Open Access Article
Open Access ArticleSynthesis of fungal polysaccharide-based nanoemulsions for cancer treatment
Archna Dhasmana†
 *a,
Pooja Dobhal†a,
Abhilekh Satia,
Ayushi Santhanam
*a,
Pooja Dobhal†a,
Abhilekh Satia,
Ayushi Santhanam a,
Subham Preetam
a,
Subham Preetam b,
Sumira Malik*cde,
Nupur Joshia,
Sarvesh Rustagif and
Ravi K. Deshwalg
b,
Sumira Malik*cde,
Nupur Joshia,
Sarvesh Rustagif and
Ravi K. Deshwalg
aHimalayan School of Biosciences, Swami Rama Himalayan University, 248104, Dehradun, India. E-mail: archnadhasmana@srhu.edu.in; poojadobhal656@gmail.com; abhilekhsati09@gmail.com; ayushisanthanam@gmail.com; nupurjoshi@srhu.edu.in
bDaegu Gyeongbuk Institute of Science and Technology (DGIST), Daegu, 42988, Republic of Korea. E-mail: sspritamrath93@gmail.com
cAmity Institute of Biotechnology, Amity University Jharkhand, Ranchi, Jharkhand 834001, India. E-mail: smalik@rnc.amity.edu
dSchool of Applied and Life Sciences, Uttaranchal University, 22 Dehradun 248007, Uttarakhand, India
eUniversity Center for Research & Development (UCRD) Chandigarh University, NH-05 Chandigarh- Ludhiana Highway, Mohali, Punjab 140413, India
fDepartment of Food Technology, School of Agriculture, Maya Devi University, Dehradun, Uttarakhand, India
gSRM University, Lucknow- Deva Road, Uttar Pradesh, India. E-mail: ravideshwal.ibst@srmu.ac.in
First published on 25th April 2025
Abstract
Long valued for their therapeutic qualities, shiitake mushrooms (Lentinula edodes) are a staple of traditional Asian medicine and cuisine. They are high in bio-actives such as polysaccharides, proteins, lipids, vitamins, minerals, sterols, and phenolic compounds, which exhibit immunomodulatory, anticancer, antibacterial, anti-inflammatory, and anti-oxidant properties. Despite these advantages, the limited bioavailability and stability of shiitake's bio-active components often restrict their therapeutic use. Recent advances in nanotechnology have led to the development of nanoemulsions to encapsulate bioactives, which enhanced their bioavailability, stability, and therapeutic efficacy. In this study, we developed a biopolymeric blend of zein and chitosan as a nanoemulsion for the encapsulation of crude shiitake extract. Focusing on the synthesis and refinement of bio-compatible nanoemulsion formulations, this study investigates the medicinal potential of shiitake mushrooms and their nanoemulsions using several in vitro assays: the DPPH assay for anti-oxidant activity; the BSA denaturation assay for anti-inflammatory activity; the MIC test for antimicrobial activity; and the MTT assay for anticancer activity. This study aimed to attain three main goals: synthesis of nanoemulsions, biochemical analysis of shiitake extracts, and in vitro characterization of the therapeutic efficacy of the resulting formulations. This study found that shiitake nanoemulsions showed significantly improved bio-availability and therapeutic efficacy, suggesting promising applications in pharmaceuticals, nutraceuticals, cosmetics, medicine, and the food industry.
1. Introduction
Shiitake mushrooms (Lentinula edodes), traditionally consumed as food, are rich in bioactive compounds such as polysaccharides (lentinan and β-glucan), proteins, lipids, vitamins (B and D), minerals, sterols, and phenolic compounds and hence have been used to treat various ailments.1 In traditional Asian medicine, shiitake are known to wield immunomodulatory, anticancer, antimicrobial, anti-inflammatory, anti-oxidant and cholesterol-lowering properties.2 Owing to these diverse therapeutic benefits, shiitake mushrooms are increasingly in demand as a functional food and are gaining recognition as an extraordinary potent source of novel therapeutic agents in modern medicine.3 However, a major limitation in their application is the poor bioavailability and stability of their bioactive components, but these challenges can be overcome through advanced drug-delivery systems.Nanotechnology offers a promising solution to improving the bioavailability, stability, delivery efficacy, and therapeutic potential of bioactive molecules via nanocarriers such as nanoemulsions. Nanoemulsions are ultra-fine oil-in-water or water-in-oil dispersions with minuscule droplet sizes ranging from 20 to 200 nanometres. Their large surface area facilitates the enhanced absorption and improved bioavailability of encapsulated compounds.4 The desired therapeutic components derived from the mushrooms can be encapsulated within nanoemulsions for their targeted delivery with minimal loss of function and efficacy.5,6
In cancer therapy and imaging, nanoemulsions exhibit unique and dynamic properties, which make them promising candidates for targeted drug delivery and cancer cell imaging.7,8 A wide range of advanced techniques are employed for nanoemulsion fabrication, e.g., high-pressure homogenization, ultrasonication and microfluidization, which require high frequency or applied force to form uniformly dispersed and stable bi-phase nanodroplets.9
In addition to the synthesis method, the selection of emulsifying agents affects the physiochemical properties and bioavailability of encapsulated drug components.10 Both natural and synthetic polymers have been used to stabilize nanoemulsions and enhance drug loading and retention, thereby improving the therapeutic index and efficacy even at low dosages. The retention time of shiitake and flavours were noticeably enhanced after encapsulating in cyclodextrin and maltodextrin powders.6
In a preliminary study, gelatin and carboxymethylcellulose microcapsules containing shiitake essential oil (SEO) were synthesized via complex coacervation to prevent oxidation and enable controlled release.11 Subsequently, the development of shiitake polysaccharide water-in-oil nanoemulsion with a droplet size of 144.5 nm significantly enhanced stability, absorption and anti-tumor activity, indicating its potential in the health, food and pharmaceutical sectors.12
Among all the polymers, chitosan is a non-toxic, protective-layer, film-forming, and biodegradable bio-polymer used in biomedical applications.13 Its structure contains aromatic rings with one or more hydroxyl groups and positively charged amino groups, enabling strong ionic interactions to form films and barrier protective layers.12,13 However, the application of pure chitosan-based films is limited owing to the films' chemical instability in acidic and alkaline environments and their swelling behavior in water.14,15 Therefore, blending with other polymers or ceramics is often required to improve the stability and flexibility of the chitosan film coating as per the desired purposes.
Zein, a biodegradable polymer derived from corn, exhibits strong moisture resistance and film-forming capabilities, and has been explored for various applications. For instance, chitosan–zein (Cs–Zn) coated PGE nanoliposomes loaded with Pulicaria gnaphalodes extract have been used in the meat industry as active packaging materials.16 In another study, chitosan/zein/lemon essential oil (LEO) composite films (C/Z/L films) with varying oil concentrations improved the anti-oxidant and antibacterial properties of the mushrooms during refrigerated storage, at 4 °C for 12 days.17 These studies demonstrated that zein dispersion with chitosan improves mechanical properties while reducing moisture permeability.17–19 As mentioned above, shiitake essential oils and polysaccharides are readily oxidized and degraded under extreme light, heat, and oxygen conditions, but their encapsulation within film coatings can enhance their stability—an approach widely applied in the food, pharmaceutical, cosmetic, and agricultural industries.20,21 These studies proven that many active compounds are susceptible to environmental degradation, coated with the biopolymer-based films having antibacterial, anti-oxidant, and other functional properties ensure the stability and controlled release of bioactives.13,22
Hence, the encapsulation of shiitake bio-actives in nanoemulsions could revolutionize their applicability in food, cosmetic and biomedical industries. The aim of our study is to develop a novel polymeric blend for encapsulating shiitake bioactives in the form of nanoemulsions to enhance their bioavailability and stability via a cutting-edge nanotechnology approach. By investigating the therapeutic efficacy of shiitake encapsulated within zein–chitosan nanoemulsions, we seek to develop and optimize nanoemulsion formulations and evaluate their applicability as novel advanced drug delivery systems. This research demonstrates shiitake-zein–chitosan nanoemulsions as a novel, superior therapeutic system that can be potentially used as a potent delivery system for clinical and nutraceutical applications.
2. Materials and methods
Dried shiitake mushrooms were procured from a local market, and all reagents used for sample preparation and characterization were of high purity grade. To prepare the stock solution, 50 mg of dried mushroom powder was dissolved in 50 mL of 0.1 M acetic acid at 50 °C for 2 hours under constant stirring. After complete dissolution, the extract was filtered to remove the undissolved impurities or bulky particles and the filtrate was stored at 4 °C for subsequent in vitro testing and nanoemulsion preparation. The shiitake-loaded zein–chitosan nanoemulsion was formulated using a liquid–liquid dispersion method.23 In brief, fresh stock solution (1 mg mL−1) of the shiitake extract diluted into variable dilutions having concentration (20, 40, 60, 80, 100 μg mL−1), 1% w/v zein solution dissolved in 90% ethanol, and 2% w/v chitosan solution dissolved in 1% v/v acetic acid were prepared under aseptic conditions and kept at RT (27 °C). Subsequently, the zein solution was mixed with different concentrations of the shiitake extract under continuous stirring at 500 rpm up to 1 hour, and the samples were then diluted with glycerol (0.6 mL for 100 mL solution) as a plasticizer. Later, the outer coating of chitosan was prepared by adding it dropwise into the zein-shiitake blend in various ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 3
1, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), followed by mixing them at 80 °C and 500 rpm for 1–2 hours and freeze-drying to ensure thorough mixing and encapsulation.
1), followed by mixing them at 80 °C and 500 rpm for 1–2 hours and freeze-drying to ensure thorough mixing and encapsulation.
2.1 Characterization of the fabricated drug loaded scaffold
For uniform dispersion and dermal sorption, the spreadability of the prepared nanoemulsion was measured using the standard protocol given in the literature.27 In brief, the samples were placed between the two glass slides. The upper slide was connected to a balance via a hook, and the lower slide was fixed to a wooden plate. The following formula was used for evaluating the spreadability of the prepared nanoemulsion gel:
| S = M × L/T, |
The functional groups and chemical interactions between the nanoemulsion constituent parts were investigated using FTIR spectral analysis.28 For this qualitative examination, crushed pellets of the sample were prepared with potassium bromide (at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 mg), and an FTIR spectrophotometer (Thermo Nicolet, USA) was used to record absorbance in the range of 4000–400 cm−1.
900 mg), and an FTIR spectrophotometer (Thermo Nicolet, USA) was used to record absorbance in the range of 4000–400 cm−1.
2.2 In vitro antioxidant assay
The DPPH assay was used to evaluate the antioxidant activity of the prepared shiitake extract dilutions in triplicate. The assay measured the ability of antioxidants to scavenge the stable free radical DPPH.29 In brief, all the reagents and test samples (chitosan–zein (CZ) emulsion, shiitake crude extract and SCZ nanoemulsions) were added to separate test tubes as follows: negative control (1000 μL DPPH, 200 μL ethanol, and 800 μL Tris–HCl), positive control (1000 μL DPPH, 200 μL ascorbic acid, and 800 μL Tris–HCl), and test groups (1000 μL DPPH, 200 μL test samples, and 800 μL Tris–HCl). The mixtures were incubated for 30 minutes in the dark, and their absorbance was measured at 517 nm. The inhibition activity ratio and IC50 were calculated using the following formula:2.3 In vitro anti-inflammatory assay
The anti-inflammatory activity of the shiitake extracts and their nanoemulsion was determined using the BSA denaturation assay.30 Different concentrations of chitosan–zein (CZ) emulsion, shiitake crude extract, SCZ nanoemulsions and standard NSAID (diclofenac) (20, 40, 60, 80, and 100 μg mL−1) were prepared. The reaction mixture was prepared by adding 1 mL of BSA solution to 1 mL of each dilution of the shiitake extract, diclofenac, and nanoemulsions. The mixture was incubated at 37 °C for 15 minutes and then heated at 70 °C for 5 minutes. The optical density (OD) of the solutions was measured at 660 nm to calculate the percentage of denaturation using the following equation:2.4 In vitro antimicrobial activity
The antimicrobial activity of the test samples was tested against fungal as well as Gram positive and negative bacterial strains using the broth dilution method.31 Freshly prepared nutrient broth was inoculated with 1 μg or 1 μL of pure microbial culture (positive control). Serial dilutions of the crude mushroom extract and its nanoemulsions stock solution (20, 40, 60, 80 and 100 μg mL−1) at a concentration of 1 mL were added to test tubes containing 5 mL of nutrient broth, inoculated with 5 μL of pure microbial culture. All the reagents were mixed well and incubated at 37 °C for 12 hours (E. coli and P. aeruginosa) and 48 hours (S. aureus) duration. After the incubation period, the absorbance of the samples was measured at 620 nm to determine antibacterial activity, where the absorbance of the sample indicates bacterial growth in the medium. However, the anti-fungal activity of the samples was tested against the fungal strain Candida albicans, cultured in potato dextrose broth, (PDB) followed by incubation at 35 °C for 48 hours. The effectiveness of the shiitake extract and nanoemulsion was interpreted as half maximal inhibitory concentration (IC50), minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC), shows complete inhibition of microbial visibility and was calculated using the linear regression method. In brief, for MIC determination, appropriate controls were set up: negative control (containing only the medium, without drug and no bacterial inoculation) taken as baseline for no growth; positive control (containing only the medium, bacterial inoculation without drug) represents maximum growth control or baseline for growth. Here, we used the absorbance of the negative control to calculate the MIC of the test sample in the equation y = mx + c, derived using the linear regression method.2.5 In vitro anticancer activity
The anticancer activity of the shiitake extracts and nanoemulsion was assessed using the standard MCF-7 breast cancer cell line (ATCC number HTB-22), procured from the university's cancer lab.32 Cancer cells were cultured in a cell specific nutrient growth medium by placing the tissue culture plates or flask in the CO2 incubator at 37 °C with 5% CO2 supply until the sub-confluent stage was reached. Once the cultured cells reached the sub-confluent stage, they were harvested via the trypsinization method, and cell viability was measured using the trypan blue exclusion assay. The cytotoxic effect of the extracts and nanoemulsions was determined using a 96-well tissue culture plate method. In brief, samples (negative control: no treatment; positive control: cisplatin (15 μg mL−1); test samples – shiitake crude extract and SCZ nanoemulsions of variable concentration: 20 μg mL−1) at a concentration of 15 μM were added into the wells containing 100 μL cell culture medium and 5 μL cell suspension (1 × 103 cells per μL). All the components were mixed well, and the cell culture plates were incubated in a CO2 incubator at 37 °C for 24 and 72 hours. After incubation, cell viability of the incubated samples was assessed using the MTT assay by measuring absorbance at 570 nm. All the samples were prepared in triplicate, and quantitative results were statistically analyzed (mean ± SD) to evaluate significant differences and calculate IC50 using the linear regression method. Subsequently, the cell proliferation rate was assessed through microscopic examination after 72 hours of incubation.3. Results
3.1 Verification of nanoemulsion formation
The zein–chitosan blend yielded optimal results when mixed in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, producing a more homogeneous nanoemulsion compared with other formulations. The resulting shiitake-encapsulated nanoemulsions (SCZ-1–5) with variable concentrations of the shiitake extract (20, 40, 60, 80 and 100 μg mL−1, respectively) were further optimized by measuring the droplet size, pH, viscosity, and chemical interaction between the components using standard techniques to ensure stability against coalescence and phase separation (Fig. 1A). The microscopic images showed the formation of nanometer range emulsion droplets; however, the droplet size consistently decreased from 629 nm to 181 nm with increasing concentration of the shiitake crude extract (20–100 μg mL−1) owing to greater separation of the oil phase in the SCZ emulsified solution (Fig. 1B). The high-resolution microscopic study of FESEM and TEM images (Fig. 1C) of nanoemulsion showed the ultrastructure and confirmed the encapsulation of shiitake extract with a cross-linked zein–chitosan polymeric coating. The polymeric zein–chitosan blend (CZ) showed larger droplet formation with an average size of 699 ± 53.5 nm, which made the blend comparatively less stable than the SCZ emulsions. Subsequently, other physicochemical parameters were assessed (Table 1). The pH of the SCZ nanoemulsions ranged from 6.14 to 5.48, which is suitable for maintaining the balance of the skin's natural oils and protecting it from harmful bacteria. The viscosity and spreadability of the SCZ nanoemulsions ranged from 12
1 ratio, producing a more homogeneous nanoemulsion compared with other formulations. The resulting shiitake-encapsulated nanoemulsions (SCZ-1–5) with variable concentrations of the shiitake extract (20, 40, 60, 80 and 100 μg mL−1, respectively) were further optimized by measuring the droplet size, pH, viscosity, and chemical interaction between the components using standard techniques to ensure stability against coalescence and phase separation (Fig. 1A). The microscopic images showed the formation of nanometer range emulsion droplets; however, the droplet size consistently decreased from 629 nm to 181 nm with increasing concentration of the shiitake crude extract (20–100 μg mL−1) owing to greater separation of the oil phase in the SCZ emulsified solution (Fig. 1B). The high-resolution microscopic study of FESEM and TEM images (Fig. 1C) of nanoemulsion showed the ultrastructure and confirmed the encapsulation of shiitake extract with a cross-linked zein–chitosan polymeric coating. The polymeric zein–chitosan blend (CZ) showed larger droplet formation with an average size of 699 ± 53.5 nm, which made the blend comparatively less stable than the SCZ emulsions. Subsequently, other physicochemical parameters were assessed (Table 1). The pH of the SCZ nanoemulsions ranged from 6.14 to 5.48, which is suitable for maintaining the balance of the skin's natural oils and protecting it from harmful bacteria. The viscosity and spreadability of the SCZ nanoemulsions ranged from 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 865 cps to 11
865 cps to 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 686 cps and 6.75 g cm s−1 to 5.61 g cm s−1, respectively, indicating a better sorption rate and spreadability. In previous studies, the polymeric-formulated nanoemulsion gel exhibited a pH range of 6.01–6.13, viscosity of approximately 12
686 cps and 6.75 g cm s−1 to 5.61 g cm s−1, respectively, indicating a better sorption rate and spreadability. In previous studies, the polymeric-formulated nanoemulsion gel exhibited a pH range of 6.01–6.13, viscosity of approximately 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cps, and spreadability of 5.8–6.4 g cm s−1, which are considered optimal for topical application.26,33 Moreover, the chitosan–zein polymeric blend, owing to its hydrophilic (chitosan) and hydrophobic (zein) properties, plays a key role in enhancing the sorption rate of the SCZ formulation on tissue, thereby improving the therapeutic index. Hence, SCZ nanoemulsion has potential applications in drug delivery, cosmetic formulations, and food products. The product could be considered edible considering the use of zein, a hydrophobic prolamin protein isolated from corn that is given the GRAS (generally recognized as safe) status. Conclusively, the zein–chitosan nanocomposite for phase-contrast nanoemulsion formation is favored for its compatibility, degradability, and non-toxicity as a coating material for biological applications.
000 cps, and spreadability of 5.8–6.4 g cm s−1, which are considered optimal for topical application.26,33 Moreover, the chitosan–zein polymeric blend, owing to its hydrophilic (chitosan) and hydrophobic (zein) properties, plays a key role in enhancing the sorption rate of the SCZ formulation on tissue, thereby improving the therapeutic index. Hence, SCZ nanoemulsion has potential applications in drug delivery, cosmetic formulations, and food products. The product could be considered edible considering the use of zein, a hydrophobic prolamin protein isolated from corn that is given the GRAS (generally recognized as safe) status. Conclusively, the zein–chitosan nanocomposite for phase-contrast nanoemulsion formation is favored for its compatibility, degradability, and non-toxicity as a coating material for biological applications.
| Nanoemulsion samples | SCZ-1 | SCZ-2 | SCZ-3 | SCZ-4 | SCZ-5 |
|---|---|---|---|---|---|
| % shiitake extract (μg mL−1) | 20 | 40 | 60 | 80 | 100 |
| Droplet size (nm) | 629.6 ± 10.25 | 448 ± 17.65 | 322.4 ± 11.24 | 252.1 ± 7.87 | 181.3 ± 3.67 |
| pH | 6.14 ± 0.02 | 6.07 ± 0.03 | 5.87 ± 0.01 | 5.69 ± 0.04 | 5.48 ± 0.01 |
| Viscosity (cps) | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 865 ± 2.30 865 ± 2.30 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 996.33 ± 3.66 996.33 ± 3.66 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 887.67 ± 4.33 887.67 ± 4.33 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 775.33 ± 4.84 775.33 ± 4.84 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 686.67 ± 1.66 686.67 ± 1.66 |
| Spreadability (g cm s−1) | 6.75 ± 0.02 | 6.44 ± 0.02 | 6.17 ± 0.01 | 5.92 ± 0.03 | 5.61 ± 0.03 |
3.2 FTIR analysis
The purity and presence of all the essential functional group peaks in the nanoemulsion components were verified by FTIR spectral analysis, as mentioned in the literature32 (Fig. 2). The FTIR spectra of the shiitake crude extract showed a sharp, narrow peak of hydroxyl (-OH) at 3293 cm−1, of C–H vibration at 2916 cm−1, of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, and C–H in-plane bending vibrations at 1636 cm−1 and 1422 cm−1, respectively, and of C–O stretching vibration at 1041 cm−1. Pure chitosan showed characteristic peaks of hydroxyl (-OH) and amine (-NH2) stretching at 3445 cm−1, of carbonyl (C
C, and C–H in-plane bending vibrations at 1636 cm−1 and 1422 cm−1, respectively, and of C–O stretching vibration at 1041 cm−1. Pure chitosan showed characteristic peaks of hydroxyl (-OH) and amine (-NH2) stretching at 3445 cm−1, of carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching at 1636 cm−1, of N–H bending and C–N stretching at 1588 cm−1, and of other glycosidic bonds at 1322 cm−1. Likewise, zein, a prolamin protein derived from corn, exhibited a distinct FTIR spectrum reflecting its proteinaceous nature: spectra peaks at 3440 cm−1 corresponded to N–H stretching vibrations, and 2936 cm−1 C–H stretching peaks were associated with the aliphatic side chains of amino acids. The 1635 cm−1 strong peak due to C
O) stretching at 1636 cm−1, of N–H bending and C–N stretching at 1588 cm−1, and of other glycosidic bonds at 1322 cm−1. Likewise, zein, a prolamin protein derived from corn, exhibited a distinct FTIR spectrum reflecting its proteinaceous nature: spectra peaks at 3440 cm−1 corresponded to N–H stretching vibrations, and 2936 cm−1 C–H stretching peaks were associated with the aliphatic side chains of amino acids. The 1635 cm−1 strong peak due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations in the peptide bonds, primarily indicating α-helical structures at 1072 cm−1, indicated C–O bending. In the shiitake encapsulated chitosan–zein nanoemulsion, the shift in the peak at 3440–3480 cm−1 indicated hydrogen bonding. Besides, the formation of new functional peaks between 1500 cm−1 and 1700 cm−1, corresponding to amide I and amide II groups indicates the formation of N–H and C–N bonds, respectively, suggesting stronger molecular interactions. FTIR analysis of the pure components of the nanoemulsions and the combined nanoemulsion provides valuable information about their interaction, structural integrity, and potential chemical bonding.34,35 In the previous studies, the chitosan–zein combination was used as a nanocarrier system for the delivery of bioactive components as a stabilized delivery matrix.36,37 Conclusively, the overlapping peaks, shifts, and changes in peak intensity may indicate physical blending or chemical interactions between these biopolymers with shiitake active components, which are critical for applications in fields such as food science, drug delivery, and biomaterials.
O stretching vibrations in the peptide bonds, primarily indicating α-helical structures at 1072 cm−1, indicated C–O bending. In the shiitake encapsulated chitosan–zein nanoemulsion, the shift in the peak at 3440–3480 cm−1 indicated hydrogen bonding. Besides, the formation of new functional peaks between 1500 cm−1 and 1700 cm−1, corresponding to amide I and amide II groups indicates the formation of N–H and C–N bonds, respectively, suggesting stronger molecular interactions. FTIR analysis of the pure components of the nanoemulsions and the combined nanoemulsion provides valuable information about their interaction, structural integrity, and potential chemical bonding.34,35 In the previous studies, the chitosan–zein combination was used as a nanocarrier system for the delivery of bioactive components as a stabilized delivery matrix.36,37 Conclusively, the overlapping peaks, shifts, and changes in peak intensity may indicate physical blending or chemical interactions between these biopolymers with shiitake active components, which are critical for applications in fields such as food science, drug delivery, and biomaterials.
 | ||
| Fig. 2 FTIR spectra of pure components: shiitake crude extract, chitosan, zein, and the nanoemulsion of shiitake-chitosan–zein indicating the functional group's peaks and chemical modifications. | ||
3.3 In vitro anti-inflammatory and anti-oxidant activity
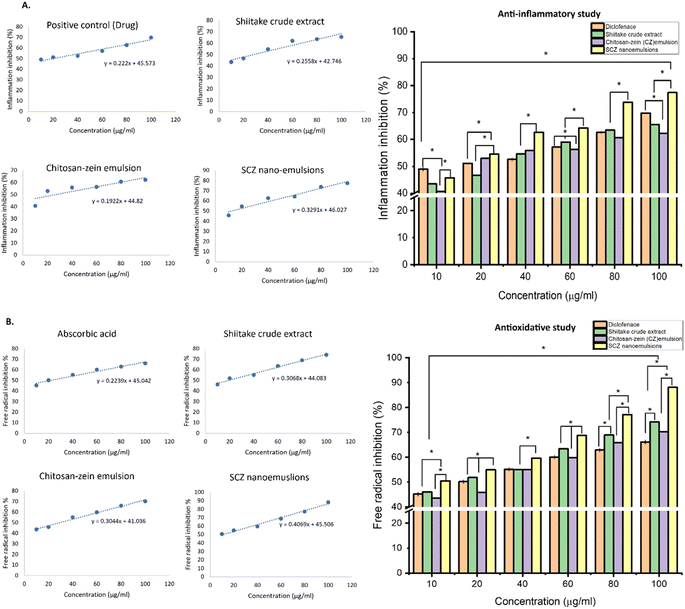
The anti-inflammatory activity of the samples (control and test) was evaluated using the BSA denaturation assay, it revealed significant improvement in the inhibition% of the shiitake in the SCZ nanoemulsion. Likewise, the synergistic effect of the shiitake along with chitosan–zein showed a concentration-dependent inhibition activity of the samples, consistently increasing with the concentration of the extract and shiitake in the polymeric nanoemulsions (SCZ1 to SCZ5) (Fig. 3A). The maximum inhibition was reported in the nanoemulsion compared with the pure shiitake extract and CZ emulsion, which verified that after entrapment, the activity of the shiitake extract significantly improved, with an even greater inhibition percentage at the same concentrations compared with the shiitake crude extract. The IC50 value calculated (Table 2) for the SCZ nanoemulsion was 12.07 μg mL−1, which was significantly lower than that (19.94 μg mL−1) of the standard drug (diclofenac), and the IC50 for the carrier matrix, i.e., the chitosan-zein emulsion, was significantly higher (26.95 μg mL−1) than that (28.35 μg mL−1) for the shiitake crude extract.| IC50 concentration (μg mL−1) | Anti-inflammation% | Anti-oxidation% |
|---|---|---|
| Standard (diclofenac and ascorbic acid) | 19.94 | 22.14 |
| Shiitake crude extract | 28.35 | 29.06 |
| Chitosan–zein emulsion | 26.95 | 29.44 |
| SCZ nanoemulsions | 12.07 | 11.04 |
Antioxidant properties assessed by the DPPH assay methods of the control and test samples, including the shiitake crude extract and SCZ nanoemulsion, showed potential free-radical inhibition activity of the compounds (Fig. 3B). The study demonstrated a concentration-dependent free radical scavenging effect of the shiitake extract, which increased with increasing the concentration from 10 to 100 μg mL−1. However, the inhibition efficiency of ascorbic acid, CZ emulsion and shiitake crude extract was significantly lower than that of the SCZ nanoemulsions at various concentrations of the encapsulated shiitake extract. The observed concentration-dependent inhibition effect of shiitake with or without polymeric encapsulation indicates that the CZ coating controls the release kinetics of the shiitake bioactive components and enhances the therapeutic effect compared with the control sample (Fig. 3B). The IC50 values (μg mL−1) (Table 2) calculated using the linear equation were as follows: 22.14 μg mL−1 for the positive control (ascorbic acid), 29.44 μg mL−1 for the CZ emulsion, 29.06 μg mL−1 for the shiitake crude extract and 11.04 μg mL−1 for the SCZ nanoemulsions. Here, the SCZ nanoemulsion with 11.04 μg mL−1 shiitake crude extract inhibited 50% of the free ionic radical generation, which is significantly lower compared with pure CZ emulsion and the shiitake crude extract. Furthermore, the aforementioned results verified that the biostability and therapeutic effect, including anti-oxidant and anti-inflammatory properties of the shiitake mushrooms improved after the encapsulation within the C–Z polymeric blend, and CZ could be the a potential natural formulation for the delivery of bioactive components. Hence, the encapsulation of the shiitake crude extract in a chitosan–zein–nanoemulsion further enhanced the free radical scavenging and inflammatory response compared with the non-encapsulated samples.23,36,38 The enhancement suggests that encapsulation increases the potency and efficacy of the shiitake bioactive components, improving their bioavailability, biocompatibility, and stability. These findings support the potential therapeutic use of shiitake mushrooms and SCZ nanoemulsion formulations in managing inflammatory conditions.
3.4 In vitro anti-microbial activity
The antimicrobial activity of the shiitake crude extract and SCZ nanoemulsions with variable concentrations was estimated using the broth culture method, which showed a significant improvement in growth inhibition activity. A notable reduction in OD indicated the inhibition of microbial growth with the increasing concentration of shiitake, indicating the potential anti-bacterial effect against the Gram-positive bacterium S. aureus and Gram-negative bacteria E. coli and P. aeruginosa as well as an anti-fungal effect on the pathogenic yeast C. albicans (Fig. 4). However, after 24–48 h of incubation, the MIC50 and MIC90 of the CZ emulsion, shiitake crude extract and SCZ nanoemulsion estimated using the dilution method, indicated the improvement of the inhibition rate with an increasing concentration of shiitake in the nanoemulsion (Fig. 5 and Table 3). The absorbance of the control sample showed higher growth, which was significantly reduced, and SCZ inhibited microbial growth of P. aeruginosa (MIC50 = 192.25 μg mL−1), S. aureus (MIC50 = 200 μg mL−1), E. coli (MIC50 = 205.33 μg mL−1), and C. albicans (MIC50 = 210.66 μg mL−1). However, the MIC value of the shiitake and SCZ nanoemulsion was identified as the lowest concentration compared with CZ emulsion, indicating the maximum inhibitory rate against microbial cells. Here, the CZ emulsion, shiitake crude extract and SCZ nanoemulsion concentration consistently reduced the microbial growth with the following 50% inhibition rate values (MIC50, μg mL−1): P. aeruginosa = 192.94, 174.75 and 100.19 μg mL−1; E. coli = 501.87, 182.126, and 134.04 μg mL−1; S. aureus = 371.53, 188.73, and 162.9 μg mL−1; and C. albicans = 665.27, 160.91, 155.99 μg mL−1, respectively. Likewise, following 90% growth inhibition concentrations (MIC90) values were calculated for the SCZ emulsion: P. aeruginosa = 385.29 μg mL−1, E. coli = 350.73 μg mL−1, S. aureus = 438 μg mL−1 and C. albicans = 514.73 μg mL−1, with the lowest value observed for the Gram negative bacteria. Similar effects were observed for the CZ nanoemulsion MIC90 values for P. aeruginosa (552.82 μg mL−1), E. coli (1817.66 μg mL−1), S. aureus (1001.44 μg mL−1), and C. albicans (1479.93 μg mL−1). However, in the case of the shiitake crude extract, the MIC90 value for P. aeruginosa (721.24 μg mL−1) was the highest among those for E. coli (454.79 μg mL−1),S. aureus (507.97 μg mL−1), and C. albicans (529.23 μg mL−1). In the previous studies, it was reported that the medicinal mushroom shiitake, when culinary processed, showed an antimicrobial effect on a wide range of pathogenic microbial strains, including bacterial, yeast and fungal strains.31,39 In this study, the encapsulation of the shiitake crude extract demonstrated potential to inhibit microbial growth, which was improved after the encapsulation in nanoemulsion form, effectively suppressing the growth of the Gram-positive and Gram-negative bacterial strains, along with an anti-fungal effect at lower inhibitory dosages compared with the shiitake crude extract alone. However, further broad-spectrum studies are needed to elaborate on their applicability as antimicrobial agents for pathogenic or infectious studies.| Strain | P. aeruginosa | E. coli | S. aureus | C. albicans | ||||
|---|---|---|---|---|---|---|---|---|
| MIC value (μg mL−1) | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 |
| CZ emulsion | 174.75 | 552.82 | 501.87 | 1817.66 | 371.53 | 1001.44 | 665.27 | 1479.93 |
| Shiitake | 192.94 | 721.34 | 182.13 | 454.79 | 188.73 | 507.97 | 160.91 | 529.23 |
| SCZ nanoemulsion | 100.19 | 385.29 | 134.04 | 350.73 | 162.9 | 438 | 155.9 | 514.73 |
3.5 In vitro anti-cancer activity
The MTT assay was conducted to evaluate the anti-cancer activity of the chitosan–zein emulsion, shiitake crude extract and shiitake nanoemulsion against the MCF-7 cell line; the results revealed that cell viability decreased with increasing concentrations of the shiitake extract, indicating significant anticancer activity of the nanoemulsion (Fig. 6). While no substantial changes in cell viability were observed after 24 hours, a notable reduction in cell viability was evident after 48–72 hours of incubation, with increased concentrations leading to greater effects, as illustrated in (Fig. 6A). The lowest cell viability was recorded for the samples S4 and S5, indicating the maximum toxic effect of the nanoemulsions against the breast cancer line. The growth inhibition study of 72 h cell incubation (Fig. 6B) with variable concentrations of CZ emulsion, shiitake crude extract and SCZ nanoemulsion indicated the significant cytotoxic effect on the cancer cell line with increasing concentration. The IC50 value of CZ emulsion 29.57 μg mL−1 was much higher in comparison with that of the shiitake crude extract (12.74 μg mL−1) and SCZ nanoemulsion having a shiitake concentration of 3.05 μg mL−1. Therefore, the IC50 value of the test samples i.e., shiitake when entrapped in CZ emulsion, indicates the enhancement of the therapeutic index of the drug nanoemulsion, which showed a significant role, especially in improving the sorption rate of the hydrophobic drug and its targeted delivery.40 Similar microscopic images (Fig. 6C) of the cells showed that cells treated with the drug, and test samples with a minimum concentration of 20 μg mL−1, had a reduced number of viable cells with the progression of incubation time compared with the untreated cells, which exhibited a higher number of proliferated cells. Furthermore, edible mushrooms have been shown to possess anticancer effects in both in vitro and in vivo studies but have not been put to the test in human clinical trials.5,41,42 In order to fully comprehend the therapeutic potential of mushrooms in oncology, more rigorous scientific research is needed, even in consideration of the encouraging preclinical and clinical data from recent research publications that have examined the shiitake mushroom's potential as an antineoplastic agent for benign or malignant (cancer) neoplastic growths.42–44 However, the significant cytotoxic and anti-cancer effects of shiitake-encapsulated SCZ nanoemulsions observed on the breast cancer cell line MCF-7 indicate their potential as a natural therapeutic agent to suppress the metastatic carcinoma cells at lower concentrations.4. Discussion
The synthesis of the zein–chitosan nanoemulsion via the liquid–liquid dispersion method demonstrates significant potential in various applications owing to its optimal formulation and stability. The 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of zein to chitosan produced a homogeneous nanoemulsion, which was further optimized by assessing the droplet size, polydispersity index (PDI), and stability. These parameters ensured proper size distribution and resistance to coalescence and phase separation, making the nanoemulsion suitable for drug delivery, cosmetic formulations, and food products. The use of zein, a GRAS (generally recognized as safe) protein, enhances the edibility and safety of the nanoemulsion, while the biocompatibility, biodegradability, and non-toxicity of the zein–chitosan nanocomposite make it an attractive option for various applications.
1 ratio of zein to chitosan produced a homogeneous nanoemulsion, which was further optimized by assessing the droplet size, polydispersity index (PDI), and stability. These parameters ensured proper size distribution and resistance to coalescence and phase separation, making the nanoemulsion suitable for drug delivery, cosmetic formulations, and food products. The use of zein, a GRAS (generally recognized as safe) protein, enhances the edibility and safety of the nanoemulsion, while the biocompatibility, biodegradability, and non-toxicity of the zein–chitosan nanocomposite make it an attractive option for various applications.
Studies verified the potential antimicrobial, anti-inflammatory, and anti-oxidant effects of shiitake as a medicinal mushroom, highlighting its role as a chemotherapeutic agent.31 The in vitro anti-oxidant activity of the shiitake nanoemulsion, as assessed through the DPPH assay, revealed a significant enhancement in antioxidant properties compared with that of the crude shiitake extract. The concentration-dependent scavenging effect observed in the study indicates that higher concentrations of the shiitake extract result in greater anti-oxidant activity. Encapsulation in the zein–chitosan nanocomposite further amplified this effect, suggesting improved bioavailability, biocompatibility, and stability of the bioactive components.23,38,45
Similarly, the in vitro anti-inflammatory activity, evaluated using the BSA denaturation assay, demonstrated that the shiitake nanoemulsion exhibited a higher inhibition percentage of BSA denaturation compared with the crude extract. This concentration-dependent inhibition underscores the potential of the nanoemulsion in managing inflammatory conditions. The lower IC50 value for the nanoemulsion compared with that for the extract indicates a more potent anti-inflammatory effect, supporting its therapeutic potential.
The in vitro antimicrobial activity, determined through absorbance measurements and MIC values, showed that the shiitake nanoemulsion effectively inhibited the growth of various microbial strains. The notable reduction in OD at specific concentrations highlights the nanoemulsion's potential as an antimicrobial agent. The MIC values for different strains, namely, E. coli, C. albicans, S. aureus, andP. aeruginosa, provide a benchmark for its efficacy against these pathogens.
Lastly, the in vitro anti-cancer activity, assessed using the MTT assay on MCF-7 cell lines, indicated a decrease in cell viability with increasing concentrations of the shiitake nanoemulsion. This suggests that the nanoemulsion formulation enhances the anti-cancer properties of the shiitake extract, potentially offering a more effective approach to cancer treatment.
In conclusion, the zein–chitosan nanoemulsion encapsulating the shiitake extract exhibits promising anti-oxidant, anti-inflammatory, antimicrobial, and anticancer activities. These findings support its potential applications in drug delivery, cosmetics, and food products, highlighting the versatility and efficacy of the nanoemulsion formulation. Further research and development could unlock additional benefits and applications, paving the way for innovative solutions in biotechnology and healthcare.
5. Conclusions
This study delves into the therapeutic potential of Lentinula edodes (shiitake mushroom) and the development of biogenic shiitake-based nanoemulsions to enhance the bioavailability and effectiveness of shiitake bio-actives, emphasizing their potential as a valuable natural health resource. A thorough analysis highlighted the significant medicinal properties of shiitake mushrooms, attributed to their rich array of bioactive compounds, such as polysaccharides, lentinan, and eritadenine.45–47 These compounds have demonstrated impressive anti-oxidant, anticancer, anti-inflammatory, immunomodulatory, and cholesterol-lowering effects, validating their traditional use in various therapeutic contexts. This research also focused on the preparation and optimization of the shiitake nanoemulsions by utilizing advanced nanoemulsion techniques to improve the dispersion, reduce the particle size, and increase the solubility of these compounds. The enhanced nanoemulsions showed superior therapeutic benefits compared with conventional shiitake extracts, including an elevated immune response, increased anti-oxidant activity, and more effective modulation of lipid metabolism.5,41 This suggests that shiitake nanoemulsions could serve as an advanced delivery system for bioactive compounds, enhancing their accessibility and efficacy in clinical and nutraceutical applications. These findings offer robust evidence of shiitake mushrooms' health benefits and highlight a promising approach to maximizing these benefits through nanoemulsion technology. Future research should focus on further refining these formulations, assessing their long-term stability and bioavailability in vivo, and exploring their applications in various health conditions. This work effectively bridges traditional medicinal knowledge with cutting-edge technological advancements, unlocking new therapeutic possibilities for natural products such as shiitake mushrooms.Data availability
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.Author contributions
Conceptualization, A. D. and P. D.; methodology, software, and validation, A. D., P. D., A. S. and Ay. S.; formal analysis, N. J.; investigation, A. D.; resources, A. S.; data curation and writing—original draft, A. D. and S. M.; writing—review and editing, S. P.; visualization, supervision, and project administration, A. D., S. R., and R. D.; funding acquisition, S. P. All the authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This research received no external funding.References
- P. C. K. Cheung, Mushrooms as Functional Foods, Wiley, 2008, DOI:10.1002/9780470367285.
- S. Wasser, Medicinal mushroom science: Current perspectives, advances, evidences, and challenges, Biomed. J., 2014, 37(6), 345, DOI:10.4103/2319-4170.138318.
- P. Roupas, J. Keogh, M. Noakes, C. Margetts and P. Taylor, The role of edible mushrooms in health: Evaluation of the evidence, J. Funct. Foods, 2012, 4(4), 687–709, DOI:10.1016/j.jff.2012.05.003.
- C. Solans, P. Izquierdo, J. Nolla, N. Azemar and M. J. Garcia-Celma, Nano-emulsions, Curr. Opin. Colloid Interface Sci., 2005, 10(3–4), 102–110, DOI:10.1016/j.cocis.2005.06.004.
- I. Ahmad, M. Arif, M. Xu, J. Zhang, Y. Ding and F. Lyu, Therapeutic values and nutraceutical properties of shiitake mushroom (Lentinula edodes): A review, Trends Food Sci. Technol., 2023, 134, 123–135, DOI:10.1016/j.tifs.2023.03.007.
- H. Shiga, H. Yoshii, H. Ohe, M. Yasuda, T. Furuta, H. Kuwahara, M. Ohkawara and P. Linko, Encapsulation of shiitake (Lenthinus Edodes) flavors by spray drying, Biosci., Biotechnol., Biochem., 2004, 68(1), 66–71, DOI:10.1271/bbb.68.66.
- D. A. Fernandes, Review on the applications of nanoemulsions in cancer theranostics, J. Mater. Res., 2022, 37(12), 1953–1977, DOI:10.1557/s43578-022-00583-5.
- D. A. Fernandes, D. D. Fernandes, A. Malik, G. N. W. Gomes, S. Appak-Baskoy, E. Berndl, C. C. Gradinaru and M. C. Kolios, Multifunctional nanoparticles as theranostic agents for therapy and imaging of breast cancer, J. Photochem. Photobiol., B, 2021, 218, 112110, DOI:10.1016/j.jphotobiol.2020.112110.
- C. G. Awuchi, S. Morya, T. A. Dendegh, C. O. R. Okpala and M. Korzeniowska, Nanoencapsulation of food bioactive constituents and its associated processes: A revisit, Bioresour. Technol. Rep., 2022, 19, 101088, DOI:10.1016/j.biteb.2022.101088.
- Q. Huang, H. Yu and Q. Ru, Bioavailability and delivery of nutraceuticals using nanotechnology, J. Food Sci., 2010, 75(1) DOI:10.1111/j.1750-3841.2009.01457.x.
- Y. Yuan, M. F. Li, W. S. Chen, Q. Z. Zeng, D. X. Su, B. Tian and S. He, Microencapsulation of shiitake (Lentinula edodes) essential oil by complex coacervation: formation, rheological property, oxidative stability and odour attenuation effect, Int. J. Food Sci. Technol., 2018, 53, 1681–1688 CrossRef CAS.
- T. Li, X. Han, R. Bao, Y. Hao and S. Li, Preparation and properties of water-in-oil shiitake mushroom polysaccharide nanoemulsion, Int. J. Biol. Macromol., 2019, 140, 343–349, DOI:10.1016/J.IJBIOMAC.2019.08.134.
- J. Baranwal, B. Barse, A. Fais, G. L. Delogu, and A. Kumar, Biopolymer: A Sustainable Material for Food and Medical Applications, 2022, DOI:10.3390/polym14050983.
- I. Aranaz, A. R. Alcántara, M. C. Civera, C. Arias, B. Elorza, A. Heras Caballero and N. Acosta, Chitosan: an overview of its properties and applications, Polymers, 2021, 13(19), 3256, DOI:10.3390/polym13193256.
- M. Yadav, B. Kaushik, G. K. Rao, C. M. Srivastava and D. Vaya, Advances and challenges in the use of chitosan and its derivatives in biomedical fields: A review, Carbohydr. Polym. Technol. Appl., 2023, 5, 100323, DOI:10.1016/j.carpta.2023.100323.
- A. Mehdizadeh, S.-A. Shahidi, N. Shariatifar, M. Shiran and A. Ghorbani-HasanSaraei, Evaluation of Chitosan-zein Coating Containing Free and Nano-encapsulated Pulicaria gnaphalodes (Vent.) Boiss . Extract on Quality Attributes of Rainbow Trout, J. Aquat. Food Prod. Technol., 2021, 30(1), 62–75, DOI:10.1080/10498850.2020.1855688.
- X. Wang, Y. Sun, Z. Liu, X. Huang, F. Yi, F. Hou and F. Zhang, Preparation and characterization of chitosan/zein film loaded with lemon essential oil: Effects on postharvest quality of mushroom (Agaricus bisporus), Int. J. Biol. Macromol., 2021, 192, 635–643, DOI:10.1016/J.IJBIOMAC.2021.10.068.
- L. Zhang, K. Li, D. Yu, J. M. Regenstein, J. Dong, W. Chen and W. Xia, Chitosan/zein bilayer films with one-way water barrier characteristic: Physical, structural and thermal properties, Int. J. Biol. Macromol., 2022, 200, 378–387, DOI:10.1016/J.IJBIOMAC.2021.12.199.
- W. Huang, F. Yao, S. Tian, M. Liu, G. Liu and Y. Jiang, Recent Advances in Zein-Based Nanocarriers for Precise Cancer Therapy, Pharmaceutics, 2023, 15(7), 1820, DOI:10.3390/pharmaceutics15071820.
- X. Fan, et al., Oregano essential oil encapsulated in zein-pectin-chitosan nanoparticles to improve the storage quality of Harbin red sausage, Int. J. Biol. Macromol., 2024, 266, 131322, DOI:10.1016/J.IJBIOMAC.2024.131322.
- I. Ahmad, M. Arif, M. Xu, J. Zhang, Y. Ding and F. Lyu, Therapeutic values and nutraceutical properties of shiitake mushroom (Lentinula edodes): A review, Trends Food Sci. Technol., 2023, 134, 123–135, DOI:10.1016/j.tifs.2023.03.007.
- K. Y. Perera, A. K. Jaiswal and S. Jaiswal, Biopolymer-Based Sustainable Food Packaging Materials: Challenges, Solutions, and Applications, Foods, 2023, 12(12), 2422, DOI:10.3390/foods12122422.
- D. Pauluk, A. K. Padilha, N. M. Khalil and R. M. Mainardes, Chitosan-coated zein nanoparticles for oral delivery of resveratrol: Formation, characterization, stability, mucoadhesive properties and antioxidant activity, Food Hydrocolloids, 2019, 94, 411–417, DOI:10.1016/J.FOODHYD.2019.03.042.
- S. K. Chinnaiyan, R. Pandiyan, S. Natesan, S. Chindam, A. K. Gouti and A. Sugumaran, Fabrication of basil oil Nanoemulsion loaded gellan gum hydrogel—evaluation of its antibacterial and anti-biofilm potential, J. Drug Delivery Sci. Technol., 2022, 68, 103129, DOI:10.1016/J.JDDST.2022.103129.
- A. Wulansari, M. Jufri and A. Budianti, Studies on the formulation, physical stability, and in vitro antibacterial activity of tea tree oil (Melaleuca alternifolia) nanoemulsion gel, Int. J. Appl. Pharm., 2017, 9, 135–139, DOI:10.22159/ijap.2017.v9s1.73_80.
- W. Ullah, A. Nawaz, M. Akhlaq, K. U. Shah, M. S. Latif, A. A. Doolaanea and M. Alfatama, Transdermal delivery of gatifloxacin carboxymethyl cellulose-based patches: Preparation and characterization, J. Drug Delivery Sci. Technol., 2021, 66, 102783, DOI:10.1016/J.JDDST.2021.102783.
