 Open Access Article
Open Access ArticleBiodegradability of poly(ester-thioether)s containing chiral biomass via a Michael-type thiol-ene click reaction†
Ryota Imamura and
Akinori Takasu *
*
Division of Soft Materials, Graduated School of Engineering, Nagoya Institute of Technology, Gokiso-cho, Showa-ku, Nagoya 466-8555, Japan. E-mail: takasu.akinori@nitech.ac.jp
First published on 17th April 2025
Abstract
We prepared dianhydrosugar-based diacrylates: isosorbide bis(acrylate) (ISDA) and isomannide bis(acrylate) (IMDA) from the corresponding dianhydrosugars. Using a scandium triflate as the catalyst, the chemoselective dehydration of D-, L-, and meso-tartaric acids with 2-mercaptoethanol selectively synthesized three dithiols: L-bis(2-mercaptoethyl)tartrate (META), D-META, and meso-META. The thiol-Michael click polyaddition of ISDA or IMDA with the three dithiols proceeded in N,N-dimethylformamide (40 °C) using triethylamine as the catalyst, yielding the expected six poly(ester-thioether) diastereomers (Mn, 8.2 × 103 to 9.2 × 103; molecular weight distribution (Mw/Mn), 1.29–1.51). The synthesized poly(ester-thioether)s showed a single glass transition temperature (Tg) between −8 and 14 °C. In biodegradation measurements, monitored by biochemical oxygen demand (BOD) values in active sludge, poly(IMDA-alt-meso-META), poly(ISDA-alt-L-META), and poly(ISDA-alt-D-META) showed lower biodegradability. In contrast, poly(IMDA-alt-L-META), poly(IMDA-alt-D-META), and poly(ISDA-alt-meso-META) showed 16% to 28% biodegradation after 30 days, indicating diastereomeric effects on biodegradability. Since enzymatic hydrolysis using lipase showed a similar trend to the BOD tests, we concluded that biodegradability depends on the stereochemistry along the polymer backbone.
Introduction
In this decade, the practical applications of polymer materials have been increasing year by year. Simultaneously, environment concerns regarding these useful polymeric materials are also increasing. Between the 1980s and 2000s, many researchers studied biodegradable polymers; however, their high cost limited practical use in daily life. The urgent requirement to address the microplastic pollution problem has now prompted polymer chemists to reconsider biodegradable polymers and their modifications. Since Tokiwa et al. (1990) reported the biodegradation of polyesters using several esterases, including lipase,1 the most reliable biodegradable polymers are aliphatic polyesters, including poly(alkylene succinate),2 poly(3-hydroxybutyric acid) (PHB),3 and poly(lactic acid).4 These were synthesized via polycondensation under severe reaction conditions following the traditional Carothers procedure.5,6 Although this classical polycondensation procedure under severe conditions has limited the incorporation of functionalities into biodegradable polyesters, we have reported the scandium triflate [Sc(OTf)3]-catalyzed dehydration polycondensation of dicarboxylic acids and diols under mild conditions, even at room temperature.7 This low-temperature polycondensation enabled us to use several dicarboxylic acids with extra functionalities, including maleic acid,8 bromosuccinic acid,8 lactic acid,9 and tartaric acid,10 to produce polyesters containing carbon–carbon double bonds, pendent bromo, methyl, and hydroxy groups, respectively. This procedure is expected to be a powerful method for synthesizing new biodegradable polyesters.As an alternative synthetic route for polyesters, successive click reactions, invented by Sharpless, using monomers containing ester linkages have been reported.11–14 We synthesized polyesters containing cyclic triazole units15 and poly(ester-thioether)s16 via copper(I)-catalyzed alkyne–azide click reactions (CCAC) and thiol-ene click reactions, respectively. As an extension of our work on click poly(ester-thioether)s,17–20 we further demonstrated click polymerization using biomass-derived monomers, including L-malic acid,21 1,4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3,6-dianhydrosugars,22 and tartaric acid.23 During this research, we found that biodegradation might depend on the stereochemistry23 along the polymer backbone. This research background prompted us to click-polymerize 1,4
3,6-dianhydrosugars,22 and tartaric acid.23 During this research, we found that biodegradation might depend on the stereochemistry23 along the polymer backbone. This research background prompted us to click-polymerize 1,4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3,6-dianhydrosugar-based diacrylates and tartaric acid-based dithiols to afford a series of poly(ester-thioether)s with different stereochemistries (Fig. 1).
3,6-dianhydrosugar-based diacrylates and tartaric acid-based dithiols to afford a series of poly(ester-thioether)s with different stereochemistries (Fig. 1).
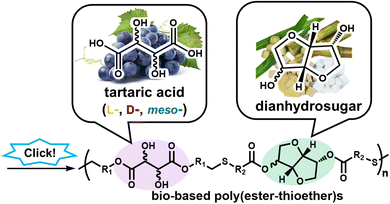 | ||
| Fig. 1 Synthesis of poly(ester-thioether)s containing dianhydrosugar and tartaric acid units via Michael-type thiol-ene click polymerization. | ||
In this article, we described the preparation of six diastereomeric poly(ester-thioether)s with similar molar masses and molecular dispersity indices, obtained from the polyaddition of two dianhydrosugar-containing diacrylate monomers and three tartaric acid-based dithiols using triethylamine as the catalyst. The dianhydrosugar-based diacrylates: isosorbide bis(acrylate) (ISDA)24 and isomannide bis(acrylate)(IMDA), were prepared from 1,4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3,6-dianhydroglucitol and 1,4
3,6-dianhydroglucitol and 1,4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3,6-dianhydromannitol. Using scandium triflate [Sc(OTf)3] as the catalyst, the chemoselective dehydration of D-, L-, and meso-tartaric acids with 2-mercaptoethanol yielded three dithiols: L-bis(2-mercaptoethyl)tartrate (META), D-META, and meso-META. We then performed biodegradation tests in activated sludge and enzymatic hydrolysis using lipase25,26 to examine whether the diastereomers showed different biodegradation behaviors. This work provides a new guideline suggesting that the stereochemistry of monomers affects their biodegradability, as most biomass-based substances contain chiral centers.
3,6-dianhydromannitol. Using scandium triflate [Sc(OTf)3] as the catalyst, the chemoselective dehydration of D-, L-, and meso-tartaric acids with 2-mercaptoethanol yielded three dithiols: L-bis(2-mercaptoethyl)tartrate (META), D-META, and meso-META. We then performed biodegradation tests in activated sludge and enzymatic hydrolysis using lipase25,26 to examine whether the diastereomers showed different biodegradation behaviors. This work provides a new guideline suggesting that the stereochemistry of monomers affects their biodegradability, as most biomass-based substances contain chiral centers.
Experimental section
Materials
L- (>99%), D- (>99%), and meso-tartaric acids (>90%) (L-TA, D-TA, and meso-TA), isomannide (IM) (>98%), isosorbide (IS) (>98%), allyl alcohol (>99%), mercaptoacetic acid (>95%), acryloyl chloride (>98%), 2-mercaptoethanol (>98%), scandium (iii) trifluromethanesulfonate [Sc(OTf)3] (98%), triethylamine (Et3N) (>99%), and 2,2′-azobis(isobutyronitrile) (AIBN) (>98%) were obtained from Tokyo Chemical Industry (Tokyo, Japan) and used as received. Lipase, immobilized on Immobead 150 from Pseudomonas cepacia, was purchased from Sigma-Aldrich, Inc. (Missouri, USA) and used as delivered. Common reagents and solvents were purchased from Japanese companies and used without further purification.Analytical measurements
1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded at 27 °C using a Bruker Analytik DPX400 apparatus, with tetramethylsilane as the internal standard (0.0 ppm). The optical rotation measurement of METAs was measured using a JASCO P-1010 polarimeter in tetrahydrofuran at 25 °C to determine specific rotations [α]25D. The number-average molecular weight (Mn) and molecular weight distribution (Mw/Mn) of the poly(ester-thioether)s were determined using Shodex KD-803 and KD-804 columns in a size exclusion chromatography (SEC) system consisting of a JASCO PU-2028 pump, a JASCO RI-2031 differential refractometer, and an intelligent column oven (JASCO CO-2065 Plus). N,N-Dimethylformamide (DMF) containing 0.05 wt% lithium bromide was used as the eluent, with a flow rate of 0.5 mL min−1 and a temperature of 40 °C. The calibration of Mn and Mw/Mn was performed using a series of poly(methyl methacrylate) samples as standards. Differential scanning calorimetry (DSC) measurements were conducted using a DSC7020 equipment (HITACHI, Japan). During the first heating, the scan was carried out from 30 °C to 90 °C at a rate of 10 °C min−1, followed by cooling to −90 °C. The second scan was then performed from −90 °C to 90 °C at a scan rate of 10 °C min−1 to determine the Tgs. The scans were performed under a nitrogen gas flow.Preparation of monomers
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–, 1.3 and 10.3 Hz), 6.20 (dd, 2H, CH2
CH–, 1.3 and 10.3 Hz), 6.20 (dd, 2H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–, 10.4 and 17.4 Hz), 6.49 (dd, 2H, CH2
CH–, 10.4 and 17.4 Hz), 6.49 (dd, 2H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–, 1.3 and 17.3 Hz) (Fig. S1†). 13C NMR (100 MHz, CDCl3, δ, ppm): 70.51 (C-1 and C-6), 73.85 (C-2 and C-5), 80.53 (C-3 and C-4), 127.58 (CH2
CH–, 1.3 and 17.3 Hz) (Fig. S1†). 13C NMR (100 MHz, CDCl3, δ, ppm): 70.51 (C-1 and C-6), 73.85 (C-2 and C-5), 80.53 (C-3 and C-4), 127.58 (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 131.90 (CH2
CHCO–), 131.90 (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 165.49 (CH2CHCO–) (Fig. S2†).
CHCO–), 165.49 (CH2CHCO–) (Fig. S2†).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–, 6.3 and 10.6 Hz), 6.15 (dd, 2H, CH2
CH–, 6.3 and 10.6 Hz), 6.15 (dd, 2H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–, 10.5 and 16.7 Hz), 6.45 (dd, 2H, CH2
CH–, 10.5 and 16.7 Hz), 6.45 (dd, 2H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–, 1.4 and 16.0 Hz) (Fig. S3†). 13C NMR (100 MHz, CDCl3, δ, ppm): 70.40 (C-1), 73.49 (C-6), 74.11 (C-2), 78.13 (C-5), 80.89 (C-4), 85.99 (C-3), 127.71 (CH2
CH–, 1.4 and 16.0 Hz) (Fig. S3†). 13C NMR (100 MHz, CDCl3, δ, ppm): 70.40 (C-1), 73.49 (C-6), 74.11 (C-2), 78.13 (C-5), 80.89 (C-4), 85.99 (C-3), 127.71 (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO–), 131.83 (CH2
CHCO–), 131.83 (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO-endo–), 131.91 (CH2
CHCO-endo–), 131.91 (CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO-exo–) 165.15 (CH2CHCO-endo–), 165.47 (CH2CHCO-exo–) (Fig. S4†).
CHCO-exo–) 165.15 (CH2CHCO-endo–), 165.47 (CH2CHCO-exo–) (Fig. S4†).Synthesis of poly(ester-thioether)s via Michael-type thiol-ene polyaddition
The Michael-type thiol-ene polyadditions of IMDA and ISDA with META (L-, D-, meso-) were carried out in a dry flask. As an example, IMDA (405.0 mg, 1.5 mmol) and L-META (381.1 mg, 1.5 mmol) were dissolved in 3 mL of dry DMF in a 10 mL-eggplant flask under N2 in a glovebox. Et3N (41.6 μL, 0.3 mmol) was then added dropwise. The solution was maintained at 40 °C for 24 h, after which the product was precipitated with CHCl3 followed by n-hexane. The polymer was dried at 40 °C to obtain poly(IMDA-alt-L-META) as a polymeric material. For the other five poly(ester-thioether)s using ISDA, D-META, and meso-META, the corresponding polymers were obtained using a similar procedure. The structures and properties of the poly(ester-thioether)s were confirmed by NMR and SEC and thermally characterized by DSC.Biochemical oxygen demand (BOD) testing
According to the reported procedure,23 10 mg of the corresponding poly(ester-thioether) in 5 mL of CHCl3 solution was added to a test bottle (n = 2 per polymer) and dried at 25 °C for 2 days. Then, activated sludge, obtained from the Nagoya City Waterworks and Sewerage Bureau, was added at a concentration of 30 mg L−1 to an incubation medium containing (mg L−1) K2HPO4 (218), KH2PO4 (85), Na2HPO4 (334), NH4Cl (5), CaCl2 (28), MgSO4·7H2O (23), and FeCl3·6H2O (0.25), with the pH adjusted to 7.4. This aqueous solution was then added to each test bottle to achieve a polymer concentration of 100 mg L−1. The test bottles were maintained at 25 °C for 30 days in a constant-temperature bath, and the atmospheric pressure in the bottles were measured daily. The biochemical oxygen demand (BOD) at 25 °C was determined in duplicate by measuring oxygen consumption using BOD equipment (model 200F, TAITEC Co., Koshigaya-shi, Japan) according to ISO guidelines (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 851). The degree of biodegradability (%) of the polymer was determined as [(BODsample − BODblank)/TOD] × 100, where BODsample and BODblank represent the actual measured BOD values of the polymer and the blank medium, respectively, and TOD is the theoretical oxygen demand of the poly(ester-thioether) when fully oxidized.
851). The degree of biodegradability (%) of the polymer was determined as [(BODsample − BODblank)/TOD] × 100, where BODsample and BODblank represent the actual measured BOD values of the polymer and the blank medium, respectively, and TOD is the theoretical oxygen demand of the poly(ester-thioether) when fully oxidized.
Enzymatic hydrolysis test by total organic carbon (TOC) measurement
According to the reported procedure,25,26 a 5 mg of each sample (n = 2 per polymer) in a trace amount of CHCl3 was transferred to separate test tubes and evaporated over 2 days. Immobilized lipase from Pseudomonas cepacia (50 units) was added to one of the tubes, while the other tube served as a control without lipase to confirm the enzyme's effect. Standard buffer (pH 6.86 ± 0.02) (2.0 mL) was added to both tubes. The targeted TOC values were monitored three times for each sample, and the average value was determined. The samples were incubated at 37 °C for 24 h. After the aqueous test medium was filtered through a membrane filter, the total organic carbon (TOC) was recorded using a total organic carbon analyzer (TOC-2300, HIRANUMA Co., Mito, Japan). The TOC measurements were performed in triplicate for each sample, and the average value was determined.Results and discussion
First, we prepared the monomers for the Michael-addition click polymerization. Esterification of IM with 2.5 equiv. acryloyl chloride was performed in dry DCM using TEA as the base catalyst at room temperature for 2 h (Scheme 1), which afforded the targeted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adduct of IM and acryloyl chloride (IMDA) (50% yield). The IS-based diacrylate was synthesized similarly to give the corresponding 1
2 adduct of IM and acryloyl chloride (IMDA) (50% yield). The IS-based diacrylate was synthesized similarly to give the corresponding 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adduct (ISDA) (45% yield). The 1H- and 13C NMR spectra indicated the expected chemical structures of IMDA (Fig. S1 and S2†) and ISDA (Fig. S3 and S4†). Chemoselective esterification of L-TA with 4.0 equiv. 2-mercaptoethanol was performed under solvent-free conditions using Sc(OTf)3 as the catalyst at 50 °C for 48 h (Scheme 1), which afforded the expected 1
2 adduct (ISDA) (45% yield). The 1H- and 13C NMR spectra indicated the expected chemical structures of IMDA (Fig. S1 and S2†) and ISDA (Fig. S3 and S4†). Chemoselective esterification of L-TA with 4.0 equiv. 2-mercaptoethanol was performed under solvent-free conditions using Sc(OTf)3 as the catalyst at 50 °C for 48 h (Scheme 1), which afforded the expected 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adduct of L-TA and 2-mercaptoethanol (L-META) in 30% yield. D-TA and meso-TA-based dithiol monomers were synthesized using a similar method to afford the corresponding 1
2 adduct of L-TA and 2-mercaptoethanol (L-META) in 30% yield. D-TA and meso-TA-based dithiol monomers were synthesized using a similar method to afford the corresponding 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 adducts (D-META and meso-META) in 25% and 20% yields, respectively. The 1H- and 13C NMR spectra confirmed the targeted structures of L-META, D-META, and meso-META (Fig. S5–S10†). To verify whether racemization occurred, we performed optical rotation measurements of the METAs in tetrahydrofuran at 25 °C. The specific rotations [α]25D of L-META, D-META, and meso-META were +10.42, −10.42, and +0.18, respectively, indicating that the stereochemistries of the tartaric acids were preserved during the Sc(OTf)3-catalyzed esterification.
2 adducts (D-META and meso-META) in 25% and 20% yields, respectively. The 1H- and 13C NMR spectra confirmed the targeted structures of L-META, D-META, and meso-META (Fig. S5–S10†). To verify whether racemization occurred, we performed optical rotation measurements of the METAs in tetrahydrofuran at 25 °C. The specific rotations [α]25D of L-META, D-META, and meso-META were +10.42, −10.42, and +0.18, respectively, indicating that the stereochemistries of the tartaric acids were preserved during the Sc(OTf)3-catalyzed esterification.
 | ||
| Scheme 1 Preparation of diacrylates containing dianhydrosugars and dithiols containing tartaric acids via esterification. | ||
Next, we demonstrated the Michael polyaddition of IMDA and ISDA with three types of META (L-, D-, and meso-) as shown in Scheme 2. The monomer solution was stirred in dry DMF at 40 °C for 24 h, catalyzed by TEA (10 mol% relative to the monomer). The results are summarized in Table 1. After the reaction, the reaction mixtures were diluted with CHCl3 and poured into an excess of n-hexane, a poor solvent, to precipitate a polymeric syrup. The synthesized polymers were soluble in general organic solvents (CHCl3, THF, DMF, etc.) but not in water. The 1H and 13C NMR spectra confirmed the corresponding poly(IMDA-alt-META)s (Fig. 2 and S11–S15) and poly(ISDA-alt-META)s (Fig. S16–S21†), indicating that the polyaddition occurred via a Michael-type thiol-ene click mechanism, with a total initial monomer concentration [M]0 of 1.0 M.
| Entry | Diacrylatea | Dithiola | Mnb (kg mol−1) | Mw/Mnb | DPc | Tgd (°C) | Yield (%) |
|---|---|---|---|---|---|---|---|
| a Conditions: in DMF at 40 °C for 24 h using TEA (10% relative to monomers).b Determined by SEC measurement based on a linear PMMA standard in DMF.c DP is the degree of polymerization, calculated by Mn/M1, where M1 is the molecular weight of the monomer unit.d Determined by DSC. | |||||||
| 1 | IMDA | L-META | 8.2 | 1.51 | 15 | 14 | 87 |
| 2 | IMDA | D-META | 9.2 | 1.31 | 17 | 5 | 80 |
| 3 | IMDA | meso-META | 8.9 | 1.29 | 16 | −5 | 93 |
| 4 | ISDA | L-META | 9.2 | 1.34 | 17 | 5 | 94 |
| 5 | ISDA | D-META | 8.9 | 1.32 | 16 | −6 | 99 |
| 6 | ISDA | meso-META | 9.0 | 1.34 | 16 | −8 | 99 |
SEC measurements showed that the Mn of the poly(ester-thioether)s ranged from 8.2 × 103 to 9.2 × 103, with a molecular weight distribution (Mw/Mn) of 1.29–1.51 (entries 1–6 in Table 1, see also Fig. S22 and S23†). The yields were almost quantitative, ranging from 80% to 99%. The yields were independent of the monomer used in this polymerization, and polymers with similar molecular weights were obtained. DSC measurements of all the poly(ester-thioether)s indicated a single glass transition temperature (Tg) in each scan, with no endothermic traces, revealing the absence of crystalline regions which melt under the examined circumstances (Fig. S24 and S25†). The Tg values ranged from −8 to 14 °C, with poly(IMDA-alt-L-META) exhibiting the highest Tg. We also synthesized poly(ester-thioether)s containing dianhydrosugars and tartaric acids via radical thiol-ene click polymerization. In this case, the combination of IM and L-TA units also showed the highest Tg of 21 °C (Scheme S1 and Table S1†). The differences in Tg are probably due to the particular diastereomers involved. The poly(ester-thioether) synthesized from the combination of IM and L-TA may facilitate stronger hydrogen bonding between polymer chains, which restricts molecular motion and increases the Tg.
In the biochemical oxygen demand (BOD) measurement of the synthesized poly(ester-thioether)s in active sludge (pH 7.4), poly(IMDA-alt-L-META), poly(IMDA-alt-D-META), and poly(IMDA-alt-meso-META) all showed biodegradability, with 28%, 15%, and 6% of degree of biodegradation (BOD/TOD, %), respectively, over a 30 days test period (Fig. 3). Aniline was used as a reference compound for the positive control in this BOD measurement (Fig. S26†). Using the same measurement procedure, poly(ISDA-alt-L-META), poly(ISDA-alt-D-META), and poly(ISDA-alt-meso-META) showed biodegradability with 3%, 9%, and 16% degradation, respectively (Fig. 3). The biodegradability of polymers containing IM followed the order: poly(IMDA-alt-L-META) (28%), poly(IMDA-alt-D-META) (15%), and poly(IMDA-alt-meso-META) (6%). In contrast, for the polymers containing IS, biodegradability followed the order: poly(ISDA-alt-meso-META) (16%), poly(ISDA-alt-D-META) (9%), and poly(ISDA-alt-L-META) (3%). The biodegradability of six similar diastereomeric poly(ester-thioether)s, including poly(L-ATA-alt-MAIM) synthesized via a radical mechanism, showed the same trend (see Table S1†). In the SEC charts of poly(L-ATA-alt-MAIM) before and after BOD (biodegradation) test (see Fig. S27†), the peak maximum shifted to the lower molecular weight region after the test, indicating scission of the poly(L-ATA-alt-MAIM) main chains. These results suggest that the stereochemistry of the diastereomers as monomers affect their biodegradability. Poly(IMDA-alt-L-META) also exhibited the highest values for both glass transition temperature and biodegradability, demonstrating enhanced thermal properties and improved biodegradability.
 | ||
| Fig. 3 BOD/TOD profiles of the biodegradation of poly(IMDA-alt-META) and poly(ISDA-alt-META) (L-, D-, and meso-) in active sludge. | ||
We also evaluated the enzymatic hydrolysis of the same poly(ester-thioether)s using total organic carbon (TOC) measurement. Enzymatic degradability was evaluated by measuring the carbon content of the degradation products produced during enzymatic degradation. Each polymer was placed in a buffer solution, and the samples with and without enzyme were incubated at 37 °C for 24 h to measure the TOC values. All samples showed higher TOC values in the presence of lipase, confirming that enzymatic degradation occurred (Fig. 4). The extent of enzymatic degradation was determined by subtracting the TOC value of the sample without enzyme from that with enzyme, thereby excluding the hydrolysis component (Fig. 4; red arrow marks). The TOC values for poly(IMDA-alt-L-META) (197.1 ppm) and poly(IMDA-alt-D-META) (169.7 ppm) were higher than that of poly(IMDA-alt-meso-META) (79.0 ppm). In contrast, poly(ISDA-alt-meso-META) (105.6 ppm) exhibited a higher TOC value than poly(ISDA-alt-L-META) (70.5 ppm) and poly(ISDA-alt-D-META) (51.2 ppm). These results showed a trend similar to the corresponding BOD results, suggesting that diastereomers affect the substrate specificity of the enzyme, thereby affecting biodegradability.
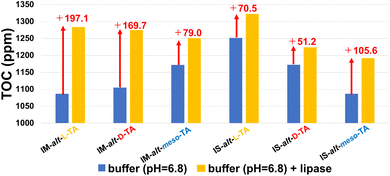 | ||
| Fig. 4 Results of TOC measurement test for poly(ester-thioether)s (runs 1–6) after incubation at 37 °C for 24 h in buffer solution (pH 6.8) with (yellow bars) and without (blue bars) lipase. | ||
Previous studies21–23 have shown that poly(ester-thioether)s containing tartarate units show differences in biodegradability depending on the stereochemistry (diastereoselectivity) of the tartarate unit.23 On the other hand, poly(ester-thioether)s containing dianhydrosugars units do not show stereochemistry-dependent differences in biodegradation, and their BOD values are lower than those of poly(ester-thioether)s containing tartrate units.22 Based on our reported results and the findings of this study, we can draw two conclusions. First, tartarate units are preferentially degraded in this biodegradation test, with biodegradability varying depending on the stereochemistry of the tartarate unit. Second, the biodegradation of tartrate units is affected by differences in the stereochemistry (diastereoselectivity) of the dianhydrosugars, namely isomannide and isosorbide. We also performed semiempirical molecular calculation using the MM2 method on the repeating units of the six poly(ester-thioether) diastereomers (Fig. S28†). The projections suggest that hydrolysis of the ester bonds in tartarate units are influenced by the steric hindrance of neighboring dianhydrosugar units.
Conclusions
Using the dianhydrosugars as starting materials, we prepared the diacrylates: isosorbide bis(acrylate) (ISDA) and isomannide bis(acrylate) (IMDA). Chemoselective esterification of tartaric acids with 2-mercaptoethanol, using scandium triflate [Sc(OTf)3] as the chemoselective esterification catalyst, resulted in the formation of three dithiol monomers: L-bis(2-mercaptoethyl)tartrate (META), D-META, and meso-META. These were synthesized by esterifying D-, L- and meso-tartaric acid with the primary alcohol. Subsequent triethylamine-catalyzed thiol-Michael polyaddition of the dianhydrosugar-based diacrylates, ISDA and IMDA, with the tartaric acid-based dithiols yielded the expected six diastereomeric poly(ester-thioether)s (Mn, 8.2 × 103 to 9.2 × 103; Mw/Mn 1.29–1.51). A biodegradation test, evaluated through BOD measurements, revealed that for poly(ester-thioether)s containing IM, biodegradability followed the order: poly(IMDA-alt-L-META) (28%), poly(IMDA-alt-D-META) (15%), and poly(IMDA-alt-meso-META) (6%). In contrast, for poly(ester-thioether)s containing IS, the biodegradability followed the order: poly(ISDA-alt-meso-META) (16%), poly(ISDA-alt-D-META) (9%), and poly(ISDA-alt-L-META) (3%). An enzymatic hydrolysis test, monitored by TOC measurements, yielded results consistent with the BOD findings. In this study, poly(IMDA-alt-L-META) also exhibited the highest values for both glass transition temperature and biodegradability, demonstrating improvements in both biodegradability and thermal properties. These results suggest that the stereochemistry of the diastereomers as monomers affects their biodegradability. These results could accelerate the development of new biodegradable polymers via click polymerization, as well as biomass-based polymers.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no competing interest.Acknowledgements
A. T. is grateful to the Takahashi Industrial and Economic Research Foundation for their financial support and continuous encouragement.References
- Y. Tokiwa, T. Ando, T. Suzuki and K. Takeda, Biodegradation of Synthetic-Polymers Containing Ester Bonds, ACS Symp. Ser., 1990, 433, 136–148 CrossRef CAS.
- Y. Tokiwa and A. Jarerat, Microbial degradation of aliphatic polyesters, Macromol. Symp., 2003, 201, 283–289, DOI:10.1002/masy.200351131.
- H. Nishida and Y. Tokiwa, Distribution of Poly(3-Hydroxybutyrate)-Degrading Anaerobic-Bacteria, Kobunshi Ronbunshu, 1993, 50(10), 739–746, DOI:10.1295/koron.50.739.
- Y. Tokiwa, M. Konno and H. Nishida, Isolation of silk degrading microorganisms and its poly(L-lactide) degradability, Chem. Lett., 1999,(4), 355–356, DOI:10.1246/cl.1999.355.
- H. R. Kricheldorf, M. Rabenstein, D. Langanke, G. Schwarz, M. Schmidt, M. Maskos and R. P. Krüger, Ring-closing polycondensations, High Perform. Polym., 2001, 13(2), S123–S136, DOI:10.1088/0954-0083/13/2/312.
- M. Wrzecionek, T. Golofit and A. Gadomska-Gajadhur, Carothers and Flory-Stockmayer Theory as a new perspective on currently ineffective poly(glycerol sebacate) synthesis and crosslinking, Polymer, 2024, 302, ARTN127077, DOI:10.1016/j.polymer.2024.127077.
- A. Takasu, Y. Iio, Y. Oishi, Y. Narukawa and T. Hirabayashi, Environmentally benign polyester synthesis by room temperature direct polycondensation of dicarboxylic acid and diol, Macromolecules, 2005, 38(4), 1048–1050, DOI:10.1021/ma0475378.
- A. Takasu, Y. Iio, T. Mimura and T. Hirabayashi, Room-temperature polycondensation of dicarboxylic acids and diols catalyzed by water-stable lewis acids, Polym. J., 2005, 37(12), 946–953, DOI:10.1295/polymj.37.946.
- A. Takasu, Y. Narukawa and T. Hirabayashi, Direct dehydration polycondensation of lactic acid catalyzed by water-stable Lewis acids, J. Polym. Sci., Part A:Polym. Chem., 2006, 44(18), 5247–5253, DOI:10.1002/pola.21639.
- A. Takasu, Y. Shibata, Y. Narukawa and T. Hirabayashi, Chemoselective dehydration polycondensations of dicarboxylic acids and diols having pendant hydroxyl groups using the room temperature polycondensation technique, Macromolecules, 2007, 40(2), 151–153, DOI:10.1021/ma062514+.
- A. C. Heiner, K. M. Bishop, G. M. Musgrave, A. K. Huber, L. M. Cushman, J. S. Bates and C. Wang, Soil Compostability of Ester-based Thiol–ene Photopolymer Networks, ACS Sustainable Chem. Eng., 2024, 12(24), 9067–9077, DOI:10.1021/acssuschemeng.4c01018.
- J. C. Worch and A. P. Dove, Click Step-Growth Polymerization and E/Z Stereochemistry Using Nucleophilic Thiol-yne/-ene Reactions: Applying Old Concepts for Practical Sustainable (Bio)Materials, Acc. Chem. Res., 2022, 55(17), 2355–2369, DOI:10.1021/acs.accounts.2c00293.
- S. Ji, L. Wang, S. Feng and L. Li, Spontaneous Amino-Acetoacetate Click Polymerization: A Powerful Tool toward Closed-Loop Recyclable Poly(vinylogous urethane)s, ACS Appl. Polym. Mater., 2024, 6(17), 10718–10726, DOI:10.1021/acsapm.4c01858.
- A. Maity, J. Chen, N. Wilson-Faubert, A. Laventure and A. Nazemi, Harnessing Activated Alkyne-Hydroxyl “Click” Chemistry for Degradable and Self-Healing Poly(urea vinyl ether ester)s, Macromolecules, 2024, 57(1), 305–316, DOI:10.1021/acs.macromol.3c01574.
- Y. Nagao and A. Takasu, “Click Polyester”: Synthesis of Polyesters Containing Triazole Units in the Main Chain via Safe and Rapid “Click” Chemistry and Their Properties, J. Polym. Sci., Part A:Polym. Chem., 2010, 48(19), 4207–4218, DOI:10.1002/pola.24206.
- Y. Nagao, A. Takasu and A. R. Boccaccini, Anode-Selective Electrophoretic Deposition of a Bioactive Glass/Sulfone-Containing Click Polyester Composite, Macromolecules, 2012, 45(8), 3326–3334, DOI:10.1021/ma300396p.
- D. Zhang and M. J. Dumont, Synthesis, characterization and potential applications of 5-hydroxymethylfurfural derivative based poly(β-thioether esters) synthesized via thiol-Michael addition polymerization, Polym. Chem., 2018, 9, 743–756, 10.1039/c7py02052j.
- D. Zhang, M. J. Dumont and A. Cherestes, An efficient strategy for the synthesis of 5-hydroxymethylfurfural derivative based poly(β-thioether ester) via thiol-Michael addition polymerization, RSC Adv., 2016, 6, 83466–83470, 10.1039/C6RA17532E.
- F. Cheng, T. Su, K. Luo, Y. Pu and B. He, The polymerization kinetics, oxidation-responsiveness, and in vitro anticancer efficacy of poly(ester-thioether)s, J. Master Chem. B, 2019, 7, 1005–1016, 10.1039/C8TB02980F.
- S. L. Luyer, P. Guegan and N. Illy, Complete depolymerization of poly(ester-alt-thioether)s under mild conditions into AB functional monomers, RSC Sustainability, 2024, 2, 510–520, 10.1039/D3SU00320E.
- Y. Sato and A. Takasu, Synthesis of L-Malic Acid Based Poly(ester-thioether)s via Thiol-Ene Click Polymerization and Their Biodegradability, Chemistryselect, 2021, 6(35), 9503–9507, DOI:10.1002/slct.202101160.
- S. Takeuchi and A. Takasu, Synthesis and Biodegradability of Poly(ester-urethane)s via the Thiol-Michael Polyaddition of Dianhydro Sugar-Based Diacrylates, ACS Appl. Polym. Mater., 2022, 4(6), 4486–4494, DOI:10.1021/acsapm.2c00466.
- R. Imamura, K. Oto, K. Kataoka and A. Takasu, Synthesis and Biodegradability of Tartaric Acid-Based Poly(ester- thioether)s via Thiol-Ene Click Polymerization, ACS Omega, 2023, 8(26), 23358–23364, DOI:10.1021/acsomega.2c07627.
- X. Yang, H. Xie, Z. Xu, J. Feng, Q. Fu, H. Li and Y. Jia, Malononitrile-involved Michael addition polymerization: An efficient and facile route for cyano-rich polyesters with programmable thermal and mechanical properties, J. Polym. Sci., 2021, 59(9), 813–823, DOI:10.1002/pol.20210074.
- M. Okada, M. Yamada, M. Yokoe and K. Aoi, Biodegradable polymers based on renewable resources. V.: Synthesis and biodegradation behavior of poly(ester amide)s composed of 1,4:3,6-dianhydro-D-glucitol, α-amino acid, and aliphatic dicarboxylic acid units, J. Appl. Polym. Sci., 2001, 81(11), 2721–2734, DOI:10.1002/app.1718.
- M. Okada, K. Tsunoda, K. Tachikawa and K. Aoi, Biodegradable polymers based on renewable resources IV.: Enzymatic degradation of polyesters composed of 1,4:3.6-dianhydro-D-glucitol and aliphatic dicarboxylic acid moieties, J. Appl. Polym. Sci., 2000, 77(2), 338–346 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Supporting information representative spectral and analytical data. See DOI: https://doi.org/10.1039/d5ra01104c |
| This journal is © The Royal Society of Chemistry 2025 |


