Open Access Article
Open Access ArticleEfficient wastewater treatment and biomass co-production using energy microalgae to fix C, N, and P
Han Yuanac,
Pengxinyue Huanga,
Jiang Yu *acd,
Pu Wang*b,
Hong Bin Jiangb,
Yinying Jiangac,
Siwei Dengae,
Zhi Huangac,
Jie Yua and
Weiwei Zhua
*acd,
Pu Wang*b,
Hong Bin Jiangb,
Yinying Jiangac,
Siwei Dengae,
Zhi Huangac,
Jie Yua and
Weiwei Zhua
aDepartment of Environmental Science and Engineering, College of Architecture and Environment, Sichuan University, Chengdu, 610065, PR China. E-mail: yuj@scu.edu.cn
bSuining Ecological Environmental Monitoring Centre of Sichuan Province, Suining Sichuan 629000, China
cInstitute of New Energy and Low Carbon Technology, Sichuan University, Chengdu, 610065, PR China
dYibin Institute of Industrial Technology, Sichuan University, Yibin 644000, PR China
eSoil and Groundwater Pollution Prevention Research Institute, Sichuan Academy of Eco-Environmental Sciences, 610046, Chengdu, P. R. China
First published on 30th April 2025
Abstract
Distillery wastewater (DWW), characterized by high organic and nutrient loads, represents a significant environmental challenge, requiring effective and sustainable treatment solutions. This study investigates an innovative approach that integrates microalgae cultivation with DWW treatment for simultaneous bioremediation and biomass production, while also exploring carbon sequestration. We analyzed the growth characteristics, nutrient removal efficiency, protein accumulation, and ultrastructural changes of Chlamydomonas reinhardtii and Scenedesmus dimorphus under varying nitrogen-to-phosphorus (N/P) ratios in diluted DWW. Optimal nutrient removal and biomass accumulation were achieved at N/P concentrations of 46/11.5 mg L−1. C. reinhardtii showed particularly high nutrient removal rates, with significantly high removal rates for total nitrogen, total phosphorus, and chemical oxygen demand. S. dimorphus, under the same conditions, demonstrated exceptional protein accumulation and also effectively removed pollutants. Both species showed enhanced performance under these conditions, with microalgal cell organelles remaining structurally intact, and chloroplasts and thylakoid layers well-developed. The study also explored carbon sequestration potential using varying CO2 concentrations, where C. reinhardtii exhibited enhanced biomass accumulation at 3000 ppm CO2. This integrated approach offers an effective and economically feasible solution for distillery wastewater treatment, simultaneously enabling biomass production and carbon capture. The dual benefits of bioremediation and bioenergy generation position this technology as a promising pathway for sustainable resource management and environmental protection.
1. Introduction
With the rapid development of the brewing industry, distillery wastewater (DWW), characterized by its high pollution load and treatment difficulty, has become a significant environmental challenge.1 DWW is acidic and contains high concentrations of chemical oxygen demand (COD), biological oxygen demand (BOD), heavy metals (HMs), and endocrine-disrupting chemicals (EDCs), as well as being rich in nutrients such as nitrogen and phosphorus.2–5 If untreated DWW is discharged into the environment, it not only reduces sunlight penetration in water bodies, decreases dissolved oxygen (DO) levels, and lowers photosynthetic efficiency but also triggers eutrophication and disrupts ecosystem balance.6Physical and chemical methods, as traditional wastewater treatment approaches, offer rapid treatment but come with high costs and the potential for secondary pollution.7–12 Microalgae technology has gained significant attention due to its low cost and high efficiency in removing nutrients, as well as its ability to facilitate biomass resource recovery for multiple uses.13,14 For example, Li et al.15 and Shrasti Vasistha et al.16 demonstrated that using different algal species to treat DWW resulted in increased biomass production and improved nutrient removal efficiency. However, most research is limited to monoculture systems, and the advantages of mixed culture systems have yet to be fully explored.
Microalgae are photosynthetic autotrophic microorganisms. Due to their rapid growth, efficient photosynthesis, and diverse metabolites, they have garnered significant attention in wastewater treatment and bioenergy development.17,18 Organic and inorganic pollutants in distillery wastewater (DWW) can serve as carbon sources and nutrients for microalgae, respectively. Combining microalgae cultivation with DWW treatment can significantly enhance the removal efficiency of nitrogen and phosphorus while reducing cultivation costs.19 Studies have shown that optimizing the nitrogen-to-phosphorus (N/P) ratio can markedly improve nutrient removal effectiveness.20,21 Additionally, microalgae convert inorganic carbon into carbohydrates through photosynthesis and release O2, creating a novel and efficient carbon sequestration model that has become a hot research topic in air pollution control.22 The carbon content of microalgae is typically 0.5 kg C kg−1 dry weight (DW), and their average carbon capture capacity is 1.8 kg CO2 kg−1 DW.23 Furthermore, microalgae are 10 to 50 times more effective at carbon sequestration than terrestrial plants.24 The CO2 content in industrial flue gas can reach 10% to 30%, and using it as a carbon source for microalgae cultivation can not only lower cultivation costs but also achieve the dual goals of CO2 fixation and environmental benefits.
In light of the insufficient focus on mixed cultures and the optimization of N/P concentrations in existing studies,25 this research investigates Chlamydomonas reinhardtii and Scenedesmus dimorphus. The growth characteristics, nutrient removal, protein accumulation, and ultrastructural changes of these species were systematically examined at various N/P concentrations. Additionally, the study explores the carbon sequestration mechanism in relation to different concentrations of exogenous CO2. The findings are expected to offer new insights for the treatment and resource utilization of distillery wastewater (DWW), while also achieving the comprehensive goals of efficient microalgal cultivation and high-value product extraction.
2. Methods and methods
2.1. Microalgae resources and wastewater components
C. reinhardtii and S. dimorphus were both obtained from the Algae Culture Laboratory at the College of Life Sciences, Sichuan University. These two microalgae strains were cultured in modified WC (Woods Hole Chu #10) medium, with potassium phosphate tribasic monohydrate (K2HPO4·3H2O) (1.55 mg P L−1) and sodium nitrate (NaNO3) (14 mg N L−1) as phosphorus and nitrogen sources, respectively. Before the formal experiment began, the microalgae were first acclimated and cultured in WC medium to ensure that the microalgae were in the logarithmic growth phase. The composition of the WC medium is detailed in Table 1.| S. No. | Components | Concentration (g L−1) | Volume (mL) |
|---|---|---|---|
| 1 | CaCl2·2H2O | 1.165 | 1 |
| 2 | MgSO4·7H2O | 95.890 | 1 |
| 3 | NaHCO3 | 14.162 | 1 |
| 4 | K2HPO4·3H2O | 5.991 | 1 |
| 5 | NaNO3 | 0.423 | 1 |
| 6 | Trace elements | 1 | |
| Na2EDTA | 4.36 | ||
| FeCl3·6H2O | 3.15 | ||
| CuSO4·5H2O | 0.01 | ||
| ZnSO4·7H2O | 0.022 | ||
| CoCl2·6H2O | 0.01 | ||
| MnCl2·4H2O | 0.18 | ||
| NaMoO4 | 0.006 | ||
| H3BO13 | 1.00 |
This study utilized distillery wastewater (DWW) from the Huangjia Baxian Distillery in Shuangliu County, Chengdu, Sichuan Province, which produces lager beer. Before microalgae cultivation, the DWW was first filtered through a 0.45 μm pore-sized cellulose acetate filter paper, then sterilized by autoclaving at 121 °C for 30 minutes, and stored sealed at 4 °C for later use. The composition of the DWW is shown in Table 2. The values presented in Table 3 for raw wastewater, after air floated, and after autoclaved, are all from the same sample, but are presented as a mean of 3 measurements.
| Parameters | Raw wastewater | After air floated | After autoclaved |
|---|---|---|---|
| pH | 3.62 | 6.92 | 6.87 |
| COD (mg L−1) | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2021 | 1200 |
| TN (mg L−1) | 2125 | 52 | 46 |
| TP (mg L−1) | 592 | 13 | 11.5 |
| CO2 concentration (ppm) | Biomass productivity (mg L−1 d−1) | CO2 fixation rate (mg L−1 d−1) |
|---|---|---|
| 380 | 27.02 | 44.59 |
| 3000 | 50.95 | 84.07 |
| 6000 | 7.98 | 13.16 |
| 9000 | 0.64 | 1.05 |
Various dilution ratios of the DWW (undiluted: 100%, v/v; 2-fold: 50%, v/v; 4-fold: 25%, v/v; 8-fold: 12.5%, v/v) were tested. Preliminary experiments revealed that microalgae exhibited no growth in the undiluted wastewater medium due to the high alcohol concentration, which caused algal toxicity. Based on the results of the preliminary experiments, a 2-fold dilution was selected as the optimal dilution ratio for this study.
2.2. Experimental setup and operation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Cultivation conditions included static growth under 2000 lux illumination (12L:12D cycle) at 20 ± 1 °C, with manual shaking (15 seconds, 3 times daily).
1. Cultivation conditions included static growth under 2000 lux illumination (12L:12D cycle) at 20 ± 1 °C, with manual shaking (15 seconds, 3 times daily).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio of 4
P ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by weight. The experimental period lasted for 17 days, during which six different nitrogen-to-phosphorus concentration gradients were tested for C. reinhardtii, S. dimorphus, and their co-cultivation. The different N
1 by weight. The experimental period lasted for 17 days, during which six different nitrogen-to-phosphorus concentration gradients were tested for C. reinhardtii, S. dimorphus, and their co-cultivation. The different N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratios were achieved by adjusting the concentration of NaNO3 and K2HPO4·3H2O. The experimental groups had N/P concentrations of 11.6/2.9, 46/11.5, 69/17.25, 103.5/25.9, 155.6/38.9, and 600/150 mg L−1, respectively. All cultures were performed in triplicate. Two different sizes of Erlenmeyer flasks were used. 150 mL Erlenmeyer flasks were used with 50 μL of inoculum and 50 mL of growth medium, and 250 mL Erlenmeyer flasks were used with 50 μL of inoculum and 130 mL of growth medium. The 150 mL flasks were used for all tests except for the cultures used for the nutrient analysis, which were grown in the larger 250 mL flasks to allow for a larger volume to be available for analysis. The cultivation conditions were the same as in Section 2.2.1, with a light intensity of 2000 lx at the surface of the culture medium, a temperature of 25 ± 2 °C, and a light cycle of 12 L:12 D, and static cultivation. The position of the Erlenmeyer flasks was rotated daily, and the cultures were manually shaken for 15 seconds, 3 times daily until the microalgae were uniformly suspended in the medium.
P ratios were achieved by adjusting the concentration of NaNO3 and K2HPO4·3H2O. The experimental groups had N/P concentrations of 11.6/2.9, 46/11.5, 69/17.25, 103.5/25.9, 155.6/38.9, and 600/150 mg L−1, respectively. All cultures were performed in triplicate. Two different sizes of Erlenmeyer flasks were used. 150 mL Erlenmeyer flasks were used with 50 μL of inoculum and 50 mL of growth medium, and 250 mL Erlenmeyer flasks were used with 50 μL of inoculum and 130 mL of growth medium. The 150 mL flasks were used for all tests except for the cultures used for the nutrient analysis, which were grown in the larger 250 mL flasks to allow for a larger volume to be available for analysis. The cultivation conditions were the same as in Section 2.2.1, with a light intensity of 2000 lx at the surface of the culture medium, a temperature of 25 ± 2 °C, and a light cycle of 12 L:12 D, and static cultivation. The position of the Erlenmeyer flasks was rotated daily, and the cultures were manually shaken for 15 seconds, 3 times daily until the microalgae were uniformly suspended in the medium.To ensure the supply of nutrients, 10 mL of original culture medium was added to the 150 mL Erlenmeyer flask samples every other day to compensate for the algae cell liquid taken for analysis.
2.3. Analytical method
The dry cell weight (DCW) of the microalgae biomass was estimated every other day over 17 days to study the growth curve. The specific growth rate (μ, number of divisions per day) was calculated using the following formula:29
 | (1) |
 | (2) |
The nutrient removal rate was calculated using eqn (3) based on the measured nutrient content.
 | (3) |
 | (4) |
 | (5) |
3. Results and discussion
3.1. The growth characteristics of microalgae in distillery wastewater
Growth rate is a very important factor to be determined for microalgal species, which determines the amount of biomass available and the amount of carbon fixed.32. The growth trends and specific growth rates of microalgae are shown in Fig. 1a–c, with the specific growth rates shown in Fig. 1d. The different N/P conditions resulted in different growth trends for all cultures. C. reinhardtii (Fig. 1a) showed a rapid increase in biomass, entering the exponential growth phase around days 7 to 9 and reaching a maximum of 730 mg L−1 at day 9 in the 46/11.5 condition, while S. dimorphus (Fig. 1b) and the co-culture (Fig. 1c) showed slower growth. These results indicate that a diluted DWW with a N/P ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 can support microalgae cultivation. Although the optimal N/P ratio for microalgal biomass production is generally considered to be 16
1 can support microalgae cultivation. Although the optimal N/P ratio for microalgal biomass production is generally considered to be 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,33,34 the optimal ratio for microalgae still having good growth performance was found to be different in this experiment by 2-fold dilution and maintaining a DWW environment with N/P of approximately 4
1,33,34 the optimal ratio for microalgae still having good growth performance was found to be different in this experiment by 2-fold dilution and maintaining a DWW environment with N/P of approximately 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. When the initial nitrogen content in the medium exceeds that of phosphorus, microalgae growth primarily depends on nitrogen, which is required for protein synthesis. In the 46/11.5 group, by day 9, when the biomass reached its maximum, the total phosphorus in the medium had decreased sharply, suggesting a reduction in the availability of phosphorus. This could lead to phosphorus starvation, and reduced photosynthetic efficiency,20 explaining the decline in growth after day 9.
1. When the initial nitrogen content in the medium exceeds that of phosphorus, microalgae growth primarily depends on nitrogen, which is required for protein synthesis. In the 46/11.5 group, by day 9, when the biomass reached its maximum, the total phosphorus in the medium had decreased sharply, suggesting a reduction in the availability of phosphorus. This could lead to phosphorus starvation, and reduced photosynthetic efficiency,20 explaining the decline in growth after day 9.
As total nitrogen and other nutrients in the culture medium were consumed, microalgae growth in all groups rapidly entered a decline phase. In the 600/150 (N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) concentration group, S. dimorphus (Fig. 1b) showed a significant reduction in maximum biomass, reaching a value of 250 mg L−1, compared to other treatment groups which reached over 500 mg L−1. This indicates that high nitrogen and phosphorus concentrations significantly inhibited the growth of S. dimorphus. Compared to monoculture conditions, the growth curves under co-culture conditions were more irregular, and the biomass was lower, particularly at later time points, suggesting a competitive relationship between the two microalgae species under these conditions. This suggests that under high nutrient conditions, interspecies competition may play a larger role than under low nutrient conditions.
P) concentration group, S. dimorphus (Fig. 1b) showed a significant reduction in maximum biomass, reaching a value of 250 mg L−1, compared to other treatment groups which reached over 500 mg L−1. This indicates that high nitrogen and phosphorus concentrations significantly inhibited the growth of S. dimorphus. Compared to monoculture conditions, the growth curves under co-culture conditions were more irregular, and the biomass was lower, particularly at later time points, suggesting a competitive relationship between the two microalgae species under these conditions. This suggests that under high nutrient conditions, interspecies competition may play a larger role than under low nutrient conditions.
Although the biomass in the 600/150 concentration group was higher than in the 11.6/2.9 low-phosphorus treatment group, it was still lower than in the other treatment groups, and resulted in a lower overall maximum biomass compared to other groups. This is because phosphorus is considered a limiting factor for growth under low phosphorus conditions; the limited phosphate availability not only reduces the efficiency of the Calvin cycle and carbon fixation capacity, but also restricts the uptake of phosphate, thereby affecting their growth and metabolic activities.35 In contrast, when phosphorus is not limiting, the phosphate uptake is not restricted.
The specific growth rates of all cultures are shown in Fig. 1d. In general, the specific growth rate of microalgae initially increased and then decreased over time, with all cultures showing a similar maximum specific growth rate (of approximately 0.28 d−1), but cultures grown under lower N and P concentrations showing reduced maximum specific growth rates. When the TN/TP ratio was 11.6/2.9, the specific growth rates of C. reinhardtii, S. dimorphus, and the co-cultivation group were at their lowest (0.186, 0.174, and 0.168 d−1, respectively), suggesting that in this condition, both N and P may be limiting for growth, although the low P concentration may be playing a larger role. When the N/P concentrations was 69/17.25, the specific growth rates of S. dimorphus and the co-cultivation group began to decrease from day 8 onwards. This may be due to the initial rapid growth and phosphorus uptake, which led to a severe imbalance in the N/P concentrations and the depletion of phosphorus more quickly. Additionally, as the initial nutrient concentration increased, the co-culture became more susceptible to the effects of total phosphorus (TP), possibly due to interspecies competition for the available phosphorus.
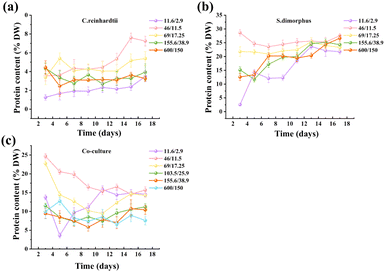
3.2. Protein synthesis characteristics of diluted distillery wastewater from microalgae culture
The protein content of C. reinhardtii and S. dimorphus, expressed as a percentage of dry biomass (% DW), showed significant variations under different nitrogen-to-phosphorus (N/P) ratios in diluted brewery wastewater (DWW). For C. reinhardtii, protein content peaked on day 15 (7.6% DW) at an N/P ratio of 46/11.5 mg L−1 (Fig. 2a). Under low N/P conditions (11.6/2.9 mg L−1), the maximum protein content was only 3.5% DW, likely due to phosphorus limitation inhibiting ATP-dependent ribosomal activity. This conclusion was supported by TEM observations of mitochondrial vacuolization and membrane rupture (Fig. 5a).36,37 At high N/P ratios (600/150 mg L−1), protein synthesis decreased further to 3.6% DW, potentially caused by cell swelling (Fig. 7) and enzyme activity inhibition in acidified environments from excess CO2.S. dimorphus demonstrated superior protein accumulation capacity, reaching 27.6% DW at the optimal N/P ratio of 46/11.5 mg L−1 (Fig. 2b). This performance correlated with its high total nitrogen removal efficiency (95.43%, Fig. 3b). The species preferentially allocated nitrogen to protein synthesis over lipid storage, a metabolic preference aligning with its ecological role in eutrophic wastewater remediation.38 Intact chloroplasts and well-organized thylakoid layers under optimal N/P conditions (Fig. 6b) enhanced photosynthetic carbon fixation, directly supporting amino acid biosynthesis.39 However, protein content at the N/P concentration ratio of 600/150 mg L−1 was lower than that at 46/11.5 mg L−1, reflecting that at high nitrogen concentration, overnutrition led to metabolic disorders, cell division was blocked, and biomass and protein content were lower.40
In co-culture systems, the maximum protein content (25.6% DW at 46/11.5 mg L−1) was intermediate between those of the two monocultures (Fig. 2c), suggesting phosphorus competition between species. While S. dimorphus dominated phosphorus uptake (showing comparable total phosphorus removal to its monoculture, Fig. 3d), C. reinhardtii likely experienced protein synthesis inhibition due to phosphorus limitation. In addition, the significant decline in protein content during co-culture may result from competition between carbon (C) and sulfur (S) for ATP-dependent assimilation pathways. For example, sulfur is essential for cysteine and methionine synthesis, which are critical for protein formation. Under nutrient-limited conditions, microalgae prioritize sulfur allocation to antioxidant systems (e.g., glutathione) over protein synthesis, as observed in Chlamydomonas by Aburai et al.41 The N/P ratio of 46/11.5 mg L−1 optimized nutrient supply and metabolic requirements, promoting efficient protein synthesis while maintaining organelle integrity.42 This ratio provided sufficient phosphorus for ATP and NADPH production during nitrogen assimilation without causing nutrient inhibition.43
