 Open Access Article
Open Access ArticleAssessing the safety of thermal mineral water for cosmetic applications: an integrated approach using physicochemical, cheminformatics, and bioinformatics techniques
Vesna Milanković *a,
Jelena Djuriš
*a,
Jelena Djuriš b,
Aleksandra Tubić
b,
Aleksandra Tubić c,
Jasmina Agbaba
c,
Jasmina Agbaba c,
Sofija Forkapić
c,
Sofija Forkapić d and
Milica Lukić
d and
Milica Lukić b
b
aFVRM Consulting, Koste Jovanovica 21, Belgrade, Serbia. E-mail: milankovicvesna@fvrmconsulting.rs
bDepartment of Pharmaceutical Technology and Cosmetology, Faculty of Pharmacy, University of Belgrade, Vojvode Stepe 450, Belgrade, Serbia
cDepartment of Chemistry, Biochemistry and Environmental Protection, Faculty of Sciences, University of Novi Sad, Trg Dositeja Obradovica 3, Novi Sad, Serbia
dDepartment of Physics, Faculty of Sciences, University of Novi Sad, Trg Dositeja Obradovica 3, Novi Sad, Serbia
First published on 28th May 2025
Abstract
Thermal and mineral waters represent a complex multifunctional natural resource that has been used for various purposes throughout human history. The physico-chemical characterization of thermal and mineral waters is a comprehensive process that integrates knowledge and practice from different scientific fields. When used in direct contact with human skin, whether for bathing or for use in topical products, a toxicological analysis of thermal and mineral waters must also be performed. This work is an example of a multidisciplinary approach to investigate the safety of concrete thermal and mineral water from the Pannonian Basin for use in cosmetics. A detailed physicochemical characterization was performed together with the subsequent safety assessment of the final cosmetic product, coupled with cheminformatics and bioinformatics tools used to predict physicochemical properties, pharmacokinetics, determination of descriptors to assess bioactive potential and evaluation of possible biological pathways and interactions. The results show that the tested thermal and mineral water is a promising resource for use in cosmetic products that can help maintain skin integrity and improve its condition. The toxicological evaluation showed that the tested water is acceptable as an ingredient in a face cream for adults, excluding pregnant and breastfeeding women. The results are discussed in detail and guidance and comments on outstanding issues are provided.
1. Introduction
Mineral and thermal waters are sourced from groundwater – defined as free water subject to gravity that is present below the Earth's surface in pores and fractures of rock formations.1–3 The interaction of groundwater, rocks, gases and living organisms is responsible for the specific chemical composition of each groundwater. If the water has more than 1 g l−1 of dissolved solids, unique chemical composition, specific constituents, radioactive elements or an elevated temperature, that differs from “ordinary”, low mineralized water, it is referred to as mineral water (MW). Groundwater with a temperature of at least 20 °C at the outflow is called thermal water (TW). If both conditions are met and the groundwater contains more than 1 g per l total dissolved solids (TDS) and has a temperature of more than 20 °C, it is defined as thermal and mineral water (TMW).3 MWs are widely used in the water industry as drinking and refreshing waters. They are also a good source of constituents (free gases, metals, bacterial cultures, algae and other complex compounds) and can be used directly as geothermal sources to produce clean energy (thermal energy and electricity generators).4–7 Probably the most important aspect of TMWs use relates to human health treatments (balneotherapy) and their beneficial effects on dermatological (atopic dermatitis, psoriasis) and musculoskeletal (rheumatoid arthritis, osteoarthritis) conditions have been demonstrated.8,9 In addition, TMWs are considered promising dermocosmetic ingredients that are already used by various cosmetic brands.In formulations of cosmetic products containing water, purified water is usually used, from which dissolved substances, electrolytes and microorganisms have been removed by a suitable method. This is the easiest way to meet the legal requirements for the quality and safety of the cosmetic product.10 However, there are no explicit regulations on the type of water used in the manufacture of cosmetic products in terms of a list of permitted or prohibited waters. For the safe use of TMWs in cosmetic production a detailed toxicological analysis must be carried out and the safety of the cosmetic product manufactured with TMW must be assessed. Compared to purified water, TMW contains additional compounds that should not be considered impurities but equivalent components and should be toxicologically evaluated.
Each TMW is characterized by a unique chemical composition and specific interactions between its constituents.11–13 The traditional characterization of TMWs was mainly based on the analysis and description of their inorganic content.14 However, the natural organic matter (NOM) and organic compounds present in certain MWs are important in terms of quantity and variety of identified structures. In recent years, numerous authors have supported the NOM and organic fractions of TMWs as the main contributors to the healing mechanisms.15,16 The sum of all components in TMW represents the total mineralization of the water and can be divided into macro-components (primary and secondary) and micro-components – all components other than macro-components, which occur in traces or very low concentrations.17 The primary macro-components (1–1000 mg l−1) that determine the mineralization and chemical composition of the water include Na+, Ca2+, Mg2+, Cl−, HCO3−, SO42−, SiO2. The secondary macro-components (0.01–10 mg) that can define groundwater types under certain conditions include K+, F−, Sr2+, Fe2+, Fe3+, CO32−, F−, B3+ and nitrogen compounds.17 The micro-components existing in concentration 0.0001–0.1 mg l−1 include Al3+, As3−, Ba2+, Br−, Cd2+, Cr3+, Co2+, Cu2+, Ge4+, I−, Pb2+, Li+, Mn2+, Mn3+, Ni2+, PO43+, Rb+, Se2−, Ti4+, Zn+, and micro-components present in concentration less than 0.001 mg l−1 include Be2+, Bi3+, Cs+, Ga3+, Au+, Au3+, In3+, La3+, Pt2+, Pt4+, Ra2+, Sc3+, Ag+, Tl3+, Th4+, Sn2+, Sn4+.17 In addition to the mentioned main constituents, TMWs contain radionuclides, NOM, dissolved gases and various microorganisms.
The inorganic content of TMWs is mainly used for their classification, like in the following one:18
Water with bicarbonate – if bicarbonate content is >600 mg l−1.
Water with sulfate – if sulfate content is >200 mg l−1.
Water with chloride – if chloride content is >200 mg l−1.
Water with calcium – if calcium content is >150 mg l−1.
Water with magnesium – if magnesium content is >50 mg l−1.
Water with fluoride – if fluoride content is >1 mg l−1.
Water with iron – Bivalent iron content greater than 1 mg l−1.
Acid water – if the CO2 content is >250 mg l−1.
Water with sodium – if sodium content is >200 mg l−1.
A systematic review of 678 MWs and 2390 TWs in selected European countries11 has shown that the total dissolved solids (TDS) content is generally below 1 g l−1, and for TWs the range is between 1–14.5 g l−1. Highly mineralized groundwaters with >14.5 g l−1 are very rare and occur exclusively in Hungary. The Pannonian Basin, which includes Hungary, Slovakia, Romania, Slavonia (part of Croatia) and Serbia, is rich in TMW springs with a particularly high NOM content in the water. Analysis of the NOM content (humic substances (HS) and aromatic compounds) of Hungarian thermal waters showed that with increasing water temperature the concentration of HS decreases, while the content of aromatic compounds increases.15,19 The aromatic hydrocarbons occurred at a threshold temperature of 80 °C, phenols at 90 °C and fatty acids were detected in the hottest waters (>90 °C).15
The occurrence of NOM in the natural environment is the result of a continuous chemical, physical and microbiological transformation of biomolecules (plants and animals) and is not yet clearly understood. Their nature and physical/chemical properties depend on the source, location and season.20 NOM can be found in almost all terrestrial and aquatic environments, and they are defined as a complex mixture of organic components consisting of groups of heterogeneous macromolecules of aromatic and aliphatic hydrocarbons with different molecular weights and sizes, hydrophobicity and functional groups.21,22 The NOM present in TMWs can be divided into particulate organic matter (POM), dissolved organic matter (DOM) and colloids (Fig. 1). POM includes bacteria, plankton and mineral constituents coated with organic material, with an average particle size between 50 μm and 2 mm.24 DOM is the fraction of dissolved POM smaller than 0.45 mm25 which can be subdivided into non-HS (components with recognizable chemical structure belonging to organic groups such as amino acids, hydrocarbons, carbohydrates, fats, waxes and resins) and HS (complex heterogeneous substances26 without recognizable structure and physical and chemical properties).
 | ||
| Fig. 1 Graphic presentation of NOM classification.23 | ||
HS are considered hydrophobic, i.e. rich in aromatic carbon, phenolic structures and conjugated double bonds, while non-HS are considered hydrophilic, i.e. rich in aliphatic carbon and nitrogen-containing compounds.27 HS make up 80–90% of NOM and include humic fragments known as humic acids (HA), which are insoluble in water at acidic pH (<2); fulvic acids (FA), soluble in water at acidic to alkaline pH; and humin, which is an insoluble residue.28,29 Due to differences in acidity and hydrophobicity it is difficult to make a clear distinction between HA, FA and humin as part of the heterogeneous supramolecular NOM system. HS in waters contain much more FA than HA, in contrast to HS from soil, which are predominantly composed of HA.30 The fact that the different sources of HS are responsible for the variation of constituents differing in structures and physico-chemical properties justifies the lack of unified model that can describe and predict the dynamic properties and fate of HS both in the aqueous phase and in soil.31 In order to fully understand and possibly predict the behaviour of HS, it is necessary to perform a specific experimental case-by-case study of the structure and physical and chemical properties, involving in a first step their isolation and purification and later the application of different analytical physical–chemical, spectroscopic and chromatographic methods.
TWs coming from great depths can have a different content of natural radionuclides conditioned by the nature and composition of the rocks, the age of the water, flow conditions and geochemical processes. In addition to radionuclides that belong to natural radioactive series (uranium and thorium series), other natural radionuclides of terrestrial or cosmogenic origin (40K, 3H, 14C) may be present as well as anthropogenic radionuclides (3H, 14C, 137Cs, 90Sr, 131I, transuranium elements). A particular risk to the health safety of water is posed by radon, a naturally occurring radioactive gas, a progeny of radium, which, due to its high solubility in water, can reach high concentrations in groundwater.
This work aims to study a specific TMW originating from the area of Vojvodina (Republic of Serbia) as part of Pannonian Basin, specific for its high NOM content. A toxicological evaluation and safety assessment was carried out based on comprehensive and measured parameters characterizing investigated TMW. In order to explore the possibility to use this TMW as a cosmetic ingredient and to assess its safety as a component of a cosmetic product, a detailed study of its physico-chemical properties was carried out. In addition, a cheminformatics and bioinformatics study of important NOMs was performed and the contribution of NOM, which is usually neglected, was discussed in detail.
2. Materials and methods
2.1. Materials
The investigated TMW originates from Novi Bečej (Vojvodina, southern part of the Pannonian Basin, the northern region of the Republic of Serbia) and was taken from a well at a depth of 560 m. The temperature of the TMW at the outlet is 40 °C. The groundwater of the entire Pannonian Basin is, as reported in the literature, specific due to their high NOM content and is used for balneological purposes in all the countries mentioned.32–352.2. Determination of physico-chemical properties of the TMW
The water samples were analyzed for their physico-chemical properties: temperature, pH, mineralization, conductivity, total dry residue, radioactivity, alkalinity, concentration of inorganic and organic components, pesticides, and acute toxicity.The pH measurements were carried out using a portable WTW InoLab device.36 The conductivity of the TMW was measured using the Hannah model HI 933000 field conductivity meter.37 The total dry residue was determined using the standard method APHA, AWWA, WEF, 2017 (ref. 38) and water alkalinity (p- and m-alkalinity) was determined according to the standard method APHA, AWWA, WEF 2012.39
The NOM content in the TMW was determined using the following parameters: total organic carbon (TOC), dissolved organic carbon (DOC), UV254 absorbance, UV278 absorbance and specific UV absorbance (SUVA). The TOC content and the dissolved organic carbon (DOC) content (after filtration of the water through a 0.45 μm membrane filter) were determined using the Elementar LiquiTOCII. The method involves pyrolytic oxidation of the sample at 850 °C.40
The content of organic substances that absorb UV radiation was determined by measuring the UV absorbance at 254 nm and 278 nm in a 1 cm cuvette on a UV spectrophotometer (PG Instruments Ltd T80+ UV/VIS, UK) according to standard methods.38 The value of specific UV absorbance (SUVA) is calculated from the values of UV254 and DOC.
The pesticide content was determined using gas chromatography with electron capture detectors (GC/μECD, Agilent Technologies, 6890N, USA) and gas chromatography with mass detectors (GC/MSD, Agilent Technologies 7890, USA).41
2.3. Determination of the radioactivity of TMW
First step in radioactivity control of TMW was the gross alpha and beta activity measurements by liquid scintillation counting LSC method respecting the requirements of reference document.42,43 The qualitative and quantitative analysis of the radionuclide content in water was then performed by gamma-spectrometry method using low-level HPGe detectors and standard procedure of radioactivity preconcentration and efficiency calibration for a specific geometry and sample matrix.44–46 Due to the problem with bumbling of TMW and an active testing of radon concentration in water with RAD7 radon monitor the LSC method was used instead, and samples were prepared by mixing with two-phase scintillation cocktail UltimaGoldF.47,48 Finally, the content of beta emitters tritium and strontium in water were analyzed by LSC technique applying the adequate validated protocols: distillation for 3H determination,49 and Cherenkov radiation detection for direct determination of 90Sr.502.4. Characterization of NOM
The qualitative and quantitative characterization of NOM present in TMW was performed by fractionation on columns filled with XAD resins (Sigma-Aldrich).51 After filtration (0.45 μm filter) and acidification to pH 2 (cc HCl (Sigma-Aldrich)), 2 liters of raw water were passed through a column filled with XAD-8 resin. The adsorbed substances were eluted from the XAD-8 resin with 250 ml of 0.1 M NaOH (Sigma-Aldrich). The eluate was then acidified to pH 1 (cc HCl), settled and centrifuged after 24 hours. The supernatant represents the fulvic acid fraction (FAF). The precipitate obtained after centrifugation was dissolved in 100 ml 0.1 M NaOH (HAF – HA fraction). After passage through the XAD-8 resin, the water was passed through the XAD-4 resin. A hydrophilic acid fraction (HPI-A) was obtained by elution with 250 ml 0.1 M NaOH. The fraction that remains after passing water through the resins is the non-acidic hydrophilic fraction (HPI-NA).2.5. Determination of acute toxicity of TMW
The screening of acute toxicity of TMW was performed using the Artemia salina toxicity test (ARC test), which is widely used to detect the toxic effects of various substances such as arsenic,52 mercury and chromium,53 drugs, herbicides, pesticides and insecticides.54 The role of this test in detecting the toxic effect of wastewater55 is particularly important as the organism used is tolerant to chloride ions and high salinity. In 2017, the International Organization for Standardization/Technical Specification (ISO/TS) 2078 standardized this test to investigate the toxic effect of nanomaterials in different aquatic ecosystems.The test was performed according to the protocol of Vanhaecke and Persoone56 with minor modifications. Lyophilized eggs of Artemia salina (JBL Artemio Pur) were incubated for 48 hours in distilled water with the addition of sea salt (33 g L−1), under constant lighting (100 W) and aeration at a temperature of 25 °C. After hatching, stage II and III larvae were transferred to wells (10 to 15 larvae per well) with TMW samples taken on the day of the experiment, to which sea salt (33 g per 1 l water) was added. The toxic effect of different concentrations of TMW (99%, 75%, 50%, 25% and 12.5%) and of negative and positive controls was tested. The negative control was distilled water with added sea salt (33 g L−1), the positive control was potassium dichromate (K2Cr2O7, Sigma-Aldrich) in concentrations of 10 to 50 μg ml−1. The microtiter plates were incubated at 25 °C and the number of live and dead individuals was determined after 24, 48 and 72 hours of incubation to determine the acute toxic effect. The test was performed in triplicate.
2.6. Cheminformatic and bioinformatic analysis
The cheminformatics analysis was conducted using the SwissADME platform,57 a widely recognized online tool for predicting physico-chemical properties, pharmacokinetics, drug-likeness, and medicinal chemistry friendliness of chemical compounds. The selection of HA and FA for cheminformatic and bioinformatic evaluation was based on their predominance in the NOM composition of the tested TMW, as confirmed by fractionation results. Representative structures were selected from the PubChem database58 to reflect typical molecular features attributed to environmental humic and fulvic acids and to enable structure-based descriptor calculation. It is important to note that cheminformatic analysis was conducted on selected model structures rather than on the full natural mixtures directly extracted from the tested water. This approach allows for the estimation of relevant molecular descriptors that support safety and functionality assessment in a cosmetic context.The molecular structures of the two substances analyzed, HA and FA, were inputted in the form of SMILES strings, which were derived from Pubchem database. SwissADME provided insights into the molecular properties of HA and FA, including their solubility, lipophilicity, polar surface area, and other descriptors crucial for assessing their potential as bioactive ingredients in cosmetic formulations. The analysis included parameters particularly relevant for dermal application, such as topological polar surface area (TPSA), lipophilicity (multiple log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Po/w models), skin permeability (log
Po/w models), skin permeability (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kp), and water solubility using ESOL, Ali, and SILICOS-IT models. These descriptors are commonly used in dermocosmetic science to estimate local bioavailability, retention in the stratum corneum, and the likelihood of systemic exposure. The SwissADME output was interpreted descriptively, with particular attention to how these physicochemical features align with general criteria for safe and effective bioactive compounds in topical cosmetic formulations.
Kp), and water solubility using ESOL, Ali, and SILICOS-IT models. These descriptors are commonly used in dermocosmetic science to estimate local bioavailability, retention in the stratum corneum, and the likelihood of systemic exposure. The SwissADME output was interpreted descriptively, with particular attention to how these physicochemical features align with general criteria for safe and effective bioactive compounds in topical cosmetic formulations.
The bioinformatics analysis focused on evaluating the biological pathways and interactions associated with the two substances, HA and FA, based on their modulated genes. The list of expressed genes associated with HA and FA was obtained from the Comparative Toxicogenomics Database (CTD),59 a resource for understanding chemical–gene–disease interactions. This gene list was then analyzed using the Gene Ontology (GO) enrichment analysis tool available at South Dakota State University's Bioinformatics website.60 The analysis provided functional annotations and pathway enrichment information, highlighting key biological processes, molecular functions, and cellular components influenced by the substances. These insights were further validated against literature to ensure biological relevance.
2.7. Safety assesment of TMW as used in cosmetic product
Safety assessment of a cosmetic product is based on the safety assessment of its ingredients as followed by the cosmetic regulation regarding its safety.10,61 According to this principle, safety assessment of TMW was conducted through hazard identification and exposure assessment of its compounds. Hazard identification was conducted by identifying compounds of the water in relation to their permitted concentrations in drinking water.62 Exposure assessment was carried out based on the guidelines,61 as if TMW was a part of a face cream used by adults. Risk characterization was determined by calculating the Margin of Safety (MoS): MoS = PoD/SED, where PoD (Point of Departure) is a dose–response point that marks the beginning of a low dose extrapolation.61 As a PoD value No Observed Adverse Effect Level (NOAEL) is generally used, although a Lowest Observed Adverse Effect Level (LOAEL) and Benchmark Dose Low Level (BMDL) can also be used, depending on the available toxicology data. PoD value was estimated from the existing available toxicology data that can be found in literature.SED is Systemic Exposure Dosage and can be calculated based on the amount of the cosmetic product used daily: SED (mg per kg body weight (bw) per day) = A (mg per kg bw per day) × C (%)/100 × DA (%)/100. A (mg per kg bw per day) is relative daily exposure – daily exposure to a cosmetic product per kg body weight based upon the amount applied and frequency of application was chosen ad a default value given in the Notes of Guidance for the testing of cosmetic ingredients and their safety evaluation.61
3. Results and discussion
3.1. Results of physico-chemical properties
The high values of conductivity and total dry residue shown in Table 1 indicate high mineralization of the water.| Parameter | Value |
|---|---|
| Temperature (°C) | 40 |
| Total dry residue (mg l−1) | 3053 |
| pH | 8.02 |
| Conductivity (μS cm−1, 20 °C) | 4810 |
| Hardness (mg CaCO3) | 167 |
| Total alkalinity (mmol l−1) | 51.8 |
The pH value of TMW is 8.02, classifying it as slightly alkaline. A hardness of 167 mg CaCO3 does not characterize the TMW as water with high hardness. TMW has a characteristic, slightly intense smell and a light-yellow colour, which is typical for water with a high HS content. In Table 2 chemical composition with concentration of cations, anions and trace elements is presented. TMW is rich in sodium, magnesium and bicarbonate and can be classified as water with bicarbonate (>600 mg l−1), magnesium (>50 mg l−1) and sodium (>200 mg l−1) based on the concentrations of these ions.18 The tested water has a rich composition of macro and micro compounds (sodium, magnesium, potassium, calcium) that are most directly involved in the structure of the skin,63 which may be important in terms of potential effects when TMW is applied to the skin.64 Magnesium and zinc ions have been reported to promote the migration of human skin fibroblasts and keratinocytes and accelerate extracellular matrix remodeling, while calcium plays an important role in regulating keratinocyte differentiation and its influx into keratinocytes improves the skin's barrier function.65,66
| Compound | Value | Compound | Value | Compound | Value |
|---|---|---|---|---|---|
| Na (mg l−1) | 595 | Mn (mg l−1) | 0.008 | Ba (mg l−1) | 0.288 |
| K (mg l−1) | 7.19 | Fe (mg l−1) | 0.336 | F− (mg l−1) | <0.1 |
| Ca (mg l−1) | 23.7 | As (mg l−1) | <0.003 | HCO3− (mg l−1) | 3128.68 |
| Mg (mg l−1) | 63.8 | Ni (mg l−1) | <0.002 | CN− (mg l−1) | <0.02 |
| Br− (μg l−1) | <5 | Zn (mg l−1) | <0.023 | SiO2 (mg l−1) | 21.2 |
| Cl− (mg l−1) | 171 | Cd (mg l−1) | <0.00015 | Sr (mg l−1) | 0.2272 |
| SO42+ (mg l−1) | 118 | Cr total (mg l−1) | 0.0075 | Sn (mg l−1) | <0.0010 |
| S2− (mg l−1) | <0.5 | Cu (mg l−1) | 0.013 | Au (mg l−1) | 0.0013 |
| NO32− (mg N per l) | 1.16 | Pb (mg l−1) | <0.006 | B (mg l−1) | 0.5647 |
| NO2− (mg N per l) | <0.005 | Hg (mg l−1) | <0.0005 | Sb (mg l−1) | <0.0001 |
| NH3 (mg NH3 per l) | 13.4 | Se (mg l−1) | 0.005 | Be (mg l−1) | <0.0001 |
| Orthophosphate (mg P per l) | 0.295 | Al (mg l−1) | <0.001 |
The content of trace elements and other elements also could be associated with the beneficial effects of TMWs on the skin. The results obtained for the tested TMW show a significant presence of strontium, silica (SiO2), zinc, copper and boron. The tested TMW has the same strontium concentration as one of the widely known MW used in commercial cosmetic products with anti-inflammatory and antioxidant properties.67 In an ex vivo study with the commercially used water, selenium and strontium salts were shown to have a modulating effect on the reduction of IL-6 cytokine production at the intra- and extracellular level.68,69 Also, the combination of ions such as magnesium, calcium, chlorine, manganese, sulphur and strontium reduces AD-like inflammation in hairless mice through immunomodulation and redox balance.70,71 A clinical study conducted with SiO2-rich MW has shown a positive effect in terms of skin barrier restoration and anti-itching potential.72 Another in vitro study with SiO2-rich TW confirmed the empirical clinical benefit of this water in hyperkeratotic conditions such as psoriasis and atopic dermatitis.73 Copper and zinc are considered important factors in promoting skin healing74 and are used in the form of salts in topical products to soothe and calm irritated skin. Zinc has been shown to modulate the different phases of scar formation in injured tissue.75 In combination with copper, zinc promotes the regeneration of elastic fibres in the dermis, which can lead to a reduction in wrinkles.64,76 The tested TMW is rich in boron, in similar quantities to other known TMW already used as an ingredient in cosmetic products. The results of a 24-hour in vitro incubation of keratinocytes with boron and manganese salts showed accelerated wound healing compared to the control medium.77 The theoretical assumption of all the above-mentioned positive effects of the compounds of the tested TMW is useful. Still, it would be of great importance to evaluate and quantify them through an in vivo testing or a clinical study.
Knowledge of the composition of the macro and micro compounds is also important for the development of cosmetic formulations with regard to a possible interaction with other ingredients and general product stability. Due to the high mineralization of the tested TMW, special attention must be given to the selection of the functional ingredients of the cosmetic formulation, such as emulsion-forming ingredients, rheology modifiers, preservatives and chelating agents. The specific odor and slightly yellow color of the TMW can be a challenge when it comes to achieving the desired sensory properties of the cosmetic formulation. Tables 3 and 4 show the concentrations of organochlorine pesticides and organic compounds in the water tested.
| Compound (μg l−1) | Value | Compound (μg l−1) | Value | Compound (μg l−1) | Value | Compound (μg l−1) | Value | Compound (μg l−1) | Value |
|---|---|---|---|---|---|---|---|---|---|
| Alpha-BHC | <0.005 | Heptachlor | <0.003 | Endosulfan I | <0.003 | Pentachlorobenzene | 0.007 | Aldrin | <0.003 |
| Beta-BHC | <0.005 | Heptachlor epoxide | <0.003 | Endosulfan II | <0.003 | Hexachlorobenzene | <0.01 | Dieldrin | <0.006 |
| Gamma-BHC | <0.003 | Endrin | <0.006 | 4.4′-DDT | <0.0005 | Simazine | <0.02 | Alachlor | <0.02 |
| Delta-BHC | <0.003 | Endrin aldehyde | <0.0006 | 4.4′-DDD | <0.002 | Trifluralin | <0.008 | Chlorpyrifos | <0.02 |
| Atrazine | <0.02 | Endosulfan sulfate | <0.003 | 4.4′-DDE | <0.005 |
| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Phenols (mg l−1) | <0.037 | Toluene (μg l−1) | <0.5 |
| PAHs (μg l−1) | <0.06 | TOC (mg C per l) | 122.3 |
| Benzo(a)pyrene (μg l−1) | <0.01 | DOC (mg C per l) | 111.5 |
| Trihalomethanes (μg l−1) | <4 | UV254 (cm−1) | 2.117 |
| Benzene (μg l−1) | <0.5 | UV278 (cm−1) | 1.575 |
| Ethylbenzene (μg l−1) | <0.5 | SUVA (l mg−1 m−1) | 4.12 |
| Xylene (μg l−1) | <0.5 |
Organochlorine pesticides (OCPs) are agrochemicals used over long periods of time, mainly in agriculture. They have the potential to accumulate in the food chain, posing a major threat to human health and the environment worldwide.78 Their concentration is very important for water quality. The results of the analysis of the content of OCPs in the tested TMW shows that these compounds are not present according to the parameters of the legislation on the quality of water intended for human consumption.62 The content of organic compounds whose presence in the tested water would be considered chemical pollution, such as polycyclic aromatic hydrocarbons (PAHs), benzene and others, is presented in Table 4. The results show that no undesirable organic pollutants are present.
The NOM content is significant, as indicated by high values for TOC and DOC as well as the UV absorption of the tested TMW. The value of specific UV absorption (SUVA) indicates the predominant presence of hydrophobic aromatic compounds such as HA and FA in the structure of the nom of tested TMW.
HS contained in cosmetic or medical products can originate from both artificial and natural resources. So far, peat has been widely used as a natural source of HS in different balneological treatments and topical products. Current knowledge about the health-promoting and cosmetic use of peat is mainly based on the effect of HS, which form the main group of active ingredients.79 Unlike raw peat, whose composition is variable and dependent on many factors, HS can be studied precisely in terms of quantity and quality.79,80 In an animal study in rats, topical administration of a poultice containing HA and FA accelerated wound healing by increasing angiogenesis and fibroblast formation and reducing the distribution of inflammatory cells.81 In another animal study in rats, a 0.5% HA gel accelerated wound healing, presumably due to anti-inflammatory and stimulatory effects on fibroblasts and vascular promotion.82 A study indicating protective hydrophobic components of HA, viable conformational arrangements and bioactive molecule content suggests innovative applicability of HS in dermatology to protect the skin from environmental irritants and as an anti-inflammatory agent.83 When formulating safe cosmetic products with a high NOM water phase, special care must be given to avoid the formation of trihalomethanes, a class of disinfection by-products known to be formed during the chlorination of high NOM drinking water.84
3.2. Results of radioactivity tests
During the direct measurement of radon concentration in water with an active device RAD7 (Durridge Company, USA), it was not possible to remove gas from the water by bubbling, indicating that the water sample was taken after degassing, and it was not possible to detect the presence of radon in the water. This assertion is confirmed by another method in which the water sample is mixed directly with a scintillator (LSC method), where the radon concentration in the sample was below the detection limit (MDA = 0.2 Bq l−1 for 50 min time of measurement). The results obtained for radon activity and gross alpha and gross beta activity are shown in Tables 5 and 6 respectively.| Gross alpha activity [Bq l−1] | Gross beta activity [Bq l−1] |
|---|---|
| 0.011 ± 0.003 | 0.24 ± 0.03 |
| Radionuclide | Activity concentration [Bq l−1] | Radionuclide | Activity concentration [Bq l−1] |
|---|---|---|---|
| 137Cs | <0.012 | 40K | 0.23 ± 0.03 |
| 226Ra | 0.50 ± 0.04 | 222Rn | 0.23 |
| 228Ra | 0.44 ± 0.04 | 3H | <2.2 |
| 238U | <0.1 | 90Sr | <0.32 |
A major problem with the LSC method is quenching and obtaining lower counts than the real ones for yellow-coloured samples of tested TMW due to the presence of iron compounds.85 Therefore, it is necessary to use the gamma-spectrometric method for quantitative determination of radionuclides, and the obtained values are given in Table 6. The measured activity concentrations of other produced radionuclides are below the detection limit.
The activity concentrations of tritium and strontium are below the detection limit, which is usual for water that comes from a great depth and does not mix with surface waters.
The gamma spectrometric analysis of the content of radionuclides in the TMW sample revealed low activity concentrations of the natural radionuclides 226Ra and 228Ra, which are close to the derived concentrations for radioactivity in water intended for human consumption86 and which are calculated based on a dose of 0.1 mSv and an average annual water intake of 730 l.
Very low concentrations of radionuclide activity and the absence of tritium 3H, strontium 90Sr and radon 222Rn were detected, which indicates the suitability of this TMW as an ingredient of a cosmetics products. However, caution should be made when using TWs for these purposes as, depending on the geology of the area, groundwater can often contain radon above the recommended level of 100 Bq l−1 for the monitoring of radioactivity in drinking water.87 Various studies carried out in the EU Member States87 have shown that the radon concentration in groundwater can vary between 1 and 50 Bq l−1 for wells in sedimentary rock, between 10 and 300 Bq l−1 for wells dug into the ground and between 100 Bq l−1 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Bq l−1 in granite and metamorphic rock with high uranium concentrations. Of course, there are large differences in radon concentrations in groundwater and monitored wells within geological regions, as well as in the usual seasonal variations of radon (at the end of fall and in winter, these concentrations are mainly due to pressure and temperature variations).
000 Bq l−1 in granite and metamorphic rock with high uranium concentrations. Of course, there are large differences in radon concentrations in groundwater and monitored wells within geological regions, as well as in the usual seasonal variations of radon (at the end of fall and in winter, these concentrations are mainly due to pressure and temperature variations).
3.3. Results of the determination of the acute toxicity
The Artemia Salina test on water crayfish is an animal test and as such was used to assess the acute toxicity of TMW to evaluate it for recreational purposes (swimming pool). The result of the mentioned test can be considered as an additional source of information for evaluating the suitability of TMW for use in cosmetic products. The results obtained showed that the tested TMW in the ARC test had no toxic effect on Artemia salina.Since the Artemia salina test evaluates acute toxicity, it would be useful to perform additional toxicity tests. Water itself as a raw material for the manufacture of cosmetic products is difficult to subject to a standard combination of genotoxicity tests (Bacterial Reverse Mutation Test (Ames) (Organization for Economic Co-operation and Development (OECD) 471),88 In vitro Micronucleus Test (OECD 487)89 or Reconstructed Skin Micronucleus Test (RSMN)90) used in the testing of cosmetic raw materials and products due to its aggregate state. However, the tests mentioned can also be performed on finished cosmetic products if required.
Even though the detected TMW compounds are expected to have positive effects, it should be kept in mind that they can pose a risk to human health. In this context, the values of parameters whose concentrations must not exceed the limits laid down in the legislation62 are sodium, iron, ammonia and DOM. When discussing the toxicological acceptability of tested TMW as a cosmetic ingredient, these parameters should be evaluated in terms of their systemic toxicity (acute toxicity, dermal and oral absorption, mutagenicity and genotoxicity, carcinogenicity, subchronic and chronic oral toxicity, reproductive toxicity and teratogenicity, endocrine disrupting activity) together with their skin and eye irritation and skin sensitization. The presence of other detected components can be considered safe as their concentrations are considered acceptable for human ingestion, but their presence should be questioned due to the possibility of causing skin irritation and sensitization.
3.4. Fractionation of the NOM results
Results obtained by fractionation of NOM isolated from tested TMW are presented in Fig. 2.Both the elemental composition and the group composition can be used to characterize HS. The elemental composition of HS is usually about 40–60% C, 30–50% O, 4–5% H, 1–4% N, 1–2% S and 0–0.3% P.91 If the group composition of HS is used to characterize them, it is possible to determine their possible chemical interactions and structural properties. HA consists mainly of phenolic groups, quinones, aromatic rings with conjugated double bonds and long-chain carboxylic acids (C12–C18), that make them hydrophobic. FA consists mainly of ether, amine and aldehyde functional groups, aliphatic carbons, nitrogen-containing compounds such as proteins, sugars and amino acids and have a higher content of polar groups (carboxyl groups), that make FA hydrophilic. The presence of different functional groups gives the specific NOM the possibility to form both hydrophilic and lipophilic interactions with different parts of the molecule, that forms the basis for many possible interactions with the environment. The results obtained for the tested TMW show that the NOM are predominantly hydrophobic and that the FAF fraction of FA dominates in it (61%), while the content of HAF fractions of HA is significantly lower (13%). The hydrophilic fraction consists equally of the acidic (HPI-A) and the hydrophilic non-acidic fraction (HPI-NA) with 13% each (Fig. 2).
3.5. Results of the cheminformatic and bioinformatic analysis of HA and FA
The application of cheminformatics and bioinformatics tools can facilitate the exploration and utilization of natural products in various industries, including health and personal care products. Such tools can be used to study the molecular composition and structural features and predict the biological activities that are critical for the desired effect. HA and FA, with their complex structures and diverse bioactive properties, are promising candidates for such analysis. This evaluation can help to explore the development of potential humic-based products, how they could be used and what effects HA and FA could have to guide the early stages of product development.Table 7 provides an overview of the chemical properties of HA and FA that are critical to understanding their potential as bioactive ingredients in cosmetic formulations. HA are complex mixtures consisting of a variety of compounds with different structures. For this analysis, we selected the representative structures of HA and FA from known databases. HA has a simpler structure, and a lower molecular weight (227.17 g mol−1) compared to FA (308.24 g mol−1). Both acids have high solubility, with HA being very soluble and FA being soluble, with HA having higher solubility in various measurements. In terms of lipophilicity, FA has a slightly higher log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Po/w consensus value (0.53) compared to the negative value of HA (−0.56), suggesting that FA is slightly more lipophilic. Both compounds have a high topological polar surface area and exhibit high gastrointestinal absorption, but neither is permeable to the blood–brain barrier and acts as a P-glycoprotein substrate. In addition, neither compound inhibits the major cytochrome P450 enzymes (CYP1A2, CYP2C19, CYP2C9, CYP2D6, CYP3A4), suggesting a minimal risk of drug–drug interactions. The skin permeation potential, indicated by log
Po/w consensus value (0.53) compared to the negative value of HA (−0.56), suggesting that FA is slightly more lipophilic. Both compounds have a high topological polar surface area and exhibit high gastrointestinal absorption, but neither is permeable to the blood–brain barrier and acts as a P-glycoprotein substrate. In addition, neither compound inhibits the major cytochrome P450 enzymes (CYP1A2, CYP2C19, CYP2C9, CYP2D6, CYP3A4), suggesting a minimal risk of drug–drug interactions. The skin permeation potential, indicated by log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kp values, is low for both acids, with FA having a slightly lower skin permeability. These properties underline the suitability of HA and FA as safe and effective bioactive ingredients for topical application in cosmetic products, especially in formulations targeting skin health and protection.
Kp values, is low for both acids, with FA having a slightly lower skin permeability. These properties underline the suitability of HA and FA as safe and effective bioactive ingredients for topical application in cosmetic products, especially in formulations targeting skin health and protection.
The descriptors obtained via SwissADME support the suitability of HA and FA for topical application. Their high water solubility, combined with low skin permeability (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kp values of −7.65 for HA and −7.98 for FA), suggests limited systemic absorption, which is desirable for dermally applied ingredients. The relatively high TPSA values (120 Å2 for HA and 134 Å2 for FA) are consistent with low transdermal bioavailability and favor retention in the superficial skin layers. Their balanced lipophilicity, as indicated by consensus log
Kp values of −7.65 for HA and −7.98 for FA), suggests limited systemic absorption, which is desirable for dermally applied ingredients. The relatively high TPSA values (120 Å2 for HA and 134 Å2 for FA) are consistent with low transdermal bioavailability and favor retention in the superficial skin layers. Their balanced lipophilicity, as indicated by consensus log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Po/w values (−0.56 for HA and 0.53 for FA), points to the potential for interaction with both hydrophilic and lipophilic components of the stratum corneum.
Po/w values (−0.56 for HA and 0.53 for FA), points to the potential for interaction with both hydrophilic and lipophilic components of the stratum corneum.
HA has been reported to chelate a variety of metal ions, including both heavy metals and trace elements. Metals such as copper (Cu), zinc (Zn), lead (Pb), cadmium (Cd), nickel (Ni), iron (Fe), calcium (Ca), magnesium (Mg), aluminum (Al), uranium (U) and various lanthanides are known to form complexes with HS. Chelate formation occurs primarily through the interaction of metal ions with functional groups such as carboxyl and phenolic groups, which are abundant in HA.92 In addition to metal ions, HS can also interact with metal oxides, metal hydroxides and complex minerals. The mechanisms of these interactions are described as ion exchange, surface adsorption, chelation, coagulation and peptization, whereby organometallic compounds are also formed. Of the different functional groups present in the molecular structure of HS, the oxygen-containing carboxyl and phenolic groups are the most important for metal–NOM bonding,93 which is strongly influenced by pH. At high pH, there is increased deprotonation of functional groups, giving them a negative charge (phenolic groups are likely to be deprotonated), resulting in an increased number of available binding sites for ligands. Carboxyl groups dissociate at lower pH values than the phenolic groups, leading to a negative charge on the surface of the molecular structure with many different possible outcomes for the counterions with which they can interact. Surface complexation models have been developed to predict metal speciation in the environment (NICA and Donnan models).94 The ability of HA to bind metal ions is approximately equal to the number of titratable H+ ions divided by the valence of the metal ion.95 FA has more oxygen-containing functional groups than HA, so it binds metal ions more easily96 and forms stronger bonds compared to other HS.97 The interactions of HS with heavy metals are important as they can immobilize heavy metals in the environment and reduce their environmental toxic effects. HA has also been reported to affect the uptake of micronutrients by plants98,99 In contrast to the reported positive effects, the question remains whether the HA–metal complexes could pose a potential threat, as certain organometallic complexes may be more toxic than their corresponding parent compounds.
FA has a unique structure that contributes to its special properties. It is involved in the transport of nutrients and minerals into cells and has antioxidant, anti-inflammatory and immunomodulatory properties. Due to its numerous functional groups, including carboxyl, hydroxyl, ketone and phenolic groups, FA can act as a natural electrolyte, maintaining the electrical balance of cells and promoting efficient nutrient absorption. In addition, the structural diversity and high oxygen content provide significant antioxidant capabilities, allowing FA to scavenge free radicals and protect cells from oxidative damage.100
The antioxidant effect of HS is mainly attributed to the presence of phenol and quinone functional groups.101 Depending on the redox state of the system, HS can act as electron donors or acceptors.102 Phenolic components, which include mono- and polyhydroxylated benzene units, have antioxidant properties.103 Numerous studies on quantification of the antioxidant activity of HS originating from different environments can provide information on the parameters important for the process. Aquatic HS have a higher electron donor capacity than terrestrial HS with comparable aromaticity.104 The most active representative, peat FA enriched with phenolic and sugary HS, has a pronounced antioxidant activity against peroxyl radicals, with antioxidant capacity values reaching those of ascorbic acid and vitamin E.101 In contrast to positive antioxidant effect, quinone groups as intermediates can also lead to possible formation of toxicologically unwanted compounds, which is a question of interest for further analysis.
In the field of agriculture, a comprehensive study on the antioxidant activity of HS has shown that it can help eliminate oxidative damage caused by various substances. For example, HA mitigated the negative effects of Cd-induced oxidative damage in wheat roots through the regulation of growth, osmotic adjustment, radical accumulation and the action of antioxidant systems.105 The results of in vitro studies conducted with HA from natural sources showed the potential of these substances as promising immunity-enhancing agents when they are involved in redox regulation and recharge the antioxidant defense mechanism by reabsorbing radicals.106 In addition, HS, when used as additives in agriculture, have been reported to boost antioxidant levels in plants.
There are numerous studies in literature that emphasize the potential clinical relevance of HA and FA.107–112 Antimicrobial, antifungal, anti-inflammatory, antiviral, antibacterial and UV-protective effects are reported as biological effects. The antiviral spectrum of HS includes hepatitis B,113 Epstein Barr virus,110 Coxsackie virus A9,111 herpes simplex virus type 1.112 The anti-inflammatory effect was demonstrated in the rat paw edema model.114 FA at a concentration of 0.5% improves the wound healing process due to its unique anti-inflammatory and neovascularizing properties at the skin wound site.81 A study in mice showed that topical FA is a potential therapeutic agent for atopic dermatitis.115 A study in rats showed that oral administration of FA appears to prevent stress-induced oxidative damage to the rat stomach caused by water avoidance.116 In a double-blind study, a carbohydrate-derived FA showed potent activity against fungal pathogens with lower minimum inhibitory concentration values, while the inflammatory response mediated by the cytokine interleukin 6 was significantly inhibited.117 Carbohydrate-derived FA was highly active against all oral bacteria tested, including Porphyromonas gingivalis, with a sessile minimum inhibitory concentration of 0.5%. This concentration has been shown to kill biofilms of multiple species by approximately 90%, a value comparable to chlorhexidine.118
The expression of several genes has been reported to be modulated by HA and FA, potentially affecting various biological signaling pathways and cellular processes (Table 8).
| Interacting genes | |
|---|---|
| Humic acid | NOS2, PTGS2, PLG, BAX, BCL2, CASP9, IL1B, RB1, AHR, AKT1, CASP8, CAT, CYP1A1, HSP90AA1, HSP90AB1, MAPK1, MAPK3, MMP9, NOS3, TNF, TP53, CASP3, CASP6, CCND1, CDK2, CDK4, CYCS, HMOX1, ITGA2, JUN, MMP2, NFE2L2, NFKBIA, PTK2, RELA and VEGFA |
| Fulvic acid | MAPK8, MAPT, PTGS2, APP, BMP2, BMP6, CCL11, COL1A1, COL2A1, CXCL8, FAS, FLT3, GBP3, IL12RB1, IL13, IL13RA1, IL6, INHBC, IRF8, ITGA2, ITGAM, MAPK1, MAPK3, MAPK9, MS4A2, RELA and SELL |
Fig. 3 represents fold enrichment of various pathways in relation to the number of genes involved that are modulated by HA. The colors indicate the significance levels (−log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 of FDR – false discover rate), and the size of the points represents the number of genes associated with each pathway. Identified pathways can be grouped into cancer-related pathways (small cell lung cancer, pancreatic cancer, prostate cancer), inflammatory pathways (IL-17 signaling, apoptosis, fluid shear stress and atherosclerosis), infection pathways (measles, hepatitis B and C, Epstein–Barr virus, Salmonella, HPV) as well as metabolic and resistance pathways (AGE-RAGE signaling in diabetes, endocrine resistance). For topical applications of cosmetic products containing HA, the relevance lies in its potential to modulate the IL-17 signaling pathway. Given the role of IL-17 in skin inflammation and immune response, HA could reduce local inflammation, enhance skin barrier function, and provide therapeutic benefits in conditions like dermatitis or acne. This aligns with the anti-inflammatory properties suggested by its interaction with pathways related to immune modulation and infection response. The listed modulated genes were also used to construct a protein–protein interaction (PPI) network using the STRING database.119 The obtained PPI network (Fig. 4) is represented by the proteins (nodes, labeled with its gene name) and interactions between these proteins (lines or edges).
10 of FDR – false discover rate), and the size of the points represents the number of genes associated with each pathway. Identified pathways can be grouped into cancer-related pathways (small cell lung cancer, pancreatic cancer, prostate cancer), inflammatory pathways (IL-17 signaling, apoptosis, fluid shear stress and atherosclerosis), infection pathways (measles, hepatitis B and C, Epstein–Barr virus, Salmonella, HPV) as well as metabolic and resistance pathways (AGE-RAGE signaling in diabetes, endocrine resistance). For topical applications of cosmetic products containing HA, the relevance lies in its potential to modulate the IL-17 signaling pathway. Given the role of IL-17 in skin inflammation and immune response, HA could reduce local inflammation, enhance skin barrier function, and provide therapeutic benefits in conditions like dermatitis or acne. This aligns with the anti-inflammatory properties suggested by its interaction with pathways related to immune modulation and infection response. The listed modulated genes were also used to construct a protein–protein interaction (PPI) network using the STRING database.119 The obtained PPI network (Fig. 4) is represented by the proteins (nodes, labeled with its gene name) and interactions between these proteins (lines or edges).
There are 35 proteins in the network with 547 interactions. Key proteins affected by the HA can be grouped into the following categories:
(1) Inflammation and immune response proteins: TNF (Tumor Necrosis Factor), IL1B (Interleukin 1 Beta), NF-kB (Nuclear Factor Kappa-light-chain-enhancer of activated B cells), and NOS2 (Nitric Oxide Synthase 2). These proteins are critical regulators of inflammation. HA's interactions with these proteins suggest it could have potent anti-inflammatory effects. This makes HA a promising ingredient in products designed to soothe skin irritation, reduce redness, and manage inflammatory skin conditions like acne or rosacea.
(2) Oxidative stress and antioxidant defense proteins: CAT (Catalase), SOD2 (Superoxide Dismutase 2), GPX1 (Glutathione Peroxidase 1), and NFE2L2 (Nuclear Factor, Erythroid 2 Like 2) that play a key role in the body's defense against oxidative stress. HA may enhance the activity or expression of these antioxidant enzymes, providing protection against free radicals.
(3) Cell growth, proliferation and apoptosis regulation proteins: BCL2 (B-cell lymphoma 2), CASP3 (Caspase 3), CASP6 (Caspase 6), CDK2 (Cyclin-Dependent Kinase 2), CDK4 (Cyclin-Dependent Kinase 4), AKT1 (Protein Kinase B), MAPK1 (Mitogen-Activated Protein Kinase 1), and JUN (c-Jun) involved in regulating cell survival, proliferation, and programmed cell death (apoptosis). HA's interactions with these proteins could promote balanced cell turnover, potentially aiding in skin renewal and rejuvenation. This is beneficial in cosmetic formulations aimed at reducing signs of aging, such as wrinkles and fine lines, and promoting overall skin health.
(4) Skin barrier and regeneration proteins: MMP2 (Matrix Metalloproteinase 2), MMP9 (Matrix Metalloproteinase 9), COL1A1 (Collagen Type I Alpha 1 Chain), ITGA2 (Integrin Subunit Alpha 2), and PTK2 (Protein Tyrosine Kinase 2). These proteins are involved in skin matrix remodelling, collagen production, cell adhesion, and migration. Humic acid's influence on these proteins suggests it could help maintain the structural integrity of the skin, improve skin elasticity, and support wound healing. This makes it potentially useful in anti-aging creams, firming serums, and products for skin repair and rejuvenation.
(5) Detoxication and environmental stress response proteins: CYP1A1 (Cytochrome P450 1A1) – protein involved in metabolizing and detoxifying environmental pollutants and chemicals. HA's interaction with CYP1A1 suggests it could help protect the skin from environmental stressors such as pollutants and UV radiation.
(6) Stress response proteins: HSP90AB1 (Heat Shock Protein 90 Alpha Family Class B Member 1), HSP70 (Heat Shock Protein 70), and other HSPs (Heat Shock Proteins). Since that these proteins play a role in protecting cells under stress conditions, such as heat, UV radiation, or pollution, HA may upregulate them, enhancing the skin's resilience to external stressors and preventing damage, which is valuable in sunscreens, after-sun care, and pollution-protection skincare products.
(7) Angiogenesis and microcirculation proteins: VEGFA (Vascular Endothelial Growth Factor A) which is involved in blood vessel formation could help enhance skin healing and rejuvenation by improving microcirculation. HA's interaction with VEGFA suggests it could promote a healthy blood supply to the skin.
Fig. 5 represents fold enrichment of various pathways in relation to the number of genes modulated by FA.
Identified pathways can be grouped into inflammatory pathways (IL-17 signaling, TNF signaling, Th1 and Th2 cell differentiation, Th17 cell differentiation, and toll-like receptor signaling), infection-related pathways (pertussis, Yersinia infection, Kaposi sarcoma-associated herpesvirus infection, pathogenic Escherichia coli infection, and tuberculosis), metabolic and disease pathways (AGE-RAGE signaling in diabetic complications, lipid and atherosclerosis, Alzheimer's disease), and cell signaling pathways (cytokine–cytokine receptor interaction, Fc epsilon RI signaling). The involvement of FA in the IL-17 signaling pathway, which is also significantly modulated by HA, suggests its synergistic anti-inflammatory effect, which could be exploited in the products for topical application on the skin. Additionally, FA's modulation of other immune-related pathways, such as TNF signaling and Th cell differentiation, further supports its role in controlling inflammation and enhancing the skin's immune defense mechanisms. This complements HA's similar effects on immune modulation, potentially making the combination of both acids particularly effective in soothing inflamed skin, managing acne, and protecting against environmental stressors. The enriched infection-related pathways suggest that FA may have antimicrobial properties, helping to defend the skin against various pathogens. This complements the potential of HA to influence infection response pathways, making the combination beneficial for maintaining a healthy skin microbiome and reducing the risk of infections or irritation caused by bacteria and viruses.
The listed modulated genes were also used to construct a PPI network using the STRING database.119 The obtained PPI network (Fig. 6) contains 26 proteins in the network with 186 interactions.
Key proteins affected by the HA can be grouped into the following categories:
(1) Inflammation and immune response proteins: IL6 (Interleukin 6), IL13 (Interleukin 13), CXCL8 (C-X-C Motif Chemokine Ligand 8), RELA (v-rel avian reticuloendotheliosis viral oncogene homolog A), and FAS (Fas Cell Surface Death Receptor). These proteins play significant roles in regulating inflammation and the immune response. FA's interactions with these proteins suggest it could have potent anti-inflammatory effects, like HA.
(2) Skin barrier and regeneration proteins: COL1A1 (Collagen Type I Alpha 1 Chain), COL2A1 (Collagen Type II Alpha 1 Chain), ITGA2 (Integrin Subunit Alpha 2), and ITGAM (Integrin Subunit Alpha M) which are involved in skin matrix remodeling, collagen production, and cell adhesion. FA's interaction with these proteins suggests it could help maintain the structural integrity of the skin, improve skin elasticity, and support wound healing, much like HA.
(3) Oxidative stress and antioxidant defense proteins: MAPK1 (Mitogen-Activated Protein Kinase 1), MAPK3 (Mitogen-Activated Protein Kinase 3), and MAPK8 (Mitogen-Activated Protein Kinase 8) – proteins important in cellular responses to oxidative stress. FA may help modulate the MAPK signaling pathways, enhancing the skin's ability to counteract oxidative damage caused by environmental stressors.
(4) Cell growth, proliferation and apoptosis regulation proteins: BMP2 (Bone Morphogenetic Protein 2), BMP6 (Bone Morphogenetic Protein 6), and APP (Amyloid Precursor Protein). As they are involved in regulating cell differentiation, growth, and apoptosis FA's interactions with them could support balanced skin cell turnover, contributing to skin renewal and rejuvenation.
(5) Stress response and immune modulation proteins: IRF8 (Interferon Regulatory Factor 8) and SELP (Selectin P). These proteins are involved in regulating immune responses and mediating cellular responses to stress. FA's effects on these proteins suggest it may enhance the skin's resilience against external stressors, providing a protective effect against pollution or UV radiation.
(6) Detoxification and antimicrobial response proteins: INHBC (Inhibin Subunit Beta C) and MS4A2 (Membrane Spanning 4-Domains A2) which are involved in detoxification processes and responses to external microbial threats. FA may help the skin detoxify and protect itself against harmful microbes, which could be advantageous in formulations aimed at reducing acne-causing bacteria and maintaining a healthy skin microbiome.
Based on performed analysis, it could be assumed that HA and FA could exert a synergistic effect when used together due to their combined interactions with overlapping signalling pathways and proteins related to inflammation, oxidative stress, cell growth and skin barrier function. Both acids modulate the IL-17 signalling pathway, collagen-related proteins (COL1A1) and oxidative stress proteins (MAPK1, MAPK3), enhancing their anti-inflammatory, antioxidant and skin-repairing properties.
3.6. Results of the safety assessment of TMW as cosmetic ingredient
Compared to purified water, TMW contains additional compounds that should be evaluated toxicologically as equal ingredients. When discussing the potential systemic toxicity of TMW, compounds whose concentrations do not exceed the limits defined as safe for human consumption62,87 can be considered safe. There is no need for their further analysis, as the exposure from drinking water is much higher than the exposure from cosmetic products. The compounds whose concentration exceeds the limits defined as safe for human consumption62 are sodium, iron, ammonium and DOM and require further risk assessment in case of their potential use through dermal application. Potentially present humic-metal complexes were not discussed, as well as the possible contribution of quinone groups.Sodium is present in body fluids as an electrolyte (Na+). It is an abundant cation in the extracellular fluid. Cases of excess sodium in the body due to dietary sodium intake are not common. Acute toxicity may occur with high sodium exposure through ingestion or other means. High levels of sodium in the body can lead to an expansion of extracellular fluid and an increase in blood pressure.122 The World Health Organization (WHO) recommends that adults consume less than 5 g of sodium chloride per day (equivalent to less than 2 g of sodium per day).123 The European Food Safety Authority (EFSA) Panel considers that 2 g of sodium per day is a safe and adequate intake for the general adult population of the EU.124 Consumption of a high-salt diet resulted in hypertension in female Sprague-Dawley rats.125 Also, in rats fed a low-salt diet (sodium chloride 157 mg per kg bw per day), there was no corresponding increase in blood pressure with age. The effects of sodium ion on reproduction were studied in three strains of pregnant rats (SHR, WKY and Sprague-Dawley) fed a diet containing either 0.4 or 8.0% sodium chloride (208 and 4196 mg sodium per kg bw per day) throughout gestation and lactation.126 Their offspring were also fed a low-sodium or high-sodium diet, the pregnancy rate was lower in the high-sodium groups. SHR pups fed the high sodium diet from high salt mothers had significantly higher blood pressure at 11.5 weeks than the offspring of all other groups and had high morbidity and mortality from peripheral capillary hemorrhage and stroke. In all rat strains studied, the maternal high-salt diet caused postnatal growth depression. No effects on development were observed in the offspring of pregnant mice, rats or rabbits given oral doses of sodium chloride equivalent to 189, 147 or 147 mg sodium per kg bw on days 6–15 (mice and rats) or 6–18 (rabbits) of gestation.
Iron is an essential biological component important for the synthesis of hemoglobin and therefore involved in oxygen transport. Iron exists in its ionic form in two oxidation states (Fe2+ and Fe3+), which can be converted into each other by the uptake or release of an electron in the cascade of oxidative reactions that can trigger oxidative damage in cells. An acute oral dose of 60 mg Fe per kg bw can be fatal. Symptoms of acute poisoning include nausea, vomiting, lethargy, then an asymptomatic phase of up to 24 hours, followed by gastrointestinal perforation, coma, convulsions, cardiovascular collapse and liver and kidney failure.127,128 Oral doses below 10–20 mg Fe per kg bw per day do not cause acute systemic toxicity.129 Side effects of oral iron supplementation have been reported with gastrointestinal, cardiovascular and metabolic disturbances. Ferrous sulfate heptahydrate was evaluated for oral toxicity in a combined OECD repeated dose and reproductive/developmental toxicity screening test in rats under GLP at doses of 0, 30, 100, 300 and 1000 mg per kg per day.130 Based on extramedullary hematopoiesis of the spleen in males at 300 mg kg−1 and elevated levels of inorganic phosphate in females at 300 mg kg−1, the oral NOAEL for repeated dose toxicity was 100 mg per kg per day, equivalent to 20 mg Fe per kg bw per day for both sexes. In an OECD TG422 study, ferric chloride was administered by oral gavage at doses of 0, 125, 250 or 500 mg per kg per day for up to 42 days in male rats and 42–54 days in female rats; deaths were observed at 500 mg kg−1. The NOAEL was set at 125 mg per kg per day for males and females, which corresponds to 55 mg Fe per kg.130 Most Ames tests performed with iron salts are negative. Iron salts are not mutagenic in vivo.130 No increase in tumor incidence was observed in rats consuming ferric chloride in drinking water at doses of up to 320–340 mg per kg bw per day (110–117 mg Fe per kg bw per day) for two years. Epidemiologic studies have provided no evidence of increased cancer risk in human populations with increased dietary iron intake or clinical supplementation.130 The risk for various cancers may be increased, if at all, only by increased intake of heme iron, not total or supplemental iron.131 Although sensitization tests have yielded mixed results, there is no convincing or reliable evidence of iron sensitization.130
In an aqueous solution, the majority (>90% below pH 8) of ammonia is present in the protonated form ammonium (NH4+) at equilibrium.132 Ammonia is an indirect source of nitrogen for living organisms. All mammalian species produce ammonia in amounts of 3–4 g per day, 43–57 mg per kg bw per day for a 70 kg adult,133 through the bacterial breakdown of nucleic acids and amino acids in the gut. Most of the endogenously produced ammonium in the gastrointestinal tract is then absorbed and transported to the liver.132 Positively charged ammonium has limited mobility in the organism, but ammonia in equilibrium with ammonium under physiologic conditions can diffuse across cell membranes, including the blood–brain barrier, and distribute throughout tissues.133 An LD50 of 1300 mg per kg bw (equivalent to 437 mg ammonium per kg bw) was observed in male mice after a single administration of ammonium chloride in water.134 Ammonia is considered safe in terms of developmental or reproductive toxicity or carcinogenicity and has low acute oral toxicity.132 Repeated exposure to ammonium chloride in laboratory animals leads to secondary effects of hyperchloremic metabolic acidosis (decreased bw, decreased pH in blood and urine, increased serum calcium levels associated with bone demineralization, enlargement of the kidneys and hypertrophy of the adrenal glands). These secondary effects are also observed with other substances that induce metabolic acidosis and are therefore not specifically related to ammonium.132 In rats chronically exposed to ammonium sulfate, a slight increase in liver and kidney weight was observed at 415 mg per kg bw per day, which is considered of little toxicological relevance.132
FA is taken orally in the form of various dietary supplements that are available on the market. It is not easy to assess the toxicology of an FA, as chemical and structural differences may occur depending on the source of FA extraction. However, several studies have been conducted with FA extracted from different sources, which can provide an important basis for an evaluation. In an acute oral toxicity study of FA from peat (OECD 423), the animals tested were found to be safe at a dose of 2000 mg per kg bw per day.135 The results of the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) test using the same FA show that FA does not have cytotoxicity, suggesting its compatibility with mammalian cells.135 Subacute oral repeated dose toxicity studies with the same FA derived from peat were conducted (OECD 407) in rodents for 28 days, with a single dose of 1000 mg kg−1 tested. The results showed that FA had neither a toxic nor a lethal effect.135 In a 60-day subchronic oral study in rats, FA from weathered coal was tested at doses of 0, 200, 1000 or 5000 mg per kg per day. The NOAEL was determined to be the highest dose tested (5000 mg per kg bw per day, which corresponds to a purified FA of 625 mg bw per day).136 The same FA was used in an in vitro bacterial reverse mutation test, an in vivo mammalian erythrocyte micronucleus test, and a mouse sperm abnormality test, which were selected together to evaluate the genotoxicity of FA, and the results indicated that FA had no genotoxic effect.136 The U.S. Environmental Protection Agency (EPA) considers that dietary exposure to FA does not raise carcinogenicity concerns, and no cancer risk assessment is required.137 In a developmental toxicity study of carbohydrate-derived FA in rats, doses of 0 or 100–120 mg per kg per day were used from 3 days before fertilization to 14 days of age. The NOAEL for development was higher than the dose used in the study (either 100 or 120 mg per kg bw per day).137 In the human clinical toxicity evaluation study, FA is considered safe at a daily dose of 1.8 g per adult.138
In healthy volunteers, FA derived from carbohydrates did not induce sensitization when applied topically.139 In an acute skin study with New Zealand albino rabbits, minimal eye and minimal skin irritation was observed with the use of carbohydrate-based FA.138 a 7-day dermal study was conducted with female mice in which no adverse effects were observed.137
HA can be considered a precursor of FA and can therefore be used as a substitute. In veterinary medicine, HA is used in horses, ruminants, pigs and poultry in oral doses of 500 to 2000 mg per kg bw per day for the treatment of diarrhea, dyspepsia and acute intoxications.140 HA are of low toxicity after oral administration. The LD50 in rats is greater than 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 mg per kg bw.140 HA have not been shown to be mutagenic.137 FA and HA preparations derived from a lignite deposit were not mutagenic or clastogenic in the bacterial reverse mutation test, the in vitro mammalian chromosome aberration test and the in vivo mammalian micronucleus test, and the NOAEL in male and female rats was 2000 mg per kg bw per day, the highest dose tested, after 90 days of continuous exposure by gavage in rats.141 In a 30-day toxicity study in rats, oral doses of 100 mg per kg bw per day of concentrated HA or its sodium salt had no effect on behavior and caused no clinical disturbances. The same results were obtained in dogs receiving daily doses of 300 mg per kg bw for 90 days.140 Groups of 10 pregnant rats were treated orally with 5000 mg sodium humate per kg bw per day on days 5 to 17 of gestation or with 1000 mg per kg bw per day on days 5 to 9 or on days 11 to 15 of gestation. No teratogenic effects were observed.140
500 mg per kg bw.140 HA have not been shown to be mutagenic.137 FA and HA preparations derived from a lignite deposit were not mutagenic or clastogenic in the bacterial reverse mutation test, the in vitro mammalian chromosome aberration test and the in vivo mammalian micronucleus test, and the NOAEL in male and female rats was 2000 mg per kg bw per day, the highest dose tested, after 90 days of continuous exposure by gavage in rats.141 In a 30-day toxicity study in rats, oral doses of 100 mg per kg bw per day of concentrated HA or its sodium salt had no effect on behavior and caused no clinical disturbances. The same results were obtained in dogs receiving daily doses of 300 mg per kg bw for 90 days.140 Groups of 10 pregnant rats were treated orally with 5000 mg sodium humate per kg bw per day on days 5 to 17 of gestation or with 1000 mg per kg bw per day on days 5 to 9 or on days 11 to 15 of gestation. No teratogenic effects were observed.140
Skin irritation and sensitization due to topical application of HA have not been reported in the literature. On the contrary, one case of anti-inflammatory effect of HA from silt-sulfide mud preparations in allergic contact dermatitis in mice has been reported.142
| Compound | Concentration in the product (%) | SED | PoD (mg per kg bw per day) | MoS |
|---|---|---|---|---|
| Sodium | 0.0476 | 0.005745 | 157 (ref. 126) | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 664 664 |
| Iron | 0.0000269 | 0.00000325 | 20 (ref. 132) | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 076 076![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 923 923 |
| Ammonia | 0.001072 | 0.000129 | 68 (ref. 143) | 263![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 566 566 |
| Humic acid | 0.0012 | 0.00014 | 100 (ref. 141) | 357![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 143 143 |
| Fulvic acid | 0.00546 | 0.000657 | 100 (ref. 138) | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 103.5 103.5 |
The risk characterization was performed by calculating the SED and the MoS. For Relative daily exposure a value of 24.14 mg per kg bw per day was taken in a first-tier approach, according to the guidelines.61 For dermal absorption (DA), 50% value is used in a first-tier worst case scenario. For oral absorption, 50% of the administered dose is used as the default value in absence of other data and therefore PoD was divided with a factor 2. Calculated MoS values are higher than 100, based on the worst-case scenario for dermal and oral absorption that indicates no toxicological concern for each substance assessed.
4. Conclusion
The detailed analysis and observation of all aspects of the physico-chemical properties including the beneficial effects and toxicological acceptability of TMW represents a comprehensive multidisciplinary process. For the investigated TMW originated from Vojvodina (Republic of Serbia), the resulting physico-chemical properties are promising regarding the expected biological activity. The combination of prominent inorganic (SiO2, zinc, copper, boron) and organic (HA, FA) compounds could have a positive effect on the maintenance of skin integrity and protection against environmental stressors. Through their synergistic effects, they could be highly effective in multifunctional skin care products targeting various skin issues such as aging, inflammation and environmental damage. TMW is a complex system with multiple possibilities for its use as a biologically active material on human skin. It would be beneficial to confirm obtained positive effects of TMW through a clinical study.As far as the toxicological safety assessment is concerned, the measurements of radioactivity, organochlorine pesticides and heavy metals are acceptable as for drinking water. For the substances whose concentration exceeds the acceptable levels for drinking water, summary toxicological profiles were drawn up and exposure was assessed for each compound, excluding the humic-metal complexes potentially present. The results of the first-tier safety assessment with MoS values above 100 indicate safe use of the investigated TMW in a face cream used by adults. The toxicological profiles of the substances evaluated did not include enough data on the reproductive and developmental toxicity of the individual substances, so that TMW could not be assessed as safe for use in pregnant and lactating women. The possibility of a theoretical evaluation of the humic substance-metal complexes and quinones that may be present according to the concept of the threshold of toxicological concern (TTC) should be considered. The toxicological risk assessment of TMW is a comprehensive and challenging task with many issues to be addressed, from qualitative and quantitative research to experimental and theoretical toxicological evaluation.
Data availability
All data sources used for the preparation of manuscript entitled “Assessing the safety of thermal mineral water for cosmetic applications: an integrated approach using physicochemical, cheminformatics, and bioinformatics techniques” prepared for the submission to RSC Advances are listed and described in the Materials and methods section of the manuscript with full URL links given in the References section. The potential publication of the manuscript has been approved by all authors. Authors would like to deny any conflict of interest.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the Ministry of Science, Technological Development and Innovation, Republic of Serbia through two grant agreements with the University of Belgrade Faculty of Pharmacy No. 451-03-136/2025-03/200161 and 451-03-137/2025-03/200161.References
- D. Hess, McKnight's Physical Geography: A Land-Publishers Scape Appreciation, Pearson, New York, 11th edn, 2014 Search PubMed.
- L. A. Shiklomanov, World Freshwater Resources, in Water in Crisis: A Guide to World's Freshwater Resources, ed. P. H. Gleick, Oxford University Press, New York, 1993, pp. 13–24 Search PubMed.
- A. Porowski, Mineral and thermal waters, in Environmental Geology, A Volume in the Encyclopedia of Sustainability Science andTechnology, Second Edition, ed. J. W. LaMoreaux, Springer, New York, New York, 1st edn, 2019, ch. 3, pp. 149–181, DOI:10.1007/978-1-4939-8787-0_978.
- V. Goldberg, A. Dashti, R. Egert, B. Benny, T. Kohl and F. Nitschke, Challenges and Opportunities for Lithium Extraction from Geothermal Systems in Germany—Part 3: The Return of the Extraction Brine, Energies, 2023, 16(16), 5899, DOI:10.3390/en16165899.
- M. Dupuy, E. Garel, E. Chatton, T. Labasque, A. Mattei, S. Santoni, V. Vergnaud, L. Aquilina and F. Huneau, Using natural gas content of groundwater to improve the understanding of complex thermo-mineral spring systems, J. Hydrol., 2024, 634, 130956, DOI:10.1016/j.jhydrol.2024.130956.
- S. Boutarfa, M. M. Senoussi, D. Gonzalez-Silvera, J. Á. López-Jiménez and M. Aboal, The Green Microalga Coelastrella thermophila var. globulina (Scenedesmaceae, Chlorophyta) Isolated from an Algerian Hot Spring as a Potential Source of Fatty Acids, Life, 2022, 12, 560, DOI:10.3390/life12040560.
- S. Y. Yildiz, Exploring the Hot Springs of Golan: A Source of Thermophilic Bacteria and Enzymes with Industrial Promise, Curr. Microbiol., 2024, 81(4), 101, DOI:10.1007/s00284-024-03617-9.
- A. Nasermoaddeli and S. Kagamimori, Balneotherapy in Medicine: A Review, Environ. Health Prev. Med., 2005, 10, 171–179 CrossRef PubMed.
- T. Bender, G. Bálint, Z. Prohászka, P. Géher and I. K. Tefner, Evidence-based hydro- and balneotherapy in Hungary—a systematic review and meta-analysis, Int. J. Biometeorol., 2014, 58(3), 311–323, DOI:10.1007/s00484-013-0667-6.
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products, https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32009R1223.
- D. Elster, T. Szőcs, N. Gál, B. Hansen, D. D. Voutchkova, J. Schullehner, J. Lions, L. Martarelli, E. Giménez-Forcada, J. Á. Díaz-Muñoz, E. Malcuit, G. Schubert, G. Hobiger and N. Rman, Terminologies and characteristics of natural mineral and thermal waters in selected European countries, Geologija, 2022, 65, 21–46 CrossRef.
- M. Szikszay, J.-M. Teissedre, U. Barner and E. Matsui, Geochemical and isotopic characteristics of spring and groundwater in the State of São Paulo, Brazil, Dev. Water Sci., 1982, 16, 23–32 Search PubMed.
- Y. Zhang, X. Zhou, H. Liu, M. Yu, K. Hai, M. Tan and D. Huo, Hydrogeochemistry, Geothermometry, and Genesis of the Hot Springs in the Simao Basin in Southwestern China, Geofluids, 2019, 2019, 1–23 CAS.
- C. Varga, Problems with classification of spa waters used in balneology, Health, 2010, 2, 1260–1263 CrossRef.
- J. Fekete, C. Sajgó, Á. Kramarics, Z. Eke, K. Kovács and Z. Kárpáti, Aquathermolysis of humic and fulvic acids: simulation of organic matter maturation in hot thermal waters, Org. Geochem., 2012, 53, 109–118 CrossRef CAS.
- C. González-Barreiro, B. Cancho-Grande, P. Araujo-Nespereira, J. A. Cid-Fernández and J. Simal-Gándara, Occurrence of soluble organic compounds in thermal waters by ion trap mass detection, Chemosphere, 2009, 75, 34–47 CrossRef PubMed.
- V. Dragišić, General Hydrogeology, Faculty of Mining and Geology, Belgrade, 1997 Search PubMed.
- Directive 2009/54/EC of the European Parliament and of the Council of 18 June 2009 on the exploatation and marketing of natural mineral waters, https://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A32009L0054.
- Z. Kárpáti, C. Sajgó, I. Vető, G. Klopp and I. Horváth, Organic matter in thermal waters of the Pannonian Basin– a preliminary report on aromatic compounds, Org. Geochem., 1992, 30, 701–712 CrossRef.
- P. D. Brooks, J. Chorover, Y. Fan, S. E. Godsey, R. M. Maxwell, J. P. McNamara and C. Tague, Hydrological partitioning in the critical zone: Recent advances and opportunities for developing transferable understanding of water cycle dynamics, Water Resour. Res., 2015, 51, 6973–6987 CrossRef.
- A. Matilainen, M. Vepsäläinen and M. Sillanpää, Natural organic matter removal by coagulation during drinking water treatment: A review, Adv. Colloid Interface Sci., 2010, 159, 189–197 CrossRef CAS PubMed.
- H. Feng, Y. Liang and X. M. Hu, Natural organic matter (NOM), an underexplored resource for environmental conservation and remediation, Mater. Today Sustainability, 2022, 19, 100159 CrossRef.
- H. Abu Hasan, M. H. Muhammad and N. I. Ismail, A review of biological drinking water treatment technologies for contaminants removal from polluted water resources, J. Water Proc. Eng., 2020, 33, 101035 Search PubMed.
- C. A. Cambardella and E. T. Elliott, Particulate Soil Organic-Matter Changes across a Grassland Cultivation Sequence, Soil Sci. Soc. Am. J., 1992, 56, 777–783 CrossRef.
- A. Zsolnay, Dissolved organic matter: Artefacts, definitions, and functions, Geoderma, 2003, 113, 187–209 CrossRef CAS.
- J. Lehmann and M. Kleber, The contentious nature of soil organic matter, Nature, 2015, 528, 60–68 CrossRef CAS PubMed.
- H. C. Kim and M. J. Yu, Characterization of natural organic matter in conventional water treatment processes for selection of treatment processes focused on DBPs control, Water Res., 2005, 39, 4779–4789 CrossRef CAS PubMed.
- L. T. Sein, J. M. Varnum and S. A. Jansen, Conformational Modeling of a New Building Block of Humic Acid: Approaches to the Lowest Energy Conformer, Environ. Sci. Technol., 1999, 33, 546–552 Search PubMed.
- K. A. Thorn, W. S. Goldenberg, S. J. Youngerand and E. J. Weber, Humic and fulvic acids: isolation, structure, and environmental role, ACS Symp. Ser., 1996, 651, 299 CrossRef CAS.
- V. Uyak and I. Toroz, Modeling the formation of chlorination by-products during enhanced coagulation, Environ. Monit. Assess., 2006, 121, 501–515 CrossRef PubMed.
- R. Angelico, C. Colombo, E. Di Iorio, M. Brtnický, J. Fojt and P. Conte, Humic Substances: From Supramolecular Aggregation to Fractal Conformation—Is There Time for a New Paradigm?, Appl. Sci., 2023, 13, 2236 CrossRef CAS.
- S. Borović, T. Marković, O. Larva, Ž. Brkić and V. Mraz, Mineral and Thermal Waters in the Croatian Part of the Pannonian Basin, in Mineral and Thermal Waters of Southeastern Europe, ed. P. Papić, Environmental Earth Sciences, Springer, New York, 2016, pp. 31–45 Search PubMed.
- M. Rosca, C. Bendea and A. M. Vîjdea, Mineral and Thermal Waters of Romania, in Mineral and Thermal Waters of Southeastern Europe, ed. P. Papić, Environmental Earth Sciences, Springer, New York, 2016, pp. 97–114 Search PubMed.
- Cs. Varga, M. László, G. Gerencsér, Z. Gyöngyi and K. Szendi, Natural UV-protective organic matter in thermal water, J. Photochem. Photobiol., B, 2015, 144, 8–10 Search PubMed.
- S. Vidović and S. Djurić, Utilisation of geothermal waters and geothermal energy in Vojvodina, presented in International Geothermal Days Poland, 2004 Search PubMed.
- Institute for Standardization of Serbia, SRPS H.Z1.111:1987 - Testing of Industrial Waters – Measurement of pH – Potentiometric Method, Serbia, 1987 Search PubMed.
- Institute for Standardization of Serbia, SRPS EN 27888:2009 – Water Quality – Determination of Electrical Conductivity (ISO 7888:1985), Serbia, 2009 Search PubMed.
- E. W. Rice, R. B. Baird and A. D. Eaton, Standard Methods for the Examination of Water and Wastewater, Method 2540 B, APHA Press, Washington, 23rd edn, 2017 Search PubMed.
- E. W. Rice, R. B. Baird and A. D. Eaton, Standard Methods for the Examination of Water and Wastewater, APHA Press, Washington, 22nd edn, 2012 Search PubMed.
- International Organization for Standardization, Water Quality—Guidelines for the Determination of Total Organic Carbon (TOC) and Dissolved Organic Carbon (DOC) ISO 8245:1999, Geneva, 1999 Search PubMed.
- E. W. Rice, R. B. Baird and A. D. Eaton, Standard Methods for the Examination of Water and Wastewater, Method 6630B, APHA Press, Washington, 22nd edn, 2017 Search PubMed.
- ASTM International, D7283:17 Standard Test Method for Alpha and Beta Activity in Water by Liquid Scintillation Counting, ASTM International, The United States, 2017 Search PubMed.
- N. Todorović, J. Nikolov, B. Tenjović, I. Bikit and M. Vesković, Establishment of a method for measurement of gross alpha/beta activities in water from Vojvodina region, Radiat. Meas., 2012, 47(11–12), 1053–1059 Search PubMed.
- International Organization for Standardization, Water Quality – Gamma-Ray Emitting Radionuclides – Test Method Using High Resolution Gamma-Ray Spectrometry ISO 10703:2021, Geneva, 2021 Search PubMed.
- D. Mrdja, S. Forkapić, J. Hansman, J. Knezević Radić, D. Velimirović and K. Demirhan, Low-level gamma ray counting on environmental samples, J. Environ. Radioact., 2024, 278, 107511 CrossRef CAS PubMed.
- European Atlas of Natural Radiation, ed. G. Cinelli, M. De Cort and T. Tollefsen, Publication Office of the European Union, Luxembourg, 2019, DOI:10.2760/46388.
- J. Nikolov, N. Todorović, I. Bikit, T. P. Pantić, S. Forkapić, D. Mrda and K. Bikit, Radon in thermal waters in South-east part of Serbia, Radiat. Prot. Dosim., 2014, 160(1–3), 239–243 Search PubMed.
- U.S. Environmental Protection Agency, Determination of Radon in Drinking Water by Liquid Scintillation Counting Method 913.0 (913:0:1991), The United States, 1991 Search PubMed.
- ASTM International, D4107:20 Standard Test Method for Tritium in Drinking Water, ASTM International, The United States, 2013 Search PubMed.
- D. D. Rao, S. T. Mehendarge, S. Chandramouli, A. G. Hegde and U. C. Mishra, Application of Cherenkov radiation counting for determination of 90Sr in environmental samples, J. Environ. Radioact., 2000, 48(1), 49–57 CrossRef CAS.
- M. R. D. Mergen, B. Jefferson, S. A. Parsons and P. Jarvis, Magnetic ion-exchange resin treatment: impact of water type and resinused, Water Res., 2008, 42, 1977–1988 CrossRef CAS PubMed.
- K. V. Brix, R. D. Cardwell and W. J. Adams, Chronic toxicity of arsenic to the Great Salt Lake brine shrimp, Artemia franciscana, Ecotoxicol. Environ. Saf., 2003, 54, 169–175 CrossRef CAS PubMed.
- M. Leis, L. Manfra, L. Taddia, M. Chicca, P. Trentini and F. Savorelli, A comparative toxicity study between an autochthonous Artemia and a non-native invasive species, Ecotoxicology, 2014, 23, 1143–1145 CrossRef CAS PubMed.
- K. Kuwabara, A. Nakamura and T. Kashimoto, Effect of petroleum oil, pesticides, PCBs and other environmental contaminants on the hatchability of Artemia salina dry eggs, Bull. Environ. Contam. Toxicol., 1980, 25, 69–74 CrossRef CAS PubMed.
- P. K. Krishnakumar, A. P. Dineshbabu, G. Sasikumar and G. S. Bhat, Toxicity evaluation of treated refinery effluent using brine shrimp (Artemia salina) egg and larval bioassay, Fish. Technol., 2007, 44, 85–92 CAS.
- P. Vanhaecke and G. Persoone, Standardized short-term toxicity test with Artemia nauplii methodology and evaluation (ARC-test), Environ. Sci. Biol. Med., 1984, 106, 370–376 Search PubMed.
- SwissADME, http://www.swissadme.ch/about.php, (accessed August 2024).
- Pubchem database, https://pubchem.ncbi.nlm.nih.gov/, (accessed August 2024).
- Comparative Toxicogenomics Database (CTD), https://ctdbase.org/, (accessed August 2024).
- South Dakota State University's Bioinformatics websitehttps://bioinformatics.sdstate.edu/go/, (accessed August 2024).
- European Comission, Scientific Committee on Consumer Safety (SCCS), Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation – 12th Revision, 2023 Search PubMed.
- Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption, https://eur-lex.europa.eu/eli/dir/2020/2184/oj/eng.
- B. Elitok, Possible Effects of Balneotherapeutic Methods in the Treatment of Psoriasis and Eczema, Clin. Gastroenterol. Int., 2018, 1, 1002 Search PubMed.
- M. Tarnowska, S. Briançon, J. Resende de Azevedo, Y. Chevalier and M. A. Bolzinger, Inorganic ions in the skin: Allies or enemies?, Int. J. Pharm., 2020, 591, 119991, DOI:10.1016/j.ijpharm.2020.119991.
- F. Yang, Y. Xue, F. Wang, D. Guo, Y. He, X. Zhao, F. Yan, Y. Xu, D. Xia and Y. Liu, Sustained release of magnesium and zinc ions synergistically accelerates wound healing, Bioactive Materials, 2023, 26, 88–101 CrossRef CAS PubMed.
- M. Denda, S. Fuziwara and K. Inoue, Influx of Calcium and Chloride Ions into Epidermal Keratinocytes Regulates Exocytosis of Epidermal Lamellar Bodies and Skin Permeability Barrier Homeostasis, J. Invest. Dermatol., 2003, 121(2), 362–367 CrossRef CAS PubMed.
- S. Seite, Thermal waters as cosmeceuticals: La Roche-Posay thermal spring water example, Clin. Cosmet. Invest. Dermatol., 2013, 6, 23–28 CrossRef PubMed.
- P. Celerier, P. Litoux, B. Dreno and A. Richard, Modulatory Effects of Selenium and Strontium Salts on Keratinocyte-Derived Inflammatory Cytokines, Arch. Dermatol. Res., 1995, 287, 680–682 CrossRef CAS PubMed.
- M. L. Mourelle, C. P. Gómez and J. L. Legido, Unveiling the Role of Minerals and Trace Elements of Thermal Waters in Skin Health, Appl. Sci., 2024, 14, 6291 CrossRef CAS.
- J. Bajgai, A. Fadriquela, J. Ara, R. Begum, M. F. Ahmed, C. S. Kim, S. K. Kim, K. Y. Shim and K. J. Lee, Balneotherapeutic efects of high mineral spring water on the atopic dermatitis-like inflammation in hairless mice via immunomodulation and redox balance, BMC Complement. Med. Ther., 2017, 17, 481 CrossRef PubMed.
- S. Cacciapuoti, M. A. Luciano, M. Megna, M. Napolitano, C. Patruno, M. C. Annunziata, E. Scala, R. Colicchio, C. Pagliuca, P. Salvatore and G. Fabbrocini, The Role of Thermal Water in Chronic Skin Diseases Management: A Review of the Literature, J. Clin. Med., 2020, 9, 3047, DOI:10.3390/jcm9093047.
- M. O. Ferreira, P. C. Costa and M. F. Bahia, Effect of São Pedro Do Sul Thermal Water on Skin Irritation, Int. J. Cosmet. Sci., 2010, 32, 205–210 CrossRef CAS PubMed.
- A. S. Oliveira, C. V. Vaz, A. Silva, S. Correia, R. Ferreira, L. Breitenfeld, J. Martinez-de-Oliveira, R. Palmeira-de-Oliveira, C. Pereira and M. T. Cruz, et al, In Vitro Evaluation of Potential Benefits of a Silica-Rich Thermal Water (Monfortinho Thermal Water) in Hyperkeratotic Skin Conditions, Int. J. Biometeorol., 2020, 64, 1957–1968 CrossRef PubMed.
- I. Tenaud, I. Sainte-Marie, O. Jumbou, P. Litoux and B. Dréno, In vitro modulation of keratinocyte wound healing integrins by zinc, copper and manganese, Br. J. Dermatol., 1999, 140, 26–34 Search PubMed.
- P. H. Lin, M. Sermersheim, H. Li, P. H. U. Lee, S. M. Steinberg and J. Ma, Zinc in wound healing modulation, Nutrients, 2018, 10, 1–20 Search PubMed.
- M. G. Mahoney, D. Brennan, B. Starcher, J. Faryniarz, J. Ramirez, L. Parr and J. Uitto, Extracellular matrix in cutaneous ageing: The effects of 0.1% copper-zinc malonate-containing cream on elastin biosynthesis, Exp. Dermatol., 2009, 18(3), 205–211, DOI:10.1111/j.1600-0625.2008.00783.x.
- N. Chebassier, E. H. Ouijja, I. Viegas and B. Dreno, Stimulatory Effect of Boron and Manganese Salts on Keratinocyte Migration, Acta Derm. Venereol., 2004, 84, 191–194 CrossRef CAS PubMed.
- S. Afful, A. K. Anim and Y. Serfor-Armah, Spectrum of organochlorine pesticide residues in fish samples from the Densu Basin, Res. J. Environ. Earth Sci., 2010, 2, 133–138 Search PubMed.
- E. M. Błońska-Sikora, M. Klimek-Szczykutowicz, M. Michalak, K. Kulik-Siarek and M. Wrzosek, Potential Possibilities of Using Peat, Humic Substances, and Sulfurous Waters in Cosmetology, Appl. Sci., 2024, 14, 6912 CrossRef.
- F. García-Villén, R. Sánchez-Espejo, E. Carazo, A. Borrego-Sánchez, C. Aguzzi, P. Cerezo and C. Viseras, Characterisation of Andalusian peats for skin health care formulations, Appl. Clay Sci., 2018, 160, 201–205 CrossRef.
- N. Gheibi, F. Samiee-Rad, M. Sofiabadi, E. Mosayebi and Z. Shalbaf, The Effect of Combining Humic and Fulvic Acids Poultice on Wound Healing in Male Rats, J. Cutan. Aesthetic Surg., 2023, 17(2), 105–111, DOI:10.4103/JCAS.JCAS_92_23.
- F. Samiee-Rad, S. F. Hosseini Sedighi, A. Taherkhani and N. Gheibi, Evaluation of Healing Effects of Poultice Containing 0.5% Fulvic Acid on Male White-Male Rats with Skin Ulcer, J. Cutan. Aesthetic Surg., 2022, 15, 40–47 CrossRef PubMed.
- M. Verrillo, M. Parisi, D. Savy, G. Caiazzo, R. Di Caprio, M. A. Luciano, S. Cacciapuoti, G. Fabbrocini and A. Piccolo, Antiflammatory activity and potential dermatological applications of characterized humic acids from a lignite and a green compost, Sci. Rep., 2022, 12, 2152, DOI:10.1038/s41598-022-06251-2.
- S. Tak and B. P. Vellanki, Natural organic matter as precursor to disinfection byproducts and its removal using conventional and advanced processes: state of the art review, J. Water Health, 2018, 16, 681–703 CrossRef PubMed.
- I. Stojković, B. Tenjović, J. Nikolov and N. Todorović, Possibilities and limitations of color quench correction methods for gross alpha/beta measurements, Appl. Radiat. Isot., 2017, 122, 164–173 CrossRef PubMed.
- Council Directive 2013/51/EURATOM of October 2013 laying down requirements for the protection of public health with regard to radioactive substances in water intended for human consumption, https://eur-lex.europa.eu/eli/dir/2013/51/oj.
- European Commission, Commission Recommendation of 20 December 2001 on the protection of the public against exposure to radon in drinking water supplies (notified under document number C(2001) 4580), https://eur-lex.europa.eu/legal-content/HR/TXT/?uri=CELEX:32001H0928.
- OECD Guidelines for the Testing of Chemicals, Test No. 471, Bacterial Reverse Mutation Test, 2020, DOI:10.1787/9789264071247-en.
- OECD Guidelines for the Testing of Chemicals, Test No. 487, In Vitro Mammalian Cell Micronucleus Test, 2023, DOI:10.1787/9789264264861-en.
- European Commission, EU Reference Laboratory for alternatives to animal testing (EURL ECVAM), 3D Reconstructed Human Skin Micronucleus assay (RSMN), under validation, received, 2020, https://tsar.jrc.ec.europa.eu/test-method/tm2020-04.
- I. Sutzkover-Gutman, D. Hasson and R. Semiat, Humic substances fouling in ultrafiltration processes, Desalination, 2010, 261, 218–231 CrossRef CAS.
- I. V. Volkov and E. V. Polyakov, Complexation of Humic Acids with Trace Elements: Methods and Approaches, J. Anal. Chem., 2023, 78, 1575–1602 CrossRef CAS.
- A. G. Kalinichev and R. J. Kirkpatrick, Molecular dynamics simulation of cationic complexation with natural organic matter, Eur. J. Soil Sci., 2007, 58(4), 909–917 Search PubMed.
- J. Adusei-Gyamfi, B. Ouddane, L. Rietveld, J. P. Cornard and J. Criquet, Natural organic matter-cations complexation, and its impact on water treatment: A critical review, Water Res., 2019, 160, 130–147 CrossRef CAS PubMed.
- H. Van Dijk, Cation binding of humic acids, Geoderma, 1971, 5, 53–67 Search PubMed.
- C. Song, S. Sun, J. Wang, Y. Gao, G. Yu, Y. Li, Z. Liu, W. Zhang and L. Zhou, Applying fulvic acid for sediment metals remediation: Mechanism, factors, and prospect, Front. Microbiol., 2023, 13, 1084097, DOI:10.3389/fmicb.2022.1084097.
- E. Gillispie, E. Andujar and M. L. Polizzotto, Chemical controls on abiotic and biotic release of geogenic arsenic from Pleistocene aquifer sediments to groundwater, Environ. Sci.: rocesses Impacts, 2016, 18, 1090–1103 CAS.
- Y. Chen, Organic matter reactions involving micronutrients in soils and their effect on plants, in Humic Substances in Terrestrial Ecosystems, ed. A. Piccolo, Elsevier, Amsterdam, 1996, pp. 507–530 Search PubMed.
- M. Haghighi, M. Kafi, P. Fang and L. Gui-Xiao, Humic acid decreased hazardous of cadmium toxicity on lettuce (Lactuca Sativa L.), J. Fruit Ornam. Plant Res., 2020, 72, 49–61 CrossRef.
- M. Swat, I. Rybicka and A. Gliszczyńska-Świgło, Characterization of Fulvic Acid Beverages by Mineral Profile and Antioxidant Capacity, Foods, 2019, 8, 605 CrossRef CAS PubMed.
- O. I. Klein, N. A. Kulikova, A. I. Konstantinov, M. V. Zykova and I. V. Perminova, A Systematic Study of the Antioxidant Capacity of Humic Substances against Peroxyl Radicals: Relation to Structure, Polymers, 2021, 13, 3262 CrossRef CAS PubMed.
- M. V. Zykova, I. A. Schepetkin, M. V. Belousov, S. V. Krivoshchekov, L. A. Logvinova, K. A. Bratishko, M. S. Yusubov, S. V. Romanenko and M. T. Quinn, Physicochemical characterization and antioxidant activity of humic acids isolated from peat of various origins, Molecules, 2018, 23, 753 Search PubMed.
- C. A. Rice-Evans, N. J. Miller and G. Paganga, Structure antioxidant activity relationships of flavonoids and phenolic acids, Free Radical Biol. Med., 1996, 20, 933–956 CrossRef CAS PubMed.
- M. Aeschbacher, C. Graf, R. P. Schwarzenbach and M. Sander, Antioxidant Properties of Humic Substances, Environ. Sci. Technol., 2012, 46(9), 4916–4925 Search PubMed.
- E. Yildiztugay, C. Ozfidan-Konakci, F. Elbasan, A. Yildiztugay and M. Kucukoduk, Humic acid protects against oxidative damage induced by cadmium toxicity in wheat (Triticum aestivum) roots through water management and the antioxidant defence system, Bot. Ser., 2019, 43, 161–173 CrossRef.
- J. Vašková, B. Veliká, M. Pilátová, I. Kron and L. Vaško, Effects of humic acids in vitro, In Vitro Cell. Dev. Biol.: Anim., 2011, 47, 376–382, DOI:10.1007/s11626-011-9405-8.
- J. Vašková, M. Stupák, M. Vidová Ugurba, D. Žatko and L. Vaško, Therapeutic Efficiency of Humic Acids in Intoxications, Life, 2023, 13, 971 CrossRef PubMed.
- J. Winkler and S. Ghosh, Therapeutic Potential of Fulvic Acid in Chronic Inflammatory Diseases and Diabetes, J. Diabetes Res., 2018, 10, 5391014, DOI:10.1155/2018/5391014.
- C. Wu, A. Lyu and S. Shan, Fulvic Acid Attenuates Atopic Dermatitis by Downregulating CCL17/22, Molecules, 2023, 28, 3507 CrossRef CAS PubMed.
- NIH, National Institute of health, division of microbiology and infectious disease screening and testing program, in Broad-spectrum antiviral efficacy of natural product humates now trademarked viracillin, 2002, https://www.viracillin.com/pdfs/4214a_National_Institutes_of_Health.pdf, accessed December 2024.
- R. Klöcking and M. Sprössig, Antiviral properties of humic acids, Experientia, 1972, 28, 607–608 CrossRef PubMed.
- V. Cagno, M. Donalisio, A. Civra, C. Cagliero, P. Rubiolo and D. Lembo, In vitro evaluation of the antiviral properties of Shilajit and investigation of its mechanisms of action, J. Ethnopharmacol., 2015, 166, 129–134 CrossRef PubMed.
- K. Pant, A. K. Yadav, P. Gupta, A. S. Rathore, B. Nayak and S. K. Venugopal, Humic acid inhibits HBV-induced autophagosome formation and induces apoptosis in HBV-transfected Hep G2 cells, Sci. Rep., 2016, 6, 34496 CrossRef CAS PubMed.
- R. Junek, R. Morrow, J. I. Schoenherr, R. Schubert, R. Kallmeyer and S. Phull, et al., Bimodal effect of humic acids on the LPS-induced TNF-alpha release from differentiated U937 cells, Phytomedicine, 2009, 16, 470–476 CrossRef CAS PubMed.
- C. Wu, A. Lyu and S. Shan, Fulvic Acid Attenuates Atopic Dermatitis by Downregulating CCL17/22, Molecules, 2023, 28, 3507 CrossRef CAS PubMed.
- S. G. Gurel, I. Sogut, C. Hurdag, A. Gurel, A. Tutar and E. Cikler-Dulger, Effect of fulvic acid on gastric mucosa damage caused by chronic water avoidance stress, Biotech. Histochem., 2022, 97, 199–206 CrossRef CAS PubMed.
- J. Gandy, J. P. Meeding, J. R. Snyman and C. E. Van Rensburg, Phase 1 clinical study of the acute and subacute safety and proof-of-concept efficacy of carbohydrate-derived fulvic acid, Clin. Pharmacol.: Adv. Appl., 2012, 4, 7–11 CAS.
- L. Sherry, E. Millhouse, D. F. Lappin, C. Murray, S. Culshaw, C. J Nile and G. Ramage, Investigating the biological properties of carbohydrate derived fulvic acid (CHD-FA) as a potential novel therapy for the management of oral biofilm infections, BMC Oral Health, 2013, 13, 47, DOI:10.1186/1472-6831-13-47.
- STRING database, https://string-db.org/, (accessed August 2024).
- K. Linauskienė, L. Malinauskienė and A. Blažienė, Metals Are Important Contact Sensitizers: An Experience from Lithuania, BioMed Res. Int., 2017, 1–5, DOI:10.1155/2017/3964045.
- M. Marinovich, M. S. Boraso, E. Testai and C. L. Galli, Metals in cosmetics: A posteriori safety evaluation, Regul. Toxicol. Pharmacol., 2014, 69, 416–424 CrossRef CAS PubMed.
- Scientific Advisory Committee on Nutrition, Salt and Health, The Stationery Office, Norwich, 2003 Search PubMed.
- European Medicines Agency Committee for Human Medicinal Products, Questions and answers on sodium used as an excipient in medicinal products for human use, 2017, EMA/CHMP/338679/2014, https://www.ema.europa.eu/en/documents/scientific-guideline/questions-and-answers-sodium-used-excipient-medicinal-products-human-use_en.pdf.
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA), Dietary reference values for sodium, 2019, DOI:10.2903/j.efsa.2019.5778, https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2019.5778.
- L. K. Dahl, Possible role of salt intake in the development of essential hypertension, in Essential Hypertension: an International Symposium, ed. K. D. Bock and P. T. Cottier, Springer Berlin, Heidelberg, 1960, pp. 53–65 Search PubMed.
- V. Karr-Dullien and E. Bloomquist, The influence of prenatal salt on the development of hypertension by spontaneously hypertensive rats (SHR), Proc. Soc. Exp. Biol. Med., 1979, 160, 421–425 CrossRef CAS PubMed.
- J. C. Merrill, J. P. Morton and S. D. Soileau, Metals, in Principles and Methods of Toxicology, ed. A. Wallace Hayes, Taylor and Francis, London, 5th edn, 2001, pp. 649–698 Search PubMed.
- EFSA, Opinion of the Scientific Panel on Dietetic Products, Nutrition and Allergies on a request from the Commission related to the Tolerable Upper Intake Level of Iron, EFSA J., 2004, 2(11), 125, DOI:10.2903/j.efsa.2004.125.
- Institute of Medicine (US) Panel on Micronutrients, Dietary Reference Intakes: Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodide, Iron, Manganese Molybdenum, Nickel, Silicon, Vanadium and Zinc, National Academy Press (US), Washington D.C., 2001, Search PubMed.
- OECD, SIDS Initial Assessment Report for SIAM 24, Paris, France, 17-20 April 2007, Iron Salts Category, https://hpvchemicals.oecd.org/ui/Search.aspx.
- A. Parlesak, T. Theresa Masino, K. D. Reis, C. Filskov Petersen, J. J. Christensen, T. Olsen and I. Tetens, Preparatory work for the update of the tolerable upper intake levels for iron, EFSA Supporting Publ., 2024, 21(2), 8661E, DOI:10.2903/sp.efsa.2024.EN-8661.
- EFSA, Health risk of ammonium released from water filters, EFSA J., 2010, 10, 2918, DOI:10.2903/j.efsa.2012.2918.
- Agency for Toxic Substances and Disease Registry (ATSDR), Toxicological Profile for Ammonia, U.S. Department of Health and Human Services, Atlanta, Georgia, 2004, p. 269 Search PubMed.
- OECD, SIDS Initial Assessment Report for SIAM 17 Arona, Italy, 11-14 November 2003, Ammonium chloride, https://hpvchemicals.oecd.org/ui/Search.aspx.
- K. Gnananath, K. S. Nataraj, B. G. Rao, K. P. Kumar, M. H. Mahnashi, Md. Anwer, A. Umar, Z. Iqbal and M. A. Mirza, Exploration of fulvic acid as a functional excipient in line with the regulatory requirement, Environ. Res., 2020, 187, 109642 CrossRef CAS PubMed.
- C. Dai, X. Xiao, Y. Yuan, G. Sharma and S. Tang, A Comprehensive Toxicological Assessment of Fulvic Acid, J. Evidence-Based Integr. Med., 2020, 1–11 Search PubMed.
- D. Lieu, Fulvic Acid; Human Health Risk Assessment and Ecological Effects Assessment to Support Proposed Exemption from the Requirement of a Tolerance when Used as an Inert Ingredient in Pesticide Formulations, US Environmental Protection Agency, 2020, https://www.regulations.gov/document/EPA-HQ-OPP-2018-0152-0006 Search PubMed.
- C. E. J. Van Rensburg, The antiinflammatory properties of humic substances: a mini review, Phytother. Res., 2015, 29, 791–795, DOI:10.1002/ptr.5319.
- J. J. Gandy, J. P. Meeding, J. R. Snyman and C. E. J. Van Rensburg, Phase 1 clinical study of the acute and subacute safety and proof-of-concept efficacy of carbohydrate-derived fulvic acid, Clin. Pharmacol., 2012, 7–11 Search PubMed.
- The European Agency for the Evaluation of the Medicinal Products, Committee for Veterinary Medicinal Products, Humic Acids and Their Sodium Salts, Summary Report, EMEA, Amsterdam, 1999 Search PubMed.
- T. S. Murbach, R. Glávits, J. R. Endres, A. E. Clewell, G. Hirka, A. Vértesi, E. Béres and I. Pasics Szakonyiné, A toxicological evaluation of a fulvic and humic acids preparation, Toxicol Rep, 2020, 7, 1242–1254 Search PubMed.
- Y. Zhernov, A. Zhdanova, N. Avvakumova and M. Khaitov, Application of topical peloid-derived humic acid suppresses allergic contact dermatitis in mice mode, World Allergy Organ. J., 2020, 13, 120 Search PubMed.
- The European Chemicals Agency (ECHA), Ammonia, anhydrous, Registration Doosier, https://echa.europa.eu/hr/registration-dossier/-/registered-dossier/15557/7/1 (accessed July 2024).
| This journal is © The Royal Society of Chemistry 2025 |



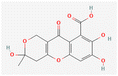
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC1C(C2C(
CC1C(C2C(


