Open Access Article
Open Access ArticleMulticomponent synthesis of novel functionalized spiroindenopyridotriazine-4H-pyrans†
Atefeh Homaee,
Mohammad Bayat and
Hajar Hosseini
and
Hajar Hosseini
Department of Chemistry, Faculty of Science, Imam Khomeini International University, Qazvin, Iran. E-mail: s.hajarhoseini@yahoo.com; h.hosseini@sci.ikiu.ac.ir; Tel: +98 (28)33780040
First published on 5th March 2025
Abstract
A very convenient one-pot method for the synthesis of new biologically active compounds, spiro-4H-pyran derivatives, has been developed. This approach, enables the incorporation of three pharmacophoric cores: pyran, pyridine and triazine scaffolds, within a single molecular skeleton using an efficient sequential multicomponent reaction. The starting materials include ninhydrin, cyanoacetohydrazide, ethyl cyanoacetate, aromatic aldehydes, pyrazolone and malononitrile. In this process, spiropyran derivatives were produced with high efficiency through three consecutive reactions. The first step involves the synthesis of 1,6-diaminopyridinone via a three-component approach using readily available starting materials. The second step, the synthesis of pyridotriazine, occurs in a domino way without the need to separate the intermediate product of the first step. Finally, the third step involves the synthesis of new spiro-4H-pyran derivatives through a one-pot three-component reaction. This method offers several advantages, including fast and simple purification of products, the use of safe solvents, no need for metallic and toxic catalysts, high atomic economy and excellent chemoselectivity of the reactions.
Introduction
One of the goals of organic synthesis is the search for efficient conversion of simple raw materials into complex functional products using methods that combine environmental and economic aspects. In this regard, the use of multicomponent reactions (MCRs) has been developed as a powerful synthetic tool to achieve this purpose. MCRs have significant advantages over linear multistep processes. In these reactions, by changing each component, a large number of products can be created around a common framework. Also, unlike linear processes, there is no need for purification after each transformation. Therefore, due to the saving of time, cost and consumption of many chemical solvents, multicomponent reactions have been introduced as one of the most attractive topics in green chemistry.1–9 Indeed, performing multicomponent reactions in which cyclization is possible as a process step, is a powerful strategy for the facile synthesis of diverse heterocyclic frameworks. Increasing attention to diversity-oriented synthesis, which is easily achievable in multicomponent reactions, has increased interest to this approach.10–14The compounds containing the spirocyclic moiety have prominent medicinal activities and are present in the structure of many natural molecules with diverse biological effects. The presence of a spiro center has a significant impact on biological activities due to the rigidity of the molecule structure. The three-dimensional nature of spiro compounds and natural bioactive structures (e.g. proteins) makes such compounds have more effective interactions with binding sites in proteins, which is an important feature in the drug development process.15–18
Meanwhile, spiro-4H-pyrans exhibit various biological properties, including: antibacterial, anti-anaphylactic, antifungal, antitumor and antirheumatic activities.19–21 The structure of some synthetic products with biological properties is shown in Fig. 1.20,22–25
Some spiro structures have also been found to act as antioxidants (AO), compounds that prevent damage from reactive oxygen and nitrogen species (ROS and RNS). These types can be produced naturally in living cells or caused by some biological disorders such as anxiety. AOs are free radical scavengers and therefore protect proteins, DNA and lipids from oxidative degradation.25 Among the compounds whose antioxidant properties have been investigated is spiro-4H-pyran (VII), which is very similar to the products synthesized in this work.26
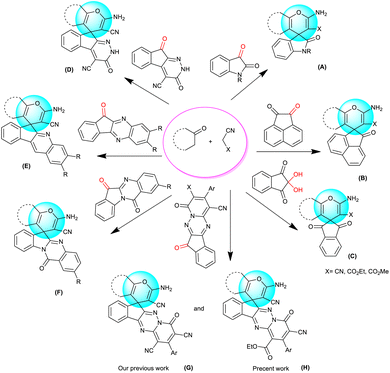
In publications, there are several reports on the reaction of cyclic compounds containing active carbonyl moiety (isatin, ninhydrin, acenaphtoquinone) with malononitrile (or ethyl/methyl cyanoacetate) and various CH-acids in order to synthesize fused spiro-4H-pyrans through multicomponent reactions (Fig. 2(A–C)).27–34
In recent years, novel carbonyl structures have been synthesized as a starting material for the preparation of new spiro-4H-pyrans. As shown in Fig. 2, these compounds include indenopyridazine (D),18 indenoquinoxaline (E)35–40 and indoloquinazoline (F).24,41,42 Recently, we used indenopyridotriazine as the active carbonyl component for multicomponent synthesis of polysubstituted fused spiro-4H-pyran derivatives (G,H).43 Most of the methods reported in the synthesis of spiro-4H-pyrans are associated with disadvantages such as the use of toxic solvents, expensive and metallic catalysts and the difficulty of the synthetic steps.27,30,36,44
In this research, we succeeded in synthesizing new products from the spiro-4H-pyrans family by using multicomponent reactions compatible with green chemistry. In these structures, the frameworks of spiropyran and pyridotriazine are connected to each other, which according to the available references, each one is a part of the biologically privileged cores.
Results and discussion
First, the pyridinone products 4 were synthesized from the reaction of cyanoacetohydrazide 1, ethyl cyanoacetate 2 and benzaldehyde derivatives 3 according to the previously reported method (Scheme 1).45 Subsequently, pyridotriazine 6 was obtained by adding ninhydrin 5 in acetic acid to the pyridinone intermediate (Scheme 2).46 Finally, the target spiropyran compounds 9, were prepared by investigating the reaction of pyridotriazine 6 with malononitrile 7 and pyrazolone 8.Optimization of the conditions
To find the optimal conditions for these reactions, dihydroindeno[1,2-e]pyrido[1,2-b][1,2,4]triazine-1-carboxylate 6a, malononitrile 7 and 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one 8 were used as model substrates under different conditions. As shown in Table 1, performing the reaction in water did not yield the desired product (entry 1, Table 1). Similarly, using organic solvents such as methanol, acetonitrile and dimethylformamide resulted in either low efficiency or no formation of spiro-4H-pyran product 9a (entries 2–4 respectively, Table 1). No product was obtained when ethanol was used at room temperature (entry 5, Table 1). However, at 78 °C within 6 hours, product 9a was synthesized with 86% yield (entry 6, Table 1). The use of acidic or basic catalysts under these condition reduced the efficiency of the final product (entry 7–9, Table 1). A detailed analysis of these reactions showed that the TLC of the final mixture, revealed a spot corresponding to pyridone. This observation suggests that some triazines decompose into their constituent components, probably due to the presence of the catalyst.| Entry | Solvent | Catalyst | Time (h) | Temp (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Reagents and conditions: 6a, 7, 8 (1 mmol), catalyst (0.1 mmol), solvent (10 mL).b Isolated yield based on 9a. | |||||
| 1 | H2O | — | 10 | 100 | No reaction |
| 2 | MeOH | — | 8 | 64 | 25 |
| 3 | MeCN | — | 6 | 82 | 33 |
| 4 | DMF | — | 12 | 120 | No reaction |
| 5 | EtOH | — | 10 | 25 | No reaction |
| 6 | EtOH | — | 6 | 78 | 86 |
| 7 | EtOH | Piperidine | 6 | 78 | 45 |
| 8 | EtOH | NEt3 | 6 | 78 | 35 |
| 9 | EtOH | HOAc | 6 | 78 | Trace |
Based on the information obtained from Table 1, we successfully synthesized a series of new spiro-4H-pyran derivatives 9a–g using triazines 6a–g, malononitrile 7 and pyrazolone 8 as starting materials in ethanol at reflux conditions without the use of any catalyst (Scheme 3). The target products, spiroindenopyridotriazine-pyranopyrazole 9a–g, were obtained with high efficiency (78–90%) and their information is given in Table 2.
