Open Access Article
Open Access ArticleSuperior solubility of anhydrous quercetin and polymer physical mixtures compared to amorphous solid dispersions†
Xu Maab,
Hong Sub,
Yongming Liu c,
Fenghua Chen
c,
Fenghua Chen *b and
Rongrong Xue
*b and
Rongrong Xue *b
*b
aCollege of Chemistry and Materials Science, Fujian Normal University, Fuzhou, 350117, Fujian, China
bSchool of Resources and Chemical Engineering, Sanming University, Sanming, 365004, Fujian, China. E-mail: fenghuachen@fjsmu.edu.cn; rongrongxue@fjsmu.edu.cn
cSchool of Education and Music, Sanming University, Sanming, 365004, Fujian, China
First published on 27th March 2025
Abstract
Quercetin (QUE) is a functional flavonoid molecule with low water solubility. The study of its amorphous solid dispersions (ASDs) is still insufficient. This work reveals that the solubility of the physical mixtures of anhydrous QUE and polymers (PVP or soluplus) is better than that of the ASDs of QUE and the corresponding polymers. Gel-like phase separation occurring during the QUE ASDs dissolution process (weak in ASDs with PVP and strong in ASDs with soluplus) reduces the driving force for QUE release, which makes research on the QUE ASDs insufficient, and can be avoided in the QUE physical mixtures with polymers. Combination of metastable polymorph and polymer is a feasible strategy for improving the solubility of poorly water-soluble molecules whose ASDs encounter the gel-like phase separation.
1. Introduction
Natural products are important candidates for the prevention and treatment of diseases.1,2 Quercetin (QUE, Scheme 1, 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one, C15H10O7) is a natural compound that belongs to the flavonoids family and has a wide range of medical properties such as anti-inflammatory and anticancer.3 QUE is widely present in fruits and vegetables, such as onion, fennel leaves, tea, cranberries, cherries, tartary buckwheat, Capparis spinosa, and others, and has been approved for widespread use as a food supplement or functional food.4 QUE has a very low solubility in water,5 resulting in poor bioavailability and limiting its applications. It is of great significance to prepare and select the suitable polymorphism of poorly soluble pharmaceutical compounds to improve their solubility and bioavailability.6 More than 60% of marketed pharmaceuticals are poorly soluble, and more than two-thirds of promising new chemical entities fail to enter the clinical setting due to solubility issues.7 Amorphous solids generally exhibit higher solubility and faster dissolution than crystalline forms.8,9 Amorphous solid dispersion (ASDs) technology, which uses polymers as a carrier, is considered as the most promising method to solve the solubility problem of poorly soluble drugs.10,11Given that QUE is one of the most studied flavonoids, it is surprising that QUE ASDs have not been reported as much (Table 1). Quercetin dihydrate (QUE-DH) is the main form sold on the market,20 and is the main raw materials for preparing QUE ASDs. QUE-DH is not suitable for preparing QUE ASDs, especially for ball milling method,18 because water is usually not a good component in ASDs. And the contents of QUE in the reported ASDs are not high. PVP (Scheme 1) is one of the most common used polymers in the preparations of QUE ASDs, and the QUE contents in the ASDs of QUE and PVP are not more than 25%.14,17 Soluplus (Scheme 1) has been applied in various pharmaceutical formulations owing to its inert nature and ability to form nanomicelles.21 Both PVP and soluplus are safe for oral, which is suitable for preparing the QUE ASDs. The ASDs of QUE and soluplus do not show a good solubility,16 but the reason is unknown.
| Raw QUE | Polymer | Methods | QUE content/% | Ref |
|---|---|---|---|---|
| a QUE dihydrate: QUE-DH, anhydrous QUE: QUE-AH. | ||||
| Hydrate | PVP K25 | Evaporating | — | 12 |
| Hydrate | Cellulose derivative | Spray dry | 50 | 13 |
| QUE-DH | Poloxamer 407, PEG 8000, PVP K40 | Solvent evaporation, freeze drying | 25 | 14 |
| Mixture | Cellulose derivative | Spray dry | 10 | 15 |
| QUE-DH | PEG 6000, PVP VA64, soluplus, poloxamer 188 | Hot melt extrusion | 12.5 | 16 |
| QUE-DH | HPMC-AS | Co-precipitation | 10 | 17 |
| QUE-DH | PVP K30 | Evaporation | 25 | 17 |
| QUE-DH | Chitosan oligosaccharide | Ball milling | 50 | 18 |
| QUE-AH | PEG | Melt-mixing | 40 | 19 |
In this work, QUE ASDs with PVP or soloplus and their physical mixtures (PMs) were prepared to explain the insufficient reports of QUE ASDs and develop suitable formulations to improve the solubility of QUE. The solubility of the PMs of anhydrous QUE (QUE-AH) and polymer is better than that of ASDs with the same QUE content and polymer. Gel-like phase separations are observed in the dissolution process of ASDs limiting the dissolution, which might be caused by the strong interactions between QUE and polymer in the ASDs which can be observed from the IR and fluorescence spectroscopy.
The PMs of metastable QUE-AH and polymer can avoid the gel-like phase separation and show a high solubility of QUE. Combination of metastable polymorph and polymer is a feasible strategy for improving the solubility of poorly water-soluble molecule.
2. Experimental section
2.1 Materials
Quercetin dihydrate (QUE-DH, C15H10O7·2H2O, 97%), anhydrous quercetin (QUE-AH, C15H10O7, 95%), and PVP K30 were purchased from Aladdin. Soluplus (repacked of the product of BASF SE) was purchased from Shanghai King Chemical. All reagents were used without treatment.2.2 Methods
3. Results
3.1 Stability, hygroscopicity and solubility of QUE
QUE is generally considered as a molecule with poor stability.22 It is essential to ensure the stability of QUE during the formulation preparation. The macro-thermogravimetric curve of commercial QUE-DH shows a weight loss of 9.7% within 30 minutes in air at 100 °C (Fig. 1a), which is close to the theoretical water content 10.7% of QUE-DH. The mass of QUE after dehydration tends to be constant, indicating that QUE has good thermal stability under the experimental condition.23 If heated in air to the temperature up to 150 °C, the mass of sample still decreases after the loss of water (Fig. S1†), indicating a slight oxidation or decomposition of QUE molecules.24 The commercial PVP and soluplus are also stable at 100 °C (Fig. 1a), with the water adsorbed during storage lost, the water content of PVP and soluplus is 5.4% and 3.6%, respectively.The moisture absorption processes of QUE-AH, dry PVP and soluplus at room temperature and ambient humidity of ∼60% were monitored (Fig. 1b). The moisture absorption capacity of QUE-AH is weak, and the absorbed water content was 0.8% in 90 minutes and 1.7% (Fig. S2†) in one week, respectively, which indicates that the conversion from QUE-AH to QUE-DH is slow in air. PVP and soluplus, especially PVP, have a faster moisture absorption rate and a stronger moisture absorption capacity than QUE-AH. The absorbed water content of PVP was up to 10% in 90 minutes, and that of soluplus reached 2.3%.
The aqueous solubility of QUE is puzzling. The measured solubility of QUE at 25 °C ranges from 0.39 to 920 μg mL−1,25–27 and the computational or fitted values are in the range from 3.2 to 38 μg mL−1.5,28 The solubility of poorly soluble molecules is easy to be influenced by various factors including measurement method (ultrasonic,29 suspension time,30 and filtration) and reagents (polymorph, purity, and stability31 etc.). The typical process of QUE solubility measurement including supernatant preparation, supernatant treatments (filtrated with membranes and diluted with organic solvents), working curve establishment of UV-visible spectroscopy or HPLC method, and concentration test. QUE solubility measurement was proposed here by using UV-visible spectroscopy and aqueous working curve, because the dilution with organic solvents and HPLC method can dissolve the QUE nanoparticles which would interfere with the test accuracy. The QUE aqueous solution was stable within 3 days (Fig. S3†). QUE-DH was used to prepare the standard solutions due to its stability and low-hygroscopic, and the QUE concentration was calculated based on the macro-thermogravimetric result (Fig. 1a). The UV-vis spectra of QUE solutions (Fig. S4†) are similar as the reported ones,32 and the absorption of the solutions at 367 nm showed a good linear relationship with its concentration in the range of 1.8–12.5 μg mL−1. The working curve of QUE aqueous solutions was established (Fig. 1c) for the non-destructive UV-vis spectroscopy test of QUE, whose molar absorption coefficient is only ∼80% of that of QUE 50 vol% water–ethanol solutions (Fig. S5†). The QUE concentration would decrease significantly after the QUE aqueous solution filtrated with PES membranes (Fig. 1d), each filtration process causing approximately a 0.10 decrease of the absorbance (equivalent to a concentration decrease of 3 μg mL−1), which is critical for the accurate determination of QUE solubility, thus, in this work a static method was employed to minimize the entry of small particles into the solutions to make the supernatant suitable for the UV-vis spectroscopy tests directly. The aqueous solubility of QUE-DH tested by this method is 3.5 ± 0.2 μg mL−1 at 25 °C and 6.7 ± 0.2 μg mL−1 at 37 °C.
We think that the method proposed in this work has the following advantages. (I) The method is simple, consistent with the Occam's razor principle. (II) The solvent used is only water. There is no issue of different molar coefficients in different solvents and the dissolution of insoluble QUE particles. (III) QUE aqueous solubility in the common temperatures must be included in the range of working curve, providing a valuable reference for the accuracy of solubility measurements.
3.2 Preparation and characterizations of three QUE polymorphs
The commercial QUE-AH transformed into QUE-DH after years' storage (Fig. S6†). Fresh QUE-AH was prepared by heating QUE-DH in air at 100 °C for 1 hour, and its PXRD pattern shows the characteristic diffraction peaks at 12.8°, 16.8°, and 26.5° (Fig. 2b), which is same as that of the reported raw QUE-AH19 and the dehydration product of QUE-DH.23 The peak widths at half-height of QUE-AH are broader than those of QUE-DH, indicating a crystallinity decrease during the dehydration process.
QUE-AM was the product of neat ball milling of QUE-AH. The PXRD pattern of QUE-AM has no obvious diffraction peaks, only showing very broad humps, which is the characteristic of amorphous phase. To our best know, the complete amorphization of QUE has not been reported yet.
The optical and SEM images of QUE-DH, QUE-AH and QUE-AM are shown in Fig. 2c. QUE-DH, QUE-AH and QUE-AM are all powders. QUE-DH and QUE-AH are light yellow, and QUE-AM is brown yellow. QUE-DH is micro rod-like crystals observed from the SEM image, and the morphology of QUE-AH is similar as that of QUE-DH. There are some cracks observed in the micro rod-like crystals of QUE-AH, which may be caused by the dehydration process. QUE-AM is micro irregular particles composed of small particles.
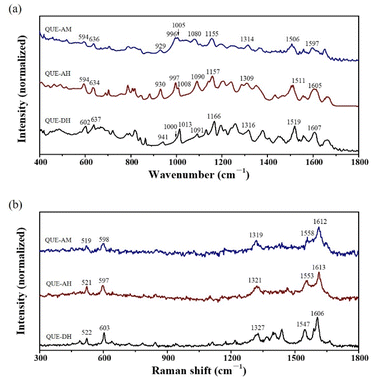 | ||
| Fig. 3 Vibrational spectra of QUE-DH, QUE-AH, and QUE-AM. (a) IR, and (b) mid-frequency Raman spectra (in the range of 300–1800 cm−1). | ||
| IR peaks (cm−1) | Assignments | ||
|---|---|---|---|
| AM | AH | DH | |
| a In-plane vibrations: ν – stretching; δ – bending; out-of plane vibrations: γ – torsional; ω – wagging vibrations; A – phenyl ring in chromone, B – phenyl ring, C – pyrone ring.33 | |||
| 594 | 594 | 602 | γ(C) + γ(A) + δ(COH)A5 + δ(COH)B3′,4′ + ω(COH)A7 + γ(B) |
| 636 | 634 | 637 | γ(C) + γ(A) + δ(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
| 929 | 930 | 941 | γ(B) + γ(A) + ν(C–O)C3 + δ(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) + ν(C–O)B3′ O) + ν(C–O)B3′ |
| 996 | 997 | 1000 | δ(A) + δ(B) + δ(CH)A6,8 |
| 1005 | 1008 | 1013 | δ(A) + δ(C) + δ(B) + δ(CH)A6,8 |
| 1080 | 1090 | 1091 | δ(C) + δ(A) |
| 1155 | 1157 | 1166 | δ(CH)A8 + δ(OH)A7 + δ(A) |
| 1314 | 1309 | 1316 | ν(B) + δ(OH)B4′ |
| 1506 | 1511 | 1519 | ν(A) + δ(OH)A5 + δ(CH)A8 + ν(C–O)A7 |
| 1597 | 1605 | 1607 | ν(A) + ν(C) + ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
Raman spectra of QUE have a poor signal-to-noise ratio and show few signals (Fig. 3b). Low-frequency Raman spectroscopy is usually a fast method to identify polymorphs,34 but QUE polymorphs cannot be identified due to their poor signal-to-noise ratio (Fig. S8†). Raman spectra of QUE in literature sometimes shows a poor signal-to-noise ratio and sometimes has a relatively good signal-to-noise ratio,35,36 which might be caused by the impurity of QUE. QUE molecule contains a lot of hydroxyl group, its Raman activity is not strong, the presence of fluorescence also makes it difficult to obtain its high-quality Raman spectra, and moreover, QUE is not stable under laser due to its instability. 5 typical bands in the mid-frequency Raman spectra (in the range of 300–1800 cm−1) of QUE are listed (Table S1†), however, it is failed to distinguish QUE polymorphs based on the current poor signal-to-noise ratio.
The IR and Raman spectra can be used for explaining from structure why QUE-AM can be obtained from QUE-AH instead of QUE-DH through neat ball milling. 6 among the 10 IR peaks selected (594, 636, 929, 996, 1005, 1155 cm−1) of QUE-AM cannot be distinguished from QUE-AH, while only 3 IR peaks (636, 996, 1314 cm−1) of QUE-AM are similar as those of QUE-DH. 4 among 5 Raman bands selected (519, 598, 1319, 1612 cm−1) of QUE-AM are similar as those of QUE-AH, and no band of QUE-AM is similar as that of QUE-DH. Both IR and Raman spectra indicate that the structure of QUE-AM is more similar to that of QUE-AH than QUE-DH. Thus, we proposed that the polymorph transition from QUE-AH to QUE-AM is easier than that from QUE-DH to QUE-AM based on their structure similarity.
 | ||
| Fig. 4 (a) Dissolution profiles of QUE polymorphs at 37 °C, (b) PXRD patterns of QUE-AM after stored for a certain time. | ||
The stability order is QUE-DH > QUE-AH > QUE-AM, which can be concluded from above dissolution profiles. Theoretically, QUE-DH and QUE-AH are stable under sealed storage condition. QUE-AM can maintain amorphous state for 7 days with sealed storage condition, and would slightly recrystallize into QUE-AH (with characteristic diffraction peaks at 12.9° and 26.6°) after 30 or 60 days' storage (Fig. 4b). Although QUE-AM primarily remained amorphous after storage, the stability of QUE-AM prepared by neat ball milling of QUE-AH was not enough to support its use as a pharmaceutical formulation.
3.3 Preparation and characterizations of QUE ASDs
Under the dissolution experiment condition, the PVP concentrations of samples BM-PVP-0.5, BM-PVP-0.2, and BM-PVP-0.1 are 0.1, 0.4, and 0.9 mg mL−1, respectively. The dissolution profiles of BM-PVP samples at 37 °C (Fig. 6b) show that all of them could maintain the supersaturated concentration of QUE within 48 h. The QUE concentration at 48 h of BM-PVP-0.8 is 10.9 ± 0.7 μg mL−1, indicating that this PVP content is insufficient to stabilize QUE-AM. And the QUE concentrations at 48 h of BM-PVP-0.5, BM-PVP-0.4, and BM-PVP-0.3 were close, which are 25.5 ± 1.0, 30.6 ± 0.8, and 39.3 ± 0.8 μg mL−1, respectively. The amount of PVP (>0.5) contained in these three samples can effectively inhibit the recrystallization of QUE-AM. The QUE concentration at 48 h of BM-PVP-0.2 can be up to 77 ± 7 μg mL−1, which is significantly higher than that of BM-PVP-0.3. BM-PVP-0.1 completely dissolved once deionized water was added in the dissolution experiment. However, during the dissolution process, unlike other BM-PVP systems, some orange flakes were observed (Fig. 6c) in the BM-PVP-0.2 and BM-PVP-0.1 systems, which might be attributed to the oxidation of QUE.
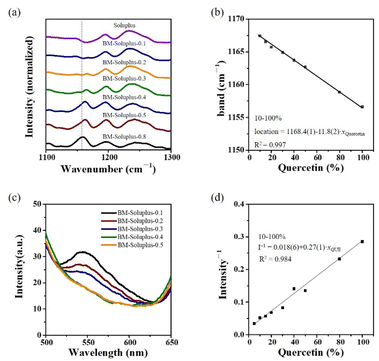
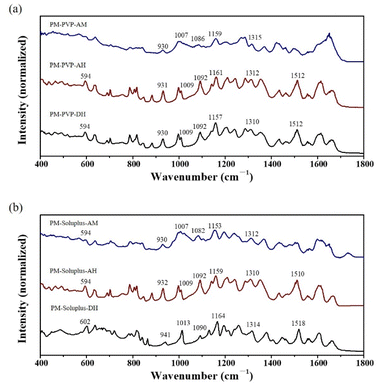
The dissolution profiles of BM-PVP could be explained by structure characterizations. After careful comparisons of the IR and Raman spectra of QUE-AM, BM-PVP and PVP (Fig. 3 and S10†), we found that most of the IR and Raman signals remain unchanged, and only the IR peak at 1155 cm−1 (corresponding to the vibrational mode of δ(CH)A8 + δ(OH)A7 + δ(A)) has a notable shift (>8 cm−1). The vibrational mode of δ(OH)A7 is related to the interaction between the hydroxyl group at A7 position of QUE and PVP. As the QUE contents of BM-PVP decrease, a significant red shift of δ(OH)A7 is observed (Fig. 7a), indicating that the hydrogen bond between QUE and PVP become strong. A good linear relationship between the peak position of δ(OH)A7 and the QUE content is observed for BM-PVP with the QUE content in the range of 0.05–0.8 (Fig. 7b), which can be used to roughly estimate the QUE contents in the ASDs.
| δ(OH)A7 = 1167–12·xQUE (0.05 ≤ xQUE ≤ 0.8), R2 = 0.995. |
The molecular weight of QUE is 302 g mol−1, and PVP K30 is ∼40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. When the molar ratio of QUE to PVP is 1
000. When the molar ratio of QUE to PVP is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the mass fraction of QUE is 0.8%, which is much lower than that of BM-PVP prepared in this work. When the QUE content of BM-PVP is high, the mainly existence form of QUE is amorphous particles, and the interaction between QUE molecules is the main interaction in BM-PVP. As the QUE content decreases, the interactions between QUE and PVP become more, and the interactions between QUE molecules become less.
1, the mass fraction of QUE is 0.8%, which is much lower than that of BM-PVP prepared in this work. When the QUE content of BM-PVP is high, the mainly existence form of QUE is amorphous particles, and the interaction between QUE molecules is the main interaction in BM-PVP. As the QUE content decreases, the interactions between QUE and PVP become more, and the interactions between QUE molecules become less.
Fluorescence spectroscopy can be utilized to analyze the molecule existence form of QUE in its ASDs. QUE-DH and QUE-AH have fluorescence (Fig. S11†),37,38 and QUE-AM does not have fluorescence. BM-PVP-0.4 and BM-PVP-0.3 have negligible fluorescence, whereas BM-PVP-0.2 has observable fluorescence, and BM-PVP-0.1 shows pronounced fluorescence (Fig. 7c). According to the ACQ phenomenon, the emergence of fluorescence of QUE ASDs means the formation of solid dispersions with QUE existing in molecular form (referred as molecular solid dispersions).
The ACQ phenomenon can be fitted with the Stern–Volmer equation.
| IoI−1 = 1 + KSV[Q] |
Io is the fluorescence intensity in the absence of the quencher (Q), and I is the fluorescence intensity in the presence of the quencher at a given concentration. KSV is the Stern–Volmer quenching constant. A higher value of KSV indicates a more efficient quenching. [Q] is the concentration of the quencher. The reciprocal of fluorescence intensity of BM-PVP shows a good linear relationship with xQUE (in the range of 0.05–0.5) (Fig. 7d).
| I−1 = 0.015 + 0.41·xQUE (0.05 ≤ xQUE ≤ 0.5), R2 = 0.992. |
The fluorescence spectra of BM-PVP indicate that molecular solid dispersions of QUE become pronounced when xQUE ≤ 0.2. We think that the emergence of molecular solid dispersions can significantly improve the solubility, which is confirmed by the higher solubility of BM-PVP-0.2. However, molecular solid dispersions are more readily oxidized than ASDs, as observed during the dissolution process of BM-PVP-0.2 (appearance of the orange flakes). Although BM-PVP-0.1 and BM-PVP-0.2 show a superior dissolution property, they are not suitable for ASDs application. Considering the dissolution performance and stability, BM-PVP-0.5, BM-PVP-0.4, and BM-PVP-0.3 are suitable candidates as ASDs.
Unfortunately, the dissolution profiles of BM-soluplus show that the QUE concentration are less than 8 μg mL−1 over 24 h (Fig. 8b). The apparent solubility of BM-soluplus-0.2 at 24 h is only 1.7 μg mL−1, significantly lower than the solubility of QUE-DH. We found that a gel-like phase separation (Fig. 8c) happened immediately once BM-soluplus-0.2 was added into water. Therefore, soluplus is not suitable for the preparation of the ASDs of QUE.
The peak corresponding to the δ(OH)A7 of BM-soluplus gradually red shift with the increase of soluplus content (Fig. 9a). And the peak position of δ(OH)A7 show a good linear relationship (Fig. 9b) with the content of QUE (from BM-PVP-0.1 to QUE-AM).
| δ(OH)A7 = 1168–12·xQUE (0.1 ≤ xQUE ≤ 1), R2 = 0.997. |
Compared with BM-PVP, BM-soluplus exhibits a higher peak position of δ(OH)A7 at the same QUE concentration, indicating a stronger binding interaction between QUE and soluplus.
BM-soluplus-0.4 does not show any fluorescence signal, while BM-soluplus-0.3 displays a significant fluorescence signal (Fig. 9c). Compared with BM-PVP, the emergence of molecular solid dispersions of QUE is at a higher QUE concentration, which confirms the stronger binding interaction between QUE and soluplus.
The fluorescence intensity of BM-soluplus against the content of QUE is well fitted with the Stern–Volmer equation (Fig. 9d).
| I−1 = 0.018 + 0.27·xQUE (0.1 ≤ xQUE ≤ 1), R2 = 0.984. |
The KSV of BM-soluplus is smaller than that of BM-PVP, indicating a less efficient quenching, which means that the interaction between QUE molecules in BM-soluplus is weaker than that in BM-PVP. Conversely, the interaction between QUE and polymer in BM-soluplus is stronger than that in BM-PVP.
3.4 Characterizations of physical mixtures of the three QUE polymorphs with PVP or soluplus
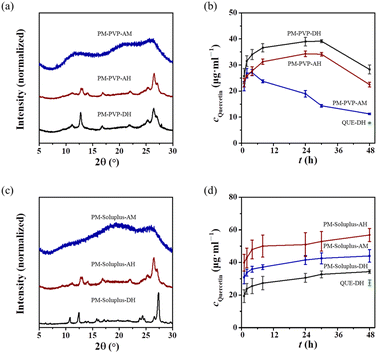
Physical mixtures (PMs) are commonly used as the reference samples in the ASDs field to demonstrate the superior efficacy of the ASDs. QUE-AH and QUE-AM are metastable forms, which can be stabilized by polymers. The apparent solubility of QUE-AH and QUE-AM can be maintained at a high level in the presence of polymers, which can prevent the occurrence of gel-like phase separation as in QUE ASDs.The PXRD patterns of the PMs of the three QUE polymorphs with equivalent PVP (PM-PVP, named as PM-PVP-QUE polymorph) show that QUE-AH and QUE-AM maintain their structure after physically mixed with PVP, while QUE-DH transforms into QUE-AH completely (Fig. 10a). Thus, PM-PVP-QUE DH (PM-PVP-DH) and PM-PVP-QUE AH (PM-PVP-AH) are the mixtures of QUE-AH and PVP with different water contents.
The dissolution profiles of PM-PVP-DH and PM-PVP-AH do not have significant difference with a deviation less than 10% (Fig. 10b). The apparent solubility of PM-PVP-AH (30.6 ± 0.8 μg mL−1 at 48 h) is 20% higher than that of BM-PVP-0.5, indicating that the PM-PVP has a slightly improved performance compared with the ASDs. The dissolution profile of PM-PVP-QUE AM (PM-PVP-AM) is better than that of QUE-AM, but still encounters the recrystallization of QUE-AM, with its apparent solubility continuously decreasing (11.2 ± 0.3 μg mL−1, similar as that of QUE-AM). Theoretically, the solubility of QUE ASDs is superior to that of QUE-AH. While BM-PVP-0.5 has a significant solubilization effect on QUE, it may be confronted with the problem of gel-like phase separation, which leads to a solubility performance inferior to PM-PVP-AH.
The PMs of the three QUE polymorphs with equivalent soluplus (PM-soluplus, named as PM-soluplus-QUE polymorph) do not show significant polymorphism transformation (Fig. 10c). The apparent solubility of PM-soluplus-DH (34.3 ± 1.2 μg mL−1 at 48 h) is slightly higher than that of QUE-DH (27.6 ± 1.7 μg mL−1) with the same concentration of soluplus (Fig. 10d), which might be caused by soluplus absorbing water from QUE-DH owing to its hygroscopicity. The overall dissolution profiles indicate that the order of solubilization is PM-soluplus-AH > PM-soluplus-AM > PM-soluplus-DH. The apparent solubility of PM-soluplus-AH (57 ± 4 μg mL−1 at 48 h) achieves an 8.5-fold increase compared to QUE-DH and a 2.1-fold increase compared to QUE-DH with the same concentration of soluplus.
IR spectroscopy was utilized to determine the existing form of QUE in the PMs (Fig. 11). Briefly, QUE polymorphs can be identified by their characteristic peaks: 1080, 1506, 1597 cm−1 of QUE-AM, 1309, 1511 cm−1 of QUE-AH, and 602, 941, 1013, 1166, 1519 cm−1 of QUE-DH. The presences of peaks at 1310 and 1512 cm−1 of PM-PVP-DH indicate the presence of QUE-AH (differences <4 cm−1), with no signal of QUE-AM and QUE-DH, suggesting a complete transformation from QUE-DH to QUE-AH during mixing. PM-PVP-AH only shows the IR peaks at 1312 and 1512 cm−1, confirming the existence of QUE-AH. The characteristic IR peak at 1082 cm−1 of PM-PVP-AM identifies its amorphous feature. IR spectra do not show a strong interaction between QUE and PVP or soluplus, indicating the existence form of QUE in PMs is particles.
The optical images of the PMs samples (Fig. 12) are different from those of ASDs. PVP and soluplus are white powders exhibiting pronounced granularity. BM-PVP-0.5 and BM-soluplus-0.5 are brown yellow, and the colour of BM-soluplus-0.5 is deeper than that of BM-PVP-0.5, which might be caused by the strong interaction between QUE and soluplus. PM-PVP-AH and PM-soluplus-AH are light yellow similar to that of QUE-AH, indicating that QUE-AH does not change during the physical mixing process. There are some aggregated spheres existed in PM-PVP-AH and PM-soluplus-AH, which might be caused the physical adhesions.
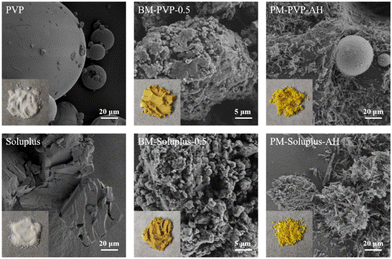 | ||
| Fig. 12 Optical and SEM images of PVP, BM-PVP-0.5, PM-PVP-AH, grinded soluplus, BM-soluplus-0.5, and PM-soluplus-AH. | ||
As shown in the SEM images (Fig. 12), raw PVP is composed of micro spheres with different size mainly in the range 1–100 μm. Soluplus was used after grinding, and the grinded soluplus is irregular particles with size mainly in the range of 10–100 μm. BM-PVP-0.5 and BM-soluplus-0.5 show the similar SEM images with irregular particles composed of small particles. PM-PVP-AH is clearly composed of QUE-AH rod-like crystals and PVP spheres. There are only a small amount of QUE-AH rod-like crystals adhering to the surface of PVP spheres. The morphology of PM-soluplus-AH is different from that of PM-PVP-AH. The surfaces of soluplus particles are fully adhered by QUE-AH rod-like crystals, indicating a strong interaction between soluplus and QUE-AH.
4. Discussion
4.1 Shortcoming of QUE metastable polymorphs
QUE-AH and QUE-AM are metastable polymorphs, which are theoretical formulation to improve the solubility of QUE. However, due to the polymorphism transformation of QUE-AH and the recrystallization of QUE-AM, they are not suitable directly as functional foods.4.2 Shortcoming of QUE ASDs
QUE ASDs with soluplus have a strong shortcoming with the occurrence of gel-like phase separation in the dissolution process, and QUE ASDs with PVP have a weak shortcoming of the occurrence of gel-like phase separation. The existence of gel-like phase separation is the main reason for the few successful reports of QUE ASDs. Herein, the apparent solubility of PM-PVP-AH exceeds that of BM-PVP-0.5, and the apparent solubility of PM-soluplus-AH exceeds that of BM-soluplus-0.5. The ASDs prepared with PVP or soluplus as the carrier is less effective, their dissolution property is even worse than that of the PMs of QUE-AH with PVP or soluplus.4.3 Advantages of QUE-AH PMs
The formulation of QUE in future can involve solid dispersions of metastable QUE-AH. The PMs of QUE-AH and polymers have the following advantages:(I) No gel-like phase separation during dissolution;
(II) A relatively high degree of supersaturation;
(III) High storage stability of QUE-AH.
(IV) Selection diversity of polymers.
It can be a feasible formulation and a promising approach for QUE solubilization.
5. Conclusions
QUE ASDs with PVP or soluplus were prepared via neat ball milling, and their properties were compared with the PMs of QUE-AH with PVP or soluplus. BM-PVP-0.5 shows a good solubility which is little smaller than that of PM-PVP-AH. BM-soluplus-0.5 shows a poor solubility which is much smaller than that of PM-soluplus-AH. Gel-like phase separation process of QUE ASDs reduce the apparent solubility of QUE, which may be caused by the strong interactions between QUE and polymer in ASDs observed from the IR and fluorescence spectra. The PMs of metastable QUE-AH and polymer can avoid this gel-like phase separation, maintain supersaturate concentration in a high level, and have no problem of polymorphism transformation, which is a good candidate for QUE formulations. It is the first time to combine metastable QUE-AH and PMs with polymer to increase QUE solubility. Combination of metastable polymorph and polymer is a feasible strategy with low cost for improving the solubility of poorly water-soluble molecules.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Abbreviations
| ASDs | Amorphous solid dispersions |
| BM-polymer-ωQUE i.e., BM-PVP-0.5 | Ball milled quercetin and polymer |
| PMs | Physical mixtures |
| PM-polymer-quercetin polymorph i.e., PM-PVP-DH | Physical mixtures of equivalent quercetin and polymer |
| PVP | Polyvinylpyrrolidone |
| QUE | Quercetin |
| QUE-AH | Anhydrous quercetin |
| QUE-AM | Amorphous quercetin |
| QUE-DH | Quercetin dihydrate |
Acknowledgements
This work was supported by the National Natural Science Foundation of China [Grant No. 22005175], and the Natural Science Foundation of Fujian Province [Grant No. 2020J01374, 2020J01383].Notes and references
- Q.-W. Zhang, L.-G. Lin and W.-C. Ye, Chin. Med., 2018, 13, 20 CrossRef PubMed
.
- Q. Lu, R. Li, Y. Yang, Y. Zhang, Q. Zhao and J. Li, Food Chem., 2022, 368, 130610 CrossRef CAS PubMed
.
- N. Georgiou, M. G. Kakava, E. A. Routsi, E. Petsas, N. Stavridis, C. Freris, N. Zoupanou, K. Moschovou, S. Kiriakidi and T. Mavromoustakos, Molecules, 2023, 28, 8141 CrossRef CAS PubMed
.
- X. Zhu, G. Ding, S. Ren, J. Xi and K. Liu, Food Chem., 2024, 458, 140262 CrossRef CAS PubMed
.
- M. H. Abraham and W. E. Acree, J. Mol. Liq., 2014, 197, 157–159 CrossRef CAS
.
- E. H. Lee, Asian J. Pharm. Sci., 2014, 9, 163–175 Search PubMed
.
- A. Paredes, P. McKenna, I. Ramoeller, Y. Naser, F. Volpe Zanutto, M. Li, M. Abbate, L. Zhao, C. Zhang, J. Abuershaid, X. Dai and R. Donnelly, Adv. Funct. Mater., 2020, 31, 1–27 Search PubMed
.
- Y. Deng, S. Liu, Y. Jiang, I. C. B. Martins and T. Rades, Pharmaceutics, 2023, 15, 2174 CrossRef CAS PubMed
.
- H. Wang, P. Zhao, R. Ma, J. Jia and Q. Fu, Drug Discovery Today, 2024, 29, 103883 CrossRef CAS PubMed
.
- S. V. Bhujbal, B. Mitra, U. Jain, Y. Gong, A. Agrawal, S. Karki, L. S. Taylor, S. Kumar and Q. Zhou, Acta Pharm. Sin. B, 2021, 11, 2505–2536 CrossRef CAS PubMed
.
- M. Pisay, S. Padya, S. Mutalik and K. B. Koteshwara, Crit. Rev. Ther. Drug Carrier Syst., 2024, 41, 45–94 CrossRef PubMed
.
- A. Costa, F. Marquiafável, M. Leite, B. Rocha, P. Bueno, P. Amaral, H. Barud and A. Berretta, J. Therm. Anal. Calorim., 2011, 104, 273–278 CrossRef CAS
.
- B. Li, S. Konecke, K. Harich, L. Wegiel, L. Taylor and K. Edgar, Carbohydr. Polym., 2013, 92, 2033–2040 CrossRef CAS PubMed
.
- P. Sang Hyun, S. Im-Sook and C. Min-Koo, Mass Spectrom. Lett., 2016, 7, 79–83 CrossRef
.
- A. D. Gilley, H. C. Arca, B. L. B. Nichols, L. I. Mosquera-Giraldo, L. S. Taylor, K. J. Edgar and A. P. Neilson, Carbohydr. Polym., 2017, 157, 86–93 CrossRef CAS PubMed
.
- X. Shi, N. Fan, G. Zhang, J. Sun, Z. He and J. Li, Pharm. Dev. Technol., 2020, 25, 472–481 CrossRef CAS PubMed
.
- Y. Wang, Y. Fang, F. Zhou, Q. Liang and Y. Deng, J. Pharm. Sci., 2021, 110, 3230–3237 CrossRef CAS PubMed
.
- J. Han, M. Tong, S. Li, X. Yu, Z. Hu, Q. Zhang, R. Xu and J. Wang, Drug Dev. Ind. Pharm., 2021, 47, 153–162 CrossRef CAS PubMed
.
- E. Van Hecke and M. Benali, Food Biosci., 2022, 49, 101868 CrossRef CAS
.
- P. Klitou, E. Parisi, S. Bordignon, F. Bravetti, I. Rosbottom, M. Dell'Aera, C. Cuocci, M. R. Chierotti, A. Altomare and E. Simone, Cryst. Growth Des., 2023, 23, 6034–6045 CrossRef CAS PubMed
.
- M. S. Attia, A. Elshahat, A. Hamdy, A. M. Fathi, M. Emad-Eldin, F.-E. S. Ghazy, H. Chopra and T. M. Ibrahim, J. Drug Delivery Sci. Technol., 2023, 84, 104519 CAS
.
- W.-F. Lai and W.-T. Wong, Crit. Rev. Food Sci. Nutr., 2022, 62, 7319–7335 CrossRef PubMed
.
- G. S. Borghetti, J. P. Carini, S. B. Honorato, A. P. Ayala, J. C. F. Moreira and V. L. Bassani, Thermochim. Acta, 2012, 539, 109–114 CrossRef CAS
.
- Z. Jurasekova, A. Torreggiani, M. Tamba, S. Sanchez-Cortes and J. V. Garcia-Ramos, J. Mol. Struct., 2009, 918, 129–137 CAS
.
- K. Srinivas, J. W. King, L. R. Howard and J. K. Monrad, J. Food Eng., 2010, 100, 208–218 CAS
.
- R. S. Razmara, A. Daneshfar and R. Sahraei, J. Chem. Eng. Data, 2010, 55, 3934–3936 CAS
.
- D. Althans, P. Schrader and S. Enders, J. Mol. Liq., 2014, 196, 86–93 CAS
.
- L. Chebil, C. Chipot, F. Archambault, C. Humeau, J. M. Engasser, M. Ghoul and F. Dehez, J. Phys. Chem. B, 2010, 114, 12308–12313 CAS
.
- D. Krishna Sandilya and A. Kannan, Ultrason. Sonochem., 2010, 17, 427–434 CrossRef PubMed
.
- D. Csicsák, E. Borbás, S. Kádár, P. Tőzsér, P. Bagi, H. Pataki, B. Sinkó, K. Takács-Novák and G. Völgyi, New J. Chem., 2021, 45, 11618–11625 Search PubMed
.
- M. Haripada, K. Cyrus and D. Juan, Curr. Pharm. Biotechnol., 2009, 10, 609–625 Search PubMed
.
- M. Buchweitz, P. A. Kroon, G. T. Rich and P. J. Wilde, Food Chem., 2016, 211, 356–364 CAS
.
- J. Hanuza, P. Godlewska, E. Kucharska, M. Ptak, M. Kopacz, M. Mączka, K. Hermanowicz and L. Macalik, Vib. Spectrosc., 2017, 88, 94–105 CrossRef CAS
.
- P. J. Larkin, M. Dabros, B. Sarsfield, E. Chan, J. T. Carriere and B. C. Smith, Appl. Spectrosc., 2014, 68, 758–776 CrossRef CAS PubMed
.
- Y. Numata and H. Tanaka, Food Chem., 2011, 126, 751–755 CrossRef CAS
.
- S. C. Uyeki, C. M. Pacheco, M. L. Simeral and J. H. Hafner, J. Phys. Chem. A, 2023, 127, 1387–1394 CrossRef CAS PubMed
.
- T. He, N. Niu, Z. Chen, S. Li, S. Liu and J. Li, Adv. Funct. Mater., 2018, 28, 1706196 CrossRef
.
- T. Prutskij, A. Deriabina, F. J. Melendez, M. E. Castro, L. Castillo Trejo, G. D. Vazquez Leon, E. Gonzalez and T. S. Perova, Chemosensors, 2021, 9, 315 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra08796h |
| This journal is © The Royal Society of Chemistry 2025 |