Open Access Article
Open Access ArticleA sustainable and efficient method for sequential extraction of lutein and lipid from deep eutectic solvent pretreated Chlorella pyrenoidosa†
Beixiao Zhang‡
*a,
Gul Muhammad‡ *abc,
Liya Denga,
Md Asraful Alam
*abc,
Liya Denga,
Md Asraful Alam a,
Anqi Zhaod,
Thomas O. Butlere,
Zhenglong Libc,
Ximing Zhang
a,
Anqi Zhaod,
Thomas O. Butlere,
Zhenglong Libc,
Ximing Zhang *bc and
Jingliang Xu
*bc and
Jingliang Xu *a
*a
aSchool of Chemical Engineering, Zhengzhou University, Zhengzhou 450001, China. E-mail: bxzhang@zzu.edu.cn; gulmuhammad@zju-qz.edu.cn; xujl@zzu.edu.cn
bCollege of Biosystems Engineering and Food Science, Zhejiang University, Hangzhou 310058, China. E-mail: zhangximing@zju.edu.cn
cInstitute of Zhejiang University-Quzhou, Quzhou 324000, China
dSchool of Life Sciences, Zhengzhou University, Zhengzhou 450001, Henan, China
eLgem/Synalgae, Achterweg 65, 1424 PP, De Kwakel, The Netherlands
First published on 30th April 2025
Abstract
Microalgae biomass is regarded as a potential feedstock for valuable compounds such as pigments, lipids and proteins. However, development of single molecule extraction processes is the most common practice. A green multiproduct extraction approach is needed for economically sustainable process development of the microalgal industry. Therefore, this study aims to investigate the sequential extraction of lutein and lipid from dry and wet Chlorella pyrenoidosa biomass pretreated with a choline chloride-based deep eutectic solvent (DES) under a sustainable biorefinery scheme. In this context, we have assessed the kinetic modeling of the solid–liquid extraction process for the aforementioned compounds, focusing on the effects of temperature and time. The maximum lutein (3.80 mg g−1) and lipid (95.0 mg g−1) contents from dry biomass were obtained at 45 °C in 40 min and at 70 °C in 90 min, respectively. From wet biomass, the maximum lutein (2.57 mg g−1) and lipid contents (87.47 mg g−1) were obtained at 35 °C in 40 min and at 70 °C in 90 min, respectively. The kinetics of the solvent-based extraction process for lutein and lipids were assessed via first-order and second-order kinetic models with an associated investigation of kinetic parameters, such as rate constants, saturation concentration and activation energies. We found that temperature is an important parameter that influences the extraction of all compounds and also has a significant impact on the kinetic parameters. Toxicity evaluation of the DES and economic assessment of DES vs. ionic liquids (ILs) were performed. The synthesis cost of the DES is lower than that of ILs, and Escherichia coli JM109 survivability assessment confirms the DES as a non-toxic solvent. The present study provides valuable insights into the sequential extraction for a high-value multiproduct biorefinery.
1. Introduction
Microalgae are photosynthetic microorganisms relying on light and nutrients to produce a vast group of compounds such as carbohydrates, proteins, lutein, and lipids.1,2 Lutein has human health benefits, such as ameliorating cardiovascular diseases, age-related macular degeneration (AMD), and cancer.3,4 Lipids with great interest are triacylglycerols (TAGs), which are used in a wide range of applications, from biodiesel to cosmetics, food ingredients, and personal care products. The largest source of TAGs is palm oil, which has led to massive deforestation.5,6 Lipids from microalgae also offer an alternative to palm oil, and omega-3 long-chain polyunsaturated fatty acids, i.e., eicosapentaenoic (EPA) and docosahexaenoic acid (DHA), are regarded as alternatives to fish oil and essential fatty acids in both human nutrition and aquaculture.6Significant progress has been achieved in extracting valuable compounds from microalgae. Various studies have suggested different extraction approaches including ball milling, French press, high-pressure homogenization (HPH), ultrasound, grinding with liquid nitrogen, enzymatic hydrolysis, and acid–alkali hydrolysis.4,7,8 However, most of these approaches focus on extracting a single product (lutein, lipids, and protein), which becomes economically unfavorable. Some studies have focused on the coproduction of high-value (carotenoids) and low-value products (protein). For example, lutein and β-carotene have been extracted using pressurised liquid extraction (PLE), which involves high pressure (103 bar) and high temperature (40–110 °C), resulting in a low yield (0.08 mg g−1).9 In another study, lutein and lipids were extracted in a single-step process where 85.0% of lutein and 58.8% of lipids were recovered from the pretreated biomass.10 However, the involvement of sophisticated methods and subsequently high energy consumption emerge as major challenges. To address these issues, a simple, fast, eco-friendly, and cost-effective method is urgently required.
To overcome the aforementioned challenges, deep eutectic solvents (DESs) have received more attention in biomass processing due to their high performance, cost-effectiveness, low toxicity, sustainability, simple preparation, biodegradability, and recyclability.11–15 In our earlier studies, biomass of several microalgal species, such as Chlorella pyrenoidosa, C. vulgaris, and Chlorococcum sp. (GN38), were pretreated using DESs.16–18 DESs-based pretreatment approach overcame the disadvantages of conventional methods, facilitating the successful extraction of lutein or lipids in an environmentally and economically friendly manner. Thus, this study attempts the sequential extraction for multiproduct biorefinery from DES-pretreated algal biomass.
The present study evaluates the implementation of sequential extraction of lutein and lipid with ethanol, as well as a combination of methanol (M) and ethyl acetate (EA) (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). As reported in our previous study, this solvent combination showed promising results for the recovery of the neutral lipids as compared to the use of a single solvent (M, EA). These neutral lipids are considered as the ideal feedstock for biodiesel.19 Moreover, this study investigates the effects of temperature and time, and the best fitting of a kinetic model for the extraction of lutein and lipids from Chlorella pyrenoidosa. The first-order kinetic model (FOKM) and second-order kinetic model (SOKM) were applied for the extraction process. The FOKM and SOKM under non-equilibrium conditions were used to simulate the solid–liquid extraction process of lutein and lipid compounds from microalgae biomass. The kinetic parameters (i.e., rate constant of extraction, saturation concentration, and activation energies) were determined to show the influence of parameters such as temperature and time on lutein and lipids extraction from dry and wet biomass and to describe the extraction mechanism of the process.
1). As reported in our previous study, this solvent combination showed promising results for the recovery of the neutral lipids as compared to the use of a single solvent (M, EA). These neutral lipids are considered as the ideal feedstock for biodiesel.19 Moreover, this study investigates the effects of temperature and time, and the best fitting of a kinetic model for the extraction of lutein and lipids from Chlorella pyrenoidosa. The first-order kinetic model (FOKM) and second-order kinetic model (SOKM) were applied for the extraction process. The FOKM and SOKM under non-equilibrium conditions were used to simulate the solid–liquid extraction process of lutein and lipid compounds from microalgae biomass. The kinetic parameters (i.e., rate constant of extraction, saturation concentration, and activation energies) were determined to show the influence of parameters such as temperature and time on lutein and lipids extraction from dry and wet biomass and to describe the extraction mechanism of the process.
2. Experimental section
2.1. Materials
Microalgae biomass (Chlorella pyrenoidosa) was provided by Yunnan Boshan Zeyuan Microalgae Health Technology Co. Ltd, China. Choline chloride (ChCl, 98%) and ethylene glycol (EG, 98%) were obtained from Macklin, China. Methanol (≤100%) and ethyl acetate (99.5%) were obtained from Thermo Fisher, China. Ethanol (99.7%) was obtained from Tianjin Fengchuan Chemical Reagent Technology Co., Ltd. The lutein standard (purity 90%) was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd.2.2. Synthesis of the DES and the toxicity test
A ChCl-based DES was synthesized using ChCl as a hydrogen bond acceptor (HBA) and EG as a hydrogen bond donor (HBD) in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 followed by heating (80 °C) and stirring with a magnetic stirrer as reported elsewhere.18 DES formation was confirmed using Fourier transform infrared (FTIR) spectroscopy (BRUKER-TENSOR II-USA) and the measurement of the spectral region was recorded between 400 and 4000 cm−1. The synthesized DES was sealed and stored in a desiccator to avoid the absorption of external water.
2 followed by heating (80 °C) and stirring with a magnetic stirrer as reported elsewhere.18 DES formation was confirmed using Fourier transform infrared (FTIR) spectroscopy (BRUKER-TENSOR II-USA) and the measurement of the spectral region was recorded between 400 and 4000 cm−1. The synthesized DES was sealed and stored in a desiccator to avoid the absorption of external water.
DES toxicity was detected according to the method reported in ref. 20, and Escherichia coli JM109 (E. coli) was chosen as a target. Furthermore, to observe the E. coli cells, 400 μL bacterial solutions of the cells grown in the standard LB medium and the medium supplemented with 150 mM of DES [ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EG (1
EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)] were observed under a 40× microscope after being dyed by crystal violet staining solution.
2)] were observed under a 40× microscope after being dyed by crystal violet staining solution.
2.3. Pretreatment of microalgae biomass and sequential extraction procedure for lutein and lipid
5 g of biomass and 66.67 g of DES (i.e., a biomass to DES ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 66.67) were added to a beaker. The solution was magnetically stirred for 5 min (pre-treatment) according to our previously optimised study.21 After that, the pretreated biomass was separated from the DES via centrifugation, washed multiple times with water and then freeze dried for 48 h.
66.67) were added to a beaker. The solution was magnetically stirred for 5 min (pre-treatment) according to our previously optimised study.21 After that, the pretreated biomass was separated from the DES via centrifugation, washed multiple times with water and then freeze dried for 48 h.
Ethanol was used for the solid–liquid extraction of lutein, and M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA (2
EA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was used for lipid extraction. 0.5 g of pretreated biomass in ethanol solvent (ratio of 1
1) was used for lipid extraction. 0.5 g of pretreated biomass in ethanol solvent (ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23.34, g
23.34, g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mL) was used in a 20 mL glass tube at 300 rpm for lutein extraction. For the kinetic studies, the experiment was carried out at three different temperatures (25, 35, and 45 °C) for lutein. After lutein extraction, the solvent was removed, and the remaining biomass was dried at room temperature for 48 h. The dried biomass was weighed, and the lipid extraction experiment was performed at 50, 60, and 70 °C. In all mentioned experiments, the time for the extraction of the lutein was assessed (10, 20, 30, 40, 50, and 60 min) along with that for lipid extraction (10, 20, 30, 60, 90, 120, and 150 min).
mL) was used in a 20 mL glass tube at 300 rpm for lutein extraction. For the kinetic studies, the experiment was carried out at three different temperatures (25, 35, and 45 °C) for lutein. After lutein extraction, the solvent was removed, and the remaining biomass was dried at room temperature for 48 h. The dried biomass was weighed, and the lipid extraction experiment was performed at 50, 60, and 70 °C. In all mentioned experiments, the time for the extraction of the lutein was assessed (10, 20, 30, 40, 50, and 60 min) along with that for lipid extraction (10, 20, 30, 60, 90, 120, and 150 min).
2.4. Biochemical analysis of lutein and lipid content
Lutein concentration in each sample was analyzed using a high-performance liquid chromatography (HPLC) system (1200 Infinity, Agilent Technologies) coupled with a variable wavelength detector, according to the previous study.18 Lutein content was calculated according to eqn (1).| Lutein content (mg g−1) = Lutein concentration (mg L−1) × Solvent volume (L)/Pretreated biomass (g) | (1) |
Lipid content was determined by the gravimetric method as mg g−1 of the dry weight biomass and calculated as follows:
| Lipid content (mg g−1) = (W2 − W1)/W0 | (2) |
For fatty acids, transesterification of extracted lipids was done according to ref. 22 and the composition was determined using our previous report.1
2.5. Kinetic modelling
In this study, FO and SOKM were used in modelling the extraction of lutein and lipid from the microalgae biomass using ethanol and M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA (2
EA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as solvents. In brief, first-order extraction (KM), which was suggested by Harouna-Oumararou et al.,23 was evaluated in such a way that the rate of leaching (re) is proportional to the driving force (Cs − Ct) and the first order rate equation corresponds to the linear driving force as shown in eqn (3).
1) as solvents. In brief, first-order extraction (KM), which was suggested by Harouna-Oumararou et al.,23 was evaluated in such a way that the rate of leaching (re) is proportional to the driving force (Cs − Ct) and the first order rate equation corresponds to the linear driving force as shown in eqn (3).| re = dCt/dt = k(Cs − Ct) | (3) |
Eqn (4) was obtained by integrating eqn (3) by using the boundary conditions as Ct = 0 at t = 0 and Ct = Ct at t = t, in such a way that plotting ln values vs. t gives the slope, which is used to determine the FO extraction rate:
| ln[Cs/(Cs − Ct)] = kt | (4) |
The Arrhenius model is used in solid–liquid extraction to investigate the relationship between the extraction rate and temperature. Therefore, the temperature depends on the extraction kinetics.24
The Arrhenius equation, as shown in eqn (5) and (6), was used to determine the kinetic parameters. KPs (Ae and Ea) calculated by plotting (ln k vs. 1/T) [eqn (5)]. Ae and Ea are obtained from the slope and intercept.
| k = Aee−(Ea/RT) | (5) |
| ln k = ln Ae − Ea/RT | (6) |
| re = dCt/dt = k(Cs − Ct)2 | (7) |
Integrating eqn (7) considering the boundary conditions Ct = 0 at t = 0 and Ct = Ct at t = t gives
| 1/(Cs − Ct) − 1/Cs = kt | (8) |
| Ct = Cs2kt/(1 + Cskt) | (9) |
Rearranging eqn (8) in a linearized form gives eqn (10) and (11) as below.
| t/Ct = t/Cs + 1/Cs2 | (10) |
| t/Ct = t/Cs + 1/m | (11) |
Using eqn (5) and (6), KPs (Aa, Ea) were found, where g (mg min)−1 is the unit of k and Ae of SO.
3. Results and discussion
3.1. DES validation and toxicity
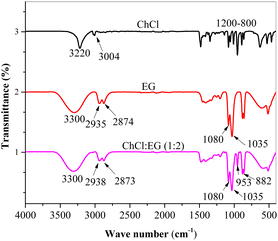
FTIR has been proven to be a very important test in confirming the formation of DES. DES was synthesized by combining ChCl and EG. FTIR spectra of ChCl, EG, and their derived DES were investigated to understand the modification as depicted in Fig. 1. In the FTIR spectra, a broad band was noticed at 3300 cm−1 in the synthesized DES, which is the characteristic of the hydrogen bonding formation and the same observation is found in the literature.25 The spectrum of the DES was dominated by EG; however, an additional characteristic band at 953 cm−1 originating from ChCl was noticed. This new band was attributed to the C–N+ stretching.26 In addition, the DES presented vibrational bands at 2938 and 2873 cm−1 corresponding to C–H stretching. Furthermore, the presence of vibrational peaks between 800 and 1100 cm−1 situated at 1080 cm−1, 1035 cm−1, and 882 cm−1 is associated with functional groups, namely, C–O stretching, C–C–O asymmetric stretching, and C–C–O symmetric stretching, respectively.26 These results confirm the successful synthesis of the ChCl:EG DES.To assess the toxicity of the ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EG (1
EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) DES, E. coli cells were grown in LB media supplemented with 150 mM to 450 mM DES from 5 h to 35 h, and compared with the standard LB media (pre-adapted cells). Experimental results (available only in the online version of ESI Fig. S1†) show that E. coli cells could grow in DES supplemented LB medium, showing that ChCl
2) DES, E. coli cells were grown in LB media supplemented with 150 mM to 450 mM DES from 5 h to 35 h, and compared with the standard LB media (pre-adapted cells). Experimental results (available only in the online version of ESI Fig. S1†) show that E. coli cells could grow in DES supplemented LB medium, showing that ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EG (1
EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) is not toxic to some extent and can be labelled as an environment friendly solvent.
2) is not toxic to some extent and can be labelled as an environment friendly solvent.
3.2. Extraction of lutein using ethanol as a solvent from dry and wet biomass
Fig. 2(a) presents the experimental results for the extraction kinetics of lutein at various temperatures (25, 35, and 45 °C) using ethanol as a solvent. A rapid increase in the lutein content at the beginning was observed (∼10 min). As the extraction process continued, a slow and steady trend remained constant until the peak point was attained, where most of the lutein was extracted. This observation can be explained on the basis of Fick's law.27 At the initial extraction stage, a high concentration gradient between the solid and liquid phases results in the high diffusion of lutein into the solvent. As the extraction process progresses, the gradient concentration decreases, resulting in an increase in the extraction yield until the maximum lutein recovery is achieved.Fig. 2(a) also shows that extracted lutein beyond the peak point in all conditions was reduced in dry and wet biomass. This observation is attributed to the decomposition of lutein due to thermal degradation or the prolonged reaction time.28–30 Lutein stability is recognized as a challenge for the food processing industry, as lutein is sensitive to light, temperature, and oxygen.31 Therefore, values beyond the peak point were excluded when simulating the lutein extraction kinetics. In fact, they do not show how quickly lutein is extracted. Instead of that, they represent the thermal degradation rate of lutein. Fig. 2(a) shows that the highest lutein extraction from dry biomass at the highest temperature of 45 °C is 3.80 mg g−1 in 40 min, and lower lutein contents of 2.82 and 2.78 mg g−1 were achieved at lower temperatures of 35 and 25 °C. In the case of wet extraction, at 35 °C, 2.57 mg per g lutein content and at 25 and 45 °C, 2.27 and 2.11 mg per g lutein contents were recorded in 40 min. These values indicate that temperature is an important extraction parameter for the recovery of lutein. The positive effect of increasing the temperature on the extraction of lutein can be a consequence of the increase of the solubility of the lutein in the solvent and the improvement of diffusion rate and mass transfer from solid to solvent.32
3.3. Kinetics of lipid extraction using M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) EA (2
EA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) 1) from microalgae
1) from microalgae
The experimental results of extraction kinetics of lipids from biomass at different temperatures (50, 60, and 70 °C) are depicted in Fig. 2(b). Fig. 2(b) shows a rapid increase in the initial 10 min of the reaction, and then a gradual rise in the lipid content was noticed. The highest lipid content was 95 mg g−1, achieved at 70 °C after 90 min. At the temperatures of 50 and 60 °C, the lipid content was 63.92 and 77.63 mg g−1 at the time of 120 min, respectively. These results show the effect of temperature on the extraction process. Meanwhile, in the wet biomass, 55.52 mg g−1, 78.66 mg g−1, and 87.47 mg g−1 lipids were achieved at 120, 60, and 90 min at 50, 60 and 70 °C, respectively.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) EA (2
EA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
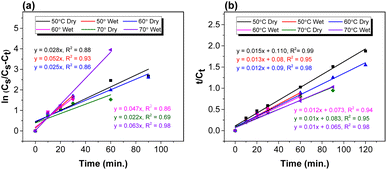
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1). The FOKM was achieved based on the experimental data of the extraction kinetics [Fig. 2(b)]. The k and R2 were determined by plotting ln Cs/(Cs − Ct) vs. t (Fig. 4a). The lipid extraction can be represented using the linear form of the first-order model. The k value decreased from 2.8 × 10−2 min−1 to 2.5 × 10−2 min−1 and 2.2 × 10−2 min−1 when the temperature was increased from 50 to 70 °C with R2 values of 0.88, 0.86, and 0.69, respectively. In contrast, in the case of wet biomass, k decreased from 5.2 × 10−2 to 4.7 × 10−2 min−1 and then increased to 6.3 × 10−2 min−1, showing no positive correlation between the temperature and the k value.
1). The FOKM was achieved based on the experimental data of the extraction kinetics [Fig. 2(b)]. The k and R2 were determined by plotting ln Cs/(Cs − Ct) vs. t (Fig. 4a). The lipid extraction can be represented using the linear form of the first-order model. The k value decreased from 2.8 × 10−2 min−1 to 2.5 × 10−2 min−1 and 2.2 × 10−2 min−1 when the temperature was increased from 50 to 70 °C with R2 values of 0.88, 0.86, and 0.69, respectively. In contrast, in the case of wet biomass, k decreased from 5.2 × 10−2 to 4.7 × 10−2 min−1 and then increased to 6.3 × 10−2 min−1, showing no positive correlation between the temperature and the k value.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) EA (2
EA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1). The SOKM was employed to examine the release kinetics of lipids from lutein free biomass using k and R2 (Fig. 4(b)). The best fits were observed when compared to the first-order and k values were determined to be 2.08 × 10−3 g (mg min)−1, 1.6 × 10−4 g (mg min)−1, and 1.1 × 10−4 g (mg min)−1 at the reaction temperatures of 50, 60 and 70 °C, respectively, with higher R2 values of 0.97, showing that the second-order model can be used to extract the lipid. However, the k values showed a decreasing trend in response to the temperature increase, which could be confirmed by the high efficiency of the solvent (M
1). The SOKM was employed to examine the release kinetics of lipids from lutein free biomass using k and R2 (Fig. 4(b)). The best fits were observed when compared to the first-order and k values were determined to be 2.08 × 10−3 g (mg min)−1, 1.6 × 10−4 g (mg min)−1, and 1.1 × 10−4 g (mg min)−1 at the reaction temperatures of 50, 60 and 70 °C, respectively, with higher R2 values of 0.97, showing that the second-order model can be used to extract the lipid. However, the k values showed a decreasing trend in response to the temperature increase, which could be confirmed by the high efficiency of the solvent (M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA, 2
EA, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). For the wet biomass, the same trend was observed, 2.3 × 10−3 g (mg min)−1, 1.5 × 10−4 g (mg min)−1, and 1.2 × 10−4 g (mg min)−1 at 50, 60 and 70 °C, respectively, with the rise of the temperature could be confirmed with the high efficiency of the used solvent.
1). For the wet biomass, the same trend was observed, 2.3 × 10−3 g (mg min)−1, 1.5 × 10−4 g (mg min)−1, and 1.2 × 10−4 g (mg min)−1 at 50, 60 and 70 °C, respectively, with the rise of the temperature could be confirmed with the high efficiency of the used solvent.3.4. Kinetic parameters determination
Related kinetic parameters were found using the Arrhenius equation discussed in Section 2.5.3.5. Fatty acid analysis of lutein extracted biomass
The results of the fatty acids are presented in Fig. 6, which shows that the major composition of fatty acids consists of C16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, C16
0, C16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, C16
2, C16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, C18
3, C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, C18
2, C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and C19
3 and C19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 in dry and wet biomass. The same fatty acids were found in other studies reported by other researchers.1,35,36 In addition, the dominant fatty acid components were C16
0 in dry and wet biomass. The same fatty acids were found in other studies reported by other researchers.1,35,36 In addition, the dominant fatty acid components were C16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 (27%), C18
0 (27%), C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (30%) and C18
2 (30%) and C18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (23%). This indicates that the major component in fatty acids lies between C16 and C18, which provides several advantages such as low viscosity, quality ignition and higher oxidative stability for longer storage.35,37,38 These types of fatty acids are the most suitable for biodiesel.1 Fig. 6 depicts the major composition of the fatty acids, including 28.0% of saturated fatty acids (SFAs) and 72.0% of polyunsaturated fatty acids (PUFAs), while for the wet biomass 32.0% SFAs and 70.0% PUFAs were noticed which was very similar to Chlorella sp. (34% SFA and 66% PUFAs) and Scenedesmus sp. culture (36.5% SFAs and 63.5% PUFAs).39 Besides, Ngangkham et al. reported 31.8% SFAs and 60.2% PUFAs from Chlorella sorokiniana.40
3 (23%). This indicates that the major component in fatty acids lies between C16 and C18, which provides several advantages such as low viscosity, quality ignition and higher oxidative stability for longer storage.35,37,38 These types of fatty acids are the most suitable for biodiesel.1 Fig. 6 depicts the major composition of the fatty acids, including 28.0% of saturated fatty acids (SFAs) and 72.0% of polyunsaturated fatty acids (PUFAs), while for the wet biomass 32.0% SFAs and 70.0% PUFAs were noticed which was very similar to Chlorella sp. (34% SFA and 66% PUFAs) and Scenedesmus sp. culture (36.5% SFAs and 63.5% PUFAs).39 Besides, Ngangkham et al. reported 31.8% SFAs and 60.2% PUFAs from Chlorella sorokiniana.40
 | ||
| Fig. 6 Fatty acid (C16–C19) fraction (a) and (c) and profile (b) and (d) of microalgae biodiesel from dry and wet biomass. | ||
3.6. Economic assessment of DES vs. ILs
The price of the solvent is a key problem in its industrialisation; whenever a new solvent is proposed, one of the most asked questions is its cost. Up to now, it can be confirmed that the success of DES lays on its simplicity of the preparation method with the parent compounds like organic salts and ionic liquids. Quaternary Ammonium Salts (QASs) and phosphonium salts are used to prepare the DES, which are cheap compared to imidazolium-based ionic liquids. One of the most attractive features of DES systems is their straightforward preparation process is just mixing, and heating without purification, which is the real benefit not only from the synthesis point of view but also from the economic point of view. The introduction of all the DES as the raw material is cheap, environmentally friendly, and economical.14 Therefore, the cost of DES and ILs was assessed using the equation reported by Dugoni et al.41| DES or ILs price = M1P1 + M2P2/M1 + M2 | (12) |
To compare the price of DES [ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EG (1
EG (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)] and ILs, ILs were selected from the previous reports,28,42 which were used for the pretreatment of microalgae biomass for carotenoid extraction. Five ILs [1-ethyl-3-methylimidazolium chloride (EMIM)Cl, 1-ethyl-3-methyl-imidazolium-ethylsulphate (EMIM) EtOSO3, 1-butyl-3-methylimidazoliumbromide, 1-butyl-3-methyl-imidazolium-chloride (BMIM) Cl, and diallylammonium diallylcarbamate (DACARB)] were used and labelled as IL-01 to IL-05. Fig. 7 shows the comparison of the DES and ILs, and it can be observed that the price of DES (18.72 USD per kg) is 13 times less than that of IL-02 [1-ethyl-3-methyl-imidazolium-ethylsulphate (EMIM EtOSO3)]. In the same way the cost of DES compared to other ILs is also less (i.e., 28, 10, 8, and 2 times). This shows a significant cost reduction, which can play a vital role in biomass valorization to extract lutein and other value-added compounds.
2)] and ILs, ILs were selected from the previous reports,28,42 which were used for the pretreatment of microalgae biomass for carotenoid extraction. Five ILs [1-ethyl-3-methylimidazolium chloride (EMIM)Cl, 1-ethyl-3-methyl-imidazolium-ethylsulphate (EMIM) EtOSO3, 1-butyl-3-methylimidazoliumbromide, 1-butyl-3-methyl-imidazolium-chloride (BMIM) Cl, and diallylammonium diallylcarbamate (DACARB)] were used and labelled as IL-01 to IL-05. Fig. 7 shows the comparison of the DES and ILs, and it can be observed that the price of DES (18.72 USD per kg) is 13 times less than that of IL-02 [1-ethyl-3-methyl-imidazolium-ethylsulphate (EMIM EtOSO3)]. In the same way the cost of DES compared to other ILs is also less (i.e., 28, 10, 8, and 2 times). This shows a significant cost reduction, which can play a vital role in biomass valorization to extract lutein and other value-added compounds.
4. Conclusion
In summary, microalgae are a promising source of lutein and other value-added chemicals for food applications. To improve the cost effectiveness of the algal biomass, a biorefinery approach was applied. Sequential extraction of lutein and lipid from DES-pretreated dry and wet biomass was attempted, representing a stepwise extraction of the compounds. The kinetic study of solid liquid extraction process of compounds were performed. Kinetic parameters showed that a lower temperature is needed for the lutein and lipid extraction using ethanol and M![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA (2
EA (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent, indicating the lower energy requirement, which can improve the economics in a large-scale process. Furthermore, the solvent demonstrated lower toxicity, as evidenced by the survival of E. coli, indicating environmental friendliness.
1) solvent, indicating the lower energy requirement, which can improve the economics in a large-scale process. Furthermore, the solvent demonstrated lower toxicity, as evidenced by the survival of E. coli, indicating environmental friendliness.
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request.Author contributions
Conceptualization: BZ, GM, JX, methodology: BZ, GM; investigation: BZ, GM, MAA, LD; supervision: JX, GM, XZ; writing—original draft: BZ, GM; writing—review & editing: BZ, GM, AZ, MAA, ZL, TB, JX; funding: JX, GM.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This work was funded by the National Natural Science Foundation of China (Grant No. 22262232515), Key R&D Special Project of Henan Province (Grant No. 251111310200) the Henan Province Science and Technology Research Program (242102321053), and the China Postdoctoral Science Foundation (Grant No. 2024M752804).References
- G. Muhammad, A. D. P. Ngatcha, Y. Lv, W. Xiong, Y. A. El-Badry, E. Asmatulu, J. Xu and M. A. Alam, Renewable Energy, 2022, 184, 753–764 Search PubMed.
- G. Muhammad, M. A. Alam, M. Mofijur, M. I. Jahirul, Y. Lv, W. Xiong, H. C. Ong and J. Xu, Renewable Sustainable Energy Rev., 2021, 135, 110209 Search PubMed.
- H. Zheng, Y. Wang, S. Li, D. Nagarajan, S. Varjani, D. J. Lee and J. S. Chang, Renewable Sustainable Energy Rev., 2022, 153, 111795 Search PubMed.
- G. Muhammad, T. O. Butler, B. Chen, Y. Lv, W. Xiong, X. Zhao, A. Solovchenko, A. Zhao, M. Mofijur, J. Xu and M. A. Alam, Biomass Convers. Biorefin., 2022, 1–22 Search PubMed.
- A. Saravanan, P. Senthil Kumar, M. Badawi, G. Mohanakrishna and T. M. Aminabhavi, Chem. Eng. J., 2023, 453, 139754 Search PubMed.
- M. J. Barbosa, M. Janssen, C. Südfeld, S. D'Adamo and R. H. Wijffels, Trends Biotechnol., 2023, 41, 452–471 Search PubMed.
- A. K. Patel, A. P. Vadrale, Y. S. Tseng, C. W. Chen, C.-D. Dong and R. R. Singhania, Bioresour. Technol., 2022, 351, 126928 Search PubMed.
- Y. Zhang, X. Kong, Z. Wang, Y. Sun, S. Zhu, L. Li and P. Lv, Renewable Energy, 2018, 125, 1049–1057 Search PubMed.
- E. Damergi, J.-P. Schwitzguébel, D. Refardt, S. Sharma, C. Holliger and C. Ludwig, Algal Res., 2017, 25, 488–495 Search PubMed.
- M. Gong, X. Li and A. Bassi, J. Appl. Phycol., 2018, 30, 1617–1627 Search PubMed.
- M. del Mar Contreras-Gamez, A. Galan-Martin, N. Seixas, A. M. da Costa Lopes, A. Silvestre and E. Castro, Bioresour. Technol., 2023, 369, 128396 Search PubMed.
- S. Spittle, D. Poe, B. Doherty, C. Kolodziej, L. Heroux, M. A. Haque, H. Squire, T. Cosby, Y. Zhang and C. Fraenza, Nat. Commun., 2022, 13, 219 Search PubMed.
- H. Zhu, X. He, Z. Xu and L. Dai, Green Chem., 2025, 27, 1278–1299 Search PubMed.
- Y. Ma, Y. Yang, T. Li, S. Hussain and M. Zhu, Green Chem., 2024, 26, 3627–3669 Search PubMed.
- G. Muhammad, J. Xu, Z. Li, L. Zhao and X. Zhang, Sci. Total Environ., 2024, 924, 171547 Search PubMed.
- W. Lu, M. A. Alam, Y. Pan, J. Wu, Z. Wang and Z. Yuan, Bioresour. Technol., 2016, 218, 123–128 Search PubMed.
- Y. Pan, M. A. Alam, Z. Wang, D. Huang, K. Hu, H. Chen and Z. Yuan, Bioresour. Technol., 2017, 238, 157–163 Search PubMed.
- G. Muhammad, J. Wang, W. Xiong, Y. Lv, S. Zhang, A. Zhao, P. Jahanbakhsh-Bonab, A. Solovchenko, J. Xu and M. A. Alam, J. Mol. Liq., 2022, 120775 Search PubMed.
- J. Wu, M. A. Alam, Y. Pan, D. Huang, Z. Wang and T. Wang, J. Taiwan Inst. Chem. Eng., 2017, 71, 323–329 Search PubMed.
- J. Torregrosa-Crespo, X. Marset, G. Guillena, D. J. Ramon and R. Maria Martinez-Espinosa, Sci. Total Environ., 2020, 704, 135382 Search PubMed.
- G. Muhammad, P. Jahanbakhsh-Bonab, W. Xiong, Y. Lv, S. Zhang, A. Zhao, J. J. Sardroodi, J. Xu and M. A. Alam, Ind. Crops Prod., 2024, 209, 117940 Search PubMed.
- W. Gu, J. M. Kavanagh and D. D. McClure, Algal Res., 2022, 61, 102564 Search PubMed.
- H. Harouna, H. Fauduet, C. Porte and Y. S. Ho, Chem. Eng. Commun., 2007, 194, 537–552 Search PubMed.
- U. Balyan and B. Sarkar, Int. J. Food Prop., 2017, 20, 372–389 Search PubMed.
- N. Delgado-Mellado, M. Larriba, P. Navarro, V. Rigual, M. Ayuso, J. García and F. Rodríguez, J. Mol. Liq., 2018, 260, 37–43 Search PubMed.
- H. Çabuk, Y. Yılmaz and E. Yıldız, Acta Chim. Slov., 2019, 66, 385–394 Search PubMed.
- K. L. Low, A. Idris and N. M. Yusof, Process Biochem., 2022, 121, 87–99 Search PubMed.
- Y. Zhu, X. Li, Y. Wang, L. Ren and Q. Zhao, Algal Res., 2021, 60, 102528 Search PubMed.
- M. Cerón-García, C. V. González-López, J. Camacho-Rodríguez, L. López-Rosales, F. García-Camacho and E. Molina-Grima, Food Chem., 2018, 257, 316–324 Search PubMed.
- C.-Y. Chen, C. Hsieh, D. J. Lee, C. H. Chang and J. S. Chang, Bioresour. Technol., 2016, 200, 500–505 Search PubMed.
- M. Ochoa Becerra, L. Mojica Contreras, M. Hsieh Lo, J. Mateos Díaz and G. Castillo Herrera, J. Funct. Foods, 2020, 66, 103771 Search PubMed.
- X. D. Fan, Y. Hou, X. X. Huang, T. Q. Qiu and J. G. Jiang, J. Agric. Food Chem., 2015, 63, 4597–4605 Search PubMed.
- P. Hobbi, O. Okoro, C. Delporte, H. Alimoradi, D. Podstawczyk, L. Nie, K. Bernaerts and A. Shavandi, Bioresour. Bioprocess., 2021, 8, 114 Search PubMed.
- M. R. González-Centeno, F. Comas-Serra, A. Femenia, C. Rosselló and S. Simal, Ultrason. Sonochem., 2015, 22, 506–514 Search PubMed.
- S. Wahidin, A. Idris and S. R. M. Shaleh, Energy Convers. Manag., 2014, 84, 227–233 Search PubMed.
- M. T. Arias-Peñaranda, E. Cristiani-Urbina, C. Montes-Horcasitas, F. Esparza-García, G. Torzillo and R. O. Cañizares-Villanueva, Bioresour. Technol., 2013, 140, 158–164 Search PubMed.
- G. Knothe, Energy Fuels, 2008, 22, 1358–1364 Search PubMed.
- M. Khalekuzzaman, M. Alamgir, M. B. Islam and M. J. P. O. Hasan, PLoS One, 2019, 14, e0225458 Search PubMed.
- J. Jena, M. Nayak, H. Pamda, N. Pradhan, C. Sarika, P. Panda, B. Rao, R. Prasad and L. Sukla, World Environ., 2012, 2(1), 1–16 Search PubMed.
- M. Ngangkham, S. K. Ratha, R. Prasanna, A. K. Saxena, D. W. Dhar, C. Sarika and R. B. N. Prasad, SpringerPlus, 2012, 1, 1–13 Search PubMed.
- G. C. Dugoni, A. Mezzetta, L. Guazzelli, C. Chiappe, M. Ferro and A. Mele, Green Chem., 2020, 22, 8680–8691 Search PubMed.
- K. S. Khoo, C. W. Ooi, K. W. Chew, S. C. Foo, J. W. Lim, Y. Tao, N. Jiang, S. H. Ho and P. L. Show, J. Chem. Eng., 2021, 411, 128510 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra00423c |
| ‡ Both authors contributed equally and will be regarded as 1st co-author. |
| This journal is © The Royal Society of Chemistry 2025 |