Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Boosting CO2 and benzene adsorption through π-hole substitution in β-diketonate Cu(II) complex within non-porous adaptive crystals†
Yoshinori Ikumuraa,
Tadashi Kawasakia,
Yuki Ishidaa,
Hirotomo Usuia,
Sayaka Uchida b,
Kazuki Kamatac,
Mikihiro Nomura
b,
Kazuki Kamatac,
Mikihiro Nomura c and
Akiko Hori
c and
Akiko Hori *a
*a
aGraduate School of Engineering and Science, Shibaura Institute of Technology, Fukasaku 307, Minuma-ku, Saitama 337-8570, Japan. E-mail: ahori@shibaura-it.ac.jp
bDepartment of Basic Sciences, School of Arts and Sciences, The University of Tokyo, 3-8-1 Komaba, Meguro-ku, Tokyo 153-8902, Japan
cGraduate School of Engineering and Science, Shibaura Institute of Technology, Toyosu 3-7-5, Koto-ku, Tokyo 135-8548, Japan
First published on 25th February 2025
Abstract
The effect of quadrupole moments in non-porous adaptive crystals of fully, partially, and non-fluorinated β-diketonate Cu(II) complexes on CO2 and hydrocarbon adsorption was systematically investigated using structurally similar models with distinct electronic properties. The fully fluorinated complex significantly enhanced CO2 adsorption, particularly at low pressures (<0.1 P/P0), achieving a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio through quadrupole interactions, where the positively polarized regions of electrostatic potentials (ESPs) on the Cu(II) center and pentafluorophenyl rings facilitated CO2 binding via its quadrupole nature. The perfluorinated complex also exhibited a stepwise vapor adsorption of benzene (C6H6), exhibiting distinct hysteresis and a 1
1 stoichiometric ratio through quadrupole interactions, where the positively polarized regions of electrostatic potentials (ESPs) on the Cu(II) center and pentafluorophenyl rings facilitated CO2 binding via its quadrupole nature. The perfluorinated complex also exhibited a stepwise vapor adsorption of benzene (C6H6), exhibiting distinct hysteresis and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 stoichiometric ratio, driven by M⋯π and π-hole⋯π interactions. In contrast, the partially fluorinated complex and non-fluorinated [Cu(dbm)2] (dbm = dibenzoylmethanido−) showed significantly reduced adsorption capabilities, reflecting the critical role of quadrupole moments and charge distribution in molecular recognition. The poor guest insertion of hexafluorobenzene (C6F6) into the perfluorinated complex highlighted the impact of electrostatic repulsion between similarly positive quadrupole moments. The gas adsorption studies further demonstrated differences in the kinetics and adsorption behavior of CO2, C2Hn (n = 2, 4, 6), and aromatic vapors, underscoring the importance of quadrupole design. These findings provide a rational framework for the development of advanced host–guest materials tailored for selective adsorption and separation applications.
3 stoichiometric ratio, driven by M⋯π and π-hole⋯π interactions. In contrast, the partially fluorinated complex and non-fluorinated [Cu(dbm)2] (dbm = dibenzoylmethanido−) showed significantly reduced adsorption capabilities, reflecting the critical role of quadrupole moments and charge distribution in molecular recognition. The poor guest insertion of hexafluorobenzene (C6F6) into the perfluorinated complex highlighted the impact of electrostatic repulsion between similarly positive quadrupole moments. The gas adsorption studies further demonstrated differences in the kinetics and adsorption behavior of CO2, C2Hn (n = 2, 4, 6), and aromatic vapors, underscoring the importance of quadrupole design. These findings provide a rational framework for the development of advanced host–guest materials tailored for selective adsorption and separation applications.
Introduction
Environmental movements to combat global warming require cost-effective methods for CO2 capture. These efforts have become increasingly critical as global emissions continue to rise, primarily resulting from industrial processes and energy production.1,2 Innovative materials and strategies are being actively explored to address these challenges. Various classes of porous materials have been investigated, including metal–organic frameworks (MOFs) and porous organic frameworks (POFs).3–8 These materials offer high surface areas, controllable pore sizes, and numerous molecular engineering pathways to tune and precisely control host–guest interaction strength through chemical modifications to their molecular building blocks. Size-based separation is currently the most effective method; however, it is not efficient for molecules with similar morphologies, such as CO2 and C2H2, which share rod-like shapes and sizes of 3–5 Å.9,10 Industrial gas streams often contain mixtures of these molecules, necessitating advanced technologies to achieve high purity.11,12 This limitation has driven the development of alternative approaches that go beyond size-based separation methods. In addition to their similar size and shape, CO2 and C2H2 are non-polar molecules with comparable equilibrium sorption parameters and related physicochemical properties.13,14 Therefore, a more rational approach to CO2/C2H2 separation is required. One promising avenue involves leveraging subtle differences in electronic properties, such as quadrupole moments, to achieve selective recognition.Microporous compounds with cross-sectional areas of 4–6 Å, suitable for small molecules, have been reported to exhibit high levels of selective hydrocarbon adsorption.15 These findings highlight the importance of precisely tuned pore environments, as even slight variations in pore size and chemistry can significantly affect selectivity. Conventional molecular recognition processes often favor C2H2 over CO2 because aromatic host frameworks16–20 typically interact preferentially with the positive quadrupole moment of C2H2 (+21.1 × 10−40 C m2)21 rather than the negative quadrupole moment of CO2 (negative, −14.3 × 10−40 C m2).22 This interaction is likely influenced by the negative quadrupole moment of aromatic centers, such as that observed in the benzene molecule (−29.0 × 10−40 C m2).23 Building on this concept, the design of host frameworks with tailored electronic properties, guided by quadrupole interactions, has become a focal point for achieving challenging separations involving non-polar molecules. Selective recognition of CO2 remains essential from both practical and theoretical perspectives. One potential solution is to invert quadrupole moments to create host materials24,25 that preferentially interact with CO2. This strategy represents a shift in molecular recognition, moving beyond size-based mechanisms to emphasize electrostatic interactions.
In this study, we investigated the adsorption of CO2 and benzene molecules in the non-porous crystals of three β-diketonate Cu(II) complexes (Scheme 1),26 each with varying degrees of fluorination on the phenyl groups. We have previously reported that a fully fluorinated complex 1 acts as a non-porous adaptive crystal (NAC),27–30 encapsulating aromatic molecules31 and small gases.32 Such encapsulation behavior underscores the versatility of NACs, enabling a direct comparison between homogeneous intermolecular interactions among host molecules and heterogeneous interactions between host and guest molecules. This highlights the selective adsorption capability of NACs, particularly for target substances. Additionally, the adsorption adaptability of NACs for non-polar guest molecules with weak intermolecular interactions provides unique insights distinct from conventional porous materials. Steric effects arising from the size of fluorine should be considered, given fluorine's van der Waals radius is approximately 1.2 times larger than hydrogen.24 Therefore, this study examines the molecular recognition behavior of structurally similar compounds, such as C6F5 and o-C6H3F2 groups (1 and 2, respectively),31,33 which share steric properties but differ in quadrupole moments.34–36 Fluorination modifies the electric quadrupole moments of the host framework, introducing electron deficiency and enabling interactions with guest molecules bearing opposite (electron-rich) quadrupole moments. This broadens the potential for capturing CO2, with an axially negative quadrupole moment.
Results and discussion
Preparation and structural information
To understand their guest-recognition behaviors, fully fluorinated complex 1 with C6F5 groups, partially fluorinated complex 2 with o-C6H3F2 groups, and the non-fluorinated [Cu(dbm)2] (dbm = dibenzoylmethanido–) were prepared. The complex motifs were selected as a notable target because dbm is a well-known ligand that forms neutral complexes when coordinated with divalent metal ions. Each complex was prepared using Cu(II) ions and the corresponding β-diketonate ligands, as previously reported protocols.26,31 This characteristic minimizes ionic intermolecular interactions within the crystal structure, allowing a clearer focus on other molecular interactions. Crystallographic and molecular structure analyses revealed that complexes 1 and 2 adopt square-planar coordination geometries with some degree of distortion (Fig. 1). The structural overlay of C, O, and Cu atoms (excluding H and F atoms) of the two complexes shows a close resemblance, with an r.m.s. deviation of 0.142 Å, making them suitable targets for discussing the structural properties and the effects of fluorine substitution (Fig. S1 and S2†). The dihedral angles between the two phenyl groups and the six-membered ring of the coordination plane are similar for complexes 1 and 2, due to the steric hindrance at the ortho positions. In complex 1, the dihedral angles are 41.79° and 59.98° for rings A1–B and A2–B, respectively (Fig. 1a),33 while in complex 2, they are 64.18° and 68.81° for rings C1–D and C2–D, respectively (Fig. 1b).31 In contrast, the dihedral angles for [Cu(dbm)2] are significantly smaller, at 0.57° and 10.50° for the two rings.37 Both crystals 1 and 2 have been confirmed to be molecular crystals without voids through Mercury's void space analysis. However, to predict potential molecular recognition pathways, we calculated the regions where voids are most likely to form. The results suggested that in both cases, potential void spaces are located between the coordination planes of the Cu(II) ions, specifically above the six-membered CuO2C3 rings and enclosed by fluorophenyl groups (Fig. S3†). Hirshfeld analysis also indicated that in crystal 2, intermolecular F⋯H/H⋯F interactions are present at the ligand sites, while the predicted void space is oriented toward the fluorophenyl planes rather than their edge regions (Fig. S4 and S5†).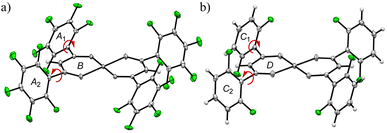 | ||
| Fig. 1 Twisted orientations of the phenyl rings and the coordination of six membered rings of (a) 1 and (b) 2, which were obtained from the single crystallographic data of 1 (CCDC 1850163) and 2 (CCDC 895496): fluorine, green; the other atoms, white.31,33 | ||
Quadrupole moments
The quadrupole moments for the principal axis (Qzz) and electrostatic potentials (ESPs) provide complementary insights into the electronic environments of the studied complexes. Qzz values quantify the electron density distribution along the principal axis, while ESPs visualize the electrostatic potential surrounding the molecule, highlighting regions of electron richness or deficiency. These properties are critical for understanding host–guest interactions, as they determine the compatibility of the host's electronic environment with the characteristics of the guest molecules. The experimental quadrupole moments referenced in the literature, along with the ESPs calculated using density functional theory (DFT)38 at the ωB97X-D/6-31G* level for related compounds, are summarized in Table 1. The degree of fluorination significantly influences both Qzz and ESPs, altering the electronic structure of the aromatic rings. Perfluorination enhances the electron-withdrawing effect, shifting the Qzz values from negative to positive. For example, Qzz values (×10−40 C m2) clearly depend on the degree of fluorination, as reported by A. Vela et al.:34 −28.6 (benzene) < −7.7 (1,4-difluorobenzene) < +1.9 (1,3,5-trifluorobenzene) < +12.3 (1,2,4,5-tetrafluorobenzene) < +31.9 (hexafluorobenzene). The quadrupole values for CO2 and C2Hn hydrocarbons {n = 2 (acetylene), 4 (ethylene), 6 (ethane)},21,35,36 which have a rod-like morphology, must account for the orientation of each axis (Qxx, Qyy and Qzz).39 This shift in electronic properties directly correlates with the increased electrophilicity of the Cu(II) centers in complex 1, as indicated by the ESP maps. These features collectively suggest that perfluorinated aromatic systems are well-suited for interactions with negative quadrupolar molecules like CO2 and benzene.| Formula | Qxx | Qyy | Qzz | ESPb | |
|---|---|---|---|---|---|
| a Ethylene shows three ESP values of −90, +15, and +18 kJ mol−1 for each C2 axes of the corresponding Qxx, Qyy, and Qzz.b ESP is calculated at Cg of Qzz. | |||||
| Benzene23,24 | C6H6 | +14.5 | +14.5 | −29.0 | −94 |
| 1,3,5-Trifluorobenzene36 | C6H3F3 | −0.87 | −1.0 | +1.9 | +5.0 |
| Hexafluorobenzene23,24 | C6F6 | −15.8 | −15.8 | +31.7 | +97 |
| Carbon dioxide22 | CO2 | +7.1 | +7.1 | −14.3 | −67 |
| Acetylene21 | C2H2 | −10.6 | −10.6 | +21.1 | +149 |
| Ethylene36,39,a | C2H4 | −10.5 | +4.9 | +5.6 | +18 |
| Ethane35 | C2H6 | +1.1 | +1.1 | −2.2 | −20 |
To understand the guest recognition behavior of fluorinated crystals of 1 and 2, ESP calculations were performed. The ESP maps were generated using DFT based on the atomic coordinates from experimentally determined crystal structures. The ESPs of each metal complex ranged from −159.01 to +206.41 kJ mol−1 for 1, and from −224.69 to +127.76 kJ mol−1 for 2 (Fig. 2 and Table 2), indicating a strong positive shift in the overall structure of 1 due to perfluorination. The highest ESP, indicated by the blue region (electron-poor), is located on the Cu atom and at the center of the pentafluorophenyl rings in 1, and on the Cu atom and at the edge of the difluorophenyl groups in 2. The lowest ESP, shown in red (electron-rich), is found in the intermediate region between O1 and O2 in both complexes, as well as at the ortho-positions of the fluorine atoms in 2. Interestingly, the ESPs at the centroid of the two asymmetric pentafluorophenyl groups in 1 are +96 kJ mol−1 for ring-A1 and +88 kJ mol−1 for ring-A2, indicating that they retain the electrostatic properties of pentafluorobenzene (+64 kJ mol−1). This relationship is also observed in 2, where the ESPs of the difluorophenyl groups are −28 kJ mol−1 for ring-C1 and −38 kJ mol−1 for ring-C2, values comparable to that of 1,3-difluorobenzene (−26 kJ mol−1). This agreement with the ESPs of fluorophenyl groups is likely due to the twisted conformation of the fluorophenyl substituents, which effectively prevents electron redistribution from the π-conjugated system. These results strongly support the notion that the positive quadrupole moment of the pentafluorophenyl rings in 1 facilitates the recognition of CO2 and benzene, both of which are negative quadrupolar molecules, within their crystals. This observation contributes to a rational approach in the design of host–guest systems.
 | ||
| Fig. 2 The energy potential maps of (a) 1 and (b) 2: the color of potential is shown between −100 kJ mol−1 (red) to +100 kJ mol−1 (blue). IsoValue is 0.002. | ||
| 1 | 2 | [Cu(dbm)2] | |
|---|---|---|---|
| Highest positive ESP (copper) | +206.41 | +127.76 | +116.71 |
| Lowest negative ESP (oxygen) | −159.01 | −224.69 | −199.12 |
| Cg of aromatic rings | +96, +88 | −28, −38 | −75, −85 |
| Cg of six-membered ring | −14, −17 | −88, −90 | −73, −79 |
Gas and vapor adsorption studies
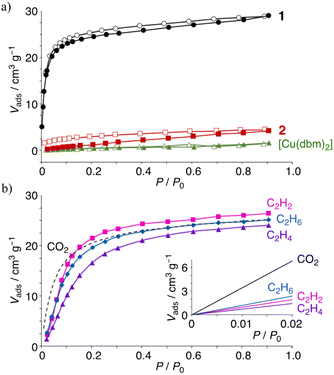
The gas adsorption/desorption behavior of the complexes was examined using microcrystalline powder samples, activated by heating at 60 °C for 3 h under vacuum. The N2 adsorption isotherms for 1 and 2 are type-III, yielding 6.97, 1.02, and 0.54 cm3 g−1 for 1, 2, and [Cu(dbm)2], respectively, at 0.91 P/P0 (Fig. S6†). The CO2 adsorption isotherms are shown in Fig. 3a. The fully fluorinated complex 1 adsorbed the highest amount of CO2, reaching 29.0 cm3 g−1 (1.13 mol mol−1), compared to 4.24 cm3 g−1 (0.12 mol mol−1) for 2 and 1.58 cm3 g−1 (0.04 mol mol−1) for [Cu(dbm)2] at 0.91 P/P0. Complex 1 exhibits a type-I isotherm, with a steep uptake at low P/P0, likely due to enhanced host–guest interactions within the crystal cavities. This corresponds to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest stoichiometric ratio. Despite the similar steric hindrance from ortho-substituted fluorine atoms in 2, which creates comparable axial space around the metal ion (Fig. 1), only minimal CO2 uptake was observed. This suggests that the molecular recognition in 1 is primarily driven by its large positive ESP and quadrupole moment. In contrast, the difluorophenyl groups in 2 has a small and negative ESP and quadrupole, which likely prevents CO2 uptake. The surface area (Vmax/cm3) and equilibrium constant (K/kPa) obtained by the Langmuir method are 13 and 15 times greater, respectively, for complex 1 than for 2. The temperature dependence of CO2 uptake for 1 was also studied (Fig. S7†). The amount of CO2 decreased with increasing temperature, as is commonly observed in other porous materials; however, at 298 K, at least 3.9 cm3 g−1 (0.15 mol mol−1 at 0.91 P/P0) of CO2 still remained adsorbed. The relatively steep uptake at low P/P0 and low temperatures (<229 K) suggests a strong contribution of quadrupole interactions to adsorption in these non-porous molecular crystals.
1 host–guest stoichiometric ratio. Despite the similar steric hindrance from ortho-substituted fluorine atoms in 2, which creates comparable axial space around the metal ion (Fig. 1), only minimal CO2 uptake was observed. This suggests that the molecular recognition in 1 is primarily driven by its large positive ESP and quadrupole moment. In contrast, the difluorophenyl groups in 2 has a small and negative ESP and quadrupole, which likely prevents CO2 uptake. The surface area (Vmax/cm3) and equilibrium constant (K/kPa) obtained by the Langmuir method are 13 and 15 times greater, respectively, for complex 1 than for 2. The temperature dependence of CO2 uptake for 1 was also studied (Fig. S7†). The amount of CO2 decreased with increasing temperature, as is commonly observed in other porous materials; however, at 298 K, at least 3.9 cm3 g−1 (0.15 mol mol−1 at 0.91 P/P0) of CO2 still remained adsorbed. The relatively steep uptake at low P/P0 and low temperatures (<229 K) suggests a strong contribution of quadrupole interactions to adsorption in these non-porous molecular crystals.
Given these results, the uptake of C2Hn hydrocarbons was studied, as hydrocarbon-rich feedstock contains some CO2, making its separation, especially from similarly sized components like C2H2, challenging. The physisorption isotherms for C2Hn hydrocarbons follow a type-I profile (Fig. 3b), although they do not align with expected values at low pressures. The volumes of physisorbed hydrocarbons are 26.4, 24.0, and 25.1 cm3 g−1 for C2H2, C2H4, and C2H6, respectively, at 0.91 P/P0 and 212 K. These maximum adsorbed amounts are close to the CO2 adsorption of 25.2 cm3 g−1, and the number of C2Hn molecules adsorbed by 1 corresponds to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio. However, the amount of adsorbed CO2 is significantly higher at P/P0 values below 0.1, indicating a potential pathway for separating CO2 from C2H2. The adsorption of C2Hn hydrocarbons in 1 may be influenced by the negative quadrupole moment along the Qxx axis on each π-plane or by adsorption driven by the negative ESP on the oxygens of the complex.
1 stoichiometric ratio. However, the amount of adsorbed CO2 is significantly higher at P/P0 values below 0.1, indicating a potential pathway for separating CO2 from C2H2. The adsorption of C2Hn hydrocarbons in 1 may be influenced by the negative quadrupole moment along the Qxx axis on each π-plane or by adsorption driven by the negative ESP on the oxygens of the complex.
To understand the higher kinetic stability of CO2 in 1 at low relative pressures, thermogravimetric (TG) studies were performed under CO2 and C2H2 gas flow at r.t. (Fig. 4). The results show a different rate of weight change over time for CO2 and C2H2, with the weight% and inserted amounts as follows: CO2 (0.53%; 0.11 mol mol−1) > C2H2 (6.7 × 10−2%; 2.2 × 10−2 mol mol−1), C2H4 (5.4 × 10−2%; 1.7 × 10−2 mol mol−1) ≫ C2H6 (6.4 × 10−4%; trace), as shown in Fig. 3 and S8.† Saturation for C2H2 in 1 was reached within 2 min, while CO2 took 4 min under the corresponding gas flow, indicating that CO2 has a higher gas intake per minute. This difference explains the higher CO2 uptake observed at low P/P0 and demonstrates that fluorination is an effective strategy for CO2 capture in non-porous crystals. The very poor insertion of C2H6 at r.t. suggests that the driving force and host–guest interactions are primarily governed by π-interactions between flat molecules, with minimal effective overlap between the quadrupole moment directions.
Similar phenomena were clearly observed for benzene (C6H6) and hexafluorobenzene (C6F6) in the adsorption studies. As reported in previous work, three C6H6 molecules are inserted into the crystal of 1 via M⋯π and π-hole⋯π interactions,26 with one and two equivalents, respectively. The M⋯π interaction refers to the interaction between the Cu center and the π-electron system of benzene, while the π-hole⋯π interaction involves the electrostatic attraction between the electron-deficient region (positive ESP) on a fluorinated aromatic ring and the π-electron system (negative ESP) of benzene. These interactions play a crucial role in stabilizing guest molecules within the host crystal and contribute to the selective adsorption properties observed in this study. The vapor adsorption isotherms of the complexes were examined using activated samples. The C6H6 isotherms for 1 show an irreversible insertion, unlike 2 and [Cu(dbm)2]. In Fig. 5a, one molecule of C6H6 is inserted at approximately 0.15 P/P0, followed by the insertion of two additional molecules around 0.5 P/P0, resulting in 1·3C6H6. The desorption isotherm exhibits unique hysteresis and a stepwise release at low pressure. The most stable crystalline state is estimated to be 1·3C6H6, which aligns with the previously observed crystal structure, suggesting that the remaining benzene molecule around 0.05 P/P0 is likely stabilized by enhanced M⋯π interactions. The lack of guest insertion in 2 clearly indicates that the three-dimensional structure of the molecule does not dominate guest recognition. Instead, the charge distribution on the complex surface plays a crucial role. The C6F6 isotherms in Fig. 5b show poor guest insertion for all complexes, providing the following insights: (1) electrostatic repulsion, due to the same positive quadrupole moments (Qzz) between the ring-As of 1 and C6F6, hinders guest insertion; (2) the weak quadrupole moments of the ring-Cs in 2 do not facilitate insertion; (3) while [Cu(dbm)2] might have potential for guest recognition, its densely packed planar molecular structure prevents guest insertion. In other word, due to the weak interactions between the pentafluorinated ligands in the crystal, 1 can expand its structure more easily to accommodate guest molecules. In contrast, [Cu(dbm)2] is less likely to expand upon crystallization due to M⋯π interactions between the Cu center in the complex and the phenyl ring of the adjusted complexes. Consequently, [Cu(dbm)2] cannot adsorb guests even when complementary guests are presented; the inference is supported by the melting points of the complexes: 216 °C for 1 < 321 °C for [Cu(dbm)2].40
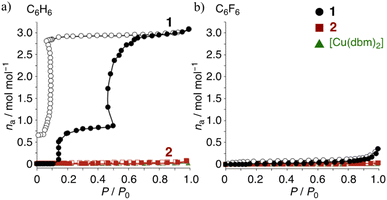 | ||
| Fig. 5 (a) Benzene and (b) hexafluorobenzene adsorption isotherms at r.t. for: 1, black circles; 2, red rectangles; non-fluorinated [Cu(dbm)2], green triangles; filled, adsorption; open, desorption. | ||
Experimental
Cu(II) complexes were obtained as crystalline solids via the reaction of the corresponding ligand and CuCl2·2H2O with NaOCH3 in MeOH, with quantitative yields.26,31 The solids were activated by heating at 60 °C for 3 hours under vacuum prior to adsorption/desorption measurements. Gas adsorption isotherms were obtained using MicrotracBEL BELSORP Mini/MAX/MAXII, and TG analysis was performed using a TA instruments TGA Q500.Conclusions
The fully fluorinated β-diketonate Cu(II) complex 1 exhibits distinctive gas and vapor adsorption properties in its crystalline state, demonstrating a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CO2 and C2Hn (n = 2, 4, 6) gas adsorption at 212 K, and a 1
1 CO2 and C2Hn (n = 2, 4, 6) gas adsorption at 212 K, and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 C6H6 vapor adsorption at r.t. Notably, the amount of CO2 adsorbed by 1 is significantly higher at low pressure (<0.1 P/P0), which is attributed to quadrupole interactions facilitated by the extended positive ESPs on the Cu(II) center and the pentafluorophenyl rings. This behavior highlights the importance of tailored electronic environments in achieving selective host–guest interactions. The stepwise insertion and release process observed for C6H6 vapor, characterized by hysteresis, reflects the contributions of M⋯π and π-hole⋯π interactions41–43 in stabilizing benzene molecules within the crystal structure. In contrast, the poor guest insertion of C6F6 into complex 1, caused by electrostatic repulsion between similarly positive quadrupole moments, highlights the importance of charge distribution in molecular recognition. Gas adsorption studies demonstrate the superiority of complex 1 over partially fluorinated complex 2 and non-fluorinated [Cu(dbm)2]. The type-I isotherm observed for CO2 and the steep uptake at low pressures reflect enhanced host–guest interactions driven by the large positive quadrupole moments in complex 1. This trend, which is absent in 2 and [Cu(dbm)2], highlights the critical role of fluorination in enabling selective adsorption. The enhanced affinity for CO2 and C6H6 in fluorinated compounds provides a novel framework for designing materials with complementary adsorption characteristics. These findings open avenues for further exploration of quadrupole modulation in gas separation technologies.
3 C6H6 vapor adsorption at r.t. Notably, the amount of CO2 adsorbed by 1 is significantly higher at low pressure (<0.1 P/P0), which is attributed to quadrupole interactions facilitated by the extended positive ESPs on the Cu(II) center and the pentafluorophenyl rings. This behavior highlights the importance of tailored electronic environments in achieving selective host–guest interactions. The stepwise insertion and release process observed for C6H6 vapor, characterized by hysteresis, reflects the contributions of M⋯π and π-hole⋯π interactions41–43 in stabilizing benzene molecules within the crystal structure. In contrast, the poor guest insertion of C6F6 into complex 1, caused by electrostatic repulsion between similarly positive quadrupole moments, highlights the importance of charge distribution in molecular recognition. Gas adsorption studies demonstrate the superiority of complex 1 over partially fluorinated complex 2 and non-fluorinated [Cu(dbm)2]. The type-I isotherm observed for CO2 and the steep uptake at low pressures reflect enhanced host–guest interactions driven by the large positive quadrupole moments in complex 1. This trend, which is absent in 2 and [Cu(dbm)2], highlights the critical role of fluorination in enabling selective adsorption. The enhanced affinity for CO2 and C6H6 in fluorinated compounds provides a novel framework for designing materials with complementary adsorption characteristics. These findings open avenues for further exploration of quadrupole modulation in gas separation technologies.
Data availability
The data supporting the findings of this study, including preparation methods, structural information, and physical properties, are available in the main article and its ESI.† Further inquiries regarding specific experimental details can be directed to the corresponding author. Additional information is available at the following sources: crystallographic data: data for compounds 1 and 2 was obtained from the Cambridge Crystallographic Data Centre under deposition numbers 1850163 and 895496, respectively. These can be obtained from the CCDC website (https://www.ccdc.cam.ac.uk/structures/). Preparation of 1: DOI: https://doi.org/10.1039/B617808A. Preparation of 2: DOI: https://doi.org/10.1039/C4CE01243G. Quadrupole moments: refer to ref. 16 and 26–28, and the Computational Chemistry Comparison and Benchmark DataBase (CCCBDB) (https://cccbdb.nist.gov/introx.asp). Adsorption data: included as part of the ESI.†Author contributions
Y. I. prepared the samples and conducted the gas studies. T. K. performed theoretical studies based on the crystal data and vapor adsorption. Y. I. (2) and H. U. assisted gas and vapor adsorption measurements, respectively, and ensured the data reproducibility. S. U. performed the TG analysis and contributed the absorption project. K. K. and M. N. provided support for the adsorption project. A. H. supervised the project and wrote the manuscript. All authors contributed to discussions and to finalizing the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Grant-in-Aid for Scientific Research B (no. 23K21122) of the JSPS KAKENHI (A. H.).Notes and references
- D. S. Sholl and R. P. Lively, Nature, 2016, 532, 435–437, DOI:10.1038/532435a.
- S. Chu, Y. Cui and N. Liu, Nat. Mater., 2016, 16, 16–22, DOI:10.1038/nmat4834.
- H.-C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673–674, DOI:10.1021/cr300014x.
- S. Kitagawa, R. Kitaura and S. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334–2375, DOI:10.1002/anie.200300610.
- S. Qiu, M. Xue and G. Zhu, Chem. Soc. Rev., 2014, 43, 6116–6140, 10.1039/C4CS00159A; S. Zhang, M. K. Taylor, L. Jiang, H. Ren and G. Zhu, Chem.–Eur. J., 2019, 26, 3205–3221, DOI:10.1002/chem.201904455.
- A. Goldman, B. Gil-Hernández, S. Millan, S. Gökpinar, C. Heering, I. Boldog and C. Janiak, CrystEngComm, 2020, 22, 2933–2944, 10.1039/D0CE00275E.
- A. Kondo, H. Kajiro, T. Nakagawa, H. Tanaka and H. Kanoh, Dalton Trans., 2020, 49, 3692–3699, 10.1039/C9DT04836G.
- X.-W. Gu, J.-X. Wang, E. Wu, H. Wu, W. Zhou, G. Qian, B. Chen and B. Li, J. Am. Chem. Soc., 2022, 144, 2614–2623, DOI:10.1021/jacs.1c10973.
- X.-P. Fu, Y.-L. Wang and Q.-Y. Liu, Dalton Trans., 2020, 49, 16598–16607, 10.1039/D0DT03349A.
- S. Mukherjee, D. Sensharma, K. J. Chen and M. J. Zaworotko, Chem. Commun., 2020, 56, 10419–10441, 10.1039/D0CC04645K.
- Y. Wang, H. Jin, Q. Ma, K. Mo, H. Mao, A. Feldhoff, X. Cao, Y. Li, F. Pan and Z. Jiang, Angew. Chem., Int. Ed., 2020, 59, 4365–4369, DOI:10.1002/anie.201915807.
- Y. Sato, M. Moździerz, K. Berent, G. Brus and M. Nomura, J. CO2 Util., 2024, 84, 102861, DOI:10.1016/j.jcou.2024.102861.
- C. R. Reid and K. M. Thomas, J. Phys. Chem. B, 2001, 105, 10619–10629, DOI:10.1021/jp0108263.
- J. R. Li, R. J. Kuppler and H. C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504, 10.1039/B802426J.
- O. T. Qazvini, R. Babarao and S. G. Telfer, Nat. Commun., 2021, 12, 197, DOI:10.1038/s41467-020-20489-2.
- (a) S. Liu, Y. Huang, Q. Dong, H. Wang and J. Duan, Inorg. Chem., 2020, 59, 9569–9578, DOI:10.1021/acs.inorgchem.0c00507; (b) L. Liu, Z. Yao, Y. Ye, Y. Yang, Q. Lin, Z. Zhang, M. O'Keeffe and S. Xiang, J. Am. Chem. Soc., 2020, 142, 9258–9266, DOI:10.1021/jacs.0c00612; (c) W. Fan, S. Yuan, W. Wang, L. Feng, X. Liu, X. Zhang, X. Wang, Z. Kang, F. Dai, D. Yuan, D. Sun and H.-C. Zhou, J. Am. Chem. Soc., 2020, 142, 8728–8737, DOI:10.1021/jacs.0c00805.
- H. Li, L. Li, R.-B. Lin, W. Zhou, Z. Zhang, S. Xiang and B. Chen, EnergyChem, 2019, 1, 100006, DOI:10.1016/j.enchem.2019.100006.
- R.-B. Lin, L. Li, H. Wu, H. Arman, B. Li, R.-G. Lin, W. Zhou and B. Chen, J. Am. Chem. Soc., 2017, 139, 8022–8028, DOI:10.1021/jacs.7b03850; F. Luo, C. S. Yan, L. L. Dang, R. Krishna, W. Zhou, H. Wu, X. L. Dong, Y. Han, T. L. Hu, M. O'Keeffe, L. L. Wang, M. B. Luo, R. B. Lin and L. Chen, J. Am. Chem. Soc., 2016, 138, 5678–5684, DOI:10.1021/jacs.6b02030.
- J. Lee, C. Y. Chuah, J. Kim, Y. Kim, N. Ko, Y. Seo, K. Kim, T. H. Bae and E. Lee, Angew. Chem., Int. Ed., 2018, 57, 7869–7873, DOI:10.1002/anie.201804442.
- B. S. Gelfand, J.-B. Lin and G. K. H. Shimizu, Inorg. Chem., 2015, 54, 1185–1187, DOI:10.1021/ic502478u.
- D. J. Gearhart, J. F. Harrison and K. L. C. Hunt, Int. J. Quantum Chem., 2003, 95, 697–705, DOI:10.1002/qua.10586.
- C. Graham, D. A. Imrie and R. E. Raab, Mol. Phys., 1998, 93, 49–56, DOI:10.1080/002689798169429.
- M. R. Battaglia, A. D. Buckingham and J. H. Williams, Chem. Phys. Lett., 1981, 78, 421–423, DOI:10.1016/0009-2614(81)85228-1.
- J. H. Williams, Crystal Engineering: How Molecules Build Solids, Morgan & Claypool Publishers, 2017 Search PubMed.
- K. Shimizu, M. F. C. Gomes, A. A. H. Pádua, L. P. N. Rebelo and J. N. C. Lopes, J. Phys. Chem. B, 2009, 113, 9894–9900, DOI:10.1021/jp903556q.
- A. Hori and T. Arii, CrystEngComm, 2007, 9, 215–217, 10.1039/B617808A.
- K. Jie, Y. Zhou, E. Li and F. Huang, Acc. Chem. Res., 2018, 51, 2064–2072, DOI:10.1021/acs.accounts.8b00255.
- M. Yan, Y. Wang, J. Chena and J. Zhou, Chem. Soc. Rev., 2023, 52, 6075–6119, 10.1039/D2CS00856D.
- T. Ogoshi, Y. Shimada, Y. Sakata, S. Akine and T. Yamagishi, J. Am. Chem. Soc., 2017, 139, 5664–5667, DOI:10.1021/jacs.7b00631.
- Y. Habuka, H. Usui, M. Okawa, T. Shinohara, H. Yuge, Y. Mao, J. Gong, G. J. Richards and A. Hori, CrystEngComm, 2024, 26, 3014–3020, 10.1039/d4ce00166d.
- A. Hori, K. Nakajima, Y. Akimoto, K. Naganuma and H. Yuge, CrystEngComm, 2014, 16, 8805–8817, 10.1039/C4CE01243G.
- (a) A. Hori, R. Gonda and I. I. Rzeznicka, CrystEngComm, 2017, 19, 6263–6266, 10.1039/C7CE01413A; (b) R. Gonda, I. I. Rzeznicka, Y. Kinoshita, S. Uchida and A. Hori, Dalton Trans., 2019, 48, 9062–9066, 10.1039/C9DT01380F.
- J. M. Crowder, H. Han, Z. Wei, E. V. Dikarev and M. A. Petrukhina, Polyhedron, 2019, 157, 33–38, DOI:10.1016/j.poly.2018.09.048.
- J. Hernández-Trujillo and A. Vela, J. Phys. Chem., 1996, 100, 6524–6530, DOI:10.1021/jp953576x.
- A. J. Russell and M. A. Spackman, Mol. Phys., 2000, 98, 867–874, DOI:10.1080/00268970050025475.
- W. Majer, P. Lutzman and W. Huttner, Mol. Phys., 1994, 83, 567–578, DOI:10.1080/00268979400101431.
- The structural information was used from CSD: CCDC 1850163 and 895496 for 1 and 2, respectively.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652, DOI:10.1063/1.464913.
- For CO2, C2H2 and C2H6, Qzz aligns with the molecule's elongation axis. In aromatics, Qzz's impact on the π-plane, perpendicular to the z-axis, is crucial for discussing molecular quadrupoles.
- A. Hori, A. Shinohe, M. Yamasaki, E. Nishibori, S. Aoyagi and M. Sakata, Angew. Chem., Int. Ed., 2007, 46, 7617–7620, DOI:10.1002/anie.200702662.
- A. S. Mahadevi and G. N. Sastry, Chem. Rev., 2013, 113, 2100–2138, DOI:10.1021/cr300222d.
- S. Yamada, Coord. Chem. Rev., 2020, 415, 213301, DOI:10.1016/j.ccr.2020.213301.
- H. Ju, B. W, M. Li, J. Hao, W. Si, S. Song, K. Mei, A. C.-H. Sue, J. Wang, C. Jia and X. Guo, J. Am. Chem. Soc., 2024, 146, 25290–25298, DOI:10.1021/jacs.4c09504.
Footnote |
| † Electronic supplementary information (ESI) available: The crystal structures and adsorption behaviors of copper complexes 1, 2, and [Cu(dbm)2]. See DOI: https://doi.org/10.1039/d4ra08463b |
| This journal is © The Royal Society of Chemistry 2025 |