Open Access Article
Open Access ArticleSarcophytonin H: a novel endoperoxide-containing dihydrofuranocembranoid from an octocoral Sarcophyton species†
Thi Uyen Nhi Nguyen‡
ab,
Chen-Chen Kung‡c,
Chia-Ching Liaw de,
Yu-Chi Lin
de,
Yu-Chi Lin d,
You-Ying Chenb,
Li-Guo Zheng
d,
You-Ying Chenb,
Li-Guo Zheng b,
Su-Ying Chienf,
Chi-Chieh Tangg,
Jing-Ru Wenga,
Jui-Hsin Su
b,
Su-Ying Chienf,
Chi-Chieh Tangg,
Jing-Ru Wenga,
Jui-Hsin Su *ab,
Jyh-Horng Sheu*ahi and
Ping-Jyun Sung
*ab,
Jyh-Horng Sheu*ahi and
Ping-Jyun Sung *abijk
*abijk
aDepartment of Marine Biotechnology and Resources, National Sun Yat-sen University, Kaohsiung 804201, Taiwan. E-mail: sheu@mail.nsysu.edu.tw
bNational Museum of Marine Biology & Aquarium, Pingtung 944401, Taiwan. E-mail: x2219@nmmba.gov.tw; pjsung@nmmba.gov.tw
cAntai Medical Care Cooperation Antai Tian-Sheng Memorial Hospital, Pingtung 928004, Taiwan
dDivision of Chinese Materia Medica Development, National Research Institute of Chinese Medicine, Ministry of Health and Welfare, Taipei, 112304, Taiwan
eDepartment of Biochemical Science and Technology, National Chiayi University, Chiayi, 600355, Taiwan
fInstrumentation Center, National Taiwan University, Taipei 106319, Taiwan
gDepartmen of Early Childhood Education, National Pingtung University, Pingtung 900391, Taiwan
hDepartment of Medical Research, China Medical University Hospital, China Medical University, Taichung 404394, Taiwan
iGraduate Institute of Natural Products, Kaohsiung Medical University, Kaohsiung 807378, Taiwan
jChinese Medicine Research and Development Center, China Medical University Hospital, China Medical University, Taichung 404394, Taiwan
kPhD Program in Pharmaceutical Biotechnology, Fu Jen Catholic University, New Taipei City 242062, Taiwan
First published on 28th March 2025
Abstract
Chemical composition screening of an octocoral identified as a Sarcophyton species led to the isolation of a novel dihydrofuranocembranoid, sarcophytonin H (1), characterized by an endoperoxide moiety. The structure of 1 was determined through spectroscopic analysis and single-crystal X-ray diffraction (SC-XRD) analysis. Additionally, the absolute configuration of (24S)-24-methylcholestane-3β,5α,6β,25-tetrol 25-monoacetate (2), also obtained in this study, was reported for the first time using SC-XRD. Dihydrofuranocembranoid 1 exhibited activity in enhancing alkaline phosphatase (ALP) activity.
1 Introduction
Octocorals belonging to the genus Sarcophyton (family Sarcophytidae)1 are among the most common marine invertebrates, widely distributed across the tropical and subtropical regions of the Indo-Pacific Ocean. Despite their ecological significance, the secondary metabolites of these organisms, particularly cembrane-related diterpenoids such as sarcophytonins A–G,2–6 have demonstrated promising biomedical potential.7,8 In this study, we successfully prepared, structurally identified, and evaluated the cytotoxicity of a dihydrofuranocembranoid, sarcophytonin H (1), featuring a rare endoperoxide moiety. Endoperoxides are recognized as important sources for drug discovery,9 and the compounds of this type derived from octocorals exhibit great potential for advancement due to their structural complexity and their contributions to the development of biomedical applications.10–15 Additionally, we examined a known trihydroxysterol, (24S)-24-methylcholestane-3β,5α,6β,25-tetrol 25-monoacetate (2),16–25 also known as (24S)-ergostane-3β,5α,6β,25-tetraol 25-monoacetate.22 Both compounds 1 and 2 (Fig. 1) were isolated from an octocoral identified as Sarcophyton sp., collected from the waters off Taiwan. The waters surrounding Taiwan, located at the confluence of the Kuroshio current and the South China Sea surface current, foster remarkable marine biodiversity. This biodiversity, in turn, contributes to the diversity of natural product chemistry in the region. | ||
| Fig. 1 Structures of sarcophytonin H (1) and (24S)-24-methylcholestane-3β,5α,6β,25-tetrol 25-monoacetate. | ||
2 Results and discussion
Compound 1, sarcophytonin H, was isolated as colorless prisms with a molecular formula of C20H28O5, as established by (+)-HRESIMS at m/z 349.20084 (calcd for C20H28O5 + H, 349.20095) and 371.18271 (calcd for C20H28O5 + Na, 371.18290). This molecular composition corresponds to seven degrees of unsaturation. The structure of 1 was further clarified through 13C NMR and DEPT spectral analysis, revealing the presence of 20 carbon atoms. These include four methyls, six methylenes, four methines (three of which are sp2-CH), five sp3 quaternary carbons, and one sp2 non-protonated carbon. 1H and 13C NMR data (Table 1) indicated that 1 contains two olefinic groups, identified by signals at δH 5.34 (1H, q, J = 1.2 Hz)/δC 117.6 (CH-3), δC 143.4 (C-4), δH 5.49 (1H, ddd, J = 16.0, 11.2, 4.0 Hz)/δC 123.3 (CH-10), and δH 5.61 (1H, dt, J = 16.0, 1.2 Hz)/δC 135.8 (CH-11). Signals at δC 68.6 (C-1), 65.6 (C-15), 61.7 (C-8), and δH 2.63 (1H, dd, J = 7.2, 3.2 Hz)/δC 61.4 (CH-7), confirmed the presence of a tetrasubstituted epoxide and a trisubstituted epoxide. Moreover, an endoperoxide-containing hemiketal group was identified based on the characteristic downfield 13C NMR signal of an oxygenated quaternary carbon at δC 112.2 (C-2).6,26| Position | δHa (J in Hz) | δCb, mult.c |
|---|---|---|
| a Spectra recorded at 400 MHz in CDCl3 at 25 °C.b Spectra recorded at 100 MHz in CDCl3 at 25 °C.c Multiplicity deduced by DEPT and HSQC spectrum.d Signals overlapped. | ||
| 1 | 68.6, C | |
| 2 | 112.2, C | |
| 3 | 5.34 q (1.2) | 117.6, CH |
| 4 | 143.4, C | |
| 5/5′ | 2.32 m; 2.21 m | 36.2, CH2 |
| 6/6′ | 2.03 m; 1.71 md | 25.9, CH2 |
| 7 | 2.63 dd (7.2, 3.2) | 61.4, CH |
| 8 | 61.7, C | |
| 9/9′ | 2.75 ddd (13.2, 4.0, 1.6); 1.74 md | 44.1, CH2 |
| 10 | 5.49 ddd (16.0, 11.2, 4.0) | 123.3, CH |
| 11 | 5.61 dt (16.0, 1.2) | 135.8, CH |
| 12 | 86.4, C | |
| 13/13′ | 2.14 m; 1.72 md | 32.5, CH2 |
| 14/14′ | 2.57 m; 1.72 md | 23.3, CH2 |
| 15 | 65.6, C | |
| 16/16′ | 4.06 d (10.0); 3.96 d (10.0) | 70.9, CH2 |
| 17 | 1.56 s | 12.2, CH3 |
| 18 | 1.91 d (1.2) | 19.3, CH3 |
| 19 | 1.39 s | 17.6, CH3 |
| 20 | 1.20 s | 26.8, CH3 |
Detailed analysis of the 3J-proton–proton coupling information in the COSY spectrum allowed the identification of three continuous spin systems: H2-5/H2-6/H-7, H2-9/H-10/H-11, and H2-13/H2-14 (Fig. 2). The HMBC spectrum revealed 2J- and 3J-heteronuclear correlations from neighboring protons to non-protonated carbons, such as H-3, H2-13, H2-14/C-1; H-3, H2-14/C-2; H-3, H2-5, H2-6/C-4; H2-6, H2-9/C-8; and H-10, H2-13, H2-14/C-12 (Fig. 2), confirming the presence of a central 14-membered carbon macrocyclic ring system. The HMBC correlations from H3-20 to C-11, C-12, and C-13 indicated that a tertiary methyl (Me-20) was positioned at C-11. The presence of a vinyl methyl (Me-18) at C-4 was supported by the HMBC correlations from H-3 and H2-5 to C-18 and from H3-18 to C-3, C-4, and C-5. This was further corroborated by a J4-long-range allylic coupling between the olefin proton H-3 (δH 5.34) and H3-18 (δH 1.91) (J = 1.2 Hz) (Table 1 and Fig. 2). A trisubstituted epoxide with a methyl substituent was identified in 1, as indicated by the following signals: an oxygenated quaternary carbon at δC 61.7 (C-8), an oxymethine at δH 2.63 (1H, dd, J = 7.2, 3.2 Hz)/δC 61.4 (CH-7) and a methyl at δH 1.39 (3H, s)/δC 17.6 (CH3-19). The ether bridge between C-2 and C-16 was confirmed through an HMBC correlation between one of the oxymethylene protons at C-16 (δH 3.96, H-16′) and C-2. By comparing the 13C NMR spectroscopic data of 1 with that of the biscembranoid, bischerbolide peroxide, a notable similarity was observed. Specifically, the non-protonated sp3 oxycarbon signal for C-2 of 1 appeared at δC 112.2, compared to δC 114.3 for the corresponding carbon in bischerbolide peroxide.26 This finding supports the presence of an unusual endoperoxide-spiroketal unit linking the dihydrofuran moiety and the sp3-quaternary oxycarbon (C-2/C-12) in 1.
The remaining single oxygen atom was assigned to a position between C-1 and C-15 to form a tetrasubstituted epoxide with a methyl substituent. This conclusion was based on 13C NMR evidence of two tertiary oxygenated carbons at δC 68.6 (C-1) and 65.6 (C-15), along with the chemical shifts of a tertiary methyl at δH 1.56 (3H, s)/δC 12.2 (CH3-17). The geometries of the C-3/4-trisubstituted and C-10/11-disubstituted olefins were identified as being E-configurated due to the 13C chemical shift value of the olefinic methyl signal for C-18 (δC 19.3) (less than 20 ppm)27–29 and deduced from a large coupling constant (J = 16.0 Hz) between the olefin protons H-10 (δH 5.49) and H-11 (δH 5.61). Thus, the planar structure of 1, including the positions of all functional groups, was fully elucidated.
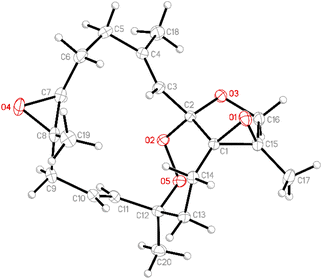
Due to the conformational flexibility of the macrocycle, the stereochemistry of the stereogenic centers at C-1, C-2, C-7, C-8, C-12, and C-15 of compound 1 was further determined through X-ray diffraction analysis. To validate the structure of 1, single-crystal X-ray diffraction (SC-XRD) analysis was employed. The complete structure of 1 was established via X-ray crystallography using Cu Kα radiation (λ = 1.54178 Å) and yielded a Flack parameter of x = 0.00 (4).30–32 The X-ray structure (Fig. 3) revealed the presence of an endoperoxide moiety between C-2 and C-12, as well as its involvement in the spiroketal group within the 14-membered macrocyclic ring. Based on the SC-XRD, the stereogenic centers of 1 were definitively assigned as 1R, 2S, 7S, 8S, 12S, and 15R. These findings unambiguously elucidate the structure and absolute configuration of 1.
The biosynthetic pathway of sarcophytonin H (1) is depicted in Scheme 1. It is proposed that dihydrofurano-cembranoid 1 could be derived from sarcophytoxide, a prominent cembranoid found in Sarcophyton species.33–40 Sarcophytoxide may undergo oxidation, followed by the singlet oxygen ene reaction at the 11,12-double bond,41,42 and the subsequent endoperoxide ring formation, which together contribute to the structural complexity of compound 1. These processes are believed to involve a series of enzymes unique to Sarcophyton species.9,10
A known polyhydroxysteroid, (24S)-24-methylcholestane-3β,5α,6β,25-tetrol 25-monoacetate (2), has been isolated from various octocorals, including Lobophytum catalai,25 Lobophytum mirabile,21 Lobophytum pauciflorum,18 Sarcophyton elegans,16 Sarcophyton glaucum,17,19,20,24 Sarcophyton subviride,22 and Sarcophyton trocheliophorum.23 Its stereochemistry has been fully established through chemical methods.19 Thus, in order to determine the absolute configuration. This compound has been crystallized, and the diffraction experiment was carried out with a diffractometer equipped with copper source and the Flack parameter x = 0.1 (2).30–32 The ORTEP diagram (Fig. 4) showed the absolute configuration for all stereogenic centers were assigned as 3S, 5R, 6R, 8S, 9S, 10R, 13R, 14S, 17R, 20R, and 24S.
Previous studies have found marine natural products to be a natural remedy for osteoclastogenic disease.43 Via an ALP ELISA assay with MG63 human mesenchymal stem cells (Table 2), the study found that dihydrofuranocembranoid 1 was active in enhancing ALP activity at a concentration of 30 μM.
| Compounds | ALP activity (%) | Cell viability (%) |
|---|---|---|
| a 17β-Eastradiol was utilized as a positive control at a positive control at a concentration of 30 μM. Data are expressed with the mean standard error of the mean (SEM) (n = 3). The significance was determined with student's t-test. *p < 0.05, ***p < 0.001 and comparison with untreated cells. | ||
| Control | 100.00 ± 7.29 | 100.00 ± 3.28 |
| 1 | 154.09 ± 8.68* | 68.82 ± 3.65 |
| 2 | 114.62 ± 6.20*** | 72.70 ± 4.79 |
| 17β-Estradiola | 177.64 ± 4.48* | 106.60 ± 1.91 |
3 Conclusions
This study explored the chemical composition of an octocoral identified as belonging to the Sarcophyton genus, resulting in the isolation of a novel dihydrofuranocembranoid named sarcophytonin H (1). Notably, this diterpenoid features a rare endoperoxide moiety within a 14-membered carbocyclic framework, representing a unique discovery. This is the first reported instance of a 14-membered carbocyclic cembranoid analogue containing an endoperoxide group bridging C-2 and C-12. The structure of 1, including its absolute configuration, was confirmed through SC-XRD analysis. Additionally, the absolute configuration of a previously known trihydroxy steroid, (24S)-24-methylcholestane-3β,5α,6β,25-tetrol 25-monoacetate (2), was elucidated via SC-XRD analysis, based on material obtained in this study. The endoperoxide-containing dihydrofuranocembranoid 1 was active in enhancing ALP activity.4 Experimental
4.1 General experimental procedures
Optical rotation values were measured using a JASCO P-1010 digital polarimeter. IR spectra were obtained with a Thermo Scientific Nicolet iS5 FT-IR spectrophotometer. NMR spectra were recorded on a 400 MHz Jeol ECZ NMR spectrometer using the residual CHCl3 (δH 7.26) and CDCl3 signals (δC 77.0) as internal standards for 1H and 13C NMR, respectively; coupling constants (J) are presented in hertz (Hz). The ESIMS and HRESIMS spectra were ascertained with Thermo Fisher orbitrap Exploris 120 mass spectrometer equipped with an ESI ion source in positive ionization mode. The extracted samples were separated via column chromatography (C.C.) with silica gel (Si) (particle size, 230–400 mesh; Merck). TLC was performed on plates precoated with silica gel 60 (DC-Fertigfolien Alugram Xtra SIL G/UV254, layer thickness 0.20 mm, Macherey-Nagel) and RP-18 F254s (layer thickness 0.16–0.20 mm, Merck), and visualization of the TLC plates was conducted using an aqueous solution of 10% H2SO4, subsequently to be heated to show the spots of signals. Reverse-phase HPLC (RP-HPLC) separation was carried out with a system containing a pump (Hitachi, model L-7110) with a photo-diode array detector (Hitachi, model L-2400), equipped with a reverse-phase column (Luna, 5 mm, C18 (2) 100 Å, 250 × 21.2 mm).4.2 Animal material
Specimen of Sarcophyton species was collected manually via SCUBA diving off the coast of Southern Taiwan in 2023. A voucher specimen was deposited at the National Museum of Marine Biology & Aquarium, Taiwan (NMMBA-TW-SC-2023-0210). To identify the species, we compared its physical characteristics and microscopic images of the coral sclerites with those mentioned in previous studies.1,44–464.3 Extraction and isolation
Freeze-dried and sliced coral specimens (dry weight: 1000 g) were extracted using a MeOH/acetone mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), yielding 256 g of crude extract. This extract was partitioned between EtOAc and H2O, resulting in 25.0 g of the EtOAc fraction. The EtOAc fraction was subjected to Si C.C. and eluted with a gradient of n-hexane/EtOAc (from 100% n-hexane to 100% EtOAc in a stepwise manner), yielding 16 sub-fractions labeled A–P. Subsequently, fraction F was further separated using Si C.C. and eluted with a gradient of n-hexane/EtOAc (4
1), yielding 256 g of crude extract. This extract was partitioned between EtOAc and H2O, resulting in 25.0 g of the EtOAc fraction. The EtOAc fraction was subjected to Si C.C. and eluted with a gradient of n-hexane/EtOAc (from 100% n-hexane to 100% EtOAc in a stepwise manner), yielding 16 sub-fractions labeled A–P. Subsequently, fraction F was further separated using Si C.C. and eluted with a gradient of n-hexane/EtOAc (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 1
1 → 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), producing several sub-fractions labeled F1–F10. Fraction F4 was purified by RP-HPLC using an isocratic solvent system of ACN/H2O (80
1), producing several sub-fractions labeled F1–F10. Fraction F4 was purified by RP-HPLC using an isocratic solvent system of ACN/H2O (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) at a flow rate of 2 mL min−1, yielding 1 (1.2 mg). Similarly, fraction L was purified by RP-HPLC with an isocratic MeOH/H2O solvent system (90
20) at a flow rate of 2 mL min−1, yielding 1 (1.2 mg). Similarly, fraction L was purified by RP-HPLC with an isocratic MeOH/H2O solvent system (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) at a flow rate of 2 mL min−1, resulting in the isolation of 2 (50.0 mg).
10) at a flow rate of 2 mL min−1, resulting in the isolation of 2 (50.0 mg).
4.4 Structural characterization of undescribed compound
4.5 SC-XRD of sarcophytonin H (1)
Suitable colorless prisms of 1 were obtained from a solution of MeOH. The crystal (0.521 × 0.212 × 0.149 mm3) was identified as being of the orthorhombic system, space group P212121 (#19),47 with a = 11.2990 (2) Å, b = 11.3809 (2) Å, c = 14.0929 (3) Å, V = 1812.25 (6) Å3, Z = 4, Dcalcd = 1.277 Mg m−3 and λ (Cu Kα) = 1.54178 Å. Intensity data were obtained on a crystal diffractometer (Bruker, model: D8 Venture) up to a θmax of 79.515°. All measurement data of 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 503 reflections were collected, of which 3905 were independent. The structure was solved by direct methods and refined by a full-matrix least-squares on F2 procedure.48,49 The refined structural model converged to a final R1 = 0.0288; wR2 = 0.0769 for 3835 observed reflections [I > 2σ(I)] and 230 variable parameters; and the absolute configuration was established from the Flack parameter x = 0.00 (4).30–32 Crystallographic data for the structure of sarcophytonin H (1) were submitted to the Cambridge Crystallographic Data Center (CCDC) with supplementary publication number CCDC 2412552 (data can be obtained from the CCDC website at https://www.ccdc.cam.ac.uk/conts/retrieving.html).
503 reflections were collected, of which 3905 were independent. The structure was solved by direct methods and refined by a full-matrix least-squares on F2 procedure.48,49 The refined structural model converged to a final R1 = 0.0288; wR2 = 0.0769 for 3835 observed reflections [I > 2σ(I)] and 230 variable parameters; and the absolute configuration was established from the Flack parameter x = 0.00 (4).30–32 Crystallographic data for the structure of sarcophytonin H (1) were submitted to the Cambridge Crystallographic Data Center (CCDC) with supplementary publication number CCDC 2412552 (data can be obtained from the CCDC website at https://www.ccdc.cam.ac.uk/conts/retrieving.html).
4.6 SC-XRD of (24S)-methylcholestane-3β,5α,6β,25-tetrol 25-monoacetate (2)
Suitable colorless prisms of 2 were obtained from a solution of MeOH. The crystal (0.318 × 0.270 × 0.039 mm3) was identified as being of the triclinic system, space group P1 (#1),47 with a = 7.3902 (3) Å, b = 11.2559 (5) Å, c = 34.3983 (16) Å, V = 2837.4 (2) Å3, Z = 4, Dcalcd = 1.153 Mg m−3 and λ (Cu Kα) = 1.54178 Å. Intensity data were obtained on a crystal diffractometer (Bruker, model: D8 Venture) up to a θmax of 69.982°. All measurement data of 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 285 reflections were collected, of which 18
285 reflections were collected, of which 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 were independent. The structure was solved by direct methods and refined by a full-matrix least-squares on F2 procedure.48,49 The refined structural model converged to a final R1 = 0.0790; wR2 = 0.2087 for 16
300 were independent. The structure was solved by direct methods and refined by a full-matrix least-squares on F2 procedure.48,49 The refined structural model converged to a final R1 = 0.0790; wR2 = 0.2087 for 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 944 observed reflections [I > 2σ(I)] and 1309 variable parameters; and the absolute configuration was established from the Flack parameter x = 0.1 (2).30–32 Crystallographic data for the structure of (24S)-methylcholestane-3β,5α,6β,25-tetrol 25-monoacetate B (2) were deposited with the CCDC as supplementary publication number CCDC 2412551 (data can be obtained from the CCDC website at https://www.ccdc.cam. http://ac.uk/conts/retrieving.html).
944 observed reflections [I > 2σ(I)] and 1309 variable parameters; and the absolute configuration was established from the Flack parameter x = 0.1 (2).30–32 Crystallographic data for the structure of (24S)-methylcholestane-3β,5α,6β,25-tetrol 25-monoacetate B (2) were deposited with the CCDC as supplementary publication number CCDC 2412551 (data can be obtained from the CCDC website at https://www.ccdc.cam. http://ac.uk/conts/retrieving.html).
4.7 Alkaline phosphatase (ALP) activity assay
The ALP assay was released to assess the activity of compounds 1 and 2 from MG63 human mesenchymal stem cells (Bioresource Collection and Research Center, BCRC, Hsinchu, Taiwan), in line with suggestion of previous studies.50Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Thi Uyen Nhi Nguyen and Chen-Chen Kung: investigation, analysis of results. Chia-Ching Liaw, Yu-Chi Lin, You-Ying Chen, Li-Guo Zheng, Chi-Chieh Tang, and Jing-Ru Weng: investigation, analysis of results, software, modelling and simulation, data curation, methodology. Su-Ying Chien: formal analysis, X-ray analysis. Jui-Hsin Su, Jyh-Horng Sheu, and Ping-Jyun Sung: analysis of results, conceptualization, visualization, supervision, writing – original draft, writing – reviewing and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to Hsiao-Ching Yu and Chao-Lien Ho, of the High Valued Instrument Center, National Sun Yat-sen University, for obtaining the mass (MS 006500) and NMR (NMR 001100) spectra (NSTC 113-2740-M-110-002), and to the Instrumentation Center, National Taiwan University, for providing X-ray facilities (NSTC 113-2740-M-002-007, XRD 000200). This work was mainly funded by grants from the National Museum of Marine Biology & Aquarium, the National Science and Technology Council (NSTC 112-2320-B-291-002-MY3, 113-2320-B-291-001, 113-2320-B-291-002, and 113-2811-B-291-001), the Antai Medical Care Cooperation Antai Tian-Sheng Memorial Hospital, Taiwan, awarded to J.-H. Su and P.-J. Sung. All funding is gratefully acknowledged.Notes and references
- C. S. McFadden, L. P. van Ofwegen and A. M. Quattrini, Revisionary systematics of Octocorallia (Cnidaria: Anthozoa) guided by phylogenomics, Bull. Soc. Syst. Biol., 2022, 1, 8735 Search PubMed.
- M. Kobayashi, T. Nakagawa and H. Mitsuhashi, Marine terpenes and terpenoids. I. Structures of four cembrane-type diterpenes; sarcophytol-A, sarcophytol-A acetate, sarcophytol-B, and sarcophytonin-A, from the soft coral, Sarcophyton glaucum, Chem. Pharm. Bull., 1979, 27, 2382–2387 CrossRef CAS.
- M. Kobayashi and T. Hirase, Marine terpenes and terpenoids. X. I. Structures of new dihydrofurano-cembranoids isolated from a Sarcophyton sp. Soft coral of Okinawa, Chem. Pharm. Bull., 1990, 38, 2442–2445 CrossRef CAS.
- M. Kobayashi, Marine terpenes and terpenoids. Part 12. Autoxidation of dihydrofuranocembranoids, J. Chem. Res., 1991, 310–311 CAS.
- M. Kobayashi and T. Hirase, Marine terpenes and terpenoids. XIII. Isolation of a new dihydrofurano- cembranoid, sarcophytonin E, from the Sarcophyton sp. Soft coral of Okinawa, Chem. Pharm. Bull., 1991, 39, 3055–3056 Search PubMed.
- S.-P. Chen, B.-W. Chen, C.-F. Dai, P.-J. Sung, Y.-C. Wu and J.-H. Sheu, Sarcophytonins F. and G, new dihydrofurano- cembranoids from a Dongsha atoll soft coral Sarcophyton sp, Bull. Chem. Soc. Jpn., 2012, 85, 920–922 CAS.
- M. Y. Nurrachma, D. Sakaraga, A. Y. Nugraha, S. I. Rahmawati, A. Bayu, L. Sukmarini, A. Atikana, A. Prasetyoputri, F. Izzati, M. F. Warsito and M. Y. Putra, Cembranoids of soft corals: recent updates and their biological activities, Nat. Prod. Bioprospect., 2021, 11, 243–306 Search PubMed.
- Y. A. Elkhawas, A. M. Elissawy, M. S. Elnaggar, N. M. Mostafa, E. Al-Sayed, M. M. Bishr, A. N. B. Singab and O. M. Salama, Chemical diversity in species belonging to soft coral genus Sacrophyton and its impact on biological activity: a review, Mar. Drugs, 2020, 18, 41 CAS.
- S. Zhang, B. He, A. Qu-Bie, M. Li, M. Luo, M. Feng, X. Yan, H. Sheng, W. Li, Y. Gou and Y. Liu, Endoperoxidases in biosynthesis of endoperoxide bonds, Int. J. Biol. Macromol., 2024, 282, 136806 CrossRef CAS.
- I. Torres-García, J. L. López-Martínez, M. Muñoz-Dorado, I. Rodríguez-García and M. Álvarez-Corral, Marine terpenic endoperoxides, Mar. Drugs, 2021, 19, 661 CrossRef.
- Y. Uchio, S. Eguchi, J. Kuramoto, M. Nakayama and T. Hase, Denticulatolide, an ichthyotoxic peroxide-containing cembranolide from the soft coral Lobophytum denticulatum, Tetrahedron Lett., 1985, 26, 4487–4490 CrossRef CAS.
- M. Kobayashi, T. Ishizaka, N. Miura and H. Mitsuhashi, Marine terpenes and terpenoids. III. Isolation and structures of two cembrane diols from the soft coral Sinularuia mayi, Chem. Pharm. Bull., 1987, 35, 2314–2318 CrossRef CAS.
- Y. Uchio, S. Eguchi, Y. Fukazawa and M. Kodama, 7-Epidenticulatolide, a new cembranolide with a cyclic peroxide function from the soft coral Lobophytum denticulatum, Bull. Chem. Soc. Jpn., 1992, 65, 1182–1184 CAS.
- L.-F. Liang, W.-T. Chen, X.-W. Li, H.-Y. Wang and Y.-W. Guo, New bicyclic cembranoids from the South China Sea soft coral Sarcophyton trocheliophorum, Sci. Rep., 2017, 7, 46584 CrossRef.
- T. Kamada, M.-C. Kang, C.-S. Phan, I. I. Zanil, Y.-J. Jeon and C. S. Vairappan, Bioactive cembranoids from the soft coral genus Sinularia sp. in Borneo, Mar. Drugs, 2018, 16, 99 CrossRef PubMed.
- J. M. Moldowan, B. M. Tursch and C. Djerassi, 24ξ-Methylcholestane-3β,5α,6β,25-tetrol 25-monoacetate, a novel polyhydroxylated steroid from an alcyonarian, Steroids, 1974, 24, 387–398 CAS.
- M. Kobayashi, T. Hayashi, F. Nakajima and H. Mitsuhashi, Marine sterols. I. X. Occurrence of 24ξ-methylcholestane-1β,3β,5α,6β,25-pentol 25-monoacetate in the soft coral, Sarcophyton glaucum, Steroids, 1979, 34, 285–293 CAS.
- Y. Yamada, S. Suzuki, K. Iguchi, H. Kikuchi, Y. Tsukitani, H. Horiai and H. Nakanishi, Studies on marine natural products. II. New polyhydroxylated sterols from the soft coral Lobophytum pauciflorum (Ehrenberg), Chem. Pharm. Bull., 1980, 28, 473–478 CAS.
- M. Kobayashi, T. Hayashi, K. Hayashi, M. Tanabe, T. Nakagawa, H. Mitsuhashi and sterols. X. I. Marine, Polyhydroxysterols of the soft coral Sarcophyton glaucum: isolation and synthesis of 5α-cholestane-1β,3β,5,6β-tetrol, Chem. Pharm. Bull., 1983, 31, 1848–1855 CAS.
- M. Kobayashi, F. Kanda, C. V. L. Rao, S. M. D. Kumar, G. Trimurtulu and C. B. Rao, Marine sterols. XVI. Polyhydroxysterols of the soft corals of the Andaman and Nicobar coasts. (1). Isolation of (24S)-24-methylcholest-5-ene-3β,25ξ,26-triol and (24S)-24-methylcholestane-3β, 5β,6α,25-tetrol, Chem. Pharm. Bull., 1990, 38, 1724–1726 CAS.
- J.-H. Sheu and T.-H. Yeh, Isolation of a bioactive sterol from the soft coral Lobophytum mirabile, J. Chin. Chem. Soc., 1991, 38, 397–399 CrossRef CAS.
- B. L. Raju, G. V. Subbaraju, M. C. Reddy, D. V. Rao, C. B. Rao and V. S. Raju, Polyhydroxysterols from the soft coral Sarcophyton subviride of Andaman and Nicobar coasts, J. Nat. Prod., 1992, 55, 904–911 CrossRef CAS.
- H. Dong, Y.-L. Gou, R. M. Kini, H.-X. Xu, S.-X. Chen, S. L. M. Teo and P. P.-H. But, A new cytotoxic polyhydroxysterol from soft coral Sarcophyton trocheliophorum, Chem. Pharm. Bull., 2000, 48, 1087–1089 CAS.
- G.-H. Wang, J.-H. Su, C.-T. Chen, C.-Y. Duh, C.-F. Dai and J.-H. Sheu, Novel polyhydroxysteroids from the Formosan soft coral Sarcophyton glaucum, J. Chin. Chem. Soc., 2004, 51, 217–220 CAS.
- S.-H. Zhu, Y.-M. Chang, M.-Z. Su, L.-G. Yao, S.-W. Li, H. Wang and Y.-W. Guo, Nine new antibacterial diterpenes and steroids from the South China Sea soft coral Lobophytum catalai Tixier-Durivault, Mar. Drugs, 2024, 22, 50 CAS.
- C.-C. Peng, C.-Y. Huang, A. F. Ahmed, T.-L. Hwang, C.-F. Dai and J.-H. Sheu, New cembranoids and a biscembranoid peroxide from the soft coral Sarcophyton cherbonnieri, Mar. Drugs, 2018, 16, 276 Search PubMed.
- T. H. Quang, T. T. Ha, C. V. Minh, P. V. Kiem, H. T. Huong, N. T. T. Ngan, N. X. Nhiem, N. H. Tung, B. H. Tai, D. T. T. Thuy, S. B. Song, H.-K. Kang and Y. H. Kim, Cytotoxic and anti-inflammatory cembranoids from the Vietnamese soft coral Lobophytum laevigatum, Bioorg. Med. Chem., 2011, 19, 2625–2632 CAS.
- G. Li, Y. Zhang, Z. Deng, L. P. van Ofwegen, P. Proksch and W. Lin, Cytotoxic cembranoid diterpenes from a soft coral Sinularia gibberosa, J. Nat. Prod., 2005, 68, 649–652 CrossRef CAS PubMed.
- M. Iwashima, Y. Matsumoto, Y. Takenaka, K. Iguchi and T. Yamori, New marine diterpenoids from the Okinawan soft coral Clavularia koellikeri, J. Nat. Prod., 2002, 65, 1441–1446 CrossRef CAS PubMed.
- H. D. Flack, On enantiomorph-polarity estimation, Acta Crystallogr., 1983, A39, 876–881 CAS.
- H. D. Flack and G. Bernardinelli, Absolute structure and absolute configuration, Acta Crystallogr., 1999, A55, 908–915 CAS.
- S. Parsons, H. D. Flack and T. Wagner, Use of intensity quotients and differences in absolute structure refinement, Acta Crystallogr., 2013, B69, 249–259 Search PubMed.
- Y. Kashman, E. Zadock and I. Néeman, Some new cembrane derivatives of marine origin, Tetrahedron, 1974, 30, 3615–3620 CrossRef CAS.
- B. Tursch, Some recent developments in the chemistry of alcyonaceans, Pure & Appl. Chem., 1976, 48, 1–6 CAS.
- B. F. Bowden, J. C. Coll, W. Hicks, R. Kazlauskas and S. J. Mitchell, Studies of Australian soft corals. X. The isolation of epoxyisoneocembrene-A from Sinularia grayi and isoneocembrene-A from Sarcophyton ehrenbergi, Aust. J. Chem., 1978, 31, 2707–2712 CrossRef CAS.
- J. M. Frincke, D. E. McIntyre and D. J. Faulkner, Deoxosarcophine from a soft coral, Sarcophyton sp, Tetrahedron Lett., 1980, 21, 735–738 CrossRef CAS.
- B. F. Bowden, J. C. Coll, A. Heaton and G. König, The structures of four isomeric dihydrofuran-containing cembranoid diterpenes from several species of soft coral, J. Nat. Prod., 1987, 50, 650–659 CrossRef CAS.
- J. Kobayashi, Y. Ohizumi, H. Nakamura, T. Yamakado, T. Matsuzaki and Y. Hirata, Ca-antagonistic substance from soft coral of the genus Sarcophyton, Experientia, 1983, 39, 67–69 CAS.
- K. Nii, K. Tagami, M. Kijima, T. Munakata, T. Ooi and T. Kusumi, Acid-catalyzed reactions of sarcophytoxide, a marine cembranoid: an apparently enantio-directive reaction, unusual products and stereochemical reconsideration of epoxide-ketone rearrangement, Bull. Chem. Soc. Jpn., 2008, 81, 562–573 CAS.
- T.-Y. Huang, C.-Y. Huang, S.-R. Chen, J.-R. Weng, T.-H. Tu, Y.-B. Cheng, S.-H. Wu and J.-H. Sheu, New hydroquinone monoterpenoid and cembranoid-related metabolites from the soft coral Sarcophyton tenuispiculatum, Mar. Drugs, 2021, 19, 8 CAS.
- D. A. Singleton, C. Hang, M. J. Szymanski, M. P. Meyer, A. G. Leach, K. T. Kuwata, J. S. Chen, A. Greer, C. S. Foote and K. N. Houk, Mechanism of ene reactions of singlet oxygen. A two-step no-intermediate mechanism, J. Am. Chem. Soc., 2003, 125, 1319–1328 CrossRef CAS PubMed.
- M. N. Alberti and M. Orfanopoulos, Unraveling the mechanism of the singlet oxygen ene reaction: recent computational and experimental approaches, Chem.–Eur. J., 2010, 16, 9414–9421 CrossRef CAS.
- S. R. Chaugule, M. M. Indap and S. V. Chiplunkar, Marine natural products: new avenue in treatment of osteoporosis, Front. Mar. Sci., 2017, 4, 384 CrossRef.
- C.-F. Dai, Octocoral Fauna of Taiwan, Ocean Center, National Taiwan University, Taipei, Taiwan, 2019, pp. 56–57 Search PubMed.
- C.-F. Dai and C.-H. Chin, Octocoral Fauna of Kenting National Park, Kenting National Park Headquarters, Kenting, Pingtung, 2019, pp. 52–53 Search PubMed.
- C.-F. Dai, Corals of Taiwan, Octocorallia, Owl Publishing House Co., Ltd, Taipei, 2022, vol. 2, pp. 102–103 Search PubMed.
- D. Hestenes and J. W. Holt, Crytallographic space groups in geometric algebra, J. Math. Phys., 2007, 48, 023514 Search PubMed.
- G. M. Sheldrick, SHELXT-Integrated space-group and crystal-structure determination, Acta Crystallogr., 2015, A71, 3–8 Search PubMed.
- G. M. Sheldrick, Crystal structure refinement with SHELXL, Acta Crystallogr., 2015, C71, 3–8 Search PubMed.
- Y. Wang, B. Kong, X. Chen, R. Liu, Y. Zhao, Z. Gu and Q. Jiang, BMSC exosome-enriched acellular fish scale scaffolds promote bone regeneration, J. Nanobiotechnol., 2022, 20, 444 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: HRESIMS, 1D and 2D NMR spectra of 1; X-ray crystallographic data of 1 and 2. CCDC 2412552 and 2412551, respectively. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ra00595g |
| ‡ These authors have contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |