Open Access Article
Open Access ArticleGreen and sustainable synthesis of chiral alcohols: the role of Daucus carota as a biocatalyst in organic chemistry
Azmat Ullah Khan,
Muhammad Shahzad,
Alia Mushtaq and
Muhammad Moazzam Naseer *
*
Department of Chemistry, Quaid-i-Azam University, Islamabad, 45320, Pakistan. E-mail: moazzam@qau.edu.pk
First published on 15th April 2025
Abstract
Chiral alcohols are essential intermediates in pharmaceuticals, agrochemicals, and advanced materials. Conventional asymmetric reduction of ketones relies on costly metal catalysts with significant environmental impact. Biocatalysis, particularly whole-cell systems, offers a sustainable alternative, providing high regio- and stereoselectivity under mild conditions. Daucus carota (carrot) roots serve as a promising biocatalyst due to their broad substrate compatibility and natural cofactor recycling ability, reducing reliance on toxic reagents and energy-intensive processes, making them both environmentally sustainable and economically viable. This review highlights the potential of D. carota for chiral alcohol synthesis while addressing challenges such as long reaction times, high biocatalyst requirements, and substrate limitations. Ongoing research focuses on optimizing reaction conditions, testing different carrot varieties, and incorporating additives to enhance efficiency and expand applicability.
1 Introduction
Chiral alcohols1 are key intermediates in synthesizing chiral auxiliaries, pharmaceuticals, agrochemicals, and advanced materials like liquid crystals.2 Chiral alcohols can be synthesized from ketones through asymmetric reduction, which can be achieved using various methods. Conventional approaches include catalysis with metal hydrides and chiral ligands,3,4 chiral chromatography for separation,5 or the use of chiral metal complexes for asymmetric reduction of prochiral compounds.6 However, these traditional methods have considerable disadvantages, such as operational complexities, unwanted byproducts, high costs, and environmental hazards.7The shift towards a bio-based economy has amplified interest in biocatalysis, making green chemistry increasingly significant. Biocatalytic reactions are highly efficient and carried out under very mild conditions, often solvent-free or carried out using water only. These conditions simplify purification and result in high regio- and stereoselectivity. These advantages highlight that biocatalysis is considered a greener alternative to traditional chemical methods, providing a more environmentally sustainable approach to organic synthesis.8–10
Baker's yeast (BY) is a widely used biocatalyst for the stereoselective reduction of ketones and ketoesters to chiral secondary alcohols.11 Baker's yeast is useful in chiral reductions, but it has certain limitations. It leads to the generation of a mixture of enantiomers, making it challenging to get the required enantiopure product. Reductions in Baker's yeast are enzyme-dependent, requiring costly cofactors like NADH or NADPH, which necessitate the regeneration of oxidized cofactors to maintain the enzyme activity.12
Plant tissues have gained attention due to their inherent stereoselectivity, mild reaction conditions, and cost-effectiveness. Compared to microbial biocatalysts such as baker's yeast, plant-based biocatalysts offer simpler handling and lower operational costs, making them attractive for large-scale applications. D. carota (carrot) has been extensively studied for its ability to catalyze the enantioselective reduction of prochiral ketones, attributed to its rich enzymatic content, particularly alcohol dehydrogenases (ADHs).13,14 Compared to other plant sources such as potato (Solanum tuberosum), apple (Malus pumila), and radish (Raphanus sativus), D. carota demonstrates superior enantioselectivity and broader functional group tolerance, making it an efficient catalyst for bioreduction applications.15 This study explores the efficiency, stereoselectivity, and substrate specificity of D. carota, shedding light on its potential as a sustainable biocatalyst for producing enantiopure alcohols. The discussion encompasses its advantages over conventional chemical methods, its application in organic synthesis, and strategies to overcome existing challenges to enhance its industrial viability.
In the enantioselective bioreduction of prochiral ketones, oxidoreductases such as alcohol dehydrogenase (ADH)16,17 play a key role in the transfer of hydride ions from NADH to the carbonyl group. Many plants were used in screening ADH activity, including celeriac (Apium graveolens), horseradish (Armoracia lapathifolia),18 and arracacha roots (Arracacia xanthorrhiza).19 Among them, D. carota (carrot) roots have shown a broad substrate scope and the highest enantioselectivity, making them particularly effective for biocatalytic processes.20 Biocatalysis in synthesizing chiral secondary alcohols can be accomplished using either isolated enzymes or whole-cell microorganisms and plants.21
Whole-cell biocatalysts,22 especially those using D. carota, offer distinct advantages over isolated enzymes. In whole cells, the enzyme and its necessary cofactors are naturally encapsulated within a microenvironment, that can perform biocatalytic action without the addition of costly external cofactors like NADH or NADPH.23 This makes whole plant cells an economically attractive option because these are economical, biodegradable, and can under mild conditions.24 Besides this, the use of comminuted (finely chopped) plant material for the preparation of active systems is quite simple. In addition to finely chopping D. carota (carrot) roots to increase surface area for biocatalytic applications, several alternative preparation techniques can enhance efficiency. Selecting specific carrot varieties, such as purple carrots, has been shown to improve biocatalytic performance in enantioselective reductions.25 The addition of surfactants like Tween® 20 can enhance enzyme accessibility and substrate solubility, leading to increased reaction rates.26 Biphasic systems, which improve substrate availability and product extraction, offer another effective approach to boost productivity in carrot-catalyzed reductions.27 By optimizing these techniques, the effectiveness of carrot-based biocatalyst systems can be improved for various bioreduction applications. D. carota roots as a whole-cell biocatalyst offer a cost-effective, environment friendly, and efficient pathway for synthesizing enantiopure chiral secondary alcohols.24,28
2 Stereospecificity in reduction via D. carota
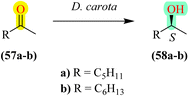
Highlighting sustainability, D. carota is one of the efficient biocatalysts for the enantioselective reduction of ketones, yielding corresponding chiral alcohols. Even though it has many enzymes, polyphenols, and carotenoids, the main enzymes responsible for bringing about these enantioselective reductions in prochiral ketones are the carbonyl reductases and dehydrogenases.29 The reducing ability of dehydrogenase30 primarily depends upon cofactors such as NADH or NADPH with which it binds. These cofactors, along with dehydrogenase, provide hydride ions (H−) to the carbonyl group, facilitating its reduction. The stereochemistry of the resulting alcohol depends on which face (Re or Si) of the nicotinamide ring approaches the carbonyl group. In D. carota, dehydrogenase donates hydride ions to the Re face of ketones, consistent with Prelog's rule, leading to the alcohol with the S configuration31 (Fig. 1).3 D. carota as a biocatalyst in organic synthesis
D. carota (carrot) has emerged as a very important biocatalyst in organic synthesis as a natural reducing agent with a lot of enzymes like reductases and dehydrogenases for the reduction of carbonyl compounds to optically active chiral alcohols.323.1 Reduction of ketones in the total synthesis of natural products
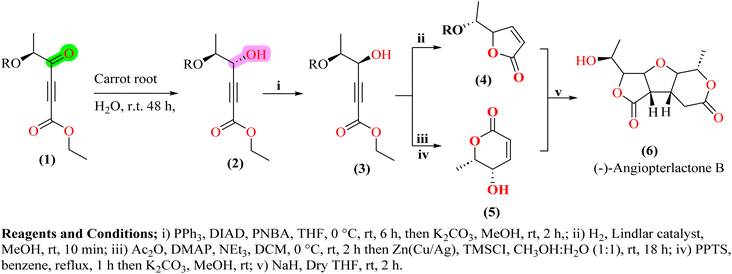
Bioreduction of ketones to chiral alcohols is an important method of natural product synthesis in biocatalysis. It offers high enantioselectivity, efficiency, and environment-friendly conditions.33 Chiral alcohols are the key intermediate or active functional groups of the bioactive molecule in natural product synthesis. It adds special selectivity to the synthesis of these compounds.34 Supporting this idea, Navnath B. Khomane35 and colleagues demonstrated the significant role of carrot roots in natural product synthesis, particularly in the enantioselective reduction of ketones. In their work, they utilized carrot roots as a natural hydrogen source to achieve the bioreduction of a propargylic ketone (1), a key intermediate in synthesizing the natural product angiopterlactone B (6). This biocatalytic process successfully generated a stereocenter with high enantioselectivity, yielding the alcohol (2) in the R configuration36 (Scheme 1).Talampanel (12) is an active antagonist (oral) of the AMPA (alpha amino-3-hydroxy-5-methyl-4-isoxazole propionate) glutamate receptor, whose overstimulation leads to excess Ca2+ influx, resulting in cell damage and death.37 It has been implicated in several neurological disorders, such as cerebral ischemia, epilepsy, ALS, and Parkinson's disease, and therefore, Talampanel (12) looks promising as a drug candidate. Anderson and coworkers38 used an environmentally safe synthesis for preparing optically pure Talampanel (12), but it had limitations due to microbiological constraints and toxicity issues affecting solution concentration. Addressing these challenges, Alvaro et al. reported the reduction of 3,4-methylenedioxy phenylacetone (8), a key intermediate in Talampanel (12) synthesis, using carrot bits in water as a green alternative39 (Scheme 2).
Many chiral amino aryl ethanols are recognized as valuable synthetic precursors for pharmaceutically important molecules. For example, (R)-Denopamine (19) contains a chiral hydroxyl group that acts as a selective β1-adrenergic receptor agonist for treating congestive heart failure.40 Similarly, the naturally occurring bioactive molecules (R)-Tembamide (18a), and (R)-Angeline (18b) have demonstrated significant hypoglycemic activity.41 While optically active (R)-Tembamide, (R)-Angeline, and (R)-Denopamine have been synthesized through various methods.42 These approaches often involve laborious chemical or biological procedures, expensive reagents, and multi-step processes that result in low yields. Overall, J. S. Yadav et al. reported an efficient synthesis of (R)-chiral azido alcohols (14) key intermediates for these molecules, from α-azido aryl ketones (13) using D. carota root in an aqueous medium43 (Scheme 3).
3.2 Bioreduction of ketones via D. carota
Considering the environmental sustainability, chiral alcohols have been synthesized via enantioselective reduction of ketones using biocatalysts. Additionally, several applications of chiral alcohols in pharmaceuticals, agrochemicals, and materials science make this procedure more effective.44(S)-(−)-1-(4-Chlorophenyl) ethanol46 (24) is an important intermediate in synthesizing antitumor drugs that are used to treat hyperproliferative conditions such as melanoma, prostate cancer, and breast cancer. It is also employed in the production of the antihistamine Clemastine. The compound (24) was synthesized using D. carota cells in a biphasic system with water and organic solvents like isooctane, acetonitrile, or 1,4-dioxane, which acted as exogenous reducing agents. However, the reduction rate was slower compared to when only water was used under similar conditions. The process was slow due to shifts in the lipophilic–hydrophilic balance and changes in the conformation of the globular enzyme involved in the reaction. Which affects both the activity and the enantioselectivity of the reduction process47 (Scheme 5).
Asymmetric synthesis of chiral alcohols has been widely adopted as a method by pharmaceutical, agrochemical, flavor, and pigment industries. Over the past 25 years, numerous methods for the asymmetric reduction of carbonyl groups have been developed, with biochemical approaches using higher plants gaining significant popularity.48 Six acetophenone derivatives (25a–c), including ortho-, meta-, and para-methoxy acetophenone, as well as ortho-, meta-, and para-bromoacetophenone, were utilized as substrates in biotransformation processes using comminuted D. carota roots. As Nakamura and Matsuda49 conducted similar studies, bromine-substituted acetophenone derivatives undergo reduction three times faster than methoxy derivatives. The highest enantiomeric excess was observed in meta-methoxy and ortho-bromo derivatives. The enantioselectivity and reduction efficiency depend primarily on the substituent's position. This enzymatic reaction (Scheme 6) predominantly followed a stereoselective pathway, resulting in the formation of (S)-alcohols (26a–f).50
The asymmetric reduction of ketones is of great biological importance because the chiral carbinol obtained from this reaction is a valuable precursor for synthesizing numerous bioactive compounds. This study explored the bioreduction of acetophenone (ACP) (27) to produce enantiomerically pure (S)-1-phenylethanol (28) and (R)-1-phenylethanol (28′) using freeze-dried carrots as a natural source of alcohol dehydrogenases (ADHs)51 (Scheme 7).
De Oliveira, C. D. S. et al.52 illustrated the bioreduction of azido acetophenones (29a–b) by employing D. carota roots as a biocatalyst, producing the corresponding chiral alcohols (30a–b) with S-configurations in good to excellent yield and excellent enantiomeric excess (Scheme 8).
A study explored how substituents on acetophenones (31a–e) would affect the rate and direction of bioreduction undertaken using cells of D. carota and Petroselinum crispum. The findings indicated that both the nature of the substituents and the choice of solvents significantly affect reaction rates and product yields. Electron-withdrawing groups like -Br and -NO2 increased the reaction rate and product yields, while the electron-donating group –OCH3 reduced them. Furthermore, reductions in isooctane showed significantly lower rates and yields compared to those in water53 (Scheme 9).
In recent years, asymmetric synthesis of chiral synthons has garnered significant attention due to their growing demand as precursors in drug and agrochemical development. While various chemical and biocatalytic reductions have been reported, challenges persist in achieving enantioselectivity. However, product recovery can be complex, and enzyme activity often requires costly cofactors (NADH, NADPH) with regeneration steps. In view of this consideration, the reduction of various prochiral ketones54 (33), such as cyclic ketones, acetophenones, β-ketoesters, azido ketones, and aliphatic ketones, was carried out using D. carota root, resulting in chiral intermediates for the synthesis of chiral drugs and agrochemicals12 (Scheme 10).
Chiral aryl vicinal diols55 with specific functional groups serve as the intermediates for synthesizing agrochemicals, pharmaceuticals, and pheromones. The biocatalytic reduction of carbonyl compounds into enantiopure secondary alcohols offers a highly chemo-, regio-, stereoselective, and non-toxic method for establishing chirality. In support of this, the asymmetric reduction of prochiral α-hydroxy aromatic ketones56 (35) was achieved using carrot (D. carota) cells, resulting in chiral aryl vicinal diols predominantly in the (R)-configuration. The reaction, carried out with small pieces of fresh carrot roots in phosphate buffer or distilled water, produced (R)-aryl vicinal diols (36) with good yields and excellent enantiomeric excess (ee) (Scheme 11).
Inspired by the remarkable selectivity demonstrated in reducing acetophenones and other ketones using D. carota (carrot) roots, Dina et al. extended this approach to reduce γ-nitroketones. Their goal was to find a more accessible and cost-effective alternative to the traditionally used baker's yeast, which often lacks enantioselectivity. By utilizing D. carota roots in an aqueous medium, a series of aromatic γ-nitroketones (37a–d) was successfully reduced to their corresponding (S)-alcohols, achieving enantiomeric excesses (ee) between 73% and 100%. This biocatalytic method not only enhanced the scope of enantiopure γ-nitroalcohols but also provided valuable intermediates for the synthesis of complex natural products, offering a green and sustainable alternative in asymmetric organic synthesis57 (Scheme 12).
In organic synthesis, chiral alcohols serve as building blocks for producing chiral auxiliaries, natural products, and pharmaceuticals. A green method for obtaining chiral alcohols is the biocatalytic asymmetric reduction of ketones,58 where carrot root pieces have been successfully employed. This reaction typically occurs under mild conditions, using water as a solvent at room temperature, and offers a simpler workup compared to other biocatalytic systems. A comparative study of the biocatalytic properties of purple, yellow, and orange carrots against substituted acetophenones (39a–f) revealed that purple carrots, an ancient variety, exhibited superior reducing activity25 (Scheme 13).
D. carota exhibits effective ketoreductase activity, cost efficiency, and ease of availability, making it a reliable and user-friendly biocatalyst for enantioselective ketone reductions. However, its long reaction time, high biocatalyst demand, and low substrate concentration pose challenges for large-scale applications. To minimize the disadvantages of bioreduction, Tween® 20 was used as a surfactant to enhance the enantioselective reduction of acetophenone derivatives (41a–e) catalyzed by D. carota. Tween® 20 significantly improved conversion and enantiomeric excess (ee) in the reduction of acetophenones by enhancing substrate solubility in water. Additionally, it influenced the enantiomeric ratio and enzyme activity.26 (Scheme 14).
In bioreduction processes, the surfactant Tween 20® enhances the solubility of hydrophobic organic substrates, which could improve the conversion rate of the reaction. By increasing solubility, the substrate disperses more uniformly throughout the reaction medium making it more accessible to the enzyme's active sites. This dispersion minimizes the formation of substrate aggregates that can limit enzyme–substrate interactions and facilitates the catalytic process. Enzymes can operate under optimal conditions, leading to faster and more complete substrate conversion. Studies have shown that this improved substrate availability directly correlates with increased reaction rates and overall process efficiency.26
Besides that, the alternative substances to Tween 20® can be used to enhance reaction efficiency and minimize the amount of biocatalyst required. Non-ionic surfactants like Triton X-100® and Tween 80® show similar benefits by improving substrate solubility and enzyme accessibility.59 Poloxamer 188, commonly used for protein stabilization, can also be used. Cyclodextrins, which are cyclic oligosaccharides are alternatives for enhancing solubility and enzyme stability in biocatalytic systems. The selection of an appropriate surfactant depends on factors such as enzyme compatibility, cost-effectiveness, and its impact on overall reaction performance.60
Nitrogen, oxygen, or sulfur in the rings of heterocyclic aromatic compounds63 provide key structural elements in a large number of natural and synthetic biologically active products. Heterocycles like acetyl-pyridines are known as aromatic components in perfumes, foods, and smoking suppressants.64 Chiral heteroaryl alcohols serve as crucial intermediates in synthesizing biologically active molecules and also as chiral ligands or auxiliaries in asymmetric addition reactions. Asymmetric reduction of heteroaryl methyl ketones is a straightforward approach, though many chemical and biological methods face limitations such as long incubation times, low substrate loading, and poor yields. In this study, the bioreduction of substituted heteroaryl ketones (51a–f) using D. carota was explored, with the dehydrogenase enzymes in D. carota selectively reducing these ketones to chiral secondary alcohols (52a–f) in good yields and high enantioselectivity65 (Scheme 16).
The quinoxaline nucleus served as a vital scaffold in the synthesis of pharmacophores with a wide range of pharmacological activities, including anti-bacterial,66,67 anti-viral,68,69 anti-HIV,70 anti-malarial,71,72 anti-cancer,73 anti-tubercular,74 and anti-leishmanial75 properties, along with potential applications in neurological disorders. Exploiting the biotransformation capabilities of D. carota, which contain alcohol dehydrogenase enzymes that selectively reduce keto compounds, quinoxaline ketones (53a–d) were effectively transformed into chiral alcohols. This reduction, in alignment with green chemistry principles, utilized alginate-immobilized D. carota homogenate beads as a biocatalyst. The resulting chiral alcohols (54a–d), characterized through X-ray crystallography, exclusively exhibited the R-configuration in high yields and exceptional enantioselectivity (98%)76 (Scheme 17).
Biotransformation proposes a greener and more viable choice to conventional chemical processes in organic synthesis. One of the earliest examples is the reduction of ketones and ketoesters to alcohol using baker's yeast. Later, it was found that various plants could also reduce prochiral compounds with different degrees of enantioselectivity, with D. carota (carrot) roots consistently yielding superior results. Utilizing the biological importance of the benzofuran moiety, D. carota roots were used to reduce benzofuran-2-yl-methyl ketone (55) to its chiral alcohol (56) in aqueous medium, highlighting the efficiency of plant-based biocatalysts in enantioselective synthesis27 (Scheme 18).
Plant-based biocatalysts offer a sustainable and environmentally friendly alternative to conventional chemical catalysts. These biocatalysts have advantages such as high selectivity, broad substrate acceptance, and operation under mild conditions.77,78 Their ability to catalyze reactions with chemo-, regio-, and stereoselectivity makes them important in pharmaceutical and chemical synthesis, reducing the need for protection and deprotection steps.79 The renewable aspect of these biocatalysts fits perfectly with the principles of green chemistry, helping to reduce toxic waste and energy use. Plant-based biocatalysis faces challenges like limited availability, lower stability, and issues with large-scale production, which makes it tough to be widely used in industry.80 Plant-derived enzymes need to be extracted and purified from their natural sources, which can result in variations in both enzyme activity and yield. Substrate and product inhibition can hinder the efficiency of reactions, necessitating further optimization strategies. Despite these challenges, recent advancements in enzyme engineering and immobilization techniques show promise for improving the industrial use of plant-based biocatalysts, positioning them as a strong contender for sustainable chemical processes.81
Plant-derived components, like hemicellulose and lignin, hinder enzymatic bioreduction by creating structural and chemical barriers that restrict enzyme accessibility, stability, and activity. Hemicellulose wraps around cellulose microfibrils, further limiting the enzymes' ability to do their job.82 On the other hand, lignin, which is a complex polyphenolic polymer, adds mechanical strength and hydrophobic properties, making it harder for enzymes to penetrate. It has aromatic rings and hydroxyl groups that interact with enzymes, creating hydrophobic interactions and hydrogen bonds. Unfortunately, this can cause enzymes to become deactivated and bind in ways that aren't productive. As a result, it is often required to use higher amounts of enzymes to achieve effective bioreduction.83 When lignin breaks down, it releases phenolic compounds like vanillin and syringaldehyde, as well as furfural and hydroxymethylfurfural (HMF) from the breakdown of hemicellulose. These compounds can hinder enzyme activity by messing with the active sites and interfering with reactions that depend on NADH and NADPH.84 Acidic degradation products, such as acetic acid, can change the microenvironments of enzymes, leading to denaturation or a decrease in their activity.
On the other hand, oligosaccharides derived from hemicellulose boost ionic strength, which can interfere with the interactions between enzymes and their substrates.85 To mitigate these challenges, many strategies can be employed, including pretreatment methods (acid, alkaline, oxidative, or enzymatic) to remove lignin and hemicellulose86 and supplementing cofactors like NADH to counteract redox imbalances.87 Implementing these approaches can enhance enzyme efficiency and improve the overall bioreduction process.
Five- and six-membered nitrogen-containing heterocyclic compounds with a quaternary stereogenic center at C3, in either (R)- or (S)-configuration, are crucial as building blocks for numerous bioactive scaffolds. Notably, they are used in the synthesis of compounds like Capromorelin,93 an orally active small molecule that mimics ghrelin, acting as a potent and selective GHS-R agonist to stimulate appetite and GH secretion, as well as in the synthesis of isonitramine94 and sibirine.95 In support of this, Romain L. et al. reported the efficient reduction of six-membered N-containing β-ketoesters and five-membered amino ketones (61) to their corresponding chiral alcohols (62) using D. carota (carrots), achieving excellent yields and high enantiomeric excess (ee)96 (Scheme 21).
Non-racemic chiral alcohols have been obtained through the asymmetric reduction of prochiral ketones using a variety of chemical and biological methods. However, achieving high yields and enantiomeric excess remains challenging due to the reliance on costly chiral reagents. To tackle this issue, the asymmetric bio-reduction of indanone (63) and tetralone (65) was carried out using D. carota (carrot) roots, yielding the corresponding enantiomerically pure (S)-alcohols97 (Scheme 22).
The bicyclo [3.3.1] nonane98,99 framework is very abundant in natural products, serving as an excellent scaffold for the synthesis of many bioactive compounds. Notably, the transformation of the bicyclo [3.3.1] nonane system into the bicyclo [5.3.1] undecane ring system represents a crucial step in taxoid synthesis.100 Bicyclo [3.3.1] nonane-2,6-dione (67–67′) was utilized in the synthesis of chiral compounds and the determination of their chiroptical properties. That was quite significant because most bioactive natural products with this moiety show optical activity.101–103 A stereoselective reduction of the racemic diketone was carried out using plant enzymes. The unreacted (+)-enantiomer was extracted from the reaction mixture with an organic solvent, while the (–)-enantiomer underwent enzymatic reduction, yielding 6-hydroxybicyclo [3.3.1]nonane-2-one (68) as the reaction product99 (Scheme 23).
3.3 Chemoselective reductions
Chiral-hydroxy carboxylic acids and their esters are used as a valuable precursor for the synthesis of many bioactive compounds.104–107 Multifunctional chiral-hydroxy carboxylic acid esters, e.g. chiral-hydroxy-but-3-enoic carboxylic acid esters are of great importance due to their ability to form new chiral centers and undergo stereoselective transformations of the adjacent alkene influenced by C-2 hydroxyl group. Both biocatalytic as well as chemical methods have been employed for the synthesis of optically pure-hydroxy-but-3-enoic carboxylic acid esters. In particular, asymmetric reduction of 4-aryl-oxo-but-3-enoic carboxylic acid esters (71a–d) was achieved using D. carota tissue culture cells, yielding 4-aryl-hydroxy-but-3-enoic carboxylic acid esters (72a–d) in high enantiomeric excess and conversion108 (Scheme 24).Dihydrochalcones, found in numerous biologically active natural products such as nothofagin, trilobatin, phlorizin, and glycyphyllin,109 play a significant role in various applications. They are particularly used as food additives due to their remarkable sweetness. Notably, trilobatin (TLB), a natural dihydrochalcone, demonstrates excellent anti-type 2 diabetic activity.110 As a result of growing recognition of dihydrochalcone's health benefits, an increased interest in the synthesis of dihydrochalcone derivatives has been observed. Given the significance of biocatalysis in organic synthesis, the biocatalytic reduction of conjugated olefins (73) to dihydrochalcones (74) using D. carota roots under mild conditions has been reported. This reaction demonstrates high selectivity, favoring 1,4-reduction over 1,2-reduction in chalcones, a preference attributed to the involvement of ene-reductase enzymes111 (Scheme 25).
3.4 Reduction of diastereomeric ketones
Carvone is a well-known example of an odoriferous compound where the two enantiomers exhibit distinct scents.112 The (4R)-(−)-carvone, characterized by a minty fragrance, is a key component of the essential oils of Mentha viridis and Mentha spicata, alongside dihydrocarveol and cis-dihydrocarvones.113 In contrast, the (4S)-(+)-carvone has a caraway scent found in the essential oils of Carum carvi L. and Anethum graveolens L. As a fragrant compound, carvone is widely used in the production of cosmetics, toothpaste, and chewing gum.114 Beyond its aromatic properties, carvone has also displayed notable biological activities, including anticancer and free radical scavenging effects,115 and showed potential as an inhibitor of acetylcholinesterase (AChE).116 Enzymatic reductions involve the transfer of reducing cofactors, with the enzyme distinguishing between substituents around the carbonyl group, resulting in enantioselectivity when the products are chiral. In the biotransformation of both enantiomers (Scheme 26) of carvone by enzymatic systems from selected plants (D. carota), dihydrocarvones were obtained.117Significant focus has been placed on enantioselective syntheses of enantiomerically pure compounds or chiral synthons, which are increasingly sought after for the advancement of modern pharmaceuticals and agrochemicals.118 The low cost, ready availability of the biocatalyst, and simplicity of the reaction conditions make biotransformation highly promising for large-scale production of valuable chiral alcohols.119 The enzymatic reduction of trans-2-methylcyclohexanone (81a–b) using fresh carrot root as a biocatalyst proceeded in a completely diastereoselective manner, yielding an equal 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of enantiomerically pure 1S, 2R- and 1S, 2S-2-methylcyclohexanol (82′a–b). In contrast, the reaction performed on racemic 2-hydroxy cyclohexanone produced a 1
1 mixture of enantiomerically pure 1S, 2R- and 1S, 2S-2-methylcyclohexanol (82′a–b). In contrast, the reaction performed on racemic 2-hydroxy cyclohexanone produced a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixture of 1S, 2R (82a–b) and 1S, 2S-1,2-cyclohexane diol32 (Scheme 27).
2 mixture of 1S, 2R (82a–b) and 1S, 2S-1,2-cyclohexane diol32 (Scheme 27).
Enol acetate (83) underwent hydrolysis with D. carota cells in water, yielding a substituted ketone (84a). Subsequent asymmetric protonation under identical conditions produced cyclohexanone (84b) in 89% yield with 45% ee, favoring the (S)-enantiomer. A diastereospecific reduction of the enantioenriched cyclohexanone (84b) selectively targeted the Re-face, affording cyclohexanol (85) with 75% yield and 100% ee after 24 hours at room temperature, highlighting the efficiency of this domino process20 (Scheme 28).
3.5 Miscellaneous
The Henry reaction was first reported in 1895, involves the reaction of aldehydes with nitroalkanes to produce β-nitro alcohols, and has garnered significant interest in medicinal chemistry due to its applications in synthesizing pharmaceutical agents, natural product precursors, and other bioactive compounds. However, using conventional bases in this reaction often leads to unwanted side products along-with the desired β-nitro alcohols. While metal and organocatalysts have been introduced to overcome this issue, their toxicity and high costs remain challenges. To address these concerns, a greener approach was developed using D. carota root enzymes, which efficiently catalyzed the Henry reaction of 2-nitrobenzaldehyde (86) and nitromethane (87), yielding β-nitro alcohol (88) in 93% yield under mild conditions (phosphate buffer, pH 7, 28 °C, 8 hours)120 (Scheme 29).Biotransformation processes using plant tissues are typically conducted in aqueous media, but their effectiveness is limited due to the low solubility of pure substrates in water, making scale-up to bioreactors unfeasible. To address this, Schewe et al. suggested the use of biphasic systems, which offer several advantages, including improved substrate solubility, easier product removal, overcoming unfavorable equilibria, and suppression of side reactions. Enantiomerically pure cyanohydrins (90), important intermediates in organic synthesis, were produced using D. carota as a biocatalyst in a biphasic system using dibutyl phthalate as a co-solvent due to its hydrophobic nature, thermal stability, resistance to photooxidation, and cost-effectiveness. Under optimized conditions, the bioreduction productivity of alcohol increased from 0.58 to 1.36 g L−1 (ref. 121) (Scheme 30).
Cenobamate (92) is a recently introduced medication for treating partial-onset seizures in adults. While the enantioselective synthesis of β-heteroaryl amino alcohols has been achieved through Ru-catalyzed asymmetric hydrogenation, many of these methods rely on costly and environmentally harmful chiral reagents, which limits their large-scale application. A more sustainable and efficient approach for producing cenobamate has been developed, utilizing a bio-reduction of β-ketotetrazole (91) with whole D. carota plant cells, yielding 70% and >99% enantiomeric excess (ee). The corresponding β-hydroxytetrazole (92) was isolated with a 60% yield and >98% ee. This is the first report of a biocatalytic reduction of β-ketotetrazole (91) using plant enzymes from D. carota root cells, that shows outstanding enantioselectivity (Scheme 31).122
Wen-JuBai et al. has systematically explored the scope of carrot-mediated reduction of Keto-derived nitrogen-heteroaromatics. The five- and six-membered nitrogen-containing heteroaromatic compounds most commonly found in FDA-approved small-molecule drugs were chosen for evaluation. A methyl group was used as a substitute for more complex ketone substituents, as outlined in the basic substrate scope. Notably, the thiazole-containing drugs often feature two substituents at the C2- and C4-positions of the thiazole ring, hence substrates with similar substitution patterns were tested. After the reduction of thiazoles (93a) substituents at the C2- and C4-positions, the alcohols (93a′) were obtained in yields of 78–95% with >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 enantiomeric ratio. However, no reactivity was observed for ketones containing imidazole (93d) or indole (93b) groups, and only a trace conversion was seen with the tetrazole-containing ketone (93c). Following the substitution patterns seen in benzimidazole-containing drugs, both mono- and di-substituted substrates (93e) at the most commonly observed positions were tested. These substrates performed well, resulting in alcohols with yields of 73–89% and >99
1 enantiomeric ratio. However, no reactivity was observed for ketones containing imidazole (93d) or indole (93b) groups, and only a trace conversion was seen with the tetrazole-containing ketone (93c). Following the substitution patterns seen in benzimidazole-containing drugs, both mono- and di-substituted substrates (93e) at the most commonly observed positions were tested. These substrates performed well, resulting in alcohols with yields of 73–89% and >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 enantiomeric ratio (Scheme 32).123
1 enantiomeric ratio (Scheme 32).123
Due to the importance of enantiomerically pure 1-phenyl ethyl alcohols as chiral auxiliaries and synthons, Wanda et al. carried out the hydrolysis of esters (94a–c) of 1-phenyl ethyl analogs to optically active alcohols through biotransformation using comminuted D. carota root tissue (Scheme 33).18
The biocatalytic potential of D. carota roots has emerged as a powerful tool in the sustainable production of chiral intermediates, which are crucial for synthesizing bioactive compounds. These enzymatic reactions proceed under mild, aqueous conditions, and eliminate the need for toxic reagents, heavy metals, or harsh reaction conditions, marking a significant advancement in green chemistry.
One of the most notable contributions of D. carota is its ability to catalyze the asymmetric reduction of prochiral ketones, such as acetophenones, and other related compounds. These reactions result in the production of chiral alcohols with high enantiomeric purity, which are essential for the synthesis of a variety of bioactive molecules. D. carota catalyzes the production of (S)-alcohols, key intermediates in pharmaceutical and agrochemical synthesis. Carrot roots also play an important role in the synthesis of (R)-chiral azido alcohols, essential for bioactive compounds like Tembamide, Angeline, and Denopamine.
The versatility of D. carota roots is further demonstrated in their ability to catalyze the reduction of a diverse range of substrates, including heteroaryl and quinoxaline ketones, as well as β-ketoesters and α-hydroxy aromatic ketones. These reactions enable the production of optically pure intermediates for the synthesis of drugs, fragrances, and other bioactive compounds, highlighting the potential of carrot-derived biocatalysts for use in industrial applications. D. carota can be effectively used in a biphasic system, in combination with co-solvents such as dibutyl phthalate, to enhance substrate solubility and increase the scalability of reactions like cyanohydrin formation. This approach significantly improves reaction yields and efficiency, demonstrating the scalability of carrot root catalysis for larger-scale industrial applications. The ability to use such systems under mild conditions further emphasizes the eco-friendly nature of this biotransformation, contributing to the growing demand for green and sustainable chemical processes.
D. carota is involved in the reduction of conjugated olefins to dihydrochalcones, using its ene-reductase enzymes. This biocatalytic reaction offers a mild, efficient method for producing valuable dihydrochalcone derivatives, which have applications in the food, cosmetic, and pharmaceutical industries. Similarly, the selective reduction of carvone enantiomers by D. carota results in dihydrocarvones, which possess distinct biological and aromatic properties, expanding the utility of carrot-derived biocatalysts in the synthesis of fragrance compounds. Moreover, D. carota root enzymes contribute to stereoselective reductions with cofactor recycling, ensuring high enantiomeric excess and excellent yields in various green chemistry applications. This characteristic makes carrot-based biocatalysis a promising approach for large-scale industrial processes, offering a sustainable and cost-effective alternative to traditional synthetic methods.
Plant-derived biocatalysts have revealed significant potential in the asymmetric reduction of ketones, yielding optically active alcohols with a high enantiomeric excess (ee). Cynara scolymus L., for instance, has been used to asymmetrically reduce various acetophenones, including 4′-haloacetophenones and 4′-nitroacetophenone, achieving (ee) values from 71.4% to 96.5% over a period of 2 to 4 days. Phoenix dactylifera L., commonly known as the date palm, has shown a remarkable ability to convert acetophenone derivatives into chiral alcohols. Reaction yields vary from 52.0% to 77.2%, with enantiomeric excess (ee) values ranging from 60.0% to 89.0%. This emphasizes its potential as a biocatalyst for asymmetric reduction. Additionally, Brassica oleracea has demonstrated impressive stereoselectivity, achieving over 99% ee in the reduction of benzyl acetoacetate to benzyl (S)-(+)-3-hydroxybutyrate, with higher selectivity. Pastinaca sativa (parsnip) has been reported to facilitate the reduction of ketones such as benzyl acetoacetate and ethyl 3-oxopentanoate, producing chiral alcohols with substantial enantiomeric excess. These findings underscore the potential of plant-based biocatalysts as sustainable and effective alternatives for stereoselective bioreduction processes.15,124
Despite its environmental benefits as a greener alternative, D. carota biocatalysis faces limitations in catalytic efficiency due to low turnover numbers and extended reaction times, making it less efficient than ketoreductases (KREDs) and alcohol dehydrogenases (ADHs), which require costly cofactors or toxic reagents. To enhance its industrial viability, optimization strategies such as enzyme immobilization (e.g., alginate/silica beads), genetic engineering (heterologous ADH expression), and reaction engineering (biphasic systems, selective surfactants) can improve stability, conversion rates, and mass transfer. Future research should focus on refining reaction kinetics and cost-effectiveness to establish D. carota as a scalable green biocatalyst.
4 Conclusion and future perspectives
D. carota roots serve as sustainable biocatalysts for enantioselective reductions of ketones, esters, and olefins. Unlike traditional chemical methods that can be costly, environmentally damaging, and complicated to operate, biocatalysis with D. carota provides a greener alternative. What's really impressive is its ability to catalyze the asymmetric reduction of prochiral ketones, producing enantiopure (S)- and (R)-chiral alcohols, which are crucial intermediates in the pharmaceutical and agrochemical industries. Moreover, it exhibits broad catalytic potential, efficiently reducing β-ketoesters, heteroaryl ketones, and conjugated olefins, positioning it as a valuable tool in sustainable organic synthesis. The whole-cell systems of D. carota naturally have enzymes and cofactors, making bioreduction efficient under mild conditions without needing extra additives. This results in high regio- and stereoselectivity due to enzyme compartmentalization, cofactor recycling, and selective substrate recognition. These benefits underscore D. carota's potential to advance green chemistry, aligning perfectly with the vision of a more sustainable and biobased economy.Future studies should focus on enzyme immobilization (e.g., alginate/silica bead encapsulation), and genetic modifications to enhance substrate range, dehydrogenase activity, and biocatalytic efficiency of D. carota, by optimizing reaction conditions such as pH, solvent choice, and temperature. Enzyme engineering and cofactor regeneration could also play a significant role in improving specificity, making it a more versatile biocatalyst for sustainable organic synthesis. Streamlining purification through in situ product removal, minimal pre-treatment, and membrane filtration can help reduce complexity, while techniques like selective precipitation and phase-specific separation can boost efficiency and cost-effectiveness. Integrating D. carota with continuous flow systems might further improve scalability and process control, making it more suitable for industrial use. Conducting economic and life cycle assessments will be essential to validate its feasibility and reinforce its position as a green and sustainable alternative in organic synthesis.
Data availability
There is no additional data available for this article.Conflicts of interest
There are no conflicts to declare.References
- B. S. Chen and F. Z. R. de Souza, Enzymatic synthesis of enantiopure alcohols: current state and perspectives, RSC Adv., 2019, 9, 2102–2115 RSC
.
- D. Caron, A. P. Coughlan, M. Simard, J. Bernier, Y. Piché and R. Chênevert, Stereoselective reduction of ketones by Daucus carota hairy root cultures, Biotechnol. Lett., 2005, 27, 713–716 CrossRef CAS PubMed
.
- E. P. Talsi and K. P. Bryliakov, Autoamplification-Enhanced Oxidative Kinetic Resolution of sec-Alcohols and Alkyl Mandelates, and its Kinetic Model, ChemCatChem, 2018, 10, 2693–2699 CrossRef CAS
.
- T. Hashimoto, Y. Shimazaki, Y. Omatsu and K. Maruoka, Indanol-Based Chiral Organoiodine Catalysts for Enantioselective Hydrative Dearomatization, Angew Chem. Int. Ed. Engl., 2018, 57, 7200–7204 CrossRef CAS PubMed
.
- A. Ghanem, M. Ahmed, H. Ishii and T. Ikegami, Immobilized β-cyclodextrin-based silica vs polymer monoliths for chiral nano liquid chromatographic separation of racemates, Talanta, 2015, 132, 301–314 CrossRef CAS PubMed
.
- M. Garbe, Z. Wei, B. Tannert, A. Spannenberg, H. Jiao, S. Bachmann, M. Scalone, K. Junge and M. Beller, Enantioselective hydrogenation of ketones using different metal complexes with a chiral PNP pincer ligand, Adv. Synth. Catal., 2019, 361, 1913–1920 CrossRef CAS
.
- T. Aboul-Fadl and F. A. Bin-Jubair, Anti-tubercular activity of isatin derivatives, Int. J. Res. Pharm. Sci., 2010, 1, 113–126 Search PubMed
.
- J. A. Rasmussen, M. Straffon, G. Dumsday and M. Zachariou, New technology for biotransformations, Aust. J. Chem., 2005, 72, 4–7 CAS
.
- A. Bruggink, A. Straathof and L. van der Wielen, A ‘fine’chemical industry for life science products: green solutions to chemical challenges, Process Integration in Biochemical Engineering, 2003, pp. 69–113 Search PubMed
.
- A. J. Straathof, S. Panke and A. Schmid, The production of fine chemicals by biotransformations, COBIOT, 2002, 13, 548–556 CAS
.
- K. Faber and K. Faber, Biotransformations in Organic Chemistry: a Textbook, Springer, 2011, vol. 6 Search PubMed
.
- J. Yadav, S. Nanda, P. T. Reddy and A. B. Rao, Efficient enantioselective reduction of ketones with Daucus carota root, J. Org. Chem., 2002, 67, 3900–3903 CrossRef CAS PubMed
.
- Z. H. Yang, R. Zeng, G. Yang, Y. Wang, L.-Z. Li, Z.-S. Lv, M. Yao and B. Lai, Asymmetric reduction of prochiral ketones to chiral alcohols catalyzed by plants tissue, J. Ind. Microbiol. Biotechnol., 2008, 35, 1047 CrossRef CAS PubMed
.
- R. Wohlgemuth, Biocatalysis—key to sustainable industrial chemistry, Curr. Opin. Biotechnol., 2010, 21, 713–724 CrossRef CAS PubMed
.
- K. Javidnia, E. Faghih-Mirzaei, R. Miri, M. Attarroshan and K. Zomorodian, Stereoselective reduction of prochiral ketones by plant and microbial biocatalysts, Indian J. Pharm. Sci., 2016, 78, 73 CrossRef CAS PubMed
.
- D. Zhu, H. T. Malik and L. Hua, Asymmetric ketone reduction by a hyperthermophilic alcohol dehydrogenase. The substrate specificity, enantioselectivity and tolerance of organic solvents, Tetrahedron: Asymmetry, 2006, 17, 3010–3014 CrossRef CAS
.
- J. Kasprzak, F. Bischoff, M. Rauter, K. Becker, K. Baronian, R. Bode, F. Schauer, H.-M. Vorbrodt and G. Kunze, Synthesis of 1-(S)-phenylethanol and ethyl (R)-4-chloro-3-hydroxybutanoate using recombinant Rhodococcus erythropolis alcohol dehydrogenase produced by two yeast species, Biochem. Eng. J., 2016, 106, 107–117 CrossRef CAS
.
- W. K. Mączka and A. Mironowicz, Enantioselective hydrolysis of 1-aryl ethyl acetates and reduction of aryl methyl ketones using carrot, celeriac and horseradish enzyme systems, Tetrahedron: Asymmetry, 2002, 13, 2299–2302 CrossRef
.
- L. H. Andrade, R. S. Utsunomiya, A. T. Omori, A. L. Porto and J. V. Comasseto, Edible catalysts for clean chemical reactions: Bioreduction of aromatic ketones and biooxidation of secondary alcohols using plants, J. Mol. Catal. B: Enzym., 2006, 38, 84–90 CrossRef CAS
.
- N. Blanchard and P. van de Weghe, Daucus carota mediated bioreduction of prochiral ketones, Org. Biomol. Chem., 2006, 4, 2348–2353 RSC
.
- C. C. de Carvalho, Whole cell biocatalysts: essential workers from nature to the industry, Microb. Biotechnol., 2017, 10, 250–263 CrossRef PubMed
.
- Y. Hasegawa, S. Adachi and R. Matsuno, Production of homochiral 1-phenylethanol through enantioselective oxidation of its racemate with whole cells of the yeast Hansenula capsulata IFO 0974, J. Ferment. Bioeng., 1997, 83, 346–351 CrossRef CAS
.
- J. Jung, H. J. Park, K.-N. Uhm, D. Kim and H.-K. Kim, Asymmetric synthesis of (S)-ethyl-4-chloro-3-hydroxy butanoate using a Saccharomyces cerevisiae reductase: Enantioselectivity and enzyme–substrate docking studies, Biochim. Biophys. Acta, Proteins Proteomics, 2010, 1804, 1841–1849 CrossRef CAS PubMed
.
- H. K. Chenault, E. S. Simon and G. M. Whitesides, Cofactor regeneration for enzyme-catalysed synthesis, Biotechnol. Genet. Eng. Rev., 1988, 6, 221–270 CrossRef CAS PubMed
.
- A. T. Omori, F. G. Lobo, A. C. G. do Amaral and C. d. S. de Oliveira, Purple carrots: Better biocatalysts for the enantioselective reduction of acetophenones than common orange carrots (D. carota), J. Mol. Catal. B: Enzym., 2016, 127, 93–97 CrossRef CAS
.
- M. Rodrigues da Costa and Á. T. Omori, Tween® 20-enhanced bioreduction of acetophenones promoted by Daucus carota root, Food Technol. Biotechnol., 2017, 55, 231–235 CAS
.
- S. Ravía, D. Gamenara, V. Schapiro, A. Bellomo, J. Adum, G. Seoane and D. Gonzalez, Enantioselective reduction by crude plant parts: reduction of benzofuran-2-yl methyl ketone with carrot (Daucus carota) bits, J. Chem. Educ., 2006, 83, 1049 CrossRef
.
- R. Verho, J. Londesborough, M. Penttilä and P. Richard, Engineering redox cofactor regeneration for improved pentose fermentation in Saccharomyces cerevisiae, Appl. Environ. Microbiol., 2003, 69, 5892–5897 CrossRef CAS PubMed
.
- T. Ema, Y. Sugiyama, M. Fukumoto, H. Moriya, J.-N. Cui, T. Sakai and M. Utaka, Highly enantioselective reduction of carbonyl compounds using a reductase purified from bakers' yeast, J. Org. Chem., 1998, 63, 4996–5000 CrossRef CAS
.
- S. Benner and A. D. Ellington, Interpreting the behavior of enzymes Purpose or pedigree?, Crit. Rev. Biochem., 1988, 23, 369–426 CrossRef CAS PubMed
.
- E. G. Weinhold, A. Glasfeld, A. D. Ellington and S. A. Benner, Structural determinants of stereospecificity in yeast alcohol dehydrogenase, Proc. Natl. Acad. Sci. U. S. A., 1991, 88, 8420–8424 CrossRef CAS PubMed
.
- F. Baldassarre, G. Bertoni, C. Chiappe and F. Marioni, Preparative synthesis of chiral alcohols by enantioselective reduction with Daucus carota root as biocatalyst, J. Mol. Catal. B: Enzym., 2000, 11, 55–58 CrossRef CAS
.
- A. M. Clark, Natural products as a resource for new drugs, Pharm. Res., 1996, 13, 1133–1141 CrossRef CAS PubMed
.
- D. J. Foley and H. Waldmann, Ketones as strategic building blocks for the synthesis of natural product-inspired compounds, Chem. Soc. Rev., 2022, 51, 4094–4120 RSC
.
- N. B. Khomane, J. S. Patel, P. K. Shirsat, P. R. Mali and H. M. Meshram, Formal synthesis of angiopterlactone B via enantioselective reduction of ketone with Daucus carota root, ChemistrySelect, 2018, 3, 1517–1520 CrossRef CAS
.
- T. K. Kotammagari, R. G. Gonnade, A. K. Bhattacharya and A. K. Bhattacharya, Biomimetic total synthesis of angiopterlactone B and other potential natural products, Org. Lett., 2017, 19, 3564–3567 CrossRef CAS PubMed
.
- E. Russo, R. Gitto, R. Citraro, A. Chimirri and G. De Sarro, New AMPA antagonists in epilepsy, Expert Opin. Invest. Drugs, 2012, 21, 1371–1389 CrossRef CAS PubMed
.
- A. T. Omori, C. d. S. de Oliveira, K. T. Andrade and M. G. Capeletto, Sassafras oil, carrot bits and microwaves: green lessons learned from the formal total synthesis of (−)-talampanel, RSC Adv., 2015, 5, 103563–103565 RSC
.
- B. A. Anderson, M. M. Hansen, A. R. Harkness, C. L. Henry, J. T. Vicenzi and M. J. Zmijewski, Application of a practical biocatalytic reduction to an enantioselective synthesis of the 5H-2, 3-benzodiazepine LY300164, J. Am. Chem. Soc., 1995, 117, 12358–12359 CrossRef CAS
.
- H. Yokoyama, T. Yanagisawa and N. Taira, Details of mode and mechanism of action of denopamine, a new orally active cardiotonic agent with affinity for β1-receptors, J. Cardiovasc. Pharmacol., 1988, 12, 323–331 CrossRef CAS PubMed
.
- A. Shoeb, R. S. Kapil and S. P. Popli, Coumarins and alkaloids of Aegle marmelos, Phytochem., 1973, 12, 2071–2072 CrossRef CAS
.
- L. C. Rocha, M. H. Seleghim, J. V. Comasseto, L. D. Sette and A. L. Porto, Stereoselective bioreduction of α-azido ketones by whole cells of marine-derived fungi, Mar. Biotechnol., 2015, 17, 736–742 CrossRef CAS PubMed
.
- J. Yadav, P. T. Reddy, S. Nanda and A. B. Rao, Stereoselective synthesis of (R)-(−)-denopamine,(R)-(−)-tembamide and (R)-(−)-aegeline via asymmetric reduction of azidoketones by Daucus carota in aqueous medium, Tetrahedron: Asymmetry, 2002, 12, 3381–3385 CrossRef CAS
.
- M. López-Iglesias, D. Méndez-Sánchez and V. Gotor-Fernández, Native proteins in organic chemistry. Recent achievements in the use of non hydrolytic enzymes for the synthesis of pharmaceuticals, Curr. Org. Chem., 2016, 20, 1204–1221 CrossRef
.
- M. M. Midland, Asymmetric reductions with organoborane reagents, Chem. Rev., 1989, 89, 1553–1561 CrossRef CAS
.
- A. M. Fournier, R. A. Brown, W. Farnaby, H. Miyatake-Ondozabal and J. Clayden, Synthesis of (−)-(S, S)-clemastine by Invertive N→ C Aryl Migration in a Lithiated Carbamate, Org. Lett., 2010, 12, 2222–2225 CrossRef CAS PubMed
.
- A. Chanysheva, E. Sheiko and V. Zorin, Asymmetric bioreduction of 4-chloroacetophenone catalyzed by Daucus carota cells in water and organic solvents, Russ. J. Gen. Chem., 2021, 91, 2953–2956 CrossRef CAS
.
- K. Ishihara, H. Hamada, T. Hirata and N. Nakajima, Biotransformation using plant cultured cells, J. Mol. Catal. B: Enzym., 2003, 23, 145–170 CrossRef CAS
.
- K. Nakamura and T. Matsuda, Asymmetric Reduction of Ketones by the Acetone Powder of Geotrichum c andidum, J. Org. Chem., 1998, 63, 8957–8964 CrossRef CAS
.
- W. K. Mączka and A. Mironowicz, Enantioselective reduction of bromo-and methoxy-acetophenone derivatives using carrot and celeriac enzymatic system, Tetrahedron: Asymmetry, 2004, 15, 1965–1967 CrossRef
.
- H. C. Kazici, E. Bayraktar and Ü. Mehmetoglu, Optimization of the asymmetric synthesis of chiral aromatic alcohol using freeze-dried carrots as whole-cell biocatalysts, Green Process. Synth., 2016, 5, 131–137 CAS
.
- A. Bruggink, R. Schoevaart and T. Kieboom, Concepts of nature in organic synthesis: cascade catalysis and multistep conversions in concert, Org. Process Res. Dev., 2003, 7, 622–640 CrossRef CAS
.
- A. Chanysheva, T. Vorobyova and V. Zorin, Relative reactivity of substituted acetophenones in enantioselective biocatalytic reduction catalyzed by plant cells of Daucus carota and Petroselinum crispum, Tetrahedron, 2019, 75, 130494 CrossRef CAS
.
- O. Rotthaus, D. Krüger, M. Demuth and K. Schaffner, Reductions of keto esters with baker's yeast in organic solvents-a comparison with the results in water, Tetrahedron, 1997, 53, 935–938 CrossRef CAS
.
- W. Yang, J.-H. Xu, Y. Xie, Y. Xu, G. Zhao and G. Q. Lin, Asymmetric reduction of ketones by employing Rhodotorula sp. AS2. 2241 and synthesis of the β-blocker (R)-nifenalol, Tetrahedron: Asymmetry, 2006, 17, 1769–1774 CrossRef CAS
.
- X. Liu, Y. Wang, H. Y. Gao and J. H. Xu, Asymmetric reduction of α-hydroxy aromatic ketones to chiral aryl vicinal diols using carrot enzymes system, Chin. Chem. Lett., 2012, 23, 635–638 CrossRef CAS
.
- D. Scarpi, E. G. Occhiato and A. Guarna, Selectivity of Daucus carota roots and baker's yeast in the enantioselective reduction of γ-nitroketones, Tetrahedron: Asymmetry, 2005, 16, 1479–1483 CrossRef CAS
.
- Y. Ni and J. H. Xu, Biocatalytic ketone reduction: a green and efficient access to enantiopure alcohols, Biotechnol. Adv., 2012, 30, 1279–1288 CrossRef CAS PubMed
.
- A. Ruiz, M. Boushehri, T. Phan, S. Carle, P. Garidel, J. Buske and A. Lamprecht, Alternative excipients for protein stabilization in protein therapeutics: overcoming the limitations of polysorbates, Pharmaceutics, 2022, 14, 2575 CrossRef PubMed
.
- G. Dhandapani, E. Wachtel, M. Sheves and G. Patchornik, Nonionic detergent micelle aggregates: An economical alternative to protein A chromatography, New Biotechnol., 2021, 61, 90–98 CrossRef CAS PubMed
.
- L. Kulishova and D. Zharkov, Solid/gas biocatalysis, BIORAK, 2017, 82, 95–105 CAS
.
- V. Aldabalde, P. Arcia, A. Gonzalez and D. Gonzalez, Enzymatic synthesis of chiral heteroaryl alcohols using plant fragments as the only biocatalyst and reducing agent, Green Chem. Lett. Rev., 2007, 1, 25–30 CrossRef CAS
.
- P. Hayoz, A. Von Zelewsky and H. Stoeckli-Evans, Stereoselective synthesis of octahedral complexes with predetermined helical chirality, J. Am. Chem. Soc., 1993, 115, 5111–5114 CrossRef CAS
.
- K. Uwai, N. Konno, S. Kitamura, S. Ohta and M. Takeshita, Purification and characterization of rat liver enzyme catalyzing stereoselective reduction of acetylpyridines, Chirality, 2005, 17, 494–500 CrossRef CAS PubMed
.
- C. S. Lakshmi, G. R. Reddy and A. B. Rao, Asymmetric reduction of heteroaryl methyl ketones using Daucus carota, Green Sustainable Chem., 2011, 1, 117–122 CrossRef CAS
.
- M. Vieira, C. Pinheiro, R. Fernandes, J. P. Noronha and C. Prudêncio, Antimicrobial activity of quinoxaline 1, 4-dioxide with 2-and 3-substituted derivatives, Microbiol. Res., 2014, 169, 287–293 CrossRef CAS PubMed
.
- P. Ramalingam, S. Ganapaty and C. B. Rao, In vitro antitubercular and antimicrobial activities of 1-substituted quinoxaline-2, 3 (1H, 4H)-diones, Bioorg. Med. Chem. Lett., 2010, 20, 406–408 CrossRef CAS PubMed
.
- D. H. Soliman, Synthesis, characterization, anti-bacterial and anti-fungal activities of new quinoxaline 1, 4-di-N-oxide derivatives, Int. J. Org. Chem., 2013, 3, 65 CrossRef
.
- J. A. Pereira, A. M. Pessoa, M. N. D. Cordeiro, R. Fernandes, C. Prudêncio, J. P. Noronha and M. Vieira, Quinoxaline, its derivatives and applications: A State of the Art review, Eur. J. Med. Chem., 2015, 97, 664–672 CrossRef CAS PubMed
.
- S. B. Patel, B. D. Patel, C. Pannecouque and H. G. Bhatt, Design, synthesis and anti-HIV activity of novel quinoxaline derivatives, Eur. J. Med. Chem., 2016, 117, 230–240 CrossRef CAS PubMed
.
- A. C. Shekhar, P. S. Rao, B. Narsaiah, A. D. Allanki and P. S. Sijwali, Emergence of pyrido quinoxalines as new family of antimalarial agents, Eur. J. Med. Chem., 2014, 77, 280–287 CrossRef PubMed
.
- A. Gil, A. Pabón, S. Galiano, A. Burguete, S. Pérez-Silanes, E. Deharo, A. Monge and I. Aldana, Synthesis, biological evaluation and structure-activity relationships of new quinoxaline derivatives as anti-Plasmodium falciparum agents, Molecules, 2014, 19, 2166–2180 CrossRef PubMed
.
- O. O. Ajani, M. T. Nlebemuo, J. A. Adekoya, K. O. Ogunniran, T. O. Siyanbola and C. O. Ajanaku, Chemistry and pharmacological diversity of quinoxaline motifs as anticancer agents, Acta Pharm., 2019, 69, 177–196 CrossRef CAS PubMed
.
- E. Vicente, R. Villar, S. Perez-Silanes, I. Aldana, R. C. Goldman and A. Monge, Quinoxaline 1, 4-di-N-oxide and the potential for treating tuberculosis, Infect. Disord.: Drug Targets, 2011, 11, 196–204 CAS
.
- J. Guillon, I. Forfar, V. Desplat, S. B. Fabre, D. Thiolat, S. Massip, H. Carrie, D. Mossalayi and C. Jarry, Synthesis of new 4-(E)-alkenylpyrrolo [1, 2-a] quinoxalines as antileishmanial agents by Suzuki-Miyaura cross-coupling reactions, J. Enzyme Inhib. Med. Chem., 2007, 22, 541–549 CrossRef CAS PubMed
.
- S. H. Meshram, T. Ramesh, J. B. Nanubolu, A. K. Srivastava, B. R. Adari and N. Sahu, Green synthesis of enantiopure quinoxaline alcohols using Daucus carota, Chirality, 2019, 31, 312–320 CrossRef CAS PubMed
.
- G. A. Behrens, A. Hummel, S. K. Padhi, S. Schätzle and U. T. Bornscheuer, Discovery and protein engineering of biocatalysts for organic synthesis, Adv. Synth. Catal., 2011, 353, 2191–2215 CrossRef CAS
.
- B. M. Nestl, S. C. Hammer, B. A. Nebel and B. Hauer, New generation of biocatalysts for organic synthesis, Angew. Chem., Int. Ed., 2014, 53, 3070–3095 CrossRef CAS PubMed
.
- K. Kavita, A. Kulkarni and S. Hussein, Application of Biocatalyst in Chemical Engineering, Int. J. Adv. Eng. Technol., 2011, 2, 506–508 Search PubMed
.
- R. De Regil and G. Sandoval, Biocatalysis for biobased chemicals, Biomolecules, 2013, 3, 812–847 CrossRef PubMed
.
- T. Pohl, E. Nägele and H. Waldmann, Biocatalysts as chemo-and regioselecting tools in organic synthesis, Catal. Today, 1994, 22, 407–426 CrossRef CAS
.
- M. E. Himmel, S.-Y. Ding, D. K. Johnson, W. S. Adney, M. R. Nimlos, J. W. Brady and T. D. Foust, Biomass recalcitrance: engineering plants and enzymes for biofuels production, Science, 2007, 315, 804–807 CrossRef CAS PubMed
.
- J. L. Rahikainen, J. D. Evans, S. Mikander, A. Kalliola, T. Puranen, T. Tamminen, K. Marjamaa and K. Kruus, Cellulase–lignin interactions—the role of carbohydrate-binding module and pH in non-productive binding, Enzyme Microb. Technol., 2013, 53, 315–321 CrossRef CAS PubMed
.
- E. Palmqvist and B. Hahn-Hägerdal, Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition, Bioresour. Technol., 2000, 74, 25–33 CrossRef CAS
.
- J. Zaldivar, A. Martinez and L. O. Ingram, Effect of selected aldehydes on the growth and fermentation of ethanologenic Escherichia coli, Biotechnol. Bioeng., 1999, 65, 24–33 CrossRef CAS PubMed
.
- R. Kumar, G. Mago, V. Balan and C. E. Wyman, Physical and chemical characterizations of corn stover and poplar solids resulting from leading pretreatment technologies, Bioresour. Technol., 2009, 100, 3948–3962 CrossRef CAS PubMed
.
- Y. H. P. Zhang, M. E. Himmel and J. R. Mielenz, Outlook for cellulase improvement: screening and selection strategies, Biotechnol. Adv., 2006, 24, 452–481 CrossRef CAS PubMed
.
- A. R. Chanysheva and V. V. Zorin, Enantioselective bioreduction of heptan-2-one and octan-2-one catalyzed by Daucus carota cells, Indian J. Chem. B, 2020, 59, 1381–1383 Search PubMed
.
- J. Liang, E. Mundorff, R. Voladri, S. Jenne, L. Gilson, A. Conway, A. Krebber, J. Wong, G. Huisman and S. Truesdell, Highly enantioselective reduction of a small heterocyclic ketone: biocatalytic reduction of tetrahydrothiophene-3-one to the corresponding (R)-alcohol, Org. Process Res. Dev., 2010, 14, 188–192 CrossRef CAS
.
- P. Bianchi, R. F. Varela, D. A. Bianchi, M. Kemppainen, A. M. Iribarren and E. Lewkowicz, Selection of microbial biocatalysts for the reduction of cyclic and heterocyclic ketones, Process Biochem., 2017, 58, 137–144 CrossRef CAS
.
- Y. Chen, B. Ma, S. Cao, X. Wu and Y. Xu, Efficient synthesis of Ibrutinib chiral intermediate in high space-time yield by recombinant E. coli co-expressing alcohol dehydrogenase and glucose dehydrogenase, RSC Adv., 2019, 9, 2325–2331 RSC
.
- N. V. Machado and A. T. Omori, Enantioselective reduction of heterocyclic ketones with low level of asymmetry using carrots, Biocatal, 2021, 39, 475–480 CrossRef CAS
.
- P. A. Carpino, B. A. Lefker, S. M. Toler, L. C. Pan, J. R. Hadcock, E. R. Cook, J. N. DiBrino, A. M. Campeta, S. L. DeNinno and K. L. Chidsey-Frink, Pyrazolinone-piperidine dipeptide growth hormone secretagogues (GHSs): discovery of capromorelin, Bioorg. Med. Chem., 2003, 11, 581–590 CrossRef CAS PubMed
.
- M. Wanner and G. Koomen, Synthesis of (±)-nitramine,(±)-isonitramine and (±)-sibirine via Diels-Alder reactions, Tetrahedron Lett., 1989, 30, 2301–2304 CrossRef CAS
.
- M. Fujii, K. Kawaguchi, K. Nakamura and A. Ohno, Stereoselective Synthesis of (±)-Isonitramine and (±)-Sibirine, Chem. Lett., 1992, 1493–1496 CrossRef CAS
.
- R. Lacheretz, D. G. Pardo and J. Cossy, Daucus carota mediated-reduction of cyclic 3-oxo-amines, Org. Lett., 2009, 11, 1245–1248 CrossRef CAS PubMed
.
- J. S. Yadav, G. S. Reddy, G. Sabitha, A. D. Krishna, A. R. Prasad, K. V. Rao and A. B. Rao, Daucus carota and baker's yeast mediated bio-reduction of prochiral ketones, Tetrahedron: Asymmetry, 2007, 18, 717–723 CrossRef CAS
.
- P. Camps, R. El Achab, J. Morral, D. Muñoz-Torrero, A. Badia, J. E. Baños, N. M. Vivas, X. Barril, M. Orozco and F. J. Luque, New tacrine− huperzine A hybrids (huprines): Highly potent tight-binding acetylcholinesterase inhibitors of interest for the treatment of Alzheimer's disease, J. Med. Chem., 2000, 43, 4657–4666 CrossRef CAS PubMed
.
- A. Zilinskas and J. Sereikaite, Stereoselective bioreduction for the resolution of racemic mixtures of bicyclo [3.3.1] nonane-2, 6-dione using vegetables, J. Mol. Catal. B: Enzym., 2013, 90, 66–69 CrossRef CAS
.
- G. Mislin and M. Miesch, Synthesis of Polyfunctionalized Bicyclo [5.3.1] undecadiene Ring Systems Using a Two-Carbon Ring-Expansion of Cyclobutene Intermediates, Eur. J. Org Chem., 2001, 2001, 1753–1759 CrossRef
.
- E. Butkus, A. Žilinskas, S. Stončius, R. Rozenbergas, M. Urbanová, V. r. Setnička, P. Bouř and K. Volka, Synthesis and chiroptical properties of enantiopure tricyclo [4.3. 0.03, 8] nonane-4, 5-dione (twistbrendanedione), Tetrahedron: Asymmetry, 2002, 13, 633–638 CrossRef CAS
.
- S. Stoncius, E. Butkus, A. Žilinskas, K. Larsson, L. Öhrström, U. Berg and K. Wärnmark, Design and Synthesis of a C 2-Symmetric Self-Complementary Hydrogen-Bonding Cleft Molecule Based on the Bicyclo [3.3.1] nonane and 4-Oxo-5-azaindole Framework. Formation of Channels and Inclusion Complexes in the Solid State, J. Org. Chem., 2004, 69, 5196–5203 CrossRef CAS PubMed
.
- E. Orentas, G. Bagdžiūnas, U. Berg, A. Žilinskas and E. Butkus, Enantiospecific synthesis and chiroptical properties of bicyclic enones, Eur. J. Org. Chem., 2007, 2007, 4251–4256 CrossRef
.
- W. Adam, M. Lazarus, C. R. Saha-Möller and P. Schreier, Biocatalytic synthesis of optically active α-oxyfunctionalized carbonyl compounds, Acc. Chem. Res., 1999, 32, 837–845 CrossRef CAS
.
- H. Kunz, W. Sager, D. Schanzenbach and M. Decker, Carbohydrates as chiral templates: Stereoselective Strecker synthesis of D-α-amino nitriles and acids using O-pivaloylated d-galactosylamine as the auxiliary, Liebigs Ann. Chem., 1991, 1991, 649–654 CrossRef
.
- A. Chadha and B. Baskar, Biocatalytic deracemisation of α-hydroxy esters: high yield preparation of (S)-ethyl 2-hydroxy-4-phenylbutanoate from the racemate, Tetrahedron: Asymmetry, 2002, 13, 1461–1464 CrossRef CAS
.
- H. Yu and H. Simon, Hydroxylation of and halogen addition to the carbon carbon double bond of (R)-2-hydroxy-3-enoic acids, Tetrahedron, 1991, 47, 9035–9052 CrossRef CAS
.
- B. Baskar, S. Ganesh, T. Lokeswari and A. Chadha, Highly stereoselective reduction of 4-Aryl-2-oxo but-3-enoic carboxylic esters by plant cell culture of Daucus carota, J. Mol. Catal. B: Enzym., 2004, 27, 13–17 CrossRef CAS
.
- Y. Nakamura, S. Watanabe, N. Miyake, H. Kohno and T. Osawa, Dihydrochalcones: evaluation as novel radical scavenging antioxidants, J. Agric. Food Chem., 2003, 51, 3309–3312 CrossRef CAS PubMed
.
- Y. L. Shi, Y.-P. Zhang, H. Luo, F. Xu, J.-M. Gao, J.-S. Shi and Q. H. Gong, Trilobatin, a natural food additive, exerts anti-type 2 diabetes effect mediated by Nrf2/ARE and IRS-1/GLUT2 signaling pathways, Front. Pharmacol, 2022, 13, 828473 CrossRef CAS PubMed
.
- V. Haritha, S. V. Satyanarayana, B. R. Adari and B. S. Reddy, Chemoenzymatic reduction of double bonds from chalcones using Daucus carota roots, Biocatal. Agric. Biotechnol., 2023, 51, 102770 CrossRef CAS
.
- A. Sivropoulou, S. Kokkini, T. Lanaras and M. Arsenakis, Antimicrobial activity of mint essential oils, J. Agric. Food Chem., 1995, 43, 2384–2388 CrossRef CAS
.
- H. J. Bouwmeester, M. C. Konings, J. Gershenzon, F. Karp and R. Croteau, Cytochrome P-450 dependent (+)-limonene-6-hydroxylation in fruits of caraway (Carum carvi), Phytochem, 1999, 50, 243–248 CrossRef CAS
.
- C. C. De Carvalho and M. M. R. Da Fonseca, Carvone: Why and how should one bother to produce this terpene, Food Chem., 2006, 95, 413–422 CrossRef CAS
.
- S. Sabir, D. Singh and J. Rocha, In vitro antioxidant activity of S-carvone isolated from Zanthoxylum alatum, Pharm. Chem. J., 2015, 49, 187–191 CrossRef CAS
.
- K. A. Wojtunik-Kulesza, K. Targowska-Duda, K. Klimek, G. Ginalska, K. Jóźwiak, M. Waksmundzka-Hajnos and Ł. Cieśla, Volatile terpenoids as potential drug leads in Alzheimer's disease, Open Chem., 2017, 15, 332–343 CAS
.
- W. Mączka, D. Sołtysik, K. Wińska, M. Grabarczyk and A. Szumny, Plant-mediated biotransformations of S (+)- and R (–)-carvones, Appl. Sci., 2018, 8, 2605 CrossRef
.
- C. Lamberth, Latest research trends in agrochemical fungicides: any learnings for pharmaceutical antifungals?, ACS Med. Chem. Lett., 2022, 13, 895–903 CrossRef CAS PubMed
.
- G. de Gonzalo, A. R. Alcántara, P. Domínguez de María and J. M. Sánchez-Montero, Biocatalysis for the asymmetric synthesis of Active Pharmaceutical Ingredients (APIs): This time is for real, Expert Opin. Drug Discovery, 2022, 17, 1159–1171 CrossRef CAS PubMed
.
- C. Acharya, A. Achari and P. Jaisankar, Daucus carota root enzyme catalyzed Henry reaction: A green approach, Tetrahedron Lett., 2018, 59, 663–666 CrossRef CAS
.
- W. Xiong, X. Wang and L. Kong, Design and application of a biphasic system that enhances productivity of Daucus carota-catalyzed asymmetric reduction, Biotechnol. Lett., 2015, 37, 1703–1709 CrossRef CAS PubMed
.
- V. Haritha, P. Deepthi, R. Gundamalla, K. Nagesh, S. V. Satyanarayana, A. B. Rao, S. Balasubramanian and B. V. S. Reddy, Biocatalytic enantioselective synthesis of cenobamate, an antiepileptic drug, Chirality, 2024, 36, e23660 CrossRef CAS PubMed
.
- W. J. Bai, M. A. Estrada, J. A. Gartman and A. S. Judd, Enantioselective Bioreduction of Medicinally Relevant Nitrogen-Heteroaromatic Ketones, ACS Med. Chem. Lett., 2023, 14, 846–852 CrossRef CAS PubMed
.
- M. S. Nedjimi and L. Sekhri, Asymmetric Bioreduction of Prochiral Ketones Catalyzed by Cynara scolumus L, Terfezia sp and Phoenix dactylifera, Biomed. Pharmacol. J., 2016, 9, 469–476 CrossRef
.
| This journal is © The Royal Society of Chemistry 2025 |