Open Access Article
Open Access ArticlePreparation and synergistic effect of aluminum hydroxide nanoplates on the fire resistance and thermal stability of the intumescent flame retardant epoxy composite
Truong Cong Doanha,
Nhung Hac Thibc,
Hong Tham Nguyenb,
Ho Thi Oanhb,
Tien Dat Doanbc,
Nguyen Duc Tuyenb,
Minh-Tan Vua and
Mai Ha Hoang *bc
*bc
aHanoi University of Industry, 298 Cau Dien, Bac Tu Liem, Hanoi, 12000, Vietnam
bInstitute of Chemistry, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Cau Giay, Hanoi, 10072, Vietnam. E-mail: hoangmaiha@ich.vast.vn
cGraduate University of Science and Technology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Cau Giay, Hanoi, 10072, Vietnam
First published on 20th May 2025
Abstract
Aluminum hydroxide nanoplates (nATH), with an average particle size of about 350–450 nm and a thickness of 30 nm, were successfully synthesized through a hydrothermal process using an Al(OH)3 gel precursor. The ATH nanoplates were then surface-treated with organic compounds and incorporated into an intumescent flame-retardant epoxy system containing polyethyleneimine-modified ammonium polyphosphate (APP@PEI). Among them, the combination of APP@PEI and PEI-treated nATH (nATHPEI) exhibited the highest synergistic effect on the fire resistance and thermal stability of epoxy resin due to the superior dispersion of the nanoplates. Additionally, the optimal mass ratio of two flame retardant additives was examined. As a result, a nanocomposite containing 3 wt% nATHPEI and 7 wt% APP@PEI exhibited the best flame resistance and thermal-oxidative stability. This nanocomposite reached a V-0 rating in the UL-94 vertical burning test with a high limiting oxygen index value of 31.1%, and a substantial char yield of 17.98% at 900 °C. Char residues of samples were analyzed by Fourier transform infrared spectroscopy, X-ray diffraction, and scanning electron microscopy coupled with energy dispersive X-ray analysis to investigate the reasonable flame retardant mechanism. The results demonstrated that the formation of highly thermally stable aluminum phosphates played an important role in the augmentation of the flame retardancy in the condensed phase. Furthermore, tension and Izod impact tests indicated that the presence of nATHPEI notably increased the mechanical properties of composite loading APP@PEI. This combination provides a promising approach for intumescent flame retardant applications in epoxy resin.
1 Introduction
Epoxy resin (EP), a type of thermosetting resin, has attracted conspicuous attention for the last decades because of its great solvent resistance, high mechanical strength, superior adhesion, excellent electrical insulation, low shrinkage, easy processing, and low cost. Hence, EP has been widely applied in several fields, including electronic and electrical materials, binders, coatings, and fiber-reinforced composite materials.1–3 However, its flammability remains a major disadvantage, severely restricting its potential for further application. Thus, it is essential to improve the flame retardancy of EP resin. In recent years, researchers have developed various solutions to address this issue. Among them, the introduction of flame retardant additives is one of the most effective approaches to enhance the fire resistance of EP resin.4–8Intumescent flame retardants (IFRs), an important class of halogen-free flame retardant materials, offer several significant benefits, such as non-toxicity, low smoke emission, and resistance to dripping.9 Typically, IFRs include three main components: acid, carbon, and gas sources.10 IFRs form a voluminous intumescent char layer that insulates heat and oxygen, effectively protecting underlying polymer matrices from flames. Ammonium polyphosphate (APP), a crucial phosphorus-based flame retardant, serves as an acid source in IFR formulations. This flame retardant often has excellent char-forming effects when combined with other components.11–14 However, APP often has poor dispersibility and compatibility with polymer matrices, which can negatively impact mechanical properties of polymers, thereby hindering its performance in engineering applications. In recent years, many efforts have been focused on investigating APP-based IFR systems.8,15–18 Meanwhile, APP modified with hyperbranched polyethyleneimine (APP@PEI) which acts as a hardener for thermosets,19,20 is considered an effective IFR system for EP resins.
Aluminum hydroxide (ATH) is the most extensively used eco-friendly inorganic filler, offering both flame retardant and smoke inhibitory effects for a variety of polymers, including polyethylene, ethylene–propylene–diene monomer rubber (EPDM), ethylene vinyl acetate, epoxy, and polyvinyl chloride, etc.21–25 Research on the use of nanoparticles as flame retardant additives has made significant progress in addressing the challenges of high inorganic additive loading and poor dispersion. Notably, studies on using ATH nanoparticles as flame retardants have yielded promising results. In comparison to commercial ATH, ATH nanoparticles exhibit better dispersion and interaction with polymers, which improves the mechanical properties and fire resistance of nanocomposites.26–30 However, even in nanoparticle form, high loading of ATH is still necessary to achieve the desired flame retardant efficiency. Therefore, a combination of ATH with other flame retardants is crucial to create synergistic effects, reducing the overall amount of additives required.
Recently, some studies on the synergistic flame-retardant effect between PEI modified APP and other fillers have been reported. For instance, Liao et al.31 grafted APP with tannic acid (TA) using PEI as a bridging agent (TPAPP). When 10% TPAPP was added to polylactic acid (PLA), the limiting oxygen index (LOI) value of the composite increased from 20% (pure PLA) to 27.8%. APP@PEI was also combined with novel DOPO derivatives (DOPO – 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide) in an EP matrix.32 The report indicated that some derivatives exhibited the good synergistic flame-retardant effect with APP@PEI. Wan et al.33 prepared a new type of multi-layered nanomaterial coating APP, APP–PEI@MXene@ZIF-67. Subsequently, this material was introduced into thermoplastic polyurethane as a flame retardant filler. Compared with the pure polyurethane, the heat and smoke of the composite containing 20 wt% of this core–shell flame retardant released during combustion significantly reduced. Kang et al.34 deposited APP and PEI into sisal fiber lumens by combining the water/nutrient transport mechanism of plant fiber with layer-by-layer self-assembly technology. When blending this modified fiber (30 wt%) with polypropylene, LOI value of the composite increased from 19.4% (unmodified) to 23.2%.
In this paper, aluminum hydroxide hexagonal nanoplates were synthesized using the hydrothermal method. Additionally, the surface of these nanoplates was treated with organic compounds, including oleic acid, Tween 80, and polyethyleneimine to improve their dispersion in an epoxy matrix. Next, EP nanocomposites were prepared by the thermal curing process, using ATH nanoplates and APP@PEI as flame retardant fillers. The flame retardancy, thermo-oxidative stability, and mechanical properties of these nanocomposites were investigated by the UL-94 vertical burning, limiting oxygen index test, thermal gravimetric analysis, tensile, and unnotched Izod impact measurements.
2 Experimental
2.1 Materials
Epoxy resin (LR 385, density: 1.16–1.20 g cm−3, viscosity: 700–1050 mPa s, epoxy equivalent: 160–170 g per equivalent, epoxy value: 0.58–0.64 equivalent/100 g) and hardener LH-386 (density: 0.93–0.97 g cm−3, viscosity: 40–90 mPa s, amine value: 480–550 mg KOH per g) were bought from Hexion Specialty Chemicals, Germany. Commercial APP (form II) was supplied by China. Aluminum nitrate nonahydrate (Al(NO3)3·9H2O, ≥98%), branched polyethyleneimine (PEI, 99%, MW 300), polysorbate 80 (Tween 80, micellar avg. mol. wt 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, average mol. wt 1310), and oleic acid (90%) were provided by Sigma-Aldrich. High-purity NaOH and absolute ethanol were used directly without additional purification.
000, average mol. wt 1310), and oleic acid (90%) were provided by Sigma-Aldrich. High-purity NaOH and absolute ethanol were used directly without additional purification.
2.2 Synthesis of aluminum hydroxide nanoplates
Aluminum hydroxide nanoplates (nATH) were synthesized using a hydrothermal method. The procedure was as follows: 60 g Al(NO3)3·9H2O was dissolved in 640 mL distilled water to obtain a homogeneous solution. Next, 500 mL of 1 M NaOH was slowly dropped to this aluminum salt solution, and pH of the reaction mixture was maintained at 5. After stirring continuously for 1 h, the gel-like precipitates were collected by centrifugation and washed many times with water to remove the excess precursors. The resulting gel was then dispersed in 320 mL of 1 M NaOH solution to adjust the pH to 9. The mixture was transferred to a 500 mL Teflon vessel, which was sealed in a steel bomb reactor. This reactor was placed in a UM 400 Memmert electric oven (Germany) and heated to 80, 90, and 100 °C with a heating rate of 2 °C min−1. The reaction systems were then maintained at the temperatures for 24–72 h. Finally, the white solid was obtained by centrifuging, washing several times with distilled water, and drying at 80 °C for 12 h.2.3 Surface modification of aluminum hydroxide nanoplates
The surface of nATH was modified using oleic acid (OA), Tween 80 (Tw80), and PEI. The modifying agents (3% of the nATH mass) were dissolved in a certain amount of ethanol. Next, nATH powder was added to the solution and the mixture was continuously stirred at 60 °C for 2 h. The ethanol was then removed by rotary evaporation and the final products was labeled nATHOA, nATHTw80, and nATHPEI.2.4 Preparation of APP@PEI
Ammonium polyphosphate was modified with polyethyleneimine (APP@PEI) based on previous literature.17 The preparation process of APP@PEI was carried out as follows: first, a solvent mixture was prepared by combining 210 mL ethanol and 10 mL distilled water. Then, 3.5 g PEI was completely dissolved in 20 mL of this solvent mixture under N2 atmosphere. Meanwhile, 10 g APP and 200 mL the solvent mixture were added to a three-neck flask, stirred, and heated to 90 °C under N2 atmosphere. Afterwards, the PEI solution was introduced into the flask, and the reaction system was continuously stirred at 90 °C for 2 h under N2 atmosphere. The resulting white solid was then filtered and washed several times with ethanol to eliminate excess PEI. Finally, the sample was dried overnight in a vacuum oven at 80 °C to obtain APP@PEI powder.2.5 Preparation of flame retardant epoxy nanocomposites
The EP composites were prepared through a traditional curing process, with a hardener to epoxy resin mass ratio of 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. The preparation involved the following steps: a certain amount of the flame retardant additives was dispersed in the hardener through stirring and ultrasonication. Afterwards, EP resin was added to the mixture with gentle stirring. When the mixture became homogeneous, it was poured into a silicone mold prepared according to standard dimensions of measurements. After curing at room temperature for 24 h and at 60 °C for 12 h, the EP composites were evaluated properties. The composite formulations are provided in Table 1.
100. The preparation involved the following steps: a certain amount of the flame retardant additives was dispersed in the hardener through stirring and ultrasonication. Afterwards, EP resin was added to the mixture with gentle stirring. When the mixture became homogeneous, it was poured into a silicone mold prepared according to standard dimensions of measurements. After curing at room temperature for 24 h and at 60 °C for 12 h, the EP composites were evaluated properties. The composite formulations are provided in Table 1.
| Samples | Compositions (wt%) | |||||
|---|---|---|---|---|---|---|
| EP | APP@PEI | nATH | nATHOA | nATHTw80 | nATHPEI | |
| a IFR: APP@PEI. | ||||||
| EP | 100 | — | — | — | — | — |
| 10APP@PEI/EP | 90 | 10 | — | — | — | — |
| 3nATH/7IFRa/EP | 90 | 7 | 3 | — | — | — |
| 3nATHOA/7IFR/EP | 90 | 7 | — | 3 | — | — |
| 3nATHTw80/7IFR/EP | 90 | 7 | — | — | 3 | — |
| 3nATHPEI/7IFR/EP | 90 | 7 | — | — | — | 3 |
| 1nATHPEI/9IFR/EP | 90 | 9 | — | — | — | 1 |
| 5nATHPEI/5IFR/EP | 90 | 5 | — | — | — | 5 |
| 10nATHPEI/EP | 90 | — | — | — | — | 10 |
2.6 Characterizations
UL-94 vertical combustion test (UL94-V) was carried on a GT-MC35F-2 horizontal and vertical flame chamber (Gester, China) based on ASTM D3801. Limiting oxygen index values were determined by using a Yasuda 214 oxygen index flammability tester (Japan) following ASTM D2863-13. The sizes of samples for both measurements were 125 mm × 13 mm × 3.2 mm.The dispersion of nATH before and after the surface organization were observed using an Eclipse Ts2 Inverted Routine Microscope (Nikon, Japan). 3 wt% of nATH, nATHOA, nATHTw80, or nATHPEI were dispersed in the hardener by stirring and ultrasonication. Next, epoxy resin was added to the mixture and stirred until homogeneous. The mixture was then spread into a very thin layer on glass slides. They were cured at room temperature for 24 h and at 60 °C for 12 h before observation.
Fourier transform infrared (FT-IR) spectra were obtained on a L1600400 Spectrum two DTGS spectrometer (PerkinElmer, USA) by the KBr pellet method. The measurements were performed in a wavenumber range of 450–4000 cm−1 with a resolution of 1 cm−1. X-ray diffraction (XRD) data were collected on a D8 Advance diffractometer (Bruker, Germany) using Cu Kα radiation (λ = 1.5406 Å) source in a 2θ range from 10° to 70° with a step size of 0.02°.
Scanning electron microscopy (FE-SEM) and energy dispersive X-ray spectroscopy (EDX) measurements were conducted on an S-4800 instrument (Hitachi, Japan) at acceleration voltages of 5 kV and 20 kV, respectively. Before conducting SEM measurements, the nATH samples and the fracture surface of the nanocomposites were sputter coated with a platinum layer in an enclosed chamber for 40 s and 60 s, respectively, to enhance conductivity and image quality. The distribution of C, O, N, Al, and P elements in the nanocomposites was analyzed using an energy dispersive X-ray spectrometer (Horiba 7593-H) coupled with the SEM (SEM-EDX).
Thermogravimetric analysis (TGA) were carried out with a LABSYS Evo STA analyzer (Setaram, France) at a heating rate of 10 °C min−1 under an air atmosphere from 50 °C to 900 °C.
Tensile properties of epoxy composites were determined according to ISO 527 using a AI-7000M Testing Machine (Gotech, Taiwan) at a tensile rate of 20 mm min−1. The tested specimens had dumbbell-shaped with a gauge length of 50 mm. Unnotched Izod impact strength of the materials were measured following ASTM D4812 using a mechanical test equipment (TestResources, USA). The samples had a dimension of 64 mm × 13 mm × 3 mm. The results for each formulation represented the average of five measurements, accompanied by error values.
3 Results and discussion
3.1 Morphology and structural characterization of nano aluminum hydroxide
 | ||
| Fig. 1 XRD patterns of nano ATH synthesized at the different temperatures: (a) 80 °C, (b) 90 °C, and (c) 100 °C. | ||
On the other hand, the hydrothermal temperature has also a strong impact on the morphology and particle size of nATH, as shown in Fig. 2. The product obtained at 80 °C is polygonal nanoplates with irregular sizes (Fig. 2a). At higher temperatures, the ATH crystals exist at the uniform hexagonal nanoplate structure. A statistical analysis of particle sizes from the SEM images indicates that the average particle diameter increases from 386 ± 21 nm at 90 °C (Fig. 2b and c) to 437 ± 26 nm at 100 °C (Fig. 2d). Nevertheless, the average thickness of the as-synthesized nATHs at 90 and 100 °C is the similar, approximately 30 nm. Additionally, as shown in Table 2, the reaction yield at 90 °C is the highest, approximately 82.5%. Based on these results, the hydrothermal temperature of 90 °C was suitable for synthesizing ATH nanoplates with a high yield, uniform morphology, and consistent particle size.
 | ||
| Fig. 2 SEM images of as-synthesized nano ATHs: nATH-1 (a), nATH-2 (b and c), nATH-3 (d), nATH-4 (e), and nATH-5 (f). | ||
| Samples | Temp. (°C) | Time (h) | Product | Morphology | Yield (%) |
|---|---|---|---|---|---|
| nATH-1 | 80 | 72 | 77.7% gibbsite, 22.3% bayerite | Polygonal nanoplates | 75.6 |
| nATH-2 | 90 | 72 | Gibbsite | Hexagonal nanoplates | 82.5 |
| nATH-3 | 100 | 72 | Gibbsite | Hexagonal nanoplates | 80.8 |
| nATH-4 | 90 | 24 | Amorphous gel with minor gibbsite | Amorphous with minor hexagonal nanoplates | 8.2 |
| nATH-5 | 90 | 48 | Gibbsite | Hexagonal nanoplates | 32.3 |
3.2 Results of ATH nanoparticle surface modification
In order to improve the compatibility of the as-synthesized ATH nanoplates with polymer matrix, the surface of this material was treated by organic compounds. The FT-IR spectra of untreated nano Al(OH)3 (nATH), oleic acid-treated nATH (nATHOA), Tween 80-treated nATH (nATHTw80), and PEI-treated nATH (nATHPEI) are shown in Fig. 4. In all spectra, the characteristic absorption peaks of Al(OH)3 are observed at 3397–3623 cm−1 (O–H stretching vibrations), 1023, and 559 cm−1 (Al–O stretching vibrations).36 Furthermore, the FT-IR spectra of nATHOA and nATHTw80 exhibit new peaks at about 2925 and 2855 cm−1, attributed to the –CH2 symmetric and asymmetric stretching vibrations,37 respectively, in the structure of oleic acid and Tween 80. Meanwhile, peaks at 2951 and 2843 cm−1 in the FT-IR spectrum of nATHPEI also correspond to the –CH2 stretching vibrations in PEI. Additionally, a peak at 1573 cm−1 is assigned to the N–H bending vibrations of PEI.38 These results indicate that the surface of ATH nanoplates is successfully coated with the modifying agents. | ||
| Fig. 4 FT-IR spectra of nATH before and after modification with organic compounds: (a) nATH, (b) nATHOA, (c) nATHTw80, and (d) nATHPEI. | ||
The dispersibility of nATH in the epoxy matrix before and after surface modification is illustrated in Fig. 5. It is evident that untreated ATH nanoplates tend to agglomerate into large clusters (Fig. 5a) due to their poor compatibility with the organic polymer matrix. However, the agglomerate of the nanoparticles is remarkably reduced after the surface modification. Among the tested samples, nATHPEI demonstrates the best dispersion within the polymer matrix, with distinct separation of nanoplates, as shown in Fig. 5d. This improvement is attributed to the surface modification of nATH with PEI which contains numerous amine groups (–NH2, –NH) capable of reacting with the oxirane rings of the epoxy resin, thereby significantly enhancing the compatibility of nATHPEI with the polymer. In addition, the dispersion of nATHPEI in nanocomposites was observed by SEM-EDX mapping measurements. It can be seen that the Al elements in the 3nATHPEI/7IFR/EP nanocomposite are concentrated in certain areas (Fig. 5e), suggesting the agglomerate of untreated nATH in the epoxy resin. In contrast, Fig. 5f manifests a uniform distribution of Al elements in 3nATHPEI/7IFR/EP, implying that nATHPEI is well-dispersed in the intumescent flame retardant epoxy composite.
3.3 Flame retardancy
The flame retardancy of EP and its composites was evaluated using UL94-V and LOI tests, and the results are summarized in Table 3. EP is a combustible material, which burns out after the first ignition. The introduction of APP@PEI to epoxy resin significantly improved the fire resistance of the polymer. Specifically, the 10APP@PEI/EP composite passes a UL-94 V-0 rating, and its LOI value (28.9%) increases notably compared to the EP sample (21.1%). The flame retardant property of nanocomposites loading APP@PEI and nATH was also investigated, with a sample total content of fillers (10 wt%). In comparison to the untreated nATH, the combination of APP@PEI and nATH surface-treated with the organic compounds enhances the flame retardancy of the composite. The flame resistance of 3nATH/7IFR/EP slightly diminishes compared to 10APP@PEI/EP, with a UL-94 V-1 rating and an LOI value of 28.5%. This result can be explained by the agglomeration of the untreated ATH nanoplates, which have high surface energy and poor compatibility with the organic polymer matrix. In contrast, the LOI values of 3nATHOA/7IFR/EP, 3nATHTw80/7IFR/EP, and 3nATHPEI/7IFR/EP increased to 30.7, 29.4 and 31.1%, respectively. It can be seen that PEI-treated nano ATH provides the highest fire retardant performance. This can be attributed to the good dispersion of nATHPEI in epoxy resin, which is proved by the SEM-EDX mapping and microscope images (Fig. 5).| Samples | UL94-V (3.2 mm) | LOI (%) | Tensile strength (MPa) | Impact strength (kJ m−2) | ||
|---|---|---|---|---|---|---|
| Rating | t1a (s) | t2b (s) | ||||
| a t1: self-extinguishing time after the first ignition.b t2: self-extinguishing time after the second ignition.c NR: no rating. | ||||||
| EP | NRc | Burnt out | — | 21.1 | 83.69 ± 0.2 | 45.92 ± 0.1 |
| 10APP@PEI/EP | V-0 | 3.3 | 5.8 | 28.9 | 59.84 ± 0.6 | 12.57 ± 0.3 |
| 3nATH/7IFR/EP | V-1 | 0.9 | 13.6 | 28.5 | 58.82 ± 0.7 | 14.54 ± 0.6 |
| 3nATHOA/7IFR/EP | V-0 | 1.0 | 2.5 | 30.7 | 66.15 ± 0.3 | 21.54 ± 0.2 |
| 3nATHTw80/7IFR/EP | V-0 | 0.8 | 3.2 | 29.4 | 64.22 ± 0.5 | 19.83 ± 0.5 |
| 3nATHPEI/7IFR/EP | V-0 | 0.6 | 0.8 | 31.1 | 67.80 ± 0.2 | 22.68 ± 0.1 |
| 1nATHPEI/9IFR/EP | V-0 | 3.4 | 2.6 | 29.8 | 62.89 ± 0.1 | 20.15 ± 0.2 |
| 5nATHPEI/5IFR/EP | V-1 | 3.1 | 15.4 | 28.1 | 68.44 ± 0.5 | 23.46 ± 0.4 |
| 10nATHPEI/EP | NR | Burnt out | — | 22.8 | 71.65 ± 0.4 | 29.70 ± 0.3 |
Additionally, the effect of nATHPEI content on the flame retardancy of the composite containing APP@PEI was investigated. As indicated in Table 3, substituting a small amount of APP@PEI (≤3 wt%) with nATHPEI results in a significant enhancement in the fire resistance of the composite. The self-extinguishing times after the flame applications in the UL94-V test notably reduce (t1 = 0.6 s and t2 = 0.8 s), and the LOI value grows to 31.1% when 3 wt% of APP@PEI is replaced with nATHPEI. However, with a higher nATHPEI content, the flame retardancy of the nanocomposite tends to diminish. The 5nATHPEI/5IFR/EP nanocomposite achieves only a V-1 UL-94 rating (t1 = 3.1 s and t2 = 15.4 s) with an LOI value of 28.1%. The enhancement in flame resistance of the composite containing APP@PEI when combined with an appropriate amount of nATHPEI is attributed to the synergistic flame retardant effect between APP@PEI and nATH.
3.4 Thermal oxidative behavior
TGA and DTG curves of EP and composites 10APP@PEI/EP, 10nATHPEI/EP and 3nATHPEI/7IFR/EP under air atmosphere are illustrated in Fig. 6, and the detailed data are summarized in Table 4. The EP resin undergoes two degradation stages under air atmosphere, with only 0.12 wt% remaining at 900 °C. For 10APP@PEI/EP, Tmax1 and Tmax2 values of this composite decrease significantly compared to the reference sample. However, the residue of 10APP@PEI/EP at 900 °C is approximately 14.75 wt%, much higher than that of the EP resin. These results can be attributed to the formation of phosphoric and polyphosphoric acids from the decomposition of APP. These acids then accelerate the decomposition of EP (dehydration and carbonization) to produce an intumescent char layer, which effectively protects the internal polymer matrix from the flame.15 In comparison with the EP resin, Tonset and Tmax1 values of the 10nATHPEI/EP nanocomposite decline by 5.34 and 17.19 °C, respectively. This reduction can be attributed to the endothermic dehydration of Al(OH)3 at around 220 °C.22,28 However, Tmax2 of this nanocomposite rises sharply by 40.28 °C.| Samples | Tonseta (°C) | Tmax1b (°C) | Tmax2b (°C) | Residue at 900 °C (wt%) |
|---|---|---|---|---|
| a Tonset: temperature at mass loss of 5 wt%.b Tmax: temperature at maximum degradation rate. | ||||
| EP | 317.54 | 368.31 | 714.15 | 0.12 |
| 10APP@PEI/EP | 316.24 | 339.72 | 659.15 | 14.75 |
| 10nATHPEI/EP | 312.2 | 351.12 | 754.43 | 7.62 |
| 3nATHPEI/7IFR/EP | 314.21 | 341.65 | 679.76 | 17.98 |
Moreover, the char yield of 10nATHPEI/EP at 900 °C is 7.62 wt%, slightly higher than the calculated residue of 6.63 wt%. These data suggest that the thermal oxidative stability of EP is enhanced by the addition of nATHPEI. Compared to 10APP@PEI/EP, the Tonset value of the 3nATHPEI/7IFR/EP nanocomposite shows a slight decrease. This phenomenon can be explained by the early decomposition of nano Al(OH)3. However, the Tmax values of this nanocomposite are higher than those of the 10APP@PEI/EP composite. Notably, the Tmax2 value increases by approximately 20.61 °C. 3nATHPEI/7IFR/EP also exhibits a reduced thermal decomposition rate and achieves higher char yield beyond 500 °C compared to 10APP@PEI/EP. This improvement can be attributed to the synergistic interaction between nATHPEI and APP@PEI, which generates highly thermally stable aluminum phosphate compounds. These compounds reinforce the char structural surface of the nanocomposite, thereby effectively slowing the degradation of the nanocomposite at high temperatures. The results explain to the enhancement in the fire resistance of the 3nATHPEI/7IFR/EP nanocomposite compared to the 10APP@PEI/EP composite.
3.5 Flame-retardant mechanism
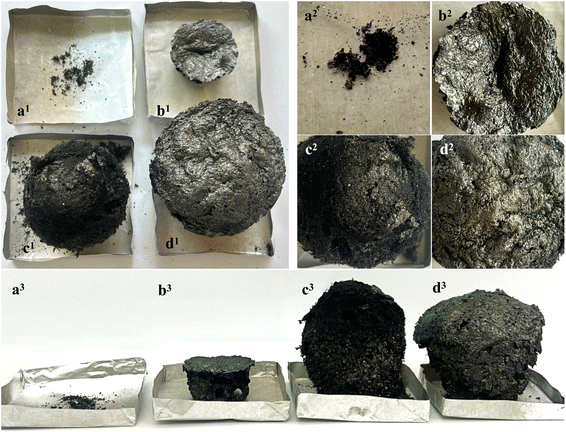
Neat EP, 10nATHPEI/EP, 10APP@PEI/EP, and 3nATHPEI/7IFR/EP were calcined to 900 °C at a rate of 10 °C min−1, using samples of the same weight. The char residues of samples were then analyzed using FT-IR, XRD, and EDX to investigate the synergistic flame-retardant effect between APP@PEI and nATHPEI. Fig. 7 depicts the char structure of the samples after calcination. It can be observed that the char yield of neat EP remains insignificant (Fig. 7a), which agrees with the TGA data. By contrast, the introduction of flame retardants remarkably promotes char formation. Among flame-retardant composites, 10nATHPEI/EP leaves the smallest char volume, however, its char structure is remarkably robust (Fig. 7b). The 10APP@PEI/EP composite forms a significantly larger porous char structure compared to 10nATHPEI/EP (Fig. 7c). However, this char layer is comparatively soft and prone to damage. The intumescence of the 3nATHPEI/7IFR/EP nanocomposite after calcination is similar to that of the 10APP@PEI/EP composite; however, its char layer surface becomes noticeably more continuous and compact, as observed in Fig. 7d. The observation confirms that incorporating a small amount of nATHPEI into the APP@PEI/EP composite helps to reinforce the char structure of the composite, effectively preventing the underlying materials from further combustion.The chemical composition of char residues was analyzed by FT-IR, X-ray diffraction, and SEM-EDS. Fig. 8 illustrates FT-IR spectra and XRD patterns of the composites 10APP@PEI/EP, 10nATHPEI/EP, and 3nATHPEI/7IFR/EP after the calcination. As displayed in Fig. 8A, all spectra appear peaks at 2926 and 2854 cm−1 (–CH2 stretching), 1744 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), and 1625 cm−1 (C
O stretching), and 1625 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching),17,38–40 indicating that the aromatization and carbonization occur during the decomposition of the composites. In addition, the FT-IR spectrum of residues of 10APP@PEI/EP exhibits a peak at 1024 cm−1 attributed to P–O stretching,8,41 suggesting the formation of the phosphorus-rich char structure upon the presence of APP@PEI. For 10nATHPEI/EP, the FT-IR spectrum reveals the existence of alumina (Al2O3) at 1018–1163 cm−1 (Al–O stretching) and 514 cm−1 (O–Al–O bending),42 which is produced from the dehydration of nATH. However, the absorption peaks of Al2O3 disappear in the FT-IR spectrum of 3nATHPEI/7IFR/EP. Instead, the intensity of the absorption peak at 1030 cm−1 increases attributed to the overlap of P–O vibrations in char structure and a metal phosphate compound. Additionally, a new peak is observed at 1119 cm−1, corresponding to PO2 in a chain structure, where two oxygen atoms from the phosphorous bonded to aluminum atoms.43 These findings demonstrate that, in addition to the phosphorus-rich char structure, metal phosphate compounds are formed during the combustion of the 3nATHPEI/7IFR/EP nanocomposite through the interaction between the decomposition products of nATH and APP.
C stretching),17,38–40 indicating that the aromatization and carbonization occur during the decomposition of the composites. In addition, the FT-IR spectrum of residues of 10APP@PEI/EP exhibits a peak at 1024 cm−1 attributed to P–O stretching,8,41 suggesting the formation of the phosphorus-rich char structure upon the presence of APP@PEI. For 10nATHPEI/EP, the FT-IR spectrum reveals the existence of alumina (Al2O3) at 1018–1163 cm−1 (Al–O stretching) and 514 cm−1 (O–Al–O bending),42 which is produced from the dehydration of nATH. However, the absorption peaks of Al2O3 disappear in the FT-IR spectrum of 3nATHPEI/7IFR/EP. Instead, the intensity of the absorption peak at 1030 cm−1 increases attributed to the overlap of P–O vibrations in char structure and a metal phosphate compound. Additionally, a new peak is observed at 1119 cm−1, corresponding to PO2 in a chain structure, where two oxygen atoms from the phosphorous bonded to aluminum atoms.43 These findings demonstrate that, in addition to the phosphorus-rich char structure, metal phosphate compounds are formed during the combustion of the 3nATHPEI/7IFR/EP nanocomposite through the interaction between the decomposition products of nATH and APP.
 | ||
| Fig. 8 FT-IR spectra (A) and XRD patterns (B) of char residues of 10APP@PEI/EP (a), 10nATHPEI/EP (b), and 3nATHPEI/7IFR/EP (c) after calcination at 900 °C. | ||
No discernible diffraction peaks are visible in the XRD pattern of residues of 10APP@PEI/EP, implying that the char structure of this material exists mainly in the amorphous phase. For 10nATHPEI/EP, the existence of Al2O3 in the residue is confirmed by peaks at 2θ ∼ 37.69°, 45.72°, and 67.24° (ICDD PDF #00-003-0915). However, the characteristic diffraction peaks of Al2O3 no longer exist in the char structure of 3nATHPEI/7IFR/EP. Instead, the XRD pattern of this char residue exhibits the appearance of aluminum orthophosphate (AlPO4) at 2θ ∼ 20.32°, 21.60°, 22.91°, and 35.48° (ICDD PDF #01-070-4689). Additionally, a small amount of aluminum metaphosphate ([Al(PO3)3]n) – a trivalent cation long chain polyphosphate could be formed in the char structure of this nanocomposite with the characteristic diffraction peak at 2θ ∼ 20.45° (ICDD PDF #00-013-0430).44,45
The result proves that the entire amount of Al2O3 produced by the dehydration of nATH reacts with decomposition products of APP to generate highly thermally stable aluminum phosphate compounds.
Fig. 9 depicts the results of SEM-EDX analyses of the char residues. To ensure uniform composition, the char residues of the composites were ground before conducting measurements. In addition to the similar char layer of the 10APP@PEI/EP composite (Fig. 9a), new compounds are formed and primarily concentrated on the char structure of the 3nATHPEI/7IFR/EP nanocomposite (Fig. 9c). This phenomenon aligns with the previously discussed XRD analysis, confirming the presence of aluminum phosphate crystals in the nanocomposite char structure. The EDX patterns identify elements such as C, O, P, and Al in the char. In contrast, the N element is absent from the char residues, suggesting that NH3 and N-containing non-combustible gases are released during the material degradation process.46–48
 | ||
| Fig. 9 SEM images and EDX spectra of the char residues of 10APP@PEI/EP (a and a′), 10nATHPEI/EP (b and b′), and 3nATHPEI/7IFR/EP (c and c′) after calcination at 900 °C. | ||
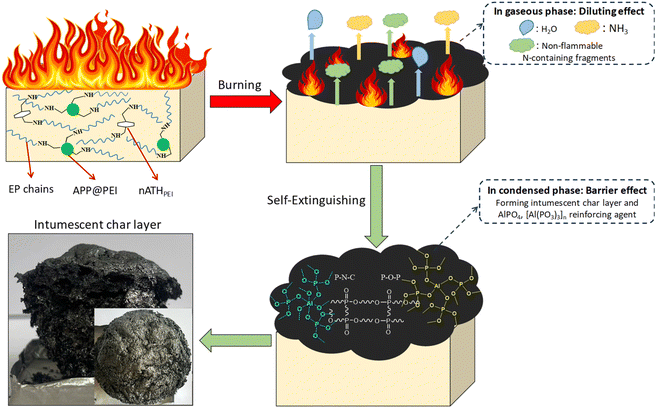
Based on the above discussions and previous studies, the flame retardant mechanism of the 3nATHPEI/7IFR/EP can be described in Fig. 10. At low temperature, APP, PEI, and amine hardener of EP begin to decompose, releasing NH3 and H2O, which serves as a blowing gas for the intumescent flame-retardant system. Simultaneously, nano Al(OH)3 undergoes the endothermic decomposition, producing H2O and Al2O3. Subsequently, phosphoric acid and its poly-/ultra-/pyro-derivatives are generated through the chain scission of APP.49–51 These derivatives promote dehydration, esterification, and aromatization reactions between EP matrix and APP, resulting in a phosphorus – rich aromatic structure. Besides, part of phosphoric acid reacts with Al2O3 to create aluminum phosphates, such as AlPO4 and [Al(PO3)3]n, the highly thermally stable compounds. As the temperature increases, P–N–C groups are formed, but eventually decompose into N-containing non-combustible gases at higher temperatures.17 However, the P–O–P, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C
C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O – containing aromatic ring structures remain in the char layer, and the presence of aluminum phosphates has the reinforcing effect this char structure. As a result, a better thermal stable char layer is formed, acting as a barrier that inhibits the heat transfer from the flame to the interior material and limits the diffusion of flammable decomposition products into the combustion zone. Therefore, the condensed-phase flame retardant mechanism of the nanocomposite demonstrates higher effectiveness.
O – containing aromatic ring structures remain in the char layer, and the presence of aluminum phosphates has the reinforcing effect this char structure. As a result, a better thermal stable char layer is formed, acting as a barrier that inhibits the heat transfer from the flame to the interior material and limits the diffusion of flammable decomposition products into the combustion zone. Therefore, the condensed-phase flame retardant mechanism of the nanocomposite demonstrates higher effectiveness.
3.6 Mechanical properties
Yield tensile and Izod impact strengths (unnotched) of EP resin and its composites are presented in Table 3. It is clear that incorporating APP@PEI negatively affects the mechanical properties of the EP resin. Specifically, the tensile and impact strengths of 10APP@PEI/EP are reduced by 28.50% and 72.63%, respectively, compared to the neat EP resin. Although the compatibility of APP within the EP matrix improves after modification with PEI, clustering of the microparticles still occurs (Fig. 11a, b and d). Besides, the large particle size of this filler is another factor causing the deterioration in the mechanical properties of the composite. Replacing part of APP@PEI with untreated ATH nanoplates does not improve the mechanical performance of the composite. The agglomeration of the untreated ATH nanoplates is obviously observed in Fig. 5e and 11c. In addition, defects shown in Fig. 11c indicate the poor compatibility of this inorganic filler with the polymer matrix. As a consequence, the reinforcing property of the nanostructured materials is not effectively harnessed. In contrast, the use of surface treated ATH nanoplates considerably enhances the mechanical properties of 10APP@PEI/EP. Among them, nATHPEI provides the best reinforcement effect for the composite. The tensile and impact strengths of 3nATHPEI/7IFR/EP heighten by roughly 13.30% and 80.43%, respectively, compared to 10APP@PEI/EP. Fig. 5f and 11e confirm the uniform dispersion of nATHPEI within the nanocomposite. The good interfacial adhesion between the material with the EP matrix is also evident in Fig. 11e. These observations account for the improvement in mechanical properties of 3nATHPEI/7IFR/EP. On the other hand, the influence of nATHPEI content on the mechanical properties of nanocomposites was also evaluated. The results exhibit that both the tensile and impact strengths of the nanocomposites gradually grow as the proportion of APP@PEI replaced by nATHPEI rises from 1 wt% to 10 wt%. Nevertheless, considering the discussions above, it can be concluded that the mixture of 3 wt% nATHPEI and 7 wt% APP@PEI is the potential formulation for developing an epoxy nanocomposite system with high flame retardant efficiency and good mechanical property. | ||
| Fig. 11 The fracture surface images of 3nATH/7IFR/EP (a–c) and 3nATHPEI/7IFR/EP (d and e) at different magnifications. | ||
4 Conclusions
In summary, hexagonal gibbsite nanoplates were successfully prepared from an Al(OH)3 gel precursor via a hydrothermal method. The average particle diameter and thickness of the as-synthesized nATH were approximately 350–450 nm and 30 nm, respectively. Additionally, these nanoplates were introduced into an intumescent flame-retardant epoxy system. It was found that PEI-treated nATH provided the best synergistic flame-retardant effect for the epoxy composite containing APP@PEI. Furthermore, the optimal content of nATHPEI was also determined. As a result, the nanocomposite containing 3 wt% nATHPEI and 7 wt% APP@PEI exhibited the highest fire resistance and thermal oxidative stability, achieving a UL-94 V-0 rating with a LOI value of 31.1% and a high char yield of 17.98% at 900 °C. The synergistic flame-retardant mechanism of the mixture of APP@PEI and nATHPEI could be occurred in both the condensed and gaseous phases. In the condensed phase, APP@PEI promoted the formation of a phosphorus-rich char layer, which acted as a physical barrier to heat and mass transfer. Moreover, the interaction between nATHPEI and APP@PEI produced aluminum phosphate compounds, which played a crucial role in reinforcing the char structure of the composite. In the gaseous phase, non-flammable gases could be released during decomposition, such as NH3, H2O, and other nitrogen-containing fragments, acted as blowing agents for the intumescent char layer and as inert diluents. Besides, the presence of nATHPEI significantly improved the tensile and impact strengths of the composite loading APP@PEI.Data availability
All data generated or analyzed during this study are included in the article. The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request. No restrictions apply to the data, and all data can be made available to the scientific community for future research upon request.Author contributions
Conceptualization, Mai Ha Hoang; data analysis, Truong Cong Doanh, Nhung Hac Thi; methodology, Truong Cong Doanh, Nhung Hac Thi, Mai Ha Hoang; project administration, Mai Ha Hoang; validation, Hong Tham Nguyen, Ho Thi Oanh, Tien Dat Doan, Nguyen Duc Tuyen; writing – original draft, Truong Cong Doanh, Nhung Hac Thi; writing – review & editing, Mai Ha Hoang, Minh-Tan Vu, Truong Cong Doanh, and Nhung Hac Thi. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This research was supported by Vietnam Academy of Science and Technology under grant number KHCBHH.02/24-25.References
- Y. Yang, D.-Y. Wang, R.-K. Jian, Z. Liu and G. Huang, Prog. Org. Coat., 2023, 175, 107316 CrossRef CAS.
- B. Xu, Q. Zhang, H. Zhou, L. Qian and S. Zhao, Composites, Part B, 2023, 263, 110832 CrossRef CAS.
- H. Niu, G. Wu, X. Wang, H. Ding and Y. Hu, Polym. Degrad. Stab., 2023, 211, 110333 CrossRef CAS.
- Y. Sui, X. Dai, P. Li and C. Zhang, Macromol. Mater. Eng., 2021, 306, 2100239 CrossRef CAS.
- Y. Sui, H. Sima, W. Shao and C. Zhang, Composites, Part B, 2022, 229, 109463 CrossRef CAS.
- Y. Sui, X. Dai, P. Li and C. Zhang, Eur. Polym. J., 2021, 156, 110601 CrossRef CAS.
- Y. He, X. Cui, Z. Liu, F. Lan, J. Sun, H. Li, X. Gu and S. Zhang, Polym. Degrad. Stab., 2023, 218, 110579 CrossRef CAS.
- X. Chen, Y. Ma, S. Liu, A. Zhang, W. Liu and S. Huang, Adv. Ind. Eng. Polym. Res., 2025, 8, 48–62 CAS.
- B. Xu, L. Shao, J. Wang, Y. Liu and L. Qian, Polym. Degrad. Stab., 2020, 181, 109281 CrossRef CAS.
- P. Wen, X. Feng, Y. Kan, Y. Hu and R. K. K. Yuen, Polym. Degrad. Stab., 2016, 134, 202–210 CrossRef CAS.
- S. Khanal, Y. Lu, S. Ahmed, M. Ali and S. Xu, Polym. Test., 2020, 81, 106177 CrossRef CAS.
- G. Turgut, M. Dogan, U. Tayfun and G. Ozkoc, Polym. Degrad. Stab., 2018, 149, 96–111 CrossRef CAS.
- B. Yuan, A. Fan, M. Yang, X. Chen, Y. Hu, C. Bao, S. Jiang, Y. Niu, Y. Zhang, S. He and H. Dai, Polym. Degrad. Stab., 2017, 143, 42–56 CrossRef CAS.
- Y. Zhan, X. Wu, S. Wang, B. Yuan, Q. Fang, S. Shang, C. Cao and G. Chen, Polym. Degrad. Stab., 2021, 191, 109684 CrossRef CAS.
- Y. Tan, Z. B. Shao, X. F. Chen, J. W. Long, L. Chen and Y. Z. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 17919–17928 CrossRef CAS PubMed.
- Y. Tan, Z.-B. Shao, L.-X. Yu, J.-W. Long, M. Qi, L. Chen and Y.-Z. Wang, Polym. Chem., 2016, 7, 3003–3012 RSC.
- Y. Tan, Z.-B. Shao, L.-X. Yu, Y.-J. Xu, W.-H. Rao, L. Chen and Y.-Z. Wang, Polym. Degrad. Stab., 2016, 131, 62–70 CrossRef CAS.
- W.-J. Hu, Y.-M. Li, Y.-R. Li and D.-Y. Wang, J. Therm. Anal. Calorim., 2022, 148, 1841–1851 CrossRef.
- F. N. Nguyen and J. C. Berg, Composites, Part A, 2008, 39, 1007–1011 CrossRef.
- A. M. Saks, J. C. Berg and F. N. Nguyen, J. Adhes. Sci. Technol., 2007, 21, 1375–1393 CrossRef.
- Y. Pan, L. Han, Z. Guo and Z. Fang, J. Appl. Polym. Sci., 2016, 134, 44551 CrossRef.
- N. H. Thi, T. N. Nguyen, H. T. Oanh, N. T. T. Trang, D. Q. Tham, H. T. Nguyen, T. Van Nguyen and M. H. Hoang, J. Appl. Polym. Sci., 2020, 138, e50317 CrossRef.
- Y. Y. Yen, H. T. Wang and W. J. Guo, J. Appl. Polym. Sci., 2013, 130, 2042–2048 CrossRef CAS.
- M. Sabet, A. Hassan and C. T. Ratnam, J. Reinf. Plast. Compos., 2013, 32, 1122–1128 CrossRef.
- J. F. Long, Z. L. Zhou, T. Wu, Y. Q. Li and S. Y. Fu, Compos. Commun., 2023, 39, 101562 CrossRef.
- S. Elbasuney, Powder Technol., 2017, 305, 538–545 CrossRef CAS.
- V. K. Abitha, A. V. Rane, R. Uday, N. Samarth, A. V. Rane and V. Kamble, Adv. Eng. Forum, 2015, 14, 3–18 Search PubMed.
- Z. Qin, D. Li, Q. Li and R. Yang, Mater. Des., 2016, 89, 988–995 CrossRef CAS.
- M. Ejaz, M. M. Azad, A. u. R. Shah, S. K. Afaq and J.-i. Song, Cellulose, 2022, 29, 1775–1790 CrossRef CAS.
- R. Suchita, C. Mukesh, N. Manoj, B. Sneha, M. Smita and A. Anupam, Int. J. Mater. Res., 2023, 114, 678–688 CrossRef.
- M. Liao, H. Chen, L. Deng, X. Wei, Z. Zou, H. Wang, S. Chen and Z. Zhu, React. Funct. Polym., 2023, 192, 105735 CrossRef CAS.
- C. T. Duc, L. C. Nguyen, P. B. Van, H. T. Nguyen, T. A. D. Thi, G. Le-Nhat-Thuy, Q. G. N. Thi, P. H. Thi, T. A. Nguyen, Q. V. Tran, H. T. Quang, M. H. Hoang and T. N. Van, RSC Adv., 2024, 14, 5264–5275 RSC.
- M. Wan, C. Shi, L. Chen, L. Deng, Y. Qin, H. Che, J. Jing, J. Li and X. Qian, Polym. Degrad. Stab., 2024, 226, 110821 CrossRef CAS.
- F. Kang, H. Han, D. He, M. Yi, R. Wang and M. Zhou, Ind. Crops Prod., 2025, 223, 120176 CrossRef CAS.
- X. Zhang, X. Zhang, T. R. Graham, C. I. Pearce, B. L. Mehdi, A. T. N'Diaye, S. Kerisit, N. D. Browning, S. B. Clark and K. M. Rosso, Cryst. Growth Des., 2017, 17, 6801–6808 CrossRef CAS.
- X. Du, Y. Wang, X. Su and J. Li, Powder Technol., 2009, 192, 40–46 CrossRef CAS.
- S. Y. Qin, J. Feng, L. Rong, H. Z. Jia, S. Chen, X. J. Liu, G. F. Luo, R. X. Zhuo and X. Z. Zhang, Small, 2014, 10, 599–608 CrossRef CAS PubMed.
- H. Liu, Y. Zhou, Y. Yang, K. Zou, R. Wu, K. Xia and S. Xie, Appl. Surf. Sci., 2019, 471, 88–95 CrossRef CAS.
- W. Song, X. Wang, Q. Wang, D. Shao and X. Wang, Phys. Chem. Chem. Phys., 2015, 17, 398–406 RSC.
- J. Ding, S. Tang, X. Chen, M. Ding, J. Kang, R. Wu, Z. Fu, Y. Jin, L. Li, X. Feng, R. Wang and C. Xia, Chem. Eng. J., 2018, 344, 594–603 CrossRef CAS.
- S. Wang, Q. Fang, C. Liu, J. Zhang, Y. Jiang, Y. Huang, M. Yang, Z. Tan, Y. He, B. Ji, C. Qi and Y. Chen, Eur. Polym. J., 2023, 187, 111897 CrossRef CAS.
- A. Amirsalari and S. F. Shayesteh, Superlattices Microstruct., 2015, 82, 507–524 CrossRef CAS.
- K. Lertjiamratn, P. Praserthdam, M. Arai and J. Panpranot, Appl. Catal., A, 2010, 378, 119–123 CrossRef CAS.
- A. Castrovinci, G. Camino, C. Drevelle, S. Duquesne, C. Magniez and M. Vouters, Eur. Polym. J., 2005, 41, 2023–2033 CrossRef CAS.
- P. Khalili, K. Y. Tshai, D. Hui and I. Kong, Composites, Part B, 2017, 114, 101–110 CrossRef CAS.
- Z.-B. Shao, C. Deng, Y. Tan, M.-J. Chen, L. Chen and Y.-Z. Wang, Polym. Degrad. Stab., 2014, 106, 88–96 CrossRef CAS.
- Z. B. Shao, C. Deng, Y. Tan, M. J. Chen, L. Chen and Y. Z. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 7363–7370 CrossRef CAS PubMed.
- Z.-B. Shao, C. Deng, Y. Tan, L. Yu, M.-J. Chen, L. Chen and Y.-Z. Wang, J. Mater. Chem. A, 2014, 2, 13955–13965 RSC.
- J. S. Wang, D. Y. Wang, Y. Liu, X. G. Ge and Y. Z. Wang, J. Appl. Polym. Sci., 2008, 108, 2644–2653 CrossRef CAS.
- J.-S. Wang, Y. Liu, H.-B. Zhao, J. Liu, D.-Y. Wang, Y.-P. Song and Y.-Z. Wang, Polym. Degrad. Stab., 2009, 94, 625–631 CrossRef CAS.
- L. Liu, Y. Zhang, L. Li and Z. Wang, Polym. Adv. Technol., 2011, 22, 2403–2408 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |