Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Eco-friendly synthesis of Ag/CeO2 and CuO/CeO2 nanocomposites using Curcuma longa extract and assessment of their antioxidant, antifungal, and cytotoxic activities†
Khaled M. Elattar *a,
Abeer A. Ghoniemb,
Fatimah O. Al-Otibi
*a,
Abeer A. Ghoniemb,
Fatimah O. Al-Otibi cd,
Abdulaziz S. Fakhouri
cd,
Abdulaziz S. Fakhouri de,
Yosra A. Helmyf,
WesamEldin I. A. Saber
de,
Yosra A. Helmyf,
WesamEldin I. A. Saber *b,
Mahmoud A. E. Hassang and
Ashraf Elsayedh
*b,
Mahmoud A. E. Hassang and
Ashraf Elsayedh
aUnit of Genetic Engineering and Biotechnology, Faculty of Science, Mansoura University, El-Gomhoria St., Mansoura, 35516, Egypt. E-mail: khaledelattar2@yahoo.com; khaledelattar2@mans.edu.eg
bMicrobial Activity Unit, Department of Microbiology, Soils, Water and Environment Research Institute, Agricultural Research Center, Giza 12619, Egypt. E-mail: abeer.abdelkhalik@yahoo.com; wesameldin.saber@arc.sci.eg; wiasaber@gmail.com
cBotany and Microbiology Department, Faculty of Science, King Saud University, Riyadh 11451, Saudi Arabia. E-mail: falotibi@ksu.edu.sa
dCenter of Excellence in Biotechnology Research, King Saud University, Riyadh, 11451, Saudi Arabia
eDepartment of Biomedical Technology, College of Applied Medical Sciences, King Saud University, Riyadh, 12372, Saudi Arabia. E-mail: afakhouri@ksu.edu.sa
fDepartment of Veterinary Science, College of Agriculture, Food, and Environment, University of Kentucky, Lexington, KY 40546, USA. E-mail: yosra.helmy@uky.edu
gAnimal Production Research Institute (APRI), Agricultural Research Center, Giza 12619, Egypt. E-mail: m.hassan55213@gmail.com
hBotany Department, Faculty of Science, Mansoura University, Mansoura 35516, Egypt. E-mail: ashraf-badawy@mans.edu.eg
First published on 17th April 2025
Abstract
This work focused on the biosynthesis of Ag/CeO2 and CuO/CeO2 nanocomposites (NCs) using Curcuma longa extract. The nanocomposites were efficiently characterized using different techniques such as FTIR, UV-visible spectroscopy, zeta potential, DLS, TEM, SEM, EDX, and XRD analyses. The C. longa extract provided high phenolic and flavonoid contents, while demonstrating strong antioxidant action at IC50 = 0.042 mg mL−1. In particular, both nanocomposites exhibited privileged antifungal activity against Macrophomina phaseolina with superiority to CuO/CeO2 (MIC = 29 µg mL−1) over Ag/CeO2 (MIC = 49 µg mL−1). TEM analyses confirmed the adverse effect of nanocomposites on the fungal cell wall. The CuO/CeO2 structure led to mitochondrial and cytoplasmic damage in MCF-7 cells (IC50 = 0.5071 µg mL−1) according to cytotoxicity tests; however, the Ag/CeO2 NC resulted in significant nuclear damage and an increased occurrence of autophagy events. The nanocomposites showed cytotoxic properties by causing oxidative stress, leading to damage of the genomic material and defects in cell structure, suggesting potential therapeutic applications.
1. Introduction
Curcumin, a polyphenol from turmeric (Curcuma longa L), has a long history of medicinal use. Traditionally used to treat various ailments, curcumin's primary bioactive component has garnered significant scientific attention.1 Research has consistently highlighted curcumin's potential therapeutic benefits in neurological-, respiratory-, cardiovascular-, metabolic-, and autoimmune disorders. Its anti-oxidant, anti-inflammatory, and anti-cancer behavior makes it a promising candidate for applications in these areas. This review will delve deeper into the scientific evidence supporting curcumin's therapeutic potential and explore its promising role in modern medicine.2Curcumin is a symmetric molecule with a heptadiene sequence and two aromatic rings substituted with hydroxyl and methoxy groups.3 To elucidate curcumin's natural function, these functional groups are considered, including the o-methoxy phenolic group, α,β-unsaturated β-diketone moiety, and 7-carbon chain.4 Curcumin's therapeutic activity arises from its promiscuous interaction with various cellular processes. The proteins of interest are multifunctional, encompassing growth factors, transcription factors, inflammatory mediators, pro- and anti-apoptotic pathways, and enzymes, thereby categorizing curcumin as a pleiotropic molecule in disease contexts.5 Additionally, curcumin exhibits antioxidant activity and modulates immunological signaling.5 Over the past decades, curcumin has garnered significant attention from scientists, particularly in biology, pharmacology, and nutraceutical science.6,7 Notably, its antibacterial properties have also been examined.8
It has been established that curcumin from the turmeric plant depends on the processing methods used when being extracted. For example, the American Spice Trade Association and the Food and Drug Administration set standard limits regarding the types and amounts of banned compounds present in commercial turmeric powder.9,10 Turmeric powder consists of 60–70% total carbohydrates, 6–13% moisture, 6–8% total protein, 5–10% total over, 3–7% total minerals, 3–7% volatile oil, 2–7% total dietary fiber and 1–9% curcuminoids.10 Newer studies have isolated over 235 other constituents of turmeric; besides curcumin, it also contains phenolic compounds and terpenoids.11
Biomolecules with self-therapeutic properties have gained increasing attention for nanoparticle synthesis because of their biocompatibility, biodegradability, repeatability, and non-toxic nature.12–14 These characteristics include the individual surface area of nanoparticles and raises the reactivity of the nanoparticles.15,16 However, most conventional methods of synthesizing nanoparticles involve some drawbacks such as high energy depletion, the toxicity of the chemicals used, and the need for high-tech equipment. Hence, more stress has been attached to green synthesis since it is a relatively environmentally friendly synthesis technique.17,18
Nanotechnology is a revolutionary technology with the possibility of affecting most sectors. It provides new approaches for controlling biotic and abiotic stresses at the molecular level.19 The opportunity to create engineered nanoparticles facilitates new strategies in plant disease control.20 The application of nanoparticles mentioned in several studies would enhance the effectiveness of crop protection against phytopathogens.21
CuO nanoparticles (NPs) have fair antibacterial efficacy against Pseudomonas aeruginosa and Aeromonas hydrophila.22 These CuO NPs also interact with cancer cells.22 Based on this CuO NPs were synthesized and characterized using Curcuma extract.12 Porous curcumin-copper nanoparticles (Cur/Cu NPs) show antifungal proprieties against Fusarium oxysporum f.sp. ciceri. Subsequent studies23 on copper-containing nanoparticles exhibited pro-active broad-spectrum antimicrobial properties, along with anti-inflammatory and cytotoxic effects. Other nanoparticle materials in the current research include the utility of nanoparticles for wound healing and anticancer activities.24 Another study shows the efficacy of Cuf-TMB@PDA nanoparticles in wound healing.25 Recently, the action of nanoceria-curcumin against cancer cells has been demonstrated.26 In addition the green synthesis of Ag NPs using Curcuma extract was characterized.27
Numerous studies extend the initial findings involving metal oxide nanoparticle anticancer and antimicrobial properties while examining synthesis methods, characterization techniques, and potential therapeutic applications. Metal oxide nanoparticles exhibit potential anticancer characteristics against hepatocellular carcinoma HepG2, which confirms our findings regarding Ag/CeO2 and CuO/CeO2 nanocomposite cytotoxicity.28 The antibacterial and anticancer activities of AgAu bimetal-doped CeO2 nanoparticles originate from their ionic-liquid-functionalized biogenic synthesis.29 The green synthesized CeO2–CuO nanocomposites have anti-cancer properties against Saos-2 osteosarcoma cells,30 which is similar to our CuO/CeO2 NC research. Cerium oxide particles were used to develop combined antibacterial and anticancer nanotherapeutic systems, which strengthens the understanding of metal oxide nanoparticle antimicrobial and anticancer properties.31 Meanwhile, research on metal-based nanoparticles through biological synthesis shows anticancer activities for hepatocellular carcinoma cells.32 Green synthesis of cerium oxide nanoparticles shows therapeutic effectiveness and further provides mechanistic anticancer actions.33
Building upon these earlier studies, this work focuses on synthesizing Ag/CeO2 and CuO/CeO2 nanocomposites (NCs) through a green technique involving the utility of Curcuma longa extract as a reducing-, stabilizing-, and capping agent. The generated nanocomposites were characterized by UV-Vis, FTIR, XRD, SEM, TEM, zeta potential, and EDX. Additionally, the biological functions of the nanocomposites were investigated, including antioxidant activity, anti-pathogenicity against Macrophomina phaseolina, and cytotoxicity against the breast cancer cell line. The use of Ag combined with CuO and CeO2 in nanocomposite structure demonstrates novelty because the compounds provide separate yet synergistic therapeutic characteristics. The antimicrobial properties of Ag, along with the cytotoxic effects of CuO, are matched with CeO2's antioxidant character and anticancer functions. Through metal oxide synergies, this nanocomposite combination demonstrates better therapeutic potential, which promotes its use for cancer therapy along with infection control applications. Through this approach, nanomaterial production benefits from sustainable methods that no longer require harmful chemicals and excessive energy usage.
In addition, certain disadvantages need to be acknowledged regarding C. longa extract utilization during nanoparticle synthesis. The extract's chemical composition remains inconsistent due to multiple influencing variables, including extraction practices, environmental components, and plant types. The changing composition of the extract affects the predictable outcomes and the reliable results of nanoparticle synthesis. The scaling up of C. longa extract as an eco-friendly and economical method requires solutions because standardizing the extract and maintaining process control during large-scale production will be difficult. The bioactivity potential of C. longa extract shows promise, but its effects could prove less predictable than standardized synthetic manufacturing methods. The research limitations can be solved by improving extraction protocols and studying scale-up methods for synthesis production.
2. Materials and methods
2.1. Instruments and reagents
The nanocomposites underwent analysis using multiple testing methods, including FTIR, UV-Vis spectroscopy, zeta potential analysis, SEM-EDX, TEM, and XRD for their structural assessment and morphological and elemental investigations. Sonication combined with centrifugation served as essential tools for preparing and processing the nanomaterials during the experimental procedure. The detailed instrumental data can be found in the ESI File “Section S1”.†The study obtained all chemicals and reagents from trusted suppliers, including Sigma Aldrich (USA), Fluka (Romania), Biomedical Inc. (USA), El-Nasr Pharmaceutical Chemicals (Egypt), and PIOCHEM (Egypt). The experiment required silver nitrate together with copper sulfate alongside cerium oxide, Folin-Ciocalteau reagent, gallic acid, DPPH, and additional analytical reagents (Section S2†).
2.2. Preparation of turmeric extract
Turmeric (Curcuma longa) powder was obtained from a local market in Mansoura city, Egypt. A solution of turmeric powder (10 g) in ethanol (100 mL, 70%) was stirred for 2 h at room temperature. The mixture was soaked overnight at room temperature with occasional stirring. The mixture was then filtered, and the extract was immediately stored in a dark glass vessel under cooling to maintain its quality.34 The plant extract was freshly used for the preparation of nanocomposites and other analyses (FTIR and UV-visible spectroscopy) and tests (phytochemical profile, antioxidant, and antimicrobial assessments).2.3. Green synthesis of nanocomposites
The silver nanoparticles were prepared by adding dropwise turmeric extract (50 mL, 13.44 mg mL−1) to 50 mL AgNO3 (1 mM, prepared in deionized water). The mixture was stirred at 55 °C until the solution color changed to brown. Similarly, copper nanoparticles were prepared by dropwise addition of turmeric extract (50 mL, 13.44 mg mL−1) to the 50 mL copper sulfate (1 mM, prepared in deionized water) with stirring at 70 °C until a notable color change was accomplished (≈5 h). A suspension of cerium dioxide was prepared in ethanol (10 mL, 1 mM) and sonicated for 1 h at 60 °C. To prepare Ag/CeO2 and CuO/CeO2 NCs, the cerium dioxide suspension was gradually added to the formed solutions of silver and copper nanoparticles with continuous stirring at room temperature. The mixtures were stirred for 4 hours under heating at 60 °C followed by sonication at 60 °C for 3 h to enable the formation of core–shell nanoparticles and interaction between nanoparticles. For SEM, EDX, and XRD analysis, the mixtures were centrifuged to collect the solid nanocomposites. The solid precipitates were washed with ethanol and deionized water to remove contaminants. The solid nanomaterials were dried at 60 °C for 24 hours.352.4. Quantification of phytochemical analysis
The total phenolic contents were assessed using the Folin–Ciocalteau assay.36,37 For the assay, 100 µL of the solution was combined with 5 mL of dilution Folin–Ciocalteau reagent (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) along with the addition of 4 mL sodium carbonate solution (7.5%). Distilled water was added to reach 10 mL volume, which was then incubated in dark conditions at 40 °C for 30 minutes. Measurement of the absorbance occurred at 765 nm using a spectrophotometer. The measurement of phenolic content relied on a standard gallic acid curve (0–100 mg L−1) from which the samples obtained their values through standard curve interpolation. The analysis showed concentrations of gallic acid equivalents as mg GAE per g DW in the dried weight of the tested samples. The total flavonoid contents were determined using the aluminum chloride assay.38,39 A 100 µL sample was taken, followed by the addition of 4 mL distilled water and 0.3 mL of sodium nitrite solution (5%) and left to stand for 5 minutes. Then, 0.3 mL of aluminum chloride solution (10% in ethanol) was added to the solution before incubating it for another 5 minutes. Subsequently, 2 mL of 1 M sodium hydroxide solution was added and thoroughly mixed. The mixture received 10 mL of distilled water before heat incubation at room temperature for 15 minutes. The absorbance of the orange solution was measured at wavelength 510 nm using a spectrophotometer. The flavonoid content in the samples was measured using a standard curve of catechin (0–100 mg L−1), which allowed the calculation of results from sample absorbance data via the standard curve equations. The results were evaluated through milligrams of catechin equivalents, which were expressed per gram of dried sample (mg QE per g DW). All assays were calculated using standard curves (gallic acid standard curve for the estimation of phenolic contents (y = 0.0062×, R2 = 0.987), and catechin standard curve for the estimation of flavonoid contents (y = 0.0028×, R2 = 0.988)) (Section S3†). The analysis was performed in three replicates for C. longa extract and nanocomposites. The values were expressed as the mean value ± standard deviation (mean ± SD).
10) along with the addition of 4 mL sodium carbonate solution (7.5%). Distilled water was added to reach 10 mL volume, which was then incubated in dark conditions at 40 °C for 30 minutes. Measurement of the absorbance occurred at 765 nm using a spectrophotometer. The measurement of phenolic content relied on a standard gallic acid curve (0–100 mg L−1) from which the samples obtained their values through standard curve interpolation. The analysis showed concentrations of gallic acid equivalents as mg GAE per g DW in the dried weight of the tested samples. The total flavonoid contents were determined using the aluminum chloride assay.38,39 A 100 µL sample was taken, followed by the addition of 4 mL distilled water and 0.3 mL of sodium nitrite solution (5%) and left to stand for 5 minutes. Then, 0.3 mL of aluminum chloride solution (10% in ethanol) was added to the solution before incubating it for another 5 minutes. Subsequently, 2 mL of 1 M sodium hydroxide solution was added and thoroughly mixed. The mixture received 10 mL of distilled water before heat incubation at room temperature for 15 minutes. The absorbance of the orange solution was measured at wavelength 510 nm using a spectrophotometer. The flavonoid content in the samples was measured using a standard curve of catechin (0–100 mg L−1), which allowed the calculation of results from sample absorbance data via the standard curve equations. The results were evaluated through milligrams of catechin equivalents, which were expressed per gram of dried sample (mg QE per g DW). All assays were calculated using standard curves (gallic acid standard curve for the estimation of phenolic contents (y = 0.0062×, R2 = 0.987), and catechin standard curve for the estimation of flavonoid contents (y = 0.0028×, R2 = 0.988)) (Section S3†). The analysis was performed in three replicates for C. longa extract and nanocomposites. The values were expressed as the mean value ± standard deviation (mean ± SD).
2.5. Antioxidant activity
The antioxidant potential was determined using the DPPH˙ method with standard ascorbic acid.40 Dilutions of each of the samples were made by serial dilutions in methanol. An equal volume of each experimental sample to be tested was mixed with 0.135 mM of DPPH˙ solution. Each sample was then incubated for 30 min in the dark at room temperature and the absorbance was read at 517 nm. The remaining DPPH˙ (%) was estimated as follows (eqn (1)):| % DPPH˙ remaining = [DPPH˙]T/[DPPH˙]T = 0 × 100 | (1) |
To draw a calibration curve, the percentage of remaining DPPH˙ was plotted against the sample concentration in mg mL−1, and the IC50 was estimated. The lower the IC50 value to which the reagents were obtained, the higher the antioxidant activity of the investigated sample.41 The analysis was performed in three replicates, and the values were expressed as the mean value ± standard deviation (mean ± SD) (Section S4†).
2.6. Antifungal activity
2.7. Cytotoxic activity
 | (2) |
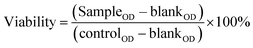 | (3) |
For significant cytotoxicity, a viability threshold of less than 70% was considered, as recommended in previous studies.45
2.8. Statistical procedure and software
The evaluation of mean data involved the use of Statistical Package for Social Sciences (SPSS, version 21) software. The p-value ≤ 0.05 served as the threshold for statistical significance to establish meaningful differences between experimental groups (Section 5).3. Results and discussion
3.1. Mechanism of formation of nanocomposites
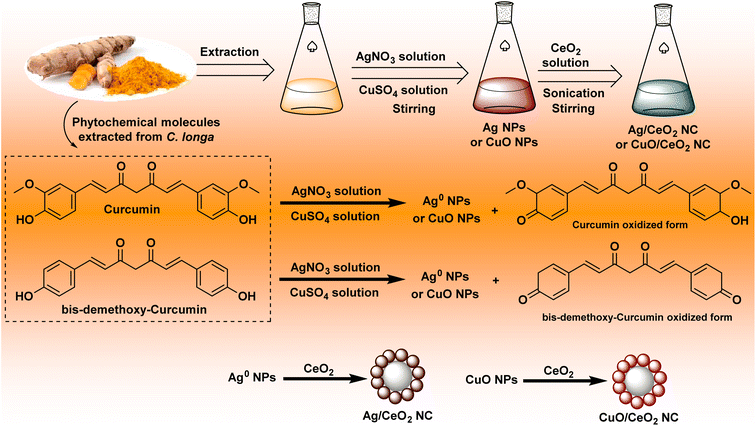
The formation of nanocomposites using C. longa extract involved several steps such as reduction and capping by C. longa extract, interaction with CeO2 NPs, reshaping, and growth of cerium dioxide shell (Fig. 1). The creation of Ag/CeO2 and CuO/CeO2 NCs has some common features. However, emphasizing their differences offers a better understanding of the entire process of each.| Ag+ + C14H14O4 → Ag0 + CO2 + H2O + other byproducts | (4) |
| Cu2+ + C14H14O4 → CuO + CO2 + H2O + other byproducts | (5) |
Curcumin and other extract components bind to the newly formed metal nanoparticles (Ag NPs and CuO NPs), preventing agglomeration and stabilizing them.47 This capping influences their size, shape, and dispersity. The interaction can be described by the Langmuir adsorption isotherm (eqn (6)):
| θ = K-ads × C/(1 + K-ads × C) | (6) |
| AgNO3 + CeO2 + C14H14O4 + other extract components → Ag/CeO2 NC + CO2 + H2O + byproducts | (7) |
| CuSO4 + CeO2 + C14H14O4 + other extract components → CuO/CeO2 NC + CO2 + H2O + byproducts + SO42− | (8) |
Curcumin displays slightly higher reducing power for Ag+ compared to Cu2+, potentially prompting the relative rates of metal ion reduction during the nanoparticle formation.51 The surface chemistry and charge distribution of Ag NPs and CuO NPs might vary, leading to variations in their interaction with and deposition on the CeO2 clusters.52 The different characters between both metals could impact the favorite pathway of formation (physical deposition vs. interdiffusion) and the final structure of the nanocomposite.53
3.2. Characterization of nanocomposites
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) groups present in curcumin (the primary bioactive component of turmeric) or an alternative component incorporated into ester or carboxyl acid groups. The (C
O) groups present in curcumin (the primary bioactive component of turmeric) or an alternative component incorporated into ester or carboxyl acid groups. The (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) groups of aromatic rings appeared close to 1681, 1626, 1592, and 1572 cm−1, implying the presence of aromatic rings, possibly curcumin or alternative aromatic compounds in the mixture, and a conjugated double bond. The absorption band for C–N stretching near 1512 cm−1 can be attributed to aromatic amines or nitrogen-rich heterocyclic rings. The range of absorptions between 1455 and 1131 cm−1 are attributed to various C–O functional groups, including alcohols, phenols, or esters found in curcumin and other extract components. The fragrance of curcumin and other potential bioactive compounds in the solution remain consistent. The bioreduction of curcumin depends on its hydroxyl-, carbonyl-, and aromatic functional groups which provide two roles: metal ion electron donation and nanoparticle surface stability. The carbonyl groups at 1741 cm−1 and aromatic rings at 1681, 1626, 1592, and 1572 cm−1 in curcumin powerfully contribute to nanocomposite stability by binding with metal ions and affecting the resulting electronic structure.
C) groups of aromatic rings appeared close to 1681, 1626, 1592, and 1572 cm−1, implying the presence of aromatic rings, possibly curcumin or alternative aromatic compounds in the mixture, and a conjugated double bond. The absorption band for C–N stretching near 1512 cm−1 can be attributed to aromatic amines or nitrogen-rich heterocyclic rings. The range of absorptions between 1455 and 1131 cm−1 are attributed to various C–O functional groups, including alcohols, phenols, or esters found in curcumin and other extract components. The fragrance of curcumin and other potential bioactive compounds in the solution remain consistent. The bioreduction of curcumin depends on its hydroxyl-, carbonyl-, and aromatic functional groups which provide two roles: metal ion electron donation and nanoparticle surface stability. The carbonyl groups at 1741 cm−1 and aromatic rings at 1681, 1626, 1592, and 1572 cm−1 in curcumin powerfully contribute to nanocomposite stability by binding with metal ions and affecting the resulting electronic structure.
 | ||
| Fig. 2 FTIR spectra and UV-visible spectroscopy of the tested samples. (a) FTIR spectra of C. longa extract, Ag/CeO2, and CuO/CeO2 NCs. (b) Absorbance of the samples at different wavelengths. | ||
The FTIR spectra of Ag/CeO2 and CuO/CeO2 NCs displayed absorption patterns related to residual organic compounds (C–H stretch) at 2969, 2925, and 2857 cm−1 for Ag/CeO2, and 3013, 2966, 2925, and 2854 cm−1 for CuO/CeO2. These absorptions likely originate from stabilizers or adsorbed hydrocarbons used during the synthesis process. The characteristic Ce–O stretching extreme of 1033 cm−1 in the FTIR spectrum of Ag/CeO2 NCs confirmed the existence of cerium oxide. Like the Ag/CeO2 case, the FTIR spectrum of CuO/CeO2 NCs showed a combination of functional groups linked at the same time to the metallic oxides (CuO and CeO2). The emergence of the Ce–O stretching absorption band indicated the presence of CeO2. The presence of the Ce–O stretching band at 1033 cm−1 in both Ag/CeO2 and CuO/CeO2 NCs is a strong indication of the presence of cerium oxide (CeO2), as reported in the literature.54,55 The FTIR spectra of both types of nanocomposites revealed a complex mixture of functional groups, suggesting the presence of residual organic materials and potential interactions between the metal oxides and organic components. This is consistent with previous studies of Ag/CeO2 catalysts prepared using microwave-assisted biosynthesis.56 The FTIR spectra from Ag/CeO2 and CuO/CeO2 nanocomposites show minor spectral changes that indicate identical interactions between their functional groups of carbonyl and aromatic substances. The spectra demonstrate that organic components including curcumin alongside additional phytochemicals stabilize the nanoparticles through their C–O (various) and C–H (organic moieties) active bands.
Curcumin's chromophores, responsible for light absorption, can undergo two main types of electronic transitions: n → π* and π → π*. The n → π transition occurs when an electron is excited from a non-bonding orbital (n) to an antibonding pi orbital (π*). This transition typically requires less energy compared to π → π* transitions and often results in weaker light absorption, i.e. a lower absorbance value.57 During bioreduction, curcumin donates electrons to metal ions, which can modify the energy spectrum levels of the curcumin and metal and move absorption bands toward red wavelengths. The chromophores of curcumin typically undergo n → π* (less energy, weaker absorption) and π → π* transitions (more energy, stronger absorption) in the UV-visible range.58 The red shift could be due to phytochemicals donating electrons to metal ions during bioreduction, altering the chromophore's electronic structure and affecting both n → π* and π → π* transitions.59
CuO/CeO2 NC exhibits a larger redshift than Ag/CeO2 NC due to a more robust interaction between curcumin and Cu ions because Cu has a higher electron affinity than Ag. The nanocomposite shows improved interactions between curcumin molecules that would modify their chromophore electronic environment and their absorption behavior. The aggregated state modifies n → π* and π → π* transitions which ultimately results in spectral redshift. The research evidence indicates that C. longa extract demonstrates promising potential for biologically reducing metal ions into nanoparticles while the detected spectral redshift explains the electron transfer behavior during metal oxide nanocomposite synthesis.
While Ag/CeO2 and CuO/CeO2 NCs exhibit comparable hydrodynamic sizes (622.9 d nm for Ag/CeO2 and 602.9 d nm for CuO/CeO2), CuO/CeO2 NC exhibit an excessive uniform atom size circulation as demonstrated by a low PDI value (0.027 compared to 0.073 for Ag/CeO2) (Fig. 3c and d). The conduction principles of both types of nanoparticles are comparable (2.05 mS cm−1 for Ag/CeO2 and 1.83 mS cm−1 for CuO/CeO2) and propose a similar ionic dispersion. Frequently, this difference in zeta potential and atom size may influence the stability and communication of the nanoparticles in different objectives. Our finding indicate a slightly positive zeta probability for the same type of nanocomposites, which is consistent with previous findings for Ag/CeO2 NC.60,61 The developed positively charged solution has potential applications in dye degradation networks where electrostatic forces enable the breakdown of negatively charged pollutants. We detected strong electrostatic repulsion between Ag/CeO2 atoms because Ag/CeO2 demonstrates a higher zeta capability than CuO/CeO2. The results support the zeta potential theory that elevated zeta potential values provide enhanced colloidal stability, although such nanoparticles would benefit from excellent dispersal properties in applications. Both Ag/CeO2 and CuO/CeO2 NCs have an equivalent hydrodynamic size of approximately 600 nanometers, which is in line with the expectations of this type of nanomaterial.62 The low PDI value (0.027) for CuO/CeO2 compared to Ag/CeO2 (0.073) indicates an excessive uniform atom size dispersion, and circulation which is desirable for several purposes. The current characteristic may be advantageous for functions where stable atom sizes are essential, such as for targeted drug distribution or catalysis. For functions where stable atom sizes are essential, such as for targeted drug distribution or catalysis, this characteristic may be advantageous. The current analysis suggests that CuO/CeO2 NC may have a positive zeta capability.63 The present condition is stable with the general shift in the composition of metallic oxide nanoparticles with a constructive veneer charge planned in the presence of a metallic oxide chemical bond. The DLS value of CuO/CeO2 NC (602.9 d nm) is comparable with the size demonstrated in previous literature for similar nanomaterials.60,61
EDX analysis of the synthesized Ag/CeO2 NC showed the presence of oxygen and cerium at higher intensity and silver at moderate intensity while zinc was observed as an impure element at a very low intensity. The EDX analysis of CuO/CeO2 NC synthesized using hydrothermal method pointed out that oxygen, copper, and cerium exhibited high-intensity peaks in the X-ray spectrum, whereas potassium was an impurity of low intensity. The EDX data obtained for Ag/CeO2 and CuO/CeO2 NCs (Fig. 6) aligns well with the findings reported in the literature. Our observation of the dominant oxygen, cerium, and silver is consistent with previous studies on Ag/CeO2 NC.67,68 The detection of zinc as a minor impurity is in line with the potential for contamination during the synthesis process, as reported in the literature.69 The dominance of oxygen, copper, and cerium in the CuO/CeO2 NC is expected based on its composition. The presence of potassium as a minor impurity could be attributed to the synthesis conditions or the use of specific precursors.69 Briefly, the EDX data obtained in this study is consistent with the literature on Ag/CeO2 and CuO/CeO2 NCs. The prosperous combination of the desired elements and the identification of minor impurities provide valuable information on the synthesis process and the properties of these materials.
The XRD pattern of the CuO/CeO2 NC has the following characteristic peaks belonging to the face-centered cubic (fcc) CuO and CeO2: (111), (200), (220), (311), (222), (400), (331), (420), (422), and (440) (Fig. 7b). The heights of these peaks indicate that there is a favored orientation of the crystallites within the nanocomposite (Table S5†). These values of d-spacing are in close agreement with the standard values of fcc copper oxide and cerium oxide, indicating the existence of these phases in the nanocomposite. The characterized broadening of the diffraction peaks gives evidence of the nanocrystalline structure of the synthesized material and the approximate diameter of the crystallites is 10–20 nm. The presence of CeO2 was confirmed owing to peaks corresponding to reference patterns (ICSD # 00-050-0543). A few peaks coincide with CuO (ICSD reference code # 01-073-0603), meaning that there is also a copper oxide phase. The determined d-spacing values correspond well to the earlier patterns for CeO2 and CuO. Based on the XRD results, we can confirm the synthesis of CuO/CeO2 NC with a polycrystalline nature. Knowledge of the crystal phases as well as their preferred orientation plays a significant role in defining the structure and possible uses of this nanocomposite.
The XRD data collected for the Ag/CeO2 and CuO/CeO2 NCs examined in this study are supported by the data available in the literature. This observation of the CeO2 phase having major peaks matching (111), (200), (220), and other planes of the crystal face matches previous work.70 The appearance of peaks related to Ag at reduced intensity indicates that the Ag has replaced some of the Ce sites in the CeO2 perovskite structure as confirmed in another related study.71 The values of d-spacing patterns estimated for the Ag/CeO2 NC correspond with the standard patterns of CeO2, indicating that CeO2 is the main crystalline phase in the nanocomposite. The XRD diffraction pattern of the CuO/CeO2 NC confirms the existence of Cu and Ce phases.72 The observed d-spacing values also correspond to the standard lattice parameters for the two identified phases, CuO and CeO2. The relative intensity of the peak indicates that the preferred orientation of the crystallites in the nanocomposite is identical to Ag/CeO2 NC. In general, the results of the XRD analysis observed in this work correlate with the previous reports concerning Ag/CeO2 and CuO/CeO2 NCs. The material's structural characteristics and potential applications were elucidated by identifying the crystal phases and preferred orientation.
3.3. Phytochemical contents
The phytochemical screening (Fig. 8a and Table S6†) of the extract of turmeric and of Ag/CeO2 and CuO/CeO2 NCs showed moderate to good content of phenolic and flavonoid compounds. The total flavonoids were estimated to be 155 ± 9.7 mg CE per g and there was 215.3 ± 11.4 mg GAE per g in the turmeric extract. Phenolic content analysis of the NCs showed that Ag/CeO2 NC had 91.64 ± 8.46 mg CE per g flavonoids, 148 mg CE per g; the turmeric extract had 92.89 ± 13.40 mg CE per g flavonoids, and the CuO/CeO2 NC had 174.1 ± 7.46 mg GAE per g phenolic content. The phytochemicals present in all the nanocomposites and extracts used in this study were presented in appreciated quantity. The slight decrease in phytochemical content of the Ag/CeO2 and CuO/CeO2 NCs compared to the turmeric extract was most probably due to the contribution of the phytochemicals in the bioreduction process of metal ions.73 Phenolic and flavone compounds have high antioxidant activity because of their high electron donation capacities. This characteristic renders the turmeric extract used in this work suitable for the bioreduction process of metal ions to metallic nanoparticles. The bioreduction process results in the transformation of phytochemical components into oxidized or dehydrogenated forms.74In this work, the participation of phenolic compound phytochemicals has been described for the green synthesis of metal nanoparticles. The higher level of phytochemicals in the turmeric extract compared to the nanocomposites can provide evidence that a major part of these compounds was involved in the bioreduction process. The action of phytochemicals with metal ions during the synthesis process may affect the nanoparticle attributes, including size, shape, and stability. This means that although the phytochemical content is lower in the nanocomposites, their bioactivity may be retained, at least in part, by some components or interactions with the nanoparticles or the other phytochemicals.75 Therefore, a decline in the phytochemical content of both Ag/CeO2 and CuO/CeO2 NCs is believed to have been triggered by the bioreduction of metal ions.
3.4. Antioxidant activity
The antioxidant activity of turmeric extract and the nanocomposites was assessed by DPPH assays (Fig. 8b). The IC50 represents the concentration of the samples that are required to scavenge DPPH radical by 50%. Turmeric extract had the lowest IC50 value at a concentration of 0.042 mg mL−1 (Fig. S2 and Table S7†), signifying that it was the most active antioxidant among the samples. The IC50 of Ag/CeO2 NC is even higher (0.242 mg mL−1) than the IC50 of the turmeric extract, indicating a lower antioxidant capacity. The IC50 value for CuO/CeO2 NC (0.081 mg mL−1) is lower than Ag/CeO2 NC but higher than that of turmeric extract. In this case, turmeric extract is the most potent antioxidant agent, followed by CuO/CeO2 NC, while Ag/CeO2 NC was the least active agent to scavenge the free radicals.The antioxidant activity of the turmeric extract and nanocomposites are comparable to that of ascorbic acid (IC50 = 0.022 mg mL−1), confirming their strong antioxidant properties. On the other hand, the highest antioxidant activity was observed with turmeric extract, with a scavenging activity of 56.99% at 0.053 mg mL−1. Ag/CeO2 NC nanocomposite exhibited significant scavenging activity of 25.67% at 0.1 mg mL−1. CuO/CeO2 NC also displayed notable scavenging activity of 63.10% at 0.117 mg mL−1 (Fig. 8b). All samples exhibited concentration-dependent antioxidant activity, with turmeric extract exhibiting the highest scavenging activity at all concentrations. Both Ag/CeO2 and CuO/CeO2 NCs displayed significant antioxidant activity, suggesting that the incorporation of the metal oxide nanoparticles did not completely diminish the antioxidant properties of the turmeric extract.
The antioxidant activity of both turmeric extract and the Ag/CeO2 and CuO/CeO2 NCs can be attributed to their ability to neutralize free radicals, chelate metal ions, and enhance antioxidant activities.76 Turmeric extract is rich in phytochemicals such as curcuminoids77–79 and directly scavenges free radicals, chelates metal ions, and improves antioxidant activity. The nanocomposites likely retain some phytochemicals from the turmeric extract, although they may also exhibit synergistic effects due to the combination of metal oxides and cerium oxide. Additionally, the surface properties of the nanoparticles and their potential to reduce metal ions could contribute to their antioxidant activity. The results of the antioxidant activity agree with the results of phytochemical contents, indicating the participation of phytochemicals in the bioreduction process to form metal nanoparticles and oxidized or dehydrogenated forms of the phytochemicals of the turmeric extract.
3.5. Antifungal activity
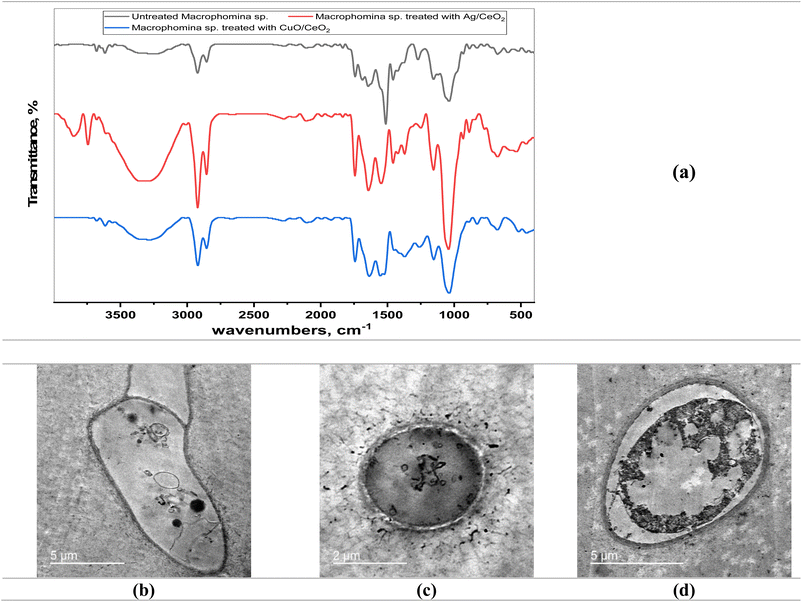
The growth patterns of M. phaseolina appear in Fig. S3,† both with and without nanocomposite treatment. M. phaseolina maintains its typical network growth pattern before treatment, as an essential reference point to assess antifungal efficacy. The extensive growth shows that no antimicrobial treatment has been applied, which underscores the necessity for antifungal interventions. The application of Ag/CeO2 NC to fungal samples caused significant growth reduction while creating visible inhibited areas at their application points. The antimicrobial effect occurs thanks to silver (Ag) nanoparticles interacting with cerium oxide (CeO2) to produce reactive oxygen species (ROS) along with supporting redox reactions. Plant protection through sustainable antifungal treatment could be achieved by using Ag/CeO2 NC. The antifungal efficiency of CuO/CeO2 NC became evident as they created distinct inhibition zones because the ROS that was produced led to fungal cell damage and stopped fungal expansion. The combination of nanoparticles in both materials demonstrates valuable prospects to protect plants from M. phaseolina infection.
Both nanocomposites exhibit different concentrations of potency because CuO/CeO2 shows increased catalytic behavior for reactive oxygen species generation that produces oxidative stress and cell damage. TEM analysis confirms membrane disruption because it shows visible cell wall deformities and both nanoparticle penetration and potential internal turgor pressure loss. The combined findings show that the antifungal effects of CuO/CeO2 NCs result from an interaction of oxidative stress and dual damage effects on cell walls and fungal cell structure.87 Future experimental research needs to incorporate both ROS measurement tests and membrane stability examinations to prove that oxidative stress exists when validating these results. The integration of nanocomposite antifungal agents with traditional antifungal therapies should be studied because such combinations could improve effectiveness without boosting resistance. Assessing nanocomposites for agricultural sustainability88 requires a complete environmental impact evaluation that considers their persistence time along with their toxic effects on beneficial organisms and their ability to biodegrade.
3.6. Cytotoxic activity
The TEM images of cells treated with CuO/CeO2 NC showed several morphological changes (Fig. 11B). The nucleus appeared nearly normal, suggesting that DNA integrity was not severely compromised. However, the mitochondria exhibited signs of atrophy, indicating impaired energy production.94 Additionally, the cytoplasm was vacuolated, and the endoplasmic reticulum was disrupted, suggesting cellular damage and dysfunction.95 The TEM images of cells treated with Ag/CeO2 NC revealed even more severe morphological changes (Fig. 11C). The nucleus appeared necrotic with chromatin condensation, indicating severe cellular damage.96 The cytoplasm was also vacuolated, and numerous lysosomes were observed, suggesting increased autophagy activity.97
TEM demonstrates that both CuO/CeO2 NC and Ag/CeO2 NC exert cytotoxic effects on MCF-7 cells. CuO/CeO2 NC induced mitochondrial damage and cytoplasmic alterations, while Ag/CeO2 NC caused severe nuclear damage and autophagy. The observed morphological changes suggest that both nanocomposites may induce cell death through multiple mechanisms, including mitochondrial dysfunction, oxidative stress, and autophagy.98,99
MCF-7 breast cancer cells show different mechanisms of cytotoxicity against CuO/CeO2 and Ag/CeO2 nanocomposites.100 Cellular energy production through the mitochondria encounters damage from CuO/CeO2 NC, which generates ROS101 to cause oxidative stress. Copper-based CuO releases free copper ions that activate Fenton reactions to worsen the oxidative damage. Oxidative stress becomes stronger when CeO2 combines its redox-active properties with the process,102 which results in apoptotic or necrotic cell death. The cytotoxic mechanism of Ag/CeO2 NC includes severe nuclear damage leading to DNA fragmentation and chromatin condensation.103 Silver nanoparticles (Ag NPs) display genomic toxicity through DNA breakages that similarly damage cell proteins, causing destructive changes in nuclear structures.104 The combination of CeO2 nanoparticles seems to strengthen the cellular process of autophagy, which may result in autophagic cell death when unregulated. The joint activity between Ag and CeO2 produces increased cell-killing power within Ag/CeO2 NC. The different ways in which copper oxide (CuO) and silver (Ag) act on cellular components combined with their separate ion release methods explain their varied toxic effects on cells. CuO/CeO2 induces extensive oxidative stress,105 but Ag/CeO2 mainly affects DNA integrity and causes autophagic cell destruction through its actions.106 Some nanocomposite combinations using these metal oxides become more toxic than single compound elements. This discovery leads to valuable knowledge for better cancer treatment methods.107,108
4. Conclusion
This study presents a green synthesis of Ag/CeO2 and CuO/CeO2 NCs using C. longa extract. Characterization confirmed the formation of well-dispersed nanoparticles with spherical or slightly elongated morphology. The interaction between the extract and metal ions during bioreduction was evident from the UV-visible spectroscopy data. The nanoparticles exhibited comparable hydrodynamic sizes and slightly positive zeta potentials. The Ag/CeO2 and CuO/CeO2 NCs retained significant antioxidant activity similar to the turmeric extract according to DPPH assays, suggesting their potential for various applications. The observed antioxidant activity of the nanocomposites can be due to the incidence of phytochemicals and the synergistic effects of the metal oxides. On the other hand, this study highlights the potential of green synthesis of nanocomposites as antifungal agents and for cancer therapy. CuO/CeO2 and Ag/CeO2 NCs showed antifungal activity against pathogenic M. phaseolina. CuO/CeO2 NC was more effective than Ag/CeO2 NC, with MIC values of 29 µg mL−1 and 49 µg mL−1, respectively. TEM analysis verified that the nanocomposites likely interacted with the fungal cell wall, causing damage and inhibiting growth. Specifically, CuO/CeO2 and Ag/CeO2 NCs showed stronger cytotoxicity against MCF-7 breast cancer cells than turmeric extract. CuO/CeO2 was particularly effective, with an IC50 value of 0.5071 µg mL−1. TEM analysis revealed that these nanocomposites caused damage to the mitochondria, cytoplasm, and the nucleus, suggesting their potential to disrupt cellular functions and induce cell death. More investigation is needed to fully elucidate their action mechanisms and evaluate their potential as anticancer agents.Future research should address these specified points when building upon the Ag/CeO2 and CuO/CeO2 NCs applications. Research should examine the antioxidant-, antifungal-, and anticancer processes operated by these nanocomposites by studying their effects at the organism and biochemical levels. Safety evaluation with therapeutic measurements needs to be conducted through in vivo experiments to assess these nanocomposites for clinical applications. Scientists should assess the prolonged effects of human cell exposure and ecosystem changes due to exposure to these nanomaterials. The complete commercial implementation of these nanocomposites requires studies on large-scale manufacturing processes and assessments of their environmental stability.
Data availability
The datasets generated and/or analyzed during the current study are available in the ESI.† Additional data are available from the corresponding author upon reasonable request.Conflicts of interest
There is no conflict of interest to declare.Acknowledgements
Researchers would like to thank and acknowledge the support of the Researchers Supporting Project Number (RSPD2025R901), King Saud University, Riyadh, Saudi Arabia.References
- G. Asghari, A. Mostajeran and M. Shebli, Res. Pharm. Sci., 2010, 4, 55–61 Search PubMed.
- Y. He, Y. Yue, X. Zheng, K. Zhang, S. Chen and Z. Du, Molecules, 2015, 20, 9183–9213 CrossRef CAS PubMed.
- D. López-Malo, C. A. Villarón-Casares, J. Alarcón-Jiménez, M. Miranda, M. Díaz-Llopis, F. J. Romero and V. M. Villar, Antioxidants, 2020, 9, 48 CrossRef PubMed.
- A. Zielińska, H. Alves, V. Marques, A. Durazzo, M. Lucarini, T. F. Alves, M. Morsink, N. Willemen, P. Eder and M. V. Chaud, Medicina, 2020, 56, 336 CrossRef PubMed.
- M. Barchitta, A. Maugeri, G. Favara, R. Magnano San Lio, G. Evola, A. Agodi and G. Basile, Int. J. Mol. Sci., 2019, 20, 1119 CrossRef CAS PubMed.
- R. R. Kotha and D. L. Luthria, Molecules, 2019, 24(16), 2930 CrossRef CAS PubMed.
- J. F. de Souza, K. da Silva Pontes, T. F. R. Alves, C. T. de Barros, V. A. Amaral, K. M. de Moura Crescencio, A. C. Rios, F. Batain, E. B. Souto and P. Severino, J. Mol. Liq., 2020, 306, 112861 CrossRef.
- H. Bagheri, F. Ghasemi, G. E. Barreto, R. Rafiee, T. Sathyapalan and A. Sahebkar, Biofactors, 2020, 46, 5–20 CrossRef CAS PubMed.
- A. Plotto, INPhO Post Harvest Compendium, Food and Agriculture Organizations of United Nations. FAO, Rome, Italy, 2004, pp. 2–8 Search PubMed.
- S. S. Hettiarachchi, S. P. Dunuweera, A. N. Dunuweera and R. G. Rajapakse, ACS Omega, 2021, 6, 8246–8252 CrossRef CAS PubMed.
- S. Li, W. Yuan, G. Deng, P. Wang, P. Yang and B. B. Aggarwal, Chemical composition and product quality control of turmeric (Curcuma longa L.), Pharm. Crops, 2011, 2, 28–54 CrossRef CAS.
- M. Sathiyabama and S. Muthukumar, Int. J. Biol. Macromol., 2020, 153, 297–304 CrossRef CAS PubMed.
- A. Elsayed, G. M. El-Shamy and A. A. Attia, Egypt. J. Bot., 2022, 62, 507–522 Search PubMed.
- A. Aboelnga, H. Salaheldin and A. Elsayed, Egypt. J. Chem., 2024, 67, 555–562 Search PubMed.
- Y.-N. Chang, M. Zhang, L. Xia, J. Zhang and G. Xing, Materials, 2012, 5, 2850–2871 CrossRef CAS.
- N. H. Ghazala, A. H. Mohamedin, M. O. Abdel-Monem and A. Elsayed, Egypt. J. Chem., 2024, 67, 587–600 Search PubMed.
- S. Ying, Z. Guan, P. C. Ofoegbu, P. Clubb, C. Rico, F. He and J. Hong, Environ. Technol. Innovation, 2022, 26, 102336 CrossRef CAS.
- M. Taha, A. Elsayed, M. Abbas, H. Fakhry and E. M. Ali, Egypt. J. Chem., 2024, 67, 513–526 CrossRef.
- P. L. Kashyap, X. Xiang and P. Heiden, Int. J. Biol. Macromol., 2015, 77, 36–51 CrossRef CAS PubMed.
- J. Chen, L. Wu, M. Lu, S. Lu, Z. Li and W. Ding, Front. Microbiol., 2020, 11, 365 CrossRef PubMed.
- Y. Hao, P. Fang, C. Ma, J. C. White, Z. Xiang, H. Wang, Z. Zhang, Y. Rui and B. Xing, Environ. Res., 2019, 170, 1–6 CrossRef CAS PubMed.
- K. Saravanakumar, A. Sathiyaseelan, A. V. A. Mariadoss, H. Xiaowen and M.-H. Wang, Int. J. Biol. Macromol., 2020, 153, 207–214 CrossRef CAS PubMed.
- X. Ma, S. Zhou, X. Xu and Q. Du, Front. Surg., 2022, 9, 905892 CrossRef PubMed.
- S. Singh, A. S. Prasad and S. Rajeshkumar, J. Int. Soc. Prev. Community Dent., 2023, 13, 450–457 CrossRef PubMed.
- Y. Zhou, P. Sun, Y. Cao, J. Yang, Q. Wu and J. Peng, J. Nanobiotechnol., 2023, 21, 474 CrossRef CAS PubMed.
- N. Zholobak, A. Shcherbakov, O. Ivanova, V. Reukov, A. Baranchikov and V. Ivanov, J. Photochem. Photobiol., B, 2020, 209, 111921 CrossRef CAS PubMed.
- K. Shameli, M. B. Ahmad, A. Zamanian, P. Sangpour, P. Shabanzadeh, Y. Abdollahi and M. Zargar, Int. J. Nanomed., 2012, 5603–5610 CrossRef CAS PubMed.
- M. Younas, M. Zubair, M. Rizwan, M. A. Khan, K. M. Hussaini, R. Mumtaz, M. Azeem, T. Abbas, M. A. Irshad and S. Ali, J. Mol. Struct., 2023, 1288, 135756 CrossRef CAS.
- P. Nithya and M. Sundrarajan, J. Photochem. Photobiol., B, 2020, 202, 111706 CrossRef CAS PubMed.
- W. M. Salih, R. Sabah, D. A. Kadhim, H. A. Kadhum and M. A. Abid, Inorg. Chem. Commun., 2024, 159, 111730 CrossRef CAS.
- H. Lu, L. Wan, X. Li, M. Zhang, A. Shakoor, W. Li and X. Zhang, Int. J. Nanomed., 2022, 17, 5733 CrossRef PubMed.
- M. Younas, M. Rizwan, M. Zubair, A. Inam and S. Ali, Ecotoxicol. Environ. Saf., 2021, 223, 112575 CrossRef CAS PubMed.
- M. Khan, Z.-u.-R. Mashwani, M. Ikram, N. I. Raja, A. H. Mohamed, G. Ren and A. A. Omar, Nanomaterials, 2022, 12, 2117 CrossRef CAS PubMed.
- A. A. Ghoniem, K. M. Elattar, F. O. Al-Otibi, A. Elsayed, M. S. El-Hersh, A. Y. El-Khateeb, Y. A. Helmy and W. I. Saber, RSC Adv., 2024, 14, 7088–7111 RSC.
- M. M. Hammouda, A. A. Alanazi and K. M. Elattar, ChemistrySelect, 2024, 9, e202401385 CrossRef CAS.
- O. Folin and V. Ciocalteu, J. Biol. Chem., 1927, 73, 627–650 CrossRef CAS.
- J. C. Sánchez-Rangel, J. Benavides, J. B. Heredia, L. Cisneros-Zevallos and D. A. Jacobo-Velázquez, Anal. Methods, 2013, 5, 5990–5999 RSC.
- A. M. Shraim, T. A. Ahmed, M. M. Rahman and Y. M. Hijji, Lwt, 2021, 150, 111932 CrossRef CAS.
- J. Zhishen, T. Mengcheng and W. Jianming, Food Chem., 1999, 64, 555–559 CrossRef CAS.
- D. D. Kitts, A. N. Wijewickreme and C. Hu, Mol. Cell. Biochem., 2000, 203, 1–10 CrossRef CAS PubMed.
- I. Parejo, C. Codina, C. Petrakis and P. Kefalas, J. Pharmacol. Toxicol. Methods, 2000, 44, 507–512 CrossRef CAS PubMed.
- S. Castaldi, M. Masi, F. Sautua, A. Cimmino, R. Isticato, M. Carmona, A. Tuzi and A. Evidente, Biomolecules, 2021, 11, 1728 CrossRef CAS PubMed.
- P. W. Sylvester, Drug Design and Discovery: Methods and Protocols, 2011, pp. 157–168 Search PubMed.
- H. Liu, S. Iketani, A. Zask, N. Khanizeman, E. Bednarova, F. Forouhar, B. Fowler, S. J. Hong, H. Mohri and M. S. Nair, Nat. Commun., 2022, 13, 1891 CrossRef CAS PubMed.
- A. P. Wilson, Animal Cell Culture: a Practical Approach, 1986, pp. 183–216 Search PubMed.
- K. Indira Priyadarsini, Curr. Pharm. Des., 2013, 19, 2093–2100 Search PubMed.
- D. A. Selvan, D. Mahendiran, R. S. Kumar and A. K. Rahiman, J. Photochem. Photobiol., B, 2018, 180, 243–252 CrossRef PubMed.
- I. Celardo, M. De Nicola, C. Mandoli, J. Z. Pedersen, E. Traversa and L. Ghibelli, ACS Nano, 2011, 5, 4537–4549 CrossRef CAS PubMed.
- Z. Ren, N. Liu, B. Chen, J. Li and D. Mei, J. Phys. Chem. C, 2018, 122, 27402–27411 CrossRef CAS.
- J. Xiong, X. Mei, J. Liu, Y. Wei, Z. Zhao, Z. Xie and J. Li, Appl. Catal., B, 2019, 251, 247–260 CrossRef CAS.
- J. Venkatas, A. Daniels and M. Singh, Nanomaterials, 2022, 12, 3201 CrossRef CAS PubMed.
- G. Pacchioni, Phys. Chem. Chem. Phys., 2013, 15, 1737–1757 RSC.
- J. A. Stewart and R. Dingreville, Acta Mater., 2020, 188, 181–191 CrossRef CAS.
- A. Martınez-Arias, M. Fernández-Garcıa, J. Soria and J. Conesa, J. Catal., 1999, 182, 367–377 CrossRef.
- L. Xue, C. Zhang, J. Wu, Q.-Y. Fan, Y. Liu, Y. Wu, J. Li, H. Zhang, F. Liu and S. Zeng, Appl. Catal., B, 2022, 304, 120951 CrossRef CAS.
- F. Yang, J. Huang, T. Odoom-Wubah, Y. Hong, M. Du, D. Sun, L. Jia and Q. Li, Chem. Eng. J., 2015, 269, 105–112 CrossRef CAS.
- Y. Wu and W. Zhu, Chem. Soc. Rev., 2013, 42, 2039–2058 RSC.
- S. H. Sumrra, A. U. Hassan, W. Zafar, Z. H. Chohan and K. A. Alrashidi, J. Fluoresc., 2024, 1–18 Search PubMed.
- T. Naghdi, S. Faham, T. Mahmoudi, N. Pourreza, R. Ghavami and H. Golmohammadi, ACS Sens., 2020, 5, 3770–3805 CrossRef CAS PubMed.
- A. A. Alanazi, W. I. Saber, M. A. AlDamen and K. M. Elattar, Int. J. Biol. Macromol., 2024, 280, 135862 CrossRef CAS PubMed.
- A. Sajid, R. Javed, Q. Manzoor, A. Sajid, A. Saleem, F. Imtiaz, S. Ahmed and H. Nadeem, Chem. Afr., 2024, 1–8 Search PubMed.
- M. Javad Farhangi, A. Es-Haghi, M. E. Taghavizadeh Yazdi, A. Rahdar and F. Baino, J. Funct. Biomater., 2021, 12, 53 CrossRef CAS PubMed.
- H.-X. Liu, Z. Gao, H. Yan, S.-Q. Li, W.-W. Wang, X. Qin, H. Sun, J. Cui and C.-J. Jia, Sci. China Chem., 2023, 66, 2590–2599 CrossRef CAS.
- N. M. Eldadamony, A. A. Ghoniem, A. A. Al-Askar, A. A. Attia, M. S. El-Hersh, K. M. Elattar, H. Alrdahi and W. I. Saber, Int. J. Biol. Macromol., 2024, 269, 132109 CrossRef CAS PubMed.
- S. Chang, M. Li, Q. Hua, L. Zhang, Y. Ma, B. Ye and W. Huang, J. Catal., 2012, 293, 195–204 CrossRef CAS.
- M. M. Hammouda, K. Shalabi, A. A. Alanazi, K. M. Elattar, M. A. Azzam and M. M. Rashed, RSC Adv., 2023, 13, 32532–32546 RSC.
- S. Sagadevan, S. Vennila, A. R. Marlinda, Y. Al-Douri, M. Rafie Johan and J. Anita Lett, Appl. Phys. A, 2019, 125, 1–9 CrossRef.
- C.-M. Hung, Aerosol Air Qual. Res., 2008, 8, 447–458 CrossRef CAS.
- G. Mondragon-Galicia, R. Perez-Hernandez, C. Gutierrez-Wing and D. Mendoza-Anaya, Phys. Chem. Chem. Phys., 2011, 13, 16756–16761 RSC.
- R. Vennila, A. H. Banu, P. Kamaraj, S. Devikala, M. Arthanareeswari, T. Pushpamalini, J. G. Buela, D. Priya and R. Sivasankari, Mater. Today: Proc., 2018, 5, 8683–8690 CAS.
- R. A. Ismail, S. A. Abid and A. A. Taha, Lasers in Manufacturing and Materials Processing, 2019, vol. 6, pp. 126–135 Search PubMed.
- J. M. Zamaro, N. C. Pérez, E. E. Miró, C. Casado, B. Seoane, C. Téllez and J. Coronas, Chem. Eng. J., 2012, 195, 180–187 CrossRef.
- T. Ahmad, M. A. Bustam, M. Irfan, M. Moniruzzaman, H. M. A. Asghar and S. Bhattacharjee, Biotechnol. Appl. Biochem., 2019, 66, 698–708 CrossRef CAS PubMed.
- K. M. Elattar, A. A. Ghoniem, F. O. Al-Otibi, M. S. El-Hersh, Y. A. Helmy and W. I. Saber, Appl. Sci., 2023, 13, 10110 CrossRef CAS.
- K. M. Elattar, F. O. Al-Otibi, M. S. El-Hersh, A. A. Attia, N. M. Eldadamony, A. Elsayed, F. Menaa and W. I. Saber, Heliyon, 2024, 10, e28359 CrossRef CAS PubMed.
- İ. Gulcin and S. H. Alwasel, Processes, 2022, 10, 132 CrossRef.
- S. Chanda and T. Ramachandra, Res. Rev. J. Pharmacol., 2019, 9, 16–23 CAS.
- D. R. Emam, A. M. Alhajoj, K. M. Elattar, N. A. Kheder and A. A. Fadda, Molecules, 2017, 22, 971 CrossRef PubMed.
- A. A. Fadda, F. A. Badria and K. M. El-Attar, Med. Chem. Res., 2010, 19, 413–430 CrossRef CAS.
- M. Ikram, S. Hayat, M. Imran, A. Haider, S. Naz, A. Ul-Hamid, I. Shahzadi, J. Haider, A. Shahzadi and W. Nabgan, Carbohydr. Polym., 2021, 269, 118346 CrossRef CAS PubMed.
- X.-w. LU, J.-c. QIAN, C. Feng, X.-z. LI and Z.-g. CHEN, Trans. Nonferrous Metals Soc. China, 2012, 22, 1418–1422 CrossRef CAS.
- P. Joshi, S. Bonde, S. Gaikwad, A. Gade, K. Abd-Elsalam and M. Rai, J. Bionanosci., 2013, 7, 378–385 CrossRef CAS.
- S. Khan, M. I. Shinwari, A. Haq, K. W. Ali, T. Rana, M. Badshah and S. A. Khan, Pakistan J. Bot., 2018, 50, 1591–1598 CAS.
- R. Britto-Hurtado and M. Cortez-Valadez, in Green Functionalized Nanomaterials for Environmental Applications, Elsevier, 2022, pp. 83–127 Search PubMed.
- A. Kalia, K. A. Abd-Elsalam and K. Kuca, J. Fungi, 2020, 6, 222 CrossRef CAS PubMed.
- C. Pagnout, A. Razafitianamaharavo, B. Sohm, C. Caillet, A. Beaussart, E. Delatour, I. Bihannic, M. Offroy and J. F. Duval, Commun. Biol., 2021, 4, 678 CrossRef CAS PubMed.
- Q. Yu, B. Zhang, J. Li, B. Zhang, H. Wang and M. Li, Free Radic. Biol. Med., 2016, 99, 572–583 CrossRef CAS PubMed.
- A. Acharya and P. K. Pal, NanoImpact, 2020, 19, 100232 CrossRef.
- H. H. M. San, K. P. Alcantara, B. P. I. Bulatao, W. Chaichompoo, N. Nalinratana, A. Suksamrarn, O. Vajragupta, P. Rojsitthisak and P. Rojsitthisak, Polymers, 2022, 14, 1835 CrossRef CAS PubMed.
- A. Altemimi, N. Lakhssassi, A. Baharlouei, D. G. Watson and D. A. Lightfoot, Plants, 2017, 6, 42 CrossRef PubMed.
- Z. Yu, Q. Li, J. Wang, Y. Yu, Y. Wang, Q. Zhou and P. Li, Nanoscale Res. Lett., 2020, 15, 115 CrossRef CAS PubMed.
- C. Carlson, S. M. Hussain, A. M. Schrand, L. K. Braydich-Stolle, K. L. Hess, R. L. Jones and J. J. Schlager, J. Phys. Chem. B, 2008, 112, 13608–13619 CrossRef CAS PubMed.
- S. Raza, A. Ansari, N. N. Siddiqui, F. Ibrahim, M. I. Abro and A. Aman, Sci. Rep., 2021, 11, 10500 CrossRef CAS PubMed.
- N. Joza, G. Y. Oudit, D. Brown, P. Bénit, Z. Kassiri, N. Vahsen, L. Benoit, M. M. Patel, K. Nowikovsky and A. Vassault, Mol. Cell Biol., 2005, 25, 10261–10272 CrossRef CAS PubMed.
- W. A. Keshk, W. S. Elseady, N. I. Sarhan and D. H. Zineldeen, Metab. Brain Dis., 2020, 35, 637–647 CrossRef CAS PubMed.
- P. Scaffidi, T. Misteli and M. E. Bianchi, Nature, 2002, 418, 191–195 CrossRef CAS PubMed.
- Y.-D. Wen, R. Sheng, L.-S. Zhang, R. Han, X. Zhang, X.-D. Zhang, F. Han, K. Fukunaga and Z.-H. Qin, Autophagy, 2008, 4, 762–769 CrossRef CAS PubMed.
- V. Bhatia and S. Sharma, J. Neurol. Sci., 2021, 421, 117253 CrossRef CAS PubMed.
- J. J. Wu, C. Quijano, E. Chen, H. Liu, L. Cao, M. M. Fergusson, I. I. Rovira, S. Gutkind, M. P. Daniels and M. Komatsu, Aging, 2009, 1, 425 CrossRef CAS PubMed.
- A. Sajid, R. Javed, Q. Manzoor, A. Sajid, A. Saleem, F. Imtiaz, S. Ahmed and H. Nadeem, Chem. Afr., 2024, 7, 2103–2110 CrossRef CAS.
- A. Sajid, M. Amjad, Q. Manzoor, S. Wazir, A. Sajid, N. Alwadai, M. Iqbal and N. Tamam, Int. J. Biol. Macromol., 2024, 274, 133194 CrossRef CAS PubMed.
- J. S. Erlichman and J. C. Leiter, Antioxidants, 2021, 10, 547 CrossRef CAS PubMed.
- H. Shamsi, R. Yari and A. Salehzadeh, Sci. Rep., 2024, 14, 10284 CrossRef CAS PubMed.
- B.-H. Mao, J.-C. Tsai, C.-W. Chen, S.-J. Yan and Y.-J. Wang, Nanotoxicology, 2016, 10, 1021–1040 CrossRef CAS PubMed.
- S. Vihodceva, A. Šutka, M. Iesalnieks, L. Orlova, A. Pludonis, M. Otsus, M. Sihtmäe, H. Vija, A. Nefedova and A. Ivask, Environ. Sci.: Nano, 2025, 12, 276–291 RSC.
- A. B. Engin and A. Engin, Curr. Drug Metab., 2019, 20, 720–741 CrossRef CAS PubMed.
- M. Afaq, A. Sajid, Q. Manzoor, F. Imtiaz, A. Sajid, R. Javed, A. Ahmad, N. Alwadai, W. Mnif and M. Iqbal, Mater. Sci. Eng. B, 2025, 312, 117847 CrossRef CAS.
- A. Sajid, Q. Manzoor, A. Sajid, A. Nazir, M. A. Mumtaz, N. Fatima, S. Z. Alshawwa, M. Iqbal and U. Younas, Biocatal. Agric. Biotechnol., 2023, 47, 102599 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra00739a |
| This journal is © The Royal Society of Chemistry 2025 |