- M. S. Latif, F. F. Al-Harbi, A. Nawaz, S. A. Rashid, A. Farid, M. A. Mohaini, A. J. Alsalman, M. A. A. Hawaj and Y. N. Alhashem, Formulation and Evaluation of Hydrophilic Polymer Based Methotrexate Patches: In Vitro and In Vivo Characterization, Polymers, 2022, 14(7), 1310, DOI:10.3390/polym14071310.
- S. Chen, Y. Han, L. Jian, W. Liao, Y. Zhang and Y. Gao, Fabrication, characterization, physicochemical stability of zein-chitosan nanocomplex for co-encapsulating curcumin and resveratrol, Carbohydr. Polym., 2020, 236, 116090, DOI:10.1016/j.carbpol.2020.116090.
- H. Fu, R. Huang, J. Li, Z. Lin, F. Wei and B. Lin, Multifunctional cinnamaldehyde-tannic acid nano-emulsion/chitosan composite film for mushroom preservation, Food Hydrocolloids, 2023, 145, 109111, DOI:10.1016/J.FOODHYD.2023.109111.
- A. Lekouaghet, A. Boutefnouchet, C. Bensuici, L. Gali, K. Ghenaiet and L. Tichati, In vitro evaluation of antioxidant and anti-inflammatory activities of the hydroalcoholic extract and its fractions from Leuzea conifera L. roots, S. Afr. J. Bot., 2020, 132, 103–107, DOI:10.1016/j.sajb.2020.03.042.
- K. Kupcova, I. Stefanova, Z. Plavcova, J. Hosek, P. Hrouzek and R. Kubec, Antimicrobial, Cytotoxic, Anti-Inflammatory, and Antioxidant Activity of Culinary Processed Shiitake Medicinal Mushroom (Lentinus edodes, Agaricomycetes) and Its Major Sulfur Sensory-Active Compound-Lenthionine, Int. J. Med. Mushrooms, 2018, 20(2), 165–175, DOI:10.1615/IntJMedMushrooms.2018025455.
- X. Yu, et al., Zein nanoparticles as nontoxic delivery system for maytansine in the treatment of non-small cell lung cancer, Drug Delivery, 2020, 27(1), 100–109, DOI:10.1080/10717544.2019.1704942.
- A. Nawaz, M. S. Latif, M. A. Alnuwaiser, S. Ullah, M. Iqbal, M. Alfatama and V. Lim, Synthesis and Characterization of Chitosan-Decorated Nanoemulsion Gel of 5-Fluorouracil for Topical Delivery, Gels, 2022, 8(7), 412, DOI:10.3390/gels8070412.
- K. Yang, Y. Li, H. Zheng, X. Luan, H. Li, Y. Wang, Q. Du, K. Sui, H. Li and Y. Xia, Adsorption of Congo red with hydrothermal treated shiitake mushroom, Mater. Res. Express, 2020, 7(1), 015103, DOI:10.1088/2053-1591/ab5ff3.
- X. Luo, S. Wu, M. Xiao, H. Gu, H. Zhang, J. Chen, Y. Liu, C. Zhang and J. Zhang, Advances and Prospects of Prolamine Corn Protein Zein as Promising Multifunctional Drug Delivery System for Cancer Treatment, Int. J. Nanomed., 2023, 18, 2589–2621, DOI:10.2147/IJN.S402891.
- S. Dahiya, R. Rani, D. Dhingra, S. Kumar and N. Dilbaghi, Conjugation of epigallocatechin gallate and piperine into a zein nanocarrier: Implication on antioxidant and anticancer potential, Adv. Nat. Sci.:Nanosci. Nanotechnol., 2018, 9(3), 035011, DOI:10.1088/2043-6254/aad5c1.
- T. C. Finimundy, G. Scola, F. J. Scariot, A. J. Dillon, S. Moura, S. Echeverrigaray, J. P. Henriques and M. Roesch-Ely, Extrinsic and Intrinsic Apoptotic Responses Induced by Shiitake Culinary-Medicinal Mushroom Lentinus edodes (Agaricomycetes) Aqueous Extract against a Larynx Carcinoma Cell Line, Int. J. Med. Mushrooms, 2018, 20(1), 31–46, DOI:10.1615/IntJMedMushrooms.2018025400.
- D. Pauluk, A. K. Padilha, N. M. Khalil and R. M. Mainardes, Chitosan-coated zein nanoparticles for oral delivery of resveratrol: Formation, characterization, stability, mucoadhesive properties and antioxidant activity, Food Hydrocolloids, 2019, 94, 411–417, DOI:10.1016/j.foodhyd.2019.03.042.
- R. Hearst, D. Nelson, G. McCollum, B. C. Millar, Y. Maeda, C. E. Goldsmith, P. J. Rooney, A. Loughrey, J. R. Rao and J. E. Moore, An examination of antibacterial and antifungal properties of constituents of Shiitake (Lentinula edodes) and Oyster (Pleurotus ostreatus) mushrooms, Complement. Ther. Clin. Pract., 2009, 15(1), 5–7, DOI:10.1016/j.ctcp.2008.10.002.
- V. Kumar, V. Garg and H. Dureja, Nanoemulsion for delivery of anticancer drugs, Cancer Adv., 2022, 5, e22016, DOI:10.53388/2022522016.
- B. Balakrishnan, Q. Liang, K. Fenix, B. Tamang, E. Hauben, L. Ma and W. Zhang, Combining the anticancer and immunomodulatory effects of astragalus and shiitake as an integrated therapeutic approach, Nutrients, 2021, 13(8), 2564, DOI:10.3390/nu13082564.
- M. Yang, H.-K. Jang and H. Kang, How do shiitake and reishi mushrooms work on lung cancer?: A high throughput screening of 3D cell culture, Cancer Res., 2023, 83(7_Supplement), 4553, DOI:10.1158/1538-7445.AM2023-4553.
- S. K. Panda, G. Sahoo, S. S. Swain and W. Luyten, Anticancer Activities of Mushrooms: A Neglected Source for Drug Discovery, Pharmaceuticals, 2022, 15(2), 176, DOI:10.3390/ph15020176.
- C. Hobbs, Medicinal Mushrooms: an Exploration of Tradition, Healing & Culture, Botanica Press, Santa Cruz, USA, 2nd edn, 1995 Search PubMed.
- F. Xue, M. Zhao, X. Liu, R. Chu, Z. Qiao, C. Li and B. Adhikari, Physicochemical properties of chitosan/zein/essential oil emulsion-based active films functionalized by polyphenols, Future Foods, 2021, 3, 100033, DOI:10.1016/j.fufo.2021.100033.
- Y. Yuan, M. F. Li, W. S. Chen, Q. Z. Zeng, D. X. Su, B. Tian and S. He, Microencapsulation of shiitake (Lentinula edodes) essential oil by complex coacervation: formation, rheological property, oxidative stability and odour attenuation effect, Int. J. Food Sci. Technol., 2018, 53(7), 1681–1688, DOI:10.1111/ijfs.13752.
- S. P. Wasser, Shiitake (Lentinus edodes), in Encyclopedia of Dietary Supplements, CRC Press, 2004, pp. 653–664. doi: DOI:10.1081/E-EDS-120024880.
Footnote |
| † Sharing first authorship. |
| This journal is © The Royal Society of Chemistry 2025 |