 Open Access Article
Open Access ArticleQuinoline derivatives' biological interest for anti-malarial and anti-cancer activities: an overview
Nagesh Dhanaji Chavan
,
S. Sarveswari
 and
V. Vijayakumar
and
V. Vijayakumar
 *
*
Department of Chemistry, School of Advanced Sciences, Vellore Institute of Technology, Vellore 632016, India. E-mail: chavannagesh605@gmail.com; vvijayakumar@vit.ac.in; kvpsvijayakumar@gmail.com
First published on 27th August 2025
Abstract
Quinoline is a heterocyclic compound that plays a fundamental role in the study of chemical compounds, highlighting its importance in drug development. Its basic framework is crucial for synthesizing new pharmaceutical substances and facilitating the development of innovative drugs with significant therapeutic potential. A thorough examination of quinoline and its various derivatives, which exhibit a wide range of biological activities, greatly contributes to groundbreaking drug discoveries that can transform the medical industry. This review presents a carefully curated collection of references related to quinoline, covering a unique range of quinoline-based medications currently available on the market, including antimalarial and anticancer drugs. It provides a comprehensive overview of the therapeutic potential of these compounds. Furthermore, the review aims to clarify and explain the complex biological properties of quinoline derivatives, particularly their antimalarial and anticancer activities. This insight will enhance our understanding of the therapeutic potential and efficacy of these compounds in combating malaria and cancer, diseases that affect millions of people worldwide.
1 Introduction
Quinoline, with the chemical formula C9H7N, is an aromatic heterocyclic compound composed of a pyridine ring fused to a benzene ring.1 Quinoline and its derivatives have several industrial, pharmaceutical, and biological applications (shown in Fig. 1). It can be obtained from natural sources like coal tar and bone oil or synthesized artificially for pharmacological purposes. Due to its versatility, reactivity, and low toxicity, quinoline is a valuable building block in synthesising various medically significant drugs, making it attractive for pharmaceutical formulations.2 A comprehensive review of recent advancements in the synthesis of hybrid antimalarial and anticancer drugs emphasizes the urgent need for innovative candidates with dual action and the importance of studying their in vitro and in vivo activity. Notable examples include chloroquine and primaquine, along with hybrids involving quinoxaline 1,4-di-N-oxide and their subsequent evaluation, and fluorescent probes.3 Quinoline derivatives have demonstrated activity as antimalarial agents,4 antibacterial5 and antifungal drugs,6 antiviral agents,7 anti-inflammatory compounds,8 anticancer drugs,9 and in treating neurological disorders,10 cardiovascular diseases,11 and as anti-protozoal agents.12 They are also used in photodynamic therapy13 and exhibit activities related to neuroscience,14 antimicrobial15 and various enzyme inhibitions, such as α-glucosidase and α-amylase.16,17 This study aims to provide a thorough and current overview of the significant research on physiologically relevant quinoline derivatives targeting malaria and cancer. It clarifies the wide range of studies conducted in this rapidly developing field. The primary goal is to present a concise, yet comprehensive summary of the biological studies and discoveries related to antimalarial and anticancer activities, enhancing our understanding of this emerging area of research.2 Preparation of quinoline
Quinoline preparation involves various methodologies (shown in Fig. 2) that produce a range of substituted derivatives of this aromatic compound, which belongs to the class of heterocyclic compounds. One of the strategies utilized for this purpose is the Skraup synthesis,18,19 a process that entails the combination of aniline with glycerol, sulphuric acid, and an oxidizing agent. Upon initiation of this chemical transformation, an intermediate compound known as cinchoninic acid is formed, subsequently undergoing cyclization to culminate in the synthesis of quinoline. Another synthetic route, namely the Combes quinoline synthesis,20a entails the mixing of aniline with β-diketones in the presence of an acid catalyst. This reaction sequence gives rise to an enamine as an intermediate species, which then proceeds to cyclize and dehydrate, eventually yielding quinoline.20b In contrast, the Friedländer synthesis21,22 involves the reaction between 2-aminobenzylamine and a carbonyl compound under the influence of an acid catalyst, ultimately leading to cyclization and dehydration reactions that result in the production of quinoline. Similarly, the Conrad–Limpach reaction23 primarily involves the condensation of aniline with an α,β-unsaturated carbonyl compound, typically a β-ketoester, in the presence of an acid catalyst. The α,β-unsaturated carbonyl compound undergoes conjugate addition with aniline, followed by cyclization and dehydration processes, which collectively contribute to the formation of the quinoline ring. Lastly, the Doebner–Miller reaction utilizes aniline and an α,β-unsaturated carbonyl compound in the presence of an acid catalyst. This multi-step reaction mechanism comprises conjugate addition, cyclization, and dehydration steps, ultimately culminating in the synthesis of quinoline. The selection of the most appropriate synthetic approach is contingent upon several factors, including the nature of the starting materials and the specific substituents desired in the final product.3 Biological interest
Heterocyclic compounds have long been a useful source for medication research and discovery. Examples of these compounds are quinoline, indole, coumarin, purine, pyrimidine, thiazole, imidazole, tetrazole, and flavones. Among these, the quinoline ring has emerged as an exceptionally versatile and propitious scaffold, showcasing an extensive spectrum of pharmacological potential. Quinoline-based compounds have demonstrated a wide range of therapeutic activities, including effects on the central nervous system (CNS), the cardiovascular system, anticancer, antifungal, antimycobacterial, antiviral, anti-protozoal, antimalarial, antioxidant, analgesic, anti-inflammatory, as well as anthelmintic properties. This multifaceted utility is attributed to the unique structural features of the quinoline ring, which facilitate specific interactions with biological targets. Medicinal chemists have well developed these characteristics to chemist novel compounds, firmly establishing the quinoline scaffold as a foundational element in the ongoing quest for innovative pharmaceutical agents. Its extensive pharmacological versatility underscores its pivotal role in scientific endeavours, with researchers continuously exploring quinoline-derived molecules to address a wide spectrum of health-related challenges. Some anti-malarial drugs shown in Fig. 3 are already available on market.Chloroquine has been pivotal in malaria therapy, particularly against specific plasmodium species. However, the rise of chloroquine-resistant P. falciparum has diminished its effectiveness in numerous areas. In 2023, the global chloroquine market was approximately USD 87.7 million, expected to grow to USD 117.0 million by 2033, at a CAGR (Compound Annual Growth Rate) of 3.0%. This expansion is attributed to the rising incidence of malaria and the increased demand for generic pharmaceuticals. In 2023, there were around 263 million malaria cases globally, leading to about 597![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 fatalities. Despite alternative treatments, chloroquine persists in use, particularly in regions with limited resistance. Hydroxychloroquine, a chloroquine derivative, has been employed for decades in malaria prevention and treatment, especially in chloroquine-sensitive areas. Additionally, hydroxychloroquine is prescribed for autoimmune disorders such as rheumatoid arthritis and lupus erythematosus. The global hydroxychloroquine market has seen notable growth recently. In 2023, it was valued at approximately USD 2.07 billion, anticipated to reach USD 2.86 billion by 2028, experiencing a CAGR of 6.6%. This increase is fuelled by the drug's effectiveness against malaria and its growing application in autoimmune disease management. Malaria remains a significant global health concern. The World Health Organization reported an estimated 228 million malaria cases worldwide in 2018, with children under five constituting 67% of malaria-related deaths. In the U.S., over 1.3 million individuals suffer from rheumatoid arthritis, with projections suggesting that by 2040, about 78 million adults will have received a doctor's diagnosis of arthritis. These statistics highlight the considerable patient demographics that could benefit from hydroxychloroquine.24,25
000 fatalities. Despite alternative treatments, chloroquine persists in use, particularly in regions with limited resistance. Hydroxychloroquine, a chloroquine derivative, has been employed for decades in malaria prevention and treatment, especially in chloroquine-sensitive areas. Additionally, hydroxychloroquine is prescribed for autoimmune disorders such as rheumatoid arthritis and lupus erythematosus. The global hydroxychloroquine market has seen notable growth recently. In 2023, it was valued at approximately USD 2.07 billion, anticipated to reach USD 2.86 billion by 2028, experiencing a CAGR of 6.6%. This increase is fuelled by the drug's effectiveness against malaria and its growing application in autoimmune disease management. Malaria remains a significant global health concern. The World Health Organization reported an estimated 228 million malaria cases worldwide in 2018, with children under five constituting 67% of malaria-related deaths. In the U.S., over 1.3 million individuals suffer from rheumatoid arthritis, with projections suggesting that by 2040, about 78 million adults will have received a doctor's diagnosis of arthritis. These statistics highlight the considerable patient demographics that could benefit from hydroxychloroquine.24,25
Quinacrine, initially engineered for antimalarial purposes, has demonstrated potential as an anticancer therapeutic agent through mechanisms including the inhibition of NF-κB, activation of the p53 signaling pathway, and induction of autophagy. It is currently being researched in relation to various malignancies such as ovarian, breast, and lung cancers, thus representing a substantial patient demographic—ovarian cancer alone impacts over 313![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 women on a global scale annually. Camptothecin, an alkaloid derived from plants, exerts its anticancer effects through the inhibition of topoisomerase I, thereby disrupting the processes of DNA replication. Its derivatives, irinotecan and topotecan, have received FDA approval for the treatment of colorectal, lung, and ovarian cancers. The global market for irinotecan was estimated at approximately USD 1.1 billion in 2022, underscoring its established significance in the field of oncology. Colorectal cancer, one of the primary indications for irinotecan, affects over 1.9 million individuals worldwide each year, thereby further emphasizing the clinical importance of this category of compounds.26
000 women on a global scale annually. Camptothecin, an alkaloid derived from plants, exerts its anticancer effects through the inhibition of topoisomerase I, thereby disrupting the processes of DNA replication. Its derivatives, irinotecan and topotecan, have received FDA approval for the treatment of colorectal, lung, and ovarian cancers. The global market for irinotecan was estimated at approximately USD 1.1 billion in 2022, underscoring its established significance in the field of oncology. Colorectal cancer, one of the primary indications for irinotecan, affects over 1.9 million individuals worldwide each year, thereby further emphasizing the clinical importance of this category of compounds.26
Quinoline derivatives are instrumental in combating diseases such as malaria and cancer via distinct molecular mechanisms, utilizing their aromatic nitrogen-containing framework for target-specific interactions.27 In the context of malaria, quinoline derivatives, including chloroquine and quinine, exert their effects within the acidic digestive vacuole of Plasmodium falciparum. During the process of hemoglobin digestion, the parasite liberates toxic free heme (Fe2+-protoporphyrin IX). Ordinarily, this heme is metabolized into inert hemozoin crystals. Quinoline compounds intercalate with heme, resulting in the formation of stable complexes that inhibit the crystallization process.28 The accumulation of heme and drug–heme complexes leads to the generation of reactive oxygen species (ROS) and the disruption of membrane integrity, culminating in the demise of the parasite. Resistance mechanisms frequently entail diminished drug accumulation attributable to mutations in vascular transporters such as PfCRT.29 In the realm of cancer, quinoline derivatives exert their effects through various pathways. Some derivatives function as topoisomerase inhibitors, thereby obstructing the relaxation of DNA that is requisite for replication and transcription.30 Others, including camptothecin analogs featuring quinoline scaffolds, intercalate into DNA or bind to ATP-binding sites of kinases, thereby impeding pro-survival signaling pathways (e.g., PI3K/AKT, EGFR).31 Certain quinoline derivatives also promote apoptosis by instigating mitochondrial dysfunction or an overproduction of ROS, thereby disrupting redox homeostasis within cancer cells. Structural modifications, such as substitutions at specific ring positions, augment target selectivity and bioavailability, rendering them promising candidates as anticancer agents.32 Consequently, quinoline derivatives function as potent pharmacophores by undermining essential survival mechanisms in both parasites and cancer cells through heme binding, DNA interaction, and enzyme inhibition, thereby underscoring their extensive therapeutic potential.
3.1 Antimalarial quinoline derivatives
Mahajan et al. (2007),33 prepared new analogues of quinolinyl thiourea and tested them in a lab setting for antimalarial activity. Having a substantial inhibitory IC50 value of 1.2 µM against the strain of Plasmodium falciparum that developed resistance to chloroquine, 1-(2-(methylamino)ethyl)-3-(quinolin-4-yl)thiourea (1) was the most effective chemical. This active ingredient is significant for its ability to selectively target parasites without causing harm to HeLa cells. Considering the growing issues relating to drug resistance, this suggests that it could be used as an alternative antimalarial medicine. The study revealed that quinoline derivatives with a diethylamine (1a) side chain exhibited moderate antimalarial activity (IC50 = 2.2 µM), while those with a dimethylamino group showed improved activity (IC50 = 1.2 µM), indicating that smaller dialkylamino groups enhance efficacy. A compound with a primary amino group showed slightly lower activity (IC50 = 3.3 µM), and an ethyl-substituted analogue had similar potency (IC50 = 1.8 µM), suggesting that flexible side chains with terminal amino functionalities favour activity. Overall, dialkylamino side chains improved potency, whereas bulky groups like phenyl reduced it. The mechanism remains speculative, with links to CQ-like activity and apoptosis in MCF-7 cells.Shiraki et al. (2011),34 synthesized the 5-aryl-8-aminoquinoline molecules have proven to be quite effective against drug-resistant types of malaria. Compounds N4-(6-methoxy-4-methyl-2-(trifluoromethyl)-5-(4-(trifluoromethyl)phenyl)quinolin-8-yl) pentan-1,4-diamine (2), N4-(6-methoxy-5-(3-methoxyphenyl)-4-methyl-2-(trifluoromethyl)quinolin-8-yl)pentan-1,4-diamine (3), and N4-(6-methoxy-4-methyl-5-(m-tolyl)-2-(trifluoromethyl)quinolin-8-yl)pentane-1,4-diamine (4) showed IC50 values ranging from 5 to 8, based on the IC50 data. Furthermore, in mouse experiments, these molecules showed lower toxicity when compared to currently available treatments. They have additionally shown metabolic stability. But it is unknown if these compounds were evaluated particularly for their hemolytic potential in connection with an absence of glucose-6-phosphate dehydrogenase (G6PD).
Radini et al. (2016),35 synthesized quinoline derivatives showed moderate to strong antimalarial activity against Plasmodium falciparum. For these compounds, the IC50 values ranged from 0.014 to 5.87 µg mL−1. When compared with the malaria-fighting drug chloroquine (CQ), compounds ethyl (Z)-4-(2-hydrazineylidene-1,2-dihydroquinolin-3-yl)-6-methyl-2-thioxo-1,2,3,4-tetrahydro pyrimidine-5-carboxylate (5), 3-(5-acetyl-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidin-4-yl)quinolin-2(1H)-one (6), and 5-(2-chloro quinolin-3-yl)-1,3,4-oxadiazole-2-thiol (7) have significant antimalarial activity against Plasmodium falciparum.
Pan et al. (2018),36 produced during the period of a 17-year investigation of more than 2000 plant extracts, 175 compounds with antiplasmodial activities against Plasmodium falciparum were found. Prosopis glandulosa var. glandulosa leaves were found to contain prosopilosidine and isoprosopilosidine, which had strong antimalarial activity against strains of P. falciparum. Lippia javanica's lippialactone shown efficacy against the D10 strain, and the roots of Eurycoma longifolia produced the powerful anti-P. falciparum compounds erycomanone and pasakbumin B. The antimalarial efficiency of gallocatecin surpassed that of mefloquine, indicating the potential of using natural chemicals to treat malaria more effectively. This thorough investigation demonstrates how nature can produce potent antimalarial remedies from a variety of plant sources, including marine plants. Hu et al. (2017),37 the main drugs used to treat the disease. It makes sense to mix quinolines with other compounds to create new, stronger antimalarial drugs with less side effects. Compound 1-(2,7-bis(trifluoromethyl)quinolin-4-yl)-2-(2-ethyl-4-methyl-1H-imidazol-1-yl)ethan-1-ol (8) shown the highest activity among a collection of molecules that included imidazole and 2,8 di(trifluoromethyl)quinoline. These compounds displayed properties against several P. falciparum strains. On the other hand, the hybrids produced by combining imidazole with 4-chloroquinoline demonstrated little to moderate activity in contrast to the parent compound chloroquine. Furthermore, new metal complexes that were created by combining eight aminoquinolines with five substituted uracils showed anti-P. falciparum characteristics. Furthermore, when 1,3,5 triazine derivatives were mixed with well-known medications like chloroquine, the resulting compounds have antimalarial properties.
