 Open Access Article
Open Access ArticleAqueous two-phase system (ATPS): from basic science to applications
Xunan Zhangab,
Mingxue Hana,
Shuang Hana and
Wei Zong*ab
aCollege of Chemistry and Chemical Engineering, Qiqihar University, Qiqihar, 161006, China. E-mail: 03007@qqhru.edu.cn
bHeilongjiang Industrial Hemp Processing Technology Innovation Center, Qiqihar University, Qiqihar 161006, China
First published on 25th March 2025
Abstract
Aqueous two-phase system (ATPS) epitomize a remarkable phenomenon where two immiscible phases manifest through the amalgamation of at least two water-soluble components at precise concentrations. Revered as an economically sustainable and environmentally harmonious technique for liquid–liquid separation, this method boasts extensive utility in the isolation and refinement of biomolecules, owing to its innate simplicity, cost-effectiveness, and compatibility with biological systems. The principal aim of this article is to provide a comprehensive overview of the fundamental principles governing phase formation in ATPS. It endeavors to elucidate the influential factors dictating this phenomenon and expound upon the construction of phase diagrams, which serve as pivotal tools in comprehending and manipulating ATPS behavior. Furthermore, the article delves into the diverse domains that reap benefits from ATPS applications. These encompass, yet transcend, the extraction of metal ions, elimination of pharmaceutical residues, environmental restoration, strides in biomedical sciences, and the recovery of dyes. In this pursuit, it strives to illuminate the manifold strengths and limitations of ATPS, offering a holistic understanding of its potential and existing challenges.
1. Introduction
The concept of environmental conservation has garnered substantial global attention, with the chemical processing industry assuming a crucial role in safeguarding the environment. In this context, the adoption of environmentally friendly techniques and the utilization of green solvents hold paramount importance in separation and extraction processes. These innovative approaches enable the isolation and purification of biomolecules while preserving their inherent biological activity. Contemporary separation technologies predominantly encompass chromatographic separation, capillary electrophoresis, membrane filtration, and precipitation. Undoubtedly, these techniques offer significant technical advantages. However, they are not exempt from notable challenges, including exorbitant investment costs, intricate production processes, maintenance complexities associated with production lines, and the thermal degradation of thermally unstable compounds.1 In comparison to the extraction processes employed in the aforementioned techniques, liquid–liquid extraction demonstrates certain distinct advantages. Liquid–liquid extraction is a widely employed technique for separation and extraction,2 which has found use not only in the pharmaceutical and chemical industries but also in various other fields, particularly for handling unstable substances.3 This method is characterized by its high selectivity, broad applicability, ease of operation, and excellent reproducibility. However, the inherent use of organic solvents during the liquid–liquid extraction process presents challenges, including increased costs and potential hazards to both environmental and human health.4 In this context, the use of an aqueous two-phase system (ATPS) represents a significant advancement in liquid–liquid extraction techniques, holding considerable promise for environmental protection.5,6 Its efficient separation and extraction capabilities can play a pivotal role in enhancing water quality, promoting resource recovery, and advancing sustainable development initiatives.ATPS arises from the incompatibility between combinations of polymers, salts, and surfactants when their concentrations surpass certain thresholds in water.7 The concept of biphasic systems first emerged in 1896 when a Dutch microbiologist discovered that aqueous solutions of gelatin and agar or starch could form ATPS.8 However, it was not until 1955 that Albertsson successfully mixed hydroxyapatite precipitate with an aqueous polyethylene glycol solution, resulting in a clear biphasic system in which chloroplasts were extracted into the polyethylene glycol phase.9 When molecules or particles are introduced into a polymer system, they disperse themselves automatically in different proportions within the two phases, without inducing inherent phase separation. Upon reaching equilibrium, these substances distribute themselves based on their affinity for a particular phase. This pioneering application of the biphasic system in cell particle separation experiments demonstrated that biphasic separation is a surface-dependent method and can complement centrifugation techniques.10–12 The utilization of ATPS has garnered significant attention and has found applications in various fields such as biochemistry, molecular biology, cell biology, and biotechnology.13–15 The timeline of the discovery, development, and application of ATPS since the beginning of the 21st century is depicted in Fig. 1. The use of ATPS can be traced back to the 1950s when they were initially employed for the purification of biological materials. Since then, there have been significant advancements in characterizing and applying ATPS, leading to the development of numerous innovative technologies. ATPS has played a significant role in the separation of biomolecules, including proteins, antibodies, viruses, antibiotics, cells, DNA, and hormones.16 From a research perspective, the technological advancements and emerging applications of ATPS hold immense significance and offer potential for future commercialization.17 However, challenges still exist, including an incomplete understanding of the separation mechanisms in ATPS, concerns regarding operational costs in industrial processes, limited predictive models, and difficulties in scaling up the engineering recovery of desired substances.18 These difficulties need to be gradually overcome by scientific means in order to promote the development of the research field of ATPS.
 | ||
| Fig. 1 The evolution of ATPS-based technologies.13 | ||
This review endeavors to illuminate the profound significance of ATPS within the realm of chemistry. It embarks upon an exploration of the intricate nuances involved in the construction of such ATPS, encompassing the elucidation of phase diagrams, the classification of biphasic types, and an examination of the factors that exert influence over their formation. Subsequently, the discourse assumes a comprehensive nature, delving into a multifaceted investigation of the manifold applications of ATPS across diverse domains. These applications encompass the detection and quantification of metal ions and pollutants, the purification and separation of proteins and enzymes, the realm of environmental remediation, and even extend to the realm of fabricating artificial cells. Through this comprehensive analysis, a deeper understanding of the potential of ATPS emerges, open up new avenues of scientific exploration and technological innovation.
2. Properties of ATPS
ATPS represents a prevalent modality in liquid–liquid extraction technology that arises from the fusion of water-soluble polymers, aqueous solutions, and salts or dissimilar water-soluble polymers possessing distinct chemical structures. This amalgamation gives birth to a system imbued with remarkable attributes, namely biocompatibility, versatility, scalability, operational simplicity, environmental friendliness, cost-effectiveness, and temporal efficiency. Within the realm of ATPS, the equilibrium distribution of targeted products can be deftly regulated by manipulating various factors, including but not limited to the phase volume ratio, temperature, pH and other influential elements. In contrast, ATPS extraction not only serves as a means of purification and separation but also transcends the limitations inherent in traditional organic liquid–liquid extraction methodologies. It endows researchers and practitioners with unparalleled effectiveness, economic viability, operational simplicity, and an absence of deleterious pollution. Consequently, ATPS extraction has emerged as a transformative force, making substantial contributions across a diverse array of domains, ranging from wastewater treatment, soil enhancement, and food production to pharmaceuticals, agriculture, textile dyes, and metal processing.19,20In ATPS, the separation objectives are achieved through the selective distribution of substances within the system.21 Fig. 2a depicts a schematic diagram of the phase separation process.22 When polymers are mixed with salts in specific proportions, the target substances, influenced by surface charge interactions, intermolecular forces, or environmental factors, undergo selective distribution between the upper and lower phases, facilitating the separation of materials.23 The distribution of target molecules between the two phases follows Nernst's law, represented by the equation as below.
| K = Ct/Cb | (1) |
2.1 Phase diagram
The construction of ATPS is typically achieved through turbidity titration, a method that involves mixing solutions of two substances at specific concentrations in predetermined ratios.25 The mixed solution is vigorously stirred and maintained at a constant temperature until it becomes turbid. This process is repeated, and by calculating the composition of each substance, the mixed solution is diluted below the turbidity point by adding a certain amount of deionized water. By repeating these steps, the phase diagram of the ATPS can be plotted successfully.26 Fig. 2b provides a schematic diagram of the ATPS, illustrating the content and phase volumes of the two components.24 The vertical axis represents the upper phase-enriched solution, while the horizontal axis represents the lower phase-aggregated solution. The boundary that connects the two-phase regions is known as the binodal curve, which divides the entire space into two regions.27 Above the binodal curve, the concentration of the two-phase components can form a distinct biphasic region. In the lower-left region, the concentration of the two-phase components can form a homogeneous solution, known as the monophasic region. The numerical values at points P, T, and B represent the initial phase concentrations of the two substances, the upper phase concentration at equilibrium, and the lower phase concentration at equilibrium, respectively. When point P falls within the region above the binodal curve, a well-defined interface between the two phases spontaneously forms. When point P coincides with the TB line, the system reaches equilibrium, and the TPB line represents the tie line. Each component is associated with a tie line length (TLL), which is a thermodynamic parameter reflecting the differences in thermodynamic properties of the ATPS phase under constant pressure and temperature. TLL provides a parameter for determining the overall composition of the ATPS and its impact on the extraction process. The TLL can be calculated using eqn (2). As point P moves towards the lower-left direction, the tie line gradually shortens, leading to a decrease in the phase difference between the two phases. When point P precisely falls on point C (the minimum phase concentration), the length of the tie line becomes zero, and the entire system reverts to a monophasic state. Each tie line is parallel, and the intersection point C, connecting the midpoint of the tie lines and the binodal curve, represents the critical point of the biphasic system.8| TLL = [(Ct1 − Cb1)2 + (Ct2 − Cb2)2]1/2 | (2) |
When the ATPS reaches equilibrium, the slope of the tie line (STL) is defined as per eqn (3):
 | (3) |
Among these, Ct1 represents the concentration of the substance in the upper phase, Ct2 signifies the slight concentration of the lower phase substance in the upper phase, Cb1 denotes the slight concentration of the lower phase substance in the lower phase, and Cb2 represents the concentration of the substance in the lower phase.
The TLL functions as a visual indicator of the incongruity among the constituents within ATPS. It is frequently employed to depict the distribution patterns between the two phases. As the initial concentration of the constituents progressively diverges from the critical point, the TLL elongates, denoting heightened disparities between the two phases and a more conspicuous inclination towards distinct phase formation. Conversely, the gradient of the STL is frequently utilized in experimental inquiries as an ancillary instrument for tie line representation.
2.2 Categorization of ATPS
The categories of ATPS primarily encompass polymer–polymer ATPS, polymer–salt ATPS, ionic liquid–salt ATPS, small molecule organic solvent–salt ATPS and polyelectrolyte complexes–polyelectrolyte complexes/polymer/salt/surfactant ATPS et al.28–33 Among them, polymer–polymer ATPS frequently feature combinations such as polyethylene glycol (PEG)/polymer, dextran (DEX)/polymer, and polypropylene glycol (PPG)/polymer. Polymer–salt ATPS commonly involve combinations like PEG/salt, PPG/salt, and potassium phosphate/polymer. Furthermore, in recent years, there has been a surge in attention and exploration of innovative materials, including amino acids and hydrogels, for the construction of ATPS, employing them as fundamental constituents.34,35Typically, the phase demarcation in polymer/polymer ATPS arises from the discrepant hydrophobicity between the polymers or the hindered ability to form a homogeneous single phase due to steric hindrance, resulting in the stable coexistence of two distinct phases. The underlying principle behind the formation of polymer/salt ATPS primarily involves the salting-out effect of salts, whereby the capacity of salts with identical anionic (or cationic) species to induce phase separation is contingent upon factors such as the valence of the cation (or anion) and the Gibbs free energy.36 Ionic liquids, renowned for their stability and well-defined physicochemical properties, are often employed in conjunction with salts to construct ATPS, and the mechanism of phase separation in such systems is intimately linked to the salting-out effect.37 Regarding the phase separation mechanism of small molecule organic solvent/salt ATPS, it is relatively intricate when compared to other systems. The phase demarcation in these systems is influenced by variables such as pH value, temperature, and the interplay between the organic solvent and the salt in their competition for water molecules. In comparison to alternative separation techniques, ATPS offer a host of advantages, including simpler phase compositions, higher water content, swifter mass transfer rates, and ease of implementation on an industrial scale.
2.3 The primary factors influencing the equilibrium of ATPS
Within ATPS, the constituents of the two phases manifest distinct levels of hydrophilicity and reduced interfacial tension. As a result, the equilibrium of the system is prone to the influence of multiple factors, encompassing polymer molecular weight, polymer hydrophobicity, type of salt employed, salt concentration, temperature, and pH of the system.Presently, numerous researchers have extensively investigated the influence of polymers on the phase behavior and distribution characteristics within ATPS. As the relative molecular weight of the polymer increases, the available free volume within the high phase diminishes, resulting in the preferential allocation of biomolecules to the phase with lower salt concentration, thereby leading to a decrease in the distribution coefficient. Notably, when similar concentration salts are mixed with higher relative molecular weight PEG, the region of two-phase formation expands. In an experimental study conducted under pH = 4.70 and temperature conditions of 298.15 K, PEG samples with molecular weights of 2000, 3000, 4000, 6000, and 8000 were employed in conjunction with zinc sulfate to construct ATPS. The findings of this study demonstrated that the increase in PEG molecular weight directly influences the extent of the two-phase region, facilitating the formation of well-defined ATPS and expanding the area of liquid–liquid biphasic separation. Moreover, analysis of the tie-line data revealed that as the molecular weight of the polymer increases, the tie-line length correspondingly elongates, indicating that lower molecular weight polymers tend to concentrate within the bottom phase (Fig. 3a).39
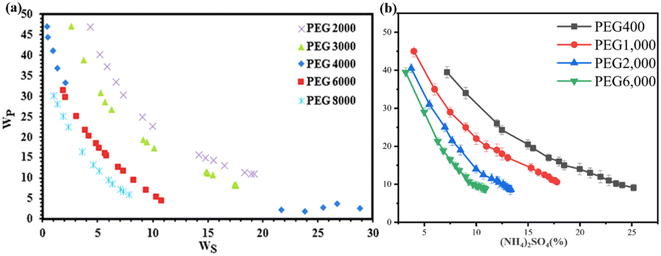 | ||
| Fig. 3 (a) Experimental binodal data for the phase diagram of PEG + ZnSO4 + water system at pH = 4.70, T = 298.15 K, and various molecular masses of PEG (2000, 3000, 4000, 6000, 8000).39 (b) Phase diagrams of PEG with different molecular weights and (NH4)2SO4 in the ATPS in the presence of Tl(I) and U(VI) (both were 10 mg L−1).40 | ||
The heavy metals thallium (Tl(I)) and uranium (U(VI)) in wastewater are very harmful, and they have serious toxic effects on the environment and human health. To determine the most suitable polymer for the extraction of Tl(I) and U(VI), a range of PEG molecules with different molecular weights (400, 1000, 4000, and 6000) were utilized in an ATPS in conjunction with a fixed salt, (NH4)2SO4. Fig. 3b illustrates the phase diagram of the ATPS, where PEG, (NH4)2SO4, Tl(I), and U(VI) (at a concentration of 10 mg L−1) were subjected to the cloud point method to generate the diagram. In the figure, the curve represents the boundary line separating the homogeneous mixture region below it, which indicates a lack of phase separation, from the two-phase zone above it, where the ATPS is formed by the combination of Tl(I), U(VI), (NH4)2SO4, and PEG. The figure also highlights the phase-separating capabilities of polymers with different molecular weights. Keeping the proportion of (NH4)2SO4 constant, higher molecular weight PEG exhibited a greater tendency for phase separation. Conversely, when the mass fraction of PEG was fixed, an increase in PEG molecular weight led to enhanced phase separation ability, accompanied by a decrease in the salt content. This can be attributed to electrostatic repulsion within the ATPS and the reduced solubility of water-soluble ions and high molecular weight PEG molecules. Additionally, higher molecular weight PEG demonstrated increased hydrophobicity. Under a constant PEG concentration, an increase in PEG molecular weight corresponded to a decrease in the amount of salt required to form the ATPS.40
In both system types represented in Fig. 4a-i and a-ii, a consistent trend in the curve can be observed, with only minor differences in the diagram. When using PEG with higher molecular weight, a slightly larger “operational region” is achieved. Consequently, the ATPS formed by PEG 8000 exhibits superior phase separation capabilities and can be considered a more effective method of separation. This result is expected since an increase in the molecular weight of the polymer corresponds to increased hydrophobicity and reduced water solubility.38 Sun et al. propose the use of PEG-based ATPS as a promising strategy for the extraction and removal of metal ions from industrial wastewater, providing a viable alternative to traditional organic-ATPS. The study also investigated the extraction mechanism (Fig. 4b-i).41 It was observed that as the Na2SO4 concentration increased, there was a significant improvement in the extraction percentage of Cr(VI) ions in the PEG-rich phase. This finding suggests that the increased hydrophobicity of the PEG-rich phase facilitated the partitioning of Cr(VI) ions in the ATPS (Fig. 4b-ii). The experimental results further validate that hydrophobic interactions play a crucial role in the transportation of Cr(VI) ions into the PEG-rich phase of the ATPS. Additionally, this study provides novel insights into the microscopic mechanisms governing the separation of metal ions in polymer-based ATPS.
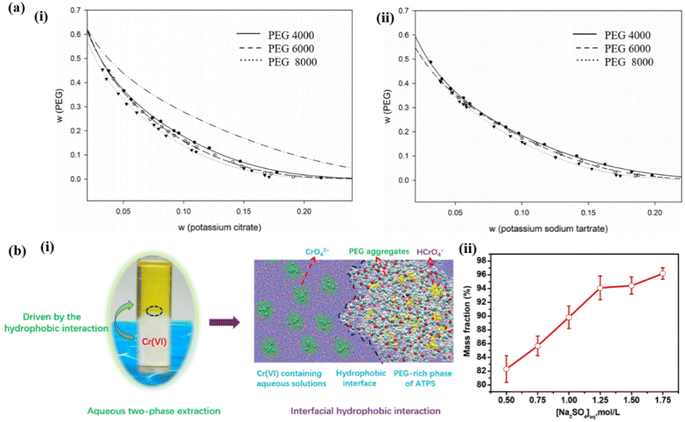 | ||
| Fig. 4 (a-i) Binodal experimental data for (a) PEG (4000, 6000, 8000) + potassium citrate systems at T = 298.15 K. (a-ii) Binodal experimental data for: PEG (4000, 6000, 8000) + potassium sodium tartrate systems at T = 298.15 K.38 (b-i) Schematic of hydrophobic interaction in driving the partitioning of metal ions in a PEG-based ATPS. (b-ii) Effect of Na2SO4 concentration on the extraction percentage of Cr(VI) in the PEG-rich phase of ATPS.41 | ||
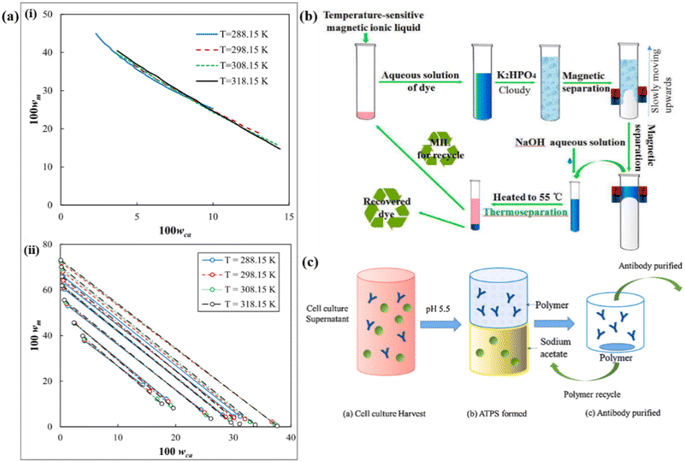 | ||
| Fig. 5 Comparison of the (a-i) binodal curves and (a-ii) tie-lines for isopropanol + trisodium citrate + water ternary systems at T = 288.15, 298.15, 308.15, and 318.15 K.43 (b) Schematic removal and recovery process of triphenylmethane dye.44 (c) Process of antibody IgG201 partition by pH-ATPS.45 | ||
Another notable development is the discovery of temperature-sensitive magnetic ionic liquids by Yao et al.44 They synthesized a thermoresponsive ionic liquid biphasic system called Magnetic Ionic Liquid ATPS (MILATPs) using PPG400 (Fig. 5b). This system combines the advantages of rapid extraction, solvent-free processes, and the unique magnetic responsiveness of magnetic ionic liquids. The thermoresponsive MILATPs enable efficient recovery of high-purity MIL and dyes through heating, achieving a high dye recovery rate of over 93%. The MILs can be reused for multiple cycles with consistent high extraction efficiency. This innovative approach has been successfully employed in the treatment of actual industrial dyeing wastewater, demonstrating its potential for separation and recovery of dye wastewater in industrial processes. Overall, temperature plays a significant role in ATPS formation, with its effects varying depending on the specific ATPS system. Understanding the influence of temperature is crucial for optimizing ATPS processes and developing innovative applications in various industries, including the treatment of dye wastewater.
Similar results were reported in previous research by Chen et al., where different pH values affected the phase diagrams and tie lines of ATPS.45 When the pH value exceeds 5.5, there is a slight decrease in the distribution coefficient and recovery rate. This can be attributed to varying degrees of protonation at different pH values. At pH 5.5, there is electrostatic repulsion between the positively charged IgG201 antibodies and the negatively charged polymers. At a pH of 6.0, the distribution coefficient of PADB4.91 is lower than that of PMDB3.36 at pH 5.5. The process described by Chen et al.45 involved introducing the supernatant of a cell culture into the ATPS consisting of the polymer and sodium acetate. The upper phase, which contained the antibody, was collected. The polymer was subsequently reclaimed through pH adjustment (Fig. 5c). Overall, pH plays a crucial role in ATPS by affecting protein dissociation, isoelectric points, charge distribution, and salt dissociation. Understanding the impact of pH is important for optimizing ATPS systems and designing efficient separation and recovery processes.
3. Application of ATPS
ATPS epitomize a separation technique that embodies a remarkable amalgamation of operational simplicity, profound selectivity, economic viability, and vast potential across a plethora of applications. Over the course of decades, this method has been widely employed in the extraction, fractionation, refinement, and enrichment of biomolecules, eliciting substantial interest from both the industrial and academic spheres. Looking ahead, our primary endeavor shall revolve around providing a comprehensive exposition spanning diverse domains, encompassing the retrieval of heavy metals, identification of residual pharmaceuticals, environmental remediation, emulation of biomimetic phenomena at the cellular and organelle levels, partitioning and purification of biological macromolecules, as well as the reclamation of dyes.3.1 Heavy metal ionic detection and separation
In recent years, fueled by the swift industrial progress, a surge in elevated concentrations of heavy metal ions, surpassing the prescribed regulatory thresholds, has been observed in a substantial portion of industrial wastewater.47 Noteworthy metals found in the effluents discharged by electroplating, petroleum, and papermaking industries include mercury, arsenic, bismuth, sodium, lead, magnesium, manganese, zinc, cobalt, chromium, copper, iron, nickel, molybdenum, cadmium, and titanium.48 Beyond the essential metal ions requisite for human sustenance, the majority of heavy metals pose considerable risks to human health. A striking example lies in the fact that metals constitute one-fifth of the composition of electronic products. In the absence of stringent regulations, these metallic wastes can be illicitly disposed of, infiltrating the delicate fabric of groundwater reserves. So, the advent of diverse techniques aimed at the separation and retrieval of heavy metal ions has proliferated. Among these pioneering methods, ATPS have emerged as an instrumental force in fostering environmental safety, owing to their cost-effectiveness, remarkable efficiency in extraction, and non-toxic attributes.49Within this context, it is worth highlighting the significant strides made by amino acid-based ATPS in the realm of metal ion leaching and extraction. Cai et al. demonstrated the successful utilization of biocompatible amino acids to leach and segregate metal ions from discarded cathode powders of lithium batteries.50 For the first time, an ATPS comprising serine and poly(propylene glycol) (Ser-PPG400-H2O) was employed for metal recovery from spent batteries. Employing relatively mild temperature conditions (70 °C) and a solid-to-liquid ratio (S/L) of 10/1 (g L−1) between the lithium-ion battery negative electrode powder and the leaching solution composed of amino acids, the leaching efficiency of each metal surpassed 80%, with a remarkable leaching rate of 98% for Co(II). By introducing thiocyanate ions into the ATPS system, selective extraction of Co(II) was accomplished with an extraction efficiency of 97% and a purity exceeding 95%. The utilization of amino acids, possessing notable environmental advantages and affordable prices, renders them invaluable for constructing amino acid-based ATPS for metal ion extraction, thereby exhibiting substantial prospects in the field of waste battery recycling. In response to the high cost and limited sustainability of metal recovery from lithium-ion batteries, Septioga et al.51 have proposed ATPS composed of a hydrophobic DES and water, effectively addressing these challenges. The presence of water significantly enhances metal leaching efficiency, while the extraction of lithium into the aqueous phase is attributed to an in situ back-extraction phenomenon. Moreover, proton transfer occurs when 4,4,4-trifluoro-1-phenyl-1,3-butadione (HBTA)-trioctylphosphine oxide (TOPO) is mixed with water, facilitating cobalt chelation. This biphasic system offers a novel and efficient strategy for the direct recovery of lithium from lithium-ion batteries.
Removal of heavy metals is a necessary work to protect health and environment. Hamta and Dehghani52 constructed ATPS to extract Hg(II), Zn(II) and Co(II) by using PEG-6000 and Na2CO3. Na2CO3 has high water solubility and strong salting out effect. Potassium iodide (KI) was selected as extractor, and the effects of its dosage, pH of salt solution, temperature and initial concentration of metal ions on extraction efficiency were systematically studied, which provided new experimental data and theoretical reference for ATPS extraction of heavy metals. This environment-friendly heavy metal extraction technology has potential industrial application value. To address the significant challenge posed by the high viscosity of ATPS, Sadeghi and Pazuki53 selected two deep eutectic solvents (DESs): choline chloride/urea and betaine/urea. They employed these solvents in conjunction with dipotassium hydrogen phosphate to construct ATPS aimed at the separation of hexavalent chromium and other heavy metals. Notably, the betaine/urea system exhibited superior biphasic behavior and a more extensive two-phase region, resulting in enhanced separation efficiency for hexavalent chromium. Hexavalent chromium demonstrated a propensity to migrate to the upper phase, while lead, cobalt, nickel, and cadmium favored the lower phase. This system is well-suited for the separation of heavy metals from industrial wastewater.
3.2 Detection of pharmaceutical residues
The medical industry, pharmaceutical sector, wastewater treatment facilities, as well as the aquatic and agricultural domains, collectively contribute to the release of residual pharmaceutical waste into the environment. If these pharmaceutical compounds remain undegraded or unremoved during the wastewater treatment process, they can find their way into surface water, groundwater, and even contaminate drinking water, thereby posing significant hazards to human health.54 Among the myriad forms of environmental pollution, the contamination arising from antibiotics assumes a particularly urgent status that demands immediate attention. Antibiotics prevalent in the environment encompass macrolides, fluoroquinolones, sulfonamides, and other classes of compounds. The eradication of antibiotics has emerged as a paramount concern for researchers.55 In Table 1, a comprehensive overview is provided, encapsulating the extraction efficacy of diverse ATPS employed for the elimination of residual drugs from water sources and food matrices. The adoption of ATPS has laid a robust theoretical groundwork for the extraction of fluoroquinolones, tetracyclines, chloramphenicol, amoxicillin, and other antibiotics from aquatic environments, thereby contributing to notable advancements in the realm of environmental conservation.| Sample | Drug | ATPS | Average extraction Efficiency(%) | Ref. |
|---|---|---|---|---|
| Water | Sulfonamide | IL-based ATPS | >89 | 56 |
| Water | Fluoroquinolone | POELE20-NaH2PO4 | >90 | 57 |
| Water | Tetracycline | Ethanol/2-propanol-ammonium sulfate | 87.7 | 58 |
| Fermentation broth | Tetracycline | Polyethylene glycol and cholinium-based salts | >80 | 59 |
| Water | Roxithromycin | [Bmim]BF4/Na2CO3 | 90.7 | 60 |
| Procine blood serum | Hormone estradiol | Ionic liquid cholinium alaninate and citrate salt | 89.7 | 61 |
| Fermentation broth | Chloramphenicol | Ethyl lactate/salts | >95 | 10 |
| Medical wastewater | Amoxicillin | PEG/K2HPO4 | 96.4 | 62 |
Roxithromycin (ROX) is a representative member of the macrolide class of antibiotics that acts by inhibiting protein synthesis in bacteria. It binds to the 50S subunit of bacterial ribosomes, impeding peptide translocation within the human body. Additionally, due to its structural similarity to chloroplasts and bacteria, ROX may also interfere with translation processes in plant chloroplasts. In their study, Li et al. proposed a novel ATPS composed of a hydrophilic ionic liquid, 1-butyl-3-methylimidazolium tetrafluoroborate ([Bmim]BF4) and Na2CO3.60 They employed molecular fluorescence spectrophotometry to extract and separate ROX from water samples. Ionic liquids, known for their green and non-volatile nature, have found applications in various fields. Under optimized conditions, the ATPS achieved an average extraction efficiency of 90.7% for ROX, and the detection limit reached 0.03 μg mL−1 (Fig. 6a). The ATPS exhibited high efficiency, rapidity, non-toxicity, and simplicity, making it a promising technique for the separation and enrichment of hydrophobic antibiotics, with broad prospects in the field of antibiotic extraction.
 | ||
| Fig. 6 (a) Schematic of separating ROX in ILATPS.60 (b) Schematic of the separation and determination of trace fluoroquinolone antibiotics.57 | ||
Ciprofloxacin (CIP), norfloxacin (NRF), and pefloxacin (PEF) belong to the fluoroquinolone class of broad-spectrum antibiotics. These antibiotics have antibacterial activity that is 1000 times stronger than sulfonamide drugs and are widely used due to their easy absorption, low cost, and high concentration in the blood.63 However, they can accumulate in plant roots, leading to contamination of soil and water. Sun et al. developed a method using polyoxyethylene lauryl ether (POELE20)/NaH2PO4 ATPS in combination with high-performance liquid chromatography (HPLC) for the simultaneous separation, concentration, and quantification of trace amounts of CIP, NRF, and PEF in environmental samples.57 The study investigated the effects of NaH2PO4 concentration, POELE20 concentration, and temperature on the extraction efficiency and enrichment factor of the antibiotics. The results demonstrated that the combination of POELE20/NaH2PO4 ATPS and HPLC is suitable for the determination of trace amounts of CIP, NRF, and PEF in environmental samples, with potential applicability in the analysis of these antibiotics in food samples (Fig. 6b).
3.3 Environmental monitoring and remediation
In recent years, there has been increasing concern about the ecological and human health risks posed by diesel pollution incidents. Researchers have focused on developing remediation techniques for diesel-contaminated soil. Diesel fuel is a mixture of saturated hydrocarbons and aromatic hydrocarbons, known for their toxic and carcinogenic properties. Shang et al. proposed a method for remediating diesel-contaminated soil using ATPS composed of alkoxyethanol and salt.64 The study investigated the effects of various factors such as temperature, stirring speed, stirring time, and solid-to-liquid ratio on the efficiency of diesel removal. The ATPS demonstrated excellent reusability and achieved a diesel removal efficiency of over 97.18% (Fig. 7a). Importantly, minimal residual organic solvents remained on the soil surface after the remediation process. This approach offers significant advantages over conventional surfactant washing and organic solvent extraction methods. | ||
| Fig. 7 (a) Schematic of remediate diesel-contaminated soil by alkoxyethanol ATPS.64 (b) Schematic of diesel removal and recovery from heavily diesel-contaminated soil based on three-liquid-phase equilibria of diesel + 2-butyloxyethanol + water.65 (c) Schematic of heavy metals removal from soil/sediments washing effluents via biocompatible ATPS.66 | ||
In another study by Shang et al.,65 a ternary ATPS consisting of diesel fuel, 2-butoxyethanol, and water was employed for the remediation of diesel-contaminated soil. The low molecular weight of 2-butoxyethanol provided benefits such as low viscosity, easy degradation, and rapid phase separation. This system combined the advantages of washing, removal, and recovery of diesel fuel from contaminated soil. The method achieved a remarkable removal efficiency of 87.5% for soil contaminated with a high concentration of diesel fuel (226, 723 mg kg−1), with a recovery rate of 73.8%. The ATPS could be continuously used during the separation and extraction process, eliminating the need for complex separation steps. The separated diesel fuel easily transitioned to the upper phase, simplifying purification procedures. The processed soil contained minimal residual organic solvent, making it suitable for plant cultivation (Fig. 7b). The recovered diesel fuel showed minimal differences in composition and performance compared to commercial diesel, demonstrating its high reusability. This study provides valuable insights into the remediation of petroleum-contaminated soil and holds promise for practical applications.
Heavy metal pollution in soil mainly comes from industries such as mining, metal processing and electroplating. These contaminants are non-biodegradable and persist in the soil for a long time, requiring effective detection and remediation methods. Álvarez et al.66 studied the separation effects of different inorganic and organic salts such as ammonium nitrate, ammonium acetate and sodium potassium tartrate. Among them, the molar Gibbs free energy of the tartrate anion ΔGhyd = −1102 kJ mol−1, The ability to interact with water molecules is more than three times that of nitrate (ΔGhyd = −300 kJ mol−1) and acetate (ΔGhyd = −365 kJ mol−1) anions, which is closer to the origin of the quadratic phase diagram than the existing two-node curve. Therefore, from these data, sodium potassium tartrate can be used as the most effective separator. Biodegradable nonionic surfactants Tween 80 and Tergitol 15S9 were investigated. The partitioning ratio and extraction rate of Tergitol 15S9 are higher than that of Tween 80, which is related to the stronger hydrophobicity of Tergitol 15S9. Three empirical equations were then used to correlate the experimental data, and the ATPS based on potassium sodium tartrate was used for the extraction of heavy metals (cadmium, copper and zinc). The metal is directly bound to the water molecule through the first hydration coordination sphere, where oxygen provides the desired pair of electrons, and the coordination water can form hydrogen bonds with other water molecules (this would be the second hydration sphere). Given that the cadmium–oxygen interaction is weaker than the zinc–oxygen or copper–oxygen interaction (Bond Dissociation Enthalpy BDE of Cd–O = 98 kJ mol−1 versus BDE of Zn–O = 159 kJ mol−1 and BDE of Cu–O = 287 kJ mol−1), it is clear that the presence of sodium potassium tartrate changes the intermolecular forces. The tie lines of the system were characterized and successfully correlated by Othmer-Tobias and Bancroft models. The results showed that the metals were preferentially allocated to the salt-rich phase, and the extraction rate of some metals was over 80% without complexing agent. SuperPro Designer 8.5v software was used to simulate a plant treating contaminated soil and the results showed that the data could be valuable for decision-making on the recovery and reuse of washing solutions during soil/sediment remediation.
3.4 Biomedical applications
ATPS is a promising separation technique known for its simplicity, selectivity, cost-effectiveness, and biocompatibility. In the field of biomedicine, ATPS technology finds applications in various areas such as protein and enzyme separation and purification, concentration and purification of viruses, nucleic acids, and plant proteins, isolation of microbial and animal cells, bioremediation, drug release, and more.67–69Hung et al. utilized a biocompatible polymer-based ATPS consisting of DEX and PEG for the rapid fabrication of scaffold-free corneal tissue.70 The ATPS facilitated the formation of complete quasi-tissue structures composed of corneal epithelial cells or endothelial cells at the liquid–liquid interface, which could be easily collected. These corneal cell structures expressed their essential physiological characteristics, retained their viability, and exhibited regenerative potential in vitro (Fig. 8a). This research highlights the valuable utility of ATPS in bioremediation applications.71
 | ||
| Fig. 8 (a) Schematic illustrations of the cell construct formation process.70 (b) Spheroid formation using DEX-in-PEG ATPS pattern.72 (c) Schematic illustration of preparation and stimulation of ATPS-liposomes.73 (d) Schematic illustration of bioseparate phycocyanin from Phormidium tergestinum using ATPS.74 | ||
Conventional two-dimensional cell culture systems fail to replicate the three-dimensional structures of biological tissues and organs, which are crucial for cellular interactions and physiological functions. Han employed a density-controlled ATPS composed of PEG and DEX to generate uniform and controllable spherical structures.72 The phase separation of PEG/DEX was visualized, and cells were initially confined within the DEX-rich phase and later trapped at the phase interface. Over time, the cells gathered at the top of the DEX phase, forming tightly packed cellular spheroids or loosely aggregated cell clusters based on their characteristics (Fig. 8b). This technique has implications in artificial cell research. In the realm of drug release, Zhang et al. utilized PEG/DEX ATPS as a synthetic cytoplasm to achieve hierarchical drug release effects. ATPS comprising PEG and DEX was encapsulated within pH-sensitive liposomes (PSLs).73 Unlike conventional PSLs, the ATPS-PSLs exhibited a distinctive hierarchical release profile, resulting in extended release duration and enhanced uptake rate of the drug. This system demonstrated superior inhibition efficiency compared to conventional PSLs and proved to be a stable drug delivery system (Fig. 8c).
Obtaining high-value bioproducts from renewable resources has gained interest, and Tan et al. employed ATPS composed of PEG and salts for the purification of phycocyanin (PC).74 The study assessed the distribution efficiency of PC within the ATPS and evaluated the influence of phase composition, pH, crude loading, and neutral salts on the purification factor and recovery rate. PC was selectively partitioned toward the bottom phase of the system containing potassium phosphate. Under optimized conditions, PC from P. tergestinum was partially purified up to 5.34-fold with a yield of 87.8%. This study demonstrated the potential of ATPS as an approach for the purification of PC from P. tergestinum (Fig. 8d).
Phenolic compounds are secondary metabolites of plants, exhibit a range of biological and pharmacological properties, including antioxidant, anti-inflammatory and anticancer activities, making them widely applicable in the food and pharmaceutical industries. Currently, various methods such as solvent extraction and pressurized fluid extraction are employed to isolate these compounds from natural matrices, followed by purification through ATPS. Souza et al.75 have pioneered the development of ATPS composed of DES formed from choline chloride ([Ch]Cl) and carbohydrates, in conjunction with acetonitrile and water. Their findings revealed that the system's ability to form distinct phases is contingent upon the number and type of hydroxyl groups present in the carbohydrates constituting the DES. Moreover, this DES-based system demonstrates a larger two-phase region compared to systems utilizing [Ch]Cl and carbohydrates independently. Furthermore, the application of this novel ATPS for the distribution study of phenolic compounds enables the selective separation of various phenolic species, providing an economically viable platform for the recovery of phenolic compounds from real matrices.
3.5 Dye reclamation
Dyes are organic compounds used for coloring various materials, and they are categorized as soluble or insoluble dyes. Soluble dyes include acid dyes, basic dyes, direct dyes, and reactive dyes, while insoluble dyes include azo dyes, disperse dyes, sulfur dyes, and vat dyes. These dyes find applications in industries such as textiles, cosmetics, food, paper, and plastics.76 However, the wastewater generated during these processes often contains residual dyes, and discharging such wastewater into water bodies can lead to colored and toxic water, negatively impacting aquatic ecosystems and potentially posing health risks to humans.To address the issue of dye removal from wastewater, various methods have been developed, and one such method is the use of ATPS. ATPS is considered an economically viable, efficient, and non-toxic technique for dye reclamation. In ATPS, polymers such as PEG, polypropylene glycol (PPG), and polyethylene oxide (PEO) are commonly used for dye separation.62 Mageste et al. proposed the use of ATPS prepared by mixing aqueous solutions of polymers or copolymers with aqueous salt solutions (Na2SO4 and Li2SO4) to investigate the distribution of the natural dye carmine in ATPS.77 The study examined the partition coefficient of carmine dye under different conditions, including the pH of the system, molar mass of the polymer, hydrophobicity, length of the system tie-line, and the nature of the electrolyte. The results revealed a significant dependence of the carmine partition coefficient on the electrolyte's nature and the pH of the system, with values reaching as high as 300. Carmine dye tended to concentrate in the polymer-rich phase, indicating enthalpic interactions between carmine and the pseudopolycation formed by cation adsorption along the macromolecule chain. As the enthalpic interaction weakened, entropic forces became dominant in the dye transfer process, leading to a decrease in the carmine partition coefficient. The study identified conditions that resulted in the maximum partitioning of carmine into the top phase, which included high concentrations of PEO and Li2SO4, along with low pH values within the explored ranges. This finding highlights the potential of ATPS-based two-phase extraction for the purification of carmine dye.
4. Conclusion and future perspective
In this review, we have discussed the profound significance of ATPS within the domain of chemistry. The formation mechanism of the phase diagram, the classification of the types and the factors affecting the formation of ATPS are investigated and introduced respectively. Then, we have introduced the application of ATPS in different fields in detail. These applications include the detection of metal ions and contaminants, purification and separation of proteins and enzymes, environmental remediation, and even the construction of artificial cells in the field of biochemistry. At the same time, many typical examples of novel design are listed, all of which show the excellent application of the ATPS. Through the comprehensive discussion of the full text, the boundless potential of ATPS is demonstrated, and the field will pave the way for scientific exploration and technological innovation.ATPS is indeed a versatile extraction methodology with numerous advantages and applications in various fields. However, there are certain limitations and challenges that need to be addressed for its widespread industrial implementation. One of the main obstacles encountered in industrial applications of ATPS is the prolonged phase separation time caused by high-viscosity polymers. High-viscosity polymers can slow down the phase separation process, leading to longer processing times and reduced efficiency. Efforts are being made to develop low-viscosity polymers or optimize the system conditions to accelerate phase separation and improve overall process performance. Another challenge is the corrosion of metal pipelines by high-concentration salt solutions used in ATPS. The high salt content in ATPS can be corrosive to metal equipment, which poses a problem for large-scale industrial operations. Strategies such as using corrosion-resistant materials or designing appropriate protective coatings can help mitigate this issue and ensure the long-term reliability of ATPS systems. Post-processing and product recovery can also be challenging in ATPS. After the separation process, it is essential to recover the desired components efficiently and effectively. Developing effective recovery systems, such as extraction techniques or membrane processes, is crucial to maximize product yield and minimize waste. Integration of other technologies, such as adsorption or membrane filtration, can provide innovative approaches for product recovery mechanisms in ATPS. Continued research and development efforts are necessary to unlock the full potential of ATPS in various industries.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 22407068), the Basic Research Program for Excellent Young Teachers in Heilongjiang Provincial Universities (YQJH2023101).References
- R. M. Smith, J. Chromatogr. A, 2003, 1000, 3–27 CrossRef CAS PubMed.
- M. Polyakova, A. L. Diekmann and M. Grünewald, Chem. Ing. Tech., 2020, 92, 1941–1952 CrossRef CAS.
- S. C. Ribeiro, G. A. Monteiro, J. M. Cabral and D. M. Prazeres, Biotechnol. Bioeng., 2002, 78, 376–384 CrossRef CAS PubMed.
- P. G. Mazzola, A. M. Lopes, F. A. Hasmann, A. F. Jozala, T. C. V. Penna, P. O. Magalhaes, C. O. Rangel-Yagui and A. Pessoa Jr, J. Chem. Technol. Biotechnol., 2008, 83, 143–157 CrossRef CAS.
- Z. Yang, J. Wang, H. Wang, H. Xie, Y. Zhang and M. Wang, J. Food Sci., 2025, 90, e17674 CrossRef CAS PubMed.
- S. V. Smirnova, I. V. Mikheev and V. V. Apyari, Talanta, 2024, 269, 125504 CrossRef CAS PubMed.
- S. Al-Saidi, F. S. Mjalli, M. Al-Azzawi, B. Abutarboosh, M. A. AlSaadi and T. Al-Wahaibi, Sep. Sci. Technol., 2022, 58, 61–74 CrossRef.
- A. L. Grilo, M. Raquel Aires-Barros and A. M. Azevedo, Sep. Purif. Rev., 2016, 45, 68–80 CrossRef.
- P. A. Albertsson, Adv. Protein Chem., 1970, 24, 309–341 CrossRef CAS PubMed.
- M. E. Zakrzewska, A. V. M. Nunes, A. R. Barot, A. Fernández-Castané, Z. P. Visak, W. Kiatkittipong and V. Najdanovic-Visak, Fluid Phase Equilib., 2021, 539, 113022 CrossRef CAS.
- M. Lv, J. J. Zheng, L. Zulu, Y. Wang, K. Kayama, R. Wei and Z. Su, Food Chem., 2025, 464, 141727 CrossRef CAS PubMed.
- Q. Qu, Z. Zhu, M. Zhao, H. Wang, W. Cui, X. Huang, Z. Yuan, Y. Zheng, N. Dong, Y. Liu, H. Wang, C. Dong, Z. Zhang and Y. Li, Food Chem.:X, 2025, 26, 102338 CAS.
- A. G. Teixeira, R. Agarwal, K. R. Ko, J. Grant-Burt, B. M. Leung and J. P. Frampton, Adv. Healthcare Mater., 2017, 7, 701036 Search PubMed.
- A. C. L. Leite, T. P. Nascimento, M. N. C. da Cunha, Y. Mehari, E. Berger, D. Scheich, N. Lingg and A. Jungbauer, Int. J. Biol. Macromol., 2024, 275, 133581 CrossRef CAS PubMed.
- B. Wang, L. Zhang and X. Lu, J. Environ. Chem. Eng., 2025, 13, 115373 CrossRef CAS.
- F. Gebhard, J. Hartmann and S. Hardt, Soft Matter, 2021, 17, 3929–3936 RSC.
- W. Baran, E. Adamek, J. Ziemianska and A. Sobczak, J. Hazard. Mater., 2011, 196, 1–15 CrossRef CAS PubMed.
- S. F. Darani, F. G. Ahsaie, G. Pazuki and S. Abdolrahimi, J. Mol. Liq., 2021, 322, 114542 CrossRef CAS.
- P. H. Le, D. N. Dao, T. Q. Huynh, T. T. T. Tran and V. Nguyen, Nat. Prod. Res., 2023, 37, 154–158 CrossRef CAS PubMed.
- R. Hatti-Kaul, Mol. Biotechnol., 2000, 19, 269–277 CrossRef PubMed.
- D. da Silveira Leite, R. C. de Assis, J. T. Domingues, P. L. Gutierrez Carvalho, M. C. M. de Castro, G. H. da Cruz, I. C. Gonçalves Silva and G. D. Rodrigues, J. Water Proc. Eng., 2021, 42, 102138 CrossRef.
- M. Singla and N. Sit, ChemBioEng Rev., 2023, 10, 65–80 CrossRef CAS.
- Y. M. S. Amaral, O. S. da Silva, R. L. de Oliveira and T. S. Porto, Prep. Biochem. Biotechnol., 2020, 50, 619–626 CrossRef CAS PubMed.
- M. Iqbal, Y. Tao, S. Xie, Y. Zhu, D. Chen, X. Wang, L. Huang, D. Peng, A. Sattar, M. A. Shabbir, H. I. Hussain, S. Ahmed and Z. Yuan, Biol. Proced. Online, 2016, 18, 18 CrossRef PubMed.
- T. Macko, R. Brüll and H. Pasch, Chromatographia, 2003, 57, S39–S43 CrossRef.
- C. Ma, X. D. Chen, Y. Q. Kong and L. M. Che, Adv. Mater. Res., 2012, 554–556, 286–294 CAS.
- K. S. M. S. Raghavarao, T. V. Ranganathan, N. D. Srinivas and R. S. Barhate, Clean Technol. Environ. Policy, 2003, 5, 136–141 CrossRef CAS.
- R. S. Amjad, M. Asadollahzadeh, R. Torkaman and M. Torab-Mostaedi, Sci. Rep., 2022, 12, 17302 CrossRef CAS PubMed.
- X. H. Zhang, H. N. Cui, J. J. Zheng, X. D. Qing, K. L. Yang, Y. Q. Zhang, L. M. Ren, L. Y. Pan and X. L. Yin, Food Res. Int., 2023, 163, 112278 CrossRef CAS PubMed.
- F. Dumas, J.-P. Benoit, P. Saulnier and E. Roger, J. Colloid Interface Sci., 2021, 599, 642–649 CrossRef CAS PubMed.
- Y. Yin, T. Liu, B. Wang, B. Yin, Y. Yang, T. P. Russell and S. Shi, Adv. Funct. Mater., 2022, 32, 2108895 CrossRef CAS.
- T. Yao, C. Feng, X. Shi and J. Song, Sep. Purif. Technol., 2025, 360, 131001 CrossRef CAS.
- M. T. Zafarani-Moattar, H. Shekaari and S. Asadollahi, Sci. Rep., 2025, 15, 5395 CrossRef CAS PubMed.
- C. Cai, T. Hanada, A. T. N. Fajar and M. Goto, Ind. Eng. Chem. Res., 2022, 61, 5306–5313 CrossRef CAS.
- Q. Cheng, J. Chen, C. Wan, Y. Song and C. Huang, ACS Appl. Mater. Interfaces, 2022, 14, 50434–50443 CrossRef CAS PubMed.
- B. Shahrokhi, M. Pirdashti and S. M. Arzideh, J. Dispersion Sci. Technol., 2021, 43, 1603–1611 CrossRef.
- F. F. Magalhaes, M. M. Pereira, R. D. C. S. D. Sousa, A. P. M. Tavares, J. A. P. Coutinho and M. G. Freire, Green Chem. Eng., 2022, 3, 10 Search PubMed.
- K. Wysoczanska and E. A. Macedo, J. Chem. Eng. Data, 2016, 61, 4229–4235 CrossRef CAS.
- M. Pirdashti, Z. Heidari, N. Abbasi Fashami, S. M. Arzideh and I. Khoiroh, J. Chem. Eng. Data, 2021, 66, 1425–1434 CrossRef CAS.
- Y. Huang, D. Chen, S. Chen, M. Su and G. Yuvaraja, J. Cleaner Prod., 2021, 297, 126452 CrossRef CAS.
- P. Sun, K. Huang, J. Lin and H. Liu, Ind. Eng. Chem. Res., 2018, 57, 11390–11398 CAS.
- Y. Wang, X. Xu, Y. Yan, J. Han and Z. Zhang, Thermochim. Acta, 2010, 501, 112–118 CrossRef CAS.
- M. Mokarizadeh, E. Nemati-Kande and R. Azizi Adeh, J. Chem. Eng. Data, 2021, 66, 2050–2060 CrossRef CAS.
- T. Yao, Y. Gan, Q. Li, M. Tan and X. Shi, J. Cleaner Prod., 2021, 328, 129648 CAS.
- J. Chen, Z. Ding, H. Pan and X. Cao, Talanta, 2017, 174, 256 CAS.
- X. Chen, Y. Guo, T. Yang, J. Wan and X. Cao, Process Biochem., 2022, 113, 125–133 CAS.
- I. N. S. Kahar, N. Othman, N. F. M. Noah and S. S. Suliman, Environ. Sci. Pollut. Res. Int., 2023, 30, 66445–66472 CrossRef CAS PubMed.
- A. Ya Fedorov, A. V. Levina and M. I. Fedorova, IOP Conf. Ser.: Mater. Sci. Eng., 2022, 1212, 012012 CrossRef.
- G. Bettio B, L. L. Okumura, D. S. Zacché, F. O. Chagas and M. C. Hespanhol, Electroanalysis, 2021, 33, 1081–1087 CrossRef CAS.
- C. Cai, A. T. N. Fajar, T. Hanada, R. Wakabayashi and M. Goto, ACS Omega, 2023, 8, 3198–3206 CrossRef CAS PubMed.
- K. Septioga, A. T. N. Fajar, R. Wakabayashi and M. Goto, ACS Sustainable Resour. Manage., 2024, 1, 2482–2491 CrossRef CAS.
- A. Hamta and M. R. Dehghani, J. Mol. Liq., 2017, 231, 20–24 CrossRef CAS.
- A. Sadeghi and G. R. Pazuki, Sci. Rep., 2025, 15, 3948 CrossRef CAS PubMed.
- K. Kümmerer, J. Antimicrob. Chemother., 2003, 52, 5–7 CrossRef PubMed.
- X. Yang, S. Zhang, W. Yu, Z. Liu, L. Lei, N. Li, H. Zhang and Y. Yu, Talanta, 2014, 124, 1–6 CrossRef CAS PubMed.
- I. Mohammadi, S. Ghorbanidehkordi and A. Hallajisani, Int. J. Environ. Sci. Technol., 2022, 19, 11291–11300 CrossRef CAS.
- Z. Sun, Y. Lu, L. Zhu, W. Liu, Y. Qu, N. Lin and P. Yu, J. Chem. Technol. Biotechnol., 2019, 94, 2917–2927 CrossRef CAS.
- Y. Wang, J. Han, X. Xu, S. Hu and Y. Yan, Sep. Purif. Technol., 2010, 75, 352–357 CrossRef CAS.
- J. F. B. Pereira, F. Vicente, V. C. Santos-Ebinuma, J. M. Araújo, A. Pessoa, M. G. Freire and J. A. P. Coutinho, Process Biochem., 2013, 48, 716–722 CrossRef CAS.
- C.-X. Li, J. Han, Y. Wang, Y.-S. Yan, X.-H. Xu and J.-M. Pan, Anal. Chim. Acta, 2009, 653, 178–183 CrossRef CAS PubMed.
- C. Díaz-Quiroz, J. F. Hernández-Chávez, G. Ulloa-Mercado, F. Deive, P. Gortáres-Moroyoqui and R. M. Molina-Barrios, Environ. Sci. Pollut. Res., 2020, 27, 28536–28544 CrossRef PubMed.
- S. Alsaidi, F. Mjalli, M. Al-Azzawi, B. Abutarboosh, M. Al-Saadi and T. Al Wahaibi, Sep. Sci. Technol., 2022, 58, 1–14 Search PubMed.
- Z. Li, J. Wang, J. Chang, B. Fu and H. Wang, Sci. Total Environ., 2023, 857, 159172 CrossRef CAS PubMed.
- Z. Shang, P. Xu, H. Yue, D. Feng, T. Zhu and X. Li, Environ. Sci. Pollut. Res. Int., 2022, 29, 25810–25823 CrossRef CAS PubMed.
- Z. Shang, P. Xu, Z. Ke, M. Yao and X. Li, J. Hazard. Mater., 2023, 442, 130061 CrossRef CAS PubMed.
- M. S. Álvarez, M. A. Longo, A. Rodríguez and F. J. Deive, J. Water Proc. Eng., 2023, 53, 103851 CrossRef.
- H. Y. Leong, X. Q. Fu, P. L. Show, S. J. Yao and D. Q. Lin, J. Sep. Sci., 2022, 45, 2064–2076 CrossRef CAS PubMed.
- E. Quagliarini, L. Digiacomo, S. Renzi, D. Pozzi and G. Caracciolo, Nano Today, 2022, 47, 101657 CrossRef CAS.
- R. D. Field, M. A. Jakus, X. Chen, K. Human, X. Zhao, P. V. Chitnis and S. K. Sia, Angew Chem. Int. Ed. Engl., 2022, 61, e202116515 CrossRef CAS PubMed.
- L. T. Hung, S. H. L. Poon, W. H. Yan, R. Lace, L. Zhou, J. K. W. Wong, R. L. Williams, K. C. Shih, H. C. Shum and Y. K. Chan, ACS Biomater. Sci. Eng., 2022, 8, 1987–1999 CrossRef CAS PubMed.
- L. H. Law, J. Huang, P. Xiao, Y. Liu, Z. Chen, J. H. C. Lai, X. Han, G. W. Y. Cheng, K. H. Tse and K. W. Y. Chan, J. Controlled Release, 2023, 354, 208–220 CrossRef CAS PubMed.
- C. Han, S. Takayama and J. Park, Sci. Rep., 2015, 5, 11891 CrossRef PubMed.
- X. Zhang, W. Zong, H. Bi, K. Zhao, T. Fuhs, Y. Hu, W. Cheng and X. Han, Eur. J. Pharm. Biopharm., 2018, 127, 177–182 CAS.
- J. S. Tan, S. Abbasiliasi, J. Lalung, Y. J. Tam, P. Murugan and C. K. Lee, Prep. Biochem. Biotechnol., 2021, 51, 260–266 CrossRef CAS PubMed.
- I. N. Souza, L. C. V. Rodrigues, C. M. F. Soares, F. S. Buarque, R. L. Souza and S. Lima Á, Molecules, 2024, 29, 4383 CAS.
- L. V. T. D. Alencar, L. M. S. Passos, R. N. Soriano, R. N. Bharagava, L. F. R. Ferreira and R. L. de Souza, in Fate and Transport of Subsurface Pollutants, 2021, ch. 3, pp. 35–55 Search PubMed.
- A. B. Mageste, L. R. de Lemos, G. M. Ferreira, C. da Silva Mdo, L. H. da Silva, R. C. Bonomo and L. A. Minim, J. Chromatogr. A, 2009, 1216, 7623–7629 CAS.
| This journal is © The Royal Society of Chemistry 2025 |

