Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
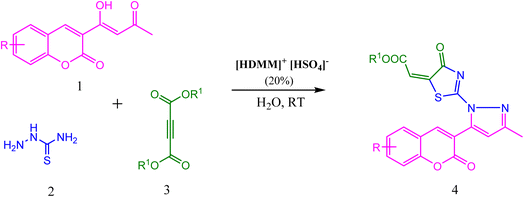
Brønsted acidic surfactant [HDMM]+ [HSO4]−: a green microreactor assembly for stereoselective synthesis of novel thiazolyl-pyrazole-chromen-2-ones in water†
Mayuri V. Patila,
Pradeep M. Mhaldarb,
Vrushali M. Mahadika,
Rinku Ghanta c,
Madhulata Shukla
c,
Madhulata Shukla d,
Suraj A. Sonawanee,
Suresh K. Ghotekar
d,
Suraj A. Sonawanee,
Suresh K. Ghotekar f,
Gajanan S. Rashinkar
f,
Gajanan S. Rashinkar a and
Dattaprasad M. Pore
a and
Dattaprasad M. Pore *a
*a
aDepartment of Chemistry, Shivaji University, Kolhapur-416004, Maharashtra, India
bShrimant Bhaiyyasaheb Rajemane Mahavidyalaya, Mhaswad Tal; Man, Dist, Satara 415509, India
cDiamond Harbour Women's University, Sarisha, South 24 Parganas (S), West Bengal 743368, India
dGram Bharti College Ramgarh, Veer Kunwar Singh University, Kaimur, Bihar, 821110, India
eRajaram Mahavidyalaya, Kolhapur-416004, Maharashtra, India
fCentre for Herbal Pharmacology and Environmental Sustainability, Chettinad Hospital and Research Institute, Chettinad Academy of Research and Education, Kelambakkam, Tamil Nadu 603103, India. E-mail: p_dattaprasad@rediffmail.com
First published on 28th April 2025
Abstract
A novel Brønsted acidic surfactant was synthesized and employed as a catalyst for a one-pot multi-component reaction. Small angle X-ray scattering (SAXS) analysis was performed, which confirmed that the micelles exhibited an average diameter of 3.1 nm and average inter-micellar distance of 0.49 nm. Ground state density functional theory (DFT) calculation was performed on the surfactant molecule to optimize the geometrical structure. A series of novel thiazolyl-pyrazole-chromen-2-one derivatives were efficiently synthesized through a convenient one-pot multi-component reaction of substituted 3-acetoacetyl coumarins, thiosemicarbazide and dialkyl acetylene dicarboxylates in water using a novel hexadecyl methyl morpholinium hydrogen sulfate [HDMM]+ [HSO4]− as surfactant. Operational simplicity, stereoselective synthesis, quick access to the desired products, high purity and good to excellent yields are the key advantages of this approach. This work remarkably highlights the dual novelty as a new class of thiazolyl-pyrazole-chromen-2-one derivatives as well as a [HDMM]+ [HSO4]− surfactant.
1 Introduction
In recent years, the development of sustainable reaction processes is a topic of great interest.1 The effort for this began with the use of water as a green solvent in organic transformation.2–9 Water is an abundant renewable resource and avoids the production of environmentally harmful waste and high process costs. Additionally, on many occasions, it activates the functional groups by forming hydrogen bonds.10 Poor solubility of the reactants is one of the limitations while using aqueous media. Hence, ‘on water’ approach is the most suitable instead of using polar aprotic solvent. The ‘on water’ system involves a surfactant that induces the reaction through micelle in an aqueous medium. The presence of surfactant significantly improves the solubility of hydrophobic compounds in water.The construction of bioactive scaffolds is known to be the focus of research in organic synthesis. Designing new drugs from hybrid molecules using different pharmacophores may offer remarkable biological activities. Sulfur–nitrogen containing heterocyclic compounds, specifically thiazoles and their derivatives, are a medicinally and pharmaceutically important class of heterocycles. Thiazolidin-4-ones are important moieties in synthetic reactions and exhibit various biological activities, including anticancer,11 anti-inflammatory,12 antimicrobial,13 anticonvulsant,14 antifungal,15 antitubercular,16 anti-HIV,17 analgesics,18 antimalarial,19 HIV inhibitory activity,20,21 and hyperglycemic22 reversal activity.23
Conversely, pyrazole and its derivatives exhibit various therapeutic activities24,25 such as anti-inflammatory,26 antihypertensive,27 antimicrobial,28 antidiabetic,29 and anticancer30 activities. Celecoxib and pyrazofurin, which are known as blockbuster drugs, incorporate pyrazole rings into their core structures (Fig. 1). Additionally, compounds of this class play a vital role in organic syntheses.31,32
 | ||
| Fig. 1 Representative examples of bioactive compounds containing coumarin, pyrazole and thiazolidinone scaffolds. | ||
As far as various classes of heterocycles are concerned, coumarins are eminent owing to their excellent pharmacological properties. Coumarins, owing to their structural diversity, are considered an efficient candidate in pharmaceutical chemistry and exhibit a range of biological activities.33–35 Anisucoumaramide and clauhainanin-A isolated from Clausena anisum-olens and Clausena hainanensis exhibit remarkable pharmacological properties.36,37 Coumarin derivatives obtained from natural sources include dicoumarol, warfarin, acenocoumarol, and coumachlor. They are widely used to decrease blood coagulation.38 In addition, various molecules containing a coumarin skeleton have applications as photosensitizers,39 fluorescent chemosensors40 for light energy harvesting,41 and electroluminescent materials42 and in soaps, perfumes, and detergents.43
Considering the importance of thiazolidine-4-one, pyrazole, and coumarin derivatives, and as a part of our endeavour towards the synthesis of a new class of biologically potent heterocyclic hybrids using a green chemistry protocol, herein, we report a highly efficient method for the diversity-oriented synthesis of thiazolyl-pyrazole-chromen-2-ones via a one-pot reaction of acetoacetyl coumarin, thiosemicarbazide, and dialkyl acetylene dicarboxylate in the presence of hexadecyl methyl morpholinium hydrogen sulphate ([HDMM]+ [HSO4]−) as an efficient and reusable Brønsted acidic surfactant catalyst (Scheme 1).
2 Results and discussion
The catalyst significantly affects various factors in organic synthesis; hence, it is a key parameter. The scientific community is continuously making efforts to develop organic transformations in aqueous medium using a benign catalytic system and avoiding toxic catalysts. Hence, initial attention was focused on the design and synthesis of a novel catalyst, viz Brønsted acidic surfactant (Fig. 2).The synthesis of 4-hexadecyl-4-methylmorpholin-4-ium hydrogen sulphate, [HDMM]+ [HSO4]−, is depicted in Scheme 2. The quaternization of 4-methylmorpholine with 1-bromo hexadecane in acetone is carried out by refluxing the reaction mixture at 60 °C for 24 h to afford 4-hexadecyl-4-methylmorpholin-4-ium bromide, [HDMM]+ [Br]−, followed by anion exchange with conc. H2SO4 in dry toluene at 80 °C for 24 h furnished 4-hexadecyl-4-methylmorpholin-4-ium hydrogen sulphate, [HDMM]+ [HSO4]−, a Brønsted acidic surfactant. The absence of bromide ions was examined by testing the reaction of the surfactant with AgNO3. The synthesized catalyst was confirmed by FTIR, 1H, 13C NMR, CMC, and TGA analyses. The obtained spectroscopic data fully agreed with the structure of the surfactant catalyst.
The critical micelle concentration (CMC) of a surfactant solution was determined by applying the conductometric method. Fig. 3 illustrates the plot of CMC with coordinates equivalent conductance (k) versus surfactant concentration. The CMC of [HDMM]+ [HSO4]− was found to be 0.0037 mol dm−3.
The thermo gravimetric analysis (TGA) was performed at temperature ranging from 25 to 1000 °C under aerobic conditions at 10 °C min−1 (Fig. 4). Initially, a weight loss of 4.515% was observed in the temperature range of 25–200 °C owing to the loss of physically adsorbed water from the catalyst. Further, the large weight loss of 75.61% in the range of 200–330 °C is attributed to the exothermic decomposition of the organic moiety. The third weight loss of 18.81% is due to the decomposition of residual carbonaceous species.
2.1 Small-angle X-ray scattering (SAXS) analysis
To elucidate the micelle diameter and the intermicellar spacing of [HDMM]+ [HSO4]−, small-angle X-ray scattering (SAXS) analysis was performed. SAXS data for the samples were obtained using a Xenocs SA-France instrument, employing CuKα X-rays with a wavelength of 1.54 Å. The sample was placed in a quartz capillary, and measurements were collected for ten minutes using line collimation. The sample-to-detector distance was 2500 millimeters. The detectors used were an Eiger R 1 M equipped with a vacuum lining and a high-resolution hybrid pixel photon counting system featuring a pixel size of 75 μm. The sample was subjected to 15-minute exposure intervals, during which scattered waves were recorded across a momentum transfer range of q = 0.04 to 4 nm−1 (eqn (1)):
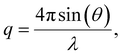 | (1) |
Fig. 5a and b show the intensity (I) vs. scattering vector (q) plots obtained from the scattering data of the SAXS measurements of [HDMM]+ [HSO4]− surfactant micelle assembly in water. X-ray scattering was observed at q = 12.74 nm−1. The intermicellar separation distance (dBragg) was determined to be 0.49 nm (eqn (2)):
 | (2) |
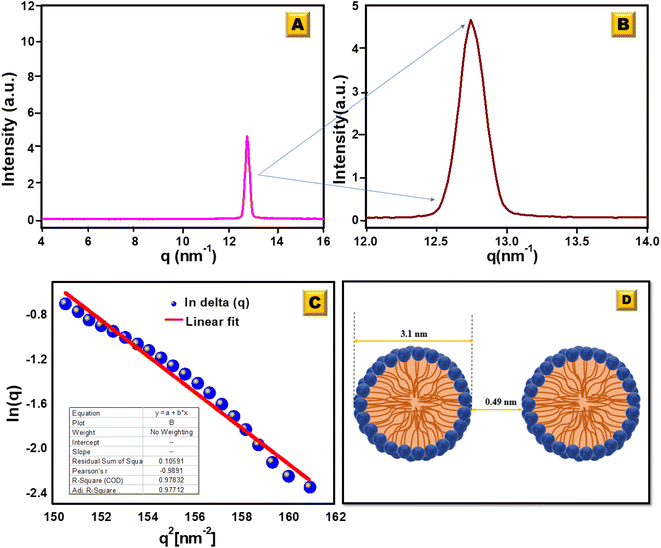 | ||
| Fig. 5 (A) SAXS analysis for the [HDMM]+ [HSO4]− surfactant in water. (B) Scattered peak position. (C) Guinier's plot. (D) Spacing between the micelle and diameter of the micelle. | ||
To determine the size of the spherical micelle system, the radius of gyration (RG) was calculated using Guinier's plot (Fig. 5c). Guinier's plot is obtained from the equation given below:
 | (3) |
The radius of the gyration (RG) was 0.69 nm evaluated from the slope of ln[Δ(q)] vs. q2 plot. The straight line of the data points in the Guinier plot indicates the uniform size of the micelles formed in water. This confirms the introduction of a homogeneous distribution of equal-sized micelles. The average radius of the spherical particle (R) was derived from the radius of gyration (RG) using the equation  . The average radius of the particle was detected to be 1.55 nm and the diameter is 3.1 nm. The average distance of separation was found to be 0.49 nm.44
. The average radius of the particle was detected to be 1.55 nm and the diameter is 3.1 nm. The average distance of separation was found to be 0.49 nm.44
2.2 Molecular geometry optimization
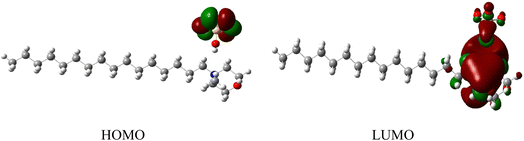
Ground state Density Functional Theory (DFT) calculation was performed on the surfactant molecule to optimize the geometrical structure. The DFT calculation was carried out at Becke's three-parameter functional and Lee–Yang–Parr hybrid functional (B3LYP) levels using the Gaussian 16 program. The 6-31G++(d,p) basis set was used for geometry optimization.45,46 Calculations were performed in the gaseous phase. The optimized structure of the surfactant molecule is shown in Fig. 6. The optimized structure depicts weak hydrogen bonding of lengths 2.16 Å, 2.13 Å, and 1.98 Å existing between the oxygen of the anion and the hydrogen of the cation moiety. Hydrogen bonding is shown by dotted lines in Fig. 6. The Mulliken charge distribution of the optimized molecule calculated at the same level of calculation is shown in Fig. 7. | ||
| Fig. 7 Mulliken charge distribution on the surfactant molecule [HDMM]+ [HSO4]− (the green color represents the positively charged atom, while the brown color represents the negatively charged atoms). | ||
The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) of a surfactant molecule are shown in Fig. 8. HOMO is located on the non-bonding orbital of the oxygen atom of the anion moiety, while LUMO is localized mainly on the cyclohexane ring of the cation moiety.
2.3 Optimization study
After these major achievements, attention was turned towards exploring the catalytic efficiency of the synthesized catalyst, [HDMM]+ [HSO4]−, to synthesize thiazolyl-pyrazole-chromen-2-one derivatives. The optimization of the catalyst was carried out using the model reaction of acetoacetyl coumarin, thiosemicarbazide and dimethyl acetylene dicarboxylate (DMAD) at room temperature. No significant yield was obtained for the reaction without a catalyst (Table 1, entry 1). Therefore, K2CO3, NH2–SO3H, p-TSA, L-proline, cetyltrimethyl ammonium bromide (CTAB), sodium dodecyl sulphate (SDS), Triton X-100, sodium dioctyl sulfosuccinate (SDOSS), benzethonium chloride [BZT]+ Cl−, [BZT]+ AlCl4− and [HDMM]+ [HSO4]− are screened for model reaction (Table 1, entries 2–12).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | Catalyst | Catalyst load (mol%) | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: acetoacetyl coumarin (1 mmol), thiosemicarbazide (1 mmol), dialkyl acetylene dicarboxylate (1 mmol), specific catalyst, water (5 mL), RT.b Isolated yield. | ||||
| 1 | — | — | 12–24 | 20 |
| 2 | K2CO3 | 20 | 20 | 40 |
| 3 | NH2–SO3H | 20 | 6.2 | 78 |
| 4 | p-TSA | 20 | 5 | 80 |
| 5 | L-Proline | 20 | 7 | 70 |
| 6 | CTAB | 20 | 18 | 55 |
| 7 | SDS | 15 | 24 | 58 |
| 8 | Triton X 100 | 20 | 24 | 61 |
| 9 | SDOSS | 20 | 20 | 48 |
| 10 | [BZT]+ Cl− | 20 | 11 | 55 |
| 11 | [BZT]+ AlCl4− | 20 | 6 | 75 |
| 12 | [HDMM]+ HSO4− | 10 | 4 | 80 |
| 13 | [HDMM]+ HSO4− | 15 | 3 | 83 |
| 14 | [HDMM]+ HSO4− | 20 | 2.5 | 89 |
| 15 | [HDMM]+ HSO4− | 25 | 3 | 90 |
K2CO3 as a catalyst provides a low yield of the desired product (Table 1, entry 2). The yield was sufficiently increased for the reactions in the acid catalysts (Table 1, entries 3–5, and 11). Commercially available surfactants, viz cetyltrimethyl ammonium bromide (CTAB), sodium dodecyl sulfate (SDS), Triton X-100, sodium dioctyl sulfosuccinate (SDOSS) and benzethonium chloride [BZT]+ Cl−, gave moderate yield in aqueous medium (Table 1, entries 6–10). Pleasingly, [HDMM]+ [HSO4]− exhibited a high yield (Table 1, entry 14). Thus, amongst the catalysts screened, [HDMM]+ [HSO4]− was found to be superior for performing the reaction at room temperature.
The effect of catalyst loading was also studied for the model reaction. The 20 mol% of [HDMM]+ [HSO4]− was found to be tolerable in promoting this reaction (Table 1, entries 12–15). Catalyst loading greater than 20% did not positively influence both the yield and reaction time.
The reaction mixture of acetoacetylcoumarin and thiosemicarbazide was converted into a homogeneous solution by adding a surfactant. The formation of white turbid emulsion confirms the formation of micelles or colloidal aggregates [Fig. 9(I)]. Finally, an orange precipitate was obtained after the addition of DMAD, indicating the completion of the reaction. The formation of spherical emulsion droplets (microbubbles) in the aqueous medium was confirmed by taking an optical microscopic image [Fig. 9(II)].
Notably, the workup of the reaction was carried out by simple filtration and recrystallization in hot ethanol to produce an extremely pure product. Based on spectral information, the structure of the product was confirmed (4a). Infrared analysis of compound 4a exhibited absorption bands at 1719, 1687 and 1347 cm−1 due to lactone (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), imine (C
O), imine (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), and (C–S) stretching frequencies, respectively. The 1H NMR and 13C NMR spectra of 4a concluded that the product was isometrically pure. The 1H NMR study of methyl (E)-2-(2-(3-methyl-5-(2-oxo-2H-chromen-3-yl)-1H-pyrazol-1-yl)-4-oxothiazol-5(4H)ylidene) acetate 4a showed that the singlet of vinylic proton at δ = 6.73 ppm clearly indicates the E-configuration of the exocyclic double bond of the thiazolidinone ring.47–49 The presence of vinylic proton above 6.90 ppm supported the Z-configuration.50 The peaks at δ 2.34 ppm and 3.87 ppm are due to methyl and methoxy protons, respectively. The singlet corresponding to the methine proton of pyrazole and coumarin is observed at δ 6.69 and 8.41 ppm, respectively. The aromatic protons of the coumarin skeleton were observed at δ 7.37–8.02 ppm. 13C NMR analysis also confirmed the structural identity, with resonance observed at δ 15.89 (–CH3), 52.06 (–OMe), 100.42 (pyrazol–CH), 114.81, 116.15, 118.59, 118.77, 124.82, 126.10, 129.20 (coumarin Ar-CH), 129.79, 132.29, 140.71 (pyrazole C–N), 141.77 (pyrazole C
N), and (C–S) stretching frequencies, respectively. The 1H NMR and 13C NMR spectra of 4a concluded that the product was isometrically pure. The 1H NMR study of methyl (E)-2-(2-(3-methyl-5-(2-oxo-2H-chromen-3-yl)-1H-pyrazol-1-yl)-4-oxothiazol-5(4H)ylidene) acetate 4a showed that the singlet of vinylic proton at δ = 6.73 ppm clearly indicates the E-configuration of the exocyclic double bond of the thiazolidinone ring.47–49 The presence of vinylic proton above 6.90 ppm supported the Z-configuration.50 The peaks at δ 2.34 ppm and 3.87 ppm are due to methyl and methoxy protons, respectively. The singlet corresponding to the methine proton of pyrazole and coumarin is observed at δ 6.69 and 8.41 ppm, respectively. The aromatic protons of the coumarin skeleton were observed at δ 7.37–8.02 ppm. 13C NMR analysis also confirmed the structural identity, with resonance observed at δ 15.89 (–CH3), 52.06 (–OMe), 100.42 (pyrazol–CH), 114.81, 116.15, 118.59, 118.77, 124.82, 126.10, 129.20 (coumarin Ar-CH), 129.79, 132.29, 140.71 (pyrazole C–N), 141.77 (pyrazole C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 142.72, 146.05 (coumarin –CH), 153.06 (
N), 142.72, 146.05 (coumarin –CH), 153.06 (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–COOMe), 153.24 C
CH–COOMe), 153.24 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C thiazole), 161.09 (C
C thiazole), 161.09 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N thiazole), 163.91 (–CO lactone), 166.12 (–CO ester) and 170.89 (–CO–amide). The molecular ion peak of compound 4a was found in the mass spectrum at m/z = 395.39 [M]+, corresponding to the molecular formula C19H13N3O5S. All the above spectroscopic data clearly indicate the formation of the target product.
N thiazole), 163.91 (–CO lactone), 166.12 (–CO ester) and 170.89 (–CO–amide). The molecular ion peak of compound 4a was found in the mass spectrum at m/z = 395.39 [M]+, corresponding to the molecular formula C19H13N3O5S. All the above spectroscopic data clearly indicate the formation of the target product.
With improved reaction conditions in hand, we expanded the scope of the reaction using various structurally diverse acetoacetyl coumarin derivatives with dimethyl and diethyl acetylene dicarboxylates (DMAD and DEAD) [Table 2]. Interestingly, acetoacetyl coumarin with electron-withdrawing and electron-donating substituents was almost inevitably transformed into its respective targets with an excellent yield (Table 2, product 4c–4i).
| a Reaction conditions: acetoacetylcoumarin (1 mmol), thiosemicarbazide (1 mmol), dialkyl acetylene dicarboxylate (1 mmol), catalyst: [HDMM]+ [HSO4]− (20%), water (5 mL), room temperature. |
|---|
 |
Eventually, the competency of the reaction was examined using an acetoacetyl coumarin derivative synthesized from 2-hydroxy naphthaldehyde, and it was found that the reaction performed well with a good yield (Table 2, product 4j).
A plausible mechanism for the formation of thiazolyl-pyrazole-chromen-2-ones is depicted in Scheme 3. Initially, the condensation of thiosemicarbazide 2 with acetoacetyl coumarin 1 results in the formation of Knorr-pyrazole skeleton 5. The sulphur atom of 6 as thiol after prototropic tautomeric shift attacks one of the ethynyl carbons of DMAD 7 in a Michael addition manner to yield S-alkylated intermediate 8, followed by an intramolecular amidation reaction to yield product 9. Overall, the reaction generates one C–S, one C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and two C–N bonds. Simultaneously, thiazole and pyrazole heterocycles were developed successively.
N, and two C–N bonds. Simultaneously, thiazole and pyrazole heterocycles were developed successively.
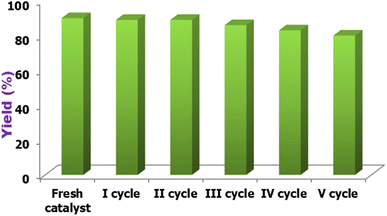
Because catalyst reusability is an essential economic consideration, recovery and reusability experiments were conducted for the reaction of acetoacetyl coumarin, thiosemicarbazide, and dimethyl acetylene dicarboxylate. Following the reaction, the product was filtered and washed multiple times with 25 mL of water. The collected filtrate was concentrated on a rotary evaporator to 5 mL, and the filtrate remaining in the flask was washed with diethyl ether before being reused immediately by adding substrates in the next cycle, with no additional purification. As shown in Fig. 10, the catalyst can be reused five times without a significant decrease in catalytic activity.
3 Conclusion
In conclusion, we developed a new, facile, one-pot multi-component synthesis of substituted thiazolyl-pyrazole-chromen-2-ones from acetoacetyl coumarin, thiosemicarbazide, and dialkyl acetylene dicarboxylate in the presence of [HDMM]+ [HSO4]− as an effective Brønsted acidic surfactant. The small angle X-ray scattering (SAXS) analysis and ground state Density Functional Theory (DFT) calculation confirmed the size and geometrical structure of the surfactant molecule. This approach provides various advantages, such as the use of water as a universal solvent, short reaction time, good yields, wide substrate scope, easy work-up, and furnishing a pure product without tedious column chromatography. This transformation established the formation of four bonds: one C–S, one C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and two C–N bonds. It also generated thiazole and pyrazole heterocycles. The synthesized derivatives containing three heterocyclic rings may be beneficial in drug discovery.
N, and two C–N bonds. It also generated thiazole and pyrazole heterocycles. The synthesized derivatives containing three heterocyclic rings may be beneficial in drug discovery.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Mayuri Patil: conceptualization, methodology, writing – original draft preparation. Pradeep Mhaldar: methodology, software, data curation, Vrushali Mahadik: visualization, writing – reviewing and editing Rinku Ghanta and Madhulata Shukla: software, validation. Suraj Sonawane: writing – reviewing and editing Suresh K. Ghotekar: visualization, writing – reviewing and editing Gajanan Rashinkar: supervision, writing original draft Dattaprasad Pore: supervision, visualization, writing – reviewing and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
MVP is thankful to Shivaji University, Kolhapur for providing Golden Jubilee Research Fellowship (Ref. No. SU/CUDC/UGK/GJRF/16/2019-20/763) of the Department of Industrial Chemistry.References
- (a) R. T. Baker and W. Tumas, Science, 1999, 284, 1477 CrossRef CAS; (b) P. T. Anastas and J. C. Warner; Green Chemistry: Theory and Practice, Oxford University Press, Oxford, 1998 Search PubMed; (c) J. Haggin, Chem. Eng. News, 1994, 72, 22 Search PubMed; (d) E. M. Kirschner, Chem. Eng. News, 1994, 72, 13 Search PubMed; (e) D. L. Illman, Chem. Eng. News, 1994, 72, 22 Search PubMed.
- N. Azizi, A. K. Amiri, R. Baghi, M. Bolourtchian and M. M. Hashemi, Monatsh. Chem., 2009, 140, 1471 CrossRef CAS.
- P. A. Grieco, Organic Synthesis in Water, Blackie Academic and Professional, 1998 Search PubMed.
- C. J. Li, Chem. Rev., 1993, 93, 2023 CrossRef CAS.
- C. J. Li, Chem. Rev., 2005, 105, 3095 CrossRef CAS PubMed.
- C. J. Li and T. H. Chan, Organic Reaction in Aqueous Media, Wiley New York, 1997 Search PubMed.
- M. C. Pirrung and K. D. Sarma, J. Am. Chem. Soc., 2004, 126, 444 CrossRef CAS PubMed.
- A. Shaabani, A. Rahmati, A. H. Rezayan, M. Darvishi, Z. Badri and A. Sarvari, QSAR Comb. Sci., 2007, 26, 973 CrossRef CAS.
- W. Wei, C. C. K. Keh, C. J. Li and R. S. Varma, Environ. Sci. Policy, 2004, 6, 250 CAS.
- A. Chanda and V. V. Fokin, Chem. Rev., 2009, 109, 725 Search PubMed.
- H. Zhou, S. Wu, S. Zhai, A. Liu, Y. Sun, R. Li, Y. Zhang, S. Ekins, P. W. Swaan, B. Fang, B. Zhang and B. Yan, J. Med. Chem., 2008, 51, 1242 CrossRef CAS PubMed.
- M. G. Vigorita, R. Ottana, F. Monforte, R. Maccari, M. T. Monforte, A. Trovato, M. F. Taviano, N. Miceli, G. D. Luca, S. Alcaro and F. Ortuso, Bioorg. Med. Chem., 2003, 11, 999 CrossRef CAS PubMed.
- S. Bondock, W. Khalifa and A. A. Fadda, Eur. J. Med. Chem., 2007, 42, 948 CrossRef CAS PubMed.
- S. V. K. Archana and A. Kumar, Indian J. Pharm. Sci., 2003, 65, 358 Search PubMed.
- K. Omar, A. Geronikaki, P. Zoumpoulakis, C. Camoutsis, M. Sokovic, A. Ciric and J. Glamoclija, Bioorg. Med. Chem., 2010, 18, 426 CrossRef CAS PubMed.
- K. Babaoglu, M. A. Page, V. C. Jones, M. R. McNeil, C. Dong, J. H. Naismithc and R. E. Lee, Bioorg. Med. Chem. Lett., 2003, 13, 3227 CrossRef CAS PubMed.
- H. Chen, T. Yang, S. Wei, H. Zhang, R. Li, Z. Qin and X. Li, Bioorg. Med. Chem. Lett., 2012, 22, 7041 CrossRef CAS PubMed.
- G. C. Look, J. R. Schullek, C. P. Holmes, J. P. Chinn, E. M. Gordon and M. A. Gallop, Bioorg. Med. Chem. Lett., 1996, 6, 707 CrossRef CAS.
- F. A. R. Ruiz, R. N. Garcia-Sanchez, S. V. Estupinan, A. Gomez-Barrio, D. F. T. Amado, B. M. Perez-Solorzano, J. J. Nogal-Ruiz, A. R. Martinez- Fernandez and V. V. Kouznetsov, Bioorg. Med. Chem., 2011, 19, 4562 CrossRef PubMed.
- H. Chen, L. Hao, M. Zhu, T. Yang, S. Wei, Z. Qin, P. Zhang and X. Li, Bioorg. Med. Chem. Lett., 2014, 24, 3426 CrossRef CAS PubMed.
- A. Zarghi, T. Zebardast, B. Daraie and M. Hedayati, Bioorg. Med. Chem., 2009, 17, 5369 CrossRef CAS PubMed.
- N. Rahuja, A. Mishra, S. Gautam, A. K. Tamrakar, R. Maurya, S. K. Jain and A. K. Srivastava, Int. J. Pharm. Sci. Rev. Res., 2013, 22, 121 Search PubMed.
- S. Raza, S. P. Srivastava, D. S. Srivastava, A. K. Srivastava, W. Haq and S. B. Katti, Eur. J. Med. Chem., 2013, 63, 611 CrossRef CAS PubMed.
- R. Perez-Fernandez, P. Goya and J. Elguero, ARKIVOC, 2014, 233 Search PubMed.
- P. D. Sauzem, P. Machado, M. A. Rubin, G. Da, S. Sant Anna, H. B. Faber, A. H. De Souza, C. F. Mello, P. Beck, R. A. Burrow, H. G. Bonacorso, N. Zanatta, M. A. P. Martins and D. Patricia, Eur. J. Med. Chem., 2008, 43, 1237 CrossRef CAS PubMed.
- M. N. A. Nasr and S. A. Said, Arch. Pharm., 2003, 336, 551 CrossRef CAS PubMed.
- G. Turan-Zitouni, P. Chevallet, F. S. Killic and K. Erol, Eur. J. Med. Chem., 2000, 35, 635 CrossRef CAS PubMed.
- Z. A. Kaplancikli, G. Turan-Zitouni, A. Ozdemir, G. Revial and K. Guven, Phosphorus, Sulfur Silicon Relat. Elem., 2007, 182, 749 CrossRef CAS.
- R. B. Pathak and S. C. Bahel, J. Indian Chem. Soc., 1980, 57, 1108 CAS.
- N. Shankaraiah, V. Devaiah, K. L. Reddy, A. Juvekar, S. Sen, N. Kurian and S. Zingde, Bioorg. Med. Chem. Lett., 2008, 18, 1468 CrossRef PubMed.
- S. Lee and S. B. Park, Org. Lett., 2009, 11, 5214 CrossRef CAS PubMed.
- J. Orrego-Hernandez, J. Cobo and J. Portilla, Eur. J. Org Chem., 2015, 23, 5064 CrossRef.
- M. Moreno-Manas and R. Pleixats, Advances in Heterocyclic Chemistry, Academic Press, San Diego, CA, 1996, vol. 53, p. 1 Search PubMed.
- D. Egan, E. O Kennedy, E. Moran, D. Cox, E. Prosser and R. D. Thornes, Drug Metab. Rev., 1990, 22, 503 CrossRef CAS PubMed.
- (a) C. Kontogiorgis and D. Hadjipavlou-Litina, J. Enzyme Inhib. Med. Chem., 2003, 18, 63 CrossRef CAS PubMed; (b) C. Spino, M. Dodier and S. Sotheeswaran, Bioorg. Med. Chem. Lett., 1998, 8, 3475 CrossRef CAS PubMed; (c) I. Kempen, D. Papapostolou, N. Thierry, L. Pochet, S. Counerotte, B. Masereel, J. M. Foidart, M. J. Reboud-Ravaux, A. Noel and B. Pirotte, Br. J. Cancer, 2003, 88, 1111 CrossRef CAS PubMed; (d) S. Vilar, E. Quezada, L. Santana, E. Uriarte, M. Yanez, N. Fraiz, C. Alcaide, E. Cano and F. Orallo, Bioorg. Med. Chem. Lett., 2006, 16, 257 CrossRef CAS PubMed; (e) E. Quezada, G. Delogu, C. Picciau, L. Santana, G. Podda, F. Borges, V. Garcia-Moraes, D. Vina and F. Orallo, Molecules, 2010, 15, 270 CrossRef CAS PubMed.
- Y. Wang, B. Li, S. Liu, Z. Wen, J. Yang, H. Zhang and X. Hao, J. Nat. Prod., 2017, 80, 798 CrossRef CAS PubMed.
- Y. Ma, C. Zhang, W. Zhao, S. Shi, D. He, P. Zhang, X. Liu, T. Han, Y. Fu and Y. Liu, Nat. Prod. Res., 2018, 32, 2159 Search PubMed.
- D. A. Horton, G. T. Bourne and M. L. Smythe, Chem. Rev., 2003, 103, 893 CrossRef CAS PubMed.
- H. Kohjiro, S. Kazuhiro, O. Yasuyo, S. Akira, S. Sadaharu and A. Hironori, Chem. Commun., 2001, 6, 569 Search PubMed.
- G. He, X. Zhang, C. He, X. Zhao and C. Duan, Tetrahedron, 2010, 66, 9762 Search PubMed.
- (a) J. M. Lang and H. G. Drickamer, J. Phys. Chem., 1993, 97, 5058 CrossRef CAS; (b) A. R. A. Palmans, P. Smith and C. Weder, Macromolecules, 1999, 32, 4677 CrossRef CAS.
- (a) M. A. Tlenkopatchev, S. Fomine, L. Fomina, R. Gavino and T. Ogawa, Polym. J., 1997, 29, 622 CrossRef CAS; (b) S. Fomine, C. Delgado, L. Fomina, R. Gavino and T. Ogawa, Macromol. Chem. Phys., 1997, 198, 3065 Search PubMed; (c) S. Fomine, L. Fomina, C. Sanchez, A. Ortiz and T. Ogawa, Polym. J., 1997, 29, 49 CrossRef CAS; (d) S. Fomine, H. Perez, L. Fomina, M. Tlenkopatchev and T. Ogawa, Macromol. Chem. Phys., 1997, 198, 1679 CrossRef CAS; (e) S. Fomine, E. Rivera, L. Fomina, A. Ortiz and T. Ogawa, Polymer, 1998, 39, 3551 CrossRef CAS.
- O. R. Gottlieb, K. Herrmann, R. D. H. Murray, G. Ohloff and G. Pattenden, fortschr. Chem. Org. Naturst., 2012, 35 Search PubMed.
- M. V. Patil, P. M. Mhaldar, S. N. Tayade, G. S. Rashinkar and D. M. Pore, J. Mol. Liq., 2022, 359, 119305 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B:Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS PubMed.
- V. A. Bakulev, V. S. Berseneva, N. P. Belskaia, Y. Y. Morzherin, A. Zaitsev, W. Dehaen, I. Luyten and S. Toppet, Org. Biomol. Chem., 2003, 1, 134 RSC.
- M. S. Mirakmahaleh, K. Rad-Moghadam, H. Kefayati and S. Falakro, Mol. Diversity, 2020, 25, 109 CrossRef PubMed.
- Z. Ahani, M. Nikbin, M. Maghsoodlou, F. Farhadi-Ghalati, J. Valizadeh, H. Beyzaei and M. Moghaddam-Manesh, J. Iran. Chem. Soc., 2018, 15, 2423 CrossRef CAS.
- A. A. Hassan, A. A. Aly, M. Ramadan, N. K. Mohamed, H. N. Tawfeek and M. Nieger, Monatsh. Chem., 2020, 151, 1453 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra00894h |
| This journal is © The Royal Society of Chemistry 2025 |