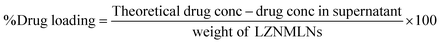Open Access Article
Open Access ArticleLuliconazole–niacinamide lipid nanocarrier laden gel for enhanced treatment of vaginal candidiasis: in vitro, ex vivo, in silico and preclinical insights
Bhabani Sankar Satapathy*a,
Ameeduzzafar Zafar *b,
Musarrat Husain Warsic,
Sritam Beherad,
Dibya Iochan Mohantye,
Md Ali Mujtaba
*b,
Musarrat Husain Warsic,
Sritam Beherad,
Dibya Iochan Mohantye,
Md Ali Mujtaba fg,
Mahaprasad Mohantyh,
Atul Kumar Upadhyayi and
Mohammad Khalidj
fg,
Mahaprasad Mohantyh,
Atul Kumar Upadhyayi and
Mohammad Khalidj
aGITAM School of Pharmacy, GITAM Deemed to be University, Hyderabad Campus, Telangana-502329, India
bDepartment of Pharmaceutics, College of Pharmacy, Jouf University, Sakaka 72341, Al-Jouf, Saudi Arabia. E-mail: zzafarpharmacian@gmail.com
cDepartment of Pharmaceutics and Industrial Pharmacy, College of Pharmacy, Taif University, Taif 21944, Saudi Arabia
dNityananda College of Pharmacy, Biju Patnaik University of Technology, Sergarh, Balasore, Odisha, India
eCentre for Nanomedicine, Department of Pharmaceutics, School of Pharmacy, Anurag University, Hyderabad, Telangana Pin 500088, India
fDepartment of Pharmaceutics, Faculty of Pharmacy, Northern Border University, Arar, Saudi Arabia
gCenter for Health Research, Northern Border University, Arar, Saudi Arabia
hSchool of Pharmaceutical Sciences, Siksha ‘O’ Anusandhan University, Odisha, India
iDepartment of Biotechnology, Thapar Institute of Engineering and Technology, Patiala, Punjab, India
jDepartment of Pharmacognosy, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al-Kharj 11942, Saudi Arabia
First published on 19th February 2025
Abstract
A lipid-based nanocarrier system is a novel technique for the delivery of poorly soluble drugs through topical delivery. This study developed a dual-drug (luliconazole: LZ, and niacinamide: NM) loaded lipid nanocarrier (LN)-laden gel for the treatment of vaginal candidiasis. The LNs were prepared using cholesterol and soya-α-lecithin through a thin-film hydration technique. The average vesicle size, polydispersity index, and zeta potential of the optimized LZNMLNs were 126.40 ± 1.30 nm, 0.276, and −34.6 ± 0.8 mV, respectively, and the formulation showed the sustained release of both drugs over an extended period. Selected LZNMLNs were incorporated into a bio-adhesive gel. The optimized LZNMLNs-gel showed excellent viscosity, spreadability, and bio-adhesiveness. The optimized LZNMLNs-gel exhibited significantly higher permeation of LZ (1.46-fold) and NM (1.55-fold) than LZNM gel. The optimized LZNMLNs-gel showed significantly higher in vitro antifungal activity (ZOI = 34 ± 2 mm) than commercial Candid V gel (18 ± 1 mm). The optimized LZNMLNs-gel did not show any cytotoxicity against vaginal epithelial cells. The bioavailability of LZNMLNs-gel was significantly (P < 0.05) increased (1.94-fold for LZ and 1.33-fold for NM) compared to Candid V, with a decrease in total clearance indicating sustained release of the drug, which may lead to the maintenance of therapeutic concentration for an extended period. In vivo antifungal activity showed that the optimized LZNMLNs-gel completely treated the infection on the 7th day of treatment in an induced rabbit model, compared to the commercial gel (Candid V gel, 10 days). Based the findings, it can be concluded that LN-laden gel is an alternative carrier for improvement of the topical delivery of drugs for the treatment of vaginal candidiasis.
Introduction
Vaginal candidiasis is a worrying medical challenge as it cannot be treated by conventional therapy.1 This infection is very invasive and can lead to serious systemic complications unless treated at an early stage. Superficial Candida infections subsequently lead to invasive candidiasis, which is very challenging to treat and particularly life-threatening in immune-compromised patients.2 Around 70% of women globally currently suffer from vulvovaginal candidiasis.3 Until now, the treatment of vulvovaginal infections has typically involved the use of antifungal drugs in conventional dosage forms, i.e., creams, suppositories, and tablets. However, traditional treatment approaches have some limitations, i.e., sub-therapeutic effectiveness and low patient compliance.4 Further, localized topical formulations suffer from several limitations, such as a rapid vaginal washout, low absorption, and low bioavailability, with undesirable adverse effects across the vaginal area.5In view of this, nanotechnology-based bio-adhesive gel (nanogel) topical formulation is an effective novel strategy to improve the topical vaginal delivery of therapeutic agents.6 Bio-adhesive gel laden with lipoidal nanocarriers (LNs, liposomes) is being heavily explored to improve the topical delivery of therapeutic agents for the treatment of topical microbial (fungal or bacterial) infections.7 LNs are nanosized lipoidal vesicles composed of biocompatible, non-toxic phospholipid and cholesterol outer layers enclosing an aqueous compartment. Owing to their nano-size and unique hydrophilic and lipophilic properties, LNs have emerged as a promising modality for the delivery of drugs into the body.8,9 LNs can protect drugs from harsh vaginal environments, target them precisely at the desired site in a controlled manner, and can also modulate their pharmacokinetic (PK) profile.10 Conversely, bio-adhesive gel possesses the ability to adhere across the vaginal epithelium for an extended period and can prolong the residence time of the drug at the infected area.11–13
Luliconazole (LZ), a synthetic imidazole (azole), has been widely used to combat severe fungal infections.14 It is a broad-spectrum antifungal agent, which selectively inhibits the cytochrome P 450 14-α-demethylase enzyme. It interrupts the alteration of lanosterol to ergosterol and hampers the formation of the fungus cell wall. LZ is poorly water-soluble, which limits its dermal delivery.15 LZ has been useful for treating superficial fungal infections, genital candidiasis, and chronic mucocutaneous candidiasis. However, following oral administration, there is considerable variation in the LZ absorption concentration and peak plasma concentration. Topical application of the drug through semisolid dosage forms also results in limited systemic availability.16 Thus, the delivery of LZ through a nanocarrier-based topical formulation could be the preferred strategy to boost its topical delivery and therapeutic effectiveness.
Niacinamide (NM), a vitamin B3 amide, is a well-known dietary supplement with a wide range of therapeutic applications. Currently, it is being widely investigated as a potential therapeutic agent to treat skin diseases. It has various therapeutic, antioxidant, anti-inflammatory, and immunomodulatory properties.17 Recent studies have documented that NM actively protects skin cells from oxidative stress.18 Topical delivery of NM improved tissue regeneration in an excisional full-thickness skin wound.19 Clinical evidence of NM also controls skin aging and pigmentation of skin.20,21 Thus, delivery of NM along with LZ in a suitable dosage form would improve tissue regeneration and promote the recovery of inflamed tissue in vaginal infection. Hence, co-delivery of LZ- and NM-loaded nanogels would show superior therapeutic efficacy compared to conventional dosage forms. The drug-loaded nanogel would thus maintain higher localized drug availability and would prompt the healing of inflamed tissue while reducing undesirable side effects.
The present study was aimed at developing an LZ- and NM-loaded lipoidal nanocarrier-laden bio-adhesive gel (LZNMLNsG) to treat vaginal candidiasis. The LZLNMLN formulation was developed using the thin-film hydration technique. The LZLNMLNs were evaluated for vesicle characteristics, drug loading and entrapment efficiency. The optimized LZNMLN formulation was incorporated in Carbopol 934P, HPMC-E15M, and neem-gum-based gel systems. The LZLNMLN gel was evaluated for various gel characteristics, in vitro release, ex vivo permeation, in vitro antifungal activity, and cytotoxicity study. A further in vivo (pharmacokinetic and pharmacodynamics) study was performed in a rabbit model. An in silico docking study was performed to analyze the possible molecular mechanism of LZNMLNs-gel in vaginal candidiasis.
Material and experimental
Materials
LZ and NM were purchased from HiMedia (Maharashtra, India). HPMC E15M was purchased from Dow Chemical Ltd, USA. Soya-α-lecithin (SL) and cholesterol (CHL) were procured from SRL Pvt. Ltd (Maharashtra, India). Butylated hydroxyl toluene (BHT), ascorbic acid, propylene glycol (PG), Sabouraud dextrose agar, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), Dulbecco's Modified Eagle's Medium, and fetal bovine serum were procured from HiMedia (Hyderabad, India).Development of experimental lipid nanocarriers
TLZNMLNs were prepared as per the composition given in Table 1 by using the thin-film hydration method.22,23 Measured amounts of SL, CHL, and LZ were dissolved in 10 mL of chloroform and transferred to a 250 mL round-bottom flask (RBF). BHT (2% w/v) was added to the solution to prevent lipid peroxidation. RBF was placed in a rotary evaporator (Rotavap, Heidolph, Germany) to evaporate the chloroform. The resultant thin lipid film was formed and stored overnight in a desiccator. Hydration of the dried lipid film was undertaken using a phosphate buffer solution (pH 7.4) containing a measured amount of NM. Then the dispersion was subjected to ultrasonication (digital ultrasonic cleaner, Mumbai, India) for 30 min (30 s intervals) for the formation of unilamellar nanosize vesicles. The resultant formulation was kept for 1–2 h at 25 °C to allow the discrete nano-vesicles to regain their native form. Then the vesicle dispersion was subjected to centrifugation at 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm (45 min at 4 °C). The resultant sediment was collected in a Petri dish and stored at −4 °C followed by lyophilization for 8–10 h (laboratory freeze dryer, Mumbai, India) to obtain freeze-dried LZNMLNs.
000 rpm (45 min at 4 °C). The resultant sediment was collected in a Petri dish and stored at −4 °C followed by lyophilization for 8–10 h (laboratory freeze dryer, Mumbai, India) to obtain freeze-dried LZNMLNs.
| Formulation code | Ratio of CHL and SL | Amount of drug (mg) | % Drug loading | EE | |||
|---|---|---|---|---|---|---|---|
| LZ | NM | LZ | NM | LZ | NM | ||
| a LZ, luliconazole; NM, niacinamide; LZNMLN, luliconazole- and niacinamide-loaded lipid nanocarriers; SL, soya-L-α-lecithin; CHL, cholesterol; EE, entrapment efficiency. Data show mean ± SD (n = 3). | |||||||
| LZNMLNs-1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
10 | 10 | 5.38 ± 0.40 | 3.96 ± 0.20 | 59.9 ± 0.50 | 51.3 ± 1.41 |
| LZNMLNs-2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
10 | 10 | 10.35 ± 0.60 | 9.13 ± 0.70 | 72.1 ± 1.71 | 70.1 ± 0.70 |
| LZNMLNs-3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
10 | 10 | 7.89 ± 0.30 | 7.95 ± 0.40 | 63.2 ± 0.70 | 67.2 ± 0.21 |
Vesicle characterization
The average vesicle size (Z-avg), polydispersity index (PDI), and zeta potential of the LZNMLNs were determined with a zeta sizer (Nano ZS 90, Malvern Instruments Ltd, UK). The lyophilized LZNMLN formulation was diluted with deionized water followed by sonication for 5–7 min to make agglomerate-free nano-vesicles. The sample was then placed into a cuvette and analyzed at 25 ± 0.5 °C using the dynamic light scattering method.23,24Entrapment efficiency (EE) and drug loading
The lyophilized LZNMLN (5 mg) formulation was dispersed in a mixture of acetonitrile and ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v). The nano-dispersion was sonicated and centrifuged at 16
1 v/v). The nano-dispersion was sonicated and centrifuged at 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min. The dispersion was filtered, and the concentration of LZ and NM was analyzed with a UV spectrophotometer (Shimadzu 1800, USA) at 296 nm and 260 nm. The % drug loading and EE were calculated as follows:
000 rpm for 5 min. The dispersion was filtered, and the concentration of LZ and NM was analyzed with a UV spectrophotometer (Shimadzu 1800, USA) at 296 nm and 260 nm. The % drug loading and EE were calculated as follows:Fourier-transform infrared spectroscopy (FTIR)
This study identified possible drug–excipient interactions by analyzing the primary wavenumbers of the functional groups.25 The spectra of pure LZ, NM, SL, CHL, BHT, physical mixtures, and optimized LZNMLNs were analyzed using an FTIR spectrophotometer (Shimadzu IR Prestige-21, Mumbai, India). The samples were scanned between 4000 and 500 cm−1 at 25 °C. The spectra were recorded and compared with each other.Differential scanning calorimetry (DSC)
A DSC instrument (Mettler Toledo DSC-1, Switzerland) was used to measure the change in enthalpy in the samples with respect to temperature.23,25 Weighed amounts of pure LZ, NM, and optimized LZNMLNs were separately packed into an aluminum pan and placed inside a DSC chamber under a dynamic nitrogen environment (50 mL min−1). The sample was scanned between 30 and 200 °C at a rate of 10 °C min−1.X-ray diffraction analysis (XRD)
An X-ray diffractometer (D8 Discover, Bruker, Germany) was employed to study the XRD analysis of the sample to explain the crystallinity/amorphous nature of the sample.26 The LZ, NM, and optimized LZNMLNs were separately used to fill the sample holder, making the thin layer. The sample was scanned between 5 and 70° at the 2-theta level (1° min−1 scanning speed).Field emission scanning electron microscopy (FESEM)
FESEM is a powerful method for the high-resolution surface imaging of lipoidal nanocarriers.27,28 Briefly, lyophilized optimized LZNMLNs were spread over a piece of carbon tape across a stub and vacuum-dried, followed by the application of a platinum coating over the surface to improve the resolution, and analyzed with a scanning electron microscope (FESEM, Carl Zeiss Microscopy GmbH, Jena, Germany).Cryo-transmission electron microscopy (cryo-TEM)
The optimized LZNMLNs were dispersed in deionized water, sonicated (5 min), and immediately vitrified over a cryo-stage inside liquid ethane. About 4 μL of sample suspension was applied over a clean grid and preserved under liquid nitrogen. The sample was analyzed using an FEI Tecnai G2 X-Twin Cryo-TEM microscope (FEI, Hillsboro, OR, USA) at 200 kV accelerated voltage.29In vitro drug release and release kinetics
The dialysis method was used to analyze the release of LZ and NM from the LZNMLNs. In brief, a measured 10 mg of LZNMLNs (containing 1.03 mg of LZ and 0.79 mg of NM) was placed in a dialysis bag (MWCO: 3.5 kDa) and submerged in 50 mL of phosphate buffer saline (PBS, pH 7.2).30 The release medium was stirred at 100 rpm and maintained at 37 °C throughout the experiment. Aliquots of 1 mL of release medium were taken at pre-fixed time intervals, and the same volume of PBS was added to the beaker to maintain a constant volume. The concentration was analyzed using a UV-visible spectrophotometer at 296 nm for LZ and 260 nm for NM against PBS as a blank. The drug release data of the LZNMLNs was plotted against time according to different models, viz., zero-order, first-order, Higuchi, and Korsmeyer–Peppas, and R2 values were calculated for each model for identification of the best-fitting model.31Preparation of LZNMLN-loaded gel
LZNMLN-loaded gel was prepared using Carbopol 934P, HPMC E15M and Azadirachta indica gum polymers as per the composition given in Table 2. Different concentrations of Carbopol 934P were dispersed under constant magnetic stirring (300 rpm). Then the required quantity of HPMC-E15M was mixed into the Carbopol 934P and stirred overnight. Then Azadirachta indica gum was dispersed in mildly heated distilled water. Then it was mixed with the Carbopol934P/HPMC HPMC-E15M dispersion under continuous stirring. Ascorbic acid and propylene glycol (PG) were added to the dispersion system. The required amount of LZNMLNs (1% w/w) was dispersed using 0.1% Span 80 and added to the aqueous polymeric dispersion system while stirring continuously. Finally, the pH of LZNMLN-loaded gel was adjusted using 10% w/v NaOH solution.| Composition | LZNMLNs2G1 | LZNMLNs2G2 | LZNMLNs2G3 |
|---|---|---|---|
| Carbopol 934P (%w/w) | 1 | 0.8 | 0.5 |
| HPMC E15M (%w/w) | 0.5 | 1.5 | 1 |
| Azadirachta indica gum (% w/w) | 1 | 0.5 | 1.5 |
| Propylene glycol | 5 mL | 5 mL | 5 mL |
| Ascorbic acid | 4 mg | 4 mg | 4 mg |
| LZNMLNs2 | 30 mg | 30 mg | 30 mg |
| Span 80 (%) | 0.5 | 0.5 | 0.5 |
| 10% NaOH solution | q.s | q.s | q.s |
| Double distilled water | 50 mL (q.s.) | 50 mL (q.s.) | 50 mL (q.s.) |
Characterization of LZNMLNs-gel
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol (1 mL, 1
ethanol (1 mL, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Then the mixture was centrifuged at 5000 rpm for 15 min, and the supernatant was collected. Then the supernatants were dried under a vacuum dryer. The dried samples were reconstituted with a mobile phase (acetonitrile
1). Then the mixture was centrifuged at 5000 rpm for 15 min, and the supernatant was collected. Then the supernatants were dried under a vacuum dryer. The dried samples were reconstituted with a mobile phase (acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol) and 50 μL of carbamazepine solution (internal standard). For the analysis, 20 μL of the sample was injected into the LCMS/MS column (Discovery C18, 15 cm × 2.1 mm ID, 5 μM). Using Phoenix WinNonlin software, various pharmacokinetic parameters i.e., Cmax, Tmax, the area under the curve (AUC), half-life (t1/2), mean time of residence (MRT), the area under the first moment curve (AUMC), and elimination rate (Kel), were determined.43
methanol) and 50 μL of carbamazepine solution (internal standard). For the analysis, 20 μL of the sample was injected into the LCMS/MS column (Discovery C18, 15 cm × 2.1 mm ID, 5 μM). Using Phoenix WinNonlin software, various pharmacokinetic parameters i.e., Cmax, Tmax, the area under the curve (AUC), half-life (t1/2), mean time of residence (MRT), the area under the first moment curve (AUMC), and elimination rate (Kel), were determined.43Results
Vesicle characterization
The average vesicle size (Z-avg) of the LZNMLN formulations was analyzed and found to be 184.60 ± 5.01 nm for LZNMLNs1, 126.40 ± 1.30 nm for LZNMLNs2, and 211.3 ± 8.1 nm for LZNMLNs3. The PDI of the formulations was found to be 0.801 for LZNMLNs1, 0.376 for LZNMLNs2, and 0.621 for LZNMLNs3. The zeta potential of the formulations was found to be −39.61 mV for LZNMLNs1, −34.6 mV for LZNMLNs2, and −26.16 mV for LZNMLNs3. The vesicle size and PDI of LZNMLNs2 were found to be lower (PDI < 0.5) than for the other formulations (LZNMLNs1 and LZNMLNs3).Drug loading and entrapment efficiency
The drug loading and EE of LZ and NM in the LZNMLNs were analyzed, and the results are depicted in Table 1. The loading of LZ ranged from 5.38% (LZNMLNs1) to 10.35% (LZNMLNs2). However, the loading of NM ranged from 3.96% (LZNMLNs1) to 9.13% (LZNMLNs2). The EE of LZ was found to range from 59.9 ± 0.5% (LZNMLNs1) to 72.1 ± 1.7 (LZNMLNs2). However, the EE of NM ranged from 51.3 ± 14% (LZNMLNs1) to 70.1 ± 0.7% (LZNMLNs2).Selection of optimized LZNMLNs
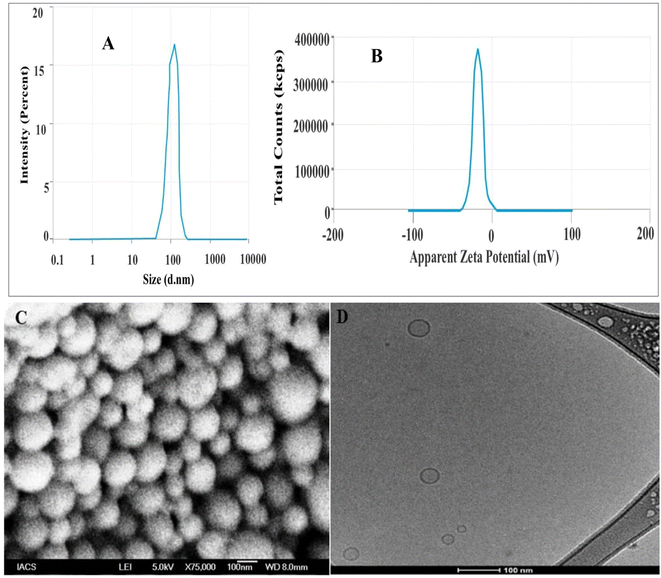
The optimized LZNMLN formulation was selected based on optimum parameters value, i.e., Z-avg, drug loading %, and EE. Among the formulations, LZNMLNs2 was selected as the optimized batch, showing Z-avg of 126.40 ± 1.30 nm, PDI of 0.376, and EE of −34.6 mV (Fig. 1A and B). The loadings of LZ and NM in LZNMLNs2 were found to be 10.35%, and 9.13%, respectively, with satisfactory EE as well (Table 1). Based on the characterization data, LZNMLNs2 was used for further study.FESEM analysis
The surface morphology of the LZNMLNs2 is displayed in Fig. 1C. The shape of LZNMLNs2 displayed a spherical and smooth surface. No agglomeration was found throughout the sample.Cryo-TEM analysis
The cryo-TEM image of LZNMLNs2 showed a spherical lipid layer with an inner aqueous compartment (Fig. 2D). Furthermore, the outer surface is smooth and spherical without any perforations, confirming its stability.FTIR-ATR analysis
The FTIR spectra of the LZ, NM, SL, CHL, BHT, physical mixtures, and optimized LZNMLNs are shown in Fig. 2A. Characteristic peaks of LZ were observed at 905.41 cm−1 (C–Cl stretching), 1644.98 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C alkene stretching), 2200 cm−1 (CN stretching), and 2830.09 cm−1 (C–H aliphatic stretching), confirming its purity. Similarly, the FTIR spectrum of NM showed characteristic peaks at 1672.95 cm−1 (C
C alkene stretching), 2200 cm−1 (CN stretching), and 2830.09 cm−1 (C–H aliphatic stretching), confirming its purity. Similarly, the FTIR spectrum of NM showed characteristic peaks at 1672.95 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 3353.6 cm−1 (N–H stretching), and 1614.13 cm−1 (N–H bending), confirming its purity. In the FTIR spectrum of the physical mixture (SL, CHL, BHT, and drugs), characteristic peaks were found at 1742.37 cm−1 corresponding to C
O stretching), 3353.6 cm−1 (N–H stretching), and 1614.13 cm−1 (N–H bending), confirming its purity. In the FTIR spectrum of the physical mixture (SL, CHL, BHT, and drugs), characteristic peaks were found at 1742.37 cm−1 corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the ester in SL, 2924.52 cm−1 for O–H stretching in CHL, 1644.98 cm−1 for C
O stretching of the ester in SL, 2924.52 cm−1 for O–H stretching in CHL, 1644.98 cm−1 for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H stretching in LZ, 3359.39 cm−1 for N–H stretching in NM, etc. This showed no significant alteration in the characteristic peaks of the drugs or excipients in the physical mixture of ingredients, confirming the absence of incompatibility. The FTIR spectrum of LZNMLNs2 further showed that the characteristic peaks of the drugs at 2852.2 cm−1 (C–H aliphatic stretching in LZ) and 1646.91 cm−1 (N–H bending or C
H stretching in LZ, 3359.39 cm−1 for N–H stretching in NM, etc. This showed no significant alteration in the characteristic peaks of the drugs or excipients in the physical mixture of ingredients, confirming the absence of incompatibility. The FTIR spectrum of LZNMLNs2 further showed that the characteristic peaks of the drugs at 2852.2 cm−1 (C–H aliphatic stretching in LZ) and 1646.91 cm−1 (N–H bending or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching in NM) were retained with only slight shifting, indicating no interaction between drugs and excipients.
O stretching in NM) were retained with only slight shifting, indicating no interaction between drugs and excipients.
DSC analysis
The physiochemical state, such as crystalline behavior or degradation profile, including any chemical interactions in the formulation, was evaluated using DSC. The thermogram showed a clear, sharp, single endothermic peak for pure LZ at 153.3 °C and for NM at 133.84 °C, corresponding to their melting points, which confirms the crystalline nature of the drugs (Fig. 2B). However, in the thermogram of LZNMLNs2, clear sharp peaks were absent, which indicated encapsulation of the drugs inside the lipoidal core/bilayer matrix and loss of crystallinity. Additionally, no additional endothermic peaks appeared in LZNMLNs2, not even in the molten state, which supported the absence of any chemical interactions between drugs and lipid components.XRD analysis
The XRD diffractogram of pure LZ showed characteristic peaks at 17.38°, 19.82°, 21.08°, 23.5°, and 27.37° with corresponding intensities of 2455 (58), 2202 (54), 1778 (41), 1516 (119), and 2125 (55), respectively. Similarly, NM characteristic peaks were detected at 14.80°, 14.61°, 25.69°, and 27.18° with corresponding intensities of 2759 (358), 2127 (171), 1998 (193), and 1496 (66), respectively (Fig. 2C). These sharp peaks with high intensity confirmed the crystalline nature of both LZ and NM. However, the diffractogram of LZNMLNs2 did not show the sharp and distinct peaks of LZ and NM; rather, diminished peaks with a significant reduction in peak height and area were observed. The overall analysis indicated the amorphisation of LZ and NM into the LZNMLNs2 matrix.In vitro drug release and release kinetics
The in vitro release study of LZ and NM from LZNMLNs2 was done by the dialysis bag method in simulated vaginal fluid (PBS, pH 4.2), and the results are shown in Fig. 3A. Both drugs (LZ and NM) were appropriately released from LZNMLNs2 without any burst release. The release of LZ and NM from LZNMLNs2 was 75.71 ± 2.79% and 67.61 ± 2.65%, respectively, in 12 h. The study showed that LZ and NM were released from LZNMLNs2 in a sustained manner without any fluctuation (burst release). This revealed that both drugs were successfully encapsulated into the lipid matrix. The first-order kinetic release model was found to give the best fit (R2 values of 0.9925 and 0.9921 for LZ and NM), which suggests that the drug release might follow the process of diffusion and erosion from the nano-lipoidal vesicles. | ||
| Fig. 3 (A) In vitro drug release study of LZ and NM from optimized LZNMLNs2. (B) % Swelling of different LZNMLNsG at different time intervals. | ||
Development and characterization of experimental LZNMLN gel
The optimized LZNMLNs (LZNMLNs2) were successfully incorporated into a gel system using a gelling polymer (Carbopol and HPMC) and evaluated. Azadirachta indica gum was used to aid sufficient bio-adhesion. The results of all evaluation parameters are depicted in Table 3. The pH of all LZNMLNs2-loaded gels (LZNMLNs2G) was found to range from 4.32 ± 0.10 to 4.56 ± 0.22, which is very close and compatible with vaginal pH (4.5). The viscosity of the gels was in the range of 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231 ± 68 cps (LZNMLNs2G1) to 43
231 ± 68 cps (LZNMLNs2G1) to 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126 ± 84 cps (LZNMLNs2G2) (Table 3). The viscosity of the gel increased with an increase in polymer concentration. The spreadability of the gels was found to be 6.82 ± 0.12 g cm per sec for LZNMLNs2G1, 5.03 ± 0.42 g cm per sec for LZNMLNs2G2, and 5.92 ± 0.24 g cm per sec for LZNMLNs2G3 (Table 3). The spreadability of gel depended on viscosity (polymer concentration) of the gel. The extrudability of the gel was analyzed and found to be (91.04 ± 0.17% for LZNMLNs2G1, 78.2 ± 0.78% for LZNMLNs2G2), and 83.43 ± 1.5% for LZNMLNs2G3. The bio-adhesive strength of all developed gels was found to be satisfactory at 19.61 ± 2.31 g for LZNMLNs2G1, 26.32 ± 1.02 g for LZNMLNs2G2, and 21.23 ± 1.14 g for LZNMLNs2G3 (Table 3).
126 ± 84 cps (LZNMLNs2G2) (Table 3). The viscosity of the gel increased with an increase in polymer concentration. The spreadability of the gels was found to be 6.82 ± 0.12 g cm per sec for LZNMLNs2G1, 5.03 ± 0.42 g cm per sec for LZNMLNs2G2, and 5.92 ± 0.24 g cm per sec for LZNMLNs2G3 (Table 3). The spreadability of gel depended on viscosity (polymer concentration) of the gel. The extrudability of the gel was analyzed and found to be (91.04 ± 0.17% for LZNMLNs2G1, 78.2 ± 0.78% for LZNMLNs2G2), and 83.43 ± 1.5% for LZNMLNs2G3. The bio-adhesive strength of all developed gels was found to be satisfactory at 19.61 ± 2.31 g for LZNMLNs2G1, 26.32 ± 1.02 g for LZNMLNs2G2, and 21.23 ± 1.14 g for LZNMLNs2G3 (Table 3).
| Formulation | pH | Viscosity (cps) | Mucoadhesive strength (gf) | Spreadability (g cm per sec) | Extrudability (%) |
|---|---|---|---|---|---|
| a Excellent.b satisfactory. | |||||
| LZNNLNs2G1 | 4.34 ± 0.12 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 231 ± 68 231 ± 68 |
19.61 ± 2.31 | 6.82 ± 0.12 | 91.04 ± 0.17b |
| LZNNLNs2G2 | 4.32 ± 0.10 | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126 ± 84 126 ± 84 |
26.32 ± 1.02 | 5.03 ± 0.42 | 78.2 ± 0.78a |
| LZNNLNs2G3 | 4.56 ± 0.22 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 092 ± 92 092 ± 92 |
21.23 ± 1.14 | 5.92 ± 0.24 | 83.43 ± 1.5b |
Swelling study
The swelling study of the gels was determined, and the results are depicted in Fig. 3B. LZNMLNs2G2 showed satisfactory swelling, i.e., 620.56 ± 25.29%, compared to LZNMLNs2G1 (408.34 ± 17.19%) and LZNMLNs2G3 (516.32 ± 19.99%) over an 8 h study period.Selection of optimized LZNMLNs2G
The LZNMLNs2G2 was selected as an optimized formulation based on viscosity, spreadability, extrudability, bio-adhesive strength, and swelling, and thus it was selected for further analysis of in vitro and in vivo efficacy.Rheological properties
The flow curve of LZNMLNs2G2 and the commercial formulation showed satisfactory rheological characteristics (Fig. 4A). The viscosity of the gel decreases by increasing the shear rate. It exhibited a shear-thinning system, i.e., pseudo-plastic flow (non-Newtonian flow). | ||
| Fig. 4 (A) Rheological graph (flow curve) of (LZNMLNs2G3) and commercial (Candid V gel), showing the shear thinning system. (B) Ex vivo permeation of LZ and NM from LZNMLNs2G3 and LZNM gel. | ||
Ex vivo permeation study
The ex vivo permeation of LZ and NM from LZNMLNs2G2 was studied using the excised goat mucosa, and results are represented graphically in Fig. 4B. The % permeation of LZ and NM from LZNMLNs2G2 was found to be 61.08 ± 3.59% (1832.60 ± 107.61 μg) and 56.74 ± 1.87% (1702.17 ± 56.09 μg), respectively. The % permeation of LZ and NM from plain LZNM-gel was found to be 41.74 ± 2.98% (1252.17 ± 89.35 μg) and 36.52 ± 2.65% (1095.65 ± 79.56 μg), respectively. The flux of LZ and NM from LZNMLNs2G2 was found to be 246.32 ± 14.46 μg cm−2 h−1 and 228.78 ± 7.54 μg cm−2 h−1, respectively. It was observed that LZNMLNs2G2 exhibited a 1.46-fold higher permeation of LZ than plain LZNM gel and a 1.55-fold higher permeation of NM than plain LZNM gel.In vitro antimicrobial efficacy evaluation against Candida albicans
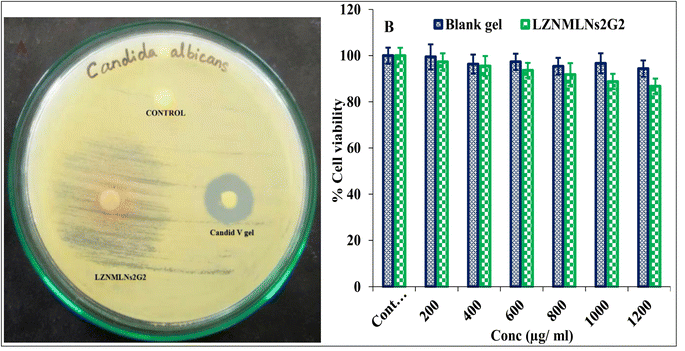
The antimicrobial activity of LZNMLNs2G2 (1 μg mL−1 drug concentration) against Candida albicans was studied and compared with Candid V gel (clotrimazole, 1% w/w) and control (DMSO) (Fig. 5A). The ZOI of LZNMLNs2G2 and Candid V gel was analyzed and found to be 34 ± 2 mm and 18 ± 1 mm, respectively. In general, antimicrobial potency is classified as sensitive (ZOI ≥ 11 mm), intermediate (ZOI, 8–10 mm), or resistant (ZOI < 7 mm).47 The area of ZOI around LZNMLNs2G2 was a little hazy compared to the ZOI of Candid V gel, which could be attributed to the slower/sustained release of drugs from the LZNMLNs2G2. This revealed that LZNMLNs2G2 exhibited significantly (P < 0.05) higher antimicrobial activity than Candid V gel against Candida albicans.48Cytotoxicity study in vaginal epithelial cells
The cytotoxicity of LZNMLNs2G2 and blank gel was evaluated by the MTT assay method on vaginal epithelial cells (ATCC PCS-480-010), and the results are depicted in Fig. 5B. A negligible reduction in the cell viability was observed in vaginal epithelial cells with LZNMLNs2G2, i.e., 91.83 ± 4.91%, 88.78 ± 3.31%, and 86.75 ± 3.29%, was observed at 800 μg mL−1, 1000 μg mL−1, and 1200 μg mL−1, respectively. Thus, the results showed no significant reduction in cell proliferation even after 24 h of incubation at various tested concentrations. The results indicated that LZNMLNs2G2 is non-toxic even at higher concentrations. The blank gel (no drugs) also did not adversely affect vaginal epithelial cells, indicating the biocompatible nature of the excipients used for the preparation of LZNMLNs2G2.Pharmacokinetic study (PK)
A pharmacokinetic study of the LZNMLNs2G2 and commercial gel (Candid V) was performed on the rabbits and the plasma concentration vs. time graph is depicted in Fig. 6A and the PK data is given in Table 4. The PK graph clearly showed higher residence of LZ and NM in plasma delivered through LZNMLN2G2 compared to LZNM gel. The Tmax, Cmax, AUC0-t, Kel, AUC0-inf, AUMC0-t, AUMC0-inf, half-life and MRT of LZ from LZNMLNs2G2 were found to be 4 h, 927.63 ± 38.667 ng mL−1, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 110.88 ± 143.87 ng h mL−1, 0.087 ± 0.021 h−1, 11
110.88 ± 143.87 ng h mL−1, 0.087 ± 0.021 h−1, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 828.00 ± 103.87 ng h mL−1, 83
828.00 ± 103.87 ng h mL−1, 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 542.28 ± 172.18 ng h2 mL−1, 144
542.28 ± 172.18 ng h2 mL−1, 144![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 276.01 ± 202.12 ng h2 mL−1, 7.88 ± 0.21 h, and 8.26 ± 0.24 h, respectively. However, the Tmax, Cmax, AUC0-t, Kel, AUC0-inf, AUMC0-t, AUMC0-inf, half-life and MRT of NM from LZNMLNs2G2 were found to be 4 h, 769.21 ± 43.67 ng mL−1, 6944.10 ± 78.93 ng h mL−1, 0.097 ± 0.25 h−1, 7888.45 ± 92.71 ng h mL−1, 54
276.01 ± 202.12 ng h2 mL−1, 7.88 ± 0.21 h, and 8.26 ± 0.24 h, respectively. However, the Tmax, Cmax, AUC0-t, Kel, AUC0-inf, AUMC0-t, AUMC0-inf, half-life and MRT of NM from LZNMLNs2G2 were found to be 4 h, 769.21 ± 43.67 ng mL−1, 6944.10 ± 78.93 ng h mL−1, 0.097 ± 0.25 h−1, 7888.45 ± 92.71 ng h mL−1, 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 446.91 ± 192.13 ng h2 mL−1, 86
446.91 ± 192.13 ng h2 mL−1, 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 823.81 ± 198.02 ng h2 mL−1, 7.13 ± 0.26 h and 7.84 ± 0.27 h, respectively. LZNMLNs2G2 exhibited significantly higher PK parameters than conventional LZNM gel. The AUC0-t of LZ and NM from the LZNMLNs2G2 were 1.94-fold and 1.33-fold higher than those of Candid V gel (commercial gel). LZNMLNs2G2 also exhibited a significantly higher MRT (1.49-fold for LZ and 1.39-fold for NM) compared to Candid V gel. This indicated sustained release of LZ and NM from LZNMLNs2G2, maintaining the therapeutic concentration over an extended period. The significantly lower elimination rate of LZ and NM from LZNMLNs2G2 compared to Candid V (0.157 ± 0.032 h−1) further revealed the prolonged in vivo residence time of drugs from LZNMLNs2G2, which would be beneficial in enhancing the effectiveness of the treatment.
823.81 ± 198.02 ng h2 mL−1, 7.13 ± 0.26 h and 7.84 ± 0.27 h, respectively. LZNMLNs2G2 exhibited significantly higher PK parameters than conventional LZNM gel. The AUC0-t of LZ and NM from the LZNMLNs2G2 were 1.94-fold and 1.33-fold higher than those of Candid V gel (commercial gel). LZNMLNs2G2 also exhibited a significantly higher MRT (1.49-fold for LZ and 1.39-fold for NM) compared to Candid V gel. This indicated sustained release of LZ and NM from LZNMLNs2G2, maintaining the therapeutic concentration over an extended period. The significantly lower elimination rate of LZ and NM from LZNMLNs2G2 compared to Candid V (0.157 ± 0.032 h−1) further revealed the prolonged in vivo residence time of drugs from LZNMLNs2G2, which would be beneficial in enhancing the effectiveness of the treatment.
| Parameters | Marketed gel (candid V) | LZNMLNs2G2 (LZ) | LZNMLNs2G2 (NM) |
|---|---|---|---|
| a Abbreviations: Cmax: maximum plasma drug concentration; Tmax: time taken in hours to attain maximum plasma drug concentration; AUC: area under the plasma concentration time curve; AUMC: area under the first moment curve; Kel: elimination rate constant; MRT: mean residence time. | |||
| Tmax (h) | 2 | 4 | 4 |
| Cmax (ng mL−1) | 781.13 ± 55.07 | 927.63 ± 38.667 | 769.21 ± 43.67 |
| AUC0-t (ng h mL−1) | 5199.60 ± 56.18 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 110.88 ± 143.87 110.88 ± 143.87 |
6944.10 ± 78.93 |
| Kel (h−1) | 0.157 ± 0.032 | 0.087 ± 0.021 | 0.097 ± 0.25 |
| AUC0-inf (ng h mL−1) | 5346.78 ± 76.23 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 828.00 ± 103.87 828.00 ± 103.87 |
7888.45 ± 92.71 |
| AUMC0-t (ng h2 mL−1) | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 349.84 ± 1121.32 349.84 ± 1121.32 |
83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 542.28 ± 172.18 542.28 ± 172.18 |
54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 446.91 ± 192.13 446.91 ± 192.13 |
| AUMC0-inf (ng h2 mL−1) | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 819.15 ± 172.19 819.15 ± 172.19 |
144![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 276.01 ± 202.12 276.01 ± 202.12 |
86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 823.81 ± 198.02 823.81 ± 198.02 |
| Half-life (h) | 4.41 ± 0.13 | 7.88 ± 0.21 | 7.13 ± 0.26 |
| MRT (h) | 5.64 ± 0.16 | 8.26 ± 0.24 | 7.84 ± 0.27 |
In vivo efficacy evaluation in vaginal candidiasis model
The in vivo efficacy of LZNMLNs2G2 compared to Candid V gel and blank gel was examined in female rabbits after the induction of vaginitis. The results are depicted in Fig. 6B. LZNMLNs2G2 exhibited a considerable decrease (to <0.05) in an infection on the 7th day of treatment as compared to the Candid V gel on the 10th day. However, the blank gel group (Group III) did not show any significant improvement in infection. Following topical application across the infected area, LZNMLNs2G2-treated rabbits showed complete recovery within 7 days of treatment.Docking analysis
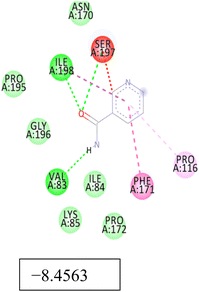
Promising in vitro/in vivo antimicrobial results prompted us to investigate the affinities of LZ and NM for key proteins linked to Candida albicans. For LZ, higher binding affinity was observed for all the selected Candida proteins, among which docking with 14-α-demethylase (5TZ1) resulted in the highest docking score of −13.44 kcal mol−1 (Table 5). Similarly, binding affinities of −9.46 kcal mol−1, −11.50 kcal mol−1 and −10.99 kcal mol−1 were observed for Als3 adhesin (4LE8), Crystal Structure of Enolase1 (7v67), and thymidylate synthase (5UIV) with LZ. Further, a higher binding affinity (−7.17 kcal mol−1) was observed for NM against 5UIV. The overall docking study rationalized the in vitro and in vivo antimicrobial test results.Discussion
The goal of the current study was to improve the transepithelial delivery of LZ and NM through LN gel. Different batches of LZNMLNs with varying ratios of lipids (CHL and SL) at fixed concentrations of the drugs were prepared using the thin-film hydration method. The optimized LZNMLN (LZNMLNs2) formulation was selected based on Z-avg, drug loading %, and EE. The optimized formulation exhibited PDI < 0.5 (0.378), indicating the monodispersed or narrow size distribution of the vesicles. PDI > 0.5 indicates the broad size distribution of the vesicles of particles.49The FESEM image showed the spherical shape and smooth morphology of LZNMLNs2 (Fig. 1C). The spherical size of the LNs would help to increase contact and adhesion with the vaginal wall. The smooth surface of the LNs may decrease irritation and enhance comfort during application.50 In addition, spherically shaped particles increase drug permeation through the vaginal epithelium and improve drug absorption. They also allow the sustained release of encapsulated drugs over an extended period.51
The FESEM result was further supported by Cryo-TEM analysis. Cryo-TEM is an irreplaceable tool for the clear visualization of internal morphology and lamellarity. In the cryo-TEM of LZNMLNs2, clear unilamellar vesicles, without any structural damage were observed (Fig. 1D). The cryo-TEM image indicated that vesicle lamellarity did not significantly alter the suspension state in terms of size, shape or architecture.52
The FTIR spectra showed the absence of any major physical/chemical interaction between LZ/NM and other lipids/excipients used in formulating the LNs. However, a few minor shifts in characteristic peaks of drugs and lipids in the LZNMLNs were detected, which could be attributed to the presence of van der Waals, electrostatic, or dipole–dipole interactions between the components during the formulation process. Such minor physical interactions would be rather beneficial in holding the drugs together in the LNs (Fig. 2A). Similar types of observation have been reported for curcumin- and tetrandrine-loaded LNs.53
A DSC study was performed to analyze the crystallinity of the therapeutic agents in the LNs.54 The sharp single endothermic peaks of pure LZ and NM indicated their purity and crystallinity (Fig. 2B). However, the absence of sharp endothermic peaks of LZ and NM in LZNMLNs2 indicated encapsulation of drugs inside the LNs with loss of their crystallinity or a change into an amorphous form, which would be helpful for improving their solubility.55
Similarly, the diffractograms of pure LZ and NM showed sharp, intense peaks indicating their crystalline nature, as supported by various reports.56,57 However, in the diffractogram of LZNMLNs2, LZ and NM did not show sharp intense peaks (but diminished peaks) (Fig. 2C). This revealed that LZ and NM might lose crystallinity or change into an amorphous form. Induction of amorphization would be helpful for improving the solubility, bioavailability, and therapeutic activity of the drug.56 In addition, the low intensity or absence of LZ and NM peaks in the LNs indicated the suitable encapsulation of the drugs into the LN matrix, which would be helpful in protecting the drugs from degradation and providing a sustained release profile.58,59
The in vitro release of LZ and NM from LZNMLNs2 was studied in simulated vaginal fluid (PBS, pH 4.2) using a dialysis bag. LZ and NM exhibited sustained release over the 24 h of the study (Fig. 3A). Owing to the hydrophilic nature of NM, it might be encapsulated deep inside the aqueous core of the LNs, which might result in slower release than for LZ. This result was confirmed by a previously published report of a tetracaine-hydrochloride-loaded lipid vesicle.60 The sustained release of drugs from the LNs would maintain the therapeutic concentration over an extended period and would increase absorption as well as decrease side effects. It would also help to increase patient compliance by decreasing the dose and dosing frequency and related adverse effects.
Furthermore, among the kinetic models, the Higuchi kinetics model was found to be the best fit because it showed the highest R2 value and followed the non-Fickian diffusion mechanism (erosion- and diffusion-controlled release) for the drug release pattern.61
LZNMLNs2-gels were successfully prepared using Carbopol-934P, HPMC-E15M, and Azadirachta indica gum. Azadirachta indica gum (a natural adhesive polymer) was used as a bio-adhesive agent to increase retention at the vaginal site. Additionally, Azadirachta indica gum possesses suitable antimicrobial properties, which would further synergize the antifungal potency. LZNMLNs2G2 (0.5% w/v neem gum, 0.8% w/v Carbopol-934P, and 1.5% w/v HPMC-E15M) was chosen as the optimized gel because it exhibited optimum gel characteristics (Table 2). Because of its excellent bio-adhesion, viscosity, and optimum swelling (Fig. 3B), LZNMLNs2G2 would remain for a long time on the application site, leading to an increase in permeation and therapeutic efficacy.62
LZNMLNs2G2 exhibited a shear-thinning system (Fig. 4A), i.e., the viscosity of the gel decreased with increasing shear rate. This indicates that the gel followed pseudo-plastic flow (non-Newtonian flow).63 This system may be easily spread over the application area and evenly distributed as well as controlling drug release. This system would be beneficial for effective in vivo application across the vaginal area.
An ex vivo permeation study of LZ and NM from LZNMLNs2G2 was performed, and the results are shown in Fig. 4B. LZNMLNs2G2 showed a significantly higher permeation of LZ and NM in 12 h than plain LZNM gel. The nanosize of LZNMLNs2, which provides a high surface area to the diffusion medium, led to high permeation of LZ and NM compared to plain LZNM gel. In addition, the higher permeation of drugs from LZNMLNs2G2 could be attributed to the high adhesion potential of the nanogel, which provided sufficient time for the drugs to come out of the gel matrix and permeate across the vaginal epithelium.64 This finding agreed with a previously published report of clotrimazole-loaded LN gel for vaginal delivery.65
The antifungal activity of LZNMLNs2G2 against Candida albicans was determined using the agar well diffusion method. LZNMLNs2G2 exhibited significantly (P < 0.05) higher activity (ZOI: 34 ± 2 mm) than the commercial gel (Candid V gel, 2%, ZOI: 18 ± 1 mm) (Fig. 5A). The high antifungal activity of LZNMLNs2G2 might be due to the nanosize of the drug-loaded LNs, which provide a high surface area and might increase permeation of the fungal cell membrane, leading to enhanced activity. Moreover, the sustained release of medicaments from LZNMLNs2G2 over an extended period might be responsible for achieving higher antifungal activity than Candid V gel. A similar type of observation was reported in fluconazole-loaded LN gel, where a significantly higher ZOI (34 mm) was shown by LN gel than by plain gel (20 mm).66
A cytotoxicity study of LZNMLNs2G2 was undertaken to determine any potential toxicity to vaginal epithelial cells upon topical application. LZNMLNs2G2 and blank gel did not show any toxicity to vaginal epithelial cells, as evidenced by an MTT assay (Fig. 5B). Even at the highest concentration of drugs, no significant toxic effect on cells was observed (86.75 ± 3.29% cell viability at 1200 μg mL−1). This revealed the non-toxic nature of LZNMLNs2G2 and its suitability for in vivo application for vaginal infections.
The PK parameters of LZ and NM for LZNMLNs2G2 after application into rabbits were analyzed and compared to Candid V gel, as depicted in Table 4 and Fig. 6A. LZNMLNs2G2 showed a significantly higher Cmax AUC, AUMC, MRT, and half-life (t1/2) of LZ and NM compared to Candid V gel. The MRT and low elimination rate of LZNMLNs2G2 revealed the good in vivo stability of the drugs. Additionally, it may be attributed to the nanosize of the particles, the higher stability of the encapsulated drugs, the bio-adhesive characteristic of the gel and the sustained released of drugs into the vaginal microenvironment. It can be revealed that the nanogel could be a good carrier for the topical treatment of vaginal infection.
LZNMLNs2G2 showed significantly better in vivo activity than the commercial gel (Candid V gel) in the disease-induced rabbit model. Infection-induced rabbits exhibited complete recovery from vaginal infection after 7 days of treatment with LZNMLNs2G2 (Fig. 6B). LZNMLNs2G2 exhibited high activity owing to an increase in solubility, protection of the drugs from untimely degradation, and an increase in the vaginal epithelial permeation, which led to an increase in bioavailability and therapeutic efficacy.
Docking data revealed higher docking scores for both LZ and NM against all selected Candida proteins. However, the docking score of LZ was considerably higher than that of NM. Appropriate molecular interaction between the drugs and selected fungal protein further rationalized our approach for the co-delivery of both drugs in LN carriers.
Conclusion
The present research developed an LZNM-loaded LN gel as a novel topical delivery method to improve therapeutic efficacy against vaginal candidiasis. LZNMLNs2 was found to be nanosized and spherical, with a high loading of LZ and NM. LZNMLNs2G2 showed excellent viscosity, spreadability, bio-adhesion, and swelling index, as well as significantly higher ex vivo vaginal epithelial permeation. Impressive in vitro antifungal activity of LZNMLNs2G2 was observed against Candida albicans cells. An MTT assay showed the nontoxic and biocompatible nature of the LZNMLNs2G2 formulation. In vivo studies showed higher therapeutic effectiveness of LZNMLNs2G2 than commercial gel (Candid V gel) in vaginitis-induced rabbits. Based on the in vitro, in vivo, and in silico results, LZNMLNs2G2 may be considered a suitable option for the treatment of vaginal candidiasis. The outcome of this study will lay the foundations for further research on comparative efficacy analysis of LZNMLNs2G2 in other in vivo vaginitis models, toxicity studies, and in vitro/in vivo correlation analysis toward its future clinical translation.Ethical statement
The study complied with the ARRIVE guidelines, and was carried out in accordance with the U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines, EU Directive 2010/63/EU for animal experiments.Data availability
All the data for the manuscript is available in the manuscript.Author contributions
Bhabani Sankar Satapathy: methodology, conceptualization, investigation, software, resources, writing – original draft, Ameeduzzafar Zafar: software, writing – original draft, writing – review & editing; Musarrat Husain Warsi: data curation, writing – review & editing Sritam Behera: investigation, resources; Dibya lochan Mohanty: methodology, resources; Md Ali Mujtaba: writing – review & editing, visualization. Mahaprasad Mohanty: methodology, writing – review & editing. Atul Kumar Upadhyay: data curation, writing – review & editing. Mohammad Khalid: writing – review & editing, data curation.Conflicts of interest
No conflict of interestAcknowledgements
The authors extend their appreciation to Taif University, Saudi Arabia, for supporting this work through project number (TU-DSPP-2024-136).References
- A. Aklilu, M. Woldemariam, A. Manilal, G. Koira, R. M. Alahmadi, G. Raman, A. Idhayadhulla and M. Yihune, Aerobic vaginitis, bacterial vaginosis, and vaginal candidiasis among women of reproductive age in Arba Minch, southern Ethiopia, Sci. Rep., 2024, 14(1), 9813 Search PubMed.
- A. Ahuja and M. Bajpai, Nanoformulations Insights: A Novel Paradigm for Antifungal Therapies and Future Perspectives, Curr. Drug Delivery, 2024, 21(9), 1241–1272 CrossRef CAS PubMed.
- C. de, J. Orlandi Sardi, D. R. Silva, P. C. Anibal, J. J. de Campos Baldin, S. R. Ramalho, P. L. Rosalen, M. L. Macedo and J. F. Hofling, Vulvovaginal candidiasis: epidemiology and risk factors, pathogenesis, resistance, and new therapeutic options, Curr. Fungal Infect. Rep., 2021, 15, 32–40 CrossRef.
- C. Chayachinda, M. Thamkhantho, T. Rekhawasin and C. Klerdklinhom, Sertaconazole 300 mg versus clotrimazole 500 mg vaginal suppository for treating pregnant women with acute vaginal candidiasis: a double-blinded, randomized trial, BMC Pregnancy Childbirth, 2024, 24(1), 1–8 CrossRef.
- P. Kumar, D. K. Sharma and M. S. Ashawat, Topical creams of piperine loaded lipid nanocarriers for management of atopic dermatitis: development, characterization, and in vivo investigation using BALB/c mice model, J. Liposome Res., 2022, 32(1), 62–73 CrossRef CAS.
- Z. Vanić, M. W. Jøraholmen and N. Škalko-Basnet, Nanomedicines for the topical treatment of vulvovaginal infections: Addressing the challenges of antimicrobial resistance, Adv. Drug Delivery Rev., 2021, 178, 113855 CrossRef.
- V. De Leo, F. Milano, A. Agostiano and L. Catucci, Recent advancements in polymer/liposome assembly for drug delivery: From surface modifications to hybrid vesicles, Polymers, 2021, 13(7), 1027 CrossRef CAS.
- K. Ghosal, A. Pani, T. Chowdhury, A. Kundu and S. Thomas, Multi-vesicular Liposome and its Applications: A Novel Chemically Modified Approach for Drug Delivery Application, Mini-Rev. Med. Chem., 2024, 24(1), 26–38 CrossRef CAS.
- N. M. Badawi, M. A. Elkafrawy, R. M. Yehia and D. A. Attia, Clinical comparative study of optimized metronidazole loaded lipid nanocarrier vaginal emulgel for management of bacterial vaginosis and its recurrence, Drug Delivery, 2021, 28(1), 814–825 CrossRef CAS PubMed.
- B. Mukherjee, S. Das, S. Chakraborty, B. Sankar Satapathy, P. Jyoti Das, L. Mondal, C. Mobaswar Hossain, N. Shekhar Dey and A. Chaudhury, Potentials of polymeric nanoparticle as drug carrier for cancer therapy: with a special reference to pharmacokinetic parameters, Curr. Drug Metab., 2014, 15(6), 565–580 CrossRef CAS PubMed.
- C. M. Caramella, S. Rossi, F. Ferrari, M. C. Bonferoni and G. Sandri, Mucoadhesive and thermogelling systems for vaginal drug delivery, Adv. Drug Delivery Rev., 2015, 92, 39–52 CrossRef CAS PubMed.
- H. Labie and M. Blanzat, Hydrogels for dermal and transdermal drug delivery, Biomater. Sci., 2023, 11(12), 4073–4093 RSC.
- S. A. Mustfa, E. Maurizi, J. McGrath and C. Chiappini, Nanomedicine approaches to negotiate local biobarriers for topical drug delivery, Adv. Ther., 2021, 4(1), 2000160 CrossRef.
- L. Song, F. Cao, S. Niu, M. Xu, R. Liang, K. Ding, Z. Lin, X. Yao and D. Liu, Population Pharmacokinetic/Pharmacodynamic Analysis of the Glucokinase Activator PB201 in Healthy Volunteers and Patients with Type 2 Diabetes Mellitus: Facilitating the Clinical Development of PB201 in China, Clin. Pharmacokinet., 2024, 63(1), 93–108 CrossRef CAS PubMed.
- L. Zhang, D. Pornpattananangku, C. M. Hu and C. M. Huang, Development of nanoparticles for antimicrobial drug delivery, Curr. Med. Chem., 2010, 17(6), 585–594 CrossRef CAS PubMed.
- M. Rani, K. Parekh, T. Mehta and A. Omri, Formulation development and characterization of luliconazole loaded− mesoporous silica nanoparticles (MCM− 48) as topical hydrogel for the treatment of cutaneous candidiasis, J. Drug Delivery Sci. Technol., 2024, 91, 105250 CrossRef CAS.
- Y. C. Boo, Mechanistic basis and clinical evidence for the applications of nicotinamide (niacinamide) to control skin aging and pigmentation, Antioxidants, 2021, 10(8), 1315 CrossRef CAS PubMed.
- Z. Zhen, X. Xiong, Y. Liu, J. Zhang, S. Wang, L. Li and M. Gao, NaCl inhibits citrinin and stimulates Monascus pigments and monacolin K production, Toxins, 2019, 11(2), 118 CrossRef CAS PubMed.
- S. A. Esfahani, M. Khoshneviszadeh, M. R. Namazi, A. Noorafshan, B. Geramizadeh, E. Nadimi and S. T. Razavipour, Topical nicotinamide improves tissue regeneration in excisional full-thickness skin wounds: A stereological and pathological study, Trauma Mon., 2015, 20(4), e18193 Search PubMed.
- C. Marques, F. Hadjab, A. Porcello, K. Lourenço, C. Scaletta, P. Abdel-Sayed, N. Hirt-Burri, L. A. Applegate and A. Laurent, Mechanistic insights into the multiple functions of niacinamide: Therapeutic implications and cosmeceutical applications in functional skincare products, Antioxidants, 2024, 13(4), 425 CrossRef CAS PubMed.
- M. Sanati and S. A. Yavari, Liposome-integrated hydrogel hybrids: Promising platforms for cancer therapy and tissue regeneration, J. Controlled Release, 2024, 368, 703–727 CrossRef CAS PubMed.
- B. S. Satapathy, L. A. Kumar, G. Pattnaik and B. Barik, Lomustine Incorporated Lipid Nanostructures Demonstrated Preferential Anticancer Properties in C6 Glioma Cell Lines with Enhanced Pharmacokinetic Profile in Mice, Acta Chim. Slov., 2021, 68(4), 970–982 CrossRef CAS PubMed.
- B. S. Satapathy, P. K. Sahoo, S. Pattnaik, A. K. Nayak, L. Maharana and R. N. Sahoo, Conveyance of sofosbuvir through vesicular lipid nanocarriers as an effective strategy for management of viral meningitis, RSC Adv., 2023, 13(47), 33500–33513 RSC.
- L. Tu, J. Zeng, X. Bai, Z. Wu, J. Wu and S. Xu, Nanoliposome-Mediated Encapsulation of Chlorella Oil for the Development of a Controlled-Release Lipid-Lowering Formulation, Foods, 2024, 13(1), 158 CrossRef CAS PubMed.
- C. Singh, K. Rao, N. Yadav, N. Bansal, Y. Vashist, S. Kumari and P. Chugh, A Review: Drug Excipient Incompatibility by Ftir Spectroscopy, Curr. Pharm. Anal., 2023, 19(5), 371–378 CrossRef CAS.
- W. Li, M. Chountoulesi, L. Antoniadi, A. Angelis, J. Lei, M. Halabalaki, C. Demetzos, S. Mitakou, L. A. Skaltsounis and C. Wang, Development and physicochemical characterization of nanoliposomes with incorporated oleocanthal, oleacein, oleuropein and hydroxytyrosol, Food Chem., 2022, 384, 132470 CrossRef CAS PubMed.
- H. Bakeshlou, S. Pirsa, F. Mohtarami and M. Bener, Investigating the Structural and Antimicrobial Properties of Wheat Gluten Nanocomposite Film Containing Zinc Oxide Nanoparticles and Quercetin Nanoliposomes, J. Environ. Polym. Degrad., 2024, 1–20 Search PubMed.
- M. Lei, G. Ma, S. Sha, X. Wang, H. Feng, Y. Zhu and X. Du, Dual-functionalized liposome by co-delivery of paclitaxel with sorafenib for synergistic antitumor efficacy and reversion of multidrug resistance, Drug Delivery, 2019, 26(1), 262–272 CrossRef CAS PubMed.
- X. Zhang, Z. Wu, W. Zhang, L. Wang, P. Zhao, X. Lv, P. Guo and J. Chen, Surface modification by chitosan for improving stability and antioxidative activity of astaxanthin-loaded liposomes, LWT–Food Sci. Technol., 2024, 198, 116033 CrossRef CAS.
- S. Gholizadeh, H. Almasi, S. Amjadi, M. Moradi, N. Ghadiri Alamdari, S. Salmasi and E. Divsalar, Development and characterization of active packaging system based on zein nanofibers mat incorporated with geraniol-loaded nanoliposomes, Food Sci. Nutr., 2024, 12, 5373–5387 CrossRef CAS PubMed.
- T. Li, C. J. Nowell, D. Cipolla, T. Rades and B. J. Boyd, Direct comparison of standard transmission electron microscopy and cryogenic-TEM in imaging nanocrystals inside liposomes, Mol. Pharm., 2019, 16(4), 1775–1781 CrossRef CAS PubMed.
- B. Wang, X. C. Yu, S. F. Xu and M. Xu, Paclitaxel and etoposide co-loaded polymeric nanoparticles for the effective combination therapy against human osteosarcoma, J. Nanobiotechnol., 2015, 13, 1 CrossRef CAS PubMed.
- B. Pyrak, K. Rogacka-Pyrak, T. Gubica and Ł. Szeleszczuk, Exploring cyclodextrin-based nanosponges as drug delivery systems: Understanding the physicochemical factors influencing drug loading and release kinetics. nt, J. Mol. Sci., 2024, 25(6), 3527 CrossRef CAS PubMed.
- D. Sudipta, P. K. Haldar and G. Pramanik, Formulation and evaluation of herbal gel containing Clerodendrum infortunatum leaves extract, Int. J. PharmTech Res., 2011, 3, 140–143 Search PubMed.
- Y. Kimura, S. Shimizu and K. Haraguchi, Gelation and anomalous viscosity dynamics in aqueous dispersions of synthetic hectorite, NPG Asia Mater., 2022, 14(1), 27 CrossRef CAS.
- G. Sharma, M. K. Saini, K. Thakur, N. Kapil, N. K. Garg, K. Raza, V. G. Goni, A. Pareek and O. P. Katare, Aceclofenac cocrystal nanoliposomes for rheumatoid arthritis with better dermatokinetic attributes: a preclinical study, Nanomed, 2017, 12(6), 615–638 CrossRef CAS PubMed.
- I. L. Dejeu, L. G. Vicaș, L. L. Vlaia, T. Jurca, M. E. Mureșan, A. Pallag, G. H. Coneac, I. V. Olariu, A. M. Muț, A. S. Bodea and G. E. Dejeu, Study for evaluation of hydrogels after the incorporation of liposomes embedded with caffeic acid, Pharmaceuticals, 2022, 15(2), 175 CrossRef CAS PubMed.
- A. Mousavi Moghadam, M. Vafaie Sefti, M. Baghban Salehi and A. Dadvand Koohi, Preformed particle gel: evaluation and optimization of salinity and pH on equilibrium swelling ratio, J. Pet. Explor. Prod. Technol., 2012, 2, 85–91 CrossRef CAS.
- A. Ortega, A. B. da Silva, L. M. da Costa, K. C. Zatta, G. R. Onzi, F. N. da Fonseca, S. S. Guterres and K. Paese, Thermosensitive and mucoadhesive hydrogel containing curcumin-loaded lipid-core nanocapsules coated with chitosan for the treatment of oral squamous cell carcinoma, Drug Delivery Transl. Res., 2023, 13(2), 642–657 CrossRef CAS PubMed.
- S. Salah, G. E. Awad and A. I. Makhlouf, Improved vaginal retention and enhanced antifungal activity of miconazole microsponges gel: Formulation development and in vivo therapeutic efficacy in rats, Eur. J. Pharm. Sci., 2018, 114, 255–266 CrossRef CAS PubMed.
- S. Austermeier, M. Pekmezović, P. Porschitz, S. Lee, N. Kichik, D. L. Moyes, J. Ho, N. K. Kotowicz, J. R. Naglik, B. Hube and M. S. Gresnigt, Albumin neutralizes hydrophobic toxins and modulates Candida albicans pathogenicity, mBio, 2021, 12(3), 10–128 CrossRef PubMed.
- S. Mukherjee; P. Malik and T. K. Mukherjee, in Experimental Mammalian Cell Culture-Based Assays, ed. Mukherjee, T. K., Malik, P. and Mukhopadhyay, S., Practical Approach to Mammalian Cell and Organ Culture. Springer, Singapore, 2023, doi: DOI:10.1007/978-981-19-1731-8_16-1.
- A. Rajput, G. Shevalkar, K. Pardeshi and P. Pingale, Computational Nanoscience and Technology, OpenNano, 2023, 12, 100147 CrossRef CAS.
- X. Zhao, D. Sun, A. Zhang, H. Huang, Y. Li and D. Xu, Candida albicans-induced activation of the TGF-β/Smad pathway and upregulation of IL-6 may contribute to intrauterine adhesion, Sci. Rep., 2023, 13(1), 579 CrossRef CAS PubMed.
- K. Atz, L. Cotos, C. Isert, M. Håkansson, D. Focht, M. Hilleke, D. F. Nippa, M. Iff, J. Ledergerber, C. C. Schiebroek and V. Romeo, Prospective de novo drug design with deep interactome learning, Nat. Commun., 2024, 15(1), 3408 CrossRef CAS PubMed.
- P. R. Murumkar, M. K. Sharma, P. Gupta, N. M. Patel and M. R. Yadav, Selection of suitable protein structure from protein data bank: an important step in structure-based drug design studies, Mini-Rev. Med. Chem., 2023, 23(3), 246–264 CrossRef CAS PubMed.
- C. Sharma, S. Gulati, N. Thakur, B. P. Singh, S. Gupta, S. Kaur, S. K. Mishra, A. K. Puniya, J. P. S. Gill and H. Panwar, Antibiotic sensitivity pattern of indigenous lactobacilli isolated from curd and human milk samples, 3 Biotech., 2017, 7(1), 53 CrossRef PubMed.
- J. W. Song, Y. S. Liu, Y. R. Guo, W. X. Zhong, Y. P. Guo and L. Guo, Nano-Liposomes Double Loaded with Curcumin and Tetrandrine: Preparation, Characterization, Hepatotoxicity and Anti-Tumor Effects, Int. J. Mol. Sci., 2022, 23(12), 6858 CrossRef CAS PubMed.
- B. Hoseini, M. R. Jaafari, A. Golabpour, A. A. Momtazi-Borojeni, M. Karimi and S. Eslami, Application of ensemble machine learning approach to assess the factors affecting size and polydispersity index of liposomal nanoparticles, Sci. Rep., 2023, 13(1), 18012 CrossRef CAS PubMed.
- H. Nsairat, D. Khater, U. Sayed, F. Odeh, A. Al Bawab and W. Alshaer, Liposomes: structure, composition, types, and clinical applications, Heliyon, 2022, 8(5), e09394 CrossRef CAS PubMed.
- J. Kuntsche, J. C. Horst and H. Bunjes, Cryogenic transmission electron microscopy (cryo-TEM) for studying the morphology of colloidal drug delivery systems, Int. J. Pharm., 2011, 417(1–2), 120–137 CrossRef CAS PubMed.
- S. Handali, E. Moghimipour, M. Kouchak, Z. Ramezani, M. Amini and K. A. Angali, New folate receptor targeted nano liposomes for delivery of 5-fluorouracil to cancer cells: strong implication for enhanced potency and safety, Life Sci., 2019, 227, 39–50 CrossRef CAS PubMed.
- B. Zhai, Q. Wu, W. Wang, M. Zhang, X. Han, Q. Li, P. Chen, X. Chen, X. Huang, G. Li, Q. Zhang, R. Zhang, Y. Xiang, S. Liu, T. Duan, J. Lou, T. Xie and X. Sui, Preparation, characterization, pharmacokinetics and anticancer effects of PEGylated β-elemene liposomes, Cancer Biol. Med., 2020, 17(1), 60–75 CAS.
- A. Khare, I. Singh, P. Pawar and K. Grover, Design and Evaluation of Voriconazole Loaded Solid Lipid Nanoparticles for Ophthalmic Application, J. Drug Delivery, 2016, 2016, 6590361 Search PubMed.
- Z. Rahman, A. S. Zidan and M. A. Khan, Non-destructive Methods of Characterization of Risperidone Solid Lipid Nanoparticles, Eur. J. Pharm. Biopharm., 2010, 76, 127–137 CrossRef CAS PubMed.
- T. Fawcett, S. Kabekkodu, J. Blanton, C. Crowder and T. Blanton, Simulation Tools and References for the Analysis of Nanomaterials, Adv. X-Ray Anal., 2015, 58, 108–120 Search PubMed.
- S. Banerjee, S. Roy, K. Nath Bhaumik, P. Kshetrapal and J. Pillai, Comparative study of oral lipid nanoparticle formulations (LNFs) for chemical stabilization of antitubercular drugs: physicochemical and cellular evaluation, Artif. Cells, Nanomed., Biotechnol., 2018, 46(sup1), 540–558 CrossRef CAS PubMed.
- S. Pande, Liposomes for drug delivery: review of vesicular composition, factors affecting drug release and drug loading in liposomes, Artif. Cells, Nanomed., Biotechnol., 2023, 51(1), 428–440 CrossRef CAS PubMed.
- N. Akombaetwa, A. B. Ilangala, L. Thom, P. B. Memvanga, B. A. Witika and A. B. Buya, Current Advances in Lipid Nanosystems Intended for Topical and Transdermal Drug Delivery Applications, Pharmaceutics, 2023, 15(2), 656 CrossRef CAS PubMed.
- Y. Jiang, F. Li, Y. Luan, W. Cao, X. Ji, L. Zhao, L. Zhang and Z. Li, Formation of drug/surfactant catanionic vesicles and their application in sustained drug release, Int. J. Pharm., 2012, 436(1–2), 806–814 CrossRef CAS PubMed.
- Y. Fu and W. J. Kao, Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems, Expert Opin. Drug Delivery, 2010, 7(4), 429–444 CrossRef CAS PubMed.
- T. Osmałek, A. Froelich, B. Jadach, A. Tatarek, P. Gadziński, A. Falana, K. Gralińska, M. Ekert, V. Puri, J. Wrotyńska-Barczyńska and B. Michniak-Kohn, Recent Advances in Polymer-Based Vaginal Drug Delivery Systems, Pharmaceutics, 2021, 13(6), 884 CrossRef PubMed.
- T. Sanz Taberner, A. Martín-Villodre, J. M. Pla-Delfina and J. V. Herráez, Consistency of Carbopol 971-P NF gels and influence of soluble and cross-linked PVP, Int. J. Pharm., 2002, 233(1–2), 43–50 CrossRef CAS PubMed.
- C. K. Enggi, H. T. Isa, S. Sulistiawati, K. A. R. Ardika, S. Wijaya, R. M. Asri, S. A. Mardikasari, R. F. Donnelly and A. D. Permana, Development of thermosensitive and mucoadhesive gels of cabotegravir for enhanced permeation and retention profiles in vaginal tissue: A proof of concept study, Int. J. Pharm., 2021, 609, 121182 CrossRef CAS PubMed.
- M. Ning, Z. Gu, H. Pan, H. Yu and K. Xiao, Preparation and in vitro evaluation of liposomal/niosomal delivery systems for antifungal drug clotrimazole, Indian J. Exp. Biol., 2005, 43(2), 150–157 CAS.
- R. Nanda, R. S. Narang and J. K. Narang, Design and Characterization of Fluconazole Loaded Elastic Liposome Based Gel for Treatment of Keratomycosis, FABAD J. Pharm. Sci., 2022, 47(2), 161–174 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |