 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Insight into the time-dependent corrosion protection of mild steel by green ionic liquids: a combined electrochemical, surface, and computational study
M. Alharbia,
Ruby Aslam*b,
Ajahar Khanc,
Khalid A. Alamry d,
Yas Al-Hadeethie,
Elena Bekyarovaf,
S. Alqahtanig and
Mahmoud A. Hussein
d,
Yas Al-Hadeethie,
Elena Bekyarovaf,
S. Alqahtanig and
Mahmoud A. Hussein *dh
*dh
aUniversity of Jeddah, Collage of Science, Department of Physics, Jeddah, Saudi Arabia
bSchool of Civil and Hydraulic Engineering, Chongqing University of Science and Technology, Chongqing 401331, China. E-mail: drrubyaslam@gmail.com
cDepartment of Food and Nutrition, Bionanocomposite Research Center, Kyung Hee University, 26 Kyungheedae-ro, Dongdaemun-gu, Seoul 02447, South Korea
dChemistry Department, Faculty of Science, King Abdulaziz University, Jeddah 21589, Saudi Arabia. E-mail: mahussein74@yahoo.com; maabdo@kau.edu.sa
eDepartment of Physics, Faculty of Science, King Abdulaziz University, Jeddah 21589, Saudi Arabia
fDepartment of Chemical & Environmental Engineering, Center for Nanoscale Science and Engineering, University of California at Riverside, Riverside, CA 92521-0403, USA
gEstidama for Project Engineering, 7th Floor, Almurjanah Tower, Jeddah, Saudi Arabia
hChemistry Department, Faculty of Science, Assiut University, Assiut, 71516, Egypt
First published on 17th March 2025
Abstract
This study reports the development of two green ionic liquids (ILs), namely, choline tyrosinate and choline prolinate, abbreviated as ChoTyr and ChoPro, respectively, as eco-friendly corrosion inhibitors for mild steel (MS) in acidic media. The obtained ILs were characterized by 1H-NMR and FT-IR spectroscopy. The anti-corrosive performance of the synthesized ILs was investigated by the weight loss and electrochemical methods (PDP, EIS) in 5% HCl solution at different immersion times of 24, 48, and 72 h at 313 K. The results indicated inhibition efficiencies of 96.9% for ChoTyr, 92.9% for ChoPro in static conditions, 95.5% for ChoTyr, and 91.5% for ChoPro in dynamic conditions after 72 h immersion. The inhibition performance increases as immersion time increases due to the enhanced adsorption of ILs molecules onto the steel surface, forming a protective film. SEM analyses exhibited a smooth surface without corrosion when ILs were present, while untreated steel showed extensive degradation. FT-IR and UV-vis spectroscopic analyses confirmed ILs adsorption. At the same time, theoretical calculations employed using density functional theory corroborated the experimental observations, suggesting stronger adsorption and a higher inhibitory potential of ChoTyr versus ChoPro. These findings proved ChoTyr and ChoPro ILs to be sustainable corrosion inhibitors, providing valuable implications for industry applications in acid pickling and cleaning areas.
1. Introduction
Corrosion inhibitors have received significant attention as potential means to reduce metal dissolution by forming a protective layer on the steel surface, thereby mitigating issues like material loss, expensive repairs, and safety.1,2 Recently, ionic liquids (ILs) have gained significant attention as promising green corrosion inhibitors with high thermal stability, tunable molecular structure, and environmental friendliness.3–6 Compared with traditional inhibitors, ILs have high efficiency even at low concentrations with low toxicity. El-Nagar et al.7 examined the corrosion inhibition activity of the imidazolium-based ILs on carbon steel in HCl solution and found that the corrosion rate decreased significantly from 5.95 (μg cm−2 min−1) (blank) to 0.66, 0.56, and 0.44 (μg cm−2 min−1) in the presence of 100 ppm of studied ILs at 293 K. Selim et al.8,9 explored the corrosion inhibiting performance of ionic liquids in inhibiting carbon steel corrosion in 1.0 M HCl. These ionic liquids acted as mixed inhibitors, exerting a maximum corrosion inhibition efficiency of more than 95%. According to another report, the 1-butyl-3-methylimidazolium trifluoromethyl sulfonate([BMIm]TfO) based IL at 800 ppm concentration exhibited 75% efficiency against carbon steel corrosion in the 3.5% NaCl solution.10 Lignin-based ILs were used to prevent corrosion of MS in 0.01 M NaCl, with the choline syringe being the most effective, reducing the corrosion current density from 1.66 μA cm−2 (blank) to 0.066 μA cm−2 after 24 h immersion.11 The anti-corrosive performance of 1-methyl-1propyl-piperidinium bromide (MPPB) on Al-6061-10 vol% SiC composite in 0.05 M HCl showed increased %IE with higher MPPB concentration but decreased with rising temperature.12 Moreover, Aslam et al. have previously reported the corrosion inhibition performances of a few green ILs like proline nitrate,13 l-alanine methyl ester nitrate,14 ILs derived from amino acid-ester salts,15 glycine and glutamic acid-based ILs,16 and choline/amino acids based ILs17,18 under static conditions and for immersion time of up to 6 h. Although previous studies provided valuable insights into the short-term performance of choline/amino acid-based ILs as corrosion inhibitors, a comprehensive evaluation of their long-term effectiveness under static and dynamic conditions was lacking. This study aims to investigate their corrosion inhibition performance over extended periods and in dynamic environments for a more realistic assessment.Amino acids, as the building blocks of proteins, have great potential in synthesising ILs owing to their natural biodegradability and bioactivity. They are low-cost, commercially available at high purity, and may be cations or anions contributing to IL properties.19 The nature of amino acid, cation–anion and length of alkyl-chain decides the physicochemical and biological properties of ILs. Through molecular engineering, it is possible to tailor the structure of ILs to yield characteristics that minimize toxicity and improve environmental compatibility.20 Choline is an essential micronutrient critical for lipid metabolism and transport. Biodegradability, biocompatibility, and cytotoxicity are enhanced in cholinium-based ILs, which promote biocompatibility and biodegradability.21 Choline–amino acid ionic liquids are a combination of choline's low toxicity and biodegradability with the versatile properties of amino acids so that they can result in environmentally sustainable materials with promising applications in many fields.22–24
The study reports the synthesis of two green ILs derived from choline hydroxide and L-tyrosine and L-proline amino acids, abbreviated as ChoTyr and ChoPro, respectively. Further, the molecular structures of ILs were confirmed using proton nuclear magnetic resonance (1H-NMR) spectroscopy and Fourier transform infrared (FT-IR) spectroscopy. The corrosion inhibition performance of these ILs in 5% HCl solution for MS under static and dynamic conditions up to 72 h was studied at 313 K. Various analyses, such as weight loss, electrochemical techniques, and surface analysis, were performed to investigate the inhibition efficiency and elucidate the adsorption mechanism. Theoretical studies using density functional theory (DFT) were also adopted to link molecular properties of ILs to their inhibition performances. Yazdani et al.21 studied the toxicity and biodegradability of ten choline–amino acid ionic liquids (ILs), reporting low toxicity (EC50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 160–1120 mg L−1) and high microbial biocompatibility with minimal impact on Gram-positive and Gram-negative bacteria. The reported ILs exhibited over 60% biodegradability within 28 days, confirming their environmental sustainability. Based on the literature, the ILs under investigation can be considered biodegradable and non-toxic. Therefore, this work aims to demonstrate that ChoTyr and ChoPro ILs are promising green corrosion inhibitors that can find potential industrial applications and contribute to sustainable corrosion management strategies.
160–1120 mg L−1) and high microbial biocompatibility with minimal impact on Gram-positive and Gram-negative bacteria. The reported ILs exhibited over 60% biodegradability within 28 days, confirming their environmental sustainability. Based on the literature, the ILs under investigation can be considered biodegradable and non-toxic. Therefore, this work aims to demonstrate that ChoTyr and ChoPro ILs are promising green corrosion inhibitors that can find potential industrial applications and contribute to sustainable corrosion management strategies.
2. Experimental section
2.1 Chemicals and materials
Choline hydroxide (46 wt%), L-proline (≥99%), and L-tyrosine (≥98%) were procured from Sigma-Aldrich, while acetone (>99.0%) and ethanol (>99.9%) were obtained from Merck. All the chemicals were used as received, and the solutions were prepared using double-distilled water. The composition of MS (A1020) under investigation was determined using an optical emission spectrometer (Shimadzu PDA-7000) and reported as follows: (wt%), C (0.28%), Si (0.22%), Mn (0.73%), P (0.015%), S (0.006%), N (0.007%), and remaining Fe. The MS specimens of 3 cm × 2 cm × 1 cm were used for weight loss, UV-vis and FT-IR analysis. Before use, the samples were prepared with SiC (grit size #100 to #3000) papers to give a homogenous surface. After polishing, the specimens were degreased in acetone, rinsed with distilled water, and thoroughly dried. For corrosion studies, a 37% commercial HCl solution was diluted with distilled water to prepare a 5% HCl solution. The inhibitors were applied in the prepared solution at a concentration of 3 × 10−3 M (determined based on our previous study34).2.2 Synthesis of the materials
Synthesis of both types of ILs involved the coupling of choline to amino acid anions, prolinate and tyrosinate, as described in a previously described method.19 Firstly, an aqueous solution of 0.06 mol of the respective amino acid was prepared, and a 4 M aqueous solution of choline hydroxide was added dropwise at room temperature, keeping the corresponding amino acid in slight excess (approximately 10 mol%).The reaction was allowed to proceed overnight at 276 K. After that; water was eliminated under reduced pressure at 323 K using a rotary evaporator. The reaction was mixed well with an acetonitrile-methanol solvent mixture (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) to remove leftover amino acids, causing the unreactive amino acids to precipitate. The resulting solution was filtered, and the filtrate was further heated at 323 K to evaporate the solvents. Fig. 1(a–c) shows the molecular structures, 1H-NMR and FT-IR spectra of the synthesized ILs, and the obtained data is given in Table 1, along with chemical formulas and molecular weights.
1, v/v) to remove leftover amino acids, causing the unreactive amino acids to precipitate. The resulting solution was filtered, and the filtrate was further heated at 323 K to evaporate the solvents. Fig. 1(a–c) shows the molecular structures, 1H-NMR and FT-IR spectra of the synthesized ILs, and the obtained data is given in Table 1, along with chemical formulas and molecular weights.
| ILs | Chemical formula | Molecular mass | FTIR | 1H NMR |
|---|---|---|---|---|
| ChoPro | C10H22N2O3 | 218.29 | 3437 cm−1 (OH/NH stretching vibration), 2968, 2884 cm−1 (CH stretching vibration), 1683 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration), 1138 cm−1 (C–N stretching vibration) and 1075 cm−1 (choline C–O–C stretching O stretching vibration), 1138 cm−1 (C–N stretching vibration) and 1075 cm−1 (choline C–O–C stretching |
(400 MHz, DMSO-d6, δ, ppm): OH– 0.80, CH2 & CH2 = 3.99 & 3.62, CH3 (for 9H) = 3.25, NH = 3.28, 4CH2 = 3.50, 1.75, 1.78 & 4.10 |
| ChoTyr | C14H24N2O4 | 284.35 | 3428 cm−1 (OH/NH stretching vibration), 2919, 2844 cm−1 (C–H stretching vibration), 1596 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration), 1500 cm−1 (C O stretching vibration), 1500 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic stretching), 1171 cm−1 (C–N stretching vibration, choline quaternary amine) and 1280 cm−1 (phenolic O–H bending), 1094 cm−1 (choline C–O–C stretching) C aromatic stretching), 1171 cm−1 (C–N stretching vibration, choline quaternary amine) and 1280 cm−1 (phenolic O–H bending), 1094 cm−1 (choline C–O–C stretching) |
(400 MHz, DMSO-d6, δ, ppm): 3.45 (s, OH), 3.75 (s, OH), 4.22 (s, NH2), 6.62 (dd, 2H), 6.64 (dd, 2H), 2.90 (d, 2H), 2.74 (d, 2H), 2.45 (s, 9H), 2.15 (d, 2H) |
2.3 Inhibition performance evaluation
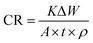 | (1) |
 | (2) |
 | (3) |
 | (4) |
3. Results and discussion
3.1 Corrosion inhibition assessment
The mass loss tests are used to calculate the % IE by comparing the average weight loss of MS in the absence and the presence of fixed IL concentrations, i.e., 3 × 10−3 M. Under static and dynamic conditions and over immersion periods of 24, 48, and 72 h, the corrosion rates and inhibition efficiencies of the studied ILs on MS were compared and evaluated. The results are shown in Fig. 2(a–c). | ||
| Fig. 2 (a) Immersion time vs. CR plot in the absence of ILs. (b and c) Immersion time vs. % IE plot in the presence of ILs under (b) static and (c) dynamic conditions. | ||
In the absence of inhibitors, the corrosion rates of MS at 313 K were determined to be 10.3 mm per year, 9.0 mm per year, and 8.89 mm per year under static conditions after 24, 48, and 72 h of immersion, respectively, as shown in Fig. 2(a). Under dynamic conditions without inhibitors, these values increased slightly to 13.0 mm per year, 11.4 mm per year, and 9.2 mm per year due to the influence of flow (Fig. 2(a)). On adding the ILs at a concentration of 3 × 10−3 M, the corrosion rates were decreased, and the maximum decreased was obtained as 0.47 mm per year and 0.83 mm per year in case of ChoTyr and ChoPro, respectively at 72 h immersion (Fig. 2(a)) under dynamic conditions. However, in static conditions, a further substantial decline in corrosion rates was exhibited, with the lowest 0.27 mm per year and 0.64 mm per year in the case of ChoTyr and ChoPro after 72 h immersion, as shown in Fig. 2(a). This reduction confirms the ILs' effectiveness in mitigating corrosion. The % IE increased by adding ILs across varying immersion times, as shown in Fig. 2(b) and (c). The order of % IE was static conditions > dynamic conditions in the case of both ILs. The observed trends validate that the ILs act as efficient corrosion inhibitors, forming protective layers on the MS surface that restrict corrosive interactions. Notably, the inhibition efficiency was higher with ChoTyr than with ChoPro IL. This trend suggests a more substantial protective effect of ChoTyr on the MS surface under identical experimental conditions. This may be due to structural differences between studied ILs. Tyrosine contains a phenolic –OH group, an aromatic ring, amine (–NH2) and carboxylic (–COOH) groups. The phenolic group enhances its ability to form strong hydrogen bonds and π–π interactions with the steel surface, contributing to better adsorption. Moreover, proline has a non-aromatic pyrrolidine ring and lacks the π-electron system present in tyrosine.
EIS data in the form of Nyquist and Bode plots for both uninhibited and ILs inhibited solutions are shown in Fig. 3(a–f). In inhibitor-free solutions at 24, 48 and 72 h, the impedance spectra display a single depressed capacitive loop, indicating that the corrosion process in an acidic solution is primarily driven by a charge-transfer mechanism.32 The Bode diagrams demonstrate a single time constant at all frequencies. Nova 2.1.4 software was used to determine the impedance characteristics. The collected EIS data were fitted with the electrical equivalent circuit reported in our previous publication.33,34 Among the obtained parameters (Table 2), Rs stands for solution resistance, Rp for polarization resistance (sum of Rct (charge transfer resistance), Rd (diffuse layer resistance), and the resistance of any accumulated species such as corrosion products/molecules/ions, etc.),35 and finally, Rf represents the film resistance. A constant phase element (CPE) is incorporated into the circuit to achieve a more accurate fit, as the capacitive loops are imperfect.36
| Immersion time | CPEdl | CPEf | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rs (Ω cm2) | Ydl (sn Ω−1 cm−2) ×10−4 | nd | Rf (Ω cm2) | Yf (sn Ω−1 cm−2) ×10−3 | nf | Rp (Ω cm2) | Rtotal = Rp + Rf | Cdl (F cm−2) ×10−4 | IE% | χ2 | slope | −ϕ0 | |
| 5% HCl | |||||||||||||
| 24 h | 0.71 | 0.87 | 1 | 18.25 | 1 | 0.90 | 5.5 | 23.8 | 0.87 | — | 0.004 | −0.68 | 53.8 |
| 48 h | 0.71 | 1 | 1 | 13.7 | 2 | 0.74 | 5.9 | 19.6 | 1 | — | 0.002 | −0.70 | 64.3 |
| 72 h | 0.77 | 2.12 | 1 | 6.01 | 0.72 | 0.58 | 6.8 | 12.8 | 2.32 | — | 0.001 | −0.73 | 67.3 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||||
| [ChoPro] | |||||||||||||
| 24 h | 0.77 | 2.39 | 1 | 6.05 | 0.74 | 0.53 | 239.8 | 245.8 | 0.23 | 90.3 | 0.001 | −0.70 | 63.7 |
| 48 h | 0.72 | 0.31 | 1 | 9.14 | 0.76 | 0.54 | 186.3 | 195.4 | 0.31 | 89.9 | 0.003 | −0.71 | 66.1 |
| 72 h | 1.29 | 0.60 | 0.99 | 8.93 | 0.32 | 0.66 | 138.5 | 147.4 | 0.60 | 91.3 | 0.002 | −0.76 | 67.5 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||||
| [ChoTyr] | |||||||||||||
| 24 h | 0.74 | 0.23 | 1 | 66.07 | 0.86 | 0.48 | 281.5 | 347.5 | 0.23 | 93.1 | 0.003 | −0.71 | 65.9 |
| 48 h | 0.67 | 0.184 | 1 | 67.88 | 0.09 | 0.72 | 303.7 | 371.5 | 0.18 | 94.7 | 0.007 | −0.82 | 78.9 |
| 72 h | 0.70 | 0.191 | 1 | 32.53 | 0.14 | 0.71 | 272.7 | 305.2 | 0.191 | 95.8 | 0.009 | −0.77 | 75.3 |
CPE has two elements: a constant (Y0) and a phase shift indicator (n). Eqn (5) can be used to express the impedance of CPE.38
 | (5) |
| Cdl = Y0(ωmax)n−1 | (6) |
Ydl and Yf (Table 2) represent the non-ideal capacitance of the double layer and the protective film, respectively.39 Their decrease with immersion time indicates that over time, the IL molecules adsorb more effectively onto the steel surface, forming a denser and more stable film that reduces the ability of ions to penetrate through it. Rp and Rt are key indicators of corrosion resistance, with higher values reflecting better inhibition performance.40 The increase in Rp and Rt indicates that the ILs effectively inhibit the charge transfer reactions involved in corrosion by blocking active sites on the metal surface. Cdl is inversely proportional to the thickness of the electrical double layer or the dielectric constant of the medium at the metal/electrolyte interface. The adsorption of ILs creates a thicker barrier layer, which reduces Cdl.41 This is consistent with the fact that IL forms a protective film that separates the metal from the corrosive environment.
As illustrated in the Bode impedance and phase angle graphs (Fig. 3(b, d and f)), the impedance shifts up, and the phase angle (−ϕ0) moves closer to −90° in the presence of ILs following the trend ChoTyr > ChoPro. This indicates enhanced corrosion protection as a higher impedance reflects greater surface resistance to corrosion. A phase angle near −90° suggests the formation of a protective film. Notably, this effect becomes stronger with increasing IL concentrations, confirming the concentration-dependent efficiency of the inhibitors.
Fig. 4(a–c) shows the potentiodynamic polarization curves, which demonstrate the corrosion behavior of MS in 5% HCl solution without and with ILs at varying immersion times under static conditions. Table 3 shows the obtained polarization data, such as anodic (βa) and cathodic (βc) Tafel slopes, the corrosion potential (Ecorr) and the current density (Icorr). Adding ILs significantly suppresses both anodic and cathodic reactions compared to the untreated 5% HCl solution.
 | ||
| Fig. 4 Polarization curves for MS at various immersion times at 313 K in the absence and presence of 3 × 10−3 M ILs. | ||
| ILs | Ecorr vs. Ag/AgCl (V) | βa (V dec−1) | −βc (V dec−1) | Rp (Ω cm−1) | Icorr (μA cm−2) | IE% |
|---|---|---|---|---|---|---|
| 24 h | ||||||
| 5% HCl | −0.44 | 13.6 | 8.7 | 13.0 | 1490 | — |
| [ChoPro] | −0.48 | 9.2 | 7.2 | 176.6 | 149 | 90.0 |
| [ChoTyr] | −0.47 | 16.4 | 8.7 | 204.7 | 84.1 | 94.3 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| 48 h | ||||||
| 5% HCl | −0.47 | 7.7 | 7.4 | 21.5 | 1330 | — |
| [ChoPro] | −0.48 | 7.5 | 9.0 | 359.1 | 72.1 | 94.6 |
| [ChoTyr] | −0.46 | 17.2 | 9.3 | 252.8 | 64.7 | 95.1 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| 72 h | ||||||
| 5% HCl | −0.45 | 12.8 | 9.2 | 19.4 | 1020 | — |
| [ChoPro] | −0.45 | 16.2 | 7.3 | 414.9 | 44.0 | 95.7 |
| [ChoTyr] | −0.49 | 28.8 | 9.8 | 310.6 | 36.2 | 96.4 |
This behaviour indicates that both ILs are mixed-type inhibitors, which can inhibit both anodic (metal dissolution) and cathodic (H2 evolution) reactions of the corrosion process. These results align with previous studies.13,14,42–44 An analysis of the Icorr values shows that the MS immersed in untreated 5% HCl solution has a much larger corrosion current density than ILs inhibited solutions for each immersion time studied (24, 48 and 72 h). After 72 h immersion at 313 K, ChoTyr showed the best corrosion inhibition efficiency, 96.4%. The significant decrease in corrosion rate is evidence of ChoTyr serving as an efficient inhibitor of MS corrosion in acid media.
3.2. Statistical analysis
Statistical analysis was carried out using two-way ANOVA to compare ILs effect on IE and differences within the techniques (Weight Loss, PDP, and EIS) with the results presented in Table 4. ANOVA was performed using OriginPro 8.0 at a significance level p < 0.05. The p-value associated with ILs (0.0017) shows there's a significant difference in IE between ChoPro and ChoTyr, suggesting that the type of IL has a significant impact on corrosion inhibition performance of MS. On the other hand, the p-value for different techniques (0.21) is greater than 0.05, indicating no significant difference in IEs obtained using different techniques for a specific IL. Therefore, the corrosion inhibition performance is influenced by the IL type rather than measurement technique.| Degree of freedom | Sum of squares | Mean square | F Value | P Value | |
|---|---|---|---|---|---|
| Between ILs | 1 | 45.0 | 45.0 | 14.9 | 0.0017 |
| Between WL, EIS and PDP | 2 | 10.5 | 5.2 | 1.7 | 0.210 |
| Model | 3 | 55.5 | 18.5 | 6 | 0.007 |
| Error | 14 | 42.1 | 3.0 | — | — |
| Total | 17 | 97.6 | — | — | — |
3.3 Spectroscopic insights into metal–inhibitor interactions
UV-vis spectra of ChoTyr and ChoPro ILs before and after immersion of MS for 24 h present noticeably different profiles that suggest adsorption on the metal surface. The spectrum of ChoPro showed an absorbance of 0.34 at 270 nm. This absorption arises from the π–π* electronic transition in the conjugated system of the molecule. Two peaks were detected: 324 nm (absorbance 0.253) and 269 nm (absorbance 0.355) after the adsorption of ChoPro on the metal surface. It indicates the interaction and adsorption of ChoPro onto the MS surface. In another case, when analyzing the spectra of pure ChoTyr, a prominent peak at 280 nm with an absorbance of 2.45 was detected (Fig. 5(a)). This absorption corresponds to the π–π* electronic transition in the benzene ring of tyrosine. The high absorbance value (2.45) suggests that tyrosine has a strong light absorption capability in this region due to its aromatic structure. After adsorption onto the MS surface, the peak shifts slightly from 280 to 274 nm. The appearance of a new peak at 222 nm could be due to a new electronic transition arising from the adsorption of the ChoTyr complex onto the steel surface. This could result from a charge transfer between the metal and the inhibitor molecules, forming a protective layer on the steel surface.17,42 | ||
| Fig. 5 (a) UV-vis spectra of pure ILs and ILs containing solution after immersing MS for 24 h. (b) FT-IR spectra of ILs adsorbed on the metal surface. | ||
FT-IR spectroscopy is a powerful analytical technique widely used in corrosion inhibition studies to gain insights into the chemical interactions between inhibitors and metal surfaces.45 The study was done after immersing the MS for 24 h in acidic solution with both studies' ILs (Fig. 5(b)). When comparing the FT-IR spectra of pure ChoPro (Fig. 1(c)) with ChoPro absorbed on the metal surface, all the similar peaks were observed however following shift in the peaks was noticed: N–H stretching vibration and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration were observed at 3430 cm−1 and 1734 cm−1, respectively. Furthermore, the 1424 cm−1 and 2928 cm−1 peaks reflected N–H bending vibration and N–CH3 stretching vibration, respectively. Moreover, the following peak shifts were observed when comparing pure ChoTyr IL (Fig. 1(c)) with the adsorbed IL spectra (Fig. 5(b)) on the metal surface: 3409 cm−1 (N–H stretching vibration), 1734 cm−1 (C
O stretching vibration were observed at 3430 cm−1 and 1734 cm−1, respectively. Furthermore, the 1424 cm−1 and 2928 cm−1 peaks reflected N–H bending vibration and N–CH3 stretching vibration, respectively. Moreover, the following peak shifts were observed when comparing pure ChoTyr IL (Fig. 1(c)) with the adsorbed IL spectra (Fig. 5(b)) on the metal surface: 3409 cm−1 (N–H stretching vibration), 1734 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration), 2928 cm−1 (N–CH3 stretching vibration), 1427 cm−1 (N–H bending vibration). Further peaks were also observed at approximately 1373 cm−1 for C–N stretching vibration and at 2846 cm−1 for C–H stretching vibration. This shows that when ILs are adsorbed on a metal surface, interactions between the compound molecules and the metal surface usually result in the formation of a thin protective layer, which causes these shifts in the wavenumbers of FT-IR peaks.
O stretching vibration), 2928 cm−1 (N–CH3 stretching vibration), 1427 cm−1 (N–H bending vibration). Further peaks were also observed at approximately 1373 cm−1 for C–N stretching vibration and at 2846 cm−1 for C–H stretching vibration. This shows that when ILs are adsorbed on a metal surface, interactions between the compound molecules and the metal surface usually result in the formation of a thin protective layer, which causes these shifts in the wavenumbers of FT-IR peaks.
3.4 Surface morphological studies
The Atomic force microscopy (AFM) analysis in the form of 2D and 3D images of the MS coupons reflected the surface topography features under different conditions Fig. 6(a–h). The surface appeared smooth and uniform before immersing the MS in the corrosive medium, as shown in the 2D and 3D AFM images in Fig. 6(a). The surface roughness parameters, such as average roughness (Ra) and the root mean square roughness (Rq), were assessed at 11.4 nm and 14.9 nm, respectively. | ||
| Fig. 6 2D and 3D AFM images of MS (a) before immersion in HCl; after immersion in HCl for 24 h (b) without inhibitor (c) with ChoPro (d) ChoTyr. | ||
The low parameter values suggest that the MS surface in the dry state was in excellent condition, without imperfections on the surface. Upon immersion in a 5% HCl solution without the addition of inhibitors, the surface has been considerably degraded because of the aggressive corrosive attack of the acidic environment, as exhibited in Fig. 6(b). The AFM images of the untreated MS surface imply a highly damaged morphology, increasing the roughness parameters. Ra and Rq showed maximum values at 181 and 221 nm, respectively. The observed increase in surface roughness further confirmed the corrosive interaction between MS and acidic solution, developing pits, grooves, and other surface irregularities. On the other hand, ILs showed excellent protective effects, as confirmed by AFM topography examination and illustrated in Fig. 6(c and d). The MS surface treated with ILs was much smoother, and the integrity of the substrate was primarily intact. Notably, the surface roughness values were reduced by both inhibitors, which indicated their efficacy in suppressing corrosion to form protective layers on the MS surface. However, ChoTyr IL (Fig. 6(d)) out-performed ChoPro (Fig. 6(c)). MS surface using ChoPro exhibited Ra and Rq values of 71.7 nm and 90.8 nm, respectively. The reduction in the roughness values in the case of ChoTyr IL was much higher, i.e., with values of Ra as 25.7 nm and Rq as 33.1 nm, indicating better corrosion inhibition efficiency. The decrease in surface roughness can mainly be ascribed to the effective adsorption and film-forming ability46 of ChoTyr IL.
SEM images of the MS surface before and after immersion in the absence and presence of ILs are presented in Fig. 7(a–d). Fig. 7(a) displays the MS surface after polishing without any imperfections. Fig. 7(b) shows the MS surface subjected to 5% HCl without inhibitors. There is a significant amount of surface damage resulting from the corrosive effect of HCl (Fig. 7(b)). The SEM images Fig. 7(c) and (d) show few defects and a smoother surface when the MS is treated with ILs, suggesting that a protective layer has formed layers that reduce the acid effect.18 Among both the ILs, ChoTyr (Fig. 7(c)) showed higher protection than ChoPro (Fig. 7(d)), possibly due to the aromatic phenol group in ChoTyr, which promotes its adsorption on the MS surface under acidic conditions.
 | ||
| Fig. 7 SEM images of MS (a) before immersion in HCl; after immersion in HCl for 24 h (b) without inhibitor (c) with ChoPro (d) ChoTyr. | ||
3.5 Theoretical study
DFT analysis is one of the most essential aspects for evaluating processes of corrosion inhibitions. As shown in Fig. 8(b–d), the HOMO–LUMO and ESP structures are obtained from the quantum chemical calculations with the geometric structures (Fig. 8(a)). From Fig. 8(b), it can infer that the HOMO is predominantly localized on the aromatic phenol moiety of tyrosine. This is due to the high electron density of the aromatic π-system and the lone pair of electrons on the oxygen atom of the hydroxyl (–OH) group, making these regions the most likely to donate electrons. In the case of ChoPro IL, it is located on the nitrogen atom in the pyrrolidine ring or on the lone pairs of proline carboxyl groups. In both cases, the LUMO is likely localized on the choline group, particularly around the positively charged quaternary ammonium nitrogen, as apparent from Fig. 8(c).This enables the molecule to accept electron density from the metal during adsorption. Inhibitors with higher EHOMO (less negative, destabilized) are likely to have more significant potential for donating electrons to an electron-deficient species, such as a metal surface.36 In contrast, a small ELUMO (more negative, more stable) indicates the inhibitor's ability toward electron acceptance. The Egap is likely to be decreased in ChoTyr due to the presence of the aromatic phenol group with a delocalized π-electron system. A smaller Egap signifies better reactivity and adsorption ability. Similar values of both the located HOMO and LUMO orbitals trigger a lower electronegativity (χ) (Table 4), meaning that ChoTyr becomes a better electron donor with a lower EHOMO and ELUMO value owing to the decarboxylated aromatic ring. A greater Egap (Table 5) leads to a higher hardness (η)37,47 and a smaller electrophilicity index (ω), indicating a decrease in reactivity, such as that displayed by ChoPro. Lower χ and η result in a higher ΔN (Table 5), meaning ChoTyr transfers more electrons to the metal, forming a stronger protective layer. Fig. 8(d) shows electron surface representations using electrostatic potential mapping (ESP). The red area indicates the high electron density region. In contrast, the blue area indicates areas that have fewer electrons.48 The green/yellow region represents neutral regions.
| EHOMO | ELUMO | ΔE (eV) | I (eV) | A (eV) | χ (eV) | η (eV) | ω | ΔN | |
|---|---|---|---|---|---|---|---|---|---|
| ChoPro | −5.77 | 1.04 | 6.81 | −1.04 | 5.77 | −2.36 | 3.40 | 0.82 | 1.06 |
| ChoTyr | −5.57 | −0.28 | 5.29 | 0.28 | 5.57 | −2.92 | 2.64 | 1.61 | 1.47 |
The presence of phenol contributes to forming more pronounced and localized red areas in ChoTyr IL. Besides, the presence of an aromatic group increases its adsorption ability on metal surfaces compared to ChoPro IL. Both molecules have similar blue regions near the choline moiety, indicating comparable electrophilic potential.
The Mulliken charge analysis (Table 6) indicates a significant difference between the electron distribution in ChoTyr and ChoPro, supporting their contrasting efficiencies as corrosion inhibitors. In ChoTyr, the O3 atom of phenol has an outstanding negative charge (−0.615) along with the delocalized electron density of an aromatic ring that facilitates an increased donation of electrons and the adsorption on the metal surface. In addition, the carboxyl oxygen atoms (O1 and O2) and amine nitrogen (N2) sites were electron donors with significant negative charges (−0.517, −0.628, −0.560, respectively). The quaternary ammonium nitrogen (N1) has a relatively moderate negative (−0.360) charge associated with it, while the neighbouring carbon (C9 = +0.347) has a positive charge, which would favour electron acceptance upon adsorption. Therefore, this equilibrium between nucleophilic and electrophilic character with its delocalized π-electron system gives ChoTyr excellent efficacy in providing a stable covering over the surface of the metal. In ChoPro, the electron density is more localized, with the pyrrolidine nitrogen (N2) bearing a notable negative charge (−0.449) and the carboxyl oxygen atoms (O2 and O3) similarly showing high negative charges (−0.628 and −0.579). However, the lack of an aromatic ring limits the molecule's ability to delocalize charge and interact strongly with the metal surface. The quaternary ammonium nitrogen (N1) has a lower negative charge (−0.314) compared to ChoTyr, and the adjacent carbon (C5 = +0.531) is similarly positive, facilitating some degree of electron acceptance. The overall electron distribution in ChoPro indicates a less effective adsorption potential due to weaker electron donation and the absence of aromatic delocalization.
| Atom label | ChoPro | Atom label | ChoTyr |
|---|---|---|---|
| C1 | +0.137 | C1 | −0.119 |
| C2 | −0.036 | C2 | +0.125 |
| C3 | −0.174 | C3 | −0.036 |
| C4 | −0.151 | C4 | −0.112 |
| C5 | +0.531 | C5 | +0.540 |
| C6 | +0.046 | C6 | −0.012 |
| C7 | −0.097 | C7 | −0.112 |
| C8 | −0.120 | C8 | +0.119 |
| C9 | −0.008 | C9 | +0.347 |
| C10 | −0.158 | C10 | −0.144 |
| H16 | +0.099 | C11 | −0.136 |
| H17 | +0.094 | C12 | −0.201 |
| H18 | +0.128 | C13 | −0.147 |
| H19 | +0.135 | C14 | −0.115 |
| H20 | +0.296 | H21 | +0.296 |
| H21 | +0.132 | H22 | +0.092 |
| H22 | +0.147 | H23 | +0.097 |
| H23 | +0.134 | H24 | +0.134 |
| H24 | +0.136 | H25 | +0.140 |
| H25 | +0.143 | H26 | +0.131 |
| H26 | +0.135 | H27 | +0.134 |
| H27 | +0.023 | H28 | +0.139 |
| H28 | +0.048 | H29 | +0.135 |
| H29 | +0.061 | H30 | +0.138 |
| H30 | +0.066 | H31 | +0.134 |
| H31 | +0.062 | H32 | +0.060 |
| H32 | +0.065 | H33 | +0.079 |
| H33 | +0.046 | H34 | +0.077 |
| H34 | +0.170 | H35 | +0.068 |
| H35 | +0.136 | H36 | +0.059 |
| H36 | +0.134 | H37 | +0.078 |
| H37 | +0.141 | H38 | +0.069 |
| N1 | −0.314 | H39 | +0.187 |
| N2 | −0.449 | H40 | +0.191 |
| O1 | −0.529 | H41 | +0.303 |
| O2 | −0.628 | H42 | +0.143 |
| O3 | −0.579 | H43 | +0.139 |
| H44 | +0.140 | ||
| N1 | −0.360 | ||
| N2 | −0.560 | ||
| O1 | −0.517 | ||
| O2 | −0.628 | ||
| O3 | −0.615 | ||
| O4 | −0.479 |
3.6. Inhibition mechanism
The corrosion inhibition mechanism of ChoTyr and ChoPro ILs for MS in 5% HCl is primarily based on their adsorption onto the steel surface, forming a protective barrier. These adsorb through electrostatic interactions and chemical adsorption, aided by tyrosine (specifically, the phenolic and amino groups) and proline (particularly the carboxyl and pyrrolidine groups) functional groups that can form coordinate bonds with the metal surface. This protective film physically insulates the steel surface from corrosive ions, like H+ and Cl−, inhibiting corrosion.13,49 The inhibition behavior of these molecules can be explained based on quantum mechanical parameters. The quantum parameters such as HOMO, LUMO, and electrophilicity index etc. indicated that these inhibitors are electron donors and play a significant role in forming a strong protective layer. These ILs inhibit anodic and cathodic corrosion processes and efficiently inhibit corrosion in acidic media.4. Conclusions
This work offers a thorough assessment of the corrosion inhibition efficacy of ChoTyr and ChoPro for mild steel under acidic conditions, utilizing a multidisciplinary methodology that combines experimental methods and computational analysis. Weight loss assessments across different immersion durations validated that both inhibitors significantly reduce corrosion. The inhibitory efficacy remained stable over prolonged durations, signifying the durability of the protective coating on the metal surface. ChoTyr IL demonstrated higher efficiency, i.e., 96.86%, than ChoPro, i.e., 92.73% after 72 h immersion, highlighting its superior long-term performance. Electrochemical techniques further supported the results of the weight loss experiment, suggesting that both inhibitors significantly reduce the corrosion rate of MS, with ChoTyr exhibiting superior efficiency. Quantum chemical studies yielded essential insights into the molecular interactions of the inhibitors with the metal surface. ESP mapping further supports these findings, showing more intense red regions in ChoTyr due to the aromatic phenol group, which enhances its nucleophilic potential and interaction with the metal surface.Future studies must focus on hybrid formulations containing ILs and other sustainable corrosion inhibitors, as they might yield synergistic effects, hence potentially providing superior corrosion protection. Additionally, this study only focused on two individual ILs for the corrosion protection of MS in acidic media. Expanding this research to include a broader spectrum of ILs, encompassing different structural variations and functional groups, would be beneficial in identifying the most effective candidates for corrosion inhibition. It would also be interesting to test them on different types of metals including alumina, copper, and stainless steel to provide valuable data on their versatility and applicability across industries. Another critical aspect for future exploration is the effect of environmental factors on the stability and efficiency of ILs.
Data availability
The authors confirm that all data was included in the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the University of Jeddah, Jeddah, Saudi Arabia, under grant no. UJ-23-SRP-2. The authors, therefore, thank the University of Jeddah for its technical and financial support.References
- O. Lahodny-Šarc and F. Kapor, Mater. Corros., 2002, 53, 264–268 CrossRef.
- V. S. Sastri, Green Corrosion Inhibitors: Theory and Practice, Wiley, 2011 Search PubMed.
- S. Singh and P. D. Promila, Int. J. Chem. Stud., 2017, 5, 1497–1503 Search PubMed.
- E. Andrzejewska, A. Marcinkowska and A. Zgrzeba, Polimery, 2017, 62, 344–352 CrossRef CAS.
- L. Souza, E. Pereira, L. Matlakhova, V. A. F. Nicolin, S. N. Monteiro and A. R. G. de Azevedo, J. Mater. Res. Technol., 2023, 22, 2186–2205 CrossRef CAS.
- Y. Zhao, X. Wang, Z. Li, H. Wang, Y. Zhao and J. Qiu, J. Phys. Chem. B, 2024, 128, 1079–1090 CrossRef CAS PubMed.
- R. A. El-Nagar, N. A. Khalil, Y. Atef, M. I. Nessim and A. Ghanem, Sci. Rep., 2024, 14, 1889 CrossRef CAS PubMed.
- Y. A. Selim, M. Abd-El-Raouf, K. Zakaria, A. Z. Sayed, Y. M. Moustafa and A. M. Ashmawy, Sci. Rep., 2024, 14, 10766 CrossRef CAS PubMed.
- Y. A. Selim, M. A. - El-Raouf, K. Zakaria, A. Z. Sayed, Y. M. Moustafa and A. M. Ashmawy, Egypt. J. Pet., 2024, 33, 269–281 Search PubMed.
- M. A. Abbas, A. S. Ismail, K. Zakaria, A. M. El-Shamy and S. Z. El Abedin, Sci. Rep., 2022, 12, 12536 CrossRef CAS PubMed.
- S. Monaci, D. Minudri, L. Guazzelli, A. Mezzetta, D. Mecerreyes, M. Forsyth and A. Somers, Molecules, 2023, 28, 5568 CrossRef CAS PubMed.
- N. Kedimar, P. Rao and S. A. Rao, J. Appl. Electrochem., 2023, 53, 1473–1489 CrossRef CAS.
- R. Aslam, M. Mobin, Huda, M. Shoeb, M. Murmu and P. Banerjee, J. Ind. Eng. Chem., 2021, 100, 333–350 CrossRef CAS.
- R. Aslam, M. Mobin, Huda, M. Murmu, P. Banerjee and J. Aslam, J. Mol. Liq., 2021, 334, 116469 CrossRef CAS.
- R. Aslam, M. Mobin, Huda, I. B. Obot and A. H. Alamri, J. Mol. Liq., 2020, 318, 113982 CrossRef CAS.
- S. Zehra, M. Mobin, R. Aslam, H. Lgaz and I.-M. Chung, J. Mol. Struct., 2021, 1240, 130505 CrossRef CAS.
- M. Mobin, R. Aslam, R. Salim and S. Kaya, J. Colloid Interface Sci., 2022, 620, 293–312 CrossRef CAS PubMed.
- R. Aslam, J. Zhao, X. Sun, X. Zhou, Q. Wang, J. Aslam and Z. Yan, Sustainable Chem. Pharm., 2024, 39, 101614 CrossRef CAS.
- S. De Santis, G. Masci, F. Casciotta, R. Caminiti, E. Scarpellini, M. Campetella and L. Gontrani, Phys. Chem. Chem. Phys., 2015, 17, 20687–20698 RSC.
- Y. Li, F. Yang, Y. Li, M. Cai, H. Li, X. Fan and M. Zhu, J. Mol. Liq., 2022, 360, 119539 CrossRef CAS.
- A. Yazdani, M. Sivapragasam, J. M. Leveque and M. Moniruzzaman, J. Microb. Biochem. Technol., 2016, 08, 415–421 CAS.
- A. Foulet, O. Ben Ghanem, M. El-Harbawi, J.-M. Lévêque, M. I. A. Mutalib and C.-Y. Yin, J. Mol. Liq., 2016, 221, 133–138 CrossRef CAS.
- R. Caparica, A. Júlio, A. R. Baby, M. E. M. Araújo, A. S. Fernandes, J. G. Costa and T. Santos de Almeida, Pharmaceutics, 2018, 10, 288 CrossRef CAS PubMed.
- R. Caparica, A. Júlio, M. E. M. Araújo, A. R. Baby, P. Fonte, J. G. Costa and T. Santos de Almeida, Biomolecules, 2020, 10, 233 Search PubMed.
- Standard Guide for Laboratory Immersion Corrosion Testing of Metals, 2021, NACE TM0169/G31−21 Search PubMed.
- Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements, 1999, G102–89 Search PubMed.
- M. S. S. Carranza, Y. I. A. Reyes, E. C. Gonzales, D. P. Arcon and F. C. Franco, Heliyon, 2021, 7, e07952 CrossRef CAS PubMed.
- Spartan '18, Wavefunction, Inc., Irvine, CA, https://www.wavefun.com Search PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- P. C. Hariharan and J. A. Pople, Theor. Chim. Acta, 1973, 28, 213–222 CrossRef CAS.
- A. Klamt and G. Schüürmann, J. Chem. Soc., Perkin Trans. 2, 1993, 799–805 RSC.
- Y. Qiang, S. Zhang, L. Guo, X. Zheng, B. Xiang and S. Chen, Corros. Sci., 2017, 119, 68–78 CrossRef CAS.
- R. Aslam, A. Aslam, Q. Wang, M. Mobin and Z. Yan, J. Mol. Struct., 2025, 1327, 141193 CrossRef CAS.
- M. Alharbi, R. Aslam, A. Khan, K. A. Alamry, M. A. Hussein, Y. Al-Hadeethi, E. Bekyarova and S. Alqahtani, Results Chem., 2025, 13, 101985 CrossRef.
- R. Hsissou, F. Benhiba, O. Dagdag, M. El Bouchti, K. Nouneh, M. Assouag, S. Briche, A. Zarrouk and A. Elharfi, J. Colloid Interface Sci., 2020, 574, 43–60 CrossRef CAS PubMed.
- A. Nahlé, R. Salim, F. El Hajjaji, M. R. Aouad, M. Messali, E. Ech-chihbi, B. Hammouti and M. Taleb, RSC Adv., 2021, 11, 4147–4162 RSC.
- S. Kaya and C. Kaya, Mol. Phys., 2015, 113, 1311–1319 CrossRef CAS.
- B. Hirschorn, M. E. Orazem, B. Tribollet, V. Vivier, I. Frateur and M. Musiani, Electrochim. Acta, 2010, 55, 6218–6227 CrossRef CAS.
- M. E. Orazem and B. Tribollet, Electrochemical Impedance Spectroscopy, Wiley, 2008 Search PubMed.
- H. Kaesche, Corrosion of Metals: Physicochemical Principles and Current Problems, Springer Berlin Heidelberg, Berlin, Heidelberg, 2003 Search PubMed.
- J. Ye, R. Huang, X. Wang, Q. Bao, J. Mu, K. Pan, W. Ji, Z. Wei and H. Liu, Ind. Eng. Chem. Res., 2023, 62, 14427–14440 CrossRef CAS.
- M. Gobara, A. Saleh and I. Naeem, Mater. Res. Express, 2020, 7, 016517 CrossRef CAS.
- Y. Atef and A. Ghanem, IOP Conf. Ser.:Mater. Sci. Eng., 2020, 975, 012014 CAS.
- X. Gao, Y. Wu, Q. Huang, Y. Jiang, D. Ma and T. Ren, J. Mol. Liq., 2021, 324, 114732 CrossRef CAS.
- K. R. Ansari, D. S. Chauhan, M. A. Quraishi and T. A. Saleh, J. Colloid Interface Sci., 2020, 564, 124–133 CrossRef CAS PubMed.
- K. Yasakau, Corros. Mater. Degrad., 2020, 1, 345–372 CrossRef.
- S. Kaya, C. Kaya and N. Islam, Phys. B, 2016, 485, 60–66 CrossRef CAS.
- D. T. P. Politzer, Chemical Applications of Atomic and Molecular Electrostatic Potentials, Springer US, Boston, MA, 1981 Search PubMed.
- A. E. Al-Rawajfeh, K. M. B. Alharmali, A. H. Tarawneh, C. A. Igwegbe, A. S. Abdalrhman, M. Talibi and A. Alnumani, Results Surf. Interfaces., 2024, 17, 100285 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |



