhibit minor changes in intensity or broadening. This could result from structural rearrangements or surface modifications following Ba2+ adsorption.
Conclusion
Current study demonstrates that sulfonic acid functionalization at the metal centers of MIL-101(Cr) significantly enhances its barium ion adsorption capacity (141.9 mg g−1) compared to the unmodified framework (54 mg g−1). The optimized MIL-101-SO3H exhibits excellent removal efficiency (99% at pH = 4), with adsorption following the Langmuir isotherm model. Characterization studies confirm that the sulfonation strategy preserves the MOF's structural integrity while introducing effective binding sites for Ba2+. Looking forward, this work suggests three key directions for further development: investigation of long-term stability and reusability under various environmental conditions, molecular-level studies of binding mechanisms through computational modeling, and scale-up trials to evaluate practical wastewater treatment applications. These future studies would build upon the fundamental insights gained here while addressing important considerations for real-world implementation. The metal-center functionalization approach presented here offers a promising strategy for designing MOF-based adsorbents tailored for heavy metal removal, with potential extensions to other environmentally relevant contaminants.Data availability
Relevant data supporting the key findings of this study are available within the article.Conflicts of interest
The authors declare no competing interests.Acknowledgements
The authors would like to thank University of Tehran and University of Sistan and Baluchestan for supporting this research.References
- Y. Peng, H. Huang, D. Liu and C. Zhong, ACS Appl. Mater. Interfaces, 2016, 8, 8527–8535 CrossRef CAS PubMed.
- H. A. Aziz, M. F. Ghazali, Y.-T. Hung and L. K. Wang, in Handbook of Advanced Industrial and Hazardous Wastes Management, CRC Press, 2017, pp. 463–482 Search PubMed.
- J. Kravchenko, T. H. Darrah, R. K. Miller, H. K. Lyerly and A. Vengosh, Environ. Geochem. Health, 2014, 36, 797–814 CrossRef CAS PubMed.
- A. K. Fard, G. McKay, R. Chamoun, T. Rhadfi, H. Preud'Homme and M. A. Atieh, Chem. Eng. J., 2017, 317, 331–342 CrossRef CAS.
- Z. Majidnia, A. Idris, M. Majid, R. Zin and M. Ponraj, Appl. Radiat. Isot., 2015, 105, 105–113 CrossRef CAS PubMed.
- M. R. Abukhadra, S. M. Ali, A. M. El-Sherbeeny, A. T. A. Soliman and A. E. E. Abd Elgawad, Inorg. Chem. Commun., 2020, 119, 108053 CrossRef CAS.
- X. Zhang, Z. Chen, X. Liu, S. L. Hanna, X. Wang, R. Taheri-Ledari, A. Maleki, P. Li and O. K. Farha, Chem. Soc. Rev., 2020, 49, 7406–7427 RSC.
- J.-P. Zhang, H.-L. Zhou, D.-D. Zhou, P.-Q. Liao and X.-M. Chen, Natl. Sci. Rev., 2018, 5, 907–919 CrossRef CAS.
- S. Ma and H.-C. Zhou, Chem. Commun., 2010, 46, 44–53 RSC.
- X. Zhao, Y. Wang, D. S. Li, X. Bu and P. Feng, Adv. Mater., 2018, 30, 1705189 CrossRef PubMed.
- M. Ranocchiari and J. A. van Bokhoven, Phys. Chem. Chem. Phys., 2011, 13, 6388–6396 RSC.
- P. Kumar, V. Bansal, K.-H. Kim and E. E. Kwon, J. Ind. Eng. Chem., 2018, 62, 130–145 CrossRef CAS.
- S. D. Taherzade, M. Abbasichaleshtori and J. Soleimannejad, RSC Adv., 2022, 12, 9023–9035 RSC.
- X. Zhao, L. Pei, Y.-N. Zhang, H. Huang, X. Zheng, B. Liu and M. Tong, Green Chem. Eng., 2022, 3, 405–412 CrossRef.
- P. Liu, P. Yang, J. Yang and J. Gu, Chem. Commun., 2021, 57, 5822–5825 RSC.
- H. Belarbi, L. Boudjema, C. Shepherd, N. Ramsahye, G. Toquer, J.-S. Chang and P. Trens, Colloids Surf., A, 2017, 520, 46–52 CrossRef CAS.
- H. Zhang, X. Hu, T. Li, Y. Zhang, H. Xu, Y. Sun, X. Gu, C. Gu, J. Luo and B. Gao, J. Hazard. Mater., 2022, 429, 128271 CrossRef CAS PubMed.
- T. Loiseau, C. Serre, C. Huguenard, G. Fink, F. Taulelle, M. Henry, T. Bataille and G. Férey, Chem. - Eur. J., 2004, 10, 1373–1382 CrossRef CAS PubMed.
- M. Ma, H. Noei, B. Mienert, J. Niesel, E. Bill, M. Muhler, R. A. Fischer, Y. Wang, U. Schatzschneider and N. Metzler-Nolte, Chem. - Eur. J., 2013, 19, 6785–6790 CrossRef CAS PubMed.
- S. Bhattacharjee, C. Chen and W.-S. Ahn, RSC Adv., 2014, 4, 52500–52525 RSC.
- Z. Zhang, S. Huang, S. Xian, H. Xi and Z. Li, Energy Fuels, 2011, 25, 835–842 CrossRef CAS.
- M. Y. Zorainy, M. G. Alalm, S. Kaliaguine and D. C. Boffito, J. Mater. Chem. A, 2021, 9, 22159–22217 RSC.
- Z. Li, X. Liu, W. Jin, Q. Hu and Y. Zhao, J. Colloid Interface Sci., 2019, 554, 692–704 CrossRef CAS PubMed.
- M. Zou, M. Dong and T. Zhao, Int. J. Mol. Sci., 2022, 23, 9396 CrossRef CAS PubMed.
- M. Shafiei, M. S. Alivand, A. Rashidi, A. Samimi and D. Mohebbi-Kalhori, Chem. Eng. J., 2018, 341, 164–174 CrossRef CAS.
- L. Bromberg, Y. Diao, H. Wu, S. A. Speakman and T. A. Hatton, Chem. Mater., 2012, 24, 1664–1675 CrossRef CAS.
- S. Kayal, B. Sun and A. Chakraborty, Energy, 2015, 91, 772–781 CrossRef CAS.
- J. Chen, X. Chen, Z. Zhang, Z. Bao, H. Xing, Q. Yang and Q. Ren, Mol. Catal., 2018, 445, 163–169 CrossRef CAS.
- M.-L. Chen, S.-Y. Zhou, Z. Xu, L. Ding and Y.-H. Cheng, Molecules, 2019, 24, 3718 CrossRef CAS PubMed.
- A. E. Khudozhitkov, S. S. Arzumanov, D. I. Kolokolov, O. A. Kholdeeva, D. Freude and A. G. Stepanov, Chem. - Eur. J., 2019, 25, 5163–5168 CrossRef CAS PubMed.
- B. Tan, Y. Luo, X. Liang, S. Wang, X. Gao, Z. Zhang and Y. Fang, Ind. Eng. Chem. Res., 2019, 58, 2983–2990 CrossRef CAS.
- Y. Jin, J. Shi, F. Zhang, Y. Zhong and W. Zhu, J. Mol. Catal. A: Chem., 2014, 383, 167–171 CrossRef.
- Z. Hasan, J. W. Jun and S. H. Jhung, Chem. Eng. J., 2015, 278, 265–271 CrossRef CAS.
- J. Hou, F. AlGhunaimi, T. P. Huang and N. Aljuryyed, 2024.
- Y. Peng, H. Huang, D. Liu and C. Zhong, ACS Appl. Mater. Interfaces, 2016, 8, 8527–8535 CrossRef CAS PubMed.
- G. Férey, C. Mellot-Draznieks, C. Serre, F. Millange, J. Dutour, S. Surblé and I. Margiolaki, Science, 2005, 309, 2040–2042 CrossRef PubMed.
- S. S. Mortazavi, A. Abbasi, M. Masteri-Farahani and F. Farzaneh, ChemistrySelect, 2019, 4, 7495–7501 CrossRef CAS.
- Z. Li, S. Zhao, H. Wang, Y. Peng, Z. Tan and B. Tang, Colloids Surf., B, 2019, 178, 1–7 CrossRef CAS PubMed.
- H. Deng, C. J. Doonan, H. Furukawa, R. B. Ferreira, J. Towne, C. B. Knobler, B. Wang and O. M. Yaghi, science, 2010, 327, 846–850 CrossRef CAS PubMed.
- B. Smith, 2016.
- Y. Pan, B. Yuan, Y. Li and D. He, Chem. Commun., 2010, 46, 2280–2282 RSC.
- H. Gad, Y. Lasheen and T. El-Zakla, Radiochemistry, 2013, 55, 589–595 CrossRef CAS.
- M. Xu, G. Wei, N. Liu, L. Zhou, C. Fu, M. Chubik, A. Gromov and W. Han, Nanoscale, 2014, 6, 722–725 RSC.
- M. Torab-Mostaedi, A. Ghaemi, H. Ghassabzadeh and M. Ghannadi-Maragheh, Can. J. Chem. Eng., 2011, 89, 1247–1254 CrossRef CAS.
- O. Celebi, Ç. Üzüm, T. Shahwan and H. N. Erten, J. Hazard. Mater., 2007, 148, 761–767 CrossRef CAS PubMed.
- D. J. Yang, Z. F. Zheng, H. Y. Zhu, H. W. Liu and X. P. Gao, Adv. Mater., 2008, 20, 2777–2781 CrossRef CAS PubMed.
- M. Chávez, L. De Pablo and T. García, J. Hazard. Mater., 2010, 175, 216–223 CrossRef PubMed.
- K. Savka, Y. Kilivnik, I. Mironyuk, H. Vasylyeva, O. Sych, M. Karbovanets and M. Yevych, Chem. Phys. Impact, 2023, 6, 100151 CrossRef.
- Y.-M. Zhao, M. Sun, L. Cheng, K.-Y. Wang, Y. Liu, J.-Y. Zhu, S. Zhang and C. Wang, J. Hazard. Mater., 2022, 425, 128007 CrossRef CAS PubMed.
- A. Modwi, H. Idriss, L. Khezami, A. Albadri, M. Ismail, A. A. Assadi and P. Nguyen-Tri, Diamond Relat. Mater., 2023, 135, 109830 CrossRef CAS.
- Y. Liu, F.-Q. Shi, X. Hao, M.-Y. Li, L. Cheng, C. Wang and K.-Y. Wang, J. Hazard. Mater., 2023, 458, 132038 CrossRef CAS PubMed.
- B.-M. Jun, C. M. Park, J. Heo and Y. Yoon, J. Environ. Manage., 2020, 256, 109940 CrossRef CAS PubMed.
- J. Gao, S. Fei, Y.-L. Ho, R. Matsuda, H. Daiguji and J.-J. Delaunay, J. Phys. Chem. C, 2021, 125, 17786–17795 CrossRef CAS.
- S. Fei, A. Alizadeh, W.-L. Hsu, J.-J. Delaunay and H. Daiguji, J. Phys. Chem. C, 2021, 125, 26755–26769 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |