Open Access Article
Open Access ArticleEvolution in the synthesis of 1,4-benzothiazines over the last decade (2014 to 2024)
Hemant Kumar Rundla
,
Shivani Soni
,
Sunita Teli
,
Shikha Agarwal
 * and
Lokesh Kumar Agarwal
*
* and
Lokesh Kumar Agarwal
*
Department of Chemistry, Mohanlal Sukhadia University, Udaipur, Rajasthan 313001, India. E-mail: shikhaagarwal@mlsu.ac.in; lokeshkumar@mlsu.ac.in; hema00886@gmail.com
First published on 24th February 2025
Abstract
1,4-Benzothiazine (1,4-BT) is a heterocyclic compound consisting of a benzene ring fused with a thiazine ring, incorporating both nitrogen and sulfur atoms. The fusion of the benzene and thiazine frameworks enhances its biological properties, making it a valuable scaffold for designing innovative heterocyclic systems. This versatile and significant member of the heteroarene family bridges synthetic organic chemistry with medicinal, pharmaceutical, and industrial applications. This structural motif demonstrates remarkable potential for accommodating a wide range of substrates and functionalizations, giving rise to diverse biological activities such as antipsychotropic, antiviral, antithyroid, antimicrobial, antifungal, antitubercular, antioxidant, and anti-inflammatory properties. Numerous derivatives have been synthesized as target structures in drug development. This review highlights various synthetic approaches to prepare 1,4-BTs. Well-established methods, such as the reactions of 2-aminothiophenol (2-ATP) with alkenes, enaminones, carboxylic acids, esters, furan-2,3-dione, aroylmethylidene malonate and 1,3-dicarbonyl compounds, are summarized. Additionally, the miscellaneous syntheses of 1,4-BTs were also outlined. These methods have utilized various catalysts, including nanocatalysts and metal-based catalysts, under diverse reaction conditions for efficient synthesis. The deep analysis of the synthesis of 1,4-BTs will grasp the scientific community towards their synthetic aspects and further advances in the field.
1. Introduction
Heterocyclic compounds are a fundamental category of organic compounds that contain atoms such as nitrogen, sulfur, within their ring structures. These compounds are of immense significance due to their abundance in nature and their vital roles in various biological processes.1 Many natural products, such as vitamins, hormones, alkaloids, and antibiotics, are heterocyclic in nature. In addition, heterocyclic compounds are crucial in the pharmaceutical, agricultural, and industrial sectors, where they serve as drugs, herbicides, dyes, and agents for corrosion inhibition and material stabilization.2,3 Out of all the heterocyclic compounds containing nitrogen and sulfur, benzothiazines are significant due to their potent biological activities and their widespread use as a critical structural component in various bioactive molecules.4Benzothiazine is a type of heterocyclic compound prepared by the fusion of a benzene ring with a thiazine ring via a carbon–carbon bond. Thiazines are six-membered heterocyclic structures that incorporate both nitrogen and sulfur in the same ring. They exist in various regioisomeric forms, such as 1,2-benzothiazines, 2,1-benzothiazines, 1,3-benzothiazines, 3,1-benzothiazines, 1,4-benzothiazine (1,4-BT), 2,3-benzothiazines, and 3,2-benzothiazines, all of which have been developed and evaluated for their pharmacological potential5 (Fig. 1). Among them, 1,4-BT (Fig. 2) has drawn increasing worldwide attention for their versatile applications.6,7 Several distinct 1,4-BT have been isolated from different natural sources, including conicaquinones A and B,8 xanthiside,9 pheomelanins,10,11 and xanthizone,12 all of which serve as examples of 1,4-BT derivatives in nature (Fig. 3).
The 1,4-BT ring system is considered a central structure in S, N-heterocycles, due to its wide-ranging pharmacological and biological effects, including antidiabetic,13 anti-hypertensive,14 analgesic,15,16 antifungal,17–19 anti-HCV,20 calcium antagonist,21 antibacterial,22 antioxidant,23 anti-inflammatory,24 antitubercular,25 anticancer,26–32 antipsychotropic,33 immunomodulator,34 antiviral,35 antithyroid,36 cardiovascular activity,37 antimalarial,38 antipyretic,39 herbicidal, and pesticidal39 activities. They also serve as the central structure in various drugs, including antibiotic and cholesterol-lowering drug40 (Fig. 4).41–44 Moreover, they are widely known to serve in the fields of dyes, photographic developers, and ultraviolet absorption.45,46 These moieties are advantageous for synthetic applications, due to the wide substrate scope and chemo-selectivity. They are commonly synthesized by condensing 2-aminothiophenol (2-ATP) with alkene, 1,3-dicarbonyls, enaminones, employing oxidative coupling or other methodologies. In recent decades, significant progress can be seen in the literature for the synthesis and exploration of the biological activities of 1,4-BT derivatives.35,39,46 However, existing reviews have often presented fragmented information, underscoring the need for a comprehensive and cohesive summary of recent advancements. This article provides an overview of various synthetic approaches to 1,4-BT derivatives, focusing on the use of 2-ATP with a range of substrates, including alkenes, carboxylic acids and their derivatives, furan-2,3-dione, enaminones, ketones, epoxides, and 1,3-dicarbonyl compounds. Special emphasis is placed on their synthetic methodologies developed over the past decade, highlighting diverse reaction conditions and strategies employed to construct these biologically significant scaffolds.
2. Synthesis of 1,4-BT derivatives
The synthesis of 1,4-BT derivatives has been widely studied using classical reactions, typically starting from 2-ATP. In this review, we have explored a variety of synthetic routes involving 2-ATP as a major precursor, which is then interacted with diverse substrates like alkenes, acids and their derivatives, ketones, aroylmethylidene malonates, epoxides, furan-2,3-dione, enaminones, 1,3-dicarbonyl compounds, and many more. Additionally, the miscellaneous synthesis of 1,4-BT was outlined with various substrates such as benzothiazole, o-quinone, 2-bromothiophenol, and others.2.1. Reactions of 2-ATP with numerous substrates
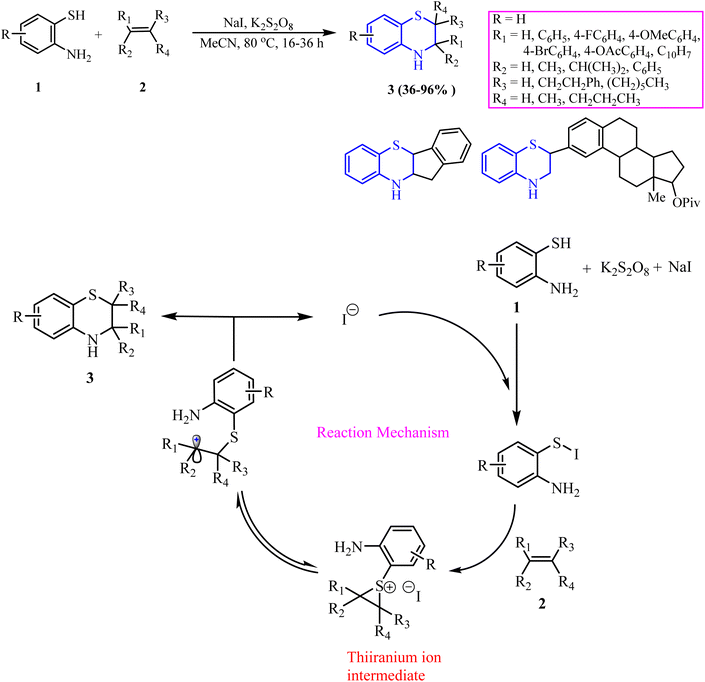
This section focuses on the synthesis of 1,4-BT using 2-ATP with diverse substrates, including alkenes, acids and their derivatives, ketones, epoxides, furan-2,3-dione, enaminones, aroylmethylidene malonates, dihydrodimethoxyfuran and 1,3-dicarbonyl compounds. The reactions are carried out under various conditions using a variety of catalysts such as nanocatalysts, metal catalysts, ionic liquid catalysts, and others.Teli et al.48 developed a method to synthesize 1,4-BT derivatives by reacting 2-ATP (1) and styrene (2) in a micellar nanoreactor medium. In this method, styrene (1 mmol) (2) and NBS (1.5 mmol) were initially dissolved in an aqueous cetylpyridinium bromide (CPB) solution (5 mL). The reaction mixture was stirred at 30 °C for 20 h, forming phenacyl bromides (4) through a hydrobromination–oxidation cascade. Afterward, these phenacyl bromides were reacted with 2-ATP (1 mmol) (1), and the reaction was allowed to proceed for 2–4 h, producing 2-aryl-1,4-BT (5) in yields of 65–72%. The mechanism began with micelle-entrapped NBS facilitating bromination through cation–π interaction, forming a bromonium intermediate (I). A regioselective epoxide ring opening yielded a stable intermediate (II), which was then oxidized to phenacyl bromide (4). This intermediate underwent micelle-assisted condensation with 1,2-ATP, ultimately leading to the formation of 1,4-BT. The merit of this method is that it is environmentally friendly and does not require organic solvents. A demerit of this synthetic route is the long reaction time and necessity of column chromatography for purification (Scheme 2).
The same group50 developed an effective methodology for the synthesis of different kinds of 2-arylbenzothiazine derivatives via a redox condensation reaction between 2-ATP (1) and different types of ketones, catalyzed by TFA (10 mol%) in DMSO solvent at 80 °C for 16 h via Umpolung strategy. When 2-ATP (1) reacted with aryl methyl ketone (1 mmol) (8), the dimeric 2-arylbenzothiazines (9) were formed in moderate to quite excellent yield (58–85%) while substituted aryl propiophenone (8) produced heteropropellanes (10) in good yield (67–79%) but when reacted with α-substituted alkyl phenyl ketones (8), 3,3-dialkyl-1,4-BT (11) were obtained 78–82% (in high yields). The authors tried to synthesize other 1,4-BT using butyrophenone, valerophenone and hexyl phenyl ketone, but the desired products were not form due to their instability in air. However, when R2 group was replaced with an electron-withdrawing group (CN, CO2Et) (12), the target molecules were synthesized in good yields (70–82%) (13). In this protocol, yields of desired product depends upon the size and electronic effect of the alkyl group of the ketones (Scheme 4).
Ya-mei Lin et al.51 synthesized 1,4-BT derivatives using a radical approach. The reaction of 2-ATP (0.375 mmol) (1) and α, β-unsaturated ketones (0.250 mmol) (14) in MeOH with Cs2CO3 (0.125 mmol) as the base at 110 °C for 10 h gave the product in moderate to excellent yields (73–90%) (15) while linear ketones (0.375 mmol) (8) and 2-ATP (1.5 equiv.) (1) were used in DMAc solvent, in 64–87% yields (11). While for reaction of fluoroalkyl ketones (0.375 mmol) (12) with 2-ATP (0.250 mmol) (1), the reaction in DMAc with DBU (0.500 mmol) as the base under air at RT for 24 h resulted in 44–91% moderate to high yields (16). In the proposed mechanism, the process began with in situ generation of thiyl radical (I) from 2-ATP (1) upon heating in the presence of air. This radical quickly reacted with the enol, formed via ketone isomerization, yielding the carbon radical intermediate (II). A subsequent single-electron transfer led to the formation of III, which underwent intramolecular condensation to afford the final product. The pros of this protocol include high efficiency, sustainability through the use of an eco-benign and transition metal-free approach, and yields from moderate to excellent (Scheme 5).
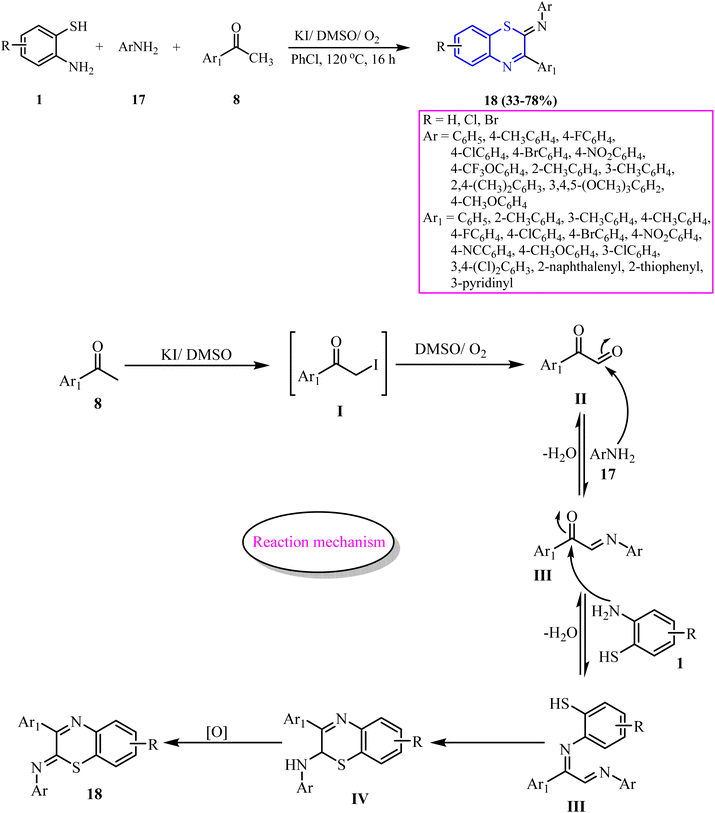
Jinwu Zhao et al.52 reported a three-component, transition-metal-free, aerobic method for synthesizing 1,4-BT via the oxidative cyclization/coupling of 2-ATP (0.2 mmol) (1), anilines (0.3 mmol) (17), and various methyl ketones (0.3 mmol) (8) in KI (0.04 mmol), DMSO (0.8 mmol), and chlorobenzene (1.0 mL) as the solvent, under an oxygen atmosphere. The reaction was conducted at 120 °C for 16 h, yielding low to good results (33–78%) (18). In the proposed mechanism, methyl ketone (8) underwent α-iodination with KI/DMSO to form I, which was oxidized via Kornblum oxidation (oxygenation) to substituted glyoxal (II). Further this reacted with aniline (17) via aldimine condensation to form imine intermediate (III), which subsequently underwent intramolecular nucleophilic addition with 2-ATP (1), yielding annulation intermediate IV. Finally, oxidative dehydrogenation converted IV into the final product (18). However, the method has limitations, that includes the use of column chromatography and a relatively tedious workup process (Scheme 6).
Shajeelammal et al.54 synthesized a novel 1-(3-methyl-4H-benzo[1,4]thiazin-2-yl) ethenone (20) by the reaction of equimolar mixture of 2-ATP (1) and acetylacetone(19) using methanol as a solvent at RT for 2 days to give excellent yield (98%). In these reaction conditions, 2-ATP readily oxidized to bis(o-aminophenyl)disulfide (V) and reacted with acetylacetone to form an enamine intermediate (IX) which was easily cyclized by the nucleophilic attack and formed novel 1,4-BT derivative. The easily available reactants, high yield and operational simplicity are the characteristics of this method but a long reaction time was considered a major limitation of this method (Scheme 7, Method 2).
1-(3-Methyl-4H-1,4-benzothiazin-2-yl)ethenone (20) synthesized by Hadda and co-authors55 through the direct condensation of 2-ATP (40 mmol) (1) and penta-2,4-dione (20 mmol) (19) in ethanol solvent at RT with stirring for 24 h. Finally, the desired product formed with low yield (36%). However, in this process, by-product 2-[(2-aminophenyl)dithio]aniline (12% yield) and 1-(2-methyl-2,3-dihydro-1,3-benzothiazol-2-yl)acetone (5% yield) were also obtained. Low yield and long reaction time are the drawbacks of this protocol. The product showed antifungal activity against the albedinis strain (Scheme 7, Method 3).
M. R. Sangvikar et al.56 developed a convenient one-pot procedure for synthesizing 1,4-BT derivatives (20) through the oxidative cyclocondensation of 2-ATP (10 mmol) (1) and 1,3-dicarbonyl compounds (10 mmol) (19). This reaction was catalyzed by m-CPBA/2-IBX (10 mmol) in acetonitrile as the solvent, carried out at 70 °C for 45–75 min, and produced moderate to excellent yields (49–89%). The mechanism involved the oxidation of 2-ATP to disulfide (V) by oxidants. The resulting disulfide (V) then underwent a reaction with 1,3-dicarbonyl (19), leading to intramolecular cyclization and the formation of 1,4-BT. The plus points of this method are its simplicity, short reaction time, efficient, and the use of an eco-friendly oxidizing agent (Scheme 7, Method 4).
Aminul Islam et al.57 developed a one-pot and efficient method for synthesizing 1,4-BT derivatives (20) by reacting 1 mmol 2-ATP (1) with 1 mmol 1,3-dicarbonyl compounds (19) in the presence of polyethylene glycol (PEG-200) at 80 °C for 4 h. The process is simple, metal-free, eco-friendly, and produced excellent yields (76–98%) under mild conditions. The reaction mechanism started with PEG-200 protonating the carbonyl carbon, intensifying carbonyl electrophilicity. The 2-ATP attacked, forming an imine, which tautomerized and underwent oxidative cyclization to give the final product. PEG-200, a green and biocompatible solvent, played a crucial role in facilitating metal-free organic reactions. However, the need for column chromatography in the purification step is a limitation of this approach (Scheme 7, Method 5).
Arun Goyal58 synthesized 1,4-BT derivatives (20) through oxidative cyclization by condensing 2-ATP (0.01 mmol) (1) with 1,3-dicarbonyl compounds (0.01 mmol) (19) in dimethyl sulfoxide (5 mL) under reflux for 50–60 min. The product was obtained in low yields (37–42%). In the reaction mechanism, 2-ATP (1) was first oxidized by DMSO to form a disulfide (V), which then reacted with 1,3-dicarbonyl (19) to produce an enamine intermediate (X) that cyclized via S–S bond cleavage, yielding the desired product. The antimicrobial activity of the synthesized compounds, including antibacterial and antifungal properties, was evaluated using a method called the agar well diffusion (Scheme 7, Method 6).
The regioselective synthesis of benzo[b]indeno[1,2-e][1,4]thiazin-11(10H)-ones (22) was developed by Mor and Khatri59 using reaction of 3 mmol 2,5-dibromo-2-substituted phenyl-1H-indene-1,3(2H)-diones (21) with 3 mmol 5-substituted-2-ATP (1) in the presence of freshly dried ethanol solvent at reflux for 7.5–10 h, then reaction proceeded through the cyclocondensation with 42–60% yields. In the plausible mechanism, first, the NH2 group of 2-ATP attacked the carbonyl carbon (C-3) of 21. Then, the SH group of 2-ATP reacted intramolecularly at C-2 of 21, leading to the elimination of HBr and the formation of intermediate II, which later produced desired product (22) with removal of water. This protocol is regioselective, efficient, green, reagent-free and catalyst-free. The drawback of this method is the tedious separation of desired products (Scheme 8, Method 1).
Mor and Nagoria60 reported a method for synthesizing 1,4-BT derivatives (22) through the equimolar reaction of 2-aryl-2-bromo-1H-indene-1,3(2H)-diones (21) with 2-ATP (1) in 30 mL of dry ethanol. The reaction was refluxed for 10 h on a water bath, producing yields, 82–95%. This protocol is efficient, green, reagent-free and catalyst-free. A disadvantage of this method is the need for column chromatography (Scheme 8, Method 2).
2.2. Miscellaneous synthesis of 1,4-BTs
The miscellaneous synthesis of 1,4-BT in this part involves employing diverse starting materials such as 2-bromothiophenol, benzothiazole, o-quinone, substituted 2-(2-(2-aminophenyl)disulfanyl)benzenamines, 2-aminobenzothiazoles, and anilines.Jiang et al.72 developed an iodide-catalyzed, aerobic method for synthesizing 1,4-BT derivatives (18) through multiple C–H functionalization with elemental sulfur. The reaction involved reacting various acetophenone (0.4 mmol) (8), substituted aniline (0.4 mmol) (17), and elemental sulfur (0.8 mmol) with DMSO (0.8 mmol) and chlorobenzene as the solvent, along with KI (0.1 mmol) as the catalyst at 150 °C for 16 h. The oxidative cyclization resulted in lower to good product yields (22–72%). The reaction mechanism initiated with the condensation of acetophenone (8) and aniline (17), forming imine intermediate (I). Under oxidative conditions (KI/DMSO/O2), the methyl group in I was oxidized and reacted with another aniline molecule, forming intermediate II, which was detected by GC-MS. Subsequently, elemental sulfur participated in an electrophilic attack at the ortho position of aniline, leading to intermediate III. This further transformed into sulfurated imine IV through elimination of Sn−1 and deprotonation. Finally, intermediate IV underwent cyclization and aromatization of intermediate V under oxidative conditions, yielded the final product. This method works under transition metal-free conditions, employing dioxygen as a green oxidant (Scheme 20).
An efficient copper-mediated C–S and C–N Ullmann coupling reaction for the synthesis of 1,4-BT derivatives (52) was reported by Lingyu Zhang et al.73 The reaction of 2-bromothiophenol (0.5 mmol) (50) with N-alkyl-substituted chloroacetamide (1.2 equiv.) (51) occurred in the presence of Cu(acac)2 (10% mol) as a catalyst, K2CO3 (2.6 equiv.) as a base, and DMF as a solvent at 135 °C under N2 for 18 h. This reaction led to the formation of the desired products in yields from 17–82%. In the mechanism, initially, a nucleophilic substitution reaction took place between 2-bromothiophenol and N-alkyl-substituted chloroacetamide in the presence of K2CO3, resulting in intermediate I. This intermediate was subsequently subjected to oxidative addition with Cu(I), forming intermediate II. Ligand exchange and hydrogen bromide elimination under basic conditions led to the transformation of intermediate II into intermediate III, which finally converted into the desired product through reductive elimination. The demerits of this procedure are the need for column chromatography and long reaction time (Scheme 21).
Suliman et al.74 reported a method for the synthesis of 1,4-BT derivatives (55) through the cleavage reaction of benzothiazole (0.5 mmol) (53) with cyclopentane-1,3-dione (1 mmol) (54) in the presence of potassium dihydrogen phosphate (KH2PO4) as a base (1 mmol) in a DMSO/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent mixture. The reaction was stirred for 48 h under air at 80 °C, yielding moderate results (40–60%). This approach is environmentally friendly, proceeds under mild conditions, and does not require any transition metals. However, the long reaction time and the need for column chromatography are the demerits of this method (Scheme 22).
1) solvent mixture. The reaction was stirred for 48 h under air at 80 °C, yielding moderate results (40–60%). This approach is environmentally friendly, proceeds under mild conditions, and does not require any transition metals. However, the long reaction time and the need for column chromatography are the demerits of this method (Scheme 22).
Londhe et al.75 developed a novel method for synthesizing 1,4-BT derivatives (20) using a biomimetic strategy. This approach involved the cyclocondensation of substituted 2-(2-(2-aminophenyl)disulfanyl)benzenamines (1 mmol) (56) with 1,3-dicarbonyl compounds (2 mmol) (19) in the presence of a supramolecular catalyst, β-cyclodextrin (β-CD) (1 mmol), in water (20 mL), at neutral pH. The reaction was carried out under stirring at 60 °C for 50 min, yielding the desired products in excellent yields (70–91%). In the proposed reaction mechanism, aqueous β-CD served as a host, offering a hydrophobic cavity and a hydrophilic exterior, facilitating the formation of non-covalent, reversible supramolecular complexes with 1,3-dicarbonyl compounds (19). This enhanced solubilization effect increased the availability of the 1,3-dicarbonyl compound for interaction with 2-(2-(2-aminophenyl)disulfanyl)benzenamine (56), which was encapsulated within the hydrophobic cavity of β-CD. The reaction proceeded via the formation of an enamine intermediate (I), followed by intramolecular cyclization, ultimately led to the desired product (20). The advantages of this approach include its one-pot, rapid execution, environmental friendliness, and cost-effectiveness, though the necessity for column chromatography for purification is a demerit (Scheme 23).
 | ||
| Scheme 23 Synthesis of 1,4-BT using substituted 2-(2-(2-aminophenyl)disulfanyl)benzenamines and 1,3-dicarbonyl compounds. | ||
The bioinspired synthetic route to 1,4-BT derivatives, reported by Halloran et al.,76 involved the selective addition of sulfur nucleophiles to o-quinones. The process began with a regioselective reaction between o-quinone (0.5 mmol) (57) and N-Boc-L-cysteine ester (0.75 mmol) (58) in the presence of magnesium bromide ethyl etherate (MgBr2·Et2O) (0.125 mmol), DIPEA (1.5 mmol), and dry, degassed dioxane (5 mL) at RT for 1.5 h, producing a sulfur-catechol adduct as the crude product (59). This crude material was dissolved in DCM/TFA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (10 mL) and stirred for 6 h at RT, which removed the Boc group. Further, the catechol was oxidized using phenyl iodoacetic acid (PIDA) (1 equiv.) in dry, degassed DCM (20 mL) for 1.5 h at RT, generating an o-quinone in situ. The quinone underwent condensation and tautomerization, yielding 1,4-BT derivatives (60) with moderate to good yields (36–79%). On the other hand, when reacting 3,5-di-tert-butyl-ortho-quinone (1 mmol) with N-methyl cysteine methyl ester (1.5 mmol) (61), the final step was conducted in DCM/MeOH (4
1) (10 mL) and stirred for 6 h at RT, which removed the Boc group. Further, the catechol was oxidized using phenyl iodoacetic acid (PIDA) (1 equiv.) in dry, degassed DCM (20 mL) for 1.5 h at RT, generating an o-quinone in situ. The quinone underwent condensation and tautomerization, yielding 1,4-BT derivatives (60) with moderate to good yields (36–79%). On the other hand, when reacting 3,5-di-tert-butyl-ortho-quinone (1 mmol) with N-methyl cysteine methyl ester (1.5 mmol) (61), the final step was conducted in DCM/MeOH (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) solvent to yield N-methyl benzothiazine 53% (63). The need for column chromatography is a demerit of this approach (Scheme 24).
1) solvent to yield N-methyl benzothiazine 53% (63). The need for column chromatography is a demerit of this approach (Scheme 24).
Sun and Bao77 developed an efficient metal-free method for synthesizing 1,4-BT derivatives via oxidative ring expansion of 2-aminobenzothiazoles with alkenes. Initially, substituted 2-aminobenzothiazoles (0.1 mmol) (62) were treated with iodobenzene diacetate [PhI(OAc)2] (0.2 mmol) in DCM (1 mL) under an argon atmosphere at 85 °C for 18 h. This led to the oxidative ring-opening of the 2-aminobenzothiazoles, followed by a [4 + 2] cycloaddition with various alkenes (0.3 mmol) (2), resulting in the formation of cyanamide-containing dihydro-1,4-BT derivatives (63) with moderate to good yields (31–97%). A demerit of this procedure is the need for flash column chromatography (Scheme 25).
3. Conclusion
1,4-BT derivatives have emerged as a crucial motif in organic synthesis, with significant applications in medicinal, pharmaceutical, and industrial fields. This review presents a comprehensive exploration of the recent methods employed for the preparation of 1,4-BT derivatives over the past decade. Most of the mentioned protocols are based on simple, cost-effective, and environmentally friendly approaches, providing high yields, extensive functionalization, and easy workups with minimal use of hazardous reagents and recyclable catalysts under ambient reaction conditions. However, some methods involve the use of harmful solvents and harsh reaction conditions that can negatively impact the environment. These reactions raise environmental concerns and highlight the need for more sustainable and eco-friendly methods in the field. This review aims to offer researchers insights into overcoming new challenges in creating straightforward, economical, and green methods for synthesizing 1,4-BT derivatives to serve mankind.Abbreviations
| [PhI(OAc)2] | Iodobenzene diacetate |
| 1,4-BT | 1,4-Benzothiazine |
| 2-ATP | 2-Aminothiophenol |
| 2-IBX | 2-Iodoxybenzoic acid |
| Anti-HCV | Anti-Hepatitis C virus |
| Boc | tert-Butyloxycarbonyl |
| CHCl3 | Chloroform |
| COX-2 | Cyclooxygenase-2 |
| CPB | Cetylpyridinium bromide |
| Cs2CO3 | Caesium carbonate |
| Cu(acac)2 | Copper(II) acetylacetonate |
| CuO | Copper oxide |
| DBU | 1,8-Diazabicyclo[5.4.0]undec-7-ene |
| DCM | Dichloromethane |
| DIPEA | N,N-Diisopropylethylamine |
| DMAc | N,N-Dimethylacetamide |
| DMF | Dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| HCN | Hydrogen cyanide |
| HEPES | 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid |
| K2CO3 | Potassium carbonate |
| KH2PO4 | Potassium dihydrogen phosphate |
| KI | Potassium iodide |
| KOH | Potassium hydroxide |
| m-CPBA | meta-Chloroperbenzoic acid |
| MeOH | Methanol |
| NBC | Nanobiocatalyst |
| NBS | N-Bromosuccinimide |
| PEG | Polyethylene glycol |
| PIDA | Phenyl iodoacetic acid |
| RT | Room temperature |
| TFA | Trifluoroacetic acid |
| β-CD | β-Cyclodextrin |
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors confirmed that this article has no conflict of interest.Acknowledgements
The authors are grateful to the Department of Chemistry, M.L.S.U. Udaipur, Rajasthan, India, for providing necessary library facilities. Hemant Kumar Rundla acknowledges University Grants Commission, New Delhi for providing Junior Research Fellowship.References
- E. Irrou, Y. A. Elmachkouri, V. Varadharajan, H. El Monfalouti, T. Hökelek, J. T. Mague, H. Ouachtak, E. M. Essassi, M. L. Taha and N. K. Sebbar, Synthesis, X-ray, spectroscopic characterizations, DFT calculations, Hirschfeld surface analyses, molecular docking, and molecular dynamic simulations of some 1, 4-benzothiazine-1, 1-dioxide derivatives as human kinase CK2 inhibitors, J. Mol. Struct., 2024, 1312, 138477 CrossRef CAS
.
- S. Mor, S. Nagoria, S. Sindhu and V. Singh, Synthesis and biological activities of 1, 4-benzothiazine derivatives: an overview, Chem. Biol. Interface, 2017, 7(1), 1–18 Search PubMed
.
- S. L. Schreiber, Target-oriented and diversity-oriented organic synthesis in drug discovery, Science, 2000, 287(5460), 1964–1969 CrossRef CAS PubMed
.
- S. J. Teague, A. M. Davis, P. D. Leeson and T. Oprea, The design of leadlike combinatorial libraries, Angew. Chem., Int. Ed., 1999, 38(24), 3743–3748 CrossRef CAS PubMed
.
- F. Ahmad Saddique, M. Ahmad, A. Kanwal, S. Aslam and A. Fawad Zahoor, Recent trends toward the synthesis of fused-benzothiazines and their derivatives, Synth. Commun., 2021, 51(3), 351–378 CrossRef CAS
.
- S. Gupta, Synthesis and biological evaluation of new 1, 4-thiazine containing heterocyclic compounds, Jordan J. Chem., 2009, 4(3), 209–221 CAS
.
- D. Armenise, G. Trapani, V. Arrivo, E. Laraspata and F. Morlacchi, Research on potentially bioactive aza and thiaza polycyclic compounds containing a bridgehead nitrogen atom. III. Synthesis and antimicrobial activity of some 1, 4-benzothiazines and pyrrolobenzothiazines, J. Heterocycl. Chem., 2000, 37(6), 1611–1616 CrossRef CAS
.
- A. Aiello, E. Fattorusso, P. Luciano, M. Menna, G. Esposito, T. Iuvone and D. Pala, Conicaquinones A and B, two novel cytotoxic terpene quinones from the Mediterranean ascidian Aplidium conicum, Eur. J. Org Chem., 2003, 2003(5), 898–900 CrossRef
.
- Y.-T. Ma, M.-C. Huang, F.-L. Hsu and H.-F. Chang, Thiazinedione from Xanthium strumarium, Phytochemistry, 1998, 48(6), 1083–1085 CrossRef CAS
.
- M. L. Alfieri and L. Panzella, The multifaceted opportunities provided by the pheomelanin-inspired 1, 4-benzothiazine chromophore: a still-undervalued issue, Molecules, 2023, 28(17), 6237 CrossRef CAS PubMed
.
- A. Takasaki, D. Nezirević, K. Årstrand, K. Wakamatsu, S. Ito and B. Kågedal, HPLC analysis of pheomelanin degradation products in human urine, Pigm. Cell Res., 2003, 16(5), 480–486 CrossRef CAS PubMed
.
- A. A. Mahmoud, A. A. Ahmed, S. S. Al-Shihry and O. Spring, A new heterocyclic glucoside from the fruits of Xanthium pungens, Nat. Prod. Res., 2005, 19(6), 585–589 CrossRef CAS PubMed
.
- H. Tawada, Y. Sugiyama, H. Ikeda, Y. Yamamoto and K. Meguro, Studies on antidiabetic agents. IX.: a new aldose reductase inhibitor, AD-5467, and related 1, 4-benzoxazine and 1, 4-benzothiazine derivatives: synthesis and biological activity, Chem. Pharm. Bull., 1990, 38(5), 1238–1245 CrossRef CAS PubMed
.
- R. Fringuelli, L. Milanese and F. Schiaffella, Role of 1, 4-benzothiazine derivatives in medicinal chemistry, Mini-Rev. Med. Chem., 2005, 5(12), 1061–1073 CrossRef CAS PubMed
.
- B. K. Warren and E. E. Knaus, Pyridine and reduced pyridine analogues of 10H-pyrido [3, 4-b][1, 4] benzothiazines with analgesic activity, Eur. J. Med. Chem., 1987, 22(5), 411–415 CrossRef CAS
.
- S. Dubey, J. Seda and E. Knaus, 2-Methyl-1, 2, 3, 4-tetrahydro-10H-pyrido| 4, 3-b|| 1, 4| benzothiazines with analgesic activity, Eur. J. Med. Chem., 1984, 19(4), 371–373 CAS
.
- R. Fringuelli, F. Schiaffella, F. Bistoni, L. Pitzurra and A. Vecchiarelli, Azole derivatives of 1, 4-benzothiazine as antifungal agents, Bioorg. Med. Chem., 1998, 6(1), 103–108 CrossRef CAS PubMed
.
- A. Macchiarulo, G. Costantino, D. Fringuelli, A. Vecchiarelli, F. Schiaffella and R. Fringuelli, 1, 4-Benzothiazine and 1, 4-benzoxazine imidazole derivatives with antifungal activity: a docking study, Bioorg. Med. Chem., 2002, 10(11), 3415–3423 CrossRef CAS PubMed
.
- F. Schiaffella, A. Macchiarulo, L. Milanese, A. Vecchiarelli and R. Fringuelli, Novel ketoconazole analogues based on the replacement of 2, 4-dichlorophenyl group with 1, 4-benzothiazine moiety: design, synthesis, and microbiological evaluation, Bioorg. Med. Chem., 2006, 14(15), 5196–5203 CrossRef CAS PubMed
.
- R. T. Hendricks, J. B. Fell, J. F. Blake, J. P. Fischer, J. E. Robinson, S. R. Spencer, P. J. Stengel, A. L. Bernacki, V. J. Leveque and S. Le Pogam, Non-nucleoside inhibitors of HCV NS5B polymerase. Part 1: synthetic and computational exploration of the binding modes of benzothiadiazine and 1, 4-benzothiazine HCV NS5b polymerase inhibitors, Bioorg. Med. Chem. Lett., 2009, 19(13), 3637–3641 CrossRef CAS PubMed
.
- N. Szentandrássy, D. Nagy, B. Hegyi, J. Magyar, T. Bányász and P. P Nanasi, Class IV antiarrhythmic agents: new compounds using an old strategy, Curr. Pharm. Des., 2015, 21(8), 977–1010 CrossRef PubMed
.
- S. Sabatini, G. W. Kaatz, G. M. Rossolini, D. Brandini and A. Fravolini, From phenothiazine to 3-phenyl-1, 4-benzothiazine derivatives as inhibitors of the Staphylococcus aureus NorA multidrug efflux pump, J. Med. Chem., 2008, 51(14), 4321–4330 CrossRef CAS PubMed
.
- M. G. Katselou, A. N. Matralis and A. P. Kourounakis, Developing potential agents against atherosclerosis: design, synthesis and pharmacological evaluation of novel dual inhibitors of oxidative stress and squalene synthase activity, Eur. J. Med. Chem., 2017, 138, 748–760 CrossRef CAS PubMed
.
- J. Gowda, A. Khader, B. Kalluraya, P. Shree and A. Shabaraya, Synthesis, characterization and pharmacological activity of 4-{[1-substituted aminomethyl-4-arylideneamino-5-sulfanyl-4, 5-dihydro-1H-1, 2, 4-triazol-3-yl] methyl}-2H-1, 4-benzothiazin-3 (4H)-ones, Eur. J. Med. Chem., 2011, 46(9), 4100–4106 CrossRef CAS PubMed
.
- S. A. Coughlin, D. W. Danz, R. G. Robinson, K. M. Klingbeil, M. P. Wentland, T. H. Corbett, W. R. Waud, L. A. Zwelling, E. Altschuler and E. Bales, Mechanism of action and antitumor activity of (S)-10-(2, 6-dimethyl-4-pyridinyl)-9-fluoro-3-methyl-7-oxo-2, 3-dihydro-7H-pyridol [1, 2, 3-de]-[1, 4] benzothiazine-6-carboxylic acid (WIN 58161), Biochem. Pharmacol., 1995, 50(1), 111–122 CrossRef CAS PubMed
.
- S. Mir, A. M. Dar and B. A. Dar, Synthetic strategies of benzothiazines: a mini review, Mini-Rev. Org. Chem., 2020, 17(2), 148–157 CrossRef CAS
.
- Y. Jacquot, L. Bermont, H. Giorgi, B. Refouvelet, G. L. Adessi, E. Daubrosse and A. Xicluna, Substituted benzopyranobenzothiazinones. Synthesis and estrogenic activity on MCF-7 breast carcinoma cells, Eur. J. Med. Chem., 2001, 36(2), 127–136 CrossRef CAS PubMed
.
- R. Gupta, M. Jain, R. Rathore and A. Gupta, Synthetic and spectral investigation of fluorinated phenothiazines and 4H-1, 4-benzothiazines as potent anticancer agents, J. Fluorine Chem., 1993, 62(2–3), 191–200 CrossRef CAS
.
- R. Gupta, A. Thomas, R. Gautam and V. Gupta, One-pot synthesis of new fluorinated 4H-1, 4-benzothiazines as possible anticancer agents, J. Fluorine Chem., 1989, 44(1), 1–14 CrossRef CAS
.
- R. Gupta and R. Kumar, Synthesis of 6-trifluoromethyl-4H-1, 4-benzothiazines as possible anticancer agents, J. Fluorine Chem., 1986, 31(1), 19–24 CrossRef CAS
.
- A. Amin, A. Ji, B. A. Bhat, D. Murtaza, A. A. Hurrah, I. A. Bhat, S. Parveen, S. Nisar and P. K. Sharma, Anti-lung Cancer Activity of Synthesized Substituted 1, 4-Benzothiazines: An Insight from Molecular Docking and Experimental Studies, Anti-Cancer Agents Med. Chem., 2024, 24(5), 358–371 CrossRef CAS PubMed
.
- Alishba, I. Ali, S. Hameed, K. M. Khan, U. Salar, M. Taha, N. Sadeghian, P. Taslimi, B. Tuzun and D. Özerkan, Exploring Benzo [b][1, 4] Thiazine Derivatives: Multitarget Inhibition, Structure–Activity Relationship, Molecular Docking, and ADMET Analysis, ChemistrySelect, 2024, 9(38), e202404087 CrossRef CAS
.
- S. Inoue, K. Hasegawa, K. Wakamatsu and S. Ito, Comparison of antimelanoma effects of 4-S-cysteaminylphenol and its homologues, Melanoma Res., 1998, 8(2), 105–112 CrossRef CAS PubMed
.
- L. Del Corona, G. Signorelli, A. Pinzetta and G. Coppi, Synthesis and immunostimulating activity of new 1, 4-benzothiazine derivatives, Eur. J. Med. Chem., 1992, 27(4), 419–423 CrossRef CAS
.
- O. O. Ajani, Functionalized 1, 4-benzothiazine: a versatile scaffold with diverse biological properties, Arch. Pharm., 2012, 345(11), 841–851 CrossRef CAS PubMed
.
- K. Hasegawa, S. Ito, S. Inoue, K. Wakamatsu, H. Ozeki and I. Ishiguro, Dihydro-1, 4-benzothiazine-6, 7-dione, the ultimate toxic metabolite of 4-S-cysteaminylphenol and 4-S-cysteaminylcatechol, Biochem. Pharmacol., 1997, 53(10), 1435–1444 CrossRef CAS PubMed
.
- V. Cecchetti, V. Calderone, O. Tabarrini, S. Sabatini, E. Filipponi, L. Testai, R. Spogli, E. Martinotti and A. Fravolini, Highly potent 1, 4-benzothiazine derivatives as KATP-channel openers, J. Med. Chem., 2003, 46(17), 3670–3679 CrossRef CAS PubMed
.
- M. Maheshwari and A. Goyal, A review: synthesis and medicinal importance of 1, 4-benzothiazine analogs, Asian J. Pharm. Clin. Res., 2015, 8(2), 41–46 CAS
.
- A. Rai, A. K. Singh, V. Raj and S. Saha, 1, 4-Benzothiazines-a biologically attractive scaffold, Mini-Rev. Med. Chem., 2018, 18(1), 42–57 CAS
.
- Y. Pawar, A. Sonawane, P. Nagle, P. Mahulikar and D. More, Synthesis of 1, 4-benzothiazine compound containing isatin moieties as antimicrobial agent, Int. J. Curr. Pharm. Res., 2011, 3(3), 47–51 CAS
.
- G. Trapani, A. Latrofa, M. Franco and G. Liso, Synthesis of 2-substituted-N-carboxymethyl-1, 5-benzothiazepin-4-ones and-1, 4-benzothiazin-3-ones and their evaluation as angiotensin converting enzyme inhibitors, Farmaco, 1995, 50(2), 107–112 CAS
.
- F. Schiaffella, A. Macchiarulo, L. Milanese, A. Vecchiarelli, G. Costantino, D. Pietrella and R. Fringuelli, Design, synthesis, and microbiological evaluation of new Candida albicans CYP51 inhibitors, J. Med. Chem., 2005, 48(24), 7658–7666 CrossRef CAS PubMed
.
- N. Gautam, N. Ajmera, S. Gupta and D. C. Gautam, Synthesis, spectral characterization and biological evaluation of 4H-1, 4-benzothiazines, their sulfones and ribofuranosides, Eur. J. Chem., 2012, 3(1), 106–111 CrossRef CAS
.
- V. Ambrogi, G. Grandolini, L. Perioli, M. Ricci, C. Rossi and L. Tuttobello, Synthesis, antibacterial and antifungal activities of several new benzo-naphtho-and quinolino-1, 4-thiazine and 1, 5-thiazepine derivatives, Eur. J. Med. Chem., 1990, 25(5), 403–411 CrossRef CAS
.
- S. B. Munde, S. P. Bondge, V. E. Bhingolikar and R. A. Mane, A facile synthesis of 1, 4-benzothiazines under solvent free conditions, Green Chem., 2003, 5(2), 278–279 RSC
.
- P. K. Sharma, A. Amin and M. Kumar, A review: medicinally important nitrogen sulphur containing heterocycles, Open Med. Chem. J., 2020, 14(1), 49–64 CrossRef CAS
.
- N. E. Alom, N. Kaur, F. Wu, S. J. Saluga and W. Li, Catalytic Regio- and Stereoselective Alkene Sulfenoamination for 1, 4-Benzothiazine Synthesis, Chem.–Eur. J., 2019, 25(28), 6902–6906 CrossRef CAS PubMed
.
- B. Teli, M. M. Wani, S. Jan, H. R. Bhat and B. A. Bhat, Micelle-mediated synthesis of quinoxaline, 1, 4-benzoxazine and 1, 4-benzothiazine scaffolds from styrenes, Org. Biomol. Chem., 2024, 22(32), 6593–6604 RSC
.
- T. B. Nguyen and P. Retailleau, Umpolung Strategy for α, α′-Functionalization of Ketones with 2-Aminothiophenols: Stereoselective Access to Spirobis (1, 4-benzothiazines), Adv. Synth. Catal., 2020, 362(18), 3824–3829 CrossRef CAS
.
- T. B. Nguyen and P. Retailleau, Umpolung Strategy with 2-Aminothiophenols: Access to 2-Arylbenzothiazine Derivatives from Alkyl Aryl Ketones, Adv. Synth. Catal., 2020, 362(23), 5380–5384 CrossRef CAS
.
- Y.-m. Lin, G.-p. Lu, R.-k. Wang and W.-b. Yi, Radical route to 1, 4-benzothiazine derivatives from 2-aminobenzenethiols and ketones under transition-metal-free conditions, Org. Lett., 2016, 18(24), 6424–6427 CrossRef CAS PubMed
.
- J. Zhao, Z. Luo and J. Xu, Synthesis of 1, 4-Benzothiazines via KI/DMSO/O2-Mediated Three-Component Oxidative Cyclization/Coupling, Adv. Synth. Catal., 2020, 362(10), 1988–1992 CrossRef CAS
.
- S. Bhattacharya, P. Ghosh and B. Basu, Graphene oxide (GO): an efficient carbocatalyst for the benign synthesis of functionalized 1, 4-benzothiazines, Tetrahedron Lett., 2017, 58(10), 926–931 CrossRef CAS
.
- J. Shajeelammal, R. Minitha and R. Ravindran, One pot synthesis of 1-(3-methyl-4H-benzo [1, 4] thiazin-2-yl) ethanone and its antimicrobial properties, Bull. Pure Appl. Sci., Sec. C, 2018, 37(1), 143–147 CrossRef
.
- T. B. Hadda, F. Z. Khardli, M. Mimouni, M. Daoudi, A. Kerbal, H. Salgado-Zamora, N. Gandhare, A. Parvez and S. Lahsasni, Impact of geometric parameters, charge, and lipophilicity on bioactivity of armed quinoxaline, benzothiaole, and benzothiazine: pom analyses of antibacterial and antifungal activity, Phosphorus Sulfur Silicon Relat. Elem., 2014, 189(6), 753–761 CrossRef
.
- M. Sangvikar, G. Phadnaik, M. Bhosle, D. Mane and R. Mane, A convenient synthetic protocol for the synthesis of 2, 3-disubstituted 1, 4-benzothiazines, Rasayan J. Chem., 2016, 9(4), 686–691 CAS
.
- A. Islam, R. Singha and P. Ghosh, Polyethylene Glycol (PEG-200): An Efficient, Green and Biocompatible Reaction Medium for the Metal-Free Synthesis of Functionalized 1, 4-Benzothiazines, ChemistrySelect, 2023, 8(2), e202203780 CrossRef CAS
.
- A. Goyal, Spectral evaluation and antimicrobial activity of synthesized 4H-1, 4-benzothiazines, Asian J. Pharm. Clin. Res., 2021, 66–69 CrossRef CAS
.
- S. Mor and M. Khatri, Regioselective synthesis of benzo [b] indeno [1, 2-e][1, 4] thiazin-11 (10 aH)-ones and antimicrobial evaluation thereof, Synth. Commun., 2022, 52(13–14), 1526–1536 CrossRef CAS
.
- S. Mor and S. Nagoria, Efficient and convenient synthesis, characterization, and antimicrobial evaluation of some new tetracyclic 1, 4-benzothiazines, Synth. Commun., 2016, 46(2), 169–178 CrossRef CAS
.
- R. Baharfar and S. Mohajer, Synthesis and Characterization of Immobilized Lipase on Fe3O4 Nanoparticles as Nano biocatalyst for the Synthesis of Benzothiazepine and Spirobenzothiazine Chroman Derivatives, Catal. Lett., 2016, 146, 1729–1742 CrossRef CAS
.
- M. Saadouni, M. Galai, S. Aoufir Yel Skal, S. Boukhris, A. Hassikou, M. Touhami, A. Guenbour, A. El Khlifi, H. Oudda and N. Habbadi, Experimental and quantum chemical and Monte Carlo simulations studies on the corrosion inhibition of mild steel in 1 M HCl by two benzothiazine derivatives, J. Mater. Environ. Sci., 2018, 9, 2493–2504 CAS
.
- A. Jaafar, A. Khalaf, H. Abdallah, T. Roisne and A. Hachem, Reaction of 2-aminothiophenol with 2, 5-dihydro-2, 5-dimethoxyfuran: a facile route to a new dihydrobenzothiazine derivative, Mediterr. J. Chem., 2014, 3(2), 831–837 CrossRef
.
- M. Bahta and N. Ahmed, Design and synthesis of 1, 4-benzothiazine hydrazide as selective and sensitive colorimetric and turn-on fluorometric sensor for Hg2+ detection in aqueous medium, J. Photochem. Photobiol., A, 2018, 357, 41–48 CrossRef CAS
.
- S. M. Gomha, Z. A. Muhammad, H. M. Gaber and M. M. Amin, Synthesis of Some Novel Heterocycles Bearing Thiadiazoles as Potent Anti-inflammatory and Analgesic Agents, J. Heterocycl. Chem., 2017, 54(5), 2708–2716 CrossRef CAS
.
- J. P. Wan, S. Cao, C. Hu and C. Wen, Iodine-catalyzed, ethyl-lactate-mediated synthesis of 1, 4-benzothiazines via metal-free cascade enaminone transamination and C–H sulfenylation, Asian J. Org. Chem., 2018, 7(2), 328–331 CrossRef CAS
.
- N. Pokhodylo, R. Martyak, M. Rogovyk, V. Matiychuk and M. Obushak, Synthesis of Oxazine, Thiazine, and Quinoxaline Derivatives Containing a Benzyl Fragment from 3-Aryl-2-Bromopropanoic Acids and Their Esters, Russ. J. Org. Chem., 2021, 57(4), 532–539 CrossRef CAS
.
- Alishba, U. Ahmed, M. Taha, N. A. Khan, U. Salar, K. M. Khan, A. Anwar and R. Siddiqui, Potential anti-amoebic effects of synthetic 1, 4-benzothiazine derivatives against Acanthamoeba castellanii, Heliyon, 2024, 10(1), e23258 CrossRef CAS PubMed
.
- N. Tret'yakov, M. Dmitriev and A. Maslivets, Synthesis of Spiro [1, 4-benzothiazine-2, 2'-pyrroles] by the Reaction of Pyrrolo [2, 1-c][1, 4] oxazinetriones with 2-Aminobenzenethiol, Russ. J. Org. Chem., 2020, 56, 935–938 CrossRef
.
- N. Tretyakov, M. Dmitriev, A. Shein and A. Maslivets, Opening of the Furandione Ring with o-Aminothiophenol: Synthesis of 2 H-1, 4-Benzothiazine-2, 3 (4 H)-dione, Russ. J. Org. Chem., 2019, 55, 716–718 CrossRef CAS
.
- M. Meenakshi, P. Antojenifer, M. Karthikeyan, C. Prahalathan and K. Srinivasan, Synthesis and biological evaluation of new 1, 4-benzothiazine derivatives as potential COX-2 inhibitors, J. Heterocycl. Chem., 2022, 59(2), 351–358 CrossRef CAS
.
- J. Jiang, X. Tuo, Z. Fu, H. Huang and G.-J. Deng, Three-component synthesis of 1, 4-benzothiazines via iodide-catalyzed aerobic C–H sulfuration with elemental sulfur, Org. Biomol. Chem., 2020, 18(17), 3234–3238 RSC
.
- L. Zhang and S. Zhang, Copper-catalyzed one-pot synthesis of 1, 4-benzothiazine-3-ones, Tetrahedron Lett., 2023, 130, 154761 CrossRef CAS
.
- A. M. Y. Suliman, Y. Li, S. Zhang and Y. Yuan, A cleavage reaction of benzothiazole with cyclic 1, 3-dicarbonyls in the presence of potassium dihydrogen phosphate, J. Chem. Res., 2015, 39(11), 657–660 CrossRef CAS
.
- B. S. Londhe, S. L. Padwal, M. R. Bhosale and R. A. Mane, Novel synthesis of 1, 4-benzothiazines in water accelerated by β-cyclodextrin, J. Iran. Chem. Soc., 2016, 13, 443–447 CrossRef CAS
.
- M. W. Halloran, E. Li, K. V. N. Esguerra and J.-P. Lumb, A bioinspired synthesis of 1, 4-benzothiazines by selective addition of sulfur nucleophiles to ortho-quinones, J. Org. Chem., 2023, 88(4), 2561–2569 CrossRef CAS PubMed
.
- Q. Sun and X. Bao, Facile preparation of dihydro-1, 4-benzothiazine derivatives via oxidative ring-expansion of 2-aminobenzothiazoles with olefins, Chem. Commun., 2022, 58(13), 2216–2219 RSC
.
| This journal is © The Royal Society of Chemistry 2025 |