3.3. Nutrient removal performance of cultured microalgae in diluted distillery wastewater
Phosphorus is a fundamental macronutrient for microalgae, and potassium hydrogen phosphate (K2HPO4) can be directly transported into microalgal cells through membrane transport proteins, where it participates in the synthesis of nucleic acids, adenosine triphosphate (ATP), and membrane phospholipids,48 and may be removed from the system through phosphate precipitation. The changes in TP absorption rates in the culture media of each experimental group are shown in Fig. 3c, while the final TP removal rates and removal efficiencies are presented in Fig. 3d. As mentioned in ref. 49, previous studies have indicated that phosphorus absorption decreases under low nitrogen conditions. This study found that under the lowest N/P concentration (11.6/2.9 mg L−1), C. reinhardtii showed a very high phosphorus absorption rate (Fig. 3c), and a final TP removal efficiency of close to 100% after 17 days (Fig. 3d). In the 46/11.5 group, S. dimorphus removed 7.84 mg L−1 of TP, with a final removal efficiency of 68.94% after 17 days. This may be because when the culture medium contains an appropriate level of phosphorus, phosphorus-starved algal cells may accumulate more phosphorus, possibly through a hunger-driven absorption mechanism.50
When the N/P concentration increased to 600/150 mg L−1, the high nutrient environment caused significant stress to S. dimorphus, leading to a reduced biomass, which led to a release of phosphates, and an increase in TP concentration (Fig. 3d).51 The TP removal efficiency decreased with increasing initial N and P concentration. Throughout the experimental period, C. reinhardtii exhibited a higher TP removal efficiency at most initial concentrations than S. dimorphus, particularly at lower nutrient concentrations (Fig. 3d), with C. reinhardtii showing much higher TP removal at the lowest N and P concentrations. Under co-culture conditions, the TP removal effect was consistent with that of S. dimorphus, suggesting that S. dimorphus plays a dominant role in wastewater nutrient utilization and may inhibit C. reinhardtii's ability to absorb TP.
In the distillery wastewater with an initial N/P concentrations of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, S. dimorphus and the co-culture group showed a higher COD removal efficiency than C. reinhardtii. The final COD values at this N/P concentrations were 260 mg L−1 for C. reinhardtii, and 160 and 140 mg L−1 for S. dimorphus and the co-culture, respectively, as shown in Fig. 4d. However, at high initial N/P concentrations (600/150 group), the final COD removal ability of C. reinhardtii was more stable, and C. reinhardtii and the co-culture group showed a higher final COD removal, compared to S. dimorphus, as shown in Fig. 4d. This indicates that C. reinhardtii has a higher COD removal ability at high-N/P and low-N/P concentrations, suggesting that it may be more suitable for treating undiluted distillery wastewater.
1, S. dimorphus and the co-culture group showed a higher COD removal efficiency than C. reinhardtii. The final COD values at this N/P concentrations were 260 mg L−1 for C. reinhardtii, and 160 and 140 mg L−1 for S. dimorphus and the co-culture, respectively, as shown in Fig. 4d. However, at high initial N/P concentrations (600/150 group), the final COD removal ability of C. reinhardtii was more stable, and C. reinhardtii and the co-culture group showed a higher final COD removal, compared to S. dimorphus, as shown in Fig. 4d. This indicates that C. reinhardtii has a higher COD removal ability at high-N/P and low-N/P concentrations, suggesting that it may be more suitable for treating undiluted distillery wastewater.
3.4. Ultrastructural changes of distillery wastewater after microalgae culture
Fig. 5 shows the ultrastructure of C. reinhardtii (a) and S. dimorphus (b) when cultured under the low N/P conditions (11.6/2.9 mg L−1). Under these low N/P conditions, mitochondrial cavitation and membrane rupture were observed in C. reinhardtii cells (Fig. 5a), which can impair oxygen respiration and disrupt energy conversion, potentially contributing to the reduced growth observed under these conditions. This may be attributed to excessive phosphorus leading to the overaccumulation of polyphosphate within the cells, which disrupts membrane permeability and enzyme function.53 Compared to C. reinhardtii, the effects of low nutrient concentrations on the organelles of S. dimorphus were more pronounced (Fig. 5b): the chloroplast structure appeared significantly deformed, and the thylakoid lamellae were disorganized or even indistinct, severely impairing photosynthesis. The cell wall exhibited severe wrinkling, with partial degradation, and some organelles appeared to be disintegrated or dissolved, resulting in cavitation. Under nitrogen and phosphorus deficiency, both the photosynthetic function of the chloroplast and the respiratory function of the mitochondria were damaged, reducing the performance of this culture as compared to C. reinhardtii as seen in Fig. 1 and 2.Fig. 6 shows the ultrastructure of C. reinhardtii (a) and S. dimorphus (b) when cultured under optimal N/P conditions (46/11.5 mg L−1). Under these optimal N/P concentrations, C. reinhardtii exhibited the best growth and resource utilization of nitrogen and phosphorus among the experimental groups, as shown in Fig. 1 and 2. Under this condition, the chloroplasts appeared to be larger, and the pyrenoid within the chloroplasts increased in size, surrounded by a starch sheath, suggesting that the cells were actively fixing carbon and storing the results of that carbon fixation. The thylakoid layers were distinct and well-organized. Mitochondria also appeared to be larger, with intact structures, including inner and outer membranes (Fig. 6a). These findings suggest that these nutrient conditions can enhance cellular photosynthesis and respiration, as has been observed by the higher growth and protein content of these cultures. In comparison, S. dimorphus showed more pronounced impacts of environmental conditions on its cellular ultrastructure (Fig. 6b). Although its growth and nutrient utilization of nitrogen and phosphorus were also optimal under this condition, its overall performance was inferior to that of C. reinhardtii. The thylakoid layers in S. dimorphus were less dense and looser compared to those in C. reinhardtii, indicating a lower photosynthetic capacity. These observations are consistent with the functional data, and shows that the growth characteristics of S. dimorphus under these conditions are consistent with its cellular structure and remain less favorable than those of C. reinhardtii.
Fig. 7 shows the ultrastructure of C. reinhardtii (a) and S. dimorphus (b) when cultured under high N/P conditions (600/150 mg L−1). Under these high-nutrient conditions, the cells of both C. reinhardtii and S. dimorphus were larger than those in the low (Fig. 5) and optimal (Fig. 6) experimental groups, possibly due to excessive nitrogen and phosphorus absorption, leading to cell swelling (Fig. 7a and b). Most organelles in the microalgae cells appeared severely damaged and deformed, with indistinguishable structures, leaving the cells on the verge of death. Vacuoles appeared abnormally abundant, with reduced or dissolved contents. Some chloroplasts shrank or deformed, the thylakoid layer structures became disordered, and severe plasmolysis occurred. These morphological changes indicate that the cultures were under considerable stress, which has likely contributed to their reduced growth and nutrient uptake performance.
4. Biological carbon sequestration mechanism of C. reinhardtii cultivated by DWW
From the observations in the previous experimental stages, the dry weight of C. reinhardtii was consistently higher than that of S. dimorphus under the low and optimal conditions, as shown in Fig. 1a and b, with similar performance at high N/P concentrations. Therefore, in this stage, the study simulates the CO2 concentration in flue gas emissions from power plants, based on the atmospheric CO2 concentration in natural environments, to explore the use of different CO2 concentrations as an external carbon source for cultivating C. reinhardtii. This preliminary investigation into the carbon sequestration capacity of C. reinhardtii lays the groundwork for utilizing distillery wastewater to cultivate bioenergy microalgae and achieve efficient carbon fixation.4.1. Effects of different CO2 concentrations on the growth of Chlamydomonas reinhardtii
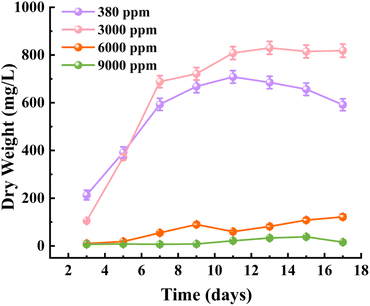
CO2 concentration directly affects the photosynthetic efficiency and growth of microalgae. While microalgae can tolerate a certain range of CO2 concentrations, concentrations beyond this range significantly inhibit growth and carbon sequestration capacity. Therefore, determining the optimal CO2 concentration is critical for achieving maximum biomass and protein yields for specific microalgae species,54 and is important for practical industrial applications. In this experimental phase, the initial inoculum concentration was relatively low (4.22 mg L−1) to ensure a clear analysis of growth rates, since high initial concentrations can affect the overall growth curve. The results showed that under atmospheric CO2 levels (380 ppm), C. reinhardtii rapidly entered the exponential growth phase, with biomass reaching 704 mg L−1 on day 11 and a specific growth rate of 0.465 d−1, as shown in Fig. 8a. At 3000 ppm CO2, C. reinhardtii exhibited slower growth during the first three days but then quickly transitioned into the exponential growth phase, achieving a biomass of 825 mg L−1 on day 13 with a specific growth rate of 0.406 d−1, as shown in Fig. 8a. This indicates that 3000 ppm CO2 enhances biomass accumulation. However, under 6000 ppm and 9000 ppm CO2 conditions, C. reinhardtii experienced a 1–7 days adaptation period, which was characterised by a slow growth, followed by even slower growth. Notably, biomass accumulation was negligible in the 9000 ppm group, as shown in Fig. 8a.Wang et al.55 found that decreasing CO2 concentrations within the range of 50% to 6% caused changes in tryptophan metabolites due to CO2 deficiency, resulting in division inhibition and growth stagnation, with high CO2 levels showing negative effects on growth, consistent with what has been found in the current study at high CO2 levels.
At low CO2 concentrations, the solubility of CO2 in water and the affinity of Rubisco for CO2 are relatively low.56 While higher CO2 concentrations provide more carbon sources, they simultaneously increase carbonic acid formation, leading to a decrease in pH, as shown in Fig. 8b, where the pH dropped from 5.54 to 4.72 as CO2 concentrations increased from 380 ppm to 9000 ppm. While the pH of the microalgal culture medium plays a role in regulating nutrient absorption, photosynthetic activity, and carbon fixation efficiency,57 the optimal pH range is difficult to maintain when using CO2 as an external carbon source, due to increased carbonic acid formation which results in a decrease in pH. Optimal or slightly alkaline conditions promote the conversion of CO2 to HCO3−, allowing microalgae to concentrate HCO3− within their cells via bicarbonate pumps. This process, facilitated by carbonic anhydrase or the Calvin cycle, enhances CO2 capture and promotes microalgal growth.58,59 However, under acidic conditions, chloroplast stroma acidification impairs the conversion of HCO3− to CO2, inactivating key enzymes of the Calvin cycle. This ultimately inhibits microalgal growth and reduces carbon assimilation efficiency.60,61
4.2. Effects of different CO2 concentrations on protein content of Chlamydomonas reinhardtii
As shown in Fig. 9, the protein content trends in the 380 ppm and 3000 ppm groups were similar, with both increasing with cultivation time, and reaching a maximum around day 13, followed by a slow decline. At a CO2 concentration of 3000 ppm, C. reinhardtii exhibited the maximum protein content, peaking at 45.28 mg L−1 on day 13, consistent with the maximum dry weight achieved in this group, as shown in Fig. 8a. This might be attributed to the availability of an appropriate carbon source, which promoted C. reinhardtii's cellular metabolism and growth, thereby enhancing the synthesis of primary metabolites such as proteins.In contrast, the 6000 ppm and 9000 ppm groups both showed the lowest protein content, with the 6000 ppm group reaching a value of only 3.54 mg L−1 at day 17, and the values for the 9000 ppm group being similarly negligible at all time points, as shown in Fig. 9. Studies have shown that elevated CO2 environments can enhance the transcription and translation of related genes and proteins (e.g., Rubisco), thereby boosting photosynthesis, carbon metabolism, protein synthesis, and lipid accumulation. However, excessively high CO2 concentrations can inhibit microalgal growth.62,63 This inhibition is primarily due to the increased acidity of the culture medium caused by high CO2 levels, which suppresses the normal physiological activities of C. reinhardtii, as linked to the pH changes shown in Fig. 8b. Consequently, the synthesis of intracellular components such as proteins is reduced, and cell division and growth are slowed or obstructed64 in C. reinhardtii.
4.3 Carbon sequestration capacity analysis of C. reinhardtii
The CO2 fixation rate of C. reinhardtii under different CO2 concentrations is shown in Table 3, At 3000 ppm CO2, the CO2 fixation rate of C. reinhardtii reached its peak (84.07 mg L−1 d−1), consistent with the observed peak biomass productivity (50.95 mg L−1 d−1). This indicates that moderate CO2 enrichment enhances photosynthetic efficiency and carbon assimilation, likely due to sufficient carbon availability for the Calvin cycle.65 At 6000 and 9000 ppm CO2, the fixation rates dropped sharply to 13.16 and 1.05 mg L−1 d−1, respectively. This decline correlates with the pH reduction (from 5.54 to 4.72, Fig. 8b), which disrupts cellular pH homeostasis and inhibits Rubisco enzyme activity.66 Additionally, excessive CO2 may induce oxidative stress, further impairing metabolic functions.67 The fixation rate at 3000 ppm (84.07 mg L−1 d−1) aligns with studies on Chlorella vulgaris (50–150 mg L−1 d−1), validating the feasibility of using C. reinhardtii for carbon capture in DWW. However, at 9000 ppm, C. reinhardtii is severely inhibited, in contrast to some extremophilia such as Scenedesmus sp., which can tolerate up to 100% CO2,68 highlighting species-specific adaptability. The results suggest that C. reinhardtii can be integrated into industrial flue gas treatment systems (typically 10–25% CO2)69 after dilution to 3000 ppm. This approach not only reduces CO2 emissions but also generates biomass for bioenergy production, achieving dual environmental and economic benefits.5. Conclusions
This study demonstrates that coupling distillery wastewater (DWW) treatment with microalgae cultivation for CO2 fixation offers a promising approach for simultaneous bioremediation, biomass production, and carbon capture. Specifically, an N/P concentration of 46/11.5 mg L−1 enabled efficient nutrient removal and resulted in high biomass and protein accumulation for both C. reinhardtii and S. dimorphus. However, excessive or insufficient nutrients significantly inhibited microalgal growth and performance, leading to reduced nutrient uptake, and resulted in structural changes such as cell wall separation, abnormal vacuole proliferation, and chloroplast contraction, as was shown in the experimental data.Although the carbon sequestration results obtained in this study with microalgae are preliminary, the observed increase in biomass at specific CO2 concentrations, particularly at 3000 ppm, indicates a potential for future carbon capture strategies using microalgae and DWW. Future research should explore the optimization of CO2 delivery systems and bioreactor designs to maximize microalgal carbon fixation, as well as address any limitations associated with scale-up, specifically with respect to high-density cultures. In conclusion, this study provides an innovative and economically feasible method for DWW treatment by integrating microalgae cultivation with CO2 fixation, highlighting the potential of microalgae for both resource recovery and environmental protection. Future work should focus on optimizing the N/P ratios and CO2 delivery, testing the process on undiluted DWW, and exploring the economic feasibility of this method for practical applications in wastewater treatment and bioenergy production.
Data availability
All data supporting this study are included within the article.Author contributions
All authors contributed to the study conception and design. The experiment was carried out by Han Yuan, Xinyue Huangpeng, Zhi Huang. Material preparation, data collection and analysis were performed by Han Yuan, Jiang Yu, Siwei Deng, Yinying Jiang. The first draft of the manuscript was written by Han Yuan. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was supported by the National Key Research and Development Program (No. 2018YFC1802605); the Science and Technology Project of Sichuan University-Suining City Strategic Cooperation “Unveiling the List and Leading the Way” (2024CDSN-09); the Science and Technology Innovation Project of the Sichuan Academy of Eco-Environmental Sciences (No. ZX-2023106).Notes and references
- Y. Ravikumar, S. A. Razack, J. Yun and G. Zhang, Recent advances in Microalgae-based distillery wastewater treatment, Environ. Technol. Innovation, 2021, 24, 101839 CrossRef CAS.
- J. Fito, N. Tefera, H. Kloos and S. W. Van Hulle, Physicochemical Properties of the Sugar Industry and Ethanol Distillery Wastewater and Their Impact on the Environment, Sugar Tech, 2019, 21, 265–277 CrossRef CAS.
- I. Johnson, C. Krishnan and M. Kumar, Enhancing nutrient bioavailability in distillery wastewater through electrochemical oxidation for microalgal growth: insights on biomass yield, nutrient utilisation, and VFA-assisted carbon capture, Algal Res., 2024, 83, 103734 CrossRef.
- H. Santana, et al., Microalgae cultivation in sugarcane vinasse: selection, growth and biochemical characterization, Bioresour. Technol., 2017, 228, 133–140 CrossRef CAS PubMed.
- S. Krishnamoorthy, P. Manickam and V. Muthukaruppan, Evaluation of distillery wastewater treatability in a customized photobioreactor using blue-green microalgae—laboratory and outdoor study, J. Environ. Manage., 2019, 234, 412–423 CrossRef CAS PubMed.
- S. Ratna, S. Rastogi and R. Kumar, Current trends for distillery wastewater management and its emerging applications for sustainable environment, J. Environ. Manage., 2021, 290, 112544 CrossRef CAS PubMed.
- Y. Song, et al., The promising way to treat wastewater by microalgae: approaches, mechanisms, applications and challenges, J. Water Process Eng., 2022, 49, 103012 CrossRef.
- J. Wang, Q. Tian, W. Zeng, G. Qiu and L. Shen, Insights about fungus-microalgae symbiotic system in microalgae harvesting and wastewater treatment: a review, Renewable Sustainable Energy Rev., 2023, 182, 113408 CrossRef CAS.
- A. Azimi, et al., Removal of Heavy Metals from Industrial Wastewaters: A Review, ChemBioEng Rev., 2017, 4, 37–59 CrossRef.
- A. Bhatt, et al., Assessing sustainability of microalgae-based wastewater treatment: environmental considerations and impacts on human health, J. Environ. Manage., 2024, 354, 120435 CrossRef PubMed.
- W. Dou, X. Peng, L. Kong and X. Hu, A review on the removal of Cl(-I) with high concentration from industrial wastewater: approaches and mechanisms, Sci. Total Environ., 2022, 824, 153909 CrossRef CAS PubMed.
- Y. Wu, J. Yu and Z. Huang, Migration of total petroleum hydrocarbon and heavy metal contaminants in the soil–groundwater interface of a petrochemical site using machine learning: impacts of convection and diffusion, RSC Adv., 2024, 14, 32304 RSC.
- J. T. Zhang, et al., Microalgal-bacterial biofilms for wastewater treatment: operations, performances, mechanisms, and uncertainties, Sci. Total Environ., 2024, 907, 167974 CrossRef CAS PubMed.
- M. Zubair, et al., Biological nutrient removal and recovery from solid and liquid livestock manure: recent advance and perspective, Bioresour. Technol., 2020, 301, 122823 CrossRef CAS PubMed.
- F. Li, et al., Cultivation of Chlorella vulgaris in membrane-treated industrial distillery wastewater: growth and wastewater treatment, Front. Environ. Sci., 2021, 9, 770633 CrossRef.
- S. Vasistha, et al., Microalgae on distillery wastewater treatment for improved biodiesel production and cellulose nanofiber synthesis: a sustainable biorefinery approach, Chemosphere, 2023, 315, 137666 CrossRef CAS PubMed.
- A. Devi, et al., Microalgae: a green eco-friendly agents for bioremediation of tannery wastewater with simultaneous production of value-added products, Chemosphere, 2023, 336, 139192 CrossRef CAS PubMed.
- A. Zhang, S. Fang and M. Ge, et.al., Microalgae-derived hydrogels/membranes for phosphorus removal and recovery from aquaculture tailwater: waste utilization and phosphorus recycling, Bioresour. Technol., 2024, 409, 131246 CrossRef CAS PubMed.
- C. Zhang, S. Li and S. H. Ho, Converting nitrogen and phosphorus wastewater into bioenergy using microalgae-bacteria consortia: a critical review, Bioresour. Technol., 2021, 342, 126056 CrossRef CAS PubMed.
- X. Y. Liu, et al., Performance and mechanism of Chlorella in swine wastewater treatment: roles of nitrogen-phosphorus ratio adjustment and indigenous bacteria, Bioresour. Technol., 2022, 358, 127402 CrossRef CAS PubMed.
- Q. Wang, et al., Growth enhancement of biodiesel-promising microalga Chlorella pyrenoidosa in municipal wastewater by polyphosphate-accumulating organisms, J. Cleaner Prod., 2019, 240, 118148 CrossRef CAS.
- R. Wang, X. Wang and T. Zhu, Research progress and application of carbon sequestration in industrial flue gas by microalgae: a review, J. Environ. Sci., 2025, 152, 14–288 CrossRef CAS PubMed.
- M. Derakhshandeh, T. Atici and U. Tezcan Un, Evaluation of Wild-Type Microalgae Species Biomass as Carbon Dioxide Sink and Renewable Energy Resource, Waste Biomass Valorization, 2021, 12, 105–121 CrossRef CAS.
- R. Prasad, et al., Role of microalgae in global CO2 sequestration: physiological mechanism, recent development, challenges, and future prospective, Sustainability, 2021, 13(23), 13061 CrossRef CAS.
- Z. He, et al., Cultivation of Chlorella pyrenoidosa and Scenedesmus obliquus in swine wastewater: nitrogen and phosphorus removal and microalgal growth, Process Saf. Environ. Prot., 2023, 179, 887–895 CrossRef CAS.
- M. Birkholz, et al., Separation of heterotrophic microalgae Crypthecodinium cohnii by dielectrophoresis, Front. Bioeng. Biotechnol., 2022, 10, 855035 CrossRef PubMed.
- M. Bhandari, S. Kharkwal and S. K. Prajapati, Recycling drinking water RO reject for microalgae-mediated resource recovery, Resour. Conserv. Recycl., 2023, 188, 106699 CrossRef CAS.
- Z. He, et al., Cultivation of Scenedesmus obliquus and Chlorella pyrenoidosa in Municipal Wastewater Using Monochromatic and White LED as Light Sources, Waste Biomass Valorization, 2021, 12, 4873–4883 CrossRef.
- M. Levasseur, P. A. Thompson and P. J. Harrison, Physiological acclimation of marine phytoplankton to different nitrogen sources, J. Phycol., 1993, 29, 587–595 Search PubMed.
- C. V. G. López, et al., Protein measurements of microalgal and cyanobacterial biomass, Bioresour. Technol., 2010, 101(19), 7587–7591 CrossRef PubMed.
- M. A. Kassim and T. K. Meng, Carbon dioxide (CO2) biofixation by microalgae and its potential for biorefinery and biofuel production, Sci. Total Environ., 2017, 584–585, 1121–1129 CrossRef CAS PubMed.
- M. Derakhshandeh, T. Atici and U. Tezcan Un, Evaluation of Wild-Type Microalgae Species Biomass as Carbon Dioxide Sink and Renewable Energy Resource, Waste Biomass Valorization, 2020, 12, 105–121 CrossRef.
- J. Li, et al., Enhancement of ammonium removal from landfill leachate using microalgae by an integrated strategy of nutrient balance and trophic mode conversion, Algal Res., 2022, 61, 102572 CrossRef.
- S. M. Z. Hossain, et al., Soft-computing modeling and multiresponse optimization for nutrient removal process from municipal wastewater using microalgae, J. Water Process Eng., 2022, 45, 102490 CrossRef.
- X. Lian, et al., A new microalgal negative carbon technology for landfill leachate treatment: Simultaneous removal of nitrogen and phosphorus, Sci. Total Environ., 2024, 948, 174779 CrossRef CAS PubMed.
- C. Oz Yasar, L. Fletcher and M. A. Camargo-Valero, Effect of macronutrients (carbon, nitrogen, and phosphorus) on the growth of Chlamydomonas reinhardtii and nutrient recovery under different trophic conditions, Environ. Sci. Pollut. Res., 2023, 30, 111369–111381 CrossRef CAS PubMed.
- M. Kamalanathan, et al., Impacts of nitrogen and phosphorus starvation on the physiology of Chlamydomonas reinhardtii, J. Appl. Phycol., 2016, 28, 1509–1520 CrossRef CAS.
- K. Jothibasu, et al., Impact of microalgal cell wall biology on downstream processing and nutrient removal for fuels and value-added products, Biochem. Eng. J., 2022, 187, 108642 CrossRef CAS.
- M. P. Sinetova, A. G. Markelova and D. A. Los, The effect of nitrogen starvation on the ultrastructure and pigment composition of chloroplasts in the acidothermophilic microalga Galdieria sulphuraria, Russ. J. Plant Physiol., 2006, 53, 153–162 CrossRef CAS.
- Y. Wang, et al., Response of energy microalgae Chlamydomonas reinhardtii to nitrogen and phosphorus stress, Environ. Sci. Pollut. Res., 2018, 25, 5762–5770 CrossRef CAS PubMed.
- N. Aburai, et al., Mutual supply of carbon and nitrogen sources in the co-culture of aerial microalgae and nitrogen-fixing bacteria, Algal Res., 2023, 70, 103001 CrossRef.
- W. Cao, et al., The response to inorganic phosphates forms for mariculture wastewater treatment with microalgae: biochemical component synthesis, cellular morphology and phosphorus storage, Process Saf. Environ. Prot., 2024, 188, 480–491 CrossRef CAS.
- K. Kumari, et al., Nitrogen, phosphorus and high CO2 modulate photosynthesis, biomass and lipid production in the green alga Chlorella vulgaris, Photosynth. Res., 2021, 148, 17–32 CrossRef CAS PubMed.
- Y. Su, et al., Revisiting carbon, nitrogen, and phosphorus metabolisms in microalgae for wastewater treatment, Sci. Total Environ., 2021, 762, 144590 CrossRef CAS PubMed.
- X. Li, et al., Role of nitrogen transport for efficient energy conversion potential in low carbon and high nitrogen/phosphorus wastewater by microalgal-bacterial system, Bioresour. Technol., 2022, 351, 127019 CrossRef CAS PubMed.
- M. Giordano and L. Prioretti Sulphur and Algae: Metabolism, Ecology and Evolution. The Physiology of Microalgae, 2016, pp. 185–209 Search PubMed.
- D. Singh, L. Nedbal and O. Ebenhöh, Modelling phosphorus uptake in microalgae, Biochem. Soc. Trans., 2018, 46, 483–490 CrossRef CAS PubMed.
- C. Zhang, S. Li and S. H. Ho, Converting nitrogen and phosphorus wastewater into bioenergy using microalgae-bacteria consortia: a critical review, Bioresour. Technol., 2021, 342, 126056 CrossRef CAS PubMed.
- E. M. Salgado, A. F. Esteves, A. L. Gonçalves and J. Pires, Microalgal cultures for the remediation of wastewaters with different nitrogen to phosphorus ratios: process modelling using artificial neural networks, Environ. Res., 2023, 231, 116076 CrossRef CAS PubMed.
- J. I. Navarro, et al., Elemental and macromolecular plasticity of Chlamydomonas reinhardtii (Chlorophyta) in response to resource limitation and growth rate, J. Phycol., 2024, 60, 418–431 CrossRef PubMed.
- W. Cao, Y. Xing, D. Xing, Y. Zhao, M. Gao, C. Jin and L. Guo, The response to inorganic phosphates forms for mariculture wastewater treatment with microalgae: biochemical component synthesis, cellular morphology and phosphorus storage, Process Saf. Environ. Prot., 2024, 188, 480–491 CrossRef CAS.
- D. Hu, J. Zhang, R. Chu, Z. Yin, J. Hu, Y. K. Nugroho, Z. Li and L. Zhu, Microalgae Chlorella vulgaris and Scenedesmus dimorphus co-cultivation with landfill leachate for pollutant removal and lipid production, Bioresour. Technol., 2021, 342, 126003 CrossRef CAS PubMed.
- Q. Wu, L. Guo, X. Li and Y. Wang, Effect of phosphorus concentration and light/dark condition on phosphorus uptake and distribution with microalgae, Bioresour. Technol., 2021, 340, 125745 CrossRef CAS PubMed.
- R. K. Mohapatra, D. Padhi, R. Sen and M. Nayak, Bio-inspired CO2 capture and utilization by microalgae for bioenergy feedstock production: a greener approach for environmental protection, Bioresour. Technol. Rep., 2022, 19, 101116 CrossRef CAS.
- Z. Wang, J. Cheng, Y. Sun, D. Jia, Y. Tang, W. Yang and K. Cen, Comprehensive understanding of regulatory mechanisms, physiological models and key enzymes in microalgal cells based on various concentrations of CO2, Chem. Eng. J., 2023, 454, 140233 CrossRef CAS.
- A. Mustafa, B. G. Lougou, Y. Shuai, Z. Wang and H. Tan, Current technology development for CO2 utilization into solar fuels and chemicals: a review, J. Energy Chem., 2020, 49, 96–123 CrossRef.
- V. A. Thiviyanathan, P. J. Ker, S. G. Hoon Tang, E. P. P. Amin, W. Yee, M. A. Hannan, Z. Jamaludin, L. D. Nghiem and T. M. I. Mahlia, Microalgae biomass and biomolecule quantification: optical techniques, challenges and prospects, Renewable Sustainable Energy Rev., 2024, 189, 113926 CrossRef CAS.
- M. A. Kassim and T. K. Meng, Carbon dioxide (CO2) biofixation by microalgae and its potential for biorefinery and biofuel production, Sci. Total Environ., 2017, 584–585, 1121–1129 CrossRef CAS PubMed.
- R. K. Goswami, S. Mehariya and P. Verma, Advances in microalgae-based carbon sequestration: current status and future perspectives, Environ. Res., 2024, 249, 118397 Search PubMed.
- A. Tarafdar, G. Sowmya, K. Yogeshwari, R. Gurdeep, T. Negi, M. K. Awasthi, A. Hoang, R. Sindhu and R. Sirohi, Environmental pollution mitigation through utilization of carbon dioxide by microalgae, Environ. Pollut., 2023, 328, 121623 CrossRef CAS PubMed.
- J. Zeng, Z. Wang and G. Chen, Biological characteristics of energy conversion in carbon fixation by microalgae, Renewable Sustainable Energy Rev., 2021, 152, 111661 CrossRef CAS.
- Y. Chen, C. Xu and S. Vaidyanathan, Influence of gas management on biochemical conversion of CO2 by microalgae for biofuel production, Appl. Energy, 2020, 261, 114420 CrossRef CAS.
- K. Li, J. Cheng, H. Lu, W. Yang, J. Zhou and K. Cen, Transcriptome-based analysis on carbon metabolism of Haematococcus pluvialis mutant under 15% CO2, Bioresour. Technol., 2017, 233, 313–321 CrossRef CAS PubMed.
- A. A. Lomeu, O. B. de Oliveira Moreira, M. A. L. de Oliveira and H. V. de Mendonca, Applying ozone in cattle wastewater to maximize lipid production in microalgae biomass, Bioenergy Res., 2023, 16, 2489–2501 Search PubMed.
- A. J. Simkin, P. E. López-Calcagno and C. A. Raines, Feeding the world: improving photosynthetic efficiency for sustainable crop production, J. Exp. Bot., 2019, 70, 1119–1140 CrossRef CAS PubMed.
- H. Peng, J. Fu and Y. Huang, et al., Deciphering mechanism of sulfide stress on microalgae and overcoming its inhibition by regulation growth acclimatization period, Chem. Eng. J., 2024, 495, 153045 Search PubMed.
- P. R. Yaashilaa, A. Saravanan and P. S. Kumar, et al., Role of microbial carbon capture cells in carbon sequestration and energy generation during wastewater treatment: a sustainable solution for cleaner environment, J. Environ. Manage., 2024, 52, 799–820 Search PubMed.
- A. Guldhe, V. Bhola, I. Rawat and F. Bux, Carbon Dioxide Sequestration by Microalgae: Biorefinery Approach for Clean Energy and Environment, Bioresour. Technol., 2015, 7, 147–154 Search PubMed.
- E. Daneshvar, R. J. Wicker, P. L. Show and A. Bhatnagar, Biologically-mediated carbon capture and utilization by microalgae towards sustainable CO2 biofixation and biomass valorization – a review, Sci. Total Environ., 2022, 427, 130884 CAS.
| This journal is © The Royal Society of Chemistry 2025 |