Open Access Article
Open Access ArticleCopper-promoted oxidative mono- and di-bromination of 8-aminoquinoline amides with HBr and DMSO†
Changdong Shao *,
Jingyi Liu,
Yanan Shen,
Li Li,
Chen Ma,
Zhengsong Hu,
Yuhe Kan
*,
Jingyi Liu,
Yanan Shen,
Li Li,
Chen Ma,
Zhengsong Hu,
Yuhe Kan ,
Ping Chen
,
Ping Chen and
Tingting Zhang*
and
Tingting Zhang*
Jiangsu Provincial Key Laboratory for Chemistry of Low-Dimensional Materials, School of Chemistry and Chemical Engineering, Huaiyin Normal University, Huai'an, 223300, Jiangsu, China. E-mail: shaochangdong@hytc.edu.cn
First published on 21st March 2025
Abstract
An efficient and convenient method for oxidative mono- and di-bromination of 8-aminoquinoline amides is presented, utilizing hydrogen bromide (HBr) as the brominating reagent and dimethyl sulfoxide (DMSO) as a mild oxidant. Copper salts act as Lewis acid catalysts, facilitating the bromination process. The formation of C5-monobrominated products is promoted by copper sulfate (CuSO4·5H2O), whereas the generation of C5, C7-dibrominated products necessitates the participation of copper nitrate (Cu(NO3)2·3H2O). A wide range of substrates bearing diverse functional groups undergo smooth transformation, resulting in brominated products with good to excellent yields.
Introduction
Brominated aromatics are one of the most important types of building blocks and are ubiquitous in natural products, pharmaceutically important molecules, pesticides, and flame retardants.1 Meanwhile, due to the emergence of transition metal-catalysed cross-coupling reactions, aryl bromides are frequently employed as synthetic intermediates in the construction of carbon–carbon and carbon–heteroatom bonds.2 Therefore, bromination of arenes and heteroarenes is still an important transformation in modern organic synthesis. Currently, the most commonly used bromination approach is electrophilic aromatic substitution using molecular bromine as the reagent. Nevertheless, molecular bromine is in fact extremely toxic and corrosive, posing significant handling challenges. Furthermore, its use often necessitates harsh conditions, leading to the generation of hazardous waste and undesirable polybrominated byproducts. In this context, many improved brominating reagents have been developed by bonding Br to C, N, or O atoms. However, low atom economy and high cost have limited their application.3 Given the considerations of green chemistry, atom economy, operational simplicity and selectivity, continuous investigations for the development of new brominating reagents and bromination methodologies are of great importance.Oxidative bromination, which is inspired by the enzyme-catalysed halogenation in nature, has become a powerful tool for the synthesis of bromo-containing molecules in recent years.4 With the assistance of various oxidants, such as molecular oxygen, hydrogen peroxide, persulfates, peracids, hypervalent iodine, selectfluor, metal-based oxidants, and others, a variety of less hazardous brominating reagents have been successfully used for the electrophilic as well as radical bromination of arenes. Among these brominating reagents, hydrogen bromide (HBr) has been proven to be a better one due to its low cost, ready availability, low toxicity, high atom economy, ease of handling, and reducibility.5 Moreover, hydrogen bromide is also a by-product of the Br2-based bromination and can be re-oxidized by stoichiometric amounts of oxidants, thereby achieving a significantly high atomic economy. Dimethyl sulfoxide (DMSO), an extremely important polar aprotic solvent both in lab and industrial settings, has been extensively employed as a reagent, catalyst, and more importantly, as an oxidant in organic synthesis.6 Therefore, the combination of hydrogen bromide and dimethyl sulfoxide has been widely used as an efficient brominating system for the bromination of many kinds of organic molecules.7
Quinoline derivatives have received continuous attention due to their widespread presence in natural products, significant biological and pharmaceutical activities, and important usage in material science.8 Given the significance of quinolines, considerable effort has been dedicated to the development of effective synthesis methodologies.9 Among them, the bromination of 8-aminoquinoline at the C5 position has received considerable attention. A number of transition metal-catalysed and metal-free bromination reactions of 8-aminoquinolines have been well documented over the past few years (Scheme 1a).10 However, compared with numerous C5 bromination reactions, only a few reports in the literature have described the dibromination reaction at the C5 and C7 positions. Xie and co-workers reported an electrochemically oxidative dibromination of 8-aminoquinoline amides at the C5 and C7 positions using NBS as the halogen source (Scheme 1b).11a Zhang's group developed a copper-catalysed mono- and di-bromination of 8-aminoquinoline amides by tuning the amount of NaBr/PhI(OAc)2 (Scheme 1c).11b Despite notable progress in the bromination of quinolines, the continued reliance on toxic and expensive oxidants or brominating reagents makes these reactions not only uneconomical but also suffering from low atom economy.
Recently, we have developed a copper-promoted C5-selective monobromination reaction of 8-aminoquinoline amides, employing both activated and unactivated alkyl bromides as the brominating agents, with DMSO serving as the solvent (Scheme 1d).12 As a continuation of our efforts to explore practical and eco-friendly halogenation reaction, we now report a copper-promoted mono- and di-bromination of 8-aminoquinoline amides, employing readily available HBr/DMSO as the brominating system (Scheme 1e).
Results and discussion
We began our investigation into the regioselective bromination of 8-aminoquinoline amides with an evaluation of a range of reaction conditions employing N-(quinolin-8-yl)benzamide (1a) as the model substrate (Table 1). Initially, 1a was treated with 40% aqueous HBr (2.5 equiv.) in the presence of CuSO4·5H2O (20 mol%) in DMSO (1.0 mL) at 100 °C for 12 h. To our delight, the C5-brominated product 2a was obtained in 73% yield and the dibrominated product 3a was not observed (Table 1, entry 1). According to Yoshida's and Jiao's report,13 the bromide cation, which is generated in situ from DMSO and HBr, would coordinate with excess DMSO to form Br+(DMSO)n, thereby dramatically reducing the electrophilic bromination reactivity. Therefore, a series of organic solvents such as acetonitrile (MeCN), ethyl acetate (EA), tetrahydrofuran (THF), 1,2-dichloroethane (DCE), and N,N-dimethylformamide (DMF) were screened with 0.5 mL of DMSO as the mild oxidant (Table 1, entries 2–6). MeCN was proved to be the best solvent for this reaction, achieving a yield of 93% (Table 1, entry 2), which suggests that the polarity of the solvent benefits the yield of 2a. However, when DMF was used as the solvent, only 34% of 2a was obtained (Table 1, entry 6), indicating that alkaline solvent inhibits the progress of the reaction. Subsequently, the bromination reaction was examined with a range of catalysts, including Cu(OAc)2·H2O, CuCl2·2H2O, CuBr2, CuCl, and CuBr (Table 1, entries 7–11), and the results indicated that these copper-based catalysts exhibit comparable catalytic efficiency. Based on preliminary results, the effects of reagent dosage, temperature, and reaction time on the C5 bromination reaction were investigated, and the results are listed in entries 12–19. The desired product 2a was obtained in 83% yield upon reducing the catalyst loading to 10 mol% (Table 1, entry 12). Notably, the bromination reaction still proceeds satisfactorily in the absence of CuSO4·5H2O, affording 2a with 77% yield (Table 1, entry 13). These results suggest that copper catalyst promotes the bromination and an electrophilic substitution pathway may be involved. The quantity of HBr can be reduced to 2.0 equivalents without affecting the yield, while using 1.5 equivalents of HBr, the yield can also reach up to 90% (Table 1, entries 14–15). A volume of 0.2 mL of DMSO is sufficient for the reaction, and a further reduction in the quantity of DMSO leads to a significant decrease in the yield of 2a (Table 1, entries 16–17). The yield decreased to 69% when the temperature was reduced from 100 °C to 80 °C (Table 1, entry 18). Finally, the C5-bromination reaction could be finished within 6 hours (Table 1, entry 19).| Entry | Catalyst | HBr (eq.) | DMSO (mL) | Solvent | Yieldb (%) 2a/3a |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol, 1.0 eq.), HBr, DMSO, catalyst (20 mol%), solvent (1.0 mL), stirred under air at 100 °C for 12 h.b 1H NMR yield with dibromomethane as the internal standard, isolated yield in parentheses.c Catalyst loading is 10 mol%.d Stirred at 80 °C.e 6 hours. | |||||
| 1 | CuSO4·5H2O | 2.5 | — | DMSO | 73/NR |
| 2 | CuSO4·5H2O | 2.5 | 0.5 | MeCN | 93/NR |
| 3 | CuSO4·5H2O | 2.5 | 0.5 | EA | 76/NR |
| 4 | CuSO4·5H2O | 2.5 | 0.5 | THF | 58/NR |
| 5 | CuSO4·5H2O | 2.5 | 0.5 | DCE | 11/NR |
| 6 | CuSO4·5H2O | 2.5 | 0.5 | DMF | 34/NR |
| 7 | Cu(OAc)2·H2O | 2.5 | 0.5 | MeCN | 89/NR |
| 8 | CuCl2·2H2O | 2.5 | 0.5 | MeCN | 83/NR |
| 9 | CuBr2 | 2.5 | 0.5 | MeCN | 87/NR |
| 10 | CuCl | 2.5 | 0.5 | MeCN | 80/NR |
| 11 | CuBr | 2.5 | 0.5 | MeCN | 79/NR |
| 12c | CuSO4·5H2O | 2.5 | 0.5 | MeCN | 83/NR |
| 13 | — | 2.5 | 0.5 | MeCN | 77/NR |
| 14 | CuSO4·5H2O | 2.0 | 0.5 | MeCN | 93/NR |
| 15 | CuSO4·5H2O | 1.5 | 0.5 | MeCN | 90/NR |
| 16 | CuSO4·5H2O | 2.0 | 0.2 | MeCN | 93/NR |
| 17 | CuSO4·5H2O | 2.0 | 0.1 | MeCN | 82/NR |
| 18d | CuSO4·5H2O | 2.0 | 0.2 | MeCN | 69/NR |
| 19e | CuSO4·5H2O | 2.0 | 0.2 | MeCN | 93(90)/NR |
| 20e | Cu(NO3)2·3H2O | 2.0 | 0.2 | MeCN | 31/45 |
| 21e | Cu(NO3)2·3H2O | 2.0 | 0.6 | MeCN | 28/52 |
| 22e | Cu(NO3)2·3H2O | 2.0 | 0.8 | MeCN | 23/57 |
| 23e | Cu(NO3)2·3H2O | 2.0 | 1.0 | MeCN | 23/58 |
| 24e | Cu(NO3)2·3H2O | 3.0 | 0.2 | MeCN | 74/15 |
| 25e | Cu(NO3)2·3H2O | 3.0 | 1.2 | MeCN | 12/79 |
| 26e | Cu(NO3)2·3H2O | 4.0 | 1.6 | MeCN | NR/90(86) |
| 27e | Cu(CO2CF3)2·xH2O | 4.0 | 1.6 | MeCN | 82/NR |
| 28e | Cu(OTf)2 | 4.0 | 1.6 | MeCN | 73/NR |
| 29e | CuSO4·5H2O | 4.0 | 1.6 | MeCN | 84/NR |
| 30e | — | 4.0 | 1.6 | MeCN | 73/NR |
Interestingly, during the screening of copper catalysts for the bromination reactions, it was observed that the use of Cu(NO3)2·3H2O resulted in the formation of C5, C7-dibrominated product 3a in a yield of 45%, as well as C5-monobrominated compound 2a in a yield of 31%, under the similar reaction conditions to those described in entry 19 (Table 1, entry 20). Through the judicious modulation of the concentrations of DMSO and HBr, it was observed that an augmentation in the concentration of DMSO was conducive to the formation of dibrominated products (Table 1, entries 21–23). Conversely, a solitary increase in the HBr concentration resulted in the predominance of monobrominated products (Table 1, entry 24). The optimal molar volume ratio of HBr to DMSO was determined to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Table 1, entry 22). When both HBr and DMSO were increased simultaneously at this optimal ratio, the dibrominated product could be obtained with a yield of 90% (Table 1, entries 25–26). Finally, when alternative copper catalysts were employed or no catalyst was incorporated, only C5-monobrominated product was formed (Table 1, entries 27–30). Consequently, a facile copper-promoted mono- and di-bromination of 8-aminoquinoline amides with HBr and DMSO was established.
2 (Table 1, entry 22). When both HBr and DMSO were increased simultaneously at this optimal ratio, the dibrominated product could be obtained with a yield of 90% (Table 1, entries 25–26). Finally, when alternative copper catalysts were employed or no catalyst was incorporated, only C5-monobrominated product was formed (Table 1, entries 27–30). Consequently, a facile copper-promoted mono- and di-bromination of 8-aminoquinoline amides with HBr and DMSO was established.
Having secured the optimal reaction conditions for mono- and di-bromination of 8-aminoquinoline amides with HBr and DMSO, as detailed in Table 1, entries 19 and 26, we subsequently explored into the substrate scopes and limitations of our devised catalytic system across a diverse range of 8-aminoquinoline amides. As illustrated in Table 2, it was gratifying to observe that the majority of monobromination reactions of substrates with aryl, alkyl, and heterocyclic moieties proceeded smoothly under standard conditions, yielding the corresponding products in good to excellent yields, ranging from 72% to 97%. A variety of functionalized benzamides, including those with simple groups on the phenyl ring such as methyl (1b-1c), methoxy (1d), tert-butyl (1e), and trifluoromethyl (1f), were well tolerated, affording products with yields of 87–97%. In recent years, a significant proportion of currently available pharmaceuticals, as well as those undergoing clinical trials, contain halogen atoms, which have been demonstrated to exert a pronounced influence on the bioactivity and physicochemical properties of small molecules.14 It is worthy of note that halogen atoms (F, Cl, Br and I) exhibited excellent compatibility with the bromination reaction (1g–1l). Furthermore, despite the presence of HBr, the ester (1m) and cyano (1n) groups remained intact during the reaction, ensuring their successful retention in the products.
| a Reaction conditions: 1 (0.2 mmol, 1.0 eq.), HBr (2.0 eq.), DMSO (0.2 mL), CuSO4·5H2O (20 mol%), MeCN (1.0 mL), stirred under air at 100 °C for 6 h. Isolated yield. |
|---|
 |
Meanwhile, the reaction also proceeded smoothly with multisubstituted benzamides as substrates, delivering the desired products in yields ranging from good to excellent (1o–1p). Encouragingly, amides bearing fused aryl (1q) and heteroaryl (1r–1s) groups furnished the C5-brominated products in yields of 93–96%, with no detectable side products substituting on the naphthalene, furan, or thiophene rings. Additionally, this protocol was compatible with aliphatic amides (1t–1y), including small rings under considerable ring stress, which endured the reaction entirely (1v–1w). The protocol was also successfully extended to sulphonamide derivatives, yielding the target product in 72% yield (1z). Finally, substituted 8-aminoquinoline amides (1aa–1ab) and other heterocycle (1ac) substrates were tested. However, it was found that only methoxy-substituted compound (1aa) underwent monobromination with 95% yield. Neither the methyl-substituted compound (1ab) nor the heterocyclic substrate (1ac) yielded the corresponding brominated products.
Next, the substrate scope of the dibromination reaction was further investigated. As shown in Table 3, the reaction exhibited excellent functional group tolerance, transforming benzamides with both electron-donating and electron-withdrawing groups into the corresponding dibrominated products in yields ranging from moderate to good. Specifically, benzamides with methyl (1c), methoxy (1d), tert-butyl (1e), trifluoromethyl (1f), fluorine (1i), chlorine (1j), ester (1m), and cyano (1n) groups on their benzene rings underwent smooth reactions, predominantly forming the desired dibrominated products in good yields. Unfortunately, due to the absence of aromatic conjugation in the reactant structure, the aliphatic amide yielded exclusively the monobrominated product under the optimal conditions (1x).
| a Reaction conditions: 1 (0.2 mmol, 1.0 eq.), HBr (4.0 eq.), DMSO (1.6 mL), Cu(NO3)2·3H2O (20 mol%), MeCN (1.0 mL), stirred under air at 100 °C for 6 h. Isolated yield. |
|---|
 |
In subsequent studies, an attempt was made to adapt the bromination reaction conditions for chlorination with hydrochloric acid and iodination with hydroiodic acid. However, the results were unsatisfactory, as the substrate (1a) failed to convert into the desired halogenated products for both chlorination and iodination. Subsequently, the scalability of the bromination reaction was explored in order to demonstrate its synthetic utility. Utilising 5 mmol of 1a, the desired mono- and di-substituted brominated products were isolated in 86% and 80% yield, respectively. Moreover, the bromo amide could be readily hydrolysed under basic conditions to yield bromine-substituted 8-aminoquinolines. Finally, Suzuki coupling,10d Heck reaction,15 and Sonogashira reaction10b from 2a enabled the synthesis of C5-functionalized 8-aminoquinoline amides. In addition, the azoloquinoline derivative 11 can be prepared from 3a according to literature procedures (Scheme 2).11b
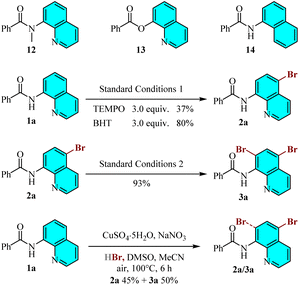
In order to gain more insight into the reaction mechanism, a series of control experiments were carried out (Scheme 3). Under the standard reaction conditions, quinolinamide analogs 12 and 13, along with naphthylamide 14, did not result in the formation of brominated products. While the precise rationale remains to be elucidated, this observation underscores the importance of the free NH group in amides and the presence of nitrogen on the quinoline scaffold. Subsequently, radical inhibition experiments were conducted to exclude the possibility of a radical mechanism. When radical scavengers, such as TEMPO (2,2,6,6-tetramethyl-1-piperidinyloxy) or BHT (butylated hydroxytoluene), were added to the reaction mixture under optimal conditions, the desired product 2a was still obtained, albeit in reduced yields of 37% and 80% respectively, suggesting that a radical pathway is not the primary mechanism. Furthermore, when the monobrominated product 2a was subjected to the standard dibromination conditions as the starting material, the desired compound 3a could be isolated in 93% yield. Finally, the dibromination product 3a was obtained in 50% yield accompanied by the monobromination product 2a in 45% yield when CuSO4·5H2O and 0.2 mmol NaNO3 were used instead of Cu(NO3)2·3H2O under the standard conditions 2. This experimental results show the effect of anions in the copper catalyst on the catalytic capacity.
In consideration of the experimental findings and previous reports,16 we propose that an aromatic electrophilic substitution mechanism is involved in the bromination process. As illustrated in Scheme 4, HBr is oxidized by the mild oxidant DMSO, potentially producing Br+(DMSO)n, bromodimethylsulfonium, or DMS·Br2. Subsequently, the bromide reagents react with the 8-aminoquinoline substrates to form the monobrominated products, which are accompanied by the generation of HBr that is recycled for further oxidation by DMSO. With the assistance of the more effective Cu(NO3)2·3H2O catalyst, the monobrominated products undergo further bromination to yield the dibrominated products. It is speculated that copper catalysts may facilitate the formation of the electrophilic Br+ reagent during the bromination step, and the anionic species present in these catalysts have a significant influence on the selectivity. However, further studies are needed to elucidate the detailed mechanism.
Experimental
General procedure for the monobromination reaction
A 35 mL sealed tube equipped with a stir bar was charged with 8-amidequinolines (1, 0.2 mmol, 1.0 equiv.), CuSO4·5H2O (0.04 mmol, 20 mol%), HBr (0.4 mmol, 2.0 equiv.), DMSO (0.2 mL), and MeCN (1.0 mL). The tube was sealed with a Teflon cap under air, then the mixture was stirred at 100 °C for 6 h. After completion, the reaction mixture was diluted with ethyl acetate (20 mL) and washed with saturated sodium bicarbonate and brine successively. The organic layer was dried over anhydrous sodium sulfate and concentrated in vacuo. The residue was purified on preparative thin layer chromatography (PTLC) to afford the desired product 2.General procedure for the dibromination reaction
A 35 mL sealed tube equipped with a stir bar was charged with 8-amidequinolines (1, 0.2 mmol, 1.0 equiv.), Cu(NO3)2·3H2O (0.04 mmol, 20 mol%), HBr (0.8 mmol, 4.0 equiv.), DMSO (1.6 mL), and MeCN (1.0 mL). The tube was sealed with a Teflon cap under air, then the mixture was stirred at 100 °C for 6 h. After completion, the reaction mixture was diluted with ethyl acetate (20 mL) and washed with saturated sodium bicarbonate and brine successively. The organic layer was dried over anhydrous sodium sulfate and concentrated in vacuo. The residue was purified on preparative thin layer chromatography (PTLC) to afford the desired product 3 and the incidental monosubstituted product 2.Conclusions
In conclusion, a straightforward, convenient, and environmentally benign method for synthesizing C5-brominated and C5, C7-dibrominated 8-aminoquinoline amides via a copper-promoted, regioselective oxidative bromination process employing HBr and DMSO has been devised. This transformation boasts high efficiency, exhibits broad functional group tolerance, and delivers the desired products in yields ranging from good to excellent.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Changdong Shao: conceptualization, methodology, resources, project administration, writing – original draft, funding acquisition. Jingyi Liu: investigation, data curation. Yanan Shen: investigation, data curation. Li Li: investigation, data curation. Chen Ma: investigation, data curation. Zhengsong Hu: resources, data curation. Yuhe Kan: resources, data curation. Ping Chen: resources, data curation. Tingting Zhang: validation, supervision, writing – review & editing, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21801088, 22104042), the Natural Science Foundation of Jiangsu Province (BK20181065), and the Natural Science Foundation of the Jiangsu Higher Education Institutions (18KJB150006, 19KJB150005).Notes and references
- (a) G. W. Gribble, Chem. Soc. Rev., 1999, 28, 335–346 RSC; (b) P. M. Pauletti, L. S. Cintra, C. G. Braguine, A. A. D. S. Filho, M. L. A. E. Silva, W. R. Cunha and A. H. Januário, Mar. Drugs, 2010, 8, 1526–1549 CrossRef CAS PubMed; (c) B.-G. Wang, J. B. Gloer, N.-Y. Ji and J.-C. Zhao, Chem. Rev., 2013, 113, 3632–3685 CAS; (d) N. Oztaskin, S. Goksu, Y. Demir, A. Maras and İ. Gulcin, Molecules, 2022, 27, 7426 CAS; (e) 1e Fire and Polymers: Hazards Identification and Prevention, ed. G. L. Nelson, American Chemical Society, Washington, DC, 1990, vol. 425 Search PubMed.
- (a) 2a Cross-Coupling Reactions: a Practical Guide, ed. N. Miyaura and S. L. Buchwald, Springer, Berlin Heidelberg, 2002 Search PubMed; (b) C. C. C. Johansson Seechurn, M. O. Kitching, T. J. Colacot and V. Snieckus, Angew. Chem., Int. Ed., 2012, 51, 5062–5085 CAS.
- (a) I. Saikia, A. J. Borah and P. Phukan, Chem. Rev., 2016, 116, 6837–7042 CAS; (b) M. Eissen and D. Lenoir, Chem.–Eur. J., 2008, 14, 9830–9841 CAS.
- (a) F. H. Vaillancourt, E. Yeh, D. A. Vosburg, S. Garneau-Tsodikova and C. T. Walsh, Chem. Rev., 2006, 106, 3364–3378 CAS; (b) A. Podgoršek, M. Zupan and J. Iskra, Angew. Chem., Int. Ed., 2009, 48, 8424–8450 CrossRef PubMed; (c) C. Schnepel and N. Sewald, Chem.–Eur. J., 2017, 23, 12064–12086 CAS; (d) R. Van Kerrebroeck, T. Horsten and C. V. Stevens, Eur. J. Org Chem., 2022, 2022, e202200310 CrossRef CAS; (e) D.-B. Jiang, F.-Y. Wu and H.-L. Cui, Org. Biomol. Chem., 2023, 21, 1571–1590 RSC.
- (a) J. Wang, W. Wang and J.-H. Li, Green Chem., 2010, 12, 2124–2126 RSC; (b) J. Wang, J.-Y. Zeng, Y. Huang, K.-A. Yang, J.-H. Xu, J.-H. Li and W. Du, ACS Omega, 2024, 9, 486–493 CrossRef CAS PubMed; (c) G. I. Nikishin, N. I. Kapustina, L. L. Sokova, O. V. Bityukov and A. O. Terentev, RSC Adv., 2018, 8, 28632–28636 RSC; (d) W. Song, M. Ma, W. Zhang, R. Feng, C. Lu, H.-Y. Zhang, Y. Zhang and J. Zhao, Org. Biomol. Chem., 2024, 22, 4145–4152 RSC; (e) L. Qiao, X. Cao, K. Chai, J. Shen, J. Xu and P. Zhang, Tetrahedron Lett., 2018, 59, 2243–2247 CrossRef CAS; (f) V. H. Tillu, P. D. Shinde, A. V. Bedekar and R. D. Wakharkar, Synth. Commun., 2003, 33, 1399–1403 CrossRef CAS; (g) K. Khosravi and S. Kazemi, Chin. Chem. Lett., 2012, 23, 387–390 CAS; (h) D. Liang, X. Li, C. Wang, Q. Dong, B. Wang and H. Wang, Tetrahedron Lett., 2016, 57, 5390–5394 CAS; (i) A. Steiner, J. D. Williams, O. De Frutos, J. A. Rincón, C. Mateos and C. O. Kappe, Green Chem., 2020, 22, 448–454 CAS; (j) L. Gavara, T. Boisse, B. Rigo and J.-P. Hénichart, Tetrahedron, 2008, 64, 4999–5004 CAS; (k) Y. Wang, H. Simon, X. Chen, Z. Lin, S. Chen and L. Ackermann, Angew. Chem., Int. Ed., 2022, 61, e202201595 CrossRef CAS PubMed.
- (a) X. Wu and K. Natte, Adv. Synth. Catal., 2016, 358, 336–352 CAS; (b) X. W. Wu, 6b Solvents as Reagents in Organic Synthesis: Reactions and Applications, Wiley, 1st edn, 2017 Search PubMed; (c) Z. Tashrifi, M. M. Khanaposhtani, B. Larijani and M. Mahdavi, Adv. Synth. Catal., 2020, 362, 65–86 CAS; (d) H.-L. Cui, Molecules, 2022, 27, 8480 CAS; (e) H. Lu, Z. Tong, L. Peng, Z. Wang, S.-F. Yin, N. Kambe and R. Qiu, Top. Curr. Chem., 2022, 380, 55 CAS.
- (a) C. Liu, R. Dai, G. Yao and Y. Deng, J. Chem. Res., 2014, 38, 593–596 CAS; (b) S. Song, X. Li, X. Sun, Y. Yuan and N. Jiao, Green Chem., 2015, 17, 3285–3289 CAS; (c) M. Karki and J. Magolan, J. Org. Chem., 2015, 80, 3701–3707 CrossRef CAS PubMed; (d) G. S. Sorabad and M. R. Maddani, New J. Chem., 2019, 43, 6563–6568 RSC; (e) J. Zhou, D. Tang and M. Bian, Synlett, 2020, 31, 1430–1434 CrossRef CAS; (f) Y. Jin, J. Yang, X. Feng, J. Li, J. Xu, X. Chen, S. Wang, Y. Lv and J. Yu, J. Flow Chem., 2020, 10, 369–376 CrossRef CAS; (g) Y. Yuan, Y. Liang, S. Shi, Y. Liang and N. Jiao, Chin. J. Chem., 2020, 38, 1245–1251 CrossRef CAS.
- (a) J. P. Michael, Nat. Prod. Rep., 2008, 25, 166–187 RSC; (b) B. S. Matada, R. Pattanashettar and N. G. Yernale, Bioorg. Med. Chem., 2021, 32, 115973 CrossRef CAS PubMed; (c) G. Lewinska, J. Sanetra and K. W. Marszalek, J. Mater. Sci.: Mater. Electron., 2021, 32, 18451–18465 CrossRef CAS PubMed.
- (a) S. M. Prajapati, K. D. Patel, R. H. Vekariya, S. N. Panchal and H. D. Patel, RSC Adv., 2014, 4, 24463–24476 RSC; (b) A. Weyesa and E. Mulugeta, RSC Adv., 2020, 10, 20784–20793 RSC; (c) T. Iwai and M. Sawamura, ACS Catal., 2015, 5, 5031–5040 CrossRef CAS; (d) A. Corio, C. Gravier-Pelletier and P. Busca, Molecules, 2021, 26, 5467 CrossRef CAS PubMed.
- (a) L. Qiao, X. Cao, K. Chai, J. Shen, J. Xu and P. Zhang, Tetrahedron Lett., 2018, 59, 2243–2247 CrossRef CAS; (b) X. Yang, Q.-L. Yang, X.-Y. Wang, H.-H. Xu, T.-S. Mei, Y. Huang and P. Fang, J. Org. Chem., 2020, 85, 3497–3507 CAS; (c) C. Sen, T. Sahoo and S. C. Ghosh, ChemistrySelect, 2017, 2, 2745–2749 CrossRef CAS; (d) X.-X. Chen, J.-X. Wang, J.-T. Ren, H. Xie, Y. Zhao, Y.-M. Li and M. Sun, Synlett, 2017, 28, 1840–1844 CrossRef CAS; (e) J.-Y. Jiao, Y.-J. Mao, A.-W. Feng, X.-F. Li, M.-T. Li and X.-H. Zhang, Tetrahedron, 2017, 73, 1482–1488 CrossRef CAS; (f) H. Qiao, S. Sun, F. Yang, Y. Zhu, J. Kang, Y. Wu and Y. Wu, Adv. Synth. Catal., 2017, 359, 1976–1980 CrossRef; (g) X. He, Y. Xu, L. Kong, H. Wu, D. Ji, Z. Wang, Y. Xu and Q. Zhu, Org. Chem. Front., 2017, 4, 1046–1050 RSC; (h) N. S. Rao, G. M. Reddy, B. Sridhar and M. H. Sarma, Eur. J. Org Chem., 2017, 2017, 438–442 CrossRef CAS; (i) J. Chen, T. Wang, Y. Liu, T. Wang, A. Lin, H. Yao and J. Xu, Org. Chem. Front., 2017, 4, 622–626 RSC; (j) H. S. Dutta, B. Khan, A. A. Khan, Raziullah, A. Ahmad, R. Kant and D. Koley, ChemistrySelect, 2017, 2, 6488–6492 CrossRef CAS; (k) D. R. Motati, D. Uredi and E. B. Watkins, Chem. Sci., 2018, 9, 1782–1788 RSC; (l) X. He, Y. Jiang, H. Zhou and Q. Zhu, ChemistrySelect, 2021, 6, 5191–5194 CrossRef CAS; (m) B. Huang, Y. Zhao, C. Yang, Y. Gao and W. Xia, Org. Lett., 2017, 19, 3799–3802 CrossRef CAS PubMed; (n) Y. Du, Y. Liu and J.-P. Wan, J. Org. Chem., 2018, 83, 3403–3408 CrossRef PubMed; (o) X. Lin, C. Zeng, C. Liu, Z. Fang and K. Guo, Org. Biomol. Chem., 2021, 19, 1352–1357 RSC; (p) Y. Long, L. Pan and X. Zhou, Molecules, 2019, 24, 535 CrossRef PubMed.
- (a) J. Hou, K. Wang, C. Zhang, T. Wei, R. Bai and Y. Xie, Eur. J. Org Chem., 2020, 2020, 6382–6386 CrossRef CAS; (b) J. Xu, X. Zhu, G. Zhou, B. Ying, P. Ye, L. Su, C. Shen and P. Zhang, Org. Biomol. Chem., 2016, 14, 3016–3021 RSC.
- (a) C. Shao, T. Xu, C. Chen, Q. Yang, C. Tang, P. Chen, M. Lu, Z. Hu, H. Hu and T. Zhang, RSC Adv., 2023, 13, 6993–6999 CAS; (b) C. Shao, C. Ma, L. Li, J. Liu, Y. Shen, C. Chen, Q. Yang, T. Xu, Z. Hu, Y. Kan and T. Zhang, Beilstein J. Org. Chem., 2024, 20, 155–161 CAS.
- (a) Y. Ashikari, A. Shimizu, T. Nokami and J. Yoshida, J. Am. Chem. Soc., 2013, 135, 16070–16073 CAS; (b) S. Song, X. Sun, X. Li, Y. Yuan and N. Jiao, Org. Lett., 2015, 17, 2886–2889 CrossRef CAS PubMed.
- E. H. Y. Li, B. Sana, T. Ho, D. Ke, F. J. Ghadessy, H. A. Duong and J. Seayad, Front. Bioeng. Biotechnol., 2022, 10, 1032707 Search PubMed.
- Y. Li, L. Zhu, X. Cao, C. Au, R. Qiu and S. Yin, Adv. Synth. Catal., 2017, 359, 2864–2873 CrossRef CAS.
- (a) K. Mislow, T. Simmons, J. T. Melillo and A. L. Ternay, J. Am. Chem. Soc., 1964, 86, 1452–1453 CrossRef CAS; (b) M. B. Floyd, M. T. Du, P. F. Fabio, L. A. Jacob and B. D. Johnson, J. Org. Chem., 1985, 50, 5022–5027 CrossRef CAS; (c) G. Megyeri and T. Keve, Synth. Commun., 1989, 19, 3415–3430 CrossRef CAS; (d) G. Majetich, R. Hicks and S. Reister, J. Org. Chem., 1997, 62, 4321–4326 CrossRef CAS PubMed; (e) L. H. Choudhury, T. Parvin and A. T. Khan, Tetrahedron, 2009, 65, 9513–9526 CrossRef CAS; (f) L. Voskressensky, N. Golantsov and A. Maharramov, Synthesis, 2016, 48, 615–643 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra00492f |
| This journal is © The Royal Society of Chemistry 2025 |