 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Preparation of Fe–N co-doped carbon-based catalysts and their influence on tetracycline degradation properties†
Yang Tao‡
a,
Tiyang Xiao‡b,
Qing Fub,
Bin Miaoc,
Sanying Hou *b,
Guowen Peng*a,
Yiyang Xiongb and
Manzhen Tangb
*b,
Guowen Peng*a,
Yiyang Xiongb and
Manzhen Tangb
aSchool of Resource Environment and Safety Engineering, University of South China, Hengyang 421001, China. E-mail: 852376775@qq.com
bSchool of Chemistry and Chemical Engineering, University of South China, Hengyang 421001, China. E-mail: tysgying@163.com
cSchool of Mechanical and Aerospace Engineering, Nanyang Technological University, Singapore 639798, Singapore
First published on 7th March 2025
Abstract
A high-performance Fe–N co-doped carbon-based catalyst was prepared to activate PMS by a one-step pyrolysis using ferbam, melamine and sucrose. The best catalyst Fe–N/C exhibited high catalytic activity against PMS at an ultra-low catalyst dosage (50 mg L−1), and the TC decomposition rate reached 92.3% within 30 min. The results demonstrated that graphite N, pyridine N, Fe0 and Fe3C were the main active substances for catalyzing PMS. Free radical quenching analysis and EPR experiments further proved that the O2˙− served as the main active oxygen species. Finally, the environmental hazard of the intermediate product was studied by ECOSAR system.
1. Introduction
At present, antibiotics have been widely used not only in the prevention and treatment of bacterial infectious diseases in the medical field, but also used as growth inhibitors in agriculture, animal husbandry and aquaculture.1,2 Antibiotics can induce bacterial resistance and contribute to its spread, posing harm to both the ecological environment and human health. At the same time, it also affects the structure and function of microbial population in the water body, thereby harming the growth and development of organisms in the water body, and causing damage to the human urinary tract, kidney and other organs.3 Therefore, the efficient degradation of antibiotic wastewater has become an important process.Due to its non-selective degradation of organic pollutants, the advanced oxidation process (AOP) based on peroxymonosulfate (PMS) has attracted more and more attention in water treatment research. Compared with the traditional Fenton process, SO4˙− produced by persulfate activation owned a longer half-life (30–40 μs),4 higher oxidation potential (E0 = 2.5–3.1 V), broader pH (2.0–8.0) and better oxidation selectivity.5 However, PMS itself exhibited insufficient oxidative capacity against organic pollutants, and some methods such as ultrasonic,6,7 ultraviolet,8,9 carbon substrate derived catalysts,10,11 transition metal ion-guided catalysts (Co2+, Fe2+, or Ni2+)12 and their cooperative derivative catalysts have been employed in activating PMS.13 Doping transition metals and nitrogen in carbon-based materials has been considered to be an effective strategy for preparing catalysts that could activate PMS. These catalysts were commonly denoted as M–N–C catalysts.14 Nitrogen doping could enhance the surface alkalinity of the carbon material and increase the electrochemical activity. This, in turn, strengthened the adsorption of PMS and improved the efficiency of electron transfer from the catalysts to the contaminants. The addition of transition metals further regulated the electronic structure, improved the catalytic performance, and promoted the recycling of the catalyst.15 Among them, iron-based catalysts are widely used for the modification of carbon-based catalysts due to their wide availability and low cost. It had been shown that the iron doping (such as single-atom, cluster, particle) could significantly improve the low reaction rate caused by the deficiency of active sites and poor structural stability in carbon-based catalysts.16–20 For instance, the Fe–CN-650 prepared by Zeng et al.21 via Fe3+ and 1,10-phenanthroline ligand could remove 100% of SMX in 20 min with excellent cycling performance. Compared with the FeN4 ligand, FeN3O1 showed better interaction with PMS. However, metal–ligand based catalyst-loaded catalysts suffer from the disadvantage of high temperature sintering, which reduces the dispersion and exposure of active iron sites. It is worth noting that metal particles possess a more stable structure, especially Fe3C. Xiao et al.22 Nitrogen-doped nanotube catalysts encapsulating Fe/Fe3C particles synthesized by supramolecular self-assembly possessed superior degradation performance for TC with reduced iron loss.
However, the Fe–N/C catalysts reported so far still encounters some constraints. Their preparation procedures are complex, leading to high cost and insufficient synergistic catalytic activity. The issue arises from the large and aggregational Fe-containing particles, which hinder effective interaction between nanoparticles and active nitrogen species.23–25 Therefore, it is urgent to adopt efficient and facile preparation strategies to rationally design three-dimensional porous Fe–N/C catalysts with well distributed Fe-nanoparticles for PMS-activation.
Hence, in this study, a high-performance Fe–N co-doped carbon-based catalyst (Fe–N/C) with iron source (ferbam) was prepared by one-step pyrolysis strategy for activating PMS to the tetracycline hydrochloride (TC) degradation. The elemental structure and variations of the catalysts were investigated thoroughly and the catalytic sites were explored in conjunction with a series of degradation experiments, including temperature, catalyst dosage, contamination concentration, PMS dosage and common interfering factors in natural water environment. The types of free radicals were corroborated by radical trapping experiments and electron paramagnetic resonance spectroscopy, and the main degradation mechanisms were derived. Finally, possible routes in TC degradation were investigated and the toxicity of intermediates was analyzed.
2. Experimental
2.1. Synthesis of catalysts
The Fe–N/C catalysts were prepared using a simple grinding and calcination process as shown in Fig. 1. Typically, 0.11 g ferbam, 2 g melamine and 0.3 g sucrose were added to the mortar and grind to dry thoroughly. The dried samples were placed in a quartz boat and with N2 at a heating rate of 5 °C min−1 to 900 °C for 2 h, which was represented as Fe–N/C. Under the same conditions, N/C and C were prepared with the same method but without the addition of iron or nitrogen sources. In addition, by varying the calcination temperature to 800 and 1000 °C and the amount of melamine of 1and 3 g, some comparison samples were prepared, which denoted as Fe–N/C-800, Fe–N/C-1000, Fe–N1/C and Fe–N3/C.2.2. Catalytic experiment
Typically, the reaction temperature was 25 °C. In a 100 ml oxidation system, the obtained Fe–N/C catalysts were added to a certain concentration of tetracycline solution and dispersed by sonication. After the addition of PMS, the timed extraction of the sample was started. The samples were filtered by a 0.45 μm polytetrafluoroethylene (PTFE) filter and the absorbance was determined by UV-1900i with a wavelength of 357 nm. Quenchers including tert-butanol (TBA), furfuryl alcohol (FFA), methanol (MeOH), and p-benzoquinone (PBQ) were used to verify the oxidation mechanism. All experimental tests were repeated three times to minimize possible errors.2.3. Assessment standards
Calculation formula of TC degradation rate:
 | (1) |
C0 represents the initial concentration of tetracycline and Ct is the concentration of tetracycline remaining at time t.
3. Results and discussion
3.1. Characterization of catalysts
The morphology of the catalysts (Fe–N/C, N/C and C) was characterized by scanning electron microscopy (SEM). Fig. S1a and b† depicted that a graphene-like structure was formed after the N doping, and the carbon nanotubes (CNTs) were further occurred after Fe/N co-doping. The element mapping results in Fig. S1(d–g)† and EDS spectra (Fig. S1h†) confirmed that C, O, N and Fe elements were successfully doped and evenly distributed in the Fe–N/C catalyst.The material structure and crystal composition of the different catalysts were measured by X-ray diffraction (XRD) (Fig. 2a). The C and N/C catalysts exhibited two similar diffraction peaks at 26.5 and 44°, which corresponded to the (002) and (101) planes of graphitic carbon, respectively.26 For the Fe–N/C catalyst, diffraction peaks were occurred in the range of 37.83, 40.15, 43.38 and 44.94°, which represented the Fe3C phase, illustrating the successful doping of Fe elements.27
 | ||
| Fig. 2 (a) XRD patterns, (b) Raman spectra, (c) nitrogen adsorption–desorption curves (d) pore size distributions of catalysts. | ||
The degree of defects in the catalysts was investigated by Raman spectroscopy (Fig. 2b). All catalysts showed two characteristic peaks near 1355 cm−1 and 1575 cm−1, which corresponded to the positions of defective carbon (D-band) and graphitic carbon (G-band), respectively. The ratio of ID/IG was commonly used to assess the degree of defects in the carbon structure.28 The ID/IG of Fe–N/C, N/C and C catalysts were 1.055, 1.499 and 0.997, respectively. The Fe–N/C exhibited the lower ID/IG value than N/C due to the loading of iron on the surface of carbon substrate, which could destroy the graphene-like structure of the carbon material partially. C catalyst had the lowest ID/IG value, suggesting that the degree of disorder in the carbon material might increase due to the embedding of N and Fe and lead to the creation of additional defect sites in the carbon network.29,30
The N2 adsorption–desorption curves of C, N/C and Fe–N/C were given in Fig. 2c. The C catalyst showed a typical type I isotherm, indicating a rich microporous structure was formed in C materials,31 and a typical type IV curve was occurred in Fe–N/C and N/C catalyst, respectively, illustrating the mesoporous characteristics of the catalysts. Table S1† illustrated that the total pore volume of N/C (0.5631 cm3 g−1) was increased compared to C, which might be related to the melamine as a blowing agent to produce abundant pore structures through gas bubbling. Meanwhile, the Fe–N/C possessed the highest porosity and average pore size. However, C showed an extremely poor pore distribution compared to Fe–N/C and N/C (Fig. 2d). In addition, Fe–N/C exhibited smaller specific surface area (341.2839 m2 g−1) compared to N/C (361.9232 m2 g−1). It was shown that iron doping resulted in a reduction in the specific surface area of the catalyst due to clogging of the pores.32
The elemental composition of the Fe–N/C was discussed by X-ray photoelectron spectrum (XPS). Fig. 3a showed that S 2p (168.46 eV), C 1s (285.36 eV), N 1s (400.35 eV), O 1s (532.24 eV), and Fe 2p (710.17 eV) were existed in Fe–N/C. However, the S content was so low (Table S2†), which could be ignored, corresponding with the SEM results (Fig. S1h†). As shown in Fig. 3b, four peaks located at 398.55, 400.11, 401.23 and 404.02 eV, which represented pyridine N, pyrrole N, graphite N and oxidized N, respectively,33 with corresponding contents of 34.04, 28.92, 26.6 and 10.43%, respectively. Graphite N was more resistant to high temperatures and activated PMS by promoting electron transfer to produce reactive species. At the same time, pyridine N also promoted π-electron transfer on its adjacent sp2 carbon, generating more free radicals.34 Fe 2p spectra (Fig. 3c) showed six peaks of 707.49, 710.89, 715.29, 720.179, 723.89 and 728.69 eV, which was regarded as Fe0 (2p3/2), Fe2+ (2p3/2), Fe3+ (2p3/2), Fe0 (2p1/2), Fe2+ (2p1/2) and Fe3+ (2p1/2), with contents of 17.06, 16.20, 13.39, 8.53, 8.10 and 6.69%, respectively.
3.2. Catalytic activity of catalysts
The adsorption degradation experiments of C, N/C and Fe–N/C were conducted. As shown in Fig. 4a, the adsorption efficiency of C, N/C and Fe–N/C were 0.01, 4.5, and 10%, respectively, which indicated that the adsorption capability of catalysts were very weak. For PMS alone, the TC removal efficiency was 30% within 30 min, suggesting that the degradation of TC by PMS alone was limited, which could be caused by the self-decomposition of PMS. When N element was doped, the degradation effect reaches 65% in 30 min. This might be related to the high electronegativity of N atom, which could change the charge distribution of C atom. In addition, the catalytic effect was the best when Fe and N were doped at the same time, and the removal effect of TC reached 92.3% within 30 min, which attributed to the Fe and N co-doping altered chemical inertness of the carbon substrate. Furthermore, the performance of Fe–N/C for TC degradation was compared with other different catalytic materials that have been reported in previous literature. As illustrated in Table S3,† home-made Fe–N/C displayed excellent catalytic performance of PMS with TC removal rate of 92.3% in 30 min at ultra-low catalyst dosage (50 mg L−1) and high TC concentration (30 mg L−1) condition.
The influence of temperature on the TC removal properties in Fe–N/C/PMS systems was further investigated in Fig. 5a. The decomposition efficiency of TC had a small influence when the temperature of the system increased from 25 °C to 45 °C, indicating that the catalytic ability of the system was not affected by temperature. Considering the above results, the experiments in the next part of the study would be carried out under the optimal condition of 0.5 g per L PMS, 0.05 g per L Fe–N/C, 30 mg per L TC and at 25 °C.
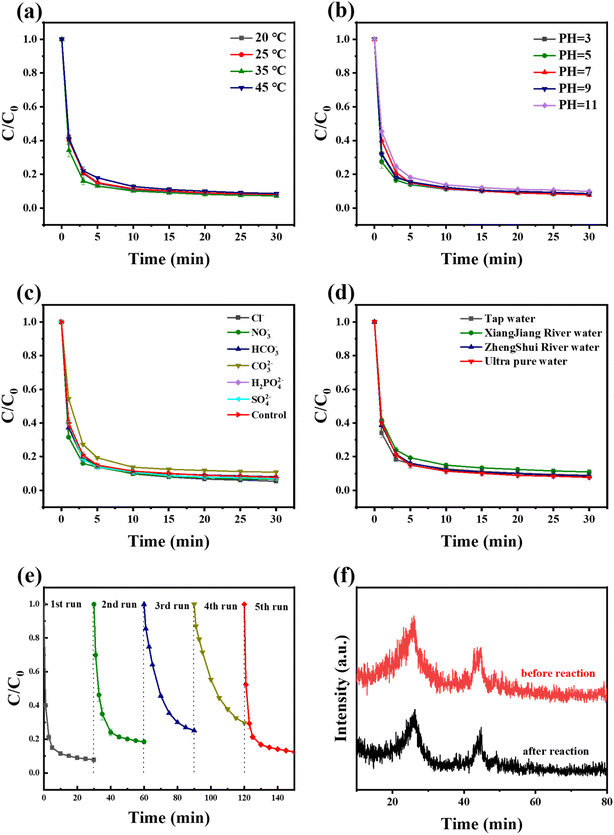 | ||
| Fig. 5 Effect factors for Fe–N/C/PMS: (a) temperature, (b) pH, (c) inorganic ions, (d) different water bodies and (e) five cycles experiment of Fe–NC (f) XRD changes of the used Fe–N/C. | ||
In Fe–N/C/PMS system, the initial pH of the system was a prominent factor for the degradation performance which was shown in Fig. 5b. In a wide range of pH (3–11), all of the degradation rate of TC achieved above 89%. However, when the pH of the solution was 11, the removal efficiency of TC was most affected. This might be attributed to the excessive OH−, which make the surface of the catalyst negatively charged. Additionally, the decomposition of PMS under alkaline conditions was also a factor. This decomposition was not conducive to the reaction inside the system.37
The presence of inorganic anions in the water environment might react with the active species generated by PMS activation and influenced the degradation efficiency of organic pollutants. The effects of Cl−, NO3−, HCO3−, CO32−, H2PO42−, SO42− on the TC decomposition were investigated. It could be found in Fig. 5c that the TC decomposition was promoted when Cl− was introduced into the reaction system, and the removal efficiency of TC reached 94.5%. The higher the concentration of Cl−, the more active chlorine free radicals (˙Cl, Cl2, HO–Cl) generated,38 which improved the decomposition of TC. In addition, the hydrolyzed solution of CO32− was alkaline and exhibited an inhibitory effect on the reaction, which was in keeping with the results of pH interference experiments. However, other ions (SO42−, NO3−, etc.) had no significant influence on the decomposition of TC, suggesting that the catalyst system had practical adaptability.
The effects of water bodies to the Fe–N/C/PMS system were investigated in Fig. 5d. The Fe–N/C catalysts exhibited stable TC removal ability in Zhengshui River water, tap water and ultrapure water. Although the removal efficiency of TC in Xiangjiang water was inhibited to some extent, it still remained above 85%, which reflected that the system had a great anti-interference performance to the actual water body.
The influence of different pollutants (methylene blue (MB), levofloxacin (LVX)) on the degradation profiles of Fe–N/C/PMS system was explored. As shown in Fig. S3.† The results showed that 80.6% of LVX and 100% of MB could be removed by the Fe–N/C/PMS system within 30 min. This illustrates that the Fe–N/C catalyst were more effective in the degradation of MB.The catalytic difference might be caused by the differences in the physicochemical properties and substituent groups of the organic pollutants.
 | ||
| Fig. 6 (a) Effect of selective scavengers on TC removal and the EPR spectra of (b) DMPO–˙OH and DMPO–SO4˙−, (c) TEMP–1O2, (d) DMPO–O2˙−. | ||
The presence of different reactive oxygen species in the Fe–N/C/PMS system was verified by electron paramagnetic resonance experiments (EPR). ˙OH, SO4˙− and O2˙− were captured by DMPO as spin trapping agent. TEMP was used as a spin catcher to detect 1O2. As shown in Fig. 6b, adding the DMPO to a blank water system with catalysts exhibited no EPR signal. On the contrary, when the Fe–N/C and PMS was present, weak signals belonging to DMPO–SO4˙− and DMPO–˙OH were detected. It could be seen that the stronger signal, with strength of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, corresponded to ˙OH, while the weaker signal around ˙OH was attributed to SO4˙−. This suggests that Fe–N/C/PMS promoted the presentation of SO4˙− and ˙OH during the activation of PMS. On the other hand, in Fig. 6c, three characteristic peaks of TEMP–1O2 were detected, which proved the existence of 1O2. Fig. 6d showed that six characteristic signal peaks were detected in the reaction system, suggesting the presence of O2˙−. Hence, it could be concluded that both the 1O2 and free radical pathways were involved in the degradation of TC in the system. Especially, in the free radical pathway, O2˙− contributed a major role in the oxidative degradation of TC compared with ˙OH and SO4˙−, which attributed to that oxygen in the air got electrons to form O2˙−, activating PMS to degrade TC.
1, corresponded to ˙OH, while the weaker signal around ˙OH was attributed to SO4˙−. This suggests that Fe–N/C/PMS promoted the presentation of SO4˙− and ˙OH during the activation of PMS. On the other hand, in Fig. 6c, three characteristic peaks of TEMP–1O2 were detected, which proved the existence of 1O2. Fig. 6d showed that six characteristic signal peaks were detected in the reaction system, suggesting the presence of O2˙−. Hence, it could be concluded that both the 1O2 and free radical pathways were involved in the degradation of TC in the system. Especially, in the free radical pathway, O2˙− contributed a major role in the oxidative degradation of TC compared with ˙OH and SO4˙−, which attributed to that oxygen in the air got electrons to form O2˙−, activating PMS to degrade TC.
Recent studies have also shown that the electron transfer from the target pollutant to the oxidant in carbon-based materials can accelerate the decomposition of the target pollutant.39,40 In order to investigate whether the electron transfer exists in the Fe–NC system, CV curves of catalysts and open circuit voltage (OCP) were explored to investigate the electrochemical performance of the catalysts. As shown in Fig. S5(a),† Fe–N/C possessed the largest inner circle area, indicating that it exhibited the largest electrochemical active surface area (ECSA).22 Furthermore, open circuit voltage test (OCP) of the system in Fig. S5(b)† showed that the initial potential of Fe–N/C was about 0.41 V, which increased to about 0.98 V after the addition of PMS, and then decreased significantly after the addition of TC, suggesting that the intermediate (Fe–N/C/PMS*, which has a higher oxidation potential) triggered the electron transfer path.
 | ||
| Fig. 7 XPS diagram of the before and after reaction of Fe–N/C: (a) full spectrum diagram, (b) N 1s and (c) Fe 2p. | ||
The possible TC decomposition mechanism in the Fe–N/C/PMS system was depicted in Fig. 8. At all, Fe–N/C/PMS exhibited excellent catalytic properties, mainly benefiting from the graphite N, pyridine N and Fe species (Fe0 and Fe3C). In addition, hydrolysis of PMS could produce free radicals. These active sites facilitated the activation of PMS, generating strong oxidative groups that degraded and mineralized TC into small molecules and water.
| Fe0 + 2HSO5− → Fe2+ + 2OH− + 2SO4˙− | (2) |
| Fe0 → Fe2+ + 2e− | (3) |
| Fe2+ + HSO5− → Fe3+ + SO4˙− + OH− | (4) |
| OH− + SO4˙− → ˙OH + SO4˙2− | (5) |
| Fe2+ + O2 → Fe3+ + O2˙− | (6) |
| 2Fe3+ + Fe0 → 3Fe2+ | (7) |
The acute and chronic toxicity of the intermediates was calculated by ECOSAR software as shown in Table S7.† According to the GHS Toxicological Classification System, the concentration of LC50/EC50/ChV represented their degree of hazard.48 Its concentration below 1 mg L−1 is red (very toxic), between 1 and 10 mg L−1 is orange (toxic), between 10 and 100 mg L−1 is blue (harmful) and greater than100 mg L−1 is green (harmless). For acute toxicity, most intermediates and TC were little toxic. In terms of chronic toxicity, daphnia showed greater toxicity than TC, such as P12, P14, P32. Overall, most of the intermediates had much lower toxicity and might eventually be degraded to non-toxic water or CO2, suggesting that the system possessed some environmental value.
4. Conclusion
In this study, Fe–N/C catalyst was successfully prepared by one simple calcination strategy for activating PMS to degrade tetracycline. The optimal Fe–N/C displayed an improved catalytic performance, which can be ascribed to the accelerating effect of reactive species (graphite N, pyridine N, Fe0 and Fe3C) on the PMS activation. In addition, the Fe–N/C also exhibited good catalytic performance in natural water, illustrating the widespread prospects practical application of Fe–N/C. The ROS burst experiments and EPR showed that the Fe–NC/PMS system produced ˙OH, SO4˙−, O2˙−, 1O2 and electronic transfer and the main ROS were O2˙−. Three possible degradation pathways were explored by LC-MS, and the acute and chronic toxicity of TC and its degradation intermediates was analyzed using ECOSAR software. The process proposed offers a carbon-based catalyst with high catalytic activity, which will be expected to develop new ideas for the application of M–N–C catalyst in PMS activation.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 22109068, 42377076), the Hunan Provincial Natural Science Foundation of China (2023JJ50129), the Undergraduate Innovation and Entrepreneurship of China (No. S202410555079), the Undergraduate Innovation and Entrepreneurship of Hunan Province (No. D202305131656247626, S202410555335, S202410555341).References
- M. Brigante and M. Avena, Microporous Mesoporous Mater., 2016, 225, 534–542 CrossRef CAS.
- Q. Zhang, G. Ying, C. Pan, Y. Liu and J. Zhao, Environ. Sci. Technol., 2015, 49(11), 6772–6782 CrossRef CAS PubMed.
- X. Xu and X. Li, Chemosphere, 2020, 78(4), 430–436 CrossRef PubMed.
- S. Zhang, S. Song, P. Gu, R. Ma, D. Wei, G. Zhao, T. Wen, R. Jehan, B. Hu and X. Wang, J. Mater. Chem. A, 2019, 7(10), 5552–5560 RSC.
- X. Duan, Z. Ao, L. Zhou, H. Sun, G. Wang and S. Wang, Appl. Catal., B, 2016, 188, 98–105 CrossRef CAS.
- Y. Pang, Y. Ruan, Y. Feng, Z. Diao, K. Shih, L. Hou, D. Chen and L. Kong, Chemosphere, 2019, 228, 412–417 CrossRef CAS PubMed.
- L. Yang, J. Xue, L. He, L. Wu, Y. Ma, H. Chen, H. Li, P. Peng and Z. Zhang, Chem. Eng. J., 2019, 378, 122146 CrossRef CAS.
- X. He, A. de la Cruz and D. Dionysiou, J. Photochem. Photobiol., A, 2013, 251, 160–166 CrossRef CAS.
- S. Verma, S. Nakamura and M. Sillanpää, Chem. Eng. J., 2016, 284, 122–129 CrossRef CAS.
- X. Duan, H. Sun, Y. Wang, J. Kang and S. Wang, ACS Catal., 2015, 5(2), 553–559 CrossRef CAS.
- G. Fang, C. Liu, J. Gao, D. Dionysiou and D. Zhou, Environ. Sci. Technol., 2015, 49(9), 5645–5653 CrossRef CAS.
- G. Anipsitakis and D. Dionysiou, Environ. Sci. Technol., 2004, 38(13), 3705–3712 CrossRef CAS PubMed.
- C. Chen, L. Liu, Y. Li, W. Li, L. Zhou, Y. Lan and Y. Li, Chem. Eng. J., 2020, 384, 123257 CrossRef CAS.
- J. Kang, X. Duan, C. Wang, H. Sun, X. Tan, M. Tade and S. Wang, Chem. Eng. J., 2018, 332, 398–408 CrossRef CAS.
- S. Wu, H. Liu, C. Yang, X. Li, Y. Lin, K. Yin, J. Sun, Q. Teng, C. Du and Y. Zhong, Chem. Eng. J., 2020, 392, 123683 CrossRef CAS.
- S. Hou, H. Hu, Q. Fu, T. Xiao, J.-Q. Xie, S.-H. Chan, M. He, B. Miao and L. Zhang, Sep. Purif. Technol., 2024, 333, 125980 CrossRef CAS.
- X. Peng, J. Wu, Z. Zhao, X. Wang, H. Dai, L. Xu, G. Xu, Y. Jian and F. Hu, Chem. Eng. J., 2022, 427, 130803 CrossRef CAS.
- M. Wu, Y. Tao, Y. Liu, S. Hou, T. Xiao, Q. Fu, G. Peng and L. Shi, Sep. Purif. Technol., 2025, 354, 129225 CrossRef CAS.
- S. Li, Y. Ke, X. Zhang, S. Wu and Z. Xie, Sep. Purif. Technol., 2024, 338, 126570 CrossRef CAS.
- Z. Liu, A. Kang, Z. Du, H. Chen and X. Yao, J. Environ. Chem. Eng., 2023, 11, 111576 CrossRef CAS.
- Y. Zeng, J. Deng, N. Zhou, W. Xia, Z. Wang, B. Song, Z. Wang, Y. Yang, X. Xu, G. Zeng and C. Zhou, Small, 2024, 20, 2311552 CrossRef CAS PubMed.
- T. Xiao, Y. Tao, S. Hou, H. Wang, J.-Q. Xie, Y. Chang, Q. Fu, K. Du and S. Zhou, Sep. Purif. Technol., 2025, 356, 129916 CrossRef CAS.
- M. M. Mian, G. Liu and H. Zhou, Sci. Total Environ., 2020, 744, 140862 CrossRef CAS PubMed.
- B.-T. Zhang, Z. Yan, J. Zhao, Z. Chen, Y. Liu, M. Fan and W. Du, Water Res., 2023, 243, 120340 CrossRef CAS PubMed.
- P. Tirira, S. Mirehbar, S. Fernández-Velayos, P. Herrasti, N. Menéndez, F. J. Recio and I. Sirés, Mater. Today Sustain., 2023, 24, 100581 Search PubMed.
- Y. Li, B. Xing, X. Wang, K. Wang, L. Zhu and S. Wang, Energy Fuels, 2019, 33(12), 12459–12468 CrossRef CAS.
- K. Liu, X. Kang, X. He, P. Wei, Y. Wen and X. Li, Nanoscale, 2019, 11(18), 9155–9162 RSC.
- V. Tsaneva, W. Kwapinski, X. Teng and B. Glowacki, Carbon, 2014, 80, 617–628 CrossRef CAS.
- W. Oh, G. Lisak, R. Webster, Y. Liang, A. Veksha, A. Giannis, J. Moo, J. Lim and T. Lim, Appl. Catal., B, 2018, 233, 120–129 CrossRef CAS.
- M. Mian and G. Liu, Chem. Eng. J., 2020, 392, 123681 CrossRef CAS.
- C. Ding, T. Liu, X. Yan, L. Huang, S. Ryu, J. Lan, Y. Yu, W. Zhong and X. Yang, Nano-Micro Lett., 2020, 12(1), 63 CrossRef CAS PubMed.
- B. Ren, J. Miao, S. Wang, Y. Xu, Z. Zhai, X. Dong and Z. Liu, Colloids Surf., A, 2021, 618, 126439 CrossRef CAS.
- C. Wang, J. Kang, P. Liang, H. Zhang, H. Sun, M. Tadé and S. Wang, Environ. Sci.:Nano, 2017, 4(1), 170–179 RSC.
- W. Sun, K. Pang, F. Ye, M. Pu, C. Zhou, C. Yang and Q. Zhang, Sep. Purif. Technol., 2022, 284, 120197 CrossRef CAS.
- X. Li, S. Zhang, M. Yu, H. Xu, J. Lv, S. Yang, X. Zhu and L. Li, J. Taiwan Inst. Chem. Eng., 2021, 128, 209–219 CrossRef CAS.
- L. Wang, J. Li, X. Liu, J. Zhang, P. Zeng and Y. Song, Chemosphere, 2023, 324, 138264 CrossRef CAS PubMed.
- Z. Yang, Z. Wang, G. Liang, X. Zhang and X. Xie, Chem. Eng. J., 2021, 426, 131777 CrossRef CAS.
- H. Fu, S. Ma, P. Zhao, S. Xu and S. Zhan, Chem. Eng. J., 2019, 360, 157–170 CrossRef CAS.
- Y. Liu, Y. Zhang, L. Zhang, W. Hayat, X. Wu, H. Meng and Q. Wu, Chem. Eng. J., 2024, 490, 151585 CrossRef CAS.
- F. Ye, Y. Su, R. Li, W. Sun, M. Pu, C. Yang, W. Yang, H. Huang, Q. Zhang and J. W. C. Wong, Appl. Catal., B, 2023, 337, 122992 CrossRef CAS.
- J. Qu, K. Li, Q. Wang, W. Tong, G. Zhang, Q. Hu, Y. Tao, Z. Jiang and Y. Zhang, J. Cleaner Prod., 2024, 441, 140881 CrossRef CAS.
- Q. Hu, J. Cao, W. Yang, Z. Xu, P. Song, M. Jia, Y. Zhang, H. Peng and A. Wu, Sep. Purif. Technol., 2021, 259, 118059 CrossRef CAS.
- X. Zhu, Y. Wang, R. Sun and D. Zhou, Chemosphere, 2013, 92(8), 925–932 CrossRef CAS PubMed.
- M. Ouyang, X. Li, Q. Xu, Z. Tao, F. Yao, X. Huang, Y. Wu, D. Wang, Q. Yang, Z. Chen and Z. Pi, J. Colloid Interface Sci., 2020, 566, 33–45 CrossRef CAS PubMed.
- M. Nie, Y. Li, L. Li, J. He, P. Hong, K. Zhang, X. Cai, L. Kong and J. Liu, Appl. Surf. Sci., 2021, 535, 147655 CrossRef CAS.
- Y. Chen, Y. Ma, J. Yang, L. Wang, J. Lv and C. Ren, Chem. Eng. J., 2017, 307, 15–23 CrossRef CAS.
- L. Shi, L. Liang, J. Ma and J. Sun, Superlattices Microstruct., 2013, 62, 128–139 CrossRef CAS.
- H. Wu, Z. Tan, B. Miao, H. Hu and S. Hou, J. Environ. Chem. Eng., 2023, 11, 109204 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra00189g |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |





