Open Access Article
Open Access ArticleSustainable antimicrobial formulations: vitamin-E based emulsions stabilized by plant-derived saponin from Acacia concinna†
Wasefa Begum a,
Rajlakshmi Laha
a,
Rajlakshmi Laha b,
Sk Mehebub Rahaman
b,
Sk Mehebub Rahaman a,
Monohar Hossain Mondal
a,
Monohar Hossain Mondal c,
Somasri Dam
c,
Somasri Dam *b,
Bidyut Saha
*b,
Bidyut Saha *a and
Ujjwal Mandal
*a and
Ujjwal Mandal *a
*a
aDepartment of Chemistry, The University of Burdwan, 713104, WB, India
bDepartment of Microbiology, The University of Burdwan, 713104, WB, India
cChemical Sciences Laboratory, Government General Degree College, Singur, Hooghly, 712409, WB, India. E-mail: umandal@chem.buruniv.ac.in; b_saha31@rediffmail.com; sdam@microbio.buruniv.ac.in
First published on 17th February 2025
Abstract
The present study reports the formulation, characterization and antimicrobial studies of a stable vitamin-E-based o/w emulsion with saponin extracted from Acacia concinna. Saponins are plant-based natural surfactants and emulsifiers exhibiting antimicrobial activities against different fungi and bacteria. By embracing the gentle and natural profile of saponins, we can harness their potential benefits to ensure safer and sustainable developments. Vitamin-E, also known as a tocopherol, is a fat-soluble antioxidant that protects cells against damage caused by different external factors, like pollution, free radicals and toxins. Its anti-inflammatory properties promote healing of the affected area by reducing redness, itching, swelling, irritation and discomfort. Keeping all these properties in mind, an emulsion was formulated using saponin and vitamin-E. The emulsion, characterized using different spectrochemical methods, demonstrated its enhanced stability and commendable ability. It was found to remain stable at neutral pH and up to 60 °C, making it suitable for topical applications. Antimicrobial study of the o/w emulsion (SE) showed specific and efficient antifungal activity against strains of Aspergillus flavus and Candida albicans. This natural, gentle, and antioxidant-rich emulsion offers a promising alternative for targeted antifungal treatments for skin, hair and nails, warranting further studies of its in vivo efficacy.
1. Introduction
In the current world scenario, escalating pollution and disruption to ecosystems have created conditions that make human beings more susceptible to infectious diseases. Environmental factors like the rise in global temperature and humidity not only facilitate the growth of harmful microorganisms but also interrupt the natural balance between host and microbes, leading to a rise in several infections.1,2 In addition, the COVID-19 pandemic has accelerated the risk of secondary infections as well as antimicrobial resistance (AMR) globally.3,4 The reasons behind this AMR crisis are mainly the overuse and misuse of antibiotics, the improper disposal of bio-medical waste, and poor infection control practices, as well as the lack of development of new antimicrobials.5 Additionally, altered hygiene practices during the pandemic have disrupted the delicate microbial balance of the body, leading to increased infections.6Overall, post-pandemic conditions have become more vulnerable for humans, thus requiring a new and sustainable approach with regard to more natural and gentle antimicrobial agents in place of conventional synthetic antibiotics. Although the process is time consuming, the crisis has prompted an urgent need for innovative antimicrobial strategies, which is prompting researchers to explore alternative sources of antimicrobial agents. In this respect, there are several plants which are precious resources for natural products with promising therapeutic properties, which may increase human wellbeing.7 Saponins, a complex group of glycosidic compounds, derived from various plant species, have a unique chemical structure, biological activity and significant potentiality for the required novel mechanism of action.8,9 Several reports have demonstrated the antimicrobial activity of saponins against a range of microorganisms.10–13
Basically, saponins are excellent surface-active agents (bio-surfactants) consisting of one or more hydrophilic sugar moieties covalently attached to a hydrophobic backbone. Their natural origin and biodegradability reduce their toxicity and negative impact on the environment, making them safer alternatives.14 Saponins have a widespread range of applications across various industries, such as food, cosmetics, pharmaceuticals, biotechnology, agriculture, and textiles. In cosmetics, saponins are used for their cleansing, foaming, and emulsifying properties, while in pharmaceuticals they improve solubility, bioavailability, and stability, and show other activities as well.15,16
In this present study, we separately investigate the antimicrobial activity of pure saponin extracted from Acacia concinna (Shikakai) and a saponin-based oil-in-water emulsion of vitamin-E, to achieve an inclusive interpretation. The aim is to develop a clear idea of the potentiality of saponin–vitamin-E (SE) emulsion in the formulation of more natural antimicrobial derma care products for cosmetic and therapeutic applications.
Recently, oil-in-water emulsions have become essential in the food supplement, pharmaceutical and cosmetic industries, owing to their promising capability and efficiency for delivering water-insoluble bio-actives, including drugs, natural antioxidants, essential nutrients, vitamins, polyunsaturated omega-3 (ω-3) oils and various steroids and carotenoids.17 Basically, in oil-in-water emulsion, small oil droplets are found to disperse throughout an aqueous medium. In most cases, they form a thermodynamically unstable system and are observed to break down in several ways, such as flocculation, coalescence, phase separation, and Ostwald ripening.18 Thus, a stabilizer, known as an emulsifier, is needed to increase their stability and improve their functional performance. In this case, surfactants play a promising role and, being amphiphilic, their presence in the emulsion results in the formation of a protective coating (Fig. 1) around the oil droplets, which prevents droplet aggregation by means of repulsive forces.19,20
 | ||
| Fig. 1 (a) Oil-in-water emulsion of saponin with vitamin-E; (b) structure of saponin moiety; (c) structure of vitamin-E. | ||
There are several reports regarding a variety of emulsifiers, such as synthetic surfactants or biopolymers, but increasing concern about wellbeing, environmental sustainability and safety are provoking consumers into using commercial products with more natural ingredients with the same or greater efficacy in place of their synthetic congeners.21 As synthetic emulsifiers may be harmful, expensive and irritant towards human health and the environment, the quest for natural alternatives, i.e., plant-based emulsifiers, has become the center of attention among researchers. Several research articles have reported that saponins from various sources act as potential natural emulsifiers with the ability to stabilize emulsions.22,23 Some of them are already commercially available for application in related industries like food or cosmetics.24,25
Compared to other emulsions, we have used water, vitamin-E oil and a natural surfactant saponin, which is indeed a sustainable approach towards the environment and human health. Shikakai saponin was found to be capable of forming the desired microdroplets and it remained stable under different environmental conditions, like elevated temperature and different pH values. The main stabilizing factor in this case is the electrostatic factor, which prevents droplet aggregation.26 Thus, all these facts support its utility in commercial products, like creams, lotions, shampoos and other topical products.27,28
In this study the use of vitamin-E as the oil phase is also a sustainable approach because of the enormous and miraculous functions of vitamin-E itself. Vitamin-E works as a great antioxidant as well as a potent calming agent for the skin. Its antioxidant properties neutralize harmful free radicals, reducing inflammation and oxidative stress, which in turn calm redness, itching, swelling, irritation and discomfort in affected skin.29 It also is known to boost immunity to prevent infection in the human body, thus playing a very crucial role in maintaining healthy skin, nails and hair. Its anti-inflammatory and antioxidant properties promote healing of the affected area and hence may potentiate the efficacy of the antifungal formulation.30
Gram-negative bacterial strains Pseudomonas aeruginosa and Escherichia coli, Gram-positive strains Staphylococcus aureus and Bacillus subtilis, and pathogenic fungi Aspergillus flavus and Candida albicans were used for screening the antimicrobial activity of pure saponin and saponin–vitamin-E emulsion. All these strains are pathogenic in nature, causing infections in the skin, respiratory tract, bloodstream, urinary tract, gastrointestinal tract, ears, eyes, central nervous systems, bones, joints etc.31,32
Aspergillus flavus and Candida albicans can have a significant impact on skin health, leading to a range of symptoms and complications. Aspergillus flavus can cause skin nodules, lesions, and scars as hair follicles are affected, leading to inflammation, redness and pus-filled bumps, while Candida albicans can cause candidiasis, fungal acne, eczema, and intertrigo. Treatment of such infections typically involves antifungal medications, such as topical creams and ointments, oral medication, and antifungal shampoo.33 Saponins are generally gentle, non-irritant and non-allergic to human skin, which makes them suitable for skin-related products for normal as well as sensitive skin.28
Modern consumers increasingly seek gentle, natural and functional ingredients in medicinal and cosmetic products. In this regard, it is worth mentioning the term ‘cosmeceuticals’, which refers to skincare products that merge the benefits of cosmetics with those of pharmaceuticals.34 In our work we have tried to produce a saponin–vitamin-E emulsion formulation, which has excellent antifungal activity along with the proven benefits of vitamin-E.
2. Materials and methods
2.1. Materials
The natural surfactant ‘saponin’ was extracted from fruit pericarps of soap-pod tree or shikakai (Acacia concinna). Detailed extraction purification and characterization studies were published in our previous article.16 Vitamin-E (98%) was obtained from SRL and sodium phosphate buffer from Sigma. All other chemicals used were of analytical grade (AR) and purchased from renowned chemical manufacturers with the highest available purity. The required solutions were prepared using double distilled water of conductivity grade.2.2. Instrumentation
The emulsion was prepared using a ‘Digital Ultrasonic MC-109 SPL’. A ‘JASCO FTIR-3500’ spectrometer was used for FTIR spectral study of extracted saponin and the saponin–vitamin-E emulsion. Optical micrographs were obtained using a ‘Weswox FM-2000’. The ζ-potential of the emulsion was determined using a Malvern Zetasizer Nano ZS series. The droplet size distribution was also studied with the same instrument.2.3. Preparation of saponin–vitamin-E emulsion
The preparation of the emulsion is a crucial process in various applications and the method for preparation is a vital step, which impacts the stability and property of the emulsion. In this study, the oil-in-water emulsion comprises two immiscible liquid phases: vitamin-E as the oil phase and an aqueous saponin solution as the aqueous phase, where saponin acts as the emulsifier and the emulsion was prepared by employing three processes: sonication, centrifugation and magnetic stirring. Although centrifugation and magnetic stirring are energy-efficient processes, the droplet diameter was found to be a minimum in the case of sonication.35,36An efficient emulsion was formulated by investigating different concentrations of aqueous saponin solutions with vitamin-E oil. Aqueous surfactant solutions were prepared, ranging from 0.06% (w/w) (0.6 mg mL−1, CMC of saponin) to 1% (w/w) of saponin. At CMC concentration, the formulated emulsion was found to be visibly unstable. The pH of the saponin solutions were adjusted to 7 using 10 mM of sodium phosphate buffer. An equal volume of vitamin-E was mixed with each 2 mL of aqueous saponin solution. The overall colloidal dispersion was then sonicated using the Digital Ultrasonic MC-109 SPL for 15 min followed by hand shaking for about 5 more min and then it was kept at rest for 15 min to produce stable emulsions.37
3. Characterization and stability testing of saponin–vitamin-E emulsion
The emulsion was characterized and the impacts of different environmental conditions that are supposed to affect emulsion stability were investigated. These results are very important to justify its utility for commercial purposes.3.1. Optical micrographs and droplet size distribution
The freshly prepared solutions were first kept at rest for 15 min. Optical images were then taken using a ‘Weswox FM-2000’ microscope with 100× magnification. The droplet sizes were calculated by treating the images in ImageJ.Ink software.The mean droplet diameter (d32) of emulsions with different saponin concentrations was determined at neutral pH 7 (Fig. 2). It is evident from the obtained plot that the particle size of the emulsions steadily decreased with saponin concentration increasing from 0.1% (w/w) to 0.5% (w/w); above this concentration the d32 values remained more or less constant. The result also matches those of other previously reported studies.38 This can be explained by the fact that at a fixed concentration of vitamin-E oil, the size of the droplets formed during emulsification is known to depend directly on saponin concentration. At lower saponin concentration, d32 gradually decreases as the emulsifier concentration increases, because the droplet diameter is limited by the total amount of surface area that can be covered. At higher concentration, the constant d32 value can be explained by the fact that there is sufficient emulsifier present to cover all the droplet surfaces; under such conditions, the droplet size depends mainly on the droplet distribution and mechanical design along with the pressure used.39 The microscopic analysis shown in Fig. 2 clearly depicts the presence of an improved dispersion of vitamin-E oil droplets over the aqueous phase. In the case of droplet size distribution, a uniform pattern is essential for emulsion stability, while polydispersity results in increased coalescence and reduced stability.40 This demonstrates the emulsification capacity of the extracted natural surfactant saponin. It is observed that the profile of the distribution is distinguished by three types of population: large, small and very small droplets. The droplet diameter ranges between 2 and 16 μm, with about 70% of the droplets having a diameter of 4–6 μm. The calculated mean droplet diameter ‘d32’ was found to be 5.54 μm. The system thus is of monomodal type with a colloidal micronized grade emulsion.26,40
 | ||
| Fig. 2 Variation in droplet diameter of prepared emulsions with increasing concentration of saponin after 15 min of standing (calculated mean droplet diameter ‘d32’ 5.54 μm). | ||
3.2. FTIR spectral analysis
FTIR spectral studies for both pure extracted saponin (Fig. 3A) and SE emulsion (Fig. 3B) were undertaken using the instrument mentioned earlier. Due to strong electrostatic interactions between vitamin-E oil and saponin, significant changes in the emulsion spectra can be observed. The strong and broad peak at 3320 cm−1 in the pure saponin spectrum (Fig. 3A), due to the presence of surface hydroxyl groups (–OH) in the saponin moiety, is missing from the emulsion spectrum (Fig. 3B), indicating the formation of a strong electrostatic bond between the vitamin and saponin using this –OH group. Additionally, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching frequency is found to have shifted from 1635 cm−1 (in pure saponin) to 1743 cm−1 in the emulsion, which suggests such a strong interaction. The frequency for the ether functional group (the oligosaccharide linked with the sapogenins) in pure saponin at 1064 cm−1 and pure vitamin-E at 1093 cm−1 was found to have shifted to 1161 cm−1 in the emulsion.16,41 The aliphatic C–H groups present in both vitamin-E and saponin, with characteristic peaks at 2924 cm−1 and 2854 cm−1 in their pure state, were found to be intact.16,42 The methyl bending peak of the vitamin-E moiety was also found to be intact in the emulsion spectrum at 1462 cm−1.41 All the above data from the FTIR spectral analysis are excellent evidence in favor of strong saponin–vitamin-E interaction and the formation of a stable emulsion.
O stretching frequency is found to have shifted from 1635 cm−1 (in pure saponin) to 1743 cm−1 in the emulsion, which suggests such a strong interaction. The frequency for the ether functional group (the oligosaccharide linked with the sapogenins) in pure saponin at 1064 cm−1 and pure vitamin-E at 1093 cm−1 was found to have shifted to 1161 cm−1 in the emulsion.16,41 The aliphatic C–H groups present in both vitamin-E and saponin, with characteristic peaks at 2924 cm−1 and 2854 cm−1 in their pure state, were found to be intact.16,42 The methyl bending peak of the vitamin-E moiety was also found to be intact in the emulsion spectrum at 1462 cm−1.41 All the above data from the FTIR spectral analysis are excellent evidence in favor of strong saponin–vitamin-E interaction and the formation of a stable emulsion.
3.3. Studies of emulsion stability
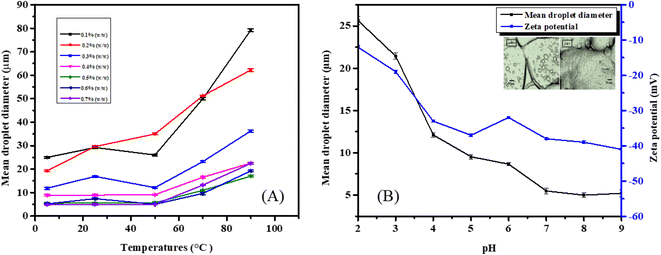
From the plot in Fig. 4A, it is evident that emulsions containing lower concentrations of natural emulsifier saponin (0.1–0.3% (w/w)) undergo coalescence, resulting increased droplet size, while emulsions with higher concentrations of saponin showed minimal change in droplet size. Eventually the emulsions containing 0.6% (w/w) and 0.7% (w/w) saponin also showed an impaired droplet size at elevated temperature. This can be explained by the fact that at higher temperature, the weak bonds between oil and emulsifier break down, leading to coalescence and a further increase in droplet diameter. Another notable finding is that the number of droplets is found to decrease with increasing temperature. The emulsion with saponin concentration of 0.5% (w/w) showed the best thermal stability over a varied temperature range. This is indeed an excellent property of the emulsion for its potential use in pharmaceuticals and the cosmetics industry.43,44
The ζ-potential value for the emulsion was found to be −12 mV at pH 2, while a high negative value of −41 mV was found at pH 9 (Fig. 4B). The highly negative ζ-potential value can be attributed to the presence of carboxylic acid groups in the saponin moiety. The carboxylic groups have pKa values around pH 3.5, where a very sharp decrease in ζ-potential value is observed. At higher pH values, the functional group remains negatively charged (R–COO−) while at acidic pH range it remains in protonated form (R–COOH).44 The presence of such a strong negative charge on the droplets plays an important role in determining the functional properties of the emulsion itself. The high negative surface charges enhance the electrostatic repulsion between droplets, preventing coalescence and aggregation, leading to stabilization of the emulsion as well as influencing the droplet size.38
The d32 values for the SE were found to have a maximum value at lower pH, which gradually decreased with decreasing H+ concentration (Fig. 4B). This factor can be explained by the fact that under strongly acidic conditions all the ionic sites of both saponin and vitamin-E were protonated, leading to electrostatic repulsion, which indeed increases the droplet size. Additionally, the absorption of H+ may contribute to the increased droplet diameter. At neutral pH, the strong electrostatic attraction between the surface –OH groups and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups comes into play, leading to a smaller droplet diameter; the same explanations can also be applied at higher pH ranges.38,45
O groups comes into play, leading to a smaller droplet diameter; the same explanations can also be applied at higher pH ranges.38,45
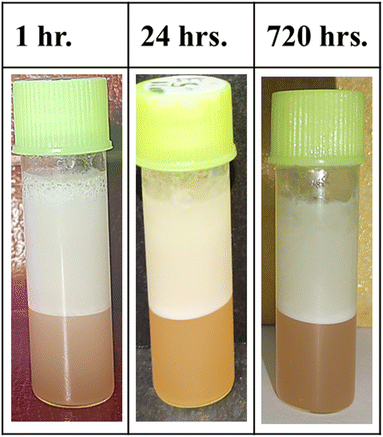
A good shelf-life for an emulsion is the most important and desirable characteristic for its potential utilization in commercial food, beverages, pharmaceuticals or cosmetic applications.26 We thus investigated the stability of the emulsion (0.5% w/w at pH 7) under both refrigerated and ambient conditions. The whole investigation was carried out for a long period of three months. Although no visible changes in either emulsion were observed, optical micrographs for both were taken at 15 days intervals. Interestingly both emulsions were found to show almost no change in droplet sizes up to 45 days, indicating the stability of their droplet growth (Fig. 5A and B).
 | ||
| Fig. 5 (A) Variation in mean droplet diameter over the time period of 90 days; (B) optical images of the SE emulsions over the time period of 90 days. | ||
After 60 days, the mean droplet diameter of the emulsion kept at ambient temperature started increasing by a very insignificant value. At 90 days the calculated d32 value implied its fairly stable nature. While the refrigerated emulsion showed almost no change in d32 value. The results show that the emulsion kept at ambient temperature is a little less stable than the one preserved in a refrigerator. A possible explanation for this phenomenon is that the saponin surfactant may have chemically degraded when stored at room temperature for such prolonged periods and thus its surface activity might have decreased.47,48
3.4. Determination of emulsifying properties
The emulsification ability of the o/w emulsion was studied after 24 h and 720 h, following the method established by Bugoz-Díaz et al.49,50 The emulsions were prepared using equal amounts of vitamin-E oil and saponin aqueous solutions of 0.5% w/w concentration. The emulsion was first prepared and kept standing for 1 h. The emulsification capacities (CE24 and CE720) and emulsion stability (SE) were calculated using established eqn (1) and (2):| CEx = (HE/HT) × 100 | (1) |
| SEx = (CEx/CE0) × 100 | (2) |
4. Study of antimicrobial activity
The antimicrobial efficacy of aqueous saponin solution and saponin–vitamin-E emulsion was investigated using the agar diffusion method, which is a widely accepted technique for evaluating antimicrobial activity against a panel of bacterial and fungal strains.13 The test microorganisms included Gram-negative bacterial strains Pseudomonas aeruginosa and E. coli, Gram-positive bacterial strains Staphylococcus aureus and Bacillus subtilis, and pathogenic fungal strains Aspergillus flavus and Candida albicans. Conventional broad-spectrum antibiotics streptomycin (100 μg mL−1) and amphotericin B (100 μg mL−1) served as positive controls for bacterial and fungal assays, respectively. The concentration of the aqueous saponin solution was 0.5% w/w, and a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 oil in the aqueous phase was used for the emulsion, where the concentration of the aqueous phase was also 0.5% w/w.
1 oil in the aqueous phase was used for the emulsion, where the concentration of the aqueous phase was also 0.5% w/w.
For the bacterial assay, 100 μL of log-phase culture inoculum was uniformly spread onto Mueller–Hinton agar plates (38 g L−1) using sterile cotton swabs. Subsequently, 3–4 mm diameter wells were created in the agar surface using a sterile cork borer and 50 μL of both aqueous saponin solution and saponin emulsion were added to each well. The plates were incubated at 37 °C for 24 h, with all experiments conducted in triplicate to ensure reliable results.
5. Results and discussion of antimicrobial studies
The saponin sample was found to show slight inhibitory activity against the Gram-negative bacterium Pseudomonas aeruginosa, with a zone of inhibition of 14 ± 0.15 mm (Fig. 6C), as shown in Table 2. However, it exhibited no inhibitory effect on the other three bacterial strains tested, i.e. Escherichia coli, Staphylococcus aureus, and Bacillus subtilis (Fig. S1, ESI†), while it showed significant antifungal activity, inhibiting the growth of both Aspergillus flavus and Candida albicans, as is evident from the notable inhibition zones of 14.9 ± 0.2 mm and 26 ± 0.15 mm, presented in Table 2 (Fig. 6A and B).| Bacterial strain(s)/fungal strain(s) | Volume of each sample given (μL) | Zone of inhibition (mm in diameter) for positive control | Zone of inhibition (mm in diameter) for aq. saponin solution |
|---|---|---|---|
| Pseudomonas aeruginosa | 50 | 19 ± 0.15 | 14 ± 0.15 |
| Staphylococcus aureus | 50 | 14.3 ± 0.1 | 0 |
| Bacillus subtilis | 50 | 18 ± 0.2 | 0 |
| Escherichia coli | 50 | 20.1 ± 0.1 | 0 |
| Aspergillus flavus | 50 | 6.2 ± 0.1 | 14.9 ± 0.2 |
| Candida albicans | 50 | 12 ± 0.11 | 26 ± 0.15 |
In the case of the emulsion, it exhibited potent antifungal properties, effectively inhibiting the growth of both Aspergillus flavus and Candida albicans, as indicated by substantial inhibition zones of 26.1 ± 0.2 mm and 18 ± 0.15 mm, as presented in Table 3 (Fig. 6D and E). Conversely, the sample failed to impede the growth of the four tested bacterial strains, Bacillus subtilis, Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus, as shown in Table 3 (Fig. S2, ESI†).
| Bacterial strain(s)/fungal strain(s) | Volume of each sample given (μL) | Zone of inhibition (mm in diameter) for positive control | Zone of inhibition (mm in diameter) for aq. SE emulsion |
|---|---|---|---|
| Aspergillus flavus | 50 | 13 ± 0.1 | 26.1 ± 0.2 |
| Candida albicans | 50 | 14.5 ± 0.11 | 18 ± 0.15 |
| Pseudomonas aeruginosa | 50 | 17 ± 0.15 | 0 |
| Staphylococcus aureus | 50 | 19 ± 0.1 | 0 |
| Bacillus subtilis | 50 | 20 ± 0.2 | 0 |
| Escherichia coli | 50 | 16 ± 0.1 | 0 |
Thus, both the saponin aqueous solution and saponin emulsion exhibited antifungal activity against pathogenic fungal strains Aspergillus flavus and Candida albicans. Due to the pure saponin aqueous solution showing poor activity against Pseudomonas aeruginosa with an insignificant zone of inhibition, the emulsion failed to show any activity against it. Hence, the saponin emulsion exhibited a selective inhibitory effect on the growth of two infective fungal strains, indicating its potential application in targeting specific fungal infections. In general, selective activity enables targeted therapy, minimizing harm to beneficial microorganisms and maintaining the delicate bacterial balance in the body, thus reducing side effects as well as improving the efficacy of the product. Additionally, this specificity can help combat antimicrobial resistance by targeting specific pathogens, decreasing the probability of resistance development. Thus, the saponin emulsion offers a natural, biodegradable and sustainable solution to synthetic antifungals, reducing their environmental impact.51,52
There are several studies where it has been reported that saponin inhibits fungal growth by rupturing the cell membrane by making it more permeable. This occurs due to the diverse structural pattern of saponins by which they are able to interact with fungal cell membrane sterols, forming complexes to generate pores, resulting in enhanced permeability to the cell and further leading to cell lysis.10,53 Additionally, the small particle size of the emulsion facilitates attachment to the microbial cell membrane. The ζ-potential data in this study addressed the potential negative charge on droplet surface, which also facilitates interaction with the fungal cell.54
6. Future prospects
The formulation of an antifungal cream utilizing an SE emulsion presents a promising approach to combating microbial infection and promoting skin health too. For the formulation of antifungal creams or ointments, a suitable cream base, such as an optimal mixture of emulsifiers, thickening agents, emollients and humectants, is essential for its performance as well as balanced moisturization.55 Here, saponin itself acts as the emulsifier in the formula with antifungal properties, while thickening agents such as xanthan gum or carboxy methyl cellulose can be used.56,57 The desired texture and stability of the formula can be achieved by adjusting the ratio of emulsifier and thickening agents.55 Further research is required to explore potential saponin-based creams by choosing appropriate thickening agents for the cream base, the optimal saponin concentration along with vitamin-E or other components for skin moisturization and product performance, and suitable preservatives for product conservation over a prolonged period.Incorporating the o/w emulsion into a nanotechnology-based targeted drug delivery system could enhance its efficacy in antifungal activity, addressing the growing need for effective nature-based treatments against microbial infections. Schreiner et al. investigated an improved topical formulation for the delivery of lipophilic vitamin-E, where small droplets of saponin–tocopherol nano-emulsion enhanced skin permeability.58
7. Conclusions
The ancient Indian Ayurvedic scriptures acknowledged the therapeutic properties of saponin-rich plants, demonstrating an early understanding of their significance. Their versatility makes them a precious asset in several industries, with ongoing research uncovering new applications and uses. By embracing the natural and gentle profile of saponins, we can harness their potential benefits for ensuring safer and more sustainable methods.7Shikakai (Acacia concinna) emerges as a compelling source for saponin extraction, distinguished by its unique combination of sustainability and practicality. The plant's natural abundance in specific Indian regions, coupled with its historical utilization in traditional Ayurvedic practices, provides a strong foundation for its suitability. Notably, Shikakai presents a cost-effective alternative to synthetic surfactants while upholding environmental principles through its renewable and biodegradable nature. These attributes collectively position Shikakai as a promising candidate for diverse applications, spanning personal care products to industrial processes.
Shikakai saponin was found to be capable of the formation of microdroplets with mean droplet diameter d32 of 5.54 μm, which remained stable under different conditions like elevated temperature (up to 60 °C) and neutral pH values. Droplet coalescence happened at highly acidic pH. Additionally, the highly negative ζ-potential value supports the formation of smaller droplets as well as higher emulsion stability. The shelf-life data address its potentiality for commercial utilization. Most importantly, the emulsion exhibited potential antifungal properties with significant inhibition zones of 26.1 ± 0.2 mm and 18 ± 0.15 mm for Aspergillus flavus and Candida albicans, respectively.
Extensive studies on the SE emulsion supported its exceptional stability and desirable emulsion characteristics, laying a strong foundation for its potential applications. Antimicrobial studies revealed significant activity against two infectious fungal strains, confirming the emulsion as an innovative formulation poised to make a substantial impact in natural remedies for various future applications in the pharmaceuticals and cosmetic industries. Its remarkable stability, unique structure and properties ensure its consistency and efficacy. Furthermore, the presence of vitamin-E adds an antioxidant property to the emulsion, making it an excellent candidate for dealing with fungus-affected skin and scalp issues by developing various formulations such as topical creams, ointments, and shampoos for treating fungal infections.
Abbreviations
| O/W | Oil in Water |
| AMR | Antimicrobial Resistance |
| CMC | Critical Micelle Concentration |
| NS | Natural Surfactant |
| SE Emulsion | Saponin-Vitamin-E Emulsion |
Data availability
The data supporting this article will be available on request from the authors.Author contributions
Wasefa Begum: conceptualization, methodology, investigation, formal analysis, data curation, writing – original draft, writing – review & editing, software, visualization; Rajlakshmi Laha: investigation, data curation, formal analysis, writing – original draft; Sk Mehebub Rahaman: investigation, formal analysis, software; Monohar Hossain Mondal: data curation, resources, writing – review & editing; Somasri Dam: data curation, resources, formal analysis, writing – review & editing, validation; Bidyut Saha: conceptualization, resources, formal analysis, supervision, writing – review & editing, validation; Ujjwal Mandal: conceptualization, data curation, resources, formal analysis, project administration, supervision, writing – review & editing, validation.Conflicts of interest
Authors wish to confirm that there are no known conflicts of interest associated with this publication.Acknowledgements
The authors acknowledge The University of Burdwan and Govt. General Degree College, Singur for providing infrastructural facilities. Dr Sujit Roy, Department of Botany, The University of Burdwan, is also acknowledged for his kind co-operation.References
- O. Uwishema, D. S. Masunga, K. M. Naisikye, F. G. Bhanji, A. J. Rapheal, R. Mbwana, A. Nazir and J. Wellington, Int. J. Surg., 2023, 109, 167–170 CrossRef PubMed.
- B. Das, B. Kumar, W. Begum, A. Bhattarai, M. H. Mondal and B. Saha, Chem. Afr., 2022, 5, 459–480 CrossRef CAS.
- A. S. Suleiman, M. A. Islam, M. S. Akter, M. R. Amin, A. A. Werkneh and P. Bhattacharya, J. Infect. Public Health, 2023, 16, 1562–1590 CrossRef PubMed.
- S. Rehman, J. Infect. Public Health, 2023, 16, 611–617 CrossRef PubMed.
- J. Rodríguez-Baño, G. M. Rossolini, C. Schultsz, E. Tacconelli, S. Murthy, N. Ohmagari, A. Holmes, T. Bachmann, H. Goossens, R. Canton, A. P. Roberts, B. Henriques-Normark, C. J. Clancy, B. Huttner, P. Fagerstedt, S. Lahiri, C. Kaushic, S. J. Hoffman, M. Warren, G. Zoubiane, S. Essack, R. Laxminarayan and L. Plant, J. Global Antimicrob. Resist., 2021, 25, 5–7 CrossRef PubMed.
- X.-E. Zhang, P. Zheng, S.-Z. Ye, X. Ma, E. Liu, Y.-B. Pang, Q.-Y. He, Y.-X. Zhang, W.-Q. Li, J.-H. Zeng and J. Guo, J. Inflamm. Res., 2024, 17, 1057–1082 CrossRef CAS PubMed.
- U. Bildziukevich, M. Wimmerová and Z. Wimmer, Pharmaceuticals, 2023, 16, 386 CrossRef CAS PubMed.
- M. H. Mondal, S. Malik and B. Saha, Tenside Surfactants Deterg., 2017, 54, 378–384 CrossRef CAS.
- M. H. Mondal, S. Malik, A. Garain, S. Mandal and B. Saha, Tenside Surfactants Deterg., 2017, 54, 519–529 CAS.
- Z. Yu, X. Wu and J. He, Eur. Food Res. Technol., 2022, 248, 783–795 CrossRef CAS.
- M. Jarzębski, P. Siejak, W. Smułek, F. Fathordoobady, Y. Guo, J. Pawlicz, T. Trzeciak, P. Ł. Kowalczewski, D. D. Kitts, A. Singh and A. Pratap Singh, Molecules, 2020, 25, 2696 CrossRef PubMed.
- X. Gao, J. Tang, J. Zhao, Y. Zhang, L. Zhu, Z. Cai, X. Zuo, M. Chen, J. Wang and Y. Shen, J. Dispersion Sci. Technol., 2024, 1–11 Search PubMed.
- P. Sharma, V. Singh, S. K. Maurya, M. A. Kamal and N. K. Poddar, Curr. Bioact. Compd., 2021, 17, 59–68 CrossRef.
- Surfactants from Renewable Resources, ed. M. Kjellin and I. Johansson, Wiley, 2010 Search PubMed.
- M. H. Mondal, W. Begum, A. Bhattarai, D. Kumar, B. Singh and B. Saha, in Applications of Next Generation Biosurfactants in the Food Sector, Elsevier, 2023, pp. 57–89 Search PubMed.
- W. Begum, B. Saha and U. Mandal, Chem. Afr., 2024, 7, 2539–2552 CrossRef CAS.
- J. Teixé-Roig, G. Oms-Oliu, I. Odriozola-Serrano and O. Martín-Belloso, Foods, 2023, 12, 1502 CrossRef PubMed.
- X. Li, K. Li, Y. Shen, F. Niu and Y. Fu, Colloids Surf., A, 2016, 504, 442–448 CrossRef CAS.
- S. M. Rahaman, N. Khatun, P. Pal, T. Mandal, A. Patra, M. Nandi and B. Saha, Nanoscale Adv., 2024, 6, 1688–1703 RSC.
- T. Mandal, S. M. Rahaman, B. Saha, N. Khatun, A. Patra, A. Mukherjee, M. Nandi, D. Dhak, S. Roy and B. Saha, New J. Chem., 2024, 48, 10112–10125 RSC.
- N. Tamang, P. Shrestha, B. Khadka, M. H. Mondal, B. Saha and A. Bhattarai, Polymers, 2021, 14, 127 CrossRef PubMed.
- P. Yatham, Y. Dahat, A. Khan, R. Baishya, A. K. Srivastava and D. Kumar, in Nanopharmaceutical Advanced Delivery Systems, Wiley, 2021, pp. 217–235 Search PubMed.
- W. Smułek, A. Makiej, M. Jarzębski, A. Zdarta, M. Jeszka-Skowron, F. Ciesielczyk, T. Jesionowski, J. Zdarta and E. Kaczorek, Rev. Adv. Mater. Sci., 2023, 62, 20220337 Search PubMed.
- M. Jarzębski, W. Smułek, Y. Umutoniwase, S. Niyobuhungiro, S. Shirodkar, P. O. Huomachi, J. Perła-Kaján, A. Szwajca and K. Pal, Food Hydrocolloids, 2024, 156, 110352 CrossRef.
- I. Dammak, P. J. do A. Sobral, A. Aquino, M. A. das Neves and C. A. Conte-Junior, Compr. Rev. Food Sci. Food Saf., 2020, 19, 2721–2746 CrossRef PubMed.
- T. B. Schreiner, M. M. Dias, M. F. Barreiro and S. P. Pinho, J. Agric. Food Chem., 2022, 70, 6573–6590 CrossRef CAS PubMed.
- K. G. O. Bezerra, H. M. Meira, B. O. Veras, T. C. M. Stamford, E. L. Fernandes, A. Converti, R. D. Rufino and L. A. Sarubbo, Processes, 2023, 11, 879 CrossRef CAS.
- A. Jolly, H. Kim, J.-Y. Moon, A. Mohan and Y.-C. Lee, Ind. Crops Prod., 2023, 205, 117489 CrossRef CAS.
- J. Chen, Y. Liu, Z. Zhao and J. Qiu, Int. J. Cosmet. Sci., 2021, 43, 495–509 CrossRef CAS PubMed.
- C. Aparecida Sales de Oliveira Pinto, T. Elyan Azevedo Martins, R. Miliani Martinez, T. Batello Freire, M. Valéria Robles Velasco and A. Rolim Baby, 2021.
- C. A. Roberts and J. E. Buikstra, Bacterial Infections, in Ortner's Identification of Pathological Conditions in Human Skeletal Remains, Elsevier, 2019, pp. 321–439 Search PubMed.
- S. Doron and S. L. Gorbach, in International Encyclopedia of Public Health, Elsevier, 2008, pp. 273–282 Search PubMed.
- A. Michalczyk and P. Ostrowska, J. Med. Mycol., 2021, 31, 101118 CrossRef PubMed.
- A. Agrawal, B. Ahirwar and K. Agrawal, in Specialized Plant Metabolites as Cosmeceuticals, Elsevier, 2024, pp. 191–220 Search PubMed.
- S. Qamar, B. Bhandari and S. Prakash, Food Res. Int., 2019, 116, 1374–1385 CrossRef CAS PubMed.
- L. Zhou, W. Zhang, J. Wang, R. Zhang and J. Zhang, Ultrason. Sonochem., 2022, 82, 105885 CrossRef CAS PubMed.
- S. Zhang, L. Tian, J. Yi, Z. Zhu, E. A. Decker and D. J. McClements, Food Hydrocolloids, 2020, 109, 106136 CrossRef CAS.
- X. Xu, Q. Sun and D. J. McClements, Food Hydrocolloids, 2019, 89, 396–405 CrossRef CAS.
- Z. Zhu, Y. Wen, J. Yi, Y. Cao, F. Liu and D. J. McClements, J. Colloid Interface Sci., 2019, 536, 80–87 CrossRef CAS PubMed.
- M. Deng, H. Chen, L. Xie, K. Liu, X. Zhang and X. Li, LWT--Food Sci. Technol., 2022, 156, 113042 CrossRef CAS.
- W. Pang, J. Wu, Q. Zhang and G. Li, RSC Adv., 2017, 7, 55536–55546 RSC.
- J. Kiefer, K. Frank, F. Zehentbauer and H. Schuchmann, Biosensors, 2016, 6, 13 CrossRef PubMed.
- H. Salminen, S. Bischoff and J. Weiss, J. Food Sci., 2020, 85, 1213–1222 CrossRef CAS PubMed.
- T. Ralla, H. Salminen, J. Tuosto and J. Weiss, Int. J. Food Sci. Technol., 2018, 53, 1381–1388 CrossRef CAS.
- Y. Ma, Y. Gao, X. Zhao, Y. Zhu, F. Du and J. Hu, Chem. - Eur. J., 2018, 24, 11703–11710 CrossRef CAS PubMed.
- S. M. Dahlawi, W. Nazir, R. Iqbal, W. Asghar and N. Khalid, RSC Adv., 2020, 10, 39700–39707 RSC.
- N. Taarji, C. A. Rabelo da Silva, N. Khalid, C. Gadhi, A. Hafidi, I. Kobayashi, M. A. Neves, H. Isoda and M. Nakajima, Food Chem., 2018, 246, 457–463 CrossRef CAS PubMed.
- Y. Yang, M. E. Leser, A. A. Sher and D. J. McClements, Food Hydrocolloids, 2013, 30, 589–596 CrossRef CAS.
- C. Burgos-Díaz, R. Pons, M. J. Espuny, F. J. Aranda, J. A. Teruel, A. Manresa, A. Ortiz and A. M. Marqués, J. Colloid Interface Sci., 2011, 361, 195–204 CrossRef PubMed.
- C. Burgos-Díaz, T. Wandersleben, M. Olivos, N. Lichtin, M. Bustamante and C. Solans, Food Hydrocolloids, 2019, 87, 847–857 CrossRef.
- Y. Huang, X. Guo, Y. Wu, X. Chen, L. Feng, N. Xie and G. Shen, Signal Transduct. Targeted Ther., 2024, 9, 34 CrossRef PubMed.
- A. N. Konwar, S. N. Hazarika, P. Bharadwaj and D. Thakur, Curr. Microbiol., 2022, 79, 330 CrossRef CAS PubMed.
- M. Wei, H. Yu, Y. Guo, Y. Cheng, Y. Xie and W. Yao, Food Control, 2021, 130, 108337 CrossRef CAS.
- S. E. Herrera-Rodríguez, R. J. López-Rivera, E. García-Márquez, M. Estarrón-Espinosa and H. Espinosa-Andrews, Food Sci. Biotechnol., 2019, 28, 441–448 CrossRef PubMed.
- A. Stolić-Jovanović, M. Martinović and I. Nešić, Acta Fac. Med. Naissensis, 2022, 39, 57–65 CrossRef.
- V. Krstonošić, L. Dokić, I. Nikolić and M. Milanović, Food Hydrocolloids, 2015, 45, 9–17 CrossRef.
- C. Arancibia, S. Bayarri and E. Costell, Food Biophys., 2013, 8, 122–136 CrossRef.
- T. B. Schreiner, A. Santamaria-Echart, G. Colucci, P. Plasencia, P. Santos Costa, M. M. Dias, S. P. Pinho and M. Filomena Barreiro, J. Mol. Liq., 2023, 391, 123371 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra08297d |
| This journal is © The Royal Society of Chemistry 2025 |