Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Thiamine hydrochloride (VB1) in aqueous media catalyzed the synthesis of polysubstituted quinolines via a one-pot strategy: a combined experimental and theoretical investigation†
Mina Hajipour,
Hossein Mehrabi * and
Hamid Reza Masoodi
* and
Hamid Reza Masoodi
Department of Chemistry, Vali-e-Asr University of Rafsanjan, Rafsanjan, Iran. E-mail: h.mehrabi@vru.ac.ir
First published on 24th April 2025
Abstract
In this work, an efficient one-pot three-component reaction of 2-cyano-N-methylacetamide, arylglyoxals, and arylamines in the presence of thiamine hydrochloride in H2O under reflux conditions was designed for the synthesis of 4-amino-2-benzoylquinoline-3-carboxamide. In this protocol, various synthetic methods such as Knoevenagel/Michael/cyclization cascade reactions were used to introduce different functional groups, such as amino and carboxamide groups, on the quinoline ring system in a single step. In addition to operational simplicity and absence of tedious separation procedures, this method offered the advantages of catalyst reusability and high product yields. Characterization techniques such as nuclear magnetic resonance spectroscopy, infrared spectroscopy, and CHN analysis were used to confirm the structure and purity of the synthesized compounds. In addition to the experimental results, the influence of solvent on the stability of compounds was investigated using DFT calculations at the B3LYP/6-311++G(d,p) level. Compared with solvent-free conditions, the stability of compounds was amplified in the presence of solvents and increased in the order of H2O > DMF > CH3CN > EtOH > THF. This trend was also in agreement with the experimental results. Theoretical data confirmed that the reaction performed best in water medium. Moreover, some electronic properties of these compounds, such as band gap, first ionization energy, electron affinity, electronic chemical potential, electrophilicity index, hardness and softness, were theoretically estimated in the presence of various solvents.
1. Introduction
The ability of multi-component reactions (MCRs) to concurrently integrate numerous reactants into a single reaction step has garnered significant interest in the field of chemical synthesis. In contrast to conventional organic reactions, which often include two or three reactants, MCRs enable the convergence of three or more components, leading to the efficient creation of complex molecular structures.1–4 From a biological standpoint, MCRs have a number of benefits that make them desirable tools for drug development.5Nitrogen-containing heterocyclic compounds are essential in organic chemistry, playing crucial roles in biological synthesis and drug development.6 These compounds are found in nature and have diverse pharmacological activities, including anticancer, anti-HIV, antimalarial, and anti-tubercular properties.7 Their unique structure and diverse properties make them valuable in the fields of medicine, pharmaceuticals, materials science, and agriculture, urging their further exploration.8–10
Quinoline, an N-heterocyclic compound with a nitrogen-containing ring structure, was first isolated by Friedlieb Ferdinand Runge in 1834. Its unique structure and versatile applications have fascinated chemists for centuries.11,12 As a versatile organic synthesis building block with a unique benzene-pyridine ring structure, quinoline is a crucial precursor for the synthesis of various compounds owing to its wide reactivity and functionality.13 Quinoline's versatility facilitates diverse transformations, introducing functional groups at different positions and enabling the creation of novel compounds with desired features through nucleophilic and electrophilic substitution processes.14 Quinoline's structure is crucial for its biological activities,15,16 and naturally occurring alkaloids, such as quinine and quinidine, contain a quinoline moiety, which has been used for medicinal applications for centuries (Fig. 1).17 Quinoline derivatives have shown promise in drug development owing to their ability to modify their structure, leading to improved drug candidates. They have been used in treating various diseases and in the synthesis of dyes, agrochemicals, and advanced materials, such as conducting polymers and luminescent compounds.18
4-Aminoquinoline is a form of quinoline with an amino group at the 4-position of the quinoline. The compound has been used as a precursor for the synthesis of its derivatives.19 Most frequently used medications for treating malaria have a 4-aminoquinoline scaffold.20 The well-known antimalarial medication chloroquine (CQ) has a 4-aminoquinoline scaffold (Fig. 1). As an antimalarial, it works against the asexual form of the malaria parasite in the stage of its life cycle within the red blood cell.21 The structure related to 4-aminoquinoline, chloroquine, was identified in 1934.22 This drug is on the list of essential drugs of the World Health Organization and is available as a generic drug.23 Additionally, CQ has been shown to have antiviral properties against human HIV-1 (ref. 24 and 25) and the agents that cause severe acute respiratory syndrome (SARS).26 Amodiaquine (AMQ) can be mentioned among other compounds of 4-aminoquinoline (Fig. 1). This compound is used in the treatment of malaria, including Plasmodium falciparum malaria resistant to chloroquine.27,28 AMQ was first synthesized in 1948.29 This medicine is on the list of essential medicines of the World Health Organization. AMQ has become an important drug in combination therapy for the treatment of malaria in Africa. Moreover, 4-aminoquinoline derivatives are used as anti-asthmatic, anti-bacterial, anti-fungal, and anti-inflammatory agents.19 4-Aminoquinoline compounds can be synthesized in various important ways including the condensation of appropriate amines with substituted quinolines, fusion of 4,7-dichloroquinoline with an amino group of side chains, methylation or formylation routes for N-methyl derivatives, butoxide-mediated synthesis from alkyl nitriles and aminobenzaldehydes, and imidoylative Sonogashira coupling followed by cyclization.30–32 In summary, the development of new 4-aminoquinoline compounds with high antimalarial potency and low toxicity is an ongoing area of research.
Quinoline-3-carboxamide is an important heterocyclic scaffold extensively studied in medicinal chemistry. It consists of a pyridine ring system fused with a benzene ring, substituted with a carboxamide group at the 3-position. This scaffold allows for various structural modifications and substitutions, leading to a diverse range of quinoline-3-carboxamide derivatives with potential biological activities. They have been shown to have a wide range of applications including as antimicrobial, antiviral, and anticancer agents.33 Quinoline-3-carboxamide compounds such as roquinimex (Linomide) (Fig. 1) exhibit immunomodulatory effects, including anti-inflammatory and anti-allergic properties, enhancing cell-mediated immunity and improving tumor surveillance.34 Quinoline-3-carboxamide compounds have been reported as potential inhibitors of ATM kinase and key mediators of the DNA damage response (DDR), which make these compounds valuable in cancer treatment.35 Quinoline-3-carboxamide derivatives have been shown to activate natural killer (NK) cells via the aryl hydrocarbon receptor, which increases their cytotoxicity against tumor cells and augments their immunoregulatory effects on dendritic cells.36 The synthesis of quinoline-3-carboxamide derivatives can be achieved through various methods including the Doebner–von Miller, Skraup, Vilsmeier–Haack, Combes, Friedlander, and Knorr synthesis, as well as copper-catalyzed reactions and cyclization reactions involving different starting materials.23 In summary, research on compounds with this scaffold is a promising approach for developing new chemotherapeutic agents.
Therefore, to broaden the scope of quinoline derivatives, we decided to design a series of new polysubstituted quinolines, where benzoyl, carboxamide, and amino groups were introduced at the positions of two, three, and four from the quinoline moiety, respectively. The introduction of different functional groups on quinoline moiety, especially carboxamide and amino groups, may further alter the properties of quinoline derivatives for pharmacological and biological purposes.
Thiamin hydrochloride (VB1) has been employed as an ecofriendly, cheap, nontoxic, easily accessible, and remarkable catalyst for the one-pot multi-component synthesis of various heterocyclic compounds.37–40 VB1 has also been used as a catalyst in organic transformations such as Knoevenagel condensation, Michael addition and cyclization.41,42
In continuation of our previous works to establish the one-pot multi-component strategies for the synthesis of the new heterocycles with potential biological activities,43,44 herein, we report the synthesis of 4-amino-2-benzoylquinoline-3-carboxamide derivatives by the reaction of 2-cyano-N-methylacetamide, arylglyoxals, and arylamines in the presence of VB1. Moreover, the influence of solvent on the stability and electronic properties of compounds was theoretically investigated using DFT calculations.
2. Results and discussion
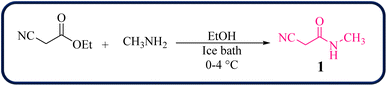
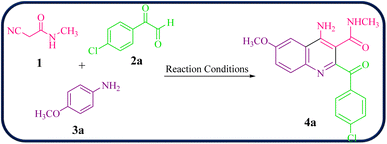
To initiate our study, 2-cyano-N-methylacetamide 1 was achieved via the reaction of ethyl cyanoacetate and methyl amine in the presence of EtOH at 0–4 °C for 2 hours (Scheme 1). The 2-cyano-N-methylacetamide 1 compound was identified by comparison of its physical and spectral data with those of authentic samples.45Then, the reaction of 2-cyano-N-methylacetamide 1 (1.0 mmol), 4-chlorophenylglyoxal (2a, 1.0 mmol), and 4-methoxyaniline (3a, 1.0 mmol) was performed under various reaction conditions for the synthesis of 4-amino-2-(4-chlorobenzoyl)-6-methoxy-N-methylquinoline-3-carboxamide 4a as a model reaction to establish the best reaction conditions (Table 1).
| Entry | Solvent | Catalyst (mol%) | Temp. a(°C) | Yield b(%) |
|---|---|---|---|---|
| a Reaction conditions: solvent = 5 mL; reaction time = 12 h.b Isolated yield.c Reaction time = 6 h. | ||||
| 1 | — | VB1 (10) | r.t. | N.R. |
| 2 | DMF | VB1 (10) | r.t. | N.R. |
| 3 | EtOH | VB1 (10) | r.t. | N.R. |
| 4 | CH3CN | VB1 (10) | r.t. | N.R. |
| 5 | THF | VB1 (10) | r.t. | N.R. |
| 6 | H2O | VB1 (10) | r.t. | 40 |
| 7 | H2O | — | r.t. | N.R. |
| 8 | DMF | VB1 (10) | Reflux | Trace |
| 9 | EtOH | VB1 (10) | Reflux | Trace |
| 10 | CH3CN | VB1 (10) | Reflux | Trace |
| 11 | THF | VB1 (10) | Reflux | Trace |
| 12 | H2O | VB1 (10) | Refluxc | 65 |
| 13 | H2O | VB1(15) | Refluxc | 75 |
| 14 | H2O | VB1 (20) | Refluxc | 75 |
| 15 | H2O | TBAB (15) | Reflux | 55 |
| 16 | H2O | DMAP·HCl (15) | Reflux | 50 |
| 17 | H2O | AlCl3 (15) | Reflux | 30 |
| 18 | H2O | CuCl2 (15) | Reflux | 35 |
| 19 | H2O | ZnO (15) | Reflux | 30 |
To obtain optimal reaction conditions, several factors including solvent, catalyst, and temperature were investigated, the results are presented in Table 1. The reaction was investigated in different solvents such as H2O, EtOH, CH3CN, THF, and DMF, and under solvent-free conditions at room temperature in the presence of 10 mol% VB1. No reaction occurred under solvent-free conditions and in the other solvents except H2O, and it was found that H2O is the best solvent for this reaction (Table 1, entry 6, yield 40%). Moreover, the desired reaction was performed without a catalyst in H2O at room temperature. It was found that 4a was not obtained after 12 h (Table 1, entry 7). Then, we observed that the reaction temperature also has an important influence on the reaction. Therefore, the reaction was carried out at room temperature in H2O for 12 h, the product was formed in 40% yield, but under reflux conditions for 6 h, the product was formed in 65% yield (Table 1, entries 6 and 12). Of course, in other solvents, in the presence of VB1 as the catalyst, the yield of the product was negligible under reflux conditions (Table 1, entries 8–11).
In addition to VB1 as the catalyst, other quaternary ammonium halides such as tetrabutylammonium bromide (TBAB) and N,N-dimethylaminopyridine hydrochloride (DMAP·HCl) were also tested under similar reaction conditions (Table 1, entries 15 and 16), but only 50–55% of product yields were obtained. Moreover, the reaction in the presence of catalysts including AlCl3, CuCl2 and ZnO under similar reaction conditions (Table 1, entries 17–19) did not achieve good yields.
Then, the same reaction was carried out in the presence of catalysts including AlCl3, CuCl2 and ZnO under similar reaction conditions (Table 1, entries 15–17), but the product yields were only 30–35%. Finally, we also observed that the mol% of VB1 as a catalyst could have an important influence on the reaction (Table 1, entries 12–14). When a larger amount of VB1 (15 mol%) was tested, a higher yield of 75% was obtained in H2O under reflux conditions (Table 1, entry 13). Notably, no change was detected in the yield after adding more amounts of VB1 (20 mol%) in H2O under reflux conditions (Table 1, entry 14). Thus, the optimized reaction conditions to prepare 4a was the use of 2-cyano-N-methylacetamide 1 (1.0 mmol), 4-chlorophenylglyoxal (2a, 1.0 mmol), and 4-methoxyaniline (3a, 1.0 mmol) in the presence of VB1 (15 mol%) in H2O under reflux conditions for 6 h (Table 1, entry 13).
Encouraged by these results, we further employed different arylglyoxals and arylamines with 2-cyano-N-methylacetamide to confirm the universality of this procedure under the optimized reaction condition (Table 2). In all cases tested, the reaction went smoothly, giving desired products in good yields. As can be seen from Table 2, the electronic effects and the nature of substituents on arylglyoxal 2 and arylamine 3 resulted in products with different reaction yields. Various substrates 2 and 3 with different substituents R1 and R2 on the aromatic rings were examined. To our delight, both electron-rich and electron-deficient groups (R1 and R2) in substrates 2 and 3 successfully afforded the desired products in good yields. Among them, if both substituents R1 and R2, or one of the substituents on arylglyoxals and arylamines, are electron-withdrawing groups, product 4a–i is synthesized with a relatively high yield. Moreover, the steric hindrance of substituents on substrates has no significant effect on the rate of the reactions.
Moreover, the reusability of the catalyst was examined using the reaction of 2-cyano-N-methylacetamide 1, 4-chlorophenylglyoxal 2a, and 4-methoxyaniline 3a under the optimized reaction conditions. For this purpose, after completion of the reaction, the solvent was removed under reduced pressure. The reaction mixture was triturated with ethyl acetate and filtered. The catalyst was collected from the residue, washed with ethyl acetate, dried and used for the next cycle. We observed that the catalyst could be run for five times without any appreciable decrease in yield (Fig. 2).
To the best of our knowledge, all the synthesized compounds 4a–i are unknown and were characterized by IR, 1H and 13C-NMR and CHN analysis. For instance, the IR spectrum of 4-amino-2-(4-chlorobenzoyl)-6-methoxy-N-methylquinoline-3-carboxamide 4a showed bands at 3452 cm−1 and 3257 cm−1 for NH2, 3183 cm−1 for NH, 1704 cm−1 and 1684 cm−1 for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and 1617 cm−1 and 1512 cm−1 for C
O, and 1617 cm−1 and 1512 cm−1 for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic groups. In the 1H NMR spectrum of compound 4a, a doublet signal at δ = 2.75 ppm with a coupling constant of 5.82 Hz for the methyl, a singlet signal at δ = 3.72 ppm for the methoxy group, a doublet signal at δ = 7.06 ppm with a coupling constant of 5.82 Hz for NH proton, and a singlet signal, which was integrated, for two protons at δ = 7.34 ppm for the NH2 protons were observed. Moreover, the aromatic protons resonated in the region δ = 7.68-8.20 ppm. The 13C NMR spectrum of compound 4a showed 17 distinct signals in agreement with the proposed structure.
C aromatic groups. In the 1H NMR spectrum of compound 4a, a doublet signal at δ = 2.75 ppm with a coupling constant of 5.82 Hz for the methyl, a singlet signal at δ = 3.72 ppm for the methoxy group, a doublet signal at δ = 7.06 ppm with a coupling constant of 5.82 Hz for NH proton, and a singlet signal, which was integrated, for two protons at δ = 7.34 ppm for the NH2 protons were observed. Moreover, the aromatic protons resonated in the region δ = 7.68-8.20 ppm. The 13C NMR spectrum of compound 4a showed 17 distinct signals in agreement with the proposed structure.
We propose a plausible mechanism for the one-pot reaction between arylglyoxal, arylamine, and 2-cyano-N-methylacetamide in the presence of VB1 (Scheme 2). Initially, the catalyst VB1 activates arylglyoxal 2 through the NH proton (intermediate A), so that the electrophilic nature of the carbonyl group increases, thereby facilitating the nucleophilic attack by arylamine 3 through the Knoevenagel condensation and forming intermediate B. The intermediate B, an iminone, which is further activated by the catalyst undergoes a nucleophilic attack by 2-cyano-N-methylacetamide 1 to obtain intermediate C. The intermediate C by an intramolecular cyclization reaction affords intermediate D, which is followed by oxidation and hydrogen shift to obtain the target molecule 4.
In order to investigate the stability of compounds in the presence of chosen solvents, the formation energy (ΔE) was theoretically obtained according to the following formula:
| ΔE = Ecompound − ΣEmon |
Subsequently, the influence of solvents on the electronic properties of compounds was also investigated. As given in Tables S6–S11,† the band gap (Eg), first ionization energy (IE), electron affinity (EA), electronic chemical potential (μ), electrophilicity index (ω), hardness (η) and softness (σ) were estimated in the presence of solvents.
The conductivity of compounds can be evaluated using the energy band gap as follows:
| Eg = ELUMO − EHOMO |
The first ionization energy (IE) and electron affinity (EA) were also estimated using Koopmans' theorem46 according to IE = –EHOMO and EA = –ELUMO. As observed from Tables S6–S11,† the IE values of compounds increase in the following order: THF > EtOH > CH3CN > DMF > H2O. An opposite trend was found for EA values.
The electronic chemical potential is usually used to examine the escaping tendency of the electron in the system. The electronic chemical potential (μ) and the electronegativity (χ) can be measured by electron affinity and first ionization energy as follows:47
One can see from Tables S6–S11† that the absolute value of μ in solvents is enhanced in the order of THF > EtOH > CH3CN > DMF > H2O.
The ability of the compound to respond to an electric field and acquire an electric dipole moment depends on its polarizability. The global chemical hardness (η) and chemical softness (σ) can be used to measure the polarizability. The definitions of these quantities are as follows:48
A small band gap automatically means small excitation energies to the manifold of excited states. Thus, soft compounds, with a small band gap, will be more polarizable than hard cases.49 The global chemical hardness of compounds in solvents is amplified in the order of THF > EtOH > CH3CN > DMF > H2O.
The concept of electrophilicity index (ω), proposed by Parr et al.,50 is a measure of the propensity of electron acceptors to acquire the maximal number of electrons from the environment. It can be calculated using μ and η parameters as follows:
As can be seen in Tables S6–S11,† the ω values in solvents increase in the order of H2O > DMF > CH3CN > EtOH > THF.
3. Experimental
3.1. General
All chemicals of high-grade quality were purchased from Aldrich and Merck and used without further purification. All products were obtained by reaction at reflux in water as the solvent. The reactions were monitored by TLC and all yields refer to isolated products. All melting points were obtained using a Barnstead Electro thermal 9200 apparatus and were uncorrected. IR spectra were recorded using a Bruker FT-IR Equinax-55 spectrophotometer in KBr with absorption in cm−1. NMR spectra were recorded using a Varian model UNITY Inova 500 MHz spectrometer (1H: 500 MHz; 13C: 125 MHz) in DMSO-d6 using TMS as an internal standard. Elemental analysis was performed using a Carlo Erba EA 1108 instrument.3.2. Synthetic procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as an eluent to obtain pure compounds 4a–i (65-85%).
1) as an eluent to obtain pure compounds 4a–i (65-85%).
3.2.1.1. 4-Amino-2-(4-chlorobenzoyl)-6-methoxy-N-methylquinoline-3-carboxamide (4a). Yellow oil; FT-IR (KBr, cm−1): 3452 and 3257 (NH2), 3183 (NH), 1704 and 1684 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1617 and 1512 (C
O), 1617 and 1512 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic groups); 1H NMR (500 MHz, DMSO-d6) δ: 2.75 (d, J = 5.82 Hz, 3H, CH3), 3.72 (s, 3H, OCH3), 7.06 (d, J = 5.82 Hz, 1H, NH), 7.34 (s, 2H, NH2), 7.62 (d, J = 8.40 Hz, 1H, ArH), 7.68 (dd, 1J = 6.85, 2J = 3.55 Hz, 2H, ArH), 7.74 (dd, 1J = 7.80, 2J = 4.1 Hz, 2H, ArH), 7.76 (d, J = 8.40 Hz, 1H, ArH), 8.20 (d, J = 2.70 Hz, 1H, ArH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 27.18, 71.11, 113.91, 114.75, 120.51, 125.33, 126.33, 128.65, 129.44, 131.54, 131.66, 133.32, 147.04, 158.99, 162.41, 166.89, 196.67 ppm. Anal. calcd for C19H16ClN3O3 (369.81): C, 61.71; H, 4.36; N, 11.36; found: C, 61.06; H, 4.12; N, 11.94%.
C aromatic groups); 1H NMR (500 MHz, DMSO-d6) δ: 2.75 (d, J = 5.82 Hz, 3H, CH3), 3.72 (s, 3H, OCH3), 7.06 (d, J = 5.82 Hz, 1H, NH), 7.34 (s, 2H, NH2), 7.62 (d, J = 8.40 Hz, 1H, ArH), 7.68 (dd, 1J = 6.85, 2J = 3.55 Hz, 2H, ArH), 7.74 (dd, 1J = 7.80, 2J = 4.1 Hz, 2H, ArH), 7.76 (d, J = 8.40 Hz, 1H, ArH), 8.20 (d, J = 2.70 Hz, 1H, ArH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 27.18, 71.11, 113.91, 114.75, 120.51, 125.33, 126.33, 128.65, 129.44, 131.54, 131.66, 133.32, 147.04, 158.99, 162.41, 166.89, 196.67 ppm. Anal. calcd for C19H16ClN3O3 (369.81): C, 61.71; H, 4.36; N, 11.36; found: C, 61.06; H, 4.12; N, 11.94%.
3.2.1.2. 4-Amino-2-(4-chlorobenzoyl)-N,6-dimethylquinoline-3-carboxamide (4b). Yellow oil; FT-IR (KBr, cm−1): 3451 and 3258 (NH2), 3185 (NH), 1707 and 1681 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1617 and 1513 (C
O), 1617 and 1513 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic groups); 1H NMR (500 MHz, DMSO-d6) δ: 2.33 (s, 3H, CH3), 2.72 (d, J = 4.70 Hz, 3H, CH3), 6.95 (d, J = 4.70 Hz, 1H, NH), 7.20 (s, 2H, NH2), 7.23 (s, 1H, ArH), 7.32 (d, J = 8.30 Hz, 2H, ArH), 7.35 (d, J = 8.90 Hz, 1H, ArH), 7.59 (d, J = 8.90 Hz, 1H, ArH), 7.64 (d, J = 8.40 Hz, 2H, ArH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 21.38, 29.40, 113.41, 115.54, 121.19, 124.32, 125.32, 129.19, 130.27, 131.56, 131.87, 135.28, 139.48, 149.33, 159.37, 163.12, 209.20 ppm. Anal. calcd for C19H16ClN3O2 (353.81): C, 64.50; H, 4.56; N, 11.88; found: C, 65.12; H, 4.87; N, 11.29%.
C aromatic groups); 1H NMR (500 MHz, DMSO-d6) δ: 2.33 (s, 3H, CH3), 2.72 (d, J = 4.70 Hz, 3H, CH3), 6.95 (d, J = 4.70 Hz, 1H, NH), 7.20 (s, 2H, NH2), 7.23 (s, 1H, ArH), 7.32 (d, J = 8.30 Hz, 2H, ArH), 7.35 (d, J = 8.90 Hz, 1H, ArH), 7.59 (d, J = 8.90 Hz, 1H, ArH), 7.64 (d, J = 8.40 Hz, 2H, ArH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 21.38, 29.40, 113.41, 115.54, 121.19, 124.32, 125.32, 129.19, 130.27, 131.56, 131.87, 135.28, 139.48, 149.33, 159.37, 163.12, 209.20 ppm. Anal. calcd for C19H16ClN3O2 (353.81): C, 64.50; H, 4.56; N, 11.88; found: C, 65.12; H, 4.87; N, 11.29%.
3.2.1.3. 4-Amino-6-chloro-2-(4-chlorobenzoyl)-N-methylquinoline-3-carboxamide (4c). Yellow oil; FT-IR (KBr, cm−1): 3452 and 3258 (NH2), 3185 (NH), 1706 and 1681 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1616 and 1489 (C
O), 1616 and 1489 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic groups); 1H NMR (500 MHz, DMSO-d6) δ: 2.72 (d, J = 4.72 Hz, 3H, CH3), 7.03 (d, J = 4.72 Hz, 1H, NH), 7.31 (s, 2H, NH2), 7.35 (dd, 1J = 6.65, 2J = 2.25 Hz, 2H, ArH), 7.59 (d, J = 7.65 Hz, 1H, ArH), 7.60 (d, J = 7.85 Hz, 1H, ArH), 7.73 (dd, 1J = 6.55, 2J = 2.15 Hz, 2H, ArH), 8.26 (d, J = 1.95 Hz, 1H, ArH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 29.43, 115.19, 120.54, 122.47, 125.82, 126.00, 127.16, 130.07, 132.77, 138.42, 141.75, 143.26, 147.62, 159.59, 162.88, 192.01 ppm. Calcd for C18H13Cl2N3O2 (374.22): C, 57.77; H, 3.50; N, 11.23; found: C, 58.39; H, 3.73; N, 10.74%.
C aromatic groups); 1H NMR (500 MHz, DMSO-d6) δ: 2.72 (d, J = 4.72 Hz, 3H, CH3), 7.03 (d, J = 4.72 Hz, 1H, NH), 7.31 (s, 2H, NH2), 7.35 (dd, 1J = 6.65, 2J = 2.25 Hz, 2H, ArH), 7.59 (d, J = 7.65 Hz, 1H, ArH), 7.60 (d, J = 7.85 Hz, 1H, ArH), 7.73 (dd, 1J = 6.55, 2J = 2.15 Hz, 2H, ArH), 8.26 (d, J = 1.95 Hz, 1H, ArH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 29.43, 115.19, 120.54, 122.47, 125.82, 126.00, 127.16, 130.07, 132.77, 138.42, 141.75, 143.26, 147.62, 159.59, 162.88, 192.01 ppm. Calcd for C18H13Cl2N3O2 (374.22): C, 57.77; H, 3.50; N, 11.23; found: C, 58.39; H, 3.73; N, 10.74%.
3.2.1.4. 4-Amino-2-benzoyl-6-chloro-N-methylquinoline-3-carboxamide (4d). Yellow oil; FT-IR (KBr, cm−1): 3448 and 3253 (NH2), 3181 (NH), 1704 and 1679 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1605 and 1492 (C
O), 1605 and 1492 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic groups); 1H NMR (500 MHz, DMSO-d6) δ: 2.73 (d, J = 4.67 Hz, 3H, CH3), 6.99 (d, J = 4.67 Hz, 1H, NH), 7.25 (s, 2H, NH2), 7.34 (d, J = 7.95 Hz, 2H, ArH), 7.41 (t, J = 8.90 Hz, 2H, ArH), 7.51 (t, J = 6.50 Hz, 1H, ArH), 7.59 (d, J = 8.80 Hz, 1H, 16ArH), 7.75 (d, J = 8.80 Hz, 1H, ArH), 8.26 (d, J = 1.90 Hz, 1H, ArH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 25.92, 115.10, 123.25, 123.79, 124.82, 125.21, 126.28, 129.82, 138.99, 144.20, 148.77, 156.10, 158.81, 159.67, 162.62, 192.64 ppm. Calcd for C18H14ClN3O2 (339.78): C, 63.63; H, 4.15; N, 12.37; found: C, 64.27; H, 4.31; N, 12.03%.
C aromatic groups); 1H NMR (500 MHz, DMSO-d6) δ: 2.73 (d, J = 4.67 Hz, 3H, CH3), 6.99 (d, J = 4.67 Hz, 1H, NH), 7.25 (s, 2H, NH2), 7.34 (d, J = 7.95 Hz, 2H, ArH), 7.41 (t, J = 8.90 Hz, 2H, ArH), 7.51 (t, J = 6.50 Hz, 1H, ArH), 7.59 (d, J = 8.80 Hz, 1H, 16ArH), 7.75 (d, J = 8.80 Hz, 1H, ArH), 8.26 (d, J = 1.90 Hz, 1H, ArH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 25.92, 115.10, 123.25, 123.79, 124.82, 125.21, 126.28, 129.82, 138.99, 144.20, 148.77, 156.10, 158.81, 159.67, 162.62, 192.64 ppm. Calcd for C18H14ClN3O2 (339.78): C, 63.63; H, 4.15; N, 12.37; found: C, 64.27; H, 4.31; N, 12.03%.
3.2.1.5. 4-Amino-2-(4-bromobenzoyl)-6-chloro-N-methylquinoline-3-carboxamide (4e). Yellow oil; FT-IR (KBr, cm−1): 3453 and 3259 (NH2), 3187 (NH), 1705 and 1682 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1608 and 1493 (C
O), 1608 and 1493 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic groups); 1H NMR (500 MHz, DMSO-d6) δ: 2.72 (d, J = 4.40 Hz, 3H, CH3), 7.02 (d, J = 4.40 Hz, 1H, NH), 7.31 (s, 2H, NH2), 7.35 (dd, 1J = 8.90, 2J = 2.30 Hz, 2H, ArH), 7.59 (dd, 1J = 6.70, 2J = 2.17 Hz, 2H, ArH), 7.66 (d, J = 8.85 Hz, 1H, ArH), 7.73 (d, J = 8.85 Hz, 1H, ArH), 8.26 (d, J = 1.95 Hz, 1H, ArH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 29.40, 115.43, 119.49, 121.20, 126.02, 127.61, 129.19, 129.67, 132.78, 137.60, 144.80, 156.65, 157.97, 158.41, 160.13, 194.90 ppm. Calcd for C18H13BrClN3O2 (418.68): C, 51.64; H, 3.13; N, 10.04; found: C, 52.11; H, 3.19; N, 9.74%.
C aromatic groups); 1H NMR (500 MHz, DMSO-d6) δ: 2.72 (d, J = 4.40 Hz, 3H, CH3), 7.02 (d, J = 4.40 Hz, 1H, NH), 7.31 (s, 2H, NH2), 7.35 (dd, 1J = 8.90, 2J = 2.30 Hz, 2H, ArH), 7.59 (dd, 1J = 6.70, 2J = 2.17 Hz, 2H, ArH), 7.66 (d, J = 8.85 Hz, 1H, ArH), 7.73 (d, J = 8.85 Hz, 1H, ArH), 8.26 (d, J = 1.95 Hz, 1H, ArH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 29.40, 115.43, 119.49, 121.20, 126.02, 127.61, 129.19, 129.67, 132.78, 137.60, 144.80, 156.65, 157.97, 158.41, 160.13, 194.90 ppm. Calcd for C18H13BrClN3O2 (418.68): C, 51.64; H, 3.13; N, 10.04; found: C, 52.11; H, 3.19; N, 9.74%.
3.2.1.6. 4-Amino-6-bromo-2-(4-bromobenzoyl)-N-methylquinoline-3-carboxamide (4f). Yellow oil; FT-IR (KBr, cm−1): 3449 and 3256 (NH2), 3184 (NH), 1703 and 1679 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1606 and 1490 (C
O), 1606 and 1490 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic groups); 1H NMR (500 MHz, DMSO-d6) δ: 2.72 (d, J = 4.65 Hz, 3H, CH3), 7.02 (d, J = 4.65 Hz, 1H, NH), 7.31 (s, 2H, NH2), 7.48 (dd, 1J = 6.70, 2J = 2.20 Hz, 2H, ArH), 7.54 (dd, 1J = 6.70, 2J = 2.17 Hz, 2H, ArH), 7.66 (d, J = 8.75 Hz, 1H, ArH), 7.73 (d, J = 8.70 Hz, 1H, ArH), 8.27 (d, J = 2.00 Hz, 1H, ArH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 29.41, 115.18, 119.84, 124.11, 126.01, 127.16, 132.10, 132.33, 132.56, 132.79, 138.01, 142.10, 150.47, 160.15, 162.91, 196.01 ppm. Calcd for C18H13Br2N3O2 (463.13): C, 46.68; H, 2.83; N, 9.07; found: C, 46.92; H, 2.88; N, 8.64%.
C aromatic groups); 1H NMR (500 MHz, DMSO-d6) δ: 2.72 (d, J = 4.65 Hz, 3H, CH3), 7.02 (d, J = 4.65 Hz, 1H, NH), 7.31 (s, 2H, NH2), 7.48 (dd, 1J = 6.70, 2J = 2.20 Hz, 2H, ArH), 7.54 (dd, 1J = 6.70, 2J = 2.17 Hz, 2H, ArH), 7.66 (d, J = 8.75 Hz, 1H, ArH), 7.73 (d, J = 8.70 Hz, 1H, ArH), 8.27 (d, J = 2.00 Hz, 1H, ArH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 29.41, 115.18, 119.84, 124.11, 126.01, 127.16, 132.10, 132.33, 132.56, 132.79, 138.01, 142.10, 150.47, 160.15, 162.91, 196.01 ppm. Calcd for C18H13Br2N3O2 (463.13): C, 46.68; H, 2.83; N, 9.07; found: C, 46.92; H, 2.88; N, 8.64%.
3.2.1.7. 4-Amino-8-bromo-N-methyl-2-(4-methylbenzoyl)quinoline-3-carboxamide (4g). Yellow oil; FT-IR (KBr, cm−1): 3453 and 3259 (NH2), 3186 (NH), 1704 and 1681 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1614 and 1491 (C
O), 1614 and 1491 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic groups); 1H NMR (500 MHz, DMSO-d6) δ: 2.33 (s, 3H, CH3), 2.73 (d, J = 4.97 Hz, 3H, CH3), 6.92 (d, J = 4.97 Hz, 1H, NH), 7.17 (s, 2H, NH2), 7.21 (d, J = 7.35 Hz, 1H, ArH), 7.23 (d, J = 8.30 Hz, 1H, ArH), 7.32 (d, J = 8.30 Hz, 2H, ArH), 7.38 (t, J = 7.65 Hz, 1H, ArH), 7.65 (d, J = 8.30 Hz, 2H, ArH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 21.37, 29.42, 115.55, 120.52, 124.32, 125.33, 128.61, 130.26, 136.71, 139.48, 142.40, 149.34, 153.46, 155.04, 159.35, 163.11, 197.11 ppm. Calcd for C19H16BrN3O2 (398.26): C, 57.30; H, 4.05; N, 10.55; found: C, 56.71; H, 3.82; N, 10.69%.
C aromatic groups); 1H NMR (500 MHz, DMSO-d6) δ: 2.33 (s, 3H, CH3), 2.73 (d, J = 4.97 Hz, 3H, CH3), 6.92 (d, J = 4.97 Hz, 1H, NH), 7.17 (s, 2H, NH2), 7.21 (d, J = 7.35 Hz, 1H, ArH), 7.23 (d, J = 8.30 Hz, 1H, ArH), 7.32 (d, J = 8.30 Hz, 2H, ArH), 7.38 (t, J = 7.65 Hz, 1H, ArH), 7.65 (d, J = 8.30 Hz, 2H, ArH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 21.37, 29.42, 115.55, 120.52, 124.32, 125.33, 128.61, 130.26, 136.71, 139.48, 142.40, 149.34, 153.46, 155.04, 159.35, 163.11, 197.11 ppm. Calcd for C19H16BrN3O2 (398.26): C, 57.30; H, 4.05; N, 10.55; found: C, 56.71; H, 3.82; N, 10.69%.
3.2.1.8. 4-Amino-6-chloro-2-(4-methoxybenzoyl)-N-methylquinoline-3-carboxamide (4h). Yellow oil; FT-IR (KBr, cm−1): 3453 and 3258 (NH2), 3185 (NH), 1706 and 1681 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1616 and 1494 (C
O), 1616 and 1494 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic groups); 1H NMR (500 MHz, DMSO-d6) δ: 2.72 (d, J = 4.65 Hz, 3H, CH3), 3.79 (s, 3H, OCH3), 6.91 (d, J = 4.65 Hz, 1H, NH), 6.94 (d, J = 8.70 Hz, 1H, ArH), 7.09 (dd, 1J = 6.90, 2J = 2.15 Hz, 2H, ArH), 7.14 (s, 2H, NH2), 7.18 (s, 1H, ArH), 7.30 (d, J = 8.75 Hz, 1H, ArH), 7.69 (dd, 1J = 6.85, 2J = 2.07 Hz, 2H, ArH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 29.43, 55.85, 114.04, 115.28, 120.66, 126.15, 129.08, 131.64, 131.74, 132.90, 138.84, 145.32, 149.61, 159.14, 160.39, 163.18, 193.91 ppm. Calcd for C19H16ClN3O3 (369.81): C, 61.71; H, 4.36; N, 11.36; found: C, 61.27; H, 4.28; N, 11.52%.
C aromatic groups); 1H NMR (500 MHz, DMSO-d6) δ: 2.72 (d, J = 4.65 Hz, 3H, CH3), 3.79 (s, 3H, OCH3), 6.91 (d, J = 4.65 Hz, 1H, NH), 6.94 (d, J = 8.70 Hz, 1H, ArH), 7.09 (dd, 1J = 6.90, 2J = 2.15 Hz, 2H, ArH), 7.14 (s, 2H, NH2), 7.18 (s, 1H, ArH), 7.30 (d, J = 8.75 Hz, 1H, ArH), 7.69 (dd, 1J = 6.85, 2J = 2.07 Hz, 2H, ArH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 29.43, 55.85, 114.04, 115.28, 120.66, 126.15, 129.08, 131.64, 131.74, 132.90, 138.84, 145.32, 149.61, 159.14, 160.39, 163.18, 193.91 ppm. Calcd for C19H16ClN3O3 (369.81): C, 61.71; H, 4.36; N, 11.36; found: C, 61.27; H, 4.28; N, 11.52%.
3.2.1.9. 4-Amino-N,6-dimethyl-2-(4-methylbenzoyl)quinoline-3-carboxamide (4i). Yellow oil; FT-IR (KBr, cm−1): 3453 and 3259 (NH2), 3180 (NH), 1707 and 1682 (C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1617 and 1514 (C
O), 1617 and 1514 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic groups); 1H NMR (500 MHz, DMSO-d6) δ: 2.23 (s, 3H, CH3), 2.33 (s, 3H, CH3), 2.73 (d, J = 4.5 Hz, 3H, CH3), 6.92 (s, 1H, NH), 7.09 (d, J = 8.6 Hz, 1H, ArH), 7.17 (s, 2H, NH2), 7.22 (d, J = 7.40 Hz, 1H, ArH), 7.32 (d, J = 8.65 Hz, 2H, ArH), 7.44 (d, J = 8.55 Hz, 1H, ArH), 7.65 (d, J = 8.25 Hz, 2H, ArH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 21.30, 21.37, 29.47, 115.56, 119.58, 124.31, 125.32, 127.13, 129.24, 129.61, 130.25, 132.95, 136.20, 139.46, 149.32, 159.72, 163.13, 195.47 ppm. Calcd for C20H19N3O2 (333.39): C, 72.05; H, 5.74; N, 12.60; found: C, 71.59; H, 5.57; N, 12.36%.
C aromatic groups); 1H NMR (500 MHz, DMSO-d6) δ: 2.23 (s, 3H, CH3), 2.33 (s, 3H, CH3), 2.73 (d, J = 4.5 Hz, 3H, CH3), 6.92 (s, 1H, NH), 7.09 (d, J = 8.6 Hz, 1H, ArH), 7.17 (s, 2H, NH2), 7.22 (d, J = 7.40 Hz, 1H, ArH), 7.32 (d, J = 8.65 Hz, 2H, ArH), 7.44 (d, J = 8.55 Hz, 1H, ArH), 7.65 (d, J = 8.25 Hz, 2H, ArH) ppm; 13C NMR (125 MHz, DMSO-d6) δ: 21.30, 21.37, 29.47, 115.56, 119.58, 124.31, 125.32, 127.13, 129.24, 129.61, 130.25, 132.95, 136.20, 139.46, 149.32, 159.72, 163.13, 195.47 ppm. Calcd for C20H19N3O2 (333.39): C, 72.05; H, 5.74; N, 12.60; found: C, 71.59; H, 5.57; N, 12.36%.
3.3. Computational section
The energetic and geometrical properties have been optimized using Gaussian 09 suite of programs51 at the B3LYP/6-311++G(d,p) level. The compounds were investigated in gas phase and in water (H2O), acetonitrile (CH3CN), tetrahydrofuran (THF), ethanol (EtOH) and N,N-dimethylformamide (DMF) as solvents. For modeling the solvation effects, the polarizable continuum model (PCM) is usually used.52–55 The PCM creates a solute cavity using a set of overlapping spheres, so that meaningful data can be obtained.56 In this study, the influence of solvent on the stability and electronic properties of compounds was examined using the PCM during the optimization of structures of compounds at the B3LYP/6-311++G(d,p) level of theory. The frequency calculations were also used to estimate the thermodynamic functions of compounds such as zero-point energy (EZPE), thermal energy (EThermal), enthalpy (H) and Gibbs free energy (G).4. Conclusions
In summary, we have designed an efficient protocol for the synthesis of 4-amino-2-benzoylquinoline-3-carboxamides via a one-pot three-component reaction of 2-cyano-N-methylacetamide, arylglyoxals, and arylamines using catalytic amounts of VB1. These new series of quinoline derivatives which contain a carboxamide group at the 3-position and an amino group at the 4-position are of great importance in medicinal chemistry. The use of readily available starting materials, simple reaction conditions, cheaply available and reusable catalysts, easy work-up procedure, and the high yield of products are the key features of this MCR protocol. Further studies and medicinal applications of these compounds are being investigated and will be reported in due course.Moreover, the influence of solvent on the stability of compounds was theoretically investigated. As compared to solvent-free conditions, the stability of compounds is amplified in the presence of solvents, and increases in the order of H2O > DMF > CH3CN > EtOH > THF. This trend is in agreement with experimental results. Theoretical data confirm that the reaction performs best in water. Moreover, some electronic properties of these compounds, such as band gap, first ionization energy, electron affinity, electronic chemical potential, electrophilicity index, hardness and softness, were theoretically estimated in the presence of solvents.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Mina Hajipour: writing – original draft, methodology, formal analysis, data curation. Hossein Mehrabi: writing – review & editing, validation, supervision, project administration, conceptualization. Hamid Reza Masoodi: writing – review, software, resources, investigation, data curation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are thankful to the Office of Graduate Studies of Vali-e-Asr University of Rafsanjan for partially supporting this work.References
- I. Ugi, A. Dömling and W. Hörl, Multicomponent reactions in organic chemistry, Endeavour, 1994, 18, 115–122 CrossRef CAS.
- R. Javahershenas and S. Nikzat, Recent advances in the multicomponent synthesis of heterocycles using tetronic acid, RSC Adv., 2023, 13, 16619 RSC.
- R. Javahershenas, H. Mei, M. Koley, V. A. Soloshonok and A. Makarem, Recent Advances in the Multicomponent Synthesis of Heterocycles Using 5-Aminotetrazole, Synthesis, 2024, 56, 2445–2461 CrossRef CAS.
- R. Javahershenas, A. Makarem and K. D. Klika, Recent advances in microwave-assisted multicomponent synthesis of spiro heterocycles, RSC Adv., 2024, 14, 5547 RSC.
- M. A. Mironov, Multicomponent reactions and combinatorial chemistry, Russ. J. Gen. Chem., 2010, 80, 2628–2646 CrossRef CAS.
- A. Mermer, T. Keles and Y. Sirin, Recent studies of nitrogen containing heterocyclic compounds as novel antiviral agents: A review, Bioorg. Chem., 2021, 114, 105076 CrossRef CAS PubMed.
- N. Kerru, L. Gummidi, S. Maddila, K. K. Gangu and S. B. Jonnalagadda, A review on recent advances in nitrogen-containing molecules and their biological applications, Molecules, 2020, 25, 1909 CrossRef CAS PubMed.
- Y. Ju and R. S. Varma, Aqueous N-heterocyclization of primary amines and hydrazines with dihalides: microwave-assisted syntheses of N-azacycloalkanes, isoindole, pyrazole, pyrazolidine, and phthalazine derivatives, J. Org. Chem., 2006, 71, 135–141 CrossRef CAS PubMed.
- P. D. Leeson and B. Springthorpe, The influence of drug-like concepts on decision-making in medicinal chemistry, Nat. Rev. Drug Discovery, 2007, 6, 881–890 CrossRef CAS PubMed.
- N. Kerru, S. Maddila and S. B. Jonnalagadda, Design of carbon-carbon and carbon-heteroatom bond formation reactions under green conditions, Curr. Org. Chem., 2019, 23, 3154–3190 CrossRef CAS.
- S. Jain, V. Chandra, P. K. Jain, K. Pathak, D. Pathak and A. Vaidya, Comprehensive review on current developments of quinoline-based anticancer agents, Arabian J. Chem., 2019, 12, 4920–4946 CrossRef CAS.
- L. M. Nainwal, S. Tasneem, W. Akhtar, G. Verma, M. F. Khan, S. Parvez and M. M. Alam, Green recipes to quinoline: A review, Eur. J. Med. Chem., 2019, 164, 121–170 CrossRef CAS PubMed.
- R. V. Solomon and H. Lee, Quinoline as a privileged scaffold in cancer drug discovery, Curr. Med. Chem., 2011, 18, 1488–1508 CrossRef PubMed.
- A. Weyesa and E. Mulugeta, Recent advances in the synthesis of biologically and pharmaceutically active quinoline and its analogues: a review, RSC Adv., 2020, 10, 20784–20793 RSC.
- B. S. Matada, R. Pattanashettar and N. G. Yernale, A comprehensive review on the biological interest of quinoline and its derivatives, Bioorg. Med. Chem., 2021, 32, 115973 CrossRef CAS PubMed.
- S. Kumar, S. Bawa and H. Gupta, Biological Activities of Quinoline Derivatives, Mini-Rev. Med. Chem., 2009, 9, 1648–1654 CrossRef CAS PubMed.
- Y. Q. Hu, C. Gao, S. Zhang, L. Xu, Z. Xu, L. S. Feng and F. Zhao, Quinoline hybrids and their antiplasmodial and antimalarial activities, Eur. J. Med. Chem., 2017, 139, 22–47 CrossRef CAS PubMed.
- S. Narwal, S. Kumar and P. K. Verma, Synthesis and therapeutic potential of quinoline derivatives, Res. Chem. Intermed., 2017, 43, 2765–2798 CrossRef CAS.
- S. A. Bourne, K. De Villiers and T. J. Egan, Three 4-aminoquinolines of antimalarial interest, Acta Crystallogr., Sect. C:Cryst. Struct. Commun., 2006, 62, o53–o57 CrossRef PubMed.
- J. S. Wiesner, R. Ortmann, H. Jomaa and M. Schlitzer, New antimalarial drugs, Angew. Chem., Int. Ed., 2003, 42, 5274–5293 CrossRef CAS PubMed.
- Z. Liang and G. You, Chloroquine and Hydroxychloroquine, as Proteasome Inhibitors, Upregulate the Expression and Activity of Organic Anion Transporter 3, Pharm, 2023, 15, 1725–1738 CAS.
- G. C. Cook and A. Zumla, Manson's Tropical Diseases, Elsevier Health Sciences, Twenty 2nd edn, 2009 Search PubMed.
- World Health Organization, World Health Organization Model List of Essential Medicines; 21st List 2019 (No. WHO/MVP/EMP/IAU/2019.06) Search PubMed.
- A. Savarino, J. R. Boelaert, A. Cassone, G. Majori and R. Cauda, Effects of chloroquine on viral infections: an old drug against today's diseases, Lancet Infect. Dis., 2003, 3, 722–727 CrossRef CAS PubMed.
- J. R. Boelaert, J. Piette and K. Sperber, The potential place of chloroquine in the treatment of HIV-1-infected patients, J. Clin. Virol., 2001, 20, 137–140 CrossRef CAS.
- A. Savarino, L. Di Trani, I. Donatelli, R. Cauda and A. Cassone, New insights into the antiviral effects of chloroquine, Lancet Infect. Dis., 2006, 6, 67–69 CrossRef PubMed.
- A. Nair, B. Abrahamsson, D. M. Barends, D. W. Groot, S. Kopp, J. E. Polli and J. B. Dressman, Biowaiver monographs for immediate release solid oral dosage forms: amodiaquine hydrochloride, J. Pharm. Sci., 2012, 101, 4390–4401 CrossRef CAS PubMed.
- J. E. Bennett, R. Dolin and M. J. Blaser, Mandell, Douglas, Bennett's Principles and Practice of Infectious Diseases E-Book: 2-Volume Set, Elsevier health sciences, 2019 Search PubMed.
- I. Ahmad, T. Ahmad and K. Usmanghani, Amodiaquine hydrochloride, Anal. Profiles Drug Subst. Excipients, 1992, 21, 43–73 Search PubMed.
- P. L. Reddy, S. I. Khan, P. Ponnan, M. Tripathi and D. S. Rawat, Design, synthesis and evaluation of 4-aminoquinoline-purine hybrids as potential antiplasmodial agents, Eur. J. Med. Chem., 2017, 126, 675–686 CrossRef CAS.
- K. Singh, H. Kaur, K. Chibale and J. Balzarini, Synthesis of 4-aminoquinoline–pyrimidine hybrids as potent antimalarials and their mode of action studies, Eur. J. Med. Chem., 2013, 66, 314–323 CrossRef CAS.
- J. W. Collet, K. Ackermans, J. Lambregts, B. U. Maes, R. V. Orru and E. Ruijter, Modular Three-Component Synthesis of 4-Aminoquinolines via an Imidoylative Sonogashira/Cyclization Cascade, J. Org. Chem., 2018, 83, 854–861 CrossRef CAS.
- H. Govender, C. Mocktar and N. A. Koorbanally, Synthesis and Bioactivity of Quinoline-3-carboxamide Derivatives, J. Heterocycl. Chem., 2018, 55, 1002–1009 CrossRef CAS.
- J. F. He, L. H. Yun, R. F. Yang, Z. Y. Xiao, J. P. Cheng, W. X. Zhou and Y. X. Zhang, Design, synthesis, and biological evaluation of novel 4-hydro-quinoline-3-carboxamide derivatives as an immunomodulatory, Bioorg. Med. Chem. Lett., 2005, 15, 2980–2985 CrossRef CAS PubMed.
- S. Ravi, S. Barui, S. Kirubakaran, P. Duhan and K. Bhowmik, Synthesis and characterization of quinoline-3-carboxamide derivatives as inhibitors of the ATM kinase, Curr. Top. Med. Chem., 2020, 20, 2070–2079 CrossRef CAS PubMed.
- M. Ott, E. Avendaño-Guzmán, E. Ullrich, C. Dreyer, J. Strauss, M. Harden and S. Nessler, Laquinimod, a prototypic quinoline-3-carboxamide and aryl hydrocarbon receptor agonist, utilizes a CD155-mediated natural killer/dendritic cell interaction to suppress CNS autoimmunity, J. Neuroinflammation, 2019, 16, 1–14 CrossRef PubMed.
- I. R. Siddiqui, P. Rai, Rahila and A. Srivastava, Synthesis of fused pyridines in the presence of thiamine hydrochloride as an efficient and reusable catalyst in aqueous conditions, New J. Chem., 2013, 37, 3798–3804 RSC.
- J. Liu, M. Lei and L. Hu, Thiamine hydrochloride (VB1): an efficient promoter for the one-pot synthesis of benzo[4,5]imidazo[1,2-a]pyrimidine and [1,2,4]triazolo[1,5-a] pyrimidine derivatives in water medium, Green Chem., 2012, 14, 840–846 RSC.
- R. . M. R. Sirigireddy, S. Shaik, M. Gundluru, S. R. Cirandur, F. Alonso-Marroquin, R. R. Kakarla, C. G. R. Nallagondu and T. M. Aminabhavi, Fused chromeno-pyrano-pyrimidinediones: Multi-component green synthesis and electrochemical properties, J. Mol. Liq., 2024, 396, 123950 CrossRef CAS.
- S. Singh, A. Gupta and K. K. Kapoor, Facile one-pot multicomponent synthesis of highly functionalized tetrahydropyridines using thiamine hydrochloride as an organocatalyst, Synth. Commun., 2020, 50, 1056–1063 CrossRef CAS.
- K. Pradhan, P. Bhattacharyya, S. Paul and A. R. Das, Tetrahedron Lett., 2012, 53, 5840–5844 CrossRef CAS.
- M. Lei, L. Ma and L. Hu, One-pot synthesis of 1H-benzimidazole derivatives using thiamine hydrochloride as a reusable organocatalyst, Synth. Commun., 2012, 42, 2981–2993 CrossRef CAS.
- F. Alizadeh-Bami, H. Mehrabi and F. Safari, Design, synthesis, and anticancer evaluation of novel imidazo[1,2-c]thieno[3,2-e]pyrimidin derivatives, Tetrahedron, 2023, 144, 133594 CrossRef CAS.
- M. Ahmadusefi-Sarhadi, H. Mehrabi, F. Alizadeh-Bami and J. T. Shaw, A convenient one-pot synthesis of novel benzo[g]benzo[4,5]imidazo[2,1-b]quinazolines through Knoevenagel/Michael/Cyclization cascade reaction, Tetrahedron Lett., 2024, 137, 154933 CrossRef CAS.
- N. Cheikh, N. Bar, N. Choukchou-Braham, B. Mostefa-Kara, J. F. Lohier, J. Sopkova and D. Villemin, Efficient synthesis of new butanolides by subsequent reactions: application for the synthesis of original iminolactones, bis-iminolactones and bis-lactones, Tetrahedron, 2011, 67, 1540–1551 CrossRef CAS.
- T. A. Koopmans, Über die zuordnung von wellenfunktionen und eigenwerten zu den einzelnen elektronen eines atoms, Physica, 1934, 1, 104–113 CrossRef.
- R. S. Mulliken, A new electroaffinity scale; together with data on valence states and on valence ionization potentials and electron affinities, J. Chem. Phys., 1934, 2, 782–793 CrossRef CAS.
- R. G. Parr and R. G. Pearson, Absolute hardness: Companion parameter to absolute electronegativity, J. Am. Chem. Soc., 1983, 105, 7512–7516 CrossRef CAS.
- R. G. Pearson, Absolute electronegativity and hardness correlated with molecular orbital theory, Proc. Natl. Acad. Sci. U. S. A., 1986, 83, 8440–8441 CrossRef CAS.
- R. G. Parr, L. V. Szentpàly and S. Liu, Electrophilicity index, J. Am. Chem. Soc., 1999, 121, 1922–1924 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- J. Tomasi, B. Mennucci and R. Cammi, Quantum mechanical continuum solvation models, Chem. Rev., 2005, 105, 2999–3094 CrossRef CAS PubMed.
- S. Asadi, H. R. Masoodi and H. Mehrabi, Theoretical insights of solvent effect on tautomerism, stability, and electronic properties of 6-ketomethylphenanthridine, J. Phys. Org. Chem., 2022, 35, e4294 CrossRef CAS.
- Y. Kurosaki, R. Nakanishi, M. Saeki and H. Ohba, Reaction pathways for palladium(I) reduction in laser-induced particle formation of Pd: An ab initio molecular orbital study, Chem. Phys., 2023, 569, 111857 CrossRef CAS.
- T. E. Da-yang and C. H. Lai, Potential energy surfaces of the Cu2+ (NH3)n=1−10 clusters in solvent phase: A DFT study, Chem. Phys., 2023, 570, 111902 CrossRef CAS.
- R. E. Skyner, J. L. McDonagh, C. R. Groom, T. V. Mourik and J. B. O. Mitchell, A review of methods for the calculation of solution free energies and the modelling of systems in solution, Phys. Chem. Chem. Phys., 2015, 17, 6174–6191 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra01461a |
| This journal is © The Royal Society of Chemistry 2025 |