Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Indomethacin-incorporated microemulsion-laden contact lenses for improved ocular drug delivery and therapeutic efficacy†
Kashvi Panchala,
Yashkumar Patela,
Harshilkumar Jania,
Mittal Dalalb,
Vijay R. Chidrawarc,
Deepanjan Datta d,
Popat Mohite
d,
Popat Mohite e,
Abhijeet Purie,
Ketan Ranch*a and
Sudarshan Singh
e,
Abhijeet Purie,
Ketan Ranch*a and
Sudarshan Singh *fg
*fg
aDepartment of Pharmaceutics and Pharmaceutical Technology, L. M. College of Pharmacy, Ahmedabad, Gujarat 380009, India. E-mail: kashvi38@gmail.com; ranchketan@gmail.com; yash.patel@lmcp.ac.in; harshil.jani@lmcp.ac.in
bDepartment of Pharmacology, L. M. College of Pharmacy, Ahmedabad, Gujarat 380009, India. E-mail: mittal.dalal@lmcp.ac.in
cSchool of Pharmacy and Technology Management, SVKM's Narsee Monjee Institute of Management Studies (NMIMS), Deemed-to-University, Green Industrial Park, TSIIC, Jadcherla, Hyderabad 509301, India. E-mail: vijay.chidrawar@gmail.com
dDepartment of Pharmaceutics, Manipal College of Pharmaceutical Sciences, Manipal Academy of Higher Education, Manipal, Karnataka 576104, India. E-mail: deepanjandtt@gmail.com
eAETs St. John Institute of Pharmacy and Research, Palghar, Maharashtra 401404, India. E-mail: mohitepb@gmail.com; abhijeetp@sjipr.edu.in
fOffice of Research Administration, Chiang Mai University, Chiang Mai 50200, Thailand
gFaculty of Pharmacy, Chiang Mai University, Chiang Mai 50200, Thailand. E-mail: sudarshan.s@cmu.ac.th
First published on 14th May 2025
Abstract
Conventional eye drops are associated with several limitations, including rapid drug clearance and low bioavailability, with only about 5% of the administered drug reaching the cornea to demonstrate therapeutic efficacy. Thus, to address these challenges, indomethacin (IND)-loaded microemulsion (Me)-embedded soft contact lenses (CLs) were developed to improve ocular drug delivery. The D-optimal mixture design was employed to optimize the composition of the Me formulation. Independent variables included Capmul MCM (oil phase), Smix (Tween 80/isopropyl alcohol), and water, while dependent variables were the globule size, transmittance (%), and drug release profile. The optimized Me exhibited a globule diameter of 45.69 ± 1.85 nm and a transmittance of 99.4% ± 1.59%. The globule dispersion index (PDI) was 0.33 ± 0.06, and the zeta potential (ZP) was 0.657 ± 0.012 mV. Soft CLs were developed using free radical polymerization and fortified with the Me loaded with the drug through direct loading and soaking techniques. Direct loading achieved a swelling percentage of 96.37% ± 1.8% and a transmittance of 96.5% ± 0.3%, while soaking resulted in 97.57% ± 1.4% swelling and 97.3% ± 1.3% transmittance. A drug content of 19.76 ± 0.23 μg per lenses for direct loading and 29.8 ± 0.2 μg per lenses for soaking demonstrated that the efficacy of the soaking technique was higher than that of direct loading. Moreover, drug release studies showed that the Me-laden lenses prepared using the direct loading technique released 44.00% ± 0.53% to 53.00% ± 0.59% of the drug within the first 6 h, while the lenses prepared via soaking released 62.58% ± 1.56% to 97.64% ± 1.52% of the drug within the first 6 h, followed by regulated drug release for up to 28 h, maintaining clarity and drug loading. Ocular irritancy test results indicated negligible irritation, suggesting that the Me-laden lenses are a safe and effective platform to manage ocular inflammation with the controlled delivery of IND.
1 Introduction
Although conventional eye drops are a flexible form of ocular dosing, they frequently exhibit serious drawbacks, such as quick removal from the pre-corneal region as a result of lacrimation, tear dilution, and tear turnover.1 Additionally, the presence of tissue barriers, such as the conjunctiva, cornea, sclera, and lenses, further reduces the bioavailability of the drug in the cornea to about 5%, necessitating frequent administration to maintain therapeutic levels.2 These challenges underscore the need for alternative approaches to enhance the pre-corneal residence time and trans-corneal permeability for improving therapeutic outcomes.The pre-corneal residence time and trans-corneal permeability are key parameters governing the effectiveness of the therapeutic regimen. Thus, several attempts have been made to address the challenges associated with conventional eye drops, including enhancing the pre-corneal residence time of drugs. These strategies involve the use of excipients that increase viscosity and/or mucoadhesion. From the perspective of drug delivery systems, semisolid formulations such as ointments (such as indomethacin eye ointment) and liquid formulations with higher viscosities have been fabricated. However, because their high viscosity causes blurred vision, they are mostly limited to night use.3 Alternatively, the use of nano-formulations, including polymeric nanoparticles,4 Me,5 liposomes,6 solid lipid nanoparticles (indomethacin),7 nanostructured lipid carrier indomethacin,8 and self-micro-emulsifying drug delivery systems9 incorporated in in situ gels can enhance the pre-corneal residence time and trans-corneal permeability. Solid formulations such as CLs can also accommodate nano-formulations to enhance the pre-corneal residence time.10
Microemulsions provide several benefits, such as improved biomaterial wettability, high drug-loading capacity, ease of production, and thermodynamic stability.11 For instance, researcher developed bimatoprost-loaded Me-laden CLs to treat glaucoma, demonstrating a two-fold improvement in drug uptake and sustained release profiles compared to conventional soaking techniques, without altering the critical lens properties.11 Similarly, Yanchu Li et al. studied the effect of Mes on ofloxacin uptake in CLs for treating conjunctivitis, showing the enhanced drug loading, release kinetics, and physical features of the lenses.12 In another study, Datta et al. and Ning Wei et al. developed cyclosporine-loaded microneedle CLs and timolol-loaded Me-laden silicone CLs, respectively, for glaucoma management, achieving enhanced drug loading and prolonged drug release without affecting the swelling and transmittance features of the lenses.13,14
To manage ocular inflammation conditions, corticosteroids (prednisolone acetate, dexamethasone, and triamcinolone acetonide) and non-steroidal anti-inflammatory drug (bromfenac, nepafenac, diclofenac, and indomethacin) are widely used.15,16 Indomethacin (IND) is a BCS class II drug with low aqueous solubility. As a topical non-steroidal anti-inflammatory drug, IND is commonly used to treat various ocular inflammatory conditions, including anterior segment inflammation,7 uveitis,17 conjunctivitis,18 macular edema,19 cystoid macular edema,20 and post-operative pain following cataract surgery.21 This drug works by inhibiting cyclooxygenase enzymes and blocking prostaglandin synthesis, thereby reducing inflammation.22 Topical administration, especially via eye drops, is the favored technique for delivering IND to the eye due to its simplicity, minimal systemic side effects, and high patient compliance.23,24 Eye drops are the major delivery route for treating anterior segment diseases such as inflammation due to their ability to target the site directly.7 However, drawbacks including frequent instillation, rapid clearance and loss due to nasolacrimal drainage lead to low ocular bioavailability and patient non-compliance when IND is delivered via the ophthalmic route. Alternatively, IND-Me addresses the low aqueous solubility issue and enhances the solubility of IND, which can accommodate a greater drug content in Me compared to eye drops. However, the short residence time of Me is still an issue; hence, in this study, we incorporated Me into CLs without compromising the critical quality attributes of the lenses, which can affect patient compliance. The Me-loaded CLs can accommodate IND above the daily therapeutic dose, which can solve the frequent instillation, and thus increase patient compliance. This strategy can be beneficial for delivering the BCS class II and class IV drugs to the eye in a sustained manner.
These studies emphasize the potential of Me-loaded CLs as a superior drug delivery system, offering enhanced drug uptake, sustained release, and improved retention compared to conventional techniques. Consequently, the research objective was to develop and optimize IND-Me to enhance the drug solubility and loading. The optimized IN-Me, integrated into soft CLs for ocular drug delivery, aimed to enhance the bioavailability, prolong the drug release, and preserve the fundamental physical features of the CLs. The success of this approach can provide a valuable solution for improving the treatment of ocular inflammatory disorders, particularly where conventional eye drop formulations fall short.
2 Materials and methods
Indomethacin (Mw: 357.787 g mol−1; > 98%) was provided as a gift sample from Cadila Healthcare Ltd, Gujarat, India. Hydroxyethyl methacrylate (HEMA; 130.14 g mol−1), isopropyl myristate (0.85 g cm−3), polyethylene glycol 400 (1.13 g cm−3), propylene glycol (1.04 g cm−3), isopropyl alcohol (786 kg m−3), triethanolamine oleate (1.0 g cm−3), polysorbate 20 (1.1 g cm−3), polysorbate 60 (1.08 g cm−3), polysorbate 80 (1.06 g cm−3), sorbitan mono-oleate (0.986 g cm−3), sorbitan trioleate (0.956 g cm−3), sorbitan monolaurate (1.032 g cm−3) and methacrylic acid (MAA; 1.02 g cm−3) were procured from Sigma-Aldrich, Massachusetts, United States. Ethylene glycol di-methacrylate (EGDMA; 198.22 g mol−1) and diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide (Duracur®) were obtained from Tokyo Chemicals (India) Pvt. Ltd, Hyderabad, India. Octadec-9-enoic acid (0.88 g cm−3) was acquired from S.D. Fine Chemicals, Gujarat, India. Propylene glycol dicaprylate (Captex 200) (0.929 g cm−3), triglycerides of caprylic/capric acid (Captex 355) (0.960 g cm−3), and glyceryl monocaprylate (Capmul MCM) (0.950 g cm−3) were purchased from Abitec Corporation. Octanoic acid (Caprylic oil) (0.91 g cm−3) and PEG-8 caprylic/capric glycerides (Mayasol) were obtained from Subhash Chemical Industries, Maharashtra, India. Polyoxyethylene glycol (Lutrol F68) (1.14 g cm−3) was sourced from Signet Chemical Corporation Pvt. Ltd, Maharashtra, India. Caprylocaproyl polyoxyl-8 glycerides (Labrasol ALF), polyglycol mono- and di-esters of 12-hydroxystearic acid 30% PEG (Solutol HS 515), and oleoyl polyoxyl-6-glycerides (Labrafil M 1944 CS) were procured from Gattefossé SAS. All other reagents were of analytical grade.2.1. Screening of oil, surfactant, and cosurfactant
The solubility of indole-3-carbinol (IND) in a wide range of oils was evaluated. These oils included isopropyl myristate, oleic acid, Captex 200, Captex 355, Capmul MCM, caprylic oil, Labrafac PG, and Labrafil M 1944 CS, and surfactants such as Span 20, Tween 20, Span 80, Span 85, Tween 80, Tween 60, Lutrol F68, Labrasol ALF, Solutol HS 515, and Mayasol. Furthermore, cosurfactants such as PEG 400, propylene glycol, isopropyl alcohol, triethanolamine oleate, and PEG-7 glyceryl cocoate were also considered in the assessment.25 Excess IND was added to each type of oil, surfactant, and cosurfactant in sealed glass vials. Subsequently, the saturated solutions were agitated in an orbital shaker (Remi CIS-18 Plus, Germany) at 100 rpm and 37 °C ± 1 °C for day. Upon reaching equilibrium, the compositions were centrifuged at 8000 rpm for 20 min (cooling microcentrifuge; Sorvall Legend X1; Thermo Scientific; USA). Then, the supernatant was then, and its IND concentration was determined using UV-visible spectroscopy (Shimadzu UV-1900, Japan). The density of the oil was considered, and a linear equation (y = 0.0279x + 0.0144; r2 = 0.997) was obtained from the calibration curve (detailed data are presented in ESI File; Tables S1, S2, Fig. S1 and S2†). The components exhibiting high solubility for IND were selected to develop the pseudo-ternary phase diagram.2.2. Construction of pseudo-ternary phase diagrams
The water titration process was employed to construct pseudo-ternary phase diagrams that delineate the concentration range of components within the Me region.25,26 Four phase diagrams were created by altering the surfactant (Tween 80) to cosurfactant (isopropyl alcohol) ratios as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 4
2, and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Subsequently, the oil phase (Capmul MCM) was combined with the surfactant mixture in weight ratios ranging from 10
1. Subsequently, the oil phase (Capmul MCM) was combined with the surfactant mixture in weight ratios ranging from 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 to 0
0 to 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. These mixtures were gradually diluted with deionized water while being stirred moderately on a magnetic stirrer at 50 rpm and ambient temperature. Subsequently, water was added dropwise until turbidity or phase separation occurred. The point at which the mixture turned turbid and biphasic indicated the termination of water addition. Transparent, single-phase systems were classified as Mes, which are characterized by low viscosity and a clear appearance. The critical points between the Me region and other phase regions were quantified by the transition from a clear state to a turbid one and vice versa. The quantity of water used was recorded to calculate the final percentages of water, oil, and surfactant/cosurfactant. Subsequently, these percentages were used to construct the pseudo-ternary phase diagrams. The results obtained from the water titration technique were exported to ProSim version 1.9, a program specifically designed for constructing pseudo-ternary phase diagrams.
10. These mixtures were gradually diluted with deionized water while being stirred moderately on a magnetic stirrer at 50 rpm and ambient temperature. Subsequently, water was added dropwise until turbidity or phase separation occurred. The point at which the mixture turned turbid and biphasic indicated the termination of water addition. Transparent, single-phase systems were classified as Mes, which are characterized by low viscosity and a clear appearance. The critical points between the Me region and other phase regions were quantified by the transition from a clear state to a turbid one and vice versa. The quantity of water used was recorded to calculate the final percentages of water, oil, and surfactant/cosurfactant. Subsequently, these percentages were used to construct the pseudo-ternary phase diagrams. The results obtained from the water titration technique were exported to ProSim version 1.9, a program specifically designed for constructing pseudo-ternary phase diagrams.
2.3. Preparation of Me
Indomethacin-loaded Me formulations were developed using a spontaneous emulsification technique, as previously reported.27 Initially, Capmul MCM (oil), Tween 80 (surfactant), and isopropyl alcohol (co-surfactant) were thoroughly blended with magnetic stirring at 37 °C ± 2 °C. Subsequently, IND was admixed in this mixture, and deionized water was slowly added dropwise with vigorous vortexing for 1 to 2 min to achieve a uniform blend. Notably, no precipitates were evident in the final drug-loaded formulations. Post-preparation of microemulsion purification was done by means of filtration, as reported previously in the literature.28 The microemulsion was passed through a disposable syringe filter (0.22 μm Millipore filter) into 10 mL glass crew capped vials. Not more than 10 mL of microemulsion was filtered through each disposable syringe filter.2.4. Optimization of indomethacin Me using D-optimal mixture design
The D-optimal mixture design was utilized to optimize the compositions of the Me formulations. After identifying the Me region from the pseudo-ternary phase diagram, batches were prepared using the D-optimal design approach. Specifically, variations in the levels of Capmul MCM (oil phase), the ratio of Tween 80 to isopropyl alcohol (surfactant–cosurfactant combination, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and deionized water were selected within the Me region. The response varying for these batches included globule size (Y1 in nm), percent transmittance of diluted Me (Y2), and percent drug release after 6 h from Me-laden CLs prepared via direct loading. The experimental design, together with the predefined levels, was implemented using the Design-Expert software version 12 (State-Ease Inc., Minneapolis, Minnesota, USA) to create the design space. The independent and dependent variables used in the D-optimal mixture design are listed in Table 1. The composition of oil, Smix, and water is presented in Table S3.†
1), and deionized water were selected within the Me region. The response varying for these batches included globule size (Y1 in nm), percent transmittance of diluted Me (Y2), and percent drug release after 6 h from Me-laden CLs prepared via direct loading. The experimental design, together with the predefined levels, was implemented using the Design-Expert software version 12 (State-Ease Inc., Minneapolis, Minnesota, USA) to create the design space. The independent and dependent variables used in the D-optimal mixture design are listed in Table 1. The composition of oil, Smix, and water is presented in Table S3.†
| Independent variables | |||||
|---|---|---|---|---|---|
| X1 | X2 | X3 | |||
| Oil phase (Capmul MCM) (mL) | Smix (Tween 80/Iso propyl alcohol) (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (mL) 1) (mL) |
Water (mL) | |||
| Low | High | Low | High | Low | High |
| 0.100 | 0.550 | 0.350 | 0.800 | 0.100 | 0.550 |
| Dependent variables | ||
|---|---|---|
| Y1 | Y2 | Y3 |
| Globule size (nm) | Transmittance (%) of Me diluted with water | Percentage drug release after 6 h from Me-laden CLs prepared using the direct loading technique |
2.5. Characterization of Mes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min to check for any phase inversion/separation or cracking. The samples that showed signs of instability were excluded from further analysis. The stable Mes were subjected to a freeze–thaw cycle for 48 h, involving both heating and cooling phases. In the heating phase, the Mes were heated at 45 °C for 48 h, followed by cooling at 4 °C. Throughout these cycles, any phase separation, cracking, or creaming was carefully observed. The Mes that remained stable throughout all tests were deemed thermodynamically stable and suitable for further use.
000 rpm for 30 min to check for any phase inversion/separation or cracking. The samples that showed signs of instability were excluded from further analysis. The stable Mes were subjected to a freeze–thaw cycle for 48 h, involving both heating and cooling phases. In the heating phase, the Mes were heated at 45 °C for 48 h, followed by cooling at 4 °C. Throughout these cycles, any phase separation, cracking, or creaming was carefully observed. The Mes that remained stable throughout all tests were deemed thermodynamically stable and suitable for further use.2.6. Fabrication of hydrogel-based CLs
Hydrogel CLs were developed using the free radical polymerization technique, as reported in the literature.32 The previously reported pre-monomer mixture composed of 46.7% (w/w) of HEMA, 0.8% (w/w) of MAA, 1% (w/w) of EGDMA, and 52% (w/w) of water was utilized for this study.32 This mixture yielded hydrogel-based CLs of desirable and adequate quality considering swelling, transparency and strength. To ensure thorough mixing and removal of air bubbles, the monomer mixture was sonicated for 10 min in a bath sonicator (LMU6, LAB MAN, Mumbai, India). Following sonication, 50 μL of the pre-monomer mixture was dispensed onto a female mould using a micropipette. Subsequently, a male mould was positioned over the female mould to establish the lens shape. The assembled moulds were exposed to 366 nm ultraviolet (UV) radiation from a UV cabinet (CLE-UV by Coral Labtech Enterprises, Vapi, India) for a duration of 20 min to initiate and complete the polymerization process of the hydrogel lenses. After curing, the lenses were carefully removed from the moulds and placed in vials containing 2 mL of simulated tear fluid (STF; pH 7.4) as the storage solution.2.7. Loading indomethacin into CLs
The pure IND and IND-loaded Mes were incorporated into CLs using two techniques, i.e., the conventional soaking technique and the conventional direct loading technique. In the soaking technique, blank CLs were immersed in 10 mL of STF containing 10 mg of IND, or in a mixture of 2 mL IND-loaded Me and 8 mL STF for 24 h. In the direct loading technique, a drug suspension with 1 mg per mL IND or 1 mL of IND-loaded Me (1 mg mL−1) was added directly to the pre-monomer mixture during the fabrication of the lenses. Then the lenses were cured using UV light at 366 nm for 20 min to complete polymerization, in which the monomers were converted to polymers. These approaches enable efficient drug loading into CLs for potential ocular applications.11,12 The composition of Me in each CL loaded using the soaking technique (SM-ME-CL) and direct loading technique (DL-ME-CL) is shown in Table 2.| Microemulsion-laden CLs prepared by soaking technique | Composition of microemulsion in each Me-laden CLs | ||
|---|---|---|---|
| Oil (mL) | Smix (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Tween 80/Isopropyl alcohol) (mL) 1) (Tween 80/Isopropyl alcohol) (mL) |
Water (mL) | |
| ME-CL-1 | 0.229 | 0.535 | 0.235 |
| ME-CL-2 | 0.322 | 0.572 | 0.104 |
| ME-CL-3 | 0.323 | 0.350 | 0.326 |
| ME-CL-4 | 0.437 | 0.350 | 0.212 |
| ME-CL-5 | 0.550 | 0.350 | 0.100 |
| ME-CL-6 | 0.100 | 0.573 | 0.326 |
| ME-CL-7 | 0.322 | 0.572 | 0.104 |
| ME-CL-8 | 0.436 | 0.463 | 0.100 |
| ME-CL-9 | 0.173 | 0.426 | 0.400 |
| ME-CL-10 | 0.100 | 0.350 | 0.550 |
| ME-CL-11 | 0.229 | 0.535 | 0.235 |
| ME-CL-12 | 0.100 | 0.800 | 0.100 |
| ME-CL-13 | 0.100 | 0.350 | 0.550 |
| ME-CL-14 | 0.100 | 0.573 | 0.326 |
| ME-CL-15 | 0.323 | 0.350 | 0.326 |
| ME-CL-16 | 0.125 | 0.676 | 0.197 |
2.8. Characterization of Me-laden contact lens
 | (1) |
The optical transmittance of the CLs should ideally remain unaffected after the incorporation of Me. As reported, the transmittance of the Me-loaded CLs was evaluated using a UV spectrophotometer.34 After soaking the hydrated CLs in STF at pH 7.4 for 24 h, the lenses were placed in a quartz cuvette. Then, the cuvette was inserted into a UV-vis spectrophotometer, where the transmittance was recorded in the wavelength range of 200–800 nm.
3 Results and discussion
The present work aimed to enhance the ocular drug delivery of IND using Me-laded CLs. Me can accommodate a higher amount of IND compared to conventional eye drops. We developed and optimized IND-Me using the D-optimal design. This Me was loaded in CLs using two different loading techniques. Subsequently, Me-laden CLs were further evaluated for their critical quality attributes.3.1. Solubility study of indomethacin
The screening of the oil phase is essential in Me formulations to ensure the maximum drug loading, prevent drug precipitation, and improve the stability of the Me effects on droplet size and system stability. The solubility study was conducted employing the established methodology to identify suitable oils, surfactants, and co-surfactants. The findings revealed that among the tested excipients, Capmul MCM exhibited the highest solubility for IND in the oil phase. Tween 80 was selected as the surfactant, and isopropyl alcohol was chosen as the co-surfactant, as illustrated in Fig. 1a–c. Capmul MCM functions as both a solubilizer and an emulsifier. The ability of Capmul MCM to stabilize the emulsion enhanced the dispersion of IND in the oil phase, thereby increasing its solubility. The reduction in surface tension using Tween 80 facilitated the formation of smaller droplets in the emulsion, which increased the overall surface area for drug dissolution and absorption. The isopropyl alcohol solubilization process involved modifying the interfacial properties and improving the miscibility of the components in the formulation. Thus, the synergy among Capmul 337 MCM, Tween 80 and IPA may be the reason for the higher solubility. Building on the findings presented previously, Capmul MCM, Tween 80, and isopropyl alcohol were selected to develop the pseudo-ternary phase diagram necessary for formulating the Me system. This selection was made due to the exceptional solubilizing properties of these components, which are crucial for ensuring the effective delivery of IND within the Me-loaded CL formulation.3.2. Pseudo-ternary phase diagram
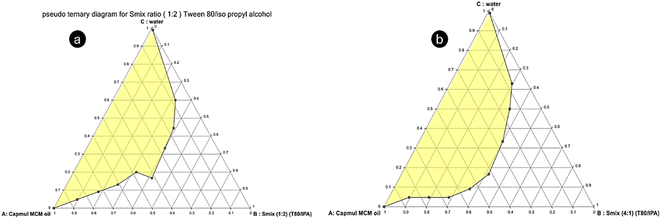
The pseudo-ternary phase diagram was created using the chosen components, including oil (Capmul MCM), Smix (a blend of Tween 80 and isopropyl alcohol), and water. Various ratios of surfactant to co-surfactant (Smix) were employed, specifically 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 4
2, and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, following the established procedure (Fig. 2 and 3). The resulting phase diagrams revealed the Me region, marked by a transparent phase, which was crucial for the subsequent preparation of Me. Upon analysis, it was observed that the Smix ratios of 1
1, following the established procedure (Fig. 2 and 3). The resulting phase diagrams revealed the Me region, marked by a transparent phase, which was crucial for the subsequent preparation of Me. Upon analysis, it was observed that the Smix ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 4
2, and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 exhibited a relatively narrow Me region, leading to their exclusion from further consideration. In contrast, the Smix ratio of 2
1 exhibited a relatively narrow Me region, leading to their exclusion from further consideration. In contrast, the Smix ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 demonstrated a larger Me region. Consequently, the pseudo-ternary phase diagram constructed with an Smix ratio of 2
1 demonstrated a larger Me region. Consequently, the pseudo-ternary phase diagram constructed with an Smix ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was employed as the basis for the subsequent development of the IND-loaded Me. Similar to the findings by Ning Wei et al., this higher surfactant-to-co-surfactant ratio enhanced the stability and solubilization of IND, supporting its selection.14
1 was employed as the basis for the subsequent development of the IND-loaded Me. Similar to the findings by Ning Wei et al., this higher surfactant-to-co-surfactant ratio enhanced the stability and solubilization of IND, supporting its selection.14
3.3. Statistical data analysis using D-optimal mixture design
The objective for utilising the D optimal mixture design was to distinguish between batches originating from the microemulsion region and characterize the latter, rather than taking batches outside the microemulsion region. Conventional statistical designs, such as factorial and response surface designs, are insufficient for studying variables that are essentially components of a mixture. The D-optimal design allows the investigation of the interaction between different microemulsion components and their proportions in determining the desired dependent variables selected in this study. By applying the D-optimal mixture design, the observed responses for the formulated compositions were concurrently fitted to the response surface model. The data showed the observed responses (Y1 to Y3) for the IND Me-laden CLs. The response obtained from the D-optimal design is shown in Table 3.| Component 1 (X1) | Component 2 (X2) | Component 3 (X3) | Response1 (Y1) | Response 2 (Y2) | Response 3 (Y3) | |
|---|---|---|---|---|---|---|
| Run | A: oil | B: Smix | C: water | Globule size | Transmittance of Me diluted with water | Drug release after 6 h from Me-laden CLs prepared using the direct loading method |
| Units | mL | mL | mL | nm | (%) | (%) |
| 1 | 0.229 | 0.535 | 0.235 | 110 | 11.1% | 48.07% |
| 2 | 0.322 | 0.572 | 0.104 | 120 | 12.2% | 51.81% |
| 3 | 0.323 | 0.350 | 0.326 | 140 | 14.3% | 52.67% |
| 4 | 0.437 | 0.350 | 0.212 | 166.5 | 16.6% | 49.45% |
| 5 | 0.550 | 0.350 | 0.100 | 170.9 | 17.8% | 47.28% |
| 6 | 0.100 | 0.573 | 0.326 | 45 | 97.8% | 44.5% |
| 7 | 0.322 | 0.572 | 0.104 | 120 | 75.2% | 51.81% |
| 8 | 0.436 | 0.463 | 0.100 | 180.1 | 18.2% | 47.9% |
| 9 | 0.173 | 0.426 | 0.400 | 59.54 | 95.6% | 49.02% |
| 10 | 0.100 | 0.350 | 0.550 | 48.47 | 96.2% | 46.9% |
| 11 | 0.229 | 0.535 | 0.235 | 110 | 11.1% | 48.07% |
| 12 | 0.100 | 0.800 | 0.100 | 41.76 | 98.2% | 44.7% |
| 13 | 0.100 | 0.350 | 0.550 | 48.47 | 96.2% | 46.9% |
| 14 | 0.100 | 0.573 | 0.326 | 45 | 97.8% | 44.5% |
| 15 | 0.323 | 0.350 | 0.326 | 120 | 14.7% | 52.67% |
| 16 | 0.125 | 0.676 | 0.197 | 68.77 | 97.4% | 44.7% |
The non-linear model produce for globule size was found to be significant with an F-value of 110.77 and p value of < 0.0001 with the R2 value of 0.9940. Therefore, among the formulated batches, only batches ME-6, 9, 10, 12, 13, 14, and 16 had a globule size of less than 70 nm. Batches ME-5 and 8 had the most significant negative effect, with globule sizes of 170.5 nm and 180 nm, respectively. This result was further confirmed from the regression analysis report that the Me batches with a lower oil phase and higher surfactant content showed better results. As the proportion of surfactant in the Me increased, the stabilization of the oil phase improved. A higher surfactant concentration allows more oil to be stabilized, leading to a decrease in globule size. This rise in the surfactant phase results in a notable reduction in globule size. The detailed ANOVA and regression analysis for globule size is shown in Table S4.†
The microemulsion data revealed that batches ME-6, ME-9, ME-10, ME-12, ME-14, and ME-16 exhibit excellent stability and clarity, with high % transmittance values (∼95–98%) even after 10-fold dilution, indicating well-formed and stable microemulsions. Batches ME-4, ME-5, ME-8, and ME-15 show moderate stability, with transmittance values after dilution in the range of 14% to 18%. In contrast, ME-1, ME-2, ME-3, ME-7, and ME-11 exhibit significant reductions in transmittance upon dilution (as low as 11.1%), reflecting lower stability or incomplete microemulsion formation. Stability is strongly influenced by composition, with higher Smix and balanced oil-to-water ratios promoting a superior performance, while oil-heavy or imbalanced formulations tend to reduce the stability and clarity. Overall, optimizing the Smix and water content is critical for achieving stable microemulsions.
The linear model created for the response (Y2) showed an F-value of 6.63 and a p-value of 0.0057, demonstrating that the model was significant. P-values less than 0.05 suggested that the model terms were significant, whereas p-values greater than 0.1 indicated that the model terms were insignificant. The R2 value of 0.7682 implied a good signal to noise ratio. Thus, among the 16 batches, only batches ME-6, 9, 10, 12, 13, 14 and 16 exhibited significantly better transmittance (%), which was greater than 90%. In contrast, the other batches showed a negative effect on transmittance (%) exhibiting less than 80% transmittance.
The design space indicated that batches with more than 80% transmittance were o/w-type Mes, given that their external phase was miscible with water. In contrast, the batches that exhibited a negative response were not water-miscible, indicating they were w/o-type Mes. The batches located near the shaded region of the pseudo-ternary diagram were classified as w/o type due to their greater proportion of oil phase. Consequently, the levels of X1, X2, and X3 significantly influenced response 2 (Y2). The design space further suggested that higher levels of X2 and X3 would result in a positive transmittance when diluted with water, given that an increased level of X2 (Smix) effectively stabilizes X1 (oil phase). The detailed ANOVA table and regression analysis of transmittance are shown in Table S5.†
An equation with coded factors can be used to predict the response at specified levels of each factor. The linear model yielded an F-value of 17.40 with a p-value of 0.0001, demonstrating that the model was statistically significant. The R2 value was determined to be 0.8969. Therefore, batches ME-6, 9, 10, 12, 13, 14 and 16 implied significantly positive results. The drug release (%) after 6 h was less than 50%, which indicated a significantly positive and acceptable sustained drug release from the Me-loaded CLs prepared using the direct loading technique. The objective of the Me-loaded CLs was to achieve prolonged drug release. The response (Y3) suggested the impact of X2 (Smix) on the sustained drug release (%). As the concentration of X2 increases, a significant decline in (Y3) drug release (%) was observed. Thus, it was proposed that the surfactant traps the drug and oil globules within the micellar structure of the polymeric membrane of the p-HEMA hydrogel CLs. The detailed ANOVA table and regression analysis for drug release at 6 h are shown in Table S6.†
 | ||
| Fig. 4 Contour plot for the response (Y1) (a), 3D plots of the response (Y1) (b), and contour plot for the response (Y2) (c). | ||
 | ||
| Fig. 5 3D contour plot for the response (Y2) (a), 3D plots of the response (Y3) (b), and contour plot for the response (Y3) (c). | ||
Using the Design Expert software version 12, the contour plot design space was generated, which revealed the impact of X1(oil), X2 (Smix) and X3 (water) on the dependent variable response (Y3) drug release (%) after 6 h from the Me-laden CLs prepared by the direct loading technique. The desired response Y3 can be achieved by keeping the concentration of X1 in range of, X2 and X3. Additionally, the desired drug release (%) within 6 h should not exceed 46%. This goal can be attained with the following concentrations: X1 (oil phase) in the range of 0.102 mL and 0.112 mL, X2 (Smix: surfactant/cosurfactant) in the range of 0.371 mL to 0.709 mL, and X3 (water) in the range of 0.525 mL to 0.178 mL. The contour plot and 3D surface plot for drug release at 6 h are shown in Fig. 5(b) and (c), respectively.
3.4. Optimization of Me using D-optimal design
Statistical analysis was conducted to examine the effect of X1 (oil), X2 (surfactant), and X3 (water) on Y1, Y2, and Y3. Considering all constraints, the D-optimal mixture design identified the optimal formulation with a desirability of 1.0. By selecting on the grid, the values of X1(oil): 0.104 mL, X2 (Smix): 0.795 mL and X3 (water): 0.1 mL for the desired globule size (nm) Y1: 39.57 nm, Y2 transmittance (%) of 100.59% and Y3 drug release (%) of 45.0603% can be achieved. The overlay plot for optimization of Me is shown in Fig. 6 and Table S7.†3.5. Characterization of Me
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (Pluronic F68
4 (Pluronic F68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PEG 200) demonstrated greater stability compared to those with a 1
PEG 200) demonstrated greater stability compared to those with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 ratio. Specifically, the ME-3 [1
6 ratio. Specifically, the ME-3 [1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6] and of ME-4 [1
6] and of ME-4 [1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6] batches showed phase separation under thermal stress, whereas the ME-1[1
6] batches showed phase separation under thermal stress, whereas the ME-1[1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4] batch passed all the tests, which is likely due to the stabilizing effect of Pluronic F68. This suggests that a higher proportion of Pluronic F68 enhances the stability by better encapsulating the oil globules and preventing phase separation.12 Similarly, the results reported by Ning Wei et al. demonstrated that Me batches with an increased surfactant concentration were more stable. In their study, batches with a 1
4] batch passed all the tests, which is likely due to the stabilizing effect of Pluronic F68. This suggests that a higher proportion of Pluronic F68 enhances the stability by better encapsulating the oil globules and preventing phase separation.12 Similarly, the results reported by Ning Wei et al. demonstrated that Me batches with an increased surfactant concentration were more stable. In their study, batches with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of Smix (Tween 20) failed under freeze–thaw cycles due to the insufficient surfactant needed to stabilize the oil droplets. However, batches ME-3 (1
1 ratio of Smix (Tween 20) failed under freeze–thaw cycles due to the insufficient surfactant needed to stabilize the oil droplets. However, batches ME-3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and ME-4 (1
2) and ME-4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), with a higher surfactant-to-oil ratio, remained thermodynamically stable.14 These findings reinforce the role of the surfactant concentration in maintaining stability, given that a higher Smix ratio supports the formation of stable Mes under stress conditions. Thus, it can be concluded that the surfactant combination of Tween 80 and isopropyl alcohol, in a 2
2), with a higher surfactant-to-oil ratio, remained thermodynamically stable.14 These findings reinforce the role of the surfactant concentration in maintaining stability, given that a higher Smix ratio supports the formation of stable Mes under stress conditions. Thus, it can be concluded that the surfactant combination of Tween 80 and isopropyl alcohol, in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, was crucial in stabilizing the oil phase. Consequently, the Me formulations with a higher proportion of surfactant and a lower oil phase exhibited excellent stability.
1 ratio, was crucial in stabilizing the oil phase. Consequently, the Me formulations with a higher proportion of surfactant and a lower oil phase exhibited excellent stability.3.6. Characterization of Me-loaded CLs
The results indicated that 100% ethanol significantly (p < 0.05) induced opacity in the cornea on exposure for 10 min compared to that of the negative control (total of three corneas were used for each group). Further, the opacity induced by the solution used for preparing the Me-laden contact indicated an insignificant difference compared to the negative control (0.9% w/v NaCl). This clearly demonstrates that the solution did not induce any corneal irritation and appears to be safe with respect to opacity. Corneal opacity was seen in the positive control for irritancy. The negative control showed no opacity, which is nonirritant. The test formulation showed negligible and minimal opacity. This data revealed that the formulation is non-irritant given that it did not show any opacity. Following the irritancy study, the same eye was preserved in a 10% buffered formalin solution. The corneas were embedded in paraffin, sectioned using a microtome, and stained with hematoxylin. Examination of the cornea was conducted under a light microscope at 100× magnification. Fig. 7 presents the histopathological images of the cornea including A. negative control, B. positive control, and C. test formulation.
Fig. 7B depicts the effect of ethanol application, which caused epithelial cell disruption and disruption of the tight junctions between epithelial cells, leading to their detachment, shedding and swelling due to ethanol-induced osmotic stress. Damage to the epithelium compromises the protective barrier property of the cornea, and further loss of the epithelial layer leads to increased light scattering, contributing to corneal opacity. Keratocytes play a pivotal role in maintaining the extracellular matrix, similar to collagen and glycosaminoglycans, which provide corneal transparency and strength. Thus, the local exposure of ethanol to the cornea leads to the loss of keratocytes, eventually negatively affecting stromal repair. Further, damage to keratocytes can lead to stromal edema and disorganization of collagen fibers, reducing transparency and leading to opacity.46,47 In the negative control (Fig. 7a) and test groups (Fig. 7c), the appearance of a normal number of keratocytes, no signs of stroma swelling and intact epithelium indicated low possibility of opacity. The histology of the cornea exposed to the negative control showed a normal epithelium and stroma with keratocytes. Moreover, the histology of the cornea exposed to the positive control showed desquamation of the corneal epithelium, blood capillaries, and edematous stroma with inflammatory cells. In addition, the histology of the cornea exposed to the test formulation showed an intact corneal epithelium and stroma substantia propria with keratocytes. Therefore, this study suggests the compatibility of the formulation, which can be further tested in clinical trials.
4 Conclusions
This study effectively demonstrated the use of CLs loaded with an indomethacin Me for managing ocular inflammation. This promising approach for treating ocular inflammation enables sustained and controlled drug release, improved bioavailability, and reduced dosing frequency. The optimized Me system, utilizing Capmul MCM as the oil, Tween 80 as the surfactant, and isopropyl alcohol as a co-surfactant, demonstrated favourable Me properties, including a desirable globule size and transmittance (%) and enhanced stability. Furthermore, the Me-laden CLs exhibited indomethacin release for up to 28 h with the sustained drug delivery of IND. The ocular irritation studies revealed negligible irritation, indicating the safety and efficacy of the Me-laden CLs for prolonged drug delivery. The D-optimal mixture design confirmed the optimization of the Me system to improve the therapeutic effectiveness in managing ocular inflammation. Nevertheless, additional in vivo studies with animals wearing the CLs for prolonged durations (weeks) are required to evaluate conjunctival redness (hyperaemia), staining responses, and any changes in the cornea. Their potential clinical applications include treating conditions such as dry eye syndrome, uveitis, and post-surgical inflammation, providing an alternative to conventional eye drops. Additionally, their enhanced patient compliance and targeted delivery can make them a valuable option in ophthalmic therapeutics. From a commercial perspective, the microemulsion preparation process is robust, reproducible, and amenable to large-scale production, ensuring consistency and cost-effectiveness for industrial manufacturing. Similarly, the scalability of the contact lens manufacturing process has been progressed over the years with the advancement in polymer science, allowing the seamless integration of the optimized microemulsion formulation into standard manufacturing procedures. Given the extensive and growing global population of contact lens wearers, this formulation presents substantial market potential. The developed microemulsion-loaded contact lenses offer enhanced therapeutic delivery, while maintaining optimal lens comfort and transparency, providing a valuable competitive advantage in the ophthalmic market. These lenses align with the growing demand for advanced drug delivery systems in ophthalmic care, presenting opportunities for the pharmaceutical and medical device industries to develop novel, marketable ophthalmic treatments. The Me-loaded CLs show potential as an optimal approach for delivering BCS class II and IV drugs to treat ocular conditions.Data availability
Data can be made available from the corresponding authors upon request.Author contributions
Data curation and investigation: KP, YP, HKJ, MD; writing original draft and writing–review & editing: HKJ, YP; conceptualization, methodology, supervision, and writing–review & editing: KR and SS; and formal analysis: VRC, PM, AP, and DD.Conflicts of interest
The authors declare no conflicts of interest.Abbreviations
| Indomethacin | IND |
| Hydroxyethyl methacrylate | HEMA |
| methacrylic acid | MAA |
| Ethylene glycol di-methacrylate | EGDMA |
| Capric acid | Captex 355 |
| Glyceryl monocaprylate | Capmul MCM |
| Polyoxyethylene glycol | Lutrol F68 |
| Caprylocaproyl polyoxyl-8 glycerides | Labrasol ALF |
| Oleoyl polyoxyl-6-glycerides | Labrafil M 1944 CS |
| Oil-in-water | o/w |
| Polydispersity index | PDI |
| Dynamic light scattering | DLS |
| Simulated tear fluid | STF |
| Lenses prepared by the soaking technique | IND-SL-CL |
| Lenses prepared by the direct loading technique | IND-DL-CL |
| Contact Lens | CL |
| Microemulsion | Me |
Acknowledgements
This work was partially supported by the CMU Proactive Researcher Scheme (2023), Chiang Mai University, for Sudarshan Singh. Moreover, Ketan Ranch would like to acknowledge L. M. College of Pharmacy, India, for providing necessary facilities.References
- A. L. Onugwu, C. S. Nwagwu, O. S. Onugwu, A. C. Echezona, C. P. Agbo, S. A. Ihim, P. Emeh, P. O. Nnamani, A. A. Attama and V. V. Khutoryanskiy, J. Controlled Release, 2023, 354, 465–488 Search PubMed
.
- P. Franco and I. De Marco, Polymers, 2021, 13, 1102 Search PubMed
.
- V. Popescu, A. Soceanu, S. Dobrinas and G. Stanciu, Ovidius Univ. Ann. Chem., 2012, 23, 87–91 Search PubMed
.
- U. D. Laddha and S. J. Kshirsagar, J. Drug Delivery Sci. Technol., 2021, 61, 102112 Search PubMed
.
- M. Dhaval, J. Devani, R. Parmar, M. M. Soniwala and J. Chavda, J. Drug Delivery Sci. Technol., 2020, 55, 101373 Search PubMed
.
- V. Y. Londhe and S. Sharma, J. Drug Delivery Sci. Technol., 2022, 67, 102951 Search PubMed
.
- S. P. Balguri, G. R. Adelli and S. Majumdar, Eur. J. Pharm. Biopharm., 2016, 109, 224–235 Search PubMed
.
- R. Gudhka, S. Dawre and P. Devarajan, Recent Pat. Nanomed., 2015, 5, 38–47 Search PubMed
.
- B. Grassiri, Y. Zambito and A. Bernkop-Schnürch, Adv. Colloid Interface Sci., 2021, 288, 102342 Search PubMed
.
- K. Leng, B. Guan, W. Liu, C. Jiang, S. Cong, B. Peng and Y. Tao, Nanomaterials, 2024, 14, 1004 Search PubMed
.
- W. Xu, W. Jiao, S. Li, X. Tao and G. Mu, J. Drug Delivery Sci. Technol., 2019, 54, 101330 Search PubMed
.
- Y. Li, C. Huang, X. Yang and X. Zhang, J. Biomater. Sci., Polym. Ed., 2020, 31, 1566–1579 Search PubMed
.
- D. Datta, G. Roy, P. Garg and V. V. K. Venuganti, J. Drug Delivery Sci. Technol., 2022, 70, 103211 Search PubMed
.
- N. Wei, H. Dang, C. Huang and Y. Sheng, J. Dispersion Sci. Technol., 2021, 42, 742–750 Search PubMed
.
- M. Ahuja, A. S. Dhake, S. K. Sharma and D. K. Majumdar, AAPS J., 2008, 10, 229 Search PubMed
.
- D. Singhal, A. Nanda, S. Kanungo, K. Sahoo and S. Mohapatra, Indian J. Ophthalmol., 2022, 70, 425–433 Search PubMed
.
- B. B. Sand and E. Krogh, Acta Ophthalmol., 1991, 69, 145–148 Search PubMed
.
- C. Baudouin, J.-P. Nordmann, P. Denis, C. Creuzot-Garcher, C. Allaire and C. Trinquand, Graefe's Arch. Clin. Exp. Ophthalmol., 2002, 240, 929–935 Search PubMed
.
- A. Russo, C. Costagliola, L. Delcassi, F. Parmeggiani, M. R. Romano, R. dell'Omo and F. Semeraro, Mediators Inflammation, 2013, 2013, 1–11 Search PubMed
.
- K. Miyake, Surv. Ophthalmol., 1984, 28, 554–568 Search PubMed
.
- M. Weber, L. Kodjikian, F. E. Kruse, Z. Zagorski and C. M. Allaire, Acta Ophthalmol., 2013, 91, e15–e21 Search PubMed
.
- C. Gunaydin and S. S. Bilge, Eurasian J. Med., 2018, 50, 116–121 Search PubMed
.
- F. A. Maulvi, K. M. Ranch, A. R. Desai, D. T. Desai and M. R. Shukla, in Remington: the Science and Practice of Pharmacy, Elsevier, 2020, pp. 565–575, DOI:10.1016/B978-0-12-820007-0.00028-3
.
- L. Battaglia, L. Serpe, F. Foglietta, E. Muntoni, M. Gallarate, A. Del Pozo Rodriguez and M. A. Solinis, Expert Opin. Drug Delivery, 2016, 13, 1743–1757 Search PubMed
.
- P. I. Siafaka, E. Ş. Çağlar, H. Sipahi, M. Charehsaz, A. Aydın and N. Üstündağ Okur, Pharm. Dev. Technol., 2021, 26, 765–778 Search PubMed
.
- A. Mahran, S. Ismail and A. A. Allam, Pharmaceutics, 2021, 13, 444 Search PubMed
.
- Y. Ma, J. Yang, Y. Zhang, C. Zheng, Z. Liang, P. Lu, F. Song, Y. Wang and J. Zhang, Drug Delivery, 2022, 29, 111–127 Search PubMed
.
- P. S. Darole, D. D. Hegde and H. A. Nair, AAPS PharmSciTech, 2008, 9, 122–128 Search PubMed
.
- B. Xu and T. Liu, J. Drug Delivery Sci. Technol., 2020, 57, 101792 Search PubMed
.
- L. M. Abd El Wahab, E. A. Essa, G. M. El Maghraby and M. F. Arafa, AAPS PharmSciTech, 2022, 24, 1 Search PubMed
.
- M. A. Moreno, M. P. Ballesteros and P. Frutos, J. Pharm. Sci., 2003, 92, 1428–1437 Search PubMed
.
- F. A. Maulvi, D. H. Lakdawala, A. A. Shaikh, A. R. Desai, H. H. Choksi, R. J. Vaidya, K. M. Ranch, A. R. Koli, B. A. Vyas and D. O. Shah, J. Controlled Release, 2016, 226, 47–56 Search PubMed
.
- F. A. Maulvi, R. J. Patil, A. R. Desai, M. R. Shukla, R. J. Vaidya, K. M. Ranch, B. A. Vyas, S. A. Shah and D. O. Shah, Acta Biomater., 2019, 86, 350–362 Search PubMed
.
- L. Moore and J. T. Ferreira, Contact Lens and Anterior Eye, 2006, 29, 115–122 Search PubMed
.
- D. Chawnani, K. Ranch, C. Patel, H. Jani, S. Jacob, M. M. Al-Tabakha and S. H. S. Boddu, J. Biomater. Sci., Polym. Ed., 2024, 35(18), 2884–2908 Search PubMed
.
- F. A. Maulvi, S. S. Singhania, A. R. Desai, M. R. Shukla, A. S. Tannk, K. M. Ranch, B. A. Vyas and D. O. Shah, Int. J. Pharm., 2018, 548, 139–150 Search PubMed
.
- J. K. Guillory, J. Med. Chem., 2007, 50, 590 Search PubMed
.
- Test No. 437: Bovine Corneal Opacity and Permeability Test Method for Identifying I) Chemicals Inducing Serious Eye Damage and Ii) Chemicals Not Requiring Classification for Eye Irritation or Serious Eye Damage, OECD, 2023 Search PubMed.
- V. Dave, S. Paliwal, S. Yadav and S. Sharma, Sci. World J., 2015, 2015, 432376 Search PubMed
.
- A. Walles Hayes, Principles and Methods of Toxicology, CRC Press, 2007, p. 44 Search PubMed
.
- M. S. Algahtani, M. Z. Ahmad and J. Ahmad, Bioengineering, 2022, 9, 384 Search PubMed
.
- C. Torres-Luna, N. Hu, A. Koolivand, X. Fan, Y. Zhu, R. Domszy, J. Yang, A. Yang and N. S. Wang, Pharmaceutics, 2019, 11(6), 262 Search PubMed
.
- R. C. Racovita, M. D. Ciuca, D. Catana, C. Comanescu and O. Ciocirlan, Nanomaterials, 2023, 13, 2311 Search PubMed
.
- W. Dongqi, Y. Daiyin, W. Junda, Z. Yazhou and Z. Chengli, J. Pet. Explor. Prod. Technol., 2022, 12, 2735–2746 Search PubMed
.
- D. Gulsen and A. Chauhan, Int. J. Pharm., 2005, 292, 95–117 Search PubMed
.
- J. Liu, C. Guo, P. Hao, P. Wang, L. Li, Y. Wang and X. Li, BMC Ophthalmol., 2022, 22, 33 Search PubMed
.
- C.-C. Chen, S.-W. Liou, C.-C. Chen, W.-C. Chen, F.-R. Hu, I.-J. Wang and S.-J. Lin, PLoS One, 2011, 6, e19111 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra01046b |
| This journal is © The Royal Society of Chemistry 2025 |