| Entry | Triazine | Product | Yield (%) | M.P. (°C) |
|---|---|---|---|---|
| a The reactions were done using pyridotriazine (1 mmol), pyrazolone (1 mmol), malononitrile (1 mmol), EtOH (10 mL), reflux. | ||||
| 1 |  |
 |
86 | 253–255 |
| 2 | 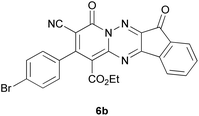 |
 |
82 | 266–268 |
| 3 | 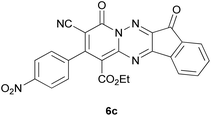 |
 |
90 | 260–262 |
| 4 |  |
 |
78 | 275–278 |
| 5 |  |
 |
80 | 295–297 |
| 6 |  |
 |
82 | 245–247 |
| 7 |  |
 |
85 | 282–284 |
Structure determination
The structure of the synthesized products 9a–g is clearly were confirmed by evaluating their spectral data, including IR, 1H NMR, 13C NMR and mass spectroscopic analyses (see the ESI†).As a representative example, we were investigated the proton and carbon spectrum data of compound 9a (Fig. 3). In the 1H NMR spectrum of 9a, the NH2 group appears at δ 7.96 ppm (this peak is exchangeable with D2O). Two methyl groups appear at δ 1.07 and 1.40 ppm (triplet and singlet respectively). A quartet signal at δ 4.15–4.20 ppm corresponds to the methylene group. Other signals in the range of 7.40 to 8.16 are related to the aromatic protons. In the 1H-decoupled 13C NMR spectrum of 9a, 32 distinct resonances confirm the proposed structure. The key signals include two methyl carbons (2CH3, at δ 12.3, 13.7 ppm), the spiro carbon (Cspiro, at δ 47.7 ppm), the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNH2 and CH2 carbons (at δ 56.4 and 61.6 ppm) respectively. The carbonyl groups are observed at δ 163.9 and 160.1 ppm. Two signals at δ 95.5, 96.9 are assigned to C–COO and C
CNH2 and CH2 carbons (at δ 56.4 and 61.6 ppm) respectively. The carbonyl groups are observed at δ 163.9 and 160.1 ppm. Two signals at δ 95.5, 96.9 are assigned to C–COO and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O (Fig. 3). In the FT-IR spectrum, absorption bands of 9a appeared at 3365, 2199, 1727, 1682 cm−1 due to NH2, CN and 2CO groups respectively. The mass spectrum of 9a did not display the molecular-ion peak (m/z 678) but the observation of the [M − 29]+ peak and the fragmentation pattern confirm the proposed structure.
C–O (Fig. 3). In the FT-IR spectrum, absorption bands of 9a appeared at 3365, 2199, 1727, 1682 cm−1 due to NH2, CN and 2CO groups respectively. The mass spectrum of 9a did not display the molecular-ion peak (m/z 678) but the observation of the [M − 29]+ peak and the fragmentation pattern confirm the proposed structure.
Scope and limitations
To further expand the scope of the reaction, we also tested various aromatic aldehydes (bearing electron-donating or electron-withdrawing groups) and 2-cyanoacetamide, ethyl-2-cyanoacetate, and malononitrile38 under the optimized conditions. However, reactions with aldehydes such as 2-nitro and 2-hydroxybenzaldehyde did not result the desired product. With these derivatives, the synthesis of primary pyridone, in pure form was unsuccessful. TLC analysis indicated, the formation of two-component product likely resulting from the reaction of cyanoacetohydrazide with aldehyde. This outcome suggests that the amide group is hydrolyzed in ethanol under the reaction conditions. When malononitrile was used as the substrate spirane products were obtained with good to high yields.With the established conditions in hand, we are continuing to explore the reaction's scope. Our ongoing research focuses on synthesizing diverse spirane products using other CH-acids, alkyl-2-cyanoacetate and pyrazolone derivatives. This work aims to enhance method for creating various complex spiro compounds.
We began our synthesis (of model substrates) with the preparation of a series of spiroindenopyridotriazine-4H-pyrans in just three steps on gram scale. This simple and scalable method significantly improves the ability to obtain useful quantities of these molecules for further research and potential applications.
Proposed mechanism
A plausible mechanism for the formation of spiropyrans 9 (as an example 9a) is shown in Scheme 4. At first, from the reaction of ninhydrin 5 and pyridone 4, during two sequential condensation steps between the amino groups and the carbonyl groups, the 6-membered triazine ring is closed. It is clear that, the amine group attached to nitrogen participates first due to its higher nucleophilicity and attacks to the middle carbonyl group of ninhydrin, which is more electrophile. Then, the intermediate 10 is formed through the Knoevenagel condensation between pyridotriazine 6 and malononitrile 7. The Michael addition of pyrazolone 8 to adduct 10, generates intermediate 11, which undergoes intramolecular cyclization via the nucleophilic attack of oxygen on the nitrile group to form intermediate 12. Finally, intermediate 12 undergoes imine-enamine tautomerization to afford the target product 9a.23,43Experimental
Materials
All Reagents and solvents for this work were purchased from Merck and Aldrich chemical companies and were used as received. The 1H NMR (300 MHz) and 13C NMR (75.4 MHz) spectra were run on a Bruker DRX-300 AVANCE instrument using DMSO-d6 as solvent. The chemical shift values were recorded on a δ scale in ppm and the coupling constants (J) given in Hertz. All melting points were determined on an electrothermal 9100 apparatus. IR spectra (KBr discs) were recorded with Bruker Tensor 27 spectrometer and the values were expressed as![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) max in cm−1. Mass spectra data were measured with by an Agilent 5975C VL MSD with Triple-Axis detector operating at an ionization potential of 70 eV. The progress of the reaction and the purity of products were checked by TLC analytical silica gel plates (Merck 60 F250).
max in cm−1. Mass spectra data were measured with by an Agilent 5975C VL MSD with Triple-Axis detector operating at an ionization potential of 70 eV. The progress of the reaction and the purity of products were checked by TLC analytical silica gel plates (Merck 60 F250).
Typical procedure for the synthesis of pyridotriazines (6a–g)
Firstly, 1,6-diaminopyridine-2-one compounds 4 were easily prepared via a three-component reaction between cyanoacetohydrazide 1, benzaldehyde derivatives 3 and ethyl cyanoacetate 2 according to reported method in our previous work.40 Then, a stoichiometric ratio (1 mmol) of ninhydrin 5 and acetic acid (5 mL) was added to the above mixture in a 25 mL round-bottomed flask connected to a reflux condenser, in a one-pot cascade manner. The resulting mixture was magnetically stirred in an oil-bath (50 °C, for 60 min). The completion time of the reaction was monitored by TLC using EtOAc/n-hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent. The reaction mixture was cooled to room temperature. As a result, an orange solid was obtained. The solid was separated, washed with water (2 × 10 mL) and subsequently with Et2O (2 × 10 mL) to give the pure pyrido[1,2,4]triazines products 6.
1) as the eluent. The reaction mixture was cooled to room temperature. As a result, an orange solid was obtained. The solid was separated, washed with water (2 × 10 mL) and subsequently with Et2O (2 × 10 mL) to give the pure pyrido[1,2,4]triazines products 6.
Synthetic procedure of spiroindenopyridotriazine-4H-pyrans (9a–g)
Indenopyrido[1,2,4]triazine 6 (1 mmol), malononitrile 7 (1 mmol), pyrazolone 8 (1 mmol) and 10 mL EtOH were placed in a 25 mL round-bottomed flask mounted on a magnetic stirrer-heater. The reaction mixture was refluxed for 6 hours. After completion of the reaction as determined by TLC, the cooled insoluble crude product was collected by simple filtration and subsequently washed with warm ethanol to give the novel spiro-4H-pyrans 9 without the need for chromatography column or recrystallization.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 166.1 (CO2Et); MS (EI, 70 eV): m/z (%) = 334 (35) [M + 2]+, 333 (21) [M + 1]+, 332 (100) [M]+, 303 (5), 286 (13), 251 (13), 229 (21), 187 (5), 162 (12), 138 (7), 111 (4), 75 (4), 58 (3), 43 (5).
O), 166.1 (CO2Et); MS (EI, 70 eV): m/z (%) = 334 (35) [M + 2]+, 333 (21) [M + 1]+, 332 (100) [M]+, 303 (5), 286 (13), 251 (13), 229 (21), 187 (5), 162 (12), 138 (7), 111 (4), 75 (4), 58 (3), 43 (5).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 148.5 (C
N), 148.5 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 154.9 (CAr-NO2), 155.8 (N–C–N), 157.9 (N–C
N), 154.9 (CAr-NO2), 155.8 (N–C–N), 157.9 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 163.9 (CO2Et), 183.5 (C
O), 163.9 (CO2Et), 183.5 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNH2), 61.6 (OCH2), 95.5 (C-COO), 96.9 (C
CNH2), 61.6 (OCH2), 95.5 (C-COO), 96.9 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O), 111.4 (C–CN), 115.9 (CN), 117.7 (CN), 120.5, 125.3, 125.6, 126.9, 128.9, 129.5, 129.7, 131.4, 133.5, 135.0, 137.1, 138.7, 144.4 (Ar), 144.9 (C–C6H5Cl), 146.5 (Me–C
C–O), 111.4 (C–CN), 115.9 (CN), 117.7 (CN), 120.5, 125.3, 125.6, 126.9, 128.9, 129.5, 129.7, 131.4, 133.5, 135.0, 137.1, 138.7, 144.4 (Ar), 144.9 (C–C6H5Cl), 146.5 (Me–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 153.4 (C
N), 153.4 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 154.8 (C
N–N), 154.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 156.0 (N–C–N), 157.4 (O–C–N), 160.1 (N–C
N), 156.0 (N–C–N), 157.4 (O–C–N), 160.1 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 160.8 (CNH2), 163.9 (CO2Et); MS (EI, 70 eV): m/z (%) = 649 (0.6) [M − 29]+, 621 (0.1), 553 (0.4), 523 (0.5), 495 (2), 461 (2), 409 (4), 352 (4), 324 (3), 293 (4), 239 (14), 189 (28), 137 (18), 109 (81), 81 (52), 43 (100), 29 (22).
O), 160.8 (CNH2), 163.9 (CO2Et); MS (EI, 70 eV): m/z (%) = 649 (0.6) [M − 29]+, 621 (0.1), 553 (0.4), 523 (0.5), 495 (2), 461 (2), 409 (4), 352 (4), 324 (3), 293 (4), 239 (14), 189 (28), 137 (18), 109 (81), 81 (52), 43 (100), 29 (22).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNH2), 61.7 (OCH2), 95.5 (C–COO), 96.8 (C
CNH2), 61.7 (OCH2), 95.5 (C–COO), 96.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O), 111.3 (C–CN), 115.9 (CN), 117.7 (CN), 120.5, 123.8, 124.2, 126.8, 129.5, 129.9, 131.4, 131.9, 133.6, 133.8, 137.1, 138.2, 144.4 (Ar), 144.9 (C–C6H5Br), 146.5 (Me–C
C–O), 111.3 (C–CN), 115.9 (CN), 117.7 (CN), 120.5, 123.8, 124.2, 126.8, 129.5, 129.9, 131.4, 131.9, 133.6, 133.8, 137.1, 138.2, 144.4 (Ar), 144.9 (C–C6H5Br), 146.5 (Me–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 153.4 (C
N), 153.4 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 154.8 (C
N–N), 154.8 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 156.0 (N–C–N), 157.4 (O–C–N), 160.1 (N–C
N), 156.0 (N–C–N), 157.4 (O–C–N), 160.1 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 160.8 (CNH2), 163.9 (CO2Et); m/z (%) = 647 (0.03), 503 (0.01), 459 (0.03), 414 (0.4), 399 (0.1), 355 (0.4), 315 (4), 269 (2), 224 (13), 178 (15), 135 (57), 91 (100), 43 (60), 15 (6).
O), 160.8 (CNH2), 163.9 (CO2Et); m/z (%) = 647 (0.03), 503 (0.01), 459 (0.03), 414 (0.4), 399 (0.1), 355 (0.4), 315 (4), 269 (2), 224 (13), 178 (15), 135 (57), 91 (100), 43 (60), 15 (6).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNH2), 61.8 (OCH2), 95.5 (C–COO), 96.7 (C
CNH2), 61.8 (OCH2), 95.5 (C–COO), 96.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O), 111.0 (C–CN), 115.7 (CN), 117.8 (CN), 120.5, 124.0, 124.3, 126.9, 129.5, 131.4, 131.5, 137.1, 137.8, 138.4, 141.4, 144.4 (Ar), 145.0 (C–C6H5NO2), 146.9 (Me–C
C–O), 111.0 (C–CN), 115.7 (CN), 117.8 (CN), 120.5, 124.0, 124.3, 126.9, 129.5, 131.4, 131.5, 137.1, 137.8, 138.4, 141.4, 144.4 (Ar), 145.0 (C–C6H5NO2), 146.9 (Me–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 148.4 (C–NO2), 153.5 (C
N), 148.4 (C–NO2), 153.5 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 154.0 (C
N–N), 154.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 156.0 (N–C–N), 157.6 (O–C–N), 160.3 (N–C
N), 156.0 (N–C–N), 157.6 (O–C–N), 160.3 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 160.9 (CNH2), 163.7 (CO2Et); MS (EI, 70 eV): m/z (%) = 578 (0.08), 551 (0.2), 523 (0.2), 495 (0.1), 467 (0.09), 416 (0.8), 368 (1), 343 (2), 313 (1), 287 (11), 261 (29), 230 (48), 204 (56), 174 (9), 127 (52), 78 (81), 43 (100), 18 (38).
O), 160.9 (CNH2), 163.7 (CO2Et); MS (EI, 70 eV): m/z (%) = 578 (0.08), 551 (0.2), 523 (0.2), 495 (0.1), 467 (0.09), 416 (0.8), 368 (1), 343 (2), 313 (1), 287 (11), 261 (29), 230 (48), 204 (56), 174 (9), 127 (52), 78 (81), 43 (100), 18 (38).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CN), 61.6 (OCH2), 95.5 (C–COO), 97.1 (C
C–CN), 61.6 (OCH2), 95.5 (C–COO), 97.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O), 111.6 (C–CN), 115.8 (CN), 116.1 (d, 2JCF = 21.8 Hz), 117.8 (CN), 120.4, 124.2, 126.8, 126.9, 129.5, 130.3 (d, 3JCF = 8.3 Hz), 130.4 (d, 4JCF = 6.0 Hz), 131.4, 137.1, 138.2, 144.4 (Ar), 145.0 (C–C6H5F), 146.4 (Me–C
C–O), 111.6 (C–CN), 115.8 (CN), 116.1 (d, 2JCF = 21.8 Hz), 117.8 (CN), 120.4, 124.2, 126.8, 126.9, 129.5, 130.3 (d, 3JCF = 8.3 Hz), 130.4 (d, 4JCF = 6.0 Hz), 131.4, 137.1, 138.2, 144.4 (Ar), 145.0 (C–C6H5F), 146.4 (Me–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 153.4 (C
N), 153.4 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 155.0 (C
N–N), 155.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 156.1 (N–C–N), 157.4 (O–C–N), 160.1 (N–C
N), 156.1 (N–C–N), 157.4 (O–C–N), 160.1 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 160.8 (CNH2), 161.4 (d, 1JCF = 240.5 Hz), 164.0 (CO2Et); MS (EI, 70 eV): m/z (%) = 596 (0.06), 550 (0.1), 523 (0.1), 488 (0.4), 443 (0.2), 416 (0.6), 388 (0.4), 346 (0.2), 313 (0.1), 301 (3), 255 (4), 212 (1), 174 (21), 145 (7), 105 (16), 77 (100), 51 (42), 28 (23).
O), 160.8 (CNH2), 161.4 (d, 1JCF = 240.5 Hz), 164.0 (CO2Et); MS (EI, 70 eV): m/z (%) = 596 (0.06), 550 (0.1), 523 (0.1), 488 (0.4), 443 (0.2), 416 (0.6), 388 (0.4), 346 (0.2), 313 (0.1), 301 (3), 255 (4), 212 (1), 174 (21), 145 (7), 105 (16), 77 (100), 51 (42), 28 (23).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNH2), 61.4 (OCH2), 95.5 (C–COO), 96.9 (C
CNH2), 61.4 (OCH2), 95.5 (C–COO), 96.9 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O), 111.6 (C–CN), 114.1 (CN), 116.1 (CN), 114.6, 120.4, 124.0, 126.5, 126.7, 126.8, 129.4, 131.3, 131.4, 137.0, 138.0, 144.3, 144.9 (Ar), 145.9 (Me–C
C–O), 111.6 (C–CN), 114.1 (CN), 116.1 (CN), 114.6, 120.4, 124.0, 126.5, 126.7, 126.8, 129.4, 131.3, 131.4, 137.0, 138.0, 144.3, 144.9 (Ar), 145.9 (Me–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 153.2 (C
N), 153.2 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 155.7 (C
N–N), 155.7 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 156.1 (N–C–N), 157.1 (O–C–N), 159.7 (CAr-OMe), 160.6 (N–C
N), 156.1 (N–C–N), 157.1 (O–C–N), 159.7 (CAr-OMe), 160.6 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 160.7 (CNH2), 164.1 (CO2Et).
O), 160.7 (CNH2), 164.1 (CO2Et).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CNH2), 61.6 (OCH2), 95.5 (C–COO), 97.0 (C
CNH2), 61.6 (OCH2), 95.5 (C–COO), 97.0 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O), 111.3 (C–CN), 115.7 (CN), 117.7 (CN), 120.5, 124.2, 126.6, 126.8, 127.5, 129.5, 130.0, 130.8, 131.3, 133.3, 136.5, 137.1, 138.3, 144.4 (Ar), 144.9 (C–C6H5Cl), 146.5 (Me–C
C–O), 111.3 (C–CN), 115.7 (CN), 117.7 (CN), 120.5, 124.2, 126.6, 126.8, 127.5, 129.5, 130.0, 130.8, 131.3, 133.3, 136.5, 137.1, 138.3, 144.4 (Ar), 144.9 (C–C6H5Cl), 146.5 (Me–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 153.4 (C
N), 153.4 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 154.3 (C
N–N), 154.3 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 156.0 (N–C–N), 157.4 (O–C–N), 160.2 (N–C
N), 156.0 (N–C–N), 157.4 (O–C–N), 160.2 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 160.8 (CNH2), 163.8 (CO2Et).
O), 160.8 (CNH2), 163.8 (CO2Et).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–CN), 61.6 (OCH2), 95.6 (C–COO), 97.1 (C
C–CN), 61.6 (OCH2), 95.6 (C–COO), 97.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O), 111.7 (C–CN), 115.8 (CN), 116.1, 117.8 (CN), 120.5, 124.2, 126.7, 126.9, 129.6, 130.3, 130.4, 131.4, 137.1, 138.3, 144.5, 145.0 (Ar), 146.4 (Me–C
C–O), 111.7 (C–CN), 115.8 (CN), 116.1, 117.8 (CN), 120.5, 124.2, 126.7, 126.9, 129.6, 130.3, 130.4, 131.4, 137.1, 138.3, 144.5, 145.0 (Ar), 146.4 (Me–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 153.4 (C
N), 153.4 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–N), 155.1 (C
N–N), 155.1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 156.1 (N–C–N), 157.4 (O–C–N), 160.1 (N–C
N), 156.1 (N–C–N), 157.4 (O–C–N), 160.1 (N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 160.8 (CNH2), 161.4, 164.0 (CO2Et).
O), 160.8 (CNH2), 161.4, 164.0 (CO2Et).Conclusion
In summary, we have developed an attractive and green approach for the synthesis of novel highly functionalized fused spiro-2-amino-4H-pyrans by sequential multicomponent reaction between pyridotriazines, pyrazolone as cyclic CH-acid and malononitrile in ethanol under mild conditions. To reach the target products, 1,6-diaminopyridine-2-one compounds were firstly prepared, then their reaction was done with ninhydrin. As a result of this condensation, pyridotriazine derivatives were synthesized as starting material for the main reaction. These two processes were carried out as a four-component cascade reaction. This method not only provides high efficiency and reaction speed, but also prevent the use of dangerous solvents or catalysts. Other promising points for the introduced synthesis include: clean reaction profile, ease of product purification, good to high yields and high atom economy.Data availability
The data supporting this article are provided in the ESI.†Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
Financial support of this research from Imam Khomeini International University, Iran is gratefully acknowledged.Notes and references
- Q. Guan, L. L. Zhou and Y. B. Dong, J. Am. Chem. Soc., 2023, 145, 1475 CrossRef CAS PubMed.
- S. E. John, S. Gulati and N. Shankaraiah, Org. Chem. Front., 2021, 8, 4237 RSC.
- Y. E. Ryzhkova, M. N. Elinson, O. I. Maslov and A. N. Fakhrutdinov, Molecules, 2021, 26, 6839 CrossRef CAS PubMed.
- A. Shaabani, R. Mohammadian, R. Afshari, S. E. Hooshmand, M. T. Nazeri and S. Javanbakht, Mol. Divers., 2021, 25, 1145 CrossRef CAS PubMed.
- J. D. Sunderhaus and S. F. Martin, Chem. Eur J., 2009, 15, 1300 CrossRef CAS PubMed.
- M. O. Rodrigues, M. N. Eberlin and B. A. D. Neto, Chem. Rec., 2021, 10, 2762 CrossRef PubMed.
- L. R. Wen, Z. R. Li, M. Li and H. Cao, Green Chem., 2012, 14, 707 RSC.
- M. Karami, A. Hasaninejad, H. Mahdavi, A. Iraji, S. Mojtabavi, M. A. Faramarzi and M. Mahdavi, Mol. Divers., 2021, 26, 2393 CrossRef PubMed.
- C. Nicolaou, D. J. Edmonds and P. G. Bulger, Angew. Chem. Int. Ed., 2006, 45, 7134 CrossRef PubMed.
- S. Sharma, V. Vaishali, N. Devi and V. Singh, Chem.–Asian J., 2024, 20, e202400862 CrossRef.
- D. Singh, S. Sharma, R. K. Thakur, V. Vaishali, S. Nain, Jyoti, C. C. Malakar and V. Singh, Tetrahedron, 2024, 152, 133809 CrossRef CAS.
- S. Sharma, C. C. Malakar and V. Singh, Chem.–Asian J., 2020, 9, 1857 CrossRef CAS.
- S. Sharma, D. Singh, S. Kumar, V. Vaishali, R. Jamra, N. Banyal, K. Deepika, C. C. Malakar and V. Singh, Beilstein J. Org. Chem., 2023, 19, 231 CrossRef CAS PubMed.
- S. S. Mritunjay, P. Singh, A. Gupta, V. Singh, L. Singh and A. Kumar, ChemistrySelect, 2024, 9, e202402133 CrossRef CAS.
- V. F. Batista, D. C. Pinto and A. M. S. Silva, Expert Opin. Drug Discovery, 2022, 17, 603 CrossRef CAS PubMed.
- K. Hiesinger, D. Darin, E. Proschak and M. Krasavin, J. Med. Chem., 2021, 64, 150 CrossRef CAS PubMed.
- Y. J. Zheng and C. M. Tice, Expert Opin. Drug Discovery, 2016, 11, 831 CrossRef PubMed.
- Y. Zheng, C. M. Tice and S. B. Singh, Bioorg. Med. Chem. Lett., 2014, 24, 3673 CrossRef CAS PubMed.
- L. Emami, L. Moezi and L. Amiri-Zirtol, et al., Mol. Divers., 2022, 26, 3129 CrossRef CAS PubMed.
- M. Saigal, P. Irfan, M. Khana and M. M. Khan, ACS Omega, 2019, 4, 16794 CrossRef CAS PubMed.
- D. C. Wang, Y. M. Xie and C. Fan, Chin. Chem. Lett., 2014, 25, 1011 CrossRef CAS.
- R. X. Liu, Y. N. Liang, X. X. Ren and Q. Q. Wu, et al., Curr. Org. Synth., 2023, 20, 870 CrossRef CAS PubMed.
- F. Safari, H. Hosseini, M. Bayat and A. Ranjbar, RSC Adv., 2019, 9, 24843 RSC.
- Z. Sadeghian, M. Bayat and F. Safari, J. Mol. Struct., 2022, 1250, 131759 CrossRef CAS.
- M. Valko, D. Leibfritz, J. Moncol, M. T. D. Cronin, M. Mazur and J. Telser, Int. J. Biochem. Cell Biol., 2007, 39, 44 CrossRef CAS PubMed.
- K. Acosta-Quiroga, C. Rojas-Peña, L. S. Nerio, M. Gutiérrez and E. Polo-Cuadrado, RSC Adv., 2021, 11, 21926 RSC.
- L. Jalili-Baleh, N. Mohammadi, M. Khoobi, L. Mamani, A. Foroumadi and A. Shafiee, Helv. Chim. Acta, 2013, 96, 1601 CrossRef CAS.
- P. Saluja, K. Aggarwal and J. M. Khurana, Synth. Commun., 2013, 43, 3239 CrossRef CAS.
- L. Mohammadi, M. M. Heravi, A. Saljooqi and P. Mohammadi, Sci. Rep., 2022, 12, 22281 CrossRef CAS PubMed.
- C. B. Li, L. S. Huang, R. S. Wu and D. Z. Xu, ChemistrySelect, 2019, 4, 1635 CrossRef CAS.
- M. M. Li, C. S. Duan, Y. Q. Yu and D. Z. Xu, Dyes Pigm., 2018, 150, 202 CrossRef CAS.
- K. Nikoofar and S. M. Dizgarani, Monatsh. Chem., 2015, 8, 1161 CrossRef.
- S. Mal and M. Jana, Arkivoc, 2022, 2022, 361 Search PubMed.
- D. Suven, RSC Adv., 2020, 10, 18875 RSC.
- R. Singh, D. Bhardwaj and M. R. Saini, RSC Adv., 2021, 11, 4760 RSC.
- P. Khanna, L. Khanna, S. J. Thomas, A. M. Asiri and S. S. Panda, Curr. Org. Chem., 2018, 22, 67 CrossRef CAS.
- S. F. Hojati, A. Amiri and E. Fardi, Appl. Organomet. Chem., 2020, 34, 1 CrossRef.
- M. R. Poor Heravi and F. Norouzy, Res. Chem. Intermed., 2017, 43, 4265 CrossRef CAS.
- G. Mohammadi Ziarani, Z. Kheilkordi and F. Mohajer, J. Iran. Chem. Soc., 2020, 17, 247 CrossRef CAS.
- N. H. Nasab and J. Safari, Polyhedron, 2019, 164, 74 CrossRef.
- Z. Sadeghian and M. Bayat, Med. Chem. Res., 2022, 31, 497 CrossRef CAS.
- Z. Sadeghian, M. Bayat, M. Eskandari and F. Safari, Results Chem., 2024, 9, 101636 CrossRef CAS.
- M. Rezaei and M. Bayat, RSC Adv., 2023, 13, 31488 RSC.
- D. M. Patel, P. J. Patel and H. M. Patel, Eur. J. Org Chem., 2022, 46, e202201119 CrossRef.
- H. Hosseini and M. Bayat, RSC Adv., 2018, 8, 27131 RSC.
- M. Shokoohian, N. Hazeri, M. T. Maghsoodlou and M. Lashkari, Polycyclic Aromat. Compd., 2022, 42, 1 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra01048a |
| This journal is © The Royal Society of Chemistry 2025 |