DOI:
10.1039/D5RA01031D
(Review Article)
RSC Adv., 2025,
15, 10337-10364
Hydrogel composite scaffold repairs knee cartilage defects: a systematic review
Received
12th February 2025
, Accepted 20th March 2025
First published on 8th April 2025
Abstract
Currently, the incidence of cartilage defects in knee joints owing to different causes is increasing every year, and surgery is the most common treatment strategy. In general, tissue engineering materials that mimic the structural, mechanical, and biological properties of natural bone have been widely used, with hydrogels being particularly prominent due to their good biocompatibility as well as adjustable mechanical properties. However, the inherent limitations of monolithic materials in mimicking the complex zonal organization of articular cartilage have driven significant progress in composite scaffold engineering. Herein, we briefly outline the structure of the knee joint and several common surgical approaches for knee cartilage defects. We also discuss the physical properties, functions, and preparation methods of common hydrogel composite scaffolds according to their different types. Finally, we review their application in knee cartilage defects and summarize and discuss their future prospects.
1. Introduction
The knee joint is the largest weight-bearing joint in the human body and the most prone to articular cartilage damage due to factors such as excessive frequency of use, complex structure, and strong weight-bearing. Once the articular cartilage is damaged, it is difficult to repair and susceptible to degenerative changes in the joints.1,2 The surfaces of connecting bones in a joint are covered with a layer of articular cartilage, which is often worn away by the shear forces generated by joint movement. Articular cartilage has no blood vessels or lymphoid tissue; its blood supply comes mainly from the synovial fluid and subchondral bone in the joint cavity. As a result, it is difficult to completely regenerate articular cartilage, and if it is damaged, it can lead to permanent damage.3,4 Knee cartilage injury is a common orthopedic disease. Its main clinical manifestations are joint pain and activity dysfunction. According to the International Cartilage Repair Association for grading articular cartilage injury standards, articular cartilage defect exists when the diameter of the defect is >3 cm. This type of defect cannot be completely self-repaired and also further damages the surrounding cartilage tissues and the surrounding wall of the bone, causing joint inflammation or articular cartilage collapse, eventually evolving into osteoarthritis.5 Relevant studies have found that the incidence of knee cartilage damage in adults with non-symptomatic knee osteoarthritis (mean age = 62.3 years) is 69%, and the incidence of knee cartilage defects in patients with symptomatic knee osteoarthritis (mean age = 66 years) is >80%.6,7 Currently, clinical surgery is used as the main treatment for knee cartilage defects.8
Tissue engineering is a multidisciplinary field involving cells, biomaterials, cell–material interactions, and their characterization through different surface technologies, which can be combined to obtain better treatments that facilitate the regeneration of new tissues.9 Hydrogels, as a material, are tunable, degradable, biocompatible, and modifiable, offering numerous advantages in tissue engineering and cell delivery applications.10,11 However, due to their inherently poor mechanical properties, they are not suitable for applications requiring high strength.12 Additionally, the rapid biodegradation of hydrogels significantly limits their broader application in the field of tissue engineering.13 Therefore, improving them through different methods can further enhance application in tissue engineering.14 Hydrogel composite systems are an approach for creating materials that are superior to any single hydrogel, obtained by combining the functions and properties of different hydrogel materials. In past decades, various composite designs have been proposed to enhance the properties of hydrogels, which include particles, anisotropic fillers, and fiber-hydrogel composite systems.15,16 These reinforcements are immobilized in a hydrogel matrix through physical or chemical interactions to enhance the hydrogel properties. In hydrogel composites, multiple polymer networks interpenetrate with each other, resulting in mechanical anchoring behavior, which can significantly affect the rheology, degradation rate, permeability, and mechanical properties of the hydrogel.17
Owing to the rapid progress and extensive utilization of hydrogel composite scaffolds in biomedical arenas, they hold significant potential in cartilage tissue engineering. In this comprehensive review, we commence by methodically outlining the structure of the knee joint. Thereafter, we dissect the principles and recent research advancements of frequently employed surgical techniques for treating knee cartilage defects. These encompass microfracture, chondroplasty, osteochondral autotransplantation, autologous matrix-induced chondrogenesis, and osteochondral allograft. Subsequently, we engage in an in-depth discussion regarding different varieties of hydrogel composite scaffolds, namely, natural hydrogel composite scaffolds, nanoparticle composite hydrogel scaffolds, nanotube composite hydrogel scaffolds, and microsphere composite hydrogel scaffolds. We explore their physical attributes, functional characteristics, and associated preparation procedures. Eventually, we appraise the extensive employment of hydrogel composite scaffolds in the treatment of knee cartilage defects and conclude with a summary and projection of future trends and directions.
2. Cartilage structure of the knee joint
Knee joint is the interconnection of the thigh and lower leg and consists of the patella, distal femur and proximal tibia. On the articular surface of all three bones, there is a layer of smooth, transparent cartilage.18 The cartilage of knee joint is mainly composed of surface cartilage, intermediate cartilage, deep cartilage and subchondral bone.19 Surface cartilage is located in the outermost layer, which is the site of direct contact between joints and has a smooth surface. Intermediate and deep cartilages are located below the surface cartilage and composed mainly of collagen fibers and proteoglycans, which provide elasticity and cushioning. The subchondral bone is located at the base of the cartilage and provides support and nutrition to it.20,21
Histologically, articular hyaline cartilage, including patellar cartilage, femoral condylar cartilage, and tibial plateau cartilage, is a semi-solid, supportive connective tissue mainly composed of chondrocytes and cartilage matrix. The main chemical components are cartilage mucin, water, and type II collagen fibers in the highest content, which are cross-linked to form a lattice scaffold wherein water and mucins are embedded. This structure is extremely important for the mechanical stability of cartilage required for weight bearing.22,23 Chondrocytes, collagen fibers, and ECM together form the different regions of articular cartilage, dividing it into a four-layered structure of superficial, intermediate, deep, and calcified layers.24,25 Most of the collagen fibers in the superficial layer are parallel to the articular surface, and this layer has the lowest content of proteoglycan and chondrocytes but is rich in water. The intermediate layer is the transition layer; the collagen fibers are distributed in a staggered fashion. The deep layer is the radiolucent layer; the collagen fibers are roughly distributed perpendicularly to the articular surface in a radial pattern, and this layer is rich in proteoglycan, with the lowest content of water and the highest content of collagen fibers, which is the largest and thickest and the most important component of articular cartilage. The deepest layer is the calcarine cortex, where the collagen fibers are arranged in a reticular pattern and extend toward the cortical bone surface (Fig. 1).26,27
 |
| | Fig. 1 Anatomy of knee cartilage. | |
3. Knee cartilage defects and repair
Knee cartilage defects, such as the medial femoral condyle, lateral femoral condyle, or patellofemoral compartment, may be caused by mechanical factors (e.g., trauma or repetitive microtrauma) or biological factors (e.g., exfoliative osteochondritis dissecans or osteonecrosis).28 The pain, swelling, and dysfunction caused by knee cartilage defects are more severe compared to anterior cruciate ligament injuries or even knee arthroplasty.29 Defects in the weight-bearing portion of the femoral condyle result in increased cartilage wear at the margins of the lesion and reduced contact area, edge loading, and increased cartilage stress in the adjacent region resulting from full-length cartilage defects are considered to predispose this tissue to degenerative changes.30,31 These defects usually result in the production of type I collagen in the fibrocartilage,32 which is characterized by poor elasticity, stiffness, and wear properties, making it susceptible to arthritis.
The classification according to the Anderson Orthopedic Research Institution (AORI) criteria divides the types of bone defects into three categories: type I bone defects, with only mild loss of osteophytes, no osteolysis or prosthetic subsidence, and an intact metaphysis; type II bone defects, which can also be classified as unilateral or bilateral, with metaphyseal femoral condylar or tibial plateau bone defects, with a small amount of osteolytic defect at the distal end of the femoral epicondyle, upward displacement of the joint line or sinking of the femoral end prosthesis, shortening of the metaphysis, and sinking of the tibial end prosthesis below the fibular head. Type III bone defects affect the attachment of structures such as the surrounding ligaments, with the defect or prosthesis sinking to the level of the cavernous tuberosity and reaching or exceeding the level of the femoral condyles, resulting in a large segmental defect in the metaphysis, which is often associated with lateral collateral ligament injuries.33,34 The goal of surgical treatment of these defects is to reconstruct the hyaline cartilage on the articular surface to achieve a properly aligned and stabilized joint, thereby relieving symptoms and reducing the risk of further progressive arthritis. The surgical approaches that exist in clinical practice are microfracture, chondroplasty, osteochondral autografting, autologous matrix-induced chondrogenesis, and osteochondral allografts (Table 1).
Table 1 Clinical surgical approaches to repair cartilage defects in knee joints
| Types |
Advantages |
Disadvantages |
| Microfractures |
Easy to operate; can effectively reduce acute or chronic knee cartilage defect pain symptoms |
Poor abrasion resistance; may increase subchondral cyst formation and bone fragility; affects re-treatment |
| Chondroplasty |
Preserves the part of damaged cartilage, which can reduce the damage to normal cartilage; it can promote the proliferation and repair of chondrocytes, helping to restore cartilage functions |
Limited by cartilage repair capacity, results may be limited in cases of large cartilage defects; higher surgical complexity; longer postoperative recovery time |
| Osteochondral autograft transplant |
Better graft survival; effective in restoring texture, thickness and elasticity of knee cartilage |
Limited area, scope, and number of extractions; prone to osteomalacia in the cartilage donor area |
| Autologous matrix-induced chondrogenesis |
Less risk of immune reaction and rejection caused by allografts or synthetic materials; higher success rate of chondrogenesis; based on the body's natural process of cartilage regeneration, which promotes chondrocyte proliferation and differentiation by providing structural support and cell growth factors |
Longer recovery period; patients may experience short-term pain and discomfort |
| Osteochondral allografts |
Adequate source of grafts; good biomechanics |
May cause immune rejection and complications |
3.1 Microfracture
Microfracture technique is a commonly used treatment for intra-articular cartilage lesions in the knee joint involving the formation of multiple perforations or microfractures in the subchondral bone plate, which penetrates the cartilage defect, causing bleeding and subsequent fibrin clot formation, defect filling and covering the exposed bone surface, thus allowing bone marrow MSCs to migrate into the clot from the bone marrow underneath to the articular surface to form a bone and fibrous cartilage mixture and promote the formation of fibrocartilage repair tissue.31 The biochemical and biomechanical properties are poor compared to normal hyaline cartilage, and although early short-term improvement in symptoms has been shown, there are drawbacks related to the durability and longevity of fibrocartilage repair.35 Studies have reported an 80% survival rate at an average of 9.4 years after microfracture of focal cartilage humeral head defects with a minimum of 5 years of follow-up.35 Evaluating results 10–14 years after the treatment of microfractures of focal cartilage defects in the knee showed a poorer prognosis.36
To overcome the mechanical limitations of microfracture methods, nanofracture techniques have been gradually developed, which have the advantage of a denser distribution of microfractures, lesser damage to the subchondral layer at the defect site, and no thermal damage to osteocytes during the procedure.37 At the same time, deeper subchondral penetration leads to more efficient filling of the defect with extravasated blood and a higher proportion of fibrocartilage occurs at the site of injury.38 In subchondral bone remodeling after the treatment of total cartilage defects with nanofractures in an animal knee model, it was found that nanofractures resulted in deeper subchondral bone penetration, less trabecular fragmentation and compaction, and better restoration of normal subchondral bone structure at six months.39
In conclusion, microfracture is the cheapest and simplest treatment and yields significant clinical results in the short to medium term treatment of smaller cartilage defects.35 If microfracture fails, other cartilage repair options can still be used to treat cartilage lesions.8
3.2 Chondroplasty
Chondroplasty can be performed by repairing knees having cartilage defects with healthy cartilage. This method involves debridement by machinery to remove the free edges to stabilize the lesion and potentially stimulate healing, but it can lead to persistent fissures and uneven surface formation.40,41 Radiofrequency thermal ablation uses a plasma energy field for debridement, which can produce a more homogeneous articular surface, thus providing a better sliding surface.42,43 One study reviewed patients who underwent BMAC chondroplasty and examined the efficacy of delaying further intervention. Results showed that only 5 of 23 procedures (21.7%) required postoperative interventions during the two-year follow-up period, and only one request for a total knee arthroplasty was made, which has not yet been completed, demonstrating that BMAC chondroplasty may be an effective method for delaying total knee arthroplasty.44 It has been suggested that chondroplasty with plasma layers used in conjunction with appropriate settings and techniques could be a safe surgical method for the treatment of intra-articular ICRS grade 2 and 3 lesions in the knee, with the primary goal of slowing the progression of cartilage lesions and improving patient prognosis, and that plasma layers may be a reasonable option for the treatment of patients with grade 2 and 3 lesions.40 Despite the high prevalence of chondroplasty, there are limited short- and long-term outcome data. To date, the procedure has only been described as a primary treatment or concomitant surgical intervention for osteoarthritis (OA), most commonly, subtotal meniscectomy.45
3.3 Osteochondral autograft transplant (OAT)
Osteochondral autograft transplant (OAT) is an attractive treatment for small-to medium-sized cartilage and osteochondral lesions of the knee. Cartilage and subchondral bone are typically obtained from the autologous non-weight-bearing articular surface to repair cartilage defects, and a healthy peg post containing articular cartilage, cartilage tidemarks, and subchondral bone is transplanted into an area of the injury with matching size.46 The advantages of this technique include the use of articular hyaline cartilage rather than fibrocartilage to repair the defect and maintenance of the height and shape of the joint.47 The bony portion of the graft usually heals completely with the surrounding bone, whereas the cartilage surface, although viable, may not heal completely with the surrounding bone.48 Autografts can provide excellent skeletal support for overlying hyaline cartilage, and the use of single or multiple grafts to achieve repair can cover recipient defects, with the space between osteochondral plugs filled with fibrocartilage, while the donor site may be empty and show fibrous tissue filling.49 An evaluation showed that the time from symptom onset to surgery, number and size of lesions, location and quality of the surrounding cartilage, and concomitant meniscal damage influence postoperative outcomes.50 In a sample study of 1139 patients (532 for open OAT and 607 for arthroscopic OAT), the size of the defect in open OAT was three times larger than that in arthroscopic OAT (2.96 ± 0.76 vs. 0.97 ± 0.48 cm2). Regarding defect site, the medial femoral condyle (MFC) was the most common (75.4%), followed by lateral femoral condyle (LFC, 12.1%) and patella (6.7%). Clinical outcomes were generally favorable for open and arthroscopic OAT; however, open OAT allowed the treatment of lesions approximately three times the size of arthroscopic OAT.46 OAT demonstrates a significantly lower rate of symptomatic recurrence at 10 years postoperatively compared to the microfracture technique.51
3.4 Autologous matrix-induced chondrogenesis (AMIC)
Since the fibrin network of blood clots derived from microfractures containing MSCs may not be stable enough to withstand the biomechanics of the knee joint, this predisposes it to premature breakdown under normal knee loading.52 Microfracture techniques are usually recommended for small cartilage defects, but autologous matrix-induced chondrogenesis (AMIC) technique offers an effective solution for larger defects.53 AMIC is a matrix-assisted bone marrow stimulation technique that combines natural collagen I/III with microfracture for the treatment and repair of osteochondral defects.54 The matrix stabilizes and protects the bone marrow clots generated by MSC-producing microfractures with a superficial dense layer that acts as a smooth barrier surface to prevent MSCs from spreading into the joint and a deep porous layer that promotes cellular uptake and adhesion.55 The procedure employs cell-free microstructured scaffolds to fill the defect after debridement and stimulation, and adherence of the covering with sutures or fibrin glue to capture bone marrow cells and stem cells, providing a scaffolding network on which cartilage can grow, which requires neither cartilage harvesting nor in vitro cell expansion.56 Analysis with at least two years of follow-up showed that the subjective IKDC score was better in the AMIC group than in the microfracture group. In addition, MOCART scores from MRI scans and acceptable defect fill rate results showed AMIC to be superior to MFx.56 After enrolling 101 patients aged 12 to 60 years with a mean follow-up of 30 months, AMIC was proved clinically and radiologically effective after at least 2 years, with improvements in all SF-36, KOOS and IKDC domains and a higher mean MOCART 2.0 score.57 Randomized controlled trials have shown that AMIC prevents the regression of common outcomes in microfractures, with 5 year outcomes significantly better than those for microfractures.58
The AMIC technique provides mechanical stability to knee cartilage defects and allows stromal cells to differentiate into chondrocytes, making it a relatively simple and attractive treatment. However, further research and refinement of this technique are needed for intermediate to late clinical outcomes.
3.5 Osteochondral allografts (OCA)
Osteochondral allografts (OCA) complement other revision procedures that present poor quality articular cartilage repair tissue or poor quality subchondral bone.59 OCA utilize fresh, fresh-frozen, or cryopreserved allograft specimens to fill defects, and due to the presence of intact hyaline cartilage, fresh OCA do not require a blood group match or a human leukocyte antigen match. At the same time, they preclude the need to obtain material from non-weight-bearing areas and can cover virtually all sizes of defects. They also provide the possibility of restoring complex defect surfaces with no lesions without causing donor site morbidity.60,61 OCA are commonly used not only for femoral condylar defects but also for tibial plateau and patellofemoral defects.62 Long-term (>10 years) graft survival has been shown to be between 70% and 91%, with excellent therapeutic results in medium to large cartilage lesions.63 However, the short shelf life and high cost of fresh samples may limit their availability, cryoprocessing may result in up to 95% chondrocyte loss, the use of OCA may lead to recipient rejection, and smaller or thinner grafts may fail to heal and consolidate.
4. Hydrogel composite scaffolds
Hydrogel composite systems are one of the most suitable strategies for combining and assembling various hydrogel functions and properties. They are fabricated as hydrogel composite scaffolds with improved mechanical properties and biological functions that cannot be achieved by any single hydrogel and modified into different types of composite scaffolds to address limitations according to different tissue engineering needs.
4.1 Natural-synthetic hydrogel composite scaffolds
Natural-synthetic composite hydrogels are prepared by block copolymerization or physical interactions between natural and synthetic polymers and have good biocompatibility and mechanical properties (Table 2). Such scaffolds provide the support structure required for cell growth and tissue repair and are capable of gradual degradation to promote new tissue generation. The performance of scaffolds can be modulated by adjusting the composition and structure of the material. A wide variety of natural and synthetic hydrogels made from gelatin, collagen, alginate, hyaluronic acid and polyethylene glycol have been reported.96 For example, filipin protein assembled with tannic acid (TA), polyvinyl alcohol (PVA) and chitosan combined by physical cross-linking in a KOH/urea solubilization system to prepare new dual-network hydrogels show improved hydrogel properties and enhanced bone defect regeneration.97,98 Lee et al.’s crosslinked gelatin, prepared by enzyme-crosslinked hydroxyphenylpropionic acid (GHPA) coupling, significantly induced MSC endothelial cell growth and differentiation and was also reported to reduce the activation of host macrophages.99 Gelatin methacrylate-based (GelMA) hydrogels have been widely used to construct bone repair material systems due to their tunable mechanical properties, excellent photocrosslinking ability, good biocompatibility, and ability to promote bone differentiation and vascularization.100 However, due to the lack of osteogenic activity, GelMA hydrogels were combined with other types of materials with osteogenic activity, such as bioceramics, bioglasses, biomimetic scaffolds, inorganic ions, biomimetic periosteums, growth factors, and two-dimensional (2D) nanomaterials to improve the osteogenic capacity of current composites. Lu et al. designed a new GelMA-HAMA/nHAP composite hydrogel as a drug delivery system where EXO was encapsulated in the hydrogel and released slowly for better osteogenesis. The results of in vitro and in vivo studies showed that this controlled and biocompatible EXOs/GelMA-HAMA/nHAP composite hydrogel could effectively promote bone regeneration by coupling osteogenesis and angiogenesis.101 However, further studies and optimization are needed to improve the performance and adaptability of the scaffolds to address issues such as scaffold stability and immune response.
Table 2 Types and characteristics of natural and synthetic hydrogels
| Types |
Material |
Application |
Advantages |
Disadvantages |
Ref. |
| Natural hydrogel |
Gelatin |
Drug delivery |
High drug loading capacity; control the release rate; biocompatible; Good drug stability |
Affected by temperature; the mechanical properties need to be improved |
64–69 |
| Tissue engineering |
Good biocompatibility; good air permeability, which can ensure the supply of oxygen and nutrients to cells in tissue engineering and promote the growth and differentiation of cells; it can be used to carry and release bioactive factors; adjustable physical properties; strong plasticity and manufacturability |
Low mechanical strength; water solubility; lack of orientation; it takes a long time to achieve tissue repair and regeneration |
| Regenerative medicine |
It has good biocompatibility and can reduce foreign body rejection; bioactive substances (such as growth factors and extracellular matrix components) can be added to promote cell attachment, proliferation and differentiation, which can help tissue regeneration; it has a good three-dimensional structure and can provide a microenvironment that supports and guides cell growth |
Relatively low mechanical strength; rapid biodegradation; the cost is higher; poor stability in the aqueous environment |
| Cell culture |
Good biocompatibility; three-dimensional support structures are available; regulation performance; injectability |
Limited mechanical properties; poor dynamic stability; degradation rates are inconsistent |
| Hyaluronic acid |
Drug delivery |
Low toxicity; good biodegradability; promotes drug absorption and stabilizes the effect of the drug |
Low viscosity and high dynamic viscosity; affect the flow performance of the drug; the preparation process is complex and costly |
70–74 |
| Tissue engineering |
High biocompatibility; promotes tissue regeneration; it can prevent chronic inflammatory reactions |
Rapid degradation rate; poor mechanical strength; susceptible to the decomposition and attack of a variety of enzymes; the activity in vivo is short |
| Regenerative medicine |
Good biocompatibility; promotes tissue regeneration and cell regeneration; biodegradable; it strengthens tissue gaps |
Weak machinery; high cost |
| Cell culture |
It can support cell proliferation, cell differentiation and embryonic development and promote cell-to-cell communication |
Lack of indicative, directed alteration of differentiation; the cost is higher |
| Alginic acid |
Drug delivery |
High biocompatibility; good degradability; controlled-release medications; protects the drug from degradation and stabilizes the effects of the drug; having bioadhesion can increase drug adhesion and adsorption capacity |
It is easy to lose drugs under acidic conditions, and it is somewhat restrictive to some drugs, which may reduce their activity |
75–78 |
| Tissue engineering |
Adjusts the rheological properties of the gel; high biocompatibility; supportive cell adhesion and proliferation; promotes tissue regeneration |
Rapid biodegradation; the mechanical strength is relatively low, and the stability and mechanical properties of the material need to be further improved |
| Regenerative medicine |
Good biocompatibility; degradability; supportive cell adhesion and proliferation; may promote tissue regeneration |
Poor stability |
| Cell culture |
Sertoli cell attachment and proliferation; it can promote cell-to-cell communication; regulates cell differentiation and function |
Due to the gelling properties and pore structure of alginic acid, it may affect the morphology and diffusion of cells |
| Collagen |
Drug delivery |
Good biocompatibility, good biodegradability, strong effect on drug stability, can reduce toxicity, and controllable release of drugs |
Unstable at a certain temperature and pH; not suitable for all drugs, antigenic |
79–81 |
| Tissue engineering |
Good biodegradability; supportive cell adhesion and proliferation; provision of structural frameworks; the composition is similar to that of the extracellular matrix |
The biological activity is short and the stability is poor |
| Regenerative medicine |
Good biocompatibility; biodegradable; sertoli cell attachment and proliferation; promotes tissue regeneration |
Poor machinery |
| Cell culture |
Sertoli cell attachment and proliferation; it can promote cell-to-cell communication; regulates cell differentiation and function |
The cost is higher |
| Synthetic hydrogel |
Polyethylene glycol |
Drug delivery |
Good biocompatibility, biodegradability, high stability, releasable drugs, increases the stability and solubility of drugs; the way the drug is delivered can be adjusted by changing its molecular weight and chain structure |
Immunogenicity is present and may elicit an immune response |
82–84 |
| Tissue engineering |
Good biocompatibility, adjustable structure and physical properties of scaffolds, supports cell adhesion and proliferation, and promotes tissue regeneration; its biological activity and cellular affinity can be enhanced by chemical modifications |
Slow biodegradation, poor mechanical properties, lack of cell signaling and biological activity |
| Regenerative medicine |
Biocompatible, biodegradable, supports cell attachment and proliferation, provides physical support and structural framework; its biological activity and tissue regeneration ability can be enhanced by chemical modification |
It can lead to immune and inflammatory responses, and the biological stability and safety of the material need to be considered |
| Polylactic acid |
Drug delivery |
Biodegradable, good stability, reduced toxicity, controllable release of drugs |
Instability at a certain temperature and pH may lead to inactivation or inconsistent release of the drug |
85–87 |
| Tissue engineering |
It has high compatibility, good biodegradability, and supports cell and tissue growth |
The rate of degeneration should be controlled to avoid affecting tissue regeneration |
| Regenerative medicine |
Biodegradable, supports cell and tissue growth and regeneration, and promotes healing |
Degradation rates and mechanical properties are inconsistent |
| Polyglycolic acid |
Drug delivery |
High biocompatibility, good biodegradability, controllable release of drugs, and improved drug bioavailability |
The molecular structure is prone to breakage, making it difficult to produce a sustainable release effect |
88–90 |
| Tissue engineering |
Good biocompatibility and biodegradability |
Acids are produced during the degradation process in the body |
| Regenerative medicine |
Good biocompatibility and biodegradability |
The biodegradation process is slow and can cause stress on the regenerating tissue |
| Polyethylene oxide |
Drug delivery |
It is biocompatible, degradable, can form stable water–soluble complexes, and can regulate the release rate and behavior of drugs |
Solubility and stability are affected by factors such as pH, temperature, and concentration |
67, 91 and 92 |
| Tissue engineering |
Biocompatible, degradable, three-dimensional structure that supports and guides cell and tissue growth |
Cytotoxicity |
| Regenerative medicine |
Biocompatible, degradable, three-dimensional structure that supports and guides cell and tissue growth |
The degradation products are toxic |
| Polycaprolactone |
Drug delivery |
Good biocompatibility, good biodegradability, controllable drug release, adjustable melting temperature, easy processing |
The rate of drug release is slower and needs to be modulated in combination with other materials or modified methods |
93–95 |
| Tissue engineering |
High biocompatibility, good biodegradability, and good mechanical properties of materials |
During the biodegradation process, the mechanical stability of the material is gradually lost |
| Regenerative medicine |
High biocompatibility, good biodegradability, adjustable melting temperature, can form complex three-dimensional structure; the mechanical properties and morphological stability of materials also provide good support for regenerative medicine |
The rate of degradation is slower and may affect the regeneration and repair of tissues |
4.2 Nanoparticle composite hydrogel scaffolds
Nanoparticles have been widely used in local drug delivery systems not only for their excellent bioactivity, promotion of bone marrow stem cell adhesion and expression of bone-related genes but also their enhanced pharmacological effects.102,103 Conventional hydrogels have limited mechanical strength and are prone to fracture, further limiting their functionality, while combination with nanoparticles expands the range of hydrogel applications. As a result, nanoparticle hydrogel hybrids typically exhibit better bioactive reservoir capacity and provide appropriate controlled release rates of drugs and factors. In addition, the incorporation of nanoparticulate elements into the hydrogel network expands the range of achievable mechanical properties while improving localized nanoparticle retention.104,105 Hydrogels are often combined with nanoparticles such as organic polymer nanoparticles, inorganic nanoparticles, and metal/metal oxide nanoparticles for superior performance.106 The preparation of nanoparticle-composite hydrogel scaffolds usually involves mixing nanoparticles with a hydrogel prepolymer, followed by a gelation reaction to form a gel structure. Nanoparticles can increase the mechanical strength and stability of the scaffolds and also modulate the performance of the scaffolds by adjusting the type, concentration and distribution of nanoparticles. In addition, nanoparticles can be surface-modified to achieve functionalization of the scaffolds, leading to properties such as drug slow release and biorecognition. Common organic polymer nanoparticles include polyvinyl alcohol, poly(methyl methacrylate), polylactic acid, polyacrylic acid, and polystyrene, and inorganic nanoparticles include hydroxyapatite, silica, silicate, and calcium phosphate. Metal/metal oxide nanoparticles include gold, silver, and iron oxide, which are widely used due to their corrosion and oxidation resistance, high specific surface area, surface chemistry and functionalization.107 The incorporation of magnetic metal nanoparticles leads to higher viability and osteogenic differentiation of MSCs.108 For example, the doping of hydroxyapatite in filipin hydrogels promoted the osteogenic differentiation of human MSCs.109 In another study, multifunctional nanocomposite hydrogels were constructed by covalently binding silver (Ag+) core-embedded mesoporous silica nanoparticles (Ag@MSN) to bone morphogenetic protein-2 (BMP-2), which was encapsulated into silk fibroin methacryloyl (SilMA) and photo-crosslinked to form an Ag@MSN-BMP-2/SilMA hydrogel to preserve the biological activity of BMP-2 and delay its release. These hydrogels possess synergistic osteogenic and antibacterial effects to promote bone defect repair. Ag@MSN-BMP-2/SilMA exhibited good biocompatibility in vitro and in vivo owing to its interconnected porosity and improved hydrophilicity.110 The enhancement of material properties can be realized by combining hydrogels and NPs. At the same time, the limitations of hydrogel scaffolds, such as poor mechanical strength and lack of bioactivity, can be overcome (Fig. 2).16 Nanoparticle composite hydrogel scaffolds have a wide range of applications and can be used to improve therapeutic efficacy in medicine and biology.
 |
| | Fig. 2 Application of nanoparticle composite hydrogel scaffolds. (A) Metal nanoparticles (NPs) with hydrogel preparation. Reprinted with permission from ref. 107. Copyright 2019 MDPI, Basel, Switzerland. (B) Morphology of magnetic hydrogels. Reprinted with permission from ref. 108. Copyright 2024 Springer Japan KK. (C) Schematic of the preparation method of SF/HAP composite hydrogels. Reprinted with permission from ref. 109. Copyright 2017 AAAS Science Partner Journal Program. (D) Schematic of Ag@MSN-BMP-2/SilMA hydrogel to promote the healing of tissue defects. Reprinted with permission from ref. 110. Copyright 2023 Oxford University Press. | |
4.3 Nanotube composite hydrogel scaffolds
Nanotube composite hydrogel scaffolds are biomaterials obtained by fusing inorganic nanomaterials nanotubes with biomaterials hydrogels, and they are widely used in biomedical fields such as tissue engineering and biosensing.111 Single-walled carbon nanotubes (SWCNTs) and multi-walled carbon nanotubes (MWCNTs) are the two most widely used types of carbon nanotubes, which have tunable chemical and mechanical properties such as electrical conductivity, biocompatibility, and nanoscale size.64,112 The high specific surface area and porous structure of nanotubes facilitate cell attachment, proliferation, and differentiation. Bioactivity on the surface of nanotubes can induce the differentiation of chondrocytes to chondrocyte-like cells and promote the regeneration and repair of cartilage tissues.113 Hydrogel scaffolds, with their soft structure and high degree of hydration, can mimic the softness and porous structure of human cartilage, while the addition of nanotubes can enhance the mechanical properties of the scaffolds, provide additional mechanical support, and guide the growth and distribution of cartilage tissues, which can help to form functional cartilage tissues. Meanwhile, the porous structure and high specific surface area of the nanotubes endow them with good drug adsorption and release properties. The scaffolds can carry drugs or growth factors, and by controlling the rate and mode of release, the proliferation and differentiation of chondrocytes and the repair and regeneration of cartilage tissue can be enhanced.114 Studies have shown that nanotubes have good compatibility with chitosan, which not only improves the mechanical properties of the material but also its wettability, and combination with chitosan has little effect on the pore structure but increases the number of pores, thus promoting cell survival.115 Kazemi-Aghdamfen et al. synthesized chitosan-modified halloysite nanotubes (mHNTs), and then Icariin Icariin was loaded into mHNT as a bone inducer to produce a sustained drug release system. In addition, nanocomposite chitosan/mHNTs hydrogels were prepared by sol–gel transformation, which reduced the gelation time and temperature and enhanced the mechanical strength of the resulting scaffolds, and MSCs were encapsulated into the hydrogels; in vitro viability assays showed the scaffolds to be biocompatible. In addition, mHNT embedded in the scaffolds led to enhanced proliferation and bone differentiation of the encapsulated cells.116 The nanocomposite of polycaprolactone-polyethylene glycol-polycaprolactone/gelatin and nanotubes (PCEC/Gel/HNT) promotes stem cell (hDPSC) differentiation. The presence of nanotubes increases the surface and its roughness, facilitating scaffold–cell interaction.117 It is important to note that there may be the possibility that the addition of functionalized carbon nanotubes to composite hydrogels yields two competing effects. The addition of carbon nanotubes increases the energy storage modulus of the composite hydrogel since the stiffness of the carbon nanotubes is much greater than the stiffness of the matrix. By contrast, when the concentration of carbon nanotubes increases, the abundance of functional groups in the composite hydrogel prevents the rapid and complete cross-linking of the polymer chains, while the slow and incomplete cross-linking of the hydrogel network leads to an increase in the gelation time and decrease in the storage modulus. Therefore, larger carbon nanotube concentrations do not necessarily lead to an increase in storage modulus (Fig. 3).118
 |
| | Fig. 3 Application of nanotube composite hydrogel scaffolds. (A) Injectable chitosan hydrogel embedding modified halloysite nanotubes for bone tissue engineering. Reprinted with permission from ref. 116. Copyright 2021 Elsevier Ltd. (B) Nanotube composite hydrogel scaffolds. Reprinted with permission from ref. 118. Copyright 2019 Mater Sci Eng C Mater Biol Appl. | |
4.4 Microsphere composite hydrogel scaffolds
Polymer microspheres have high potential for tissue engineering and biomaterial applications and can be used to synergistically repair and rebuild tissues by encapsulating cells, cytokines, and drugs. Alternatively, microsphere composite hydrogel scaffolds refer to a material constructed by compositing microspheres with a hydrogel material, which has better organoplastic properties and can be prepared into scaffolds with different shapes and structures.119 Microsphere composite gel scaffolds can improve the stability, bioactivity and biocompatibility of cells and factors by controlling the microsphere size, porosity, and surface reaction structure.120 Microspheres with certain strength can be used as rigid anchors in soft hydrogels to form more physical cross-linking points, which can further increase the mechanical strength of the whole system and solve the problems of fragility or breakage of hydrogels in biomedical applications.121 These physical cross-linking points can further increase the internal porosity of the hydrogel, thus improving the affinity of the hydrogel for the cells and enabling the cells to stretch in the 3D hydrogel microenvironment.122 The unique surface topology of microspheres dispersed in hydrogels can increase cell affinity; the spheres facilitate cell–cell interactions but lack initial adhesion to natural or artificial ECMs, whereas hydrogels are ECM-like structures that promote initial cell adhesion. Therefore, microspheres not only improve the internal structure of hydrogels but also provide more internal space for cell proliferation and migration, thus providing favorable conditions for cellular respiration and transport of nutrients and metabolic wastes.123 Polymer microspheres have good mechanical properties, biocompatibility, and degradability but poor hydrophilicity.124 Lin et al. used PLGA microspheres carrying growth factors in combination with methoxypoly(ethylene glycol)-poly(poly(alanine)) (mPA) hydrogel, and results showed the ability to provide excellent 3D microenvironment for the promotion of cartilage-forming phenotypes. This synthesized composite hydrogel system was also able to regulate chondrocyte biosynthesis and differentiation activity.88 Ji et al. newly developed a composite scaffold consisting of porous chitosan (CS) microspheres and hydroxypropyl chitin (HPCH) hydrogel, in which dimethyloxalylglycine (DMOG) was encapsulated in a heat-sensitive HPCH hydrogel (HD), and tyrosine (KGN) coupled to porous CS microspheres (CSK-PMS) was employed as a controlled drug delivery system that can effectively create the M2 macrophage microenvironment and coordinate osteochondral regeneration.125 However, microspheres prepared by current techniques have a single internal and external structure, high dispersion and low yield, which can affect cell and drug delivery scalability and biocompatibility126 (Fig. 4).
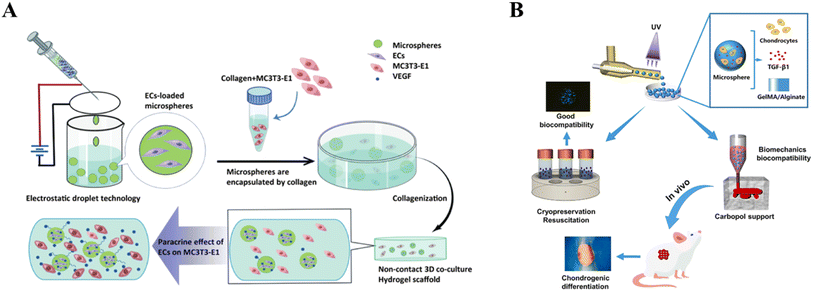 |
| | Fig. 4 Application of microsphere composite hydrogel scaffolds. (A) Indirect co-culture of type I collagen composite hydrogel scaffolds loaded with calcium alginate microspheres. Reprinted with permission from ref. 127. Copyright 2024 PLOS. (B) Microsphere composite hydrogel scaffolds for articular cartilage repair. Reprinted with permission from ref. 128. Copyright 2023 Elsevier Ltd. | |
5. Preparation of hydrogel composite scaffolds
Hydrogel composites have attracted widespread attention due to their superior properties. The preparation of hydrogel composite scaffold materials with good biocompatibility, tunable degradability, and mechanical strength to improve their efficacy in clinical applications has become a major goal. The selection of crosslinking and fabrication strategies for hydrogel scaffolds must align with target tissue requirements, balancing mechanical performance, biocompatibility, and structural fidelity. Innovations in hybrid techniques, dynamic materials, and precision manufacturing will accelerate the translation of hydrogel-based scaffolds into clinically viable multifunctional platforms for complex tissue regeneration (Table 3). Herein, we present the main preparation methods of hydrogel composite scaffolds.
Table 3 Comparison of performance of hydrogel composite scaffolds' preparation methods
| Parameter |
Physical cross-linking |
Chemical cross-linking |
Photocrosslinking |
3D printing |
| Mechanical strength |
Low (kPa range) |
High (MPa range) |
Moderate-high |
Moderate-high (process-dependent) |
| Biocompatibility |
Excellent |
Moderate (post-purification) |
Good (low phototoxicity) |
Moderate (process-dependent) |
| Degradation control |
Short-term (days-weeks) |
Long-term (months-years) |
Tunable (covalent + photolysis) |
Programmable (multi-material) |
| Structural complexity |
Low (homogeneous) |
Moderate (pre-molded) |
High (light-patterned) |
Extremely high (3D microarchitecture) |
| Clinical potential |
Injectable fillers |
Long-term implants |
Minimally invasive in situ gels |
Customized tissue substitutes |
5.1 Physical cross-linking
Physical cross-linking method is one of the commonly used techniques to prepare polymer scaffolds.127 Physical hydrogels are formed utilizing physical interactions, which involves non-covalent interactions (e.g., hydrogen bonds, ionic bonds), to allow polymers to form an ordered network structure. Compared to chemical cross-linking, physically cross-linked hydrogels usually have better solubility and can be released in a controlled manner under changing conditions such as temperature or pH. However, the prepared physical hydrogels usually have poor mechanical properties and structural stability, which are the main limitations of this crosslinking method.128,129 There are several common physical cross-linking methods, including freeze-thawing, ionic interactions, and hydrogen bonding.130–132 Freeze-thawing method forms microcrystalline structures through multiple freeze–thaw cycles, which reduces the gaps between polymer chains, increases the concentration of polymers, and forms interconnected network structures, with no toxic effect on cells and no effect on the biocompatibility and biodegradability of polymerized hydrogels.133 Nie et al. used cyclic freeze-thawing to prepare chitosan/gelatin hydrogels with biphasic calcium phosphate nanoparticles (BCP-NPs) as scaffolds for bone tissue engineering (CGB); the prepared scaffolds showed good cytocompatibility and induced bone regeneration in bone marrow mesenchymal stem cell (BMSC) culture.134 Ionic interaction, alternatively, is a method of gelling the polyelectrolyte solution by the addition of divalent and trivalent ions, in which multivalent ions form crosslinked structures with oppositely charged groups in the polymer.132 Hydrogen bonding is an important physical interaction that can lead to the formation of tertiary and secondary structures in hydrogels. This interaction is based on the degree of protonation present in the chemical environment and protonation of polar functional groups. Through hydrogen bonding interactions, soft materials can be endowed with self-healing properties. Physical cross-linking networks of composite hydrogels created through intramolecular or intermolecular hydrogen bonding increase the heat resistance of the composite hydrogels and decrease the rate of swelling and biodegradation.135 The main drawback of these physical cross-linking methods is the lack of sufficient strength and mechanical stability (Fig. 5).
 |
| | Fig. 5 Physical cross-linking. (A) Physical cross-linking to construct chitosan/PVA bi-network hydrogels. Reprinted with permission from ref. 49. Copyright 2002 Elsevier Ltd. (B) Reversible linkages based on supramolecular interactions and dynamic covalent bonds. Reprinted with permission from ref. 132. Copyright 2020 KeAi Publishing. | |
5.2 Chemical cross-linking
Chemically crosslinked hydrogels are covalently crosslinked by the addition of chemical reagents, which endows the hydrogel higher mechanical strength. In contrast to physical cross-linking, chemically cross-linked hydrogels are formed by irreversible covalent bonding, which are known as permanent hydrogels, and their swelling properties depend mainly on the concentration of the cross-linking agent.136 Free radical polymerization is one of the methods that often uses vinyl monomers or their functionalized forms with free radical initiators and cross-linking agents added. The advantage of this method is that a wide range of monomers can be used for the synthesis of biologically active hydrogels, but it requires photostimulation or thermal stimulation to initiate cross-linking, which can cause cytotoxicity.137 Tanasa et al. prepared nanocomposite hydrogels that stimulate soft tissue regeneration by the free radical polymerization of magnetite nanoparticles modified by acrylamide monomers and double bonds.138 Schiff base reaction is used to form hydrogels through the reaction between amino and aldehyde groups, and the reaction of natural polymers with natural or synthetic polymers containing amino groups produces hydrogels with high reaction rates. Alginate/gelatin hydrogel scaffolds embedded in chitosan microspheres containing curcumin were formed in situ by the Schiff base reaction for bone tissue regeneration.139 The Diels–Alder reaction, alkyne–azide cycloaddition reaction, and thiophene reaction are also commonly used for the preparation of composite scaffolds in hydrogels, but their slow kinetics have hindered their development. Enzymatic reactions can be used to prepare polymers containing enzyme-responsive regions such as tyrosine, tyramine, aminophenol, and dopamine, and rapid in situ gelation occurs during oxidation using hydrogen peroxide. Commonly used cross-linking agents include glutaraldehyde (GA), formaldehyde (FA), curcumin, and genipin (GE).140 However, the use of cross-linking agents may cause toxicity, and to overcome the side effects of polymers and improve stability, the use of non-cytotoxic natural cross-linking agents may be an option (Fig. 6).141
 |
| | Fig. 6 Chemical cross-linking preparation of GelMA-SFMA hydrogels. Reprinted with permission from ref. 141. Copyright 2024 Springer Nature. | |
5.3 Photocrosslinking
Photocrosslinking is generally considered a form of chemical cross-linking, except in specific cases where physical cross-linking may occur. It is based on a free radical polymerization reaction initiated by a photoinitiator, which crosslinks polymer molecules together to form a 3D network structure by means of a crosslinking agent, capable of controlling the position and timing of the crosslinks in a physiological environment.142 Photocrosslinking consists of initiation, propagation and termination steps performed under UV-visible light irradiation and photoinitiators.143,144 Free radicals dissociate from the photoinitiator upon irradiation and attack the vinyl group on the macromolecular precursor, and light irradiation creates covalent bonds that crosslink the hydrogel network within seconds to minutes. Selection of suitable photoinitiators can inhibit the undesirable cytotoxic effects of cell encapsulation.145 A number of key characteristics need to be considered when selecting a photoinitiator, such as the ability to generate free radicals, absorption spectrum, water solubility, molar extinction coefficient, and stability. Visible photoinitiators are relatively less cytotoxic and have high water solubility compared to UV photoinitiators.146 Paek et al. developed a GelMA photocrosslinking system based on a pore plate with tunable hydrogel mechanical properties to modulate PTH-mediated osteogenic fate. In response to PTH, hMSCs in a high stiffness microenvironment upregulated osteogenic differentiation.147 Another study demonstrated the visible light-induced photocrosslinking of methacrylate-κ-carrageenan (MA-κ-CA) mixed with bioactive silica nanoparticles (BSNPs) for the fabrication of 3D composite hydrogels using digital light processing (DLP) printing, which showed high cellular viability, no cytotoxicity, and significantly enhanced osteogenic differentiation.148 Photocrosslinking requires the addition of photosensitizers to activate the cross-linking reaction; however, for some sensitive cells or biomolecules, using photosensitizers may adversely affect their activity or function. Photocrosslinking requires the use of specific wavelengths and intensities of light to activate the cross-linking reaction, and if the light conditions are not appropriate or stable, it may result in suboptimal cross-linking (Fig. 7).149
 |
| | Fig. 7 SF-IMA/PIL hydrogel photocrosslinking preparation. Reprinted with permission from ref. 149. Copyright 2024 American Chemical Society. | |
5.4 3D printing
In the preparation process of hydrogel composite scaffolds, 3D printing technology can improve the production efficiency, consistency and reproducibility of the prepared hydrogel composite scaffolds owing to advantages such as printing design refinement, accelerated production speed, reduced manual operation, and improved precision of preparation, which cannot be achieved by the traditional manual production methods.150 The data associated with 3D printing technology comes from 3D model data based on computer design. After slicing the 3D model into 2D code information, 3D printers can recognize it. The principle of 3D printing technology is broadly defined as the layer by layer stacking of materials to achieve free-form fabrication, but there are different categories of 3D printing molding principles.151 Unlike traditional hydrogel scaffolds, 3D printing technology allows the preparation of a variety of complex and fine structures, such as nano or particle-sized channels and pores, for natural cell adhesion and differentiation of cells through hydrogel composite scaffolds, accelerating bio-integration of real tissue growth and cell transplantation. In addition, 3D printing technology can control the porosity of the scaffolds, pore size and orientation, etc., to achieve the creation of an ideal acid-base balance, cell supply, and generative growth environment, and to improve the efficiency of tissue regeneration and success rate of cell regeneration (Table 4).161 For example, Jiang et al. used 3D bioprinting to construct scaffolds containing mesenchymal stem cells (MSCs) in GelMA/graphene oxide (GO) complexes and found that GO enhanced cell proliferation, but it had no significant effect on cell viability. In vitro experiments showed that GO promoted substance–cell interactions and expression of osteogenesis-related genes. In vivo experiments showed that GO decreased degradation time of the material and increased deposition of calcium nodules, and incorporation of GO created a suitable microenvironment to promote the differentiation of exogenous MSCs loaded in vitro and in vivo for bone defects repair compared to pure GelMA.162 nHA/PLA/dECM/β-CD, synthesized by Li et al. through 3D printing technology, was found to be a good choice for bone defects repair. Composite scaffolds showed promise in inducing bone regeneration, preventing infection, and promoting jaw defect repair.163 Bartnikowski et al. prepared multilayered hydrogel composites by 3D printing, casting functionalized gelatin methacrylamide (GelMA) or GelMA with hyaluronic acid methacrylate (HAMA) on pure alginate or alginate/hydroxyapatite (HAp) composites; the incorporation of hydroxyapatite increased the elastic modulus of the printed hydrogel composites, while HAMA in the GelMA hydrogels improved cartilage formation.164 Currently, the available hydrogel cross-linking methods are severely limited, with only photo- and ionic-cross-linking strategies applicable to 3D printing, and the materials and printing systems for these methods are not able to meet the stringent requirements for tissue engineering applications. Meanwhile, most hydrogel composites are simply mixed with different weight ratios of components, which leads to severe agglomeration of reinforcing materials within the hydrogel matrix and affects the material properties (Fig. 8).165,166
Table 4 3D printing to make hydrogel composites
| 3D printing method |
Reinforcement/loading |
Base materials |
Performances |
Application |
Ref. |
| DIW |
LAP |
2-Hydroxyethyl methacrylate |
Activation (e.g., protein) treatment is not required to promote robust growth compliance that directs the spatial attachment of fibroblasts (3T3) and osteoblast pre-cells, thereby cultivating the latter's ability to direct long-term bone differentiation |
Tissue engineering |
152 |
| RGD-PDL |
(2-Hydroxyethyl) methacrylate (pHEMA) |
Allows direct formation of cellular networks and organotypic structures |
Regenerative medicine |
153 |
| Glycidyl methacrylate |
HAc |
Improved mechanical properties |
Tissue engineering |
154 |
| SLM |
Alg-PCL |
Alginate |
Ideal for fast adaptation to a range of interchangeable melts and high-viscosity materials |
Additive manufacturing |
155 |
| FDM |
PLA |
Gelatin-chitosan |
Enhance mechanical stability, biocompatibility and biological activity |
Tissue engineering |
156 |
| PEEK |
MeHA-HAp |
Promotes the adhesion and proliferation of mesenchymal stem cells, aids in osteogenic differentiation and extracellular matrix mineralization |
Biomedicine |
157 |
| GMHA |
Poly(ethylene glycol)-block-poly(β-caprolactone) scaffold (mPEG-PCL), hydroxyapatite (HAp) powder and grafting RGD peptide to the surface of the scaffold |
Promotes healing of kneecap cartilage defects in rabbits |
Tissue engineering |
158 |
| Extrusion bioprinting |
Curcumin |
Gelatin methacrylate (GelMA)/chitosan nanoparticles (CSNPs) |
Effectively protects against gram-positive and gram-negative bacteria, promotes cell proliferation and reduces microbial infections in wounds |
Wound healing |
159 |
| hpcECM |
SF and G-TA |
It has improved and tunable biomechanical properties for encapsulated cells as well as high biological activity |
Soft tissue engineering |
160 |
 |
| | Fig. 8 3D printing technology for hydrogel composites. (A) Laser-based hydrogel 3D printing system. Reprinted with permission from ref. 165. Copyright 2012 Elsevier Ltd. (B) Nozzle-based hydrogel 3D printing system. Reprinted with permission from ref. 166. Copyright 2017 Elsevier Ltd. (C) Inkjet printer-based hydrogel 3D printing system. Reprinted with permission from ref. 166. Copyright 2017 Elsevier Ltd. | |
6. Application of hydrogel composite scaffold in knee cartilage defects
When patients' knee cartilage is damaged, traditional treatment mainly relies on the repair and regeneration of their own cartilage. However, the limited ability to repair cartilage defects often leads to aggravation of the condition and even requires knee replacement surgery. The hydrogel composite scaffold material can provide a suitable three-dimensional scaffold structure for chondrocyte settlement and tissue regeneration as it can be tightly integrated with the damaged cartilage area, acting as a bridge so that cells can grow and settle in the defective area and create a microenvironment conducive to the regeneration of chondrocytes. The composite scaffold is able to degrade and transform into the extracellular matrix on its own, forming a complete group structure with the cells and providing good mechanical support. In clinical application, it can be customized according to the shape, size and location of the patient's knee cartilage defect to better fit the defect site and improve the accuracy and completeness of repair. Some hydrogel composite stents can be implanted into the body through minimally invasive injections to reduce surgical trauma and complications, shorten the patient's recovery time, and improve treatment experience. It can also be combined with existing knee cartilage repair technologies such as arthroscopic technology and microfracture technology to give full play to their respective advantages and further improve the treatment effect. In recent years, an increasing number of clinical studies have been conducted to apply hydrogel composite scaffolds in the treatment of knee cartilage defects.
6.1 Medial femoral condyle
Cartilage lesions of the medial femoral condyle are the most common knee joint site other than the femoral patellofemoral joint. Cartilage injuries frequently occur in the main stress zone of the medial femoral condyle due to mechanical stress and kinematic orientation.167 Dashtdar et al. formed medial femoral condyle defects in both knees of 24 adult New Zealand white rabbits, transplanted a novel polyvinyl alcohol (PVA)-chitosan composite hydrogel-mesenchymal stem cell (MSC) and evaluated cartilage repair at six months. Significant tissue repair was observed on treatment with PVA chitosan-MSC. In addition, the saffron O staining and glycosaminoglycan (GAG) content of the MSC-treated group was significantly higher than that of the scaffold-only or untreated control group, suggesting that PVA-chitosan composite hydrogel scaffolds can be used as a carrier of MSC for the treatment of cartilage defects.168 Lu et al. investigated the use of biodegradable hydrogel composite scaffolds based on macro-oligomer (poly(ethylene glycol)) fumarate (OPF) in medial femoral condylar osteochondral defects, in which three different scaffolds were implanted to provide growth factors for repairing osteochondral tissue in a rabbit model. The bilayer OPF hydrogel composite scaffold showed potential as a spatially-guided multiple growth factor release carrier for osteochondral tissue repair.169 Using a goat model, autologous chondrocytes immobilized in MPEG-PLGA scaffolds via fibrin hydrogel produced a 6 mm round, full-length cartilage defect in both medial femoral condyles, which was evaluated for cartilage repair potential and showed favorable cartilage repair response170 (Fig. 9). Various approaches utilizing advanced hydrogel scaffolds and stem cell therapies demonstrate promise for repairing cartilage lesions in the medial femoral condyle. These advancements support the potential for developing effective treatments for cartilage defects, although further research is needed to optimize these techniques for clinical application.
 |
| | Fig. 9 CT analysis of the cartilage repair effect of a hydrogel composite scaffold loaded with growth factors on medial femoral condylar defects. (A) Cross section of osteochondral defect, cortex. (B) Trabeculae. (C) Cortical area after six weeks of treatment. (D) Trabeculae after six weeks of treatment. (E) Section of the sample after 12 weeks of treatment. Reprinted with permission from ref. 169. Copyright 2014 Elsevier Ltd. | |
6.2 Tibial plateau
There are few reports on hydrogel composite scaffolds for the treatment of tibial plateau defects. In one study, sodium alginate (SA)/gelatin (Gel) hydrogel scaffolds doped with different contents of nano-gravite were prepared by 3D printing technology, and their surface microstructure, hydrophilicity, and mechanical properties were evaluated. In addition, mouse bone marrow-derived mesenchymal stem cells (BMSCs) were cultured with the composite hydrogels in vitro and evaluated for proliferation and osteoblast differentiation, while a rabbit tibial plateau defect model was used to assess the osteogenic potential of the composite hydrogels in vivo. Results showed that the Gel/SA/nano-ATP composite hydrogels exhibited better mechanical properties and printability when the nano-ATP content was increased. They showed excellent bioactivity, and a significant mineralization effect was observed on the surface after incubation in simulated body fluid (SBF) for 14 d. The Gel/SA/nano-ATP composite hydrogel also showed good biocompatibility and could effectively promote bone regeneration in vivo (Fig. 10).171
 |
| | Fig. 10 Histological analysis of gel/SA/nano-ATP composite hydrogel scaffolds in tibial plateau defects in rabbits 4–12 weeks after implantation. (A) Photographs of the five experimental groups during surgery and 4–12 weeks postoperatively. (B). Observation of H&E staining. (C) Observation of the new bone formed in the composite hydrogel. (D) Quantitative analysis of Masson staining. Reprinted with permission from ref. 171. Copyright 2021 Dove Press. | |
6.3 Meniscus
The meniscus is a fibrocartilaginous tissue that is important for the stability of the knee joint. However, it has low self-healing ability; thus, damage to it always leads to articular cartilage degeneration. Li et al. performed total meniscus replacement in a rabbit model using a 3D printed network hydrogel composite scaffold to investigate whether hydrogel composite scaffolds can prevent articular cartilage degeneration, promote tissue degeneration, and to explore its mechanical properties at three months and six months postoperatively. Results showed that the hydrogel composite scaffold slowed down articular cartilage degeneration, promoted tissue regeneration, and facilitated articular cartilage regeneration.172 Baysan et al. fabricated a new type of meniscus hydrogel composite scaffold made of chitosan, loofah mat, and PHBV nanofibers, and in vitro analyses showed no cytotoxic effects, allowing cells to attach, proliferate, and migrate inside the scaffold, making it a promising meniscus tissue engineering material.173 Chitosan-collagen hydrogel composite scaffolds consisting of 3D-printed polylactic acid (PLA) struts and nanofibrous cellulose showed no cytotoxic effects on rabbit mesenchymal stem cells, enabling cells to attach, proliferate, and migrate through the inner regions of the scaffolds, which can be used for meniscal cartilage tissue engineering174 (Fig. 11).
 |
| | Fig. 11 Hydrogel composite scaffold repairs meniscus. (A) Examining the gross changes in articular cartilage after the replacement of the meniscus with a PG–Pg scaffold. Reprinted with permission from ref. 172. 2024 ESSKA a.s.b.l. (B) Histological sections of hydrogel composite scaffolds loaded with mesenchymal cells stained for H&E and type I collagen/type II collagen. Reprinted with permission from ref. 173. Copyright 2022 Elsevier Ltd. | |
6.4 Others
A composite scaffold for cartilage defect repair was developed by incorporating a carbon nanotube-doped octapeptide hydrogel into a 3D-printed PCL scaffold (FEK/C-S), where CNTs were fabricated in a network of a previously introduced non-immunogenic octapeptide (FEFEFKFK) to form a novel hydrogel denoted as FEK/C. Then, FEK/C was applied to 3D-printed PCL scaffolds to form a composite scaffold denoted as FEK/C-S. The composite scaffolds promoted cartilage and subchondral bone regeneration in a rabbit knee defect model.175 Long et al. developed an ultrasonication-induced silica-gel-collagen composite hydrogel (COL + S(S)), which demonstrated excellent stability during cell culture. It promoted SOX9 gene expression at an early stage and sulfated glycosaminoglycan (sGAG) deposition in the absence of any exogenous growth factors. In addition, well-integrated articular hyaline cartilage, very similar to natural articular cartilage, was demonstrated in a rabbit knee defect model.176 Wu et al. developed a TGF-β3-loaded TGF-β3-containing methacrylate sericin-protein sealant (Sil-MA), which is biocompatible and has good adhesive properties to promote chondrocyte migration and differentiation. Importantly, this TGF-β3-loaded Sil-MA hydrogel bridges the gap between the cartilage layer of the scaffold and the surrounding cartilage, which then guides the growth of new cartilage toward the surrounding natural cartilage and replaces the degraded cartilage layer in the early stages of knee repair. Using this composite, osteochondral regeneration and superior lateral integration were achieved in vivo.177 Li evaluated the ability of filipin protein (SF) hydrogel scaffolds doped with chitosan (CS) nanoparticles (NPs) to repair cartilage defects in the knee joint. The developed TGF-β 1@CS/BMP-2@SF hydrogel promoted the chondrogenic capacity of BMSCs in vivo and in vitro by releasing TGF-β1 and BMP-2.178 In another study, a solution containing TGF-β1 was photocrosslinked to form a hydrogel layer immobilized on the upper side of an RP scaffold. Using a scaffold designed for fused deposition modeling, the damaged articular cartilage and subchondral bone were repaired and regenerated in an in vivo trial in the knee of a Lanyu miniature pig over a 12 month period.179 In another study, osteochondral regeneration induced by TGF-β-loaded photocrosslinked hyaluronic acid hydrogel infiltrated in a composite scaffold fabricated by the fused deposition of hydroxyapatite and poly(ethylene glycol)-block-poly(ε-caprolactone) was investigated in an in vivo trial on rabbit knees; the histological sections showed that 12 weeks after implantation of the TGF-β1-loaded hydrogel and scaffolds, the bone and cartilage defects produced in the knee joints were completely healed and the regenerated cartilage was hyaline cartilage158 (Fig. 12).
 |
| | Fig. 12 Effect of composite scaffolds on the repair of articular cartilage and subchondral bone defects in rabbits. (A) Images of rabbit knee joints after articular defect and scaffold implantation eight weeks postoperatively. (B) CT scans, defective areas are shown as red circles or red squares. Reprinted with permission from ref. 175. Copyright 2023 MDPI, Basel, Switzerland. | |
7. Conclusion
In recent years, research on hydrogel composite scaffolds in regenerative medicine and tissue engineering has achieved numerous positive outcomes. Although current research on knee cartilage defects is somewhat limited, it has exhibited great potential. Hydrogel composite scaffolds possess excellent biocompatibility and can integrate seamlessly with surrounding tissues, thus providing an ideal growth environment for promoting the regeneration and repair of chondrocytes. Treating knee joint cartilage defects with hydrogel composite scaffolds can significantly relieve patient pain and functional limitations. Moreover, hydrogel composite scaffolds have adjustable release properties. They can release beneficial cytokines and growth factors, which stimulate the proliferation and differentiation of chondrocytes, thereby promoting cartilage regeneration. Additionally, these scaffolds can serve as carriers for introducing stem cells or genes into cartilage defects, further accelerating the cartilage repair process.
However, current research also confronts several challenges and limitations. Firstly, the long-term efficacy and safety of hydrogel composite scaffolds require further evaluation. Secondly, the preparation and manipulation techniques of these scaffolds need to be refined to enhance preparation efficiency and controllability. Thirdly, more clinical studies are essential to validate the efficacy of hydrogel composite scaffolds in treating different types and degrees of cartilage defects.
To address these issues, researchers can focus on the following emerging trends. To improve the mechanical properties, exploring novel composite materials and cross-linking methods can enhance the strength and durability of hydrogel composite scaffolds, enabling them to better withstand the mechanical forces within the knee joint. For more precise drug release, advanced drug-loading and release-control technologies, such as smart hydrogels that respond to specific physiological signals (e.g., pH, temperature, or enzyme activity), can be developed, ensuring that growth factors and therapeutic agents are released at the optimal time and dosage to optimize cartilage repair. Integrating stem cell therapies with hydrogel composite scaffolds also holds great promise. By precisely regulating the microenvironment provided by the scaffolds, stem cells can be more effectively guided to differentiate into chondrocytes, thus enhancing cartilage regeneration. For instance, incorporating signaling molecules or extracellular matrix components into the scaffolds can promote the differentiation of stem cells into chondrocytes. In addition, with the assistance of advanced technologies such as bioprinting, more precise and personalized scaffolds can be fabricated to meet the specific requirements of different patients and cartilage defect types. This personalized approach is likely to improve treatment outcomes and patient satisfaction.
In conclusion, the study of hydrogel composite scaffolds in knee cartilage defects presents a promising outlook. With continuous technological progress and in-depth research, hydrogel composite scaffolds are expected to become a crucial therapeutic tool, bringing better clinical results for patients with knee cartilage defects.
Author contributions
Conceptualization, D. T. and H. S.; resources: D. T., H. S., C. Y. and Y. L.; writing – original draft preparation, D. T.; writing – review and editing, X. S. and S. R. All authors have read and agreed to the published version of the manuscript.
Conflicts of interest
The authors have no conflict of interest to declare.
Acknowledgements
This research was funded by the Zunyi Science and Technology Bureau and the First People's Hospital of Zunyi joint science and Technology Research and development fund project (No. Zun City Science and HZ word [2023] 16); Guizhou Province Science and Technology Program Project (No. Guizhou Science and Technology-ZK [2021] General 393); Guizhou Provincial Health Commission Science and Technology Foundation Project (No. gzwkj2021-259); Guizhou Science and Technology Program Project (No. Guizhou Synthetic Fruit-LC [2022] 029); Zunyi Science Plan Project (No. Zun City Science and HZ word (2022) 49); and Guizhou Province Science and Technology Program Project (No. Guizhou Science and Technology-ZK [2023] General 491).
References
- R. Rossi, U. Cottino, M. Bruzzone, F. Dettoni, D. E. Bonasia and F. Rosso, Total knee arthroplasty in the varus knee: tips and tricks, Int. Orthopaedics, 2019, 43, 151–158, DOI:10.1007/s00264-018-4116-3.
- C. Lopes, A. Vilaca, C. Rocha and J. Mendes, Knee positioning systems for X-ray environment: a literature review, Phys. Eng. Sci. Med., 2023, 46, 45–55, DOI:10.1007/s13246-023-01221-y.
- I. Khodarahmi, H. Alizai, E. Alaia and S. Gyftopoulos, MR imaging of the knee posterolateral and posteromedial corner injuries, Magn. Reson. Imaging Clin. N. Am., 2022, 30, 215–226, DOI:10.1016/j.mric.2021.11.003.
- A. L. Boden, J. Kaplan and A. Aiyer, Subtalar osteochondral lesions: diagnosis, indications, and prognosis, Foot Ankle Clin., 2024, 29, 225–233, DOI:10.1016/j.fcl.2023.07.002.
- M. Abolghasemian, S. León, P. T. H. Lee, O. Safir, D. Backstein and A. E. Gross, et al., Long-term results of treating large posttraumatic tibial plateau lesions with fresh osteochondral allograft transplantation, J. Bone Joint Surg. Am., 2019, 101, 1102–1108, DOI:10.2106/JBJS.18.00802.
- A. Guermazi, J. Niu, D. Hayashi, F. W. Roemer, M. Englund and T. Neogi, et al., Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (Framingham Osteoarthritis Study), BMJ, 2012, 345, e5339, DOI:10.1136/bmj.e5339.
- C. Ding, F. Cicuttini and G. Jones, Tibial subchondral bone size and knee cartilage defects: relevance to knee osteoarthritis, Osteoarthritis Cartilage, 2007, 15, 479–486, DOI:10.1016/j.joca.2007.01.003.
- A. J. Krych, D. B. F. Saris, M. J. Stuart and B. Hacken, Cartilage injury in the knee: assessment and treatment options, J. Am. Acad. Orthop. Surg., 2020, 28, 914–922, DOI:10.5435/JAAOS-D-20-00266.
- A. H. Pandit, N. Mazumdar and S. Ahmad, Periodate oxidized hyaluronic acid-based hydrogel scaffolds for tissue engineering applications, Int. J. Biol. Macromol., 2019, 137, 853–869, DOI:10.1016/j.ijbiomac.2019.07.014.
- A. S. Vaziri, E. Vasheghani-Farahani, S. Hosseinzadeh, F. Bagheri, M. Büchner and D. W. Schubert, et al., Genipin-cross-linked silk fibroin/alginate dialdehyde hydrogel with tunable gelation kinetics, degradability, and mechanical properties: a potential candidate for tissue regeneration, Biomacromolecules, 2024, 25, 2323–2337, DOI:10.1021/acs.biomac.3c01203.
- Y. M. Lee, Z. W. Lu, Y. C. Wu, Y. J. Liao and C. Y. Kuo, An injectable, chitosan-based hydrogel prepared by Schiff base reaction for anti-bacterial and sustained release applications, Int. J. Biol. Macromol., 2024, 269, 131808, DOI:10.1016/j.ijbiomac.2024.131808.
- W. Li, S. Yang, W. Chen, J. Yang, H. Yu and R. Lv, et al., Free-standing and flexible polyvinyl alcohol-sodium alginate-polypyrrole electrodes based on interpenetrating network hydrogels, J. Colloid Interface Sci., 2024, 664, 299–308, DOI:10.1016/j.jcis.2024.03.064.
- G. Zhong, P. Lei, P. Guo, Q. Yang, Y. Duan and J. Zhang, et al., A photo-induced cross-linking enhanced A and B combined multi-functional spray hydrogel instantly protects and promotes of irregular dynamic wound healing, Small, 2024, e2309568, DOI:10.1002/smll.202309568.
- Q. Niu, L. Huang, S. Fan, X. Yao and Y. Zhang, 3D printing silk fibroin/polyacrylamide triple-network composite hydrogels with stretchability, conductivity, and strain-sensing ability as bionic electronic skins, ACS Biomater. Sci. Eng., 2024, 10, 3489–3499, DOI:10.1021/acsbiomaterials.4c00201.
- A. K. Gaharwar, N. A. Peppas and A. Khademhosseini, Nanocomposite hydrogels for biomedical applications, Biotechnol. Bioeng., 2014, 111, 441–453, DOI:10.1002/bit.25160.
- P. Thoniyot, M. J. Tan, A. A. Karim, D. J. Young and X. J. Loh, Nanoparticle-hydrogel composites: concept, design, and applications of these promising, multi-functional materials, Adv. Sci., 2015, 2, 1400010, DOI:10.1002/advs.201400010.
- S. H. Jeong, Y. H. Koh, S. W. Kim, J. U. Park, H. E. Kim and J. Song, Strong and biostable hyaluronic acid-calcium phosphate nanocomposite hydrogel via in situ precipitation process, Biomacromolecules, 2016, 17, 841–851, DOI:10.1021/acs.biomac.5b01557.
- F. Flandry and G. Hommel, Normal anatomy and biomechanics of the knee, Sports Med. Arthroscopy Rev., 2011, 19, 82–92, DOI:10.1097/JSA.0b013e318210c0aa.
- E. A. Makris, P. Hadidi and K. A. Athanasiou, The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration, Biomaterials, 2011, 32, 7411–7431, DOI:10.1016/j.biomaterials.2011.06.037.
- A. S. Tkachenko, O. S. Maksymova, O. V. Korenkov, A. P. Voznyi and G. F. Tkach, Structure of the knee articular cartilage after the femur and tibia extra-articular injury, Wiad. Lek., 2021, 74, 1863–1868, DOI:10.36740/WLek202108115.
- J. M. Link, E. Y. Salinas, J. C. Hu and K. A. Athanasiou, The tribology of cartilage: mechanisms, experimental techniques, and relevance to translational tissue engineering, Clin. Biomech., 2020, 79, 104880, DOI:10.1016/j.clinbiomech.2019.10.016.
- J. Hubert, F. T. Beil, T. Rolvien, S. Butscheidt, S. Hischke and K. Püschel, et al., Cartilage calcification is associated with histological degeneration of the knee joint: a highly prevalent, age-independent systemic process, Osteoarthritis Cartilage, 2020, 28, 1351–1361, DOI:10.1016/j.joca.2020.04.020.
- C. D. Hoemann, C. H. Lafantaisie-Favreau, V. Lascau-Coman, G. Chen and J. Guzmán-Morales, The cartilage-bone interface, J. Knee Surg., 2012, 25, 85–97, DOI:10.1055/s-0032-1319782.
- L. Dönges, A. Damle, A. Mainardi, T. Bock, M. Schönenberger and I. Martin, et al., Engineered human osteoarthritic cartilage organoids, Biomaterials, 2024, 308, 122549, DOI:10.1016/j.biomaterials.2024.122549.
- J. Schneevoigt, C. Fabian, C. Leovsky, J. Seeger and M. Bahramsoltani, In vitro expression of the extracellular matrix components aggrecan, collagen types i and ii by articular cartilage-derived chondrocytes, Anat. Histol. Embryol., 2017, 46, 43–50, DOI:10.1111/ahe.12230.
- M. Bhattacharjee, S. Chameettachal, S. Pahwa, A. R. Ray and S. Ghosh, Strategies for replicating anatomical cartilaginous tissue gradient in engineered intervertebral disc, ACS Appl. Mater. Interfaces, 2014, 6, 183–193, DOI:10.1021/am403835t.
- C. R. Correia, R. L. Reis and J. F. Mano, Multiphasic, multistructured and hierarchical strategies for cartilage regeneration, Adv. Exp. Med. Biol., 2015, 881, 143–160, DOI:10.1007/978-3-319-22345-2_9.
- N. L. Grimm, J. M. Weiss, J. I. Kessler and S. K. Aoki, Osteochondritis dissecans of the knee: pathoanatomy, epidemiology, and diagnosis, Clin. Sports Med., 2014, 33, 181–188, DOI:10.1016/j.csm.2013.11.006.
- S. Heir, T. K. Nerhus, J. H. Røtterud, S. Løken, A. Ekeland and L. Engebretsen, et al., Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: a comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery, Am. J. Sports Med., 2010, 38, 231–237, DOI:10.1177/0363546509352157.
- F. R. Convery, W. H. Akeson and G. H. Keown, The repair of large osteochondral defects. An experimental study in horses, Clin. Orthop. Relat. Res., 1972, 82, 253–262, DOI:10.1097/00003086-197201000-00033.
- E. J. Strauss, J. U. Barker, J. S. Kercher, B. J. Cole and K. Mithoefer, Augmentation strategies following the microfracture technique for repair of focal chondral defects, Cartilage, 2010, 1, 145–152, DOI:10.1177/1947603510366718.
- W. Craig, J. W. David and H. Z. Ming, A current review on the biology and treatment of the articular cartilage defects [part I & part II], J. Musculoskelet. Res., 2003, 7, 157–181, DOI:10.1142/S0218957703001125.
- Y. Liu, Y. Zhang, J. Shen, B. Zhang, H. Ma and Y. Zhou, Metaphyseal metal sleeves for reconstruction of severe knee bone defects: excellent survival rate at a mean follow-up of 6.4 years, Orthop. Surg., 2023, 15, 3202–3208, DOI:10.1111/os.13905.
- M. Brenneis, D. A. Flevas, T. D. Bornes, S. Braun, A. Meurer and P. K. Sculco, et al., Tibial bone defect prediction based on preoperative artefact-reduced CT imaging is superior to standard radiograph assessment, Knee Surg. Sports Traumatol. Arthroscopy, 2023, 31, 4842–4850, DOI:10.1007/s00167-023-07527-4.
- R. O. Dey Hazra, J. C. Rutledge, J. A. Hanson, M. D. Hazra, M. P. Horan and K. C. Doan, et al., Mid-term outcomes of microfracture for the treatment of focal, full-thickness cartilage defects isolated to the humeral head, J. Shoulder. Elbow. Surg., 2024, 33(9), 1972–1979, DOI:10.1016/j.jse.2023.12.022.
- E. Solheim, J. Hegna, E. Inderhaug, J. Øyen, T. Harlem and T. Strand, Results at 10-14 years after microfracture treatment of articular cartilage defects in the knee, Knee Surg. Sports Traumatol. Arthroscopy, 2016, 24, 1587–1593, DOI:10.1007/s00167-014-3443-1.
- J. P. Benthien and P. Behrens, Reviewing subchondral cartilage surgery: considerations for standardised and outcome predictable cartilage remodelling: a technical note, Int. Orthop., 2013, 37, 2139–2145, DOI:10.1007/s00264-013-2025-z.
- H. Chen, C. D. Hoemann, J. Sun, A. Chevrier, M. D. McKee and M. S. Shive, et al., Depth of subchondral perforation influences the outcome of bone marrow stimulation cartilage repair, J. Orthop. Res., 2011, 29, 1178–1184, DOI:10.1002/jor.21386.
- P. Zedde, S. Cudoni, G. Giachetti, M. L. Manunta, G. Masala and A. Brunetti, et al., Subchondral bone remodeling: comparing nanofracture with microfracture. An ovine in vivo study, Joints, 2016, 4, 87–93, DOI:10.11138/jts/2016.4.2.087.
- S. R. Anderson, S. C. Faucett, D. C. Flanigan, R. A. Gmabardella and N. H. Amin, The history of radiofrequency energy and Coblation in arthroscopy: a current concepts review of its application in chondroplasty of the knee, J. Exp. Orthop., 2019, 6, 1, DOI:10.1186/s40634-018-0168-y.
- D. E. Anderson, M. B. Rose, A. J. Wille, J. Wiedrick and D. C. Crawford, Arthroscopic mechanical chondroplasty of the knee is beneficial for treatment of focal cartilage lesions in the absence of concurrent pathology, Orthop. J. Sports Med., 2017, 5, 2325967117707213, DOI:10.1177/2325967117707213.
- G. Spahn, G. O. Hofmann and L. V. von Engelhardt, Mechanical debridement versus radiofrequency in knee chondroplasty with concomitant medial meniscectomy: 10-year results from a randomized controlled study, Knee Surg. Sports Traumatol. Arthrosc., 2016, 24, 1560–1568, DOI:10.1007/s00167-015-3810-6.
- T. Tuthill, G. R. Jackson, S. F. Schundler, J. S. Lee, S. Allahabadi and L. M. Salazar, et al., Radiofrequency chondroplasty of the knee yields excellent clinical outcomes and minimal complications: a systematic review, Arthroscopy Sports Med. Rehabil., 2023, 5, 100749, DOI:10.1016/j.asmr.2023.05.006.
- A. P. K. Saleh, D. Korsog and J. Mundt, Bone marrow aspirate concentrate chondroplasty to delaying need for total knee arthroplasty: a retrospective review of a veteran cohort, Regener. Med., 2023, 18, 833–838, DOI:10.2217/rme-2023-0124.
- I. M. Dávila Castrodad, M. J. Kraeutler, S. M. Fasulo, A. Festa, V. K. McInerney and A. J. Scillia, Improved outcomes with arthroscopic bone marrow aspirate concentrate and cartilage-derived matrix implantation versus chondroplasty for the treatment of focal chondral defects of the knee joint: a retrospective case series, Arthroscopy Sports Med. Rehabil., 2021, 4, e411–e416, DOI:10.1016/j.asmr.2021.10.018.
- K. Kizaki, H. A. El-Khechen, F. Yamashita, A. Duong, N. Simunovic and V. Musahl, et al., Arthroscopic versus open osteochondral autograft transplantation (Mosaicplasty) for cartilage damage of the knee: a systematic review, J. Knee Surg., 2021, 34, 94–107, DOI:10.1055/s-0039-1692999.
- M. Marcacci, G. Filardo and E. Kon, Treatment of cartilage lesions: what works and why?, Injury, 2013, 44, S11–S15, DOI:10.1016/S0020-1383(13)70004-4.
- E. Inderhaug and E. Solheim, Osteochondral autograft transplant (mosaicplasty) for knee articular cartilage defects, JBJS Essent. Surg. Tech., 2019, 9(e34), 1–2, DOI:10.2106/JBJS.ST.18.00113.
- C. S. Ahmad, W. B. Guiney and C. J. Drinkwater, Evaluation of donor site intrinsic healing response in autologous osteochondral grafting of the knee, Arthroscopy, 2002, 18, 95–98, DOI:10.1053/jars.2002.25967.
- E. Solheim, J. Hegna and E. Inderhaug, Early determinants of long-term clinical outcome after cartilage repair surgery in the knee, J. Orthop., 2018, 15, 222–225, DOI:10.1016/j.jor.2018.01.021.
- M. Kowalczuk, V. Musahl and F. H. Fu, Cochrane in CORR®: surgical interventions (microfracture, drilling, mosaicplasty, and allograft transplantation) for treating isolated cartilage defects of the knee in adults, Clin. Orthop. Relat. Res., 2018, 476, 16–18, DOI:10.1007/s11999.0000000000000016.
- S. Bark, T. Piontek, P. Behrens, S. Mkalaluh, D. Varoga and J. Gille, Enhanced microfracture techniques in cartilage knee surgery: fact or fiction?, World J. Orthop., 2014, 5, 444–449, DOI:10.5312/wjo.v5.i4.444.
- S. Anders, M. Volz, H. Frick and J. Gellissen, A randomized, controlled trial comparing autologous matrix-induced chondrogenesis (AMIC®) to microfracture: analysis of 1- and 2-year follow-up data of 2 centers, Open Orthop. J., 2013, 7, 133–143, DOI:10.2174/1874325001307010133.
- J. Jarecki, M. K. Waśko, W. Widuchowski, A. Tomczyk-Warunek, M. Wójciak and I. Sowa, et al., Knee cartilage lesion management-current trends in clinical practice, J. Clin. Med., 2023, 12, 6434, DOI:10.3390/jcm12206434.
- M. Howell, Q. Liao and C. W. Gee, Surgical management of osteochondral defects of the knee: an educational review, Curr. Rev. Musculoskelet. Med., 2021, 14, 60–66, DOI:10.1007/s12178-020-09685-1.
- J. H. Kim, J. W. Heo and D. H. Lee, Clinical and radiological outcomes after autologous matrix-induced chondrogenesis versus microfracture of the knee: a systematic review and meta-analysis with a minimum 2-year follow-up, Orthop. J. Sports Med., 2020, 8, 2325967120959280, DOI:10.1177/2325967120959280.
- M. Peras, A. Caubère, C. Choufani, N. Passuti, G. Versier and O. Barbier, Does AMIC® provide improvements at least two years after surgery for knee osteochondral lesions? A multicentre retrospective study of 101 patients, Orthop. Traumatol. Surg. Res., 2024, 110, 103774, DOI:10.1016/j.otsr.2023.103774.
- M. Volz, J. Schaumburger, H. Frick, J. Grifka and S. Anders, A randomized controlled trial demonstrating sustained benefit of autologous matrix-induced chondrogenesis over microfracture at five years, Int. Orthop., 2017, 41, 797–804, DOI:10.1007/s00264-016-3391-0.
- C. W. Nuelle, K. Rucinski, J. P. Stannard, R. Ma, M. Kfuri and J. L. Cook, Comparison of outcomes after primary versus salvage osteochondral allograft transplantation for femoral condyle osteochondritis dissecans lesions, Orthop. J. Sports Med., 2024, 12, 23259671241232431, DOI:10.1177/23259671241232431.
- S. L. Sherman, J. Garrity, K. Bauer, J. Cook, J. Stannard and W. Bugbee, Fresh osteochondral allograft transplantation for the knee: current concepts, J. Am. Acad. Orthop. Surg., 2014, 22, 121–133, DOI:10.5435/JAAOS-22-02-121.
- G. Pisanu, U. Cottino, F. Rosso, D. Blonna, A. G. Marmotti and C. Bertolo, et al., Large osteochondral allografts of the knee: surgical technique and indications, Joints, 2018, 6, 42–53, DOI:10.1055/s-0038-1636925.
- C. E. Gross and A. Palanca, Fresh osteochondral allograft for large talar osteochondral lesions, Foot Ankle Clin., 2024, 29, 343–356, DOI:10.1016/j.fcl.2023.07.009.
- C. W. Nuelle, P. E. Gelber and B. R. Waterman, Osteochondral allograft transplantation in the knee, Arthroscopy, 2024, 40, 663–665, DOI:10.1016/j.arthro.2024.01.006.
- B. Behrouznejad, S. B. Sadat and E. Masaeli, The orchestration of sustained drug delivery by bacterial cellulose/gelatin nanocomposites reinforced with carboxylic carbon nanotubes, Carbohydr. Polym., 2024, 333, 121917, DOI:10.1016/j.carbpol.2024.121917.
- Y. Jiang, Y. Guo, H. Wang, X. Wang and Q. Li, Hydrogel coating based on dopamine-modified hyaluronic acid and gelatin with spatiotemporal drug release capacity for quick endothelialization and long-term anticoagulation, Int. J. Biol. Macromol., 2023, 230, 123113, DOI:10.1016/j.ijbiomac.2022.123113.
- M. Chen, H. Tan, W. Xu, Z. Wang, J. Zhang and S. Li, et al., A self-healing, magnetic and injectable biopolymer hydrogel generated by dual cross-linking for drug delivery and bone repair, Acta Biomater., 2022, 153, 159–177, DOI:10.1016/j.actbio.2022.09.036.
- A. Liguori, A. De Vita, G. Rossi, L. S. Dolci, S. Panzavolta and C. Gualandi, et al., A modular composite device of poly(ethylene oxide)/poly(butylene terephthalate) (PEOT/PBT) nanofibers and gelatin as a dual drug delivery system for local therapy of soft tissue tumors, Int. J. Mol. Sci., 2022, 23, 3239, DOI:10.3390/ijms23063239.
- M. Mohseni, P. Shokrollahi and J. Barzin, Impact of supramolecular interactions on delivery of dexamethasone from a physical network of gelatin/ZnHAp composite scaffold, Int. J. Pharm., 2022, 615, 121520, DOI:10.1016/j.ijpharm.2022.121520.
- M. A. Sakr, K. Sakthivel, T. Hossain, S. R. Shin, S. Siddiqua and J. Kim, et al., Recent trends in gelatin methacryloyl nanocomposite hydrogels for tissue engineering, J. Biomed. Mater. Res. A., 2022, 110, 708–724, DOI:10.1002/jbm.a.37310.
- L. E. Nita, I. Nacu, A. Ghilan, A. G. Rusu, A. M. Şerban and M. Bercea, et al., Evaluation of hyaluronic acid-polymacrolactone hydrogels with 3D printing capacity, Int. J. Biol. Macromol., 2024, 256, 128279, DOI:10.1016/j.ijbiomac.2023.128279.
- M. Hu, J. Yang and J. Xu, Structural and biological investigation of chitosan/hyaluronic acid with silanized-hydroxypropyl methylcellulose as an injectable reinforced interpenetrating network hydrogel for cartilage tissue engineering, Drug Deliv., 2021, 28, 607–619, DOI:10.1080/10717544.2021.1895906.
- L. Rethi, C. C. Wong, W. J. Liu, C. Y. Chen, P. R. Jheng and C. H. Chen, et al., Self-adaptable calcium-based bioactive phosphosilicate-infused gelatin-hyaluronic hydrogel for orthopedic regeneration, Int. J. Biol. Macromol., 2024, 256, 128091, DOI:10.1016/j.ijbiomac.2023.128091.
- W. M. Metwally, S. E. El-Habashy, L. S. El-Hosseiny, M. M. Essawy, H. M. Eltaher and L. K. El-Khordagui, Bioinspired 3D-printed scaffold embedding DDAB-nano ZnO/nanofibrous microspheres for regenerative diabetic wound healing, Biofabrication, 2023, 16, 015001, DOI:10.1088/1758-5090/acfd60.
- J. H. Galarraga, R. C. Locke, C. E. Witherel, B. D. Stoeckl, M. Castilho and R. L. Mauck, et al., Fabrication of MSC-laden composites of hyaluronic acid hydrogels reinforced with MEW scaffolds for cartilage repair, Biofabrication, 2021, 14, 014106, DOI:10.1088/1758-5090/ac3acb.
- M. Li, H. Li, X. Li, H. Zhu, Z. Xu and L. Liu, et al., A bioinspired alginate-gum arabic hydrogel with micro-/nanoscale structures for controlled drug release in chronic wound healing, ACS Appl. Mater. Interfaces, 2017, 9, 22160–22175, DOI:10.1021/acsami.7b04428.
- E. Kolanthai, P. A. Sindu, D. K. Khajuria, S. C. Veerla, D. Kuppuswamy and L. H. Catalani, et al., Graphene oxide-a tool for the preparation of chemically crosslinking free alginate-chitosan-collagen scaffolds for bone tissue engineering, ACS Appl. Mater. Interfaces, 2018, 10, 12441–12452, DOI:10.1021/acsami.8b00699.
- S. Jahangir, S. Hosseini, F. Mostafaei, F. A. Sayahpour and M. Baghaban Eslaminejad, 3D-porous β-tricalcium phosphate-alginate-gelatin scaffold with DMOG delivery promotes angiogenesis and bone formation in rat calvarial defects, J. Mater Sci. Mater Med., 2018, 30, 1, DOI:10.1007/s10856-018-6202-x.
- J. C. Schagemann, E. H. Mrosek, R. Landers, H. Kurz and C. Erggelet, Morphology and function of ovine articular cartilage chondrocytes in 3-d hydrogel culture, Cells Tissues Organs, 2006, 182, 89–97, DOI:10.1159/000093063.
- M. Caldera-Villalobos, D. A. Cabrera-Munguía, J. J. Becerra-Rodríguez and J. A. Claudio-Rizo, Tailoring biocompatibility of composite scaffolds of collagen/guar gum with metal–organic frameworks, RSC Adv., 2022, 12, 3672–3686, 10.1039/d1ra08824f.
- A. Karimizade, E. Hasanzadeh, M. Abasi, S. E. Enderami, E. Mirzaei and N. Annabi, et al., Collagen short nanofiber-embedded chondroitin sulfate-hyaluronic acid nanocomposite: a cartilage-mimicking in situ-forming hydrogel with fine-tuned properties, Int. J. Biol. Macromol., 2024, 266, 131051, DOI:10.1016/j.ijbiomac.2024.131051.
- S. Tharakan, S. Khondkar, S. Lee, S. Ahn, C. Mathew and A. Gresita, et al., 3D printed osteoblast-alginate/collagen hydrogels promote survival, proliferation and mineralization at low doses of strontium calcium polyphosphate, Pharmaceutics, 2022, 15, 11, DOI:10.3390/pharmaceutics15010011.
- F. Mirab, Y. Wang, H. Farhadi and S. Majd, Preparation of gel-liposome nanoparticles for drug delivery applications, Annu Int. Conf. IEEE Eng. Med. Biol. Soc., 2019, 2019, 3935–3938, DOI:10.1109/EMBC.2019.8856639.
- J. M. Unagolla, B. Gaihre and A. C. Jayasuriya, In vitro and in vivo evaluation of 3D printed poly(ethylene glycol) dimethacrylate-based photocurable hydrogel platform for bone tissue engineering, Macromol. Biosci., 2024, 24, e2300414, DOI:10.1002/mabi.202300414.
- S. Van Belleghem, B. Mahadik, K. Snodderly, Z. Mote, B. Jiang and J. R. Yu, et al., Dual extrusion patterning drives tissue development aesthetics and shape retention in 3D printed nipple-areola constructs, Adv. Healthc. Mater., 2021, 10, e2101249, DOI:10.1002/adhm.202101249.
- N. Moghimi, M. Kamaraj, F. Zehtabi, S. Amin Yavari, M. Kohandel and A. Khademhosseini, et al., Development of bioactive short fiber-reinforced printable hydrogels with tunable mechanical and osteogenic properties for bone repair, J. Mater. Chem. B, 2024, 12, 2818–2830, 10.1039/d3tb02924g.
- A. Asti and L. Gioglio, Natural and synthetic biodegradable polymers: different scaffolds for cell expansion and tissue formation, Int. J. Artif. Organs., 2014, 37, 187–205, DOI:10.5301/ijao.5000307.
- G. Rubí-Sans, A. Nyga, E. Rebollo, S. Pérez-Amodio, J. Otero and D. Navajas, et al., Development of cell-derived matrices for three-dimensional in vitro cancer cell models, ACS Appl. Mater. Interfaces, 2021, 13, 44108–44123, DOI:10.1021/acsami.1c13630.
- S. J. Lin, Y. C. Chan, Z. C. Su, W. L. Yeh, P. L. Lai and I. M. Chu, Growth factor-loaded microspheres in mPEG-polypeptide hydrogel system for articular cartilage repair, J. Biomed. Mater Res. A, 2021, 109, 2516–2526, DOI:10.1002/jbm.a.37246.
- H. Qian, T. Lei, L. Hua, Y. Zhang, D. Wang and J. Nan, et al., Fabrication, bacteriostasis and osteointegration properties researches of the additively-manufactured porous tantalum scaffolds loading vancomycin, Bioact. Mater., 2023, 24, 450–462, DOI:10.1016/j.bioactmat.2022.12.013.
- M. G. L. Olthof, D. H. R. Kempen, X. Liu, M. Dadsetan, M. A. Tryfonidou and M. J. Yaszemski, et al., Bone morphogenetic protein-2 release profile modulates bone formation in phosphorylated hydrogel, J. Tissue Eng. Regen. Med., 2018, 12, 1339–1351, DOI:10.1002/term.2664.
- H. N. Tran, I. G. Kim, J. H. Kim, A. Bhattacharyya, E. J. Chung and I. Noh, Incorporation of cell-adhesive proteins in 3d-printed lipoic acid-maleic acid-poly(propylene glycol)-based tough gel ink for cell-supportive microenvironment, Macromol. Biosci., 2023, 23, e2300316, DOI:10.1002/mabi.202300316.
- H. N. Tran, I. G. Kim, J. H. Kim, E. J. Chung and I. Noh, Control of maleic acid-propylene diepoxide hydrogel for 3D printing application for flexible tissue engineering scaffold with high resolution by end capping and graft polymerization, Biomater. Res., 2022, 26, 75, DOI:10.1186/s40824-022-00318-x.
- Y. Song, Q. Hu, S. Liu, Y. Wang, H. Zhang and J. Chen, et al., Electrospinning/3D printing drug-loaded antibacterial polycaprolactone nanofiber/sodium alginate-gelatin hydrogel bilayer scaffold for skin wound repair, Int. J. Biol. Macromol., 2024, 129705, DOI:10.1016/j.ijbiomac.2024.129705.
- Z. Yao, Y. Qian, Y. Jin, S. Wang, J. Li and W. E. Yuan, et al., Biomimetic multilayer polycaprolactone/sodium alginate hydrogel scaffolds loaded with melatonin facilitate tendon regeneration, Carbohydr. Polym., 2022, 277, 118865, DOI:10.1016/j.carbpol.2021.118865.
- L. Hao, Z. Tianyuan, Y. Zhen, C. Fuyang, W. Jiang and Y. Zineng, et al., Biofabrication of cell-free dual drug-releasing biomimetic scaffolds for meniscal regeneration, Biofabrication, 2021, 14, 015001, DOI:10.1088/1758-5090/ac2cd7.
- D. Seliktar, Designing cell-compatible hydrogels for biomedical applications, Science, 2012, 336, 1124–1128, DOI:10.1126/science.1214804.
- S. M. Bai, X. L. Zhang, X. L. Lv, M. Y. Zhang, X. W. Huang and Y. Shi, et al., Bioinspired mineral-organic bone adhesives for stable fracture fixation and accelerated bone regeneration, Adv. Funct. Mater., 2020, 30, 1908381, DOI:10.1002/adfm.201908381.
- S. Bi, P. Wang, S. Hu, S. Li, J. Pang and Z. Zhou, et al., Construction of physical-crosslink chitosan/PVA double-network hydrogel with surface mineralization for bone repair, Carbohydr. Polym., 2019, 224, 115176, DOI:10.1016/j.carbpol.2019.115176.
- S. H. Lee, Y. Lee, Y. W. Chun, S. W. Crowder, P. P. Young and K. D. Park, et al., In situ crosslinkable gelatin hydrogels for vasculogenic induction and delivery of mesenchymal stem cells, Adv. Funct. Mater., 2014, 24, 6771–6781, DOI:10.1002/adfm.201401110.
- B. Zhou, X. Jiang, X. Zhou, W. Tan, H. Luo and S. Lei, et al., GelMA-based bioactive hydrogel scaffolds with multiple bone defect repair functions: therapeutic strategies and recent advances, Biomater. Res., 2023, 27, 86, DOI:10.1186/s40824-023-00422-6.
- W. Lu, M. Zeng, W. Liu, T. Ma, X. Fan and H. Li, et al., Human urine-derived stem cell exosomes delivered via injectable GelMA templated hydrogel accelerate bone regeneration, Mater. Today Bio, 2023, 19, 100569, DOI:10.1016/j.mtbio.2023.100569.
- P. Horcajada, T. Chalati, C. Serre, B. Gillet, C. Sebrie and T. Baati, et al., Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging, Nat. Mater., 2010, 9, 172–178, DOI:10.1038/nmat2608.
- H. Yi, F. Ur Rehman, C. Zhao, B. Liu and N. He, Recent advances in nano scaffolds for bone repair, Bone Res., 2016, 4, 16050, DOI:10.1038/boneres.2016.50.
- D. M. Huang, J. K. Hsiao, Y. C. Chen, L. Y. Chien, M. Yao and Y. K. Chen, et al., The promotion of human mesenchymal stem cell proliferation by superparamagnetic iron
oxide nanoparticles, Biomaterials, 2009, 30, 3645–3651, DOI:10.1016/j.biomaterials.2009.03.032.
- M. Xavier, M. E. Kyriazi, S. Lanham, K. Alexaki, E. Matthews and A. H. El-Sagheer, et al., Enrichment of skeletal stem cells from human bone marrow using spherical nucleic acids, ACS Nano, 2021, 15, 6909–6916, DOI:10.1021/acsnano.0c10683.
- Y. Wang, H. Q. Lv, X. Chao, W. X. Xu, Y. Liu and G. X. Ling, et al., Multimodal therapy strategies based on hydrogels for the repair of spinal cord injury, Mil. Med. Res., 2022, 9, 16, DOI:10.1186/s40779-022-00376-1.
- H. L. Tan, S. Y. Teow and J. Pushpamalar, Application of metal nanoparticle-hydrogel composites in tissue regeneration, Bioengineering, 2019, 6, 17, DOI:10.3390/bioengineering6010017.
- S. J. Tang, Y. Yan, X. L. Lu, P. Wang, X. Q. Xu and K. Hu, et al., Nanocomposite magnetic hydrogel with dual anisotropic properties induces osteogenesis through the NOTCH-dependent pathways, NPG Asia Mater., 2024, 16, 16, DOI:10.1038/s41427-024-00535-x.
- M. H. Kim, B. S. Kim, J. Lee, D. Cho, O. H. Kwon and W. H. Park, Silk fibroin/hydroxyapatite composite hydrogel induced by gamma-ray irradiation for bone tissue engineering, Biomater. Res., 2017, 21, 12, DOI:10.1186/s40824-017-0098-2.
- Y. Mao, Y. Zhang, Y. Wang, T. Zhou, B. Ma and P. Zhou, A multifunctional nanocomposite hydrogel with controllable release behavior enhances bone regeneration, Regen. Biomater., 2023, 10, rbad046, DOI:10.1093/rb/rbad046.
- J. Prasek, J. Chomoucka, J. Hubalek, O. Jasek, V. Adamc and R. Kizek, Methods for carbon nanotubes synthesis—review, J. Mater. Chem., 2011, 21, 15872–15884, 10.1039/c1jm12254a.
- S. Bashir, I. M. Almanjahie, M. Ramzan, A. N. Cheema, M. Akhtar and F. Alshahrani, Impact of induced magnetic field on Darcy-Forchheimer nanofluid flows comprising carbon nanotubes with homogeneous-heterogeneous reactions, Heliyon, 2024, 10, e24718, DOI:10.1016/j.heliyon.2024.e24718.
- S. Same, G. Navidi, G. Samee, F. Abedi, M. Aghazadeh and M. Milani, et al., Gentamycin-loaded halloysite-based hydrogel nanocomposites for bone tissue regeneration: fabrication, evaluation of the antibacterial activity and cell response, Biomed. Mater., 2022, 17, 065018, DOI:10.1088/1748-605X/ac94ad.
- A. Pietraszek, G. Ledwójcik, J. Lewandowska-Łańcucka, W. Horak, R. Lach, A. Łatkiewicz and A. Karewicz, Bioactive hydrogel scaffolds reinforced with alkaline-phosphatase containing halloysite nanotubes for bone repair applications, Int. J. Biol. Macromol., 2020, 163, 1187–1195, DOI:10.1016/j.ijbiomac.2020.07.045.
- Y. Wang, H. Q. Lv, X. Chao, W. X. Xu, Y. Liu and G. X. Ling, et al., Multimodal therapy strategies based on hydrogels for the repair of spinal cord injury, Mil. Med. Res., 2022, 9, 16, DOI:10.1186/s40779-022-00376-1.
- F. Kazemi-Aghdam, V. Jahed, M. Dehghan-Niri, F. Ganji and E. Vasheghani-Farahani, Injectable chitosan hydrogel embedding modified halloysite nanotubes for bone tissue engineering, Carbohydr. Polym., 2021, 269, 118311, DOI:10.1016/j.carbpol.2021.118311.
- S. Same, J. Kadkhoda, G. Navidi, F. Abedi, M. Aghazadeh and M. Milani, et al., The fabrication of halloysite nanotube-based multicomponent hydrogel scaffolds for bone healing, J. Appl. Biomater. Funct. Mater., 2022, 20, 22808000221111875, DOI:10.1177/22808000221111875.
- H. Ravanbakhsh, G. Bao, N. Latifi and L. G. Mongeau, Carbon nanotube composite hydrogels for vocal fold tissue engineering: biocompatibility, rheology, and porosity, Mater. Sci. Eng., C, 2019, 103, 109861, DOI:10.1016/j.msec.2019.109861.
- X. Zhao, Y. Zhou, J. Li, C. Zhang and J. Wang, Opportunities and challenges of hydrogel microspheres for tendon-bone healing after anterior cruciate ligament reconstruction, J. Biomed. Mater. Res., Part B, 2022, 110, 289–301, DOI:10.1002/jbm.b.34925.
- Q. He, J. Zhang, Y. Liao, E. V. Alakpa, V. Bunpetch and J. Zhang, et al., Current advances in microsphere based cell culture and tissue engineering, Biotechnol. Adv., 2020, 39, 107459, DOI:10.1016/j.biotechadv.2019.107459.
- M. Ciocci, I. Cacciotti, D. Seliktar and S. Melino, Injectable silk fibroin hydrogels functionalized with microspheres as adult stem cells-carrier systems, Int. J. Biol. Macromol., 2018, 108, 960–971, DOI:10.1016/j.ijbiomac.2017.11.013.
- Z. Yuan, X. Yuan, Y. Zhao, Q. Cai, Y. Wang and R. Luo, et al., Injectable GelMA cryogel microspheres for modularized cell delivery and potential vascularized bone regeneration, Small, 2021, 17, e2006596, DOI:10.1002/smll.202006596.
- L. P. Ferreira, V. M. Gaspar and J. F. Mano, Design of spherically structured 3D in vitro tumor models-advances and prospects, Acta Biomater., 2018, 75, 11–34, DOI:10.1016/j.actbio.2018.05.034.
- P. Qi, R. Bu, H. Zhang, J. Yin, J. Chen and A. Zhang, et al., Goserelin acetate loaded poloxamer hydrogel in PLGA microspheres: core-shell di-depot intramuscular sustained release delivery system, Mol. Pharm., 2019, 16, 3502–3513, DOI:10.1021/acs.molpharmaceut.9b00344.
- X. Ji, H. Shao, X. Li, M. W. Ullah, G. Luo and Z. Xu, et al., Injectable immunomodulation-based porous chitosan microspheres/HPCH hydrogel composites as a controlled drug delivery system for osteochondral regeneration, Biomaterials, 2022, 285, 121530, DOI:10.1016/j.biomaterials.2022.121530.
- S. Zhang, A. Lin, Z. Tao, Y. Fu, L. Xiao and G. Ruan, et al., Microsphere-containing hydrogel scaffolds for tissue engineering, Chem.–Asian J., 2022, 17, e202200630, DOI:10.1002/asia.202200630.
- C. Li, G. Chen, Y. Wang, W. Xu and M. Hu, Indirect co-culture of osteoblasts and endothelial cells in vitro based on a biomimetic 3D composite hydrogel scaffold to promote the proliferation and differentiation of osteoblasts, PLoS One, 2024, 19, e0298689, DOI:10.1371/journal.pone.0298689.
- P. Yin, W. Su, T. Li, L. Wang, J. Pan and X. Wu, et al., A modular hydrogel bioink containing microsphere-embedded chondrocytes for 3D-printed multiscale composite scaffolds for cartilage repair, iScience, 2023, 26, 107349, DOI:10.1016/j.isci.2023.107349.
- M. Amiri, P. Khazaeli, A. Salehabadi and M. Salavati-Niasari, Hydrogel beads-based nanocomposites in novel drug delivery platforms: recent trends and developments, Adv. Colloid Interface Sci., 2021, 288, 102316, DOI:10.1016/j.cis.2020.102316.
- M. Zhang and X. Zhao, Alginate hydrogel dressings for advanced wound management, Int. J. Biol. Macromol., 2020, 162, 1414–1428, DOI:10.1016/j.ijbiomac.2020.07.311.
- H. K. Lau and K. L. Kiick, Opportunities for multicomponent hybrid hydrogels in biomedical applications, Biomacromolecules, 2015, 16, 28–42, DOI:10.1021/bm501361c.
- Z. Tong, L. Jin, J. M. Oliveira, R. L. Reis, Q. Zhong and Z. Mao, et al., Adaptable hydrogel with reversible linkages for regenerative medicine: dynamic mechanical microenvironment for cells, Bioact. Mater, 2020, 6, 1375–1387, DOI:10.1016/j.bioactmat.2020.10.029.
- D. M. Radulescu, I. A. Neacsu, A. M. Grumezescu and E. Andronescu, New insights of scaffolds based on hydrogels in tissue engineering, Polymers, 2022, 14, 799, DOI:10.3390/polym14040799.
- L. Nie, Q. Wu, H. Long, K. Hu, P. Li and C. Wang, et al., Development of chitosan/gelatin hydrogels incorporation of biphasic calcium phosphate nanoparticles for bone tissue engineering, J. Biomater. Sci. Polym. Ed., 2019, 30, 1636–1657, DOI:10.1080/09205063.2019.1654210.
- H. Wang, R. Yin, X. Chen, T. Wu, Y. Bu and H. Yan, et al., Construction and evaluation of alginate dialdehyde grafted RGD derivatives/polyvinyl alcohol/cellulose nanocrystals IPN composite hydrogels, Molecules, 2023, 28, 6692, DOI:10.3390/molecules28186692.
- S. Bashir, M. Hina, J. Iqbal, A. H. Rajpar, M. A. Mujtaba and N. A. Alghamdi, et al., Fundamental concepts of hydrogels: synthesis, properties, and their applications, Polymers, 2020, 12, 2702, DOI:10.3390/polym12112702.
- W. Hu, Z. Wang, Y. Xiao, S. Zhang and J. Wang, Advances in crosslinking strategies of biomedical hydrogels, Biomater. Sci., 2019, 7, 843–855, 10.1039/c8bm01246f.
- E. Tanasa, C. Zaharia, I. C. Radu, V. A. Surdu, B. S. Vasile and C. M. Damian, et al., Novel nanocomposites based on functionalized magnetic nanoparticles and polyacrylamide: preparation and complex characterization, Nanomaterials, 2019, 9, 1384, DOI:10.3390/nano9101384.
- N. Amiryaghoubi, M. Fathi, A. Safary, Y. Javadzadeh and Y. Omidi, In situ forming alginate/gelatin hydrogel scaffold through Schiff base reaction embedded with curcumin-loaded chitosan microspheres for bone tissue regeneration, Int. J. Biol. Macromol., 2024, 256, 128335, DOI:10.1016/j.ijbiomac.2023.128335.
- N. A. N. Hanafy, S. Leporatti and M. El-Kemary, Mucoadhesive curcumin crosslinked carboxy methyl cellulose might increase inhibitory efficiency for liver cancer treatment, Mater Sci. Eng., C, 2020, 116, 111119, DOI:10.1016/j.msec.2020.111119.
- X. R. Zhao, X. F. Nie, X. P. Zhang, Y. G. Sun, R. Yang and X. Y. Bian, et al., 3D printed β-sheet-reinforced natural polymer hydrogel bilayer tissue engineering scaffold, Sci. China: Technol. Sci., 2024, 67, 1170–1184, DOI:10.1007/s11431-023-2471-0.
- B. B. Rothrauff, L. Coluccino, R. Gottardi, L. Ceseracciu, S. Scaglione and L. Goldoni, et al., Efficacy of thermoresponsive, photocrosslinkable hydrogels derived from decellularized tendon and cartilage extracellular matrix for cartilage tissue engineering, J. Tissue Eng. Regen. Med., 2018, 12, e159–e170, DOI:10.1002/term.2465.
- S. L. Fenn and R. A. Oldinski, Visible light crosslinking of methacrylated hyaluronan hydrogels for injectable tissue repair, J. Biomed. Mater. Res., Part B, 2016, 104, 1229–1236, DOI:10.1002/jbm.b.33476.
- S. Ibusuki, G. J. Halbesma, M. A. Randolph, R. W. Redmond, I. E. Kochevar and T. J. Gill, Photochemically cross-linked collagen gels as three-dimensional scaffolds for tissue engineering, Tissue Eng., 2007, 13, 1995–2001, DOI:10.1089/ten.2006.0153.
- J. R. Choi, K. W. Yong, J. Y. Choi and A. C. Cowie, Recent advances in photo-crosslinkable hydrogels for biomedical applications, Biotechniques, 2019, 66, 40–53, DOI:10.2144/btn-2018-0083.
- P. Nezhad-Mokhtari, M. Ghorbani, L. Roshangar and J. Soleimani Rad, Chemical gelling of hydrogels-based biological macromolecules for tissue engineering: photo- and enzymatic-crosslinking methods, Int. J. Biol. Macromol., 2019, 139, 760–772, DOI:10.1016/j.ijbiomac.2019.08.047.
- K. Paek, S. Woo, S. J. Song, M. K. Kim, K. Yi and S. Chung, et al., A well plate-based GelMA photo-crosslinking system with tunable hydrogel mechanical properties to regulate the PTH-mediated osteogenic fate, Biofabrication, 2024, 16, DOI:10.1088/1758-5090/ad2a7e.
- S. Kumari, P. Mondal, S. Tyeb and K. Chatterjee, Visible light-based 3D bioprinted composite scaffolds of κ-carrageenan for bone tissue engineering applications, J. Mater. Chem. B, 2024, 12, 1926–1936, 10.1039/d3tb02179c.
- G. Yang, X. Chen, W. Shi, N. Chen, Y. Liu and B. Zhang, et al., Facile preparation of a photo-cross-linked silk fibroin-poly ionic liquid hydrogel with antifreezing and ion conductive properties, ACS Appl. Mater. Interfaces, 2024, 16, 1543–1552, DOI:10.1021/acsami.3c15712.
- T. Mehta, H. Aziz, K. Sen, S. Y. Chang, V. Nagarajan and A. W. K. Ma, et al., Numerical study of drop dynamics for inkjet based 3D printing of pharmaceutical tablets, Int. J. Pharm., 2024, 656, 124037, DOI:10.1016/j.ijpharm.2024.124037.
- Y. Li, X. Ren, L. Zhu and C. Li, Biomass 3D printing: principles, materials, post-processing and applications, Polymers, 2023, 15, 2692, DOI:10.3390/polym15122692.
- J. M. McCracken, B. M. Rauzan, J. C. E. Kjellman, M. E. Kandel, Y. H. Liu and A. Badea, et al., 3D-printed hydrogel composites for predictive temporal (4D) cellular organizations and patterned biogenic mineralization, Adv. Healthc. Mater., 2019, 8, e1800788, DOI:10.1002/adhm.201800788.
- A. Badea, J. M. McCracken, E. G. Tillmaand, M. E. Kandel, A. W. Oraham and M. B. Mevis, et al., 3D-printed pHEMA materials for topographical and biochemical modulation of dorsal root ganglion cell response, ACS Appl. Mater. Interfaces, 2017, 9, 30318–30328, DOI:10.1021/acsami.7b06742.
- A. Sadeghianmaryan, N. Ahmadian, S. Wheatley, H. Alizadeh Sardroud, S. A. S. Nasrollah and E. Naseri, et al., Advancements in 3D-printable polysaccharides, proteins, and synthetic polymers for wound dressing and skin scaffolding – a review, Int. J. Biol. Macromol., 2024, 266, 131207, DOI:10.1016/j.ijbiomac.2024.131207.
- R. Cornock, S. Beirne, B. Thompson and G. G. Wallace, Coaxial additive manufacture of biomaterial composite scaffolds for tissue engineering, Biofabrication, 2014, 6, 025002, DOI:10.1088/1758-5082/6/2/025002.
- C. Pasini, S. Pandini, G. Ramorino and L. Sartore, Tailoring the properties of composite scaffolds with a 3D-printed lattice core and a bioactive hydrogel shell for tissue engineering, J. Mech. Behav. Biomed. Mater., 2024, 150, 106305, DOI:10.1016/j.jmbbm.2023.106305.
- L. Ferroni, U. D'Amora, S. Leo, E. Tremoli, M. G. Raucci and A. Ronca, et al., PEEK and hyaluronan-based 3D printed structures: promising combination to improve bone regeneration, Molecules, 2022, 27, 8749, DOI:10.3390/molecules27248749.
- Y. H. Hsieh, M. F. Hsieh, C. H. Fang, C. P. Jiang, B. Lin and H. M. Lee, Osteochondral regeneration induced by TGF-β loaded photo cross-linked hyaluronic acid hydrogel infiltrated in fused deposition-manufactured composite scaffold of hydroxyapatite and poly(ethylene glycol)-block-poly(ε-caprolactone), Polymers, 2017, 9, 182, DOI:10.3390/polym9050182.
- K. Khoshmaram, F. Yazdian, Z. Pazhouhnia and N. Lotfibakhshaiesh, Preparation and characterization of 3D bioprinted gelatin methacrylate hydrogel incorporated with curcumin loaded chitosan nanoparticles for in vivo wound healing application, Biomater. Adv., 2024, 156, 213677, DOI:10.1016/j.bioadv.2023.213677.
- K. H. Schneider, B. J. Goldberg, O. Hasturk, X. Mu, M. Dötzlhofer and G. Eder, et al., Silk fibroin, gelatin, and human placenta extracellular matrix-based composite hydrogels for 3D bioprinting and soft tissue engineering, Biomater. Res., 2023, 27, 117, DOI:10.1186/s40824-023-00431-5.
- Y. Zheng, H. Zhang, Z. Wang, A. Lu, A. Yu and B. Duan, Chitin nanofibrils assisted 3D printing all-chitin hydrogels for wound dressing, Carbohydr. Polym., 2024, 334, 122028, DOI:10.1016/j.carbpol.2024.122028.
- Y. Jiang, D. Zhou and Y. Jiang, Three-dimensional bioprinted GelMA/GO composite hydrogel for stem cell osteogenic differentiation both in vitro and in vivo, J. Biomater. Appl., 2024, 38, 1087–1099, DOI:10.1177/08853282241243337.
- S. Li, Z. Liu, X. Gao, L. Cheng, Z. Xu and L. Li, et al., Preparation and properties of a 3D printed nHA/PLA bone tissue engineering scaffold loaded with a β-CD-CHX combined dECM hydrogel, RSC Adv., 2024, 14, 9848–9859, 10.1039/d4ra00261j.
- M. Bartnikowski, A. R. Akkineni, M. Gelinsky, M. A. Woodruff and T. J. Klein, A hydrogel model incorporating 3D-plotted hydroxyapatite for osteochondral tissue engineering, Materials, 2016, 9, 285, DOI:10.3390/ma9040285.
- T. Billiet, M. Vandenhaute, J. Schelfhout, S. Van Vlierberghe and P. Dubruel, A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering, Biomaterials, 2012, 33, 6020–6041, DOI:10.1016/j.biomaterials.2012.04.050.
- X. Wang, M. Jiang, Z. W. Zhou, J. H. Gou and D. Hui, 3D printing of polymer matrix composites: a review and prospective, Composites, Part B, 2017, 110, 442–458, DOI:10.1016/j.compositesb.2016.11.034.
- G. Spahn, J. Fritz, D. Albrecht, G. O. Hofmann and P. Niemeyer, Characteristics and associated factors of Klee cartilage lesions: preliminary baseline-data of more than 1000 patients from the German cartilage registry (KnorpelRegister DGOU), Arch. Orthop. Trauma. Surg., 2016, 136, 805–810, DOI:10.1007/s00402-016-2432-x.
- H. Dashtdar, M. R. Murali, A. A. Abbas, A. M. Suhaeb, L. Selvaratnam and L. X. Tay, et al., PVA-chitosan composite hydrogel versus alginate beads as a potential mesenchymal stem cell carrier for the treatment of focal cartilage defects, Knee Surg. Sports Traumatol. Arthrosc., 2015, 23, 1368–1377, DOI:10.1007/s00167-013-2723-5.
- S. Lu, J. Lam, J. E. Trachtenberg, E. J. Lee, H. Seyednejad and J. J. J. P. van den Beucken, et al., Dual growth factor delivery from bilayered, biodegradable hydrogel composites for spatially-guided osteochondral tissue repair, Biomaterials, 2014, 35, 8829–8839, DOI:10.1016/j.biomaterials.2014.07.006.
- M. Lind, A. Larsen, C. Clausen, K. Osther and H. Everland, Cartilage repair with chondrocytes in fibrin hydrogel and MPEG polylactide scaffold: an in vivo study in goats, Knee Surg. Sports Traumatol. Arthrosc., 2008, 16, 690–698, DOI:10.1007/s00167-008-0522-1.
- C. Liu, W. Qin, Y. Wang, J. Ma, J. Liu and S. Wu, et al., 3D printed gelatin/sodium alginate hydrogel scaffolds doped with nano-attapulgite for bone tissue repair, Int. J. Nanomed., 2021, 16, 8417–8432, DOI:10.2147/IJN.S339500.
- J. Li, F. Zhang, X. Ga, G. Gao and T. Guo, Total meniscus replacement with a 3D printing of network hydrogel composite scaffold in a rabbit model, Knee Surg. Sports Traumatol. Arthrosc., 2024, 32, 1187–1198, DOI:10.1002/ksa.12139.
- G. Baysan, O. Colpankan Gunes, P. Akokay, R. B. Husemoglu, P. Ertugruloglu and A. Ziylan Albayrak, et al., Loofah-chitosan and poly(-3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) based hydrogel scaffolds for meniscus tissue engineering applications, Int. J. Biol. Macromol., 2022, 221, 1171–1183, DOI:10.1016/j.ijbiomac.2022.09.031.
- O. C. Gunes, A. Kara, G. Baysan, R. Bugra Husemoglu, P. Akokay and A. Ziylan Albayrak, et al., Fabrication of 3D Printed poly(lactic acid) strut and wet-electrospun cellulose nano fiber reinforced chitosan-collagen hydrogel composite scaffolds for meniscus tissue engineering, J. Biomater. Appl., 2022, 37, 683–697, DOI:10.1177/08853282221109339.
- J. Lv, Y. Wu, Z. Cao, X. Liu, Y. Sun and P. Zhang, et al., Enhanced cartilage and subchondral bone repair using carbon nanotube-doped peptide hydrogel-polycaprolactone composite scaffolds, Pharmaceutics, 2023, 15, 2145, DOI:10.3390/pharmaceutics15082145.
- S. Long, D. Huang, Z. Ma, S. Shi, Y. Xiao and X. Zhang, A sonication-induced silk-collagen hydrogel for functional cartilage regeneration, J. Mater. Chem. B, 2022, 10, 5045–5057, 10.1039/d2tb00564f.
- X. Wu, M. Zhou, F. Jiang, S. Yin, S. Lin and G. Yang, et al., Marginal sealing around integral bilayer scaffolds for repairing osteochondral defects based on photocurable silk hydrogels, Bioact. Mater., 2021, 6, 3976–3986, DOI:10.1016/j.bioactmat.2021.04.005.
- Y. Li, Y. Liu and Q. Guo, Silk fibroin hydrogel scaffolds incorporated with chitosan nanoparticles repair articular cartilage defects by regulating TGF-β1 and BMP-2, Arthritis Res. Ther., 2021, 23, 50, DOI:10.1186/s13075-020-02382-x.
- Y. H. Hsieh, B. Y. Shen, Y. H. Wang, B. Lin, H. M. Lee and M. F. Hsieh, Healing of osteochondral defects implanted with biomimetic scaffolds of poly(ε-caprolactone)/hydroxyapatite and glycidyl-methacrylate-modified hyaluronic acid in a minipig, Int. J. Mol. Sci., 2018, 19, 1125, DOI:10.3390/ijms19041125.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *
*












