 Open Access Article
Open Access ArticlePillar[5]arene-based supramolecular assemblies application in artificial light-harvesting systems
Kaipeng Zhong *,
Wenrui Pang,
Zhancheng Yang,
Shaoju Bian and
Naicai Xu*
*,
Wenrui Pang,
Zhancheng Yang,
Shaoju Bian and
Naicai Xu*
Qinghai Key Laboratory of Advanced Technology and Application of Environmental Functional Materials, College of Chemistry and Chemical Engineering, Qinghai Normal University, Xining 810008, China. E-mail: zhongkp0430@163.com; xunc@qhnu.edu.cn
First published on 10th April 2025
Abstract
Due to the global energy crisis, many scientists have tried to solve this problem by constructing artificial light-harvesting systems (ALHSs) to mimic photosynthesis. However, achieving efficient energy transfer remains a challenge as excitons need to travel longer diffusion lengths within the donor matrix to reach the acceptor. Supramolecular assemblies based on non-covalent interactions provide diverse approaches for the preparation of ALHSs with high energy-transfer efficiency and more flexible options. Many efficient pillar[5]arene-based supramolecular ALHSs with extremely high energy transfer efficiency and the antenna effect have been successfully constructed by non covalent interactions. These ALHSs have expanded various properties on photoluminescence and photocatalysis, enabling promising applications on cell imaging, supramolecular catalysis and so on. In this review, we highlight the recent developments in pillar[5]arene-based supramolecular assemblies application in light-harvesting systems. We also provide the construction, modulation, and applications of supramolecular ALHSs, and provide a brief discussion of their research prospects, challenges, and future opportunities.
1. Introduction
With the development of the economy, the energy shortage is a worldwide problem and search for clean and renewable energy sources is a global challenge. Inspired by nature, many scientists have tried to solve this problem by mimicking photosynthesis, which is green plants and bacteria produce clean energy natural process.1–8 As we all know, photosynthesis plays a vital role in the life activities of animals and plants, it is a highly efficient process that produces energy by multistep absorb photons from a large number of tightly packed chlorophyll molecules in the pigment-protein complex to produce chemical energy. The enormous efficiency of this process helps green plants to survive in low-light conditions and capture, transfer and store solar energy.9 Drawing cues from nature, scientists have worked to construct artificial light-harvesting systems (ALHSs) to mimic mechanisms of photosynthesis for controlled over photon capture and efficient energy transfer. The construction of ALHSs not only simulates natural processes but also enables diverse functional applications across multiple cutting-edge fields, such as photocatalysis, latent fingerprints imaging, biological imaging and therapy and photoluminescent. Unfortunately, conventional chromophores have a high tendency to aggregate at high concentrations, which is detrimental to photoluminescence and reduces the utilisation of light. ALHSs should transfer excitation energy with high efficiency and have a clear antenna effect: the ratio of the emission intensity of the donor excited receptor to that of the direct excitation of the acceptor should be significantly larger than 1.10,11 Therefore, how to maximise chromophore density while minimising self-bursting remains a great challenge in the construction of ALHSs.12–20With the rapid development of supramolecular chemistry, pillar[5]arene-based functional materials constructed by non-covalent bonding self-assembly methods have been rapidly developed in recent years.21–24 As an interesting class of macrocyclic materials, pillar[5]arene can exhibit highly desirable host–guest recognition properties due to their highly symmetrical rigid structures and suitable cavity sizes can be well suited to form stable supramolecular assemblies with guest molecules by intermolecular non-covalent interactions such as hydrogen bonding, C–H⋯π, cation⋯π, π⋯π stacking, van der Waals forces and so on.25–27 Benefiting from the excellent host–guest binding capacity, pillar[5]arene-based supramolecular assemblies are widely used in the fabrication of 1D channels, 2D sheets, and 3D vesicles or porous MOF/COF materials in aqueous media for applications in stimuli-responsive supramolecular materials, anti-cancer drug delivery, multifunctional nanoplatforms, and supramolecular catalysis.28–30 The formation of such supramolecular assemblies provides favourable conditions for the construction of efficient ALHSs.31–34
Compared with covalent binding between donor and acceptor, non-covalent binding endows ALHSs more construction flexibility and relatively easier way to improve light-harvesting efficiency because the donor and acceptor chromophores can easily keep a suitable distance in this system. By rationally introducing specific guest molecules into pillar[5]arene-based supramolecular ALHSs, the strength and selectivity of the host–guest interactions can be modulate, thus promoting photochemical reactions and achieving energy transfer and storage.35,36 In addition, a various kinds of ALHSs with efficient light capture capability, high energy transfer efficiency and high stability can be constructed by modifying the alkoxy structures at the upper and lower ends of the pillar[5]arene.37–39 However, there are still some challenges in constructing efficient pillar[5]arene-based ALHSs with broad absorption ranges, high extinction coefficients, high energy transfer efficiency and high stability as well as large-scale applications.
2. ALHSs based on pillar[5]arene supramolecular assemblies
2.1 Basic principles of ALHSs
As shown in Fig. 1, the pigment molecules are held in situ by the surrounding proteins via noncovalent interactions, and during photosynthesis, the protein-pigment complex changes from a ground state to an excited state after absorbing a high-energy photon.40–42 Then, multi-step cascade energy transfer occurs between the chlorophyll molecules and subsequently transfers to the reaction center, where O2 ATP and NADPH are produced in the presence of catalytic sites. The chlorophyll molecule acts as a bridging linker among chloroplast pigments and the reaction center, which form a highly ordered structure in space that enhances the efficiency of light energy absorption.43–45 The transfer of energy in the light-harvesting complex can usually be described by the theory of Förster resonance energy transfer (FRET).46–48 | ||
| Fig. 1 Schematic diagram of chlorophyll structure.1 | ||
FRET is an electrostatically mediated nonradiative energy transfer process, plays a decisive role in the construction of efficient ALHSs. This process through the long-range dipole–dipole interaction among the fluorescent donor and the fluorescent or non-fluorescent acceptor decrease the emission intensity of the former by transferring energy to the latter (Fig. 2a).49–52 The key parameter that determines the actual utility of FRET is the energy transfer efficiency (ΦET). In experiments, the ΦET is measured by using the ratio of the fluorescence intensity or fluorescence lifetime of the donor in the absence (FD and τD) and presence of the acceptor (FDA and τDA), which is given as
 | ||
| Fig. 2 (a) Energy transfer from donor to acceptor, and (b) schematic diagram of spectral overlap between donor emission spectra and acceptor absorption spectra.1,2 | ||
FRET is based on randomly fluctuating dipole–dipole coupling interactions between the transient excited state dipole of the donor and the resonant ground state dipole of the acceptor. Thus, the ΦET is given by
This strong distance dependence allows FRET to achieve efficient energy transfer from the donor to the acceptor, a process that requires the following two critical prerequisites: (1) the fluorescence emission spectrum of the donor can effectively overlap with the absorption spectrum of the acceptor; and (2) the spatial distance between the donor and acceptor is close enough, typically less than 10 nm (Fig. 2b).53–57
The energy transfer efficiency and antenna effect are two key empirical parameters for evaluating light capture capability.49 The antenna effect quantifies a system's ability to broaden light absorption and concentrate energy through spatially organized chromophores. Under certain concentrations of donor and acceptor, the antenna effect is equal to the ratio of the emission intensity of the acceptor under donor excitation (IAFλ(D)) to that of the acceptor direct excitation of (IAFλ(A))
The energy transfer efficiency quantifies the variation in donor emission intensity at different acceptor concentrations, while the antenna effect captures changes in donor emission parameters under varying donor concentrations. Combination of ΦET and antenna effect makes evaluation of the ALHSs unequivocal and general.
2.2 Pillar[5]arene-based supramolecular ALHSs with one-step energy transfer
With the development of nanotechnology, pillar[5]arene-based water-soluble supramolecular assemblies as a new type of material using non-covalent interactions for construct of ALHSs have attracted more and more attention.58–60 To mimic the natural chlorophyll/carotenoid complex in photosynthesis, Diao et al.61 constructed a stimulus-responsive supramolecular ALHSs using β-carotene (β-CAR), chlorophyll-b (Chl-b) and carboxyl-modified pillar[5]arene (CWP5). In this work, the authors studied the host–guest interaction of water-soluble CWP5 with β-CAR and Chl-b, which could bind CWP5 in binary solvents to achieve LHCs-b complexation (Fig. 3). CWP5⊃β-CAR complexation (WCC) through the binding of β-CAR to CWP5 in water by a hydrophobic effect. The resulting amphiphilic WCC was then able to construct an supramolecular ALHSs by self-assembling in water to form a β-CAR-containing superstructure. Interestingly, LHCs-b displayed a series of unusual properties, including spontaneous growth, fusion, pH responsiveness and even some photocatalytic activity. They investigated the photocatalytic reduction of 4-nitrophenol by LHC-b and CWP5⊃β-CAR supramolecular vesicles in the presence of NaNO2. LHC-b and CWP5⊃β-CAR supramolecular vesicles catalyzed the reduction of 4-nitrophenol to 4-aminophenol within 10 and 20 min, respectively. The reported strategy paves a new method for studying the origin of bioenergy systems in organisms. | ||
| Fig. 3 (a) Chl-b containing HMS-based ALHSs, (b) CWP5, (c) β-CAR and (d) Chl-b.61 | ||
In 2022 Tang et al.62 prepared two efficient ALHSs with multiple acceptors for a single donor by a supramolecular self-assembly technique (Fig. 4). These ALHSs were prepared by supramolecular self-assembly of an anthryl-cinnamonitrile derivative (ABTA), a water-soluble pillar[5]arene (WP5) and two fluorescent dye molecules (Nile Red (NiR) and sulforhodamine (SR101)). The prepared ALHSs exhibit high energy transfer efficiency (85.7% for WP5⊃ABTA-NiR and 83.2% for WP5⊃ABTA-SR101) and have very high donor–acceptor molar ratios ([ABTA]/[NiR] = 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and [ABTA]/[SR101] = 250
1 and [ABTA]/[SR101] = 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The formed WP5⊃ABTA nanoparticles significantly enhanced the aggregation-induced emission of ABTA and acted as an excellent donor in aqueous solution to transfer the obtained energy to the acceptors NiR and SR101 with high antenna effect for the WP5⊃ABTA-NiR system and 26.1 for WP5⊃ABTA-SR101. This work provides a new approach for the preparation of efficient ALHSs in aqueous solution, which exhibits excellent light captures efficiency and shows potential in mimicking natural energy transfer process.
1). The formed WP5⊃ABTA nanoparticles significantly enhanced the aggregation-induced emission of ABTA and acted as an excellent donor in aqueous solution to transfer the obtained energy to the acceptors NiR and SR101 with high antenna effect for the WP5⊃ABTA-NiR system and 26.1 for WP5⊃ABTA-SR101. This work provides a new approach for the preparation of efficient ALHSs in aqueous solution, which exhibits excellent light captures efficiency and shows potential in mimicking natural energy transfer process.
 | ||
| Fig. 4 Description of efficient supramolecular ALHSs (WP5⊃ABTA-NiR and WP5⊃ABTA-SR101) in water.62 | ||
2.3 Pillar[5]arene-based supramolecular ALHSs with two-step sequential energy transfer
Photosynthesis in nature begins with the absorption of sunlight by a large number of antennae pigments, followed by a multi-step energy transfer that ultimately converts the absorbed energy into chemical energy.63–67 In 2019 Wang et al.68 first constructed ALHSs with two sequential energy-transfer in aqueous solution through a supramolecular self-assembly strategy based on the host–guest recognition of water-soluble pillar[5]arene (WP5) (see Fig. 5). When the water-soluble host macrocyclic WP5 is added to the aqueous TPEDA solution, a stable host–guest complex can be formed and further self-assembled to form supramolecular vesicles. Driven by hydrophobic interactions, the energy acceptor hydrophobic material eosin Y (ESY) can be successfully encapsulated into the hydrophobic fence layer of the formed WP5⊃TPEDA vesicles, enabling energy transfer from WP5⊃TPEDA to ESY. The hydrophobic dye NiR can be simultaneously encapsulated into the WP5⊃TPEDA vesicles as a second energy acceptor to achieve two sequential energy-transfer processes (ΦET = 56.28%). The resulting ALHSs can be used as a nano-reactor and efficient photocatalyst the dehalogenation of α-bromoacetophenone with yields up to 96% compared with the control group using organic dyes alone in the aqueous phase. This sequential energy-transfer ALHSs shows great potential applications in mimicking natural photosynthesis featuring facile integration and multi-step energy transfer process in aqueous solution. | ||
| Fig. 5 Schematic representation of the self-assembly of pillar[5]arene-based aqueous ALHSs with two-step sequential energy transfer.68 | ||
In 2020, Liu et al.69 constructed sequential energy-transfer. ALHSs in water via the self-assembly of a water-soluble pillar[5]arene (WP5) and a pyridinium modified tetraphenylethene (Py-TPE) derivatives to form supramolecular nanoparticles (Py-TPE⊃WP5). In this system, Py-TPE⊃WP5 acted as an energy donor, and sulforhodamine 101 and sulfonated aluminum phthalocyanine (AlPcS4) actd as the first and second acceptors, respectively. When excited with 365 nm light, the energy is transferred from Py-TPE⊃WP5 to SR101 and then to the second energy acceptor AlPcS4 through a FRET process to achieved two-step sequential energy-transfer, and the energy transfer efficiency were calculated to be 84.2% at a Py-TPE/WP5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SR101
SR101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AlPcS4 molar ratio of 20
AlPcS4 molar ratio of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 133
133![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15. This provides a new idea for the construction of ALHSs with sequential energy-transfer in the aqueous solution to better mimic the photosynthesis process in nature (see Fig. 6).
15. This provides a new idea for the construction of ALHSs with sequential energy-transfer in the aqueous solution to better mimic the photosynthesis process in nature (see Fig. 6).
 | ||
| Fig. 6 Construction of ALHSs with sequential energy transfer in water.69 | ||
2023 Xiao et al.70 used a pillar[5]arene-mediated nanoplatform with sequential energy-transfer strategy to achieve efficient ALHSs (Fig. 7). In this work, this system by incorporating the hydrophobic dye ESY into the supramolecular assemblies as a acceptor, the host–guest assembly (WP5⊃TPEP) as an energy donor can efficiently transfer excitation energy to the final acceptor SR101 with an overall energy transfer efficiency of 94%. Interestingly, the radiative rate constant (kr) of WP5⊃TPEP is almost 8 times higher, and the emission brightness (ε(λabs) × φF) is nearly doubled, demonstrating the fast and efficient energy transfer process of the constructed ALHSs. Finally, the prepared supramolecular assemblies of ALHSs were applied to LED devices to achieve tunable colour photoluminescence. This work paves a new avenue for the construction of an efficient ALHSs that has great potential for the fabrication of bright organic luminescent materials.
 | ||
| Fig. 7 Illustration of the construction of an efficient two-step ALHSs in aqueous solution based on the self-assembly of WP5 and TPEP.70 | ||
3. Applications of pillar[5]arene-based supramolecular ALHSs
3.1 Photocatalysis
Converting solar energy into carbon-neutral energy can reduce our reliance on fossil fuels and is one effective way to combat the energy crisis. In nature, for example, it is plants and other photosynthetic organisms that use sunlight to convert water and carbon dioxide into nutrients.71–77 However, the efficiency of this natural process is limited by metabolic processes that greatly reduce the efficiency of converting solar energy into other energy. ALHSs have been shown to be inherently more efficient (i.e. artificially catalysed than photosynthesis) by mimicking photosynthesis. In 2021, we proposed a novel and efficient approach to constructing an efficient ALHSs (CsPbBr3@PTY⊃EYB) by confining the luminescent perovskite quantum dots (CsPbBr3) into the supramolecular self-assembled system of thymine functionalised pillar[5]arene (PTY) and eosin Y-based derivative (EYB) for the cross-coupling reaction (Fig. 8).78 In this system, the guest molecule EYB could self-assembled with the host PTY through a host–guest interaction to form supramolecular assemblies (PTY⊃EYB). The thymine group on the host PTY could coordinated with Pb2+ ions to further form CsPbBr3 quantum dots. The CsPbBr3 quantum dots confined in the PTY⊃EYB interlayer overcome the long-standing problem of self-quenching. Furthermore, the hydrophobic CsPbBr3 quantum dot donor was loaded into the hydrophobic PTY⊃EYB assembly, thus enabling an efficient energy transfer process from the CsPbBr3 quantum dot to the nearby acceptor EYB with an energy transfer efficiency of 96.5%. This study opens up new avenues for the use of perovskite quantum dots as an energy donor to construct efficient ALHSs with greater coverage of the solar spectrum to synthesize useful chemical products and chemical fuels. | ||
| Fig. 8 Graphical representation of the self-assembly and photocatalytic reaction process of the ALHSs.78 | ||
In 2021 Yang et al.79 successfully synthesised an AIEgen-branched ALHSs using an AIEgen-functionalized [2]rotaxane with up to 21 TPE units through a controllable divergent approach (Fig. 9). In this work, the authors constructed pillar[5]arene-based AIEgen-branched ALHSs with the energy transfer efficiency of 71.6% through a multiple energy transfer process. The addition of methanol molecules as a poor solvent induced the motions of the DEP[5]A ring within the dendritic macromolecular backbone and triggered the aggregation process, which together induced its enhanced fluorescence emission. Impressively, these ALHSs proved to be effective photocatalysts for photooxidation and cross-dehydrogenative coupling reactions, and exhibited enhanced catalytic performance. The study realized the first successful synthesis of AIEgen-branched dendrimers ALHSs, which provided a feasible and practical applications such as photocatalysis.
 | ||
| Fig. 9 Pillar[5]arene-based AIEgen-branched rotaxane dendrimers to construction of novel ALHSs for photooxidation reaction.79 | ||
In 2024 Yao et al.80 successfully constructed a fluorescent supramolecular ALHSs (P5Py2/Zn/Gen)n using orthogonal self-assembly and metal ion coordination (Fig. 10). Within the supramolecular ALHSs, the host–guest interaction effectively inhibits the ACQ effect, allowing the guest molecule Gen unit acts as a light-harvesting moiety, while the (Py)2/Zn center serves as a catalytic site. Moreover, the authors founded that this ALHSs have a good stability can be reused at least 5 times without significant conversion loss in the catalytic catalysis of the reduction of p-nitrophenol to p-aminophenol. These findings provide a pathway for constructing a recyclable ALHSs that mimics the entire photosynthesis process.
 | ||
| Fig. 10 Chemical structures of terpyridine-modified-pillar[5]arene (P5Py) and di(alkyl-trimethylammonium)-modified-anthracene (Gen) and cartoon representations of the ALHSs for photocatalytic.80 | ||
3.2 Latent fingerprints imaging
Fingerprints, as an information feature that serves as a personal identity card and information base, have a high evidentiary value in criminal cases. In particular, latent fingerprints (LFPs) are invisible prints formed by sweat or oil that is left after finger contact, and could be used for mobile phone unlocking, access control and personal identification. The collection of LFPs at crime scenes is an important and broadly used operation in forensic science for the identification of individuals. In 2021, we constructed a near-infrared (NIR) emitting ALHSs (MAPbBr3@Zn(II)TCP⊃EYB-NiB) with two-step efficient sequential energy-transfer based on the in situ growing perovskite quantum dots (MAPbBr3) in the supramolecular self-assembly of carboxyl-functionalised pillar[5]arene Zn coordination polymer and two different fluorescent dyes (eosin derivatives (EYB) and Nile blue (NiB)) for near-infrared latent fingerprint sweat pores fluorescence imaging (Fig. 11).81 Notably, in this ALHSs, carboxyl passivation increased the photoluminescence quantum yield of MAPbBr3 quantum dots at 503 nm to 54.1%. Through a two-step efficient sequential energy-transfer, the energy of the MAPbBr3 quantum dots transferred to EYB and then to NiB with an energy transfer efficiency of 44.4%, which enabling near-infrared fluorescence latent fingerprint imaging. Very interestingly, a small amount of perspiration secreted from human body was found to be sufficient to cause the fluorescence quenched of the system, allowing accurate imaging of sweat pores in latent fingerprints. This study opens up a new avenue for the design of an efficient sequential energy-transfer perovskite quantum-based ALHSs for NIR third-level fingerprint imaging and other potential applications. | ||
| Fig. 11 Graphical representation of the self-assembly and sweat pore imaging of the ALHSs.81 | ||
In 2023, Xiao et al.82 proposed a red light emitting supramolecular ALHSs for LFPs imaging, specifically using macrocyclic host guest nanoparticles as energy donors and the hydrophobic dye NiR as an energy acceptor (Fig. 12). The authors first synthesised a tetraphenylene (TPE) derivative (appointed FTPE) as an AIE-type guest molecule and selected a water-soluble pillar[5]arene (WP5) to form supramolecular nanoparticles (NP) with FTPE in water, with the host–guest combination emitting intense yellow fluorescence. The authors then assembled the complex with 1% NiR to obtain a highly efficient ALHSs with red light emission, a system that was able to identify tertiary fingerprint details well. When the molar ratio of WP5⊃FTPE/NiR was 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the calculated energy transfer efficiency reached 60.9%. This study provides a new approach for the development of high-performance ALHSs with red emission in high-resolution imaging of LFPs.
1, the calculated energy transfer efficiency reached 60.9%. This study provides a new approach for the development of high-performance ALHSs with red emission in high-resolution imaging of LFPs.
 | ||
| Fig. 12 Schematic illustration of the construction of the supramolecular ALHSs based on WP5 and FTPE, as well as its application in latent fingerprint imaging.82 | ||
3.3 Biological imaging and therapy
Fluorescence imaging technology has become a fundamental tool for biomedical applications, and plays an indispensable role in the fields of biological activity process analysis and public health. ALHSs constructed based on supramolecular assemblies of pillar[5]arene with modulated luminescence colour and luminescence intensity properties, which are promising for biomedical applications. In 2022, Sun et al.83 successfully prepared two efficient ALHSs (Fig. 13) in water by supramolecular assembly of a salicylideneaniline derivative (PPA), a water-soluble pillar[5]arene (WP5) and two conventional fluorescent dye molecules (Phloxine B (PHB) and SR101). Benefitting to the inducing assembly of WP5 could tightly bind with PPA by host–guest interaction and form supramolecular nanoparticles (WP5⊃PPA). After PHB and SR101 loaded in WP5⊃PPA, the constructed ALHSs (WP5⊃PPA-PHB and WP5⊃PPA-SR101) exhibited excellent energy transfer efficiency (ΦET = 65.2% for WP5⊃PPA-PHB and ΦET = 78.9% for WP5⊃PPA-SR101) and antenna effects (AE). Significantly, cell cytotoxicity experiments displayed that WP5⊃PPA-SR101 was highly biocompatible in NIH 3T3 cells. The CLSM results further confirmed the potential application of WP5⊃PPA-SR101 in bioimaging materials due to the presence of FRET process. This study fabricated two novel and facile aqueous ALHSs with outstanding light-harvesting capability for potential applications in the field of biomaterials. | ||
| Fig. 13 (a) Supramolecular ALHSs based on WP5⊃PPA. (b) Cytocompatibility experiments 24 h incubation and (c) 48 h incubation.83 | ||
In 2024, Wang et al.84 constructed supramolecular ALHSs based on hydra-headed macrocycles hosts pillar[5]arene and guests AIE molecules for bioimaging (Fig. 14). In this system, the researchers proposed discrete macrocyclic polymers hosts for assembling AIE molecules to induce cascade luminescence enhancement in water performance via the cooperation of hydra-headed macrocycles containing two or three pillar[5]arene units (defined as P2, P3), the block polymer F127 and the guest AIE molecules (alkyl-cyano modified tetraphenylethene, alkyl-triazole-cyano modified 9,10-distyrylanthracene, defined as TPE-(CN)4 and DSA-(TACN)2) through host–guest interactions. More importantly, cascaded supramolecular assembly-induced emission enhancement (SAIEE) and high luminescence quantum yields in aqueous solution were achieved in this ALHSs. Discrete macrocyclic supramolecular assembled systems. This work integrated the advantages of supramolecular macrocycles, AIE molecules, and polymers, the proposal of discrete macrocyclic polymer hosts provided new ideas to construction of ALHSs by supramolecular assemblies, which can be used for bioimaging and other potential applications such as information security.
 | ||
| Fig. 14 Schematic illustration of ALHSs in water based on discrete macrocyclic hosts and AIE molecules as well as its applications in the fields of bioimaging.84 | ||
Photosensitizers (PS) with controllable switching have been extensively explored for activated to produce reactive oxygen species (ROS) under specific wavelength of light irradiation in recent years. Due to their inherent dynamic and reversible characteristics, host–guest interactions provide an efficient and convenient method to control the “on–off” switchability of ROS generation for photodynamic therapy. In 2023, Hu et al.85 utilized a TPE-derived pillar[5]arene host (m-TPEWP5) with AIE activity, a merocyanine derivative (MC-G) guest and Nile blue (NiB) as acceptor to construct ALHSs based on a two-step FRET process in aqueous media (Fig. 15). In this system, the energy could transfer from the m-TPEWP5 donor to the MC-G acceptor and then to the NiB acceptor with high energy transfer efficiency through a two-step sequential FRET process (ΦET = 62.5%). Under UV irradiation, the reversible isomerization of SP-G endowed the PS with an ‘‘on–off’’ function by the non-fluorescent guest SP-G converted into fluorescent MC-G. Interestingly, the in situ formed MC-G acted as a primary energy acceptor and an effective PS to produce ROS. The prepared ALHSs were successfully used for in vitro anticancer and anti-bacterial treatment through specific light irradiation. This work provides a novel approach to fabricating smart supramolecular PS materials based on ALHSs strategy.
 | ||
| Fig. 15 Schematic of the construction of ALHSs-based ROS-generation system in aqueous solution.85 | ||
3.4 Photoluminescent
Constructing multicolor photoluminescent materials with tunable properties is an attractive research objective on account of their abundant applications in materials science and biomedical engineering.86,87 In 2020, Xu et al.88 report a novel strategy to construct efficient ALHSs (Fig. 16) with an antenna effect of 90.4 in solid film. This ALHSs constructed of pillar[5]arene-basd conjugated polymer host (CPH) and conjugated ditopic guests (Gs) derived from conjugated polymer supramolecular cross-linked network through host–guest recognition. The aggregation-enhancing emission properties of the tetraphenylene group in the CPH backbone and the cross-linked structure based on the interaction of the host and guest give the CPSN an excellent performance in tunable fluorescence emission wavelengths. More importantly, the authors evidenced that the fluorescence emission of the ALHSs was readily tuned by using different guests or different the host/guest ratio, and the color gamut reached about 96% sRGB region that containing pure white light emission spots in CIE coordinates, which has potential applications in the field of photoluminescent materials.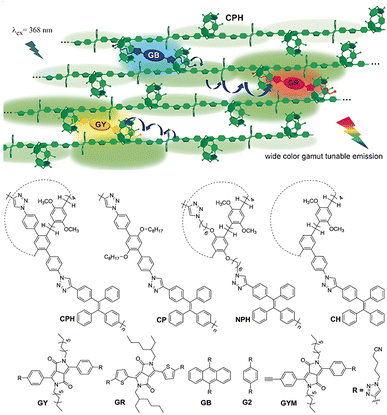 | ||
| Fig. 16 A schematic illustration of the construction of ALHSs-based the conjugated polymeric host CPH, CP, NPH, and CH; and the guests (GB, GY, GR, G2, and GYM).88 | ||
In 2024, Xiao et al.89 engineered ALHSs through the host–guest interaction between a pyridinium salt-modified cyanostilbene guest (CPy) and a water soluble pillar[5]arene host (WP5) in aqueous solution. CPy comprises a cyanostilbene (CS) moiety and a methylpyridinium unit linked by a ten-carbon alkyl spacer, rendering it AIE active (Fig. 17). Consequently, the WP5 induces the self-assembly of CPy into emissive nanoparticles, serving as an effective nano-platform and energy donor. By doping the dye DBT into these nanoparticles, highly efficient ALHSs (WP5⊃CPy-DBT) with an energy transfer efficiency of 23.5% was constructed. Additionally, this system demonstrated tunable fluorescence emission in the solid state and exhibited potential applications as a color-tunable fluorescent ink for information encryption due to their AIE properties. This study delineated a promising approach for fabricating efficient ALHSs via a simple supramolecular strategy and highlights the great potential of tunable photoluminescent supramolecular material applications.
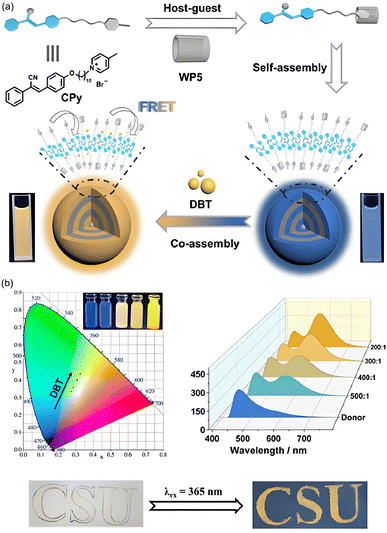 | ||
| Fig. 17 (a) Schematic representation of the construction of ALHSs in water based on WP5⊃CPy and DBT. (b) CIE chromaticity, fluorescence spectra and illustration of characters of WP5⊃CPy-DBT.89 | ||
In addtion, Xiao et al.90 fabricated a supramolecular ALHSs with sequential energy transfer for white light emitting diode (LED) device (Fig. 18). In this study, the ALHSs by non-covalent self-assembly based on pillar[5]arene (WP5) and AIE-type guests (TPED), and two fluorescent dyes (4,7-di(2-thienyl)-benzo[2,1,3]thiadiazole (DBT) and SR101) in water. Moreover, the energy transfer efficiency was calculated to be 37.2% at a WP5⊃TPED![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DBT
DBT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SR101 molar ratio of 1000
SR101 molar ratio of 1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15. Impressively, when the molar ratio of WP5⊃TPED/DBT/SR101 is 1100/2/16, the system shows white-light emission and could be coated on LED bulb for white-light emission. This study demonstrated a general strategy for the construction of sequential ALHSs though pillar[5]arene-based supramolecular assemblies in water and explored its applications in white-light LED device.
15. Impressively, when the molar ratio of WP5⊃TPED/DBT/SR101 is 1100/2/16, the system shows white-light emission and could be coated on LED bulb for white-light emission. This study demonstrated a general strategy for the construction of sequential ALHSs though pillar[5]arene-based supramolecular assemblies in water and explored its applications in white-light LED device.
 | ||
| Fig. 18 Schematic illustration of (a) the fabrication of supramolecular ALHSs, (b) photographs showing the fluorescence variation at different WP5⊃TPED/DBT/SR101 ratios, and (c) its application in white light emitting device.90 | ||
4. Conclusions
In summary, the recent research advances in artificial light-harvesting systems (ALHSs) based on pillar[5]arene supramolecular assemblies are highlighted in this review. Compared to the ALHSs employing covalent bonds between donor and acceptor, pillar[5]arene-based supramolecular assemblies have provided more flexible options for the preparation of ALHSs with the simplify synthesis process and higher light-harvesting efficiency. Although pillar[5]arene-based supramolecular ALHSs have made encouraging progress in practical applications, some of them have a lot of drawbacks, such as the low energy transfer efficiency and the relatively narrow absorption window.In photosynthetic system, solar energy is absorbed by chlorophyll molecules, and through multiple steps of energy transfer, the excited state energy is ultimately transferred to the reaction center, where charge separation occurs, enabling efficient energy transduction. At present, most of the ALHSs can achieve one-step energy transfer or two-step energy transfer, whereas the mimicry of two-step and more than two-step energy transfers is far less mature than one-step energy transfer technology. In addition, the process of photosynthesis in nature is all about multiple donors transferring energy to one acceptor. Therefore, ALHSs involving more than two-step energy transfer and multiple-donor/one-acceptor have yet to be explored.
Fluorescent molecules with wide absorption ranges are essential in the construction of efficient ALHSs. However, unlike the continuous energy band structure of inorganic semiconductors, the relatively narrow absorption window of organic fluorescent molecules leads to insufficient capture of the capacity of ALHSs. This leaves much room for improvement compared to the efficient energy transfer in photosynthesis in nature. Therefore, if the ALHSs is constructed by organic–inorganic hybrid materials, the efficiency of energy transfer will be greatly improved. Overall, we believe that the ALHSs constructed by using pillar[5]arene-based supramolecular assemblies has a broad application prospect in the fields of photoluminescent materials, photocatalysis, biomedical materials, and advanced encryption.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this reviews.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the Qinghai Kunlun Talents Program and the Qinghai Normal University Young and Middle-aged Faculty Research Fund (2025QZR09).References
- S. Kundu and A. Patra, Chem. Rev., 2017, 117, 712–757 CrossRef PubMed.
- Y. Yu, T. Ma and H. Huang, Adv. Funct. Mater., 2023, 33, 2213770 CrossRef.
- S. Bhunia, M. Mukherjeeb and P. Purkayastha, Chem. Commun., 2024, 60, 3370–3378 RSC.
- K. Bramhaiah and S. Bhattacharyya, Mater. Adv., 2022, 3, 142–172 RSC.
- M. V. Pavliuk, S. Wrede, A. Liu, A. Brnovic, S. Wang, M. Axelsson and H. Tian, Chem. Soc. Rev., 2022, 51, 6909–6935 RSC.
- V. N. Gopalakrishnan, J. Becerra, E. F. Pena, M. Sakar, F. Bélandc and T.-O. Do, Green Chem., 2021, 23, 8332–8360 RSC.
- J. Liu, Y.-Y. Ren, J. Wu, W. Xia, B.-Y. Deng and F. Wang, J. Mater. Chem. A, 2021, 9, 19346–19368 RSC.
- I. N. Chakraborty, P. Roy, A. Rao, G. Devatha, S. Roy and P. P. Pillai, J. Mater. Chem. A, 2021, 9, 7422–7457 RSC.
- Z. Zhang, Z. Zhao, L. Wu, S. Lu, S. Ling, G. Li, L. Xu, L. Ma, Y. Hou, X. Wang, X. Li, G. He, K. Wang, B. Zou and M. Zhang, J. Am. Chem. Soc., 2020, 142, 2592–2600 CrossRef CAS.
- C. Curutchet and B. Mennucci, Chem. Rev., 2017, 117, 294–343 CrossRef CAS.
- Y. Jiang and J. McNeill, Chem. Rev., 2017, 117, 838–859 CrossRef CAS PubMed.
- P.-Z. Chen, Y.-X. Weng, L.-Y. Niu, Y.-Z. Chen, L.-Z. Wu, C.-H. Tung and Q.-Z. Yang, Angew. Chem., Int. Ed., 2016, 55, 2759–2763 CrossRef CAS PubMed.
- Z. Xu, D. Gonzalez-Abradelo, J. Li, C. A. Strassert, B. J. Ravoo and D.-S. Guo, Mater. Chem. Front., 2017, 1, 1847–1852 RSC.
- X. Wei, X. Su, P. Cao, X. Liu, W. Chang, M. Li, X. Zhang and Z. Liu, Nature, 2016, 534, 69–74 CrossRef CAS.
- G. Sinawang, M. Osaki, Y. Takashima, H. Yamaguchi and A. Harada, Chem. Commun., 2020, 56, 4381–4395 RSC.
- Y. X. Hu, W. J. Li, P. P. Jia, X. Q. Wang, L. Xu and H. B. Yang, Adv. Opt. Mater., 2020, 8, 2000265 CrossRef.
- X. M. Chen, Q. Cao, H. K. Bisoyi, M. Wang, H. Yang and Q. Li, Angew. Chem., Int. Ed., 2020, 59, 10493–10497 CrossRef.
- R.-Z. Zhang, Y.-S. Bi, K.-K. Niu, S. Yu, H. Liu and L.-B. Xing, Adv. Opt. Mater., 2025, 13, 2402112 CrossRef.
- T. Xiao, W. Zhong, L. Zhou, L. Xu, X.-Q. Sun, R. B. P. Elmes, X.-Y. Hu and L. Wang, Chin. Chem. Lett., 2019, 30, 31–36 CrossRef.
- T. Xiao, H. Wu, G. Sun, K. Diao, X. Wei, Z. Y. Li, X. Q. Sun and L. Wang, Chem. Commun., 2020, 56, 12021–12024 RSC.
- Y. Zhou, K. Jie, R. Zhao and F. Huang, Adv. Mater., 2020, 32, 1904824 CrossRef CAS.
- T. Ogoshi, T. A. Yamagishi and Y. Nakamoto, Chem. Rev., 2016, 116, 7937–8002 CrossRef CAS.
- D. Xia, P. Wang, X. Ji, N. M. Khashab, J. L. Sessler and F. Huang, Chem. Rev., 2020, 120, 6070–6123 CrossRef CAS PubMed.
- Z. Li, N. Song and Y.-W. Yang, Matter, 2019, 1, 345–368 CrossRef.
- H.-B. Cheng, Y.-M. Zhang, Y. Liu and J. Yoon, Chem, 2019, 5, 553–574 Search PubMed.
- X.-S. Li, Y.-F. Li, J.-R. Wu, X.-Y. Lou, J. Han, J. Qin and Y.-W. Yang, J. Mater. Chem. A, 2020, 8, 3651–3657 RSC.
- Q. Lin, K.-P. Zhong, J.-H. Zhu, L. Ding, J.-X. Su, H. Yao, T.-B. Wei and Y.-M. Zhang, Macromolecules, 2017, 50, 7863–7871 CrossRef CAS.
- W. J. Li, W. Wang, X. Q. Wang, M. Li, Y. Ke, R. Yao, J. Wen, G. Q. Yin, B. Jiang, X. Li, P. Yin and H. B. Yang, J. Am. Chem. Soc., 2020, 142, 8473–8482 CrossRef CAS.
- T. Ogoshi, T. Kakuta and T. A. Yamagishi, Angew. Chem., Int. Ed., 2019, 58, 2197–2206 CrossRef CAS PubMed.
- G. Yu, K. Jie and F. Huang, Chem. Rev., 2015, 115, 7240–7303 CrossRef CAS PubMed.
- C. Wang, H. Li, J. Dong, Y. Chen, X. Luan, X. Li and X. Du, Chem.–Eur. J., 2022, 28, e202202050 CrossRef CAS.
- T. Xiao, L. Qi, W. Zhong, C. Lin, R. Wang and L. Wang, Mater. Chem. Front., 2019, 3, 1973–1993 RSC.
- H. Zhang, Z. Liu, F. Xin and Y. Zhao, Coord. Chem. Rev., 2020, 420, 213425 CrossRef CAS.
- W.-L. Guan, J.-F. Chen, J. Liu, B. Shi, H. Yao, Y.-M. Zhang, T.-B. Wei and Q. Lin, Coord. Chem. Rev., 2024, 507, 215717 CrossRef CAS.
- G. Sun, W. Qian, J. Jiao, T. Han, Y. Shi, X. Y. Hu and L. Wang, J. Mater. Chem. A, 2020, 8, 9590–9596 RSC.
- X.-M. Chen, X. Chen, X.-F. Hou, S. Zhang, D. Chen and Q. Li, Nanoscale Adv., 2023, 5, 1830–1852 RSC.
- Y. Zhu, L. Xu, L. Wang, H. Tang and D. Cao, Chem. Commun., 2019, 55, 5910–5913 RSC.
- K. Wang, K. Velmurugan, B. Li and X.-Y. Hu, Chem. Commun., 2021, 57, 13641–13654 RSC.
- T. Xiao, L. Zhou, X.-Q. Sun, F. Huang, C. Lin and L. Wang, Chin. Chem. Lett., 2020, 31, 1–9 CrossRef CAS.
- M. Taniguchi and J. S. Lindsey, Chem. Rev., 2017, 117, 344–535 CrossRef CAS PubMed.
- C. Pucci, C. Martinelli, A. Degl'Innocenti, A. Desii, D. D. Pasquale and G. Ciofani, Macromol. Biosci., 2021, 21, 2100181 CrossRef CAS PubMed.
- N.-W. Qiu, D.-C. Jiang, X.-S. Wang, B.-S. Wang and F. Zhou, Photosynthetica, 2019, 57, 974–984 CrossRef CAS.
- S. Pramanik and S. Mukherjee, Chem. Phys. Rev., 2023, 4, 031306 CrossRef CAS.
- D. Bokotial, K. Acharyya, A. Chowdhury and P. S. Mukherjee, Angew. Chem., Int. Ed., 2024, 63, e202401136 CrossRef CAS.
- J. Lv, J. Xie, A. G. A. Mohamed, X. Zhang, Y. Feng, L. Jiao, E. Zhou, D. Yuan and Y. Wang, Nat. Rev. Chem, 2023, 7, 91–105 CrossRef PubMed.
- Y. J. Lin, J. W. Chen, P. T. Hsiao, Y. L. Tung, C. C. Chang and C. M. Chen, J. Mater. Chem. A, 2017, 5, 9081–9089 RSC.
- H. J. Lee, Y. G. Lee, J. Kang, S. H. Yang, J. H. Kim, A. B. T. Ghisaidoobe, H. J. Kang, S.-R. Lee, M. H. Lim and S. J. Chung, Chem. Sci., 2019, 10, 1000–1007 RSC.
- J. P. Otto, L. Wang, I. Pochorovski, S. M. Blau, A. Aspuru-Guzik, Z. Bao, G. S. Engel and M. Chiu, Chem. Sci., 2018, 9, 3694–3703 RSC.
- H.-Q. Peng, L.-Y. Niu, Y.-Z. Chen, L.-Z. Wu, C.-H. Tung and Q.-Z. Yang, Chem. Rev., 2015, 115, 7502–7542 CrossRef CAS.
- X. Liu, Y. Zeng, X. Zhang, T. Yu, J. Chen and Y. Li, Sci. China Chem., 2015, 58, 390–399 CrossRef CAS.
- J. H. Lee, Y. Kim, S. Oh and W.-D. Jang, Appl. Phys. Rev., 2024, 11, 031319 CAS.
- J. Yang, M.-C. L. Yoon, H. Yoo, P. Kim and D. Kim, Chem. Soc. Rev., 2012, 41, 4808–4826 RSC.
- K. Acharyya, S. Bhattacharyya, H. Sepehrpour, S. Chakraborty, S. Lu, B. Shi, X. Li, P. S. Mukherjee and P. J. Stang, J. Am. Chem. Soc., 2019, 141, 14565–14569 Search PubMed.
- E. A. Reyes Cruz, D. Nishiori, B. L. Wadsworth, N. P. Nguyen, L. K. Hensleigh, D. Khusnutdinova, A. M. Beiler and G. F. Moore, Chem. Rev., 2022, 122, 16051–16109 Search PubMed.
- H. Jing, J. Rong, M. Taniguchi and J. S. Lindsey, Coord. Chem. Rev., 2022, 456, 214278 Search PubMed.
- Q. Zou, K. Liu, M. Abbas and X. Yan, Adv. Mater., 2016, 28, 1031–1043 Search PubMed.
- Y.-X. Hu, P.-P. Jia, C.-W. Zhang, X.-D. Xu, Y. Niu, X. Zhao, Q. Xu, L. Xu and H.-B. Yang, Org. Chem. Front., 2021, 8, 5250–5257 RSC.
- W.-J. Li, X.-Q. Wang, W. Wang, Z. Hu, Y. Ke, H. Jiang, C. He, X. Wang, Y.-X. Hu, P.-P. Jia, P. Yin, J. Chen, H. Sun, Z. Sun, L. Xu and H.-B. Yang, Giant, 2020, 2, 100020 CrossRef.
- Q. Zhang, X. Dang, F. Cui and T. Xiao, Chem. Commun., 2024, 60, 10064–10079 RSC.
- X.-H. Wang, X.-Y. Lou, T. Lu, C. Wang, J. Tang, F. Liu, Y. Wang and Y.-W. Yang, ACS Appl. Mater. Interfaces, 2021, 13, 4593–4604 CrossRef CAS.
- Y. Sun, F. Guo, T. Zuo, J. Hua and G. Diao, Nat. Commun., 2016, 7, 12042 CrossRef CAS PubMed.
- G. Sun, Z. Wang, Y. Hu, T. Sun and Y. Tang, Dyes Pigments, 2022, 197, 109913 CrossRef CAS.
- Z. Wu, H. Qian, X. Li, T. Xiao and L. Wang, Chin. Chem. Lett., 2024, 35, 108829 CrossRef CAS.
- L. Xu, R. Wang, H. Tang, L. Wang and D. Cao, J. Mater. Chem. A, 2022, 10, 11332–11339 RSC.
- W.-J. Li, H. Jiang, X.-Q. Wang, D.-Y. Zhang, Y. Zhu, Y. Ke, W. Wang and H.-B. Yang, Mater. Today Chem., 2022, 24, 100874 CrossRef CAS.
- D. Chen, T. Xiao, É. Monflier and L. Wang, Commun. Chem., 2024, 7, 88 CrossRef CAS.
- Y. Wang, N. Han, X.-L. Li, R.-Z. Wang and L.-B. Xing, ACS Appl. Mater. Interfaces, 2022, 14, 45734–45741 CrossRef CAS.
- M. Hao, G. Sun, M. Zuo, Z. Xu, Y. Chen, X.-Y. Hu and L. Wang, Angew. Chem., Int. Ed., 2020, 59, 10095–10100 CrossRef CAS PubMed.
- J.-J. Li, H.-Y. Zhang, X.-Y. Dai, Z.-X. Liu and Y. Liu, Chem. Commun., 2020, 56, 5949–5952 RSC.
- T. Xiao, H. Qian, X. Li, Z. Wu, Z.-Y. Li and X.-Q. Sun, Dyes Pigments, 2023, 215, 111289 CrossRef CAS.
- G. Sun, M. Zuo, W. Qian, J. Jiao, X.-Y. Hu and L. Wang, Green Synth. Catal., 2021, 2, 32–37 CrossRef.
- G. Sun, L. Cai, H. Cui, Y. Hu, J. Wang, M. Wang, J. Zhu, T. Sun and Y. Tang, Dyes Pigments, 2022, 201, 110257 CrossRef CAS.
- G. Sun, M. Li, L. Cai, D. Wang, Y. Cui, Y. Hu, T. Sun, J. Zhu and Y. Tang, J. Colloid Interface Sci., 2023, 641, 803–811 CrossRef CAS.
- G. Sun, M. Li, L. Cai, J. Zhu, Y. Tang and Y. Yao, Chem. Commun., 2024, 60, 1412–1415 RSC.
- K. Wang, Y. Shen, P. Jeyakkumar, Y. Zhang, L. Chu, R. Zhang and X.-Y. Hu, Curr. Opin. Green Sustainable Chem., 2023, 41, 100823 CrossRef.
- T. Xiao, D. Chen, H. Qian, Y. Shen, L. Zhang, Z.-Y. Li and X.-Q. Sun, Dyes Pigments, 2023, 210, 110958 CrossRef.
- Z. Bai, K. Velmurugan, X. Tian, M. Zuo, K. Wang and X.-Y. Hu, Beilstein J. Org. Chem., 2022, 18, 429–437 CrossRef PubMed.
- K. Zhong, S. Lu, W. Guo, J. Su, S. Sun, J. Hai, F. Chen, A. Wang and B. Wang, J. Mater. Chem. A, 2021, 9, 10180–10185 RSC.
- W.-J. Li, X.-Q. Wang, D.-Y. Zhang, Y.-X. Hu, W.-T. Xu, L. Xu, W. Wang and H.-B. Yang, Angew. Chem., Int. Ed., 2021, 60, 18761–18768 CrossRef PubMed.
- L. Zhou, Y. Zhou, L. Fang, Y. Bai, Y. Meng, L. Li, J. Yang and Y. Yao, Chin. Chem. Lett., 2024, 35, 109509 CrossRef.
- K. Zhong, S. Lu, W. Guo, J. Su, S. Sun, J. Hai and B. Wang, Chem. Commun., 2021, 57, 9434–9437 RSC.
- T. Xiao, L. Zhang, D. Chen, Q. Zhang, Q. Wang, Z.-Y. Li and X.-Q. Sun, Org. Chem. Front., 2023, 10, 3245–3251 RSC.
- G. Sun, L. Cai, Y. Zhang, Y. Hu, J. Zhu, T. Sun and Y. Tang, Dyes Pigments, 2022, 205, 110577 CrossRef.
- Y.-Q. Zhu, Z. Chen, Z.-Y. Chen, Z.-W. Zhou, Q. Bai, M.-X. Wu and X.-H. Wang, Chem.–Eur. J., 2024, e202402808, DOI:10.1002/chem.202402808.
- X. Tian, S. Li, K. Velmurugan, Z. Bai, Q. Liu, K. Wang, M. Zuo and X.-Y. Hu, Mater. Chem. Front., 2023, 7, 2484–2492 RSC.
- T. Xiao, H. Qian, Y. Shen, C. Wei, D. Ren, L. Zhang, Z.-Y. Li, L. Wang and X.-Q. Sun, Mater. Today Chem., 2022, 24, 100833 CrossRef.
- X. Li, Z. Wu, Q. Wang, Z.-Y. Li, X.-Q. Sun and T. Xiao, ChemPhysChem, 2023, 88, e202300431 CAS.
- L. Xu, Z. Wang, R. Wang, L. Wang, X. He, H. Jiang, H. Tang, D. Cao and B. Z. Tang, Angew. Chem., Int. Ed., 2020, 59, 9908–9913 CrossRef CAS.
- X. Li, Q. Zhang, X. Dang, F. Cui, Z.-Y. Li, X.-Q. Sun and T. Xiao, Energy adv., 2024, 3, 1672–1677 RSC.
- Q. Zhang, F. Cui, X. Dang, Q. Wang, Z.-Y. Li, X.-Q. Sun and T. Xiao, Chem.–Eur. J., 2024, 30, e202401426 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |



