Open Access Article
Open Access ArticleProduction of 1,4:3,6-dianhydro-α-D-glucopyranose from methyl 3,6-anhydro-α-D-glucopyranoside and its taste identification†
Zhifei Chena,
Yibo Ninga,
Lei Lia,
Xueying Caoa,
Gaolei Xia,
Dongxu Chenga,
Qingfu Wang *a,
Changtong Lu*a and
Kai Yang
*a,
Changtong Lu*a and
Kai Yang *b
*b
aTechnology Center, China Tobacco Henan Industrial Limited Company, Zhengzhou, 450002, P. R. China. E-mail: wqfhust@163.com; luchangtongfly@163.com
bSchool of Chemistry and Chemical Engineering, Shandong University of Technology, Zibo, 255049, P. R. China. E-mail: yangkai@sdut.edu.cn
First published on 10th February 2025
Abstract
1,4:3,6-Dianhydro-α-D-glucopyranose (DGP) is one of important biomass pyrolysis anhydro sugar products that derive from the cellulose and hemicellulose components. There is no reliable method for the preparation of DGP at present, which contributed to its high cost with limited market supply and restricted applied research. In this study, we provided a novel method for the synthesis of DGP from methyl 3,6-anhydro-α-D-glucopyranoside for the first time. A mild and environmentally friendly synthetic approach for 3,6-anhydro glucopyranoside was developed via the intramolecular cyclization of 6-O-tosyl glucopyranoside, promoted by a catalytic amount of TBAF. And the preparation of DGP was achieved through the stabilization effect on carbocation intermediates by HFIP in the intramolecular cyclization of 3,6-anhydro glucopyranoside. Further sensory evaluation studies revealed that DGP had a sweetness similar to that of sucrose.
1 Introduction
1,4:3,6-Dianhydro-α-D-glucopyranose (DGP) is a dehydration sugar compound with rigid, multi-oxygen-bridged, multi-ring cage-like chiral structure, without C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O or C
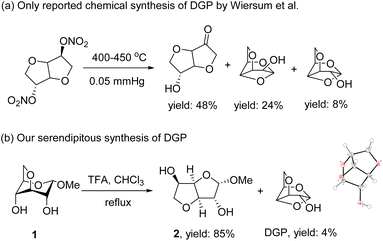
O or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C unsaturated bonds (Scheme 1).1 This unique polyether cage structure endows DGP with good water solubility and potential applications in pharmaceutical chemistry and food.2,3 DGP was first isolated and identified by Tischenko et al. in 1948 from the gasification products of wood.4,5 And it is one of important biomass pyrolysis anhydro sugar products, such as levoglucosan (LG), 1-hydroxy-3,6-dioxabicyclo[3.2.1]octan-2-one (LAC), levoglucosenone (LGO), and DGP, that derived from the cellulose and hemicellulose components.6,7 The selective production and application in organic synthesis of LG, LAC, and LGO has been widely reported.8–15 The selective production of DGP from cellulose fast pyrolysis has recently been achieved for the first time by Lu et al., achieving a 14% yield at 400 °C.3,16 However, this process remained at the laboratory research stage and has not yet been applied in industry. Additionally, the synthesis of DGP through chemical synthesis method was only discovered by Wiersum et al. during the study of the high-temperature rearrangement reaction of isosorbide dinitrate, giving a mixture containing DGP with a yield of approximately 8% (Scheme 1a).17 Therefore, the current DGP in market is still produced by collecting and separating cellulose pyrolysis gas, contributing to the high cost of DGP with limited market supply (cost 5 mg > $200, through a comprehensive search on SciFinder), and thus its application has been rarely reported.3 While the long-term goal is to achieve large-scale, cost-effective production of DGP through cellulose fast pyrolysis, developing effective conventional chemical synthesis methods suitable for laboratory-scale preparation is crucial for promoting its application in food, pharmaceuticals, and other fields. Previously, our group discovered an unintentional side production of DGP with a 4% yield during the investigation of the acid-catalytic rearrangement of methyl 3,6-anhydro-α-D-glucopyranoside 1 for the conversion to its furanose form 2 (Scheme 1b). The structure of DGP was unambiguously confirmed by X-ray crystallographic analysis (ESI, Fig. S1†). Additionally, we have preliminarily determined that DGP possessed a certain degree of sweet taste. Inspired by our previous research on the taste identification of anhydro sugar 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one (DDMP),18,19 this work aimed to further optimize reaction conditions, develop an efficient chemical synthesis method for DGP and investigate the sweetness performance of DGP for the first time.
C unsaturated bonds (Scheme 1).1 This unique polyether cage structure endows DGP with good water solubility and potential applications in pharmaceutical chemistry and food.2,3 DGP was first isolated and identified by Tischenko et al. in 1948 from the gasification products of wood.4,5 And it is one of important biomass pyrolysis anhydro sugar products, such as levoglucosan (LG), 1-hydroxy-3,6-dioxabicyclo[3.2.1]octan-2-one (LAC), levoglucosenone (LGO), and DGP, that derived from the cellulose and hemicellulose components.6,7 The selective production and application in organic synthesis of LG, LAC, and LGO has been widely reported.8–15 The selective production of DGP from cellulose fast pyrolysis has recently been achieved for the first time by Lu et al., achieving a 14% yield at 400 °C.3,16 However, this process remained at the laboratory research stage and has not yet been applied in industry. Additionally, the synthesis of DGP through chemical synthesis method was only discovered by Wiersum et al. during the study of the high-temperature rearrangement reaction of isosorbide dinitrate, giving a mixture containing DGP with a yield of approximately 8% (Scheme 1a).17 Therefore, the current DGP in market is still produced by collecting and separating cellulose pyrolysis gas, contributing to the high cost of DGP with limited market supply (cost 5 mg > $200, through a comprehensive search on SciFinder), and thus its application has been rarely reported.3 While the long-term goal is to achieve large-scale, cost-effective production of DGP through cellulose fast pyrolysis, developing effective conventional chemical synthesis methods suitable for laboratory-scale preparation is crucial for promoting its application in food, pharmaceuticals, and other fields. Previously, our group discovered an unintentional side production of DGP with a 4% yield during the investigation of the acid-catalytic rearrangement of methyl 3,6-anhydro-α-D-glucopyranoside 1 for the conversion to its furanose form 2 (Scheme 1b). The structure of DGP was unambiguously confirmed by X-ray crystallographic analysis (ESI, Fig. S1†). Additionally, we have preliminarily determined that DGP possessed a certain degree of sweet taste. Inspired by our previous research on the taste identification of anhydro sugar 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one (DDMP),18,19 this work aimed to further optimize reaction conditions, develop an efficient chemical synthesis method for DGP and investigate the sweetness performance of DGP for the first time.
2 Results and discussions
2.1 Synthesis of methyl 3,6-anhydro-α-D-glucopyranoside 1
Methyl 3,6-anhydro-α-D-glucopyranoside 1 was usually synthesized by intramolecular cyclization of methyl 6-O-tosyl-α-D-glucopyranoside 3 under strong basic conditions such as sodium hydroxide or sodium methoxide.20 Recently, Nitz et al. demonstrated a novel approach to construct various 3,6-anhydrohexosides with excellent yields by treating 6-O-tosyl-pyranosides with tetrabutylammonium fluoride (TBAF) (ESI, Scheme S1a†).21 However, the primary drawback lay in the requirement of 7.5 equivalents of TBAF. Excessive TBAF was neither environmentally friendly nor economically efficient, and presented substantial challenges in product purification, particularly in large-scale synthesis. Water washing resulted in substantial loss of certain water-soluble product 1 in presence of large amount of TBAF. Additionally, concentrating the solution and employing direct column chromatography necessitated the use of significant quantities of silica gel and eluent. When the amount of TBAF was reduced to 1.2 equivalents, there was a marked increase in reaction time and a noticeable decrease in yield (Table 1, entry 1). Considering the proposed reaction mechanism in the literature,21 we hypothesized that an excess of TBAF was unnecessary. This is based on the idea that fluoride ion could be released to promote the reaction with the addition of a small amount of weak base to neutralize the HF generated during the reaction (ESI, Scheme S1b†). Based on the above analysis, we optimized the intramolecular cyclization of 6-O-tosyl-glucopyranoside 3 promoted by a catalytic amount of TBAF, providing a more efficient synthetic approach for 3,6-anhydro glucopyranoside 1.| Entry | Conditions | Time | 1 yieldb |
|---|---|---|---|
| a All reactions were performed on 0.2 mmol scale and using anhydrous TBAF unless otherwise stated.b Isolated yield.c Using TBAF·3H2O.d Yield < 5%. | |||
| 1 | TBAF (1.2 eq.), THF/DMF, 70 °C | 64 h | 62% |
| 2 | TBAF (0.1 eq.), Na2CO3(1.2 eq.), THF, reflux | 16 h | 86%/c85% |
| 3 | TBAF (0.1 eq.), K2CO3 (1.2 eq.), THF, reflux | 12 h | 83% |
| 4 | TBAF (0.1 eq.), NaHCO3 (1.2 eq.), THF, reflux | 30 h | 75% |
| 5 | TBAF (0.1 eq.), DIPEA (1.2 eq.), THF, reflux | 48 h | traced |
| 6 | TBAF (0.1 eq.), pyridine (1.2 eq.), THF, reflux | 48 h | traced |
| 7 | TBAF (0.1 eq.), Na2CO3 (1.2 eq.), DCE, reflux | 40 h | 63% |
| 8 | TBAF (0.1 eq.), Na2CO3 (1.2 eq.), toluene, reflux | 40 h | 50% |
| 9 | TBAF (0.1 eq.), Na2CO3 (1.2 eq.), MeCN, reflux | 16 h | 79% |
| 10 | Na2CO3 (1.2 equiv.), THF, reflux | 48 h | traced |
| 11 | TBAB (0.1 eq.), Na2CO3(1.2 eq.), THF, reflux | 48 h | traced |
| 12 | NaF (0.1 eq.), Na2CO3(1.2 eq.), THF, reflux | 48 h | traced |
6-O-Tosyl-glucopyranoside 3 was synthesized following methods described in the literature.21 Subsequently, the reaction conditions for the preparation of 1 were investigated in detail (Table 1). It was discovered that using 0.1 equivalents of TBAF in conjunction with bases like NaHCO3, Na2CO3, or K2CO3, efficiently afforded the target product in high yields (entries 2–4). Conversely, the use of organic bases such as DIPEA and pyridine resulted in negligible reaction progress (entries 5–6). Considering both reaction time and yield, Na2CO3 emerged as the most effective co-catalyst. Further investigation into the impact of the reaction solvent revealed THF as the optimal choice (entries 7–9). Additional experiments demonstrated that omitting TBAF or replacement by TBAB halted the reaction (entries 10–11), underscoring the crucial role of fluoride ion. TBAF exhibited superior catalytic activity in comparison of NaF (entries 12). The equivalent catalytic efficiency of the more economical TBAF.3H2O was also confirmed (entry 2c). In summary, the optimal reaction conditions were identified as using TBAF.3H2O (0.1 eq.), Na2CO3 (1.2 eq.), with reflux in THF under nitrogen atmosphere. The high tolerance for scale-up experiment was also further verified with starting material 4 of 50 grams, giving 27.2 grams 3,6-anhydro glucopyranoside 1 with similar yield, thus supporting subsequent research (Scheme 2).
2.2 Synthesis of DGP
Possible reaction mechanisms for the conversion of 1 to DGP and 2 were proposed before optimizing the conditions for DGP generation (Fig. 1). Under acid catalysis, the bridged structure of 1 underwent ring-opening rearrangement to form the less strained fused-ring structure of furanoside 2, which has been reported in previous literature.22 On the other hand, the acetal moiety in 1 eliminated a molecule of methanol catalyzed by acid to form a glycosyloxonium ion B and C, which was then attacked by the 4-position hydroxyl to form a new acetal structure, leading to the formation of DGP.23,24 Thus, the formation of DGP occurred via an acid-catalytic intramolecular transacetalization originating from 1.Based on the aforementioned mechanistic analysis, the reaction conditions were further optimized to improve the selectivity of the DGP formation pathway and the results were summarized in Table 2. A series of Brønsted and Lewis acid catalysts in different solvents was investigated, while the yields were generally low disappointingly (entries 1–17). It was found that only product 2 was generated at room temperature, while the formation of DGP required higher temperatures and highly polar solvents. On the other hand, according to the transacetalization of 2-formylpyrrole acetals under strongly basic conditions in the literature,25 NaH was also explored as a potential catalyst. However, this investigation revealed no formation of the desired products DGP and 2 (entry 18).
| Entry | Conditions | Time | Yieldb: DGP/2 |
|---|---|---|---|
| a Reaction conditions: 3 (0.2 mmol), acid and solvent (2 mL) was stirred under argon atmosphere, and monitored by TLC until all 1 was consumed.b Isolated yield. | |||
| 1 | TFA (0.3 eq.), CHCl3, reflux | 1 h | 4%/85% |
| 2 | TFA (0.3 eq.), CHCl3, 25 °C | 48 h | 0/87% |
| 3 | TFA (0.3 eq.), MeCN, relux | 1 h | 10%/71% |
| 4 | TFA (0.3 eq.), DCE, relux | 1 h | Trace/67% |
| 5 | p-TsOH (0.3 eq.), MeCN, relux | 1 h | 9%/47% |
| 6 | p-TsOH (0.3 eq.), toluene, relux | 1 h | 5%/36% |
| 7 | p-TsOH (0.3 eq.), DCE, relux | 1 h | Trace/65% |
| 8 | p-TsOH (0.3 eq.), THF, relux | 1 h | Trace/44% |
| 9 | AcOH (1.0 eq.), CHCl3, reflux | 6 h | 0/Trace |
| 10 | HCOOH (0.3 eq.), CHCl3, reflux | 3 h | 0/55% |
| 11 | FeCl3 (0.3 eq.), MeCN, reflux | 8 h | 12%/49% |
| 12 | FeCl3 (0.5 eq.), MeCN, reflux | 8 h | 9%/52% |
| 13 | FeCl3 (0.1 eq.), MeCN, reflux | 16 h | 4%/66% |
| 14 | FeCl3 (0.3 eq.), DCE, reflux | 8 h | 0/60% |
| 15 | FeCl3 (0.3 eq.), THF, reflux | 8 h | 0/42% |
| 16 | BF3·Et2O (0.3 eq.), THF, 0 °C-reflux | 2 h | 0/21% |
| 17 | BF3·Et2O (0.3 eq.), MeCN, 0 °C-reflux | 2 h | 0/Trace |
| 18 | NaH (1.2 eq.), THF, reflux | 24 h | 0/0 |
| 19 | p-TsOH (0.3 eq.), TFE, reflux | 1 h | 9%/38% |
| 20 | p-TsOH (0.3 eq.), HFIP, reflux | 1 h | 33%/35% |
| 21 | TFA (0.3 eq.), HFIP, reflux | 1 h | 19%/40% |
| 22 | FeCl3 (0.3 eq.), HFIP, reflux | 1 h | Trace/trace |
| 23 | H2SO4 (0.3 eq.), HFIP, reflux | 1 h | 10%/25% |
| 24 | CF3SO3H (0.3 eq.), HFIP, reflux | 1 h | Trace/22% |
| 25 | p-TsOH (0.1 eq.), HFIP, reflux | 1 h | 12%/44% |
| 26 | p-TsOH (0.5 eq.), HFIP, reflux | 1 h | 17%/32% |
By analyzing the competition of the formation of 2 and DGP, we hypothesized that the stability of the carbocation intermediate B and C may be the key factor in enhancing the DGP formation pathway. In addition, strongly hydrogen bond-donating, polar solvents trifluoroethanol (TFE) and hexafluoroisopropanol (HFIP) have found numerous uses in organic synthesis due to its ability to stabilize ionic species, transfer protons, and engage in a range of other intermolecular interactions.26,27 For example, in the metal-free Markovnikov-type alkyne hydration reported by Li et al., the carbocation was strongly stabilized in TFE, which facilitated the progression of the reaction.26 Therefore, we investigated the reaction conditions using TFE and HFIP as solvent to stabilize carbocation intermediate B and C, promoted by different acid catalysts (entries 19–26). Pleasingly, when HFIP was used as the solvent and p-TsOH (0.3 eq.) as the acid catalyst, the reaction produced DGP with a 33% yield (entry 20). Although the yield was not high, it sufficed for the rapid preparation of DGP and the study of its taste identification.
To enhance the availability of high-purity materials for the taste evaluation, we have innovatively developed a vacuum sublimation purification technique for DGP, drawing upon previously reported thermogravimetric analysis.5 A sample of DGP, isolated through column separation, was subjected to sublimation using a glass sublimation apparatus under a controlled pressure of 0.15 mm Hg at 120 °C, yielding purified DGP.
2.3 Taste identification
A taste test was carried out on the DGP and 3,6-anhydrohexoside 1 and 2 (Table 3). The results showed that the DGP exhibited a sweet taste at a threshold of 14 mmol L−1 (0.2%) in water, while 1 and 2 were tasteless even at 57 mmol L−1 (1%). The sweetness of DGP relative to sucrose was further determined, and it was found to have a sweetness comparable to that of sucrose. Although DGP did not have exceptionally high sweetness, its moderate thermal volatility may provide it with potential application value in certain specific areas of sweetness adjustment.| DGP | 1 | 2 | |
|---|---|---|---|
| a Taste quality and detection thresholds of taste compounds were determined in a triangle test.b Compare DGP aqueous solutions to a 5% sucrose aqueous solution to determine the concentration at which the sample's sweetness intensity matches that of the sucrose solution and divide this concentration by the sucrose solution concentration (5%). | |||
| Taste qualitya | Sweet taste | Tasteless | Tasteless |
| Relative sweetnessb (relative to sucrose/times) | 1.0 | — | — |
| Sweet taste thresholda (% or mmol L−1 in water) | 0.2% or 14 mmol L−1 | — | — |
3 Experimental section
3.1 General considerations
Acetonitrile (CH3CN) and Dichloroethane (DCE) was dried with CaH2 and distilled. Toluene and tetrahydrofuran (THF) were dried with Na and distilled. All other commercial reagents were used as received without additional purification. 1H NMR and 13C NMR spectra were recorded on 400 MHz and 100 MHz spectrometers respectively with TMS as the internal standard, using CDCl3 or CD3OD as solvent. Data for 1H NMR spectra are reported as follows: chemical shift δ (ppm), referenced to TMS; Multiplicities are indicated as the following: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; dd, doublet of doublets; coupling constants (Hz) and integration. HRMS was recorded on an LC TOF (ES).3.2 Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 50
MeOH = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 20
1 to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) afforded the product 3. (65.5 g, 73%). 1H NMR (400 MHz, CD3OD) δ 7.77 (d, J = 8.0 Hz, 2H), 7.42 (d, J = 8.0 Hz, 2H), 4.53 (d, J = 8.0 Hz, 1H), 4.28 (dd, J = 12.0, 4.0 Hz, 1H), 4.13 (dd, J = 12.0, 8.0 Hz, 1H), 3.79–3.66 (m, 1H), 3.66–3.58 (m, 1H), 3.35–3.21 (m, 3H), 3.13–3.18 (m, 1H), 2.43 (s, 3H). 13C NMR (100 MHz, CD3OD) δ 146.5, 134.5, 131.0, 129.1, 101.2, 74.9, 73.2, 71.3, 71.1, 71.0, 55.7, 21.6.
1, v/v) afforded the product 3. (65.5 g, 73%). 1H NMR (400 MHz, CD3OD) δ 7.77 (d, J = 8.0 Hz, 2H), 7.42 (d, J = 8.0 Hz, 2H), 4.53 (d, J = 8.0 Hz, 1H), 4.28 (dd, J = 12.0, 4.0 Hz, 1H), 4.13 (dd, J = 12.0, 8.0 Hz, 1H), 3.79–3.66 (m, 1H), 3.66–3.58 (m, 1H), 3.35–3.21 (m, 3H), 3.13–3.18 (m, 1H), 2.43 (s, 3H). 13C NMR (100 MHz, CD3OD) δ 146.5, 134.5, 131.0, 129.1, 101.2, 74.9, 73.2, 71.3, 71.1, 71.0, 55.7, 21.6.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH = 50
MeOH = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 20
1 to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) afforded the product 1. (27.2 g, 82%). 1H NMR (400 MHz, CD3OD) δ 4.93 (d, J = 3.0 Hz, 1H), 4.23 (t, J = 2.4 Hz, 1H), 4.17–4.12 (m, 2H), 4.12–4.08 (m, 1H), 3.93 (dd, J = 12.0, 3.2 Hz, 1H), 3.78 (s, 1H), 3.56 (s, 3H). 13C NMR (100 MHz, CD3OD) δ 99.78, 76.9, 73.3, 72.7, 71.7, 69.9, 57.5. HRMS (ESI-TOF): calcd. for C7H12NaO5 [M + Na]+ 199.0582; found 199.0580.
1, v/v) afforded the product 1. (27.2 g, 82%). 1H NMR (400 MHz, CD3OD) δ 4.93 (d, J = 3.0 Hz, 1H), 4.23 (t, J = 2.4 Hz, 1H), 4.17–4.12 (m, 2H), 4.12–4.08 (m, 1H), 3.93 (dd, J = 12.0, 3.2 Hz, 1H), 3.78 (s, 1H), 3.56 (s, 3H). 13C NMR (100 MHz, CD3OD) δ 99.78, 76.9, 73.3, 72.7, 71.7, 69.9, 57.5. HRMS (ESI-TOF): calcd. for C7H12NaO5 [M + Na]+ 199.0582; found 199.0580.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of petroleum ether and ethyl acetate (20.0 mL) to slurry wash, then filter to obtain the product 2 (850 mg, 85%). 1H NMR (400 MHz, CDCl3) δ 5.08 (d, J = 4.4 Hz, 1H), 4.63 (t, J = 5.4 Hz, 1H), 4.45 (dd, J = 5.3, 2.8 Hz, 1H), 4.22 (dt, J = 6.9, 5.7 Hz, 1H), 4.17 (dd, J = 4.4, 2.8 Hz, 1H), 3.94 (dd, J = 9.3, 5.9 Hz, 1H), 3.61–3.54 (m, 1H), 3.52 (d, J = 3.5 Hz, 3H), 2.69–2.39 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 104.9, 87.8, 79.4, 77.9, 72.0, 71.3, 56.0. HRMS (ESI-TOF): calcd. for C7H12NaO5 [M + Na]+ 199.0582; found 199.0584.
1 mixture of petroleum ether and ethyl acetate (20.0 mL) to slurry wash, then filter to obtain the product 2 (850 mg, 85%). 1H NMR (400 MHz, CDCl3) δ 5.08 (d, J = 4.4 Hz, 1H), 4.63 (t, J = 5.4 Hz, 1H), 4.45 (dd, J = 5.3, 2.8 Hz, 1H), 4.22 (dt, J = 6.9, 5.7 Hz, 1H), 4.17 (dd, J = 4.4, 2.8 Hz, 1H), 3.94 (dd, J = 9.3, 5.9 Hz, 1H), 3.61–3.54 (m, 1H), 3.52 (d, J = 3.5 Hz, 3H), 2.69–2.39 (m, 2H). 13C NMR (100 MHz, CDCl3) δ 104.9, 87.8, 79.4, 77.9, 72.0, 71.3, 56.0. HRMS (ESI-TOF): calcd. for C7H12NaO5 [M + Na]+ 199.0582; found 199.0584.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EA = 5
EA = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2
1 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) afforded the product DGP. (279 mg, 33%). 1H NMR (400 MHz, CDCl3) δ 5.43 (s, 1H), 5.17 (t, J = 4.0 Hz, 1H), 4.22 (s, 1H), 4.12 (d, J = 4.0 Hz, 1H), 4.03 (d, J = 12.0 Hz, 1H), 3.92 (d, J = 12.0 Hz, 1H), 3.76 (s, 1H), 1.86 (s, 1H). 13C NMR (100 MHz, CDCl3) δ 101.2, 82.9, 79.5, 79.1, 75.9, 71.8. HRMS (ESI-TOF): calcd. for C6H8NaO4 [M + Na]+ 167.0320; found 167.0321. A 1.0 g sample of DGP, isolated through column separation, was subjected to sublimation using a glass sublimation apparatus under a controlled pressure of 0.15 mm Hg and a temperature of 120 °C, yielding 850 mg purified DGP.
1, v/v) afforded the product DGP. (279 mg, 33%). 1H NMR (400 MHz, CDCl3) δ 5.43 (s, 1H), 5.17 (t, J = 4.0 Hz, 1H), 4.22 (s, 1H), 4.12 (d, J = 4.0 Hz, 1H), 4.03 (d, J = 12.0 Hz, 1H), 3.92 (d, J = 12.0 Hz, 1H), 3.76 (s, 1H), 1.86 (s, 1H). 13C NMR (100 MHz, CDCl3) δ 101.2, 82.9, 79.5, 79.1, 75.9, 71.8. HRMS (ESI-TOF): calcd. for C6H8NaO4 [M + Na]+ 167.0320; found 167.0321. A 1.0 g sample of DGP, isolated through column separation, was subjected to sublimation using a glass sublimation apparatus under a controlled pressure of 0.15 mm Hg and a temperature of 120 °C, yielding 850 mg purified DGP.3.3 Sensory analyses
Training of the sensory panel and determination of taste followed the methods previously described.18,28,29 Assessors were trained to evaluate sweet taste intensity using sucrose (0.5%, 2.5%, 5% in water). Sensory analyses were performed using tap water (pH 6.5) as the solvent in a panel room at 22–25 °C in three different sessions by six panelists (3 women, 3 men) whose data were averaged. The detection thresholds of taste compounds were determined in a triangle test and the samples were presented in order of increasing concentrations (serial 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dilutions). Relative sweetness of taste compounds was determined as follows. Prepare a series of aqueous solutions with varying concentrations of the sample. Compare these to a 5% sucrose aqueous solution to determine the concentration at which the sample's sweetness intensity matches that of the sucrose solution. Then, divide this concentration by the sucrose solution concentration (5%) to obtain the relative sweetness of the sample.
1 dilutions). Relative sweetness of taste compounds was determined as follows. Prepare a series of aqueous solutions with varying concentrations of the sample. Compare these to a 5% sucrose aqueous solution to determine the concentration at which the sample's sweetness intensity matches that of the sucrose solution. Then, divide this concentration by the sucrose solution concentration (5%) to obtain the relative sweetness of the sample.
4 Conclusions
In conclusion, a reliable method for the preparation of DGP from methyl 3,6-anhydro-α-D-glucopyranoside 1 has been developed for the first time. The 3,6-anhydro glucopyranoside 1 was synthesized via an intramolecular cyclization reaction of 6-O-tosyl glucopyranoside promoted by a catalytic amount of TBAF. This new approach for the preparation of 3,6-anhydro glucopyranoside 1 proceeded under mild and environmentally friendly conditions, avoiding the use of strong bases and large excesses of TBAF. And we found that the stabilizing effect of HFIP on carbocation intermediates significantly improved the yield of the intramolecular cyclization of 3,6-anhydro glucopyranoside in the construction of DGP by systematic optimization. Further sensory evaluation studies revealed that DGP had a sweetness similar to that of sucrose. Overall, this work provided an efficient chemical synthesis method for DGP and investigated the sweet performance of DGP for the first time, providing the potential to propel research on the applications of DGP in the fields of pharmaceutical chemistry and the food sector.Data availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request. All data supporting the findings of this study are included in the article and its ESI files.† Any additional data related to this paper may be requested from the authors.Author contributions
Zhifei Chen, Qingfu Wang and Changtong Lu proposed and designed the experiment; Qingfu Wang and Kai Yang wrote the paper; Yibo Ning, Lei Li, Xueying Cao and Gaolei Xi processed the data; Kai Yang and Changtong Lu corrected the language of the paper. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The experimental work was supported by a grant from China National Tobacco Corporation (110202202005) and a grant from China Tobacco Henan Industrial Co., Ltd. (AW2022008).Notes and references
- A. Ohnishi, K. Kato, T. Hori and M. Nakayama, Carbohydr. Res., 1981, 96, 161–166 CrossRef CAS.
- M. Cerný, Chemistry of anhydro sugars, Adv. Carbohydr. Chem. Biochem., 2003, 58, 121–198 CrossRef PubMed.
- B. Hu, A. S. Cheng, Y. Li, Y. B. Huang, J. Liu, B. Zhang, K. Li, L. Zhao, T. P. Wang and Q. Lu, Chem. Eng. J., 2022, 436, 135200 CrossRef CAS.
- D. Tischenko and N. Nosova, Zh. Obshch. Khim., 1948, 18, 1193–1197 Search PubMed.
- F. Shafizadeh, R. H. Furneaux, T. T. Sievenson and T. G. Cochran, Carbohydr. Res., 1978, 61, 519–528 CrossRef CAS.
- K. Guo, Q. Cheng, J. Jiang, J. Xu and W. Tan, ACS Sustainable Chem. Eng., 2020, 8, 11099–11113 CrossRef CAS.
- J. Ethiraj, D. Wagh and H. Manyar, Energy Fuels, 2022, 36, 1189–1204 CrossRef CAS.
- L. Chen, W. C. Elias, Y. B. Yin, Z. C. Zhang and M. S. Wong, Green Chem., 2020, 22, 1968–1977 RSC.
- L. A, H. Radhakrishnan, H. Hu, H. Hu and X. Bai, Green Chem., 2020, 22, 7871–7883 RSC.
- X. Huang, S. Kudo, S. Asano and J. I. Hayashi, Fuel Process. Technol., 2021, 212, 106625 CrossRef CAS.
- Z. X. Zhang, B. Hu, Y. Li, K. Li and Q. Lu, Bioresour. Technol., 2020, 309, 123370 CrossRef CAS PubMed.
- F. Xu, J. Luo, L. Jiang and Z. Zhao, Cellulose, 2022, 29, 1463–1472 CrossRef CAS.
- X. Huang, S. Kudo, J. Sperry and J. I. Hayashi, ACS Sustainable Chem. Eng., 2019, 7, 5892–5899 CrossRef CAS.
- O. Oyola-Rivera, J. He, G. W. Huber, J. A. Dumesic and N. Cardona-Martínez, Green Chem., 2019, 21, 4988–4999 RSC.
- K. Tomishige, M. Yabusshita, J. Cao and Y. Nakagawa, Green Chem., 2022, 24, 5652–5690 RSC.
- L. Zhao, H. Fu, Y. Xia, J. Liu, B. Hu, Z. Cheng, X. Luo, D. Yan, J. Li and Q. Lu, Energy Fuels, 2024, 38, 4302–4311 CrossRef CAS.
- J. G. Batelaan, A. J. M. Weber and U. E. Wiersum, J. Chem. Soc. Chem. Commun., 1987, 18, 1397–1399 RSC.
- Z. F. Chen, G. L. Xi, Y. F. Fu, Q. F. Wang, L. L. Cai, Z. W. Zhao, Q. Liu, B. Bai and Y. P. Ma, Food Chem., 2021, 361, 130052 CrossRef CAS PubMed.
- Z. Chen, Q. Liu, Z. Zhao, B. Bai, Zh. Sun, L. Cai, Y. Fu, Y. Ma, Qi. Wang and G. Xi, RSC Adv., 2021, 11, 34456–34461 RSC.
- W. Xie, A. Lin, J. Xu and X. Wu, Asian J. Org. Chem., 2017, 6, 6–26 CrossRef CAS.
- Z. A. Morrison and M. Nitz, Org. Lett., 2020, 22, 1453–1457 CrossRef CAS PubMed.
- C. McDonnell, O. López, P. Murphy, J. G. Fernández Bolaños, R. Hazell and M. Bols, J. Am. Chem. Soc., 2004, 126, 12374–12385 CrossRef CAS PubMed.
- P. M. Aberg and B. Ernst, Acta Chem. Scand., 1994, 48, 228–233 CrossRef CAS PubMed.
- A. Sadeghi-Khomami, T. J. Forcada, C. Wilson, D. A. Sanders and N. R. Thomas, Org. Biomol. Chem., 2010, 8, 1596–1602 RSC.
- Z. Yan, R. Kang, Y. Liu and S. Lin, Chin. Chem. Lett., 2012, 23, 33–36 CrossRef CAS.
- W. Liu, H. Wang and C. J. Li, Org. Lett., 2016, 18, 2184–2187 CrossRef CAS PubMed.
- H. F. Motiwala, A. M. Armaly, J. G. Cacioppo, T. C. Coombs, K. R. K. Koehn, V. M. Norwood IV and J. Aubé, Chem. Rev., 2022, 122, 12544–12747 CrossRef CAS PubMed.
- M. J. Gwak, S. J. Chung, Y. J. Kim and C. S. Lim, Food Sci. Biotechnol., 2012, 21, 889–894 CrossRef CAS.
- M. Medel-Marabolí, F. González-Castillo, A. Bunger and C. Sáenz, J. Food Meas., 2024, 18, 2196–2204 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2416300. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ra00266d |
| This journal is © The Royal Society of Chemistry 2025 |