Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Light-activated antimicrobial coatings: the great potential of organic photosensitizers
Karolina Socha†
 a,
Ivan Gusev†a,
Patryk Mroczko
a,
Ivan Gusev†a,
Patryk Mroczko a and
Agata Blacha-Grzechnik
a and
Agata Blacha-Grzechnik *ab
*ab
aSilesian University of Technology, Faculty of Chemistry, Strzody 9, Gliwice, 44-100, Poland. E-mail: agata.blacha@polsl.pl
bSilesian University of Technology, Centre for Organic and Nanohybrid Electronics, Konarskiego 22B, Gliwice, 44-100, Poland
First published on 13th March 2025
Abstract
Contamination of inanimate surfaces with microorganisms is considered one of the routes for transmission of pathogens, which is a matter of concern not only in healthcare-related facilities, but also in public areas. Durable antimicrobial coatings have emerged as the one of most promising strategies for reducing the accumulation of microorganisms on high-touch surfaces. Light-activated antimicrobial layers are of particular interest for such a purpose, as they generate singlet oxygen and other reactive oxygen species that are effective against a broad spectrum of bacteria, viruses, and fungi. In this review, the antimicrobial coatings containing organic photosensitizers are discussed, focusing on the recent advances in the strategies for PSs' immobilization on solid surfaces. The review attempts to assess the advantages and limitations of those systems, and the challenges that still need to be overcome.
Introduction
Hospital acquired infections (HAI, nosocomial infections) remain one of the greatest challenges for healthcare systems worldwide. In Europe and the Western Pacific, the hospital-acquired infection rate is between 7.7% and 9%, ca. 11% in the Middle East, and ca. 10% in Southeast Asia. In some countries, the rate reaches up to 20%.1 HAIs result in prolonged treatment, increased number of lethal cases, and higher costs of treatment.1,2 Several routes of transmission of HAI pathogens have been identified, including direct transmission between patients/workers, or indirect routes via contaminated medical devices or high-touch surfaces.3,4 While it has been agreed that proper environmental and hand hygiene or use of protective equipment can significantly reduce HAI case rate, the suitable treatment of inanimate surfaces contaminated with microorganisms has been under longer debate.2,5,6 Moreover, the recent pandemic caused by SARS-CoV-2 showed that the presence of pathogens is also problematic for high-touch surfaces in non-healthcare-related public areas.Depending on the reduction level, the following terms are used for the microorganisms' inactivation: cleaning, sanitizing, disinfecting, and sterilizing, with the last one yielding complete removal of all forms of microbes. Mechanical (e.g. brushing & water jet), chemical (e.g. detergents, oxidizing agents, ionic surfactants, halogenated compounds), and physical (e.g. ultrasounds, UV-light, autoclave) methods are commonly applied on different stages of surface treatment. A combination of different techniques is also frequently used. The selection of proper cleaning procedure depends on a type of surface and its role, e.g. high-touch surfaces (door knobs, light switches, handrails) in healthcare units or public areas, medical device surfaces, food contact surfaces, etc.2,5
Various pathogenic microorganisms can persevere on inanimate surfaces for several weeks or even months and can be re-deposited rapidly after disinfection,2,5,7 thus the alternative preventive measure, i.e. modification of objects' surface with antimicrobial coatings, has been proposed.7,8 Such layers covering fabrics, metals, plastics, etc. should be active against a broad range of pathogens, easy to fabricate, and safe for end-users. In the area of antimicrobial coatings two groups can be distinguished: (i) anti-fouling and antiadhesive, i.e. lowering microorganisms' adhesion (e.g. poly(ethylene glycol)) and (ii) active ones, i.e. destroying already adhered microorganisms (e.g. silver- or copper-containing coatings, polycationic layers).8 In particular cases, the antimicrobial effect is connected with a release of an active agent into the environment, e.g. coatings with bactericidal ions or antibiotics on implants.9 The target use of the material is the main criterion for the selection of the type of antimicrobial coating. However, the types of pathogens, environment, mechanism of action, user safety, or biocompatibility need to be taken into account.
The subject of this review – the light-activated antimicrobial layers, falls into the category of active antimicrobial coatings. The mechanism of action is based on the production of reactive oxygen species (ROS). This type of coating has been under high research interest for many years now.8,10 Though, till now mostly inorganic-based coatings have been employed in general use, the ones consisting of organic photosensitizers possess several advantages and thus, are still widely investigated. This review aims to summarize the recent advances in the light-activated antimicrobial organic layers. In the first part, reactive oxygen species, the organic photosensitizers, and the mechanism of antimicrobial action will be introduced. Next, the recent strategies for the formation of light-activated antimicrobial layers will be reviewed. In the final part, the future outlook will be discussed, emphasizing the challenges that still need to be overcome.
Reactive oxygen species, organic photosensitizers & photodynamic antimicrobial therapy
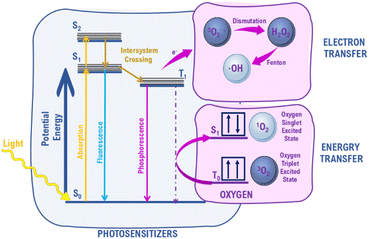
Reactive oxygen species (ROS) is a group of highly active forms of oxygen that includes superoxide anion radical, hydrogen peroxide, singlet oxygen, and hydroxyl radical.11 ROS are produced during normal oxygen metabolism, however, if the amount of ROS is significantly increased, the cell reaches a state of oxidative stress with an impairment of cellular structures. The excessive levels of ROS can cause severe damage to DNA and proteins.12In the group of ROS, singlet oxygen, O2(1Δg), is the unique molecule. It is the lowest excited state of oxygen molecule, that unlike triplet oxygen, O2(3Σg−), has no unpaired electrons on π* orbital (Fig. 1).13 The other singlet form, O2(1Σg+), rapidly decays,7,8 thus the term singlet oxygen usually refers to O2(1Δg).14,15 1O2 possess remarkable properties, such as high reactivity and strong oxidizing properties.16,17 The lifetime of singlet oxygen lies in the μs–ms range depending on the solvent type, and temperature, interacting quickly with other molecules in the surroundings.17,18 Lately, Wang et al. estimated that under everyday atmospheric conditions, i.e. 23 °C and 1 atm, singlet oxygen's lifetime in air is equal to 2.80 s and it diffuses ca. 0.992 cm.19
Photosensitization is one of the most efficient methods of singlet oxygen production. It involves the absorption of light by so called photosensitizer (PS) molecule and a transfer of energy to ground state oxygen. The absorption of energy by organic photosensitizer causes its transition from a ground singlet state, S0, to an excited singlet state, Sn (Fig. 2). This is followed by the non-radiative transition to the first high-energy singlet state, S1. The transition from the singlet excited state, S1, to a triplet excited state, T1, i.e. intersystem crossing, is crucial. These are forbidden transitions associated with a change in electron spin. Photosensitizer in the T1 state can transfer energy to triplet oxygen yielding singlet oxygen. It is also possible to observe the electron transfer process yielding other types of ROS.13,17 It has to be noted that several competitive processes may occur, e.g. fluorescence or phosphorescence, that may significantly reduce the yield of ROS production.
Photosensitizers can be generally classified into the following groups:17
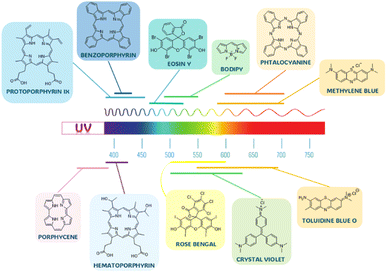
(1) Organic PSs: phenothiazines,20–22 crystal violet,23 porphyrins,24,25 porphycenes,26 phthalocyanines,27,28 chlorines,29 texaphyrins,30 indocyanine dyes,31,32 eosin y,33 boron-dipyrromethene (BODIPY),34,35 diketopyrrolopyrrole,36,37 xanthenes,38–40 squaraines,41 curcuminoids42,43 and chalcogenopyrylium dyes.44,45
(2) Inorganic PSs: metals and metal oxides such as iridium,46 gold,47 zinc oxide,48,49 and titanium oxide.50–52
(3) Heavy metal complexes: ruthenium,53,54 iridium,55,56 platinum.57
It has to be noted that the mechanism of ROS production in the case of inorganic compounds is different. The light absorption causes the generation of electron (e−)–hole (h+) pair that further undergo redox reactions yielding ROS.58
Organic and inorganic sources of ROS differ significantly in the absorption range. While inorganic ones absorb mainly in the UV region, organic photosensitizers possess also strong absorption bands in various parts of the visible region (Fig. 3).17 This is, of course, particularly advantageous for any visible-light-driven photocatalytic applications. Moreover, the chemical structure of organic PSs can be fine-tuned to control not only their absorption but also the solubility or quantum yield of singlet oxygen production.59,60
In the case of antimicrobial action, the electrostatic interaction of PS needs to be taken into account when designing photosensitizer molecules. For example, Gram-positive bacteria have a high density of negative charges on the cell membrane, due to the high content of phosphate and hydroxyl groups, thus they are more vulnerable to cationic PSs.60,61 Taking this into account, the following classification of organic PSs have been introduced:
(1) Cationic PSs based on: phenothiazines,62,63 BODIPYs,64,65 phthalocyanines,66 porphyrins,67 or porphycenes.68
(2) Anionic PSs: xanthenes,69–71 and tricarbocyanine dyes.72
(3) Neutral PSs based on: chlorin,73,74 temoporfins75,76 or curcumin.77,78
The term Photodynamic Antimicrobial Chemotherapy (PACT) was introduced for the first time in 1960s. For many years, PACT was treated as a subgroup of anticancer Photodynamic Therapy (PDT) named Antimicrobial Photodynamic Therapy (aPDT), due to their similar mechanism of action. Nowadays, both terms: PACT and aPDT are commonly used in the literature. PACT employs photosensitizers to induce phototoxic effects in microorganisms. Inactivation of microbes using photosensitizers has been reported for various Gram-positive (e.g. Staphylococcus aureus, Enterococcus faecalis, Bacillus cereus) and Gram-negative bacteria (e.g. Escherichia coli, Pseudomonas aeruginosa),79 viruses (e.g. Vesicular stomatitis),80,81 and fungi (e.g. Candida albicans).82
The detailed mechanism of photosensitizers' antimicrobial action has been under debate for many years.83 Recently, Baptista et al., proposed a classification of light-activated processes into (i) photosensitized oxidation and (ii) oxygen-independent photosensitization.84 The first one is based on the action of ROS that is generally multi-target and very efficient. As described above, reactive oxygen species are formed either via electron transfer (named as Type I aPDT) or by energy transfer (named as Type II aPDT).85,86 ROS interact with cell wall increasing the ion permeability, cause oxidation of proteins and structural changes in nucleic acids.87–90 Antioxidant enzymes can effectively protect microbes against some types of ROS, but not against 1O2. Within Type I, H2O2, O2˙− and ˙OH are produced and the last is considered as the most reactive one in this group.91 Since ROS affects microbes in a multi-target mechanism, PACT can be advantageous for dealing with multi-drug-resistant microbes.83,87
The advantage of photoantymicrobials over biocides is that they are majorly nontoxic molecules that can kill microbes at lower conentrations than biocides and covers a whole spectrum of bacteria, viruses, fungi and protozoa.92 Photoactive compounds used in PACT should also prioritize physical properties as solubility and aggregation. Limiting the aggregation effect can reduce the dose of photosensitizer needed for the therapeutic effect.93
Antimicrobial coatings based on organic photosensitizers
Similarly to the inactivation of microorganisms with non-immobilized photosensitizers, the action of light-activated antimicrobial coatings is based on the production of reactive oxygen species. Though, in this case, ROS photogeneration needs to be considered as a heterogeneous process, and additional parameters, e.g. transport of reactants to/within the layer, need to be taken into account.94Till now, inorganic photoactive species have been widely used in the light-activated antimicrobial coatings. Next to titanium dioxide, which has been investigated for many years,95–97 zinc oxide has gained more interest in the last years.3,98 The main limitation of inorganic-based antimicrobial coatings is their absorbance located mainly in the UV region and their toxicity, which is still under investigation.96,99 Some of the photoactive coatings have already been approved for commercial use and introduced to the market, e.g. by PhotoACTIVE®,100 EnvisionSQ,101 TitanoClean™,102 and Pilkington.103
One of the first works discussing the possibility of the use of organic photosensitizers in the light-activated antimicrobial coatings was a series of papers of Wilson et al., in which cellulose acetate was used as a matrix for Toluidine Blue O or Rose Bengal immobilization.104–106 So far, many papers reporting different strategies for the deposition of antimicrobial coatings based on organic PSs have been published.
Formation of organic layers, also photoactive ones, is possible using a variety of techniques that differ in the quality and properties of the resulting layer, ease of operation, control over the deposition process, or availability and costs of the equipment. For the aim of this review, the photosensitizers' immobilization techniques can be divided into two main groups resulting in (i) covalent or (ii) non-covalent interactions between PS and solid support.
A wide range of surface grafting techniques based on chemical, photochemical, electrochemical, or thermal processes can be used for the covalent attachment of organic molecules to solid surfaces. Within this group, Self-Assembled Monolayers (SAM) have been the most widely explored for many years. SAMs are formed thanks to the specific interactions between surface-anchoring groups present in organic molecules and a given type of surface, e.g. thiols – gold, alkoxysilanes – SiO2, or indium tin oxide (ITO). The self-assembly process is spontaneous and the resulting layer usually possesses a well-defined and well-organized structure.107,108 The electrochemical grafting process, on the other hand, allows for the modification of (semi)conductive surfaces only. In most cases, the electrografting process is specific for the given type of substrate, e.g. oxidative electrografting of carboxylates occurring only on carbon surface.109 The most versatile electrografting technique, in terms of surface type and organic molecules, is an electrochemical reduction of diazonium salts110 that was reported for the first time in 1992 by Pinson et al.111 It has to be noted that thanks to so-called post-functionalization techniques, the chemical structure and thus properties of grafted organic layers can be further optimized.112 In the case of functionalization of polymers, two approaches can be distinguished: either functionalized monomers are directly polymerized or the so-called reactive polymer precursor and a consecutive postpolymerization modification are used.113
The second group of techniques, i.e. resulting in non-covalent deposition of organic layers is much broader and in most cases doesn't require introduction of any specific functional groups. The best example is physical adsorption of e.g. dyes, that is governed by van der Waals forces or hydrogen bonding.114 Here, we will discuss only a few examples of techniques that are the most frequently used for the preparation of the light-activated antimicrobial coatings.
In the solution-based techniques a substrate is uniformly coated with a solution of organic compound that after drying yields film. Those methods are very common both on the laboratory- and on the industrial scale, due to rather low-costs and ease of operation. Spin coating, dip coating, drop casting, and spray coating are examples of solution processing methods. They differ in the costs, speed of coating, the complexity of the process, uniformity of resulting film, etc.115 For example, the spin coating yields films with high uniformity, only small substrates can be coated and a straightforward scalability is not possible. On the other hand, in the spray coating large substrates can be covered quite quickly and the process is scalable, but the resulting layer has low uniformity and the costs are higher. In all the above-mentioned techniques the morphology, thickness, and uniformity of the film can be controlled by optimizing process parameters.116–118
Another technique in the non-covalent deposition group is electrochemical polymerization, which is used mostly for the formation of conducting polymer films.119 In this case, the functional group that undergoes electropolymerization needs to be present in the monomer's structure and the surface material needs to be conductive and not easily-oxidized, e.g. indium-tin-oxide on glass (ITO) or platinum plate (Pt) can be used. Finally, a non-covalent immobilization of PS in a polymer matrix may be achieved with methods well-known for enzymes120 or nanoparticles121 immobilization, e.g. encapsulation or entrapment.
Below, the selected examples of light-activated coatings based on organic photosensitizers are discussed. Table 1 summarizes the examples and gives additional ones, providing a reader with all necessary information about the type of the immobilized molecule, selected immobilization strategy, pathogen type or strain with the reported inactivation efficiency, etc.
| No. | Photoactive molecule | Immobilization strategy | Antibacterial effect | Coating stability | Irradiation parameters | Ref. |
|---|---|---|---|---|---|---|
| 1 | Methylene blue (MB) | Immobilization in polystyrene | S. aureus: 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction. E. coli: 1 log reduction. E. coli: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction log reduction |
Leakage under illumination was higher than that in the dark, resulting in 0.48 μM MB | 0.5–3 h, white light, 400–700 nm, 1–3 mW cm2, light intensity (1.8–5.4 J cm−2) | 130 |
| 2 | Dispersed in polymer resins | S. aureus, E. coli: full bacterial growth; little growth; no growth – described | Not reported | 4 min, 2 min, 1 min, 0.5 min, 615–645 nm, 48 mW cm−2 | 122 | |
| 3 | Covalently bound to a silicone surface | E. coli: 1.3 log reduction (energy dose of 42 J cm−2) | Not reported | 21 min, 660 nm, 32.5 mW cm−2 | 123 | |
| 4 | Ultrasonic spraying, host–guest interaction between β-cyclodextrin and MB | Methicillin-resistant S. aureus: 99% reduction | Release of MB was observed | 10 min, 660 nm laser (30 J cm−2) | 179 | |
| 5 | Toluidine blue O (TBO) | Covalently bound to a silicone surface | E. coli: 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction (energy dose of 42 J cm−2) log reduction (energy dose of 42 J cm−2) |
Not reported | 4 min, 634 nm, 190 mW cm−2 | 123 |
| 6 | Deposited by absorption on the surfaces of the silicone and polyurethane polymers | S. aureus: >4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction after 3 min (silicone), >4 log reduction after 3 min (silicone), >4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction after 1 min (polyurethane). E. coli: >4 log reduction after 1 min (polyurethane). E. coli: >4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction after 2 min (polyurethane), 1.5 log reduction after 2 min (polyurethane), 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction after 3 min (silicone) log reduction after 3 min (silicone) |
When immersed in water or methanol, PS is not released into solution | 1–3 min, 634 nm, 1.0 W laser | 124 | |
| 7 | Immobilized in polymer matrix | C. albicans: Reduction >90% | Resisting dissolution when immersed in artificial saliva | 3 h, 635 nm, 100 mW cm−2 | 125 | |
| 8 | Covalent linkage | S. aureus: 5–6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction. E. coli: 5 log reduction. E. coli: 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction log reduction |
High charge density renders colloidal stability of the fibers | 15 min, 630 nm red LED lamp and 300–800 nm solar simulator, 250W m−2 | 184 | |
| 9 | Rose bengal (RB) | (1) Plasma treatment + acrylic acid binding to PDMS. (2) Chemical grafting of chitosan-RB | E. coli: not able to inhibit the growth; S. aureus: vitality 50% inhibition | Increase in the surface wettability of PDMS samples after surface functionalization | 60 min, incandescent lamp, 120 W | 129 |
| 10 | Immobilization in polystyrene | S. aureus: 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction. E. coli: 2.5 log reduction. E. coli: 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction. P-values for comparison with control series were 0.0029 for S. aureus and 0.0038 for E. coli log reduction. P-values for comparison with control series were 0.0029 for S. aureus and 0.0038 for E. coli |
Leakage under illumination was higher than that in the dark, resulting in 0.26 μM RB | 0.5–3 h, white light, 400–700 nm, 1–3 mW cm2, light intensity (1.8–5.4 J cm−2) | 130 | |
| 11 | Immobilization in polymeric matrix (PMMA, PC) | S. aureus: 99.998% reduction | Upon addition of RB, the porous structure was preserved only in the case of PS, whereas the RB-containing PC and PMMA had smooth surface structures | 0–60 min, white light, 1.2 mW cm−2 | 9 | |
| 12 | Cross-linking to polymer | E. coli: 99 ± 2% reduction in dark, 28-fold increase upon irradiation; MRSA: >99 ± 1% reduction in dark, 3-fold increase upon irradiation; SARS-CoV-2: 90% reduction | Not reported | 0.5-2h; 530 nm, 39 mW cm−2 | 127 | |
| 13 | Immobilized in Amberlite® by ion-exchange with chloride ions | S. aureus: 5.5–7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction. E. faecalis: 8 log reduction. E. faecalis: 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction E. coli: 5.5 log reduction E. coli: 5.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction P. aeruginosa: 8 log reduction P. aeruginosa: 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction C. albicans: 1.5–3.0 log reduction C. albicans: 1.5–3.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction (dark effect) log reduction (dark effect) |
Combination with commercial supports like cationic exchange resins enhances effectiveness | 515 nm, total light dose of 100 J cm−2 | 128 | |
| 14 | MB,RB and TBO | PSs mixed with poly(vinylidene fluoride) | E. coli: 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction (24 h) S. aureus: 5 log reduction (24 h) S. aureus: 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction (6 h) log reduction (6 h) |
Soaking the PS surfaces in PBS for 1 week – stable in the surface and their leakage to the solution is negligible | 6–24 h, white light, 1.46 mW cm−2 | 131 |
| 15 | RB and TBO | Cellulose acetate modified with PSs | S. aureus: 78.9–99.8% inhibition | Not reported | 6 h, fluorescent lamp (∼3700 lux), 28 W | 106 |
| 16 | Thionine | Grafting | S. aureus: 99.985% (∼3.82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log) reduction. E. coli: 99.99% (4 log) reduction. E. coli: 99.99% (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log) reduction log) reduction |
Presence of disperse dyes increases photostability; humidity negatively impacts stability | 60 min, 400–700 nm noncoherent light, 65 ± 5 mW cm2; xenon lamp 500 W equipped with a long-pass filter (λ ≥ 420 nm) | 132 |
| 17 | Eosin Y | Immobilized during photoinduced crosslinking of a PEG–diacrylate monomer | E. coli: 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log unit reduction. S. aureus: 4 log unit reduction. S. aureus: 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log unit reduction log unit reduction |
Not reported | 6 h, visible light (400–650 nm) | 133 |
| 18 | Erythrosine B | Solvent casting | S. aureus: 5.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log unit reduction. E. coli: undetectable Salmonella: Undetectable log unit reduction. E. coli: undetectable Salmonella: Undetectable |
Not reported; effectiveness decreased after multiple uses due to bacterial accumulation | 10–50 min; LED light; 400–800 nm | 134 |
| 19 | Anthraquinone-triazine | Surface modification of cotton fabrics (covalently) | E. coli: 99.9% inhibition | The thermal decomposition occurred mainly in a narrow temperature range of 310–370 °C | 2 days, 400–800 nm, 1380 lm, 110–130V, 400 mA | 137 |
| 20 | Meso-tetraphenylporphyrin (TPP) | Cross-linking | S. aureus: >99.9% reduction | Photostability under the tested conditions | 30 min, 360–600 nm, 50 mW cm−2 | 138 |
| 21 | Zn-porphyrin | Covalently attach | Infuenza A virus: Inactivated 99.99% | After exposure to high-intensity white light for 4 days and then subjected to a 1000 minute quantification experiment -similar levels of 1O2 production | 4 h, visible light, 90 W | 140 |
| 22 | Zinc Tetra(4 N-methylpyridyl)-porphyrine (ZnTMPyP4+) | Spray-coating or dip-coating | E. coli: 99.86% inactivation S. aureus: 99.9999% inactivation SARS-CoV-2: 99.9998% inactivation | Not reported | 1 h, 400–700 nm, S. aureus – 65 ± 5 mW cm−2, E.coli 80 ± 5 mW cm−2 | 141 |
| 23 | ZnTMPyP4+, MB and RB | Spray coating | S. aureus: 97–99.999% inactivation HCoV-229E: 99.999% inactivation | Layers showed the same level of activity even after exposure for 4 weeks to indoor ambient lighting | 1 h , 400–700 nm, S. aureus – 65 ± 5 mW cm−2, HCoV-229E – 80 mW cm−2 | 142 |
| 24 | Pd(II)-porphyrin | Electrochemical polymerization | E. coli: 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction C. albicans: 2.5 log reduction C. albicans: 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction log reduction |
Mechanically stable polymeric films | 30 min, 60 min, 350–800 nm, 90 mW cm−2 | 143 |
| 25 | Mn(III) meso-tetra(4-sulfonatophenyl) porphine chloride (MnTPPS) | Electrostatic interaction – solvent evaporation | E. coli: 83% inhibition | Not reported | 60 min, halogen bulb, 100 W | 144 |
| 26 | Hematoporphyrin | Covalent binding to stainless steel surface via esterification reaction | E.coli: decreased from 106 CFU mL−1 to 104 CFU mL−1. S. aureus: Decreased from 105 CFU mL−1 to 100 CFU mL−1 | Not reported | 2h, λmax = 520 nm GLED, <400 nm cutoff filter, 3.5 mW cm−2 | 139 |
| 27 | Para-aminophenylporphyrin derivatives | Grafting | E.coli, S. aureus: Inhibition are 37% for anionic cotton, 93.7% for neutral cotton, and 100% for cationic cotton | At much higher temperatures, grafted cotton samples showed multistep weight loss due to decomposition of photosensitizers, removal of linker groups, or degradation of polymeric material or backbone itself | 24 h, 400–800 nm, 0.16 mW cm−2 | 146 |
| 28 | Meso-arylporphyrin | Covalent linking to cellulose fabric via click-reaction | E. coli: 5.31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log CFU reduction. S. aureus: 5.3 log CFU reduction. S. aureus: 5.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log CFU reduction log CFU reduction |
Not reported | 24 h, white light, 1000 lux | 147 |
| 29 | Protoporphyrin (PpIX) | Electrospinning | E. coli: 86.6% inactivation. S.aureus: 99.8% inactivation | Thermal stability of PpIX/CA microfibers in comparison to that of CA was not significantly changed: Both had a significant onset of decomposition at ∼300 °C | 30 min, 420 nm, 65 ± 5 mW cm−2 | 148 |
| 30 | ‘One-pot, two-step’ reactions | E. coli S. aureus (The growth of the microorganisms was examined visually) | Not reported | Four 150 W tungsten bulbs, totalling 1.7 mW cm−2 | 149 | |
| 31 | Grafting | E. coli: 68.33–99.999% reduction, S. aureus: 98.50% reduction | Decreased efficacy after multiple uses due to photobleaching and bacterial accumulation | 30 min, Xenon lamp (λ ≥ 420 nm) | 150 | |
| 32 | Carboxyporphyrins | Grafting | E. coli and S. aureus: GP ≥ 0.18 photoinhibition | No change in UV-spectrum upon 48 h continuous illumination | 24 h, four 150 W tungsten bulbs, 1.7 mW cm−2 | 151 |
| 33 | Biscarbazol-triphenylamine end-capped dendrimeric zinc(II) porphyrin | Electrochemical polymerization | S. aureus: >99.9998% reduction. E. coli: 99.4% reduction | Photostability observed with reusability for at least three treatments | 15, 30, 60 min, visible light (455–800 nm), 0.5 mW cm−2 | 145 |
| 34 | Tetra-substituted diazirine porphyrin cross-linked to polyethylene terephthalate | Cross-linking | S. aureus: 97.5% inhibition | Covalent cross-linking of porphyrin to PET provides long-term stability; stable antimicrobial properties | 6 h, white LED light 75 W, 1800 lx, 59.37 J cm−2 | 152 |
| 35 | Porphyrinic metal–organic frameworks (PCN-224) grown on Ti3C2 nanosheets | Magnetron sputtering | E. coli: 99.9999% inactivation S. aureus: 99.995% inactivation | Not reported | 30 min, Xenon lamp, 420 nm filter, 500 W, 31.45 W cm−2 | 185 |
| 36 | Ethynylphenyl porphyrin | Covalent attachment to cellulosic surface | E. coli: 1-2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction. M. smegmatis: 3–4 log reduction. M. smegmatis: 3–4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction. S. aureus: 5–6 log reduction. S. aureus: 5–6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction log reduction |
Minor degradation around 210 °C (weight loss of 20%) and major decomposition above 320 °C | 30 min, 400–700 nm, white light 60 mW cm−2 | 186 |
| 37 | 5,10,15,20-Tetrakis(pentafluorophenyl)porphyrin | Polymerization | E. coli: 2.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction. S. aureus: 3.6 log reduction. S. aureus: 3.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction log reduction |
After 75 minutes of irradiation, no significant loss of efficiency | 75 min, white light, 156 mW cm−2 | 187 |
| 38 | Tetrakis(4-carboxyphenyl)porphyrin (TCPP) | Surface-initiated polymerization and cross-linking | E. coli: 64% reduction | Minimal leaching of TCPP from the surface of SiO2 beads was observed after 7 days | 24 h, 470 nm, LED, 2000 μW cm−2 | 153 |
| 39 | Cationic zinc phthalocyanines | Electrostatic interactions | E. coli: 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction. S. aureus: 6 log reduction. S. aureus: 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction. C. albicans: 6.5 log reduction. C. albicans: 6.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction log reduction |
Decomposition starts above 300 °C (Pristine cellulose crystals start to decompose above 200 °C) | 620–645, red light irradiation, 18 mW cm−2 | 188 |
| 40 | Zinc(II) phthalocyanine tetrasulfonic acid, ZnPcS | Covalent attachment by reactive dyeing | E. coli: >2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log inhibition log inhibition |
Still stable after 9 months of use | 30 min, visible light, halogen lamp, type no. CY-118A, 500 W 230 V, 50 Hz | 189 |
| 41 | Pyridine substituted phthalocyanine zinc complex | Dye-impregnated cellulose material | C. albican – 99.996% inhibition S. aureus – 99.998% inhibition E. faecalis – 99.998% inhibition | Not reported | 30 min, 60 min, LED/fluorescent lamp, 4000 lux, 270 lux | 190 |
| 42 | Phenoxy-substituted phthalocyanine zinc (PPcZn) | Incorporation in cellulose acetate | Bacteriophage Qβ: 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction log reduction |
The layer is still functional after 6 months of exposure to daylight | 4 h, visible light, 1000 lux | 182 |
| 43 | Zinc tetracarboxy-phthalocyanine | Double-grafted: Solvent evaporation, dip-coating | E. coli: 99.99% reduction. S. aureus: 99.99% reduction | Not reported | 30 min, LED, 680 nm | 191 |
| 44 | Grafting | E. coli: 99.99% reduction. S. Aureus: 99.99%reduction | Double-grafted fiber retained 99.75% of antibacterial efficacy after ten washing | 10 min, 680 nm, 15 J cm−2 | 157 | |
| 45 | Mono-substituted β-carboxy zinc phthalocyanine | Grafting | E. coli: 99%, S. aureus: 98% | Coating was decomposing with time, but at much lower rate, comparing to photosensitizer in solution | 10 min, 660–740 nm, 150 mW cm−2 | 158 |
| 46 | Pyridine zinc phthalocyanine | Grafting | A. baylyi: 3.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction. E. coli: 2.7 log reduction. E. coli: 2.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction log reduction |
No leaching in water unless pH < 2; effective at 0.008 mg cm−2 loading | 1 h, white light, 485–750 nm, 18 mW cm−2 | 156 |
| 47 | Poly(3,4-ethylenedioxythiophene) zinc phthalocyanine (ZnPc-PEDOT) | Electrochemical polymerization | ZnPc-PEDOT:S. aureus: 99.98% reduction; E. coli: 99.98% reduction, CuPc-PEDOT:S. aureus: 90% reduction, E. coli: 95% reduction | Negligible photolysis of PS observed | ZnPc-PEDOT: 30 min (S. aureus) and 90 min (E. coli), visible light 108–162 J cm−2 CuPc-PEDOT: 60 min (S. aureus) and 90 min (E. coli), visible light 108–162 J cm−2 | 162 |
| 48 | Silicon phthalocyanine derivative (AGA405) | Drop-casting | E. coli: 50% biofilm mass reduction | Not reported | 30 min, near-infrared light (18 J cm−2); 30 min incubation | 160 |
| 49 | Bis-amino Si-phthalocyanine | One step sol–gel process | P. gingivalis: 99.99% reduction | No photobleaching in experimental conditions | 15 min, CW diode laser 669 nm, 270–315 J cm−2 | 159 |
| 50 | Tetratert-butyl-substituted silicon phthalocyanine dihydroxide | Adsorption | S. aureus 99.9% inactivation, E. coli unaffected | Not reported | 1–3 h, polychromatic light (10 mW cm−2) with 610 nm cut-off filter; (108 J cm−2) | 161 |
| 51 | Chlorophyllin | Grafted to cotton fabric (Chl-fabric) or embedded in electrospinned polyacrylonitrile nanofibers (Chl-NF) | Chl-fabric: E. faecium: 99.998% reduction. S.Aureus: 99.994% reduction. F. calcivirus: 99.8% reduction. Chl-NF: E. faecium: 99.9999% reduction. S.Aureus: 99.9999% reduction. F. calcivirus: 99.8% reduction. K. pneumoniae: 99.9999% reduction after addition of MoS2 | Chl-fabric: Photostability for short-term use. Chl-NF: Higher photosensitizer loading, better inactivation | 30–60 min, 400–700 nm, 80 ± 5 mW cm−2 for lower intensities LED light 3 ± 1 mW cm−2 and LumaCare PDT light 30 ± 5 mW cm−2 | 164 |
| 52 | 8-Acetoxymethyl-2,6-dibromo-1,3,5,7-tetramethyl pyrromethene fluoroborate (Br2B-OAc) | Spin coating | S. aureus: >5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log inactivation. E. coli: 3.5 log inactivation. E. coli: 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log inactivation log inactivation |
Stable; 7% reduction in fluorescence intensity after 1 month in water and ambient light | 30 min, 480−550 nm, 1.0 mW cm−2 | 166 |
| 53 | Boron-dipyrromethane) (BODIPY) derivative | Covalent attachment to PDMS | S. aureus – 99.9% reduction | Not reported | 5 h, white light, 4000 lux | 167 |
| 54 | Paprika spice | Photopolymerization | 100% inhibition of adhered E. coli and S. aureus after 2 h and 6 h, resepctively | Thermal stability up to 300 °C | Visible light, total intensity of 170 μmol m−2s−1 | 192 |
| 55 | Quercetin | Spin coating | S. aureus: 99% reduction | Very good adhesive properties to the stainless steel substrates and a high thermal stability up to 375 °C | 2 h and 6 h, 365 nm, Xe lamp, 70 mW cm−2 | 193 |
| 56 | Curcumin | Photopolymerization | E. coli: 95% inactivation. S. aureus: 99% inactivation | Good adherence properties on an inox substrate and a high thermal stability to 375 °C | 48 h, 4 lamps, 170 μmolm−2s | 194 |
| 57 | Atom-transfer radical polymerization | E. coli: ∼2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction log reduction |
Surface morphology does not change after immersion in water | 15 min, white-light, 42 mW cm−2 | 168 | |
| 58 | Triphenylamine quinolinium hexafluorophosphate (TPAQ-PF6) | Covalently grafted | S. aureus: 91.0% reduction. P. aeruginosa: 97.0% reduction | The material retained its antibacterial efficacy even after multiple washing cycles | 60 min, white light, 40 mW cm−2 | 195 |
| 59 | Boron-functionalized polyethyleneimine (PEI-BF2) | LbL assembly | E. coli: 99% reduction. S. aureus: 99.9% reduction | No change in thickness was observed over 3–4 days, indicating the photostability, stable in the pH range of 4–10 | 12 h, visible light irradiation, 12 V, 36 W | 196 |
| 60 | Acridine | Spin-coating, spray-coating, drop-coating | E. coli: 46.0% inhibition. S. aureus: 61.2% inhibition. P. aeruginosa: 75.4% inhibition. 99.3% inhibition of all of the above after 1 month | The contact angle of the surface did not change considerably after being treated with acid, alkali, salt, and other liquids for 48 h, 2 h of UV irradiation, 2 L running water attack, and 2 h of ultrasonic shaking | 60 min, visible light | 197 |
Phenothiazines and xanthenes
Some of the most widely-explored photosensitizers, either in solution or in coatings, are methylene blue (MB), toluidine blue O (TBO), and rose bengal (RB) (Fig. 3). The first two are phenothiazine derivatives, i.e. cationic PSs, characterized by low toxicity and strongly absorbing in the range 500–750 nm. In one of works of Wainwright et al., phenothiazinium-based coatings with methylene blue were prepared by the solvent evaporation method. The resulting films showed antibacterial activity against S. epidermidis and E. coli under illumination.122 When bound covalently to a silicone surface, MB also showed good bactericidal properties. In addition, when compared to TBO, it yielded higher efficiency of inactivation of methicillin-resistant Staphylococcus aureus (MRSA).123 However, TBO deposited on polyurethane showed better bactericidal properties than on silicone and amounted to >4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log after 2 min exposure and 1.5
log after 2 min exposure and 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log after 3 min exposure respectively. Similarly, for MRSA the detection limit (reduction >4
log after 3 min exposure respectively. Similarly, for MRSA the detection limit (reduction >4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log) was reached after 3 min when silicone was employed and after 1 min when polyurethane was used. Compared to inorganic antimicrobial coatings, e.g. with titanium dioxide, it takes 4–24 hours to kill MRSA bacteria up to this level.124 It was stated by the authors that even a short-time application of TBO-containing mucoadhesive patches should make it possible to treat freshly acquired oral and pharyngeal candidiasis.125
log) was reached after 3 min when silicone was employed and after 1 min when polyurethane was used. Compared to inorganic antimicrobial coatings, e.g. with titanium dioxide, it takes 4–24 hours to kill MRSA bacteria up to this level.124 It was stated by the authors that even a short-time application of TBO-containing mucoadhesive patches should make it possible to treat freshly acquired oral and pharyngeal candidiasis.125
Rose bengal is a water-soluble anionic photosensitizer known for a high singlet oxygen quantum yield (Ф = 0.79). RB immobilized in PC (polycarbonate) and PMMA (poly(methyl methacrylate)) exhibited high antibacterial activity. The amount of S. aureus dropped by 3–3.5 orders of magnitude after 0.5 h of illumination and an additional ca. tenfold decrease was observed after 1 h of treatment.126
In the work of Wright et al., rose bengal lactone was photolinked onto a siloxane copolymer. The produced textile showed both photo-activated and contact-antimicrobial properties. The >98% inactivation was observed for S. aureus and E. coli, and was further increased in the presence of light – 3× and 28×, respectively. In addition, the removal of Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) up to 90% was observed.127 Valkov et al. showed RB cross-linked to commercially available cationic polystyrene formed antimicrobial light-activated coating, which is efficient in E. coli, Enterococcus faecalis, S. aureus, and yeast Candida albicans inactivation using green light (5–8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log).128 Finally, RB layers combined with chitosan on PDMS (poly(dimethylsiloxane)) (Fig. 4) resulted in an approximately 50% inhibition of E. coli.129
log).128 Finally, RB layers combined with chitosan on PDMS (poly(dimethylsiloxane)) (Fig. 4) resulted in an approximately 50% inhibition of E. coli.129
 | ||
| Fig. 4 Schematic illustration of the different steps for PDMS surface modification with CH. RB. Reprinted from ref. 129 Copyright (2013), with permission from Elsevier. | ||
Since most organic PS possess rather narrow absorbance bands, sunlight or indoor lighting is not fully used to produce ROS. One of the ways to overcome this problem and increase aPDT under white light is the formation of a coating containing various PSs having complementary absorption. In one of the works, MB and RB were immobilized by mixing solutions of the photosensitizers in chloroform with a polymer solution. This was followed by air evaporation of the solvent. The obtained polymer films showed significant antimicrobial properties, resulting in a 1.5–3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction in S. aureus and E. coli.130 In another work, the combination of MB, RB, and TBO resulted in E. coli inactivation up to 4
log reduction in S. aureus and E. coli.130 In another work, the combination of MB, RB, and TBO resulted in E. coli inactivation up to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log levels after 24 hours. However, a reduction of over 4
log levels after 24 hours. However, a reduction of over 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log in S. aureus was observed even after just 6 hours of irradiation. This demonstrates an inexpensive, straightforward, and contemporary approach to the preparation of antibacterial surfaces.131 In the work from Decraene et al., S. aureus suspended in phosphate-buffered saline (PBS), saliva, or horse serum was sprayed onto cellulose acetate coatings containing TBO and RB, and survival of the organism on these surfaces was determined after 6 h of exposure to a household light source. Inactivation ranged from 78.9% (in horse serum) to 99.8% (in PBS) was reported.106
log in S. aureus was observed even after just 6 hours of irradiation. This demonstrates an inexpensive, straightforward, and contemporary approach to the preparation of antibacterial surfaces.131 In the work from Decraene et al., S. aureus suspended in phosphate-buffered saline (PBS), saliva, or horse serum was sprayed onto cellulose acetate coatings containing TBO and RB, and survival of the organism on these surfaces was determined after 6 h of exposure to a household light source. Inactivation ranged from 78.9% (in horse serum) to 99.8% (in PBS) was reported.106
Thionine was grafted to the cotton fiber using cyanuric chloride (2,4,6-trichloro-1,3,5-triazine) as the coupling agent. Produced light-activated coating showed 99.985% (∼3.82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log unit reduction, P = 0.0021) effectiveness against S. aureus, and 99.99% (4
log unit reduction, P = 0.0021) effectiveness against S. aureus, and 99.99% (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log unit reduction, P ≤ 0.0001) against E. coli and 99.99% inactivation against enveloped human coronavirus 229E.132 Thionine aggregation at high concentrations reduces its photosensitizing efficiency by hindering light absorption and energy transfer, leading to lower singlet oxygen generation. The presence of dispersed dyes can improve the photostability of the system by preventing the degradation of thionine under prolonged light exposure, thus maintaining its antimicrobial activity. However, humidity negatively affects the stability of photosensitizers, as moisture can cause hydrolytic degradation and interfere with the photosensitizer's electronic properties.132
log unit reduction, P ≤ 0.0001) against E. coli and 99.99% inactivation against enveloped human coronavirus 229E.132 Thionine aggregation at high concentrations reduces its photosensitizing efficiency by hindering light absorption and energy transfer, leading to lower singlet oxygen generation. The presence of dispersed dyes can improve the photostability of the system by preventing the degradation of thionine under prolonged light exposure, thus maintaining its antimicrobial activity. However, humidity negatively affects the stability of photosensitizers, as moisture can cause hydrolytic degradation and interfere with the photosensitizer's electronic properties.132
The coating obtained by photoinduced cross-linking of a PEG–diacrylate monomer associated with the eosin Y dye showed antibacterial activity against E. coli and S. aureus under white light through ROS generation mechanisms and in the dark via antibacterial agent release from the coating.133 The solvent casting method was employed to produce a light-activated coating with erythrosine B acting as a photosensitizer. It was efficient in photodynamic inactivation of S. aureus, E. coli, and Salmonella. The authors suggest using this prototype for the development of photodynamic antibacterial and environmentally friendly active packaging material.134
Anthraquinones
Aminoanthraquinone dyes (ANQ), used for semi-permanent hair coloring and dyeing fabrics and plastics, show singlet oxygen yield equal to ca. 84%.135 Photochemical studies on natural anthraquinones have revealed that their triplet state can promote the formation of reactive species, such as the superoxide radical anion, and that they are also highly effective photosensitizers for singlet oxygen production.136 Three anthraquinone dyes (Fig. 5) covalently bonded to cotton fabric demonstrated effective antimicrobial properties under visible light exposure, achieving a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log inactivation efficiency against E. coli.137
log inactivation efficiency against E. coli.137
 | ||
| Fig. 5 Anthraquinone-triazine structures for surface modification of cotton fabrics investigated in ref. 137. | ||
Porphyrins
Porphyrin is a conjugated macrocycle made of 4 pyrrole rings with a high molar absorption coefficient (ε), excellent photostability, and biocompatibility with mammalian cells. In the work of Felgenträger et al., meso-tetraphenylporphyrin was deposited on polyurethane through a spraying and the produced coating demonstrated remarkable efficacy in the photodynamic inactivation of S. aureus – more than 99% (>3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log-steps) yield within 30 minutes irradiation.138 In another work, hematoporphyrin (HP) was covalently bonded to the surface of 316L stainless steel through an esterification reaction. The antimicrobial effect of the PSS plate was tested with S. aureus and E. coli. The formed biofilm by S. aureus was effectively inactivated (99.999%). The biofilm formation by S. aureus was efficiently inhibited for 2 days under the condition of light irradiation. The confirmation of reactive oxygen generation was done by measuring the time-dependent UV-vis spectra of DPBF (1,3-diphenylisobenzofuran) – a chemical trap for singlet oxygen (Fig. 6).139
log-steps) yield within 30 minutes irradiation.138 In another work, hematoporphyrin (HP) was covalently bonded to the surface of 316L stainless steel through an esterification reaction. The antimicrobial effect of the PSS plate was tested with S. aureus and E. coli. The formed biofilm by S. aureus was effectively inactivated (99.999%). The biofilm formation by S. aureus was efficiently inhibited for 2 days under the condition of light irradiation. The confirmation of reactive oxygen generation was done by measuring the time-dependent UV-vis spectra of DPBF (1,3-diphenylisobenzofuran) – a chemical trap for singlet oxygen (Fig. 6).139
 | ||
| Fig. 6 Set of UV-vis spectra of DPBF in ethanol in the presence of photofunctional stainless steel and under green light irradiation. Inset represents the ratio between the sajewing concentration and concentration of DPBF in time of the measurement: (a) DPBF with the PSS plate under dark conditions (b) DPBF only with the light, (c) DPBF with the PSS plate with the light. All measurements were performed with the same power of the light irradiation. Reprinted fromref. 139. Copyright (2017), with permission from Elsevier. | ||
The introduction of a central metal atom to the porphyrin core strongly alters its properties – absorbance, photoactivity, or (photo)stability. Thus, metalloporphyrins are also widely investigated for aPDT processes. For example, zinc porphyrin was attached to the surface of a melt-blown non-woven textile filter material. The resulting material exhibited remarkable performance against the Influenza A virus, achieving a 99% effectiveness. Interestingly, when the sample was exposed to high-intensity white light for 4 days and then subjected to a 1000 minutes 1O2 quantification experiment, there was a similar level of 1O2 production, which confirms the stability of the system.140 In another work, zinc 5,10,15,20-tetrakis(4 N-methylpyridyl)porphyrin (ZnTMPyP4+) tetrachloride was employed in the fabrication of light-activated layers through two distinct coating methodologies: spray-coating and dip-coating with PA6. Produced coatings were found very effective in the reduction of S. Aureus, antibiotic-resistant E. Coli, and SARS-CoV-2.141
Three types of coating, composed of the commercially-available UV-photocrosslinkable polymer N-methyl-4(4′-formyl-styryl)pyridinium methosulfateacetal poly(vinyl alcohol) (SbQ-PVA) and one of three photosensitizers- (ZnTMPyP4+), methylene blue or rose bengal, showed clear bioinhibition of S. aureus and the human coronavirus strain HCoV-229E under visible light illumination with efficiency ranging from 97–99.999% and HCoV-229E inactivation from 92–99.999%, even after exposure for 4 weeks to indoor ambient room lighting, depending on the employed photosensitizer. This is proof of the long duration of action and stability of these porphyrin derivatives, which is a great advantage when used as self-disinfecting surfaces.142 These results are extremely promising, indicating that the porphyrins can be applied (in the form of a coating) as well as in fibrous furnishings found in homes, offices, temporary housing, and medical facilities.
Funes et al. reported, two porphyrins coatings (5,10,15,20-tetra(4-N,N-diphenyl aminophenyl)porphyrin (H2P-film) and its complex with Pd(II) (PdP-film)) were created on optically transparent indium tin oxide (ITO) electrodes using the electrochemical polymerization. The resultant layers exhibited an approximate 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction in E. coli and a 2.5
log reduction in E. coli and a 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction in C. albicans cellular survival after 30 minutes of irradiation with visible light.143 Lower levels of pathogen inactivation (87% against E. Coli) were observed for micrometer-sized porous honeycomb thin films formed using hybrid complexes formed by electrostatic interaction between meso-tetra(4-sulfonatophenyl)porphine chloride Mn(III) (acid form, {MnTPPS}) and dimethyldiocta-decylammonium bromide (DODMABr). The reduction of bacteria in the light was 83%, while the reduction in the dark for honeycomb films was only 5%.144 The electrochemical polymerization via oxidation of the carbazole groups was used to obtain biscarbazol-triphenylamine end-capped dendrimeric zinc porphyrin, which successfully eliminated S. aureus and E. coli.145
log reduction in C. albicans cellular survival after 30 minutes of irradiation with visible light.143 Lower levels of pathogen inactivation (87% against E. Coli) were observed for micrometer-sized porous honeycomb thin films formed using hybrid complexes formed by electrostatic interaction between meso-tetra(4-sulfonatophenyl)porphine chloride Mn(III) (acid form, {MnTPPS}) and dimethyldiocta-decylammonium bromide (DODMABr). The reduction of bacteria in the light was 83%, while the reduction in the dark for honeycomb films was only 5%.144 The electrochemical polymerization via oxidation of the carbazole groups was used to obtain biscarbazol-triphenylamine end-capped dendrimeric zinc porphyrin, which successfully eliminated S. aureus and E. coli.145
In the work of Krausz et al., anionic, neutral, and cationic amino porphyrins (Fig. 7) have been covalently grafted onto cotton fabric using 1,3,5-triazine derivative as the linker.146 The following modifications were implemented for the click–chemistry reaction (Fig. 8): porphyrins were reacted with cyanuric chloride, enabling the substitution of the first triazine chlorine atom with an amino group, yielding porphyrin-triazine derivatives. This was followed by the complete substitution of chlorine atoms using piperidine and sodium sulfonate. Finally, alkali-treated fabrics (cellulose) were introduced into the reaction mixture, and the grafting process was followed by washing cycles to remove any unreacted photosensitizer. The resulting coatings, with neutral and anionic porphyrin as photosensitizers, were effective in the inactivation of S. aureus and E. coli. Depending on the photosensitizer charge, different degrees of bacterial inhibition were obtained. Percentages of bacterial growth inhibition are 37% for anionic cotton, 93.7% for neutral cotton, and 100% for cationic cotton. The electric charge of photosensitizers directly influences photoinactivation efficacy, and these results confirm the presence of a structure–activity relationship in the photoinactivation of Gram-positive bacteria.146 The click-chemistry approach was also used for grafting meso-arylporphyrin to cotton fabric using cellulose azidation followed by acetylenic porphyrin. Meso-arylporphyrin-appended polymers inactivated Gram-negative and Gram-positive bacteria.147
 | ||
| Fig. 7 Chemical structure of neutral, anionic, and cationic amino porphyrins investigated in ref. 146. Adapted with permission from ref. 146. Copyright 2011, American Chemical Society. | ||
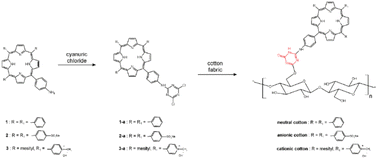 | ||
| Fig. 8 Synthetic route to photoantimicrobial cotton. Reprinted with permission from ref. 146. Copyright 2011, American Chemical Society. | ||
Wang et al. showed that electrospun microfibers of cellulose diacetate (CA) with embedded protoporphyrin IX (PpIX) can effectively inactivate S. aureus and E. coli (99.8% and 86.6% photodynamic inactivation, respectively).148 The bacteria were investigated in detail using scanning electron microscopy (SEM). The obtained SEM images confirmed the irreversible oxidative damage due to ROS (Fig. 9).148 PpIX was also used as a photosensitizer in the work of Krouit et al.149 and the work of Dong et al.150 In both cases, the covalent immobilization of PS was obtained, either in a “one-pot, two-step” esterification approach or diamide spacer, respectively. “One-pot, two-step” grafting via esterification was also proved to be effective for immobilization of carboxyporphyrins (Fig. 10) to cellulose laurate esters. It was shown that by varying the length of the alkyl chains, the problem of steric hindrance can be overcome. The grafted carboxyporphyrins showed efficiency in inhibiting bacterial growth of E. coli and S. aureus.151
 | ||
| Fig. 9 SEM images of S. aureus and E. coli on the PpIX/CA microfibers before (A and B, respectively) and after (C and D respectively) illumination. Reproduced with permission from ref. 148 Copyright 2021, Elsevier. | ||
 | ||
| Fig. 10 Structure of cellulose laurate ester modified with porphyrin investigated in (ref. 151). | ||
Cross-linked to polyethylene terephthalate tetra-substituted diazirine porphyrin formed an antimicrobial coating with bactericidal properties towards S. Aureus (1.76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log inactivation). The cross-linking was performed via a thermally triggered C–H insertion mechanism, activating the diazirine moieties to lose dinitrogen and form stable C–C bonds with the substrate, making the produced coating covalently attached and resistant to photobleaching.152
log inactivation). The cross-linking was performed via a thermally triggered C–H insertion mechanism, activating the diazirine moieties to lose dinitrogen and form stable C–C bonds with the substrate, making the produced coating covalently attached and resistant to photobleaching.152
In the work of Hunter et al., the functionalization of SiO2 with polymer brushes and crosslinking them with carboxylic acid-functionalized porphyrins (TCPP), was confirmed through various characterization techniques. The modified SiO2 beads exhibited enhanced singlet oxygen production under visible light, leading to effective antibacterial activity against E. coli.153
Phthalocyanines
Another important class of organic photosensitizers is phthalocyanines (Pcs). Thanks to extended conjugation, phthalocyanines absorb in longer wavelengths than porphyrins, thus they are widely-investigated for application in PDT.17 The photophysical and photochemical properties of Pcs can be tuned by varying central metal atoms or outer substituents.154,155 Zinc phthalocyanine is one of the most studied Pc-based photosensitizers, due to its high quantum efficiency of singlet oxygen generation. In the work of George et al., novel pyridine zinc phthalocyanine was synthesized and immobilized on filter paper via adhesion. The resulting material demonstrated ca. 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction in CFU against E. coli and A. baylyi ADP1 just after 1 h of 16 illumination with the white light of low intensity.156
log reduction in CFU against E. coli and A. baylyi ADP1 just after 1 h of 16 illumination with the white light of low intensity.156
In the recent work of Lin et al., zinc tetracarboxy-phthalocyanine was grafted to a fibrous PET that was followed by chitosan coating on the modified fiber. The resulting photoactive material was capable of significant biofilm inhibition with 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log against E. Coli and S. Aureus. Importantly, under dark conditions, the double-grafted fibers also showed high efficiency (Fig. 11). The double-grafted fiber retained 99.75% of antibacterial efficacy after ten washing.157 In another study on zinc carboxy-phthalocyanin e, coating fabric was produced. In the first layer, ε-polylysine with positive charges significantly disrupts bacterial membrane, while the second layer contains ZnPc for aPDT action. This coating efficiently inactivated E. Coli and S. Aureus by 99% and 98%, respectively. Notably, the photostability of zinc carboxy-phthalocyanine is increased when immobilized, as shown by the photobleaching tests (Fig. 12).158
log against E. Coli and S. Aureus. Importantly, under dark conditions, the double-grafted fibers also showed high efficiency (Fig. 11). The double-grafted fiber retained 99.75% of antibacterial efficacy after ten washing.157 In another study on zinc carboxy-phthalocyanin e, coating fabric was produced. In the first layer, ε-polylysine with positive charges significantly disrupts bacterial membrane, while the second layer contains ZnPc for aPDT action. This coating efficiently inactivated E. Coli and S. Aureus by 99% and 98%, respectively. Notably, the photostability of zinc carboxy-phthalocyanine is increased when immobilized, as shown by the photobleaching tests (Fig. 12).158
 | ||
| Fig. 11 Antibacterial activity of the fiber materials using colony counting method. Reprinted with permission from ref. 157. Copyright 2022, American Chemical Society. | ||
 | ||
| Fig. 12 High photostability of CPZ-EPL-Fabric (a) as shown by the much slower photo-bleaching rate compared to CPZ in methanol (b). 150 mW cm−2 energy density. Reproduced with permission from ref. 158 Copyright 2017, Elsevier. | ||
In the work of Pushalkar, silicon phthalocyanine (SiPc) was covalently attached to a sol–gel silica surface (Fig. 13). The resulting system exhibited a significant yield of 1O2 production (2.3-fold higher quantities than for modification with chlorin e6). The biofilm inactivation (>5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction) of Porphyromonas gingivalis was reported. The bacterial cultures were cultivated on hydroxyapatite discs (discs were selected to mimic the conditions of the teeth surface since hydroxyapatite is the primary mineral found in teeth), underscoring the potential of the developed device for the treatment of periodontitis.159
log reduction) of Porphyromonas gingivalis was reported. The bacterial cultures were cultivated on hydroxyapatite discs (discs were selected to mimic the conditions of the teeth surface since hydroxyapatite is the primary mineral found in teeth), underscoring the potential of the developed device for the treatment of periodontitis.159
 | ||
| Fig. 13 Bisamino Si-phthalocyanine incorporated by a sol–gel investigated in. (ref. 159) Adapted with permission from ref. 159. Copyright 2018 American Chemical Society. | ||
Silicon phthalocyanine derivative (AGA405, Fig. 14) was linked to poly(vinyl alcohol) (PVA) via boronic acid and drop-casted. The produced PVA-AGA405 coating showed antibacterial activity, sufficiently inhibiting the growth of E. coli. SEM was applied for the visualization of bacterial cells and extracellular surfaces of biofilms (Fig. 15), proving the changes in the morphology of bacteria due to ROS.160
 | ||
| Fig. 14 Silicon phthalocyanine derivative investigated in. (ref. 160) Adopted with permission from. (ref. 160) Copyright 2017, Wiley-VCH. | ||
 | ||
| Fig. 15 Representative scanning electron microscopy (SEM) images of treated and untreated biofilm samples. Reproduced with permission from ref. 160. Copyright 2017, Wiley-VCH. | ||
A straightforward adsorption of axially and peripherally substituted silicon phthalocyanines were used to modify LAPONITE® nanodiscs. It was shown that SiPc loading strongly influences the photoactivity of the system. The resulting systems were effective towards S. Aureus and no effect towards E. Coli was observed. This was explained by interactions between the surface of the modified nanodiscs and the peptidoglycan layer of Gram-positive bacteria, while for the outer membrane of Gram-negative bacteria such interactions are hindered.161
Baigorria et al. reported electropolymerized ZnPc-PEDOT and CuPc-PEDOT coating having antimicrobial activity towards S. Aureus and E. Coli, It was shown that the incubation of surfaces in 0.1 M KI solution prior to photodynamic inactivation experiment improved the efficiency of phototherapy at least two times for both types of coatings, showing the synergizing effect of singlet oxygen and iodine species.162
Chlorins
Chlorins as tetrapyrrole-based compounds stay closely related to porphyrins and phthalocyanines. Chlorins are highly effective photosensitizers, owing to their high singlet oxygen quantum yields (e.g. 89% for 2-chloro and 98% for 2,6-dichlorophenyl derivatives)163 In the recent work of Jiang et al., chlorophyllin was grafted to cotton fabric or embedded in electrospinning polyacrylonitrile nanofibers. Modified cotton fabric and nanofibers effectively generated ROS and inhibited the growth of E. faecium and S. aureus. Slightly higher effectivness, i.e. 99.9999%, was observed for nanofibres.164Other
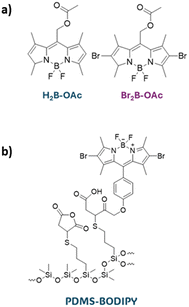
Apart from classical photosensitizer groups presented above, novel classes are being widely investigated. The main aim is to develop PS with boosted properties, e.g. absorption range, absorption coefficient, photostability, or efficiency of ROS production. Boron-dipyrromethanes (BODIPY), metal complexes, or perylenebisimides are widely explored.165 Though, most of the works on novel photosensitizers reported their efficiency in the solution phase, several works already reported their application in the light-activated antimicrobial coatings.In the work of Martinez et al., spin-coating was used for the deposition of 8-acetoxymethyl-2,6-dibromo-1,3,5,7-tetramethyl pyrromethene fluoroborate (Br2B-OAc) (Fig. 16a). The produced coating successfully inactivated S. aureus and E. coli in planktonic media for at least three cycles with short-time light exposure.166 BODIPY-derivative was covalently linked to a poly(dimethylsiloxane) material (Fig. 16b) to give material effective against S. aureus biofilm growth.167
The use of natural organic photosensitizers conduces to the formation of biocompatible layers with uniform antimicrobial properties. In the work of Santos et al., an example of a layer in which cationic polymeric biocides (SPB) were combined with a natural photosensitizer – curcumin, was described. Studies using Gram(−) and Gram(+) bacteria did not show a significant difference in their antimicrobial activity in dark or light conditions, probably due to the small amount of PS present in the bacterial suspension, which did not produce enough reactive oxygen species (ROS) to have a lethal effect on microorganisms.168
Hybrid coatings
Hybrid materials, formed by joining organic units with inorganic ones, are formed to obtain systems showing better properties than its individual counterparts. In the last years, such systems have attracted a great attention, since thanks to combining the valuable properties of their building blocks, they can be favourable for application in e.g. optics, electronics, energy storage, medicine. Similar approach can be undertaken in the case light-activated antimicrobial coatings, in which organic photosensitizers can be combined with photoactive inorganic or carbon nanomaterials (Table 2).| No. | Hybrid system | Immobilization strategy | Antibacterial effect | Irradiation parameters | Ref. |
|---|---|---|---|---|---|
| 1 | Crystal violet with cadmium-free quantum dots | Swell-encapsulation-shrink method, incorporation into polyurethane | methicillin-resistant Staphylococcus aureus: 99.98% reduction. E. coli: 99.96% reduction | 18 h for S. Aureus and 4 h for E. Coli; broad-band visible illumination at 6000 lux | 169 |
| 2 | Crystal violet, ZnO nanoparticles | Two step dipping process, incorporation in acrylic latex | E. coli: 1.97–2.51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction for CV-only (4 h). S. aureus: 1.16–2.01 log reduction for CV-only (4 h). S. aureus: 1.16–2.01![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction for CV-only (3 h, 1.34 log reduction for CV-only (3 h, 1.34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log higher for CV-ZNO) log higher for CV-ZNO) |
2 h-6 h, white light, 512 lux | 170 |
| 3 | Phloxine B, layered silicate, polyurethane | Nanocomposite supported on polytetrafluoroethylene | S. aureus: 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction log reduction |
120 s irradiation with green laser (532 nm, 100 mW) | 171 |
| 4 | Erythrosine B, layered silicate, polyurethane | Nanocomposite supported on polytetrafluoroethylene | S. aureus: Up to 10.000-fold reduction | 10 min green laser 1.5 h green LED light | 172 |
| 5 | Methylene blue, crystal violet, Au nanoparticles | Silicone surface modification | S. epidermidis: ≥2.92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction (3h). S. cerevisiae: 1.5 log reduction (3h). S. cerevisiae: 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction (3 h). MS2 Bacteriophage: 2.33 log reduction (3 h). MS2 Bacteriophage: 2.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction (4 h) log reduction (4 h) |
1 h – 5 days, fluorescent tube light, 8 W, 3500 lux | 173 |
| 6 | Protoporphyrin IX (PPIX-ED), Ag nanoparticles | (1) Bio-inspired cationic polymer bearing pendent catechols; (2) silver-loaded nanogel decorated with o-quinone groups; (3) amino modified protoporphyrin IX | B. subtilis: 14.0 mm of inhibition zone. E. coli: 17.4 mm of inhibition zone | 24 h, 380 – 750 nm, 300 W | 174 |
| 7 | Poly(3,4-ethylenedioxythiophene)-fullerene C60 (PEDOT-fullerene C60) | Electrochemical polymerization | S. aureus: >99.9% inactivation | 15, 30, or 60 min, visible light (3.1 mW cm−2, 5.6 J cm−2); different light doses compared to previous studies | 175 |
| 8 | Porphyrin-fullerene C60 dyad (TCP-C60) | Electrodeposited film | S. aureus: 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction. E. coli: 4 log reduction. E. coli: 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction log reduction |
30 min (S. aureus) 60 min (E. coli), 350–800 nm, 90 mW cm−2 | 176 |
Owusu et al. reported cadmium-free quantum dots and crystal violet conjugates immobilized via the swell-encapsulation method. The produced coating was efficient in the inactivation of E. Coli and S. Aureus with 99.96% and 99.98% reduction, respectively.169 In another work, crystal violet was combined with zinc oxide nanoparticles and deposited on the surface of polyurethane. The bactericidal activity against E. coli, P. aeruginosa, methicillin-resistant S. aureus (MRSA), and notably, highly resistant endospores of Clostridioides (Clostridium) difficile was reported.170
In the works of H. Bujdakova et al., a hybird composed of polyurethane, layered silicate (saponite) and phloxine B171 or erythrosine B,172 showed effectiveness against S. aureus. The reduction of biofilm growth associated with the surface modification of PU was also observed under dark conditions.171
Organic photosensitizers can also be accompanied with gold or silver nanoparticles to boost the antimicrobial effect. A composite material comprising crystal violet, methylene blue, and nanometer-scale gold nanoparticles was applied as a coating on medical-grade silicone. The resultant material exhibited efficacy in the treatment of S. epidermidis, S. cerevisiae, MS2 Bacteriophage, Pythium ultimum, and the filamentous fungus Botrytis cinerea.173 In the work of Bryaskov et al., bioinspired photoactive antibacterial polymer coatings on stainless steel were described. The photoactive coating, which is formed in a three-step deposition process involving a catechol-based primer for adhesion, a silver-doped nanogel for enhancing antibacterial properties, and a porphyrin-based photosensitizer that generates reactive oxygen species under visible light, showed effective antibacterial activity against B. subtilis and E. coli.174
Finally, organic photosensitizers hybrid with fullerene also show promising results. Two types of fullerene-containing organic layers were reported as light-activated antimicrobial layers. Lopez et al. reported the formation of PEDOT-fullerene C60 (Fig. 17) coating via electrochemical polymerization on ITO. The resulting layers inhibited S. Aureus by 99.9%.175 In the work of Ballatore et al., a porphyrin-fullerene dyad substituted with carbazoyl units was electrodeposited on ITO to form a layer effective against S. aureus and E. coli.176
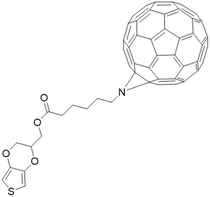 | ||
| Fig. 17 PEDOT-C60 investigated in (ref. 175). | ||
Future outlook
The significant progress in the design and application of the light-activated antimicrobial organic layers has been observed in recent years. A variety of techniques can be used for the deposition of layers containing organic photosensitizers on inanimate surfaces, including covalent and non-covalent immobilization of PS. Both, PS-only layers or those with additional components, can be produced. The early works employed mainly commercially-available well-known organic dyes. However, lately more sophisticated PSs with tailored properties are used. The non-covalent immobilization methods, e.g. drop-casting or dispersion in polymeric matrix, are usually more straightforward and less time-consuming than the covalent attachment of PSs. However, such layers are more likely to exhibit only short-term stability, due e.g. PS leakage. Though, great antimicrobial response is observed for many reported light-activated organic coatings, still several challenges need to be overcome prior to commercialization (Fig. 18).Properties of photosensitizer (PS)
The key part of light-activated antimicrobial layers is a photosensitizer that is responsible for ROS production and the ROS-related antimicrobial action. The rules for the selection or design of the proper PS are generally similar to classical PACT. Since the lower intensity of light affects the antimicrobial response, PS should possess strong absorbance in the UV-vis range (preferably broadband absorption), high efficiency of ROS production and high photostability (Fig. 18a).177 The formation of layers consisting of several PSs with complementary absorption in visible range106,131,178 can be considered as an alternative to the tedious synthesis of PS with various light-harvesting antennas. Additionally, the influence of a solid surface on the properties of deposited PS needs to be taken into account, since e.g. aggregation132,179 or change in the chemical structure of PS due to the involvement of functional groups in covalent bond formation,146,178 may strongly alter photophysical and photochemical properties of organic PS.Light absorption and oxygen transport
Light and oxygen are both crucial to initiate ROS formation. Thus, first of all, the PS's optical properties should be optimized, as described above. Second of all, the transport of light and oxygen/ROS within the layer has to be taken into account while designing light-activated layers (Fig. 18b).134 In the case of deposition of PS within polymeric matrices, polymers with high transparency and high oxygen permeability are preferable, e.g. PDMS.180 Moreover, the ROS produced by PS cannot be scavenged within/by the coating, since it would significantly lower its antimicrobial efficiency and may result in its degradation.(Photo)stability and non-toxicity of the layer
Low stability of the material is commonly a key factor limiting its practical use. When considering light-activated antimicrobial coatings, next to the photostability of the photosensitizer itself, the stability of the entire coating has to be considered: (i) its adhesion to the surface of an object, (ii) stability of other components of layer, especially against oxidation by ROS (Fig. 18c).150 The leakage of the photosensitizer should be avoided or should be minimal, to ensure the long-term effectiveness of the coating.146,181,182 Also, when the leakage of PS is observed, the exact contribution of the photoactive layer to the inactivation of microorganisms, is harder to assess, since the observed effect may be mostly due to PS being in the solution phase. Finally, the toxicity of the coating has to be assessed before the commercial use.Microbial adhesion
Another crucial aspect of the designed coating is the adhesion of microorganisms under dark conditions (Fig. 18d). The irreversible attachment of bacteria may lead to the formation of thick biofilm183 effectively limiting access to light.150 The adhesion of microorganisms depends on the properties of the surface, its roughness, hydrophobicity, charge, etc. The biofilm growth under dark conditions can be limited either by optimizing the anti-adhesive properties of the layer159,171,183 or by boosting so-called contact-killing properties,127,146,158,168 e.g. by the introduction of quaternary ammonium groups.Summary
Light-activated layers provide a unique approach to deal with pathogenic microorganisms contaminating inanimate surfaces. Thanks to the production of ROS, such coatings usually show versatile and highly-effective antimicrobial action. Organic photosensitizers possess several advantages over inorganic ones, e.g. strong visible light absorption, high yields of ROS production, or tunability. Still, there is no straightforward and easily-scalable method for the deposition of durable organic photoactive coatings on a surface irrespective of its type. Thus, the practical use is generally limited to inorganic photoactive materials. The significant progress has been made in the research on organic photosensitizers and corresponding layers in the last years. Hence, we believe that there is still a great potential for organic PSs in light-activated antimicrobial coatings, either alone or in hybrid systems.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
K. S., I. G., P. M. – writing – original draft, visualization; A. B.-G. – supervision, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was supported by National Science Centre, Poland, research project no. 2021/42/E/ST5/00110.Notes and references
- World Health Organization, Report On The Burden Of Endemic Health Care-Associated Infection Worldwide, World Health Organization, Geneva, 2011 Search PubMed.
- K. Ledwoch, S. J. Dancer, J. A. Otter, K. Kerr, D. Roposte, L. Rushton, R. Weiser, E. Mahenthiralingam, D. D. Muir and J.-Y. Maillard, J. Hosp. Infect., 2018, 100, e47–e56 CrossRef CAS PubMed.
- V. Puspasari, A. Ridhova, A. Hermawan, M. I. Amal and M. M. Khan, Bioprocess Biosyst. Eng., 2022, 45, 1421–1445 CrossRef CAS PubMed.
- A. Jabłońska-Trypuć, M. Makuła, M. Włodarczyk-Makuła, E. Wołejko, U. Wydro, L. Serra-Majem and J. Wiater, Int. J. Environ. Res. Public Health, 2022, 19, 8121 CrossRef PubMed.
- A. Kramer, I. Schwebke and G. Kampf, BMC Infect. Dis., 2006, 6, 130 CrossRef PubMed.
- L. Porter, O. Sultan, B. G. Mitchell, A. Jenney, M. Kiernan, D. J. Brewster and P. L. Russo, J. Hosp. Infect., 2024, 147, 25–31 CrossRef CAS PubMed.
- M. P. Muller, C. MacDougall, M. Lim, I. Armstrong, A. Bialachowski, S. Callery, W. Ciccotelli, M. Cividino, J. Dennis, S. Hota, G. Garber, J. Johnstone, K. Katz, A. McGeer, V. Nankoosingh, C. Richard and M. Vearncombe, J. Hosp. Infect., 2016, 92, 7–13 CrossRef CAS PubMed.
- K. Page, M. Wilson and I. P. Parkin, J. Mater. Chem., 2009, 19, 3819 RSC.
- X. Chen, J. Zhou, Y. Qian and L. Zhao, Mater. Today Bio, 2023, 19, 100586 CrossRef CAS PubMed.
- S. Noimark, C. W. Dunnill and I. P. Parkin, Adv. Drug Delivery Rev., 2013, 65, 570–580 CrossRef CAS PubMed.
- S.-H. Park, J.-H. Kim, G. Y. Chi, G.-Y. Kim, Y.-C. Chang, S.-K. Moon, S.-W. Nam, W.-J. Kim, Y. H. Yoo and Y. H. Choi, Toxicol. Lett., 2012, 212, 252–261 CrossRef CAS PubMed.
- M. A. Hayat, in Autophagy: Cancer, Other Pathologies, Inflammation, Immunity, Infection, And Aging, Elsevier, 2015, pp. 1–53 Search PubMed.
- P. R. Ogilby, Chem. Soc. Rev., 2010, 39, 3181 RSC.
- M. Bodesheim and R. Schmidt, J. Phys. Chem. A, 1997, 101, 5672–5677 CrossRef CAS.
- R. D. Scurlock, B. Wang and P. R. Ogilby, J. Am. Chem. Soc., 1996, 118, 388–392 CrossRef CAS.
- E. L. Clennan and A. Pace, Tetrahedron, 2005, 61, 6665–6691 CrossRef CAS.
- M. DeRosa, Coord. Chem. Rev., 2002, 233, 351–371 CrossRef.
- F. Thorning, P. Henke and P. R. Ogilby, J. Am. Chem. Soc., 2022, 144, 10902–10911 CrossRef CAS PubMed.
- K.-K. Wang, S. Song, S.-J. Jung, J.-W. Hwang, M.-G. Kim, J.-H. Kim, J. Sung, J.-K. Lee and Y.-R. Kim, Phys. Chem. Chem. Phys., 2020, 22, 21664–21671 RSC.
- V. G. Garcia, M. Longo, E. C. Gualberto Júnior, A. F. Bosco, M. J. H. Nagata, E. Ervolino and L. H. Theodoro, J. Periodontal Res., 2014, 49, 584–594 CrossRef CAS PubMed.
- J. Vara, M. S. Gualdesi, S. G. Bertolotti and C. S. Ortiz, J. Mol. Struct., 2019, 1181, 1–7 CrossRef CAS.
- M. Wainwright, N. J. Grice and L. E. C. Pye, Dyes Pigm., 1999, 42, 45–51 CrossRef CAS.
- K. Reszka, F. S. Cruz and R. Docampo, Chem.-Biol. Interact., 1986, 58, 161–172 CrossRef CAS PubMed.
- J. Kou, D. Dou and L. Yang, Oncotarget, 2017, 8, 81591–81603 CrossRef PubMed.
- A. Akbar, S. Khan, T. Chatterjee and M. Ghosh, J. Photochem. Photobiol., B, 2023, 248, 112796 CrossRef CAS PubMed.
- E. J. Gonzalez Lopez, S. C. Santamarina, M. G. Alvarez, D. A. Heredia and E. N. Durantini, J. Photochem. Photobiol., A, 2023, 435, 114288 CrossRef CAS.
- X. Li, B.-D. Zheng, X.-H. Peng, S.-Z. Li, J.-W. Ying, Y. Zhao, J.-D. Huang and J. Yoon, Coord. Chem. Rev., 2019, 379, 147–160 CrossRef CAS.
- J. D. Spikes, Photochem. Photobiol., 1986, 43, 691–699 CrossRef CAS PubMed.
- J. Ferreira, P. F. C. Menezes, C. Kurachi, C. Sibata, R. R. Allison and V. S. Bagnato, Laser Phys. Lett., 2008, 5, 156–161 CrossRef.
- A. D. Quartarolo, N. Russo, E. Sicilia and F. Lelj, J. Chem. Theory Comput., 2007, 3, 860–869 CrossRef CAS PubMed.
- C. Giraudeau, A. Moussaron, A. Stallivieri, S. Mordon and C. Frochot, Curr. Med. Chem., 2014, 21, 1871–1897 CrossRef CAS PubMed.
- K. Urbanska, B. Romanowska-Dixon, Z. Matuszak, J. Oszajca, P. Nowak-Sliwinska and G. Stochel, Acta Biochim. Pol., 2002, 49, 387–391 CrossRef CAS PubMed.
- D. Yan, Q. Zhao, L. Rao, J. Chen and W. Xiao, Chem. - Eur. J., 2018, 24, 16895–16901 CrossRef CAS PubMed.
- M. Pederzoli, M. Wasif Baig, M. Kývala, J. Pittner and L. Cwiklik, J. Chem. Theory Comput., 2019, 15, 5046–5057 CrossRef CAS PubMed.
- E. Bassan, A. Gualandi, P. G. Cozzi and P. Ceroni, Chem. Sci., 2021, 12, 6607–6628 RSC.
- Y. Li, X. Li, J. Yu, Z. Wan, Y. Hu, R. Huang, Y. Tao and W. Miao, Dyes Pigm., 2022, 207, 110748 CrossRef CAS.
- Y. Cai, P. Liang, Q. Tang, W. Si, P. Chen, Q. Zhang and X. Dong, ACS Appl. Mater. Interfaces, 2017, 9, 30398–30405 CrossRef CAS PubMed.
- T. M. Ebaston, F. Nakonechny, E. Talalai, G. Gellerman and L. Patsenker, Dyes Pigm., 2021, 184, 108854 CrossRef CAS.
- D. Y. T. Galdino, G. Da Rocha Leódido, C. Pavani, L. M. Gonçalves, S. K. Bussadori and M. A. B. Paschoal, Photodiagn. Photodyn. Ther., 2021, 33, 102191 CrossRef CAS PubMed.
- F. Morikawa, M. Fukuda, M. Naganuma and Y. Nakayama, J. Dermatol., 1976, 3, 59–67 CrossRef CAS PubMed.
- L. Serpe, S. Ellena, N. Barbero, F. Foglietta, F. Prandini, M. P. Gallo, R. Levi, C. Barolo, R. Canaparo and S. Visentin, Eur. J. Med. Chem., 2016, 113, 187–197 CrossRef CAS PubMed.
- K. T. Kazantzis, K. Koutsonikoli, B. Mavroidi, M. Zachariadis, P. Alexiou, M. Pelecanou, K. Politopoulos, E. Alexandratou and M. Sagnou, Photochem. Photobiol. Sci., 2020, 19, 193–206 CrossRef CAS PubMed.
- L. D. Dias, K. C. Blanco, I. S. Mfouo-Tynga, N. M. Inada and V. S. Bagnato, J. Photochem. Photobiol., C, 2020, 45, 100384 Search PubMed.
- K. A. Leonard, M. I. Nelen, T. P. Simard, S. R. Davies, S. O. Gollnick, A. R. Oseroff, S. L. Gibson, R. Hilf, L. B. Chen and M. R. Detty, J. Med. Chem., 1999, 42, 3953–3964 Search PubMed.
- T. Y. Ohulchanskyy, M. K. Gannon, M. Ye, A. Skripchenko, S. J. Wagner, P. N. Prasad and M. R. Detty, J. Phys. Chem. B, 2007, 111, 9686–9692 CrossRef CAS PubMed.
- X. Cai, K.-N. Wang, W. Ma, Y. Yang, G. Chen, H. Fu, C. Cui, Z. Yu and X. Wang, J. Nanobiotechnol., 2021, 19, 254 Search PubMed.
- P. García Calavia, G. Bruce, L. Pérez-García and D. A. Russell, Photochem. Photobiol. Sci., 2018, 17, 1534–1552 CrossRef PubMed.
- Y. Li, Y. Li, Y. Bai, R. Wang, L. Lin and Y. Sun, RSC Adv., 2020, 10, 38416–38423 RSC.
- C. Yi, Z. Yu, Q. Ren, X. Liu, Y. Wang, X. Sun, S. Yin, J. Pan and X. Huang, Photodiagn. Photodyn. Ther., 2020, 30, 101694 CrossRef CAS PubMed.
- W. Macyk, K. Szaciłowski, G. Stochel, M. Buchalska, J. Kuncewicz and P. Łabuz, Coord. Chem. Rev., 2010, 254, 2687–2701 CrossRef CAS.
- T. Robeldo, L. S. Ribeiro, L. Manrique, A. M. Kubo, E. Longo, E. R. Camargo and R. C. Borra, ACS Omega, 2022, 7, 17563–17574 CrossRef CAS PubMed.
- H. Zhang, Y. Shan and L. Dong, J. Biomed. Nanotechnol., 2014, 10, 1450–1457 Search PubMed.
- F. Heinemann, J. Karges and G. Gasser, Acc. Chem. Res., 2017, 50, 2727–2736 CrossRef CAS PubMed.
- M. A. Munegowda, A. Manalac, M. Weersink, S. A. McFarland and L. Lilge, Coord. Chem. Rev., 2022, 470, 214712 CrossRef PubMed.
- P. Szymaszek, M. Tyszka-Czochara and J. Ortyl, Eur. J. Med. Chem., 2024, 276, 116648 CrossRef CAS PubMed.
- Y. Wang, Y. Huang, S. Chen, J. Gao, Y. Zhang, Y. Duan and P. Deng, Inorg. Chem., 2023, 62, 7212–7219 CrossRef CAS PubMed.
- X. Fan, S. Lv, F. Lv, E. Feng, D. Liu, P. Zhou and F. Song, Chem.–Eur. J., 2024, 30, e202304113 CrossRef CAS PubMed.
- D. Chatterjee and S. Dasgupta, J. Photochem. Photobiol., C, 2005, 6, 186–205 CrossRef CAS.
- D. Ashen-Garry and M. Selke, Photochem. Photobiol., 2014, 90, 257–274 CrossRef CAS PubMed.
- M. Klausen, M. Ucuncu and M. Bradley, Molecules, 2020, 25, 5239 CrossRef CAS PubMed.
- R. T. Aroso, F. A. Schaberle, L. G. Arnaut and M. M. Pereira, Photochem. Photobiol. Sci., 2021, 20, 1497–1545 CrossRef CAS PubMed.
- H. Zhang, L. Xu, X. Gu, D. Yu and S. Li, RSC Adv., 2023, 13, 239–250 RSC.
- M. Wainwright, D. A. Phoenix, S. M. Burrow and J. Waring, J. Chemother., 1999, 11, 61–68 CrossRef CAS PubMed.
- I. W. Badon, J.-P. Jee, T. P. Vales, C. Kim, S. Lee, J. Yang, S. K. Yang and H.-J. Kim, Pharmaceutics, 2023, 15, 1512 CrossRef CAS PubMed.
- M. L. Agazzi, M. B. Ballatore, A. M. Durantini, E. N. Durantini and A. C. Tomé, J. Photochem. Photobiol., C, 2019, 40, 21–48 CrossRef CAS.
- M. Halaskova, A. Rahali, V. Almeida-Marrero, M. Machacek, R. Kucera, B. Jamoussi, T. Torres, V. Novakova, A. de la Escosura and P. Zimcik, ACS Med. Chem. Lett., 2021, 12, 502–507 CrossRef CAS PubMed.
- I. O. Savelyeva, K. A. Zhdanova, M. A. Gradova, O. V. Gradov and N. A. Bragina, Curr. Issues Mol. Biol., 2023, 45, 9793–9822 CrossRef CAS PubMed.
- X. Ragàs, D. Sánchez-García, R. Ruiz-González, T. Dai, M. Agut, M. R. Hamblin and S. Nonell, J. Med. Chem., 2010, 53, 7796–7803 CrossRef PubMed.
- B. M. Estevão, D. S. Pellosi, C. F. De Freitas, D. Vanzin, D. S. Franciscato, W. Caetano and N. Hioka, J. Photochem. Photobiol., A, 2014, 287, 30–39 CrossRef.
- B. Fischer, A. Krieger-Liszkay and R. Eggen, Environ. Sci. Technol., 2005, 38, 6307–6313 CrossRef PubMed.
- D. S. Pellosi, B. M. Estevão, C. F. Freitas, T. M. Tsubone, W. Caetano and N. Hioka, Dyes Pigm., 2013, 99, 705–712 CrossRef CAS.
- M. Wainwright, H. Smalley and C. Flint, J. Photochem. Photobiol., B, 2011, 105, 1–5 CrossRef CAS PubMed.
- W. Chin, P. Heng, P. Thong, R. Bhuvaneswari, W. Hirt, S. Kuenzel, K. Soo and M. Olivo, Eur. J. Pharm. Biopharm., 2008, 69, 1083–1093 CrossRef CAS PubMed.
- T. B. Thapa Magar, J. Lee, J. H. Lee, J. Jeon, P. Gurung, J. Lim and Y.-W. Kim, Pharmaceutics, 2023, 15, 1577 CrossRef CAS PubMed.
- A. Wiehe and M. O. Senge, Photochem. Photobiol., 2023, 99, 356–419 CrossRef CAS PubMed.
- R. Whelpton, A. T. Michael-Titus, S. S. Basra and M. Grahn, Photochem. Photobiol., 1995, 61, 397–401 CrossRef CAS PubMed.
- K. T. Kazantzis, K. Koutsonikoli, B. Mavroidi, M. Zachariadis, P. Alexiou, M. Pelecanou, K. Politopoulos, E. Alexandratou and M. Sagnou, Photochem. Photobiol. Sci., 2020, 19, 193–206 CrossRef CAS PubMed.
- A. Kielbik, P. Wawryka, D. Przystupski, J. Rossowska, A. Szewczyk, J. Saczko, J. Kulbacka and A. Chwiłkowska, In Vivo, 2019, 33, 1857–1864 CrossRef CAS PubMed.
- M. Wainwright, D. A. Phoenix, J. Marland, D. R. A. Wareing and F. J. Bolton, FEMS Immunol. Med. Microbiol., 1997, 19, 75–80 CrossRef CAS PubMed.
- K.-K. Wang, B.-J. Kim, S.-H. Ko, D. H. Choi and Y.-R. Kim, J. Nanomater., 2012, 2012, 1–7 Search PubMed.
- M. Eshaghi Gorji and D. Li, Food Qual. Saf., 2022, 6, fyac017 CrossRef.
- I. B. Rosseti, L. R. Chagas and M. S. Costa, Laser Med. Sci., 2014, 29, 1059–1064 CrossRef PubMed.
- C. P. Sabino, M. S. Ribeiro, M. Wainwright, C. Dos Anjos, F. P. Sellera, M. Dropa, N. B. Nunes, G. T. P. Brancini, G. U. L. Braga, V. E. Arana-Chavez, R. O. Freitas, N. Lincopan and M. S. Baptista, Photochem. Photobiol., 2023, 99, 742–750 CrossRef CAS PubMed.
- M. S. Baptista, J. Cadet, A. Greer and A. H. Thomas, Photochem. Photobiol., 2021, 97, 1456–1483 CrossRef CAS PubMed.
- M. S. Baptista, J. Cadet, A. Greer and A. H. Thomas, Photochem. Photobiol., 2023, 99, 313–334 CrossRef CAS PubMed.
- M. S. Baptista, J. Cadet, P. Di Mascio, A. A. Ghogare, A. Greer, M. R. Hamblin, C. Lorente, S. C. Nunez, M. S. Ribeiro, A. H. Thomas, M. Vignoni and T. M. Yoshimura, Photochem. Photobiol., 2017, 93, 912–919 CrossRef CAS PubMed.
- M. Wainwright and K. B. Crossley, Int. Biodeterior. Biodegrad., 2004, 53, 119–126 CrossRef CAS.
- C. Spagnul, L. C. Turner and R. W. Boyle, J. Photochem. Photobiol., B, 2015, 150, 11–30 CrossRef CAS PubMed.
- A. Anas, J. Sobhanan, K. M. Sulfiya, C. Jasmin, P. K. Sreelakshmi and V. Biju, J. Photochem. Photobiol., C, 2021, 49, 100452 CrossRef CAS.
- M. Wainwright, Dyes Pigm., 2021, 196, 109813 CrossRef CAS PubMed.
- J. Jiang, X. Lv, H. Cheng, D. Yang, W. Xu, Y. Hu, Y. Song and G. Zeng, Acta Biomater., 2024, 177, 1–19 CrossRef CAS PubMed.
- M. Wainwright, T. Maisch, S. Nonell, K. Plaetzer, A. Almeida, G. P. Tegos and M. R. Hamblin, Lancet Infect. Dis., 2017, 17, e49–e55 CrossRef PubMed.
- G. A. Meerovich, E. V. Akhlyustina, I. D. Romanishkin, E. A. Makarova, I. G. Tiganova, V. G. Zhukhovitsky, E. G. Kholina, I. B. Kovalenko, Y. M. Romanova, V. B. Loschenov and M. G. Strakhovskaya, Photodiagn. Photodyn. Ther., 2023, 44, 103853 CrossRef CAS PubMed.
- W. Browne, Coord. Chem. Rev., 2008, 252, 2470–2479 Search PubMed.
- G. Fu, P. S. Vary and C.-T. Lin, J. Phys. Chem. B, 2005, 109, 8889–8898 CrossRef CAS PubMed.
- V. Kumaravel, K. M. Nair, S. Mathew, J. Bartlett, J. E. Kennedy, H. G. Manning, B. J. Whelan, N. S. Leyland and S. C. Pillai, Chem. Eng. J., 2021, 416, 129071 CrossRef CAS PubMed.
- S. M. Imani, L. Ladouceur, T. Marshall, R. Maclachlan, L. Soleymani and T. F. Didar, ACS Nano, 2020, 14, 12341–12369 CrossRef CAS PubMed.
- P. Carvalho, P. Sampaio, S. Azevedo, C. Vaz, J. P. Espinós, V. Teixeira and J. O. Carneiro, Appl. Surf. Sci., 2014, 307, 548–557 CrossRef CAS.
- A. Magrez, L. Horváth, R. Smajda, V. Salicio, N. Pasquier, L. Forró and B. Schwaller, ACS Nano, 2009, 3, 2274–2280 CrossRef CAS PubMed.
- photoactive.fr.
- envisionsq.com.
- titanoclean.com.
- pilkington.com.
- M. Wilson, Infect. Control Hosp. Epidemiol., 2003, 24, 782–784 CrossRef PubMed.
- V. Decraene, J. Pratten and M. Wilson, Appl. Environ. Microbiol., 2006, 72, 4436–4439 CrossRef CAS PubMed.
- V. Decraene, J. Pratten and M. Wilson, Curr. Microbiol., 2008, 57, 269–273 CrossRef CAS PubMed.
- A. Ulman, Chem. Rev., 1996, 96, 1533–1554 CrossRef CAS PubMed.
- J. J. Gooding, F. Mearns, W. Yang and J. Liu, Electroanalysis, 2003, 15, 81–96 CrossRef CAS.
- D. Bélanger and J. Pinson, Chem. Soc. Rev., 2011, 40, 3995 RSC.
- J. Pinson and F. Podvorica, Chem. Soc. Rev., 2005, 34, 429 Search PubMed.
- M. Delamar, R. Hitmi, J. Pinson and J. M. Saveant, J. Am. Chem. Soc., 1992, 114, 5883–5884 CrossRef CAS.
- C. Gautier, I. López and T. Breton, Mater. Adv., 2021, 2, 2773–2810 RSC.
- E. Blasco, M. B. Sims, A. S. Goldmann, B. S. Sumerlin and C. Barner-Kowollik, Macromolecules, 2017, 50, 5215–5252 CrossRef CAS.
- A. Ketema and A. Worku, J. Chem., 2020, 2020, 1–7 Search PubMed.
- L. Wengeler, M. Schmitt, K. Peters, P. Scharfer and W. Schabel, Chem. Eng. Process.: Process Intensif., 2013, 68, 38–44 Search PubMed.
- N. Sahu, B. Parija and S. Panigrahi, Indian J. Phys., 2009, 83, 493–502 CrossRef CAS.
- A. Kaliyaraj Selva Kumar, Y. Zhang, D. Li and R. G. Compton, Electrochem. Commun., 2020, 121, 106867 CrossRef CAS.
- C. Zuo and L. Ding, Angew. Chem., Int. Ed., 2021, 60, 11242–11246 Search PubMed.
- M. Zhao, H. Zhang, C. Gu and Y. Ma, J. Mater. Chem. C, 2020, 8, 5310–5320 RSC.
- F. Asaduzzaman and S. Salmon, Mol. Syst. Des. Eng., 2022, 7, 1385–1414 Search PubMed.
- S. Mahouche-Chergui, M. Guerrouache, B. Carbonnier and M. M. Chehimi, Colloids Surf., A, 2013, 439, 43–68 Search PubMed.
- M. Wainwright, M. N. Byrne and M. A. Gattrell, J. Photochem. Photobiol., B, 2006, 84, 227–230 CrossRef CAS PubMed.
- C. Piccirillo, S. Perni, J. Gil-Thomas, P. Prokopovich, M. Wilson, J. Pratten and I. P. Parkin, J. Mater. Chem., 2009, 19, 6167 RSC.
- S. Perni, P. Prokopovich, C. Piccirillo, J. Pratten, I. P. Parkin and M. Wilson, J. Mater. Chem., 2009, 19, 2715 Search PubMed.
- R. F. Donnelly, P. A. McCarron, M. M. Tunney and A. David Woolfson, J. Photochem. Photobiol., B, 2007, 86, 59–69 CrossRef CAS PubMed.
- A. Valkov, F. Nakonechny and M. Nisnevitch, Appl. Mech. Mater., 2015, 719–720, 21–24 Search PubMed.
- T. Wright, M. Vlok, T. Shapira, A. D. Olmstead, F. Jean and M. O. Wolf, ACS Appl. Mater. Interfaces, 2022, 14, 49–56 CrossRef CAS PubMed.
- R. Gavara, R. De Llanos, V. Pérez-Laguna, C. Arnau Del Valle, J. F. Miravet, A. Rezusta and F. Galindo, Front. Med., 2021, 8, 641646 Search PubMed.
- A. M. Ferreira, I. Carmagnola, V. Chiono, P. Gentile, L. Fracchia, C. Ceresa, G. Georgiev and G. Ciardelli, Surf. Coat. Technol., 2013, 223, 92–97 CrossRef CAS.
- F. Nakonechny, A. Pinkus, S. Hai, O. Yehosha, Y. Nitzan and M. Nisnevitch, Photochem. Photobiol., 2013, 89, 671–678 CrossRef CAS PubMed.
- R. Cahan, R. Schwartz, Y. Langzam and Y. Nitzan, Photochem. Photobiol., 2011, 87, 1379–1386 Search PubMed.
- C. Jiang, S. Dejarnette, W. Chen, F. Scholle, Q. Wang and R. A. Ghiladi, Photochem. Photobiol. Sci., 2023, 22, 1573–1590 Search PubMed.
- P. Sautrot-Ba, A. Contreras, S. Abbad Andaloussi, T. Coradin, C. Hélary, N. Razza, M. Sangermano, P.-E. Mazeran, J.-P. Malval and D.-L. Versace, J. Mater. Chem. B, 2017, 5, 7572–7582 RSC.
- S. Zhu, R. H. Ukwatta, X. Cai, Y. Zheng, F. Xue, C. Li and L. Wang, Int. J. Biol. Macromol., 2023, 225, 112–122 Search PubMed.
- H.-U. Borst, J. Kelemen, J. Fabian, M. Nepra
![[s with combining breve]](https://www.rsc.org/images/entities/char_0073_0306.gif) and H. E. A. Kramer, J. Photochem. Photobiol., A, 1992, 69, 97–107 Search PubMed.
and H. E. A. Kramer, J. Photochem. Photobiol., A, 1992, 69, 97–107 Search PubMed. - L. Breloy, C. Elian, V. Alphonse, S. Lajnef, F. Peyrot, D. Jacquemin, S. Pascal, E. Devernois, T. Coradin, S. Abbad Andaloussi and D.-L. Versace, RSC Appl. Polym., 2025, 3, 222–237 RSC.
- V. Cardoso, T. Rittmeyer, R. J. Correa, G. C. Brêda, R. V. Almeida, G. Simões, B. M. De França, P. N. De Azevedo and J. S. Bello Forero, Dyes Pigm., 2019, 161, 16–23 CrossRef CAS.
- A. Felgenträger, T. Maisch, A. Späth, J. A. Schröder and W. Bäumler, Phys. Chem. Chem. Phys., 2014, 16, 20598–20607 RSC.
- K.-K. Wang, B.-J. Kim, Il- Heo, S.-J. Jung, J.-W. Hwang and Y.-R. Kim, Surf. Coat. Technol., 2017, 310, 256–262 CrossRef CAS.
- T. J. Cuthbert, S. Ennis, S. F. Musolino, H. L. Buckley, M. Niikura, J. E. Wulff and C. Menon, Sci. Rep., 2021, 11, 19029 Search PubMed.
- B. S. T. Peddinti, N. Morales-Gagnon, B. Pourdeyhimi, F. Scholle, R. J. Spontak and R. A. Ghiladi, ACS Appl. Mater. Interfaces, 2021, 13, 155–163 Search PubMed.
- C. R. Ghareeb, B. S. T. Peddinti, S. C. Kisthardt, F. Scholle, R. J. Spontak and R. A. Ghiladi, Front. Med., 2021, 8, 657837 CrossRef PubMed.
- M. D. Funes, D. A. Caminos, M. G. Alvarez, F. Fungo, L. A. Otero and E. N. Durantini, Environ. Sci. Technol., 2009, 43, 902–908 CrossRef CAS PubMed.
- Y. Wang, Y. Liu, G. Li and J. Hao, Langmuir, 2014, 30, 6419–6426 Search PubMed.
- D. A. Heredia, S. R. Martínez, A. M. Durantini, M. E. Pérez, M. I. Mangione, J. E. Durantini, M. A. Gervaldo, L. A. Otero and E. N. Durantini, ACS Appl. Mater. Interfaces, 2019, 11, 27574–27587 CrossRef CAS PubMed.
- C. Ringot, V. Sol, M. Barrière, N. Saad, P. Bressollier, R. Granet, P. Couleaud, C. Frochot and P. Krausz, Biomacromolecules, 2011, 12, 1716–1723 CrossRef CAS PubMed.
- C. Ringot, V. Sol, R. Granet and P. Krausz, Mater. Lett., 2009, 63, 1889–1891 CrossRef CAS.
- T. Wang, H. Ke, S. Chen, J. Wang, W. Yang, X. Cao, J. Liu, Q. Wei, R. A. Ghiladi and Q. Wang, Mater. Sci. Eng. C, 2021, 118, 111502 CrossRef CAS PubMed.
- M. Krouit, R. Granet, P. Branland, B. Verneuil and P. Krausz, Bioorg. Med. Chem. Lett., 2006, 16, 1651–1655 CrossRef CAS PubMed.
- J. Dong, R. A. Ghiladi, Q. Wang, Y. Cai and Q. Wei, Cellulose, 2018, 25, 1673–1686 CrossRef CAS.
- M. Krouit, R. Granet and P. Krausz, Eur. Polym. J., 2009, 45, 1250–1259 CrossRef CAS.
- S. F. Musolino, F. Shatila, G. M. O. Tieman, A. C. Masarsky, M. C. Thibodeau, J. E. Wulff and H. L. Buckley, ACS Omega, 2022, 7, 29517–29525 CrossRef CAS PubMed.
- B. Hunter, J. L. Sacco, K. Katterle, J. Kirigo, T. K. Wood, E. W. Gomez and C. W. Pester, Eur. Polym. J., 2024, 213, 113090 CrossRef CAS.
- A. M. Schmidt and M. J. F. Calvete, Molecules, 2021, 26, 2823 CrossRef CAS PubMed.
- K. Ishii, Coord. Chem. Rev., 2012, 256, 1556–1568 CrossRef CAS.
- L. George, A. Müller, B. Röder, V. Santala and A. Efimov, Dyes Pigm., 2017, 147, 334–342 CrossRef CAS.
- Y. Lin, J. Chen, Y. Mai, L. Chen, Z. Chen, G. Wang, L. Deng, P. Xu, C. Yuan, L. Jiang and M. Huang, ACS Appl. Mater. Interfaces, 2022, 14, 47003–47013 CrossRef CAS PubMed.
- J. Chen, W. Wang, P. Hu, D. Wang, F. Lin, J. Xue, Z. Chen, Z. Iqbal and M. Huang, Dyes Pigm., 2017, 140, 236–243 CrossRef CAS.
- S. Pushalkar, G. Ghosh, Q. Xu, Y. Liu, A. A. Ghogare, C. Atem, A. Greer, D. Saxena and A. M. Lyons, ACS Appl. Mater. Interfaces, 2018, 10, 25819–25829 CrossRef CAS PubMed.
- A. Galstyan, R. Schiller and U. Dobrindt, Angew. Chem. Int. Ed., 2017, 56, 10362–10366 CrossRef CAS PubMed.
- M. Grüner, L. Tuchscherr, B. Löffler, D. Gonnissen, K. Riehemann, M. C. Staniford, U. Kynast and C. A. Strassert, ACS Appl. Mater. Interfaces, 2015, 7, 20965–20971 CrossRef PubMed.
- E. Baigorria, J. E. Durantini, S. R. Martínez, M. E. Milanesio, Y. B. Palacios and A. M. Durantini, ACS Appl. Bio Mater., 2021, 4, 8559–8570 Search PubMed.
- M. Pineiro, M. M. Pereira, A. M. d’A Rocha Gonsalves, L. G. Arnaut and S. J. Formosinho, J. Photochem. Photobiol., A, 2001, 138, 147–157 CrossRef CAS.
- C. Jiang, F. Scholle, F. Jin, Q. Wei, Q. Wang and R. A. Ghiladi, Cellulose, 2024, 31, 2475–2491 Search PubMed.
- J. Zhao, W. Wu, J. Sun and S. Guo, Chem. Soc. Rev., 2013, 42, 5323 RSC.
- S. R. Martínez, Y. B. Palacios, D. A. Heredia, V. Aiassa, A. Bartolilla and A. M. Durantini, ACS Appl. Mater. Interfaces, 2021, 13, 11597–11608 CrossRef PubMed.
- W. J. Peveler, S. Noimark, H. Al-Azawi, G. B. Hwang, C. R. Crick, E. Allan, J. B. Edel, A. P. Ivanov, A. J. MacRobert and I. P. Parkin, ACS Appl. Mater. Interfaces, 2018, 10, 98–104 CrossRef CAS PubMed.
- M. R. E. Santos, P. V. Mendonça, R. Branco, R. Sousa, C. Dias, A. C. Serra, J. R. Fernandes, F. D. Magalhães, P. V. Morais and J. F. J. Coelho, ACS Appl. Mater. Interfaces, 2021, 13, 7567–7579 CrossRef CAS PubMed.
- E. G. A. Owusu, A. J. MacRobert, I. Naasani, I. P. Parkin, E. Allan and E. Yaghini, ACS Appl. Mater. Interfaces, 2019, 11, 12367–12378 Search PubMed.
- S. K. Sehmi, C. Lourenco, K. Alkhuder, S. D. Pike, S. Noimark, C. K. Williams, M. S. P. Shaffer, I. P. Parkin, A. J. MacRobert and E. Allan, ACS Infect. Dis., 2020, 6, 939–946 CrossRef CAS PubMed.
- N. C. T. Dadi, J. Bujdák, V. Medvecká, H. Pálková, M. Barlog and H. Bujdáková, Materials, 2021, 14, 7583 CrossRef CAS PubMed.
- L. Bugyna, K. Bilská, P. Boháč, M. Pribus, J. Bujdák and H. Bujdáková, Molecules, 2024, 29, 3917 CrossRef CAS PubMed.
- T. Walker, M. Canales, S. Noimark, K. Page, I. Parkin, J. Faull, M. Bhatti and L. Ciric, Sci. Rep., 2017, 7, 15298 CrossRef PubMed.
- R. Bryaskova, N. Philipova, N. Georgiev, D. Ganchev, I. Lalov and C. Detrembleur, J. Polym. Res., 2023, 30, 199 CrossRef CAS.
- E. Reynoso, A. M. Durantini, C. A. Solis, L. P. Macor, L. A. Otero, M. A. Gervaldo, E. N. Durantini and D. A. Heredia, RSC Adv., 2021, 11, 23519–23532 Search PubMed.
- M. B. Ballatore, J. Durantini, N. S. Gsponer, M. B. Suarez, M. Gervaldo, L. Otero, M. B. Spesia, M. E. Milanesio and E. N. Durantini, Environ. Sci. Technol., 2015, 49, 7456–7463 Search PubMed.
- W. Zhou, X. Jiang and X. Zhen, Biomater. Sci., 2023, 11, 5108–5128 RSC.
- A. Nyga, D. Czerwińska-Główka, M. Krzywiecki, W. Przystaś, E. Zabłocka-Godlewska, S. Student, M. Kwoka, P. Data and A. Blacha-Grzechnik, Materials, 2021, 14, 3093 CrossRef CAS PubMed.
- T. Yao, J. Wang, Y. Xue, W. Yu, Q. Gao, L. Ferreira, K.-F. Ren and J. Ji, J. Mater. Chem. B, 2019, 7, 5089–5095 Search PubMed.
- H.-X. Rao, F.-N. Liu and Z.-Y. Zhang, J. Membr. Sci., 2007, 303, 132–139 CrossRef CAS.
- M. Chen, R. Dong, J. Song, J. Qi, J. Zhang, Z. Zhao, W. Zhang, Y. Li and B. Z. Tang, Adv. Healthcare Mater., 2024, 13, 2303967 CrossRef CAS PubMed.
- N. Harada, K. Masuda, J. Nakamura and H. Uyama, Polym. J., 2021, 53, 1383–1391 CrossRef CAS.
- A. Uneputty, A. Dávila-Lezama, D. Garibo, A. Oknianska, N. Bogdanchikova, J. F. Hernández-Sánchez and A. Susarrey-Arce, Colloid Interface Sci. Commun., 2022, 46, 100560 Search PubMed.
- D. Langerreiter, K. Solin, M. Jordà-Redondo, R. Bresolí-Obach, L. Fliri, S. Nonell, M. A. Kostiainen and E. Anaya-Plaza, Mater. Today Commun., 2024, 38, 107858 CrossRef CAS.
- X. Nie, S. Wu, F. Huang, W. Li, H. Qiao, Q. Wang and Q. Wei, Chem. Eng. J., 2021, 416, 129072 CrossRef CAS.
- E. Feese, H. Sadeghifar, H. S. Gracz, D. S. Argyropoulos and R. A. Ghiladi, Biomacromolecules, 2011, 12, 3528–3539 Search PubMed.
- K. A. D. F. Castro, N. M. M. Moura, M. M. Q. Simões, J. A. S. Cavaleiro, M. D. A. F. Faustino, Â. Cunha, F. A. Almeida Paz, R. F. Mendes, A. Almeida, C. S. R. Freire, C. Vilela, A. J. D. Silvestre, S. Nakagaki and M. d. G. P. M. S. Neves, Appl. Mater. Today, 2019, 16, 332–341 Search PubMed.
- E. Anaya-Plaza, E. van de Winckel, J. Mikkilä, J. Malho, O. Ikkala, O. Gulías, R. Bresolí-Obach, M. Agut, S. Nonell, T. Torres, M. A. Kostiainen and A. de la Escosura, Chem.–Eur. J., 2017, 23, 4320–4326 Search PubMed.
- R. Bonnett, M. A. Krysteva, I. G. Lalov and S. V. Artarsky, Water Res., 2006, 40, 1269–1275 CrossRef CAS PubMed.
- N. E. Grammatikova, L. George, Z. Ahmed, N. R. Candeias, N. A. Durandin and A. Efimov, J. Mater. Chem. B, 2019, 7, 4379–4384 RSC.
- Y. Lin, J. Chen, Y. Mai, L. Chen, Z. Chen, G. Wang, L. Deng, P. Xu, C. Yuan, L. Jiang and M. Huang, ACS Appl. Mater. Interfaces, 2022, 14, 47003–47013 CrossRef CAS PubMed.
- P. Sautrot-Ba, J.-P. Malval, M. Weiss-Maurin, J. Paul, A. Blacha-Grzechnik, S. Tomane, P.-E. Mazeran, J. Lalevée, V. Langlois and D.-L. Versace, ACS Sustainable Chem. Eng., 2018, 6, 104–109 CrossRef CAS.
- M. Condat, J. Babinot, S. Tomane, J.-P. Malval, I.-K. Kang, F. Spillebout, P.-E. Mazeran, J. Lalevée, S. A. Andalloussi and D.-L. Versace, RSC Adv., 2016, 6, 18235–18245 RSC.
- M. Condat, P.-E. Mazeran, J.-P. Malval, J. Lalevée, F. Morlet-Savary, E. Renard, V. Langlois, S. Abbad Andalloussi and D.-L. Versace, RSC Adv., 2015, 5, 85214–85224 RSC.
- R. Sahu, N. Ninan, N. H. Nguyen, J. Wang, K. Vasilev, V. K. Truong and Y. Tang, Molecules, 2024, 29, 1209 CrossRef CAS PubMed.
- A. K. Gill, S. Shah, P. Yadav, A. Shanavas, P. P. Neelakandan and D. Patra, J. Mater. Chem. B, 2022, 10, 9869–9877 RSC.
- L. Kang, H. Peng, M. Yang, K. Hu, Y. Lin, Y. Zhu, H. Chen, J. Zhao, S. Han, Y. Wang, N. Wen, J. Long and H. Xi, Prog. Org. Coat., 2024, 194, 108593 CrossRef CAS.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |