Open Access Article
Open Access ArticleRational design of a lead-free Cs2Na0.4Ag0.6In0.9Bi0.1Cl6:0.15Mn2+ double perovskite for enhancing the colour rendition index of UV-pumped W-LEDs†
Dhiyaneshwari Balaji and
G. Swati *
*
Centre for Nanotechnology Research, Vellore Institute of Technology, Vellore 632014, Tamil Nadu, India. E-mail: swati.g@vit.ac.in
First published on 3rd June 2025
Abstract
Compared with lead halide perovskites, double perovskites (DPs) have drawn much attention because of their intriguing optoelectronic qualities, environmental stability, and non-toxicity. Herein, we systematically designed a Cs2Na0.4Ag0.6In0.9Bi0.1Cl6:0.15Mn2+ double perovskite by alloying Ag+ and Bi3+ at the Na+ and In3+ sites in the host lattice. Ag+ broke the parity forbidden transition of Cs2NaInCl6, and Bi3+ significantly broadened the emission spectra from 450–700 nm. Doping of Mn2+ further reduced the defect density and enhanced the radiative recombination. Broad emission owing to the 4T1 → 6A1 transition of Mn2+ ions was observed at 665 nm. Upon doping, the sample exhibited a PLQY of 44.9%. Temperature-dependent emission characteristics of the synthesized double perovskite were also studied. Even at a high temperature of 100 °C, the emission intensity was reduced only by 53%, and the material possessed a high activation energy of 0.46 eV. The optimised sample was physically blended with blue emitting BaMgAl10O17:Eu2+ to obtain complete spectral color coverage in visible light when excited with a 365 nm UV light source. CIE coordinates of (0.37, 0.39), CCT of 4457.53 K and CRI of 87.4 were obtained for the optimised sample. Cs2Na0.4Ag0.6In0.9Bi0.1Cl6:0.15Mn2+ was found to be a promising luminescent material for enhancing the color rendition index of UV-pumped white LEDs. The as-prepared double perovskite is a multifunctional material that can be used for WLEDs, photodetectors and display screens.
1. Introduction
Perovskite materials are gaining importance in optoelectronic applications, such as light emitting diodes, solar cells, photo-detectors, scintillators and lasers, owing to their tunable band gap, high light absorption ability, low defect density, high carrier mobility and large charge diffusion lengths.1–5 White light can be obtained using any of the following three methods: combining tri-color emitting (red, green and blue) LEDs, using UV-LEDs in combination with red-, blue- and green-emitting phosphors and utilising phosphor-converted LEDs. Owing to the high energy efficiency and low spectral mismatch of phosphor-converted LEDs, they are more commonly utilized for commercial purposes.6 Phosphor-converted LEDs use the combination of an InGaN blue LED chip with a YAG:Ce3+ yellow emitting phosphor or an inorganic phosphor, such as (SrMg2La2W2O12:Mn4+),7 (Ca2YSbO6:Eu3+),8 (Sr3La(BO3)3:Ce, Tb),9 (NaBaY(BO3)2:Ce3+, Tb3+),10 and (NaZnPO4:Mn).11 However, phosphor-converted LEDs have some drawbacks, such as high cost owing to the involvement of rare earth dopants and color shift due to the potential degradation of phosphor over time. A major drawback of WLEDs is the low color rendition index because the overall emission spectrum lacks a red light component.12–14The low CRI is due to the inadequate amount of the red component. CRI is defined as the scale that is used to compare the rendering of natural colors of any object in artificial white light with that of sunlight. The lower CRI with a huge CCT may cause defects in the human retina owing to the versatile emission of blue light from the semiconductor chip. Poor CRI also affects the circadian rhythm, alertness, and cognitive performance of an individual.15 Pc-LEDs also suffer from Stokes shift energy loss, narrow emission bands and phosphor degradation. To overcome the shortcomings of Pc-converted LEDs, novel materials have been explored, such as carbon quantum dots,16 lead halide perovskites,17 and metal–organic framework-based materials.18,19 Lead halide perovskites possess unique optical and electronic properties; however, owing to the inherent toxicity of lead, lead halide perovskite nanocrystals (NCs),3–5 such as (CsZnxPb1−xX3 NCs),17 ((C6H5C2H4NH3)2PbCl2Br2),20 and (CsPb(Br0.4, I0.6)3),21 are used in LEDs. The toxicity of lead halide perovskites owing to the presence of lead limits their practical applications. Tin-based perovskite material ASnX3 suffers from poor stability at room temperature owing to the unstable nature of (Sn2+).22–24 Lead free inorganic halide double perovskites A2B(I) B(III)X6 (A = Rb+ and Cs+; B(I) = K+, Na+, Ag+, and Li+; B(III) = Bi3+, In3+, and Sb3+; X = Br, I, and Cl) are non-toxic alternatives to lead- and tin-based halide perovskites. However, these emerging double perovskites face challenges, such as an indirect band gap and low photoluminescence quantum yield (<3%), owing to their parity-forbidden transitions. Cs2NaInCl6 is a potential direct band gap candidate that exhibits photo luminescence owing to self-trapped excitons, resulting in parity-forbidden transitions. To improve the photoluminescence quantum yield, various strategies, such as doping and alloying, are employed.23,25,26 Luminescence from Cs2NaInCl6 arises owing to self-trapped excitons arising from the Jahn–Teller distortion of the NaCl6 octahedron, which causes parity-forbidden transitions to be seldom allowed.
Motivated by the same, we investigate the role of alloying Ag+ and Bi3+ at Na+ and In3+ sites in the Cs2NaInCl6 lattice, respectively. Further, Mn2+ ions are used as dopants to enhance the emission in the orange-red region of the visible spectra. Optimised sample Cs2Na0.6Ag0.4In0.9Bi0.1Cl6:0.15Mn2+ was found to exhibit broadband spectra (410–800 nm) with a quantum efficiency of 44.9%. The structural and morphological properties of the DPs were explored using X-ray diffraction (XRD), Raman spectra, Field Emission Scanning Electron Microscope (FE-SEM) and High-Resolution Transmission Electron Microscope (HR-TEM). It is important to ensure the thermal stability of the luminous material to ensure that the light output from the LEDs remains reliable over an extended time. The thermal stability of the sample was tested; it was found that the emission intensity of the sample stayed up to ∼45% of its original value even at a high temperature of 100 °C. A composite of the DP sample was prepared with a blue emitting phosphor of BaMgAl10O17:Eu2+ (BAM) to obtain a broad emission in the visible spectra under UV excitation (350 nm). Various samples with varying concentrations of BAM: DP perovskite powder were prepared and spectral parameters, such as CIE coordinates, color purity, CRI and CCT, were calculated.
2. Experimental procedure
2.1. Materials and methods
Cesium chloride (CsCl, 99.9%), sodium chloride (NaCl, 99.9%), indium(III) chloride (InCl3, 99.9%), bismuth(III) acetate (CH3COOBi, 99.9%), manganese(II) chloride (MnCl2) and silver(I) acetate (CH3COOAg, 99.9%) were purchased from Sigma Aldrich and were used without any further purification.To synthesize Cs2Na0.6Ag0.4In0.9Bi0.1Cl6:0.15Mn2+, 2 mmol of NaCl, 2 mmol of InCl3, 0.8 mmol of AgCl, 0.4 mmol of BiCl3 and 0.15 mmol of MnCl2 were dissolved in a beaker with 20 ml of HCl. The reaction temperature was kept constant at 80 °C throughout the reaction. After the precursor materials were dissolved completely in HCl, a 4 mmol solution of CsCl was added to the clear solution under constant stirring, and a white precipitate was formed. For an hour, the reaction was allowed to stir. The reaction mixture was centrifuged, and the obtained filtrate was then stored in a hot air oven for 8 hours at 60 °C for drying. The schematic representation of the synthesis procedure is shown in Scheme 1. The brief procedure for the preparation of the Cs2NaInCl6 sample is described in the ESI file in Section 2.†
 | ||
| Scheme 1 Schematic of the various steps involved in the synthesis of Cs2Na0.6Ag0.4In0.9Bi0.1Cl6:0.15Mn2+ perovskite. | ||
2.2. Preparation of a composite of Cs2Na0.4Ag0.6In0.9Bi0.1Cl6:0.15Mn2+ with BaMgAl10O17:Eu2+
The optimised sample of Cs2Na0.4Ag0.6In0.9Bi0.1Cl6:0.15Mn2+ microcrystals was made into a fine powder using a motor and pestle. It was physically blended with the as-prepared blue emitting phosphor BaMgAl10O17:Eu2+ (BAM:Eu2+) using isopropyl alcohol as a solvent. After physical blending, the composite samples were allowed to dry at room temperature. BAM:Eu was prepared via a solid-state reaction process, which is explained in Section 1 in the ESI file.† The prepared DPs Cs2Na0.4Ag0.6In0.9Bi0.1Cl6:0.15Mn2+ were mixed with different ratios of as-synthesized BAM:Eu. The ratios are listed in Table 3. Photoluminescence spectroscopy was recorded for all the composite samples, and various spectral parameters, such as CRI, CIE and CCT, were calculated. The synthesis of BAM:Eu2+ phosphor is described in Section 1 of the ESI file.†2.3. Characterization tools
A Bruker D8 ADVANCE device fitted with a Cu Kα (0.1541 nm) radiation source was used to capture the powder XRD patterns of the prepared perovskite material. The elemental composition of the prepared DPs is analysed through Raman Spectroscopy (Horiba France, XploRA PLUS). Morphological and elemental analyses are performed using a field emission-scanning electron microscope equipped with EDS (FE-SEM, Thermo Fisher, FEI QUANTA 250 FEG). A high-resolution transmission electron microscope (HR-TEM, FEI–TECNAI, G2-20 TWIN, Operating voltage: 200 kV) is used to calculate the average particle size of the prepared DPs. Thermal gravimetric analysis (TGA) helps to determine the thermal stability of the DP material. The optical properties of the prepared samples were characterised using an Agilent UV-vis-NIR spectrophotometer. Steady state and time-resolved photoluminescence spectroscopy at room temperature were performed using photoluminescence spectroscopy (Edinburgh Instruments UK, FLS1000-SS-s). Temperature-dependent photoluminescence spectra for the prepared DPs were recorded using FLMS15147.2.4. Colorimetric theory
The color of any emitting material can be defined using Commission Internationale de l'Eclairage (CIE) coordinates. The CIE coordinates for ideal white light are x = 0.33 and y = 0.33. Color Correlation Temperature (CCT), CRI, LE and lifespan are also important spectral parameters that are used to evaluate artificial lightning.27| CCT = −449n3 + 3535n2 − 6823.3n + 5520.33, |
| Ra = 100 – 4.6ΔEuvw |
3. Results and discussion
3.1. Structural property
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m, with which the [AgCl6]/[NaCl6] octahedra are attached alternatively to [InCl6] to form a 3D structure.35
m, with which the [AgCl6]/[NaCl6] octahedra are attached alternatively to [InCl6] to form a 3D structure.35
Further, the calculation of the average grain size and the lattice strain of the double perovskites is carried out with the WH plot using the following formula:
β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = Kλ/D + 4ε θ = Kλ/D + 4ε![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sinθ, sinθ, |
| Sample | Crystallite size (nm) | Strain | Dislocation density (m−2) | Lattice parameter (Å) (a = b = c) |
|---|---|---|---|---|
| Cs2NaInCl6 | 132 | 1.1 × 10−3 | 0.63 | 10.51 |
| Cs2Na0.6Ag0.4InCl6 | 171 | 1.5 × 10−3 | 0.59 | 10.52 |
| Cs2Na0.6Ag0.4In0.9Bi0.1Cl6 | 65 | 1.7 × 10−4 | 0.53 | 10.48 |
| Cs2Na0.6Ag0.4In0.9Bi0.1Cl6:0.15Mn2+ | 114 | 1.3 × 10−3 | 0.43 | 10.47 |
3.2. Morphological property
 | ||
| Fig. 3 (a–d) FE-SEM image of Cs2Na0.6Ag0.4In0.9Bi0.1Cl6:0.15Mn2+, (e) elemental mapping and (f) EDS mapping of Cs2Na0.6Ag0.4In0.9Bi0.1Cl6:0.15Mn2+. | ||
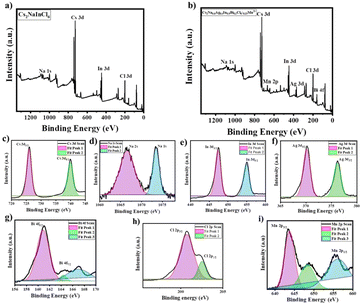 | ||
| Fig. 5 (a) XPS spectra of Cs2NaInCl6, (b) Cs2Na0.6Ag0.4In0.9Bi0.1Cl6:0.15Mn2+, (c) Cs 3d, (d) Na 1s, (e) In 3d, (f) Ag 3d, (g) Bi 4f , (h) Cl 2p and (i) Mn 2p. | ||
3.3. Thermogravimetric analysis
Fig. 6 depicts the thermogravimetric analysis of the as-prepared optimised sample of Cs2Na0.6Ag0.4In0.9Bi0.1Cl6:0.15Mn2+. TGA analysis is performed from room temperature to 800 °C with a N2 atmosphere to analyse the thermal stability of the prepared sample. The decomposition of the sample occurs in two steps/stages. Minor loss of weight (∼15%) is observed beyond 548 °C. A major weight loss of 35% occurs from 450 °C to 600 °C, beyond which an abrupt weight loss of ∼50% occurs beyond 600 °C to 800 °C. Thus, the sample exhibits good thermal stability of up to 500 °C compared to the halide-based perovskites.47,483.4. Optical properties
where α denotes the absorption coefficient, h denotes Planck's constant, C denotes the proportionality constant, v denotes the frequency of light, Eg denotes the band gap and 1/n refers to 1/2 or 2, which denotes that the material possesses either a direct or an indirect band gap, respectively.34 The pristine Cs2NaInCl6 and Cs2Na0.6Ag0.4In0.9Bi0.1Cl6:0.15Mn2+ samples possess an indirect band gap with wide optical band gaps Eg = 5.1 eV and Eg = 3.01 eV, respectively, which is very similar to the literature survey.49 As silver (Ag+) and bismuth (Bi3+) are incorporated into the lattice, there is a huge reduction in the bandgap. As the silver content in the DPs increases, there is a high possibility for an increase in the interaction between Ag 4d and the valence band of the DP lattice, which leads to a reduction in the band gap by ∼1.4 eV, as shown in Fig. S5(a).†12,50 Further, the incorporation of Bi causes a reduction in the band gap from 3.7 eV to 3.01 eV, as shown in Fig. S5(b),† which explains the redshift in the emission wavelength.34,51 After the incorporation of Mn into the optimised lattice, no change in the band gap is observed.
 | ||
| Fig. 8 PL emission spectra of (a) Cs2Na(1−x)AgxInCl6 and (b) Cs2Na0.6Ag0.4In(1−y)BiyCl6. (c) and (d) PLE and PL of Cs2Na0.6Ag0.4In0.9Bi0.1Cl6:0.15Mn2+. | ||
Keeping the concentration of Ag constant (0.4 mmol), a series of samples with variations in Bismuth were prepared, and their PL was recorded, as shown in Fig. 8(b). The optimum concentration of Bi was found to be 0.1 mmol. The addition of Bismuth causes a considerable red shift in the emission spectra from 587 to 614 nm, with the full width at half maxima (FWHM) of 204 nm.55 The incorporation of Bi3+ into the lattice helps enhance the broadband emission from 400 to 750 nm. Bi3+ ions form passivating defects inside the crystal and help to increase the PLQY of Cs2Na0.6Ag0.4In0.7Bi0.1Cl6. Table S1† shows the PLQY of all the optimised samples. Bi3+ introduction also leads to the redshift in the luminescent peak position34,50 and helps extend the excitation till ∼360 nm,55 and PLQY was found to be ∼29.4%. To further enhance the red emission, Mn2+ ion is used as a dopant in the optimised Cs2Na0.6Ag0.4In0.9Bi0.1Cl6. We prepared a series of samples with Mn concentrations ranging from 0.07 to 0.21 mmol. Beyond 0.15 mmol, the photoluminescence intensity was found to reduce, and a slight blue shift in the emission spectra was also observed, as shown in Fig. S5 in the ESI file.† After the incorporation of the optimised amount of Mn2+ (0.15 mmol) into Cs2Na0.6Ag0.4In0.9Bi0.1Cl6, a shift is observed in the emission peak at 665 nm, as shown in Fig. 8(d) and (c), which represent the PLE spectra of Cs2Na0.6Ag0.4In0.9Bi0.1Cl6:0.15Mn2+. In this lattice, Mn2+ ions primarily serve as luminescent centers, enabling strong orange-red photoluminescence and further shifting in the emission peak position of Cs2Na0.6Ag0.4In0.9Bi0.1Cl6 sample by 51 nm. The Mn2+ ions, when incorporated into the lattice, typically occupy octahedral sites and exhibit a characteristic 4T1 → 6A1 transition attributed to the spin-forbidden transition of Mn2+.24,56 After the incorporation of Mn2+ in the optimised lattice, the PLQY reached 44.9%. The broad emission peak of the prepared DPs is observed due to the Self Trapped Exciton (STE)-based emission, which can be explained using Scheme 2. Cs2NaInCl6 exhibits weak blue emission at a wavelength of 450 nm with a low PL intensity that results in optically forbidden transition, which is accompanied by dark STEs of halide double perovskites.57 The broad emission in Cs2Na0.6Ag0.4In0.9Bi0.1Cl6 is due to the inherent property of STE-based emission, which is possessed by the host lattice (transition 3). When Mn2+ is introduced into the lattice, an energy shift occurs from the STEs to 4T1 to 6A1 (transition 4). This leads to the red shift in the emission peak position, and a very distinctive peak position at 665 nm is obtained (transition 5) in Scheme 2.1
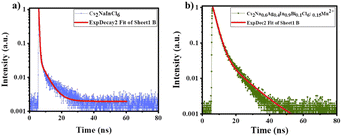
where I and I0 denote the intensities of the photoluminescence emissions at time t and at t = 0. Moreover, τ1 and τ2 are the decay constants, indicating the fast and slow lifetimes for the exponential components, respectively. A1 and A2 are known as the fitting constants or weighting constant parameters. The decay constant of the prepared DPs can be fitted using a bi-exponential equation. The average value of lifetime can be calculated by applying the Inokuti–Hirayama model using the following equation:52
The calculated average lifetime of the prepared DPs (Cs2Na0.6Ag0.4In0.9Bi0.1Cl6:0.15Mn2+) was found to be 2 μs. Decay parameters are listed in Table 2.
| Sample | τ1 | τ2 | A1 | A2 | Avg. lifetime |
|---|---|---|---|---|---|
| Cs2NaInCl6 | 316.68 | 4187.522 | 1.49 | 0.03 | 1 ns |
| Cs2Na0.6Ag0.4In0.9Bi0.1Cl6:0.15Mn2+ | 2261.15 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 643.82 643.82 |
15.36 | 0.10 | 2 ns |
where Io is the emission intensity that is observed at room temperature, IT is the emission intensity of temperature t, C is the arbitrary constant and KB is the Boltzmann constant value of 8.617 × 10−5 eV K−1. The activation energy of the synthesised material determined by calculating the slope of the plot between ln(I0/IT) − 1 and 1/KBT is 0.465 eV.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, 3
8, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, 4
7, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 5
6, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 6
5, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 7
4 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 8
3 and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 by weight%. The room temperature PL emission of all the composite samples was recorded, as shown in Fig. S6.† Various spectral parameters, such as CIE coordinates, CRI values, and CCT, were calculated, as shown in Table 3. A high CRI of ∼87 was obtained for the DP:BAM composite of 6
2 by weight%. The room temperature PL emission of all the composite samples was recorded, as shown in Fig. S6.† Various spectral parameters, such as CIE coordinates, CRI values, and CCT, were calculated, as shown in Table 3. A high CRI of ∼87 was obtained for the DP:BAM composite of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 by weight, as shown in Fig. 11. CIE color coordinates were calculated using the wavelength equidistant method. The as-prepared composite was found to have CIE coordinates of (0.37, 0.39), which is quite close to that of ideal white light (0.33, 0.33). The CIE plots of all the composites are shown in Fig. S7.† The color-correlated temperature was found to be 4453.5 K, which falls under the natural white light category. The CCT of the composite can be tuned from warm (2935 K) to cool white (6789 K).
4 by weight, as shown in Fig. 11. CIE color coordinates were calculated using the wavelength equidistant method. The as-prepared composite was found to have CIE coordinates of (0.37, 0.39), which is quite close to that of ideal white light (0.33, 0.33). The CIE plots of all the composites are shown in Fig. S7.† The color-correlated temperature was found to be 4453.5 K, which falls under the natural white light category. The CCT of the composite can be tuned from warm (2935 K) to cool white (6789 K).
| WLEDs | Mass ratio of BAM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) perovskites perovskites |
CCT/K | CIE (x,y) coordinates | CRI |
|---|---|---|---|---|
| 1 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
2935.45 | (0.44, 0.40) | 68.9 |
| 2 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
3086.65 | (0.42, 0.43) | 78.2 |
| 3 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
3198.13 | (0.41, 0.42) | 82.7 |
| 4 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
3319.18 | (0.40, 0.42) | 80.1 |
| 5 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
3599.35 | (0.38, 0.41) | 82.1 |
| 6 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
4453.52 | (0.37, 0.39) | 87.4 |
| 7 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
3758.60 | (0.34, 0.39) | 81 |
| 8 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
6789.64 | (0.27, 0.34) | 78.1 |
4. Conclusion
In this study, we successfully synthesised pristine Cs2NaInCl6 by applying a facile co-precipitation method. To enhance the photoluminescence properties, Ag and Bi alloying was performed at the Na and In sites to obtain optimised sample.Cs2Na0.6Ag0.4In0.9Bi0.1Cl6. It was observed that the incorporation of a 0.4 mmol concentration of Ag alloying enhanced the emission signal 10 times, with peak maxima at 587 nm. Further, Bi ions cause a red shift in the emission spectra from 587 to 614 nm and increase the FWHM by 204 nm. By incorporating Bi and Ag, PLQY was found to increase by 29.4%. Broad emission owing to the 4T1 → 6A1 transition of Mn2+ ions was observed at 665 nm. The sample also exhibits good thermal stability with a high activation energy of 0.5 eV, and 43% of the initial PL emission intensity stays intact even at a high temperature of 100 °C. Detailed structural, optical, morphological and compositional analyses were conducted for the pristine and optimised samples. PLQY was found to increase from 1.8% to 44.9% upon alloying and doping the pristine sample. Spectral parameters (CIE coordinates of (0.37, 0.39), CCT of 4453.52 K, and CRI of 87.4) were calculated. Thus, combined with blue-emitting phosphors, Cs2Na0.6Ag0.4In0.9Bi0.1Cl6:Mn2+ is a potential material for enhancing the CRI of WLEDs owing to its broad red emission extending in the blue region at 475 nm. The scope of future work involves developing double perovskite materials to obtain complete coverage in the visible spectra.
Data availability
The data supporting the findings of this study are available within the article and its ESI.†Author contributions
D. B.: conception, experimentation, analysis, writing manuscript; G. S.: review and final editing.Conflicts of interest
Authors declare no conflicts of interest.Acknowledgements
The authors would like to acknowledge Centre for Nanotechnology Research, VIT Vellore, for their research facilities and School of Advanced Sciences (SAS), VIT Vellore, for the financial support.References
- M. Hu, S. Li, J. Liu and J. Li, Broadband emission of double perovskite, Opt. Lett., 2019, 44, 4757–4760, DOI:10.1364/OL.44.004757.
- C. Jiawei, et al., Perovskite White Light Emitting Diodes: Progress, Challenges, and Opportunities, ACS Nano, 2021, 15(11), 17150–17174 CrossRef PubMed.
- B. Zhang, et al., Colloidal Bi-Doped Cs2Ag1-xNaxInCl6Nanocrystals: Undercoordinated Surface Cl Ions Limit their Light Emission Efficiency, ACS Mater. Lett., 2020, 2, 1442–1449 CrossRef PubMed.
- P. Han, et al., Lead-Free Sodium–Indium Double Perovskite Nanocrystals through Doping Silver Cations for Bright Yellow Emission, Angew. Chem., 2019, 131, 17391–17395 CrossRef.
- Y. Wu, X. Li and H. Zeng, Lead-Free Halide Double Perovskites: Structure, Luminescence, and Applications, Small Struct., 2021, 2(3), 2000071 CrossRef.
- J. Yao, L. Xu, S. Wang and J. Song, Metal halide perovskites-based white light-emitting diodes, JPhys Photonics, 2022, 4, 042001 CrossRef.
- S. Wang, et al., Novel SrMg2La2W2O12:Mn4+ far-red phosphors with high quantum efficiency and thermal stability towards applications in indoor plant cultivation LEDs, RSC Adv., 2018, 30191–30200, 10.1039/c8ra06134c.
- M. Liu, B. Shen, K. Wang and J. Zhong, RSC Adv., 2019, 20742–20748, 10.1039/c9ra03410b.
- X. Wu, et al., Photoluminescence, energy transfer, color tunable properties of Sr3La(BO3)3:Ce, Tb phosphors, J. Alloys Compd., 2018, 730, 521–527 CrossRef CAS.
- W. Geng, X. Zhou, J. Ding and Y. Wang, NaBaY(BO3)2:Ce3+,Tb3+: A novel sharp green-emitting phosphor used for WLED and FEDs, J. Am. Ceram. Soc., 2018, 101, 4560–4571 CrossRef CAS.
- D. Haranath, et al., Rare-earth free yellow-green emitting NaZnPO4:Mn phosphor for lighting applications, Appl. Phys. Lett., 2012, 101, 221905 CrossRef.
- S. Dan, Origin of bandgap bowing in Cs2Na1− x Ag x BiCl6 double perovskite solid-state alloys: a paradigm through scanning tunneling spectroscopy, J. Phys.: Condens. Matter, 2021, 33(48), 485701 CrossRef CAS.
- Y. Zhou, Impact of Eu3+ dopants on optical spectroscopy of Ce3+: Y3Al5O12-embedded transparent glass-ceramics, J. Am. Ceram. Soc., 2015, 98(8), 2445–2450 CrossRef CAS.
- X. B. Li, W. B. Dai, K. Nie, S. P. Li and M. Xu, Investigation on optical properties of borate Sr3Y2B4O12: Ce/Tb/Sm and its application in wLEDs, J. Lumin., 2023, 263, 120038 CrossRef CAS.
- F. Wang, H. Pan, W. Mao and D. Wang, Heliyon Optimizations of luminescent materials for white light emitting diodes toward healthy lighting, Heliyon, 2024, 10, e34795 CrossRef CAS PubMed.
- W. Chen, et al., Carbon dots embedded in lead-free luminescent metal halide crystals toward single-component white emitters, Sci. China Mater., 2022, 65, 2802–2808 CrossRef CAS.
- S. Thapa, G. C. Adhikari, H. Zhu, A. Grigoriev and P. Zhu, Zn-Alloyed All-Inorganic Halide Perovskite-Based White Light-Emitting Diodes with Superior Color Quality, Sci. Rep., 2019, 9, 18636 CrossRef CAS PubMed.
- S. B. Kundu, P. Pallavi and A. Patra, Chem.–Eur. J., 2020, 26(25), 5557–5582 CrossRef PubMed.
- S. Mukherjee and P. Thilagar, Organic White-Light Emitting Materials, Dyes Pigm., 2014, 110, 2–27 CrossRef.
- P. Cai, X. Wang, H. J. Seo and X. Yan, Bluish-white-light-emitting diodes based on two-dimensional lead halide perovskite (C6H5C2H4NH3)2PbCl2Br2, Appl. Phys. Lett., 2018, 112, 153901 CrossRef.
- Y. H. Song, et al., Design of long-term stable red-emitting CsPb(Br0.4, I0.6)3 perovskite quantum dot film for generation of warm white light, Chem. Eng. J., 2017, 313, 461–465 CrossRef.
- F. Igbari, Z.-K. Wang and L.-S. Liao, Progress of Lead-Free Halide Double Perovskites, Adv. Energy Mater., 2019, 9, 1803150 CrossRef.
- R. L. Z. Hoye, J. Hidalgo, R. A. Jagt, T. Fix and J. L. Macmanus-driscoll, The Role of Dimensionality on the Optoelectronic Properties of Oxide and Halide Perovskites, and their Halide Derivatives, Adv. Energy Mater., 2021, 2100499 Search PubMed.
- L. Zhang, et al., Investigation on lead-free Mn-doped Cs 2 NaInCl 6 double perovskite phosphors and their optical properties, Opt. Mater., 2021, 122, 111802 CrossRef.
- H. Tang, et al., Lead-Free Halide Double Perovskite Nanocrystals for Light-Emitting Applications: Strategies for Boosting Efficiency and Stability, Adv. Sci., 2021, 3, 1–23 Search PubMed.
- M. Tabassum, Q. Zia, Y. Zhou, M. J. Reece and L. Su, A review on advances in doping with alkali metals in halide perovskite materials, SN Appl. Sci., 2021, 3, 1–15 Search PubMed.
- J. Chen, et al., Perovskite White Light Emitting Diodes: Progress, Challenges, and Opportunities, ACS Nano, 2021, 15, 17150–17174 CrossRef PubMed.
- Y. Wang, et al., High color rendering index trichromatic white and red LEDs prepared from silane-functionalized carbon dots, J. Mater. Chem. C, 2017, 5, 9629–9637 RSC.
- P. Bodrogi, S. Brückner, N. Krause and T. Q. Khanh, Semantic interpretation of color differences and color-rendering indices, Color Res. Appl., 2014, 39, 252–262 CrossRef.
- A. K. Vishwakarma, et al., Emerging cool white light emission from Dy3+ doped single phase alkaline earth niobate phosphors for indoor lighting applications, Dalton Trans., 2015, 44, 17166–17174 RSC.
- N. Ohta, and A. Robertson, Colorimetry: fundamentals and applications, John Wiley & Sons, 2006 Search PubMed.
- S. Westland,The CIE System, in Handbook of Visual Display Technology, ed. J. Chen, W. Cranton, M. Fihn, Springer, Cham, 2016, DOI:10.1007/978-3-319-14346-0_11.
- H. A. Badran, et al., Study of Colorimetric properties of Ethidium bromide dye-doped PVP/DNA film, J. Phys.: Conf. Ser., 2021, 1963(1), 012102 CrossRef CAS.
- K. Peng, et al., The synthesis of lead-free double perovskite Cs2Ag0.4Na0.6InCl6 phosphor with improved optical properties via ion doping, J. Alloys Compd., 2022, 891, 161978 CrossRef CAS.
- B. Ke, et al., Homo- and Heterovalent Doping-Mediated Self-Trapped Exciton Emission and Energy Transfer in Mn-Doped Cs2Na1–xAgxBiCl6 Double Perovskites, J. Phys. Chem. Lett., 2020, 11, 340–348, DOI:10.1021/acs.jpclett.9b03387.
- M. Jeevaraj, S. Sudhahar, N. Nallamuthu, P. Devendran and S. Saravanakumar, Physica B : Condensed Matter Solution processed Mn 2 + doped Cs 2 AgInCl 6 lead free double perovskite as a potential light emitting material, Phys. B, 2023, 653, 414679 CrossRef CAS.
- G. Wang, et al., Observation of Extraordinary Vibration Scatterings Induced by Strong Anharmonicity in Lead-Free Halide Double Perovskites, Adv. Sci., 2025, 12, 2408149 CrossRef CAS.
- Q. Chen, et al., single-composition white-light emitting phosphors and multimodal optical, J. Lumin., 2023, 258, 119783 CrossRef CAS.
- P. Han, et al., Lead-Free Sodium – Indium Double Perovskite Nanocrystals through Doping Silver Cations for Bright Yellow Emission, Angew. Chem. Int. Ed., 2019, 1–6, DOI:10.1002/anie.201909525.
- X. Li, et al., Facile Melting-Crystallization Synthesis of Cs2NaxAg1–xInCl6: Bi Double Perovskites for White Light-Emitting Diodes, Inorg. Chem., 2022, 61, 5040–5047, DOI:10.1021/acs.inorgchem.1c03996.
- B. Zhou, et al., Efficient White Photoluminescence from Self- Trapped Excitons in Sb 3+/Bi 3+ -Codoped Cs 2 NaInCl 6 Double Perovskites with Tunable Dual-Emission, ACS Energy Lett., 2021, 6(9), 3343–3351 CrossRef CAS.
- M. Jeevaraj, P. Devendran, N. Nallamuthu, S. Sudhahar and M. K. Kumar, Influence of Mn2+ doping on the optical properties of Cs2AgBiCl6 double perovskite luminescent phosphors, J. Mater. Sci.:Mater. Electron., 2023, 34, 65 CrossRef CAS.
- K. Kumar, Extrinsic electronic states to tune the luminescence and bonding nature of Cs 2 NaInCl 6 double perovskite, Mater. Chem. Phys., 2024, 311, 128569 CrossRef.
- M. Kalapsazova, R. Stoyanova, E. Zhecheva, G. Tyuliev and D. Nihtianova, Sodium deficient nickel–manganese oxides as intercalation electrodes in lithium ion batteries, J. Mater. Chem. A, 2014, 2, 19383–19395 RSC.
- W. Jiang, et al., Improved optical properties of lead-free double perovskite Cs2NaBiCl6 nanocrystal via K ions doping, J. Alloys Compd., 2023, 960, 170871 CrossRef CAS.
- H. Wang, et al., Lead – free – perovskite films with broad warm - yellow emission for lighting applications, Sci. Rep., 2024, 1–14, DOI:10.1038/s41598-024-65492-5.
- L. Zhang, et al., Investigation on lead-free Mn-doped Cs2NaInCl6 double perovskite phosphors and their optical properties, Opt. Mater., 2021, 122, 111802 CrossRef CAS.
- R. Zeng, et al., Highly Efficient Blue Emission from Self-Trapped Excitons in Stable Sb 3+ -Doped Cs 2 NaInCl 6 Double Perovskites, J. Phys. Chem. Lett., 2020, 11(6), 2053–2061 CrossRef CAS PubMed.
- H. You, et al., Tunable-Color UV-Excitation Sb3+/(Bi3+, Ag+)-Doped Cs2NaInCl6 Perovskite for High-Color-Rendering Full-Spectrum w-LEDs, ACS Appl. Opt. Mater., 2023, 1, 788–794 CrossRef CAS.
- F. Liu, et al., White light emission with unity efficiency from Cs2Na1−xAgxIn1−yBiyCl6 double perovskites: the role of bismuth and silver, J. Mater. Chem. C, 2022, 14232–14241, 10.1039/d2tc02919g.
- H. You, et al., Perovskite for High-Color-Rendering Full-Spectrum w - LEDs, ACS Appl. Opt. Mater., 2023, 1(3), 788–794 CrossRef.
- J. Zhou, et al., Manipulation of Bi 3 +/In 3 + Transmutation and Mn 2 + -Doping Effect on the Structure and Optical Properties of Double Perovskite Cs 2 NaBi 1- x In x Cl 6, Adv. Opt. Mater., 2019, 1801435 CrossRef.
- H. Li, et al., Double perovskite Cs2NaInCl6 nanocrystals with intense dual-emission via self-trapped exciton-to-Tb3+ dopant energy transfer, J. Mater. Chem. C, 2022, 10, 10609–10615 RSC.
- R. Ahmad, et al., Uncovering the Role of Trioctylphosphine on Colloidal and Emission Stability of Sb-Alloyed Cs2NaInCl6 Double Perovskite Nanocrystals, ACS Appl. Mater. Interfaces, 2021, 13, 47845–47859 CrossRef.
- M. B. Gray, J. D. Majher, T. A. Strom and P. M. Woodward, Broadband White Emission in Cs2AgIn1–xBixCl6 Phosphors, Inorg. Chem., 2019, 58, 13403–13410, DOI:10.1021/acs.inorgchem.9b02299.
- L. Chen, et al., materials Mn 2 + -doped Cs 2 NaInCl 6 double perovskites and their photoluminescence properties, J. Mater. Sci., 2021, 56, 8048–8059 CrossRef.
- Y. Gao, S. Xu and B. Chen, Lead-free double perovskite Cs2NaInCl6 nanocrystals doped with Sb3+ and Tb3+ for copper ions detection in lubricating oil, Spectrochim. Acta, Part A, 2024, 308, 123683 CrossRef.
- A. M. Achari, V. Perumalsamy, G. Swati and A. Khare, SrAl2O4:Eu2+,Dy3+ Long Afterglow Phosphor and Its Flexible Film for Optomechanical Sensing Application, ACS Omega, 2023, 8, 45483–45494 CrossRef PubMed.
- P. Vanishree and G. Swati, Enhanced photoluminescence in (Ca, Zn)TiO3: Pr3+ afterglow phosphor for anti-counterfeiting application, J. Mater. Sci.:Mater. Electron., 2024, 35, 597 CrossRef.
- X. Huang, Y. Matsushita, H. Sun and N. Shirahata, Nanoscale Adv., 2022, 3091–3100, 10.1039/d2na00238h.
- P. Kanapathi, N. Ravi and T. Appadurai, Investigation on Structurally Engineered Cs2AgInCl6 via Alloying (Na+)/Doping (Cr3+, Mn2+) for Flexible Phosphor-Based White Light Devices and Latent Fingerprint Detection Application, ACS Appl. Energy Mater., 2024, 7, 10026–10036 CrossRef.
- P. Li, et al., Temperature dependent red luminescence from a distorted Mn4+ site in CaAl4O7:Mn4+, Opt. Express, 2013, 21, 18943–18948 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra01415h |
| This journal is © The Royal Society of Chemistry 2025 |