Open Access Article
Open Access ArticleKnoevenagel-IMHDA and -IMSDA sequences for the synthesis of chiral condensed O,N-, S,N- and N-heterocycles†
Mihály Kajtárab,
Sándor Balázs Király*a,
Attila Bényei c,
Attila Kiss-Szikszaia,
Anita Kónya-Ábraháma,
Lilla Borbála Horváthd,
Szilvia Bősze
c,
Attila Kiss-Szikszaia,
Anita Kónya-Ábraháma,
Lilla Borbála Horváthd,
Szilvia Bősze de,
Andras Kotschy
de,
Andras Kotschy f,
Attila Paczalf and
Tibor Kurtán
f,
Attila Paczalf and
Tibor Kurtán *a
*a
aDepartment of Organic Chemistry, University of Debrecen, Egyetem Square 1, Debrecen 4032, Hungary. E-mail: kurtan.tibor@science.unideb.hu
bDoctoral School of Chemistry, University of Debrecen, Egyetem Square 1, 4032 Debrecen, Hungary
cDepartment of Physical Chemistry, University of Debrecen, Egyetem Square 1, 4032 Debrecen, Hungary
dHungarian Research Network (HUN-REN), Research Group of Peptide Chemistry, Eötvös Loránd University, H1117 Budapest, Hungary
eDepartment of Genetics, Cell- and Immunobiology, Semmelweis University, Faculty of Medicine, Budapest, 1089, Hungary
fServier Research Institute of Medicinal Chemistry, Budapest 1031, Hungary
First published on 15th January 2025
Abstract
Domino Knoevenagel-cyclization reactions of styrene substrates, containing an N-(ortho-formyl)aryl subunit, were carried out with N-substituted 2-cyanoacetamides to prepare tetrahydro-4H-pyrano[3,4-c]quinolone and hexahydrobenzo[j]phenanthridine derivatives by competing IMHDA and IMSDA cyclization, respectively. The diastereoselective IMHDA step with α,β-unsaturated amide, thioamide, ester and ketone subunits as a heterodiene produced condensed chiral tetrahydropyran or thiopyran derivatives, which in the case of Meldrum's acid were reacted further with amine nucleophiles in a multistep domino sequence. In order to simplify the benzene-condensed tricyclic core of the targets and get access to hexahydro-1H-pyrano[3,4-c]pyridine derivatives, a truncated substrate was reacted with cyclic and acyclic active methylene reagents in diastereoselective Knoevenagel-IMHDA reactions to prepare novel condensed heterocyclic scaffolds. The chemo-, regio- and diastereoselectivity of the cyclization step were investigated and structural elucidation was aided by single crystal X-ray analysis.
Introduction
The inverse-electron-demand hetero-Diels–Alder (IEDHDA) reaction is a highly efficient method used to assemble a chiral 3,4-dihydropyran ring (Scheme 1a),1 which has been recently gaining increasing attention to prepare condensed and spirocyclic heterocyclic scaffolds of pharmacological interest stereoselectively in inter-2 or intramolecular reactions.3 For instance, George and co-workers reported the biomimetic intramolecular oxa-HDA reaction of an electron-rich 2H-chromene substrate (Scheme 1a), which afforded the polycyclic framework of the natural product busseihydroquinone E.3c The analogue thia-HDA reactions have been much less utilized for building the dihydrothiopyran ring and arylidene-thiolactams,4 amides of thiocinnamic acid5 and thiochalcones6 were reported as typical thiodienes as exemplified by Fishwick and co-workers' thia-HDA reaction with N,N-disubstituted amides of thiocinnamic acid (Scheme 1b).5 Thermal intramolecular styryl Diels–Alder (IMSDA) reactions often compete with IMHDA reactions in styrene derivatives. Although styrene is considered a poor diene, IMSDA prevails when the heterodiene has low reactivity or conditions are not suitable to enable an IMHDA reaction (Scheme 1c).7 The initial step of the IMSDA reaction involves the loss of aromaticity but a subsequent 1,3-hydrogen shift restores it by producing condensed tetralin derivatives. For instance, Andrus and co-workers utilized the IMSDA reaction to assemble the tetracyclic deoxypodophyllotoxin core with the styryl functionality acting as the diene and reacting with an electron-deficient alkyne dienophile (Scheme 1c).8The IMHDA and IMSDA reactions can be readily incorporated into domino sequences, which provide access to complex chiral condensed heterocycles of novel scaffolds and pharmacological interest from reasonably simple building blocks. While more than half of the new drugs derived from natural products or related to them in the past four decades,9 there is still a great demand for synthetic procedures, which can produce novel heterocyclic entities with versatile substitution patterns. Domino IMHDA and IMSDA sequences were proved to be powerful methods to synthesize natural products or novel heterocyclic scaffolds.10
Recently we have explored domino Knoevenagel-cyclization sequences involving different cyclisation mechanisms such as IMHDA, IMSDA, [2 + 2] cycloaddition or a multistep inverse Cadogan-type cyclization to produce novel condensed and spirocyclic heterocyclic skeletons of pharmacological relevance.11
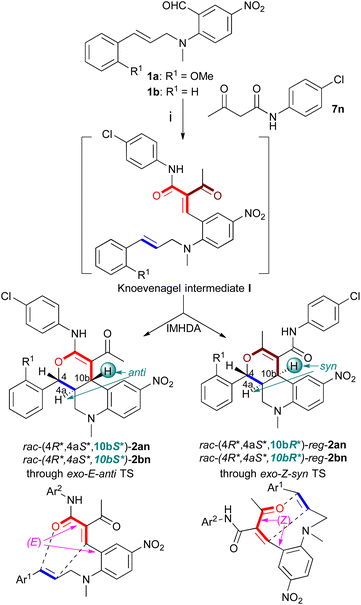
In this work, we envisaged the domino Knoevenagel-cyclization reaction of a styrene substrate (1a) with N-substituted 2-cyanoacetamides (7a–h) to prepare tetrahydro-4H-pyrano[3,4-c]quinolone (1a → E → 2) and hexahydrobenzo[j]phenanthridine derivatives (1a → E → 3) with different substitution patterns by competing IMHDA and IMSDA cyclization, respectively (Scheme 2a and b). The N-substitution of the α,β-unsaturated amide heterodiene governed the mechanism of the cyclization step whether it took place with an oxa-IMHDA reaction (E → 2) with the involvement of the amide carbonyl or an IMSDA-rearomatization sequence using the carbon–carbon double bond as a dienophile (E → 3). When reacting the styrene substrates 1a–c with active methylene reagents 7i and 7k–n containing a ketone or ester carbonyl group, oxa-IMHDA cyclization occurred resulting in further 4H-pyrano[3,4-c]quinolones with versatile substitution (Scheme 2a and b). With 2-cyanothioacetamide reagent (7j), a thia-IMHDA cyclization prevailed affording the novel tetrahydro-4H-thiopyrano[3,4-c]quinolone skeleton (7j → 4). The domino Knoevenagel-cyclization sequence of 1a with Meldrum's acid reagent (7o) produced the initial oxa-IMHDA intermediate F, which was reacted with amine nucleophiles 10a–g to induce a multistep ring-opening and fragmentation sequence of the 1,3-dioxinone ring resulting in lactone products 5.
 | ||
| Scheme 2 (a) General structures of the chiral condensed heterocyclic target compounds and (b) domino cyclization sequences of substrates 1a–c and (c) 1d. | ||
In order to simplify the tricyclic core of A by the removal of the condensed benzene ring and get access to the hexahydro-1H-pyrano[3,4-c]pyridine skeleton D (Scheme 2a), the conformationally flexible substrate 1d was prepared and reacted with cyclic and acyclic active methylene reagents in diastereoselective Knoevenagel-IMHDA reactions affording novel condensed heterocyclic scaffolds 6 (1d → G → 6) (Scheme 2c).
Results and discussion
The substrates 1a–d of the domino cyclization reactions were prepared from commercially available cinnamaldehyde derivatives in a few steps (Scheme S1–S3†).11b 2-Cyanoacetamide reagents (7a–i) with different substituents at the amide nitrogen were reacted with substrate 1a in dry EtOH using piperidine as an additive. We found that depending on the substitution of the amide nitrogen of the reagent 7a–h, the initial Knoevenagel intermediate E underwent an IMSDA or IMHDA cyclization resulting in 3 or 2, respectively (Table 1).| Entry | 1a–c | 7a–i | R2 | 2-3aa–ai | Y (%) | drc |
|---|---|---|---|---|---|---|
| a Piperidine, dry ethanol, rt, overnight.b Piperidine, dry ethanol, reflux 4 h.c Ratio of isomers rac-(6aR*,12S*,12aS*)-3/rac-(6aR*,12R*,12aS*)-epi-3 and rac-(4R*,4aS*,10bS*)-2/-(4R*,4aS*,10bR*)-epi-2 was determined by 1H-NMR integrals.d No reaction at room temperature, reaction was carried out at reflux temperature. | ||||||
| 1 | 1a | 7a |  |
3aa | 68 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 2 | 1a | 7b |  |
3ab | 93 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 3 | 1a | 7c |  |
3ac | 78 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 4 | 1a | 7d |  |
3ad, epi-3ad | 56 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 1a | 7e |  |
3ae | 57 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
| 6 | 1a | 7f |  |
2af, epi-2af | 58 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 7 | 1a | 7g |  |
2ag, epi-2ag | 67 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1d 1d |
| 3ag | 22 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0d 0d |
||||
| 8 | 1a | 7h |  |
2ah, epi-2ah | 21 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4d 4d |
| 3ah | 76 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0d 0d |
||||
| 9 | 1a | 7i | Ph | 2ai, epi-2ai | 94 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| 10 | 1b | 7i | Ph | 2bi, epi-2bi | 77 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | 1c | 7i | Ph | 2ci, epi-2ci | 70 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
With secondary amide reagents 7a–e (entries 1–5), the domino diastereoselective Knoevenagel-IMSDA reaction took place exclusively, which afforded cis-annulated tetralin derivatives rac-(6aR*,12S*,12aS*)-3aa–3ae as a single diastereomer with good to excellent yields (57–93%). The three contiguous chirality centers were introduced with full diastereoselectivity and only with the 2-cyano-N-allylacetamide reagent, was the epimeric rac-(6aR*,12R*,12aS*)-epi-3ag product observed as a minor product (entry 4). When using tertiary amide reagents (entry 6–8), the IMHDA cyclization mechanism competed with the IMSDA one. With 3-oxo-3-(piperidin-1-yl)propanenitrile (7f), only IMHDA cyclization occurred producing the C-10b epimers rac-(4R*,4aS*,10bS*)-2af (trans ring fusion) and rac-(4R*,4aS*,10bR*)-epi-2af (cis ring fusion) with 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (58%, entry 6). Both IMHDA and IMSDA cyclizations occurred with reagents 3-(morpholin-4-yl)-3-oxopropanenitrile (7g) and 3-oxo-3-(pyrrolidin-1-yl)propanenitrile (7h), the reactions of which required reflux temperature to be completed (entries 7 and 8). With reagent 7g, the epimeric mixture of the IMHDA products 2ag and epi-2ag was obtained as the major product (67%), while the IMSDA product 3ag was isolated as a single diastereomer (22%, entry 7). The reagent 7h afforded the 3ah diastereoselectively as the major product (76%), while the IMHDA product was obtained as a 1
1 ratio (58%, entry 6). Both IMHDA and IMSDA cyclizations occurred with reagents 3-(morpholin-4-yl)-3-oxopropanenitrile (7g) and 3-oxo-3-(pyrrolidin-1-yl)propanenitrile (7h), the reactions of which required reflux temperature to be completed (entries 7 and 8). With reagent 7g, the epimeric mixture of the IMHDA products 2ag and epi-2ag was obtained as the major product (67%), while the IMSDA product 3ag was isolated as a single diastereomer (22%, entry 7). The reagent 7h afforded the 3ah diastereoselectively as the major product (76%), while the IMHDA product was obtained as a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 epimeric mixture of 2ah and epi-2ah (entry 8).
1 epimeric mixture of 2ah and epi-2ah (entry 8).
As a related reagent, benzoylacetonitrile (7i) was tested with substrates 1a–c (entries 9–11) when the heterodiene moiety of the Knoevenagel intermediate E contained a more reactive ketone carbonyl group, which reacted exclusively by the IMHDA cyclization mechanism. The products were isolated as a mixture of C-10b epimers favoring the rac-(4R*,4aS*,10bS*) diastereomer with trans ring annulation over the rac-(4R*,4aS*,10bR*) one. The epimeric products were not separated but the major isomer rac-(4R*,4aS*,10bS*)-2ai crystallized from the solution and thus its planar structure and relative configuration were also confirmed by single crystal X-ray diffraction analysis (CCDC no. 2283893, Fig. 1).
 | ||
| Fig. 1 ORTEP view at 50% probability level of (a) rac-(4R*,4aS*,10bS*)-2ai (CCDC no. 2283893) and (b) rac-(4aS*,5R*,9bR*)-6p (CCDC no. 2401371). For rac-(4R*,4aS*,10bS*)-2ai, the solvent molecule is omitted for clarity. | ||
The relative configuration of the IMHDA products 2 was determined by using the 3J4a-H,10b-H coupling constant and characteristic NOE correlations (Table S1†). For the (4R*,4aS*,10bS*)-2 having trans ring junction, the 3J4a-H,10b-H coupling constant values fall typically in the range of 9.0–12.0 Hz and 10b-H/4-H NOE correlation indicated the cis orientation of the axial methine protons. In contrast, the cis ring junction of (4R*,4aS*,10bR*)-epi-2 gave rise to 3.1–4.7 Hz 3J4a-H,10b-H coupling constant values and 10b-H/4a-H NOE correlation. For tetralin derivatives 3, the small value of the 3J6a-H,12a-H coupling constant in the range of 3.7–3.0 Hz allowed determining the cis annulation of the tetralin ring, which could be also confirmed by the 6aH/12a-H NOE correlation (Fig. S3–S17†). Since the quaternary C-12 chirality center has no proton, its relative configuration could be only assigned on the basis of NOE correlation among the 12a-H and carboxamide substituent (Fig. S11 and S14†). The relative configuration of the C-12 and C-12a chirality centers is governed by the stereospecificity of the cyclization step; an (E) configuration of the carbon–carbon double bond of the Knoevenagel intermediate E results in the product (6aR*,12S*,12aS*)-3 with cis orientation of the 12a-H and the carboxamide group, while (Z) configuration affords (6aR*,12R*,12aS*)-epi-3 with trans arrangement of the 12a-H and the carboxamide group.12
The domino reaction of the sulfur-containing active methylene reagent 2-cyanothioacetamide (7j) with 1a produced the initial Knoevenagel intermediate E that contained a reactive α,β-unsaturated thiocarbonyl heterodiene (Scheme 3) favoring the thia-IMHDA cyclization. The thia-IMHDA cyclization (E → 4aj) occurred diastereoselectively with quantitative yield, affording the tetrahydro-4H-thiopyrano[3,4-c]quinolone derivative rac- (4R*,4aS*,10bS*)-4aj with trans ring junction. The tricyclic S,N-scaffold of rac-4aj represents a new condensed heterocyclic entity, which was reported earlier only as part of condensed pentacycles.4b
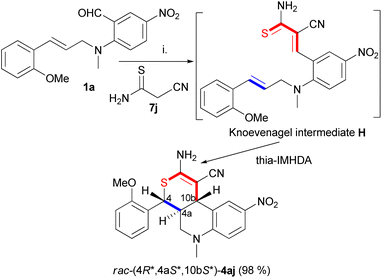 | ||
| Scheme 3 Domino Knoevenagel-thia-IMHDA reaction of 1a with 2-cyanoacethioacetamide. (i) Piperidine, dry ethanol, rt, overnight. | ||
The domino Knoevenagel-cyclization sequence was also extended to active methylene reagents containing ester groups (Table 2). The Knoevenagel intermediate obtained in the reaction of ethyl acetoacetate (7k) and substrates 1a and 1b had an α,β-unsaturated ketone heterodiene, which reacted in a diastereoselective oxa-IMHDA reaction to afford rac-(4R*,4aS*,10bS*)-2ak and -2bk (entries 12 and 13, Table 2). With ethyl nitroacetate (7l) reagent, the ester carbonyl contributed to the heterodiene and only the formation of (4R*,4aS*,10bS*)-2ak was observed by an oxa-IMHDA cyclization step with low yield (entry 14). The low yield is probably due to a competing inverse Cadogan-type cyclization sequence initiated by a nitro-IMHDA reaction, which we observed as a novel multistep cascade in the reaction of 2H-chromene derivatives with methyl nitroacetate.11c In entry 14, this cascade probably did not lead to stable intermediates and products, which lowered the yield by decomposition. With the methyl (phenylsulfonyl)acetate reagent (7m), the Knoevenagel intermediates cyclized with the oxa-IMHDA mechanism using the ester carbonyl group for the heterodiene and the rac-(4R*,4aS*,10bR*)-epi-2am and -epi-2bm products formed with full diastereoselectivity (entries 15 and 16).
Based on our previous density functional theory (DFT) calculations on related substituted 2H-chromene derivatives, the (4R*,4aS*,10bS*)- and (4R*,4aS*,10bR*)-epi-diastereomers formed through exo-E-anti (entries 12–14) and exo-Z-syn (entries 15 and 16) transition states, respectively.11c
Since the reagent N-(4-chlorophenyl)-3-oxobutanamide (7n) can be considered an amide analogue of the methyl acetoacetate (7k) forming a conjugating ketone and an amide carbonyl group in the Knoevenagel intermediate obtained with 1a and 1b, it was unexpected that both the α,β-unsaturated amide and ketone subunits reacted in oxa-IMHDA cyclization, affording a mixture of regioisomeric products 2an/reg-2an and 2bn/reg-2bn (entries 17 and 18, Table 3). Rac-(4R*,4aS*,10bS*)-2an and -2bn were produced diastereoselectively as the major product with an oxa-IMHDA reaction of the α,β-unsaturated amide heterodiene of the Knoevenagel intermediate through an exo-E-anti transition state. The regioisomeric rac-(4R*,4aS*,10bR*)-reg-2an and -2bn formed also diastereoselectively with cis-ring annulation and participation of the α,β-unsaturated ketone moiety as a diene of the oxa-IMHDA step. The stereochemistry of the products suggested that the initial Knoevenagel intermediate had (E) configuration for the carbon–carbon double bond and exo-Z-syn and exo-E-anti transition states led to trans- and cis-ring annulations in the oxa-IMHDA step with participation of amide- or ketone-type heterodienes, respectively. In IMHDA reactions, the exo/endo notations of the transition state refer to the relative orientation of the heterodiene and linker connecting the heterodiene and the dienophile subunits. If the linker is located below or above the heterodiene, the transition state is endo. The E/Z and anti/syn notations are defined in the scheme of Table 3.
| Entry | Products | Y (%) | Ratio | |
|---|---|---|---|---|
| a Piperidine, dry EtOH, reflux 4 h. | ||||
| 17 | rac-(4R*,4aS*,10bS*)-2an | rac-(4R*,4aS*,10bR*)-reg-2an | 37 | 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 18 | rac-(4R*,4aS*,10bS*)-2bn | rac-(4R*,4aS*,10bR*)-reg-2bn | 67 | 1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
The Meldrum's acid (7o) is a frequently utilized convenient active methylene reagent in domino Knoevenagel-IMHDA reactions to prepare condensed tetrahydro-α-pyrones, since the reactive 1,3-dioxinone moiety of the IMHDA product can be readily removed by fragmenting it with water or alcohols. This approach was exploited in the synthesis of condensed tetrahydropyran-2H-2-one natural products,13 and Tietze and co-workers also used it for the preparation of hirsutin14 and (+)-camptothecin.15 However, there are no examples when the fragmentation is initiated by the nucleophilic attack of primary or secondary amines at the lactone carbonyl of the condensed 1,3-dioxinone moiety of the IMHDA product, by which we could introduce a C-1 carboxamide group to the 4-aryl-hexahydro-2H-pyrano[3,4-c]quinolin-2-one skeleton in a multistep sequence (Table 4).
| Entry | Amines 10a–g | 5a–g | Yield (%) | dr (dia1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dia2)b dia2)b |
|---|---|---|---|---|
| a Amine reagent, dry toluene, rt, 4 days.b Ratio of diasomers as determined by 1H-NMR integrals.c Inseparable diastereomeric mixture. | ||||
| 19 |  |
dia1-5a, dia2-5a | 65 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5c 5c |
| 20 | 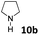 |
dia1-5b | 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
| dia2-5b | 18 | |||
| 21 | 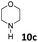 |
dia1-5c | 16 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| dia2-5c | 9 | |||
| 22 | 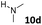 |
dia2-5d | 38 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 23 | 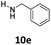 |
dia1-5e | 4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| dia2-5e | 21 | |||
| 24 |  |
dia2-5f | 24 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 25 |  |
dia2-5g | 37 | 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
In this work, we utilized secondary (10a–d) and primary amines (10e–g) as nucleophiles for the ring-opening of the 1,3-dioxinone moiety of IMHDA intermediate 2ao, which after attacking the lactam carbonyl induced a loss of an acetone molecule. The products 5a–g contained a β-dicarbonyl subunit with an enolizable C-1 chirality center (Table 4). The multistep sequences provided access to hexahydro-2H-pyrano[3,4-c]quinolin-2-one-1-carboxamides with moderate to low yields, which would not be accessible with malonamide reagents due to their low reactivity (Scheme S4†). A competitive ring-opening reaction with water is also feasible, affording the decarboxylated lactones, which may contribute the lower yields and different ratio of diastereomers (Scheme S5†). The IMHDA reaction occurred through the exo-Z-syn and exo-E-anti transition states leading to trans- and cis-ring annulations and the labile C-1 chirality center adopted the more stable relative configuration through enolization. This implies four possible diastereomers, from which only one or two were isolated in the reaction. Varying mixtures of diastereomers dia1 and dia2 having trans relative configuration of the 1-H and 10b-H, were obtained in the reaction of 1a with secondary cyclic amine nucleophiles 10a–c (entries 19–21) and benzylamine (10e) (entry 23), which could be separated by column chromatography in the case of amines 10b, 10c and 10e (Fig. S68–S82 and S86–S92†). With dimethylamine (10d), generated in situ from the hydrochloride salt, only a single diastereomer dia2-5d was isolated with 38% yield (entry 22, Fig. S83–S85†). The 3JH,H coupling constants of the methine protons and the NOE correlations were utilized to determine the absolute configuration of the four contiguous chirality centers (Table S2†). When using primary methylamine (10f), dia2-5f with trans orientation of the 1-H and 10b-H protons and cis ring junction was isolated (entry 24, Fig. S93 and S94†). The reaction of the primary allylamine (10g) afforded a single diastereomer dia2-5g with 37% yield (Fig. S95 and S96†).
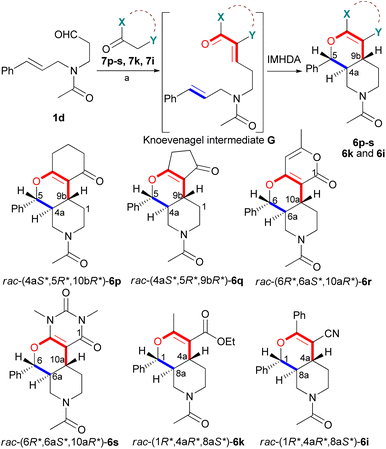
In order to prepare further simplified hexahydro-1H-pyrano[3,4-c]pyridine analogues of pharmacological relevance,16 which lack the condensed benzene ring and represent novel heterocyclic skeletons, the truncated substrate 1d was prepared from cinnamaldehyde in four steps (Schemes S1–S3† and Table 5). Substrate 1d had an N-acetyl group instead of the N-methyl, which increased the stability in the presence of the propanal subunit. The aliphatic substrate 1d had lower reactivity in the domino Knoevenagel-IMHDA sequences compared to aromatic substrates 1a–c and thus reactions were carried out with microwave activation at 120 °C. When using the regular thermal conditions of the domino cyclizations, refluxing ethanol in the presence piperidine, the reaction of 1d and 1,3-cyclohexanedione (7p) stopped at the stage of the Knoevenagel intermediate and prolonged reflux resulted in decomposition.
| Entry | 7a–r | Structure of the reagent | Product | Yield (%) |
|---|---|---|---|---|
| a Piperidine, dry EtOH, 120 °C MW 1 h. | ||||
| 26 | 7p | 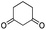 |
6p | 92 |
| 27 | 7q | 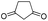 |
6q | 42 |
| 28 | 7r | 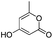 |
6r | 37 |
| 29 | 7s | 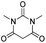 |
6s | 16 |
| 30 | 7k | 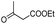 |
6k | 63 |
| 31 | 7i |  |
6i | 48 |
The microwave-assisted domino reaction of 1d with 1,3-cyclohexanedione (7p) and 1,3-cyclopentanedione (7q) afforded the trans-annulated condensed tricyclic products rac-(4aS*,5R*,10bR*)-6p and rac-(4aS*,5R*,9bR*)-6q as a single diastereomer in excellent and moderate yields, respectively (Table 5, entries 26 and 27). The reactions with cyclic reagents 4-hydroxy-6-methyl-2H-pyran-2-one (7r) and N,N-dimethylbarbituric acid (7s) resulted in the formation of rac-(6aR*,6aS*,10aR*)-6r and rac-(6aR*,6aS*,10aR*)-6s, respectively, which have the same stereochemical orientation as 6p and 6q, although the numbering and stereochemical descriptors of the chirality centers are different due to the different tricyclic core (entries 28 and 29). With acyclic reagents 7k and 7i, condensed trans-annulated bicyclic cores of 6k and 7i were produced with full diastereoselectivity. In all the products, the piperidine and dihydropyran rings had trans fusion, which was obtained through exo-Z-syn transition state of the IMHDA step.
The slow interconversion of the s-cis and s-trans conformers of the N-acetyl subunit augmented by the conformational flexibility of the piperidine ring resulted in at least two series of signals in the NMR spectra, which made the assignment of relative configuration challenging. In most cases, overlapping signals of different conformers did not allow the assignment of the relative configuration by using only NOE correlations. In the case of 6k and 6q, the key 1H-NMR signals did not overlap, and thus the relative configuration could be assigned as rac-(1R*,4aR*,8aS*) and rac-(4aS*,5R*,9bR*), respectively. The relative configuration of 6p was also confirmed independently by means of single crystal X-ray diffraction analysis (CCDC no. 2401371) as (4aS*,5R*,9bR*).
In vitro antiproliferative activity of the chiral condensed heterocyclic products was tested on U87 glioblastoma, A2058 melanoma and HT-29 colorectal adenocarcinoma human cancer cell lines, and IC50 values were determined for the most active ones by MTT method. Epi-2am, obtained in the Knoevenagel-IMHDA reaction of 1a and methyl (phenylsulfonyl)acetate reagent (7m), had IC50 values of 46 μM and 19 μM against U87 and A2058 cancer cell lines, respectively (positive control for U87: etoposide with an IC50 value of 12.0 μM).
Experimental
Materials and methods
Chemicals were purchased Puriss p.a. from commercial suppliers, and solvents were purified by distillation before use. For thin-layer chromatography (TLC), silica gel plates Merck 60 F254 were used, and compounds were visualized by irradiation with UV light. Column chromatography was performed using silica gel Merck 60 (particle size 0.063–0.200 mm). Melting points were determined on a Kofler hot-stage apparatus and are uncorrected. The NMR spectra were recorded on Bruker Avance DRX 360 MHz (1H: 360 MHz; 13C: 90 MHz), Bruker Avance II 400 (1H: 400 MHz. 13C: 100 MHz) and Bruker Avance II 500 MHz (1H: 500 MHz. 13C: 125 MHz) spectrometers using TMS as internal standard. The nuclear Overhauser effects were detected with offset-compensated and zero-quantum suppressed ROESY experiments developed by Batta et al.17 Chemical shifts were reported as δ in ppm and 3JH,H coupling constants in Hz. IR spectra were recorded on a JASCO FT/IR-4100 spectrometer and absorption bands are presented as wavenumber in cm−1. Electrospay Quadrupole Time-of-Flight HRMS measurements were performed with a MicroTOF-Q type QqTOF MS instrument equipped with an ESI source from Bruker (Bruker Daltoniks, Bremen, Germany).X-ray diffraction analysis
X-ray-quality crystals were grown by slow evaporation of the mixture of chloroform/methanol 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 solution of the compounds. A crystal well-looking in polarized light microscope was fixed under a microscope onto a Mitegen loop using high-density oil. Diffraction intensity data were collected ambient temperature using a Bruker-D8 Venture diffractometer (Bruker AXS GmbH, Karlsruhe, Germany) equipped with INCOATEC IμS 3.0 (Incoatec GmbH, Geesthacht, Germany) dual (Cu and Mo) sealed tube micro sources and a Photon II Charge-Integrating Pixel Array detector (Bruker AXS GmbH, Karlsruhe, Germany) using Mo Kα (λ = 0.71073 Å) radiation.
1 solution of the compounds. A crystal well-looking in polarized light microscope was fixed under a microscope onto a Mitegen loop using high-density oil. Diffraction intensity data were collected ambient temperature using a Bruker-D8 Venture diffractometer (Bruker AXS GmbH, Karlsruhe, Germany) equipped with INCOATEC IμS 3.0 (Incoatec GmbH, Geesthacht, Germany) dual (Cu and Mo) sealed tube micro sources and a Photon II Charge-Integrating Pixel Array detector (Bruker AXS GmbH, Karlsruhe, Germany) using Mo Kα (λ = 0.71073 Å) radiation.
High-multiplicity data collection and integration were performed using APEX3 (version 2017.3-0, Bruker AXS Inc., 2017, Madison, WI, USA) software. Data reduction and multiscan absorption correction were performed using SAINT (version 8.38A, Bruker AXS Inc., 2017, Madison, WI, USA). The structure was solved using direct methods and refined on F2 using the SHELXL program18 incorporated into the APEX3 suite. Refinement was performed anisotropically for all non-hydrogen atoms. Hydrogen atoms were placed in idealized positions on parent atoms in the final refinement.
The CIF file was manually merged using Publcif software,19 while graphics were designed using the Mercury program.20 Details of the crystal parameters, data collection, and structure refinement are given in Table SX1.† The compounds are racemates, crystallizing in monoclinic centrosymmetric space group no. 14 so the relative configuration of the chirality elements are given. ORTEP views are shown in Fig. SX1–3.† The results of the X-ray diffraction structure determinations were in accordance with the Checkcif functionality of PLATON software (Utrecht University, Utrecht, the Netherlands),21 without A or B level alert. Structural parameters, such as bond length and angle data (Tables SX2–4†), are in the expected range.
For the end-point-type tetrazolium [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, MTT] assay,23 cells were seeded during the exponential growth phase one day before the experiment. In the case of cytostasis, 5 × 103 cells/100 μl per well were seeded on a 96-well cell culture plate (Sarstedt, Nümbrecht, Germany) in CM DMEM. Cells were treated with the compounds using DMSO stock solutions (c = 20 mM) diluted in DMEM incomplete medium (ICM) (final c = 1% (v/v) for DMSO content) in the concentration range 2.56 × 10−3–100 μM. Cells were treated with the compounds for 24 h. ICM and ICM containing 0.5% (v/v) DMSO were used as controls. After incubation, cells were washed with ICM three times, and in the last step, CM was added. After culturing the cells for 72 h, 45 μl sterile-filtered MTT (Millex 0.22 μm filter, Millipore, Cork, Ireland) was added (2 mg ml−1 in ICM) to the cells. Mitochondrial enzymes reduce MTT to a formazan derivative (purple crystals). As a positive control etoposide was employed.
After 3.5 h incubation, plates were centrifuged (2000 rpm, 5 min), the supernatant was removed, and formazan crystals were dissolved in DMSO. Absorbance was determined with an ELISA plate reader (Labsystems iEMS reader, Helsinki, Finland) at λ = 540 and 620 nm. A620 values were subtracted from A540 values, and cytostatic activity was calculated with the formula: cytostasis% = 100 × (1 − Atreated cells/Acontrol cells), where Atreated cells and Acontrol cells are the average absorbance of treated and control cells. The 50% inhibitory concentration (IC50) values were determined from the dose–response curves. The curves were calculated using Microcal OriginPro (version: 2018) software (OriginLab, Northampton, MA, USA).
General methods for the domino Knoevenagel-cyclization reactions
Method A (MW): to a standard 10 ml volume cylindrical Pyrex® reaction vessel, 1d, 7p–7s, 7k, 7i, piperidine and 2 ml of dry ethanol were added. The vessel was sealed with PEEK snap cap and standard PTFE-coated silicone septum and warmed up to 120 °C and stirred for 1 hour. The solvent was removed in vacuo, and the crude was triturated with 3 ml of cold ethanol, and the crystals were filtered and washed with 2 ml of cold ethanol. If no precipitate formed, the crude was purified with column chromatography.Method B: in a flame-dried three-necked round-bottom flask equipped with a reflux condenser and a CaCl2 drying tube, benzaldehyde derivative 1a (100 mg) and Meldrum's-acid 7o (1.2 equivalent) were dissolved in toluene (5 ml). Primary or secondary amines (10a–g, 1.2 equivalent) were added to the mixture and stirred for four days at room temperature. After completion, the solvent was removed in vacuo, and the crude product was purified by column chromatography.
Methods C and D: in a flame-dried three-necked round-bottom flask equipped with a reflux condenser and a CaCl2 drying tube, benzaldehyde derivative 1a–c (100 mg) and active methylene reagent 7a–n, (1.2 equivalent) were dissolved in ethanol (5 ml). Piperidine (1 equivalent) was added to the solution, and it was stirred overnight at room temperature (method C) or refluxed for 4 hours (method D). The mixture was concentrated in vacuo and purified by column chromatography.
Synthetic procedures and characterization of the products
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) affording rac-(6aR*,12S*,12aS*)-3aa as yellow powder (68%), which decomposes above 240 °C. Rf = 0.46 (hexane/chloroform/acetone 10
1) affording rac-(6aR*,12S*,12aS*)-3aa as yellow powder (68%), which decomposes above 240 °C. Rf = 0.46 (hexane/chloroform/acetone 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).1H-NMR (500 MHz, CDCl3) δ 1.00–2.02 (m, 10H, 7′-11′-H), 2.69–2.82 (m, 1H, 6a-H), 2.98–3.03 (m, 2H, 7-H), 3.04 (s, 3H, 1′-H), 3.28–3.38 (m, 1H, 6-Hb), 3.59 (t, J = 12.9 Hz, 1H, 6-Ha), 3.85 (s, 3H, 2′-H), 3.87–3.94 (m, 1H, 6′-H), 3.96 (d, J = 3.0 Hz, 1H, 12a-H), 6.44 (d, J = 7.9 Hz, 1H, 5′-H), 6.63 (d, J = 9.3 Hz, 1H, 4-H), 6.78–6.94 (m, 2H, 9-H, 11-H), 7.19–7.31 (m, 1H, 10-H), 7.85 (d, J = 2.5 Hz, 1H, 1-H), 8.12 (dd, J = 9.3, 2.6 Hz, 1H, 3-H). 13C NMR (100 MHz, CDCl3) δ 24.5 (C-10′), 24.6 (C-9′), 24.7 (C-7), 25.4 (C-8′), 28.0 (C-6a), 32.7 (C-7′), 32.8 (C-11′), 38.6 (C-1′), 41.6 (C-6′), 50.1 (C-12a), 51.8 (C-6), 52.1 (C-12), 55.6 (C-2′), 109.8 (C-4), 110.0 (C-11), 119.5 (C-3′), 119.7 (C-1), 120.6 (C-12b), 122.8 (C-11a), 125.5 (C-10), 126.6 (C-3), 128.4 (C-9), 129.4 (C-7a), 136.2 (C-2), 150.6 (C-4′), 157.7 (C-4a), 165.8 (C-8). IR: (KBr) ν: 2989, 2929, 2848, 1684, 1670, 1601, 1576, 1515, 1492, 1465, 1431, 1303, 1275, 1260, 1224, 1183, 1152, 1106, 1085. HRMS: calcd for C27H31N4O4 [M + H]+ 475.2345, found 475.2342.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) affording rac-(6aR*,12S*,12aS*)-3ab as yellow powder (93%), which decomposes above 253 °C. Rf = 0.50 (hexane/chloroform/acetone 10
1) affording rac-(6aR*,12S*,12aS*)-3ab as yellow powder (93%), which decomposes above 253 °C. Rf = 0.50 (hexane/chloroform/acetone 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).1H NMR (500 MHz, CDCl3) δ 2.72–2.80 (m, 1H, 6a-H), 2.97–3.05 (m, 5H, 1′-H, 7-H), 3.30–3.37 (m, 1H, 6-Hb), 3.58 (t, J = 12.9 Hz, 1H, 6-Ha), 3.85 (s, 3H, 2′-H), 4.00 (d, J = 3.1 Hz, 1H, 12a-H), 4.49 (dd, J = 14.6, 5.3 Hz, 1H, 6′-Ha), 4.65 (dd, J = 14.6, 5.3 Hz, 1H, 6′-Hb), 6.61 (d, J = 9.3 Hz, 1H, 4-H), 6.78–6.88 (m, 3H, 9-H, 11-H, 5′-H), 7.20–7.35 (m, 6H, Ph-H, 10-H), 7.90 (d, J = 2.6 Hz, 1H, 1-H), 8.10 (dd, J = 9.3, 2.6 Hz, 1H, 3-H). 13C NMR (100 MHz, CDCl3) δ 24.5 (C-7), 28.0 (C-6a), 38.6 (C-1′), 41.7 (C-12a), 45.1 (C-6′), 51.8 (C-6), 52.2 (C-12), 55.6 (C-2′), 109.9 (C-4), 110.2 (C-11), 119.5 (C-3′), 119.9 (C-9), 120.4 (C-12b), 122.8 (C-11a), 125.4 (C-1), 126.7 (C-3), 128.1 (C-8′, C-10′, C-12′), 128.4 (C-10), 129.0 (C-9′, C-11′), 129.2 (C-7′), 136.3 (C-7a), 136.7 (C-2), 150.6 (C-4′), 157.7 (C-4a), 167.0 (C-8). IR: (KBr) ν: 2923, 2851, 1747, 1680, 1647, 1604, 1523, 1495, 1466, 1434, 1361, 1321, 1303, 1276, 1261, 1185, 1108. HRMS: calcd for C27H25N4O4 [M + H]+ 469.1875, found 469.1870.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Unifying the product from crystallization and chromatography afforded rac-(6aR*,12S*,12aS*)-3ac as yellow powder (78%), which decomposes at 270 °C. Rf = 0.17 (hexane/chloroform/ethyl acetate 5
1). Unifying the product from crystallization and chromatography afforded rac-(6aR*,12S*,12aS*)-3ac as yellow powder (78%), which decomposes at 270 °C. Rf = 0.17 (hexane/chloroform/ethyl acetate 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).1H-NMR (500 MHz, DMSO-d6) δ 2.66–2.75 (m, 1H, 6a-H), 2.89–2.95 (m, 1H, 7-H), 2.99 (s, 3H, 1′-H), 3.06 (s, 1H, 8′-H), 3.32–3.40 (m, 1H, 6-Hb), 3.44 (dd, J = 13.0, 5.2 Hz, 1H, 6-Ha), 3.81 (d, J = 3.2 Hz, 1H, 12a-H), 3.85 (s, 3H, 2′-H), 3.91 (dd, J = 22.7, 3.2 Hz, 2H, 6′-H), 6.75–6.83 (m, 2H, 4-H, 9-H), 7.02 (d, J = 8.2 Hz, 1H, 11-H), 7.30 (t, J = 8.2 Hz, 1H, 10-H), 7.68 (d, J = 2.3 Hz, 1H, 1-H), 8.03 (dd, J = 9.3, 2.3 Hz, 1H, 3-H), 8.67 (t, J = 5.2 Hz, 1H, 5′-H). 13C-NMR (100 MHz, DMSO-d6) δ 23.9 (C-7), 27.2 (C-6a), 29.4 (C-6′), 38.3 (C-1′), 40.6 (C-12a), 50.9 (C-6), 52.9 (C-12), 55.7 (C-2′), 80.0 (C-7′), 110.1 (C-4), 110.4 (C-11), 119.2 (C-3′), 119.4 (C-12b), 119.7 (C-9), 122.6 (C-7a), 124.9 (C-3), 126.0 (C-1), 128.1 (C-10), 128.9 (C-12a), 134.9 (C-2), 150.8 (C-4a), 157.1 (C-8), 167.5 (C-4′). IR: (KBr) ν: 3005, 2988, 2932, 2839, 1683, 1600, 1259, 1182, 1105. HRMS: calcd for C24H23N4O4 [M + H]+ 431.1719, found 431.1714.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) affording the mixture of rac-(6aR*,12S*,12aS*)-3ad and rac-(6aR*,12R*,12aS*)-epi-3ad as yellow powder (56%), Rf = 0.67 (hexane/chloroform/acetone 10
1) affording the mixture of rac-(6aR*,12S*,12aS*)-3ad and rac-(6aR*,12R*,12aS*)-epi-3ad as yellow powder (56%), Rf = 0.67 (hexane/chloroform/acetone 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The diastereomeric ratio was ∼5
1). The diastereomeric ratio was ∼5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 according to the 1H-NMR integrals.
1 according to the 1H-NMR integrals.1H-NMR (500 MHz, DMSO-d6) δ 2.64–2.72 (m, 1H, 6a-H), 2.90–2.95 (m, 2H, 7-H), 2.99 (s, 3H, 1′-H), 3.07 (s, 1H, 5′-H), 3.27–3.36 (m, 2H, 2× 6-Hb), 3.36–3.46 (m, 2H, 2× 6-Ha), 3.74–3.83 (m, 2H, 6′-H), 3.85 (s, 3H, 2′-H), 3.87 (d, J = 3.7 Hz, 1H, 12a-H, major), 5.01–5.14 (m, 2H, 2× 8′-H), 5.77–5.90 (m, 1H, 2× 7′-H), 6.78 (d, J = 9.5 Hz, 1H, 4-H), 6.84–6.89 (m, 1H, 9-H), 7.01 (dd, J = 8.0, 2.8 Hz, 1H, 11-H), 7.06 (d, J = 7.8 Hz, 1H, 11-H), 7.31 (t, J = 8.1 Hz, 1H, 10-H), 7.64 (d, J = 2.7 Hz, 1H, 1-H), 8.03 (dd, J = 9.3, 2.7 Hz, 1H, 3-H), 8.45 (t, J = 5.7 Hz, 1H, 5′-H). 13C-NMR (100 MHz, CDCl3) δ 24.4 and 27.6 (C-7), 28.0 (C-6a), 38.6 (C-1′), 41.8 and 43.2 (C-12a), 43.4 and 44.0 (C-6′), 51.8 (C-6), 52.2 (C-12), 55.6 (C-2′), 109.8 and 110.0 (C-4), 110.1 and 110.7 (C-11), 118.1 (C-3′), 119.0 (C-9), 119.3 (C-3′), 119.9 (C-7′), 120.4 (C-9), 122.8 (C-12b), 124.3 (C-7a), 125.3 (C-1), 125.5 (C-3), 126.6 (C-1), 128.2 (C-3), 128.4 and 129.0 (C-10), 132.7 (C-11a), 132.8 (C-7′), 136.2 (C-2), 150.6 (C-4a), 157.6 (C-8), 167.0 (C-4′). IR: (KBr) ν: 2924, 1671, 1601, 1524, 1492, 1467, 1437, 1319, 1290, 1257, 1185, 1109, 1050, 1023. HRMS: calcd for C24H25N4O4 [M + H]+ 433.1875, found 433.1869.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) affording rac-(6aR*,12S*,12aS*)-3ae as brown oil (57%), Rf = 0.38 (hexane/chloroform/acetone 10
1) affording rac-(6aR*,12S*,12aS*)-3ae as brown oil (57%), Rf = 0.38 (hexane/chloroform/acetone 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).1H NMR (500 MHz, acetone-d6) δ 2.76–2.84 (m, 1H, 6a-H), 2.97–3.02 (m, 2H, 7-H), 3.07 (s, 3H, 1′-H), 3.40–3.48 (m, 1H, 6′-Ha), 3.48–3.54 (m, 1H, 6-Hb), 3.60–3.66 (m, 1H, 7′-Ha), 3.68–3.75 (m, 2H, 6-H), 3.81–3.84 (m, 1H, 7′-Hb), 3.89 (s, 3H, 2′-H), 3.99 (d, J = 3.4 Hz, 1H, 12a-H), 6.76–6.81 (m, 1H, 4-H), 6.97–7.03 (m, 2H, 11-H and 4-H), 7.23–7.32 (m, 1H, 10-H), 7.50 (s, 1H, 8′-H), 7.82 (d, J = 2.3 Hz, 1H, 1-H), 8.03–8.07 (m, 1H, 3-H). 13C NMR (126 MHz, acetone-d6) δ 25.1 (C-7), 28.6 (C-6a), 38.7 (C-1′), 42.3 (C-12a), 43.7 (C-6′), 52.3 (C-6), 55.9 (C-12), 56.0 (C-2′), 60.8 (C-7′), 110.7 (C-4), 110.9 (C-11), 120.7 (C-3′), 120.7 (C-12b), 120.9 (C-9), 123.8 (C-11a), 126.0 (C-1), 126.7 (C-3), 128.9 (C-10), 130.7 (C-7a), 136.8 (C-2), 151.8 (C-4′), 158.5 (C-4a), 168.2 (C-8). IR: (KBr) ν: 2922, 1675, 1603, 1524, 1490, 1465, 1434, 1320, 1295, 1275, 1259, 1224, 1183, 1108. HRMS: calcd for C23H25N4O5 [M + H]+ 437.1824, found 437.1822.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 according to the 1H-NMR integrals. Rf = 0.07 (hexane/acetone 2
2 according to the 1H-NMR integrals. Rf = 0.07 (hexane/acetone 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).1H-NMR (400 MHz, DMSO-d6) δ 1.44–1.67 (m, 12H, 2× 3′′-H, 2× 4′′-H, 2× 5′′-H), 2.20 (bs, 1H, 4a-H), 2.56–2.64 (m, 1H, 4a-H), 2.79 (dd, J = 11.9, 5.4 Hz, 1H, 5-Hb), 2.91 (s, 3H, 3′-H), 2.98 (s, 3H, 3′-H), 3.41–3.50 (m, 5H, 2′′-H, 6′′-H, 5-Ha), 3.53 (d, J = 4.7 Hz, 1H, 10b-H from epi-2af), 3.58–3.71 (m, 4H, 2′′-H, 6′′-H), 3.76 (s, 3H, 2′-H), 3.84 (bs, 3H, 2′-H), 5.37 (d, J = 6.6 Hz, 1H, 4-H from epi-2af), 5.42 (bs, 1H, 4-H from 2af), 6.62–6.75 (m, 2H, 2× 7-H), 6.75–6.81 (m, 1H, 5′′′-H), 6.97–7.13 (m, 3-H, 3′′′-H, 2× 5′′′-H), 7.23 (d, J = 7.2 Hz, 1H, 3′′′-H), 7.30–7.46 (m, 4H, 2× 4′′′-H, 2× 6′′′-H), 7.95–8.06 (m, 2H, 2× 8-H), 8.08 (d, J = 1.9 Hz, 1H, 10-H), 8.19 (bs, 1H, 10-H). 13C-NMR (100 MHz, DMSO-d6) δ 23.8 (2× C-4′′), 25.4 and 25.5 (2× C-5′′ and 2× C-3′′), 27.2 (C-4a), 33.0 (C-10b), 33.1 (C-4a), 38.0 and 38.2 and 38.7 (C-3′), 48.1 (2× C-2′′ and 2× C-6′′), 48.3 (C-2′′ and C-6′′), 49.5 and 51.0 and 51.8 (C-6), 54.5 (C-1), 55.4 and 55.6 and 55.8 (C-3′), 60.0 (C-1), 74.5 (2× C-4), 109.5 and 109.7 and 110.0 (C-7), 110.5 and 111.3 and 111.5 (C-9), 118.7 (C-1′′′), 119.2 (C-10), 119.3 (C-6′′′), 120.5 (C-10b), 120.7 (C-6′′′), 120.8 (C-5′′′), 121.7 (C-1′), 122.0 (C-1′′′), 122.1 (C-1′), 124.8 and 124.9 (C-3′′′), 125.3 (2× C-10a), 125.5 (C-10), 125.6 and 125.9 and 126.5 (C-8), 128.6 (C-4′′′), 129.0 (C-1′′′), 129.8 and 130.4 (C-4′′′), 134.6 and 135.3 and 135.6 (C-9), 149.7 (C-6a), 150.9 (C-4a), 151.3 (2× C-6a), 156.0 (C-2′′′), 157.3 (C-2′′′), 163.0 and 164.6 and 166.9 (C-2). IR: (KBr) ν: 3005, 2989, 2936, 2848, 2169, 1574, 1530, 1492, 1453, 1437, 1300, 1275, 1260, 1178, 1112, 1077, 1051. HRMS: calcd for C26H29N4O4 [M + H]+ 461.2188, found 461.2187.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) affording the mixture of rac-(4R*,4aS*,10bS*)-2ag and rac-(4R*,4aS*,10bR*)-epi-2ag as the major product and brown oil (67%). The diastereomeric ratio was ∼4
1) affording the mixture of rac-(4R*,4aS*,10bS*)-2ag and rac-(4R*,4aS*,10bR*)-epi-2ag as the major product and brown oil (67%). The diastereomeric ratio was ∼4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 according to the 1H-NMR integrals, Rf = 0.38 (hexane/chloroform/acetone 10
1 according to the 1H-NMR integrals, Rf = 0.38 (hexane/chloroform/acetone 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).1H-NMR (500 MHz, DMSO-d6) δ 2.21 (bs, 1H, 4a-H), 2.80 (dd, J = 11.7, 5.3 Hz, 1H, 5-Hb), 2.91 (s, 3H, 3′H), 2.98 (s, 1H, 2′′-H), 3.48–3.55 (m, 3H, 5-Ha, 3′′-H), 3.60–3.66 (m, 6H, 5′′-H, 6′′-H, 10b-H from epi-2ag), 3.76 (s, 1H, 2′′-H), 3.83 (s, 3H, 2′-H), 5.38 (d, J = 6.7 Hz, 1H, 4-H from epi-2ag), 5.45 (bs, 1H, 4-H from 2ag), 6.68 (d, J = 9.2 Hz, 1H, 7-H), 7.01 (t, J = 7.4 Hz, 1H, 5′′′-H), 7.10 (d, J = 8.3 Hz, 1H, 3′′′-H), 7.36–7.47 (m, 2H, 6′′′-H, 4′′′-H), 8.01 (dd, J = 9.2, 2.6 Hz, 1H, 8-H), 8.17–8.21 (m, 1H, 10-H). 13C-NMR (125 MHz, DMSO-d6) δ 30.7 and 33.0 (C-4a), 38.1 and 38.7 (C-3′), 47.4 (C-1), 47.6 (C-5), 49.4 (C-1), 51.8 and 55.7 (C-2′′ and C-6′′), 55.8 (C-10b), 65.9 (C-2′), 74.7 (2× C-3′′, 2× C-5′′), 109.6 and 109.7 (C-7), 111.3 and 111.6 (C-9), 119.3(C-5′′′), 120.7 and 120.9 (C-10), 121.8 (C-1′), 124.8 (C-10a), 124.9 (C-8), 125.2 and 125.4 (C-1′′′), 126.6 and 129.9 (C-6′′′), 130.5 (C-4′′′), 135.4 and 135.6 (C-9), 151.3 (C-6a), 156.1 (C-2′′′), 166.9 (C-2). IR: (KBr) ν: 3004, 2988, 2848, 2348, 2175, 1602, 1570, 1522, 1491, 1457, 1431, 1397, 1361, 1311, 1275, 1260, 1232, 1220, 1177, 1114. HRMS: calcd for C25H27N4O5 [M + H]+ 463.1981, found 463.1975.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) affording rac-(6aR*,12S*,12aS*)-3ag as the minor product and yellow amorphous solid (22%), Rf = 0.48 (hexane/chloroform/acetone 10
1) affording rac-(6aR*,12S*,12aS*)-3ag as the minor product and yellow amorphous solid (22%), Rf = 0.48 (hexane/chloroform/acetone 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).1H NMR (500 MHz, DMSO-d6) δ 2.69–2.78 (m, 1H, 6a-H), 2.95–3.02 (m, 4H, 7-H, 1′-H), 3.42–3.48 (m, 4H, 10′-H, 6′-H), 3.52–3.59 (m, 2H, 6-H), 3.63 (d, J = 3.4 Hz, 4H, 7′-H, 9′-H), 3.66 (d, J = 4.1 Hz, 1H, 12a-H), 3.85 (s, 3H, 1′-H), 6.76–6.82 (m, 2H, 9-H, 4-H), 7.05 (d, J = 8.0 Hz, 1H, 11-H), 7.37 (t, J = 8.0 Hz, 1H, 10-H), 7.66 (d, J = 2.7 Hz, 1H, 1-H), 8.04 (dd, J = 9.3, 2.7 Hz, 1H, 3-H). 13C-NMR (90 MHz, DMSO-d6) δ 23.5 (C-7), 27.2 (C-6a), 38.1 (C-1′), 51.0 (C-6), 54.8 (C-12), 55.1 (C-6′, C-10′), 55.6 (C-2′), 65.9 (C-7′, 9′), 110.0 (C-4), 110.7 (C-11), 118.5 (C-3′), 119.0 (C-12b), 119.3 (C-9), 120.8 (C-11a), 125.7 (C-1), 125.9 (C-3), 128.6 (C-7a), 128.7 (C-10), 134.6 (C-2), 150.9 (C-4a), 157.3 (C-4′), 165.1 (C-8). IR: (KBr) ν: 2917, 2857, 1648, 1602, 1580, 1525 1492, 1468, 1434, 1275, 1262, 1109. HRMS: calcd for C25H27N4O5 [M + H]+ 463.1981, found 463.1974.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 according to the 1H-NMR integrals, Rf = 0.21 (chloroform).
4 according to the 1H-NMR integrals, Rf = 0.21 (chloroform).1H-NMR (400 MHz, CDCl3) δ 1.77–1.87 (m, 4H, 4′′-H, 5′′-H), 2.11–2.24 (m, 4H, 3′′-H, 6′′-H), 2.57–2.67 (m, 1H, 4a-H), 2.70–2.87 (m, 1H, 4a-H), 2.93 (dd, J = 18.4, 6.5 Hz, 1H, 5-Hb), 3.05 (s, 3H, 3′-H), 3.08 (d, J = 6.6 Hz, 1H, 10b-H from 2ah), 3.31–3.40 (m, 1H, 5-Hb), 3.63 (d, J = 4.6 Hz, 1H, 10b-H from epi-2ah), 3.65–3.79 (m, 2H, 2× 5-Ha), 3.83–3.90 (m, 6H, 2′′-H), 5.14 (d, J = 4.6 Hz, 1H, 4-H from epi-2ah), 5.30 (bs, 1H, 4-H from 2ah), 6.64 (d, J = 9.3 Hz, 1H, 7-H), 6.84 (d, J = 8.1 Hz, 1H, 6′′′-H), 6.89 (d, J = 8.0 Hz, 1H, 3′′′-H), 7.27 (dd, J = 8.8, 7.0 Hz, 2H), 4′′′-H, 5′′′-H, 7.70 (d, J = 2.6 Hz, 1H, 10-H), 8.10 (dd, J = 9.3, 2.6 Hz, 1H, 8-H). 13C-NMR (100 MHz, CDCl3) δ 23.3 (C-3′′), 24.5 (C-5′′), 27.0 (C-6′′), 28.4 and 31.1 (C-4a), 38.6 (C-3′), 41.4 (C-10b), 47.7 (C-1), 49.2 (C-2′′), 51.8 and 55.6 (C-2′), 71.5 and 71.6 (C-4), 109.9 (C-7), 110.0 (C-2′′′), 118.8 (C-1′), 119.7 (C-5′′′), 120.7 (C-10), 122.0 (C-10a), 125.9 (C-8), 126.7 (C-3′′′), 127.4 and 127.4 (C-6′′′), 128.5 and 128.7 (C-4′′′), 136.1 (C-9), 150.9 (C-6a), 157.5 (C-2′′′), 165.7 (C-2). IR: (KBr) ν: 2922, 1635, 1601, 1528, 1490, 1466, 1432, 1299, 1274, 1259, 1221, 1181, 1107, 1097. HRMS: calcd for C25H27N4O4 [M + H]+ 447.2032, found 447.2029.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) affording rac-(6aR*,12S*,12aS*)-3ah as the major product and yellow amorphous solid (76%), Rf = 0.06 (hexane/dichloromethane/acetone 8
1) affording rac-(6aR*,12S*,12aS*)-3ah as the major product and yellow amorphous solid (76%), Rf = 0.06 (hexane/dichloromethane/acetone 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).1H NMR (500 MHz, DMSO-d6) δ 1.80 (bs, 2H, 7′-H), 2.03–2.13 (m, 2H, 6′-H), 2.66–2.73 (m, 1H, 6a-H), 2.91 (dd, J = 18.4, 5.8 Hz, 2H, 7-Hb), 3.00 (s, 3H, 1′-H), 3.08 (bs, 1H, 7-Ha), 3.37–3.47 (m, 1H, 6-Ha), 3.47–3.56 (m, 3H, 6-Hb, 8′-H), 3.73 (d, J = 3.8 Hz, 1H, 12a-H), 3.85 (s, 3H, 2′-H), 6.73–6.81 (m, 2H, 9-H, 4-H), 7.03 (d, J = 8.2 Hz, 1H, 11-H), 7.33 (t, J = 8.0 Hz, 1H, 10-H), 7.54 (d, J = 1.9 Hz, 1H, 1-H), 8.01–8.06 (m, 1H, 3-H). 13C NMR (90 MHz, DMSO-d6) δ 22.6 (C-6′), 23.6 (C-7), 26.1 (C-7′), 27.3 (C-6a), 38.2 (C-1′), 38.6 (C-12a), 46.0 (C-4′), 48.2 (C-8′), 50.9 (C-6), 54.7 (C-12), 55.6 (C-2′), 109.9 (C-4), 110.4 (C-9), 118.9 (C-12b), 119.3 (C-3′), 119.8 (C-11), 121.9 (C-11a), 125.8 (C-3), 126.0 (C-1), 128.4 (C-7a), 128.6 (C-10), 134.6 (C-2), 150.9 (C-4a), 157.0 (C-8), 164.7 (C-4). IR: (KBr) ν: 2918, 2850, 1634, 1600, 1574, 1529, 1489, 1465, 1432, 1399, 1359, 1337, 1318, 1299, 1291, 1259, 1220, 1180, 1152, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 285, 1107. HRMS: calcd for C25H27N4O4 [M + H]+ 447.2032, found 447.2036.
285, 1107. HRMS: calcd for C25H27N4O4 [M + H]+ 447.2032, found 447.2036.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The diastereomeric ratio was ∼6
1). The diastereomeric ratio was ∼6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 according to 1H-NMR integrals.
5 according to 1H-NMR integrals.1H-NMR (500 MHz, DMSO-d6) δ 2.73–2.78 (m, 1H, 4a-H), 2.86 (dd, J = 11.6, 5.5 Hz, 1H, 5-Ha), 2.95 and 3.00 (s, 3H, 3′-H), 3.12 (dd, J = 13.1, 6.8 Hz, 1H, 5-Ha), 3.39–3.44 (m, 1H, 5-Hb), 3.69 (dd, J = 13.2, 4.4 Hz, 1H, 5-Hb), 3.79 (s, 3H, 2′-H), 3.83 (d, J = 5.2 Hz, 1H, 10b-H from epi-2ai), 3.87 (s, 3H, 2′-H), 3.93 (d, J = 9.0 Hz, 1H, 10b-H from 2ai), 5.56 (d, J = 6.2 Hz, 1H, 4-H from epi-2ai), 5.64 (bs, 1H, 4-H from 2ai), 6.74–6.79 (m, 2H, 7-H), 6.99 (t, J = 7.4 Hz, 1H, 5′′′-H), 7.07 (t, J = 7.5 Hz, 1H, 5′′′-H), 7.12 (dd, J = 12.5, 8.4 Hz, 2H, 3′′′-H), 7.32 (d, J = 7.4 Hz, 1H, 6′′′-H), 7.38–7.46 (m, 3H, 2× 4′′′-H, 6′′′-H), 7.48–7.60 (m, 5H, 2× 3′′-H, 5′′-H, 2× 4′′-H), 7.72–7.77 (m, 4H, 2× 2′′-H, 2× 6′′-H), 8.02 (dd, J = 9.3, 2.7 Hz, 1H, 8-H), 8.08 (dd, J = 9.2, 2.6 Hz, 1H, 8-H), 8.21 (d, J = 2.7 Hz, 1H, 10-H), 8.53 (dd, J = 2.6, 1.1 Hz, 1H, 10-H). 13C-NMR (125 MHz, DMSO-d6) δ 31.6 (C-4a), 32.6 and 37.2 (C-10b), 38.1 and 38.8 (C-3′), 49.4 and 51.6 (C-5), 55.7 and 55.8 (C-2′), 74.3 (C-4), 83.1 and 85.7 (C-1), 110.0 and 110.1 (C-7), 111.3 and 111.6 (C-3′′′), 119.2 and 120.0 (C-10a), 120.4 (C-1′), 120.8 and 120.8 (C-5′′′), 123.1 (C-10a), 124.9 and 125.2 (C-8), 125.4 (C-1′′′), 125.8 (C-10), 126.6 (C-6′′′), 128.0 (C-2′′, C-6′′), 128.5 and 128.6 (C-3′′, C-5′′), 128.8 (C-2′′, C-6′′), 130.0 (C-4′′′), 130.5(C-6′′′), 131.0 and 131.5 (C-4′′), 132.6 and 132.8 (C-1′′), 135.4 and 135.7 (C-9), 149.6 and 151.8 (C-6a), 156.0 and 169.4 (C-2). IR (KBr) ν: 2916, 2842, 2310, 2205, 1605, 1493, 1313, 1282. HRMS: calcd for C27H23N3O4Na [M + Na]+ 476.1586, found 476.1581.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The diastereomeric ratio was ∼3
1). The diastereomeric ratio was ∼3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 according to 1H-NMR integrals.
1 according to 1H-NMR integrals.1H-NMR (500 MHz, DMSO-d6) δ 2.38–2.47 (m, 1H, 4a-H), 2.69 (dd, J = 11.6, 5.6 Hz, 1H, 5-Hb), 2.75–2.81 (m, 1H, 4a-H), 2.91 and 3.02 (s, 3H, 2′′′-H), 3.06–3.14 (m, 1H, 5-Ha), 3.40 (t, J = 11.6 Hz, 1H, 5-Hb), 3.66 (dd, J = 12.9, 3.7 Hz, 1H, 5-Hb), 3.81 (d, J = 4.2 Hz, 1H, 10b-H from epi-2bi), 3.84 (d, J = 11.0 Hz, 1H, 10b-H from 2bi), 5.29 (d, J = 10.0 Hz, 1H, 4-H from 2bi), 5.39 (bd, J = 5.3 Hz, 1H, 4-H from epi-2bi), 6.73 (d, J = 9.2 Hz, 1H, 7-H), 6.77 (d, J = 9.4 Hz, 1H, 7-H), 7.38–7.48 (m, 5H, Ph), 7.48–7.60 (m, 9H, Ph, 2× 3′′-H, 4′′-H, 5′′-H), 7.73–7.82 (m, 2H, 2′′-H, 6′′-H), 8.02 (dd, J = 9.4, 2.3 Hz, 1H, 8-H), 8.07 (dd, J = 9.2, 2.2 Hz, 1H, 8-H), 8.24 and 8.56 (bs, 1H, 10-H). 13C-NMR (125 MHz, DMSO-d6) δ 32.3 (C-10b) 32.5 (C-4a), 37.2 (C-10b), 38.0 and 38.9 (C-2′′′), 39.5 (C-4a), 49.4 and 51.8 (C-5), 78.5 and 81.4 (C-4), 83.1 and 86.1 (C-1), 110.0 and 110.3 (C-7), 119.2 (C-10), 119.9 and 120.3 (C-1′′′), 123.0 (C-10a), 124.9 and 125.0 (C-8), 126.0 (C-10), 127.6 (C-2′′, C-6′′), 128.0 (C-4′′), 128.5 (C-3′′, C-5′′), 128.6 (C-2′′, C-6′′), 128.8 (C-3′′, C-5′′), 129.3 (C-4′′), 131.0 and 131.6 (C-4′), 132.5 and 132.8 (C-1′), 135.6 and 135.7 (C-1′′), 136.2 and 138.0 (C-9), 149.8 and 151.8 (C-6a), 169.3 (C-2). IR (KBr) ν: 2895, 2310, 2198, 1605, 1520, 1491, 1317, 1284. HRMS: calcd for C26H21N3O3Na [M + Na]+ 446.1481, found 446.1475.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The diastereomeric ratio was ∼4
1). The diastereomeric ratio was ∼4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 according to 1H-NMR integrals.
1 according to 1H-NMR integrals.1H-NMR (500 MHz, DMSO-d6) δ 2.66–2.75 (m, 1H, 4a-H), 2.91 (dd, J = 11.8, 5.3 Hz, 1H, 5-Hb), 2.96 and 3.02 (s, 3H, 2′-H), 3.19–3.27 (m, 1H, 5-Hb), 3.58 (t, J = 11.8 Hz, 1H, 5-Ha), 3.70 (dd, J = 13.2, 4.5 Hz, 1H, 5-Ha), 3.94 (bd, J = 4.1 Hz, 1H, 10b-H from epi-2ci), 4.00 (d, J = 10.9 Hz, 1H, 10b-H from 2ci), 5.78 (d, J = 10.0 Hz, 1H, 4-H from 2ci), 5.91 (bd, J = 3.2 Hz, 1H, 4-H from epi-2ci), 6.72–6.83 (m, 2H, 2× 7-H), 7.49–7.61 (m, 3H, 2′′-H, 4′′′-H, 6′′-H), 7.67–7.77 (m, 3H, 2× 5′′′-H, 6′′′-H), 7.77–7.83 (m, 3H, 3′′-H, 2× 5′′-H), 7.87–7.92 (m, 3H, 3′′′-H, 2× 4′′-H), 8.01–8.07 (m, 1H, 3′′′-H), 8.09 (dd, J = 9.2, 2.4 Hz, 1H, 8-H), 8.14 (d, J = 8.0 Hz, 1H, 8-H), 8.19–8.22 and 8.53–8.57 (m, 1H, 10-H). 13C-NMR (125 MHz, DMSO-d6) δ 32.1 and 37.2 (C-10b), 38.1 and 38.7 (C-2′), 39.4 (C-4a), 49.3 and 51.4 (C-5), 75.0 and 75.8 (C-4), 83.8 and 86.5 (C-1), 110.1 and 110.2 (C-7), 119.3 and 119.3 (C-10), 120.1 (C-1′), 122.7 (C-10a), 124.4 (C-3′′′), 125.0 and 125.3 (C-8), 128.0 (C-3′′, C-5′′), 128.5 and 128.6 (C-2′′, C-6′′), 128.9 (C-3′′, C-5′′), 129.8 (C-1′′), 129.9 (C-4′′), 130.1 (C-4′′), 130.7 (C-3′′′), 131.1 and 131.7 (C-4′′′), 132.3 and 132.5 (C-1′′′), 133.8 and 134.4 (C-5′′′), 135.5 and 135.6 (C-9), 149.4 and 149.7 (C-2′′′), 151.6 (C-6a), 168.9 (C-2). IR (KBr) ν: 2912, 2364, 2321, 2207, 1605, 1524, 1314, 1284. HRMS: calcd for C26H20N4O5Na [M + Na]+ 491.1331, found 491.1326.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) affording rac-(4R*,4aS*,10bS*)-4aj as yellow amorphous solid (98%), which decomposed above 250 °C. Rf = 0.11 (hexane/chloroform/acetone 5
1) affording rac-(4R*,4aS*,10bS*)-4aj as yellow amorphous solid (98%), which decomposed above 250 °C. Rf = 0.11 (hexane/chloroform/acetone 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).1H NMR (400 MHz, DMSO-d6) δ 2.51–2.58 (m, 1H, 4a-H), 2.88 (s, 3H, 4′-H), 2.93 (dd, J = 11.7, 5.4 Hz, 1H, 5-Hb), 3.11–3.19 (m, 1H, 5-Ha), 3.64 (d, J = 11.0 Hz, 1H, 10b-H), 3.84 (s, 3H, 3′-H), 4.75 (d, J = 10.6 Hz, 1H, 4-H), 6.64–6.71 (m, 3H, 2′-H, 7-H), 6.94–7.00 (m, 1H, 5′′-H), 7.08 (d, J = 7.7 Hz, 1H, 3′′-H), 7.29–7.36 (m, 1H, 4′′-H), 7.41 (dd, J = 7.6, 1.6 Hz, 1H, 6′′-H), 8.01 (dd, J = 9.1, 2.6 Hz, 1H, 8-H), 8.19 (dd, J = 2.6, 1.2 Hz, 1H, 10-H). 13C NMR (100 MHz, DMSO-d6) δ 37.6 (C-4′), 40.4 (C-10b), 41.0 (C-4), 41.8 (C-4a), 54.0 (C-5), 55.7 (C-3′), 68.1 (C-1), 109.5 (C-7), 111.7 (C-3′), 119.9 (C-10), 120.4 (C-1′), 120.9 (C-5′′), 123.4 (C-1′′), 124.3 (C-8), 125.5 (C-10a), 128.7 (C-6′′), 129.5 (C-4′′), 135.8 (C-9), 151.5 (C-6a), 157.0 (C-2′′), 160.0 (C-2). IR: (KBr) ν: 3734, 3440, 3005, 2989, 1601, 1560, 1524, 1491, 1462, 1452, 1436, 1418, 1362, 1313, 1275, 1260, 1217, 1194, 1159. HRMS: calcd for C21H21N4O3S [M + H]+ 409.1334, found 409.1330.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).1H-NMR (400 MHz, CDCl3) δ 1.21 (t, J = 7.1 Hz, 3H, 4′), 2.13 (bs, 1H, 4a-H), 2.39 (s, 3H, 5′-H), 2.76 (dd, J = 11.2, 5.8 Hz, 1H, 5-Hb), 2.92 (s, 3H, 7′-H), 3.39 (t, J = 11.2 Hz, 1H, 5-Ha), 3.73 (d, J = 12.0 Hz, 1H, 10b-H), 3.86 (s, 3H, 6′-H), 4.15–4.37 (m, 2H, 3′-H), 5.29 (bs, 1H, 4-H), 6.48 (d, J = 9.1 Hz, 1H, 7-H), 6.94 (d, J = 8.4 Hz, 1H, 3′′-H), 7.01 (t, J = 7.4 Hz, 1H, 5′′-H), 7.33 (t, J = 7.1 Hz, 2H, 6′′-H, 4′′-H), 7.63–7.70 (m, 1H, 10-H), 8.02 (dd, J = 9.1, 2.4 Hz, 1H, 8-H). 13C-NMR (100 MHz, CDCl3) δ 14.4 (C-4′), 20.4 (C-5′), 38.1 (C-7′), 38.8 (C-10b), 43.5 (C-4a), 52.7 (C-5), 55.7 (C-6′), 60.4 (C-3′), 71.4 (C-4), 102.4 (C-1), 109.4 (C-7), 110.9 (C-3′′), 120.4 (C-10), 121.4 (C-5′′), 124.4 (C-8), 126.7 (C-1′′), 127.0 (C-6′′), 129.9 (C-4′′), 137.3 (C-9), 152.0 (C-6a), 167.2 (C-2), 168.3 (C-1′). IR (KBr) ν: 2925, 2852, 2370, 2318, 1702, 1616, 1513, 1490. HRMS: calcd for C24H26N2O6Na [M + Na]+ 461.1689, found 461.1683.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).1H-NMR (500 MHz, CDCl3) δ 1.22 (t, J = 7.1 Hz, 3H, 4′-H), 2.11–2.23 (m, 1H, 4a-H), 2.40 (s, 3H, 5′-H), 2.68 (dd, J = 11.6, 5.9 Hz, 1H, 5-Hb), 2.91 (s, 3H, 6′-H), 3.25 (t, J = 11.6 Hz, 1H, 5-Ha), 3.70 (d, J = 11.2 Hz, 1H, 10b-H), 4.15–4.35 (m, 2H, 3′-H), 4.65 (d, J = 10.2 Hz, 1H, 4-H), 6.50 (d, J = 9.0 Hz, 1H, 7-H), 7.29–7.36 (m, 2H, 2′′-H, 6′′-H), 7.36–7.47 (m, 3H, 3′′-H, 4′′-H, 5′′-H), 7.63–7.71 (m, 1H, 10-H), 8.02 (dd, J = 9.0, 2.5 Hz, 1H, 8-H). 13C-NMR (125 MHz, CDCl3) δ 14.4 (C-4′), 20.2 (C-5′), 38.1 (C-6′), 38.7 (C-10b), 42.9 (C-4a), 53.4 (C-5), 60.5 (C-3′), 80.7 (C-4), 102.6 (C-1), 109.5 (C-7), 120.4 (C-10), 124.4 (C-8), 126.7 (C-10a), 126.9 (C-2′′, C-6′′), 129.1 (C-3′′, C-5′′), 129.3 (C-4′′), 137.3 (C-1′′), 137.6 (C-9), 151.9 (C-6a), 166.6 (C-2), 168.1 (C-1′). IR (KBr) ν: 2977, 2911, 2318, 1700, 1613, 1515, 1490, 1316, 1281. HRMS: calcd for C23H24N2O5Na [M + Na]+ 431.1583, found 431.1582.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).1H-NMR (400 MHz, CDCl3) δ 1.38 (t, J = 7.1 Hz, 3H, 3′-H), 2.48–2.65 (m, 1H, 4a-H), 2.88 (dd, J = 11.3, 5.9 Hz, 1H, 5-Hb), 2.96 (s, 3H, 4′-H), 3.35 (t, J = 11.3 Hz, 1H, 5-Ha), 4.13 (d, J = 11.2 Hz, 1H, 10b-H), 4.36–4.56 (m, 2H, 2′-H), 5.29 (d, J = 10.1 Hz, 1H, 4-H), 6.58 (d, J = 9.1 Hz, 1H, 7-H), 7.30–7.39 (m, 2H, 2′′-H, 6′′-H), 7.39–7.51 (m, 3H, 3′′-H, 4′′-H, 5′′-H), 7.73 (dd, J = 2.6, 1.1 Hz, 1H, 10-H), 8.08 (dd, J = 9.1, 2.6 Hz, 1H, 8-H). 13C-NMR (100 MHz, CDCl3) δ 14.2 (C-3′), 38.2 (C-4′), 40.7 (C-4a), 40.9 (C-10b), 52.2 (C-5), 63.2 (C-2′), 85.2 (C-4), 110.3 (C-7), 113.6 (C-1), 119.9 (C-4), 122.8 (C-10a), 125.3 (C-6), 127.5 (C-2′′, C-6′′), 129.4 (C-3′′, C-5′′), 130.5 (C-4′′), 133.1 (C-1′′), 137.7 (C-9), 151.0 (C-6a), 161.6 (C-2). IR (KBr) ν: 2929, 1730, 1606, 1305, 1284. HRMS: calcd for C21H21N3O6Na [M + Na]+ 434.1328, found 434.1323.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3).
3).1H-NMR (500 MHz, DMSO-d6) δ 2.57 (s, 1H, 4a-H), 2.69 (d, J = 13.3 Hz, 1H, 5-Hb), 2.95 (s, 3H 3′′-H), 3.45 (s, 3H, 1′′-H), 3.57 (s, 3H, 2′′-H), 3.85 (dd, J = 13.3, 4.4 Hz, 1H, 5-Ha), 4.39 (d, J = 3.4 Hz, 1H, 10b-H), 5.21 (bs, 1H, 4-H), 6.72 (d, J = 9.3 Hz, 1H, 7-H), 6.98 (t, J = 7.3 Hz, 1H, 5′′′-H), 7.04 (d, J = 7.3 Hz, 1H, 6′′′-H), 7.28 (d, J = 7.3 Hz, 1H, 3′′′-H), 7.36–7.45 (m, 1H, 4′′′-H), 7.60–7.74 (m, 3H, 3′-H, 4′-H, 5′-H), 7.93–8.06 (m, 3H, 2′-H, 6′-H, 8-H), 8.24 (bs, 1H, 10-H). 13C-NMR (90 MHz, DMSO-d6) δ 34.5 (C-4a), 35.2 (C-10b), 38.7 (C-3′′), 50.0 (C-5), 54.9 (C-2′′), 55.3 (C-1′′), 91.6 (C-1), 109.6 (C-7), 111.5 (C-6′′′), 120.7 (C-5′′′), 122.5 (C-1′′′), 124.0 (C-10a), 124.8 (C-8), 126.2 (C-10), 126.7 (C-2′, C-6′), 128.8 (C-3′, C-3′′′, C-5′), 130.6 (C-4′′′), 132.4 (C-4′′), 135.9 (C-9), 144.2 (C-1′), 149.5 (C-6a), 156.9 (C-2′′′), 161.1 (C-2). IR (KBr) ν: 2952, 2833, 1605, 1493, 1327, 1304, 1281. HRMS: calcd for C27H26N2O7SNa [M + Na]+ 545.1358, found 545.1353.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).1H-NMR (500 MHz, DMSO-d6) δ 2.43–2.48 (m, 1H, 4a-H), 2.63 (d, J = 12.9 Hz, 1H, 5-Hb), 2.94 (s, 3H, 2′′′-H), 3.49 (s, 3H, 1′′′-H), 3.82 (dd, J = 12.9, 3.5 Hz, 1H, 5-Ha), 4.43 (d, J = 3.5 Hz, 1H, 10b-H), 4.95 (d, J = 11.0 Hz, 1H, 4-H), 6.75 (d, J = 9.3 Hz, 1H, 7-H), 7.28–7.36 (m, 2H, 2′′-H, 3′′-H), 7.42 (bs, 3H, 3′′-H, 4′′-H, 5′′-H), 7.62 (t, J = 7.4 Hz, 2H, 3′-H, 5′-H), 7.65–7.73 (m, 1H, 4′-H), 7.94–8.05 (m, 3H, 2′-H, 6′-H, 8-H), 8.35 (bs, 1H, 10-H). 13C-NMR (100 MHz, DMSO-d6) δ 35.3 (C-10b), 35.6 (C-4a), 40.2 (C-2′′′), 50.0 (C-5), 55.1 (C-1′′′), 81.4 (C-4), 92.1 (C-1), 110.2 (C-7), 122.9 (C-10a), 124.6 (C-8), 126.7 (C-10), 126.9 (C-2′, C-6′), 127.3 (C-2′′, C-6′′), 128.8 (C-3′′, C-4′′, C-5′′), 129.3 (C-3′, C-5′), 132.4 (C-4′), 136.3 (C-9), 136.3 (C-1′′′), 144.0 (C-1′), 149.5 (C-6a), 161.0 (C-2). IR (KBr) ν: 2949, 2863, 2318, 1603, 1330, 1304, 1287. HRMS: calcd for C26H24N2NaO6S [M + Na]+ 515.1253, found 515.1247.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Unifying the product from crystallization and chromatography afforded the mixture of rac-(4R*,4aS*,10bS*)-2an and rac-(4R*,4aS*,10bR*)-reg-2an as yellow powder (37%). Rf = 0.26 (hexane/acetone 3
1). Unifying the product from crystallization and chromatography afforded the mixture of rac-(4R*,4aS*,10bS*)-2an and rac-(4R*,4aS*,10bR*)-reg-2an as yellow powder (37%). Rf = 0.26 (hexane/acetone 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The regioisomer ratio was ∼10
1). The regioisomer ratio was ∼10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 according to the 1H-NMR integrals.
7 according to the 1H-NMR integrals.1H-NMR (500 MHz, DMSO-d6) δ 2.11 and 2.24 (s, 3H, 2′-H), 2.27–2.35 (m, 1H, 4a-H), 2.74 (d, J = 13.0 Hz, 2H, 2× 5-Hb), 2.78–2.84 (m, 2H, 5-Ha, 4a-H), 2.91 and 2.96 (s, 3H, 5′-H), 3.72 (s, 3H, 4′-H), 3.79–3.90 (m, 4H, 5-Ha, 4′-H), 4.06 (d, J = 9.5 Hz, 1H, 10b-H from 2an), 4.22 (d, J = 3.1 Hz, 1H, 10b-H from reg-2an), 5.27 (bd, J = 10.9 Hz, 2H, 2× 4-H), 6.66 and 6.74 (d, J = 9.3 Hz, 1H, 7-H), 6.98–7.07 and 7.06–7.14 (m, 2H, 3′′′-H, 5′′′-H), 7.20–7.26 (m, 4H, 2′′-H, 3′′-H, 5′′-H, 6′′-H), 7.34–7.43 (m, 5H, 2′′-H, 3′′-H, 4′′′-H, 5′′-H, 6′′-H), 7.63 (d, J = 8.8 Hz, 2H, 2× 6′′′-H), 7.69–7.75 (m, 1H, 10-H), 7.95 (dd, J = 9.3, 2.5 Hz, 1H, 8-H), 8.00–8.05 (m, 2H, 8-H, 10-H), 10.07 (s, 1H, 2′-H), 13.80 (s, 1H, 3′-H). 13C-NMR (125 MHz, DMSO-d6) δ 18.5 (C-3′), 26.0 (C-2′), 33.9 (C-10b), 35.0 (C-4a), 37.9 (C-5′), 38.4 (C-10b), 38.5 (C-5′), 50.2 and 52.2 (C-5), 55.6 and 55.7 (C-4′), 89.4 and 106.5 (C-1), 109.3 and 109.8 (C-7), 111.3 (C-3′′′), 119.4 (C-10), 120.9 (C-5′′′), 121.5 (C-6′′′), 122.4 (C-10a), 122.8 (C-2′′, C-3′′, C-5′′, C-6′′), 124.3 (C-8), 124.4 (C-1′′′), 124.7 (C-8), 125.0 (C-10), 125.1 (C-10a), 127.0 (C-4′′), 127.6 (C-1′′′), 128.5 (C-3′′, C-5′′), 128.9 (C-2′′, C-6′′), 130.0 and 130.4 (C-4′′′), 135.6 and 135.7 (C-9), 136.2 and 137.9 (C-1′′), 149.9 and 152.0 (C-6a), 156.9 and 158.3 (C-2′′′), 161.0 and 167.6 (C-2), 193.1 (C-2′′). IR (KBr) ν: 2916, 2310, 1601, 1492, 1315. HRMS: calcd for C28H26ClN3O5Na [M + Na]+ 542.1459, found 542.1453.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Unifying the product from crystallization and chromatography afforded the mixture of rac-(4R*,4aS*,10bS*)-2bn and rac-(4R*,4aS*,10bR*)-reg-2bn as yellow powder (67%). Rf = 0.40 (hexane/acetone 3
1). Unifying the product from crystallization and chromatography afforded the mixture of rac-(4R*,4aS*,10bS*)-2bn and rac-(4R*,4aS*,10bR*)-reg-2bn as yellow powder (67%). Rf = 0.40 (hexane/acetone 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The regioisomer ratio was ∼10
1). The regioisomer ratio was ∼10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 according to the 1H-NMR integrals.
7 according to the 1H-NMR integrals.1H-NMR (500 MHz, DMSO-d6) δ 2.14 (s, 3H, 2′-H), 2.20–2.28 (m, 4H, 2× 3′-H, 2× 4a-H), 2.63–2.71 (m, 3H, 4a-H, 2× 5-Hb), 2.88 and 2.97 (s, 3H, 4′-H), 3.33–3.38 (m, 1H, 5-Ha), 3.79 (dd, J = 13.4, 4.3 Hz, 1H, 5-Ha), 4.03 (d, J = 11.0 Hz, 1H, 10b-H from 2bn), 4.26 (d, J = 3.4 Hz, 1H, 10b-H from reg-2bn), 4.86 (d, J = 10.1 Hz, 1H, 4-H from 2bn), 4.93 (d, J = 11.4 Hz, 1H, 4-H from reg-2bn), 6.64 (d, J = 9.2 Hz, 1H, 7-H), 6.75 (d, J = 9.3 Hz, 1H, 7-H), 7.22 (s, 3H, 2× 3′′-H, 5′′-H), 7.36 (d, J = 8.8 Hz, 3H, 3′′-H, 2× 5′′-H), 7.37–7.47 (m, 12H, 2× Ph-H, 2′′-H, 6′′-H), 7.65 (d, J = 8.8 Hz, 2H, 2′′′-H, 6′′′-H), 7.73–7.76 (m, 1H, 10-H), 7.94 (dd, J = 9.1, 2.5 Hz, 1H, 8-H), 7.99–8.04 (m, 2H, 10-H, 8-H), 10.14 (s, 1H, 2′-H), 13.82 (s, 1H, 3′-H). 13C-NMR (125 MHz, DMSO-d6) δ 18.7 (C-2′), 26.0 (C-3′), 34.0 (C-10b), 35.3 (C-4a), 37.5 (C-10b), 37.8 and 38.7 (C-4′), 40.6 (C-4a), 50.3 and 52.5 (C-5), 79.4 and 80.1 (C-4), 89.6 and 106.6 (C-1), 109.8 and 109.9 (C-7), 119.4 (C-10), 121.8 (C-2′′ and C-6′′), 122.6 (C-10a), 122.9 (C-2′′′ and C-6′′′), 124.3 and 124.7 (C-8), 125.0 (C-10), 125.1 (C-10a), 127.1 (C-4′′), 127.3 (C-3′′′ and C-5′′′), 127.7 (C-4′′), 128.4 (C-3′′, C-5′′), 128.6 (C-3′′, C-5′′), 128.9 (C-2′′′ and C-6′′′ and C-4′′′), 129.2 (C-4′′′), 135.8 and 135.9 (C-9), 136.2 and 136.8 (C-1′′′), 137.5 and 137.8 (C-1′′), 150.0 and 152.1 (C-6a), 157.8 (C-1′), 160.8 and 167.4 (C-2), 193.3 (C-1′). IR (KBr) ν: 3032, 2911, 2310, 1617, 1602, 1494, 1316, 1282. HRMS: calcd for: C27H24ClN3O4Na [M + Na]+ 512.1353, found 512.1348.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) affording the mixture of rac-(1R*,4R*,4aS*,10bR*)-dia1-5a and rac-(1S*,4R*,4aS*,10bS*)-dia2-5a as orange oil (65%). Rf = 0.49 (hexane/ethyl acetate 1
1) affording the mixture of rac-(1R*,4R*,4aS*,10bR*)-dia1-5a and rac-(1S*,4R*,4aS*,10bS*)-dia2-5a as orange oil (65%). Rf = 0.49 (hexane/ethyl acetate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). The diastereomeric ratio was ∼4
2). The diastereomeric ratio was ∼4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 according to 1H-NMR integrals.
5 according to 1H-NMR integrals.1H-NMR (500 MHz, CDCl3) δ 1.39–1.67 (m, 4H, 5′′-H, 4′′-H), 2.05–2.15 (m, 4H, 5′′-H, 3′′-H), 2.17–2.24 (m, 1H, 4a-H), 2.41–2.51 (m, 1H, 4a-H), 2.93 (s, 3H, 3′-H), 3.08 (s, 3H, 3′-H), 3.17–3.26 (m, 2H, 6′′-H), 3.36–3.47 (m, 2H, 6′′-H), 3.52 (dd, J = 12.7, 10.1 Hz, 1H, 5-Hb), 3.61 (dd, J = 12.8, 5.8 Hz, 1H, 5-Ha), 3.63–3.69 (m, 1H, 5-Hb), 3.72–3.79 (m, 1H, 5-Ha), 3.82–3.87 (m, 6H, 2× 2′-H, 2′′-Ha), 3.88 (d, J = 9.8 Hz, 1H, 1-H from dia2-5a), 3.92 (dd, J = 9.8, 5.2 Hz, 1H, 10b-H from dia2-5a), 3.96 (d, J = 10.5 Hz, 1H, 1-H from dia1-5a), 4.03 (dd, J = 11.5, 10.5 Hz, 1H, 10b-H from dia1-5a), 4.08–4.17 (m, 1H, 1-H), 5.74 (d, J = 10.5 Hz, 1H, 2′′-Ha), 5.84 (d, J = 3.1 Hz, 1H, 4-H from dia2-5a), 6.44 (d, J = 10.4 Hz, 1H, 4-H from dia1-5a), 6.57 (d, J = 9.3 Hz, 1H, 7-H), 6.89 (d, J = 8.2 Hz, 1H, 7-H), 6.95 (d, J = 8.3 Hz, 1H, 3′′′-H), 7.02 (t, J = 7.5 Hz, 1H, 3′′′-H), 7.12 (t, J = 7.5 Hz, 1H, 5′′′-H), 7.30–7.39 (m, 3H, 2× 4′′′-H, 6′′′-H), 7.54 (dd, J = 7.6, 1.6 Hz, 1H, 6′′′-H), 7.72–7.76 (m, 1H, 10-H), 7.78 (d, J = 2.6 Hz, 1H, 10-H), 7.93–7.99 (m, 1H, 8-H), 8.01 (ddd, J = 9.2, 2.6, 1.1 Hz, 1H, 8-H). 13C-NMR (125 MHz, CDCl3) δ 24.37 and 24.66 (C-5′′), 25.59 (C-4′′), 26.10 and 26.28 (C-3′′), 33.93 (C-4a), 34.20 (C-10b), 37.41 (C-4a), 38.46 (C-10b), 38.98 and 39.27 (C-3′), 43.95 (C-4′′), 46.83 (C-1), 47.00 (C-2′′), 47.72 (C-2′′), 48.01 (C-5), 50.37 and 51.61 (C-6′′), 51.70 (C-1), 55.58 and 55.82 (C-2′), 77.67 and 78.86 (C-4), 109.31 and 109.54 (C-7), 110.41 and 111.31 (C-3′′′), 121.20 and 121.25 (C-5′′′), 121.35 (C-10a), 121.78 (C-10), 122.22 (C-10a), 124.62 (C-1′′′), 124.86 (C-10), 125.23 and 125.56 (C-8), 126.18 (C-1′′′), 127.69 and 128.29 (C-6′′′), 129.77 and 130.65 (C-4′′′), 137.04 and 137.09 (C-9), 149.55 and 150.31 (C-6a), 155.36 and 157.13 (C-2′′′), 165.96 and 166.77 (C-1′), 166.84 and 167.08 (C-2). IR: (KBr) ν: 2925, 1721, 1637, 1601, 1578, 1520, 1492, 1437, 1312, 1275, 1261, 1240, 1183, 1164, 1107, 1084, 1024. HRMS: calcd for C26H30N3O6 [M + H]+ 480.2134, found 480.2129.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) affording rac-(1R*,4R*,4aS*,10bR*)-dia1-5b as the minor product and orange oil (3%), Rf = 0.46 (hexane/ethyl acetate 1
1) affording rac-(1R*,4R*,4aS*,10bR*)-dia1-5b as the minor product and orange oil (3%), Rf = 0.46 (hexane/ethyl acetate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2).
2).1H-NMR (400 MHz, CDCl3) δ 2.00–2.26 (m, 4H, 3′′-H, 4′′-H), 2.39–2.51 (m, 1H, 4a-H), 2.89–3.00 (m, 4H, 5-Hb, 3′-H), 3.42 (t, J = 11.8 Hz, 1H, 5-Ha), 3.63–3.72 (m, 1H, 2′′-Ha), 3.72–3.81 (m, 1H, 2′′-Hb), 3.86 (s, 5H, 5′′-Ha, 2′-H), 3.96 (d, J = 10.2 Hz, 1H, 1-H), 4.05 (dd, J = 11.8, 10.2 Hz, 1H, 10b-H), 4.09–4.19 (m, 1H, 5′′-Hb), 5.76 (d, J = 10.5 Hz, 1H, 4-H), 6.46 (d, J = 9.2 Hz, 1H, 7-H), 6.96 (dd, J = 8.4, 1.1 Hz, 1H, 3′′′-H), 6.98–7.08 (m, 1H, 5′′′-H), 7.32–7.43 (m, 2H, 4′′′-H and 6′′′-H), 7.77 (dd, J = 2.6, 1.4 Hz, 1H, 10-H), 8.01 (dd, J = 9.2, 2.5 Hz, 1H, 8-H). 13C-NMR (100 MHz, CDCl3) δ 24.68 (C-4′′), 26.13 (C-3′′), 37.46 (C-4a), 38.50 (C-10b), 39.30 (C-3′), 47.03 (C-5′′), 47.75 (C-2′′), 51.64 (C-5), 51.72 (C-1), 55.84 (C-2′), 78.78 (C-4), 109.32 (C-7), 111.31 (C-3′′′), 121.28 (C-5′′′), 121.81 (C-10), 122.24 (C-10a), 124.63 (C-1′′′), 125.28 (C-8), 128.31 (C-6′′′), 130.67 (C-4′′′), 137.15 (C-9), 150.30 (C-6a), 157.14 (C-2′′′), 166.75 (C-1′), 166.85 (C-2). IR: (KBr) ν: 2988, 1715, 1642, 1603, 1580, 1520, 1492, 1463, 1437, 1275, 1260, 1022. HRMS: calcd for C25H28N3O6 [M + H]+ 466.1978, found 466.1973.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) affording rac-(1S*,4R*,4aS*,10bS*)-dia2-5b as the major product and orange oil (18%), Rf = 0.46 (hexane/ethyl-acetate 1
1) affording rac-(1S*,4R*,4aS*,10bS*)-dia2-5b as the major product and orange oil (18%), Rf = 0.46 (hexane/ethyl-acetate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2).
2).1H-NMR (400 MHz, CDCl3) δ 1.61–1.78 (m, 2H, 4′′-H), 1.80–1.91 (m, 2H, 3′′-H), 2.61–2.73 (m, 3H, 2′′-H, 4a-H), 3.10 (d, J = 2.9 Hz, 3H, 3′-H), 3.47–3.65 (m, 6H, 2′′-H, 5′′-H, 5-H), 3.65 (d, J = 10.0 Hz, 1H, 1-H), 3.84 (s, 3H, 2′-H), 3.86 (dd, J = 10.0, 4.5 Hz, 1H, 10b-H), 5.86 (d, J = 3.1 Hz, 1H, 4-H), 6.57 (d, J = 9.2 Hz, 1H, 7-H), 6.90 (dd, J = 8.3, 1.1 Hz, 1H, 3′′′-H), 7.13 (ddd, J = 7.6, 6.8, 1.1 Hz, 1H, 5′′′-H), 7.34 (td, J = 7.9, 1.7 Hz, 1H, 4′′′-H), 7.58 (dd, J = 7.7, 1.7 Hz, 1H, 6′′′-H), 7.74–7.80 (m, 1H, 10-H), 8.02 (dd, J = 9.2, 2.7 Hz, 1H, 8-H). 13C-NMR (100 MHz, CDCl3) δ 24.54 (C-4′′), 25.95 (C-3′′), 33.70 (C-4a), 34.19 (C-10b), 39.05 (C-3′), 46.44 (C-2′′), 47.36 (C-5′′), 49.82 (C-1), 50.35 (C-5), 55.60 (C-2′), 77.74 (C-4), 109.54 (C-7), 110.39 (C-3′′), 121.17 (C-10a), 121.29 (C-5′′′), 124.56 (C-10), 125.60 (C-8), 126.15 (C-1′′′), 127.77 (C-6′′′), 129.80 (C-4′′′), 137.01 (C-9), 149.55 (C-6a), 155.33 (C-2′′′), 166.20 (C-1′), 167.01 (C-2). IR: (KBr) ν: 2916, 1724, 1635, 1603, 1489, 1430, 1274, 1259, 1183, 1111, 1088. HRMS: calcd for C25H28N3O6 [M + H]+ 466.1978, found 466.1974.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) affording rac-(1R*,4R*,4aS*,10bR*)-dia1-5c as the major product and orange oil (16%), Rf = 0.51 (hexane/ethyl acetate 1
1) affording rac-(1R*,4R*,4aS*,10bR*)-dia1-5c as the major product and orange oil (16%), Rf = 0.51 (hexane/ethyl acetate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2).
2).1H-NMR (500 MHz, CDCl3) δ 2.48 (ddt, J = 14.9, 10.9, 4.2 Hz, 1H, 4a-H), 2.91–2.99 (m, 4H, 3′-H, 5-Hb), 3.42 (t, J = 11.7 Hz, 1H, 5-Ha), 3.81–3.89 (m, 5H, 2′-H, 2′′-H), 3.88–4.01 (m, 6H, 3′′-H, 4′′-H, 5′′-H), 4.10 (dd, J = 11.7, 10.0 Hz, 1H, 10b-H), 4.15 (d, J = 10.0 Hz, 1H, 1-H), 5.73 (d, J = 10.3 Hz, 1H, 4-H), 6.46 (dd, J = 9.3, 1.4 Hz, 1H, 7-H), 6.97 (d, J = 8.3 Hz, 1H, 3′′′-H), 7.03 (t, J = 7.5 Hz, 1H, 5′′′-H), 7.33 (dd, J = 7.6, 1.7 Hz, 1H, 6′′′-H), 7.35–7.42 (m, 1H, 4′′′-H), 7.68–7.77 (m, 1H, 10-H), 7.92–8.06 (m, 1H, 8-H). 13C-NMR (125 MHz, CDCl3) δ 37.41 (C-4a), 38.36 (C-10b), 39.29 (C-3′), 43.56 (C-6′′), 47.69 (C-2′′), 48.60 (C-1), 51.66 (C-5), 55.87 (C-2′), 66.62 (C-3′′ and C-5′′), 78.89 (C-4), 109.36 (C-7), 111.37 (C-3′′′), 121.33 (C-5′′′), 121.58 (C-10), 122.05 (C-10a), 124.48 (C-1′′′), 125.40 (C-8), 128.29 (C-6′′′), 130.77 (C-4′′′), 137.10 (C-9), 150.33 (C-6a), 157.15 (C-2′′′), 166.44 (C-1′), 166.92 (C-2). IR: (KBr) ν: 2989, 1717, 1633, 1601, 1577, 1523, 1491, 1459, 1275, 1260, 1112, 1046. HRMS: calcd for C25H28N3O7 [M + H]+ 482.1927, found 482.1922.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) affording rac-(1S*,4R*,4aS*,10bS*)- dia2-5c as the minor product and orange oil (9%), Rf = 0.6 (hexane/ethyl acetate 1
1) affording rac-(1S*,4R*,4aS*,10bS*)- dia2-5c as the minor product and orange oil (9%), Rf = 0.6 (hexane/ethyl acetate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2).
2).1H-NMR (500 MHz, CDCl3) δ 2.69–2.76 (m, 1H, 4a-H), 3.10–3.16 (m, 4H, 6′′-Hb, 3′-H), 3.19–3.25 (m, 1H, 5′′-Hb), 3.43–3.70 (m, 4H, 5-H, 5′′-Ha, 6′′-Ha), 3.71–3.79 (m, 4H, 3′′-H, 2′′-H), 3.83 (d, J = 9.8 Hz, 1H, 1-H), 3.86 (s, 3H, 2′-H), 3.98 (dd, J = 9.8, 4.7 Hz, 1H, 10b-H), 5.88 (d, J = 3.0 Hz, 1H, 4-H), 6.60 (d, J = 9.2 Hz, 1H, 7-H), 6.93 (d, J = 8.1 Hz, 1H, 3′′′-H), 7.09–7.18 (m, 1H, 5′′′-H), 7.35–7.40 (m, 1H, 4′′′-H), 7.46–7.56 (m, 1H, 6′′′-H), 7.77–7.82 (m, 1H, 10-H), 8.04 (ddd, J = 9.2, 2.7, 0.7 Hz, 1H, 8-H). 13C-NMR (125 MHz, CDCl3) δ 33.91 (C-4a), 33.99 (C-10b), 39.01 (C-3′), 43.18 (C-2′′), 46.75 (C-1), 47.22 (C-6′′), 50.40 (C-5), 55.64 (C-2′), 66.50 (C-5′′), 66.83 (C-3′′), 77.92 (C-4), 109.69 (C-7), 110.55 (C-3′′′), 121.21 (C-10a), 121.26 (C-5′′′), 124.84 (C-10), 125.69 (C-8), 126.03 (C-1′′′), 127.54 (C-6′′′), 129.94 (C-4′′′), 137.22 (C-9), 149.53 (C-6a), 155.40 (C-2′′′), 166.29 (C-1′), 166.85 (C-2). IR: (KBr) ν: 2919, 1722, 1643, 1602, 1579, 1523, 1493, 1463, 1437, 1320, 1293, 1268, 1240, 1185, 1169, 1109, 1084, 1009. HRMS: calcd for C25H28N3O7 [M + H]+ 482.1927, found 482.1923.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) affording rac-(1S*,4R*,4aS*,10bS*)-dia2-5d as orange oil (38%), Rf = 0.11 (hexane/ethyl acetate 1
1) affording rac-(1S*,4R*,4aS*,10bS*)-dia2-5d as orange oil (38%), Rf = 0.11 (hexane/ethyl acetate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).1H-NMR (500 MHz, CDCl3) δ 2.67–2.75 (m, 1H, 4a-H), 2.79 (s, 3H, 4′-H), 3.04 (s, 3H, 3′-H), 3.11 (s, 3H, 6′-H), 3.51–3.60 (m, 1H, 5-Hb), 3.63 (dd, J = 12.6, 5.7 Hz, 1H, 5-Ha), 3.84 (s, 3H, 5′-H), 3.89 (bs, 2H, 1-H, 10b-H), 5.86 (d, J = 3.1 Hz, 1H, 4-H), 6.54–6.61 (m, 1H, 7-H), 6.87–6.96 (m, 1H, 3′′-H), 7.12–7.18 (m, 1H, 5′′-H), 7.33–7.44 (m, 1H, 4′′-H), 7.54 (dd, J = 7.7, 1.8 Hz, 1H, 6′′-H), 7.72–7.81 (m, 1H, 10-H), 7.98–8.11 (m, 1H, 8-H). 13C-NMR (125 MHz, CDCl3) δ 33.77 (C-4a), 34.18 (C-10b), 36.32 (C-4′), 38.10 (C-3′), 38.97 (C-6′), 47.22 (C-1), 50.36 (C-5), 55.59 (C-5′), 77.76 (C-4), 109.54 (C-7), 110.44 (C-3′′), 121.10 (C-10c), 121.24 (C-5′′), 124.54 (C-10), 125.61 (C-8), 126.20 (C-1′′), 127.62 (C-4′′), 129.79 (C-6′′), 137.11 (C-9), 149.55 (C-6a), 155.34 (C-1′), 166.99 (C-2′′), 167.93 (C-2). IR: (KBr) ν: 2989, 1720, 1649, 1601, 1488, 1461, 1275, 1259, 1179, 1132, 1085. HRMS: calcd for C23H26N3O6 [M + H]+ 440.1821, found 440.1816.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) affording rac-(1S*,4R*,4aS*,10bS*)-dia2-5e as the minor product (orange oil, 4%), Rf = 0.43 (hexane/ethyl-acetate 1
1) affording rac-(1S*,4R*,4aS*,10bS*)-dia2-5e as the minor product (orange oil, 4%), Rf = 0.43 (hexane/ethyl-acetate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).1H NMR (500 MHz, CDCl3) δ 2.83–2.89 (m, 1H, 4a-H), 3.08 (s, 3H, 5′-H), 3.33 (dd, J = 12.8, 7.6 Hz, 1H, 5-Hb), 3.62–3.68 (m, 1H, 5-Ha), 3.74 (d, J = 6.7 Hz, 1H, 1-H), 3.80 (s, 3H. 4′-H), 4.00–4.05 (m, 1H, 10b-H), 4.46–4.53 (m, 1H, 3′′-Hb), 4.59–4.65 (m, 1H, 3′′-Ha), 5.70 (d, J = 5.9 Hz, 1H, 4-H), 6.59 (d, J = 9.2 Hz, 1H, 7-H), 6.83–6.89 (m, 1H, 4′′-H), 6.91–6.96 (m, 1H, 3′′′-H), 7.00–7.08 (m, 1H, 5′′′-H), 7.22–7.26 (m, 1H, 6′′′-H), 7.26–7.31 (m, 3H, 3′′-H, 5′′-H), 7.31–7.36 (m, 2H, 2′′-H, 6′′-H), 7.36–7.40 (m, 1H, 4′′′-H), 7.97–8.03 (m, 1H, 10-H), 8.03–8.11 (m, 2H, 8-H, 2′-H). 13C-NMR (125 MHz, CDCl3) δ 32.85 (C-4a), 32.98 (C-10b), 39.08 (C-5′), 44.47 (C-5), 50.63 (C-3′), 51.05 (C-1), 55.63 (C-4′), 78.54 (C-4), 109.59 (C-7), 111.00 (C-3′′′), 120.26 (C-10a), 121.20 (C-5′′′), 124.17 (C-4′′), 125.60 (C-1′′′), 125.67 (C-6′′′), 127.87 (C-2′′ and C-6′′), 128.92 (C-1′′), 130.13 (C-3′′ and C-5′′), 130.26 (C-4′′′), 137.66 (C-9), 149.99 (C-6a), 156.10 (C-2′′′), 165.27 (C-1′), 168.16 (C-2). IR: (KBr) ν: 2989, 1725, 1653, 1601, 1578, 1525, 1492, 1462, 1275, 1260, 1189, 1023. HRMS: calcd for C28H28N3O6 [M + H]+ 502.1978, found 502.1977.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) affording rac-(1R*,4R*,4aS*,10bR*)-dia1-5e as the major product (orange oil, 21%), Rf = 0.18 (hexane/ethyl acetate 2
1) affording rac-(1R*,4R*,4aS*,10bR*)-dia1-5e as the major product (orange oil, 21%), Rf = 0.18 (hexane/ethyl acetate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).1H-NMR (500 MHz, DMSO-d6) δ 2.66 (dd, J = 9.3, 4.7 Hz, 1H, 4a-H), 3.07 (s, 3H, 5′-H), 3.40 (dd, J = 13.2, 9.3 Hz, 1H, 5-Hb), 3.62–3.70 (m, 2H, 10b-H, 5-Hb), 3.82 (s, 3H, 4′-H), 3.86 (d, J = 9.3 Hz, 1H, 1-H), 4.22 (dd, J = 15.1, 5.0 Hz, 1H, 3′-Hb), 4.45 (dd, J = 15.1, 5.0 Hz, 1H, 3′-Ha), 5.76 (d, J = 4.0 Hz, 1H, 4-H), 6.76 (d, J = 9.3 Hz, 1H, 7-H), 7.07–7.17 (m, 4H, 3′′′-H, 5′′′-H, 2′′-H, 6′′-H), 7.17–7.24 (m, 3H, 3′′-H, 5′′-H, 6′′′-H), 7.33–7.42 (m, 2H, 4′′′-H, 4′′-H), 7.72 (d, J = 2.7 Hz, 1H, 10-H), 8.00 (dd, J = 9.3, 2.7 Hz, 1H, 8-H), 8.57 (t, J = 5.8 Hz, 1H, 2′-H). 13C-NMR (125 MHz, DMSO-d6) δ 32.73 (C-4a), 33.32 (C-10b), 38.51 (C-5′), 42.32 (C-3′), 49.52 (C-5), 50.61 (C-1), 55.60 (C-4′), 76.36 (C-4), 109.87 (C-7), 111.18 (C-3′′′), 120.03 (C-10a), 120.37 (C-5′′′), 123.92 (C-10), 125.19 (C-8), 126.63 (C-1′′′), 126.81 (C-4′′), 127.05 (C-2′′ and C-6′′), 128.06 (C-3′′ and C-5′′), 129.61 (C-4′′′), 135.24 (C-9), 138.41 (C-1′′), 150.06 (C-6a), 155.46 (C-2′′′), 166.82 (C-1′), 166.87 (C-2). IR: (KBr) ν: 2989, 1735, 1669, 1640, 1605, 1578, 1532, 1488, 1467, 1454, 1431, 1371, 1312, 1275, 1260, 1184, 1114, 1025. HRMS: calcd for C28H28N3O6 [M + H]+ 502.1978, found 502.1976.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) affording rac-(1S*,4R*,4aS*,10bS*)-dia2-5f as orange oil (24%), Rf = 0.19 (hexane/ethyl acetate 1
1) affording rac-(1S*,4R*,4aS*,10bS*)-dia2-5f as orange oil (24%), Rf = 0.19 (hexane/ethyl acetate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).1H-NMR (400 MHz, DMSO-d6) δ 2.62 (d, J = 4.6 Hz, 3H, 3′-H), 2.66 (dd, J = 8.6, 4.7 Hz, 1H, 4a-H), 3.04 (s, 3H, 4′-H), 3.32 (dd, J = 13.3, 8.6 Hz, 1H, 5-Hb), 3.58 (dd, J = 8.4, 4.6 Hz, 1H, 10b-H), 3.64 (dd, J = 13.2, 4.9 Hz, 1H, 5-Ha), 3.86–3.79 (m, 4H, 5′-H, 1-H), 5.72 (d, J = 4.9 Hz, 1H, 4-H), 6.74 (d, J = 9.4 Hz, 1H, 7-H), 7.04–7.10 (m, 2H, 3′-H, 5′-H), 7.37–7.43 (m, 2H, 4′-H, 6′-H), 7.70 (d, J = 2.6 Hz, 1H, 10-H), 7.97 (dd, J = 9.2, 2.6 Hz, 1H, 8-H), 8.11 (d, J = 4.6 Hz, 1H, 2′-H). 13C-NMR (100 MHz, DMSO-d6) δ 33.0 (C-4a), 33.7 (C-4′), 36.8 (C-10b), 49.6 (C-5), 50.8 (C-3′), 53.0 (C-1), 55.6 (C-5′), 76.1 (C-4), 109.6 (C-3′′), 109.7 (C-5′′), 110.0 (C-7), 111.1 (C-10), 120.0 (C-10a), 120.5 (C-8), 125.4 (C-4′′), 126.6 (C-1′′), 127.2 (C-6′′), 135.3 (C-9), 150.2 (C-6a), 155.6 (C-1′), 166.9 (C-2′′), 167.7 (C-2). IR: (KBr) ν: 2990, 1716, 1650, 1599, 1484, 1460, 1279, 1242, 1182, 1137, 1080. HRMS: calcd for C22H24N3O6 [M + H]+ 426.1665, found 426.1664.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) affording rac-(1S*,4R*,4aS*,10bS*)-dia2-5g as orange oil (37%), Rf = 0.12 (hexane/ethyl acetate 2
1) affording rac-(1S*,4R*,4aS*,10bS*)-dia2-5g as orange oil (37%), Rf = 0.12 (hexane/ethyl acetate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).1H-NMR (400 MHz, acetone-d6) δ 2.77 (dt, J = 9.4, 4.3 Hz, 1H, 4a-H), 3.15 (s, 3H, 7′-H), 3.54 (dd, J = 12.9, 9.6 Hz, 1H, 5-Hb), 3.75 (dd, J = 13.0, 5.3 Hz, 1H, 5-Ha), 3.80 (d, J = 2.6 Hz, 2H, 3′-Ha), 3.84 (ddd, J = 7.2, 3.6, 1.4 Hz, 1H, 3′-Hb), 3.88 (s, 3H, 7′-H), 3.89 (dd, J = 4.5, 1.6 Hz, 1H, 10b-H), 4.94–5.01 (m, 1H, 1-H), 5.08–5.15 (m, 1H, 5′-Hb), 5.73–5.82 (m, 1H, 5′-Ha), 5.83 (d, J = 3.9 Hz, 1H, 4′-H), 6.76 (d, J = 9.3 Hz, 1H, 4-H), 7.03–7.13 (m, 1H, 7-H), 7.38 (td, J = 8.1, 1.7 Hz, 1H, 5′′-H), 7.48 (dd, J = 7.5, 1.4 Hz, 1H, 3′-H), 7.79 (d, J = 2.7 Hz, 1H, 6′′-H), 7.95–8.01 (m, 2H, 10-H, 8-H). 13C-NMR (100 MHz, CDCl3) δ 32.7 (C-4a), 33.1 (C-1), 39.1 (C-6′), 42.7 (C-5), 50.6 (C-10b), 50.9 (C-3′), 55.6 (C-7′), 78.5 (C-4), 109.5 (C-7), 110.9 (C-3′), 117.0 (C-5′), 120.1 (C-1′), 121.1 (C-5′′), 124.1 (C-10), 125.5 (C-10a), 125.6 (C-8), 127.9 (C-6′′), 130.2 (C-4′′), 133.5 (C-4′), 137.1 (C-9), 149.9 (C-6a), 156.1 (C-2′′), 165.3 (C-1′), 168.2 (C-2). IR: (KBr) ν: 2920, 2849, 1745, 1682, 1648, 1600, 1525, 1499, 1469, 1430, 1355, 1317, 1304, 1276, 1260, 1189, 1101. HRMS: calcd for C24H26N3O6 [M + H]+ 452.1821, found 452.1825.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) affording rac-(4aS*,5R*,10bR*)-6p as pale-yellow oil (92%), Rf = 0.23 (chloroform/acetone 10
1) affording rac-(4aS*,5R*,10bR*)-6p as pale-yellow oil (92%), Rf = 0.23 (chloroform/acetone 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).1H NMR (400 MHz, CDCl3) δ 0.94–1.13 (m, 2H, 1-H), 1.70 (s, 3H, 2′-H), 1.71–1.82 (m, 2H, 4a-H), 1.88–2.00 (m, 4H, 2× 9-H), 2.04 (s, 3H, 2′-H), 2.21–2.28 (m, 1H, 10b-H), 2.28–2.36 (m, 1H, 10b-H), 2.36–2.56 (m, 8H, 2× 7-H, 2× 8-H), 2.57–2.67 (m, 1H, 1-Ha), 2.67–2.76 (m, 1H, 4-Hb), 2.89–3.04 (m, 2H, 4-Ha, 4-Hb), 3.09–3.23 (m, 2H, 2-Ha, 2-Hb), 3.78–3.91 (m, 1H, 2-Ha), 4.10–4.19 (m, 1H, 4-Ha), 4.59 (d, J = 10.3 Hz, 2H, 2× 5-H), 4.65–4.74 (m, 1H, 4-Ha), 7.29–7.36 and 7.39–7.49 (m, 2× 5 H, 2× Ph). 13C NMR (100 MHz, CDCl3) δ 20.3 and 21.2 (C-7), 21.7 and 29.0 (C-2′), 29.0 and 29.4 (C-1), 30.0 (C-8), 37.3 and 37.4 (C-10), 37.4 and 37.5 (C-9b), 42.0 and 43.2 (C-4), 43.7 and 45.6 (C-4a), 46.7 and 48.3 (C-2), 81.1 and 82.0 (C-5), 113.9 and 114.2 (C-9a), 126.9, 127.2, 129.1, 129.4 and 129.5 (2× Ph), 136.5 and 136.9 (C-1′′), 168.9 and 169.3 (C-1′), 172.0 and 172.5 (C-6a), 197.7 and 198.0 (C-10). IR: (KBr) ν: 2924, 2854, 1646, 1604, 1454, 1387, 1277, 1256, 1228, 1188, 1162, 1084. HRMS: calcd for C20H24NO3 [M + H]+ 326.1756, found 326.1758.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) affording rac-(4aS*,5R*,9bR*)-6q as pale-yellow oil (42%), Rf = 0.29 (chloroform/acetone 10
1) affording rac-(4aS*,5R*,9bR*)-6q as pale-yellow oil (42%), Rf = 0.29 (chloroform/acetone 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).1H NMR (400 MHz, CDCl3) δ 1.13–1.32 (m, 2H, 1-Ha, 1-Hb), 1.68–1.77 (m, 4H, 2′-H, 4a-H), 2.04 (s, 3H, 2′-H), 2.16–2.26 (m, 1H, 4-Hb), 2.41–2.54 (m, 6H, 2× 7-H, 2× 9b-H), 2.54–2.64 (m, 4H, 2× 8-H), 2.70–2.78 (m, 2H, 2-Ha, 1-Hb), 2.78–2.92 (m, 1H, 1-Ha), 3.03–3.13 (m, 1H, 2-Hb), 3.17–3.31 (m, 1H, 2-Hb), 3.84–3.95 (m, 1H, 2-Ha), 4.17–4.28 (m, 1H, 4-Ha), 4.69–4.80 (m, 1H, 4-Ha), 4.80–4.91 (m, 2H, 2× 5-H), 7.25–7.55 (m, 10H, 2× Ph). 13C NMR (100 MHz, CDCl3) δ 21.1 and 21.7 (C-2′), 26.1 and 26.2 (C-8), 27.4 and 28.1 (C-1), 33.8 and 36.0 (C-7), 36.1 (C-9b), 41.6 and 42.3 (C-4), 42.9 and 44.2 (C-4a), 46.5 and 47.9 (C-2), 83.5 and 84.4 (C-5), 115.8 and 116.1 (C-9a), 126.9, 127.2, 129.1, 129.2, 129.3, 129.6, 129.7, 136.1, 136.5 (2× Ph), 168.9 and 169.3 (C-1′), 184.0 and 184.5 (C-6a), 203.1 and 203.3 (C-9). IR: (KBr) ν: 2923, 1681, 1618, 1425, 1396, 1280, 1248, 1224, 1131, 1063. HRMS: calcd for C19H22NO3 [M + H]+ 312.1599, found 312.1602.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) affording rac-(6R*,6aS*,10aR*)-6r as pale yellow oil (37%), Rf = 0.53 (chloroform/ethyl acetate 10
1) affording rac-(6R*,6aS*,10aR*)-6r as pale yellow oil (37%), Rf = 0.53 (chloroform/ethyl acetate 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).1H NMR (400 MHz, CDCl3) δ 1.07–1.28 (m, 2H, 2× 10-Hb), 1.74–1.87 (m, 2H, 2× 10a-H), 1.94–2.10 (m, 4H, 2′-H and 7-Hb), 2.15–2.23 (m, 6H, 2′-H and 3′-H), 2.54–2.71 (m, 2H, 10-Ha and 7-Hb), 2.71–2.81 (m, 1H, 9-Hb), 3.06–3.18 (m, 1H, 9-Hb), 3.18–3.30 (m, 1H 9-Ha), 3.85–3.97 (m, 1H, 9-Ha), 4.16–4.26 (m, 1H, 7-Ha), 4.74 (d, J = 10.4 Hz, 2H, 2× -6-H), 5.78 (s, 1H, 4-H), 7.28–7.53 (m, 10H, 2× Ph). 13C NMR (100 MHz, CDCl3) δ 19.7 (C-2′), 21.0 and 21.5 (C-3′), 28.0 and 28.7 (C-10), 30.8 and 36.8 (C-10a), 41.7 (C-7), 42.8 (C-6a), 42.9 (C-7), 44.7 (C-6a), 46.4 and 47.9 (C-9), 81.2 and 82.0 (C-6), 99.6 (C-10b), 100.0 and 100.2 (C-4), 126.2, 126.8, 127.1, 128.5, 129.0, 129.1, 129.5, 129.6, 131.6, 136.0 and 136.3 (2× Ph), 161.0 (C-1), 163.3 and 165.7 (C-4a), 168.8 and 169.2 (C-1′). IR: (KBr) ν: 3063, 2922, 2172, 1705, 1648, 1572, 1496, 1446, 1407, 1360, 1283, 1230, 1144, 1034. HRMS: calcd for C20H22NO4 [M + H]+ 340.1548, found 340.1552.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) affording rac-(6R*,6aS*,10aR*)-6s as pale-yellow oil (16%), Rf = 0.07 (chloroform/ethyl acetate 5
1) affording rac-(6R*,6aS*,10aR*)-6s as pale-yellow oil (16%), Rf = 0.07 (chloroform/ethyl acetate 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).1H NMR (400 MHz, CDCl3) δ 1.50–1.60 (m, 1H, 10-Hb), 2.12 (s, 3H, 2′-H), 2.14–2.17 (m, 3H, 2′-H), 2.27–2.41 (m, 2H, 2× 10-Ha), 2.62 (t, J = 13.0 Hz, 1H, 7-Hb), 2.73–2.81 (m, 1H, 7-Ha), 3.03–3.10 (m, 2H, 2× 10a-H), 3.17 (t, J = 13.3 Hz, 1H, 7-Hb), 3.23 (s, 3H, 3′-H), 3.25–3.31 (m, 4H, 3′-H, 9-Hb), 3.31–3.36 (m, 7H, 2× 4′-H, 9-Ha), 3.42 (d, J = 14.0 Hz, 1H, 10-Hb), 3.85 (d, J = 13.5 Hz, 1H, 9-Hb), 4.27 (d, J = 14.2 Hz, 1H, 9-Ha), 4.68 (d, J = 13.3 Hz, 1H, 7-Ha), 5.18 (d, J = 11.2 Hz, 1H, 6-H), 5.26 (d, J = 11.0 Hz, 1H, 6-H), 7.27–7.56 (m, 10H, 2× Ph). 13C NMR (100 MHz, CDCl3) δ 21.0 and 21.7 (C-2′), 28.0 (C-4′), 28.3 (C-10), 28.8 (C-3′), 29.3 (C-10), 29.8 and 30.1 (C-10a), 37.5 and 38.1 (C-6a), 41.3 (C-7), 46.2 (C-9), 80.2 and 80.8 (C-6), 90.1 and 90.8 (C-10b), 127.0, 127.6, 128.1, 128.7, 129.4, 129.4, 130.3, 136.1 and 136.9 (2× Ph), 151.0 (C-3), 155.7 (C-10), 162.4 (C-4a), 169.2 and 169.8 (C-1′). IR: (KBr) ν: 2920, 1704, 1629, 1487, 1427, 1372, 1301, 1261, 1244, 1185. HRMS: calcd for C20H24N3O4 [M + H]+ 370.1766, found 370.1771.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) affording rac-(1R*,4aR*,8aS*)-6k as pale-yellow oil (63%), Rf = 0.49 (chloroform/ethyl acetate 10
4) affording rac-(1R*,4aR*,8aS*)-6k as pale-yellow oil (63%), Rf = 0.49 (chloroform/ethyl acetate 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4).
4).1H NMR (500 MHz, acetone-d6) δ 0.95–1.06 (m, 1H, 5-Hb), 1.09–1.20 (m, 1H, 5-Hb), 1.31 (t, J = 7.1 Hz, 6H, 2× 5′-H), 1.60–1.68 (m, 4H, 7′-H, 8a-H), 1.74–1.83 (m, 1H, 8a-H), 1.98 (s, 3H, 7′-H), 2.04–2.12 (m, 1H, 6-Hb), 2.13–2.17 (m, 3H, 1′-H), 2.20–2.32 (m, 1H, 6-Hb), 2.32–2.38 (m, 2H, 2× 5-Ha), 2.50–2.66 (m, 1H, 6-Ha), 2.85 (t, J = 12.4 Hz, 1H, 8-Hb), 3.07–3.15 (m, 1H, 8-Hb), 3.21 (d, J = 13.1 Hz, 1H, 8-Ha), 3.96 (d, J = 13.5 Hz, 1H, 8-Ha), 4.06–4.13 (m, 1H, 6-Ha), 4.16–4.28 (m, 4H, 2× 4′-H), 4.62 (d, J = 13.3 Hz, 1H, 6-Ha), 4.74–4.79 (m, 2H, 2× 1-H), 7.30–7.65 (m, 10H, 2× Ph). 13C NMR (125 MHz, acetone-d6) δ 14.7 and 19.7 (C-5′), 21.1 and 21.6 (C-7′), 30.6 and 31.3 (C-5), 39.3 (2 × C-4a), 42.0 and 43.3 (C-6), 44.4 and 45.6 (C-8a), 46.9 and 48.5 (C-8), 60.1 (C-4′), 80.8 and 81.4 (C-1), 106.5 and 106.7 (C-4), 128.0, 2× 129.4, 129.5, 129.6, and 2× 139.0 (2× Ph) 161.6 and 161.7 (C-3), 167.9 and 168.0 (C-2′), 168.5 and 168.9 (C-6′). IR: (KBr) ν: 3033, 2979, 2926, 2863, 1955, 1884, 1704, 1645, 1446, 1368, 1263, 1166, 1090, 1024. HRMS: calcd for C20H26NO4 [M + H]+ 344.1861, found 344.1860.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) affording rac-(1R*,4aR*,8aS*)-6i as pale-yellow oil (48%), Rf = 0.28 (chloroform/ethyl acetate 10
1) affording rac-(1R*,4aR*,8aS*)-6i as pale-yellow oil (48%), Rf = 0.28 (chloroform/ethyl acetate 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).1H NMR (400 MHz, CDCl3) δ 1.37–1.55 (m, 2H, 2× 5-Ha), 1.71–1.82 (m, 5H, 3′-H, 2× 9a-H), 2.06 (s, 3H, 3′-H), 2.13–2.23 (m, 1H, 8-Hb), 2.23–2.36 (m, 2H, 2× 5-Hb), 2.46–2.63 (m, 3H, 2× 4a-H, 8-Hb), 2.65–2.79 (m, 1H, 6-Hb), 3.02–3.16 (m, 1H, 6-Hb), 3.20–3.34 (m, 1H, 6-Ha), 3.89–4.04 (m, 1H, 8-Ha), 4.22–4.36 (m, 1H, 8-Ha), 4.87 (d, J = 10.3 Hz, 2H, 2× 1-H), 7.29–7.48 (m, 14H, 2× Ph), 7.75 (d, J = 7.7 Hz, 2H, 2× 4′-H). 13C NMR (100 MHz, CDCl3) δ 21.2 and 21.7 (C-3′), 29.7 and 30.5 (C-5), 38.2 (C-4a), 41.4 (C-8a), 41.7 (C-8), 42.7 (C-8a), 43.4 (C-8), 46.1 and 47.7 (C-6), 81.8 and 82.7 (C-1), 86.2 and 86.8 (C-4), 118.3 and 118.6 (C-1′′), 126.8, 127.1, 128.1, 128.4, 129.1, 129.2, 129.5, 129.6, 130.9, 131.0, 132.7, 132.8, 136.2 and 136.5 (4× Ph), 165.2 and 165.5 (C-3), 169.0 and 169.3 (C-2′). IR: (KBr) ν: 3055, 3037, 3016, 3002, 2961, 2906, 2863, 2192, 1642, 1606, 1448, 1425, 1359, 1267, 1227, 1152, 1028. HRMS: calcd for C23H23N2O2 [M + H]+ 359.1759, found 359.1755.
Conclusions
A styrene substrate, containing an N-(ortho-formyl)aryl subunit, was reacted with N-substituted 2-cyanoacetamides in domino Knoevenagel-cyclization reactions, which produced tetrahydro-4H-pyrano[3,4-c]quinolone and hexahydrobenzo[j] phenanthridine derivatives selectively by competing IMHDA and IMSDA cyclizations, respectively. We found that the N-substitution of the α,β-unsaturated amide heterodiene governed the mechanism of the cyclization step whether it took place with an oxa-IMHDA reaction with the involvement of the amide carbonyl or an IMSDA-rearomatization sequence using the carbon–carbon double bond as a dienophile.The diastereoselective IMHDA step of the domino reactions took place with α,β-unsaturated amide, thioamide, ester and ketone subunits of the Knoevenagel intermediate as a heterodiene and it produced condensed chiral tetrahydropyran or -thiopyran derivatives with versatile substitution pattern. In the case of Meldrum's acid, the IMHDA product was transformed further with amine nucleophiles to induce a multistep ring-opening and fragmentation sequence of the 1,3-dioxinone ring, resulting in lactone products. Competing IMHDA pathways with contribution of amide or ester carbonyls to the heterodiene subunit were identified in the domino reactions with N-(4-chlorophenyl)-3-oxobutanamide.
A truncated substrate, lacking the condensed benzene ring, was reacted with cyclic and acyclic active methylene reagents to get access to the tricyclic and bicyclic substituted hexahydro-1H-pyrano[3,4-c]pyridine derivatives of novel skeletons in diastereoselective Knoevenagel-IMHDA reactions through exo-Z-syn transition state of the IMHDA step.
One of products, obtained by Knoevenagel-IMHDA reaction with methyl (phenylsulfonyl)acetate reagent, showed in vitro antiproliferative activity against human glioblastoma cell line with an IC50 value of 46 μM.
Data availability
CCDC 2283893 for rac-(4R*,4aS*,10bS*)-2ai and 2401371 for rac-(4aS*,5R*,9bR*)-6p contain the supplementary crystallographic data for this paper. These data can be obtained free from http://www.ccdc.cam.ac.uk/data_request/cif, or by emailing E-mail: data_request@ccdc.cam.ac.uk, or by contacting the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033. The data supporting this article are included in the Experimental section and the ESI.†Author contributions
Conceptualization, A. P., S. B. K. and T. K.; methodology, M. K., S. B. K., L. B. H. and S. B.; software, A. B.; validation, M. K., A. K.-S., A. B.; formal analysis, M. K., A. K.-Á.; investigation, M. K. and S. B. K.; resources, T. K. and A. K.; data curation, M. K, S. B. K. and T. K.; writing – original draft preparation, M. K. and S. B. K.; writing – review and editing, A. P., A. K. and T. K.; visualization, T. K., M. K.; supervision, T. K.; project administration, A. P., S. B. K. and T. K.; funding acquisition, T. K. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the National Research Development and Innovation Office (Grant numbers K-138672, K-142904) and supported by the KDP-2021 Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund. S. B. and L. B. H. are grateful for the ELTE 2018-1.2.1-NKP-2018-00005 project under the 2018–1.2.1-NKP funding scheme. The purchase of the X-ray diffractometer was co-financed by the European Regional Development Fund under the project GINOP-2.3.3-15-2016-00004. The University of Debrecen Program for Scientific Publication supported the research.Notes and references
- L. Zeng, S. Liu, Y. Lan and L. Gao, Nat. Commun., 2023, 14(1), 3511 CrossRef CAS PubMed.
- (a) N.-K. Li, B.-B. Sun, J.-B. Chen, H.-D. Yang, B.-L. Wang, J.-Q. Yu, X.-W. Wang and Z. Wang, Org. Chem. Front., 2021, 8(9), 2009–2018 RSC; (b) Y. Zheng, S. Qian, P. Xu, T. Ma and S. Huang, Adv. Synth. Catal., 2022, 364(22), 3800–3804 CrossRef CAS; (c) L. Zeng, Q. Lei, W. Rao and L. Gao, Org. Lett., 2022, 24(11), 2115–2119 CrossRef CAS PubMed; (d) A. Yesilcimen, N.-C. Jiang, F. H. Gottlieb and M. Wasa, J. Am. Chem. Soc., 2022, 144(14), 6173–6179 CrossRef CAS PubMed.
- (a) J. Lauberteaux, A. Lebrun, A. van der Lee, M. Mauduit, R. Marcia de Figueiredo and J.-M. Campagne, Org. Lett., 2019, 21(24), 10007–10012 CrossRef CAS PubMed; (b) M. Jin, C. Tang, Y. Li, S. Yang, Y.-T. Yang, L. Peng, X.-N. Li, W. Zhang, Z. Zuo, F. Gagosz and L.-L. Wang, Nat. Commun., 2021, 12(1), 7188 CrossRef CAS PubMed; (c) L. Burchill, H. P. Pepper, C. J. Sumby and J. H. George, Org. Lett., 2019, 21(20), 8304–8307 CrossRef CAS PubMed.
- (a) A. Lozynskyi, V. Matiychuk, O. Karpenko, A. K. Gzella and R. Lesyk, Heterocycl. Commun., 2017, 23(1), 1–5 CrossRef CAS; (b) M. Kiamehr, B. Alipour, L. Mohammadkhani, B. Jafari and P. Langer, Tetrahedron, 2017, 73(21), 3040–3047 CrossRef CAS; (c) N. H. Metwally and E. A. El-Desoky, ACS Omega, 2023, 8(6), 5571–5592 CrossRef CAS PubMed.
- I. T. Barnish, C. W. G. Fishwick, D. R. Hill and C. Szantay, Tetrahedron Lett., 1989, 30(33), 4449–4452 CrossRef CAS.
- G. Mlostoń, K. Urbaniak, M. Jasiński, E.-U. Würthwein, H. Heimgartner, R. Zimmer and H.-U. Reissig, Chem.–Eur. J., 2020, 26(1), 237–248 CrossRef PubMed.
- (a) J. R. Dawson and J. M. Mellor, Tetrahedron Lett., 1995, 36(49), 9043–9046 CrossRef CAS; (b) S. Sun, I. J. Turchi, D. Xu and W. V. Murray, J. Org. Chem., 2000, 65(8), 2555–2559 CrossRef CAS PubMed; (c) P. Mpaata, C. R. Miller, D. K. Bonsrah, A. B. Camp, K. M. Ballard, L. Angelie, J. Kirkland, J. Joy, W. J. Hirschi, S. J. Smith, D. H. Ess and M. B. Andrus, J. Org. Chem., 2024, 89(6), 3883–3893 CrossRef CAS PubMed.
- D. I. Saavedra, B. D. Rencher, D.-H. Kwon, S. J. Smith, D. H. Ess and M. B. Andrus, J. Org. Chem., 2018, 83(4), 2018–2026 CrossRef CAS PubMed.
- D. J. Newman and G. M. Cragg, J. Nat. Prod., 2020, 83(3), 770–803 CrossRef CAS PubMed.
- (a) M. Bakthadoss and D. Kannan, RSC Adv., 2014, 4(23), 11723–11731 RSC; (b) Y.-H. Chen, D.-H. Li and Y.-K. Liu, ACS Omega, 2018, 3(12), 16615–16625 CrossRef CAS PubMed; (c) Y.-M. Fan, L.-J. Yu, M. G. Gardiner, M. L. Coote and M. S. Sherburn, Angew. Chem., Int. Ed., 2022, 61(39), e202204872 CrossRef CAS PubMed; (d) L. Wang, S. Li, X. Xiao, W. Xu, P. Zhang and Y. Ma, Adv. Synth. Catal., 2022, 364(4), 855–864 CrossRef CAS; (e) X. Lin, X. Liu, K. Wang, Q. Li, Y. Liu and C. Li, Nat. Commun., 2021, 12(1), 4958 CrossRef CAS PubMed.
- (a) S. B. Király, A. Bényei, E. Lisztes, T. Bíró, B. I. Tóth and T. Kurtán, Eur. J. Org Chem., 2021,(45), 6161–6170 CrossRef; (b) M. Kajtár, S. B. Király, A. Bényei, A. Kiss-Szikszai, A. Kónya-Ábrahám, N. Zhang, L. B. Horváth, S. Bősze, D. Li, A. Kotschy, A. Paczal and T. Kurtán, J. Org. Chem., 2024, 89(10), 6937–6950 Search PubMed; (c) S. Balázs Király, L. Tóth, T. Kovács, A. Bényei, E. Lisztes, B. István Tóth, T. Bíró, A. Kiss-Szikszai, K. E. Kövér, A. Mándi and T. Kurtán, Adv. Synth. Catal., 2023, 365(19), 3301–3319 CrossRef.
- (a) H. Sugiura, S. Yamazaki, K. Go and A. Ogawa, Eur. J. Org Chem., 2019, 2019(1), 204–220 CrossRef CAS; (b) S. Yamazaki, H. Sugiura, S. Ohashi, K. Ishizuka, R. Saimu, Y. Mikata and A. Ogawa, J. Org. Chem., 2016, 81(22), 10863–10886 CrossRef CAS PubMed.
- (a) S. Takano, T. Ohkawa, S. I. Tamori, S. Satoh and K. Ogasawara, J. Chem. Soc., Chem. Commun., 1988, 3, 189–191 RSC; (b) L.-F. Tietze, Angew. Chem., Int. Ed. Engl., 1983, 22(11), 828–841 CrossRef; (c) S. Takano, S. Satoh and K. Ogasawara, J. Chem. Soc., Chem. Commun., 1988, 1, 59–60 RSC.
- L. F. Tietze and Y. Zhou, Angew. Chem., Int. Ed., 1999, 38(13–14), 2045–2047 CrossRef CAS.
- L. F. Tietze, M. Bischoff, T. A. Khan and D. Liu, Chem. Heterocycl. Compd., 2017, 53(4), 434–445 CrossRef CAS.
- (a) S. Cheenpracha, T. Ritthiwigrom and S. Laphookhieo, J. Nat. Prod., 2013, 76(4), 723–726 CrossRef CAS PubMed; (b) T. Kok, H. Wapenaar, K. Wang, C. G. Neochoritis, T. Zarganes-Tzitzikas, G. Proietti, N. Eleftheriadis, K. Kurpiewska, J. Kalinowska-Tłuscik, R. H. Cool, G. J. Poelarends, A. Dömling and F. J. Dekker, Bioorg. Med. Chem., 2018, 26(5), 999–1005 CrossRef CAS PubMed; (c) M. Saraceno, A. Coi and A. A. Bianucci, Int. J. Biol. Macromol., 2008, 42(4), 362–371 CrossRef CAS PubMed; (d) S. Jiang, G. Borjigin, J. Sun, Q. Li, Q. Wang, Y. Mu, X. Shi, Q. Li, X. Wang, X. Song, Z. Wang and C. Yang, Int. J. Mol. Sci., 2023, 24(20), 15457 CrossRef CAS PubMed.
- S. Boros and G. Batta, Magn. Reson. Chem., 2016, 54(12), 947–952 CrossRef CAS PubMed.
- G. Sheldrick, Acta Crystallogr., Sect. A, 2008, 64(1), 112–122 CrossRef CAS PubMed.
- S. Westrip, J. Appl. Crystallogr., 2010, 43(4), 920–925 CrossRef CAS.
- C. F. Macrae, I. Sovago, S. J. Cottrell, P. T. A. Galek, P. McCabe, E. Pidcock, M. Platings, G. P. Shields, J. S. Stevens, M. Towler and P. A. Wood, J. Appl. Crystallogr., 2020, 53(1), 226–235 CrossRef CAS PubMed.
- A. Spek, J. Appl. Crystallogr., 2003, 36(1), 7–13 CrossRef CAS.
- (a) J. A. Schulz, L. T. Rodgers, R. J. Kryscio, A. M. S. Hartz and B. Bauer, BMC Cancer, 2022, 22(1), 844 CrossRef CAS PubMed; (b) J. Ponten and E. H. Macintyre, Acta Pathol. Microbiol. Scand., 1968, 74(4), 465–486 CrossRef CAS PubMed.
- (a) Y. Liu, D. A. Peterson, H. Kimura and D. Schubert, J. Neurochem., 1997, 69(2), 581–593 CrossRef CAS PubMed; (b) T. F. Slater, B. Sawyer and U. Sträuli, Biochim. Biophys. Acta, 1963, 77, 383–393 CrossRef CAS PubMed; (c) T. Mosmann, J. Immunol. Methods, 1983, 65(1), 55–63 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2283893 and 2401371. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ra08353a |
| This journal is © The Royal Society of Chemistry 2025 |