Open Access Article
Open Access ArticleCu/Au-doped nanopolymers with multiple catalytic activities for NIR II laser-promoted nanocatalytic tumor therapy†
Xixi Wua,
Xiang Fenga,
Peng Yuc,
Jing Zhangd and
Rui Liu *b
*b
aDepartment of Radiation Oncology, The People's Hospital of Guangxi Zhuang Autonomous Region, Nanning 530000, China
bDepartment of Joint Surgery and Sports Medicine, The People's Hospital of Guangxi Zhuang Autonomous Region, Nanning 530000, China. E-mail: lr1021231810@163.com
cDepartment of Endocrinology and Metabolism, The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, Jiangxi 330006, China
dDepartment of Anesthesiology, The Second Affiliated Hospital, Jiangxi Medical College, Nanchang University, Nanchang, Jiangxi Province 330006, China
First published on 15th April 2025
Abstract
Breast cancer remains a significant global health concern owing to the limitations of conventional therapies, such as side effects, drug resistance, and high costs. Nanocatalytic therapy has emerged as a promising alternative due to its tumor specificity, spatiotemporal controllability, and noninvasiveness. However, its effectiveness is limited by endogenous antioxidants like glutathione (GSH) and the low catalytic activity of nanocatalysts. Herein, an Cu/Au-doped polypyrrole nanocatalyst, Cu-AuPP, with multiple catalytic activities is developed by sequentially polymerizing pyrrole monomers using CuCl2 and HAuCl4, followed by PEGylation. The obtained Cu-AuPP catalyzes the production of hydroxyl radicals (·OH) and facilitates the oxidation of GSH to GSSG via redox reactions mediated by multivalent Cu ions, leading to oxidative damage, mitochondrial dysfunction, and tumor cell apoptosis. Upon 1064 nm laser irradiation, these catalytic activities were enhanced by elevated temperature and electron–hole separation mediated by Au nanoclusters, resulting in more intense oxidative damage to tumor cells. Collectively, the developed Cu-AuPP nanocatalytic medicine capable of simultaneously catalyzing GSH depletion and ·OH production via several improved catalytic mechanisms, has significant promise for the treatment of malignant tumors.
1. Introduction
Breast cancer remains a major health challenge for women globally, characterized by high mortality rates.1 Conventional therapies, including surgery, radiotherapy, chemotherapy, and immunotherapy, often yield suboptimal outcomes due to issues such as invasiveness, advanced side effects, drug resistance, and high costs.2–4 Recent advancements have highlighted nanocatalytic therapy as a promising alternative owing to its tumor specificity, spatiotemporal controllability, and noninvasive features.5–7 By harnessing the unique tumor microenvironment and metabolic differences between tumor cells and normal cells, nanocatalysts demonstrate specific catalytic activity to generate reactive oxygen species (ROS) within tumor cells, consequently diminishing interference to normal physiological processes and minimizing toxicity to healthy tissues, a major drawback of traditional chemical drugs.8,9 For instance, Fe-based nanocatalysts utilize excess hydrogen peroxide (H2O2) in tumor cells as a substrate to catalyze the production of hydroxyl radicals (·OH), which selectively eradicate tumor cells in a process known as Fenton catalytic therapy.10–12 Despite its potential for tumor suppression, nanocatalytic therapy often necessitates high dosages to counteract limited catalytic activity and endogenous antioxidants in tumors, such as glutathione (GSH), thereby raising the risk of toxicity.13–15 As a result, improving the effectiveness of nanocatalytic therapy is a critical concern for ensuring safety and efficacy.To date, various strategies have been developed and implemented to improve nanocatalytic therapeutics. Studies have demonstrated that Cu-based nanocatalysts have superior catalytic activity with less dependency on harsh conditions in comparison to Fe-based nanocatalysts.16–18 Incorporating noble metals as electron acceptors at interfaces or blocks to promote ROS generation by increasing electron–hole separation is also an effective strategy to improve catalytic activity.19–21 Among these, gold (Au) with excellent electrical conductivity has received great interest.22,23 Furthermore, numerous studies have showed that a controlled increase in local temperature is contributed to catalytic processes.24,25 The merging of laser and photothermal agents permits spatiotemporal selectivity and accelerates catalytic rate, a strategy that has been applied in oncology.26 Due to the practical challenges related to the restricted tissue penetration of ultraviolet and near-infrared (NIR) lasers, NIR II laser-responsive compounds have more potential for clinical therapeutic applications.27,28 Additionally, intratumoral GSH levels increase spontaneously in response to ROS generation, alleviating oxidative stress damage in tumor cells.29 Conversely, artificially manipulating GSH levels in tumors may alleviate the constraints on oxidative stress damage and boost nanocatalytic therapy. Therefore, modulating antioxidant levels in tumors while augmenting the catalytic activity of nanocatalysts is a potent approach to enhance nanocatalytic therapy.
Polypyrrole (PPy) is a widely studied heterocyclic conjugated conductive polymer made by electrochemical or oxidant-initiated polymerization of pyrrole monomers or their derivatives.30 PPy demonstrates advantageous properties such as biocompatibility, stability, electrical conductivity, and strong optical absorption coupled with high photothermal conversion efficiency.31–33 In our prior investigation, tetrachloroauric acid (HAuCl4) was utilized as an oxidant to synthesis Au-doped PPy (AuP), which exhibited significant optical absorption and photothermal effects in the NIR I and II regions.34 However, the catalytic activity of AuP remains unknown, despite the common use of Au monomers as electron acceptors in catalytic fields. Recent research has focused on improving the physicochemical properties and physiological activities of PPy by optimizing the oxidant and stabilizer components, while introducing novel therapeutic functions.35,36 For instance, multivalent Cu-doped PPy has been successfully synthesized using copper chloride (CuCl2) as an oxidant, effectively inducing oxidative stress in tumor tissues and activating immune responses.37
Inspired by these findings, we developed here a technique to initiate the polymerization of pyrrole monomers using HAuCl4 and CuCl2 in a sequential manner (Scheme 1). This process involved the partial reduction of HAuCl4 and CuCl2 to form Au nanoclusters and Cu+ ions, which were subsequently incorporated into PPy nanostructures. The resulting composite was further modified by DSPE-PEG to produce Cu-AuPP, wherein DSPE-PEG modification improved its physiological stability. Compared to the previously reported PEGylated AuP (AuPP), Cu-AuPP not only improved photothermal conversion efficiency but also exhibited a range of catalytic activities, including GSH consumption and ROS generation. Upon reaching the tumor site, Cu+/Cu2+ ions embedded inside the nanostructures catalyzed the conversion of GSH and H2O2 into oxidized glutathione (GSSG) and ·OH, respectively, which process was further accelerated by the photothermal effect induced by the NIR II laser. Simultaneously, Au nanoclusters functioned as electron acceptors to augment catalytic activities during irradiation, leading to increased ·OH production and more intense oxidative damage to tumor cells. Collectively, this engineered Cu-AuPP showed significant promise for advancing tumor nanocatalytic therapy.
 | ||
| Scheme 1 Schematic illustration of the fabrication of Cu-AuPP and its multiple catalytic mechanism for NIR II laser-enhanced nanocatalytic tumor therapy. | ||
2. Experimental section
2.1. Materials and chemicals
Polyvinyl alcohol (PVA), pyrrole, copper chloride (CuCl2), and glutathione (GSH) were purchased from Aladdin. Tetrachloroauric acid trihydrate (HAuCl4·3H2O), methylene blue (MB), 5,5-dimethyl-1-pyrroline oxide (DMPO), and 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) were sourced from Sigma-Aldrich. 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N [amino-(polyethylene glycol) 2000] (DSPE-PEG, molecular weight: 2000) was supplied by Xi'an Ruixi Biotech Biochemistry Technology Co., Ltd. 2′,7′-Dichlorofluorescin diacetateb (DCFH-DA), calcein-AM, propidium iodide (PI), and JC-1 probes were obtained from Beyotime Biotechnology. ThiolTracker™ Violet was bought from ThermoFisher Scientific. Dulbecco's modified eagle's medium (DMEM), phosphate buffer (PBS), fetal bovine serum, and trypsin–EDTA were obtained from Gibco-BRL.2.2. Synthesis of Cu-AuP
50 mg of PVA were dissolved in 10 mL of deionized (Di) water inside a 100 mL round-bottom flask and stirred for 1 h at 90 °C. The solution was then allowed to cool naturally to room temperature, after which 75 mg mL−1 CuCl2 (10 mL) was slowly added and stirred for 0.5 h. Following that, 200 μL of pyrrole monomer was added to the mixture and stirred for 12 h. Then, 5 mM HAuCl4·3H2O solution (5 mL) was added dropwise and stirred continuously for 1 h. The precipitate was collected by centrifuging the resultant mixture for 20 min at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, and it was then rinsed three times with Di water.
000 rpm, and it was then rinsed three times with Di water.
2.3. Synthesis of Cu-AuPP
In an ice bath sonication environment, 10 mg of Cu-AuP was dissolved in 10 mL of Di water. Subsequently, 50 mg of DSPE-PEG was added, followed by mixing and sonication for 0.5 h. After stirring for 24 h, the product was centrifuged and rinsed with Di water to purify Cu-AuPP.2.4. Characterization
Transmission electron microscopy (TEM, JEM-2100UHR, Japan) was used to investigate the nanocatalyst morphology. The chemical components and crystal structures were examined using X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi, Japan), X-ray diffraction (XRD, Bruker D8, Germany), and a Fourier transform infrared spectrometer (FTIR, Nexus 470, USA). Thermal images and temperature changes were recorded using a thermal imaging camera (FlukeTi100, USA). The optical absorption was investigated using a UV-vis-NIR spectrometer (Infinite M200 Pro, Switzerland). Dynamic light scattering (DLS, NanoBrook 173Plus, USA) was used to measure dynamic particle size and zeta potential.2.5. Photothermal conversion performance
The temperature variations of Cu-AuPP was recorded over time using an infrared camera to evaluate its photothermal conversion efficiency. Cu-AuPP solutions at various concentrations (0, 6.25, 12.5, 25, and 50 μg mL−1) were subjected to a 1064 nm laser at various power densities (0.5, 1.0, 1.5, and 2.0 W cm−2) for 5 min, with pure water serving as a control. Photothermal stability was assessed by exposing Cu-AuPP (25 μg mL−1) to a 1064 nm laser (1.0 W cm−2) for five cycles of alternating 6 min of irradiation and 6 min of rest. Furthermore, the photothermal conversion efficiency was examined by exposing Cu-AuPP (25 μg mL−1) to a 1064 nm laser (1.0 W cm−2) for 15 min, and then cooling it for another 15 min.2.6. In vitro GSH depletion and ·OH generation
A DTNB probe and UV-vis-NIR spectroscopy were used to examine the GSH depletion of Cu-AuPP. Briefly, either AuPP or Cu-AuPP (25 μg mL−1) was added to a GSH solution (1 mM) and incubated at 37 °C. After 20 min of incubation, a 1064 nm laser (1.0 W cm−2) was administered for 5 minutes to the groups that needed irradiation. The mixtures were extracted and mixed with DTNB (10 mg mL−1) after 60 min of co-incubation. UV-vis-NIR spectroscopy was then used to quantify the absorbance of the resultant suspensions.The ·OH generation of Cu-AuPP was analyzed utilizing a MB probe and UV-vis-NIR spectroscopy. Briefly, either AuPP or Cu-AuPP (25 μg mL−1) was added to the mixed solutions of 7 μg mL−1 of MB and 5 mM of H2O2. The groups that needed laser treatment were exposed to 1064 nm laser irradiation (1.0 W cm−2) for 5 min after co-incubation for 0, 15, 30, 45, and 60 min. Using a UV-vis-NIR spectrometer, the absorbance of those mixtures was detected every 15 min. To further determine ·OH generation, Cu-AuPP was mixed with H2O2 (5 mM) and DMPO probe, either with or without 1064 nm laser irradiation. Electron spin resonance was then used to capture the ·OH signal.
2.7. Intracellular GSH depletion and ·OH generation
ThiolTracker™ Violet and DCFH-DA probes were employed to detect variations in GSH and ·OH levels in mouse breast cancer cells (4T1 cells). Mouse breast cancer cells (4T1 cells) were purchased from the American Type Culture Collection (ATCC). Briefly, 4T1 cells (2 × 105 cells per dish) were seeded in confocal dishes and incubated overnight. The cells were treated with pure DMEM, AuPP, or Cu-AuPP after PBS washing. The cells in the laser-treated group were subjected to a 1064 nm laser at 1.0 W cm−2 for 5 min. After 12 h of incubation, the medium was replaced with DMEM containing either ThiolTracker™ Violet (20 μM) or DCFH-DA (10 μM), and incubated for 30 min. Subsequently, the cells were rinsed with PBS, fixed with 4% paraformaldehyde, stained with DAPI, and imaged using a confocal laser scanning microscope (CLSM, FV3000, Japan).2.8. Cellular uptake
4T1 cells (2 × 105 cells per dish) were seeded in confocal dishes and incubated overnight at 37 °C. The cells were treated with Cy5-labeled Cu-AuPP (Cy5: 5 μg mL−1) diluted in DMEM after PBS washing. Following incubation periods of 1, 3, and 6 h, the old medium was removed, the samples were rinsed with PBS, and then imaged using CLSM.2.9. Cell cytotoxicity
The cytotoxicity of Cu-AuPP on 4T1 cells was assessed using the conventional cell counting kit-8 (CCK-8) test. Briefly, 4T1 cells were seeded at a density of 5 × 103 cells per well onto 96-well plates, and then incubated overnight. Fresh DMEM with various concentrations of Cu-AuPP (0, 7.5, 15, 30, and 60 μg mL−1) was added to replace the old medium. After 12 h of co-incubation, the cells in the groups needing laser treatment were subjected to a 1064 nm laser (1.0 W cm−2, 5 min), followed by an additional 12 h of incubation. Finally, the cell viability was determined with the CCK-8 procedure.The NIR II laser-promoted therapeutic effects of Cu-AuPP were investigated in 4T1 cells using calcein-AM/PI staining. 4T1 cells were seeded in 24-well plates and incubated overnight. Fresh DMEM, with or without Cu-AuPP (30 μg mL−1), was added to replace the old medium and incubated for 24 h. The cells in the laser-treated groups underwent 1064 nm laser irradiation (1.0 W cm−2, 5 min). Subsequently, each well was filled with calcein-AM and PI, and the mixture was incubated for 20 min. Following PBS washing, a fluorescence microscope was used to observe the cells.
2.10. Mitochondrial membrane potential
Mitochondrial membrane potential assay was performed using the JC-1 probe. 4T1 cells were seeded in confocal dishes at a density of 2 × 105 cells per dish and incubated overnight. The cells were then exposed to either pure DMEM or Cu-AuPP (30 μg mL−1) for 12 h. The cells in the laser-treated groups underwent 1064 nm laser irradiation (1.0 W cm−2, 5 min). Images were obtained using CLSM after the cells were stained with JC-1 dye for 15 min.2.11. Statistical analysis
All statistical analyses were performed using GraphPad Prism software. The results of statistical analysis were presented as mean ± SD. Statistical significance was calculated by one-way ANOVA analysis. The statistical significance was defined as *p < 0.05; **p < 0.01; ***p < 0.001.3. Results and discussion
3.1. Synthesis and characterization
In the manufacture of Cu-AuP, CuCl2 was employed as the primary oxidant to initiate the oxidative polymerization of pyrrole monomer, with PVA acting as a stabilizer. Subsequently, HAuCl4 was introduced as a secondary oxidant to accelerate polymerization while reducing to Au nanoclusters. Fig. 1a and c illustrated the spherical morphology of the synthesized Cu-AuP nanoparticles, which possessed a hydrodynamic diameter of approximately 125.6 nm, as shown by TEM images and DLS measurements. Following PEGylated modification, the spherical morphology of Cu-AuPP remained consistent, but its hydrodynamic diameter and zeta potential changed to approximately 169.9 nm and −5.26 mV, respectively, as indicated by the DLS data (Fig. 1b and d). The hydrodynamic diameters of Cu-AuPP in DMEM medium at different pH levels remained constant within 48 h, indicating the good stability of Cu-AuPP (Fig. S1†). The crystal structure of Cu-AuP was analyzed using XRD spectroscopy, which displayed the characteristic diffraction peaks of cubic Au (JCPDS no. 04-0784), confirming the successful reduction of HAuCl4 to Au nanoclusters (Fig. 1e). Additionally, a broad diffraction peak in the range of 24° to 35° was observed, corresponding to the intermolecular stacking structures characteristic of amorphous PPy. In the FTIR spectra, distinct peaks at 1257, 1462, and 1609 cm−1 were detected in both Cu-AuP and Cu-AuPP, corresponding to C–H, C–N, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibrations typical of PPy frameworks, indicating the presence of PPy structures (Fig. 1f).
N stretching vibrations typical of PPy frameworks, indicating the presence of PPy structures (Fig. 1f).
 | ||
| Fig. 1 TEM images of (a) Cu-AuP and (b) Cu-AuPP. (c) Size distribution and (d) zeta potential of Cu-AuP and Cu-AuPP. (e) XRD spectra of Cu-AuP. (f) FTIR spectra of Cu-AuP and Cu-AuPP. | ||
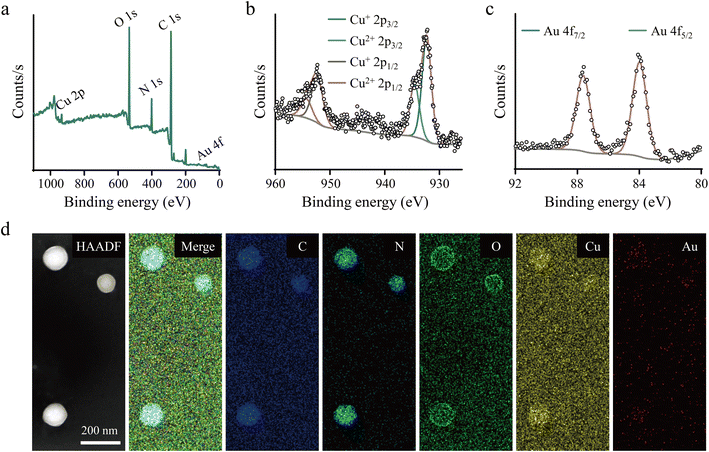
Next, XPS detection was conducted to analyze the chemical constitution of Cu-AuPP. The elements C, N, O, Au, and Cu were identified in Cu-AuP, verifying the effective co-doping of Au nanoclusters and Cu ions (Fig. 2a). High-resolution XPS spectra was used to further investigate the chemical states of these elements. Both monovalent and divalent Cu states were identifiable in the Cu 2p XPS spectra, with peaks at 932.3 eV and 934.5 eV attributed to Cu+ 2p3/2 and Cu2+ 2p3/2, respectively, and peaks at 952.3 and 954.3 eV belonging to Cu+ 2p1/2 and Cu2+ 2p1/2, respectively (Fig. 2b). The Cu+ to Cu2+ ratio in Cu-AuP was calculated as 2.98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The formation of Au nanoclusters was confirmed by the characteristic peaks at 83.9 eV and 87.6 eV corresponding to Au 4f7/2 and Au 4f5/2, respectively, in the high-resolution Au 4f spectra (Fig. 2c). Furthermore, the high-resolution C 1s spectra had four peaks at 284.0, 284.8, 286.3, and 287.8 eV, which corresponded to Cα, Cβ, C
1. The formation of Au nanoclusters was confirmed by the characteristic peaks at 83.9 eV and 87.6 eV corresponding to Au 4f7/2 and Au 4f5/2, respectively, in the high-resolution Au 4f spectra (Fig. 2c). Furthermore, the high-resolution C 1s spectra had four peaks at 284.0, 284.8, 286.3, and 287.8 eV, which corresponded to Cα, Cβ, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N/C–N+, and C
N/C–N+, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the PPy structure, respectively. Similarly, pyrrolic N, C–N+, and C
O in the PPy structure, respectively. Similarly, pyrrolic N, C–N+, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+ in PPy were identified as three peaks at 399.6, 401.2, and 402.8 eV in the high-resolution N 1s spectra (Fig. S2†). These findings confirmed the presence of the PPy nanostructure in Cu-AuP, with Cu ions predominantly existing in monovalent and divalent states, and Au forming nanoclusters. High-resolution TEM and elemental mapping images further revealed the homogeneous and overlapping distribution of C, N, O, Au, and Cu elements in Cu-AuP (Fig. 2d).
N+ in PPy were identified as three peaks at 399.6, 401.2, and 402.8 eV in the high-resolution N 1s spectra (Fig. S2†). These findings confirmed the presence of the PPy nanostructure in Cu-AuP, with Cu ions predominantly existing in monovalent and divalent states, and Au forming nanoclusters. High-resolution TEM and elemental mapping images further revealed the homogeneous and overlapping distribution of C, N, O, Au, and Cu elements in Cu-AuP (Fig. 2d).
3.2. Optical absorption and photothermal property of Cu-AuPP
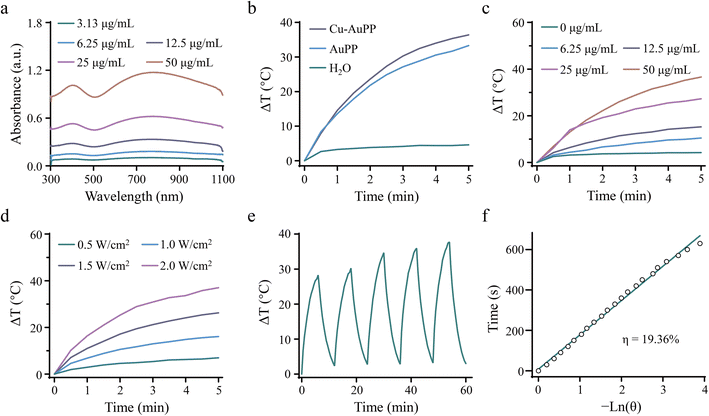
The broad absorption of Cu-AuPP in the NIR I and II regions was validated by recording its UV-vis-NIR spectra at various concentrations (Fig. 3a). The absorbance at 1064 nm displayed a linear increase with concentration, indicating the efficient optical energy harvesting capability of Cu-AuPP nanocatalysts from 1064 nm laser irradiation (Fig. S3†). A thermal camera was then employed to monitor the temperature rise (ΔT) of AuPP and Cu-AuPP solutions under 1064 nm laser irradiation. Following 5 min of laser irradiation at 1.0 W cm−2, the ΔT for AuPP and Cu-AuPP solutions were 33.32 and 36.40 °C, respectively, whereas the ΔT for H2O, serving as a control, was negligible (Fig. 3b and S4a†). Moreover, the photothermal curves of Cu-AuPP exhibited a pattern depending on both concentration and power density (Fig. 3c and d). For instance, the ΔT of Cu-AuPP solution at concentrations of 25 and 50 μg mL−1 rose to 27.26 and 36.59 °C, respectively, after 5 min of irradiation (1.0 W cm−2). The photostability of Cu-AuPP was then evaluated by a repetitive laser activation and deactivation protocol. As shown in Fig. 3e, the temperature profile of Cu-AuPP under irradiation remained stable over five consecutive on/off cycles, demonstrating good photothermal stability of Cu-AuPP. The photothermal conversion efficiency (η) of Cu-AuPP under 1064 nm laser irradiation was calculated to be 19.36%, comparable to that of previously reported conjugated polymer (20.2%)38 and PPy-based hydrogel (20.3%)39 (Fig. 3f and S4b†). Together, these findings well confirmed the prominent photothermal conversion property of Cu-AuPP.3.3. Multiple catalytic activities of Cu-AuPP
To demonstrate the multiple catalytic activities of Cu-AuPP, DTNB probe was initially utilized as a GSH indicator to assess residual GSH levels by detecting the formation of yellow 5-mercapto-2-nitrobenzoic acid (TNB), which exhibited a characteristic absorption peak at 412 nm. In comparison to AuPP, Cu-AuPP showed significantly greater GSH depletion during an identical 1 h incubation, as evidenced by the lower absorbance intensity of TNB at 412 nm (Fig. 4a). This catalytic capability was ascribed to the redox reaction between Cu2+ active sites and GSH. More importantly, laser irradiation further amplified this catalytic activity, likely owing to the facilitation of redox processes driven by electron migration mediated by Au nanoclusters (Fig. S5†). After that, the Fenton catalytic activity of Cu-AuPP, with and without 1064 nm laser irradiation, was investigated using MB as an indicator. Fig. 4b illustrated a gradual reduction in MB levels in the Cu-AuPP group, indicating the generation of ·OH through a Cu+-driven Fenton catalytic reaction. Laser-induced photoelectron generation and migration further boosted ·OH generation in the Cu-AuPP + L group. Moreover, electron spin resonance (ESR) approach was used to track the generation of ·OH by employing DMPO as a spin-trapping agent. As shown in Fig. 4c, the characteristic signal of the ·OH/DMPO adduct was identified as a quadruple signal with a relative intensity of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in the ESR spectrum, confirming laser-enhanced ·OH production via Cu-AuPP-mediated catalytic reactions.
1, in the ESR spectrum, confirming laser-enhanced ·OH production via Cu-AuPP-mediated catalytic reactions.
Encouraged by the superior catalytic activity of Cu-AuPP in a simulated tumor microenvironment, we then assessed its catalytic properties on mouse breast cancer 4T1 cells using ThiolTracker™ Violet and DCFH-DA fluorescence probes. As displayed in Fig. 4d, untreated 4T1 cells exhibited strong green fluorescence, indicating high GSH levels within tumor cells. After incubation with Cu-AuPP for 12 h, 4T1 cells demonstrated decreased fluorescence, whereas the AuPP group showed comparable fluorescent intensity to that of the control group. Importantly, the green fluorescence was further diminished in the Cu-AuPP + L group, which demonstrated the enhanced GSH depletion via hyperthermia-accelerated Cu2+-mediated redox reactions. Subsequently, the ·OH generation in 4T1 cells was determined using DCFH-DA probe, which emits green fluorescence upon oxidation by ·OH. As shown in Fig. 4e, negligible green fluorescence was found in both the control and AuPP groups, whereas the Cu-AuPP group showed increased green fluorescence attributable to Cu+/Cu2+-mediated multiple catalytic processes. Significantly, the cells treated with Cu-AuPP + L exhibited the greatest ·OH fluorescence signal, which was attributed to increased catalytic activity induced by hyperthermia.
3.4. Cellular uptake and antitumor effect
The intracellular distribution of Cy5-labeled Cu-AuPP (Cy5Cu-AuPP) was examined using CLSM. A steady rise in red fluorescence over time indicated a time-dependent cellular uptake of Cy5Cu-AuPP, as displayed in Fig. 5a. Subsequently, the therapeutic effects of Cu-AuPP under 1064 nm laser irradiation were evaluated via standard CCK-8, live/dead cell staining, and JC-1 staining assays. Following a 24 h incubation of Cu-AuPP with 4T1 cells, cell survival decreased in a concentration-dependent manner, highlighting the multiple catalytic activities of Cu-AuPP within tumor cells (Fig. 5b). Upon exposure to the 1064 nm laser, the CCK-8 assay demonstrated a notable reduction in 4T1 cell viability. Specifically, the cell survival decreased to 35.97% and 14.99% at concentrations of 30 and 60 μg mL−1, respectively, which was attributed to the NIR II laser-accelerated catalytic activity of Cu-AuPP. Additionally, fluorescence imaging of live/dead cells further confirmed the excellent antitumor efficacy mediated by Cu-AuPP and amplified by 1064 nm laser irradiation (Fig. 5c). Compared to the control, laser-only, and Cu-AuPP-only groups, the Cu-AuPP + L-treated cells showed a significant decrease in live cells (green fluorescence) and an increase in dead cells (red fluorescence) following 5 minutes of laser exposure. The JC-1 indicator, which displayed red aggregates when the mitochondrial membrane potential was normal and green when depolarized, was next used to assess probable mitochondrial malfunction after various treatments. Consistent with the live/dead staining results, the green/red fluorescence intensity ratio was elevated in the Cu-AuPP group, and further augmented after laser irradiation, indicating severe mitochondrial dysfunction due to oxidative damage (Fig. 5d).4. Conclusions
In summary, we have developed an Cu-AuPP nanocatalyst with multiple catalytic activities for NIR II laser-enhanced nanocatalytic tumor therapy. In comparison with our previously reported AuPP, Cu-AuPP resulting from the sequential polymerization of pyrrole monomers using HAuCl4 and CuCl2 exhibited a range of catalytic activities, including the generation of ·OH from excessive H2O2 and the conversion of GSH to GSSG within tumor cells. These activities were primarily ascribed to the doped multivalent Cu ions. More importantly, NIR II laser irradiation enhanced these catalytic activities, presumably due to the promotion of redox processes driven by elevated temperature and electron migration. Collectively, these catalytic reactions induced oxidative stress and damage in tumor cells, as evidenced by live/dead cell staining and mitochondrial membrane potential assays. The Cu-AuPP produced in this study offers a promising alternative for nanocatalysts by enhancing catalytic activity and depleting GSH, thus advancing the development of nanocatalytic nanomedicines for tumor therapy.Data availability
All relevant data are within the manuscript and its additional files.Author contributions
R. L. conceptualized and designed the research; X. W., X. F., and P. Y. performed the experiments and analyzed the data. J. Z. contributed to some cellular experiments. X. W. and R. L. wrote the manuscript. All authors contributed to the discussion of the results and implications, and revising of the manuscript at all stages. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the Guangxi Natural Science Foundation (No. 2024GXNSFBA010060, No. 2025GXNSFBA069534), the Guangxi First Batch of Qingmiao Talent Fund Project (PI: Xixi Wu), the Guangxi Science and Technology Base and Talent Special Project (No. AD23026097), the Natural Science Foundation of Jiangxi Province (20224ACB216009), and the Jiangxi Province Thousands of Plans (jxsq2023201105). The scheme was created with http://biorender.com.References
- N. Harbeck, F. Penault-Llorca, J. Cortes, M. Gnant, N. Houssami, P. Poortmans, K. Ruddy, J. Tsang and F. Cardoso, Nat. Rev. Dis. Primers, 2019, 5, 66 CrossRef PubMed.
- S. K. Yeo and J. L. Guan, Trends Cancer, 2017, 3, 753–760 CrossRef CAS PubMed.
- M. R. Lloyd, K. Jhaveri, K. Kalinsky, A. Bardia and S. A. Wander, Nat. Rev. Clin. Oncol., 2024, 21, 743–761 CrossRef PubMed.
- W. Zeng, H. Zhang, Y. Deng, A. Jiang, X. Bao, M. Guo, Z. Li, M. Wu, X. Ji, X. Zeng and L. Mei, Chem. Eng. J., 2020, 389, 124494 CrossRef CAS.
- W. Zeng, H. Zhang, X. Yuan, T. Chen, Z. Pei and X. Ji, Adv. Drug Delivery Rev., 2022, 184, 114241 CrossRef CAS PubMed.
- W. Feng, X. Han, R. Wang, X. Gao, P. Hu, W. Yue, Y. Chen and J. Shi, Adv. Mater., 2019, 31, 1805919 CrossRef PubMed.
- B. Yang, Y. Chen and J. Shi, Adv. Mater., 2019, 31, 1901778 CrossRef PubMed.
- Y. Kang, Z. Mao, Y. Wang, C. Pan, M. Ou, H. Zhang, W. Zeng and X. Ji, Nat. Commun., 2022, 13, 2425 CrossRef CAS PubMed.
- Y. Nie, W. Zhang, W. Xiao, W. Zeng, T. Chen, W. Huang, X. Wu, Y. Kang, J. Dong, W. Luo and X. Ji, Biomaterials, 2022, 289, 121791 CrossRef CAS PubMed.
- Z. Tang, Y. Liu, M. He and W. Bu, Angew. Chem., Int. Ed., 2019, 58, 946–956 CrossRef CAS PubMed.
- M. Huo, L. Wang, Y. Wang, Y. Chen and J. Shi, ACS Nano, 2019, 13, 2643–2653 CAS.
- Y. Wang, D. Wang, Y. Zhang, H. Xu, L. Shen, J. Cheng, X. Xu, H. Tan, X. Chen and J. Li, Bioact. Mater., 2023, 22, 239–253 CAS.
- Z. Jie, B. Xiong and J. Shi, Adv. Sci., 2024, 11, 2402801 CrossRef CAS PubMed.
- Y. Li, W. Fan, X. Gu, S. Liu, T. He, S. Gou, W. Meng, M. Li, X. Liu, Y. Ren, C. Qi and K. Cai, Adv. Funct. Mater., 2024, 34, 2313540 CrossRef CAS.
- Y. He, X. Liu, L. Xing, X. Wan, X. Chang and H. Jiang, Biomaterials, 2020, 241, 119911 CrossRef CAS PubMed.
- C. Huang, J. Tang, Y. Liu, T. Chen, J. Qi, S. Sun, H. Hao, W. Zeng, J. Zhao and M. Wu, Acta Biomater., 2023, 167, 463–472 CrossRef CAS PubMed.
- W. Li, Y. Xiao, N. Zhu, Z. Chen, Z. Jiang, B. Li, G. Yu, Z. Guo, M. Liang and W. Guo, Nano Today, 2024, 56, 102223 CrossRef CAS.
- L. Fu, Y. Wan, C. Qi, J. He, C. Li, C. Yang, H. Xu, J. Lin and P. Huang, Adv. Mater., 2021, 33, 2006892 CrossRef CAS PubMed.
- X. Meng, H. Fan, L. Chen, J. He, C. Hong, J. Xie, Y. Hou, K. Wang, X. Gao, L. Gao, X. Yan and K. Fan, Nat. Commun., 2024, 15, 1626 CrossRef CAS PubMed.
- Y. Xu, M. Fan, W. Yang, Y. Xiao, L. Zeng, X. Wu, Q. Xu, C. Su and Q. He, Adv. Mater., 2021, 33, 2101455 CrossRef CAS PubMed.
- X. Wu, Y. Li, M. Wen, Y. Xie, K. Zeng, Y. Liu, W. Chen and Y. Zhao, Chem. Soc. Rev., 2024, 53, 2643–2692 RSC.
- T. Chen, W. Zeng, C. Tie, M. Yu, H. Hao, Y. Deng, Q. Li, H. Zheng, M. Wu and L. Mei, Bioact. Mater., 2022, 10, 515–525 CAS.
- L. Cai, J. Du, F. Han, T. Shi, H. Zhang, Y. Lu, S. Long, W. Sun, J. Fan and X. Peng, ACS Nano, 2023, 17, 7901–7910 CrossRef CAS PubMed.
- J. Hao, K. Ge, G. Chen, B. Dai and Y. Li, Chem. Soc. Rev., 2023, 52, 7707–7736 RSC.
- J. Lou-Franco, B. Das, C. Elliott and C. Cao, Nano-Micro Lett., 2021, 13, 10 CrossRef PubMed.
- Y. Yao, W. Zeng, H. Ping, X. Zeng and M. Lin, View, 2021, 2, 20200042 CrossRef.
- H. Zhang, W. Zeng, C. Pan, L. Feng, M. Ou, X. Zeng, X. Liang, M. Wu, X. Ji and L. Mei, Adv. Funct. Mater., 2019, 29, 1903791 CrossRef.
- C. Li, G. Chen, Y. Zhang, F. Wu and Q. Wang, J. Am. Chem. Soc., 2020, 142, 14789–14804 CrossRef CAS PubMed.
- Y. Xiong, C. Xiao, Z. Li and X. Yang, Chem. Soc. Rev., 2021, 50, 6013–6041 RSC.
- L. Hao, C. Dong, L. Zhang, K. Zhu and D. Yu, Polymers, 2022, 14, 5139 CrossRef CAS PubMed.
- W. Zeng, X. Wu, T. Chen, S. Sun, Z. Shi, J. Liu, X. Ji, X. Zeng, J. Guan, L. Mei and M. Wu, Adv. Funct. Mater., 2021, 31, 2008362 CrossRef CAS.
- Y. Liu, Y. Yi, S. Sun, T. Wang, J. Tang, Z. Peng, W. Huang, W. Zeng and M. Wu, Small, 2024, 20, 2309206 CrossRef CAS PubMed.
- L. Wang, G. Liu, Y. Hu, S. Gou, T. He, Q. Feng and K. Cai, Nanoscale, 2022, 14, 3097–3111 RSC.
- X. Wu, H. Liang, C. Li, D. Zhou and R. Liu, RSC Adv., 2023, 13, 29061–29069 RSC.
- H. Li, Y. Li, L. Su, K. Zheng, Y. Zhang, J. Li, F. Lv, M. Huang, T. Chen, H. Zhang, Z. Shi, D. Zhu, X. Dong, W. Zeng and L. Mei, Adv. Sci., 2024, 11, 2308250 Search PubMed.
- Y. Wang, W. Zeng, H. Liang, X. Wu, H. Li, T. Chen, M. Yang, X. Wang, W. Li, F. Zhang, Q. Li, F. Ye, J. Guan and L. Mei, ACS Appl. Mater. Interfaces, 2022, 14, 50557–50568 CrossRef CAS PubMed.
- W. Zeng, M. Yu, T. Chen, Y. Liu, Y. Yi, C. Huang, J. Tang, H. Li, M. Ou, T. Wang, M. Wu and L. Mei, Adv. Sci., 2022, 9, 2201703 CrossRef CAS PubMed.
- G. Zhang, Y. Dai, D. Wang, Y. Liu, H. Lu, L. Qiu and K. Cho, Dyes Pigm., 2017, 147, 175–182 CrossRef CAS.
- S. Geng, H. Zhao, G. Zhan, Y. Zhao and X. Yang, ACS Appl. Mater. Interfaces, 2020, 12, 7995–8005 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra08591d |
| This journal is © The Royal Society of Chemistry 2025 |