Joshi et al. (2013),38 the study categorizes quinoline triazole amide analogues into two groups based on substitution at the 7-position: 7-chloro (7-Cl) and 7-cyano (7-CN) derivatives. In the 7-Cl series, a strong linear correlation (r2 = 0.84) between antimalarial activity [log(1/IC50)] and lipophilicity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) was observed, indicating that increased lipophilicity enhances compound penetration into the parasite's digestive vacuole. Conversely, no such correlation was found in the 7-CN series, suggesting that factors other than lipophilicity may influence activity. These findings highlight key structural features guiding future optimization of quinoline-based antimalarials. Synthesized A variety of amide analogs that contain quinoline triazole were synthesized and subjected to analysis to determine their effectiveness against malaria. Having IC50 values ranging from 349 to 1247 nM, compounds 2-(1-(((7-chloroquinolin-4-yl)amino)methyl)-1H-1,2,3-triazol-4-yl)-N,N-dicyclohexyl acetamide (9), 2-(1-(((7-cyanoquinolin-4-yl)amino)methyl)-1H-1,2,3-triazol-4-yl)-N,N-diethylacetamide (10), and N-benzyl-2-(1-(((7-cyanoquinolin-4-yl)amino)methyl)-1H-1,2,3-triazol-4-yl)-N-methyl acetamide (11) showed the most effectiveness among these analogs against the Plasmodium falciparum strain that is highly sensitive to chloroquine (D10). The β-haematin was most strongly inhibited by the quinoline triazoles 9, with 50% inhibitory doses of 14.7 and 8.9 mM, respectively. As a result of this, hydrophilic analogs with considerable activity and little cross-resistance to chloroquine were effectively generated. A linear connect was found among the resistance index (IC50 K1/IC50 D10) and log
P) was observed, indicating that increased lipophilicity enhances compound penetration into the parasite's digestive vacuole. Conversely, no such correlation was found in the 7-CN series, suggesting that factors other than lipophilicity may influence activity. These findings highlight key structural features guiding future optimization of quinoline-based antimalarials. Synthesized A variety of amide analogs that contain quinoline triazole were synthesized and subjected to analysis to determine their effectiveness against malaria. Having IC50 values ranging from 349 to 1247 nM, compounds 2-(1-(((7-chloroquinolin-4-yl)amino)methyl)-1H-1,2,3-triazol-4-yl)-N,N-dicyclohexyl acetamide (9), 2-(1-(((7-cyanoquinolin-4-yl)amino)methyl)-1H-1,2,3-triazol-4-yl)-N,N-diethylacetamide (10), and N-benzyl-2-(1-(((7-cyanoquinolin-4-yl)amino)methyl)-1H-1,2,3-triazol-4-yl)-N-methyl acetamide (11) showed the most effectiveness among these analogs against the Plasmodium falciparum strain that is highly sensitive to chloroquine (D10). The β-haematin was most strongly inhibited by the quinoline triazoles 9, with 50% inhibitory doses of 14.7 and 8.9 mM, respectively. As a result of this, hydrophilic analogs with considerable activity and little cross-resistance to chloroquine were effectively generated. A linear connect was found among the resistance index (IC50 K1/IC50 D10) and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P, with a more prominent slope noted for the 7-Cl series. At the hydrophobic end of the spectrum, the 7-CN series showed resistance index values like those of the 7-Cl compounds; however, at the hydrophilic end, they showed lower resistance index values.
P, with a more prominent slope noted for the 7-Cl series. At the hydrophobic end of the spectrum, the 7-CN series showed resistance index values like those of the 7-Cl compounds; however, at the hydrophilic end, they showed lower resistance index values.
Lombard et al. (2012),39 the authors categorize 4-aminoquinoline derivatives based on side chain modifications, including basic groups (diethylamino, piperidinyl, morpholino), substituted amides, aryl groups, and various linkers. SAR analysis shows that basic side chains enhance activity through better accumulation in the plasmodium digestive vacuole; longer alkyl spacers improve potency, and electron-donating groups favor π–π interactions. Compounds were tested against P. falciparum, though specific molecular targets were not identified. The presumed mechanism involves pH trapping and inhibition of haemozoin (β-haematin) formation, similar to chloroquine, disrupting haem detoxification in the parasite's food vacuole. Their synthesis, two hybrid dimers of artemisinin-4-amino-quinoline (12 and 13) were found to possess relatively weak nanomolar antiplasmodial activity against Plasmodium vinckei in vitro. On the fifth day after treatment, hybrid-dimer 12 exhibited considerable in vitro efficacy against Plasmodium vinckei, resulting in a parasitemia level of less than 1%. Computational studies suggest that the antimalarial activity of the hybrid-dimers can be ascribed to their active metabolites. In a mouse model infected with P. vinckei, hybrid-dimer 12 demonstrated potent antimalarial activity, eliminating nearly 99% of the parasites following intraperitoneal administration. Mice treated with doses of 2.5 and 7.5 mg kg−1 of hybrid-dimer 12 experienced almost complete recovery from blood parasitemia. However, recrudescence between days 7 and 18 led to the death of the mice, highlighting the necessity for further fine-tuning to prevent parasite recrudescence.
Soares et al. (2015),40 the study effectively addresses the categorization of quinoline derivatives by organizing them into structural groups such as piperazine and triazole hybrids, with clear structure–activity relationship (SAR) discussions based on substituents and linker variations. However, while figures are grouped by scaffold, they could be improved by consolidating structures according to pharmacophore or target class for broader comparative analysis. The biological target is only presumed—based on similarity to chloroquine—to involve inhibition of haemozoin formation, with no direct experimental confirmation. Furthermore, the manuscript lacks binding interaction studies such as molecular docking or target–protein interaction analysis, which are essential for understanding the molecular basis of antimalarial activity and guiding future optimization. Ten synthetic compounds generated from quinoline were tested for their efficacy against both living organisms and laboratory-grown Plasmodium falciparum and berghei. The compounds selectively expressed both CQ-sensitive and CQ-resistant strains of P. falciparum, and they showed no negative effects in HepG2 or HeLa cell lines. Examination of the hydrazine derivative 14 chemical in living creatures showed that it exhibited an activity level against the blood parasite development equivalent to that of the reference medication, chloroquine (CQ). The compound 7-chloro-4-hydrazineylquinoline (14) showed the highest LipE value of all the chemicals examined in living things, suggesting that it could be a promising molecule to investigate further in antimalarial chemotherapy.
Verma et al. (2016),41 development and assessment of N-(7-chloroquinolinyl-4-aminoalkyl) arylsulfonamides as antimalarial drugs, with an emphasis on their effectiveness against P. falciparum strains and the discovery of hemozoin. Compounds N-(2-((7-chloroquinolin-4-yl)amino)ethyl)-2,4,6 triisopropylbenzenesulfonamide (15) and N-(3-((7-chloroquinolin-4-yl)amino)propyl)-2,4,6-triisopropylbenzenesulfonamide (16) efficiently reduced the production of hemozoin and showed promise antimalarial action. To find out more about the interactions between the compounds and their targets, which include heme, m-oxo dimer, and heme detoxification protein (HDP), docking experiments have been carried out. Ferriprotoporphyrin and propionic acid side chains were essential elements for interactions with heme and m-oxo dimer; for HDP, those components were His175 and Glu126. As compared to chloroquine (CQ), the compounds' sulfonamide moiety improved the inhibition of hemozoin production. The study highlights that these substances may be further studied for their antimalarial effect by blocking the growth of hemozoin.
Teguh et al. (2013),42 the study categorizes the compounds as triazole-linked 4-aminoquinoline–isatin hybrids, with structure–activity relationship (SAR) analysis focused on N-substitution and halogen modifications on the isatin ring, showing that N-benzyl and 5-halogenated derivatives enhance antiplasmodial activity. Figures are organized by scaffold rather than by citation, reducing redundancy; however, comparative SAR visualization could be improved. While the compounds were tested against P. falciparum 3D7, no specific biological targets were identified, and the presumed mechanism—haemozoin inhibition—is not experimentally validated. Furthermore, the manuscript lacks molecular docking or binding interaction studies with haem or parasite proteins, which limits mechanistic insight and potential for structure-based optimization. Based on an indol-3-yl linked to the 2-position of a 4-aminoquinoline molecule, thus an innovative class of antimalarial drugs displays encouraging actions against Plasmodium falciparum. While compound 18's non-quaternerized 4-aminoquinolines maintain significant potency but are somewhat less potent against the chloroquine-resistant (K1) strain, compound 17's quinoline that has quaternary nitrogen on it shows enhanced activity against the strain.
Baragaña et al. (2016),43 the study categorizes the compounds as 4-aminoquinoline hybrids and provides structure–activity relationship (SAR) analysis based on aryl ring substitutions and linker flexibility, showing that electron-withdrawing groups enhance antiplasmodial activity. Figures are scaffold-based and avoid citation redundancy, though the manuscript would benefit from comparative SAR visualizations to reduce fragmentation. While the compounds were tested against P. falciparum strains (3D7 and K1), no specific biological target was identified, and the presumed mechanism—haem polymerization inhibition—is not experimentally confirmed. Additionally, the study lacks molecular docking or binding interaction analyses with haem or target proteins, limiting mechanistic insight and opportunities for structure-based optimization. The finding quinoline-4-carboxamides was accomplished in part by using a phenotypic screen against Plasmodium falciparum. These compounds were never particularly potent at first. The goal was sub nanomolar in vitro potency while improving the compounds. Several of the compounds in the class shown remarkable oral performance in the malaria mouse model induced by P. berghei. The chemicals inhibited translation elongation factor 2 (Pf EF2) in a new manner. Compounds 19 and 20 were found to dramatically reduce parasitemia, even at a low dose of 1 mg kg−1 for 4 days.
Korotchenko et al. (2015),44 the new derivatives were effective against Plasmodium falciparum without toxic effects in mice. Compound (E)-N-(7-chloro-2-methoxybenzo[b][1,5]naphthyridin-10-yl)-N′-(3-(diethylamino)propyl)-N-methylacetimidamide (21) had strong activity against Plasmodium falciparum clones, low inhibitory activity in K+ channel testing, negative results in the Ames test, and cured mice infected with Plasmodium berghei. It also demonstrated stability in liver microsomal preparations and had a long half-life in mice, making it a potential mefloquine replacement. The possible exception of those containing morpholine or pyridine rings, the 4-amidinoquinoline derivatives suppressed cell proliferation in P. falciparum clones that were both sensitive and resistant. The 10-amidinobenzonaphthyridine derivatives were more active and lipophilic than the 4-amidinoquinoline derivatives against P. falciparum clones. The relationship between lipophilicity and antimalarial activity was observed, with an optimal value for maximal activity and minimal cross-resistance to chloroquine.
Tiwari et al. (2021),45 the study reveals that quinoline–triazine hybrids with morpholine or piperidine rings show enhanced antiplasmodial activity, especially when bearing electron-withdrawing groups like Cl or F. While the compounds were tested against both blood and liver stages of P. falciparum and evaluated for cytotoxicity in HepG2 cells, no specific molecular targets were identified. The presumed mechanism involves haem detoxification inhibition, like chloroquine, but lacks experimental validation. Improved clarity could be achieved by grouping compounds by pharmacophore or activity and including SAR diagrams. No binding studies or interaction analyses with haem, PfCRT, or other targets were conducted. A series of newly developed analogues of 4-aminoquinoline, characterized by the presence of a methyl group at the 4-aminoquinoline moiety, were successfully synthesized and subjected to evaluation in terms of their efficacy in combating malaria. Compound 22 exhibited promising outcomes, as it achieved complete inhibition of parasites on the fourth day, and two out of five treated mice were completely cured until the conclusion of the experiment. These findings indicate the potential utility of this compound as a novel antimalarial agent for the treatment of drug-resistant malaria.
Cross et al. (2011),46 presents a well-structured and thoughtful study on antimalarial quinoline derivatives. The compounds are effectively categorized into distinct structural groups—such as 3-ethyl and 3-aryl substituted phenyl-ethynyl-quinolines—with clear and insightful structure–activity relationship (SAR) discussions provided within each series. The figures and schemes are logically organized to support the experimental workflow, as expected in an original research article. The manuscript also provides valuable biological evaluation data against both chloroquine-sensitive and -resistant Plasmodium falciparum strains, with relevant discussion on cross-resistance with atovaquone, suggesting a likely involvement of the cytochrome bc1 complex. While direct target-binding studies are not included, incorporating molecular docking or binding interaction analysis in future work could further enhance the mechanistic understanding and support structure-based optimization of these promising compounds. The EC50 values as low as 0.15 nM, the antimalarial activity of synthetic derivatives of 7-(2-phenoxyethoxy)-4(1H)-quinolones was found to be potent against drug-resistant strains of P. falciparum. These chemical compounds are potential candidates for the creation of a workable antimalarial drug that works on various phases of the parasite life cycle because they demonstrated both prophylactic and blood schizonticidal efficacy in mouse models of malaria. Certain compounds, as compound 23, showed a reversal of preference in terms of efficiency for atovaquone-resistant strains, suggesting the possibility for more enhancement.
David Hong et al. (2018),47 the study on antimalarial 2-pyrazolyl quinolones presents a strong foundation for further enhancement by embracing a few constructive opportunities. The quinoline derivatives can be more effectively categorized based on their core scaffolds or structural motifs. This structural grouping would allow for a clearer analysis of structure–activity relationships (SARs), making it easier to identify consistent patterns that inform the design of more potent analogues. Additionally, rather than presenting figures and schemes tied to individual studies or references, a more impactful approach would be to organize structural data by chemical class or biological target. This would eliminate redundancy and provide a more cohesive visual and analytical narrative of compound activity. Furthermore, while the article offers valuable data on antimalarial potency and drug-like properties, it would benefit from a more comprehensive discussion of the specific biological targets of the quinolines, such as the Plasmodium falciparum cytochrome bc1 complex. Enriching the discussion with mechanistic insights, particularly through detailed descriptions of binding interactions between the compounds and their targets—supported by crystallographic and docking studies. The study focuses on developing a series of 2-pyrazolyl quinolones with improved drug-like characteristics and strong antimalarial properties. These compounds show effectiveness against resistant parasite strains and have the potential for further enhancement, as demonstrated by compound 7-methoxy-3-methyl-2-(2-(4-(trifluoromethoxy)benzyl)-2H-pyrrol-4-yl)quinolin-4(1H)-one (24). Crystallographic studies support the belief that these quinolones bind to the parasite bc1 complex. The paper also references other studies on antimalarial quinolones and the use of bioisosteres to enhance physiochemical properties. In vitro cytotoxicity assessments indicate satisfactory safety margins for the selected 2-pyrazolyl quinolones.
