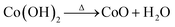Open Access Article
Open Access ArticleUltrasound-assisted synthesis of biomass-derived carbon-supported binary metal oxides for efficient adsorption of heavy metals from wastewater†
Walaa S. Gadoa,
Asmaa S. Morshedy a,
Ahmed M. Masoud
a,
Ahmed M. Masoud b,
Ard elshifa M. E. Mohammed
b,
Ard elshifa M. E. Mohammed c,
Entsar H. Tahad,
Adel A. El-Zahhar
c,
Entsar H. Tahad,
Adel A. El-Zahhar e,
Majed M. Alghamdie,
Ahmed M. A. El Naggar
e,
Majed M. Alghamdie,
Ahmed M. A. El Naggar *a and
Esraa M. El-Fawala
*a and
Esraa M. El-Fawala
aEgyptian Petroleum Research Institute (EPRI), Nasr City, 11727, Cairo, Egypt
bNuclear Materials Authority, P.O. Box 530, El Maadi, Cairo, Egypt
cDepartment of Chemistry, College of Science, Qassim University, Al-Qassim, Saudi Arabia
dDepartment of Plant Protection, Faculty of Agriculture, Ain Shams University, Egypt
eDepartment of Chemistry, Faculty of Science, King Khalid University, Abha 9004, Saudi Arabia. E-mail: drmeto1979@yahoo.com
First published on 29th April 2025
Abstract
Heavy metal contamination in water sources remains a critical environmental issue, primarily due to industrial activities, in terms of its continuous contribution to pollution through non-compliance and illegal discharge. This study presents an innovative biochar-supported binary metal oxide composite (nickel oxide (NiO) and cobalt oxide (CoO) nanoparticles) synthesized via ultrasound-assisted techniques for efficient adsorption of Zn(II) and Cd(II) ions from wastewater. By utilizing solid residues and leveraging ultrasound technology, this approach aligns with the principles of green chemistry due to utilization of a renewable biomass-based source, enhancing energy efficiency in the synthesis process, and minimizing waste production. Thereby a sustainable and innovative route for material development is explicitly demonstrated. Structural and morphological characterizations confirm the uniform integration of Nickel oxide (NiO) and cobalt oxide (CoO) particles into the biochar matrix, leading to maximum adsorption capacities of 18.9 mg g−1 for Zn(II) and 10.2 mg g−1 for Cd(II). The adsorption process follows a chemisorptive monolayer mechanism, as demonstrated by kinetic and isotherm studies, and is thermodynamically confirmed to be endothermic and spontaneous. The material also exhibits excellent reusability over five adsorption–desorption cycles. By integrating sustainable resources with innovative synthesis techniques, this work contributes to advancing wastewater remediation technologies while supporting global sustainability initiatives.
1. Introduction
Heavy metal pollution in aquatic environments has emerged as a global environmental challenge due to the extensive industrial use of metals like cadmium (Cd), zinc (Zn), nickel (Ni), and copper (Cu).1–3 These pollutants are non-biodegradable, toxic, and prone to bioaccumulation, threatening aquatic ecosystems, soil fertility, and human health.4,5 The World Health Organization (WHO) and other regulatory bodies have set stringent limits for these metals in water to mitigate their adverse effects—for instance, the permissible levels for zinc and cadmium in drinking water are 3 mg L−1 and 0.003 mg L−1, respectively.6,7 Industrial processes, including metal plating, pigment production, and battery manufacturing, discharge heavy metals into water bodies, exacerbating contamination levels.8–10 Addressing this issue requires the development of innovative and sustainable technologies that align with the principles of green chemistry.Traditional methods for heavy metal removal, such as chemical precipitation,11 ion exchange,12 membrane separation,13 solvent extraction,14 often face limitations related to cost, efficiency, and secondary waste generation. For instance, chemical precipitation can generate large volumes of sludge, requiring additional treatment and disposal, which increases operational costs. Ion exchange, while effective, is often expensive due to the high cost of resins and their regeneration. Membrane separation, although efficient, is energy-intensive and prone to fouling, leading to increased maintenance costs. Solvent extraction, on the other hand, can produce toxic organic solvents as byproducts, posing environmental risks.11–15 Adsorption has emerged as a preferred approach due to its cost-effectiveness, high efficiency, and adaptability to renewable materials.15–19 However, the search for advanced adsorbents that are both scalable and environmentally friendly remains a crucial area of research. Recent advances in materials science offer the potential to overcome these challenges by integrating renewable resources with innovative synthesis techniques.
Solid agricultural waste has emerged as a promising resource for addressing environmental and sustainability challenges.20–22 Materials such as crop residues, fruit peels, and other biomass are often discarded as waste, contributing to environmental degradation.20–22 However, these resources can be transformed into value-added materials like bio-char, which possess unique physicochemical properties suitable for environmental applications.23,24 Utilizing agricultural waste for bio-char production not only mitigates waste disposal issues but also aligns with the circular economy model by creating sustainable materials for advanced technologies.20–24
This study presents an innovative approach to heavy metal adsorption through the synthesis of a bio-char-supported binary metal oxide composite (nickel oxide (NiO) and cobalt oxide (CoO) nanoparticles). Nickel oxide (NiO) and cobalt oxide (CoO) were selected due to their well-documented high surface area, stability, and strong affinity for metal ion adsorption. NiO is known for its excellent catalytic and adsorptive properties, while CoO enhances redox interactions, improving adsorption kinetics. Their combined use creates a synergistic effect, optimizing adsorption performance. Additionally, both oxides demonstrate structural compatibility with biochar, ensuring homogeneous dispersion and maximizing active sites for heavy metal uptake. By utilizing pressed sunflower leaves as a renewable biomass source, bio-char was produced via pyrolysis and subsequently functionalized with nickel and cobalt oxides using ultrasonic homogenization. This method not only aligns with the principles of green chemistry by minimizing the use of synthetic materials but also leverages the transformative power of ultrasound technology to enhance material properties and performance. Comprehensive analyses, including adsorption kinetics, thermodynamics, and isotherm modeling, were performed for providing insights into the underlying mechanisms.
By integrating renewable resources with advanced synthesis methodologies, this study exemplifies the potential of chemistry to address pressing environmental challenges. The use of sunflower-derived bio-char underscores the importance of utilizing agricultural waste as a sustainable feedstock, while the ultrasonic-assisted synthesis emphasizes the role of innovative technologies in creating high-performance materials. These findings contribute not only to wastewater remediation but also to broader efforts in developing scalable, green, and effective solutions for environmental sustainability. This work underscores the transformative role of chemistry in achieving sustainable environmental solutions and providing a blueprint for designing next-generation adsorbents that are efficient, sustainable, and aligned with global efforts to mitigate pollution and protect natural resources.
2. Experimental
2.1. Materials
Analytical grades of chemical reagents were utilized in this study and all of them were used as-received with no further treatments. These chemicals including anhydrous zinc sulfate (ZnSO4) and Cadmium sulfate (CdSO4) were used to prepare a stock solution of cadmium and zinc ions using double-distilled water. Anhydrous nickel chloride (NiCl2) and cobalt chloride (CoCl2) were employed to synthesize the hydroxide structures of these metals. The previously mentioned reagents had a purity of 99.9% and they were purchased from Sigma-Aldrich, UK. Ammonia solution (NH4OH 35%), obtained from ADWIC-Egypt, was used as a precipitator during the process of producing metal oxide species, passing firstly through the obtainment of their metal hydroxide species. Pressed sunflower leaves were presented in this study as a raw solid biomass residual for preparing activated biochar particles to be utilized as support for the two metal oxides, generating a binary oxide composite. Pure nitric, hydrochloric, and sulfuric acids (Merck, Germany) were used during the recovery of sorbents via the desorption of metal ions.2.2. Synthesis of sorbent
The preparation of the introduced adsorbent in this research work was done through two consecutive steps. In the first one, bio-char species were prepared via pyrolysis of pressed sunflower residues at 300 °C under a nitrogen atmosphere for 2 hours. This temperature was chosen to ensure complete carbonization and enhance the porosity of the bio-char. The bio-char was thoroughly washed with deionized water and was then dried at 100 °C for 12 hours. It is important to note that no pretreatment was performed on the precursor material, as reported by Moniem et al.23In the second step, Nickel oxide (NiO) and cobalt oxide (CoO) nanoparticles were synthesized using a co-precipitation method. Nickel chloride (NiCl2) and cobalt nitrate (Co(NO3)2) solutions were mixed with ammonium hydroxide (NH4OH) as a precipitating agent under continuous stirring, forming hydroxide precursors (Ni(OH)2 and Co(OH)2). These precursors were then calcined at 400 °C for 3 hours to yield NiO and CoO nanoparticles, as described by Ali et al.24 The chemical reactions involved are as follows:
| NiCl2 + 2NH4OH → Ni(OH)2 + 2NH4Cl |
| Co(NO3)2 + 2NH4OH → Co(OH)2 + 2NH4NO3 |
Subsequently, the particles of metal oxides were coupled with the activated bio-char using ultrasonic waves, producing a composite adsorbent containing 20% of its weight as 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 of the two metal oxide particles. Particularly, calculated amounts of nickel oxide and cobalt oxide were suspended with a corresponding weight of the activated bio-char (representing 80% of the adsorbent composition) in a proper volume of ethanol under the effect of ultrasound waves for a time of 30 min using a homogenizer to ensure uniform distribution of the metal oxides within the activated bio-char matrix. The resulting composite was dried at 60 °C for 24 hours and stored for further characterization. The ultrasound-assisted synthesis method was employed to enhance the dispersion and interaction of NiO/CoO nanoparticles with biochar. The intense cavitation and microstreaming effects generated by ultrasound could facilitate uniform dispersion of metal oxides, prevented particle aggregation, and preserved the biochar's porous structure. These advantages are evident from the composite's homogeneous morphology (SEM), strong metal-support interactions (FTIR), and optimized adsorption performance. In contrast, conventional methods without ultrasound may lead to uneven metal oxide distribution, weaker binding, and reduced adsorption efficiency. The integration of ultrasound during synthesis procedures ensures superior composite properties and enhanced adsorption capacity, confirming its role in improving material functionality.
50 of the two metal oxide particles. Particularly, calculated amounts of nickel oxide and cobalt oxide were suspended with a corresponding weight of the activated bio-char (representing 80% of the adsorbent composition) in a proper volume of ethanol under the effect of ultrasound waves for a time of 30 min using a homogenizer to ensure uniform distribution of the metal oxides within the activated bio-char matrix. The resulting composite was dried at 60 °C for 24 hours and stored for further characterization. The ultrasound-assisted synthesis method was employed to enhance the dispersion and interaction of NiO/CoO nanoparticles with biochar. The intense cavitation and microstreaming effects generated by ultrasound could facilitate uniform dispersion of metal oxides, prevented particle aggregation, and preserved the biochar's porous structure. These advantages are evident from the composite's homogeneous morphology (SEM), strong metal-support interactions (FTIR), and optimized adsorption performance. In contrast, conventional methods without ultrasound may lead to uneven metal oxide distribution, weaker binding, and reduced adsorption efficiency. The integration of ultrasound during synthesis procedures ensures superior composite properties and enhanced adsorption capacity, confirming its role in improving material functionality.
2.3. Sorbent characterization
To figure out morphological, structural and surface characteristics of the produced composite structures, several analytical tools were utilized. Details of the employed instruments for determination of such features are described in Section S1, see the provided ESI† with this article.2.4. Adsorption experiments
Sorption tests were conducted to investigate the ability of CoO/NiO@bio-char to absorb Zn(II) and Cd(II) from an aqueous solution. Batch experiments were conducted using a Thermo-shaker water bath (Scientific Precision SWB 27, Waltham-USA) in a polypropylene tube. To conduct the experiments, a certain weight of sorbent (m, g) was added to a definite volume (V, L) of metal-containing aqueous solution (Co, 40 mg L−1) and shaken for 240 minutes at room temperature (25 ± 1 °C). Regarding the investigation plan, solution pH from 2–8 and the sorbent dosage from 0.5 g L−1 to 4.0 g L−1 were examined.For the desorption investigation, a similar experimental procedure was followed. The metal loading was done using the method mentioned earlier and the total metal present on the CoO/NiO@bio-char was calculated using the mass balance equation. Bimetal-loaded sorbents were mixed with a fixed volume of eluent (nitric acid, hydrochloric acid, or sulfuric acid) for 10 hours, with the sorbent dosage set at 3.0 g L−1. After filtration, the concentration in the eluate was analyzed to calculate the amount released and to find out the desorption efficiency. The recycling process of the sorbent was studied for five consecutive cycles of adsorption and desorption. During this process, CoO/NiO@bio-char was mixed with an aqueous metal solution that contained 40 mg L−1 of Zn(II) and Cd(II). The sorbent dosage was 3.0 g L−1, and the mixture was shaken for 4 hours at room temperature. The metal ions were then desorbed from the sorbent using a proper eluent with a sorbent dosage of 3.0 g L−1. The suspension was kept under agitation for 4 hours at room temperature, and the solutions were mixed and analyzed to determine the cumulative desorption of target metals.
Adsorption kinetics were monitored by periodically sampling the supernatant at different time intervals (5–600 min) and a sorbent dosage of 3.0 g L−1. The 4 hours equilibrium time was chosen based on preliminary studies indicating that adsorption rates stabilize after this period. The initial concentration interval of 20 to 120 mg L−1 was tested to explore the isotherm performance and evaluate the maximum capacity. Lagergren, pseudo-second-order, and intraparticle diffusion (Weber and Morris) kinetic equations were applied to describe the adsorption kinetic, while the uptake isotherm was modeled using Langmuir, Freundlich, Dubinin–Radushkevich (D–R), and Sips isotherm equations. The non-linear form of the applied kinetic and isotherm equations were declared in Table S1†.25–30 The main idea of the models used is found in their respective references. The fitting of the applied kinetic and isotherm models is deduced based on the average relative error (ARE), and coordination coefficient (R2) equations (Table S1†).25–30 The thermodynamic performance of the uptake process was investigated at different reaction temperatures of 25–50 ± 1 °C. The thermodynamic parameters namely; standard Gibbs free energy change (ΔG°), standard enthalpy change (ΔH°), and standard entropy change (ΔS°) were evaluated using equations listed in Table S1.†31,32
In all the experiments conducted, the initial concentration (Co, mg L−1) and the final residual concentration (Ce, mg L−1) of Zn(II) and Cd(II) were analyzed using Atomic Absorption Spectrometer GBC 932 AA (UK). Before the analysis, the samples were filtered through 0.22 mm filters. Each test was repeated three times, and only a relative error mean value of ≤5% was considered acceptable. The sorption efficiency (E%), sorption capacity (qe, mg g−1), and distribution coefficient (Kd) were calculated using eqn (1)–(3), as described in reference.
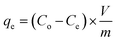 | (1) |
 | (2) |
 | (3) |
3. Results & discussion
3.1. Sorbent characteristics
Although biochar constitutes 80% of the composite, the notable alteration in its XRD signal is attributed to strong interactions between the biochar matrix and the embedded metal oxides. The incorporation of NiO and CoO modifies the local electronic structure, redistributing electron density across the carbon framework. Additionally, ultrasound-assisted dispersion promotes uniform metal oxide dispersion, influencing the overall crystalline arrangement of the composite. This interaction results in a reduction in the intensity of biochar-related peaks, primarily due to partial coverage of the carbon matrix by NiO and CoO nanoparticles and increased scattering effects from the crystalline metal oxide domains. Furthermore, the presence of highly disordered carbon regions, induced by metal oxide incorporation and ultrasonic treatment, contributes to the observed peak broadening and intensity variations. The absence of distinct X-ray diffraction (XRD) peaks corresponding to nickel (Ni) and cobalt (Co) in the composite can be attributed to several factors inherent to the material's characteristics and the limitations of the XRD technique. One primary reason is the nanocrystalline nature of the Ni and Co particles within the composite. When metal particles are reduced to the nanoscale, especially below 10 nm, their diffraction peaks tend to broaden significantly and decrease in intensity due to the effects of particle size on peak broadening. This phenomenon can render the peaks indistinguishable from the background noise, making them challenging to detect.
Additionally, if the Ni and Co particles are well-dispersed within an amorphous carbon matrix, their relative concentration might fall below the detection limit of the XRD instrument. XRD typically requires a minimum phase concentration—often around 4 weight percent—to produce discernible peaks. If the metal content is below this threshold, the corresponding peaks may not be observable. Furthermore, the formation of solid solutions or alloys between Ni and Co can lead to overlapping diffraction peaks, complicating the identification of individual metal phases. The similar crystal structures and lattice parameters of Ni and Co result in diffraction peaks occurring at nearly identical positions, causing them to merge and appear as a single broadened peak. This overlap can obscure the presence of distinct Ni and Co phases in the XRD pattern. The lack of clear Ni and Co peaks in the XRD analysis is likely due to the nanoscale size of the metal particles, their dispersion within the amorphous carbon matrix, potential low concentration below the detection limit, and the overlapping of diffraction peaks in the case of alloy formation. These factors collectively contribute to the challenges in detecting distinct Ni and Co phases using XRD in such composites.
Additional sharp peaks at higher angles (2θ = 42°, 48°, and 75.6°) correspond to specific crystallographic planes, further confirming the successful integration of NiO and CoO into the biochar matrix.33,34 To validate the crystalline phases, XRD analysis was referenced against standard JCPDS data, where NiO aligns with JCPDS Card No. 47-1049 and CoO matches JCPDS Card No. 48-1719. These results confirm the hybrid nature of the composite, demonstrating that the synergistic combination of biochar and metal oxides enhances adsorption capacity and chemical stability, making it a highly effective material for heavy metal removal applications.
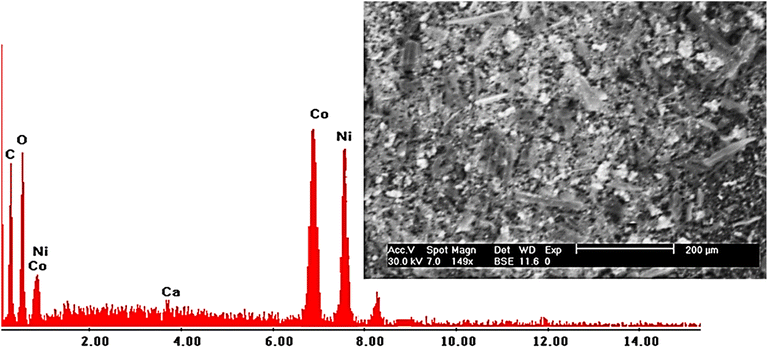
Energy-dispersive X-ray (EDX) analysis further verified the elemental composition of the composite, detecting strong signals for carbon and oxygen—the principal components of the biochar—as well as nickel and cobalt, confirming the successful incorporation of metal oxides (Fig. 4). Quantitative analysis showed that biochar constituted approximately 80% of the composite's weight, while metal oxides accounted for the remaining 20%, consistent with the preparation protocol. The coexistence of organic and inorganic phases creates a heterogeneous surface, providing diverse active sites for heavy metal adsorption and enhancing the material's functional versatility.
| Sample name | SBET (m2 g−1) | VP (cm3 g−1) | DH (nm) |
|---|---|---|---|
| Blank bio-char | 883.29 | 0.6487 | 2.9375 |
| CoO/NiO@bio-char | 232.55 | 0.1922 | 3.3062 |
The adsorption efficiency of Zn(II) and Cd(II) is strongly influenced by the mesoporous structure of the material. The mesopores in the NiO/CoO@biochar composite (average diameter: 3.31 nm) are comparable to the hydrated radii of Zn(II) (∼4.30 Å) and Cd(II) (∼4.26 Å),35 facilitating effective diffusion of metal ions into the porous network and ensuring strong adsorption interactions. If the micropore size were significantly smaller, steric hindrance would limit ion accessibility, reducing adsorption efficiency. Conversely, excessively large pores could lead to weaker binding interactions, lowering selectivity for target metal ions. This study has already established that the NiO/CoO@biochar composite possesses a mesoporous structure with an average pore diameter of 3.31 nm, as determined from BET analysis. Given that mesoporosity is the dominant characteristic, the micropore and mesopore volume differentiation was not explicitly calculated. Instead, the total pore volume measured at P/P0 = 0.99 provides a reliable estimate of the material's porosity, which is sufficient for understanding its adsorption behavior. The adsorption efficiency of Zn(II) and Cd(II) is strongly influenced by the mesoporous structure, as the hydrated ionic radii (Zn(II) ∼4.30 Å, Cd(II) ∼4.26 Å) are comparable to the composite's mesopore size. This ensures effective diffusion of metal ions without significant steric hindrance. Moreover, the adsorption mechanism involves both electrostatic attraction and surface complexation, further confirming that steric limitations are not a major factor. Therefore, focus of this study was on ensuring a balance between mesoporous structure and functionalized active sites for adsorption rather than explicitly determining micropore and mesopore sizes separately. The incorporation of metal oxides altered the biochar's structure, enhancing ion accessibility while maintaining an optimal pore environment for adsorption. Thus, it can be concluded that the optimized mesoporous structure of the composite plays a crucial role in governing adsorption kinetics and capacity.
Although blank biochar exhibits a high surface area, large pore volume, and a strongly negative surface charge, its adsorption capacity for Zn(II) and Cd(II) is limited by the relatively low density of specific active sites required for strong chemical interactions with metal ions. In contrast, the fabrication of the composite by incorporating NiO and CoO nanoparticles onto the biochar introduces additional reactive sites—such as surface hydroxyl groups—that can form strong inner-sphere complexes with heavy metal ions. These metal oxide sites not only enhance the chemical affinity for Zn(II) and Cd(II) but also create a synergistic effect where the inherent porosity of the biochar facilitates the rapid diffusion of ions to these active sites. Consequently, even though the composite displays a reduction in overall surface area and pore volume compared to the bare biochar, the enhanced chemical functionality and specific binding sites result in a markedly improved adsorption capacity. This approach has been demonstrated in previous studies, which reported that metal oxide-modified biochars significantly outperform unmodified biochar in heavy metal removal.33 Adsorption of metals in oil sands process water by a biochar/iron oxide composite: influence of the composite structure and surface functional groups.
A detailed pore size distribution (PSD) analysis revealed distinct differences between the two materials. The blank biochar exhibited a unimodal distribution, emphasizing a uniform pore structure, while the NiO/CoO composite showed a bi-modal profile (Fig. 5). This mixed pore system, characterized by both mesopores and micropores, is particularly advantageous for adsorption as it accommodates a range of ion sizes and provides diverse pathways for diffusion. The composite's bi-modal nature reflects the synergistic integration of metal oxides into the biochar framework, enhancing its structural complexity and adsorption potential (Fig. 5). The total pore volume of the NiO/CoO composite was found to be 0.1922 cm3 g−1, compared to 0.6487 cm3 g−1 for the blank biochar, aligning with the observed reduction in surface area (Table 1). The volume of micropores and mesopores was not explicitly calculated, as the dominant mesoporosity of the composite is well-characterized by its BET surface area and BJH pore size distribution. However, the total pore volume measured at P/P0 = 0.99 provides a reliable estimate of the overall porosity, which effectively reflects the material's adsorption potential. More detailed micropore-mesopore volume differentiation, such as NLDFT modeling, could provide additional insights, but given the composite's structural characteristics, such calculations were not deemed essential for this study. The observed differences in surface and pore characteristics underscore the influence of metal oxide loading on biochar's textural properties. These modifications not only alter the physical structure but also enhance the material's functional capabilities, making the NiO/CoO composite a highly effective adsorbent for heavy metal removal.
The average particle size of 830 nm for the composite reflects the successful integration of metal oxides particles which obviously have smaller sizes than those of biochar into its matrix. Hence, such addition of metal oxides of smaller particles in size to biochar structure promotes better dispersion and homogeneity, which is advantageous for adsorption applications (Table 2). Zeta potential measurements provided insights into the surface charge and stability of the materials. The NiO/CoO@biochar composite exhibited a zeta potential of −13.1 mV, while the blank biochar showed a slightly more negative value of −16.7 mV (Table 2). These values indicate moderate colloidal stability, sufficient to prevent aggregation during adsorption processes.23,24 The incorporation of nickel and cobalt oxides altered the surface chemistry of the composite, slightly reducing its negative charge but improving its capacity to interact with positively charged metal ions such as Zn(II) and Cd(II).
| Sorbent name | DLS analysis, nm | Zeta potential, mV |
|---|---|---|
| Blank biochar | 1900 | −16.7 |
| NiO/CoO@biochar | 830 | −13.1 |
These characteristics—a narrower particle size distribution and a stable, moderately negative surface charge—enhance the composite's dispersion in aqueous solutions and its ability to capture heavy metal ions. The findings underscore the role of surface charge in facilitating electrostatic interactions, further contributing to the composite's high adsorption efficiency and stability in practical applications.
3.2. Sorption properties
The effect of pH on Zn(II) and Cd(II) adsorption was studied within the range of 2.0–8.0 at a fixed sorbent dose (3.0 g L−1), shaking time (240 min), initial concentration (40 mg L−1), and room temperature. As shown in Fig. 7(I), the sorption capacity increased with pH, reaching maximum values at pH 6.0 (10.7 mg g−1 for Zn(II) and 8.5 mg g−1 for Cd(II)). Beyond pH 6.0, the adsorption capacity declined due to the formation of insoluble metal hydroxide species such as ZnO and Cd(OH)2, which reduce the availability of free metal ions for adsorption. The speciation of Zn(II) and Cd(II) in solution at varying pH levels (Fig. S1†) and the zeta potential data for the sorbent (Table 2) help explain the observed adsorption trends.
At low pH values, the high concentration of hydrogen ions (H+) competes with Zn(II) and Cd(II) for active adsorption sites, leading to suppressed adsorption efficiency.8–10 As the pH increases, the hydrogen ion concentration decreases, allowing for stronger electrostatic interactions between the negatively charged sorbent (−13.1 mV zeta potential) and cationic species (Zn2+, ZnOH+, and Cd2+). The measured negative zeta potential (Table 2) confirms that the NiO/CoO@biochar composite maintains a negatively charged surface, enhancing metal ion attraction in the optimal pH 4–6 range.
To assess potential ion exchange effects, the equilibrium pH values were recorded post-adsorption, revealing a slight pH increase from 6.00 to about 6.03 for both metal ions. These minor pH shifts suggest that adsorption was the dominant removal mechanism rather than precipitation, ensuring that metal uptake primarily occurred via electrostatic attraction and surface complexation. However, at pH > 6.0, the predominant presence of insoluble metal hydroxides (ZnO and Cd(OH)2) reduces adsorption affinity, a trend consistent with the surface complexation formation (SCF) theory.36,37 Similar findings have been reported by Gupta et al.,2 El-Said et al.,3 and others,7–10 supporting the conclusion that electrostatic interactions and surface complexation are the primary adsorption mechanisms within the optimal pH range (4–6). The stable equilibrium pH values further confirm that metal precipitation effects were minimal, ensuring that adsorption, rather than chemical precipitation, governed metal removal efficiency.
In respect to the impact of sorbent dose on adsorption process, a set of experiments were evaluated over a range of 0.5–4.0 g L−1. As illustrated in Fig. 7(II), the adsorption percentage of Zn(II) and Cd(II) increased with higher sorbent doses, attributed to the greater surface area and availability of active sites.8–10 However, the adsorption capacity (qeexp) decreased significantly from 41.9 to 9.8 mg g−1 for Zn(II) and from 16.2 to 7.5 mg g−1 for Cd(II) as the sorbent dose increased. The slight decrease in adsorption capacity at higher sorbent dosages can be attributed to the adsorbent-dose effect, where the excess of available active sites relative to metal ion concentration leads to underutilization of the sorbent's adsorption potential. Additionally, at higher doses, particle aggregation may reduce effective surface area, limiting accessibility to adsorption sites.2,3 The selected 3.0 g L−1 dose balances high removal efficiency and accurate kinetic modeling, ensuring adsorption equilibrium is achieved within a measurable timeframe. Furthermore, distribution coefficient (Kd) values, calculated from experimental data, showed significant variations with changing sorbent doses (Fig. S2†). This indicates the strong complexation of Zn(II) and Cd(II) ions with the functional groups on the CoO/NiO@bio-char surface.38–40 These results highlight the critical role of optimizing both solution pH and sorbent dose to maximize adsorption performance. Understanding the interplay of these parameters provides valuable insights into designing efficient sorption systems for heavy metal removal.38
 | ||
| Fig. 8 (I) The kinetic curve of Zn(II) and (II) Cd(II) uptake process (solution pH 5.9; temperature of 25 ± 1 °C; initial concentration of 40 mg L−1; sorbent dosage of 3.0 g L−1). | ||
The results of an experiment were analyzed using different kinetic models, namely the Pseudo-first-order, pseudo-second-order, and Weber and Morris (intraparticle diffusion) models, to understand the adsorption process better.25–28 The terms of the models are shown in Table 3. The data gathered showed that the pseudo-first-order and pseudo-second-order models had a strong correlation coefficient (R2 ≥ 0.95) for both metal ions. However, the pseudo-second-order model had the lowest average relative error (ARE = 2.1, and 1.3 for Zn(II) and Cd(II) respectively), indicating that it fits better for the adsorption process. This suggests that the uptake of Zn(II) and Cd(II) is a chemisorption process and that an electron sharing or transfer process occurs during the interaction between both metal ions and the CoO/NiO@bio-char sorbent.25–28 Notably, the calculated adsorption capacity equals 15.5, and 11.7 mg g−1 for Zn(II), and Cd(II) respectively, and the rate constant for Zn(II) and Cd(II) is 0.009 and 0.003 min−1 respectively, which reflects a higher affinity for Zn(II) ions than Cd(II) ions. In addition, the initial sorption rate (h = 2.1 and 0.5 mol g−1 h−1 for Zn(II) and Cd(II) respectively), and the half-equilibrium time (t0.5 = 7.4 and 24.8 h for Zn(II) and Cd(II) respectively) reveal that Zn(II) adsorption process exhibit a faster rate of reaction than Cd(II) adsorption process which is consistent with the experimental findings.
| Zn(II) | Cd(II) | |
|---|---|---|
| Pseudo first-order model | ||
| q1 (mg g−1) | 14.8 | 10.7 |
| k1 (min−1) | 0.1 | 0.03 |
| ARE | 5.6 | 7.07 |
| R2 | 0.95 | 0.99 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Pseudo second-order model | ||
| q2 (mg g−1) | 15.5 | 11.7 |
| k2 (min−1) | 0.009 | 0.003 |
| h (mol g−1 h−1) | 2.1 | 0.5 |
| t1/2 (h) | 7.4 | 24.8 |
| ARE | 2.1 | 1.30 |
| R2 | 0.99 | 0.99 |
The same kinetic attitude (chemisorption process) was depicted for Zn(II), and Cd(II) removal from aqueous solution using other sorbents such as Wood ash amended biochar,1 potato starch phosphate (PSP) polymer,2 Rice husk ash (RHA),3,18 cross-linked reusable polystyrene adsorbents (PS-NH-DCDEE, and PS-NH-TCE),7 bentonite/polypyrrole (B-Ppy) composite,12 and Thermal power plant fly Ash.38
Weber–Morris model is used for analyzing the experimental results to explore the diffusion mechanism of Zn(II), and Cd(II) ions using CoO/NiO@bio-char.25–28 The model terms were evaluated from the plot of sorption capacity (qt) against time0.5 (Fig. S3†) and displayed in Table S2.† As can be seen, both metal ions exhibit a multi-linear relationship, which deduced that the adsorption process is controlled by multiple mechanisms (i.e. chemical and physical mechanisms).16–18 Initially, the adsorption process is characterized by the presence of many free surface active sites and a high ion concentration, resulting in low boundary resistance and a high rate of reaction, which means a fast process.16–18 So far, chemical reaction mechanisms such as complexation mechanisms are involved during this stage. Beyond reaction equilibrium, the boundary layer resistance is increased, and most of the surface active sites are occupied with the ions therefore, the metal ions diffuse inside the pores of the sorbent, leading to a sluggish adsorption process.16,18 Physical adsorption mechanisms, such as the intra-particle diffusion mechanism, take place at this stage.16,18
The uptake isotherm is profoundly dissected by analyzing the experimental results using the following isotherm models; Langmuir, Freundlich, D–R, and Sips models.27–30 The terms of the isotherm models were evaluated and displayed in Table 4. The displayed data shows that the Zn(II) and Cd(II) ions sorption process obeys the Langmuir model whereas it exhibits the lowest (ARE) values (3.7, and 1.1) and the highest (R2) values (0.99) for both Zn(II) and Cd(II) ions respectively. This indicates that the removal is a monolayer process, and CoO/NiO@bio-char has a uniform and homogenous surface, which is consistent with the SEM analysis (Fig. 4). Similar isotherm behavior (i.e. monolayer, and homogeneous process) was elucidated for Zn(II), and Cd(II) adsorption from aqueous solution using other sorbents such as Wood ash amended biochar,1 potato starch phosphate (PSP) polymer 2, Rice husk ash (RHA),3,18 cross-linked reusable polystyrene adsorbents (PS–NH-DCDEE, and PS-NH-TCE),10 bentonite/polypyrrole (B-Ppy) composite,12 and Thermal power plant fly Ash.37
| Zn(II) | Cd(II) | Zn(II) | Cd(II) | ||
|---|---|---|---|---|---|
| Langmuir model | Freundlich model | ||||
| qm (mg g−1) | 18.9 | 10.2 | 1/nF | 0.1 | 0.1 |
| kL (L mg−1) | 1.0 | 0.7 | kF (mg g−1) (mg L−1) | 11.5 | 7.9 |
| ARE | 3.72 | 1.11 | ARE | 14.89 | 7.19 |
| R2 | 0.99 | 0.96 | R2 | 0.79 | 0.76 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| D–R model | Sips model | ||||
| qD (mg g−1) | 18.4 | 10.0 | qS (mg g−1) | 18.9 | 10.2 |
| B (mol2 kJ−2) | 0.4 | 0.5 | kS (L mg−1) | 1.0 | 0.7 |
| Ead | 1.2 | 0.99 | mS | 1.0 | 1.0 |
| ARE | 9.5 | 1.98 | ARE | 3.93 | 1.12 |
| R2 | 0.97 | 0.99 | R2 | 0.99 | 0.99 |
The sorption isotherm provides two important insights: (I) the maximum sorption capacity achieved at the sorbent saturation, and (II) the sorbent affinity for the metal ions.35,40 The data in Table 4 shows that The sorption capacities at saturation of monolayer (i.e., qm) are 18.9 and 10.2 mg g−1 for Zn(II) and Cd(II) respectively, which is roughly consistent with the experimental values of maximum sorption capacities (18.6 mg/g for Zn(II), and 10.0 mg/g for Cd(II)). The affinity coefficient (i.e., kL) for Zn(II) is higher than that for Cd(II) which confirms the higher tendency of the applied sorbent towards Zn(II) than Cd(II). The same behavior (higher affinity to Zn(II) ions than Cd(II) ions) was recognized by other sorbents such as Wood ash amended biochar,1 potato starch phosphate (PSP) polymer,2 Rice husk ash (RHA),3,18 cross-linked reusable polystyrene adsorbents (PS-NH-DCDEE, and PS-NH-TCE),10 bentonite/polypyrrole (B-Ppy) composite,12 and Thermal power plant fly Ash37 during the adsorption of heavy metal ions from aqueous solution.
Many factors, including hydration ion radii, ion hydrolysis constants, metal ion electronegativity, and hydration energy, have a significant impact on the sorbents' affinity for ions.35,42,43 The effect of ionic radii and the hydration energy (ΔHhyd) on the Zn2+, Cd2+, and Pb2+ selectivity of EDTA-modified maize cob was also mentioned by Igwe and Abia.44 According to Mihajlović et al.45 the hydrated cation radii, hydration energy, and electronegativity of the ions were shown to be correlated with the affinity of natural and modified zeolite towards various metals, with Pb2+ > Cd2+ > Zn2+ being the most preferred. According to Page et al.,46 the cation hydration energy has an impact on the REE's strength over Al and Fe(III) adsorption onto a sulfonic acid resin.
In this study, hydrated zinc and cadmium metal ions were compared in terms of their ion radius (CdH2O62+ (0.96 Å); and ZnH2O62+ (0.74 Å)) and hydration energy (Zn(II) (−2046 kJ Mol−1), and Cd(II) (−1807 kJ Mol−1)).35,47,48 It was observed that the sorption affinity is inversely proportional to the ion radius, meaning smaller ions are easier to penetrate an adsorbent. This observation has also been reported previously by Salam et al.10 Based on hydration energy, Zn(II) is expected to have a greater sorption strength than Cd(II). This matches the obtained data in the study, which shows that the sorption capacity is positively impacted by an increase in metal ions' hydration energy. The same observation has been reported by Igwe and Augustine.44 The electronegativity values of zinc and cadmium, ions are reported as 1.55 and 1.90 respectively. So the biochar affinity towards the capture of both Zn and Cd ions could be non-correlated to this parameter where it has no critical influence on its tendency to sorption of these metal ions. The hydrolysis constant (pKa) of the metal ions could be sequenced as Cd(II) 10.01 > Zn(II) 9.15. This means that the affinity of CoO/NiO@bio-char is almost affected positively with the decrease of the metal ion hydrolysis constant. This may be due to that the lower the hydrolysis constant of an ion, the higher its tendency to form hydrolysis products, and in turn more competition toward charged sorbent surfaces.10 This behavior disagrees with Nayak et al.,16 and Mihajlović et al.45 According to the Pearson Hard/Soft Acids and Bases (HSAB) theory, metal ions are classified based on their behavior as electron acceptors (acids) or electron donors (bases).35,42 This classification helps interpret the strength of metal binding. Strong acids preferentially react with strong bases, while weak acids react with weak bases.49 Zinc is considered a borderline acid, and cadmium is considered a soft acid based on the HSAB theory, with their Lewis acid strengths being 0.405 and 0.32, respectively.35,42 This means that the function group of the CoO/NiO@bio-char will have a higher affinity for zinc than for cadmium ions. The experimental results obtained in this study are in harmony with the HSAB theory, indicating that zinc ions exhibit stronger binding than cadmium ions.
It is noteworthy that the adsorption isotherm of Zn(II), and Cd(II) ions onto the blank bio-char obeyed to Langmuir isotherm model (Table S3†), which illuminated that both blank bio-char and CoO/NiO@bio-char exhibits similar isotherm behavior (i.e. monolayer, and homogeneous process). The comparison between the affinity of both carbonaceous species to Zn(II), and Cd(II) ions declares that The adsorption capacity of the composite was significantly higher than that of biochar alone, with maximum capacities of 18.9 mg g−1 for Zn(II) and 10.2 mg g−1 for Cd(II), compared to 7.0 mg g−1 and 8.5 mg g−1 for biochar, respectively. Although biochar has a higher surface area and pore volume (Table 1), the composite exhibits higher adsorption capacity due to the presence of metal oxides, which provide additional active sites for adsorption. The metal oxides also enhance the electrostatic interactions between the adsorbent and the metal ions, leading to improved adsorption performance. This illuminates that the activation process improves the texture properties of the blank bio-char. This is consistent with the insights from the sorbent characterization results (Section 3.1). Nevertheless, blank biochar sorbent exhibits a higher affinity to cadmium ions than zinc ions. This performance could be attributed to strong dependency on its function groups (acting as bases), during the adsorption process, to attract the species of Cd ions which have stronger acidic strength than Zn ions.
It is possible to examine the sorption process profile by using the dimensionless equilibrium parameter RL, which is equal to 1/(1 + KLCo).27–30 The sorption can be categorized as irreversible (RL = 0), linear (RL = 1), unfavorable (RL > 1), or favorable (0 < RL < 1).27–30 The values of RL were assessed and are presented in Fig. S4.† The data demonstrates a favorable sorption process where RL values vary between 0.01 to 0.03 for Zn(II), and from 0.02 to 0.06 for Cd(II) for all initial concentrations that were investigated.
The Sips equation, which is based on the Langmuir–Freundlich concept, includes a third adjustable parameter that is meant to enhance the accuracy of curve fitting.27–30 This is supported by the lower ARE and higher R2 values obtained. The calculated sorption capacity for Zn(II) and Cd(II) (18.9 and 10.2 mg g−1) aligns with the calculated sorption capacity from the Langmuir model. The Freundlich model represents multi-layer adsorption on heterogamous surfaces.27–30 It has the lowest coordination coefficient and the highest ARE coefficient. However, it fits well at a low initial concentration as shown in Fig. 9. The term (1/n) can be used to determine the adsorption intensity of a substance. A sorption process is considered favorable if the value of 1/n is less than one but greater than zero. If 1/n equals one, the process is irreversible. If 1/n is greater than one, then the process is unfavorable.27–30 Table 4 indicates favorable sorption for Zn(II) and Cd(II) with 1/n values of 0.1. The same conclusion was reached using the Langmuir dimensionless parameter RL.
The Dubinin–Radushkevich model, which follows the pore-filling mechanism, suggests that a multilayer adsorption process through van der Waals's forces could occur during the Zn(II) and Cd(II) ions adsorption process.27–30 This is supported by proper coordination and ARE coefficient values. The maximum sorption capacity (qad) was found to be 18.4 and 10.0 mg g−1 for Zn(II) and Cd(II) respectively, which is close to the maximum sorption capacity calculated from the Langmuir model. The mean free energy (Ead, kJ mol−1) can be used to determine the nature of the sorption process. E < 8 kJ mol−1 reflects a physisorption process, whereas 8 < E < 16 kJ mol−1 indicates a chemisorption process.27–30 In this case, the data in Table 4 suggests a physisorption process since E ≤ 1.2 kJ mol−1. Finally, isotherm investigation confirms that both metal ions' sorption process is controlled by multiple reaction mechanisms, which is consistent with the achievement of the Webber-Morris kinetic model.
To further evaluate the adsorption performance of the NiO/CoO@biochar composite, a comparative analysis with previously reported adsorbents was conducted and is summarized in Table 5. This table presents a direct comparison of adsorption capacities under various experimental conditions, including metal ion concentration, pH, contact time, and temperature, providing a meaningful assessment of our composite's efficiency. The results demonstrate that the NiO/CoO@biochar composite exhibits a competitive or superior adsorption capacity compared to other biochar-based and metal-oxide-modified adsorbents. This enhancement can be attributed to the synergistic effect of metal oxide incorporation, which increases surface functional groups, enhances electrostatic interactions, and improves adsorption affinity toward Zn(II) and Cd(II) ions. Unlike a direct correlation between BET surface area and adsorption capacity, which may not fully account for adsorption efficiency, the combination of textural properties and surface chemistry plays a critical role in metal ion uptake. Thus, the comparative analysis in Table 5 effectively contextualizes our findings within the literature and highlights the adsorption potential of NiO/CoO@biochar under practical conditions.
| Sorbent type | Co, mg L−1 | pH | Time, h | qe, mg g−1 | R |
|---|---|---|---|---|---|
| Zn(II) | |||||
| Rice husk ash (RHA) | 10–100 | 6.0 | 6 | 3.5 | 3 |
| Natural foxtail millet shell | 25–300 | 5.0 | 2 | 10.6 | 6 |
| Wood ash amended biochar | 10–150 | 4.7 | 48 | 12.1 | 8 |
| KMnO4/hematite te modified biochar | 5–100 | 6.0 | 24 | 14.2 | 9 |
| Bentonite/polypyrrole composite | 1–20 | 5.0 | 1 | 6.6 | 17 |
| Rice husk ash | 10–100 | 6.0 | 5 | 3.0 | 18 |
| Durian peel | 10–200 | 8.0 | 4 | 36.6 | 22 |
| Thermal power plant fly ash | 1–20 | 6.0 | 2 | 1.4 | 35 |
| Blank bio-char | 20–120 | 6.0 | 4 | 7.0 | PW |
| CoO/NiO@bio-char | 18.9 | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Cd(II) | |||||
| Rice husk ash (RHA) | 10–100 | 6.0 | 6 | 2.1 | 3 |
| Natural foxtail millet shell | 25–300 | 5.0 | 2 | 12.5 | 6 |
| Wood ash amended biochar | 10–150 | 4.7 | 48 | 10.2 | 8 |
| KMnO4/hematite modified biochar | 5–100 | 6.0 | 24 | 17.3 | 9 |
| Bentonite/polypyrrole composite | 1–20 | 5.0 | 1 | 3.2 | 17 |
| Rice husk ash | 10–100 | 6.0 | 5 | 5.8 | 18 |
| Thermal power plant fly ash | 1–20 | 8.0 | 2 | 4.7 | 35 |
| Blank bio-char | 20–120 | 6.0 | 4 | 8.5 | PW |
| CoO/NiO@bio-char | 10.2 | ||||
| ΔG (kJ mol−1) | ΔH (kJ mol−1) | ΔS (J mol−1 K−1) | ||||
|---|---|---|---|---|---|---|
| 20 °C | 30 °C | 40 °C | 50 °C | |||
| Zn(II) | −20.9 | −21.6 | −23.5 | −25.0 | 29.6 | 169.5 |
| Cd(II) | −15.7 | −16.2 | −16.9 | −17.9 | 10.0 | 86.3 |
| Sorbent name | DLS analysis, nm | Zeta potential, mV | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| Blank charcoal | 1900 | 3560 | −16.7 | −6.4 |
| CoNi@charcoal | 830 | 1798 | −13.1 | −1.9 |
Additionally, Raman spectroscopy of the spent adsorbent (Fig. 11(II)) provides further evidence of chemisorption through noticeable spectral shifts and the emergence of new vibrational bands. Compared to the fresh CoO/NiO@bio-char, the spent adsorbent exhibits additional peaks below 500 cm−1, which correspond to Cd–O and Zn–O bond formations, indicating strong chemical interactions between the metal ions and the adsorbent surface. Moreover, a newly observed peak around 1000 cm−1 is attributed to possible metal–metal interactions (Ni–Zn, Co–Zn, Ni–Cd, or Co–Cd) or complexation between biochar functional groups and the adsorbed metal ions. These spectral changes confirm that the adsorption process is not purely physical but involves chemical bonding, reinforcing the findings from FTIR and kinetic modeling that suggest an inner-sphere complexation mechanism. The disappearance or broadening of characteristic D- and G-band features further supports the structural modifications induced by metal ion coordination, highlighting the role of chemisorption in the removal process.
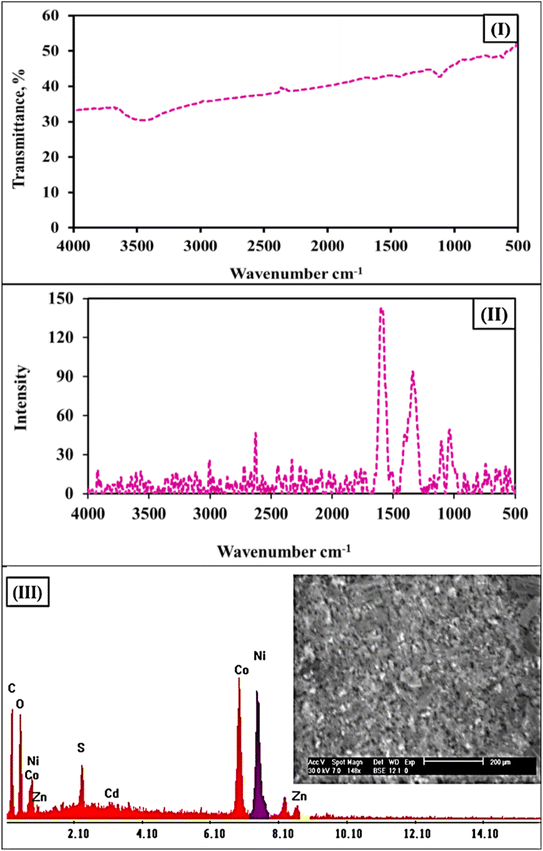
In the present study, the zeta potential analysis declares that the synthesized CoO/NiO@bio-char sorbent exhibits a negatively charged surface value, while the speciation of Zn(II) and Cd(II) ions over the different pH values of the aqueous solution (Fig. S1†) displayed a cationic species (mainly, Zn2+, ZnOH+, and Cd2+), deducing the occurrence of electrostatic interaction between the negative charge on surfaces of the carbonaceous material and the positive cation species. This proposal is confirmed by the zeta potential analysis of the carbonaceous sorbent after the adsorption process, whereas the charge negativity of the sorbent surfaces decreased from −13.1 to −1.9 mV due to their uptake of positive divalent cations. On another side, the obvious boost in the particle size after the adsorption process confirms the outer-sphere complexes between the sorbent's surfaces and Zn/or Cd ions. Furthermore, the capture of both metal ions could take place via inner-sphere complexes with the present hydroxyl function group on the surface of the composite Carbonaceous sorbent. The surface complexation mechanism could be confirmed by the noted variations in FTIR analysis for the CoO/NiO@bio-char before and after the uptake process (Fig. 2, and 11 respectively). Particularly, the indicative peak of the –OH group in FTIR spectra showed obvious changes after the adsorption process (Fig. 11), compared to the corresponding fresh adsorbent (Fig. 2), reflecting the taking part of the complex formation mechanism in the removal process. This mechanism is consistent with the findings of the kinetic analysis results, which revealed that the Pseudo-second order kinetic model is the best fit for describing the adsorption process, which reflects the chemisorption interaction through the inner surface complexation mechanism. Additionally, the newly observed peaks in Raman analysis of the spent adsorbent could further confirm chemisorption occurrence as well as the presence of metal–metal interaction between the sorbent particles and contaminating metal cations. On the other side, adsorption through the diffusion of Zn and Cd ions within the sorbent pores (pores trapped mechanism) is also present owing to the smaller radii of these ions than average pores radii of the adsorbent, as presented in Table 1 (surface characteristics).
The morphology and texture investigation of the CoO/NiO@bio-char sorbent explores that it has a uniform and porous surface. This indicates that physical sorption mechanisms could play a role, in addition to chemisorption mechanisms, particularly prolong the equilibrium state. This finding is consistent with the insights from the W-M kinetic model which explores that the intraparticle diffusion of the metal ions over the sorbent pores contributes to the adsorption process by extending the equilibrium time. SEM (Fig. 11) analysis of the spent CoO/NiO@bio-char confirmed this finding, as many of the crevices on the sorbent surface disappeared (Fig. 3). The contribution of the physical mechanism in the adsorption process could also be recognized from the thermodynamic and D-R isotherm analysis. The ΔH° values for both metal ions were found to be <40 kJ mol−1 (Table 6), indicating that van der Waals force interaction mechanisms take place during the adsorption process. D–R isotherm model results reveal that the values of Ead are <8 kJ mol−1 (Table 4), which confirms that the metal ions adsorption occurs through a physisorption process, applying van der Waals force.
In conclusion, the CoO/NiO@bio-char sorbent interacts with Zn(II) and Cd(II) ions through both physisorption and chemisorption (multiple mechanisms), depending on the adsorption phase. The pseudo-second-order kinetic model suggests an interaction-driven process, where electrostatic forces and possible surface complexation contribute to adsorption kinetics. However, thermodynamic parameters indicate that the overall process is governed by physisorption, as suggested by the relatively low activation energy (Ea) and enthalpy change (ΔH). This dual-mode behavior is common in biochar-based adsorbents, where fast kinetics (resembling chemisorption) and low binding energies (indicative of physisorption) coexist. This suggests that initial metal ion uptake is rapid and controlled by electrostatic attractions, potentially involving outer-sphere complexation or hydrogen bonding. However, as equilibrium is approached, weak physisorption forces dominate, resulting in an adsorption process that is primarily physical in nature rather than chemically irreversible. The proposed sorption mechanisms are illustrated in Fig. 12. The same mechanisms have been observed in the adsorption of Zn(II), and Cd(II) ions using potato starch phosphate (PSP) polymer,2 Wood ash amended bio-char,8 KMnO4/hematite modified corn straw bio-char (MnFeB),9 multi-walled carbon nanotubes,10 KMnO4-activated spinach waste bio-char,16 Chitosan-modified MXene (CSMX),37 and Magnesium/coconuts shell activated carbon composite (Mg-AC).51
3.3. Desorption and recycling investigation
Investigating the desorption and reusability of CoO/NiO@bio-char is essential to assess its practicality and sustainability as an adsorbent. This study evaluated the efficiency of different acidic solutions—sulfuric acid, hydrochloric acid, and nitric acid at a concentration of 1.0 M—for desorbing Zn(II) and Cd(II) ions from the loaded sorbent. Experimental parameters were maintained at a sorbent dose of 3.0 g L−1, shaking time of 240 minutes, and room temperature. The results, illustrated in Fig. 13, revealed that all acidic solutions facilitated desorption, with sulfuric acid emerging as the most effective eluent, achieving elution yields of 95.2% for Zn(II) and 93.1% for Cd(II). The superior performance of sulfuric acid may be attributed to its ability to effectively disrupt metal-sorbent interactions, making it a viable candidate for industrial applications. | ||
| Fig. 13 Zn(II), and Cd(II) desorption from loaded CoO/NiO@bio-char sorbent using different solutions (3.0 g L−1, room temperature; 240 min). | ||
The recycling performance of CoO/NiO@bio-char was further examined across five successive sorption-desorption cycles, as summarized in Table S4.† The CoO/NiO@biochar composite demonstrated high regeneration efficiency, maintaining an average adsorption efficiency of 80.8% for Zn(II) and 63.6% for Cd(II) across five cycles. The desorption efficiency remained stable at >90% using sulfuric acid as an eluent. While a slight decrease in adsorption capacity (∼5%) was noted, this is within an acceptable range for industrial applications. The minimal change in adsorption and desorption efficiencies confirms the structural stability of the composite, indicating that the active phase remains intact even after multiple cycles. These findings highlight the CoO/NiO@bio-char composite's resilience and efficiency in heavy metal ion recovery, aligning with the global demand for sustainable and reusable materials in wastewater treatment. By demonstrating high performance across multiple cycles, this material proves to be a promising candidate for scalable and environmentally friendly remediation technologies.
4. Conclusion
The synthesis of CoO/NiO@bio-char composites via pyrolysis and ultrasonic-assisted methods represents a significant advancement in sustainable materials for environmental applications. This study has demonstrated that the novel composite exhibits superior adsorption capabilities for Zn(II) and Cd(II) ions, achieving maximum adsorption capacities of 18.9 mg g−1 and 10.2 mg g−1, respectively. Comprehensive analyses have validated that the adsorption mechanism is primarily governed by chemisorption, supported by electrostatic interactions, surface complexation, and intra-particle diffusion mechanisms. Additionally, thermodynamic investigations confirmed the endothermic, spontaneous, and energetically favorable nature of the adsorption process. The findings underscore the potential of combining renewable biomass with innovative synthesis techniques to produce efficient adsorbents, aligning with the principles of green chemistry. The developed composite not only exhibited high reusability over multiple sorption-desorption cycles but also demonstrated scalability and versatility for practical wastewater remediation applications. This reflects that the present work contributes valuable insights into the design of functional materials for addressing pressing environmental challenges. The integration of sustainable practices and advanced material science in this study offers a promising pathway for the development of next-generation adsorbents, paving the way for cleaner water resources and a healthier environment.Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request. This includes: raw data: experimental results, adsorption isotherm data, kinetic profiles, thermodynamic evaluations, and characterization outputs (e.g., FTIR, XRD, SEM-EDX, Raman spectroscopy). Processed data: tabulated data used for analysis and graphical representations in the manuscript.Author contributions
Walaa Gado: materials characterization and data curation. Asmaa Morshedy: material synthesis. Ahmed Masoud: methodology, experimental studies, data interpretation. Ard elshifa Mohammed: data curation & interpretation and editing manuscript. Entsar Taha: data curation & interpretation. Adel El-Zahhar: validation, data interpretation, reviewing the original draft. Majed Alghamdi: validation, data curation, reviewing the original draft. Ahmed El Naggar: data curation & interpretation, writing & editing the manuscript, Esraa El-Fawal: data curation & interpretation and taking part in writing/editing the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University for funding this work through Large Research Project under grant number RGP 2/74/45.References
- V. K. Gupta, A. Nayak, B. Bhushan and S. Agarwal, Crit. Rev. Environ. Sci. Technol., 2015, 45, 613–668 CrossRef.
- V. K. Gupta, M. R. Ganjali, A. Nayak, B. Bhushan and S. Agarwal, Chem. Eng. J., 2012, 197, 330–342 CrossRef CAS.
- A. G. El-Said, N. A. Badawy and S. E. Garamon, Environ. Anal. Toxicol., 2018, 8, 1–5 Search PubMed.
- S. Lal, P. Singh, A. Singhal, S. Kumar, A. P. Singh Gahlot, N. Gandhi and P. Kumari, RSC Adv., 2024, 14, 3413–3446 RSC.
- A. Nayak, B. Bhushan, V. Gupta and L. Rodriguez-Turienzo, J. Environ. Chem. Eng., 2016, 4, 4342–4353 CrossRef CAS.
- S. H. Peng, R. Wang, L. Z. Yang, L. He, X. He and X. Liu, Ecotoxicol. Environ. Saf., 2018, 165, 61–69 CrossRef CAS PubMed.
- M. Dardouri, F. Ammari, A. BelHadj Amor and F. Meganem, Mater. Chem. Phys., 2018, 216, 435–445 CrossRef CAS.
- S. Cairns, S. Chaudhuri, G. Sigmund, I. Robertson, N. Hawkins, T. Dunlop and T. Hofmann, Environ. Technol. Innov., 2021, 24, 101961 CrossRef CAS.
- X. Kang, N. Geng, Y. Li, X. Li, J. Yu, S. Gao, H. Wang, H. Pan, Q. Yang, Y. Zhuge and Y. Lou, Bioresour. Technol., 2022, 363, 127817 CrossRef CAS PubMed.
- M. A. Salam, G. Al-Zhrani and S. A. Kosa, C. R. Chim., 2012, 15, 398–408 CrossRef CAS.
- M. C. Benalia, L. Youcef, M. G. Bouaziz, S. Achour and H. Menasra, Arab. J. Sci. Eng., 2022, 47, 5587–5599 CrossRef CAS.
- Z. J. Fu, S. K. Jiang, X. Y. Chao, C. X. Zhang, Q. Shi, Z. Y. Wang, M. L. Liu and S. P. Sun, Water Res., 2022, 222, 118888 CrossRef CAS PubMed.
- J. Jayaraman, J. Kumaraswamy, Y. K. S. S. Rao, M. Karthick, S. Baskar, M. Anish, A. Sharma, A. S. Yadav, T. Alam and M. I. Ammarullah, RSC Adv., 2024, 14, 34769–34790 RSC.
- M. Y. Alyapyshev, V. A. Babain, Y. A. Ustynyuk, W.-S. Chen, W.-C. Liang, C.-H. Lee, C. Chen, T. Xu, Y. Li, L. Yang, Z. Xu and Q. Sun, IOP Conf. Ser.:Mater. Sci. Eng., 2017, 167, 012005 Search PubMed.
- K. H. Hama Aziz, F. S. Mustafa, K. M. Omer, S. Hama, R. F. Hamarawf and K. O. Rahman, RSC Adv., 2023, 13, 17595–17610 RSC.
- A. Nayak, B. Bhushan, V. Gupta and P. Sharma, J. Colloid Interface Sci., 2017, 493, 228–240 CrossRef CAS PubMed.
- A. Ben Bouabdallah and N.-E. Djelali, Rev. Roum. Chim., 2015, 60, 321–330 Search PubMed.
- V. C. Srivastava, I. D. Mall and I. M. Mishra, Colloids Surf., A, 2008, 312, 172–184 CrossRef CAS.
- W. Jing, C. Yang, X. Lin, M. Tang, D. Lian, Y. Yu and D. Liu, RSC Adv., 2024, 14, 39995–40005 RSC.
- B. Wang, J. Lan, C. Bo, B. Gong and J. Ou, RSC Adv., 2023, 13, 4275–4302 RSC.
- V. K. Gupta and A. Nayak, Chem. Eng. J., 2012, 180, 81–90 CrossRef CAS.
- M. Ngabura, S. A. Hussain, W. A. W. A. Ghani, M. S. Jami and Y. P. Tan, J. Environ. Chem. Eng., 2018, 6, 2528–2539 CrossRef CAS.
- M. A. Moniem, A. M. A. El Naggar, H. A. El Sayed, M. S. Mostafa, N. M. Khalil and M. E. D. Hassan, Egypt. J. Pet., 2018, 27, 991–995 CrossRef.
- M. M. Ali, S. A. Abedelmaksoud, M. H. Taha, A. M. A. El Naggar, A. S. Morshedy and A. A. Elzoghbi, Radiochemistry, 2020, 62, 204–215 CrossRef CAS.
- Q. Hu, S. Pang and D. Wang, Sep. Purif. Rev., 2022, 51, 281–299 CrossRef.
- J. P. Vareda, J. Mol. Liq., 2023, 376, 121416 CrossRef CAS.
- J. Wang and X. Guo, Crit. Rev. Environ. Sci. Technol., 2023, 53, 1837–1865 CrossRef CAS.
- Y. Wang, C. Wang, X. Huang, Q. Zhang, T. Wang and X. Guo, Chemosphere, 2024, 349, 140736 CrossRef CAS PubMed.
- M. Mozaffari Majd, V. Kordzadeh-Kermani, V. Ghalandari, A. Askari and M. Sillanpää, Sci. Total Environ., 2022, 812, 151334 CrossRef CAS PubMed.
- M. A. Al-Ghouti and D. A. Da'ana, J. Hazard. Mater., 2020, 393, 122383 CrossRef CAS PubMed.
- T. A. Saleh, Interface Sci. Technol., 2022, 34, 65–97 Search PubMed.
- A. N. Ebelegi, N. Ayawei, D. Wankasi, A. N. Ebelegi, N. Ayawei and D. Wankasi, Open J. Phys. Chem., 2020, 10, 166–182 CrossRef CAS.
- J. Song, Z. Huang and M. Gamal El-Din, Chem. Eng. J., 2021, 421, 129937 CrossRef CAS.
- D. R. Abd El-Hafiz, S. A. El-Temtamy, M. A. Ebiad, R. A. El-Salamony, S. A. Ghoniem, A. M. A. El Naggar and T. S. Gendy, Int. J. Hydrogen Energy, 2020, 45, 9783–9794 CrossRef CAS.
- A. F. Abou-Hadid, U. A. El-Behairy, M. M. Elmalih, E. Amdeha, A. M. A. El Naggar, M. H. Taha and A. E. M. Hussein, Biomass Convers. Biorefin., 2022, 14, 10501–10516 CrossRef.
- T. Golgoli, K. Ghanemi and F. Buazar, J. Mol. Liq., 2024, 393, 123680 CrossRef CAS.
- N. R. Azeez, S. S. Salih, M. Kadhom, H. N. Mohammed and T. K. Ghosh, Green Chem. Eng., 2024, 5, 339–347 CrossRef.
- B. R. Venkata, J. Appl. Sci., 2014, 14(13), 1372–1378 CrossRef.
- R. Karthik and S. Meenakshi, Int. J. Biol. Macromol., 2015, 78, 157–164 CrossRef CAS PubMed.
- Y. Hu, X. Wang, Y. Zou, T. Wen, X. Wang, A. Alsaedi, T. Hayat and X. Wang, Chem. Eng. J., 2017, 316, 419–428 CrossRef CAS.
- A. S. Morshedy, M. H. Taha, D. M. Abd El-Aty, A. Bakry and A. M. A. El Naggar, Environ. Technol. Innov., 2021, 21, 101363 CrossRef CAS.
- J. Liao, T. Xiong, L. Ding, Y. Xie, Y. Zhang and W. Zhu, Biochar, 2022, 4, 1–18 CrossRef.
- P. T. Tho, H. T. Van, L. H. Nguyen, T. K. Hoang, T. N. Ha Tran, T. T. Nguyen, T. B. Hanh Nguyen, V. Q. Nguyen, H. Le Sy, V. N. Thai, Q. B. Tran, S. M. Sadeghzadeh, R. Asadpour and P. Q. Thang, RSC Adv., 2021, 11, 18881–18897 RSC.
- J. C. Igwe and A. Augustine, Eclét. Quím., 2007, 32, 33–42 CrossRef CAS.
- M. T. Mihajlović, S. S. Lazarević, I. M. Janković-Častvan, B. M. Jokić, D. T. Janaćković and R. D. Petrović, Chem. Ind. Chem. Eng. Q., 2014, 20, 283–293 CrossRef.
- M. J. Page, K. Soldenhoff and M. D. Ogden, Hydrometallurgy, 2017, 169, 275–281 CrossRef CAS.
- I. Persson, Pure Appl. Chem., 2010, 82, 1901–1917 CAS.
- F. I. Khalili, N. H. Salameh and M. M. Shaybe, J. Chem., 2013, 2013, 586136 CrossRef.
- H. Demey, T. Vincent and E. Guibal, Chem. Eng. J., 2018, 332, 582–595 CrossRef CAS.
- A. Andelescu, M. A. Nistor, S. G. Muntean and M. E. Rădulescu-Grad, Sep. Sci. Technol., 2018, 53, 2352–2364 CrossRef CAS.
- H. Yanagisawa, Y. Matsumoto and M. Machida, Appl. Surf. Sci., 2010, 256, 1619–1623 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Additional experimental details, mathematical models, and relevant supporting figures or tables referenced in the manuscript. See DOI: https://doi.org/10.1039/d5ra00057b |
| This journal is © The Royal Society of Chemistry 2025 |