Open Access Article
Open Access ArticleSingle step site-selective reaction to construct a Ag2Au2 ← Ag4 supramolecular assembly from hybrid N-heterocyclic carbene (NHC): synthesis, structures and optoelectronic properties†
Pooja Dasa,
Soumi Halderbc,
Partha Pratim Ray b,
Narayan Ch. Jana
b,
Narayan Ch. Jana d,
Priyanka Sahua,
Anvarhusein A. Isabe,
Rambabu Dandela
d,
Priyanka Sahua,
Anvarhusein A. Isabe,
Rambabu Dandela f,
Ramalingam Natarajan
f,
Ramalingam Natarajan g and
Joydev Dinda
g and
Joydev Dinda *a
*a
aDepartment of Chemistry, Utkal University, VaniVihar, Bhubaneswar-751004, Odisha, India. E-mail: joydevdinda@gmail.com; joydevdinda@utkaluniversity.ac.in
bDepartment of Physics, Jadavpur University, Kolkata-700032, WB, India
cDepartment of Physics, Vidyanagar College, South 24 Parganas, West Bengal 743503, India
dSchool of Chemical Sciences, National Institute of Science Education and Research, HBNI, Bhubaneswar, Odisha 752050, India
eDepartment of Chemistry, King Fahd University of Petroleum and Minerals, Dhahran 31261, Saudi Arabia
fDepartment of Chemistry, Indian Institute of Chemical Technology, Bhubaneswar-751004, Odisha, India
gDepartment of Chemistry, CSIR-Indian Institute of Chemical Biology, Kolkata-700032, West Bengal, India
First published on 23rd April 2025
Abstract
Two supramolecular complex assemblies, [Ag4(1)2][PF6]4·4MeCN 2 and Ag(I)–Au(I) mixed metal complex [Ag2Au2(1)2][PF6]4·4MeCN 3, have been prepared from 3-(pyridylmethyl)imidazo[1,5-a]pyridin-4-ylium hexafluorophosphate (1 HPF6), which is the precursor of N-heterocyclic carbene (NHC). These complexes were subsequently analyzed using various spectroscopic techniques to confirm their structural and chemical properties. Transmetallation of Au(I) onto the Ag4 macrocycle results in the formation of an Ag2Au2 macrocyclic assembly. Au(I) selectively binds with the soft donor Ccarbene, whereas Ag(I) binds with comparatively hard donor Npy (py = pyridine). The geometries of 2 and 3 were established by single-crystal X-ray diffraction studies. Both molecules form a 2D network through M–M and several non-covalent interactions. Electrical conductivity measurements revealed that Ag(I) complex 2 is better conductor than Au(I) complex 3. Optoelectronic studies revealed the utility of complexes 2 and 3 as photovoltaic devices. Furthermore, MS-junction potential measurements show that they are suitable for semiconductor devices, with complex 2 being more efficient than complex 3. Finally, in this study, we aimed to explore the scope of (i) the development of heterobimetallic supramolecular organometallic complexes (SOC), (ii) the charge transport behaviour of SOCs, and (iii) the modification of intrinsically conductive SOCs-based electronics.
Introduction
In the past few decades, extensive research on supramolecular coordination complexes (SCCs)1 has drawn significant attention within the field of coordination chemistry. The diverse areas of application have created interest in these compounds. Until now, the scope of SCCs has been explored in drug delivery,2 molecular recognition,3 catalysis,4 and the stabilization of highly reactive species,5 among other applications. The most accepted method used for the construction of SCCs is coordination-driven self-assembly.6 From the ligand point of view, multitopic ligands are capitalized upon to construct metallosupramolecular assemblies; the ligands mostly carry Werner-type O-, N- and P-donor sites. Beyond the common O-, N- and P-donor atoms, researchers have also explored C-donor ligands. This led to the development of poly-N-heterocyclic carbene (NHC) ligands,7 which serve as strong C-donor ligands for constructing supramolecular organometallic complexes (SOCs). These NHC-based SOCs are a subclass of metallosupramolecular assemblies, where metal–carbon (M–C) bonds play a crucial role in stabilizing diverse architectures.7–9 The rapidly growing class of organometallic supramolecular assemblies, referred to as supramolecular organometallic complexes (SOCs), was initially reported by Pothig and Casini.10 Further studies on the biological importance of SOCs were also reported,10 where linker-metal connections are established by M–C bonds.Nowadays, the number of supramolecular organometallic complexes (SOCs) featuring M–C bonds has grown steadily. In particular, NHC-based SOCs, which incorporate poly-NHC ligands as key structural elements, have been extensively reported, especially for group 11 metals such as silver. Coinage metals in the +1 oxidation state form linear Ccarbene–M–Ccarbene linkages that essentially construct the fundamental building blocks.8c,d Importantly, the labile Ag–Ccarbene bond facilitates rearrangement to form the thermodynamically most stable assembly (see d/b ratio is 7.8–12.68, d = donation, b = back donation of electron); as a result, diverse architectures such as rectangles, triangles,11 cylinders12 and barrels12f have been obtained. Hahn's group was the first one to exploit square- and rectangular-shaped supramolecular organometallic assemblies incorporating transition metals such as gold,13 iridium,14 platinum,15 palladium,16 and nickel.17 Although there has been significant development in supramolecular organometallic assemblies in recent years, their applications remain largely focused on host–guest chemistry. Beyond this, Mukherjee's group has developed Ag(I)–NHC-based supramolecular assemblies for picric acid sensing,18a artificial light harvesting,18b and the detection of the broad-spectrum pesticide 2,6-dichloro-nitroaniline (DCN).18c Altmann and Pöthig have reported the encapsulation of organic substrates into Ag8 and Au8 pillarplexes.19 Rit's group has also reported trinuclear and tetranuclear supramolecular organometallic assemblies of coinage metals developed by NHC ligands.20 Peris has developed Pt(II) SOCs that function as receptors for electron-deficient organic molecules such as 1,4,5,8-naphthalenetetracarboxylic dianhydride (NTCDA), 1,2,4,5-tetracyanobenzene (TCNB), and 2,4,7- trinitro-9-fluorenone (TNFLU).21 Our group also reported a helical Hg(II) SOC.22
The architecture of metallosupramolecular complexes incorporating poly-NHC ligands and transition metals is a promising strategy.8a,14 Very recently, we reported an atom-selective reaction on an Ag3 cluster, which led to an Ag2Au mixed-metal cluster stabilized by a pincer NCN-carbene.23 Herein, we have introduced a multitopic hybrid-NHC ligand bearing C- and N-donor centers, assuming that M–Ccarbene will form a linear bond and Npyridine will extend the coordination, resulting in a metallosupramolecular assembly. We have constructed an Ag4 cage-like structure tailored by the C, N-donor NHC ligand; while transmetallation leads to an Ag2Au2 heterometallic supramolecular complex, where Au(I) selectively binds with Ccarbene and Ag(I) binds with Npyridine. Thus, inspired by recent findings, we have successfully synthesized and characterized the Ag4 coordination assembly and explored its optoelectronic properties, particularly the variation in electrical conductivity in dark and light, given silver's long-standing history in photoelectric properties.24
Incorporation of a second transition metal into the supramolecular framework is also an important area of research, as the resulting coordination motifs provide opportunities to tailor the properties and functionalities of these materials to meet specific application requirements.24 Moreover, the incoming second metal selectively binds with the multitopic ligand. This site-specific bonding has been observed with several metals and facilitates the development of heterobimetallic and heterometallic systems.25 The construction of heterometallic systems is a highly promising field of research due to their elegant architectures and interesting properties. They span wide-ranging fields such as bioinorganic chemistry, materials science, photophysics artificial photosynthesis, redox- and photoactive polymers, nanoscience, catalysis, sensors, and optoelectronic chemistry, among others.26 In recent years, scientists have increasingly focused on developing smart functional materials for constructing electronic and optoelectronic devices, with the goal of achieving robust electrical conductivity. This property in a metal complex can be achieved either through a continuous chain of coordination bonds between metal centers and ligands, or via non-covalent interactions. Among these, π–π stacking interactions between organic moieties within the molecular framework play a crucial role in enhancing electrical conductivity.27 Although numerous organic ligands embedded with various transition metals have been synthesized in the search for better performing optoelectronic materials, NHC ligand framework with silver or gold metals remain largely unexplored.
Based on abovementioned findings, we present a novel NHC-based SOC constructed using a site-selective metallation approach. The ligand 3-(pyridylmethyl)imidazo[1,5-a]pyridin-4-ylium hexafluorophosphate, a multitopic NHC precursor, facilitates the formation of the Ag4 supramolecular complex 2. Furthermore, successful transmetallation of complex 2 with the gold precursor Au(SMe2)Cl resulted in Ag2Au2 supramolecular organometallic complex 3 (Chart 1). Due to the extended conjugation and several interactions within the molecular framework of complexes 2 and 3, their electrical conductivity and potential application in electronic devices were also studied.
Results and discussion
3-Picolyl wingtip imidazolium based-NHC carbene precursor (1 HPF6) was prepared (Scheme 1) following a reported procedure.28 The formylative cyclization was carried out on the Schiff base of pyridine-2-carboxaldehyde and 3-picolylamine. The formation of the carbene precursor was confirmed by the appearance of the imidazolium NCHN- proton signal at 9.54 ppm in the 1H NMR spectrum and the procarbenic signal at 147.7 ppm in the 13C NMR spectrum. The reaction of 1 HPF6 with Ag2O afforded the tetranuclear complex 2, which was followed by transmetallation with gold(I) using Au(SMe2)Cl, yielding the tetranuclear complex 3. Reaction of 2 with Au(SMe2)Cl in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratios yielded the same product 3; this establishes a site-selective reaction.
4 ratios yielded the same product 3; this establishes a site-selective reaction.
 | ||
| Scheme 1 Site selective metallation of 1 HPF6 with Ag(I) and Au(I). The letters surrounding 1 refer to the NMR assignments; see the Experimental section. | ||
Experimental section
The disappearance of the imidazolium proton signal (δ = 9.54 ppm) (Fig. S1†) in the 1H NMR spectrum and the downfield shift of the 13C NMR signal at 165.02 ppm (the signal of the free proligand appears at 147.71 ppm) (Fig. S2†) confirm the formation of complex 2 (as shown in Fig. S3 and S4†). Similarly, the disappearance of the imidazolium proton and a downfield shift of the carbenic carbon at 173.02 ppm confirm the formation of complex 3 (as shown in Fig. S5 and S6†). The formation of a self-assembled single product was confirmed by 1H diffusion-ordered NMR spectroscopy (DOSY NMR) (as shown in Fig. S7 and S8†). HR-MS analysis of the proligand 1 HPF6, showing a peak at 210.1030 m/z, supports the proposed formulation of proligand (Fig. S9†). ESI-MS analysis provided the exact stoichiometry of complexes 2 and 3. For complex 2, the charged fragment [Ag3L2][PF6]3 was identified by a prominent peak at m/z = 1175.1194 (Fig. S10†). In the case of complex 3, the fragments [AuL2] and [AuAgL2][PF6] were indicated by significant peaks at m/z = 615.1282 and 867.0345, respectively, (Fig. S11†). Complexes 2 and 3 show absorption maxima (λmax) at 271 and 277 nm, respectively (Fig. 2). The FTIR spectra (Fig. S12†) show a notable decrease in the imidazole ring streching bands (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C–N), observed at ∼1053 and 1444 cm−1 for complex 2 and ∼1038 and 1438 cm−1 for complex 3, compared to those in the corresponding NHC proligands (∼1060 and 1450 cm−1). This shift strongly supports the formation of the desired carbene complexes.
N and C–N), observed at ∼1053 and 1444 cm−1 for complex 2 and ∼1038 and 1438 cm−1 for complex 3, compared to those in the corresponding NHC proligands (∼1060 and 1450 cm−1). This shift strongly supports the formation of the desired carbene complexes.
We have further performed single-crystal X-ray diffraction measurements to determine the molecular identity of complexes 2 and 3 (Fig. 1a and c). Single-crystal X-ray diffraction analysis revealed that crystals of complexes 2 and 3 belong to the triclinic crystal system in the space group ![[P with combining macron]](https://www.rsc.org/images/entities/i_char_0050_0304.gif) 1 (as shown in Table S1†). In the asymmetric unit of complex 2, half of a dimer is observed: one silver ion coordinates with two carbene carbon atoms from distinct ligands, while the other silver ion coordinates with pyridyl nitrogen atoms (Npy, where py = pyridine) from two different ligands. By contrast, the asymmetric unit of complex 3 consists of one Ag atom and one Au atom, where the softer carbene carbon center binds to Au(I) and the comparatively harder pyridyl nitrogen binds with Ag(I).
1 (as shown in Table S1†). In the asymmetric unit of complex 2, half of a dimer is observed: one silver ion coordinates with two carbene carbon atoms from distinct ligands, while the other silver ion coordinates with pyridyl nitrogen atoms (Npy, where py = pyridine) from two different ligands. By contrast, the asymmetric unit of complex 3 consists of one Ag atom and one Au atom, where the softer carbene carbon center binds to Au(I) and the comparatively harder pyridyl nitrogen binds with Ag(I).
The Ag–Ccarbene bond lengths in complex 2 are 2.096(4) Å, while the Au–Ccarbene distances in complex 3 are slightly shorter, at 2.020(5) Å and 2.021(4) Å. The shorter Au–Ccarbene bond lengths compared to Ag–Ccarbene can indeed be attributed to relativistic effects. Ag–Npy bond distances range from 2.149(4) Å to 2.146(5) Å in both complexes (Table S2†). The Ccarbene–Ag–Ccarbene bond angle in complex 2 is 171.11(14)°, showing a greater deviation from linearity compared to the Ccarbene–Au–Ccarbene bond angle in complex 3, which is 173.45(16)°. However, the Npy–Ag–Npy angles are very similar for both complexes, at 167.90(15)° for complex 2 and 167.66(18)° for complex 3 (Table S3†). These bond distances and angles align with reported values for Ag-carbene complexes.28a In both complexes 2 and 3, two solvated CH3CN molecules bridge two Ag(I) atoms that are bonded to the pyridyl nitrogen.29 M–M weak interactions are found in complexes 2 and 3: the intermolecular Ag(1)–Ag(1) separations (3.2490(6)Å) and intramolecular Ag(2)–Ag(2) separations (3.3461(11)Å) are observed in complex 2, whereas intermolecular Au(1)–Au(1) interactions (3.2991(4)Å) and intramolecular Ag(1)–Ag(1) interactions (3.3205(13)Å) are observed in complex 3. Various metal–metal, metal–solvent, and π⋯π stacking interactions contribute to the solid-state stability and promote the formation of 1D and 2D chain networks in both complexes, as illustrated in Fig. 1b and d. The powder X-ray diffraction (PXRD) patterns of complexes 2 and 3, shown in Fig. S13,† are in good agreement with the simulated spectra, confirming the phase purity and isomorphism of complexes 2 and 3.
Both molecules 2 and 3 possess several M–M interactions; these close-shell d10–d10 interactions are expected to be a source of luminescence (as shown in Fig. 2).30 Following excitation at 273 nm, the emission maxima for complexes 2 and 3 were observed at 382 and 379 nm, respectively. Time-resolved fluorescence analysis revealed that complexes 2 and 3 exhibit relatively long emission lifetimes. Notably, complex 2 demonstrated a higher average fluorescence lifetime of 3.56 ns, whereas it is 2.31 ns in the case of complex 3, in DMSO at an excitation wavelength of 273 nm (as shown in Table S4†).23 The lifetime of 1 HPF6 could not be measured due to its low emission intensity.
The SEM images of complexes 2 and 3 reveal distinct differences in porosity (Fig. S14†). While both complexes exhibit a porous-like architecture, complex 2 demonstrates a significantly more porous structure compared to complex 3. A more porous structure can have implications for conductivity-related applications.
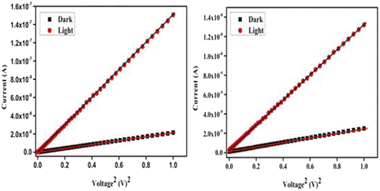
The presence of coinage metals Ag and Au and the polymeric nature of complexes 2 and 3 prompted the study of their optoelectronic properties. Metal–semiconductor (MS) junction thin-film devices were used to collect the current–voltage (I–V) data under dark conditions and white light illumination. The Schottky behaviour of the MS junction devices is evidenced by the nonlinear rectifying nature of the graphs, as shown in Fig. 3.
The photoresponse was studied under AM1.5 radiation. All measurements were carried out at room temperature with a bias voltage range of ±1 V. The On/Off ratio of complex 3 was determined to be 3.3 in the dark and 10.9 under light, while for complex 2, it improved to 3.5 in the dark and 21.5 under light, indicating the enhanced rectifying character of the silver-containing complex.
Under dark and light conditions, the conductivity of complex 2 was 4.6 × 10−9 S m−1 and 3.5 × 10−8 S m−1, respectively, measured at room temperature, which decreased to 6.5 × 10−10 S m−1 and 3.3 × 10−9 S m−1 in complex 3 (shown in Table 1). The calculated value of photosensitivity for complex 2 was 6.98, which is 1.3 times greater than that of complex 3, which showed a photosensitivity of 5.27. This substantial increase in photosensitivity indicates that the Ag complex 2 has greater light absorption properties than the Ag–Au mixed-metal complex 3. All the estimated parameters (shown in Table 1) clearly indicate that complex 2-based photovoltaic devices perform better compared to those based on complex 3, and may be applied in a broad range of photovoltaic applications.30 The shorter distances between tetranuclear units via M–M interactions and more compact π⋯π stacking interactions in complex 2, compared to complex 3, could be the reason for its superior performance as a photovoltaic device.
| Compd | Condition | On/off ratio | Conductivity (S m−1) | Ideality factor (n) | Barrier height (ϕB) eV | Series resistance, (RS) Ω | |
|---|---|---|---|---|---|---|---|
dV/d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) I I |
H(I) | ||||||
| 2 | Dark | 3.5 | 4.5 × 10−9 | 1.46 | 0.75 | 3.43 × 107 | 8.26 × 107 |
| Light | 21.5 | 3.5 × 10−8 | 1.01 | 0.63 | 3.61 × 106 | 2.90 × 107 | |
| 3 | Dark | 3.3 | 6.5 × 10−10 | 0.88 | 1.05 | 2.67 × 108 | 3.76 × 109 |
| Light | 10.9 | 3.3 × 10−9 | 0.97 | 0.89 | 1.68 × 107 | 6.27 × 107 | |
Cheung's equations were employed to study the various Schottky parameters such as ideality factor, series resistance, barrier height, etc., and to compare the behaviour of devices based on complexes 2 and 3. The value of the ideality factor (η) is very important, as it indicates the degree of ideal metal–semiconductor junction formation. The calculated values of η for both diodes under dark and illuminated conditions are listed in Table 1, where it was found that the formed MS junctions were not exactly ideal.
The deviation from ideal behaviour could be related to inhomogeneities in the Schottky barrier, along with the presence of interface states and series resistance at the junction.28 The Schottky barrier height (∅B) is calculated using an alternative set of formulae, as illustrated below.
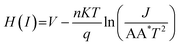 | (1) |
| H(I) = IRS + n∅B | (2) |
The dielectric constant (εr) was determined by plotting the capacitance vs. frequency graph (Fig. 6). Using the capacitance value at the saturation level, εr was calculated with the following equation.3
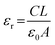 | (3) |
The diode parameters of complex 2 exhibit significantly improved charge-transfer kinetics after light soaking, compared to 3 (Table 2). Such materials hold great potential for advancing photosensitive device applications in the future.
| Compd | Condition | Mobility, μeff (cm2 V−1 s−1) | Carrier concentration N (m−3) | Lifetime (τ) (sec) | Diffusion coefficient D (m2 s−1) | Diffusion length LD (m) |
|---|---|---|---|---|---|---|
| 2 | Dark | 3.39 × 10−23 | 8.43 × 1032 | 3.06 × 1015 | 8.77 × 10−25 | 7.33 × 10−5 |
| Light | 2.37 × 10−22 | 9.31 × 1032 | 3.23 × 1014 | 6.13 × 10−24 | 6.29 × 10−5 | |
| 3 | Dark | 2.67 × 10−24 | 1.52 × 1033 | 3.48 × 1016 | 6.90 × 10−26 | 6.93 × 10−5 |
| Light | 1.44 × 10−23 | 1.45 × 1033 | 8.13 × 1015 | 3.72 × 10−25 | 7.78 × 10−5 |
Experimental section
2-Pyridine carboxaldehyde, 3-picolylamine, and Ag2O were supplied by Sigma-Aldrich, UK. All reagents used in synthesis and analysis were of analytical grade and utilized without additional purification. Solvents were dried (when necessary) following established protocols, and all experimental procedures were conducted under open air conditions. 1H NMR and 13C NMR spectra were recorded using a Bruker spectrometer at 400 and 100 MHz, respectively. For 1H and 13C NMR, chemical shifts (δ) are reported in ppm relative to the internal standard TMS, with coupling constants (J) given in Hz. Elemental analysis was carried out using a PerkinElmer 2400C elemental analyzer. A Cary 100 Bio spectrophotometer (Agilent Technologies) was used to record UV-visible spectra ranging from 200 to 800 nm. Emission spectra were obtained using a Cary Eclipse fluorescence spectrophotometer (Agilent Technologies). Time-resolved fluorescence measurements were conducted with a LifeSpec II TCSPC spectrometer, utilizing a 330 nm picosecond diode laser (EPL) as the excitation source. The FTIR spectra of the compounds were recorded using a PerkinElmer 2000 system spectrometer. Electrospray ionization mass spectra were recorded using an Agilent 6538 Ultra-High Definition (UHD) Accurate Mass Q-TOF spectrometer. The photoresponse was studied using AM1.5 radiation. All the measurements were carried out at room temperature at bias voltage range of ±1 V.Crystal structure determination
Using Bruker Smart Apex-II CCD diffractometer the Single-crystal X-ray diffraction data were collect at 273 K using Mo Kα radiation (λ = 0.71073 Å). The intensity data were processed and integrated using Bruker SAINT-plus software, with absorption corrections were made using the SADABS program.32 The structures were determined using direct methods in SHELXT 2014/5 and refined through full-matrix least squares techniques on F2 with SHELXL 2018/3,33 integrated within the OleX2 software.34 Disorder within the molecule was also modeled using OleX2. Anisotropic thermal displacement parameters were applied for non-hydrogen atoms, while hydrogen atoms were positioned in idealized geometries and refined using a riding model, with isotropic thermal parameters set at 1.2 or 1.5 times those of their parent atoms. Crystal structures and packing diagrams for the isomeric complexes were visualized and generated using Mercury 4.3.1 and POV-Ray software. Key crystallographic parameters, along with details of the structure determination and refinement, are summarized in Table S1.†Synthesis of 3-(pyridylmethyl)imidazo[1,5-a]pyridin-4-ylium hexafluorophosphate, (1 HPF6)
Pyridine 2-carboxaldehyde (1500 mg, 14 mmol) and 3-picolylamine (1500 mg, 14 mmol) were dissolved in toluene and stirred for 10 h. Then, the mixture of Schiff base (E)-1-(pyridin-2-yl)-N-(pyridin-3-ylmethylene)methanamine (2400 mg, 12 mmol) and crushed 91% paraformaldehyde powder (75.6 mg, 2.70 mmol) were stirred for 8 h. 5 ml of 4(N) HCl in Et2O were added slowly, resulting in immediate layer separation. Lower viscous yellowish layer was separated and aqueous KPF6 was added to the solution, leading to the immediate formation of a white precipitate. The precipitate was filtered and recrystallized from acetonitrile and diethyl ether. The solid product was dried. Yield was 2650 mg (7.46 mmol, 86.66%). 1H NMR (400 MHz, DMSO-d6), δ (ppm): 9.77 (s, 1H, Hk), 8.84 (s, 1H, He), 8.72 (d, J = 4.9 Hz, 1H, Ha), 8.56 (d, J = 7.1 Hz, 1H, Hh), 8.23 (s, 1H, Hi), 8.17 (d, J = 7.9 Hz, 1H, Hf), 7.79 (d, J = 9.3 Hz, 1H, Hd), 7.68 (dd, J = 7.9, 5.2 Hz, 1H, Hc), 7.22 (dd, J = 17.3, 8.1 Hz, 1H, Hb), 7.14 (t, J = 6.9 Hz, 1H, Hg), 5.82 (s, 1H, Hj). 13C NMR (100 MHz, DMSO-d6), δ (ppm): 147.71, 140.69, 132.38, 130.23, 127.43, 125.73, 125.40, 124.96, 118.72, 118.23, 118.05, 113.80, 50.75. Anal. calc. for C13H12N3PF6: C, 43.94; H, 3.38; N, 11.83; found: C, 43.71; H, 3.34; N, 11.76%. HRMS (ESI): C13H12N3 [1 H]+ = 210.1031 m/z (calcd) found: 210.1021 m/z.Synthesis of [Ag4(1)4][PF6]4·4MeCN
Proligand 1 HPF6 (200 mg, 0.56 mmol) and Ag2O (130.5 mg, 0.56 mmol) were mixed in acetonitrile. The mixture was stirred at room temperature in the dark until most of the Ag2O disappeared. The solution was filtered through a plug of Celite to remove the excess of Ag2O. The clear filtrate was then evaporated to dryness to get crude complex 2 as solid. Slow diffusion of diethyl ether into CH3CN solution of crude complex 2 yielded a crystalline material. Yield was 257.8 mg, (0.13 mmol, 78%).1H NMR (400 MHz, DMSO-d6), δ (ppm): 8.84 (s, 1H, He), 8.78 (d, J = 4.5 Hz, 1H, Ha), 8.66 (d, J = 7.2 Hz, 1H, Hd), 8.42 (s, 1H, Hi), 8.24 (d, J = 4.7 Hz, 1H, Hf), 8.20 (d, J = 6.3 Hz, 1H, Hh), 7.71 (m, J = 7.65 Hz, 1H,Hg), 7.38 (t, J = 9.1, Hz, 1H, Hb), 7.31 (t, J = 9.1 Hz, 1H, Hc), 5.82 (s, 2H, Hj). 13C NMR (100 MHz, DMSO-d6), δ (ppm): 165.02, 148.75, 148.34, 135.3 3, 128.05, 125.85, 125.60, 124.90, 122.60, 119.11, 118.32, 114.26, 50.92. Anal. calc. for C60H56N16Ag4P4F24: C, 35.81; H, 2.80; N, 11.13; found: C, 35.18; H, 2.36; N, 10.65%. ESI-MS: m/z observed at 1175.1194 for [Ag3(1)2]3PF6.Synthesis of [Ag2–Au2(1)4][PF6]4·4MeCN
At room temperature, complex 2 (300 mg, 0.16 mmol) was dissolved in 10 ml of acetonitrile. The acetonitrile solution (5 ml) of Au(SMe2)Cl (24 mg, 0.08 mmol) was added dropwise to the complex 2 solution. After 2 h of stirring, the solution changed to light yellow colour and a white precipitate of AgCl appeared. After filtration, the resulting solution was evaporated and a light yellow powder was collected. The crude product was recrystallized by slow diffusion of diethyl ether into acetonitrile solution of 3. Yield was 256 mg (0.013 mmol, 79%). 1H NMR (400 MHz, DMSO-d6), δ (ppm): 8.87 (s, 1H, He), 8.84 (d, J = 4.5 Hz, 1H, Ha), 8.72 (d, J = 7.2 Hz, 1H, Hd), 8.61 (s, 1H, Hi), 8.47 (d, J = 4.8 Hz, 1H, Hf), 8.27 (d, J = 6.3 Hz, 1H, Hh), 7.78 (m, 1H, Hg),7.52 (t, J = 9.1 Hz, 2H, Hb), 7.35 (t, J = 9.3 Hz, 1H, Hc), 5.82 (s, 2H, Hj).13C NMR (100 MHz, DMSO-d6), δ (ppm): 173.02, 148.65, 147.60, 135.20, 128.04, 125.71, 125.68, 125.60, 122.82, 118.33, 118.10, 114.63,50.98. Anal. calc. for C60H56N16Ag2Au2P4F24: C, 32.89; H, 2.58; N, 10.23; found: C, 32.32; H, 2.06; N, 9.75%. ESI-MS: m/z observed at 615.1282 for [Au(1)2]+ and 867.0345 for [[AuAg(1)2]PF6]+.Conclusion
In summary, in this manuscript we have described the synthesis, characterization and application of tetranuclear coinage metal-hybrid NHC complexes. The work also reports a heterobimetallic Ag2Au2 complex, synthesized via a selective transmetallation pathway from the tetranuclear silver-NHC congener. The compounds have furthermore been tested for photoelectric applications to create devices with novel capabilities, and it was observed that complex 2 is more efficient than the complex 3. The shorter distances between tetranuclear units via M–M interactions and more compact π⋯π stacking interactions in complex 2, compared to complex 3, could be the reason for its better performance as a photovoltaic device. Further modification of the ligand framework might lead to more impactful photoconductive properties.Data availability
The supplemental crystallographic data for this paper are contained in CCDC 2271882 (for 2), and CCDC 2271883 (for 3). These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
JD is grateful to Utkal University and RUSA 2. O for funding.References
- (a) R. Chakrabarty, P. S. Mukherjee and P. J. Stang, Chem. Rev., 2011, 111, 6810–6918 CrossRef CAS PubMed; (b) A. Goswami, S. Saha, P. K. Biswas and M. Schmittel, Chem. Rev., 2020, 120, 125–199 CrossRef CAS PubMed; (c) M. Fujita, M. Tominaga, A. Hori and B. Therrien, Acc. Chem. Res., 2005, 38, 369–378 CrossRef CAS PubMed; (d) W.-X. Gao, H.-J. Feng, B.-B. Guo, Y. Lu and G.-X. Jin, Chem. Rev., 2020, 120, 6288–6325 CrossRef CAS PubMed; (e) M. Yoshizawa and L. Catti, Acc. Chem. Res., 2019, 52, 2392–2404 CrossRef CAS PubMed; (f) D. Fujita, Y. Ueda, S. Sato, N. Mizuno, T. Kumasaka and M. Fujita, Nature, 2016, 540, 563–566 CrossRef CAS PubMed; (g) S. Hiraoka, K. Harano, M. Shiro, Y. Ozawa, N. Yasuda, K. Toriumi and M. Shionoya, Angew. Chem., Int. Ed., 2006, 45, 6488–6491 CrossRef CAS PubMed; (h) S.-C. Wang, K.-Y. Cheng, J.-H. Fu, Y.-C. Cheng and Y.-T. Chan, J. Am. Chem. Soc., 2020, 142, 16661–16667 CrossRef CAS PubMed; (i) L.-X. Cai, D.-N. Yan, P.-M. Cheng, J.-J. Xuan, S.-C. Li, L.-P. Zhou, C.-B. Tian and Q.-F. Sun, J. Am. Chem. Soc., 2021, 143, 2016–2024 CrossRef CAS PubMed.
- (a) J. Meeuwissen and J. N. Reek, Nat. Chem., 2010, 2, 615–621 CrossRef CAS PubMed; (b) S. De, K. Mahata and M. Schmittel, Chem. Soc. Rev., 2010, 39, 1555–1575 RSC; (c) J.-R. Li and H.-C. Zhou, Nat. Chem., 2010, 2, 893–898 CrossRef CAS PubMed; (d) M. Yamanaka, Y. Yamada, Y. Sei, K. Yamaguchi and K. Kobayashi, J. Am. Chem. Soc., 2006, 128, 1531–1539 CrossRef CAS PubMed; (e) Q.-F. Sun, S. Sato and M. Fujita, Angew. Chem., Int. Ed., 2014, 53, 13510–13513 CrossRef CAS; (f) J.-F. Ayme, J. E. Beves, C. J. Campbell and D. A. Leigh, J. Am. Chem. Soc., 2019, 141, 3605–3612 CrossRef CAS PubMed; (g) G. Li, Z. Zhou, C. Yuan, Z. Guo, Y. Liu, D. Zhao, K. Liu, J. Zhao, H. Tan and X. Yan, Angew. Chem., Int. Ed., 2020, 59, 10013–10017 CrossRef CAS PubMed.
- (a) P. Mal, B. Breiner, K. Rissanen and J. R. Nitschke, Science, 2009, 324, 1697–1699 CrossRef CAS PubMed; (b) F. J. Rizzuto, L. K. S. von Krbek and J. R. Nitschke, Nat. Chem. Rev., 2019, 3, 204–222 CrossRef; (c) K. Niki, T. Tsutsui, M. Yamashina, M. Akita and M. Yoshizawa, Angew. Chem., Int. Ed., 2020, 59, 10489–10492 CrossRef CAS PubMed; (d) P. Howlader, B. Mondal, P. C. Purba, E. Zangrando and P. S. Mukherjee, J. Am. Chem. Soc., 2018, 140, 7952–7960 CrossRef CAS PubMed; (e) L.-J. Wang, X. Li, S. Bai, Y.-Y. Wang and Y.-F. Han, J. Am. Chem. Soc., 2020, 142, 2524–2531 CrossRef CAS PubMed; (f) L. Xu, D. Zhang, T. K. Ronson and J. R. Nitschke, Angew. Chem., Int. Ed., 2020, 59, 7435–7438 CrossRef CAS PubMed; (g) M. Kieffer, R. A. Bilbeisi, J. D. Thoburn, J. K. Clegg and J. R. Nitschke, Angew. Chem., Int. Ed., 2020, 59, 11369–11373 CrossRef CAS PubMed.
- (a) M. Zhang, M. L. Saha, M. Wang, Z. Zhou, B. Song, C. Lu, X. Yan, X. Li, F. Huang, S. Yin and P. J. Stang, J. Am. Chem. Soc., 2017, 139, 5067–5074 CrossRef CAS PubMed; (b) A. J. McConnell, C. S. Wood, P. P. Neelakandan and J. R. Nitschke, Chem. Rev., 2015, 115, 7729–7793 CrossRef CAS PubMed; (c) M. L. Saha, X. Yan and P. J. Stang, Acc. Chem. Res., 2016, 49, 2527–2539 CrossRef CAS PubMed; (d) X. Yan, H. Wang, C. E. Hauke, T. R. Cook, M. Wang, M. L. Saha, Z. Zhou, M. Zhang, X. Li, F. Huang and P. J. Stang, J. Am. Chem. Soc., 2015, 137, 15276–15286 CrossRef CAS PubMed.
- (a) M. Yoshizawa, M. Tamura and M. Fujita, Science, 2006, 312, 251–254 CrossRef CAS PubMed; (b) D. M. Kaphan, M. D. Levin, R. G. Bergman, K. N. Raymond and F. D. Toste, Science, 2015, 350, 1235–1238 CrossRef CAS PubMed; (c) C. J. Brown, F. D. Toste, R. G. Bergman and K. N. Raymond, Chem. Rev., 2015, 115, 3012–3035 CrossRef CAS PubMed; (d) L. J. Jongkind, X. Caumes, A. P. T. Hartendorp and J. N. H. Reek, Acc. Chem. Res., 2018, 51, 2115–2128 CrossRef CAS PubMed; (e) Y. Fang, J. A. Powell, E. Li, Q. Wang, Z. Perry, A. Kirchon, X. Yang, Z. Xiao, C. Zhu, L. Zhang, F. Huang and H.-C. Zhou, Chem. Soc. Rev., 2019, 48, 4707–4730 RSC; (f) V. Martí-Centelles, A. L. Lawrence and P. J. Lusby, J. Am. Chem. Soc., 2018, 140, 2862–2868 CrossRef PubMed; (g) Q.-Q. Wang, S. Gonell, S. H. A. M. Leenders, M. Dürr, I. IvanovićBurmazović and J. N. H. Reek, Nat. Chem., 2016, 8, 225–230 CrossRef CAS PubMed; (h) J. Guo, Y.-Z. Fan, Y.-L. Lu, S.-P. Zheng and C.-Y. Su, Angew. Chem., Int. Ed., 2020, 59, 8661–8669 CrossRef CAS PubMed; (i) C. Tan, J. Jiao, Z. Li, Y. Liu, X. Han and Y. Cui, Angew. Chem., Int. Ed., 2018, 57, 2085–2090 CrossRef CAS PubMed.
- (a) T. R. Cook and P. J. Stang, Chem. Rev., 2015, 115, 7001–7045 CrossRef CAS PubMed; (b) A. M. Castilla, W. J. Ramsay and J. R. Nitschke, Acc. Chem. Res., 2014, 47, 2063–2073 CrossRef CAS PubMed.
- M. Poyatos, J. A. Mata and E. Peris, Chem. Rev., 2009, 109, 3677–3707 CrossRef CAS PubMed.
- (a) M. Fujita, Chem. Soc. Rev., 1998, 27, 417–425 RSC; (b) A. J. McConnell, C. S. Wood, P. P. Neelakandan and J. R. Nitschke, Chem. Rev., 2015, 115, 7729–7793 CrossRef CAS PubMed; (c) A. Rit, T. Pape and F. E. Hahn, J. Am. Chem. Soc., 2010, 132, 4572–4573 CrossRef CAS PubMed; (d) M.-M. Gan, J.-Q. Liu, L. Zhang, Y.-Y. Wang, F. E. Hahn and Y.-F. Han, Chem. Rev., 2018, 118, 9587–9641 CrossRef CAS PubMed.
- A. J. Boydston and C. W. Bielawski, Dalton Trans., 2006, 34, 4073–4077 RSC.
- A. Pöthig and A. Casini, Theranostics, 2019, 9, 3150–3169 CrossRef.
- (a) J. Kim, D. Nam, H. Kitagawa, D.-W. Lim and W. Choe, Nano Res., 2021, 14, 392–397 CrossRef CAS; (b) F. M. Conrady, R. Fröhlich, C. Schulte to Brinke, T. Pape and F. E. Hahn, J. Am. Chem. Soc., 2011, 133, 11496–11499 CrossRef CAS PubMed; (c) M. Schmidtendorf, T. Pape and F. E. Hahn, Angew. Chem., Int. Ed., 2012, 51, 2195–2198 CrossRef CAS PubMed; (d) C. Mejuto, G. Guisado-Barrios, D. Gusev and E. Peris, Chem. Commun., 2015, 51, 13914–13917 RSC.
- (a) D. H. Wang, B. G. Zhang, C. He, P. Y. Wu and C. Y. Duan, Chem. Commun., 2010, 46, 4728–4730 RSC; (b) C. Segarra, G. Guisado-Barrios, F. E. Hahn and E. Peris, Organometallics, 2014, 33, 5077–5080 CrossRef CAS; (c) N. Sinha, F. Roelfes, A. Hepp, C. Mejuto, E. Peris and F. E. Hahn, Organometallics, 2014, 33, 6898–6904 CrossRef CAS; (d) N. Sinha, L. Stegemann, T. T. Y. Tan, N. L. Doltsinis, C. A. Strassert and F. E. Hahn, Angew. Chem., Int. Ed., 2017, 56, 2785–2789 CrossRef CAS PubMed; (e) Y. Li, Y. Y. An, J. Z. Fan, X. X. Liu, X. Li, F. E. Hahn, Y. Y. Wang and Y. F. Han, Angew. Chem., Int. Ed., 2020, 59, 10073–10080 CrossRef CAS PubMed; (f) Y. Li, M.-Z. Han, X.-T. Chang, L. Zhang, L.-L. Ma, L.-Y. Sun, F. E. Hahn and Y.-F. Han, CCS Chem., 2024, 1–11 Search PubMed.
- C. Radloff, J. J. Weigand and F. E. Hahn, Dalton Trans., 2009, 43, 9392–9394 RSC.
- M. Schmidtendorf, C. Schulte to Brinke and F. E. Hahn, J. Organomet. Chem., 2014, 751, 620–627 CrossRef CAS.
- M. Schmidtendorf, T. Pape and F. E. Hahn, Dalton Trans., 2013, 42, 16128–16141 RSC.
- A. Sinha, F. Roelfes, A. Hepp and E. F. Hahn, Chem.–Eur. J., 2017, 23, 5939–5942 CrossRef PubMed.
- (a) F. E. Hahn, C. Radloff, T. Pape and A. Hepp, Organometallics, 2008, 27, 6408–6410 CrossRef CAS; (b) C. Radloff, F. E. Hahn, T. Pape and R. Fröhlich, Dalton Trans., 2009, 35, 7215–7222 RSC.
- (a) P. C. Purba, M. Venkateswaralu, S. Bhattacharyya and P. S. Mukherjee, Inorg. Chem., 2022, 61, 713–722 CrossRef CAS PubMed; (b) P. K. Maitra, S. Bhattacharyya, P. C. Purba and P. S. Mukherjee, Inorg. Chem., 2024, 63, 2569–2576 CrossRef CAS PubMed; (c) P. C. Purba, P. K. Maitra, S. Bhattacharyya and P. S. Mukherjee, Inorg. Chem., 2023, 62, 11037–11043 CrossRef CAS PubMed.
- (a) P. J. Altmann and A. Pöthig, J. Am. Chem. Soc., 2016, 138, 13171–13174 CrossRef CAS PubMed; (b) P. J. Altmann and A. Pöthig, Angew. Chem., Int. Ed., 2017, 56, 15733–15736 CrossRef CAS PubMed.
- R. C. Nishad, S. Kumar and A. Rit, Angew. Chem., Int. Ed., 2022, 61, e202206788 CrossRef CAS PubMed.
- S. Ibańez, M. Poyatos and E. Peris, Acc. Chem. Res., 2020, 53, 1401–1413 CrossRef PubMed.
- B. K. Rana, V. Bertolasi, S. Pal, P. Mitra and J. Dinda, J. Mol. Strur., 2013, 1049, 458–463 CrossRef CAS.
- P. Sahu, N. C. Jana, S. Barik, H. K. Kisan, A. Changotra, A. A. Isab and J. Dinda, Dalton Trans., 2024, 53, 1099–1104 RSC.
- D. M. Roundhill and . P. Fackler, Optoelectronic Properties of Inorganic Compounds, Springer, USA, 2013 Search PubMed.
- (a) S. Bente, F. Kampert, T. T. Y. Tan and F. E. Hahn, Chem. Commun., 2018, 54, 12887 RSC; (b) P. D. Dutschke, S. Bente, C. G. Daniliuc, J. Kinas, A. Hepp and F. E. Hahn, Dalton Trans., 2020, 49, 14388–14392 RSC.
- (a) C. Cesari, B. Berti, F. Calcagno, C. Lucarelli, M. Garavelli, R. Mazzoni, I. Rivalta and S. Zacchini, Organometallics, 2021, 40, 2724–2735 CrossRef CAS; (b) M. Redrado, A. Benedi, I. Marzo, A. L. G. -Otín, V. F. -Moreira and M. C. Gimeno, Chem.–Eur. J., 2021, 27, 9885–9897 CrossRef CAS PubMed; (c) G. Mo, Q. Wang, W. Lu, C. Wang and P. Li, Chin. J. Chem., 2023, 41, 335–354 CrossRef CAS.
- (a) L. S. Xie, E. V. Alexandrov, G. Skorupskii, D. M. Proserpio and M. Dincă, Chem. Sci., 2019, 10, 8558–8565 RSC; (b) E. M. Johnson, S. Iiic and A. J. Morris, ACS Cent. Sci., 2021, 7, 445–453 CrossRef CAS PubMed; (c) Y. W. Zhang, Y. Lu, L. Y. Sun, P. D. Dutschke, M. M. Gan, L. Zhang, A. Hepp, Y. F. Han and F. E. Hahn, Angew. Chem., 2023, 135, e202312323 CrossRef.
- (a) J. Dinda, T. Samanta, A. Nandy, K. D. Saha, S. K. Seth, S. K. Chattopadhyay and C. W. Bielawski, New J. Chem., 2014, 38, 1218–1224 RSC; (b) B. K. Rana, A. Nandy, V. Bertolasi, C. W. Bielawski, K. D. Saha and J. Dinda, Organometallics, 2014, 33, 2544–2548 CrossRef CAS.
- (a) V. J. Catalano and A. L. Moore, Inorg. Chem., 2005, 44, 6558–6566 CrossRef CAS PubMed; (b) V. J. Catalano and M. A. Malwitz, Inorg. Chem., 2003, 42, 5483–5485 CrossRef CAS PubMed.
- M. Das, J. Datta, A. Dey, R. Jana, A. Layek, S. Middya and P. P. Ray, RSC Adv., 2015, 5, 101582 RSC.
- A. Dey, S. Middya, R. Jana, M. Das, J. Datta, A. Layek and P. P. Ray, J. Mater. Sci.: Mater. Electron., 2016, 27, 6325–6335 CrossRef CAS.
- G. M. Sheldrick, SADABS, University of Göttingen, Germany, 1996 Search PubMed.
- G. M. Sheldrick, ActaCrystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Cryst., 2009, 42, 339–341 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2271882 and 2271883. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ra00684h |
| This journal is © The Royal Society of Chemistry 2025 |