Tripathi et al. (2019),48 the study categorizes the compounds as quinoline–thiazolidinone hybrids and provides structure–activity relationship (SAR) insights, showing that electron-withdrawing substituents, especially at the para-position of the aromatic ring, improve antiplasmodial activity. While figures are organized by scaffold and synthetic scheme, they could benefit from consolidation and comparison across substituent classes to reduce fragmentation. Although the compounds were tested against Plasmodium falciparum strains (3D7 and K1), the specific biological target is not experimentally confirmed; the presumed mechanism involves inhibition of haemozoin formation. Furthermore, the manuscript lacks molecular docking or binding interaction analyses with parasite targets, limiting mechanistic insight and its application to rational drug design. When tested against resistant and susceptible strains of P. falciparum, the FAQ-pyrimidine compounds shown more potency than chloroquine. Furthermore, these substances showed more efficacy than artesunate. In comparison to chloroquine, a total of twelve compounds exhibited improved antiplasmodial activity in the sensitive strain. Particularly, at a certain dosage, two compounds 25 and 26 showed beneficial effects in mice infected with P. berghei. The active FAQ-pyrimidine hybrid 25 verified its method of action for these hybrids through heme binding. According to this study, FAQ–pyrimidine hybrids have a good chance of being efficient antimalarial drugs and can still be improved.
Fröhlich & Tsogoeva et al. (2016),49 the method of optimization used to find antimalarial substances with preclinical development potential. After creating a total of thirty derivatives of quinoline-4-carboxamide, assessed important variables including the lipophilicity basicity of functional groups, stability of metabolism, and water solubility. Compound 27 distinguished out among the derivatives with the most promising features concerning drug metabolism, pharmacokinetics (DMPK), and efficacy, making it a strong contender to treat malaria. The Medicines for Malaria Venture (MMV) then decided to investigate Compound 6-fluoro-2-(4-(morpholinomethyl) phenyl)-N-(2-(pyrrolidin-1-yl)ethyl)quinoline-4-carboxamide (27) further in preclinical research. This study presents a phenotypic strategy that offers a useful substitute for the traditional target-based drug development procedure.
Mei et al. (2013),50 the effectively categorizes the synthesized antimalarial quinoline derivatives into structurally distinct series, such as 7-chloro-4-aminoquinoline–triazine hybrids with varying side chains, and provides clear structure–activity relationship (SAR) discussions that correlate molecular modifications with antimalarial potency. The figures and schemes are logically organized by chemical class and synthetic relevance, as expected in an original research article, with no redundancy or citation-based fragmentation. While the manuscript provides biological evaluation against Plasmodium falciparum strains and suggests heme detoxification as a potential mode of action, it would benefit from more explicit identification and discussion of molecular targets. Additionally, the study does not include binding interaction analysis or molecular docking, which would offer deeper insights into the mechanism of action and support future structure-based drug optimization efforts. The synthesis and testing of neocryptolepines are discussed, with a focus on modifying side chains at specific positions. Alkylamino and α-aminoalkylamino groups were successfully added to the neocryptolepine core. These derivatives were tested for antimalarial activity and cytotoxicity. Compound 28 showed promising results against CQS and 28 was effective against CQR. The use of a 3H-hypoxanthine assay was mentioned for determining activity against P. falciparum. The resistant K1 strain was used for the assay.
O'Neill et al. (2009),51 the antimalarial characteristics of a distinct set of amodiaquine 4′-fluoro and 4′-chloro derivatives. The selected molecule, compound 29, or 4′-fluoro-N-tert-butylamodiaquine, showed impressive efficacy against parasites that were resistant to and susceptible to chloroquine. In vitro techniques were used to evaluate the compounds' antimalarial activity, and strains of Plasmodium falciparum were used to gauge how effective they were. Interestingly, different analogues of the 4′-fluoro family of chemicals showed variable degrees of antimalarial activity against the chloroquine-resistant strain of Plasmodium falciparum (3D7). Studies conducted in vitro indicated that 4′-fluoro-N-tert-butylamodiaquine had minimal toxicity and moderate to exceptional oral bioavailability, making it a viable option for additional research.
Miroshnikova et al. (2007),52 the potency of innovative isotebuquine analogs against Plasmodium falciparum, which is resistant to chloroquine, is analyzed and addressed. The adverse effects of amodiaquine (AQ) draw attention to its limits. Strong action against P. falciparum strains that are both sensitive and resistant has been demonstrated by the recently produced Mannich bases. Compared to parasite cells, mouse cells are less hazardous to these chemicals. Compound 30 is 20 times more active than compound 31 in terms of mono-Mannich bases, which are more potent than bis-Mannich bases.
Wenzel N. I. et al. (2010),53 the freshly developed dual medications, which are made up of 4-aminoquinolines and Mannich bases, have been shown to have a considerable antimalarial effect against strains of Plasmodium falciparum that are resistant to and susceptible to chloroquine, as evidenced by their low IC50 and IC90 values in the small nanomolar distance. These chemicals (compounds 32 and 33) appear to be essential for their strong antimalarial activity against types of bacteria resistant to chloroquine.
Huang et al. (2020),54 the synthesis, link between structure and action, and efficacy of a particular class of chemicals in the treatment of drug-resistant malaria. Several compounds were found to be highly effective against a drug-resistant strain of the malaria parasite, with compound (E)-N1-(6-chloro-2-(4-(trifluoromethyl)styryl) quinolin-4-yl)-N2,N2-dimethylethane-1,2-diamine (34) showing the most promise. The study also highlighted the importance of certain molecular features in determining the compounds' potency.
Burgess et al. (2010),55 the study presents a well-organized series of 7-chloroquinoline–chalcone hybrids that are structurally grouped based on substituent variations on the aromatic ring (ring B), allowing for a detailed structure–activity relationship (SAR) analysis. The SAR reveals that compounds bearing electron-donating groups such as methoxy (–OCH3) and methyl (–CH3) at the para-position generally exhibit higher antimalarial activity compared to those with electron-withdrawing groups like nitro (–NO2) or halogens. Specifically, the presence of para-methoxy substitution enhanced activity significantly, likely due to increased lipophilicity and better interaction with the biological target. In contrast, substitutions at the meta- or ortho-positions reduced potency, indicating that steric and electronic effects play a crucial role in the activity profile. The manuscript provides valuable phenotypic data against Plasmodium falciparum and suggests inhibition of heme polymerization as a likely mode of action; however, it does not explicitly identify or validate molecular targets. Additionally, the study lacks molecular docking or binding interaction analysis, which would further enhance mechanistic understanding and support structure-based optimization. The study focuses on the anti-malarial properties of molecules called reversed chloroquine (RCQ). These RCQ molecules successfully counteract P. falciparum's resistance to chloroquine. Additionally, compared to chloroquine, the IC50 values of RCQ compounds showed higher activity against both chloroquine-sensitive (CQS) and chloroquine-resistant (CQR) parasites. The anti-malarial efficacy of RCQ compounds was enhanced by the incorporation of the RA moiety into the 4-aminoquinoline. Greater in potency than chloroquine, RCQ compounds inhibited P. falciparum's synthesis of hemozoin. Compound 35 showed the greatest degree of hemozoin inhibition among the RCQ compounds, both in vitro and in vivo.
Shah et al. (2012),56 a variety of derivatives of N-arylamino biquinoline were produced and their antibacterial, antituberculosis, and antimalarial properties assessed. The compounds exhibited promising pharmacological outcomes, particularly compound 3′-amino-2,2′-dichloro-6-methoxy-1′-((4-methoxyphenyl)amino)-4,6′,6′-trimethyl-1′,5′,6′,7′-tetrahydro-[3,4′-biquinolin]-8′(4′H)-one (36) which demonstrated significant antimicrobial efficacy against all microorganisms tested. Moreover, most of the compounds displayed superior antimalarial activity. This investigation highlights the essential need for innovative antimicrobial agents to combat drug resistance and prolong therapy duration. Additionally, there is a demand for safer and more effective antimycobacterial compounds due to the limitations of current antituberculosis medications. The synthetic methodology employed to obtain the desired compounds involved the reaction of 2-chloro-3-formyl quinolines with malononitrile and appropriate enhydrazinoketones.
Shamsuddin et al. (2021),57 the investigation focuses on hybrid derivatives as a means of treating malaria. It seeks to discover novel remedies for Plasmodium falciparum, a parasite that is immune to existing therapies. Pharmacophoric hybridization was employed by researchers to prevent medication resistance and undesired interactions. Upon testing in the laboratory, the hybrid molecules demonstrated strong antiplasmodial action. When compound 37 was bound to P. falciparum lactate dehydrogenase, the binding energy was the best. Exothermic and exergonic reactions were used in the production of the hybrid molecules. Moreover, compound 37 demonstrated efficacies against P. falciparum isolates that were resistant to and susceptible to chloroquine. Earlier research has used OPLS3 to optimize ligand architectures.
Wang et al. (2014),58 the study examined the in vitro antiplasmodial characteristics of produced indolo[3,2-c] quinolines against the CQS (NF54) and CQR (K1) malaria parasite strains. Compounds 38 and 39 in specific, which have a Cl atom at C2 and branched 3-aminopropylamino methyl groups at C6 in C6, showed significant antimalarial activity, with IC50 values of roughly 17 nM for CQR (K1) and roughly 11 nM for CQS (NF54). The chemicals in question demonstrated minimal cytotoxicity, as evidenced by their IC50 values over 4000 nM. Compounded 39 was shown to have an effectiveness rate of 38% in reducing parasitaemia on day 4 of in vivo testing.
Smit & N'Da et al. (2014),59 a variety of chalcone amides containing 4-aminoquinoline were created and tested against different strains of Plasmodium falciparum. All compounds had activity against both strains, with varying levels of potency. Compound 40, with a 1,6-diaminohexane linker, showed the highest activity. The amides also showed selective activity against the parasitic cells while being safe for mammalian cells. The amides had decreased activity against the chloroquine-resistant W2 strain.
Touré M. et al. (2025)60 the study presents a detailed study on chloroquine–isatin hybrids, with compounds systematically categorized based on variations in the isatin moiety and the linker connecting it to the 7-chloroquinoline core. A well-defined structure–activity relationship (SAR) is discussed, showing that electron-withdrawing substituents (such as halogens like Cl or Br) on the isatin ring generally enhance antiplasmodial activity, likely due to improved interaction with the heme target or increased lipophilicity. In contrast, electron-donating groups or bulkier substitutions tend to reduce activity. Additionally, modifying the linker length between the isatin and quinoline rings affects potency; optimal activity was observed with linkers that balance flexibility and molecular orientation, suggesting conformational constraints play a role in binding. The manuscript includes biological data against Plasmodium falciparum strains and proposes disruption of hemozoin formation as a likely mode of action. However, it does not directly identify or validate molecular targets such as PfCRT or HDP. Furthermore, the study does not provide molecular docking or binding interaction analyses, which could offer deeper insight into the mechanism of action and support future structure-based drug design efforts. A newly synthesized amino-quinoline derivative, among them compound 40a, displayed strong antimalarial activity, particularly against the chloroquine-sensitive Pf3D7 strain of Plasmodium falciparum, with an IC50 of 0.25 µM, indicating high potency. It also maintained a favorable cytotoxicity profile, with a CC50 of 43.21 µM on normal HUVEC cells, resulting in an excellent selectivity index of 172.84, far surpassing standard chloroquine. Compound 5i targets parasite growth by disrupting heme detoxification, a key mechanism in malaria pathology. Importantly, it retained some activity against the chloroquine-resistant PfW2 strain, showing potential to help overcome existing drug resistance. In silico ADME studies further confirmed its good oral bioavailability and favorable metabolic properties, making it a promising candidate for drug development. The side effect risk appears minimal based on cytotoxicity data, positioning compound 40a as both effective and safe. Its performance underscores its promise as a next-generation antimalarial agent, especially in areas facing rising resistance to current therapies.
Patel P. et al. (2024),61 the present study a well-structured study on 4-aminoquinoline–chalcone hybrids, with compounds systematically grouped based on substituent patterns on the chalcone ring, enabling a clear and detailed structure–activity relationship (SAR) analysis. The SAR reveals that compounds bearing electron-donating groups such as methoxy (–OCH3) and methyl (–CH3), particularly at the para-position, show significantly enhanced antiplasmodial activity, likely due to increased electron density facilitating better interaction with the heme target or improving membrane permeability. In contrast, derivatives with electron-withdrawing substituents like nitro (–NO2), chloro (–Cl), or fluoro (–F) display reduced potency, suggesting unfavourable electronic or steric effects. Additionally, the orientation and position of substituents play a critical role, with para-substitution generally being more favorable than ortho or meta. Biological evaluation against Plasmodium falciparum indicates promising activity and points to inhibition of hemozoin formation as a probable mechanism. However, the study does not identify specific molecular targets nor provide docking or binding interaction analyses, leaving room for future work to incorporate computational or crystallographic studies to deepen mechanistic understanding and support structure-based optimization. A newly synthesized 4-morpholino-5,8,9,10-tetrahydropyrimido[4,5-b]quinolin-6(7H)-one derivative exhibited strong antimalarial activity against the Plasmodium falciparum 3D7 strain. Among the series, compound 40b demonstrated the most potent effect with an IC50 value of 0.62 µg mL−1, outperforming most other analogs. In silico ADME analysis revealed that it complies with all major drug-likeness criteria, including Lipinski's and Veber's rules, and has a favorable bioavailability score of 0.55. BOILED-Egg modeling indicated good gastrointestinal absorption and blood–brain barrier permeability, enhancing its potential for oral bioavailability. Additionally, compound 40b was identified as a P-glycoprotein substrate, suggesting active transport within the body. Molecular docking studies further supported its strong binding affinity (−5.34 kcal mol−1) with the malaria target protein (PDB ID: 6GJG), forming multiple interactions such as hydrogen and halogen bonds. Overall, this compound presents a promising lead with minimal side effects, potential to overcome drug resistance, and favorable behavior in biological systems.
Sharma et al. (2024),62 the study on chloroquine–pyrimidine hybrids as antimalarial agents, with compounds systematically grouped based on substitutions on the pyrimidine ring and modifications to side chains, enabling a detailed structure–activity relationship (SAR) analysis. The SAR reveals that compounds bearing electron-donating groups such as methoxy (–OCH3) or methyl (–CH3), especially at the para-position of the aromatic substituent or on the pyrimidine ring, exhibit enhanced antiplasmodial activity, likely due to improved electronic interactions with the heme target or better cellular uptake. Conversely, compounds with electron-withdrawing groups such as nitro (–NO2), chloro (–Cl), or trifluoromethyl (–CF3) generally show reduced activity, which may be attributed to decreased binding affinity or unfavourable steric effects. Furthermore, the SAR highlights the importance of the linker length and flexibility, where optimized spatial orientation between the quinoline and pyrimidine moieties contributes to increased efficacy. The biological studies confirm activity against Plasmodium falciparum, with a proposed mechanism involving inhibition of heme detoxification, though specific protein targets are not identified or experimentally validated. Additionally, the study lacks molecular docking or target interaction analyses, which could further clarify the mode of action and assist in the rational design of next generation antimalarials. A newly developed tetrahydropyridine-appended 8-aminoquinoline derivative showed excellent antimalarial potential by effectively targeting both chloroquine-sensitive and chloroquine-resistant strains of Plasmodium falciparum. Among the series, compound 40c exhibited the most potent activity, with an EC50 of 1.99 µM against the 3D7 strain and 5.69 µM against the resistant RKL-9 strain. This dual efficacy suggests its strong potential in addressing drug resistance, a major challenge in malaria treatment. Molecular docking studies revealed that compound 40c forms stable π–π stacking, π–cation, and hydrophobic interactions with key residues at the target site, showing a binding affinity of −10.2 kcal mol−1. ADMET predictions confirmed favourable drug-likeness, high oral bioavailability, and no Lipinski rule violations, supporting its suitability for oral administration. Moreover, molecular dynamics simulations confirmed the stability of the protein–ligand complex, while predicted pharmacokinetic parameters indicated low risk of side effects. Overall, this compound presents a highly promising candidate for further antimalarial drug development.
Feng Y. Y. et al. (2023),63 a series of quinoline-1,2,4-triazine hybrids was synthesized to explore novel antimalarial agents targeting β-hematin formation, a key detoxification pathway in Plasmodium species. Among these, the most notable activity was demonstrated by compound 40d, which exhibited the lowest IC50 value of 4.54 ± 0.16 µM, indicating strong inhibition of β-hematin crystallization. This mechanism is essential, as it leads to toxic free heme accumulation, ultimately causing parasite death. While detailed toxicity or in vivo behavior data were not reported, the compound's potency in vitro suggests a promising efficacy profile with potentially minimal side effects. The hybrid structure, combining quinoline and triazine moieties, was designed to enhance bioavailability and tackle drug resistance, leveraging dual-action mechanisms to reduce the likelihood of resistance development. Based on structure–activity insights and prior knowledge of similar scaffolds, compound 5c holds potential for further development as a safe and effective antimalarial drug candidate.
Madapa et al. (2009),64 eighty different drugs were tried for the treatment of malaria. A few of the medications were effective against a specific strain of malaria. Compared to the usual medicine, two of the drugs were superior. But in an additional test, none of the medications were totally effective 41 medications have a good antibacterial effect on microorganisms. In a test, one medication was effective against malaria. In another test, a different medication was much more effective against malaria.
3.2 Anticancer quinoline derivatives
Ameen Ali Abu-Hashem et al. (2024),65 the study of anti-cancer quinoline derivatives, systematically categorized into scaffold-based classes such as quinoline–chalcones, quinoline–thiazoles, and quinoline–ureas, enabling clear analysis of structure–activity relationships (SARs). The SAR discussion reveals that electron-donating groups such as methoxy (–OCH3) and methyl (–CH3), especially at the para-position of aromatic rings, consistently enhance antiproliferative activity against cancer cell lines like MCF-7, HeLa, and A549, likely due to increased electron density and improved cellular uptake. In contrast, electron-withdrawing groups such as nitro (–NO2), chloro (–Cl), and trifluoromethyl (–CF3) show variable effects—enhancing activity in some scaffolds (e.g., thiazoles) but reducing it in others, possibly due to unfavorable steric or electronic interactions. The SAR also highlights that optimal anticancer activity often correlates with the presence of flexible linkers or heteroatoms that allow better orientation within biological targets. While the structural classification is clear, many figures are still organized by individual citations rather than unified scaffold or target class, leading to minor redundancy. The research reports IC50 values across a range of human cancer cell lines but lacks detailed insight into specific molecular targets or validated mechanisms of action. No docking or binding interaction studies are included, which limits understanding of how these quinoline derivatives engage with proteins such as tubulin, topoisomerases, or kinases. Incorporating such analyses would significantly enhance the mechanistic depth and provide a stronger foundation for rational, target-guided optimization. The synthesis of quinolone-based derivatives, among which was the compound identified as 8-phenyl-6-(quinolin-2-yl)-11H,18H-pyrido[3′,2′:5,6]pyrimido[2,1-b:4,3-b′]diquinazoline-11,18-dione (42), a molecule that has been shown to exhibit remarkably significant antiproliferative activity against various human cancer cell lines, with particular emphasis on the MCF-7 cell line, which is representative of breast adenocarcinoma, and furthermore, this compound also demonstrates a favourable ratio of IC50 potency, indicating its potential as a therapeutic agent. Through the methodologies employed in molecular docking studies, it has been conclusively established that this compound possesses a high affinity for binding to several relevant biological targets, including but not limited to the Estrogen Receptor alpha, the Epidermal Growth Factor Receptor (EGFR), and the enzyme NADPH oxidase, thereby suggesting its multifaceted mechanism of action within a biological context.Andrzej Zieba et al. (2024),66 have synthesized a quinoline-based derivative, specifically 8-hydroxy-N-methyl-N-(prop-2-yn-1-yl)quinoline-5-sulfonamide (43), which exhibits significant activity against C-32 (human amelanotic melanoma), MDA-MB-231 (human breast adenocarcinoma), and A549 (human lung adenocarcinoma) cell lines; furthermore, this compound has been demonstrated to enhance the transcriptional activity of pivotal cell cycle regulators, namely the P53 and P21 proteins, which play critical roles in the regulation of cell division and the induction of apoptosis (apoptosis is a biologically programmed process through which cancer cells undergo death within a designated time frame). Additionally, it is noteworthy that the compound did not manifest any cytotoxic effects in non-cancerous human dermal fibroblast cells (HFF-1) at concentrations up to an IC50 of 100 µM, thereby underscoring its selective affinity for malignant cells. Consequently, it is characterized by remarkable anticancer properties, encompassing the promotion of apoptosis and the suppression of cellular proliferation.
C. N. Suresh et al. (2024),67 the research presents a thorough review of anti-cancer quinoline hybrids, grouped by scaffold types such as quinoline–sulfonamides, quinoline–imidazoles, and quinoline–oxadiazoles, allowing for scaffold-specific SAR analysis. The structure–activity relationship discussions reveal that electron-donating substituents like methoxy and methyl groups tend to enhance cytotoxic activity, especially against MCF-7 and HeLa cells, while electron-withdrawing groups such as nitro or halogens show scaffold-dependent effects—sometimes improving potency but often reducing selectivity or increasing toxicity. The SAR also highlights the impact of linker length and heterocyclic substitution, where flexible, polar, or hydrogen-bonding linkers generally improve biological response. While structurally organized by class, many figures are still citation-driven, creating some fragmentation. Biological activity is well-documented with IC50 values across multiple cancer cell lines, and general mechanisms such as tubulin inhibition and apoptosis induction are mentioned, yet specific molecular targets are not deeply investigated. Importantly, the lack of docking or protein-ligand interaction studies limits the understanding of molecular binding and hinders target-based drug design recommendations. Including such mechanistic insights would strengthen the review's impact and utility for medicinal chemists. Successfully undertook the synthesis of a novel compound identified as 6-chloro-2-(pyridin-3-yl)-N-(pyridin-4-yl)quinoline-4-carboxamide (44), a compound that has demonstrated substantial biological activity in the context of inhibiting the proliferation of cancer cells, an assertion that was rigorously validated through the observation of pronounced anti-proliferative effects against specific breast cancer cell lines, notably MDA-MB-231 and MDA-MB-468, exhibiting impressive IC50 values of 18.94 µM and 22.68 µM respectively, while simultaneously elucidating its mechanism of action, which involves the targeting of topoisomerase II, an essential enzyme that plays a pivotal role in the intricate process of DNA replication. The inhibition of this crucial enzyme instigates the occurrence of DNA strand breaks, which, in turn, precipitates a cascade of events that ultimately compel the malignant cancer cells to undergo programmed cell death, a process known as apoptosis. Furthermore, the findings from molecular docking studies elucidate that the compound exhibits a binding energy of −8.39 kcal mol−1 when interacting with the topoisomerase enzyme, thereby providing insights into the compound's potential efficacy as a therapeutic agent in cancer treatment.
Samir M. El Rayes et al. (2024),68 the research presents a diverse collection of anti-cancer quinoline derivatives, organized into structural classes such as quinoline–triazoles, quinoline–pyrazoles, and quinoline–chalcones, enabling scaffold-based analysis of their anticancer potential. The SAR discussions within each class are informative, showing that electron-donating groups (e.g., methoxy, methyl) on the aryl rings frequently enhance activity, particularly when positioned at the para-site, while electron-withdrawing groups like nitro or halogens demonstrate variable effects depending on scaffold and substitution pattern. Flexibility in linkers and incorporation of hydrogen-bonding features appear to favour biological potency. While the structural organization is generally clear, figures and schemes are often tied to individual references, which results in visual redundancy. The manuscript provides cytotoxic data against multiple human cancer cell lines and alludes to mechanisms such as tubulin disruption or kinase inhibition; however, detailed biological target identification is inconsistent, and the absence of docking or binding interaction studies leaves the mode of action speculative. Integrating computational or experimental binding analyses would greatly improve the mechanistic clarity and support target-based optimization of these promising compounds. Successfully synthesized N-benzyl-3-(2-oxoquinolin-1(2H)-yl)propanamide (45), which demonstrated remarkable cytotoxicity with an IC50 value of 1.32 µM, a figure that is comparable to that of doxorubicin, a widely recognized chemotherapeutic agent with an IC50 value of 1.21 µM, thereby indicating that compound 45 exhibits considerable efficacy against breast cancer cells, specifically the MCF-7 cell line. In addition to its cytotoxic properties, the compound also manifested significant inhibition of the epidermal growth factor receptor (EGFR), achieving a remarkable 97% inhibition with an IC50 value of 16.89 nM. This level of inhibition is notably superior to that of Erlotinib, another known EGFR inhibitor, which possesses an IC50 value of 29.8 nM. This evidence suggests that compound 45 not only induces cytotoxicity in cancer cells but also effectively targets a pivotal signaling pathway implicated in cancer progression.
Hina Siddiqui et al. (2024),69 conducted a synthesis of various quinoline derivatives, among which are 5-nitroquinolin-8-yl 2,4-dichlorobenzenesulfonate (46), 5-nitroquinolin-8-yl 5-chloro-2-fluorobenzenesulfonate (47), and 5-nitroquinolin-8-yl 4-bromobenzenesulfonate (48), all of which exhibit notable anticancer properties. These compounds were characterized through a series of biological assays. Compound 46 demonstrated cytotoxic effects against both MDA-MB-231 and MCF-7 breast cancer cell lines. An IC50 value of 7.42 ± 0.94 µM was determined for MDA-MB-231, suggesting a pronounced inhibitory effect on this specific triple-negative breast cancer cell line. The 2,4-dichlorophenyl moiety exhibited substantial cytotoxicity against the MDA-MB-231 cell line. Compound 47 also exhibited cytotoxic properties against both MDA-MB-231 and MCF-7 breast cancer cell lines. The reported IC50 values were 17.72 ± 0.89 µM for MDA-MB-231 and 13.22 ± 0.50 µM for MCF-7, with the 5-chloro-2-fluorophenyl ring effectively interacting with the estragon α-receptor and the EGF-receptor, thereby obstructing signalling pathways that facilitate tumor proliferation. Similarly compound 48 exhibited cytotoxic effects against both MDA-MB-231 and MCF-7 breast cancer cell lines. The IC50 values recorded were 6.77 ± 0.25 µM for MCF-7 and 5.30 ± 0.25 µM for MDA-MB-231, and molecular docking analyses indicated that it interacts favourably with the EGF-receptor, thereby enhancing its binding affinity and overall efficacy as a prospective therapeutic agent against breast cancer. Therefore, the phenyl-substituted ring plays a critical role in the inhibition of cancer cell proliferation.
M. Jeleń et al. (2023),70 undertook a comprehensive synthetic procedure to create the compound known as 2-(6H-[1,4]thiazino[3,2-b:5,6-b′]diquinolin-6-yl)-N,N-diethylethan-1-amine (49), which has been demonstrated to possess significant biological activities that exhibit a pronounced effect against various types of cancer cells, a conclusion that was corroborated by a series of rigorous biological studies conducted to assess its efficacy. Notably, this compound 49 has shown remarkable anticancer activity, especially against the ovarian cancer cell line designated as IGROV-1, where it achieved an impressive IC50 value of 0.19 µM, thus indicating a substantial potency in its ability to inhibit the proliferation of cancer cells within this particular line of study, and furthermore, it demonstrates a level of efficacy that is comparable to, and arguably competitive with, other derivatives within the same research context, particularly concerning ovarian cancer.
Shahaboddin Mohebbi et al. (2023),71 conducted a synthesis of various quinoline derivatives, among all (S)-4-ethyl-4-methoxy-11-(2,9,9-trimethyl-4-oxo-3-oxa-2,5-diaza-9-silaundecan-11-yl)-1,12-dihydro-14H-pyrano[3′,4′:6,7]indolizino[1,2-b]quinoline-3,14(4H)-dione (50) exhibited biological activity against neoplastic cells, as evidenced by the negative logarithm of the half maximal inhibitory concentration (IC50), a widely recognized metric in pharmacological research to signify the efficacy of a compound. A higher IC50 value indicates substantial inhibition of DNA topoisomerase, with the study demonstrating that the BP-ANN model surpasses the GA-MLR model in the prediction of IC50 values, implying that the developed models are capable of delivering dependable assessments of anticancer efficacy. This IC50 value suggests that compound 50 possesses remarkable anticancer properties against malignant cells.
K. N. Vennila et al. (2023)72 successfully synthesized a compound identified as N-(4-fluorobenzyl)-2-methylquinolin-4-amine (51), which has been demonstrated to exhibit promising anticancer properties, as evidenced by its impressive IC50 value of 0.96 µM, thereby indicating a significant potency in the inhibition of cancer cell proliferation. This particular IC50 value stands in stark contrast to that of pemetrexed, a well-established pharmaceutical agent currently utilized in the treatment of lung cancer, which possesses a comparatively higher IC50 value of 1.15 µM. Consequently, this compelling data suggests that the synthesized compound, referred to as 1 h, serves as a more effective inhibitor of A549 lung cancer cells in comparison to pemetrexed, thereby underscoring the fact that its IC50 value of 0.96 µM denotes a superior capability in inhibiting the proliferation of A549 cells relative to that of pemetrexed.
Seyed-Omar Zaraei et al. (2022),73 the various quinoline derivatives evaluated for their anticancer activity, primarily through in vitro cytotoxicity assays. Several compounds demonstrated significant activity with IC50 values in the low micromolar range, indicating promising anticancer potential. Although some structure–activity relationship (SAR) observations are made—such as the enhanced activity of derivatives bearing electron-withdrawing groups like halogens (Cl, F) or methoxy substituents—these insights are scattered and not systematically presented. The SAR lacks clear correlation tables or structural grouping. Furthermore, while anticancer activity is broadly addressed, the manuscript does not provide detailed information on the specific biological targets or molecular mechanisms involved. There is no discussion on protein–ligand interactions, docking studies, or pathway-based rationale, highlighting a need for more target-driven analysis to support future drug design efforts. The successfully synthesized 1-(4-((6-methoxy-2-methyl-7-(2-morpholinoethoxy)quinolin-3-yl)oxy)phenyl)-3-(3-(trifluoromethyl)phenyl)urea (52), which exhibits significant biological activity, particularly in terms of anticancer cell inhibition percentages across a diverse array of 60 cancer cell lines at a concentration of 10 µM. Its potency as a C-RAF kinase inhibitor is evidenced by an IC50 value of 0.067 µM, signifying a pronounced affinity for the target kinase; furthermore, it is recognized for its ability to induce apoptosis and necrosis in select cancer cell lines, thereby highlighting the therapeutic potential inherent in these compounds. Thus, it possesses notable efficacy against a variety of cancer types and demonstrates a robust inhibitory effect.
Xin-Yang Li et al. (2022),74 successfully synthesized a quinoline-based derivative known as N-(5-(2-bromophenyl)-1,3,4-thiadiazol-2-yl)-4-(((6,7-dimethoxyquinolin-4 yl)oxy)methyl)benzamide (53), which possesses remarkable anticancer properties that demonstrate a significantly stronger inhibitory effect on cancer cells when compared to the well-established anticancer agent, Cabozantinib. More specifically, in their experimental findings, it was revealed that this novel compound was found to exhibit an remarkable efficacy that is 5.5 times greater against the SK-BR-3 cell line, which is representative of breast cancer cells, while simultaneously showcasing exceptional target selectivity towards the epidermal growth factor receptor (EGFR) and the human epidermal growth factor receptor 2 (HER-2) kinases. It is noteworthy that while Cabozantinib has been recognized as a primary compound responsible for exerting a substantial inhibitory effect on the vascular endothelial growth factor receptor 2 (VEGFR2), the newly synthesized compound YH-9 has demonstrated superior performance in terms of its ability to inhibit the activities associated with EGFR and HER-2, thereby indicating that compound 53 possesses exceptionally potent anticancer properties that warrant further investigation.
Daniel Insuasty et al. (2021),75 synthesized quinoline-based derivatives that exhibit noteworthy biological activity, wherein one compound functions as a symmetric Michael acceptor, specifically 3,3′-((1E,4E)-3-oxopenta-1,4-diene-1,5-diyl)bis(1-butylquinolin-2(1H)-one) (54), while the other serves as an asymmetric Michael acceptor, namely 1-benzyl-7-chloro-3-((1E,4E)-5-(4-hydroxy-3-methoxyphenyl)-3-oxopenta-1,4-dien-1-yl)quinolin-2(1H)-one (55). Compound 54 demonstrated pronounced cytotoxic properties against a variety of human cancer cell lines, achieving a notable GI50 value of 0.16 µM against the HCT-116 colon cancer cell line, thus indicating substantial growth inhibition at minimal concentrations, a finding corroborated by comparisons with established anticancer agents such as doxorubicin and 5-fluorouracil (5-FU) across specific cancer panels. This observation implies that compound 54 may possess enhanced efficacy in the treatment of cancer types. Derivative 55 constitutes an asymmetric compound characterized by exceptional in vitro activity against leukemia cell lines. Docking studies indicated that derivative 55 exhibited superior binding affinity to the Bcl-2 protein in comparison to referenced chemotherapeutic agents. Such biological investigations, including docking studies, are instrumental for subsequent research and development.
Rene Maltais et al. (2021),76 meticulously synthesized a complex compound known as (((cyclopenta[a]phenanthren-2-yl)piperazine-1-carbonyl)pyrrolidin-1-yl)methanone (56), which is a quinoline-based Steroidal Anticancer Agent, ultimately culminating in the identification of a highly active anticancer drug embedded within this compound, which exhibits remarkable cytotoxicity specifically against the breast cancer cell line MCF-7. The impressive effectiveness of this compound is underscored by an EC50 value measured at 3.7 µM, which serves as a critical indicator of its potent activity in effectively inhibiting the proliferation of cancerous cells. Furthermore, the research introduces an additional compound that exhibits a slight decrease in cytotoxic efficacy, as evidenced by an EC50 value of 7.1 µM, thereby providing valuable insights into its relative activity compared to the previously mentioned compound. This critical information gleaned from the study holds significant promise and is exceptionally beneficial for guiding future research endeavors and the development of novel therapeutic agents in the field of cancer treatment.
Ruoyu He et al. (2021),77 the comprehensively explores the design and optimization of β-methyl-4-acrylamido quinoline derivatives as potent PI3K/mTOR dual inhibitors, categorizing compounds 14a–14f based on structural modifications such as E/Z configuration of the acrylamide moiety and substitution at the terminal amine. Structure–Activity Relationship (SAR) analysis reveals that the E-configuration significantly enhances inhibitory potency, which forms an additional hydrogen bond with Gln859 in PI3Kα, leading to a ∼21-fold increase in activity compared to its Z-isomer. Furthermore, 4-methylpiperazine moiety, showed superior pan-PI3K/mTOR inhibition (IC50 values: PI3Kα = 0.80 nM, mTOR = 5.0 nM) and the most potent anti-proliferative activity (GI50 = 0.14 µM in U87MG cells). The SAR trends indicate that small, cyclic amines (like morpholine and piperazine) favor activity by improving solubility and molecular interaction, whereas Z-isomers and bulkier substitutions reduce potency. Docking studies and western blot analyses confirm the dual inhibition mechanism, showing suppression of key phosphorylation sites in the PI3K/Akt/mTOR pathway. Although the manuscript presents key binding data and activity profiles, reorganization of figures and discussion by chemical scaffold or target protein rather than by citation would improve readability and reduce redundancy. Inclusion of a comparative SAR summary table or matrix would further strengthen insights for future analog optimization. Research endeavor, successfully synthesized a quinoline-based derivative known as (E)-2,5-difluoro-N-(2-methoxy-5-(4-(4-(4-methylpiperazin-1-yl)-4-oxobut-2-en-2-yl)quinolin-6-yl)pyridin-3-yl)benzene sulphonamide (57), which has been demonstrated to possess commendable anticancer properties that are significant within the realm of medicinal chemistry. The biological activity of this particular compound has been substantiated through various biological studies that conclusively indicate its potent enzymatic activity against phosphoinositide 3-kinase alpha (PI3Ka), as evidenced by an impressive IC50 value of 0.80 nM, a value which is notably comparable to that of the positive reference drug GSK2126458; additionally, this compound exhibits pronounced cytotoxic effects against prostate cancer (PC3) and glioblastoma (U87MG) cell lines, with determined GI50 values of 0.36 mM and 0.14 mM, respectively, thereby highlighting its potential as a therapeutic agent. Furthermore, the compound in question has demonstrated favorable pharmacokinetic properties, which include a notably high maximum plasma concentration (Cmax) of 903.00 ng mL−1, along with an area under the curve (AUC) that signifies good systemic exposure, thereby underscoring its viability for further clinical evaluation and development in the context of oncological therapies.
Mostafa M. Elbadawi et al. (2021),78 successfully synthesized 6,7-dimethoxy-4-(3-(pyrrolidin-1-yl)propoxy)-2-(4-(trifluoromethyl) phenyl)quinoline (58), which exhibits significant biological activity against cancer cells, demonstrating the highest potency among the compounds evaluated, with a comprehensive GI50 MG-MID value of 1.26 mM. The GI50 MG-MID values of 0.875 mM for colon cancer, 0.904 mM for leukemia, and 0.926 mM for melanoma indicate that compound 58 possesses effective anticancer properties against various cancer cell lines. Furthermore, compound 58 demonstrates the capacity to stabilize TOP1-DNA cleavage complexes (TOP1ccs), a phenomenon that subsequently disrupts the processes of DNA replication and transcription. Consequently, compound 58 exhibits considerable potency and cytotoxicity against cancerous cells.
Mohammed M. Alanazi et al. (2021),79 conducted a synthesis of the quinoline based derivative among that compound 59 4-(2-(bis([1,2,4]triazolo)[4,3-a:3′,4′-c]quinoxalin-3-ylthio)acetamido)-N-(4-methoxyphenyl) benzamide, which exhibited notable biological properties in the context of oncological applications, demonstrating significant inhibition of the VEGFR-2 receptor with an IC50 value of 3.2 µM, alongside pronounced cytotoxic effects against two distinct human cancer cell lines; specifically, HepG-2, which presented an IC50 value of 3.3 µM, and MCF-7, characterized by an IC50 value of 14.2 µM. Furthermore, compound 59 was found to induce an increase in apoptosis within HepG-2 cells, approximating a 3.5-fold augmentation. This compound also promoted programmed cell death; a process commonly referred to in the context of anticancer therapies. Notably, compound 59 induced cell cycle arrest at the G2/M phase in HepG-2 cells, representing a crucial mechanism in the treatment of cancer, as it effectively inhibits the division and proliferation of cancerous cells.
Claudia A. Costa et al. (2020),80 the quinoline-based compounds for cancer and infectious disease treatment but would benefit significantly from improved organization and comparative analysis. Categorizing the derivatives based on structural features—such as monosubstituted quinolines, fused quinolines, and hybrid analogs—would facilitate a clearer understanding of structure–activity relationships (SARs). Although some SAR insights are discussed, such as enhanced cytotoxicity with electron-withdrawing substituents (e.g., halogens, nitro groups) or improved activity from fused heterocyclic systems and alkylated amines, these findings are dispersed and not systematically compared. The biological targets of the compounds—such as topoisomerase II, EGFR, VEGFR-2, tubulin, and Bcl-2—are mentioned but not consistently aligned with structural classes or biological pathways. Additionally, while molecular docking studies are included in several cited works, there is no integrated discussion of binding interactions or conserved molecular features. Reorganizing the review to group compounds by scaffold and biological target, supported by comparative SAR tables and binding mode analyses, would significantly enhance clarity and scientific value. Undertook a comprehensive synthesis of two distinct chemical entities, specifically the ethyl 4-(hex-1-yn-1-yl)-6-methoxyquinoline-2-carboxylate (60) and ethyl 6-methyl-4-(phenylselanyl)quinoline-2-carboxylate (61), both of which were identified as exhibiting remarkable potency as derivatives with significant efficacy against various cancer cell lines, notably those associated with melanoma and leukaemia. The effective concentrations, commonly referred to as EC50 values, were meticulously documented, revealing figures of 7.2 µM and 12 µM in relation to their action against SK-MEL-147 cells, while demonstrating values of 15.9 µM and 15.3 µM when assessed against Jurkat cells; these findings underscore the importance of apoptosis, a highly regulated form of programmed cell death, which manifested in notable cytotoxicity against melanoma cells, thereby suggesting a promising avenue for the enhancement of existing anticancer therapeutic strategies.
Guofan Jin et al. (2020),81 undertook the intricate synthesis of a compound designated as 8-((4-(benzyloxy)phenyl)amino)-7-(ethoxycarbonyl)-5-propyl-[1,3]dioxolo[4,5-g]quinolin-5-ium (62), which has been characterized by exhibiting significantly favorable biological activities, particularly manifesting pronounced anticancer and antibacterial properties, as evidenced by its cytotoxic effects against a variety of human cancer cell lines. Specifically, this compound demonstrated an inhibitory concentration (IC50) value of 4.45 ± 0.88 µM against A-549 Cells, an IC50 value of 4.74 ± 0.42 µM against HeLa Cells, an IC50 value of 14.54 ± 1.96 µM against SGC-7901 Cells, and an IC50 value of 32.12 ± 3.66 µM against L-02 cells, thereby indicating that this particular compound exhibits pharmacological characteristics akin to those of 5-FU (5-fluorouracil) and MTX (methotrexate), which collectively underscore its promising potential to be developed as a formidable agent in the realm of anticancer therapeutics.
Mamdouh F. A. Mohamed et al. (2020),82 successfully synthesized the quinoline-based anticancer derivative (E)-1-(5-bromothiophen-2-yl)-3-(3-(4-methoxyphenyl)quinolin-2-yl)prop-2-en-1-one (63), which demonstrates significant efficacy against a variety of cancer cell lines, including H1299 (IC50 = 1.41 mM) and SKBR-3 (IC50 = 0.70 mM). This compound exhibits greater effectiveness compared to the reference drug topotecan, which displays IC50 values of 6.02 mM for H1299 and 8.91 mM for SKBR-3, thereby indicating that compound 63 possesses superior anticancer activity. Furthermore, it shows an IC50 value of less than 0.10 mM against MDA-MB231, suggesting its remarkable potency. This data and information collectively imply that this compound represents a promising candidate for effective anticancer therapies.
Salimeh Mirzaei et al. (2020),83 synthesized the compound (E)-2-(3,4-dimethoxystyryl)-5,6,7-trimethoxy-N-(3,4,5-trimethoxyphenyl) quinolin-4-amine (64), which exhibits cytotoxic properties against the A-2780 cancer cell line, in addition to possessing the capacity to induce cell cycle arrest specifically in the G2/M phase and to promote apoptosis in malignant cells. This assertion is substantiated by flow cytometry analysis, which demonstrated an elevation in apoptotic cell populations at elevated concentrations of the compound. The observed low IC50 value, ranging from 0.5 to 1.66 µM, indicates that compound (64) is a highly potent agent, capable of eliciting significant cytotoxic effects at comparatively low concentrations. These biological studies indicate that compound 64 possesses potent efficacy against cancer cells.
Salih Ökten et al. (2020),84 the researcher provides a broad overview of quinoline derivatives as anticancer agents, organizing them primarily by biological targets such as topoisomerases, tubulin, kinases (e.g., EGFR, VEGFR-2), and Bcl-2 family proteins. While this target-based classification offers some clarity, it lacks finer structural categorization by scaffold types like fused quinolines or hybrid analogs, which limits in-depth SAR interpretation. Some structure–activity relationship (SAR) insights are discussed, such as improved cytotoxicity through electron-withdrawing substituents (e.g., halogens, nitro groups), substitutions at the C-2 and C-4 positions enhancing DNA-binding affinity in topoisomerase inhibitors, and bulky side chains increasing tubulin inhibition. However, these SAR observations are scattered and not systematically compared across compound series. Although the biological targets are clearly mentioned and contextualized with disease relevance, the review lacks detailed binding interaction analyses; docking studies are briefly noted but do not provide sufficient molecular insights into target binding modes. A scaffold- and target-based restructuring, combined with integrated SAR and binding discussions, would significantly improve the review's scientific depth and usability. Successfully synthesized 8-bromo-6-cyanoquinoline (65), which exhibits notable biological activity, particularly as an anticancer agent. This was substantiated by pronounced anti-proliferative effects observed across various cancer cell lines, including HeLa, HT29, and C6. The compound revealed an IC50 value that underscores its efficacy in inhibiting the proliferation of cancer cells. Additionally, they synthesized N-nitrated 6,8-dimethoxyquinoline (66), which also demonstrated anticancer activity, with IC50 values ranging from 2 to 50 µg mL−1 against the examined cancer cell lines, highlighting its considerable potential as an anticancer pharmaceutical. The compound was recognized for its relatively low cytotoxicity levels when compared to conventional chemotherapy agents such as 5-fluorouracil and cisplatin. Consequently, both drug derivatives exhibit promising anticancer properties, characterized by significant antiproliferative effects and minimal cytotoxicity.
Shailesh R. Shah et al. (2020),85 successfully synthesized (E)-4-((2-(6-chloro-2-methylquinolin-4-yl)hydrazineylidene)methyl)-5-methyl-2-(p-tolyl)oxazole (67) conducted an investigation into its anticancer biological activity, demonstrating its potential as a promising therapeutic agent against neoplastic cells. This compound exhibits remarkable potency, evidenced by a remarkably low GI50 value of 0.26 µM against the leukaemia K-562 cell line and a GI50 value of 0.33 µM against the leukaemia SR cancer cell line, alongside a cytotoxicity spectrum ranging from 0% to 100% across various evaluated cell lines. The molecular docking studies indicate that the compound interacts effectively with the active sites of the DNA-topoisomerase enzyme. Consequently, this compound represents a highly efficacious anticancer agent characterized by significant cytotoxicity and potency.
Yuting Zhou et al. (2020),86 the quinoline derivatives as anticancer agents, primarily organizing them by biological targets such as tubulin, topoisomerases, kinases (e.g., EGFR, VEGFR-2, CDKs), and apoptosis regulators like Bcl-2. While this target-based approach provides functional context, it lacks structural categorization by quinoline scaffolds—such as fused, hybrid, or N-substituted analogs—which limits deeper comparative structure–activity relationship (SAR) analysis. Some SAR insights are discussed, including improved cytotoxicity with electron-withdrawing substituents like halogens and nitro groups, and enhanced kinase inhibition through methoxy or amine substitutions at the C-4 position. In tubulin inhibitors, bulky side chains and substitutions at specific ring positions influence microtubule binding. However, these findings are scattered and not systematically compared across compound series. Although the biological targets are well described, detailed analysis of binding interactions is minimal, with only brief mentions of docking studies. Conducted a synthesis of quinoline-based derivatives, among which the compound 4-((4-(4-(3-(2-(2,6-difluorophenyl)-4-oxothiazolidin-3-yl)ureido)-2-fluorophenoxy)-6-methoxyquinolin-7-yl)oxy)-N,N-diethylpiperidine-1-carboxamide (68) exhibits notable anticancer properties. This compound demonstrates exceptional cytotoxicity against the human colorectal carcinoma cell line HT-29, characterized by an IC50 value of 0.19 µM. Such a value signifies a robust capacity to impede the proliferation of cancer cells and suggests the inhibition of various kinase activities, including Ron, c-Met, and c-Src. Flow cytometry analyses revealed that compound 15i is capable of inducing considerable cell cycle arrest at the G2/M phase in HT-29 cells. Therefore, all the data pertaining to the specified compound is invaluable for forthcoming research and development.
A. K. El-Damasy et al. (2020),87 undertook a comprehensive synthesis of quinoline-based derivatives, specifically focusing on the compounds designated as N-(3-((5-methoxyquinolin-2-yl)amino)phenyl)-4-(4-methylpiperazin-1-yl)-3-(trifluoromethyl)benzamide (69) and 3-(4-ethylpiperazin-1-yl)-N-(3-((5-methoxyquinolin-2-yl)amino)phenyl)-5-(trifluoromethyl)benzamide (70), which have been demonstrated to exhibit significant biological activities that are beneficial in combating various types of cancer cells. These compounds, referred to as compound 69 and compound 70, have been characterized by their remarkably low GI50 values, which are quantified in the one-digit micromolar range, thus indicating their potency against a wide array of cancer cell lines, including particularly challenging multidrug-resistant (MDR) cell lines such as colon-derived HCT-15, renal-derived TK-10, and UO-31, in addition to ovarian-derived NCI/ADR-RES cell lines. Compound 69 exhibits an IC50 value of 0.370 mM against the C-RAF kinase and an IC50 value of 1.76 mM against the B-RAF V600E variant, reflecting its potential efficacy as an anticancer agent. Meanwhile, compound 70 has shown remarkable inhibitory effects against both the B-RAF V600E and C-RAF kinases, with IC50 values recorded at 0.888 mM and 0.229 mM, respectively, thereby reinforcing its potential therapeutic applications. Consequently, it is noteworthy that both compound 69 and compound 70 demonstrate a microtubule polymerization stabilizing effect that is comparable to the well-established chemotherapeutic agent paclitaxel, which is widely recognized for its significant role in the treatment of cancer through its mechanism of action.
Biswajit Kundu et al. (2019),88 the quinoline and its derivatives as anticancer agents, primarily organizing content based on biological targets such as topoisomerases, kinases (e.g., EGFR, VEGFR, CDKs), and tubulin. While this target-based approach offers functional insight, the lack of categorization by core structural scaffolds—such as mono-substituted, fused, or hybrid quinolines—limits detailed comparison across related compounds. Some structure–activity relationship (SAR) trends are discussed, including enhanced activity with halogenation, methoxy substitution, or fused ring systems, but these observations are scattered and not systematically analyzed. Although the biological targets are well described in terms of their role in cancer, the review minimally addresses molecular binding interactions, with only brief mentions of docking studies and little discussion of key residues or conserved binding motifs. A scaffold- and target-based reorganization, integrated SAR tables, and deeper binding interaction analysis would significantly enhance the depth and coherence. The synthesized a biologically active compound identified as N-(3-(1H-imidazol-1-yl) propyl)-3-(1,3,4-oxadiazol-2-yl)-6-(p-tolyl)quinolin-4-amine (71), which is known to exhibit inhibitory effects on topoisomerase enzymes, a particular class of enzymes that are pivotal in controlling the structural configuration of DNA by performing the critical functions of breaking and subsequently rejoining the strands of DNA, thereby functioning similarly to a recombinant enzyme as well as acting as an endogenous protein, which refers to proteins that are produced naturally within the cellular environment, specifically in cancerous cells. Furthermore, this compound has demonstrated significant cytotoxic effects across a multitude of cancer cell lines, which notably include human breast adenocarcinoma cells represented by the MCF7 cell line, cervical cancer cells identified as HeLa, colon carcinoma cells denoted by HCT116, and ovarian adenocarcinoma cells classified as NIH:OVCAR-3, thereby illustrating its potential effectiveness in combating various forms of cancerous cells.
Vivek K. Vyas et al. (2019),89 undertook a significant endeavor in the field of medicinal chemistry by successfully synthesizing two intricate compounds, specifically benzyl 2-(4-fluorophenyl)-6-methyl quinoline-4-carboxylate (72) and 4-((benzyloxy)methyl)-6,7-dichloro-2-(4-chlorophenyl)quinoline (73), which are noteworthy for their potential biological activity. The first compound, designated as compound 72, exhibited a remarkable inhibitory concentration (IC50) value of 1.56 µM against the human dihydroorotate dehydrogenase (hDHODH), while the second compound, referred to as compound 73, demonstrated an even more favorable IC50 value of 1.22 µM against the same enzyme, thereby establishing it as the lead compound within the context of this comprehensive study. The relatively low IC50 values observed for both compounds are indicative of their ability to effectively inhibit the enzymatic activity at significantly reduced concentrations, which is a highly desirable characteristic in the development of therapeutic agents. The selection of both compounds 72 and 73 was meticulously performed based on a thorough analysis of their predictive biological activity, the absence of any discernible toxicity, and their advantageous docking scores, all of which were rigorously evaluated through an extensive three-dimensional quantitative structure–activity relationship (3D QSAR) study, followed by systematic in vitro screening against established cancer cell lines, specifically HT-29 and MDA-MB-231.
Kuldip D. Upadhyay et al. (2018),90 the compounds synthesized are based on a pyrano[3,2-c]quinoline scaffold but are not grouped systematically by scaffold modifications. The structure–activity relationship (SAR) is partially discussed, highlighting that substitutions at the 3-position of the aryl ring on the C4 position of the quinoline core play a critical role in anti-inflammatory and anticancer activity. Electron-withdrawing groups (e.g., Cl, NO2, Br) generally enhanced TNF-α and IL-6 inhibition as well as cytotoxic activity, whereas electron-donating groups (e.g., OH, OCH3) and bulky substitutions (e.g., phenoxy, indolyl) had mixed or reduced effects. The biological evaluation focuses on cytokine inhibition and cytotoxicity across various cancer cell lines but fails to correlate structural modifications with specific protein interactions or docking studies. To strengthen the manuscript, the authors should group compounds based on structural motifs, enrich the SAR analysis with clear comparative data, and include molecular docking or target-based interaction studies to elucidate binding mechanisms, thereby guiding future optimization efforts. The successfully synthesized a series of compounds, specifically 2-amino-4-(3-chlorophenyl)-6-methyl-5-oxo-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carbonitrile (74), 2-amino-6-methyl-4-(3-nitrophenyl)-5-oxo-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carbonitrile (75) 2-amino-4-(3-bromophenyl)-6-methyl-5-oxo-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carbonitrile (76) and 2-amino-6-methyl-5-oxo-4-(3-phenoxyphenyl)-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carbonitrile (77) which exhibit significant biological activities, including anticancer and anti-inflammatory properties. Notably, compound 74 demonstrates a commendable anticancer efficacy, evidenced by an antiproliferative inhibition ranging from 81% to 53% at a concentration of 1 µM against various cancer cell lines. While other derivatives also exhibit substantial activity, compound 74 is particularly distinguished by the presence of the 3-chloro substituent, which significantly enhances its potency against cancer cells and its capacity to inhibit pro-inflammatory cytokines such as TNF-α and IL-6, thereby presenting promising outcomes in the context of cancer cell treatments.
S. M. Somagond et al. (2018),91 successfully synthesized two quinoline-based derivatives, namely 4-((2-hydroxyquinolin-3-yl)methyl)-2-phenyl-2,4-dihydro-3H-1,2,4-triazol-3-one (78) and 4-((6-chloro-2-hydroxyquinolin-3-yl)methyl)-2-phenyl-2,4-dihydro-3H-1,2,4-triazol-3-one (79), both of which exhibit significant biological activity characterized by their inhibitory effects on cancer cell proliferation, as evidenced by their respective average GI50 values of 9.67 mM for compound 78 and 9.50 mM for compound 79 this compelling data suggests that these compounds not only effectively inhibit the growth of malignant cells but also possess the potential to impede the progression of cancerous conditions, thereby underscoring their relevance in the field of oncology. The information presented in this research is of paramount importance and holds considerable promise for guiding future investigations and advancements in therapeutic strategies aimed at combating cancer.
Rasha M. Aly et al. (2017),92 conducted the synthesis of quinoline derivatives followed by a comprehensive biological evaluation. Among the derivatives, 6-(5-amino-4-cyanothiophen-3-yl)-4-((3-bromophenyl)amino)quinoline-3-carboxamide (80) exhibited an IC50 value of 5.069 µM against the MCF-7 breast cancer cell line and demonstrated significant inhibition of the EGFR enzyme, with an EGFR IC50 value of 1.73 µM, thereby establishing it as one of the most potent EGFR inhibitors identified in this investigation. The synthesized compounds 80 underwent EGFR binding assays to assess their inhibitory efficacy, revealing IC50 values that were lower than those of other derivatives. Furthermore, the study incorporated a structure–activity relationship (SAR) analysis, aligning the proposed compounds with a pharmacophore model to elucidate the characteristics that enhance their biological activity. The research highlighted substantial anticancer properties and is undergoing rigorous investigations to assess its therapeutic efficacy and underlying mechanisms of action.
K. Pluta et al. (2016),93 successfully synthesized a series of quinoline-based anticancer agents, specifically the compounds known as 9-fluoro-12-(2-(1-methylpiperidin-2-yl)ethyl)-12H-benzo[5,6][1,4] thiazino[2,3-c]quinoline (81) and 12-(2-(1-methylpiperidin-2-yl) ethyl)-9-(methylthio)-12H-benzo[5,6][1,4]thiazino[2,3-c]quinoline (82), both of which exhibit significant properties that are beneficial in the realm of anticancer activity. These synthesized compounds, designated as compound 81 and compound 82, demonstrate impressive biological efficacy with an IC50 value that is notably less than 7 mg mL−1 when evaluated against a comprehensive range of tested cell lines, thereby indicating a robust anticancer effect and effectiveness against several distinct cancer cell lines, including the MCF-7 cell line associated with breast cancer and the MDA-MB-231 line, recognized as a triple-negative breast cancer model, in addition to the glioblastoma SNB-19 cell line. The incorporation of the N-methylpiperidinylethyl moiety within compounds 81 and 82 plays a crucial role and is considered a significant contributor to their pronounced biological activity against various anticancer cell types. Therefore, the data and insights provided by this research are invaluable and serve as a foundational resource for guiding future investigations and developments in the field of cancer therapeutics.
Vladimir V. Kouznetsov et al. (2016),94 a synthesis of 4-methyl-2-(pyridin-4-yl)quinoline (83), a compound that has been demonstrated to exhibit remarkable biological efficacy, as evidenced by extensive research findings. This exceptional potency was particularly highlighted in studies that revealed its significant effectiveness against cervical epithelial carcinoma (HeLa) cells, as indicated by a notably low IC50 value of merely 0.016 µM, which underscores its strong therapeutic potential. Such a striking characteristic positions this compound as being 227 times more potent than the widely recognized reference drug doxorubicin, which, in contrast, possesses a considerably higher IC50 value of 3.62 µM; furthermore, it is important to note that this compound also displays a selectivity index (SI) value of 4168.1, which suggests that it possesses the ability to combat cancer cells while minimizing the associated toxic effects typically observed with conventional chemotherapeutic agents.
Elkotamy M. S. et al. (2025),95 the quinoline-sulfonamide derivative compound 85 demonstrated impressive biological activity with strong potential as an anticancer agent. It exhibited potent EGFR inhibitory activity with an IC50 of 0.161 µM, comparable to Erlotinib (0.142 µM), indicating its efficacy as a selective kinase inhibitor. In vitro, 8c showed broad cytotoxic effects against multiple cancer cell lines, including HCT-116, MCF-7, HeLa, and HepG2. Mechanistic studies confirmed that it induces G1/S cell cycle arrest and significantly promotes apoptosis in HepG2 cells. In vivo, using a solid Ehrlich carcinoma (SEC) model, compound 85 reduced tumor volume and weight effectively, while also lowering TNF-α and COX-2 levels, suggesting anti-inflammatory benefits in cancer treatment. Furthermore, it demonstrated a high safety margin, with an LD50 of 3000 mg kg−1, classifying it as less toxic than Erlotinib.
Lavunuri S. et al. (2024),96 the quinoline-fused 1,2,3-triazole derivatives demonstrated strong anticancer potential, particularly compound 86, which showed potent cytotoxicity against breast cancer cell lines (MCF-7, MDA-MB-468, MDA-MB-231) and superior EGFR/HER2 inhibition compared to standard drugs Erlotinib and Lapatinib. The hybrid pharmacophore strategy used in their design aimed to reduce side effects and overcome drug resistance by targeting multiple cancer-related pathways. In silico pharmacokinetic studies revealed high gastrointestinal absorption and the ability to cross the blood–brain barrier. All lead compounds adhered to major drug-likeness rules (Lipinski, Veber, etc.), supporting their potential for oral use with favorable bioavailability. However, most compounds inhibited CYP1A2 and CYP2C19 enzymes, indicating possible drug–drug interaction risks, while compound 86 showed a slightly better metabolic profile with less enzyme inhibition. These findings highlight the compounds' promising therapeutic potential with balanced efficacy, bioavailability, and manageable safety considerations.
Priya M. G. et al. (2024),97 a novel quinoline-based derivative, compound 87, exhibited exceptional potential as an anti-breast cancer agent. It showed the highest EGFR-TK inhibition (87.5%) among the tested analogs and demonstrated strong cytotoxicity against MCF-7 cells (IC50 = 36.2 µM) with no toxicity toward normal MCF-12A cells, indicating excellent selectivity. In a DMBA-induced breast cancer model, it significantly reduced tumor volume, improved body weight, and restored antioxidant enzyme levels (SOD, catalase, glutathione), suggesting a role in reducing oxidative stress. Compared to standard EGFR inhibitors, which often cause side effects like hair loss, diarrhea, and neuropathy, it presented a favourable safety profile, even at higher doses (150 mg kg−1). Its hybrid structure, integrating piperazine and thiazole moieties, is designed to minimize side effects and potentially overcome drug resistance by targeting key cancer pathways. These results support its promise as a safe and effective candidate for targeted breast cancer therapy.
A. A. Aly et al. (2025),98 a newly developed brominated pyranoquinoline derivative among compound 88 them demonstrated significant anticancer and antibacterial activity, particularly against lung epithelial A549 cancer cells and Staphylococcus aureus. It exhibited the lowest IC50 (35.10 µM) in the series and showed strong inhibition of both topoisomerase II and DNA gyrase, which are key targets in cancer and microbial therapy. The compound effectively induced apoptosis (20.4% total apoptosis) and G1 cell cycle arrest, while showing limited cytotoxicity toward normal cells (IC50 = 43.28 µM), reflecting a favourable safety and selectivity profile. Importantly, it demonstrated the ability to overcome drug resistance, a common challenge in chemotherapy, due to its dual-action mechanism. In silico ADME studies predicted high gastrointestinal absorption, good oral bioavailability, and compliance with Lipinski's rules, making it suitable for drug development. With its dual inhibitory action and low side effects, the compound represents a promising candidate for further development in anticancer therapies.
All quinoline-based compounds with anti-malarial and anti-cancer activities discussed in the previous sections have been compiled and are presented in Table 1. Quinoline-derived compounds have historically been integral to the discovery of anti-malarial and anti-cancer pharmacological agents, and their significance continues to escalate considering emerging therapeutic challenges. Within the realm of anti-malarial research, the increasing prevalence of resistance to conventional agents such as chloroquine highlights the imperative for the development of innovative quinoline derivatives that exhibit enhanced efficacy and alternative mechanisms of action. Prospective research directions may encompass the engineering of hybrid molecules that integrate the quinoline scaffold with various pharmacophores to augment potency and evade resistance mechanisms. Structure-guided drug design alongside molecular modelling is anticipated to assume a critical function in the optimization of these candidate compounds. In the context of cancer research, the quinoline framework has exhibited remarkable adaptability in targeting a multitude of molecular pathways, which include DNA topoisomerases, kinases, and microtubules. The trajectory of future research is likely to focus on the formulation of selective, mechanism-oriented quinoline derivatives specifically designed for particular cancer types or molecular targets. The integration of nanotechnology and advanced drug delivery systems may further enhance bioavailability and selectivity towards tumor sites. Furthermore, quinoline-metal complexes and conjugates for theranostic applications are emerging as promising domains that synergistically combine therapeutic and diagnostic functionalities. The advantages associated with quinoline derivatives encompass their extensive spectrum of biological activity, the relative simplicity of structural modifications, and their robust binding affinity to various biological targets (e.g., heme, DNA, kinases), rendering them highly versatile agents in the therapeutic management of malaria and cancer. Additionally, these compounds frequently demonstrate favourable oral bioavailability and cost-effective synthetic pathways. Conversely, the disadvantages include potential toxicity, particularly at elevated dosages (e.g., effects on retinal or cardiac function), as well as the emergence of drug resistance, which has been notably observed in malaria due to mutations in the PfCRT gene. Furthermore, certain derivatives are also compromised by inadequate solubility and metabolic instability. In comparison to novel quinoline analogs (e.g., fused-ring systems), conventional quinolines may exhibit diminished selectivity and reduced therapeutic efficacy against resistant strains or specific tumor types.
| Structure | Target | Strain/cell lines | IC50, CC50, IG50 and EC50 | Ref. |
|---|---|---|---|---|
 |
Antimalarial agent | Plasmodium falciparum | 1.2 µM | 30 |
 |
Metabolic stability | Metabolic stability | 5 to 8 | 31 |
 |
Antimalarial agent | Plasmodium falciparum | 0.014 to 5.87 µg mL−1 | 32 |
 |
Plasmodium falciparum | — | 34 | |
 |
Antimalarial agent | Plasmodium falciparum | With 50% inhibitory doses of 14.7 and 8.9 mM | 35 |
 |
In vivo (mice) | Plasmodium vinckei | Mice treated with doses of 2.5 and 7.5 mg kg−1 | 36 |
 |
Antimalarial agent | Plasmodium falciparum, HepG2 or HeLa cell lines | 37 | |
 |
HDP interaction | P. falciparum | Heme and m-oxo dimer; for HDP, those components were His175 and Glu126 | 38 |
 |
Plasmodium falciparum | — | 39 | |
 |
In vivo | Plasmodium falciparum | To dramatically reduce parasitemia, even at a low dose of 1 mg kg−1 for 4 days | 40 |
 |
Ion channel inhibitor | Plasmodium falciparum | K+ channel testing | 41 |
 |
Antimalarial agent | P. falciparum | 43 | |
 |
Resistant strains | Resistant parasite strains | Parasite bc1 complex | 44 |
 |
Multiple strains | Strains of P. falciparum | — | 45 |
 |
DMPK study | Drug metabolism, pharmacokinetics (DMPK) | — | 46 |
 |
CQS and CQR strains | P. falciparum | — | 47 |
| CQS and CQR | ||||
 |
Chloroquine-sensitive | Plasmodium falciparum (3D7) | — | 48 |
 |
RCQ compounds | P. falciparum | Of RCQ compounds showed higher activity against both chloroquine-sensitive (CQS) and chloroquine-resistant (CQR) parasites. The anti-malarial efficacy of RCQ compounds | 52 |
 |
Plasmodium falciparum | — | 54 | |
 |
Quinolines | Quinolines against the CQS (NF54) and CQR (K1) malaria parasite strains | 55 | |
 |
Antimalarial agent | Against the chloroquine-sensitive Pf3D7 strain of Plasmodium falciparum | 0.25 µM | 57 |
 |
Antimalarial agent | Plasmodium falciparum 3D7 | 0.62 µg mL−1 | 58 |
 |
Antimalarial agent | Plasmodium falciparum 3D7 strain | 1.99 µM | 59 |
 |
Antimalarial agent | Plasmodium falciparum | 1.99 µM | 60 |
 |
Cytotoxicity | MCF-7 cell line | — | 62 |
 |
Cytotoxicity | C-32 (human amelanotic melanoma), MDA-MB-231 (human breast adenocarcinoma), and A549 (human lung adenocarcinoma) cell lines | — | 63 |
 |
Cytotoxicity | MDA-MB-231 and MDA-MB-468 | 18.94 µM and 22.68 µM | 64 |
 |
Cytotoxicity | The MCF-7 cell line | 1.32 | 65 |
 |
Cytotoxicity | MDA-MB-231 and MCF-7 breast cancer cell lines | 7.42 ± 0.94 µM | 66 |
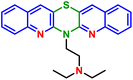 |
Cytotoxicity | IGROV-1 | 0.19 µM | 67 |
 |
Neoplastic line | Neoplastic cell line | — | 68 |
 |
Cytotoxicity | A549 lung cancer cells | 0.96 µM | 69 |
 |
C-RAF kinase inhibitor | C-RAF kinase inhibitor | 0.067 µM | 70 |
 |
Cytotoxicity | SK-BR-3 cell line | — | 71 |
 |
Cytotoxicity | HCT-116 colon cancer cell line | 0.16 µM | 72 |
| Bcl-2 protein | ||||
 |
Cytotoxicity | Breast cancer cell line MCF-7 | 7.1 µM | 73 |
 |
PI3Kα inhibito | Phosphoinositide 3-kinase alpha (PI3Ka) | 0.80 nM | 74 |
 |
Cytotoxicity | Colon cancer | 0.904 mM for leukemia, and 0.926 mM for melanoma | 75 |
 |
VEGFR-2 target | VEGFR-2 receptor | HepG-2, which presented an IC50 value of 3.3 µM, and MCF-7, characterized by an IC50 value of 14.2 µM | 76 |
 |
Cytotoxicity | SK-MEL-147 cells | 15.9 µM and 15.3 µM | 77 |
 |
Multiple cell lines | Human cancer cell lines | (IC50) value of 4.45 ± 0.88 µM against A-549 cells, an IC50 value of 4.74 ± 0.42 µM against HeLa cells, an IC50 value of 14.54 ± 1.96 µM against SGC-7901 cells, and an IC50 value of 32.12 ± 3.66 µM against L-02 cells | 78 |
 |
Cytotoxicity | Of cancer cell lines, including H1299 | 1.41 mM and SKBR-3 (IC50 = 0.70 mM | 79 |
 |
Cytotoxicity | A-2780 cancer cell line | 0.5 to 1.66 µM | 80 |
 |
Cytotoxicity | HeLa, HT29, and C6 | 2 to 50 µg mL | 81 |
 |
Cytotoxicity | Leukemia K-562 cell line | 0.26 µM | 82 |
| Leukemia SR cancer cell line | 0.33 µM | |||
 |
Cytotoxicity | Carcinoma cell line HT-29 | 0.19 µM | 83 |
 |
Cytotoxicity | Colon-derived HCT-15, renal-derived TK-10, and UO-31 | 0.888 mM and 0.229 mM | 84 |
 |
Cytotoxicity | HCT116, and ovarian adenocarcinoma cells classified as NIH:OVCAR-3 | — | 85 |
 |
Cytotoxicity | MCF-7 breast cancer cell | Of 5.069 µM | 89 |
 |
Cytotoxicity | HCT-116, MCF-7, HeLa, and HepG2 | 0.161 µM | 92 |
 |
Cytotoxicity | MCF-7, MDA-MB-468, MDA-MB-231 | — | 93 |
 |
Cytotoxicity | — | 36.2 µM | 94 |
 |
Cytotoxicity | Lung epithelial A549 cancer cells | 35.10 µM | 95 |
4 Conclusions
Among heterocyclic molecules, quinoline and its analogues are particularly important in the field of drug research and discovery. These compounds are extremely important because they are thought to be possible naturally occurring products and because they are essential scaffolds in medicinal chemistry. In recent years, there has been an increased interest in the use of quinoline derivatives as medicinal compounds for fighting diseases and various illnesses. It should be highlighted that while this extensive review aims to give a comprehensive grasp of quinolines antimalarial and anticancer activity, it might not cover all aspects entirely. Our genuine wish is that this study will be helpful to upcoming professionals in the field, inspiring new concepts and innovative approaches in the fields of synthetic and medicinal chemistry, helping them to act on the problems.Conflicts of interest
The authors report has no conflict of interest.Data availability
This review paper does not introduce any novel data, research findings, software, or code, focusing uniquely on existing literature and analyses.Acknowledgements
The authors would like to express their gratitude to the Vellore Institute of Technology Vellore, for the facility and fellowship.Notes and references
- M. Ezzati, J. Khalafy, A. P. Marjani and R. H. Prager, Aust. J. Chem., 2018, 71(6), 435–441 CrossRef CAS.
- S. Jain, V. Chanra, P. K. Jain, K. Pathak, D. Pathak and A. Vaidya, Arabian J. Chem., 2019, 12(8), 4920–4946 CrossRef CAS.
- (a) A. Marella, O. P. Tanwar, R. Saha, M. R. Ali, S. Srivastava, M. Akhter, M. Shaquiquzzaman and M. M. Alam, Saudi Pharm. J., 2013, 21(1), 1–2 CrossRef PubMed; (b) N. D. Chavan and V. Vijayakumar, RSC Adv., 2024, 14(29), 21089–21101 RSC; (c) N. D. Chavan and V. Vijayakumar, J. Mol. Struct., 2025, 1321, 139739 CrossRef CAS; (d) N. D. Chavan, S. Sarveswari and V. Vijayakumar, Sci. Rep., 2025, 15(1), 10972 CrossRef CAS PubMed.
- P. Singh and V. Kumar, Pharm, 2023, 16(10), 1358 Search PubMed.
- (a) S. Sarveswari, V. Vijayakumar, R. Siva and R. Priya, Appl. Biochem. Biotechnol., 2015, 175, 43–64 Search PubMed; (b) S. Sarveswari and V. Vijayakumar, Arabian J. Chem., 2016, 9, S35–S40 Search PubMed.
- S. Ökten, A. Aydın, U. M. Koçyiğit, O. Çakmak, S. Erkan, C. A. Andac, P. Taslimi and I. Gülçin, Arch. Pharm. Pract., 2020, 9(353), 2000086 CrossRef PubMed.
- (a) L. Jyothish Kumar, S. Sarveswari and V. Vijayakumar, Open Chem., 2018, 16(1), 1077–1088 CrossRef; (b) X. Li and Y. Song, Eur. J. Med. Chem., 2023, 260, 115772 CrossRef CAS PubMed.
- L. Senerovic, D. Opsenica, I. Moric, I. Aleksic, M. Spasić, and B. Vasiljevic, Advances in Microbiology, Infectious Diseases and Public Health, 2020, vol. 14, pp. 37–69 Search PubMed.
- S. Mukherjee and M. Pal, Curr. Med. Chem., 2013, 20(35), 4386–4410 CrossRef CAS PubMed.
- S. Jain, V. Chandra, P. K. Jain, K. Pathak, D. Pathak and A. Vaidya, Arabian J. Chem., 2019, 12(8), 4920–4946 CrossRef CAS.
- S. M. Hussaini, Expert Opin. Ther. Pat., 2016, 26(10), 1201–1221 CrossRef CAS PubMed.
- B. S. Matada, R. Pattanashettar and N. G. Yernale, Bioorg. Med. Chem., 2021, 32, 115973 CrossRef CAS PubMed.
- A. Dorababu, ChemistrySelect, 2021, 6(9), 2164–2177 CrossRef CAS.
- K. Ilina and M. Henary, Chem.–Eur. J., 2021, 27(13), 4230–4248 CrossRef CAS PubMed.
- (a) S. Chauhan, T. Umar and M. K. Aulakh, ChemistrySelect, 2023, 8(14), 202204960 CrossRef; (b) A. Krishna, V. Vijayakumar and S. Sarveswari, ChemistrySelect, 2020, 5(26), 7967–7972 CrossRef CAS; (c) K. Alla, V. Vijayakumar and S. Sarveswari, Polycyclic Aromat. Compd., 2023, 43(3), 2844–2865 Search PubMed.
- (a) A. Dorababu, Arch. Pharm., 2021, 354(3), 2000232 CrossRef CAS PubMed; (b) P. Hemanth Kumar, L. Jyothish Kumar, G. Pavithrra, R. Rajasekaran, V. Vijayakumar, R. Karan and S. Sarveswari, Res. Chem. Intermed., 2020, 46, 1869–1880 Search PubMed.
- L. J. Kumar, Y. Suresh, R. Rajasekaran, S. R. Reddy and V. Vijayakumar, J. Iran. Chem. Soc., 2019, 16, 1071–1080 CrossRef CAS.
- P. H. Kumar, M. Rambabu, V. Vijayakumar and S. Sarveswari, ACS Omega, 2023, 18(13), 11806–11812 CrossRef PubMed.
- Z. H. Skraup, Monatshefte für Chemie und verwandte Teile anderer Wissenschaften, 1880, pp. 316–318 Search PubMed.
- (a) R. H. Manske, Chem. Rev., 1942, 30(1), 113–144 CrossRef CAS; (b) M. Conrad and l. Limpach, Ber. Dtsch. Chem. Ges., 1887, 20(1), 944–948 CrossRef.
- E. A. Alyamkina, S. A. Yamashkin, N. N. Artayeva and M. A. Yurovskaya, Moscow Univ. Chem. Bull., 2010, 65, 335–340 Search PubMed.
- (a) P. Friedlaender, Ber. Dtsch. Chem. Ges., 1882, 15(2), 2572–2575 CrossRef; (b) N. D. Chavan, B. Shanmugavel, Y. Tamboli and V. Vijayakumar, ChemistrySelect, 2025, 10(10), e202500851 CrossRef CAS.
- P. Friedländer and C. F. Gohring, Ber. Dtsch. Chem. Ges., 1883, 16(2), 1833–1839 Search PubMed.
- Spherical Insights LLP, Global Chloroquine Market Revenue, Forecasts to 2033, 2025, Report ID: SI8635, pp. 1–276 Search PubMed.
- Zion Market Research, Hydroxychloroquine Market Size, Industry Analysis, Forecast 2032, 2024, Report Code: ZMR-5346 Search PubMed.
- R. Preet, P. Mohapatra, S. Mohanty, S. K. Sahu, T. Choudhuri, M. D. Wyatt and C. N. Kundu, Int. J. Cancer, 2012, 130(7), 1660–1670 Search PubMed.
- K. Wadhwa, N. Kapoor, H. Kaur, E. A. Abu-Seer, M. Tariq, S. Siddiqui, V. K. Yadav, P. Niazi, P. Kumar and S. A. Alghamdi, Mycol, 2024, 52, 335–387 Search PubMed.
- M. Foley and L. Tilley, Pharmacol. Ther., 1998, 79(1), 55–87 CrossRef CAS PubMed.
- M. Foley and L. Tilley, Int. J. Parasitol., 1997, 27(2), 231–240 Search PubMed.
- Y. Pommier, Chem. Rev., 2009, 109(7), 2894–2902 CrossRef CAS PubMed.
- O. Afzal, S. Kumar, M. R Haider, M. R. Ali, R. Kumar, M. Jaggi and S. A. Bawa, Eur. J. Med. Chem., 2015, 97, 871–910 CrossRef CAS PubMed.
- A. Saxena, S. Majee, D. Ray and B. Saha, Bioorg. Med. Chem., 2024, 103, 117681 CrossRef CAS PubMed.
- A. Mahajan, S. Yeh, M. Nell, C. E. van Rensburg and K. Chibale, Bioorg. Med. Chem. Lett., 2007, 17(20), 5683–5685 CrossRef CAS PubMed.
- H. Shiraki, M. P. Kozar, V. Melendez, T. H. H. C. Ohrt, A. J. Magill and A. J. Lin, J. Med. Chem., 2011, 54(1), 131–142 CrossRef CAS PubMed.
- I. A. Radini, T. M. Elsheikh, E. M. El-Telbani and R. E. Khidre, Molecules, 2016, 21(7), 909 CrossRef PubMed.
- W. H. Pan, X. Y. Xu, N. Shi, S. W. Tsang and H. J. Zhang, Int. J. Mol. Sci., 2018, 19(5), 1382 CrossRef PubMed.
- Y. Q. Hu, C. Gao, S. Zhang, L. Xu, Z. Xu, L. S. Feng, X. Wu and F. Zhao, 0 Eur. J. Med. Chem., 2017, 139(139), 22–47 CrossRef CAS PubMed.
- M. C. Joshi, K. J. Wicht, D. Taylor, R. Hunter, P. J. Smith and T. J. Egan, J. Med. Chem., 2013, 69, 338–347 CAS.
- M. C. Lombard, D. D. N'Da, J. C. Breytenbach, N. I. Kolesnikova, C. T. Van Ba, S. Wein, J. Norman, P. Denti, H. Vial and L. Wiesner, Eur. J. Med. Chem., 2012, 47(5), 834–841 CAS.
- R. R. Soares, J. M. da Silva, B. C. Carlos, C. C. da Fonseca, L. S. de Souza, F. V. Lopes, R. M. de Paula Dias, P. O. Moreira, C. Abramo, G. H. Viana and F. de Pila Varotti, Bioorg. Med. Chem. Lett., 2015, 25(11), 2308–2313 Search PubMed.
- S. Verma, S. Pandey, P. Agarwal, P. Verma, S. Deshpande, J. K. Saxena, K. Srivastava, P. M. Chauhan and Y. S. Prabhakar, RSC Adv., 2016, 6(30), 25584–25593 RSC.
- S. C. Teguh, N. Klonis, S. Duffy, L. Lucantoni, V. M. Avery, C. A. Hutton, J. B. Baell and L. Tilley, J. Med. Chem., 2013, 56(15), 6200–6215 CrossRef CAS PubMed.
- B. Baragaña, N. R. Norcross, C. Wilson, A. Porzelle, I. Hallyburton, R. Grimaldi, M. Osuna-Cabello, S. Norval, J. Riley, L. Stojanovski and F. R. Simeons, J. Med. Chem., 2016, 59(21), 9672–9685 CrossRef PubMed.
- V. Korotchenko, R. Sathunuru, L. Gerena, D. Caridha, Q. Li, M. Kreishman-Deitrick, P. L. Smith and A. J. Lin, J. Med. Chem., 2015, 58(8), 3411–3431 CrossRef CAS.
- V. S. Tiwari, P. Joshi, K. Yadav, A. Sharma, S. Chowdhury, A. Manhas, N. Kumar, R. Tripathi and W. Haq, ACS Omega, 2021, 6(20), 12984–12994 CrossRef CAS PubMed.
- R. M. Cross, N. M. Namelikonda, T. S. Mutka, L. Luong, D. E. Kyle and R. Manetsch, J. Med. Chem., 2011, 54(24), 8321–8327 CrossRef CAS PubMed.
- W. David Hong, S. C. Leung, S. K. Amporndanai, J. Davies, R. S. Priestley, G. L. Nixon, N. G. Berry, S. Samar Hasnain, S. Antonyuk, S. A. Ward and G. A. Biagini, ACS Med. Chem. Lett., 2018, 9(12), 1205–1210 CrossRef CAS PubMed.
- M. Tripathi, D. Taylor, S. I. Khan, B. L. Tekwani, P. Ponnan, U. S. Das, T. Velpandian and D. S. Rawat, ACS Med. Chem. Lett., 2019, 10(5), 714–719 CrossRef CAS PubMed.
- T. Fröhlich and S. B. Tsogoeva, J. Med. Chem., 2016, 59(21), 9668–9671 CrossRef.
- Z. W. Mei, L. Wang, W. J. Lu, C. Q. Pang, T. Maeda, W. Peng, M. Kaiser, S. El and T. Inokuchi, J. Med. Chem., 2013, 56(4), 1431–1442 CrossRef CAS PubMed.
- P. M. O'Neill, A. E. Shone, D. Stanford, G. Nixon, E. Asadollahy, B. K. Park, J. L. Maggs, P. Roberts, P. A. S. G. Biagini and P. G. Bray, J. Med. Chem., 2009, 52(7), 1828–1844 CrossRef PubMed.
- O. V. Miroshnikova, T. H. Hudson, L. Gerena, D. E. Kyle and A. J. Lin, J. Med. Chem., 2007, 50(4), 889–896 CrossRef CAS PubMed.
- N. I. Wenzel, N. Chavain, Y. Wang, W. Friebolin, L. Maes, B. Pradines, M. Lanzer, V. Yardley, R. Brun, C. Herold-Mende and C. Biot, J. Med. Chem., 2010, 53(8), 3214–3226 CrossRef CAS PubMed.
- G. Huang, C. Murillo Solano, J. Melendez, J. Shaw, J. Collins, R. Banks, A. K. Arshadi, R. Boonhok, H. Min, J. Miao and D. Chakrabarti, J. Med. Chem., 2020, 63(20), 11756–11785 CrossRef CAS PubMed.
- S. J. Burgess, J. X. Kelly, S. Shomloo, S. Wittlin, R. Brun, K. Liebmann and D. H. Peyton, J. Med. Chem., 2010, 53(17), 6477–6489 CrossRef CAS PubMed.
- N. M. Shah, M. P. Patel and R. G. Patel, New N-arylamino biquinoline derivatives, Eur. J. Med. Chem., 2012, 54, 239–247 CrossRef CAS.
- M. A. Shamsuddin, A. H. Ali, N. H. Zakaria, M. F. Mohammat, A. S. Hamzah, Z. Shaameri, K. W. Lam, W. F. Mark-Lee, H. K. Agustar, M. R. Mohd Abd Razak and J. Latip, Pharm, 2021, 14(11), 1174 CAS.
- N. Wang, K. J. Wicht, K. Imai, M. Q. Wang, T. A. Ngoc, R. Kiguchi, M. Kaiser, T. J. Egan and T. Inokuchi, Bioorg. Med. Chem., 2014, 22(9), 2629–2642 CrossRef CAS PubMed.
- F. J. Smit and D. D. N'Da, Bioorg. Med. Chem., 2014, 22(3), 1128–1138 CrossRef CAS PubMed.
- M. Touré, A. Gassama, O. Sambou, C. Cavé and S. Cojean, Eur. J. Med. Chem., 2025, 13, 100241 Search PubMed.
- P. Patel, B. Patel, M. Patel and P. Patel, Results Chem., 2024, 7, 101377 CrossRef CAS.
- G. Sharma and C. S. Sharma, Bioorg. Chem., 2024, 151, 107674 CrossRef CAS PubMed.
- Y. Y. Feng, C. E. Dong, R. Li, X. Q. Zhang, W. Wang, X. R. Zhang, W. W. Liu and D. H. Shi, J. Mol. Struct., 2023, 1271, 133982 CrossRef CAS.
- S. Madapa, Z. Tusi, D. Sridhar, A. Kumar, M. I. Siddiqi, K. Srivastava, A. Rizvi, R. Tripathi, S. K. Puri, G. S. Keshava and P. K. Shukla, Bioorg. Med. Chem., 2009, 17(1), 203–221 CrossRef CAS PubMed.
- A. A. Abu-Hashem, O. Hakami and N. Amri, Heliyon, 2024, 10(5), e26735 CrossRef CAS.
- A. Zieba, D. Pindjakova, M. Latocha, J. Plonka-Czerw, D. Kusmierz, A. Cizek and J. Jampilek, Molecules, 2024, 29(17), 4044 CrossRef CAS PubMed.
- C. N. Suresh, B. A. Kumar, H. B. Preetham, S. M. Srinivasa, M. S. Ali, H. A. Al-Lohedan, K. S. Kumar, C. Shivamallu, A. Jain, S. Rangappa and M. Umashankara, J. Mol. Struct., 2024, 1312, 138519 CrossRef.
- S. M. El Rayes, I. A. Ali, W. Fathalla, M. A. Ghanem, A. H. El-Sagheer and M. S. Nafie, ACS Omega, 2024, 9(30), 32789–32798 CAS.
- H. Siddiqui, F. Rizvi, W. Shehzad, M. Hassam, R. Uddin and M. I. Choudhary, Results Chem., 2024, 10, 101692 CrossRef CAS.
- M. Jeleń, B. Morak-Młodawska and R. Korlacki, J. Mol. Struct., 2023, 1287, 135700 CrossRef.
- S. Mohebbi, F. Shafiei, T. Momeni Isfahani and M. Ahmadi Sabegh, Int. J. Quantum Chem., 2024, 124(1), e27314 CrossRef CAS.
- K. N. Vennila, D. Sunny, S. Madhuri, S. Ciattini, L. Chelazzi and K. P. Elang, Bioorg. Chem., 2018, 81, 184–190 CrossRef CAS PubMed.
- S. O. Zaraei, N. N. Al-Ach, H. S. Anbar, R. El-Gamal, H. Tarazi, R. T. Tokatly, R. R. Kalla, M. A. Munther, M. M. Wahba, A. M. Alshihabi and M. K. Shehata, Eur. J. Med. Chem., 2022, 238, 114434 CrossRef CAS PubMed.
- X. Y. Li, D. P. Wang, S. Li, W. H. Xue, X. H. Qian, K. L. Liu, Y. H. Li, Q. Q. Lin, G. Dong, F. H. Meng and L. Y. Jian, Bioorg. Chem., 2022, 119, 105469 CrossRef CAS PubMed.
- D. Insuasty, S. García, R. Abonia, B. Insuasty, J. Quiroga, M. Nogueras, J. Cobo, G. L. Borosky and K. K. Laali, Arch. Pharm., 2021, 354(9), 2100094 CrossRef CAS.
- R. Maltais, J. Roy and D. Poirier, ACS Med. Chem. Lett., 2021, 12(5), 822–826 CrossRef CAS PubMed.
- R. He, B. Xu, L. Ping and X. Lv, Eur. J. Med. Chem., 2021, 214, 113249 CrossRef CAS PubMed.
- M. M. Elbadawi, W. M. Eldehna, W. Wan, K. K. Agama, Y. Pommier and M. Abe, Eur. J. Med. Chem., 2021, 215, 113261 CrossRef CAS.
- M. M. Alanazi, H. A. Mahdy, N. A. Alsaif, A. J. Obaidullah, H. M. Alkahtani, A. A. Al-Mehizia, S. M. Alsubaie, M. A. Dahab and I. H. Eissa, Bioorg. Chem., 2021, 112, 104949 CrossRef CAS PubMed.
- C. A. Costa, R. M. Lopes, L. S. Ferraz, G. N. Esteves, J. F. Di Iorio, A. A. Souza, I. M. de Oliveira, F. Manarin, W. A. Judice, H. A. Stefani and T. Rodrigues, Bioorg. Med. Chem., 2020, 28(11), 115511 CrossRef CAS PubMed.
- G. Jin, F. Xiao, Z. Li, X. Qi, L. Zhao and X. Sun, ChemMedChem, 2020, 15(7), 600–609 CrossRef CAS PubMed.
- M. F. Mohamed and G. E. Abuo-Rahma, RSC Adv., 2020, 10(52), 31139–31155 RSC.
- S. Mirzaei, F. Eisvand, F. Hadizadeh, F. Mosaffa, A. Ghasemi and R. Ghodsi, Bioorg. Chem., 2020, 98, 103711 CrossRef CAS.
- S. Ökten, A. Aydın, U. M. Koçyiğit, O. Çakmak, S. Erkan, C. A. Andac, P. Taslimi and I. Gülçin, Arch. Pharm. Chem. Life Sci., 2020, 353(9), 2000086 CrossRef PubMed.
- S. R. Shah, K. D. Katariya and D. Reddy, ChemistrySelect, 2020, 5(3), 1097–1102 CrossRef CAS.
- Y. Zhou, X. Xu, F. Wang, H. He and B. Qi, Bioorg. Chem., 2021, S106, 104511 CrossRef.
- A. K. El-Damasy, M. M. Haque, J. W. Park, S. C. Shin, J. S. Lee, E. E. Kim and G. Keum, Eur. J. Med. Chem., 2020, 208, 112756 CrossRef CAS PubMed.
- B. Kundu, S. K. Das, S. Paul Chowdhuri, S. Pal, D. Sarkar, A. Ghosh, A. Mukherjee, D. Bhattacharya, B. B. Das and A. Talukdar, J. Med. Chem., 2019, 62(7), 3428 CrossRef CAS PubMed.
- V. K. Vyas, G. Qureshi, D. Oza, H. Patel, K. Parmar, P. Patel and M. D. Ghate, Bioorg. Med. Chem. Lett., 2019, 29(7), 917–922 CrossRef CAS PubMed.
- K. D. Upadhyay, N. M. Dodia, R. C. Khunt, R. S. Chaniara and A. K. Shah, ACS Med. Chem. Lett., 2018, 9(3), 283–288 CrossRef CAS PubMed.
- S. M. Somagond, R. R. Kamble, P. P. Kattimani, S. K. Shaikh, S. R. Dixit, S. D. Joshi and H. C. Devarajegowda, ChemistrySelect, 2018, 3(7), 2004–2016 CrossRef CAS.
- R. M. Aly, R. A. Serya, A. M. El-Motwally, A. Esmat, S. Abbas and D. A. Abou El Ella, Bioorg. Chem., 2017, 75, 368–392 CrossRef CAS PubMed.
- K. Pluta, M. Szmielew, K. Suwińska and M. Latocha, J. Mol. Struct., 2016, 1122, 62–71 CrossRef CAS.
- V. V. Kouznetsov, M. L. Robles-Castellanos, F. Sojo, F. A. Rojas-Ruiz and F. Arvelo, Med. Chem. Res., 2017, 551–561 CrossRef CAS.
- M. S. Elkotamy, M. K. Elgohary, L. A. Elkelesh, M. A. Alkabbani, E. F. Khaleel, W. M. Eldehna and H. A. Abdel-Aziz, Bioorg. Chem., 2025, 157, 108247 CrossRef CAS PubMed.
- S. Lavunuri, R. V. Nadh, D. Banothu and S. K. Rapeti, Tetrahedron Lett., 2024, 150, 155282 CrossRef CAS.
- M. G. Priya, V. R. Solomon, N. Hemavathy, J. Jeyakanthan, D. Kumar and J. Mahesh, Results Chem., 2024, 7, 101359 CrossRef.
- A. A. Aly, H. A. Abd El-Naby, E. K. Ahmed, S. A. Gedamy, K. Rissanen, M. Nieger, A. B. Brown, M. G. Shehat, M. M. Shaaban and A. Atta, RSC Adv., 2025, 15(3), 1941–1956 RSC.
| This journal is © The Royal Society of Chemistry 2025 |
















































