Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enantioselective synthesis of spiro[indoline-3,1′-pyrazolo[1,2-b]phthalazine] derivatives via an organocatalytic three-component cascade reaction†
Luying Song‡
ab,
Yuhong Sun‡ab,
Renbo Anb,
Liming Wang*a and
Ying Jin *ab
*ab
aDepartment of Pharmacy, Jilin Medical University, Jilin, Jilin 132013, China. E-mail: jinying2288@163.com; 13630635312@163.com
bCollege of Pharmacy, Yanbian University, Yanji, Jilin 133000, China
First published on 21st May 2025
Abstract
An asymmetric synthesis of spiro[indoline-3,1′-pyrazolo[1,2-b]phthalazine] derivatives was first developed through an organocatalysed cascade Knoevenagel/Michael/cyclization reaction using a quinidine-derived squaramide. Under the optimized conditions, the three-component reaction of isatins, malononitrile (cyanoacetate) and phthalhydrazide yields the desired pyrazolophthalazinyl spirooxindoles in good yields (73–90%) with up to >99% enantiomer excess (ee).
Introduction
The pyrazole ring is a key moiety in numerous biologically active compounds and clinical drugs,1 such as Viagra, Celebrex, and Analginum (Fig. 1). Therefore, the synthesis of heterocyclic compounds containing the pyrazole ring is a research hotspot in the fields of organic synthesis and medicinal chemistry.On the other hand, heterocyclic fused phthalazines have been found to possess cytotoxic, antimicrobial, anticonvulsant, antifungal, anticancer, anti-inflammatory, and cardiotonic activities.2 They also exhibited potential as new luminescence materials or fluorescence probes.3
In addition, optically active spirooxindole derivatives have been extensively investigated because of their prominent activities and wide utility as synthetic intermediates for alkaloids and clinical drugs.4 The methods for synthesizing various spirooxindoles, including spiro[N-heterocycle-oxindoles], spiro[cycloalkane-oxindoles] and spiro[O-heterocycle-oxindoles] etc. have been well established.5a Over the past few years, significant progress has been made in the stereoselective construction of spirooxindole.5
Based on the biological activity of pyrazole, phthalazine, and spiroindole skeleton, according to the splicing principle, chemists have considered combining these three parts into one molecule. At present, several methods have been developed for the synthesis of racemic spiro[indoline-3,1′-pyrazolo[1,2-b]phthalazine] derivatives.6 Zhang,6a Shi6b and Kefayati6c groups reported one-pot three-component reaction catalyzed by nickel chloride, pyridine, or tetrabutylammonium fluoride (TBAF) respectively as shown in Scheme 1(a). However, there are no reports on the synthesis of optically pure pyrazolophthalazinyl spirooxindoles.
As well known, the development of synthetic methods to access optically active spiro-heterocycles would be of significance for drug discovery.7 So, we herein first reported the asymmetric Michael/cyclization cascade reaction of malononitrile (cyanoacetate), phthalhydrazide with a variety of the substituted isatins (N1, C4, C5, C6, C7 substituents) to construct spiro-heterocycles by using cinchona-squaramide catalyst (Scheme 1(b)). Bifunctional organocatalysts 1a–1i including cinchona alkaloids and Takemoto's catalysts bearing (thio)urea or squaramide8 have been applied in this cascade reaction as shown in Fig. 2.
Results and discussion
We initially screened the bifunctional catalysts in the Knoevenagel/Michael/cyclization reaction of isatin (2a), malononitrile (3a) and phthalhydrazide with DCM as a solvent in the presence of 10 mol% of catalysts 1a–1i (Table 1).| Entry | Cat. | Solvent | Cat. loading (mol%) | Yieldb (%) | %eec,d |
|---|---|---|---|---|---|
| a Reaction conditions: isatin 2a (0.1 mmol), malononitrile 3a (0.1 mmol), phthalhydrazide (0.1 mmol) and catalysts 1a–1i in solvent (1.0 mL) at rt for 24 h.b Isolated yield.c Determined by HPLC analysis (Chiralpak OJ–H).d The sign in parentheses indicates the sign of the optical rotation. | |||||
| 1 | 1a | DCM | 10 | 75 | 6 (−) |
| 2 | 1b | DCM | 10 | 83 | 92 (+) |
| 3 | 1c | DCM | 10 | 88 | 98 (−) |
| 4 | 1d | DCM | 10 | 82 | 94 (+) |
| 5 | 1e | DCM | 10 | 76 | 79 (+) |
| 6 | 1f | DCM | 10 | 80 | 97 (+) |
| 7 | 1g | DCM | 10 | 78 | 94 (−) |
| 8 | 1h | DCM | 10 | 81 | 89 (−) |
| 9 | 1i | DCM | 10 | 82 | 96 (+) |
| 10 | 1c | CHCl3 | 10 | 83 | 93 |
| 11 | 1c | DCE | 10 | 84 | 95 |
| 12 | 1c | CH3CN | 10 | 70 | 85 |
| 13 | 1c | EA | 10 | 68 | 51 |
| 14 | 1c | EtOH | 10 | 65 | 39 |
| 15 | 1c | Et2O | 10 | — | — |
| 16 | 1c | DCM | 5 | 70 | 79 |
As shown in Table 1, catalysts 1a–i smoothly catalyzed the reaction to provide the desired product in 75–88% yields (entries 1–9). Among them, quinidine-derived squaramide catalyst 1c was screened to be the optimal catalyst in terms of yield and enantioselectivity (98% ee, entry 3). In addition, Takemoto's catalyst 1f with a urea moiety and catalyst 1i bearing a squaramide moiety induced the reaction with excellent enantioselectivities (entries 6 and 9). By comparison, catalysts 1e and 1h with a thiourea moiety showed bad asymmetric induction to give decreased ee values (79% and 89%, entries 5 and 8).
Since stereoselectivity of up to 98% has been achieved under the current conditions, there is not much room for further improvement. However, we would like to investigate the effect of solvents on the stereoselectivity of the reaction, and whether lowering the loading of catalyst can maintain excellent enantioselectivity. The results showed that solvents have a significant impact on the catalytic performance of the catalyst. Among the 8 selected solvents, dichloromethane was screened as the optimal solvent for the yield and enantioselectivity (entry 3), and halogenated alkane as solvents were found to be suitable for this reaction to give 93% and 95% ees respectively (entries 10 and 11), while low enantioselectivities were obtained in ethyl acetate and EtOH (entries 13 and 14). Surprisingly, Et2O was used as solvent, the transformation did not occur even with prolonged the reaction time of 36 h (entry 15). Additionally, reducing the catalyst loading to 5 mol% led to an obvious decrease in enantioselectivity and yield (entry 16 vs. 3). Thus, the optimized conditions were determined to be DCM as the solvent with a 10 mol% loading of catalyst 1c at rt.
With the optimized conditions in hand, we explored the scope and general applicability of the protocol. A wide range of substituted isatins (N1, C4, C5, C6 and C7 substituents) and 2 types of electron-rich nucleophiles, including malononitrile and cyanoacetates, were evaluated as shown in Table 2.
| Entry | 2 (R1, R2) | R3 | Product | Yieldb (%) | %eec |
|---|---|---|---|---|---|
| a Reaction conditions: isatins 2 (0.1 mmol), 3 (0.1 mmol), phthalhydrazide (0.1 mmol) and catalyst 1c (0.01 mmol) in DCM (1.0 mL) at rt for 24 h.b Isolated yield.c Determined by HPLC analysis (Chiralpak IA, AS, AD, Chiralcel OD, OJ). | |||||
| 1 | 2a (H,H) | CN | 4a | 88 | 98 |
| 2 | 2b (H, 4-Cl) | CN | 4b | 80 | 96 |
| 3 | 2c (H, 4-Br) | CN | 4c | 87 | 92 |
| 4 | 2d (H, 5-F) | CN | 4d | 81 | 98 |
| 5 | 2e (H, 5-Cl) | CN | 4e | 82 | 94 |
| 6 | 2f (H, 5-Br) | CN | 4f | 83 | 98 |
| 7 | 2g (H, 5-NO2) | CN | 4g | 90 | 93 |
| 8 | 2h (H, 5-Me) | CN | 4h | 85 | 99 |
| 9 | 2i (H, 5-OMe) | CN | 4i | 79 | 96 |
| 10 | 2j (H, 6-Br) | CN | 4j | 80 | 97 |
| 11 | 2k (H, 7-F) | CN | 4k | 82 | 90 |
| 12 | 2l (H, 7-Cl) | CN | 4l | 78 | 91 |
| 13 | 2m (H, 7-Br) | CN | 4m | 81 | 98 |
| 14 | 2n (H, 7-Me) | CN | 4n | 80 | 95 |
| 15 | 2o (H, 7-NO2) | CN | 4o | 79 | 93 |
| 16 | 2p (Me, H) | CN | 4p | 83 | >99 |
| 17 | 2q (Bn, H) | CN | 4q | 81 | 88 |
| 18 | 2r (Bn, 5-Cl) | CN | 4r | 82 | 92 |
| 19 | 2a (H, H) | COOMe | 4s | 73 | 91 |
| 20 | 2a (H, H) | COOEt | 4t | 78 | 98 |
| 21 | 2f (H, 5-Br) | COOEt | 4u | 80 | 99 |
| 22 | 2m (H, 7-Br) | COOEt | 4v | 81 | 99 |
| 23 | 2p (Me, H) | COOEt | 4w | 82 | 99 |
All of the substrates reacted smoothly to give the corresponding products in 73–90% yield with excellent ees (88–>99%). Compared with N1-benzyl substituted isatin, no substitution or methyl substituted isatin as substrates were found to be better for the enantioselectivity (entries 1 and 16 vs. entry 17). To broaden the scope of substrates, cyanoacetates were applied instead of malononitrile in the reaction, and excellent ee values of 91–99% were obtained (entries 19–23). Notably, ethyl cyanoacetate as substrate was found to be better than methyl cyanoacetate for the enantioselectivity and yield of the reaction (entry 20 vs. entry 19). Therefore, the screened catalytic condition showed excellent general applicability for this three components cascade reaction.
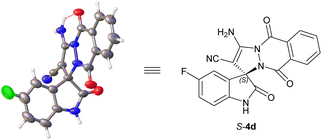
The absolute configuration of spiro[indoline-3,10-pyrazolo[1,2-b]phthalazine] products 4 were unambiguously assigned as S according to the X-ray crystal structure analysis of 4d (Fig. 3).
On the basis of the absolute stereochemistry of 4d, a plausible mechanism for the formation of spirooxindole derivative 4d was proposed according to the literature (Scheme 2).10 Isatin (2a) first condenses with malononitrile to yield the isatylidene malononitrile (A), one cyanide group of which is fixed and activated by the neighboring two squaramide N–H moieties through double H-bonding, while phthalhydrazide is activated by an interaction between the tertiaryamine moiety of 1c and the amino group of phthalhydrazide (B). Then, Si-face addition of the nucleophilic phthalhydrazide to the electron-deficient isatylidene malononitrile results in a Michael adduct intermediate (C), which in turn transfers to intermediate (D) through proton immigration. Next, the intramolecular nucleophilic addition of the amino group to the cyano group produces a cyclic imino carbanion (E), which is further transformed to the final (S)-4d through subsequent proton immigration and tautomerization (Scheme 2).
Conclusions
In summary, we described the first enantioselective Knoevenagel/Michael/cyclization cascade reaction of isatins, malononitrile (or cyanoacetates) and phthalhydrazide to produce spiro[indoline-3,10-pyrazolo[1,2-b]phthalazine] in high enantioselectivity (88–>99% ees). Moreover, we used our optimized conditions to expand upon the substrate scope of this reaction.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
L. Y. Song and Y. H. Sun are co-first authors; they contributed equally to this work. They performed the experiments, acquired the original data and cultivated the single crystal. R. B. An conducted instrumental analysis. L. M. Wang supervised the experiment, analyzed and checked all the data, provided funding supporting. Y. Jin designed the research plan, wrote the draft and revised manuscript. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by Key Project of Medicine and Health of Jilin Province (No. 20250204067YY), the Department of Education of Jilin province (No. JJKH20240595KJ), Postgraduate Innovation Program Project of Jilin Medical University (title: Synthesis of chiral 10-hydroxyphenanthrene compounds and the study of its anti-tumor activity, 2023zyc02) and Students' Program for Innovation and Entrepreneurship Training (No. S202413706026).References
- (a) J. Elguero, P. Goya, N. Jagerovic and A. M. S. Silva, Pyrazoles as drugs: facts and fantasies, Targets Heterocycl. Syst., 2002, 6, 52–98 Search PubMed; (b) S. K. Singh, P. G. Reddy, K. S. Rao, B. B. Lohray, P. Misra, S. A. Rajjak, Y. K. Rao and A. Venkatewarlu, Polar substitutions in the benzenesulfonamide ring of celecoxib afford a potent 1, 5-diarylpyrazole class of COX-2 inhibitors, Bioorg. Med. Chem. Lett., 2004, 14, 499–504 CrossRef CAS PubMed.
- (a) F. Al'-Assar, K. N. Zelenin, E. E. Lesiovskaya, P. Bezhan and B. A. Chakchir, Synthesis and pharmacological activity of 1-hydroxy-, 1-amino-, and 1-hydrazino-substituted 2, 3-dihydro-1H-pyrazolo [1, 2-a] pyridazine-5, 8-diones and 2, 3-dihydro-1H-pyrazolo [1, 2-b] phthalazine-5, 10-diones, Pharm. Chem. J., 2002, 36, 598–603 CrossRef; (b) R. P. Jain and J. C. Vederas, Structural variations in keto-glutamines for improved inhibition against hepatitis A virus 3C proteinase, Bioorg. Med. Chem. Lett., 2004, 14, 3655–3658 CrossRef CAS PubMed; (c) R. W. Carling, K. W. Moore, L. J. Street, D. Wild, C. Isted, P. Leeson, S. Thomas, D. O'Conner, R. M. McKernan, K. Quirk, S. M. Cook, J. R. Atack, K. A. Waftord, S. A. Thompson, G. R. Dawson, P. Ferris and J. L. Castro, 3-Phenyl-6-(2-pyridyl) methyloxy-1, 2, 4-triazolo [3, 4-a] phthalazines and analogues: high-affinity γ-aminobutyric acid-A benzodiazepine receptor ligands with α2, α3, and α5-subtype binding selectivity over α1, J. Med. Chem., 2004, 47, 1807–1822 Search PubMed; (d) S. Grasso, G. DeSarro, N. Micale, M. Zappala, G. Puia, M. Baraldi and C. Demicheli, Synthesis and anticonvulsant activity of novel and potent 6, 7-methylenedioxyphthalazin-1 (2 H)-ones, J. Med. Chem., 2000, 43, 2851–2859 CrossRef CAS PubMed; (e) M. Rodriguez-Ciria, A. M. Sanz, M. J. R. Yunta, F. Gomez-Contreras, P. Navarro, M. Sanchez-Moreno, S. Boutaleb-Charki, A. Osuna, A. Castineiras, M. Pardo, C. Cano and L. Campayo, Bioorg. Med. Chem., 2007, 15, 2081–2091 CrossRef CAS PubMed; (f) H. J. Wang, X. N. Zhang and Z. H. Zhang, Highly efficient three-component synthesis of 1H-indazolo [1, 2-b] phthalazinetrione derivatives catalyzed by heteropolyacids, Monatsh. Chem., 2010, 141, 425–430 CrossRef CAS; (g) P. De, M. Baltas, D. Lamoral-Theys, C. Bruyere, R. Kiss, F. Bedos-Belval and N. Saffon, Synthesis and anticancer activity evaluation of 2 (4-alkoxyphenyl) cyclopropyl hydrazides and triazolo phthalazines, Bioorg. Med. Chem., 2010, 18, 2537–2548 CrossRef CAS PubMed; (h) J. Galisteo, P. Navarro, L. Campayo, M. J. R. Yunta, F. Gomez-Contreras, J. A. Villa-Pulgarin, B. G. Sierra, F. Mollinedo, J. Gonzalez and E. Garcia-Espana, Synthesis and cytotoxic activity of a new potential DNA bisintercalator: 1, 4-Bis {3-[N-(4-chlorobenzo [g] phthalazin-1-yl) aminopropyl]} piperazine, Bioorg. Med. Chem., 2010, 18, 5301–5309 CrossRef CAS PubMed.
- H. Wu, X. M. Chen, Y. Wan, H. Q. Xin, H. H. Xu, R. Ma, C. H. Yue and L. L. Pang, Synthesis and luminescence of 7-amino-2H-indazolo [2, 1-b] phthalazine-1, 6, 11 (13H)-triones catalyzed by silica sulfuric acid, Lett. Org. Chem., 2009, 6, 219–223 Search PubMed.
- (a) R. M. Williams and R. J. Cox, Paraherquamides, brevianamides, and asperparalines: laboratory synthesis and biosynthesis. An interim report, Acc. Chem. Res., 2003, 36, 127–139 CrossRef CAS PubMed; (b) Y. T. Yang, J. F. Zhu, G. Liao, H. J. Xu and B. Yu, The development of biologically important spirooxindoles as new antimicrobial agents, Curr. Med. Chem., 2018, 25, 2233–2244 CrossRef CAS PubMed; (c) G. S. Singh and Z. Y. Desta, Isatins as privileged molecules in design and synthesis of spiro-fused cyclic frameworks, Chem. Rev., 2012, 112, 6104–6155 Search PubMed; (d) S. S. Panda, R. A. Jones, P. Bachawala and P. P. Mohapatra, Spirooxindoles as potential pharmacophores, Mini-Rev. Med. Chem., 2017, 17, 1515–1536 Search PubMed.
- (a) Y. C. Wang, A. A. Cobob and A. K. Franz, Recent advances in organocatalytic asymmetric multicomponent cascade reactions for enantioselective synthesis of spirooxindoles, Org. Chem. Front., 2021, 8, 4315–4348 Search PubMed; (b) H. Pellissier, Recent developments in asymmetric organocatalytic domino reactions, Adv. Synth. Catal., 2012, 354, 237–294 CrossRef CAS; (c) D. Cheng, Y. Ishihara, B. Tan and C. F. Barbas, Organocatalytic asymmetric assembly reactions: synthesis of spirooxindoles via organocascade strategies, ACS Catal., 2014, 4, 743–762 CrossRef CAS; (d) M. M. M. Santo, Recent advances in the synthesis of biologically active spirooxindoles, Tetrahedron, 2014, 70, 9735–9757 Search PubMed; (e) P. Chauhan, S. Mahajan, U. Kaya, D. Hack and D. Enders, Bifunctional amine-squaramides: powerful hydrogen-bonding organocatalysts for asymmetric domino/cascade reactions, Adv. Synth. Catal., 2015, 357, 253–281 CrossRef CAS; (f) R. Ardkhean, D. F. J. Caputo, S. M. Morrow, H. Shi, Y. Xiong and E. A. Anderson, Cascade polycyclizations in natural product synthesis, Chem. Soc. Rev., 2016, 45, 1557–1569 RSC; (g) G. M. Ziarani, R. Moradi and N. Lashgari, Asymmetric synthesis of chiral oxindoles using isatin as starting material, Tetrahedron, 2018, 74, 1323–1353 CrossRef; (h) T. Chanda and J. C. G. Zhao, Recent progress in organocatalytic asymmetric domino transformations, Adv. Synth. Catal., 2018, 360, 2–79 CrossRef CAS; (i) G. J. Mei and F. Shi, Catalytic asymmetric synthesis of spirooxindoles: recent developments, Chem. Commun., 2018, 54, 6607–6621 RSC; (j) S. Konda, S. Jakkampudi, H. D. Arman and J. C. G. Zhao, Enantioselective synthesis of spiro [4H-pyran-3, 3′-oxindole] derivatives catalyzed by cinchona alkaloid thioureas: Significant water effects on the enantioselectivity, Synth. Commun., 2019, 49, 2971–2982 CAS; (k) Z. Tian, J. Jiang, Z. H. Yan, Q. Q. Luo, G. Zhan, W. Huang, X. Li and B. Han, Catalytic asymmetric [3+2] cycloaddition of pyrazolone-derived MBH carbonate: highly stereoselective construction of the bispiro-[pyrazolone-dihydropyrrole-oxindole] skeleton, Chem. Commun., 2022, 58, 5363–5366 RSC; (l) L. M. Wang, H. W. Mu, Y. H. Sun, Y. Jin and W. Zhang, Asymmetric Synthesis of Spiro[4H-Chromene-3,3′-oxindoles] via a Squaramide-Organocatalytic Three-Component Cascade Knoevenagel/Michael/Cyclization Sequence, Mol. Diversity, 2025, 29, 293–302 CrossRef CAS PubMed.
- (a) X. N. Zhang, Y. X. Li and Z. H. Zhang, Nickel chloride-catalyzed one-pot three-component synthesis of pyrazolophthalazinyl spirooxindoles, Tetrahedron, 2011, 67, 7426–7430 CrossRef CAS; (b) H. Chen and D. Q. Shi, Efficient One-Pot Synthesis of Spiro[indoline-3,10-pyrazolo[1,2-b]phthalazine] Derivatives via Three-Component Reaction, J. Heterocycl. Chem., 2013, 50, 56–60 CrossRef CAS; (c) H. Kefayati, S. Khandan and S. Tavancheh, Ultrasound-assisted synthesis of pyrazolo [1, 2-b] phthalazines and dihydrospiro [indoline-3, 1'-pyrazolo [1, 2-b] phthalazines] using TBAF as an efficient phase-transfer catalyst, Russ. J. Gen. Chem., 2015, 85, 1735–1740 CrossRef; (d) J. X. Wang, X. G. Bai, C. L. Xu, Y. C. Wang, W. Lin, Y. Zou and D. Q. Shi, Ultrasound-Promoted One-Pot, Three-Component Synthesis of Spiro[indoline-3,1'-pyrazolo[1,2-b]phthalazine] Derivatives, Molecules, 2012, 17, 8674–8686 CrossRef CAS PubMed; (e) S. J. T. Rezaei, Y. Bide and M. R. Nabid, An efficient ultrasound-promoted one pot synthesis of spiroacenaphthylene pyrazolotriazole and pyrazolophthalazine derivatives, Tetrahedron Lett., 2012, 53, 5123–5126 CrossRef CAS; (f) A. Khazaei, M. A. Zolfigol, F. Karimitabar, I. Nikokar and A. R. Moosavi-Zare, N, 2-Dibromo-6-chloro-3, 4-dihydro-2 H-benzo [e] [1, 2, 4] thiadiazine-7-sulfonamide 1, 1-dioxide: An efficient and homogeneous catalyst for one-pot synthesis of 4 H-pyran, pyranopyrazole and pyrazolo [1, 2-b] phthalazine derivatives under aqueous media, RSC Adv., 2015, 5, 71402–71412 RSC.
- (a) J. J. Badillo, N. V. Hanhan and A. K. Franz, Enantioselective synthesis of substituted oxindoles and spirooxindoles with applications in drug discovery, Curr. Opin. Drug Discovery Dev., 2010, 13, 758–776 CAS; (b) F. Zhou, Y. L. Liu and J. Zhou, Catalytic asymmetric synthesis of oxindoles bearing a tetrasubstituted stereocenter at the C-3 position, Adv. Synth. Catal., 2010, 352, 1381–1407 CrossRef CAS; (c) W. B. Chen, Z. J. Wu, Q. L. Pei, L. F. Cun, X. M. Zhang and W. C. Yuan, Highly enantioselective construction of spiro [4H-pyran-3, 3′-oxindoles] through a domino Knoevenagel/Michael/cyclization sequence catalyzed by cupreine, Org. Lett., 2010, 12, 3132–3135 CrossRef CAS PubMed; (d) B. M. Trost and M. K. Brennan, Asymmetric syntheses of oxindole and indole spirocyclic alkaloid natural products, Synthesis, 2009, 18, 3003–3025 CrossRef.
- (a) T. Okino, Y. Hoashi and Y. Takemoto, Enantioselective Michael Reaction of Malonates to Nitroolefins Catalyzed by Bifunctional Organocatalysts, J. Am. Chem. Soc., 2003, 125, 12672–12673 Search PubMed; (b) J. P. Malerich, K. Hagihara and V. H. Rawal, Chiral Squaramide Derivatives are Excellent Hydrogen Bond Donor Catalysts, J. Am. Chem. Soc., 2008, 130, 14416–14417 Search PubMed.
- CCDC 2384066 (4d) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge, available online: https://www.ccdc.cam.ac.uk/structures/.
- (a) X. X. Jiang, Y. L. Sun, J. Yao, Y. M. Cao, M. Kai, N. He, X. Y. Zhang, Y. Q. Wang and R. Wang, Core Scaffold-Inspired Concise Synthesis of Chiral Spirooxindole Pyranopyrimidines with Broad-Spectrum Anticancer Potency, Molecules, 2015, 20, 15807–15826 Search PubMed; (b) C. J. Xu, H. W. Li, X. L. He, W. Du and Y. C. Chen, Asymmetric Direct Remote Michael Addition Reactions of Allyl Furfurals via Dearomative Trienamine and Tetraenamine Catalysis, Asian J. Org. Chem., 2019, 8, 1037–1040 CrossRef CAS; (c) M. Balha, B. Mondal and S. C. Pan, Organocatalytic asymmetric synthesis of dihydrofuran-spirooxindoles from benzylidene malononitriles and dioxindoles, Org. Biomol. Chem., 2019, 17, 6557–6561 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2384066. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ra01633a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |