Open Access Article
Open Access ArticleA novel boronic acid-based fluorescent sensor for the selective detection of L-lysine in food samples and cells†
Wei Gao a,
Jing Sua,
Huarui Yanga,
Xuefeng Zhaoa,
Jingjing Liua,
Zhijun Wang*a and
Qingming Wang
a,
Jing Sua,
Huarui Yanga,
Xuefeng Zhaoa,
Jingjing Liua,
Zhijun Wang*a and
Qingming Wang *b
*b
aDepartment of Chemistry, Changzhi University, Changzhi 046011, People's Republic of China
bSchool of Pharmacy, Yancheng Teachers' University, Yancheng 224051, People's Republic of China
First published on 4th April 2025
Abstract
A novel probe DFC (2-(dicyanomethylene)-2,5-dihydro-5,5-dimethyl-4-((E)-2-(5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)furan-2-yl)vinyl)furan-3-carbonitrile) was successfully designed and synthesized for the detection of L-lysine (L-Lys). The sensing behavior was characterized using absorption and fluorescence emission spectra. Upon addition of L-Lys to DFC, a rapid response time of 5 seconds was observed, accompanied by a significant 4-fold enhancement in fluorescence intensity. Additionally, DFC exhibits an impressively low detection limit of 0.14 μMol L−1. Furthermore, the applicability of DFC was demonstrated through successful detection of L-Lys in water samples, food samples, and imaging of L-lys in live HeLa cells.
Introduction
As one of the most abundant natural amino acids, L-Lysine (L-Lys) plays important roles in cell division, gene regulation, immune system regulation, metabolic function and so on.1–3 The human body's requirement for L-Lys can be met by two methods: dietary intake and in vivo synthesis. For instance, it could be produced by the Krebs–Henseleit cycle and the gluconeogenesis cycle in metabolisms.4–6 Due to its role in metabolisms, hyperlysinemia or cystinuria could be caused by the abnormal concentration of L-Lys.7,8 It has also been reported that excessive intake of L-Lys may lead to metabolic disorders and obesity.9 Therefore, it is very necessary to develop a simple, highly selective and sensitive, rapid-response method for the detection of L-Lys.The methods of electrochemistry,10 high-performance liquid chromatography,9 mass spectrometry,11 capillary electrophoresis,12 and Fourier transform infrared spectroscopy13 (FT-IR) have been used for the detection of L-Lys. However, the drawbacks of these methods bring many inconveniences. Fluorescence sensors have attracted much attention due to their simplicity, ease of use, high sensitivity and selectivity. Up to now, some types of sensors on the basis of different fluorophores or materials have been reported for L-Lys.14–21 Some of these sensors exhibit insufficient selectivity for Lys due to the interference from arginine, histidine, metal ions, anions and so on.1,14–16,18 Lysine is a strong alkaline amino acid with a isoelectric point (pI) value of 9.47.22 Reports showed that L-Lys practically worked as a strong base to deprotonate sensors and promote the equilibrium towards the anionic.16
This work describe a L-Lys probe (E)-2-(3-cyano-5,5-dimethyl-4-(2-(5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)furan-2-yl)vinyl)furan-2(5H)-ylidene)malononitrile, that can be synthesized through straightforward organic synthesis procedures for the detection of lysine (Scheme 1). Interestingly, a distinguished color change from colorless to blue was observed with the addition of L-Lys to the DFC solution. A rapid fluorescence color change for DFC in PBS (pH = 7.40, 10 mM) was found within 5 s in the presence of L-Lys. The calculated limit of detection (LOD) of DFC was 0.14 μMol L−1. The mechanism was proved that the hydroxyl oxygen of –COOH in L-Lys could react with the B atom of DFC by nucleophilic attack to form borate esters, and then aromatization of O on the furan ring with –NH2 in L-Lys via intra-ring heteroatom substitution reaction to form a stable ring with six members. The ε-NH2 of L-Lys and the cyanide groups formed a stable internal salt structure. The deprotonation and complexation mechanism was consistent with the previous reports.23 Moreover, DFC was applied for the detection of L-Lys in real water samples and food; the results were in agreement with the L-Lysine Detection Kit (48 Assay). The cell imaging indicated that DFC could penetrate the cell membrane to detect the L-Lys.
Experimental section
Materials
The amino acids such as lysine (Lys), glycine (Gly), proline (Pro), Tyr, arginine (Arg), glutamine (Gln), cysteine (Cys), methionine (Met), valine (Val), phenylalanine (Phe), histidine (His), glutamic acid (Glu), threonine (Thr), aspartic acid (Asp), alanine (Ala), dipeptide such as glutathione (GSH), metal ions and anions were obtained from commercial suppliers (Shanghai Titan Scientific Co.,Ltd). All of the materials were analytical grade and used without additional purification.Instruments
The NMR was recorded on a Bruker DRX 400 spectrometer with TMS as the internal standard and chemical shifts were expressed in ppm. HR-MS was performed with a Triple TOF TM 5600+ system. The UV-visible absorption spectrum was carried out from UV-1800 ENG 240 V. Fluorescence spectra data were recorded on an CRT-960 fluorescence spectrophotometer with a quartz cuvette (path length = 1 cm). Bioimaging of probe DFC with L-Lys was performed on a confocal laser scanning microscope L700 with HeLa cells.Spectral measurements
In order to record the UV-vis spectrum, stock solution of the DFC (25 μMol L−1) (DFC stock solution was obtained by dissolving it in DMSO, the final concentration: 10−2 mol L−1) was prepared in PBS (pH = 7.40, 10 mM), 5 μL of L-Lys was gradually added to DFC, then the UV-Vis spectra were monitored.To record fluorescence spectra, the stock solution of DFC and the L-Lys were prepared as the same as that used for UV-vis method. 25 μmol L−1 DFC was added to 2 mL PBS (pH = 7.40, 10 mM), then 2 μL of 10−3 mol per L-Lys was gradually added, then the fluorescence spectra were recorded. The excitation wavelength was 380 nm and the emission wavelength were collected at 390–650 nm. The slit widths were 5/5 nm.
Food sample treatment
8.0 g of cashew nuts, soybeans, red beans, millet, flour, mung beans, black beans were all purchased from local supermarkets (Yonghui, Changzhi). Pre-treatment of the food samples were done before the experiment by crushing the food samples into pieces and passing through a 60 mesh sieve. The samples were put in round bottom flasks, add petroleum ether and soak with stir continuously for 48 hours. And then degrease, dry and dissolve in anhydrous ethanol. At last, distilled water was added to reach 50 mL. The supernatant was centrifuged for the measurement of the concentration of L-Lys.Cell culture and imaging
Fluorescence confocal imaging
For the imaging experiment, HeLa cells at a density of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well in laser confocal culture dishes (diameter: 35 mm; glass bottom diameter: 10 mm) were incubated with 20.0 μmol L−1 DFC at 37 °C for 20 minutes prior to laser confocal imaging. To determine the effect of exogenous Lys, HeLa cells pre-incubated with 20.0 μmol L−1 DFC were washed thoroughly and subsequently incubated with Lys (20.0, 30.0, 40.0 and 50.0 μmol L−1) for 20 minutes before undergoing laser confocal imaging. All cells were washed three times with sterile PBS and imaged using an confocal laser scanning microscope L700 (excitation wavelength = 405 nm, emission collection range = 450–500 nm).
000 cells per well in laser confocal culture dishes (diameter: 35 mm; glass bottom diameter: 10 mm) were incubated with 20.0 μmol L−1 DFC at 37 °C for 20 minutes prior to laser confocal imaging. To determine the effect of exogenous Lys, HeLa cells pre-incubated with 20.0 μmol L−1 DFC were washed thoroughly and subsequently incubated with Lys (20.0, 30.0, 40.0 and 50.0 μmol L−1) for 20 minutes before undergoing laser confocal imaging. All cells were washed three times with sterile PBS and imaged using an confocal laser scanning microscope L700 (excitation wavelength = 405 nm, emission collection range = 450–500 nm).
Synthesis of DFC
Results and discussion
Detection of L-Lys in solution
The spectroscopic response of DFC to L-Lys was investigated. As shown in Fig. S2,† probe DFC exhibited a strong absorption at 310 nm and a noticeable emission peak at 560 nm, with a Stokes shift of 250 nm. The absorption of DFC at 310 nm was enhanced with the addition of L-Lys (Fig.S3†). At the same time, the emission peak of DFC shifted from 560 nm to 450 nm (about 110 nm).Fig. 1a showed the optical response between DFC and L-Lys in PBS (pH = 7.40, 10 mM). When L-Lys was added to the solution of DFC, an emission peak at 450 nm appeared. And its intensity was gradually enhanced with the increasing of L-Lys. The fluorescence intensity was enhanced about 3.79-fold when 24 μMol L−1 of L-Lys was added. The color of the solution changed from colorless to blue under a 365 nm UV lamp. Furthermore, the UV-vis titration was used to investigate the quantitative response of DFC to L-Lys. As depicted in Fig. S4,† with the increase of L-Lys (0–37.5 μMol L−1), the peak at 310 nm was gradually increased. Meantime, a weak band at 503 nm remained unchanged. These changes indicated that a reaction was occurred between DFC and L-Lys. Most importantly, the emission intensity of DFC at 450 nm showed excellent linearity with L-Lys concentrations increased from 0 to 50 μMol L−1. The equation of F450nm and [L-Lys] was plotted as Y = 23.475 + 3.322X with a correlation coefficient (R2) value of 0.99945, indicating that DFC could be used in actual sample testing (Fig. 1b). Accordingly, the detection limit was calculated as 0.15 μMol L−1, which is lower than most reported methods.26–29
In order to optimise the detection conditions, time and pH were evaluated on the fluorescence intensity of the DFC response to L-Lys. Firstly, the effect of time on the detection capability for L-Lys was investigated. The results showed that the DFC showed a negligible variation during the assay time (1 h). After the addition of L-Lys, the fluorescence intensity of DFC increased rapidly and it took about 5 s to reach the maximum value, and the maximum value was maintained unchanged within the assay time (1 h). The effect of different concentrations (10, 25 μMol L−1) of L-Lys on the reaction time was investigated and it was found that the concentration of L-Lys had no effect on the time.
To explore the impact of pH on the detection of L-Lys, we assessed the pH responses of the fluorescence intensities of DFC and the mixture of DFC and L-Lys. As shown in Fig. 2b, DFC showed a very low level of fluorescence intensity under the pH of 4.00, 6.86, 7.40, 9.18, 10.01. When L-Lys was induced, the fluorescence intensity of DFC was remarkably enhanced. The enhancement was 3.39-fold, 4.37-fold, 4.02-fold, 3.67-fold and 3.71-fold at pH = 4.00, 6.86, 7.40, 9.18, 10.01 respectively. A wide pH range of 4.0–10.0 for DFC response to L-Lys was similar to the early reports.20,30
Selectivity
To evaluate the specificity of DFC for L-Lys, a large number of related species such as amino acids (Gly, Pro, Tyr, Arg, Gln, Cys, Met, Val, Phe, His, Glu, Thr, Asp, Ala, GSH), anions (CO32−, SO42−, SO32−, ACO−, HSO4−, H2PO4−, S2O32−, HCO3−, Cl−, HSO3−, PO43−, NO3−, F−, S2−, ClO−) and metal ions (Na+, K+, Mg2+, Fe3+, Ca2+, Al3+, Zn2+, Cr3+, Cu2+, Ni2+, Cd2+, Mn2+) were tested (as showed in Fig. S5†). The results showed that only L-Lys could enhance the fluorescence intensity of DFC in PBS (pH = 7.40,10 mM), no obvious fluorescence changes were obtained in the presence of other related species. In addition, we investigated whether other related species coexisting with L-Lys could enhance the fluorescence intensity of DFC or not. From Fig. 3, when DFC (25 μMol L−1) in PBS (pH = 7.40,10 mM) was first treated with 500 μMol L−1 of amino acids, anions and metal ions, the fluorescence intensity of DFC was almost unchanged, and the fluorescence was turned on when 100 μMol L−1 of L-Lys was added to the above system. These results told us that the specific recognition of DFC for L-Lys was not affected by amino acids, anions and metal ions, and that DFC could be used for L-Lys in biological and food samples.Sensing mechanism
The sensing mechanism between DFC and L-Lys has been proposed in Scheme 2. Since the hydroxyl oxygen of –COOH in L-Lys could react with the B atom of DFC by nucleophilic attack to form borate esters. Aromatization of O on the furan ring with –NH2 in L-Lys via intra-ring heteroatom substitution reaction.31 The ε-NH2 of L-Lys and the cyanide groups formed a stable internal salt structure.20 The pyrrole 2-position C–C and 2 H-furan 2-position C–C cannot rotate, resulting in increased fluorescence intensity of DFC-Lys. DFC and L-Lys was formed a complex in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry and were identified by the Job's plot study. The mechanism was confirmed by EI-MS. After mixing DFC with 1 equal amount of L-Lys, a peak m/z = 531.2614 was obtained, which could be belonging to [DFC + L-Lys] (Cal. 531.2653) (Fig. 4). The result was used to further verify the mechanism.
1 stoichiometry and were identified by the Job's plot study. The mechanism was confirmed by EI-MS. After mixing DFC with 1 equal amount of L-Lys, a peak m/z = 531.2614 was obtained, which could be belonging to [DFC + L-Lys] (Cal. 531.2653) (Fig. 4). The result was used to further verify the mechanism.
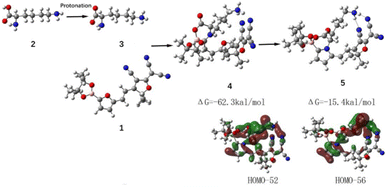
Density Functional Theory (DFT) calculations were performed at the ωb97xd/6-31g(d) level on the reaction of DFC (1) and L-Lys (2) to further clarify the sensing mechanism.32 Due to the alkalinity of the ε-amino group in L-Lys is strong, so it can capture the carboxyl group proton on L-Lys to form the compound 3. From Fig. 5, the compound 4 was formed between compound 3 and 1 by the heteroatom substitution reaction of aromatisation O on the furan ring with –NH2 in 2. The reaction energy (ΔG) of this reaction is −62.3 kcal mol−1, which is an exothermic reaction. Compound 5 was obtained by a ring–closing reaction with the reaction energy of −15.4 kcal mol−1. Moreover, the HOMO-52 and HOMO-56 of structure 5 show that hydrogen bonds are existed between the H atom of the ε-amino group on L-Lys and the N atom of the cyano group on DFC. DFT further validated our proposed mechanism.
Determination of L-Lys in different water samples
We then investigated the application of DFC in tracing the concentration of L-Lys in real samples.33,34 A certain amount of L-Lys was dissolved in river water, purified water and tap water. The fluorescence intensity of DFC (25 μMol L−1) with various concentrations (5, 10, 15, 20, 25, 30, 35, 40, 45, 50 μMol L−1) of L-Lys was investigated in these real samples. From Fig. 6, a good linear relationship between [L-Lys] and F450 nm, and the linear correlation coefficients for river water, purified water and tap water were 0.9990, 0.99963 and 0.99936, respectively. The recoveries of DFC for the detection of L-Lys ranged from 96.4% to 112% (Table 1). The good recoveries showed that DFC could be used for the detection of L-Lys in real water samples.| Sample | Added (μmol L−1) | Detected (μmol L−1) | Recovery (%) |
|---|---|---|---|
| River water | 5 | 5.39 ± 0.07 | 108 |
| 10 | 10.58 ± 0.02 | 106 | |
| 15 | 15.16 ± 0.07 | 101 | |
| 20 | 19.37 ± 0.06 | 96.8 | |
| 25 | 25.19 ± 0.07 | 105 | |
| 30 | 30.27 ± 0.26 | 101 | |
| 35 | 35.27 ± 0.06 | 101 | |
| 40 | 40.65 ± 0.11 | 102 | |
| 45 | 45.35 ± 0.15 | 101 | |
| 50 | 49.84 ± 0.28 | 99.7 | |
| Purified water | 5 | 4.82 ± 0.07 | 96.4 |
| 10 | 10.23 ± 0.17 | 102 | |
| 15 | 15.76 ± 0.09 | 105 | |
| 20 | 19.85 ± 0.10 | 99.3 | |
| 25 | 25.53 ± 0.09 | 102 | |
| 30 | 30.66 ± 0.28 | 102 | |
| 35 | 34.58 ± 0.31 | 98.8 | |
| 40 | 40.11 ± 0.15 | 100 | |
| 45 | 46.61 ± 0.19 | 104 | |
| 50 | 50.68 ± 0.05 | 101 | |
| Tap water | 5 | 5.60 ± 0.01 | 112 |
| 10 | 10.20 ± 0.36 | 102 | |
| 15 | 16.45 ± 0.17 | 110 | |
| 20 | 20.44 ± 0.03 | 102 | |
| 25 | 26.13 ± 0.06 | 105 | |
| 30 | 31.37 ± 0.16 | 105 | |
| 35 | 36.06 ± 0.20 | 103 | |
| 40 | 39.80 ± 0.26 | 99.5 | |
| 45 | 47.11 ± 0.23 | 105 | |
| 50 | 51.11 ± 0.17 | 102 |
Determination of L-Lys in food samples
There is an abundance of amino acids in food. So we chose commercially available food samples to realize the detection of L-Lys.Three parallel experiments were carried out in accordance with the experimental method, and the relative standard deviation and the recovery were calculated. The experiment was carried out three times in parallel. From Table 2, the contents in cashew nuts, soybeans, red beans, millet, flour, mung beans, black beans were different from each other. The content of L-Lys in these seven samples were 1.68, 2.65, 1.37, 0.20, -, 1.92, and 0.09 mg g−1, respectively, measured by DFC in PBS (pH = 7.40, 10 mM). To verify the accuracy of the method proposed in this paper, the L-Lysine Detection Kit (48 Assay) was used to further validate the L-Lys. The results calculated by the L-Lysine Detection Kit (48 Assay) showed that the L-Lys in these seven samples were 1.53, 2.60, 1.22, 0.06, 0.26, 1.90, and 0.04 mg g−1, respectively. A comparison of the DFC and L-Lysine Detection Kit (48 Assay) methods showed that the two methods were consistent with each other.
| Samples | L-Lysine detection kit (mg g−1) | RSD% | DFC (mg g−1) | RSD% |
|---|---|---|---|---|
| Cashew nuts | 1.53 | 0.223 | 1.68 | 3.17 |
| Soybeans | 2.60 | 0 | 2.65 | 1.04 |
| Red beans | 1.22 | 0.402 | 1.37 | 5.59 |
| Millet | 0.06 | 0 | 0.20 | 1.16 |
| Flour | 0.26 | 0 | — | — |
| Mung beans | 1.90 | 0.112 | 1.92 | 3.09 |
| Black beans | 0.04 | 0.112 | 0.09 | 0.79 |
Imaging of L-Lys in living cells
In order to expand the application of DFC, cellular fluorescence imaging for DFC was conducted through HeLa cells. First, from Fig. S6,† the minimal cytotoxicity of DFC toward HeLa cells was confirmed by the cytotoxicity experiments, and showed the high concentration was 80 μMol L−1 (84.2% cell viability). We used the DFC, which has good biocompatibility and low toxicity, for cellular fluorescence imaging. From Fig. 7, we could find that when the cells were stained with DFC (20 μMol L−1), and further treated with L-Lys (0, 20, 30, 40, 50 μM), a visible variation of fluorescence intensity was found. Moreover, the blue fluorescence in HeLa cells became strong with the increase of the L-Lys. The cell imaging indicated that DFC could penetrate the cell membrane to detect the L-Lys.Conclusions
A simple and efficient “turn-on” fluorescence sensor was developed for the highly sensitive and selective detection of L-Lys. The addition of L-Lys to the solution of DFC in PBS (pH = 7.42, 10 mM) induced a marked fluorescence enhancement (3.79-fold). No significant changes in fluorescence emission or colour were observed when the other amino acids (Gly, Pro, Tyr, Arg, Gln, Cys, Met, Val, Phe, His, Glu, Thr, Asp, Ala), metal ions, anions and GSH were added to DFC. The detection limit of DFC for Lys was 0.15 μMol L−1 based on fluorescence titration and DFC showed high stability for L-Lys in the pH range of 4.00 to 10.01. DFC was used to detect L-Lys in various water samples with recoveries ranging from 96.4% to 112%. Furthermore, the comparison of the DFC and L-Lysine Detection Kit (48 Assay) methods showed that the two methods were in agreement with each other. In addition, cellular experiments demonstrated that DFC has low toxicity and high penetration, which could be used for fluorescence imaging in HeLa cells.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (No. 2023L334); The special fund for Science and Technology Innovation Teams of Shanxi Province (202204051001034); Fund for Shanxi “1331 Project”, Horizontal Project from Shanxi Agricultural University (Shanxi Academy of Agricultural Sciences) Millet Research Institute (HX0608) and the applied teaching material construction project of Changzhi University (XN0329).Notes and references
- L. Wang, Z. L. Wei, C. Liu, W. K. Dong and J. X. Ru, Spectrochim. Acta, Part A, 2020, 239, 118496 CAS.
- A. Chéramy, M. L'Hirondel, G. Godeheu, F. Artaud and J. Glowinski, Amino Acids, 1998, 14, 63–68 CrossRef PubMed.
- J. Hao, M. Wang, S. Wang, Y. Huang and D. Cao, Dyes Pigm., 2020, 175, 108131 CrossRef CAS.
- A. M. Pettiwala and P. K. Singh, Spectrochim. Acta, Part A, 2018, 188, 120–126 CrossRef CAS PubMed.
- H. Yoshida, Y. Nakano, K. Koiso, H. Nohta, J. Ishida and M. Yamaguchi, Anal. Sci., 2001, 17, 107–112 CrossRef CAS PubMed.
- Y. Zhou, J. Won, J. Y. Lee and J. Yoon, Chem. Commun., 2011, 47, 1997–1999 RSC.
- S. Lohar, D. A. Safin, A. Sengupta, A. Chattopadhyay, J. S. Matalobos, M. G. Babashkina, K. Robeyns, M. P. Mitoraj, P. Kubisiak, Y. Garcia and D. Das, Chem. Commun., 2015, 51, 8536–8539 RSC.
- P. Felig, Annu. Rev. Biochem., 1975, 44, 933–955 CrossRef CAS.
- G. Wu, Amino Acids, 2009, 37, 1–17 CrossRef CAS PubMed.
- G. L. Luque, N. F. Ferreyra and G. A. Rivas, Talanta, 2007, 71, 1282–1287 CrossRef CAS PubMed.
- N. Burford, M. D. Eelman, D. E. Mahony and M. Morash, Chem. Commun., 2003, 146–147, 10.1039/B210570E.
- C. K. Larive, S. M. Lunte, M. Zhong, M. D. Perkins, G. S. Wilson, G. Gokulrangan, T. Williams, F. Afroz, C. Schöneich, T. S. Derrick, C. R. Middaugh and S. Bogdanowich-Knipp, Anal. Chem., 1999, 71, 389r–423r CrossRef CAS PubMed.
- G. Yao and Q. Huang, Spectrochim. Acta, Part A, 2022, 278, 121371 CrossRef CAS PubMed.
- G. Mi, M. Yang, C. Wang, B. Zhang, X. Hu, H. Hao and J. Fan, Spectrochim. Acta, Part A, 2021, 253, 119555 CrossRef CAS.
- Y. F. Shi, Y. P. Jiang, P. P. Sun, K. Wang, Z. Q. Zhang, N. J. Zhu, R. Guo, Y. Y. Zhang, X. Z. Wang, Y. Y. Liu, J. Z. Huo, X. R. Wang and B. Ding, Spectrochim. Acta, Part A, 2021, 249, 119214 CrossRef CAS.
- T. Wang, Q. Pang, Z. Tong, H. Xiang and N. Xiao, Spectrochim. Acta, Part A, 2021, 258, 119824 CrossRef CAS.
- S. Sudewi, C. H. Li, S. Dayalan, M. Zulfajri, P. V. S. Sashankh and G. G. Huang, Spectrochim. Acta, Part A, 2022, 279, 121453 CrossRef CAS PubMed.
- S. Wei, B. wang, H. Zhang, C. Wang, S. Cui, X. Yin, C. Jiang and G. Sun, Chem. Eng. J., 2023, 466, 143103 CrossRef CAS.
- J. Wu, Y. Luo, C. Cui, Q. Han and Z. Peng, Spectrochim. Acta, Part A, 2024, 309, 123840 CrossRef CAS PubMed.
- J. Yu, J. Fan, Y. Song, Y. Zhao, Z. Lin, L. Jiang and H. Li, Spectrochim. Acta, Part A, 2024, 308, 123734 CrossRef CAS.
- Y. Q. Pan, X. Xu, Y. Zhang, Y. Zhang and W. K. Dong, Spectrochim. Acta, Part A, 2020, 229, 117927 CrossRef CAS PubMed.
- H. X. Liu, R. S. Zhang, X. J. Yao, M. C. Liu, Z. D. Hu and B. T. Fan, J. Chem. Inf. Comput. Sci., 2004, 44, 161–167 CrossRef CAS PubMed.
- R. S. Bhosale, G. V. Shitre, R. Kumar, D. O. Biradar, S. V. Bhosale, R. Narayan and S. V. Bhosale, Sens. Actuators, B, 2017, 241, 1270–1275 CAS.
- Z. C. Wang, L. C. Yuan, Q. Zhang and D. W. Lei, CN202111296746.8, 2022.
- A. Oehlke, A. A. Auer, K. Schreiter, N. Friebe and S. Spange, Chem.–Eur. J., 2015, 21, 17890–17896 CrossRef CAS PubMed.
- A. Borchers and T. Pieler, Genes, 2010, 1, 413–426 CrossRef CAS PubMed.
- M. Zhang, J. Qiao, S. Zhang and L. Qi, Talanta, 2018, 182, 595–599 CrossRef CAS PubMed.
- H. Zhao, L. Li, Y. Cao, G. Gong, Y. Zhou, X. Gao, L. Pu and G. Zhao, Tetrahedron Lett., 2019, 60, 1238–1242 CrossRef CAS.
- K. Ghosh, D. Tarafdar, I. D. Petsalakis and G. Theodorakopoulos, Eur. J. Org Chem., 2017, 2017, 355–362 CAS.
- Z. Zhu, Y. Wang, H. Ding, C. Fan, Y. Tu, G. Liu and S. Pu, Luminescence, 2021, 36, 691–697 Search PubMed.
- Z. Xu, P. Deng, J. Li and S. Tang, Sens. Actuators, B, 2018, 255, 2095–2104 CAS.
- M. Xing, Y. Han, Y. Zhu, Y. Sun, Y. Shan, K. N. Wang, Q. Liu, B. Dong, D. Cao and W. Lin, Anal. Chem., 2022, 94, 12836–12844 CAS.
- T. Gao, L. Gao, J. Zhang, W. Zhou, Z. Zhang, X. Niu and T. Hu, J. Lumin., 2021, 231, 117798 CAS.
- W. Gao, H. Song, X. Wang, X. Liu, X. Pang, Y. Zhou, B. Gao and X. Peng, ACS Appl. Mater. Interfaces, 2018, 10, 1147–1154 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra00621j |
| This journal is © The Royal Society of Chemistry 2025 |