 Open Access Article
Open Access ArticleSimultaneous adsorption of sulfamethoxazole and neodymium from wastewater by a MXene-, α-aminophosphonate-, and sulfated fucan-based ternary composite based on anion-synergistic interactions†
Irfan Ijaz *a,
Aysha Bukhari*a,
Ammara Nazira,
Ezaz Gilania,
Hina Zainb,
Attia Shaheenc,
Mohammed Rafi Shaik
*a,
Aysha Bukhari*a,
Ammara Nazira,
Ezaz Gilania,
Hina Zainb,
Attia Shaheenc,
Mohammed Rafi Shaik d,
Mujeeb Khan
d,
Mujeeb Khan d and
Jilani P. Shaike
d and
Jilani P. Shaike
aSchool of Chemistry, Faculty of Basic Sciences and Mathematics, Minhaj University Lahore, Lahore 54700, Pakistan. E-mail: iffichemixt266@gmail.com; ayshabukhari.che@mul.edu.pk
bDepartment of Chemistry, University of Cincinnati, OH 45221, USA
cInstitute for Advanced Study, Shenzhen University, Shenzhen, Guangdong, P. R. China
dDepartment of Chemistry, College of Science, King Saud University, P. O. Box 2455, Riyadh 11451, Saudi Arabia
eDepartment of Biochemistry, College of Science, King Saud University, P. O. Box 2455, Riyadh 11451, Saudi Arabia
First published on 17th February 2025
Abstract
The simultaneous removal of antibiotics and heavy metal ions is of utmost importance because of their hazardous effects on the environment and humans. For the adsorption of sulfamethoxazole (SMX) and neodymium (Nd3+) in mono- and binary contaminant systems (SMX–Nd3+ and Nd3+–SMX), a novel composite was designed using sulfated fucan (FuS), MXenes, and α-aminophosphonates (AMPs) in this study. As far as we know, the concurrent adsorption of SMX and Nd3+ employing materials made of MXenes, FuS, and AMPs with this specific structure has not yet been reported. At 318 K, the FuS@MXene@AMP adsorbent demonstrated excellent adsorption capacities of 448.91 and 255.78 mg g−1 for SMX and Nd3+, respectively. The pseudo-first-order (PSO) kinetic model was the most appropriate for depicting the adsorption of SMX and Nd3+ among all the tested kinetic models. The adsorption of SMX and Nd3+ is better described by the Langmuir isotherm model with a higher value of adsorption capacity and R2. The simultaneous presence of Nd3+ and SMX promoted mutual sorption between the antibiotic and metal ions in the binary systems. The results of FTIR and XPS studies indicated that the removal mechanisms were primarily due to hydrogen bonding, complexation, electrostatic interaction, and π–π interaction.
1 Introduction
Neodymium ions (Nd3+) and antibiotics are commonly used in diagnosis and treatment, and they eventually coexist in hospital wastewater.1,2 On the one hand, Nd3+ contamination of the environment has hazardous impacts on terrestrial and aquatic ecosystems.3 On the other hand, Nd3+ is one of the five most important and strategic rare earth elements (REEs) and has been extensively employed in designing cutting-edge and clean energy technologies, such as permanent magnets,4 laser generators,5 and electronic devices.6 However, the steady supply of neodymium has been restricted, making it a hot topic. This is because of limited mineral reserves,7 growing global needs,8 and tight export restrictions from various Nd-producing countries.9 Consequently, recycling and recovering Nd3+ from hospital waste and industrial wastes that contain Nd3+, such as red mud, coal fly ash, phosphogypsum, and mine wastewater, are considered an environmentally sound approach to address the Nd3+ supply crisis and lessen the impact on the environment. Because residual SMX encourages the growth of antibiotic-resistant bacteria and the spread of antibiotic resistance genes, it presents a significant risk to human health, much like other antibiotic residues. Neodymium is present in some electronic equipment used extensively in hospitals (e.g., in hard drives, medical imaging equipment, or other electronic components). Hospital e-waste may contain Nd3+ and SMX if it is disposed improperly. Similarly, hospital effluents consisting of SMX may get mixed with industrial and municipal effluents that incorporate Nd3+ from different sources (such as manufacturing processes or electronics), which makes removal more challenging. When Nd3+ is reacted with SMX, its chemical characteristics change and its toxicity rises. Therefore, the concurrency of Nd3+ and SMX may improve the generation and spread of antibiotic resistance to a greater extent. Consequently, developing a practical strategy for the individual and simultaneous adsorption of SMX and Nd3+ from hospital effluents is essential.Different wastewater remediation approaches, like membrane filtration,10 photocatalysis,11 advanced oxidation processes,12 biodegradation, ion exchange,13 electrochemical degradation,14,15 and adsorption,16,17 have been utilized to eliminate Nd3+ and SMX from wastewater. Adsorption techniques have drawn significant attention because of their adaptability in terms of cost-effectiveness,18 material sources,19,20 high efficiency,21 absence of by-products,22 quick adsorption rates,23 and ease of operation,24 making them perfect for eliminating SMX and Nd3+ contamination.
Natural polysaccharide biopolymers have been derived from different natural sources and have attained notable attention as adsorbents in contaminant elimination because of their biodegradability,25 affordability,26 and renewable nature.27,28 Sulfated fucan is a natural polysaccharide primarily comprised of fucose.29 Egg jellies, sea cucumbers, sea urchins, and brown algae are the primary natural sources of sulfated fucan.30 Sulfated fucan is widely employed in the pharmaceutical industries, food, and therapeutic agents because of its outstanding biological activities, such as immunoregulatory,31,32 antitumor,33 anti-inflammatory,34 antiviral,35 antioxidant,29 anticoagulant,36,37 and antithrombotic properties.38 Sulfated fucan contains various moieties, such as hydroxyl, oxygen, and sulfate groups, which aid in drug binding through hydrogen bonding, complexation, and electrostatic interaction. Despite this, sulfated fucan exhibits lower adsorption capacity in the adsorption of antibiotics and Nd3+. The adsorption capability of natural polysaccharides have been enhanced by physical and chemical modifications.39–42
MXene, a novel two-dimensional (2D) material, has attracted notable interest because of its physicochemical stability,43 outstanding conductivity,43 distinctive laminated structure,44 and ample surface functionalities.45 Its applications include energy conversion, bioactive materials, and catalysis.46 Typically, MXene is written as Mn+1XnTx (n = 1, 2, 3), where Tx is the surface group (–O, –F, and –OH), M is the transition metal element, and X stands for C or/and N. Furthermore, because of its higher surface area, hydrophilic character, abundant active functional groups, and ability to offer numerous sites for direct adsorption and ion exchange of heavy metals47 and dyes, MXene has been studied as an effective adsorbent for water treatment.48,49 Adil et al. prepared a Cu/Ni-MXene composite with an adsorption capacity of 66.30 mg g−1 for the removal of Cr.50 With an adsorption capacity of 208.20 mg g−1, Ghani et al. prepared sodium intercalated Ti3C2Tx MXene for the adsorption of ciprofloxacin.51 MXene still encounters multiple issues, such as inadequate renderability, expensive, and aggregation and oxidation, which could decrease its reactivity with contaminants.
Organophosphorus molecules, like α-aminophosphonates, structurally resemble amino acids since they substitute the carboxylic group with a phosphonic acid or similar moiety. This resemblance may demonstrate their elevated reactivity with pollutants and multiple applications. Research on uranium adsorption employing trialkylphosphine oxides and tributyl phosphate indicated that the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional group demonstrated an efficient coordination bond with U(VI). To the best of our knowledge, however, there have been no reports on the concurrent adsorption of SMX and Nd3+ using sulfated fucan, MXene, and α-aminophosphonates.
O functional group demonstrated an efficient coordination bond with U(VI). To the best of our knowledge, however, there have been no reports on the concurrent adsorption of SMX and Nd3+ using sulfated fucan, MXene, and α-aminophosphonates.
In the current analysis, we have prepared α-aminophosphonates and MXene-functionalized sulfated fucan (FuS@MXene@AMP) composite by incorporating sulfated fucan, MXene, and α-aminophosphonates. The composite development philosophy in the current research revolves around maximizing the synergistic impacts of integrating sulfated fucan, MXene, and α-aminophosphonates to prepare a novel adsorbent material with improved adsorption capabilities for wastewater treatment. The introduction of α-aminophosphonates and MXene modifications efficiently introduced phosphonate, oxygen, carboxymethyl moieties, fluorine, hydroxyl, amino, and thiocarbonyl groups into sulfated fucan, which increase the density of the active sites for the adsorption of SMX and Nd3+ mono-pollutant system and binary pollutant system through hydrogen bonding, complexation, electrostatic interaction, and π–π interaction. The compositional, morphological, and structural characteristics of the FuS@MXene@AMP composites have been systematically studied utilizing FTIR, SEM, XPS, and BET analyses. Under distinct solution environments, such as temperature, pH, temperature, and concentration, the sorption performance of SMX and Nd3+ by the FuS@MXene@AMP adsorbent has been studied. The sorption characteristics have been determined by employing thermodynamic, isothermal, and kinetic models. Furthermore, employing FTIR and XPS analyses, the possible adsorption mechanism for SMX, Nd3+, and SMX + Nd3+ by the FuS@MXene@AMP adsorbent has been revealed. These results highlight a versatile approach for designing FuS-based composites with outstanding adsorption capability appropriate for real-world wastewater remediation.
2 Experimental section
2.1. Materials
Thiocarbazide, p-phthalaldehyde, and triphenylphosphite were provided by Shanghai Chemical Reagent Co., Ltd. Acetonitrile (CH3CN), titanium (Ti) powder, aluminum (Al) powder, copper(II) triflate (Cu (TOF)2), and acetone were supplied by Shanghai Aladdin Biochemical Technology Co., Ltd. Hydrofluoric acid (HF), hydrochloric acid (HCL), neodymium ions (Nd3+), and sulfamethoxazole were provided by Sigma-Aldrich.2.2. Synthesis of the Ti3AlC2 MAX phase
The detailed process for the synthesis of the Ti3AlC2 MAX phase is provided in Text S1.†2.3. Preparation of MXene
The strategy employed for the MXene preparation was based on the method reported by Chen et al..52 Briefly, 5 g of HF was added to 50 mL of HCl solution and stirred for 10 min. After 15 minutes of mixing 37 g of the MAX phase (Ti3AlC2) in 30 mL of HCl, the mixture was gradually added to the HF/HCL solution while being constantly stirred. Subsequently, the resultant product underwent a 15 hours reaction with continuous magnetic stirring at 60 °C. A dark-colored suspension was produced by centrifuging the obtained suspension at 1500 rpm after cleaning with deionized water.2.4. Preparation of sulfated fucans
According to an earlier published analysis, the body wall of sea cucumbers was used to isolate sulfated fucans (FuS). The dried body walls of sea cucumbers were ground and then incubated with papain. By precipitating enzymatic hydrolysate with 80% ethanol, FuS was produced, which was subsequently purified employing gel exclusion chromatography.532.5. Synthesis of α-aminophosphonate materials
α-Aminophosphonates were prepared using the procedures reported by Imam et al. Fig. S1† exhibits the proposed structures of the material and its preparation pathway. Thiocarbazide, triphenylphosphite, and p-phthalaldehyde, with different molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were mixed in 5 mL of acetonitrile (CH3CN) for designing AMP. The resultant solution was agitated for 15 min at room temperature. Next, a Lewis acid catalyst, copper(II) triflate (Cu (TOF)2), was immediately introduced into the above solution at a 20% (w/w) concentration. The resultant solution was filtered, cleaned with acetonitrile, and dried in air to yield α-aminophosphonate after stirring for 72 h at room temperature.
1 were mixed in 5 mL of acetonitrile (CH3CN) for designing AMP. The resultant solution was agitated for 15 min at room temperature. Next, a Lewis acid catalyst, copper(II) triflate (Cu (TOF)2), was immediately introduced into the above solution at a 20% (w/w) concentration. The resultant solution was filtered, cleaned with acetonitrile, and dried in air to yield α-aminophosphonate after stirring for 72 h at room temperature.
2.6. Synthesis of FuS@MXene@AMP composite
0.5 g of FuS and 1.3 g of α-aminophosphonates were introduced to a 6 mL MXene suspension to produce the FuS@MXene@AMP composite. The resultant solution produced a gelatinous substance after being centrifuged for 25 minutes at 3500 rpm and ultrasonically dispersed for 65 minutes. The gelatinous substance was transferred to a vacuum oven and heated for 18 hours at 70 °C to get the FuS@MXene@AMP composite. After being crushed into powder, the synthesized FuS@MXene@AMP composite was cleaned with acetone and dried for 6 hours at 55 °C. Scheme 1 depicts the mechanism for the preparation of the FuS@MXene@AMP composite.2.7. Characterizations
The functional groups of the ternary composite were determined by employing a Fourier transform infrared spectrometer (FT-IR; Nicolet NEXUS 670). X-ray photoelectron spectroscopy (XPS; PHI 5000, ULVAC-PHI, Japan) was used to analyze the adsorbent chemical states. Brunauer–Emmett–Teller (BET; ASAP 2050, America) was employed to investigate the pore size distribution and surface area of the ternary composites. Using a scanning electron microscope (SEM, FEI TF20, USA), the morphologies of the ternary adsorbent were examined.2.8. Batch sorption studies
The batch adsorption study is discussed in detail in Text S2.†2.9. Extraction process
The extraction process is discussed in detail in Text S3.†3 Result and discussion
3.1. Characterization of the FuS@MXene@AMP composite
Fig. 1(a) displays the FTIR spectra of the synthesized FuS, FuS@MXene, and FuS@MXene@AMP composite. The stretching vibration of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1237 cm−1 is attributed to the FuS peaks in the FTIR spectrum. For FuS, the C–O–C stretching vibration of the pyran ring was observed at 1025 cm−1. The peak at 3428 cm−1 corresponds to the OH group of FuS. In the FTIR spectrum of FuS@MXene, three new peaks appeared at 623, 1119, and 1645 cm−1, corresponding to the Ti–O, C–F, and C
O at 1237 cm−1 is attributed to the FuS peaks in the FTIR spectrum. For FuS, the C–O–C stretching vibration of the pyran ring was observed at 1025 cm−1. The peak at 3428 cm−1 corresponds to the OH group of FuS. In the FTIR spectrum of FuS@MXene, three new peaks appeared at 623, 1119, and 1645 cm−1, corresponding to the Ti–O, C–F, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of MXene. Xing et al. have reported similar peaks for MXene.54 In contrast to FuS@MXene, a new peak at 1619 cm−1 corresponding to the primary and secondary –NH groups appeared in the FTIR spectrum of FuS@MXene@AMP. Additionally, a peak at 2830 cm−1 verified the existence of aromatic and aliphatic C–H groups in the prepared FuS@MXene@AMP ternary composite. Peaks at 1275 cm−1 are associated with the P
O groups of MXene. Xing et al. have reported similar peaks for MXene.54 In contrast to FuS@MXene, a new peak at 1619 cm−1 corresponding to the primary and secondary –NH groups appeared in the FTIR spectrum of FuS@MXene@AMP. Additionally, a peak at 2830 cm−1 verified the existence of aromatic and aliphatic C–H groups in the prepared FuS@MXene@AMP ternary composite. Peaks at 1275 cm−1 are associated with the P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups. Imam et al. have reported similar peaks for α-aminophosphonate.55 The appearance of NH, C–H, and P
O groups. Imam et al. have reported similar peaks for α-aminophosphonate.55 The appearance of NH, C–H, and P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups justified the successful introduction of α-aminophosphonate in FuS@MXene. The FTIR peaks confirmed the presence of Ti–O, C
O groups justified the successful introduction of α-aminophosphonate in FuS@MXene. The FTIR peaks confirmed the presence of Ti–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–F, HOSO3−, P
C, C–F, HOSO3−, P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, NH, and OH groups, which increased the adsorption performance of the adsorbent. SMX and Nd3+ adsorbed on the surface of the adsorbent through hydrogen bonding, π–π interaction, and electrostatic interaction between Ti–O, C
O, NH, and OH groups, which increased the adsorption performance of the adsorbent. SMX and Nd3+ adsorbed on the surface of the adsorbent through hydrogen bonding, π–π interaction, and electrostatic interaction between Ti–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–F, HOSO3−, P
C, C–F, HOSO3−, P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, NH, and OH groups of the adsorbent and SMX and Nd3+.
O, NH, and OH groups of the adsorbent and SMX and Nd3+.
The XPS survey spectra for the FuS, FuS@MXene, and FuS@MXene@AMP composites are depicted in Fig. 1(b). In the FuS XPS spectrum, typical XPS signals of S 2p, S 2s, C 1s, and O 1s were detected. In contrast to FuS, two new XPS signals of Ti 2p and F 1s appeared in the XPS spectrum of FuS@MXene. The appearance of Ti 2p and F 1s XPS signals suggested the successful introduction of MXene in FuS. In the XPS survey spectra of FuS@MXene@AMP, two XPS peaks of P 2p and N 1s emerged. The appearance of new XPS peaks of P 2p and N 1s and the increased intensities of O 1s, S 2s, S 2p, and C 1s justified the successful preparation of the ternary composite.
Fig. 2 displays the high-resolution XPS spectra of these elements. In the high-resolution XPS C 1s survey spectrum, C–H and C–C functional groups appeared at a binding energy of 284.28 eV. Similarly, at a binding energy of 284.28 eV, the C–S, C–OH, and C–P groups emerged. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O–P, and C–O–C groups were observed at a binding energy of 287.20 eV in the C 1s XPS spectrum (Fig. 2(a)).56,57 The characteristic XPS signals of OH, C
O, C–O–P, and C–O–C groups were observed at a binding energy of 287.20 eV in the C 1s XPS spectrum (Fig. 2(a)).56,57 The characteristic XPS signals of OH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, P–O, and P–O–C were detected at 530.51, 532.31, and 532.94 eV, respectively, according to the O 1s XPS spectrum displayed in Fig. 2(b).58,59 For N 1s, two groups appeared at a binding energy of 399.57 and 401.92 eV, attributing to NH2, NH and NH2+, NH3+ groups, respectively.60 Three sulfur-containing groups corresponding to C–S, C
O, P–O, and P–O–C were detected at 530.51, 532.31, and 532.94 eV, respectively, according to the O 1s XPS spectrum displayed in Fig. 2(b).58,59 For N 1s, two groups appeared at a binding energy of 399.57 and 401.92 eV, attributing to NH2, NH and NH2+, NH3+ groups, respectively.60 Three sulfur-containing groups corresponding to C–S, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S, and S–O (2p3/2) groups emerged in the case of S 2p at binding energies of 161.23, 163.54, and 166.81 eV. XPS signals relating to C–S, C–OH, C–H, C–C, C–P, OH, C
S, and S–O (2p3/2) groups emerged in the case of S 2p at binding energies of 161.23, 163.54, and 166.81 eV. XPS signals relating to C–S, C–OH, C–H, C–C, C–P, OH, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, P–O, P–O–C, NH2, NH, and HOSO3− groups of adsorbents are observed, which offered active sites for the adsorption of SMX and Nd3+.
O, P–O, P–O–C, NH2, NH, and HOSO3− groups of adsorbents are observed, which offered active sites for the adsorption of SMX and Nd3+.
 | ||
| Fig. 2 High-resolution XPS spectra of the FuS@MXene@AMP composite before adsorption of SMX and Nd3+: C 1s (a), O 1s (b), N 1s (c), and S 2p (d). | ||
The BET-specific surface area (SSA) and pore size distribution for the FuS, FuS@MXene, and FuS@MXene@AMP composite are depicted in Fig. 3(a and b). MXene indicates characteristic microporous properties, as seen in Fig. 3(a). The FuS@MXene and FuS@MXene@AMP composite indicated type IV nitrogen adsorption/desorption isotherms with characteristic H3 hysteresis loops, suggesting the existence of mesopores and micropores. The specific surface area of the FuS@MXene@AMP composite was 428 m2 g−1, which was significantly greater than that of FuS@MXene (341 m2 g−1) and FuS (147 m2 g−1), as exhibited in Fig. 3(a). The higher surface area of the FuS@MXene@AMP composite was attributed to the presence of pores and lamellar morphology.61,62 According to Fig. 3(b), FuS exhibited pore diameters ranging from 2 to 119 nm, averaging 21.8 nm (4 V/A by BET). FuS@MXene and FuS@MXene@AMP composite had average pore diameters of 19.4 and 18.5 nm, respectively, less than that of FuS. The FuS@MXene@AMP composite is more promising than FuS@MXene and FuS in forming mesoporous structures, which is advantageous for adsorption.63 This enhanced BET surface area and pore diameters of the FuS@MXene@AMP adsorbent than FuS and FuS@MXene are anticipated to promote the diffusion of SMX and Nd3+ during the adsorption of pollutants, enabling faster and more efficient adsorption of SMX and Nd3+.
 | ||
| Fig. 3 N2 adsorption–desorption (a) and pore distribution (b) of FuS, FuS@MXene, and the FuS@MXene@AMP composite. | ||
The morphologies of FuS, FuS@MXene, and FuS@MXene@AMP are studied employing SEM, as depicted in Fig. 4(a–e). The Al layers are successfully etched from the MAX phase (Ti3AlC2), as evidenced by the accordion-like multilayer morphology (Fig. 4(a)). Fig. 4(b) indicates small-sized particles of FuS with irregular shapes. Large, irregularly shaped AMP particles are visible in Fig. 4(c). The layered structure of MXene, larger AMP particles, and tiny FuS particles are all visible in Fig. 4(d and e), which confirms the successful formation of the FuS@MXene@AMP composite. Fig. 4(e) depicts the porous surface of the composite, which is appropriate for the adsorption of Nd3+ and SMX.
3.2. Adsorption of SMX and Nd3+ by FuS@MXene@AMP composite in mono-pollutant systems
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, NH, and HOSO3− groups of the FuS@MXene@AMP adsorbent and the S
O, NH, and HOSO3− groups of the FuS@MXene@AMP adsorbent and the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and NH groups of SMX. Apart from hydrogen bonding, there were also π–π interactions between the benzene ring in the FuS@MXene@AMP adsorbent and the SMX. In this work, hydrogen bonding and π–π interaction are the dominating interactions that played a vital role in the adsorption of SMX by the adsorbent. Xue et al. have reported similar interactions in the adsorption of SMX.67 When the pH value was raised from 5.7 to 10, the FuS@MXene@AMP adsorbent's adsorption capacity for SMX was lowered gradually due to the quick conversion of the zwitterionic form (SMX0) to an anionic (SMX−) form. The anionic FuS@MXene@AMP composite and negatively charged (SMX−) exhibited electrostatic repulsion, significantly hindering SMX's adsorption onto the adsorbent. Because of π–π interactions and hydrogen bonding, SMX− could still adsorb on the negatively charged FuS@MXene@AMP surface despite this strong electrostatic repulsion.
O and NH groups of SMX. Apart from hydrogen bonding, there were also π–π interactions between the benzene ring in the FuS@MXene@AMP adsorbent and the SMX. In this work, hydrogen bonding and π–π interaction are the dominating interactions that played a vital role in the adsorption of SMX by the adsorbent. Xue et al. have reported similar interactions in the adsorption of SMX.67 When the pH value was raised from 5.7 to 10, the FuS@MXene@AMP adsorbent's adsorption capacity for SMX was lowered gradually due to the quick conversion of the zwitterionic form (SMX0) to an anionic (SMX−) form. The anionic FuS@MXene@AMP composite and negatively charged (SMX−) exhibited electrostatic repulsion, significantly hindering SMX's adsorption onto the adsorbent. Because of π–π interactions and hydrogen bonding, SMX− could still adsorb on the negatively charged FuS@MXene@AMP surface despite this strong electrostatic repulsion.
The influence of pH on Nd3+ sorption by TCFs was studied at pH values ranging from 1 to 7 due to the precipitation of REE at pH values above 7.68 Fig. 5(b) illustrates the adsorption of Nd3+ by the adsorbent as a function of pH. When the pH was below 3.0 (pHPZC), the FuS@MXene@AMP surface had a positive charge, and the adsorption of Nd3+ by the adsorbent was negligible due to the strong electrostatic repulsion between the positively charged FuS@MXene@AMP surface and Nd3+ as well as the inhabitation impact of –OH deprotonation. When the pH of the solution slowly increases to 5.0, the positively charged FuS@MXene@AMP transformed into negatively charged, and the adsorption capacity or removal rate of the adsorbent for Nd3+ was improved due to electrostatic interaction between the negatively charged FuS@MXene@AMP adsorbent and positively charged Nd3+, and more OH species may be deprotonated. The hydrolysis of Nd3+ at higher pH values of 6 and 7 may be the primary cause of the slight drop in adsorption capacity.69,70
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, NH, and OH) than the MXene and FuS, FuS@MXene exhibited superior adsorption performance for SMX and Nd3+, as depicted in Fig. S5(a and b).†
O, NH, and OH) than the MXene and FuS, FuS@MXene exhibited superior adsorption performance for SMX and Nd3+, as depicted in Fig. S5(a and b).†
 | ||
| Fig. 7 Adsorption kinetics of the FuS@MXene@AMP adsorbent for SMX; PSO, PFO (a) and IPD (b) and Nd3+; PSO and PFO (c) and IPD (d) onto the FuS@MXene@AMP. | ||
The IPD kinetic model for SMX and Nd3+ is illustrated in Fig. 7(b) and (d). Three stages comprise the adsorption process for SMX and Nd3+: a fast stage at the beginning, an intermediate slower phase, and an equilibrium phase at the end. According to Table S3,† the values of the rate constant for the first phase (kint1) were notably greater than the values of the second phase (kint2) and third phase (kint3), suggesting that the rate-controlling step for the adsorption of SMX and Nd3+ was primary film diffusion. Furthermore, in the adsorption of SMX and Nd3+, no straight lines pass through the point of origin, indicating that the adsorption reaction was not only controlled by intraparticle diffusion but also involved some other diffusion processes, such as surface diffusion.77
To evaluate the thermodynamic properties of SMX and Nd3+, various thermodynamic parameters, including enthalpy change (kJ mol−1; ΔH°), Gibbs free energy (kJ mol−1; ΔG°), and entropy change (ΔS°; J mol K−1), were calculated employing the following equation.
ΔG° = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KD KD
| (1) |
 | (2) |
| ΔG° = ΔH° + TΔS | (3) |
T (K) is the absolute temperature and R (J mol−1 K−1) is the ideal gas constant. The values of ΔS° and ΔH° were determined by utilizing the slope and intercept of the ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KD−1/T plot, respectively.
KD−1/T plot, respectively.
The thermodynamic investigation is essential to comprehending the sorption mechanism. Table S7† shows that the changes in Gibbs free energy (ΔG < 0) were negative at different temperatures, suggesting that FuS@MXene@AMP spontaneously adsorbed SMX and Nd3+. The adsorption of SMX and Nd3+ was irreversible, as demonstrated by the positive change in entropy. The adsorption process was endothermic, as indicated by the positive values of the enthalpy change.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is employed to accomplish SMX desorption.85 A 0.2 M HCl solution is employed to attain Nd3+ desorption.86 After adsorption of Nd3+ and SMX in a mono-pollutant or binary pollutant system, the spent FuS@MXene@AMP adsorbent was recovered using centrifugation, rinsed with excessive Milli-Q water, and thereafter submerged in HCl (in the case of Nd3+) or in a mixed solution of ethanol and HCl (in the case of SMX) and agitated for two hours at ambient temperature, performed three times, cleaned again with DI water, and finally vacuum-dried at 60 °C overnight. The recovered adsorbent was utilized to adsorb naproxen under identical experimental circumstances. The adsorption and desorption analyses are performed in four consecutive cycles. Eqn (4) and (5) are employed to calculate the desorption efficiency and regeneration rate (%).
1 is employed to accomplish SMX desorption.85 A 0.2 M HCl solution is employed to attain Nd3+ desorption.86 After adsorption of Nd3+ and SMX in a mono-pollutant or binary pollutant system, the spent FuS@MXene@AMP adsorbent was recovered using centrifugation, rinsed with excessive Milli-Q water, and thereafter submerged in HCl (in the case of Nd3+) or in a mixed solution of ethanol and HCl (in the case of SMX) and agitated for two hours at ambient temperature, performed three times, cleaned again with DI water, and finally vacuum-dried at 60 °C overnight. The recovered adsorbent was utilized to adsorb naproxen under identical experimental circumstances. The adsorption and desorption analyses are performed in four consecutive cycles. Eqn (4) and (5) are employed to calculate the desorption efficiency and regeneration rate (%).
 | (4) |
 | (5) |
 | ||
| Fig. 9 The influences of regeneration on FuS@MXene@AMP adsorption capacity and desorption performance (a) and its use in simulated real wastewater (b). | ||
The ability of FuS@MXene@AMP to remove SMX and Nd3+ and its regeneration capability is investigated in simulated water. The detailed process for the preparation of simulated water is provided in Text S4.†Fig. 9(b) illustrates that at a concentration of 5 g L−1 of the FuS@MXene@AMP composite, the adsorption rates for SMX and Nd3+ are up to 92%. Following regeneration, the adsorption rate of the composites for SMX and Nd3+ slightly decreased. As a result, FuS@MXene@AMP also demonstrated remarkable regeneration ability and adsorption performance in the simulated model wastewater.
 | (6) |
 | ||
| Fig. 10 Simultaneous adsorption of SMX (a) and Nd3+ (b) onto the FuS@MXene@AMP composite in SMX–Nd3+(a) and Nd3+–SMX binary solutions. | ||
Depending on the value of Rq, the simultaneous adsorption of multiple pollutants can be divided into three categories. Synergism (where Rq > 1) enhances the adsorbent's ability to adsorb the target adsorbate, antagonism (where Rq < 1), where the adsorbent's ability to adsorb the target adsorbate is reduced when a co-adsorbate is present, and non-interacting (where Rq = 1). Fig. S7(a and b)† displays the determined Rq values based on the initial SMX and Nd3+ concentrations. The co-occurrence of Nd3+ remarkably increased the adsorption of SMX in the SMX–Nd3+ pollutant systems (Rq, SMX > 1). In the antibiotic-heavy metal binary pollutant solution systems, the occurrence of Nd3+ notably enhanced the adsorption capacity of SMX, especially at higher concentrations of Nd3+. This phenomenon may be caused by several reactions during the adsorption of SMX in the presence of Nd3+. Firstly, the medium influence of Nd3+ in the SMX adsorption phenomenon enhanced the adsorption capability of SMX. Nd3+ exhibited coordination with SMX and formed bonds with OH, NH, HOSO3−, F, O, and S on the adsorbent surface, utilizing the multifunctional characteristics of the antibiotics. Multiple reports have demonstrated that these functional groups cause drugs to bind to other materials, mainly metal ions, to form antibiotic-metal ion complexes.87–90 SMX might also interact with metal hydroxide by coordination, indirectly bound to the composite, and synergistically eliminated, in addition to the phenomena entailed in the direct actions of FuS@MXene@AMP in mono-pollutant systems.61 Another effective mechanism might include the generation of an antibiotic-metal combination between SMX and Nd3+ before being adsorbed on the FuS@MXene@AMP adsorbent surface.91 Notably, at a lower concentration of Nd3+, the Rq value for SMX was less than 1. Thus, we can say that the “medium” role of metal ions (Nd3+) was not possible at lower Nd3+ concentrations. For this scenario, a minute dose of Nd3+ was first attached to SMX in the solution, generating an Nd3+ + SMX combination, then attached to the FuS@MXene@AMP composite surface to be removed.
Like SMX, the adsorbent's Nd3+ adsorption capability in the Nd3+–SMX binary system was also higher than that of the mono-pollutant system (Rq, Nd3+ > 1), as exhibited in Fig. S7(a and b).† The adsorption of other metal ions, such as Ca2+ and Al3+, in the presence of SMX was investigated. The presence of SMX synergistically enhanced the adsorption of Ca2+ and Al3+, as shown by the Rq values exceeding 1 (Fig. S8†). The presence of multiple functional groups in SMX antibiotics adsorbed on the FuS@MXene@AMP composite surface, where additional oxygenous and nitrogenous functional groups were available, potentially describes this synergistic interaction and facilitates the adsorption of Nd3+, Ca2+, and Al3+.
3.2.8.1. Possible adsorption mechanism for neodymium (Nd3+). Two types of interactions, such as metal complexation and electrostatic interactions, are involved in the adsorption of Nd3+, as exhibited in Fig. 13. XPS and FTIR analyses were conducted before or after the adsorption to describe the possible adsorption mechanism of Nd3+ by the FuS@MXene@AMP adsorbent. The FTIR peaks for Ti–O, C–F, HOSO3−, P
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, NH, and OH groups shifted from 623, 1119, 1237, 1275, 1614, and 3428 cm−1 to 614, 1108, 1223, 1255, 1602, and 3410 cm−1, respectively (Fig. S9†). The shifting of the FTIR peaks of these functional groups of the FuS@MXene@AMP adsorbent suggested the electrostatic attraction between Nd3+ and Ti–O, C–F, HOSO3−, P
O, NH, and OH groups shifted from 623, 1119, 1237, 1275, 1614, and 3428 cm−1 to 614, 1108, 1223, 1255, 1602, and 3410 cm−1, respectively (Fig. S9†). The shifting of the FTIR peaks of these functional groups of the FuS@MXene@AMP adsorbent suggested the electrostatic attraction between Nd3+ and Ti–O, C–F, HOSO3−, P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, NH, and OH. Consequently, the electrostatic attraction was a dominant force for the adsorption of Nd3+ due to the sufficient electronegativity difference between the Ti–O, C–F, HOSO3−, P
O, NH, and OH. Consequently, the electrostatic attraction was a dominant force for the adsorption of Nd3+ due to the sufficient electronegativity difference between the Ti–O, C–F, HOSO3−, P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, NH, and OH groups of adsorbents and Nd3+ metal ions. For instance, the electronegativity differences are 1.35, 3.84, 1.44, 1.25, 1.90, and 2.30 between Ti–O, C–F, HOSO3−, P
O, NH, and OH groups of adsorbents and Nd3+ metal ions. For instance, the electronegativity differences are 1.35, 3.84, 1.44, 1.25, 1.90, and 2.30 between Ti–O, C–F, HOSO3−, P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, NH, and OH groups of FuS@MXene@AMP and Nd3+, respectively. XPS analysis was further used to investigate the adsorption mechanism of Nd3+ before or after adsorption. Three prominent peaks are visible in the C 1s spectra of the FuS@MXene@AMP composite at binding energies of 284.28 (C–H and C–C), 285.69 (C–OH, C–S, and C–P), and 287.20 eV (C
O, NH, and OH groups of FuS@MXene@AMP and Nd3+, respectively. XPS analysis was further used to investigate the adsorption mechanism of Nd3+ before or after adsorption. Three prominent peaks are visible in the C 1s spectra of the FuS@MXene@AMP composite at binding energies of 284.28 (C–H and C–C), 285.69 (C–OH, C–S, and C–P), and 287.20 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O–P, and C–O–C), as exhibited in Fig. 2(a). After the adsorption of Nd3+, the binding energies shift from 284.28, 285.69, and 287.20 eV to 284.01, 285.18, and 287.00 eV, respectively (Fig. 11(a)). The findings show that the CH, C–C, C–OH, C–S, C–P, C
O, C–O–P, and C–O–C), as exhibited in Fig. 2(a). After the adsorption of Nd3+, the binding energies shift from 284.28, 285.69, and 287.20 eV to 284.01, 285.18, and 287.00 eV, respectively (Fig. 11(a)). The findings show that the CH, C–C, C–OH, C–S, C–P, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O–P, and C–O–C functional groups present in FuS@MXene@AMP adsorbed Nd3+ pollutants via electrostatic interaction and metal complexation. The high-resolution XPS spectra of the FuS@MXene@AMP composites showed three characteristic XPS peaks in the O 1s region, which correspond to the P–O–C or P
O, C–O–P, and C–O–C functional groups present in FuS@MXene@AMP adsorbed Nd3+ pollutants via electrostatic interaction and metal complexation. The high-resolution XPS spectra of the FuS@MXene@AMP composites showed three characteristic XPS peaks in the O 1s region, which correspond to the P–O–C or P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and OH groups (Fig. 2(b)). Originally, these XPS peaks were detected at the binding energies of 530.51, 532.31, and 532.94 eV, respectively.92 After Nd3+ adsorption, the three peaks were shifted to 530.31, 532.04, and 532.76 eV (Fig. 11(b)). These shifts in binding energies of P
O, and OH groups (Fig. 2(b)). Originally, these XPS peaks were detected at the binding energies of 530.51, 532.31, and 532.94 eV, respectively.92 After Nd3+ adsorption, the three peaks were shifted to 530.31, 532.04, and 532.76 eV (Fig. 11(b)). These shifts in binding energies of P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, OH, and P–O–C groups of the FuS@MXene@AMP composites confirmed the vital role of oxygen-containing groups in the adsorption of Nd3+. Two distinct peaks assigned to the NH2 or NH and NH2+ or NH3+ groups are visible in the XPS survey spectra of FuS@MXene@AMP composites in the N 1s region, as illustrated in Fig. 2(c). Before the adsorption of Nd3+, the binding energies were identified at 399.57 and 401.92 eV, respectively. After the adsorption of Nd3+, these XPS peaks were shifted to 399.43 and 401.76 eV for Nd3+ (Fig. 11(c)). The XPS survey spectra of the FuS@MXene@AMP composite in the S 2p region showed three characteristic peaks attributed to the C–S, C
O, OH, and P–O–C groups of the FuS@MXene@AMP composites confirmed the vital role of oxygen-containing groups in the adsorption of Nd3+. Two distinct peaks assigned to the NH2 or NH and NH2+ or NH3+ groups are visible in the XPS survey spectra of FuS@MXene@AMP composites in the N 1s region, as illustrated in Fig. 2(c). Before the adsorption of Nd3+, the binding energies were identified at 399.57 and 401.92 eV, respectively. After the adsorption of Nd3+, these XPS peaks were shifted to 399.43 and 401.76 eV for Nd3+ (Fig. 11(c)). The XPS survey spectra of the FuS@MXene@AMP composite in the S 2p region showed three characteristic peaks attributed to the C–S, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S, and S–O (2p3/2) groups, as shown in Fig. 2(d). Before the adsorption of Nd3+, these peaks were identified at binding energies of 161.23, 163.54, and 166.81 eV, respectively. After successful sorption of Nd3+, the three XPS peaks shifted to 161.01, 163.28, and 166.63 eV Fig. 11(d). The results suggested that the nitrogen- and sulfur-containing functional groups of the composites play a role in the adsorption of Nd3+.
S, and S–O (2p3/2) groups, as shown in Fig. 2(d). Before the adsorption of Nd3+, these peaks were identified at binding energies of 161.23, 163.54, and 166.81 eV, respectively. After successful sorption of Nd3+, the three XPS peaks shifted to 161.01, 163.28, and 166.63 eV Fig. 11(d). The results suggested that the nitrogen- and sulfur-containing functional groups of the composites play a role in the adsorption of Nd3+.
 | ||
| Fig. 11 High-resolution XPS survey spectra of C 1s (a), O 1s (b), N 1s (c), and S 2p (d) after adsorption of Nd3+. | ||
3.2.8.2. Possible adsorption mechanism for SMX. The PSO model was well fitted with the experimental uptake data of SMX, indicating that SMX was chemically adsorbed on the adsorbent surface, which may include hydrogen bonding and π–π interactions. The change in the peak positions of functional groups of the adsorbent was assessed after the adsorption of SMX through the FTIR study. The peaks in the FTIR spectrum of FuS@MXene@AMP-SMX were shifted from 623, 1119, 1237, 1275, 1614, and 3428 cm−1 to 619, 1113, 1229, 1267, 1609, and 3419 cm−1, corresponding to the Ti–O, C–F, HOSO3−, P
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, NH, and OH groups, respectively (Fig. S9†). The SMX molecules consist of SO2NH2, NH2, CH3, and C3H3NO groups, as shown in Fig. 13. The adsorption process of SMX was facilitated by the Ti–O, C–F, HOSO3−, P
O, NH, and OH groups, respectively (Fig. S9†). The SMX molecules consist of SO2NH2, NH2, CH3, and C3H3NO groups, as shown in Fig. 13. The adsorption process of SMX was facilitated by the Ti–O, C–F, HOSO3−, P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, NH, and OH groups of the adsorbent forming hydrogen bonds with SO2NH2, NH2, CH3, and C3H3NO of SMX. After the adsorption of SMX, peaks were shifted from 1580 to 1568 cm−1, attributing to the C
O, NH, and OH groups of the adsorbent forming hydrogen bonds with SO2NH2, NH2, CH3, and C3H3NO of SMX. After the adsorption of SMX, peaks were shifted from 1580 to 1568 cm−1, attributing to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C aromatic functional group, which indicated the π–π interaction between the prepared adsorbent consisting of the aromatic ring and benzene ring of SMX.93 XPS spectra results help further explore the SMX adsorption mechanisms. After SMX uptake, the binding energies of CH, C–C, C–OH, C–S, C–P, C
C aromatic functional group, which indicated the π–π interaction between the prepared adsorbent consisting of the aromatic ring and benzene ring of SMX.93 XPS spectra results help further explore the SMX adsorption mechanisms. After SMX uptake, the binding energies of CH, C–C, C–OH, C–S, C–P, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O–P, C–O–C, C–OH, C–S, and C–P were shifted from 284.28, 285.69, and 287.20 eV to 284.01, 285.36, and 287.05 eV, respectively (Fig. 12(a)). The adsorption of SMX was promoted by the CH, C–C, C–OH, C–S, C–P, C
O, C–O–P, C–O–C, C–OH, C–S, and C–P were shifted from 284.28, 285.69, and 287.20 eV to 284.01, 285.36, and 287.05 eV, respectively (Fig. 12(a)). The adsorption of SMX was promoted by the CH, C–C, C–OH, C–S, C–P, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O–P, and C–O–C functional groups of the adsorbent through hydrogen bonding and π–π interactions. The peaks of P–O–C, or P
O, C–O–P, and C–O–C functional groups of the adsorbent through hydrogen bonding and π–π interactions. The peaks of P–O–C, or P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and OH groups originally appeared at 530.51, 532.31, and 532.94 eV before adsorption in the O 1s spectra and were shifted to 530.45, 532.22, and 532.89 eV after adsorption of SMX, respectively (Fig. 12(b)), suggesting that P–O–C, or P
O, and OH groups originally appeared at 530.51, 532.31, and 532.94 eV before adsorption in the O 1s spectra and were shifted to 530.45, 532.22, and 532.89 eV after adsorption of SMX, respectively (Fig. 12(b)), suggesting that P–O–C, or P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and OH of the adsorbent form hydrogen bonding with SO2NH2, NH2, CH3, and C3H3NO of SMX. In the N 1s region, two distinct peaks corresponding to the NH2 or NH and NH2+ or NH3+ groups were observed at 399.57 and 401.92 eV. After the adsorption of SMX, these XPS peaks were shifted to 399.49 and 401.85 eV Fig. 12(c). The peaks of C–S, C
O, and OH of the adsorbent form hydrogen bonding with SO2NH2, NH2, CH3, and C3H3NO of SMX. In the N 1s region, two distinct peaks corresponding to the NH2 or NH and NH2+ or NH3+ groups were observed at 399.57 and 401.92 eV. After the adsorption of SMX, these XPS peaks were shifted to 399.49 and 401.85 eV Fig. 12(c). The peaks of C–S, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S, and S–O (2p3/2) groups were identified at binding energies of 161.23, 163.54, and 166.81 eV in the s 2p spectra before adsorption, and these peaks shifted to 161.09, 163.48, and 166.74 eV after the adsorption of SMX, respectively (Fig. 12(d)). The results indicated that the nitrogen- and sulfur-containing functional groups of FuS@MXene@AMP were involved in the adsorption of SMX through hydrogen bonding.
S, and S–O (2p3/2) groups were identified at binding energies of 161.23, 163.54, and 166.81 eV in the s 2p spectra before adsorption, and these peaks shifted to 161.09, 163.48, and 166.74 eV after the adsorption of SMX, respectively (Fig. 12(d)). The results indicated that the nitrogen- and sulfur-containing functional groups of FuS@MXene@AMP were involved in the adsorption of SMX through hydrogen bonding.
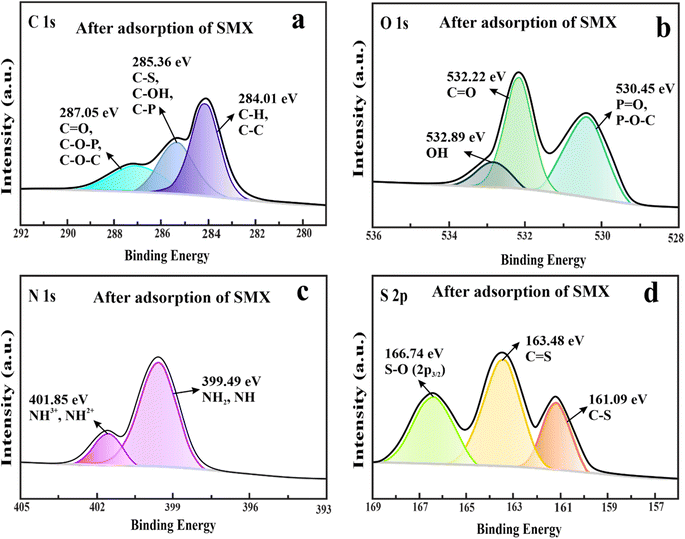 | ||
| Fig. 12 High-resolution XPS survey spectra of C 1s (a), O 1s (b), N 1s (c), and S 2p (d) after adsorption of SMX. | ||
3.2.8.3. Possible adsorption mechanism for the binary pollutant system. The co-occurrence of Nd3+ remarkably increased the adsorption of SMX in the SMX–Nd3+ pollutant systems. The occurrence of Nd3+ and SMX in the solution notably enhanced the adsorption capacity of SMX or Nd3+. This might be due to the following types of interactions:
(a) The medium effect of Nd3+ in the adsorption process increased the adsorption capacity for SMX. Specifically, in addition to interacting with Ti–O, C–F, HOSO3−, P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, NH, and OH groups on the adsorbent, parts of Nd3+ could bind with SMX.94
O, NH, and OH groups on the adsorbent, parts of Nd3+ could bind with SMX.94
(b) Firstly, SMX was adsorbed on the adsorbent surface through hydrogen bonding or π–π interaction, then SMX bound with Nd3+ through electrostatic interactions rather than directly interacting with the adsorbent like single pollutant systems.78
(c) Another effective mechanism might include the formation of an antibiotic-metal combination between SMX and Nd3+ before being adsorbed on the FuS@MXene@AMP adsorbent surface.91
FTIR and XPS analyses were used to explore the adsorption mechanism for the binary system. Fig. S9† shows the spectral changes that occur for binary pollutant systems following the adsorption of SMX + Nd3+. FTIR peaks for Ti–O, C–F, HOSO3−, P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, NH, and OH groups shifted to lower wave numbers after the SMX + Nd3+ were absorbed, suggesting that these functional groups are involved in the adsorption of SMX + Nd3+ pollutants. After the simultaneous uptake of SMX and Nd3+ from the binary pollutant system, the binding energies of CH, C–C, C–OH, C–S, C–P, C
O, NH, and OH groups shifted to lower wave numbers after the SMX + Nd3+ were absorbed, suggesting that these functional groups are involved in the adsorption of SMX + Nd3+ pollutants. After the simultaneous uptake of SMX and Nd3+ from the binary pollutant system, the binding energies of CH, C–C, C–OH, C–S, C–P, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O–P, C–O–C, C–OH, C–S, and C–P were moved from 284.28, 285.69, and 287.20 eV to 283.99, 285.02, and 286.96 eV, respectively (Fig. S10(a)†). The peaks of P–O–C, or P
O, C–O–P, C–O–C, C–OH, C–S, and C–P were moved from 284.28, 285.69, and 287.20 eV to 283.99, 285.02, and 286.96 eV, respectively (Fig. S10(a)†). The peaks of P–O–C, or P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C
O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and OH groups initially emerged at 530.51, 532.31, and 532.94 eV before adsorption in the O 1s spectra and were shifted to 530.68, 531.99, and 532.68 eV after the adsorption of SMX + Nd3+, respectively (Fig. S10(b)†). In the N 1s region, two distinct peaks corresponding to the NH2 or NH and NH2+ or NH3+ groups were seen at 399.59 and 401.92 eV. After capturing SMX + Nd3+, these XPS peaks were shifted to 399.28 and 401.59 eV (Fig. S10(c)†). The peaks of C–S, C
O, and OH groups initially emerged at 530.51, 532.31, and 532.94 eV before adsorption in the O 1s spectra and were shifted to 530.68, 531.99, and 532.68 eV after the adsorption of SMX + Nd3+, respectively (Fig. S10(b)†). In the N 1s region, two distinct peaks corresponding to the NH2 or NH and NH2+ or NH3+ groups were seen at 399.59 and 401.92 eV. After capturing SMX + Nd3+, these XPS peaks were shifted to 399.28 and 401.59 eV (Fig. S10(c)†). The peaks of C–S, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S, and S–O (2p3/2) groups were detected at 161.23, 163.54, and 166.81 eV in the S 2p spectra before adsorption, and these peaks shifted to 160.99, 163.10, and 166.01 eV after the adsorption of SMX + Nd3+, respectively (Fig. S10(d)†). The shifting of peaks in the C 1s, O 1s, N 1s, and S 2p high-resolution spectra suggested that the carbon-, oxygen-, nitrogen-, and sulfur-containing functional groups of composites play a part in the adsorption of SM + Nd3+ (Fig. 13).
S, and S–O (2p3/2) groups were detected at 161.23, 163.54, and 166.81 eV in the S 2p spectra before adsorption, and these peaks shifted to 160.99, 163.10, and 166.01 eV after the adsorption of SMX + Nd3+, respectively (Fig. S10(d)†). The shifting of peaks in the C 1s, O 1s, N 1s, and S 2p high-resolution spectra suggested that the carbon-, oxygen-, nitrogen-, and sulfur-containing functional groups of composites play a part in the adsorption of SM + Nd3+ (Fig. 13).
4 Conclusion
The efficient adsorption of SMX and Nd3+ from wastewater was conducted by preparing an unexplored FuS@MXene@AMP adsorbent. Over a wide pH range, the FuS@MXene@AMP adsorbent exhibited an excellent ability to remove SMX and Nd3+ in mono and binary pollutant systems. A pseudo-first-order (PSO) kinetic model was the most appropriate for describing the adsorption of SMX and Nd3+ among all the tested kinetic models. With a higher value of adsorption capacity and R2, the Langmuir isotherm model provided a better description of the adsorption of SMX and Nd3+. Compared to the previously reported adsorbents, FuS@MXene@AMP adsorbents demonstrated noticeably higher adsorption capacities for SMX and Nd3+. Furthermore, it was found that FuS@MXene@AMP exhibited higher adsorption performance even after undergoing 4 cycles in wastewater simulation. This result demonstrates its applicability and reusability in wastewater treatment procedures. In binary SMX + Nd3+ pollutant systems, FuS@MXene@AMP indicates excellent ability for simultaneous adsorption of coexisting Nd3+ and SMX from wastewater. The improved adsorption performance was primarily due to the synergistic interaction of SMX and Nd3+. The results of FTIR and XPS studies indicated that the removal mechanisms were primarily based on hydrogen bonding, π–π interaction, complexation, and electrostatic interactions. This research provides a practical strategy for designing new adsorbents that can efficiently adsorb heavy metals and antibiotics over a broad pH range.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Aysha Bukhari: data curation, supervision, writing original draft, reviewing, and editing, Irfan Ijaz: formal analysis, visualization, writing original draft, reviewing, and editing, Ezaz Gilani: reviewing, and editing, and methodology, Ammara Nazir: writing original draft, software, and investigation, Hina Zain: data curation and investigation, Attia Shaheen: formal analysis, and methodology, Mohammed Rafi Shaik: investigation and validation, Mujeeb Khan: reviewing, and editing and data curation, Jilani P. Shaik: formal analysis and resource.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors acknowledge the funding from Researchers Supporting Project number (RSPD2025R665), King Saud University, Riyadh, Saudi Arabia. All authors also acknowledge the School of Chemistry, Minhaj University Lahore, Pakistan, for providing a platform for this work.References
- Q. Fan, C. Sun, B. Hu and Q. Wang, Mater. Today Bio, 2023, 20, 100646 CrossRef CAS PubMed.
- S. Sun, M. Chen, X. Dao, L. Wang, X. Huang, L. Zhou and H. Hao, Sep. Purif. Technol., 2023, 306, 122537 CrossRef CAS.
- D. Liu, X. Wu, C. Hu, Y. Zeng and Q. Pang, Aquat. Toxicol., 2023, 261, 106621 CrossRef CAS PubMed.
- Y. Yang, A. Walton, R. Sheridan, K. Güth, R. Gauß, O. Gutfleisch, M. Buchert, B.-M. Steenari, T. Van Gerven and P. T. Jones, J. Sustain. Metall., 2017, 3, 122–149 CrossRef.
- N. Radwan-Pragłowska, J. Radwan-Pragłowska, K. Łysiak, Ł. Janus and T. Galek, Opt. Laser Technol., 2024, 174, 110611 CrossRef.
- T. Cheisson and E. J. Schelter, Science, 2019, 363, 489–493 CrossRef CAS PubMed.
- T. Yao, Y. Geng, J. Sarkis, S. Xiao and Z. Gao, Resour., Conserv. Recycl., 2021, 174, 105752 CrossRef CAS.
- S.-L. Liu, H.-R. Fan, X. Liu, J. Meng, A. R. Butcher, L. Yann, K.-F. Yang and X.-C. Li, Ore Geol. Rev., 2023, 157, 105428 Search PubMed.
- Y. Chen and B. Zheng, Sustainability, 2019, 11, 1288 CrossRef.
- B. Qin, B. Wang, J. Li, T. Wang and X. Xu, Sep. Purif. Technol., 2024, 342, 126905 CrossRef CAS.
- X. Chen, F. Duan, X. Yu, Y. Xie, Z. Wang, A. El-Baz, B.-J. Ni and S.-Q. Ni, Water Res., 2024, 265, 122268 CrossRef CAS PubMed.
- I. Ijaz, A. Bukhari, A. Shaheen, A. Nazir, E. Gilani, H. Zain, S. Muhammad and S. Hussain, J. Environ. Chem. Eng., 2024, 112838 CrossRef CAS.
- K. Burdzy, R. Jastrząb and D. Kołodyńska, Chem. Eng. J., 2024, 479, 147632 CrossRef CAS.
- R. Antonelli, G. R. P. Malpass and A. C. S. C. Teixeira, Sep. Purif. Technol., 2024, 330, 125290 CrossRef CAS.
- K. Lu, J. Qin, M. Hu, L. Hu, M. Mao, X. Li, Z. Lin and W. Liu, Environ. Sci.:Nano, 2024, 11, 900–910 RSC.
- Z. Qu, R. Leng, S. Wang, Z. Ji and X. Wang, Rev. Environ. Contam. Toxicol., 2024, 262, 1–37 CrossRef.
- M. Nogueira, I. Matos, M. Bernardo, L. A. C. Tarelho, A. M. Ferraria, A. M. B. do Rego, I. Fonseca and N. Lapa, J. Waste Manage., 2024, 174, 451–461 CrossRef CAS PubMed.
- S. Kathi and A. E. D. Mahmoud, Desalin. Water Treat., 2024, 100258 CrossRef.
- I. Ijaz, A. Bukhari, A. Nazir, E. Gilani, H. Zain, S. Hussain and H. Ahmad, Mater. Chem. Phys., 2024, 314, 128929 CrossRef CAS.
- J. Yu, Y. Xia, L. Chen, W. Yan, B. Liu and S. Jin, npj Clean Water, 2024, 7, 133 CrossRef CAS.
- I. Liaquat, R. Munir, N. A. Abbasi, B. Sadia, A. Muneer, F. Younas, M. F. Sardar, M. Zahid and S. Noreen, Environ. Pollut., 2024, 123922 CrossRef CAS PubMed.
- U. Tyagi and N. Anand, Waste Manag. Bull, 2024, 2, 308–325 CrossRef.
- Y. Wang, J. Wu, W. Zhang, L. Zhong, D. Zhang, S. Yan and J. Shi, React. Chem. Eng., 2024, 9, 1276–1291 RSC.
- Y. S. Giri, A. Subash and B. Kandasubramanian, Hybrid Adv., 2024, 100237 CrossRef.
- N. A. Efiana, G. Kali, A. Fürst, A. Dizdarević and A. Bernkop-Schnürch, Eur. J. Pharm. Sci., 2023, 180, 106313 CrossRef CAS PubMed.
- N. Jabeen and M. Atif, Polym. Adv. Technol., 2024, 35, e6203 CrossRef CAS.
- Q. Liu, L. Hu, C. Wang, M. Cheng, M. Liu, L. Wang, P. Pan and J. Chen, Int. J. Biol. Macromol., 2023, 225, 526–543 CrossRef CAS PubMed.
- L. Sun, Z. Jiang, B. Yuan, S. Zhi, Y. Zhang, J. Li and A. Wu, Chem. Eng. Res. Des., 2021, 174, 71–78 CrossRef CAS.
- G. Chen, L. Yu, F. Shi, J. Shen, Y. Zhang, G. Liu, X. Mei, X. Li, X. Xu and C. Xue, Carbohydr. Polym., 2024, 122345 CrossRef CAS PubMed.
- S. B. Kim, M. Farrag, S. K. Mishra, S. K. Misra, J. S. Sharp, R. J. Doerksen and V. H. Pomin, Carbohydr. Polym., 2023, 301, 120316 CrossRef CAS PubMed.
- Q. Hu, L. Li, J. Li, X. Sun, C. Yan, M. Mao, Z. Lin and W. Liu, ACS ES&T Eng., 2024, 4, 1657–1667 Search PubMed.
- D. P. Nagahawatta, N. M. Liyanage, T. U. Jayawardena, F. Yang, H. Jayawardena, M. Kurera, F. Wang, X. Fu, Y.-J. Jeon and D. P. Nagahawatta, Algae, 2023, 38, 217–240 CrossRef CAS.
- Z.-B. Wang, J. Zhang, Q. Miao, H.-Y. Cao, F. Xiong, T. Lee, A. El-Baz, L. Xie and S.-Q. Ni, Environ. Sci. Technol., 2024, 58, 21242–21250 CrossRef CAS PubMed.
- S. Li, J. Li, Z. Zhi, C. Wei, W. Wang, T. Ding, X. Ye, Y. Hu, R. J. Linhardt and S. Chen, Carbohydr. Polym., 2017, 173, 330–337 CrossRef CAS PubMed.
- U. Adhikari, C. G. Mateu, K. Chattopadhyay, C. A. Pujol, E. B. Damonte and B. Ray, Phytochemistry, 2006, 67, 2474–2482 CrossRef CAS PubMed.
- Y. Cai, W. Yang, R. Yin, L. Zhou, Z. Li, M. Wu and J. Zhao, Carbohydr. Res., 2018, 464, 12–18 CrossRef CAS PubMed.
- J. Deng, H. Wang, R. Gao, X. Ma, M. Chen, D. Xu and A. Cai, J. Environ. Manage., 2025, 373, 123552 CrossRef CAS PubMed.
- S. Chen, Y. Hu, X. Ye, G. Li, G. Yu, C. Xue and W. Chai, Biochim. Biophys. Acta, Gen. Subj., 2012, 1820, 989–1000 CrossRef CAS PubMed.
- Q. Xin, Q. Wang, K. Luo, Z. Lei, E. Hu, H. Wang and H. Wang, Carbohydr. Polym., 2024, 324, 121576 CrossRef CAS PubMed.
- Y. Wen, C. Xue, D. Ji, Y. Hou, K. Li and Y. Li, Colloids Surf., A, 2023, 656, 130531 CrossRef CAS.
- S. Wang, K. Yao, Z. He, B. Shao, J. Shen and H. Jiang, J. Environ. Chem. Eng., 2023, 11, 109188 CrossRef CAS.
- L. Su, S. Wu, W. Zhu, B. Liang, X. Zhang and J. Yang, J. Environ. Manage., 2024, 370, 122870 CrossRef CAS PubMed.
- S. Shahmoradi, M. Mirshafiei, I. Zare, M. Koohkhezri, H. M. Gholipour, F. Yazdian and E. Mostafavi, Two-Dimensional Nanomater. Polym. Nanocomposites Process. Prop. Appl., 2024, pp. 609–648 Search PubMed.
- X. Ren, Z. Liu, T. Zhang, X. Jiang, Q. Fang, Y. Li, F. Ma, R. Chen, H. Zhang and H. Ni, J. Mater. Sci., 2024, 59, 10724–10743 CrossRef CAS.
- M. Al-Shaeli, O. O. Teber, R. A. Al-Juboori, A. Khataee, I. Koyuncu and V. Vatanpour, Sep. Purif. Technol., 2024, 127925 CrossRef CAS.
- C. Wu, L. Xia, W. Feng and Y. Chen, ChemPlusChem, 2024, e202300777 CrossRef CAS PubMed.
- Y. Cai, M. Fang, X. Tan, B. Hu and X. Wang, Sep. Purif. Technol., 2024, 127975 CrossRef CAS.
- P. Gu, S. Liu, X. Cheng, S. Zhang, C. Wu, T. Wen and X. Wang, Sci. Total Environ., 2023, 169533 Search PubMed.
- S. Kim, F. Gholamirad, M. Yu, C. M. Park, A. Jang, M. Jang, N. Taheri-Qazvini and Y. Yoon, Chem. Eng. J., 2021, 406, 126789 CrossRef CAS.
- S. Adil and J.-O. Kim, Sep. Purif. Technol., 2023, 326, 124725 CrossRef CAS.
- A. A. Ghani, A. Shahzad, M. Moztahida, K. Tahir, H. Jeon, B. Kim and D. S. Lee, Chem. Eng. J., 2021, 421, 127780 CrossRef.
- R. Chen, Y. Cheng, P. Wang, Q. Wang, Z. Yang, C. Tang, S. Xiang, S. Luo, S. Huang, C. Su and Y. Wang, Chem. Eng. J., 2021, 421, 129682 CrossRef CAS.
- F. Shi, Y. Chang, J. Shen, G. Chen and C. Xue, Food Hydrocolloids, 2023, 134, 108081 CrossRef CAS.
- L. Xing, H. Cheng, Y. Li, Q. Chen and X. Liu, Chem. Eng. J., 2024, 487, 150729 CrossRef CAS.
- E. A. Imam, A. I. Hashem, A. A. Tolba, M. G. Mahfouz, I. E.-T. El-Sayed, A. I. El-Tantawy, A. A. Galhoum and E. Guibal, J. Environ. Chem. Eng., 2023, 11, 109951 CrossRef CAS.
- X. Su, X. Wang, Z. Ge, Z. Bao, L. Lin, Y. Chen, W. Dai, Y. Sun, H. Yuan and W. Yang, Chem. Eng. J., 2024, 486, 150387 CrossRef CAS.
- Y. Liu, R. Tian, S. Zhang, T. Bo, Z. Wang, J. Zhao, Y. Wang, G. Lisak, Y. Liu and M. Chang, Chem. Eng. J., 2024, 481, 148388 CrossRef CAS.
- X. Huang, P. Zeng, Y. Lu, J. Yang, M. Chen, H. Liu and X. Wang, Chem. Eng. J., 2024, 487, 150490 CrossRef CAS.
- X.-M. Cao, J.-Q. Chen, X.-R. Zhao, H. Ge, D. Liu, Q. Wu, Z.-J. Sun and Q. Zhang, Chem. Eng. J., 2024, 479, 147663 Search PubMed.
- C. Zhang, C. Ma, W. Zhang, Y. Wang, Z. U. Rehman, X. Shen and S. Yao, Chem. Eng. J., 2024, 481, 148374 Search PubMed.
- M. Wang and X. You, Chem. Eng. J., 2023, 454, 140417 Search PubMed.
- I. Ijaz, A. Bukhari, A. Nazir, E. Gilani, H. Zain, A. Shaheen, M. R. Shaik and M. Khan, Sep. Purif. Technol., 2025, 131591 Search PubMed.
- Y. Liu, C. Gao, Y. Sun, X. Hao, Z. Pi, M. Yang, X. Zhao and K. Cai, Chem. Eng. J., 2024, 151535 Search PubMed.
- M. A. Islam, D. W. Morton, B. B. Johnson and M. J. Angove, Sep. Purif. Technol., 2020, 247, 116949 Search PubMed.
- P. Sarker, X. Lei, K. Taylor, W. Holmes, H. Yan, D. Cao, M. E. Zappi and D. D. Gang, Chem. Eng. J., 2023, 454, 140082 CrossRef CAS.
- I. Ijaz, A. Bukhari, E. Gilani, A. Nazir, H. Zain, A. Shaheen, M. R. Shaik, M. Khan and M. E. Assal, Int. J. Biol. Macromol., 2024, 132690 Search PubMed.
- Y. Xue, M. Kamali, T. M. Aminabhavi, L. Appels and R. Dewil, Chem. Eng. J., 2024, 152037 Search PubMed.
- L. Singh, P. Rekha and S. Chand, Sep. Purif. Technol., 2016, 170, 321–336 CrossRef CAS.
- L. Han, Y. Peng, J. Ma, Z. Shi and Q. Jia, Sep. Purif. Technol., 2022, 285, 120378 CrossRef CAS.
- Y. Wu, B. Li, X. Wang, S. Yu, Y. Liu, H. Pang, H. Wang, J. Chen and X. Wang, Chem. Eng. J., 2019, 365, 249–258 CrossRef CAS.
- Y. Xie, Q. Rong, C. Wen, X. Liu, M. Hao, Z. Chen, H. Yang, G. I. N. Waterhouse, S. Ma and X. Wang, CCS Chem., 2024, 6, 1908–1919 CrossRef CAS.
- Y. Ma, C. Zeng, Y. Ding, J. Tang, O. Mašek, Z. Deng, R. Mu and Z. Zhang, Sep. Purif. Technol., 2024, 331, 125584 Search PubMed.
- C. Yan, J. Li, Z. Sun, X. Wang and S. Xia, Sep. Purif. Technol., 2024, 127457 Search PubMed.
- B. Luo, G. Huang, Y. Yao, C. An, P. Zhang and K. Zhao, J. Cleaner Prod., 2021, 319, 128692 CrossRef CAS.
- S. Gul, S. Hussain, H. Khan, M. Arshad, J. R. Khan and A. de Jesus Motheo, Chemosphere, 2024, 357, 141868 CrossRef CAS PubMed.
- H. Cai, M. Rong, Q. Meng, Z. Liu, Y. Zhao, C. Chen and L. Yang, Sep. Purif. Technol., 2024, 331, 125612 Search PubMed.
- Y. Chen, Y. Liu, Y. Li, L. Zhao, Y. Chen, H. Li, Y. Liu, L. Li, F. Xu and M. Li, Environ. Sci. Pollut. Res., 2020, 27, 38644–38653 CrossRef CAS PubMed.
- A. Bukhari, I. Ijaz, E. Gilani, A. Nazir, H. Zain, S. Muhammad, A. Bukhari and S. Hussain, Chem. Eng. J., 2023, 474, 145890 CrossRef CAS.
- Q. Zhao, X. Zhu and B. Chen, Chem. Eng. J., 2018, 334, 1119–1127 Search PubMed.
- M. Zhang, H. Hao, C. Shi, C. Chen, G. Zhou, J. Wang, Y. Cao and X. Han, Sep. Purif. Technol., 2023, 310, 123095 CrossRef CAS.
- K. Burdzy, Y. Ju and D. Kołodyńska, Chem. Eng. J., 2023, 461, 142059 Search PubMed.
- Y.-Y. Chen and J.-G. Yu, J. Environ. Chem. Eng., 2022, 10, 108348 CrossRef CAS.
- J. Ouyang, J. Chen, S. Ma, X. Xing, L. Zhou, Z. Liu and C. Zhang, Particuology, 2022, 62, 71–78 CrossRef CAS.
- R. Zhang, X. Zheng, B. Chen, J. Ma, X. Niu, D. Zhang, Z. Lin, M. Fu and S. Zhou, J. Cleaner Prod., 2020, 256, 120662 CrossRef CAS.
- L. Chen, M. Wang, Q. Sun, Z. Zhao, J. Han, R. Ji, X. Jiang, Y. Song, J. Xue and H. Cheng, Sep. Purif. Technol., 2024, 333, 125940 Search PubMed.
- F. An, B. Gao, X. Huang, Y. Zhang, Y. Li, Y. Xu, Z. Zhang, J. Gao and Z. Chen, React. Funct. Polym., 2013, 73, 60–65 CrossRef CAS.
- N. Yao, C. Li, J. Yu, Q. Xu, S. Wei, Z. Tian, Z. Yang, W. Yang and J. Shen, Sep. Purif. Technol., 2020, 236, 116278 CrossRef CAS.
- Y. Zhou, Y. He, Y. Xiang, S. Meng, X. Liu, J. Yu, J. Yang, J. Zhang, P. Qin and L. Luo, Sci. Total Environ., 2019, 646, 29–36 CrossRef CAS PubMed.
- N. Li, L. Zhou, X. Jin, G. Owens and Z. Chen, J. Hazard. Mater., 2019, 366, 563–572 CrossRef CAS PubMed.
- X. Jiang, J. Guo, M. Sun, Q. Sun, W. Ding, H. Li and H. Zheng, Chem. Eng. J., 2024, 486, 150391 CrossRef CAS.
- R. Zhao, W. Ding, M. Sun, L. Yang, B. Liu, H. Zheng and H. Li, Sep. Purif. Technol., 2022, 287, 120487 CrossRef CAS.
- I. Ijaz, A. Bukhari, E. Gilani, A. Nazir, H. Zain, A. Bukhari, A. Shaheen, S. Hussain and A. Imtiaz, Process Biochem., 2023, 129, 257–267 Search PubMed.
- Y. Wu, H. Zheng, H. Li, Y. Sun, C. Zhao, R. Zhao and C. Zhang, Chem. Eng. J., 2021, 426, 127208 CrossRef CAS.
- T. Zhang, M. Wang, W. Yang, Z. Yang, Y. Wang and Z. Gu, Ind. Eng. Chem. Res., 2014, 53, 14913–14920 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra08766f |
| This journal is © The Royal Society of Chemistry 2025 |







