Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Breaking barriers in cancer diagnosis: unveiling the 4Ms of biosensors
Sachin Gupta†
a,
Vijay Mishra†
 b,
Alaa A. A. Aljabali
b,
Alaa A. A. Aljabali
 *c,
Aqel Albuttid,
Rajeev Kanday
*c,
Aqel Albuttid,
Rajeev Kanday
 e,
Mohamed El-Tananif and
Yachana Mishra
e,
Mohamed El-Tananif and
Yachana Mishra
 *g
*g
aDepartment of Robotics and Control Engineering, School of Electronics and Electrical Engineering, Lovely Professional University, Phagwara, Punjab-144411, India
bSchool of Pharmaceutical Sciences, Lovely Professional University, Phagwara, Punjab-144411, India
cDepartment of Pharmaceutics & Pharmaceutical Technology, Yarmouk University, Irbid, Jordan. E-mail: alaaj@yu.edu.jo
dDepartment of Basic Health Sciences, College of Applied Medical Sciences, Qassim University, Buraydah 51452, Saudi Arabia
eSchool of Computer Science and Engineering, Lovely Professional University, Phagwara, Punjab-144411, India
fRas Al Khaimah Medical and Health Sciences University, Ras Al Khaimah, United Arab Emirates
gSchool of Bioengineering and Biosciences, Lovely Professional University, Phagwara, Punjab-144411, India. E-mail: yachanamishra@gmail.com
First published on 17th March 2025
Abstract
Cancer, an insidious affliction, continues to exact a heavy toll on humanity, necessitating early detection and nuanced comprehension of its intricacies for effective treatment. Recent strides in micro and nanoscale electronic chip fabrication have revolutionized biosensor technology, offering promising avenues for biomedical and telemedicine applications. Micro Electromechanical System (MEMS)-based integrated circuits (ICs) represent a paradigm shift in detecting chemical and biomolecular interactions pertinent to cancer diagnosis, supplanting conventional methodologies. Despite the wealth of research on biosensors, a cohesive framework integrating Material, Mechanism, Modeling, and Measurement (4M) dimensions is often lacking. This review aims to synthesize these dimensions, exploring recent breakthroughs in biosensor design and development. Categorized based on electromechanical integration, material selection, and fabrication processes, these biosensors bridge crucial knowledge gaps within the research community. A comparative analysis of sensing methods in point-of-care (PoC) technology provides insights into their practicality and efficacy. Moreover, we critically evaluate biosensor limitations, pivotal in addressing challenges hindering their global commercialization.
1. Introduction
A major problem in contemporary medicine, cancer affects millions of individuals globally. Globally, GLOBOCAN estimates that 2.2 million instances of cancer, including many forms of cancer, have been diagnosed as being caused by infections. Accurate diagnosis is essential in the battle against cancer because of its worldwide relevance. This necessitates the creation of novel techniques that can distinguish between various cancer forms and offer individualized care to every patient. The timely identification and diagnosis of cancer cells are essential for formulating successful treatment plans.1,2The generally recognized gold standard for cancer diagnosis is the enzyme-linked immunosorbent test (ELISA), which identifies protein biomarkers unique to malignancy. Additionally, molecular techniques based on genomics and proteomics, such as radioimmunoassay (RIA), immunohistochemistry (IHC), and polymerase chain reaction (PCR), are utilized in the detection of cancer. Furthermore, a variety of clinical instruments are made considerable use of, including computed tomography (CT), endoscopy, sonography, MRI, PET, and X-rays. Though the technologies and procedures listed above are effective, the majority of them are costly, time-consuming, intrusive, and only available in certain hospitals' laboratories. It may be difficult to definitively identify cancer tumors smaller than millimeters, especially when using imaging techniques. Similar issues and challenges arise when identifying early-stage cancer tumors using invasive techniques like biopsy.3
The high specificity and sensitivity of PCR procedures, which enable the identification of genomic material with as few as 10 oligomers, are among the benefits. RT-PCR analysis requires expensive equipment, a lengthy analysis, and precise control as limitations in diagnosis to prevent false positives caused by contamination. LAMP is a gene amplification method that enables quick and accurate DNA detection. A LAMP's low detection threshold, simple operation, and rapid amplification (it can generate 109 copies of DNA per h) all work in its favor. The widely recognized and often applied microscope agglutination test (MAT). Even though MAT is the gold standard for serological testing, it nevertheless demands a high degree of technical proficiency. Furthermore, ELISA has potential since it can be conducted in a greater variety of laboratories at a lower cost than the MAT test. Immunoglobulin M (IgM) and other biomarkers generated in the body may be measured using ELISA to diagnose an infection brought on by a disease. Biosensor devices are one of the most promising alternatives for the diagnosis or therapeutic monitoring of major diseases such as cancer. They are anticipated to provide fast and accurate biomedical analysis with small sample sizes and no pretreatment. Early diagnosis and monitoring of pathological conditions, particularly cancer disease, may significantly improve prognosis and survival rates through the analysis of molecular biomarkers using biosensor platforms. This would reduce the burden of disease, support social development, and increase access to healthcare for people worldwide.4–7
The rapid advancement of technology, exemplified by Lab-on-Chip (LoC) systems incorporating Micro Electromechanical Systems (MEMS) and the fabrication of nano-chips using biocompatible materials, has revolutionized Point-of-Care (PoC) telemedicine in the field of biomedicine.8
The pressing need for early disease detection and continuous monitoring of disease progression and regression cannot be overstated. Chip-based devices have emerged as vital tools capable of precisely measuring biological processes and transmitting this critical information to receivers, enabling a proactive approach by medical practitioners. In alignment with the vision of medical experts and the scientific community, there is a growing demand for the development of ready-to-use electrochemical chambers at the nanoscale, designed with a plug-and-play approach and built for biocompatibility. These chambers aim to monitor specific signals of interest for early detection through the PoC approach.9 These cutting-edge developments have enabled clinical tools and procedures to be seamlessly embedded into micro and nanoscale chips, facilitated by bio-fabrication and state-of-the-art technology.10–14
The effectiveness of a portable LoC system with an external sample extraction procedure in identifying cervical cancer from biopsy samples is demonstrated by Wormald et al. The apparatus uses loop-mediated isothermal amplification (LAMP) tests in conjunction with ion-sensitive field-effect transistor (ISFET) sensors to amplify human telomerase reverse transcriptase (hTERT) mRNA and HPV DNA. These markers were chosen due to their low to nonexistent expression in normal cervical tissue but high levels of expression in cervical cancer cells. The limit of detection for hTERT was reached with an analytical sensitivity of 102 for the molecular targets, which resolved down to a single copy per reaction for the mRNA markers. All malignant tissues and no benign tissues had hTERT mRNA identified; the pilot results suggest that the LoC might be widely used for cervical cancer screening in resource-constrained environments.15
The effectiveness of the LoC technology in combination with variant-specific isothermal amplification techniques for the identification and separation of wild-type (WT) and mutant (MT) copies of the ESR1 gene is demonstrated by Alexandrou et al. Hormone-resistant malignancies frequently increase the risk of metastatic illness, which raises death rates, particularly in places with limited access to healthcare and poor economic levels. Compared to other common industry-similar technologies like qPCR and sequencing, the LoC system that is being demonstrated here is smaller and less expensive. The suggested LoC platform offers mutational tracking of circulating tumor DNA in liquid biopsies to support patient stratification and metastatic surveillance, and it may be utilized in a point-of-care diagnostic context for breast cancer (BC).16
A groundbreaking method developed in Sunny et al.'s study, tele-cytology combined with the risk stratification model, can be a priceless PoC tool for early detection/screening in oral cancer.17 In a three-armed parallel randomized pilot research, Alacevich et al. assigned recently referred patients with financial toxicity and cancer to either normal care plus instructional materials or individual, group-certified telehealth financial counseling (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The financial toxicity (COST) and Telehealth Usability Questionnaire (TUQ) scores were used to evaluate the feasibility of recruitment, randomization, retention, baseline, and post-intervention comprehensive scores. This pilot research evaluated the viability of a PoC intervention to link adult patients experiencing financial toxicity due to cancer to financial counseling provided by telehealth.18
1). The financial toxicity (COST) and Telehealth Usability Questionnaire (TUQ) scores were used to evaluate the feasibility of recruitment, randomization, retention, baseline, and post-intervention comprehensive scores. This pilot research evaluated the viability of a PoC intervention to link adult patients experiencing financial toxicity due to cancer to financial counseling provided by telehealth.18
A typical biosensor consists of the following components: (a) bioreceptors that bind specifically to the analyte; (b) an interface architecture where a particular biological event occurs and results in a signal picked up by (c) the transducer element; the transducer signal, which can be anything from the current generated at an electrode to the in-coupling angle of a laser beam, is amplified by a detector circuit using the appropriate reference and sent for processing by, (d) computer software to be converted to a meaningful physical parameter describing the process being investigated; finally, the resultant quantity must be presented through (e) an interface to the human operator.19 Electrochemical biosensors are classified as (i) potentiometric, (ii) amperometric, (iii) impedimetric, (iv) conductometric, and (v) voltammetric based on the transduction principle. The potential difference that builds up between the reference and indicator electrodes during the recognition process in an electrochemical cell when very little or no current passes through the electrode is the basis for the potentiometric transduction operation.20
It is important to note that the potential of biosensors in the field of cancer diagnosis and monitoring is still being explored and refined. The complexity of cancer and the intricacies of its biomarkers present unique challenges that require meticulous research and development efforts. Separating and enriching the circulating tumor cells (CTCs) in advance using CTC capture methods, followed by the introduction of the enriched CTC into electrochemical chambers, the addition of substrate, and aptamer/antibody microbeads (which have catalytic properties) is a major process for cancer detection. The microbeads can catalyze the reaction, induce color change, or generate gas; by monitoring the color change, pressure change, or pressure-driven distance, the CTC can be quantified. Well-designed channels, columns, and chambers would allow for effective cell separation. It also accomplished high-throughput detection, allowing for the quick analysis of hundreds of samples. The microfluidic chip's low cost, ease of use, and little reagent usage are its most crucial features.21
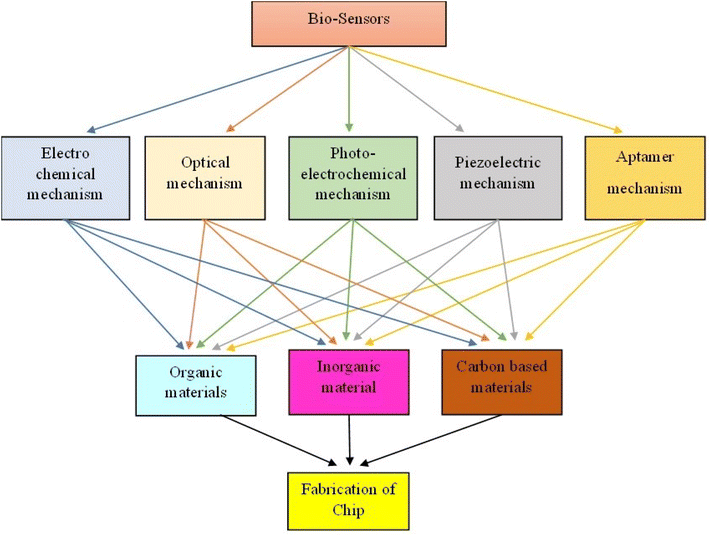
In this comprehensive review, we delve into the recent breakthroughs in biosensors and the associated processes. Notably, we explore cutting-edge research concerning the amalgamation of materialistic structures presented across distinct sections. Fig. 1 serves as an illustrative depiction of the materials and methodologies employed in the development of biosensors engineered for the detection and identification of various cancer biomarkers.
2. Architecture and functioning of biosensors for cancer detection
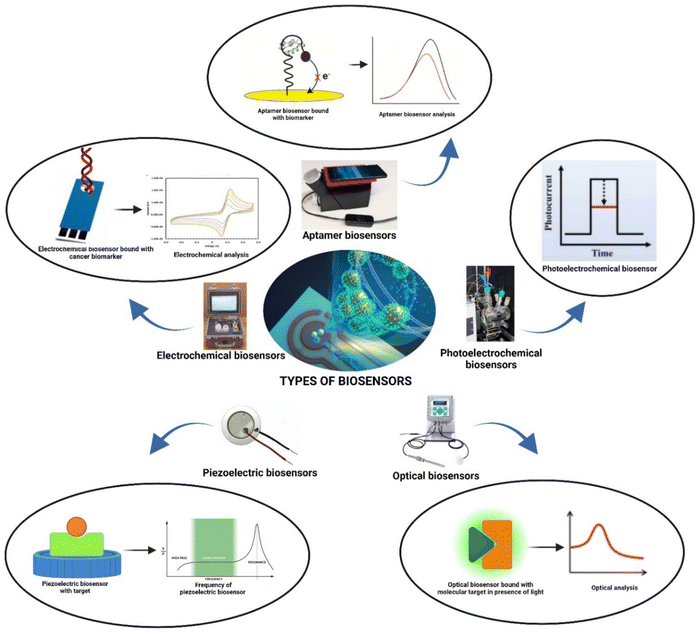
The biosensing field has experienced exponential growth, leading to the displacement of traditional methodologies for detecting an array of compositions, including DNA, proteins, and cancer-related biomarkers.22–25 Biosensors adhere to a standardized architecture, which is categorized into different transduction principles encompassing electrochemical, optical, photoelectrochemical, piezoelectric, and aptamer sensors (Fig. 2). These diverse sensor types leverage a wide array of compatible materials, facilitating the precise detection of cancer biomarkers.26–34 These advanced biosensor assemblies are meticulously designed in a specific sequence, intricately tuned to the precise detection of the biomarker in question. This strategic alignment of materials, mechanisms, and methodologies represents a significant advancement over conventional techniques, enhancing sensitivity and accuracy in detecting critical cancer biomarkers.42.1 Architecture of biosensors
While various cutting-edge technologies are continuously being created in laboratory settings, the procedures and methods for diagnosing cancer that are used in clinical practice have not evolved significantly over the past few decades. There are two distinct steps to the conventional diagnostic process. An invasive tissue biopsy of the afflicted area is first carried out. Medical imaging, such as computed tomography (CT), magnetic resonance imaging (MRI), or positron emission tomography (PET), frequently comes next. Although these techniques have received a great deal of validation and are therefore commonly used, they have some inherent drawbacks, including being intrusive, requiring laborious procedures, and being expensive. Although blood tests have made it possible to screen people more quickly and diagnose illnesses earlier, they are still quite intrusive, particularly for those who are weak. Although commercially available ELISA kits have a considerable test duration, they offer high sensitivity.35 Reagents used in radioimmunoassay are unstable and will eventually degrade, and measurement repeatability is not good. Despite the ease of use, high sensitivity, and accuracy of fluorescence immunoassay, the tedious labeling procedure makes quantitative analysis difficult. High-information biological materials and biomolecular signal systems can be obtained by Raman spectroscopy. However, for high-sensitivity quantitative and qualitative analysis, conventional Raman signal spectra are too faint to be often employed. In this sense, biosensors provide significant benefits, including quick reaction times and simpler methods.36,37The development of biosensors dedicated to cancer detection stands as a prominent and focused area of research for scientists and research communities. The multifaceted nature of biosensor engineering necessitates a synthesis of knowledge spanning diverse fields. Biosensors represent electromechanical devices with a hierarchical structure, commencing with a bioreceptor at the apex, followed by a transduction segment, and culminating in an analyte composition. These devices harness electrochemical or optical principles to amplify biological activity and convert it into discernible electrical signals through a myriad of physiochemical reactions.38,39 To address the challenges unique to the measurement of circulating proteins clinical samples, Nasrollahpour et al. developed an enzyme-free electrochemical biosensor based on electrosynthesized biocompatible WO3/poly glutamic acid nano-biocomposites. Apart from its environmentally friendly manufacturing process, the produced nano-biocomposite exhibited exceptional performance in untreated biological samples, including high protein content due to the low amine group propensity of both components. With a broad linear response between 1 ng mL−1 and 1 fg mL−1, the HER-2 concentration was detected with a nanobiosensor as low as 1 fg mL−1. Meanwhile, the procedure demonstrated optimal repeatability, stability, and specificity for HER-2 protein detection in untreated blood samples from BC patients.40
It demonstrates how to use a surface plasmon resonance-based optical fiber biosensor that is both affordable and portable for early BC detection. The HER-2 protein is highly expressed in BC, which is frequently linked with more aggressive tumour development and a worse prognosis; thus, a HER-2 aptamer may be utilized to identify the existence of HER-2 positive BC. The antigen–antibody binding approach is used to determine the presence of the HER-2 protein. Notably, the biosensor's reaction time was shown to be as quick as 5 s. The suggested biosensor provides a route to early BC prevention and shows promise for early detection of HER-2 protein in first cancer serum.41
Micro-electromechanical systems (MEMS) technology serves as a versatile platform, facilitating the customization of chips wherein various modules are intricately integrated to execute specific functions, such as the detection, injection, implantation, and tissue repair within the human body. These biosensors encompass multiple sections meticulously designed to capture proportional or linear signals in response to target analyte concentrations.42–44
Advancements in chip lithography techniques have paved the way for the precise formation of electrodes, crucial components of biosensors, using micro- and nano-scale sputtering methods. Various nanomaterials, including carbon fiber, gold nanoparticles (AuNPs), silver nanoparticles (AgNPs), and paper-based materials, are harnessed in the creation of electrode assemblies.45 In line with contemporary lithography methods, biosensor-based electrodes can be meticulously molded into diverse sizes, shapes, and fusion-based aspect ratios, thereby enhancing their capacity to detect signals.46 In one study, lithographic micro-electromechanical systems were used to create a comb of microchannel and immunosensor based on long-period fiber grating for the real-time and label-free detection of particular antigens. A microchannel long-period fiber grating (CM-LPFG) comb was used to connect the propagating core and cladding modes. The CM-LPFG-based immunosensor was made of an optical fiber sandwiched between a microchannel structure created using photoresist stacking techniques and a long-period structure. To detect specific protein antigen from the extracted protein mixtures of the cancer cell lines, a particular antibody against protein antigen was attached to an optical fiber surface and created a real-time resonance effect. Based on the diversification of a transmission loss, it was discovered that the CM-LPFG-based immunosensor for the real-time detection of label-free protein antigen is practical and sensitive, and it fulfils specific immuno-sensing objectives for lab-on-fiber (LoF) technology.47
Recent research underscores the pivotal role of material fusion in enhancing the detection of specific biomolecules. Fusion-based electrodes exhibit remarkable properties, offering linear responses across electrical, magnetic, and optical domains. These electrodes outshine conventional laboratory-based diagnostic techniques such as biopsy, chromatography, or light-based spectroscopy by several orders of magnitude.48 In summary, biosensors are poised to revolutionize the approach to the prognosis and detection of cancer-causing agents, introducing unparalleled levels of precision and efficiency.
In the scope of this review, we explore various categories of biosensors, dissecting their operational principles and assessing their success rates in identifying cancer symptoms. The comparative analysis of diverse research endeavors provides a comprehensive analytical perspective on the progress within the biosensing sector.49,50
2.2 Functioning of biosensor for identification of biomarkers
When it comes to designing biosensors, nanomaterials play a pivotal role in crafting the electrode assembly. Where the analyte and substrate converge to produce a selective signal specific to the target analyte. This critical juncture is where the transduction principle comes into play, serving as the sole mechanism responsible for extracting essential information from the generated signal. Within this framework, chemical reactions give rise to a diverse array of signals, each accompanied by various types of labels. The transduction principle serves as the discerning agent within this hypothetical cluster of signals, isolating the specific signal indicative of the target analyte. In the context of cancer-related molecule detection, various transduction processes are explored and discussed in detail.Numerous recent studies emphasize the calibration of these biosensors for re-use, tailored to specific patent situations. The ability to fine-tune biosensors provides medical practitioners with a valuable tool for continuously monitoring dynamic conditions within tissues. This approach not only improves the PoC concept but also does so at a cost-effective rate.56 A profound understanding of the electrochemical transduction principle's mechanisms for signal processing is imperative. The electrical signal processing unit scrutinizes the signal across a spectrum of transduction mechanisms, encompassing voltammetry, amperometry, potentiometry, and electrochemical impedance variations. The transition of the measured signal from the electrode to the signal conditioning module within the electrode assembly relies on careful control and manipulation of impedance matching between modules. This represents a pivotal task in the creation of multilayer-based hybrid biosensors. Throughout the electrochemical process, the strength of the physiochemical signal fluctuates within a range of 1 μA to 100 nA. Effective signal transmission hinges on the creation of a well-structured biosensor that incorporates a fusion of nanomaterials, allowing for the formation of compatible impedance matching between modular components, such as gel or nanowire strings. Consistent impedance matching ensures that the spectrum of signals can be readily observed, aiding in making informed decisions regarding cancer stage management.57
Nanomaterials exhibit diverse properties and can be categorized based on a range of attributes, including mechanical strength, temperature stability, thermal conductivity, inertness, electrical responsiveness, and physiochemical behavior. Within the realm of polymeric organic nanomaterials (ONMs), several subcategories have emerged as key players in the fabrication of cancer-based biosensing modules. These include polymer nanocomposites, molecularly imprinted polymers, dendrimers, hyperbranched polymers, nanostructured hydrogels, nanofilms derived from polymers, and covalent–organic frameworks. ONMs possess distinctive electrochemical properties, characterized by their remarkable inertness, rapid response kinetics, and robust physical integrity, making them ideal for immobilizing electrons within electrode assemblies. In contemporary research, metallic nanostructures such as nanowires, nanocages, and nanoshells composed of silver, platinum, and gold have garnered significant attention due to their exceptional sensitivity in detecting target analytes.58
Furthermore, carbon-based nanomaterials (CNMs) are emerging as prominent contenders in biosensing applications. Carbon allotropes have found utility in tissue and structural engineering, driven by their high surface-to-mass ratio. Notably, their lightweight nature, cost-effectiveness, and potential for reusability have raised considerable interest. Advances in tissue engineering and vascular structure design have enabled the shaping of carbon into various forms, including carbon nanotubes (CNTs), carbon dots (CDs), graphene-based materials, nanodiamonds, and fullerenes. The fusion of these carbon-based materials holds promise for the design of cancer-based biosensors. To delve deeper into the electrochemical transduction process facilitated by these materials and its role in enhancing biomarker specificity with heightened sensitivity and superior detection limits, please refer to Table 1.
| Material | Nanomaterial base | Target analyte | Size (nm) | Range of detection | Limit of detection | Key features | Ref. |
|---|---|---|---|---|---|---|---|
| Organic | Graphene/Ag/Au/polyaniline | PSA | 42–99 | 1 pM to 10 mM | <1.02 pM | Several organic NMs have proven effective in detecting tumor markers, such as prostate-specific antigen (PSA), human carcinoembryonic antigen (CEA), alpha-fetoprotein (AFP), human chorionic gonadotropin (hCG), human epidermal growth factor receptor-2 (HER-2), cancer antigen 125 (CA125), cancer antigen 15-3 (CA15-3, MUC1), and cancer antigen 19-9 (CA19-9) with great sensitivity and selectivity | 59 |
| Nano MIPs acrylamide monomer/PVC | P53 | 80–125 | 1 nM to 1 mM | 1 nm | As the suggested sensor has a surprisingly high sensitivity spanning a concentration range of 0–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm, as well as a high quality factor (QF) and figure of merit, it might be an important component of a viable platform for detecting low quantities of various heavy metal ions in freshwater 000 ppm, as well as a high quality factor (QF) and figure of merit, it might be an important component of a viable platform for detecting low quantities of various heavy metal ions in freshwater |
60 | |
| PNC (Phc-PSy) | CA-125 | 25–49 | 0.012–125 U mL−1 | 10–4 U mL−1 | Diagnostic procedures and developments achieved with LCs are gaining increased sensitivity due to their streamlined strategy and multiplexed detection | 61 | |
| Au/MoMA/LoPr | NSE | 24–81 | 1.01 pM–10.01 nM | 0.77 pM | Organic semiconductor nanoparticles (OSNs) have various benefits in biosensing applications, including strong fluorescence emission, variable emission wavelength, and good biocompatibility. Furthermore, by applying alternative design methodologies, a diverse spectrum of functional probes that suit distinct biosensing needs have been produced | 62 | |
| Inorganic | Graphene–palladium–platinum | PSA | 15–60 | 1–300 μm | 0.3 μM | The research investigates how organic framework emitters are incorporated into biosensor systems to improve sensitivity and selectivity for a variety of analytes. The authors compare organic framework emitters to standard inorganic emitters, highlighting benefits such as improved biocompatibility, simplicity of functionalization, and the possibility of multiplexed detection | 63 |
| Gold–graphene NCg | CEA | 30–80 | 6–100 μm | 0.5 μm | This article introduces the principles and mechanisms of electrochemiluminescence (ECL) biosensors for biomarker determination, as well as recent advances in ECL biosensors on key aspects such as new ECL reagents and materials, new biological recognition elements, and emerging construction biointerfacial strategies, with illustrative examples and a critical eye on pitfalls. It also discusses the challenges and perspectives of ECL biosensors in health analysis | 64 | |
| Gold NCgs-nanotubes | SCCA | 20–50 | 0.3–0.6 μm | 0.1 μm | Nanomaterial-based biosensor systems provide high conductivity, a wide surface area, and great electron transferability to immobilized biomolecules | 65 | |
| AuPtAg-ANCs | PSA | 50–150 | 0.17 ng mL−1 | 0.05–50 ng mL−1 | Biosensors are useful for improving therapy success and patient survival since they need minimum invasion. Carbon elements, on the other hand, have a wide range of characteristics at the nanoscale, making them ideal for biosensor manufacturing. As a result, carbon nanomaterials such as graphene and carbon nanotubes are employed as elite nanomaterials in healthcare-related biosensors | 66 | |
| Carbon allotropes | Graphene oxide nanosheet | AFP | 30–95 | 0.01–300 ng mL−1 | 6.4 pg L−1 | Electrochemical (bio)sensors made from nanodiamonds have been used to detect a wide range of species of interest, including organic chemicals, medicines, hormones, glucose, heavy metals, nitroaromatic explosives, and pesticides | 67 |
| Au NPs/NH2-GS | AFP | 17–90 | 10–198 fg mL−1 | 3.3 fg mL−1 | The surface area, porous network, hybridization, functionalization, synthesis route, combination of dimensionalities, purity levels, and mechanisms underlying carbon nanomaterial interactions all have an impact on biosensor technology applications in bioanalytical chemistry | 68 | |
| ND-Di hexadecyl phosphate film | CA19-9 | 09–30 | 0.299–108 μm | 54.5 nM | Various functionalized CNMs have a high capability for improving cell targeting of anti-cancer medicines. Because of their thermal capabilities, they have been widely utilized in cancer photothermal and photodynamic treatment, which is supplemented with laser irradiation and CNMs. The CNMs can also pass the blood–brain barrier, allowing them to treat a variety of brain ailments, including neurodegenerative diseases, by eliminating amyloid fibrils | 69 | |
| CNT-amended graphite electrode | TP53 | 10–65 | 0.05–1 nM | 18 μm | The biosensor displayed great storage stability of 63 days, as well as high accuracy in detecting ANXA2 in blood samples from LC patients, as verified by the enzyme-linked immunosorbent assay method | 70 |
Contemporary research endeavors are dedicated to the detection of potential cancer biomarkers, including exosomes, through the utilization of hybrid and meticulously structured biosensors integrated with electrochemical sensing platforms. The prevailing design emphasis is directed toward expediting the immobilization process while concurrently enhancing the conductivity detection range within biosensing modules. Experimental trials and compositional testing aimed at optimizing morphology and design have yielded substantial improvements in the nano-sensing interface. Notably, these efforts have led to the integration of a more active surface area, thereby augmenting the sensitivity and efficacy of biomarker detection. The realm of nanocomposite development is particularly noteworthy, highlighting strides in enhancing charge carrier mobility and the emulation of low band gaps within material layers. One noteworthy example is the utilization of the carbon-based material known as ‘Graphdiyne.’ This material stands out due to its remarkable charge carrier mobility, facilitating efficient charge transport, and its judiciously selected band gap, which contributes to superior performance.71 In the context of detecting CA19-9 antigens, the conductivity detection limit achieved with Graphdiyne was remarkable, nearing 0.00012 U mL−1 within a concentration range spanning from 0.00051 to 199.99 U mL−1. Additionally, ionic liquids have garnered substantial attention in this arena, primarily owing to their compatibility with carbon nanotubes. Ionic liquids exhibit an outstanding electrochemical window, enhancing the sensitivity of the transduction mechanism, and thereby surpassing the capabilities of conventional detection methods.72 These materials also possess noteworthy optical properties, further expanding their utility in the analysis of biomarkers.
Uniyal et al. fabricated a frequency-based optical biosensor.73 The overall diameter of the biosensor was varied from 4 to 6 μm and 140 mm stretching length. The IgG layer is coated with polydopamine to trap anti-IgG. It has been observed that the concentration level of anti-human IgG linearly varied from 0 to 13.99 ng mL−1 with a sensitivity range of 49 mm. The recent advancement in photonic crystal fiber-based biosensors is also gaining popularity due to improved refractive index in relation to cancer-related antigens detection.75 The hybridization of photonic crystals with plasmonic metals such as gold, silver, and copper tremendously increase the biosensor's sensitivity. However, due to poor biomolecule adsorption, these sensors lack the authenticity of results.76
Optical transduction-based biosensors are highly regarded for their exceptional sensitivity, selectivity, reusability of assembly, and non-contact measurement capabilities. These attributes have led to their widespread adoption in the design of flexible wearable biosensors, where the need for versatile and reliable biosensing platforms is paramount. The optical transduction process comprises three fundamental components: a light source, an optical chamber, and a photodetector. Within this assembly, received optical signals are meticulously transformed into electrical signals. Importantly, this generation of electrical signals is entirely contactless, eliminating the need for traditional electrodes. The linear transfer of these signals is seamlessly integrated into a signal conditioning module. In the optical transduction module, electrodes are strategically employed to partition serum samples into sub-sections, facilitating the monitoring of various physio-optical characteristics of transmitted light rays.77 These optical signals dynamically interact with biomolecules within the electrode and transduction module assembly. The alterations in optical signal characteristics, such as changes in reflection, refractive index, fluorescence, and grating, are induced by chemical reactions between the target analyte and labeled substrate. The detection of cancer cell-based signals relies on diverse gene detection methods, encompassing genetic assembly formation and protein analysis. Noteworthy biomarkers, including Squamous Cell Carcinoma Antigen (SCCA), carcinoembryonic antigen (CEA), and prostate-specific antigen (PSA), have been successfully identified through these advanced biosensing techniques. It is important to highlight that the backbone of these biosensors lies in nanostructured materials.78 The integration of biocompatible nanomaterials in various structural configurations has yielded remarkable advancements in biosensing performance. A comprehensive overview of recent advances in optical biosensors is presented in Table 2.
| Nature of material | Nanomaterial | Target analyte | Size (nm) | Range of detection | Limit of detection | Key features | Ref. |
|---|---|---|---|---|---|---|---|
| Organic | Gold electrode-MIP film | PSA | 14–16 | 0.1–1 μm | 0.1 μm | Because of the desirable biosensor qualities such as ease of use, cost, and sensitivity, novel technology has emerged as a method of employing optical features for cancer cell detection | 79 |
| CuLnZnS-QDs | NSE | 06–19 | 0.1–1.5 nM | 0.03 nM | These biosensors are very sensitive, easy to build, inexpensive, and recyclable; yet, they are typically non-selective, and controlling the sensing process at high currents is difficult | 80 | |
| Hydrogel–Ag–Au nano frame | CA-125 | 40–60 | 1–50 μm | 50 μm | Optical biosensors have superseded traditional techniques of detecting and quantifying CA-125 oncomarkers in biomedical research due to their capacity to perform multiplexed detection of several analytes and their real-time and direct monitoring capabilities | 81 | |
| Phthalocyanine-doped polystyrene shapes | CA-125 | 200–250 | 0.01–127 U mL−1 | 10−4 U mL−1 | The sensing behavior of this metal organic framework (MOF) toward cancer biomarkers has been investigated using a variety of experimental and computational methodologies. Luminescence and electrochemical investigations of MOF sensors offered quick and cost-effective systems for detecting cancer biomarkers | 82 | |
| Inorganic | Cadmium sulfide with AuNPs | NSE | 05–20 | 20–103 fg mL−1 | 4 fg mL−1 | The combination of CdS's light absorption and AuNP's plasmonic characteristics results in considerable signal amplification, allowing for the detection of extremely low concentrations of target analytes | 83 |
| MNPs | PSA | 15–21 CFU mL−1 | 14 CFU mL−1 | This biosensor might be used as a novel clinical tool for early diagnosis and prognosis of prostate cancer. Furthermore, using the concepts utilized to design a biosensor, inexpensive and simple biosensors for early detection of a variety of human ailments, including heart disease, cancer, and infectious diseases, might be produced | 84 | ||
| Au–Ag nanoclusters | PSA | 10–25 | 0.4–36.5 U mL−1 | 0.29 U mL−1 | The combination of AuNPs and AgNps improves the sensitivity and stability of the biosensor. The gold surface can easily change to attach antibodies or other PSA-specific recognition components, whilst the silver increases the sensitivity of the optical signal | 85 | |
| Gold nano frame with cadmium | CA19-9 | 05–12 | 1–70 μm | 0.64 μm | This paper shows the successful creation of a highly sensitive and selective electrochemical biosensor for detecting CA19-9, a crucial biomarker for pancreatic cancer | 86 | |
| Carbon allotropes | Europium-enriched GQDs | CEA | 06–20 | 0.5–50 μm | 0.31 μm | GQDs' strong electron transfer and facile biomolecule immobilization characteristics make them ideal for constructing effective (bio)analytical sensors. These photoluminescent QDs have exceptional biocompatibility, high current density, quick electron mobility, high water solubility, outstanding photochemical stability, and minimal cytotoxicity, making them dominant in the field of sensing | 87 |
| AuNPs enriched graphene nano frame | PSA | 09–16 | 0.51–0.4 μm | 0.51 nM | This technology differs substantially from current peptide-based PSA detection methodologies. It has great promise for usage in laboratory investigations and clinical diagnostics because it involves only a basic sample handling process and a small investment in equipment | 88 | |
| Dendrimer wire with CQDs | SCCA | 20–40 | 0.01–2 mM | 0.01 μm | Depending on the material, intended fluorescence, and expected size, these carbon-based fluorescent nanoparticles can be manufactured with a number of chemical and natural chemicals and methods. Because of their unique adjustable optoelectronic capabilities, they display size-tunable emission, excitation-dependent emission, and solvent-dependent emission | 89 | |
| MWCNTs | NSE | 05–15 | 0.05–1 ppb | 0.05 ppb | This aptasensor demonstrated high repeatability, stability, and specificity, indicating a feasible technique for detecting NSE in clinical diagnostics | 90 |
In recent developments within the realm of optical transduction-based biosensors, the exploration of photo-sensing has emerged as a focal point of research interest. This approach offers notable advantages, particularly in the facile detection of cancer biomarkers. A noteworthy study by Barve et al. has centered its attention on the identification of Neuron-Specific Enolase (NSE) as a pivotal biomarker derived from neuroendocrine cells.89 SE, an acid-based protease serum component, is extracted explicitly from neuroblastoma sources. Intriguingly, investigations have revealed the utility of the NSE biomarker in the early-stage detection of lung-related cancer cells. This innovative research underscores the potential of optical transduction-based biosensors in revolutionizing early cancer diagnosis through the precise identification of crucial biomarkers.
Wang et al. designed a net-based farmwork from the Au–Ag nanorods for target-doped photosensitive biosensors.90 In the experimental results, it was observed that the addition of a dopant in the transition composition could improve the sensitivity range and boost the amplification of the received signal with a high precision rate. Al Mahmud et al. designed a biosensor using the extract of cadmium-sulfate, which had an ultra-sensitive assembly for identifying PSA biomarkers from the sample.91 This biosensor assembly achieved ultra-sensitivity with a 1 pg mL−1 detection limitation. The response was approximately 100 times more sensitive than other categories having the same substances.
Zare et al. developed a QDs-based antibody-antigen assembly to detect CEA cancer biomarkers for effective quantitative analysis of cancer biomolecules on a spectrum form.92 Due to the proration in the quantum dots with nano frame structure, the sensitivity becomes very high with a detection limit below 80 fM. In immunoassay-based sensing methods, recent advances have been observed in label-free sensing to detect cancer-related biomarkers. Nanomaterial-based optical sensors can frame out the analyte chamber to produce a label-free spectrum of light to identify the target analyte.
Matar et al. used the fusion of SnS2-mpg-C3N4 to design a photochemical immunoassay biosensor to detect PSA biomarkers.93 This biosensor had an exceptional linear response concerning the target analyte, which varied from 55 fg mL−1 to 12 ng mL−1, and the sensitivity limit was near about 18 fg mL−1.
In addition, Foroozandeh et al. designed a nanoparticle (denoted as MoS2QDs-g-C3N4-CS-AuNPs) to build an optical sensor based on plasmon resonance with the limit of detection of 0.25 pg mL−1 and the linear range from 1 pg mL−1 to 24 ng mL−1.94 The spectroscopic methods are also embedded in the biosensors for the analysis of the biospectrum and the improvement of the quantification of target cells. Surface-enhanced Raman scattering is an ultramodern method based on the laser light spectrum.95,96 This assembly has four key modules (light source, monochromator, high-fidelity signal conditioning module, and photosensor) cascaded to study the target analyte. This method was suitable for capturing electrochemical reactions between antigen–antidot carriers with an exceptional detection limit. This scattering method exhibits excellent linear response toward studying metallic NP-based immunoassay biosensors. Surface-enhanced Raman scattering has recently gained popularity in biosensing devices due to its nanoscale fabrication. As per recent trends in optical transduction mechanisms, the amalgamation of both electrochemical transduction and optical transduction paves the way to improve biosensor designs in new horizons.97
The photoelectrochemical-based biosensors are working on the principle of irradiation of light signals. When the light assembly passes through the sample solution of the analyte, the strength of the photo signal varies. The electron and hole pairs are generated at the photo-sensing element as per the light received. It is worth using the low band gap semi-conducting materials to prepare photoelectrochemicals for improved results compared to the individual optical and electrochemical transduction-based sensors. This transduction mechanism opens a big pool of research in various dimensions, such as contactless detection, very low detection rate, and flexibility in making wireless. Recently, different exogenic materials such as Si, TiO2, CdS, and phosphate dots have been inspected and selected to make these kinds of sensors due to their spectacular performance in justifying hypothesis values on the probability index.100–112 Due to optical isolation between the electrode assembly and signal conditioning circuit, the output signal has no interference effect, and the use of light ensures the sensor's reuse on a periodic cycle. Table 3 shows the response of materials in relation to photoelectrochemical transduction.
| Material | Nanomaterial base | Target analyte | Size (nm) | Range of detection | Limit of detection | Key features | Ref. |
|---|---|---|---|---|---|---|---|
| Organic | TiO2 crystals and PAMAM dendrimers | CYFRA 21-1 | 40–60 | 10–90 pg mL−1 | 3.3 fg mL−1 | The nanoparticles were coupled with CYFRA21-1 to create a biosensor for monitoring nonsmall cell lung cancer due to their sensitive detection of CYFRA21-1 | 101 |
| 2D porphrinic-COFs on AgNPs | PSA | 50–100 | 0.5–100 ng mL−1 | 100 pg mL−1 | COFs with large surface area and porosity, high electrical conductivity, and new interactions with bioactive substances have the potential to diagnose cancer (by biosensing and bioimaging) | 102 | |
| MIPs based on CNDs-embedded COFs | SCCA | 250 | 0.025–0.4 mg mL−1 | 7 μg ML−1 | The constructed biosensor demonstrated various benefits, including superb specificity, exceptional selectivity, and great stability. The suggested approach is suitable for the quick and sensitive detection of SCCA in human serum and shows potential for clinical usage | 103 | |
| Phthalocyanine-doped polystyrene spheres | CA-125 | 100–200 | 0.01–127 U mL−1 | 4 U mL−1 | The significant suppression of the luminescence intensity of the Ni–phthalocyanine complex doped in PS matrix by varied concentrations of CA 125 was effectively used as an optical sensor for detecting CA 125 in ovarian disease blood samples | 104 | |
| Inorganic | Pd–Ag–CeO2 core–shell NPs | NSE | 40–110 | 0.4–99 ng mL−1 | 0.14 pg mL−1 | The suggested nanocomposite structure demonstrated superior catalytic performance, increased specific surface area, and acceptable stability. The developed immunosensor demonstrated great sensitivity, adequate selectivity, and acceptable stability in detecting NSE | 105 |
| Ag nanoplates | CEA | 30–90 | 0.12–6 μg mL−1 | 42 pg mL−1 | Given its simplicity, high sensitivity, outstanding repeatability, and specificity, the proposed biosensor can be deemed an effective diagnostic test for CEA measurement | 106 | |
| Nanoclusters of gold–silver | PSA | 30–55 | 0.45–38 U mL−1 | 0.25 U mL−1 | The findings suggest that integrating biosensors for cancer screening with serum PSA levels is a potential technique for enhancing prostate cancer diagnostic accuracy | 107 | |
| MNPs-tunneling magnetoresistance | NSE | 25–75 | 14–1.3 × 109 CFU mL−1 | 13 CFU mL−1 | The suggested idea of a simple tunnel magnetoresistance (TMR) sensor for real-time detection of MNPs has considerable potential applications in cancer cell localization by magnetic particle detection in tissue and cancer treatment via magnetic hyperthermia | 108 | |
| Carbon allotropes | ZnFe2O4–Ag/rGO nanocomposites | CEA | 40–80 | 1–200 ng mL−1 | 0.98 pg mL−1 | The nanohybrid's exceptional high catalytic capacity may allow it to replace current target molecule identification methods | 109 |
| Graphene | PSA | 30–90 | 0.06–101 ng mL−1 | 0.04 ng mL−1 | The suggested immunosensor demonstrated remarkable performance, with high sensitivity, selectivity, and stability. On the one hand, this form of electrochemiluminescent immunosensor should be practical for the field of cancer biomarker detection and other life science domains | 110 | |
| Europium-enriched GQDs | CEA | 45–110 | 0.6–49 μM | 0.34 μM | A novel photoluminescence (PL)-based assay for the detection of CEA has been presented, which is based on the graphene-quantum-dot (GQD) surface for Eu3+ ions. The graphene-like structures, paired with QD-like optical characteristics, point to GQDs' potential as flexible instruments in analytical science and biology | 111 | |
| Magnetic ND/GO | PSA | 50–90 | 5–249 ng mL−1 | 1.50 ng mL−1 | The developed biosensor could detect extremely low levels of PSA | 112 |
Recently, Fan et al. designed a photoelectrochemical biosensor using the TiO2/PpIX/Ag–Cu2O composition. This assembly was beneficial in identifying the CYFRA 21-1 biomarker associated with lung cancer. The hydrothermal method was used to fabricate TiO2 nanomaterials on the protoporphyrin IX (PpIX) molecules and Ag–Cu2O. This assembly has an excellent response in the visible light spectrum, so the photoelectric chemical properties are improved from other families of the same biosensors group. As per the results, this assembly attains the regenerative mechanism for electron–hole pairs, which increases the photocurrent amount from the previous threshold level. This assembly provides a high-sensitivity response with a wide range of linearity in the photospectrum.112
Esfandiari et al. developed a biosensor assembly to diagnose BC-related MDA-MB-231 biomarkers. The photoactive material composition of heterostructures (CdS/Bi2S3HSs) was fabricated to prepare the modular biosensor in cascading with signal amplification strategy for immune identification between epidermal receptors. The results show that this assembly has an excellent response during photoelectrochemical reactions. This biosensor exhibits improved sensitivity and adsorption response for recognizing the MDA-MB-231 biomarker. The linear response of this sensor module varies from 98 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cells per mL with six cells per mL detection range. The signal-to-noise ratio of this biosensor is 3.113
500 cells per mL with six cells per mL detection range. The signal-to-noise ratio of this biosensor is 3.113
Yu et al. have prepared an improved and sophisticated label-free photoelectrochemical immunoassay biosensor using the photoactive nanomaterial composition of Bi2WO6/BiOBr to detect prostate-specific antigens. This heterojunction-based biosensor composition attains various properties, such as improved photocurrent response due to immobilized dopamine current. In the optimal testing conditions, the sensor produces an improved linear response ranging from 1 pg mL−1 to 51 ng mL−1.114
The instrumentation-based parameters such as stability, selectivity, reproducibility, and the ratio of signal to noise of this assembly pave the way to develop real-time responsible sensors. The study of Han et al. led to the development of ultra-thin and diffusion-based immune sensors to detect prostate-specific antigens in the human body. The authors used Fe-loaded Bi2O2S nanosheets to diffuse photoanode crystals with a photoelectric transduction mechanism. Response of the split incubation reaction in the signal probe (PB-mAb2) helps to mark the presence of target PSA antigens. The cost of this assembly is far less than other material-based assemblies. They achieved a wide range of linear responses ranging from 0.1–105 ng mL−1 with the detection limit near 35 pg L−1. This sensor assembly leads to the designing and developing of very low-cost and real-time sensors to identify cancer-based cells.115
| Material | Nanomaterial base | Analyte | Size (nm) | Range of detection | Limit of detection | Ref. |
|---|---|---|---|---|---|---|
| Organic | Gold surface modified nano film of 2-hydroxyethyl methacrylate ethacryloylamido aspartic acid | PSA | 24 nm | 0.1–1.25 nM | 0.03 nM | 120 |
| Bilirubin-imprinted nanofilm with 2-hydroxyethyl methacrylate methacryloylamido aspartic acid | CEA | 40–90 | 1–50 μg mL−1 | 0.45 μg mL−1 | 121 | |
| Zr-amide porphyrin-based COFs | CA-125 | 40–75 | 5–60 pM | 2.3 pM | 122 | |
| CuInZnS-QDs with polydopamine and AuNPs | PSA | 30–50 | 0.1–1.5 nM | 0.03 nM | 123 | |
| Inorganic | AuNPs | CA-125 | 40–110 | 10–99 pM | 2.4 pM | 124 |
| MNPs | PSA | 60–112 | 14–1.3 × 109 CFU mL−1 | 13 CFU mL−1 | 125 | |
| Nanoclusters of gold–silver | CEA | 30–90 | 0.6–38 U mL−1 | 0.4 U mL−1 | 126 | |
| Cadmium sulfide AuNPs | CA-125 | 30–65 | 20–103 fg mL−1 | 4 fg mL−1 | 127 | |
| Carbon allotropes | CNTs-amended graphite electrode | CEA | 25–75 | 0.05–1 nM | 18 μM | 128 |
| CQDs | PSA | 40–78 | 0.714–4.286 ng mL−1 | Less than 1 ng mL−1 | 129 | |
| Carbon–silver-based multiwalled nanotubes | CEA | 80–140 | 0.25–250 ng mL−1 | 0.08 ng mL−1 | 130 | |
| Gold–silver–graphene hybrid nanosheets | PSA | 40–110 | 0.002–201 ng mL−1 | 0.45 pg mL−1 | 131 |
Bakhshpour et al. developed a multi-walled carbon nanotube (MWCNT)-based biosensor. In this transduction mechanism, two electrodes were fabricated on an aluminum nanoplate, and then the alternating voltage was applied to observe the vibration-based signal at the actuation point.130 The measured physical indication was very low, but when the frequency of the applied signal was changed, at a point, the strength of the physical signal increased to represent the target analyte during immunoassay trials. When the result of this biosensor was calibrated with a conventional ELISA test, the 95% confidence interval was reported on the probability index. Hence, this simple mechanism indicates the importance of piezoelectric transduction in relation to the development of biosensors in cancer diagnosis to the research community. The various parameters of this biosensing assembly, such as low fabrication cost, real-time response, high sensitivity, ultra-lower detection limit, reproducibility, and flexibility in the electrode assembly, attract the researchers to find new horizons.
Lino et al. developed fan-shaped and circular-shaped biosensors by using flexural plate-wave-based materials such as silico, ferromagnetic metal, and quartz-based multilayer floating structures to detect CEA.131 The structure was developed to implant a silicon-grooved reflective grating property for balancing the resonance effect. In the trials, they achieved a minimum detection limit of 6 ng mL−1, a time response of 8 min, and a high linear sensitivity response of 50 to 60 cm g−1. With the advancement in photolithography-based fabrication of nanomaterials, multi-layered biosensor electrodes with piezoelectric transduction mechanisms were developed.
Villarim et al. developed a fusion of plasmon resonance-based biosensors in association with the piezoelectric effects of metals to reduce the reaction time inside the assembly for fast response.132 The enhanced nanolayers of AuNPs and piezoelectric material NaNbO3 were fabricated to detect the immune incubation process of target PSA from the plasma. Using the density function spectrum, the confidence interval of measurement regarding linear response and sensitivity was 97% concerning the ELISA test for target PSA. This assembly sets a benchmark for the quick and meticulous detection of cancer-related proteins. The electrochemical methods adopted to study cancer biomarkers have limited transfer of electrons from the sensor to the actuation module. There is a need to resize the platforms for effect measurements.
Li et al. developed a piezo-phototropic-based flexible biosensor for recognizing PSA from the cancer-associated biological fluid.133 In addition to this transduction mechanism, a multilevel signal enhancement scheme was adopted to promote the detection of ascorbic acid from the substrate, enhancing the sensitivity limit of this biosensing module. Unlike the electrochemical and optical transduction mechanism, this assembly promotes the transfer of physical information about chemical reactions on an ultrasonic signal level, which is why the label-free detection was done with a very low interface level between the cascaded modules. This biosensor assembly achieved the detection sensitivity of PSA from 0.02–40 ng mL−1.
To detect the circulating tumor cells (CTCs), Chi et al. developed a Bi2O2S nanoflowers, which was fabricated along with 45 nm sized AuNPs.136 The composition of AuNPs/Bi2O2S coated on ITO electrode with mercapto-group functionalized aptamer (SH-Apt). During the photoelectrochemical process, it has been observed that the MCF-7 cells were specifically trapped on the prepared hydrothermal-based composition, likely on the surface of SH-Apt/AuNPs/Bi2O2S/ITO. This biosensor produces a linear response ranging from 50 to 6 × 105 cells per mL with a detection limit of 17 cells per mL. This study helps to test the serum on a clinical level. This configuration indicates the importance of the aptamer transduction mechanism in the detection of specific tumor cells related biomarkers. The response of biosensors based on the aptamer transduction mechanism is shown in Table 5.
| Material | Nanomaterial base | Target analyte | Size (nm) | Range of detection | Limit of detection | Ref. |
|---|---|---|---|---|---|---|
| Organic | 2D porphrinic-COFs on AgNPs | CEA | 80–100 | 0.5–100 ng mL−1 | 100 pg mL−1 | 137 |
| TiO2 crystals and PAMAM dendrimers | PSA | 40–89 | 10 ag mL−1 to 90 pg mL−1 | 3.3 fg mL−1 | 138 | |
| 4-Aminobenzoic acid/MWCNTs | CA-125 | 25–30 | 0.25–10 μM | 0.2 μM | 139 | |
| Gold electrode-MIPs film | NSE | 14–16 | 0.1–1 μM | 0.1 μM | 140 | |
| Inorganic | Gold–graphene NCg | PSA | 20–100 | 6–1.6 × 103 μM | 0.5 μM | 141 |
| Nanoclusters of gold–silver | PSA | 80–120 | 0.5–36 U mL−1 | 0.4 U mL−1 | 142 | |
| AuNPs | CEA | 45–95 | 10–100 pM | 2.4 pM | 143 | |
| SH-Apt/AuNPs/Bi2O2S/ITO | CTC | 40–95 | 50 to 6 × 105 cell per mL | 17 cells per mL | 144 | |
| Carbon allotropes | AuNP-rGO | PSA | 50–150 | 0.03–520 ng mL−1 | 0.041 ng mL−1 | 145 |
| ZnFe2O4–Ag/rGO nano-frame | CEA | 50–110 | 1 pg mL−1–220 ng mL−1 | 0.88 pg mL−1 | 146 | |
| Graphene oxide-chitosan-ferrocene nanoplates | SCCA | 20–100 | 0.001–9 μg mL−1 | 0.35 ng mL−1 | 147 | |
| Au–Pt-vertical graphene electrode | NSE | 40–65 | 1 fg mL−1–104 ng mL−1 | 0.8 fg mL−1 | 148 |
2.3 Comparative analysis of different transduction methods
The inherent miniaturization, mass production, and low cost of electrochemical transduction make it stand out. However, before portable, reliable, and user-friendly point-of-care biosensors for cancer diagnosis through the detection of circulating miRNA expression profiles become a reality, there are still several significant obstacles to overcome. Additionally, as a result of these difficulties, there is currently no commercial electrochemical biosensor for circulating miRNA analysis to the best of our knowledge; as a result, more work has to be done soon on validation, clinical assays, and commercialization.149 Currently, electrochemical detection is dealing with many issues that need to be resolved to create a precise and sensitive cancer identification technique. Li has examined the sensitivity ranges of many methods for determining the least residual illness of hematologic neoplasms.150Hematological neoplasms can be identified with a better degree of sensitivity thanks to electrochemical biosensors. For electrodes intended for single use or for repeated usage, the stability of the biosensor is a crucial component. An alternative but more important topic is how to appropriately assess actual samples in vivo for the advancement of electrochemical biosensors in the future. These challenges have thus far kept these approaches mostly in the experimental realm, preventing their widespread clinical use. The ideal in vivo sensor should be stable, sensitive, safe, and compatible with cells and tissues. An electrochemical biosensor needs to possess exceptional selectivity and be capable of detecting several tiny molecules, including aptamers and folic acid, as individual components. Furthermore, a powerful method in bioresearch to achieve an incredibly sensitive and discriminating identification of various compounds is the combined use of the unique identification powers of aptamers and the catalytic ability of enzymes. The identification of electroactive substances is substantially facilitated by enzymes associated with biomolecules.151
Non-toxic, low-reactivity nanomaterials that do not elicit an immune response can be used to optimize the biocompatibility of these biosensors. Suppose it is shown that nanoparticle-based electrochemical biosensors are very sensitive and effective detection instruments in in vivo experiments as well. In that case, they may eventually emerge as the standard way for diagnosing leukemia.152
Although aptasensors provide many benefits, there are still issues with aptamers that restrict the practical uses of these sensors. The applicability of aptasensors in complex biological systems is limited because the immobilization of aptamers onto surfaces might alter the aptamer conformation, which is further influenced by the makeup of the binding environment. There are still difficulties; for example, poorly designed proteins may interact non-specifically with aptamers and cover the analyte's unique binding site. Moreover, aptamers and the nucleic acids found in biological fluids may hybridize to modify the aptamer's conformation and, in turn, the analyte's binding site.
While some of the issues listed above are being addressed by developing research in the field, other areas of interest include novel techniques like the creation of aptamer-based micro-/nanochips for therapeutic applications. Additionally, aptamer microfluidic/wearable devices are anticipated to be crucial in the early diagnosis of cancer. Aptasensors have reportedly been used for cancer diagnosis in a number of different ways. These biosensors' aptamers are engineered to offer exceptional selectivity and enhanced sensitivity for the early detection of key cancer kinds, including those that impact the prostate, breast, and lung tissues of humans. Diagnostics is greatly aided by some biomarkers that are often expressed on the surfaces of these cancer cells. While many of the aptasensors that have been published are just electroanalytical and have no therapeutic use, the development of methods based on ratiometric electrochemical sensors can enhance the sensitivity, specificity, and output of cancer biomarkers. Furthermore, to build point-of-care testing (POCT), novel methods with enhanced sensitivity that are scalable and specific enough for early-stage cancer diagnosis are required. The next stage that will spur the commercial deployment of aptasensors for broad cancer diagnoses is the development of POCT. Aptasensors have several benefits, such as a low cost and relatively quick analysis time, which makes them extremely promising for clinical applications. However, there are also some drawbacks, such as the lack of long-term stability of bioelectronic chips. Thus, with more study, aptamer-based biosensors can be created as strong instruments for the accurate and efficient identification of biomarkers of different cancer cells in physiological fluids.153
Optical biosensors, including metamaterials, evanescent waves, colorimetric detection, and surface plasmon resonance, have been used in the detection of cancer. It has been demonstrated, meanwhile, that conventional techniques, such as optical coherence tomography, surface-enhanced Raman spectroscopy, colorimetric detection, and reflectometric interference petrography, call for a skilled operator to oversee the data that is being received. Furthermore, colorimetric detection is primarily dependent on the size, shape, and material type of the nanoparticles utilized, which may lead to a high degree of fault probability, even if it is simple to set up and the necessary equipment is readily available. The SERS method is likewise linked to the same drawbacks. Despite being quick, safe, and non-invasive, OCT is expensive and mainly utilized for diagnosing retinal conditions. Furthermore, temperature changes do not affect the RIfS approach. Conversely, novel methodologies such as SP PCF, slot waveguides, and metamaterials rely on quantifying the shift in resonance wavelength/frequency in accordance with the blood specimen being examined. As a result, these techniques can identify cancer early, are label-free, and do not require the assistance of an expert to do the analysis. It is also important to remember that slot biosensors can provide strong field confinement in the analyte under study, which can lead to great sensitivity. Additionally, the SP PCF sensors offer great sensitivity and design freedom. Moreover, depending on the analyte being studied, metamaterial biosensors can generate sharp resonance, which can be detected with great precision.154
Another exciting prospect for the future of optical nanobiosensors is in vivo fluorescence imaging of circulating cancer biomarkers. However, further research on the cytotoxicity of the nanoparticles will be necessary. For the time being, the emphasis will be on facilitating the synthesis and functionalization of those particles as well as enhancing their fluorescence through the combination of other enhancing processes or an increase in quantum yield.155
Many modifications are known for piezoelectric biosensors, which have been experimentally shown to be dependable. The primary disadvantages of piezoelectric biosensors are their reliance on sample preparation and intricate pretreatment procedures; however, the experimental results are encouraging, and a shift in this situation is anticipated in the near future. The development of low-cost piezoelectric materials and the ability to produce targeted materials in large quantities, such as oligonucleotides, nanoparticles, and imprinted polymers, provide promising indications for future advancements in this sector.156,157
3. Biosensors in different cancer diagnostic measurements
As per the WHO report of 2020, more than 15 million people worldwide lose their lives yearly due to various cancer-causing agents such as pathogens and carcinogens. However, each category of cancer is a matter of concern, but as per the reported number of cases, the mortality rate is higher in specific types of cancer diseases. Among the cases, the most significant number of cases are reported concerning various body organs, such as lungs, liver, gastric, breast, prostate, and cervical cancer (Table 6). The state of the art in biosensing plays a pivotal role in diagnosing these types of cancers (Fig. 3).| Targeted cancer | Studies | Key findings | Ref. |
|---|---|---|---|
| Lung cancer | Evaluating the efficacy and cardiotoxicity of EGFR-TKI AC0010 with a novel multifunctional biosensor | AC0010 (10 μM) effectively inhibited EGFR-T790M mutant NSCLC cell lines without compromising cardiomyocyte viability | 158 |
| Within 1 h, 10 μM AC0010 increased frequency and decreased amplitude of extracellular field potential along with mechanical beat | |||
| A highly sensitive sensor for carcinoembryonic antigen based on AlGaN/GaN high-electron-mobility transistors | CEA antibodies were immobilized in the sensing area of AlGaN/GaN high-electron-mobility transistors, allowing for specific identification | 159 | |
| Vangl-dependent Wnt/planar cell polarity signaling mediates collective breast carcinoma motility and distant metastasis | Vangl-dependent Wnt/PCP signaling enhances breast cancer collective cell migration regardless of tumor subtype, indicating a critical function for this system in breast cancer development | 160 | |
| This sensor can interpret Wnt/planar cell polarity (Wnt/PCP) metastatic signals to assess the etiology and symptoms of lung cancer | |||
| Liver cancer | Electrochemical immunosensor based on Fe3O4/MWCNTs-COOH/AuNPs nanocomposites for trace liver cancer marker alpha-fetoprotein detection | The combination intended to improve the sensor's electrochemical performance | 161 |
| The immunosensor performed well in identifying AFP in clinical samples, showing its potential for real-world use in early liver cancer detection | |||
| Well-ordered Au nanoarray for sensitive and reproducible detection of hepatocellular carcinoma-associated miRNA via CHA-Assisted SERS/fluorescence dual-mode sensing | This sensing technique can detect the target miR-224 over a large linear range (1 fM to 1 nM) | 162 | |
| This biosensor, with its high specificity and anti-interference capacity, can distinguish target miR-224 from other interference miRNAs | |||
| This biosensor can differentiate HCC cancer subjects from normal ones, monitor HCC patients before and after hepatectomy, and guide the various Barcelona clinic liver cancer (BCLC) phases | |||
| Self-powered logically operated fluorescent detection of hepatitis B virus (HBV) | The suggested biosensor detects HBV-DNA at a threshold of 1 fM in human serum | 163 | |
| Gastric cancer | A novel bimetallic MXene derivative QD-based ECL sensor for miRNA-27a-3p detection | The biosensor demonstrated a strong linear connection between ECL intensity and miRNA-27a-3p concentration over a wide range of 1 fM to 10 nM, with a detection limit of 1 fM | 164 |
| The sensing system, which included Mo2TiC2 QDs, SnS2 nanosheets, and lipid bilayers, exhibited a high potential for therapeutic applications | |||
| Novel sensitive electrochemical immunosensor development for the selective detection of HopQ H. pylori bacteria biomarker | The use of MWCNT/AuNP nanocomposite enables for antibody covalent bonding, which improves SPCE performance | 165 | |
| It is promising to develop cost-effective biosensors compatible with point-of-care test technologies for H. pylori detection in order to reduce its impact on mankind | |||
| This study is the first to disclose the application of HopQ as a biomarker for H. pylori in an electrochemical immunosensor, as well as the dissociation constant of HopQ/HopQ-Ab interaction | |||
| Photofuel cell-based self-powered biosensor for HER-2 detection by integration of plasmonic-metal/conjugated molecule hybrids and electrochemical sandwich structure | The localized surface plasmon resonance impact of AuNPs may clearly improve the separation efficiency of photo-generated electron/hole pair, which was favorable to the sensitivity and stability of PFC-SPB | 166 | |
| Using the open circuit voltage (EOCV) as the output signal, the PFC-SPB can detect HER-2 in the 0.1–500 pg mL−1 range, with a low detection limit of 0.02 pg mL−1 | |||
| Breast cancer | Development of an electrochemical biosensor for the detection of mammary gland carcinoma using molybdenum enhanced poly taurine nano-biofilms confirmed pathological findings | According to the desired collaboration with pathological studies, the developed biosensor is an appropriate diagnostic tool for the examination of HER-2 positive actual samples in clinical laboratories | 167 |
| A colorimetric biosensor to track Trop-2 status of tumor cells for diagnosis of breast cancer | A dual-aptamer-assisted biomimetic capture approach achieves high capture efficiency while preserving the viability and original phenotypic of captured cells, allowing for precise Trop-2 status analysis | 168 | |
| The high intrinsic peroxidase activity and excellent targeting ability of Trop-2-specific aptamer-linked TDN-PCN-222 (Fe) allow for the specific detection of Trop-2-positive tumor cells with a limit of detection (LOD) of 10 cells per mL, as well as the visual tracking of tumor cell Trop-2 status | |||
| Faraday cage-type ECL biosensor for the detection of circulating tumor cell MCF-7 | Under ideal experimental circumstances, the limit of detection was 3 cells per mL | 169 | |
| The sensor was capable of detecting genuine human blood samples, marking the first report on the identification of entire circulating tumor cells using a Faraday cage-type electrochemiluminescence biosensor | |||
| Prostate cancer | Development of a novel electrochemical biosensor based on plastic antibodies for detection of STEAP1 biomarker in cancer | The biosensor demonstrates a linear detection range from 130 pg mL−1 to 13 μg mL−1, indicating its potential for sensitive and reproducible detection of STEAP1 in medical and clinical research applications | 170 |
| The biosensor employs MIPs, synthetic receptors designed to imitate natural antibodies, resulting in great specificity for the target biomarker | |||
| A sensitive label-free biosensor based on Ag2S-sensitived Bi2WO6/BiOBr heterojunction for photoelectrochemical immunoassay of prostate specific antigen | Antigen and antibody are precisely mixed to provide quantitative PSA detection based on current changes at varying antigen concentrations | 171 | |
| Under perfect experimental circumstances, the PEC immunosensor exhibits an excellent linear relationship between 1 pg mL−1 and 50 ng mL−1, with a detection limit of 0.084 pg mL−1 | |||
| Suspended CNTs/MoS2 heterostructure field effect transistor for high performance biosensor and serum biomarker detection | The suspended CNTs/MoS2 FET demonstrated sensitive and reliable detection capabilities, with significant promise for quick clinical assessment and biomarker monitoring | 172 | |
| Cervical cancer | A new Nbd-based probe for specific colorimetric and turn-on fluorescence sensing of Cu2+ and bio-imaging applications | A fluorescence imaging investigation demonstrates that probe AH can identify Cu2+ in live HeLa cells, locust Malpighian tubules, and zebrafish | 173 |
| An ultrasensitive electrochemical DNA biosensor for monitoring human papillomavirus-16 (HPV-16) using graphene oxide/Ag/Au nano-biohybrids | The biosensor detected a qualitative difference between the probe and the target HPV-16 DNA | 174 | |
| The designed biosensor detected HPV-16 with a high sensitivity of 0.54 mA aM−1 | |||
| Design and development of an electroanalytical genosensor based on Cu-PTCA/rGO nanocomposites for the detection of cervical cancer | The genosensor demonstrated good selectivity for HPV-16 target DNA, with no appreciable interference from non-target DNA sequences | 175 |
3.1 Biosensors in lung cancer detection
Jiang et al. experimented with evaluating the response of AC0010-based aptamer biosensors for detecting lung cancer-related biomarkers such as Non-Small Cell Lung Cancer (NSCLC), one of the main cancer-causing factors responsible for mortality worldwide. This biosensor architecture integrates interdigital and microgrid electrodes to quantify the label-free response of AC0010-induced NSCLC inhibition. This biosensing assembly can produce the output in terms of real-time response on a monitoring screen by a non-invasive way of implanting a biosensor in the human body. The embedment of the digital electrode in the biosensor assembly is responsible for a linear response of 0.8–119 ng mL−1 with a detection range of 106 pg mL−1.158Huang et al. developed an effective biosensor for carcinoembryonic antigen detection, known as a serum-based biomarker in lung cancer diagnosis. This sensor is designed in a nanoscale transistor form using AlGaN/GaN material due to various miraculous properties such as high levels of electron mobility and real-time response in the transduction assembly. This optical sensor can produce a linear response from 0.03–125 U mL−1 with a limit range of 3 to 3.4 U mL−1. Due to the use of AlGaN/GaN material, the cost of this biosensor becomes much lower than the other sensing mechanisms in this category, with an improved response on a time scale.159
VanderVorst et al. developed a multi-functional biosensor assembly to scale out the progress in breast tumor cells. This sensor can translate the progress in metastasis signals such as Wnt/planar cell polarity (Wnt/PCP) to measure the advanced status of lung cancer-related causes and symptoms. This biosensor mechanism focuses on associating tetraspanin-related proteins, such as Vangl1 and Vangl2, on measuring breast tumor cell activities. The results show that the Vangl1 and Vangl2 proteins can detect the cytoskeletal regulator RhoA-based leader cells in terms of tumor growth.160
3.2 Biosensors in liver cancer detection
Wu et al. developed an advanced electrochemical biosensor for detecting liver cancer biomarkers at very early stages of progression. In this sensor assembly, authors used iron tetroxide-based nanotubes with the fusion of carboxylate carbon and AuNPs to scale out the alpha-fetoprotein level in the human subject's liver. The working strategy of this sensor assembly is very sophisticated in generating the electrical signal spectrum to replicate the monoclonal antibodies of alpha-fetoprotein level. This sensor's response was linear and had a detection limit of 1.06 pg mL−1.161Huang et al. developed an optical biosensing assembly to detect serum-based hepatocellular carcinoma and related miRNA, specifically miR-224. This sensor assembly contains well-arranged Au nanoarrays with substrate-coupled hairpin assembly for target-catalyzed serum. The miRNA was detected with a linear range of 1.0 fM to 1.3 nM based on the fluorescence-based dual-mode detection process. The detection limit of this sensor varied from 0.31 to 0.34 fM. This sensor assembly has ultra-sensing capability due to the integration of a catalyzed hairpin assembly amplification unit.162
Imbriano et al. developed a fluorescent-based smart digital biosensor for identifying hepatitis B virus DNA-based enzymatic logic network for cancer applications. This optical sensor contains an AND-based logic gate having two input electrodes (input 1: alanine aminotransferase (ALT) and input 2: lactate dehydrogenase (LDH)) and one output in the form of lactate (quenching DNA). In this assembly, as per the amount of measurement, the specific electrode generated the electric signal to present the quantity of quenching DNA labeled with BHQ2 or FAM. The detection limit of this sensor varied from 0.27 to 0.33 fM with a high level of sensitivity. The working mechanism of this sensor is a benchmark for upgradation in digital sensor development.163
3.3 Biosensors in gastric cancer detection
Li et al. developed an electrochemiluminescence biosensor to detect the miRNA-27a-3p biomarker for stomach-related cancer consequences. The authors developed the biosensor assembly using the QDs of bimetallic MXene derivative material and SnS2 nanosheets for better luminescence output with stability in electric field potential related to the oxidation process. This sensing mechanism produced excellent reproducibility of an analyte with linear response. The limit of detection was even less than 1 fM. So, overall, for detecting miRNA-27a-3p biomarkers, the fusion of Mo2TiC2 QDs and nanosheets of SnS2 composition plays a pivotal role.164Jaradat et al. developed a photo-voltammetry-based immunoassay biosensor for detecting Helicobacter pylori-based HopQ protein biomarker for early detection of cancer symptoms in gastrointestinal saliva samples of human subjects. The biosensing assembly was developed on a thin carbon sheet coated by MWCNT-COOH and AuNPs. The sensor mechanism was based on electrochemical oxidation-based impedance spectroscopy to present the target analyte. The sensor had excellent linear response ranging from 10 pg mL−1 to 100 ng mL−1 range with a detection limit of 2 pg mL−1.165 This sensor had shown good response in selectivity, stability, and reproducibility for detecting H. pylori-based HopQ protein, a potential biomarker for insight into gastro-related cancer diseases.
Bai et al. a photo fuel-based self-powered electronic biosensor for breast and gastric cancer detection. In this sensor assembly, they sandwiched the QDs of plasmonic gold and hybrid photoanode-based organic semiconductor layers to detect human epidermal growth factor receptor-2 (HEGFR-2), a potential key biomarker for detecting early breast and gastric cancer symptoms. As per the resonance effect of localized surface plasmon-based electron–hole pair generation, the photo spectrum was developed to scale out the target analyte. This assembly provides an excellent linear response ranging from 0.2–510 pg mL−1 with a detection limit of 0.03 pg mL−1.166 The self-charging of biosensors is one of the primary vital concepts that set the benchmark for development in a system on a chip module.
3.4 Biosensors in breast cancer detection
Nasrollahpour et al. developed an electrochemical synthesis-based biosensor framework for the early detection of BC-related symptoms in female human subjects. The authors fabricated the biosensing assembly using molybdenum trioxide/polytaurine nanofilms to process the serum from the breast to detect the target analyte, HEGFR-2. This sensor could assess sample fluid regarding morphological and elemental progression in cancer-causing agents. The sensor's response time was significantly less and had a high reproducibility range. The linearity range of this biosensor varied from 0.1 ng mL−1 to 0.000001 ng mL−1, with a significant detection limit of 0.000001 ng mL−1.167 This sensor has proved reliable in using biomaterials for clinical applications.Zeng et al. developed a colorimetric biosensor processing of track Trop-2 biomarker for early detection of symptoms of BC. This aptamer-based biosensor has excellent specificity and sensitivity for the target analyte with label marking of the target analyte. This sensor was fabricated using organic material-based nanoplates of Fe-based metal with a tetrahedral DNA-based nanostructure to process the target analyte. This biosensor produced excellent linearity with a 10 cells per mL detection limit adopted for BC-based clinical trials.168
Zhang et al. developed an electrochemiluminescence-based biosensor with Faraday cage technology to detect the MCF-7 BC cells in an early stage of development. In this sensor mechanism, the Faraday cage consists of two consecutive sections. The capture section and signal unit were designed by Fe3O4 NPs and GO-PTCA nanosheets, respectively, for photochemical synthesis. This aptamer-based material gave a very sensitive response to detecting the target analyte in MCF-7. The detection limit of this sensor was observed to be 3 cells per mL.169
3.5 Biosensors in prostate cancer detection
Carvalho et al. developed an electrochemical biosensor using CNTs and dendritic platinum NPs to detect STEAP1 biomarkers for prostate cancer treatment. In this biosensor, the oxidative modification of screen-printed electrodes in conjugation with lysates of neoplastic detects the prostate antigens. This electro-polymerization-based biosensor has an excellent linear response of 131 pg mL−1 to 14 μg mL−1 with a detection range of 0.3 pg mL−1. This sensor has produced supportive instrumentation factors, i.e., reproducibility, selectivity, and precision for clinical applications and system-on-chip progression.170Chang et al. developed a photoelectrochemical-based biosensor for the identification of prostate-specific antigens. This sensor was fabricated using Bi2WO6/BiOBr nanomaterials in conjunction with an Ag2S agent for sensitization. The current response of this sensor was based on the change in concentration of PSA antigen. The optical response of this assembly was observed to be linear, with a specified range of 1 pg mL−1 to 50 ng mL−1 with an improved detection limit of 0.084 pg mL−1.171 The electromechanical response of this sensor was satisfactory and comparable to traditional immunoassay methods.
Wei et al. designed a field effect-based semiconductor transistor-based high-performing biosensor to find PSA-based cancer symptoms. The authors fabricated the sensor assembly using CNTs with MoS2. The field effect mechanism is responsible for the rapid detection of target analyte due to heterostructure oxidation response on the selective electrodes. As per experimentation, the sensor has a linear response, which was varied from 10 to 13 μg μL−1 with a detection limit of 4 μg μL−1.172 The sensor has a higher stability approach for physiochemical temperaments.
3.6 Biosensors in cervical cancer detection
Anbu et al. developed a fluorescent-based sensor for detecting Cu2+ in relation to cervical cancer using naphthalimide–phenanthroimidazole. Under physiological conditions, the response of Cu2+ sustains paramagnetic ion reactions, due to which the response of this biosensor opens opportunities for fluorescent-based biosensor development. The response of this sensor was to initiate absorption through the stoichiometry process of transduction. The main benefit of this sensor was its economic factors. There was scope to improve the sensing mechanism to scale out the instrumental parameters for the target analyte.173Pareek et al. designed a DNA-based biosensor to recognize Human Papilloma Virus-16 (HPV 16) biomarkers for early detection of cervical cancer. The sensing mechanism was based on an electrochemical phenomenon. The authors fabricated the biosensor using indium tin oxide, glass rod-based electrodes, and silver-coated AuNPs to enhance the sensing capability of the sensor. The sensor produced 0.54 mA aM−1 sensitivity with a tremendous linearity range of 100 aM to 1 μM. The detection limit of this assembly was 100 aM. This sensor also paves the way to early-stage DNA-based cancer analyte estimation.174
Rawat et al. prepared an electrochemical-based Geno sensor-based biosensor to detect cervical cancer. The main target analyte was HPV 16. The biosensor was fabricated using novel perylene tetracarboxylic acid with processed copper and graphene oxides to detect the target analyte. The sensor performed very well with a detection limit of 0.06 μA μM−1 mm−2. From the PoC system design, the Geno sensors set the benchmark regarding sensitivity and selectivity.175
4. Biosensors: modelling and measurements
Cancer is one of the most common life-threatening diseases, with the most remarkable fatality rate worldwide. Early cancer diagnosis is crucial for effective and successful therapy. Traditional cancer screening diagnostic procedures are expensive, time-consuming, and unsuitable for recurrent tests. However, biomarker-based cancer diagnosis is emerging as one of the most promising approaches for early detection, disease progression monitoring, and eventual cancer treatment. Parihar et al. developed a highly sensitive, selective, and specific electrochemical aptasensor called CeO2–GO/Apt/BSA/GCE for detecting EGFR biomarkers in PBS and actual saliva, sweat, and serum samples. The sol–gel process was used to manufacture the CeO2–GO nanocomposite. The oxygen atoms (O-atoms) on the faces and edges of CeO2 and GO enable considerable and facile EGFR protein immobilization/binding. The functionalization of CeO2 improves electroconductivity and inhibits aggregation in the CeO2–GO nanocomposite. The electrochemical characterization data indicate that the CeO2–GO nanocomposite has good electrochemical properties and a large surface area, making it suitable for the development of an electrochemical aptasensor with an ultra-low limit of detection (LOD) and a wide detection range for EGFR, ranging from 25.0 fg mL−1 to 25.0 ng mL−1. The manufactured aptasensor demonstrated outstanding results in genuine samples such as saliva, sweat, and serum. Therefore, it may be employed in clinical settings for non-invasive detection of cancer biomarkers.176The synthesized material was utilized to create an electrochemical aptasensor capable of detecting epidermal growth factor receptor (EGFR) antigen. The developed sensor (Y2O3-rGO/Apt/BSA) has a broad linear detection range (10 fg mL−1 to 100 ng mL−1), a low detection limit of 0.251 fg mL−1, and an exceptional sensitivity of 51.96 μA fM−1 cm−2. The aptasensor also offers a simple and rapid way to detect EGFR antigen in biological serum samples. This work's simple and straightforward synthesis approach resulted in the manufacture of a high-purity, water-dispersible yttrium functionalized reduced graphene oxide nanocomposite for cost-effective, quicker, and ultrasensitive detection of breast cancer biomarkers utilizing electrochemical aptasensors.177
MXene-enabled electrochemical aptasensors have shown considerable potential in detecting cancer biomarkers at a femtomolar level. Furthermore, the stability, simplicity of synthesis, strong repeatability, and high specificity afforded by MXene-enabled aptasensors show potential for becoming the dominant diagnostic technique.178 The combination of carbon-based nanomaterials with highly specific aptamers (antibody mimics) can reduce sensor production costs while simultaneously improving effectiveness, precision, selectivity, and detection speed for a wide range of analytes. Furthermore, the integration of ideas and technology increases speedy design and smooth operations, therefore playing a vital role in numerous fields such as medical and healthcare services.179
Luo et al. demonstrated a locked nucleic acid (LNA) aided strand displacement reaction-based ratiometric electrochemical biosensor with greater repeatability to detect exosomal miR-21 originating from cancer with an LOD of 2.3 fM.180 Human epidermal growth factor receptor 2 (+) BT 474 (HER-2 (+) BT 474), a well-known BC biomarker, was easily identified from the first collected population using its complementary HER-2 antibody. The detection limit was as low as 4.7 × 105 exosomes per μL.181
Canbaz and co-workers covalently attached an anti-HER-3 antibody to the modified Au electrode to detect the cancer-specific biomarker HER-3.182 Voltammetry and EIS were used to characterize the stated sensors, resulting in a linear detection range of 0.2–1.4 pg mL−1. Another group used a disposable electrochemical biosensor to detect the breast cancer-specific biomarker HER-2-ECD in human serum in the early stages, with a detection range of 10–150 ng mL−1 and a detection limit of 2.1 ng mL−1. The disclosed sensor's validity was verified by screening several human proteins and other cancer biomarkers (CA15-3).183
Heidari et al. described a GCE-based sensor that detects and quantifies p53 cancer biomarkers.184 This sandwich assay used GCE/CdS/p53-Ab1 and p53-Ab2-tGO-AuNPs to identify p53 cancer biomarkers. Epithelial ovarian cancer possesses a unique biomarker, human epididymis protein 4 (HE4), which can be detected in human serum down to 2.8 pM.185
Pacheco et al. demonstrated an Au-SPE-based biosensor to diagnose carbohydrate antigen 15-3 (CA 15-3) in human serum, which can be detected at concentrations as low as 1.5 U mL−1.186 Prasad et al. have developed a low-cost disposable paper-based electrode modified with a GO-based electrochemical biosensor to identify pancreatic cancer-specific biomarkers pseudopodium-enriched atypical kinase 1, SGK269 (PEAK1).187 The immunosensor operated well with a linear range of 10 –106 pg mL−1 and a limit of detection of 10 pg mL−1. Muñoz-San Martín et al. recently announced an MBs-based microfluidic electrochemical biosensor capable of detecting tumoral hypoxia biomarker-hypoxia-inducible factor-1 alpha (HIF-1α) as low as 76 pg mL−1.188
5. Marketed biosensors
The biosensors are the future devices to detect the symptoms and progress in disorder for any illness such as cancer or other deadly disease in living beings. Various immunoassay-based biosensors are available on the market. The ORIGEN 1.5, M-series M-384 Analyzer, M-1 Analyzer, Luminex 200™ System, Sector PR2 Reader, Elecsys 1010 and 2010, as well as Modular analytics E-170 Analyzer, are the commercially available biosensor modules for measurement of cancer cells.189,190As per the current scenario, the market of biosensors is worth more than 18 million worldwide due to an increment in reported cases. Adopting biocompatible materials, fabrication techniques, innovative design, and integration techniques support the commercialization of cancer-related biosensors. The recent advances in PoC technology demand sophisticated instruments to be involved in commercializing biosensors. The biosensing research and development filed under trials and needs the approval of governing bodies such as the FDA. The research community claims a linear response of biosensors with an expanded detection limit. However, the trials of these sensors are still underway to ensure stability concerning certain physio-environmental aspects. The Luminex 200™ System is recognized as commercially viable due to its 100 times reusability.190
For commercialization, there are certain aspects on which the research community must focus, such as stability, reusability, biocompatibility, cost of trial, and approval of ethical committees and governing bodies such as the FDA. With the advancement in IoT and NEMS-based technology, the search community must focus on developing commercial wireless biosensing modules to support cutting-edge technologies.191
6. Limitations of biosensor
Although the biosensors are designed and tested for the identification of target analyte with improvement in various electromechanical instrumentation factors, various aspects need to be considered as per PoC technology. The biosensor design must be user-friendly and have an ace of embodiment with testing modules.192 There is a need to fabricate more sophisticated and flexible electrode assemblies, embed low exciters in the transduction modules to improve the sensitivity of biosensors, enhance the effort in designing a photo detection-based system on a chip module to support non-invasive electronic portable devices, incorporate new amplification techniques and improve optical response. The biosensors lack stability under trials due to multilateral factors and only focus on one biomarker at a time, so false observations can be made, so multiplex detection is desired in the transduction process to improve the accuracy of biosensor results.193–200 The research community must focus on the fusion of artificial intelligence (AI) and machine learning (ML)-based algorithms to improve the performance of a biosensing mechanism in terms of stability and producible scalability. The biosensor field is an emerging area, and various aspects need to be incorporated as per the approval of medical and governing bodies for commercialization on a global scale.201The primary obstacle in the development of transportable electrochemical paper-based biosensors is ensuring their dependability and capacity to identify many cytokines or other biomarkers concurrently in intricate biological specimens. Achieving the electrochemical transduction linked to hybridization events that do not genuinely need charge transfer processes is a difficulty in the development of electrochemical biosensors for nucleic acid BC indicators. The CNTs, graphene, graphene oxide (GO), reduced graphene oxide (rGO), graphene quantum dots (GQD), metal nanoparticles (MNPs) and nanostructures, magnetic beads, polymers, hydrogels, dendrimers, and nanocomposites are examples of nanostructured materials that are expected to be used more in the future and are becoming more and more important in their fabrication.202
When applied to actual samples, graphene-based biosensors are unable to produce the intended detection outcomes. This is primarily due to the non-specific nature of the interfacial interactions between graphene and different chemical and biological substances toward the intended targets. Furthermore, biological sample preparation—which may entail laborious and time-consuming steps like separation or preconcentration—is required prior to the completion of the tests. However, it can be difficult to disperse graphene nanoparticles in other materials, and doing so necessitates functionalizing the graphene surface, which raises the cost of producing biosensors. The identification of precise and sensitive biomarkers can significantly enhance cancer diagnosis. Despite this, not much progress has been made in the creation of extremely sensitive and repeatable biosensors for early cancer diagnosis or in the identification of novel biomarkers. Despite several publications, it might be challenging to create substrates that are highly specific to a particular cancer biomarker because most biomarkers are often present in multiple malignancies. Therefore, another significant difficulty for electrochemical biosensors is devising ways to detect many biomarkers while avoiding false positives concurrently. It is also probable that cancer biosensors do not cover the complete spectrum of biomarker concentrations due to the low concentration of the majority of cancer biomarkers.203,204
The main disadvantages of aptasensors that hinder the broader application of aptamer DNAzyme conjugate as therapeutics are noted as their toxicity, limited metabolism, and nuclease degradation in a physiological environment. There is still a long way to go before DNAzyme-assisted aptasensors can be used to identify cancer biomarkers in vivo and in complex clinical specimens. Since the majority of human malignancies contain several biomarkers, it is still difficult to detect specific biomarkers since developed DNAzyme aptasensors interfere with the variety of biomarkers present in cancer cells. Moreover, it is difficult to measure the local amounts of signals that cancer cells emit in vivo.205 The development of 3D-printed electrochemical sensors for cancer protein biomarkers often requires the printing of many components, which is hampered by the significant variance in inter-material adhesion forces and the highly restricted printing processes capable of attaining such a characteristic. Furthermore, standard 3D printing materials and procedures are not very compatible with biomolecules, especially when it comes to the high energy needed for printing processes and the high-intensity laser used in stereolithographic 3D printing, which makes it impossible to print these biomolecules directly.206
7. Clinical trials
Fig. 4 schematically represents the clinical trials on biosensors for detecting various cancers.207–2138. Conclusion and future aspects
The difficulties and advancements in the development of biosensors have made it easier to analyze cancer biomarkers. Without a doubt, developments in biosensor technology will contribute to the development of medical diagnostic equipment for use at the bedside. This review provides a quick overview of many popular sensor types, including electrochemical, optical, and piezoelectric sensors, that are often employed to identify common tumor markers. The electrochemical sensors are capable of identifying common tumor markers among them. A range of novel materials, including chelated Eu3+ materials and GO NPs, have also been developed with the specific goal of detecting the stomach cancer marker CEA. These materials provide excellent sensitivity, good selectivity, and quick analytical speeds. Early diagnosis, better patient outcomes from therapy, and enhanced quality of life are all aided by it. Moreover, optical biosensors are crucial for drug development, clinical diagnostics, and other areas. Optical biosensors offer excellent stability, great dependability, and high sensitivity. They have more significant anti-interference capabilities and greater accuracy when compared to standard electrochemical biosensors. Furthermore, adding protamine to plasma can increase the measured LOD value in the experiment employing a piezoelectric biosensor to detect PSA because protamine can bind to fibrinogen.The use of biosensors for early clinical diagnosis and prognostic monitoring in real-world settings remains challenging. First, because biomarkers are exceedingly intricate in living things, their detection does not imply the presence of cancer; genetic and environmental factors must also be considered. Second, it is difficult to distinguish the kind of cancer alone by looking for biomarkers, which is harmful to early clinical diagnosis and therapy due to gene crossing in cancer cells. Finally, taking optical sensors as an example, the bulk of those now on the market are still difficult. As a result, further work is needed to improve the biosensors' stability, reusability, and compatibility with biological fluids. In the future, they should be more compact, integrated, and mobile. If these issues are addressed, biosensors can be used in clinical laboratories and hospitals to diagnose and treat cancer at a low cost.
Data availability
No new data was generated during the preparation of this manuscript.Author contributions
Conceptualization, S. G., V. M., and Y. M.; writing—original draft preparation, S. G., V. M., A. A. A. A., A. A., R. K., and Y. M.; writing—review and editing, V. M., A. A. A. A., A. A., M. E. T., and Y. M.; supervision, V. M., and Y. M. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work received no external funding.References
- L. A. García-Hernández, E. Martínez-Martínez, D. Pazos-Solís, J. Aguado-Preciado, A. Dutt, A. U. Chávez-Ramírez, B. Korgel, A. Sharma and G. Oza, Optical detection of cancer cells using lab-on-a-chip, Biosensors, 2023, 13, 439 CrossRef PubMed.
- V. Sanko and F. Kuralay, Label-free electrochemical biosensor platforms for cancer diagnosis: recent achievements and challenges, Biosensors, 2023, 13(3), 333 CrossRef CAS PubMed.
- G. Li, J. Wu, X. Qi, X. Wan, Y. Liu, Y. Chen and L. Xu, Molecularly imprinted polypyrrole film-coated poly (3, 4-ethylenedioxythiophene): polystyrene sulfonate-functionalized black phosphorene for the selective and robust detection of norfloxacin, Mater. Today Chem., 2022, 26, 101043 CrossRef CAS.
- G. E. Chaudhry, A. M. Akim, N. Safdar, A. Yasmin, Y. Y. Sung and T. S. T. Muhammad, Cancer and disease diagnosis-biosensor as potential diagnostic tool for biomarker detection, J. Adv. Pharm. Technol. Res., 2022, 13(4), 243–247 CrossRef CAS PubMed.
- Z. Zhang, J. Zhou and X. Du, Electrochemical biosensors for detection of foodborne pathogens, Micromachines, 2019, 10(4), 222 CrossRef PubMed.
- A. E. Murillo, L. Melo-Maximo, B. Garcia-Farrera, O. S. Martínez and D. V. Melo-Máximo, et al., Development of AlN thin films for breast cancer acoustic biosensors, J. Mater. Res. Technol., 2019, 8(1), 350–358 CrossRef CAS.
- N. Cheng, D. Du, X. Wang, D. Liu, W. Xu, Y. Luo and Y. Lin, Recent advances in biosensors for detecting cancer-derived exosomes, Trends Biotechnol., 2019, 37(11), 1236–1254 CrossRef CAS PubMed.
- Q. Cui, L. Dai, J. Li, Y. Shen, H. Tao, X. Zhou and J. Xue, Contrast-enhanced ultrasound-guided sentinel lymph node biopsy in early-stage breast cancer: a prospective cohort study, World J. Surg. Oncol., 2023, 21(1), 143 CrossRef PubMed.
- A. Gajda-Walczak, A. Potęga, A. Kowalczyk, S. Sek, S. Zięba, A. Kowalik, A. Kudelski and A. M. Nowicka, New, fast and cheap prediction tests for BRCA1 gene mutations identification in clinical samples, Sci. Rep., 2023, 13(1), 7316 CrossRef CAS PubMed.
- A. Chattaraj, V. Mishra and Y. Mishra, Carbon nanotubes in the diagnosis and treatment of ovarian cancer, Indian J. Microbiol., 2024, 1–6 Search PubMed.
- I. U. Hassan, G. A. Naikoo, F. Arshad, F. Ba Omar, A. A. Aljabali, V. Mishra, Y. Mishra, M. El-Tanani, N. B. Charbe, S. R. Chava, Á. Serrano-Aroca and M. M. Tambuwala, Applications of trimetallic nanomaterials as non-enzymatic glucose sensors, Drug Dev. Ind. Pharm., 2023, 49(6), 393–404 CrossRef CAS PubMed.
- R. Khan, F. Arshad, I. U. Hassan, G. A. Naikoo, M. Z. Pedram, M. S. Zedegan, H. Pourfarzad, A. A. Aljabali, Á. Serrano-Aroca, Y. Haggag, V. Mishra, Y. Mishra, M. Birkett and M. M. Tambuwala, Advances in nanomaterial-based immunosensors for prostate cancer screening, Biomed. Pharmacother., 2022, 155, 113649 CrossRef CAS PubMed.
- B. Zolfaghari, L. Mirsadeghi, K. Bibak and K. Kavousi, Cancer prognosis and diagnosis methods based on ensemble learning, ACM Comput. Surv., 2023, 55(12), 1–34 CrossRef.
- M. Dutt, G. Hartel, R. S. Richards, A. K. Shah, A. Mohamed and S. Apostolidou, et al., Discovery and validation of serum glycoprotein biomarkers for high grade serous ovarian cancer, Proteonomics Clin. Appl., 2023, 17(4), 2200114 CrossRef CAS PubMed.
- B. W. Wormald, N. Moser, N. M. deSouza, K. T. Mantikas and K. Malpartida-Cardenas, et al., Lab-on-chip assay of tumour markers and human papilloma virus for cervical cancer detection at the point-of-care, Sci. Rep., 2022, 12(1), 8750 CrossRef CAS PubMed.
- G. Alexandrou, N. Moser, K. T. Mantikas, J. Rodriguez-Manzano and S. Ali, et al., Detection of multiple breast cancer ESR1 mutations on an ISFET based lab-on-chip platform, IEEE Trans. Biomed. Circuits Syst., 2021, 15(3), 380–389 Search PubMed.
- S. Sunny, A. Baby, B. L. James, D. Balaji, A. NV, M. H. Rana and P. Gurpur, et al., A smart tele-cytology point-of-care platform for oral cancer screening, PLoS One, 2019, 14(11), e0224885 CrossRef CAS PubMed.
- C. Alacevich, A. M. Abi Nehme, J. H. Lee, D. Li, E. M. Mobley and J. L. Close, et al., A point-of-care pilot randomized intervention to connect patients with cancer-induced financial toxicity to telehealth financial counseling, Cancer Causes Control, 2024, 35(3), 393–403 CrossRef PubMed.
- D. Grieshaber, R. MacKenzie, J. Vörös and E. Reimhult, Electrochemical biosensors-sensor principles and architectures, Sensors, 2008, 8(3), 1400–1458 CrossRef CAS PubMed.
- D. I. Sandoval Bojórquez, Z. Janićijević, B. Palestina Romero, E. S. Oliveros Mata and M. Laube, et al., Impedimetric nanobiosensor for the detection of SARS-CoV-2 antigens and antibodies, ACS Sens., 2023, 8(2), 576–586 CrossRef PubMed.
- C. Lu, J. Han, X. Sun and G. Yang, Electrochemical detection and point-of-care testing for circulating tumor cells: current techniques and future potentials, Sensors, 2020, 20(21), 6073 CrossRef CAS PubMed.
- A. A. Shalaby, C. W. Tsao, A. Ishida, M. Maeki and M. Tokeshi, Microfluidic paper-based analytical devices for cancer diagnosis, Sens. Actuators, B, 2023, 379, 133243 CrossRef CAS.
- X. Huang, Q. Lin, H. Gong, L. Lu, Q. Wei and D. Tang, Bio-inspired nanozyme with ultra-thin Fe–Bi2O2S nanosheets for in-situ amplified photoelectrochemical immunoassay of cancer-related protein, Anal. Chim. Acta, 2023, 1252, 341058 CrossRef CAS PubMed.
- H. Liang, X. Wang, F. Li, Y. Xie, J. Shen, X. Wang and Y. Huang, et al., Label-free plasmonic metasensing of PSA and exosomes in serum for rapid high-sensitivity diagnosis of early prostate cancer, Biosens. Bioelectron., 2023, 235, 115380 CrossRef CAS PubMed.
- C. Chen, Y. L. Wang, X. Lin, S. H. Ma, J. T. Cao and Y. M. Liu, Cu-MOFs/GOx bifunctional probe-based synergistic signal amplification strategy: toward highly sensitive closed bipolar electrochemiluminescence immunoassay, ACS Appl. Mater., 2023, 15(19), 22959–22966 CrossRef CAS PubMed.
- H. Hayrapetyan, T. Tran, E. Tellez-Corrales and C. Madiraju, Enzyme-linked immunosorbent assay: types and applications, ELISA: Methods and Protocols, 2023, pp. 1–17 Search PubMed.
- E. M. Khalaf, N. A. Abood, R. Z. Atta, A. A. Ramírez-Coronel, R. Alazragi and R. M. R. Parra, et al., Recent progressions in biomedical and pharmaceutical applications of chitosan nanoparticles: A comprehensive review, Int. J. Biol. Macromol., 2023, 231, 123354 CrossRef CAS PubMed.
- D. Chen, N. Chen, F. Liu, Y. Wang, H. Liang, Y. Yang and Q. Yuan, Flexible point-of-care electrodes for ultrasensitive detection of bladder tumor-relevant miRNA in urine, Anal. Chem., 2023, 95(3), 1847–1855 CrossRef CAS PubMed.
- D. Vignesh Kumar, G. Nookaraju, T. Sreeja Reddy, B. S. Panigrahi and S. N. Mohanty, Optimal Convolutional Neural Network Model for Early Detection of Lung Cancer on CT Images, in Proceedings of the International Health Informatics Conference: IHIC 2022, Springer Nature Singapore, Singapore, 2023, pp. 357–363 Search PubMed.
- T. Brito-Rocha, V. Constâncio, R. Henrique and C. Jerónimo, Shifting the Cancer Screening Paradigm: The Rising Potential of Blood-Based Multi-Cancer Early Detection Tests, Cells, 2023, 12, 935 CrossRef CAS PubMed.
- H. H. Kim, O. J. Moon, Y. H. Seol and J. Lee, A simple urine test by 3D-plus-3D immunoassay guides precise in vitro cancer diagnosis, Bioeng. Transl., 2023, 8(3), e10489 CrossRef CAS PubMed.
- A. Chellachamy Anbalagan and S. N. Sawant, Redox-labelled detection probe enabled immunoassay for simultaneous detection of multiple cancer biomarkers, Microchim. Acta, 2023, 190(3), 86 CrossRef CAS PubMed.
- L. Chi, X. Wang, H. Chen, D. Tang and F. Xue, Based photoelectrochemical immunoassay for ultrasensitive screening of carcinoembryonic antigen on hollow CdS/CdMoO4-functionalized photoanode, Talanta, 2023, 254, 124176 CrossRef CAS PubMed.
- D. K. Tsoneva, M. N. Ivanov, N. V. Conev, R. Manev, D. S. Stoyanov and M. Vinciguerra, Circulating histones to detect and monitor the progression of cancer, Int. J. Mol. Sci., 2023, 24(2), 942 CrossRef CAS PubMed.
- R. Goldoni, A. Scolaro, E. Boccalari, C. Dolci, A. Scarano and F. Inchingolo, et al., Malignancies and biosensors: A focus on oral cancer detection through salivary biomarkers, Biosensors, 2021, 11(10), 396 CrossRef CAS PubMed.
- L. Liu, H. Wang, B. Xie, B. Zhang, Y. Lin and L. Gao, Detection of alpha-fetoprotein using aptamer-based sensors, Biosensors, 2022, 12(10), 780 CrossRef CAS PubMed.
- P. Sardarabadi, A. A. Kojabad, D. Jafari and C. H. Liu, Liquid biopsy-based biosensors for MRD detection and treatment monitoring in Non-Small Cell Lung Cancer (NSCLC), Biosensors, 2021, 11(10), 394 CrossRef CAS PubMed.
- C. H. Li, M. H. Chan, Y. C. Chang and M. Hsiao, Gold nanoparticles as a biosensor for cancer biomarker determination, Molecules, 2023, 28(1), 364 CrossRef CAS PubMed.
- Z. Ashikbayeva, A. Bekmurzayeva, Z. Myrkhiyeva, N. Assylbekova, T. S. Atabaev and D. Tosi, Green-synthesized gold nanoparticle-based optical fiber ball resonator biosensor for cancer biomarker detection, Opt. Laser Technol., 2023, 161, 109136 CrossRef CAS.
- H. Nasrollahpour, A. Naseri, M. R. Rashidi and B. Khalilzadeh, Application of green synthesized WO3-poly glutamic acid nanobiocomposite for early stage biosensing of breast cancer using electrochemical approach, Sci. Rep., 2021, 11(1), 23994 CrossRef CAS PubMed.
- X. Wu, Y. Wang, J. Zhang, Y. Zhang, X. Rao and C. Chen, et al., A D-Shaped Polymer Optical Fiber Surface Plasmon Resonance Biosensor for Breast Cancer Detection Applications, Biosensors, 2023, 14(1), 15 CrossRef PubMed.
- A. Khodadoust, N. Nasirizadeh, S. M. Seyfati, R. A. Taheri, M. Ghanei and H. Bagheri, High-performance strategy for the construction of electrochemical biosensor for simultaneous detection of miRNA-141 and miRNA-21 as lung cancer biomarkers, Talanta, 2023, 252, 123863 CrossRef CAS PubMed.
- H. Sohrabi, N. Bolandi, A. Hemmati, S. Eyvazi, S. Ghasemzadeh and B. Baradaran, et al., State-of-the-art cancer biomarker detection by portable (Bio) sensing technology: A critical review, Microchem. J., 2022, 177, 107248 CrossRef CAS.
- J. Li, Y. Tong, Z. Sun, Y. Chen and Y. Wang, et al., A duplex-specific nuclease assisted photoelectrochemical biosensor based on MoS2@ ReS2/Ti3C2 hybrid for ultrasensitive detection of colorectal cancer-related piRNA-31,143, Acta Biomater., 2022, 149, 287–296 CrossRef CAS PubMed.
- T. Liyanage, B. Alharbi, L. Quan, A. Esquela-Kerscher and G. Slaughter, Plasmonic-based biosensor for the early diagnosis of prostate cancer, ACS Omega, 2022, 7(2), 2411–2418 CrossRef CAS PubMed.
- T. J. Bondancia, A. C. Soares, M. Popolin-Neto, N. O. Gomes, P. A. Raymundo-Pereira and H. S. Barud, et al., Low-cost bacterial nanocellulose-based interdigitated biosensor to detect the p53 cancer biomarker, Biomater. Adv., 2022, 134, 112676 CrossRef CAS PubMed.
- H. Y. Wen, S. F. Wang, C. H. Li, Y. T. Yeh and C. C. Chiang, Real-time and sensitive immunosensor for label-free detection of specific antigen with a comb of microchannel long-period fiber grating, Anal. Chem., 2020, 92(24), 15989–15996 CrossRef CAS PubMed.
- T. Dong, N. M. Matos Pires, Z. Yang and Z. Jiang, Advances in electrochemical biosensors based on nanomaterials for protein biomarker detection in saliva, Adv. Sci., 2023, 10(6), 2205429 CrossRef CAS PubMed.
- M. Gezimati and G. Singh, Advances in terahertz technology for cancer detection applications, Opt. Quant. Electron., 2023, 55(2), 151 CrossRef PubMed.
- A. Mohammadpour-Haratbar, S. B. A. Boraei, Y. Zare, K. Y. Rhee and S. J. Park, Graphene-based electrochemical biosensors for breast cancer detection, Biosensors, 2023, 13(1), 80 CrossRef CAS PubMed.
- K. Ondraskova, R. Sebuyoya, L. Moranova, J. Holcakova, P. Vonka, R. Hrstka and M. Bartosik, Electrochemical biosensors for analysis of DNA point mutations in cancer research, Anal. Bioanal. Chem., 2023, 415(6), 1065–1085 CrossRef CAS PubMed.
- X. Wang, S. Dong and H. Wei, Recent Advances on Nanozyme-based Electrochemical Biosensors, Electroanalysis, 2023, 35(1), e202100684 CrossRef CAS.
- J. Liu, Y. Tang, Y. Cheng, W. Huang and L. Xiang, Electrochemical biosensors based on saliva electrolytes for rapid detection and diagnosis, J. Mater. Chem. B, 2023, 11(1), 33–54 RSC.
- X. Zhang, X. Tan, P. Wang and J. Qin, Application of polypyrrole-based electrochemical biosensor for the early diagnosis of colorectal cancer, Nanomaterials, 2023, 13(4), 674 CrossRef CAS PubMed.
- S. Park, E. Cho, S. T. D. Chueng, J. S. Yoon, T. Lee and J. H. Lee, Aptameric fluorescent biosensors for liver cancer diagnosis, Biosensors, 2023, 13(6), 617 CrossRef CAS PubMed.
- A. Mohammadpour-Haratbar, Y. Zare and K. Y. Rhee, Electrochemical biosensors based on polymer nanocomposites for detecting breast cancer: Recent progress and future prospects, Adv. Colloid Interface Sci., 2022, 309, 102795 CrossRef CAS PubMed.
- D. Ozkan-Ariksoysal, Current perspectives in graphene oxide-based electrochemical biosensors for cancer diagnostics, Biosensors, 2022, 12(8), 607 CrossRef CAS PubMed.
- D. Rybak, Y. C. Su, Y. Li, B. Ding, X. Lv, Z. Li, Y. C. Yeh, P. Nakielski, C. Rinoldi, F. Pierini and J. M. Dodda, Evolution of nanostructured skin patches towards multifunctional wearable platforms for biomedical applications, Nanoscale, 2023, 15(18), 8044–8083 RSC.
- U. Laraib, S. Sargazi, A. Rahdar, M. Khatami and S. Pandey, Nanotechnology-based approaches for effective detection of tumor markers: A comprehensive state-of-the-art review, Int. J. Biol. Macromol., 2022, 195, 356–383 CrossRef CAS PubMed.
- Z. A. Alrowaili, H. M. Fathy, H. A. Elsayed, M. Aouassa, M. H. Mahmoud, K. S. El-Nasser, T. A. Taha and A. Mehaney, Heavy metals biosensor based on defective one-dimensional phononic crystals, Ultrasonics, 2023, 130, 106928 CrossRef CAS PubMed.
- I. Pani, S. Sil and S. K. Pal, Liquid crystal biosensors: A new therapeutic window to point-of-care diagnostics, Langmuir, 2023, 39(3), 909–917 CrossRef CAS PubMed.
- Z. Wang, D. Han, H. Wang, M. Zheng, Y. Xu and H. Zhang, Organic semiconducting nanoparticles for biosensor: a review, Biosensors, 2023, 13(4), 494 CrossRef CAS PubMed.
- X. Qin, Z. Zhan and Z. Ding, Progress in electrochemiluminescence biosensors based on organic framework emitters, Curr. Opin. Electrochem., 2023, 39, 101283 CrossRef CAS.
- Y. Wei, H. Qi and C. Zhang, Recent advances and challenges in developing electrochemiluminescence biosensors for health analysis, Chem. Commun., 2023, 59(24), 3507–3522 RSC.
- K. Theyagarajan and Y. J. Kim, Recent developments in the design and fabrication of electrochemical biosensors using functional materials and molecules, Biosensors, 2023, 13(4), 424 CrossRef CAS PubMed.
- N. Saini, P. Pandey, S. Wankar, M. Shirolkar, A. A. Kulkarni, J. A. Kim, T. Kim and A. Kulkarni, Carbon Nanomaterial-Based Biosensors: A Forthcoming Future for Clinical Diagnostics, in Handbook of Porous Carbon Materials, Springer Nature Singapore, Singapore, 2023, pp. 1067–1089 Search PubMed.
- L. R. Silva, J. H. Carvalho, J. S. Stefano, G. G. Oliveira, J. Prakash and B. C. Janegitz, Electrochemical sensors and biosensors based on nanodiamonds: A review, Mater. Today Commun., 2023, 35, 106142 CrossRef CAS.
- K. P. Castro, R. N. Colombo, R. M. Iost, B. G. da Silva and F. N. Crespilho, Low-dimensionality carbon-based biosensors: the new era of emerging technologies in bioanalytical chemistry, Anal. Bioanal. Chem., 2023, 415(18), 3879–3895 CrossRef CAS PubMed.
- S. Masoudi Asil, E. D. Guerrero, G. Bugarini, J. Cayme, N. De Avila and J. Garcia, et al., Theranostic applications of multifunctional carbon nanomaterials, View, 2023, 4(2), 20220056 CrossRef CAS PubMed.
- D. Chauhan, R. Chandra and S. Kumar, Hemocompatible functionalized hydrogen substituted graphdiyne based highly durable biosensor for liver cancer detection, ACS Appl. Bio Mater., 2023, 6(6), 2257–2265 CrossRef CAS PubMed.
- J. Xu, Y. Liu, K. J. Huang, R. Wang and J. Li, Cascade amplification strategy based on ultra-thin graphdiyne and CRISPR/Cas for real-time detection of tumor biomarker, Chem. Eng. J., 2023, 466, 143230 CrossRef CAS.
- M. H. Son, S. W. Park, H. Y. Sagong and Y. K. Jung, Recent advances in electrochemical and optical biosensors for cancer biomarker detection, BioChip J., 2023, 17(1), 44–67 CrossRef CAS.
- A. Uniyal, G. Srivastava, A. Pal, S. Taya and A. Muduli, Recent advances in optical biosensors for sensing applications: a review, Plasmonics, 2023, 18(2), 735–750 CrossRef CAS.
- S. Mittal, A. Saharia, Y. Ismail, F. Petruccione and A. V. Bourdine, et al., Spiral shaped photonic crystal fiber-based surface plasmon resonance biosensor for cancer cell detection, Photonics, 2023, 10(3), 230 CrossRef CAS.
- O. Rusyakina, T. Geernaert, M. Loyez, M. Lobry, K. Chah and P. Mergo, et al., Cascaded Bragg gratings in photonic crystal fiber for plasmonic cladding mode-based biosensing of HER2 protein, Sens. Actuators, B, 2023, 382, 133561 CrossRef CAS.
- R. Gala, V. V. Gandhi, N. Barooah, A. Kunwar, A. C. Bhasikuttan and J. Mohanty, Label-free optical bio-sensing of non-cancerous and cancerous tissues from mice: distinct spectroscopic features of thiazole orange, Sens. Diagn., 2023, 2(1), 147–154 RSC.
- S. Ganesh, P. Dharmalingam, S. Das, K. Venkatakrishnan and B. Tan, Mapping Immune–Tumor Bidirectional Dialogue Using Ultrasensitive Nanosensors for Accurate Diagnosis of Lung Cancer, ACS Nano, 2023, 17(9), 8026–8040 CrossRef CAS PubMed.
- A. Ghosh, S. Fathima Thanutty Kallungal and S. Ramaprabhu, 2D metal-organic frameworks: properties, synthesis, and applications in electrochemical and optical biosensors, Biosensors, 2023, 13(1), 123 CrossRef CAS PubMed.
- S. C. Sajan, A. Singh, P. K. Sharma and S. Kumar, Silicon photonics biosensors for cancer cells detection—A review, IEEE Sens. J., 2023, 23(4), 3366–3377 CAS.
- M. Pourmadadi, A. Moammeri, A. Shamsabadipour, Y. F. Moghaddam, A. Rahdar and S. Pandey, Application of various optical and electrochemical nanobiosensors for detecting cancer antigen 125 (CA-125): a review, Biosensors, 2023, 13(1), 99 CrossRef CAS PubMed.
- B. Jafari, E. Gholizadeh, M. Zhoulideh, E. Adibnia, M. Ghafariasl, S. Golmohammadi and M. Noori, Ultrasensitive and Label-free, Graphene/CaF2 Multilayer-based Biosensor for Detecting Gasses, Cancers, Viruses, and Diabetes, with Significant Improvement of Sensitivity, Quality Factor, and Figure of Merit, 2023 Search PubMed.
- B. Mohan, S. Kumar, H. Xi, S. Ma, Z. Tao and T. Xing, et al., Fabricated Metal-Organic Frameworks (MOFs) as luminescent and electrochemical biosensors for cancer biomarkers detection, Biosens. Bioelectron., 2022, 197, 113738 CrossRef CAS PubMed.
- H. Sohrabi, E. Dezhakam, A. Khataee, E. Nozohouri, M. R. Majidi, N. Mohseni, E. Trofimov and Y. Yoon, Recent trends in layered double hydroxides based electrochemical and optical (bio) sensors for screening of emerging pharmaceutical compounds, Environ. Res., 2022, 211, 113068 CrossRef CAS PubMed.
- A. Bekmurzayeva, Z. Ashikbayeva, N. Assylbekova, Z. Myrkhiyeva, A. Dauletova, T. Ayupova, M. Shaimerdenova and D. Tosi, Ultra-wide, attomolar-level limit detection of CD44 biomarker with a silanized optical fiber biosensor, Biosens. Bioelectron., 2022, 208, 114217 CrossRef CAS PubMed.
- A. Roberts and S. Gandhi, A concise review on potential cancer biomarkers and advanced manufacturing of smart platform-based biosensors for early-stage cancer diagnostics, Biosens. Bioelectron., 2022, 11, 100178 CAS.
- Z. L. Lei and B. Guo, 2D material-based optical biosensor: status and prospect, Adv. Sci., 2022, 9(4), 2102924 CrossRef CAS PubMed.
- H. Sohrabi, E. Dezhakam and A. Khataee, et al., Recent trends in layered double hydroxides based electrochemical and optical (bio) sensors for screening of emerging pharmaceutical compounds, Environ. Res., 2022, 211, 113068 CrossRef CAS PubMed.
- A. P. Carvalho, E. C. Alegria, A. Fantoni, A. M. Ferraria, A. M. B. do Rego and A. P. Ribeiro, Effect of graphene vs. reduced graphene oxide in gold nanoparticles for optical biosensors—a comparative study, Biosensors, 2022, 12(3), 163 CrossRef CAS PubMed.
- K. Barve, U. Singh, P. Yadav and D. Bhatia, Carbon-based designer and programmable fluorescent quantum dots for targeted biological and biomedical applications, Mater. Chem. Front., 2023, 7(9), 1781–1802 RSC.
- Q. Wang, B. Wang, D. Shi, F. Li and D. Ling, Cerium Oxide Nanoparticles-Based Optical Biosensors for Biomedical Applications, Adv. Rem. Sens., 2023, 2(3), 2200065 CrossRef.
- R. Al Mahmud, R. H. Sagor and M. Z. M. Khan, Surface plasmon refractive index biosensors: A review of optical fiber, multilayer 2D material and gratings, and MIM configurations, Opt. Laser Technol., 2023, 159, 108939 CrossRef.
- Y. Zare and K. Y. Rhee, Multiphase approach for calculation of tunneling conductivity of graphene-polymer nanocomposites to optimize breast cancer biosensors, Compos. Sci. Technol., 2023, 232, 109852 CrossRef CAS.
- H. A. Matar, M. A. Ibrahim and M. El-Hagary, Simple and cost-effective route for PANI-ZnO-rGO nanocomposite as a biosensor for L-arginine detection, Diam. Relat. Mater., 2023, 133, 109703 CrossRef CAS.
- A. Foroozandeh, M. Abdouss, H. SalarAmoli, M. Pourmadadi and F. Yazdian, An electrochemical aptasensor based on g-C3N4/Fe3O4/PANI nanocomposite applying cancer antigen_125 biomarkers detection, Process Biochem., 2023, 127, 82–91 CrossRef CAS.
- M. C. Sardaru, N. L. Marangoci, R. Palumbo, G. N. Roviello and A. Rotaru, Nucleic Acid Probes in Bio-Imaging and Diagnostics: Recent Advances in ODN-Based Fluorescent and Surface-Enhanced Raman Scattering Nanoparticle and Nanostructured Systems, Molecules, 2023, 28(8), 3561 CrossRef CAS PubMed.
- K. Wu, K. Lai, J. Chen, J. Yao, S. Zeng, T. Jiang, H. Si, C. Gu and J. Jiang, Ag NC and Ag NP/PorC film-based surface-enhanced raman spectroscopy-type immunoassay for ultrasensitive prostate-specific antigen detection, ACS Omega, 2023, 8(21), 18523–18529 CrossRef CAS PubMed.
- S. T. Liu, J. S. Chen, X. P. Liu, C. J. Mao and B. K. Jin, A photoelectrochemical biosensor based on b-TiO2/CdS: Eu/Ti3C2 heterojunction for the ultrasensitive detection of miRNA-21, Talanta, 2023, 253, 123601 CrossRef CAS PubMed.
- Z. Wei, H. Zhang and Z. Wang, High-Intensity Focused Ultrasound Combined with Ti3C2–TiO2 to Enhance Electrochemiluminescence of Luminol for the Sensitive Detection of Polynucleotide Kinase, ACS Appl. Mater. Interfaces, 2023, 15(3), 3804–3811 CrossRef CAS PubMed.
- J. H. Zhu, L. P. Mei, A. J. Wang, Y. Y. Song and J. J. Feng, Integration of phosphate functionalized Pt/TiO2 and Ru (bpy) 32+ sensitization for ultrasensitive assay of adenosine deaminase activity on a novel split-typed PEC aptasensor, Biosens. Bioelectron., 2023, 226, 115141 CrossRef CAS PubMed.
- S. Singh and Y. K. Prajapati, Novel bottom-side polished PCF-based plasmonic biosensor for early detection of hazardous cancerous cells, IEEE Trans. NanoBioscience, 2023, 22(3), 647–654 CAS.
- K. Xiao, R. Zhu, C. Du, X. Zhang and J. Chen, Highly sensitive and selective microRNA photoelectrochemical assay with magnetic electron donor–acceptor covalent organic framework as photoactive material and ZnSe QDs as photocurrent-polarity-switching factor, Sens. Actuators, B, 2023, 380, 133403 CrossRef CAS.
- D. Zhang, Y. Wang, W. Geng and H. Liu, Rapid detection of tryptamine by optosensor with molecularly imprinted polymers based on carbon dots-embedded covalent-organic frameworks, Sens. Actuators, B, 2019, 285, 546–552 CrossRef CAS.
- M. S. Attia, K. Ali, M. El-Kemary and W. M. Darwish, Phthalocyanine-doped polystyrene fluorescent nanocomposite as a highly selective biosensor for quantitative determination of cancer antigen 125, Talanta, 2019, 201, 185–193 CrossRef CAS PubMed.
- X. Zhang, Y. Li, H. Lv, Z. Gao and C. Zhang, et al., Electrochemical immunosensor with enhanced stability for sensitive detection of α-fetoprotein based on Pd@ Ag@ CeO2 as signal amplification label, J. Electrochem. Soc., 2018, 165(16), B931 CrossRef CAS.
- M. Constantinou, K. Hadjigeorgiou, S. Abalde-Cela and C. Andreou, Label-free sensing with metal nanostructure-based surface-enhanced Raman spectroscopy for cancer diagnosis, ACS Appl. Nano Mater., 2022, 5(9), 12276–12299 CrossRef CAS PubMed.
- R. Zhou, T. Y. Ohulchanskyy, Y. Xu, R. Ziniuk, H. Xu, L. Liu and J. Qu, Tumor-microenvironment-activated NIR-II nanotheranostic platform for precise diagnosis and treatment of colon cancer, ACS Appl. Mater. Interfaces, 2022, 14(20), 23206–23218 CrossRef CAS PubMed.
- L. Xu, S. Hu, D. Qin, Y. Wu, Z. Luo and B. Deng, An electrochemiluminescence immunosensor with double co-reaction accelerators based on Ag3PO4@ EuPO4-AgNP for detecting squamous cell carcinoma antigen, Microchim. Acta, 2023, 190(6), 223 CrossRef CAS PubMed.
- X. Zhang, A. Fan, Y. Pan, X. Liu, Z. Zhao, Y. Song and X. Zhang, Large Laser Spot-Swift Mapping Surface-Enhanced Raman Scattering on Ag Nanoparticle Substrates for Liquid Analysis in Serum-Based Cancer Diagnosis, ACS Appl. Nano Mater., 2022, 5(10), 15738–15747 CrossRef CAS.
- L. Liu, X. An, M. Schaefer, B. Yan, C. de la Torre, S. Hillmer, J. Gladkich and I. Herr, Nanosilver inhibits the progression of pancreatic cancer by inducing a paraptosis-like mixed type of cell death, Biomed. Pharmacother., 2022, 153, 113511 CrossRef CAS PubMed.
- C. M. Snyder, Synthesis of Extracellular Matrix Proteins and Enrichment of Polyunsaturated Fatty Acids Contribute to Breast and Lung Cancer Sensitivity to Silver Nanoparticles, Doctoral dissertation, Wake Forest University, 2022.
- A. Sharma, D. Shambhwani, S. Pandey, J. Singh, H. Lalhlenmawia and M. Kumarasamy, et al., Advances in lung cancer treatment using nanomedicines, ACS Omega, 2022, 8(1), 10–41 CrossRef PubMed.
- D. Fan, J. Luo, Z. Gong, J. Niu, H. Wang, D. Wu and Q. Wei, Polyacrylic acid/polyethylene glycol hybrid antifouling interface for photoelectrochemical immunosensing of CYFRA 21-1 based on TiO2/PpIX/Ag@ Cu2O composite, Talanta, 2023, 260, 124570 Search PubMed.
- M. Esfandiari, M. Khodayari, M. Shojaee, M. Kamankesh, B. Esfandiari and S. Mohajerzadeh, A laser-assisted electrochemical biosensor based on folic acid-functionalized tungsten disulfide nanosheets for label-free cancer cell detection, Sens. Actuators, B, 2023, 381, 133443 Search PubMed.
- Q. Yu, Y. Fu, K. Xiao, X. Zhang, C. Du and J. Chen, A label-free photoelectrochemical biosensor with ultra-low-background noise for lead ion assay based on the Cu2O-CuO-TiO2 heterojunction, Anal. Chim. Acta, 2022, 1195, 339456 Search PubMed.
- X. Han, S. Lin, C. Cheng, X. Han and D. Tang, Inspired by game theory: Multi-signal ouput photoelectrochemical point-of-care immunoassay based on target-triggered organic electronic barriers, Anal. Chim. Acta, 2023, 1265, 341362 CrossRef CAS PubMed.
- N. M. Bardhan, M. Radisic and M. Nurunnabi, Bioinspired materials for wearable diagnostics and biosensors, ACS Biomater. Sci. Eng., 2023, 9(5), 2015–2019 CrossRef CAS PubMed.
- G. Gupta, K. Jha and S. Chaudhary, Biosensor Assays Types and Their Roles Toward Ligand–Receptor Interactions in Drug Discovery, Assay Drug Dev. Technol., 2023, 21(5), 190–201 CrossRef CAS PubMed.
- S. Madhurantakam, Z. J. Lee, A. Naqvi and S. Prasad, Importance of IP-10 as a biomarker of host immune response: Critical perspective as a target for biosensing, Curr. Res. Biotechnol., 2023, 5, 100130 CrossRef CAS.
- N. Krathumkhet, T. Imae, F. M. Wang, C. C. Yuan, J. M. L. Gaol and N. Paradee, Electrochemical immunosensing by carbon ink/carbon dot/ZnO-labeled-Ag@ polypyrrole composite biomarker for CA-125 ovarian cancer detection, Bioelectrochemistry, 2023, 152, 108430 CrossRef CAS PubMed.
- R. Sakthivel, M. Keerthi, R. J. Chung and J. H. He, Heterostructures of 2D materials and their applications in biosensing, Prog. Mater. Sci., 2023, 132, 101024 CrossRef CAS.
- M. Chen, Y. Zhou, J. Lang, L. Li and Y. Zhang, Triboelectric nanogenerator and artificial intelligence to promote precision medicine for cancer, Nano Energy, 2022, 92, 106783 CrossRef CAS.
- S. Chupradit, S. A. Jasim, D. Bokov, M. Z. Mahmoud, A. B. Roomi and K. Hachem, et al., Recent advances in biosensor devices for HER-2 cancer biomarker detection, Anal. Methods, 2022, 14(13), 1301–1310 RSC.
- Y. Cheng, J. Xu, L. Li, P. Cai, Y. Li and Q. Jiang, et al., Boosting the piezoelectric sensitivity of amino acid crystals by mechanical annealing for the engineering of fully degradable force sensors, Adv. Sci., 2023, 10(11), 2207269 CrossRef CAS PubMed.
- M. Y. Azab, M. F. O. Hameed and S. S. Obayya, Overview of optical biosensors for early cancer detection: fundamentals, applications, and future perspectives, Biology, 2023, 12(2), 232 CrossRef CAS PubMed.
- S. Chen, P. Zhu, L. Mao, W. Wu, H. Lin, D. Xu, X. Lu and J. Shi, Piezocatalytic medicine: an emerging frontier using piezoelectric materials for biomedical applications, Adv. Mater., 2023, 35(25), 2208256 CrossRef CAS PubMed.
- C. Sun, F. Wu, D. J. Wallis, M. H. Shen, F. Yuan and J. Yang, et al., Gallium nitride: a versatile compound semiconductor as novel piezoelectric film for acoustic tweezer in manipulation of cancer cells, IEEE Trans. Electron Devices, 2020, 67(8), 3355–3361 CAS.
- Z. Li, J. Zhang, Y. Huang, J. Zhai, G. Liao, Z. Wang and C. Ning, Development of electroactive materials-based immunosensor towards early-stage cancer detection, Coord. Chem. Rev., 2022, 471, 214723 CrossRef CAS.
- Z. Yu, H. Gong, J. Xu, Y. Li, Y. Zeng, X. Liu and D. Tang, Exploiting photoelectric activities and piezoelectric properties of NaNbO3 semiconductors for point-of-care immunoassay, Anal. Chem., 2022, 94(7), 3418–3426 CrossRef CAS PubMed.
- X. Bai, W. Zhang, Y. Dai, Y. Wang, H. Sun and L. Feng, Acoustic and magnetic hybrid actuated immune cell robot for target and kill cancer cells, in 2022 International Conference on Robotics and Automation (ICRA), IEEE, 2022, pp. 7936–7941 Search PubMed.
- M. Bakhshpour, A. K. Piskin, H. Yavuz and A. Denizli, Preparation of Notch-4 Receptor Containing Quartz Crystal Microbalance Biosensor for MDA MB 231 Cancer Cell Detection, Biomed. Eng. Technol., 2022, 1, 515–533 Search PubMed.
- C. Lino, S. Barrias, R. Chaves, F. Adega, J. R. Fernandes and P. Martins-Lopes, Development of a QCM-based biosensor for the detection of non-small cell lung cancer biomarkers in liquid biopsies, Talanta, 2023, 260, 124624 CrossRef CAS PubMed.
- M. R. Villarim, D. R. Belfort and C. P. de Souza, A Surface Plasmon Resonance (SPR)-Based Biosensor Simulation Platform for Performance Evaluation of Different Constructional Configurations, Coatings, 2023, 13(3), 546 CrossRef CAS.
- Q. Li, L. Yang, S. Zhang, F. Wang, Y. Gu, X. Deng and Y. Yang, Organic–Inorganic Hybrid Perovskite Materials for Ultrasonic Transducer in Medical Diagnosis, Crystals, 2022, 12(8), 1043 CrossRef CAS.
- W. Xiang, Q. Lv, H. Shi, B. Xie and L. Gao, Aptamer-based biosensor for detecting carcinoembryonic antigen, Talanta, 2020, 214, 120716 CrossRef CAS PubMed.
- D. Ou, D. Sun, X. Lin, Z. Liang, Y. Zhong and Z. Chen, A dual-aptamer-based biosensor for specific detection of breast cancer biomarker HER2 via flower-like nanozymes and DNA nanostructures, J. Mater. Chem. B, 2019, 7(23), 3661–3669 RSC.
- L. Chi, X. Wang, H. Chen, D. Tang and F. Xue, Ultrasensitive photoelectrochemical biosensing platform based target-triggered biocatalytic precipitation reactions on a flower-like Bi2O2S super-structured photoanode, J. Mater. Chem. B, 2022, 10(48), 10018–10026 RSC.
- Y. Nur, S. Gaffar, Y. W. Hartati and T. Subroto, Applications of electrochemical biosensor of aptamers-based (APTASENSOR) for the detection of leukemia biomarker, Sens. Bio-Sens. Res., 2021, 32, 100416 CrossRef.
- Q. Zhang, R. Ma, Y. Zhang, J. Zhao, Y. Wang and Z. Xu, Dual-aptamer-assisted ratiometric SERS biosensor for ultrasensitive and precise identification of breast cancer exosomes, ACS Sens., 2023, 8(2), 875–883 CrossRef CAS PubMed.
- L. He, R. Huang, P. Xiao, Y. Liu, L. Jin, H. Liu and S. Li, et al., Current signal amplification strategies in aptamer-based electrochemical biosensor: A review, Chin. Chem. Lett., 2021, 32(5), 1593–1602 CrossRef CAS.
- S. I. Zida, C. C. Yang, Y. L. Khung and Y. D. Lin, Fabrication and characterization of an aptamer-based N-type silicon nanowire FET biosensor for VEGF detection, J. Med. Biol. Eng., 2020, 40, 601–609 CrossRef.
- J. Zhao, Z. Di and L. Li, Spatiotemporally Selective Molecular Imaging via Upconversion Luminescence-Controlled, DNA-Based Biosensor Technology, Angew. Chem., Int. Ed., 2022, 61(32), e202204277 CrossRef CAS PubMed.
- M. Barani, M. Khatami, B. Behnam, R. Rajendram, P. Kesharwani and A. Sahebkar, Aptamer-conjugated carbon nanotubes or graphene for targeted cancer therapy and diagnosis, in Aptamers Engineered Nanocarriers for Cancer Therapy, Woodhead Publishing, 2023, pp. 277–294 Search PubMed.
- Y. Fan, W. Wu, N. Xie, Y. Huang, H. Wu and J. Zhang, et al., Biocompatible engineered erythrocytes as plasmonic sensor initiators for high-sensitive screening of non-small cell lung cancer-derived exosomal miRNA in an integrated system, Biosens. Bioelectron., 2023, 219, 114802 CrossRef CAS PubMed.
- A. Díaz-Fernández and E. E. Ferapontova, Electrochemical ELASA: improving early cancer detection and monitoring, Anal. Bioanal. Chem., 2023, 415(18), 3831–3846 CrossRef PubMed.
- Z. Ji, Z. Shang, M. Sohail, P. Wang, B. Li, X. Zhang and G. Chen, A CRISPR-enabled fluorometric biosensor for the sensitive detection of heparin antidote protamine based on programmable nuclease Cas12a, Sens. Actuators, B, 2023, 374, 132709 CrossRef CAS.
- S. Gundagatti and S. Srivastava, An optimization of blocking agents for designing reliable electrochemical biosensors for ovarian cancer, Mater. Today Proc., 2023 DOI:10.1016/j.matpr.2023.04.460.
- S. Kizhepat, A. S. Rasal, J. Y. Chang and H. F. Wu, Development of two-dimensional functional nanomaterials for biosensor applications: opportunities, challenges, and future prospects, Nanomaterials, 2023, 13(9), 1520 CrossRef CAS PubMed.
- Q. Chen, H. Gao, J. Yao, B. Jiang, R. Yuan and Y. Xiang, Target/aptamer binding-induced inhibition of enzyme activity for amplified electrochemical detection of Sonic Hedgehog protein, Sens. Actuators, B, 2023, 385, 133702 CrossRef CAS.
- M. El Aamri, G. Yammouri, H. Mohammadi, A. Amine and H. Korri-Youssoufi, Electrochemical biosensors for detection of microRNA as a cancer biomarker: Pros and cons, Biosensors, 2020, 10(11), 186 CrossRef CAS PubMed.
- W. Li, Measurable Residual Disease Testing in Acute Leukemia: Technology and Clinical Significance, Exon Publications, 2022, pp. 79–100 Search PubMed.
- A. Rhouati, J. L. Marty and A. Vasilescu, Electrochemical biosensors combining aptamers and enzymatic activity: Challenges and analytical opportunities, Electrochim. Acta, 2021, 390, 138863 CrossRef CAS.
- A. Allegra, C. Petrarca, M. Di Gioacchino, G. Mirabile and S. Gangemi, Electrochemical biosensors in the diagnosis of acute and chronic leukemias, Cancers, 2022, 15(1), 146 CrossRef PubMed.
- J. I. Omage, E. Easterday, J. T. Rumph, I. Brula, B. Hill, J. Kristensen and D. T. Ha, et al., Cancer diagnostics and early detection using electrochemical aptasensors, Micromachines, 2022, 13(4), 522 CrossRef PubMed.
- M. Y. Azab, M. F. O. Hameed and S. S. Obayya, Overview of optical biosensors for early cancer detection: Fundamentals, applications and future perspectives, Biology, 2023, 12(2), 232 CrossRef CAS PubMed.
- C. Amri, A. K. Shukla and J. H. Lee, Recent advancements in nanoparticle-based optical biosensors for circulating cancer biomarkers, Materials, 2021, 14(6), 1339 CrossRef CAS PubMed.
- M. Pohanka, Overview of piezoelectric biosensors, immunosensors and DNA sensors and their applications, Materials, 2018, 11(3), 448 CrossRef PubMed.
- F. Narita, Z. Wang, H. Kurita, Z. Li, Y. Shi, Y. Jia and C. Soutis, A review of piezoelectric and magnetostrictive biosensor materials for detection of COVID-19 and other viruses, Adv. Mater., 2021, 33(1), 2005448 CrossRef CAS PubMed.
- D. Jiang, X. Wei, Y. Zhu, Y. Qiu, X. Liu, L. Kong and F. Li, et al., Evaluating the efficacy and cardiotoxicity of EGFR-TKI AC0010 with a novel multifunctional biosensor, Microsyst. Nanoeng., 2023, 9(1), 57 CrossRef CAS PubMed.
- W. Huang, S. Hu, X. Jiang, Y. Weng and Y. Liu, et al., A highly sensitive sensor for carcinoembryonic antigen based on AlGaN/GaN high-electron-mobility transistors, Nanotechnology, 2023, 34(31), 315203 CrossRef CAS PubMed.
- K. VanderVorst, C. A. Dreyer, J. Hatakeyama, G. R. Bell, J. A. Learn and A. L. Berg, et al., Vangl-dependent Wnt/planar cell polarity signaling mediates collective breast carcinoma motility and distant metastasis, Breast Cancer Res., 2023, 25(1), 52 CrossRef CAS PubMed.
- H. Wu, G. Zhang and X. Yang, Electrochemical immunosensor based on Fe3O4/MWCNTs-COOH/AuNPs nanocomposites for trace liver cancer marker alpha-fetoprotein detection, Talanta, 2023, 259, 124492 CrossRef CAS PubMed.
- X. Huang, H. Tian, L. Huang, Q. Chen, Y. Yang and R. Zeng, et al., Well-ordered Au nanoarray for sensitive and reproducible detection of hepatocellular carcinoma-associated miRNA via CHA-assisted SERS/fluorescence dual-mode sensing, Anal. Chem., 2023, 95(14), 5955–5966 CrossRef CAS PubMed.
- A. Imbriano, A. Tricase, E. Macchia, L. Torsi and P. Bollella, Self-powered logically operated fluorescent detection of hepatitis B virus (HBV), Anal. Chim. Acta, 2023, 1252, 341037 CrossRef CAS PubMed.
- M. Li, Z. Li, P. Wang and Q. Ma, A novel bimetallic MXene derivative QD-based ECL sensor for miRNA-27a-3p detection, Biosens. Bioelectron., 2023, 228, 115225 CrossRef CAS PubMed.
- H. Jaradat, A. Al-Hamry, M. Ibbini, N. Fourati and O. Kanoun, Novel sensitive electrochemical immunosensor development for the selective detection of HopQ H. pylori bacteria biomarker, Biosensors, 2023, 13(5), 527 CrossRef CAS PubMed.
- L. Bai, C. Gu, J. Liu, P. Gai and F. Li, Photofuel cell-based self-powered biosensor for HER2 detection by integration of plasmonic-metal/conjugated molecule hybrids and electrochemical sandwich structure, Biosens. Bioelectron., 2023, 220, 114850 CrossRef CAS PubMed.
- H. Nasrollahpour, B. Khalilzadeh, R. Rahbarghazi, N. Erk, M. R. Rashidi and A. Naseri, Development of an electrochemical biosensor for the detection of mammary gland carcinoma using molybdenum enhanced poly taurine nano-biofilms confirmed pathological findings, Cancer Nanotechnol., 2023, 14(1), 45 CrossRef CAS.
- T. Zeng, S. Wu, Q. Liang, H. Shi, J. Gong and N. Duan, et al., A colorimetric biosensor to track Trop-2 status of tumor cells for diagnosis of breast cancer, Sens. Actuators, B, 2023, 390, 134020 CrossRef CAS.
- J. Zhang, H. Zhou, T. Hao, Y. Yang, Q. Zhang and J. Li, et al., Faraday cage-type ECL biosensor for the detection of circulating tumor cell MCF-7, Anal. Chim. Acta, 2023, 1271, 341465 CrossRef CAS PubMed.
- M. Carvalho, R. M. Gomes, S. M. Rocha, J. Barroca-Ferreira, C. J. Maia and L. Guillade, et al., Development of a novel electrochemical biosensor based on plastic antibodies for detection of STEAP1 biomarker in cancer, Bioelectrochemistry, 2023, 152, 108461 CrossRef CAS PubMed.
- H. Chang, Q. Zhu, Y. Wu, A. Liu, M. Jiang and C. Li, et al., A sensitive label-free biosensor based on Ag2S-sensitized Bi2WO6/BiOBr heterojunction for photoelectrochemical immunoassay of prostate specific antigen, Talanta, 2023, 257, 124343 CrossRef CAS PubMed.
- J. Wei, Z. Liu, Z. Zhang, K. Lan, Y. Wang, R. Chen and G. Qin, Suspended CNTs/MoS2 heterostructure field effect transistor for high performance biosensor and its application for serum PSA detection, Sens. Actuators, B, 2023, 381, 133417 CrossRef CAS.
- S. Anbu, A. Paul, K. Surendranath, A. Sidali and A. J. Pombeiro, Naphthalimide-phenanthroimidazole incorporated new fluorescent sensor for “turn-on” Cu2+ detection in living cancer cells, J. Inorg. Biochem., 2021, 220, 111466 CrossRef CAS PubMed.
- S. Pareek, U. Jain, M. Bharadwaj, K. Saxena, S. Roy and N. Chauhan, An ultrasensitive electrochemical DNA biosensor for monitoring Human papillomavirus-16 (HPV-16) using graphene oxide/Ag/Au nano-biohybrids, Anal. Biochem., 2023, 663, 115015 CrossRef CAS PubMed.
- R. Rawat, S. Singh, S. Roy, A. Kumar, T. Goswami and A. Mathur, Design and development of an electroanalytical genosensor based on Cu-PTCA/rGO nanocomposites for the detection of cervical cancer, Mater. Chem. Phys., 2023, 295, 127050 CrossRef CAS.
- A. Parihar, P. Vishwakarma, P. Prajapati and R. Khan, Non-invasive ultra-sensitive detection of breast cancer biomarker using cerium nanoparticle functionalized graphene oxide enabled impedimetric aptasensor, Biosens. Bioelectron., 2025, 268, 116925 CrossRef CAS PubMed.
- A. Parihar and R. Khan, Yttrium functionalized reduced graphene oxide nanocomposite-based aptasensor for ultrasensitive detection of a breast cancer biomarker, ACS Appl. Nano Mater., 2023, 7(16), 18207–18218 CrossRef.
- A. Parihar, A. Singhal, N. Kumar, R. Khan, M. A. Khan and A. K. Srivastava, Next-generation intelligent MXene-based electrochemical aptasensors for point-of-care cancer diagnostics, Nano-Micro Lett., 2022, 14(1), 100 CrossRef CAS PubMed.
- A. Parihar, N. K. Choudhary, P. Sharma and R. Khan, Carbon nanomaterials-based electrochemical aptasensor for point-of-care diagnostics of cancer biomarkers, Mater. Today Chem., 2023, 30, 101499 CrossRef CAS.
- L. Luo, L. Wang, L. Zeng, Y. Wang, Y. Weng, Y. Liao, T. Chen, Y. Xia, J. Zhang and J. Chen, A ratiometric electrochemical DNA biosensor for detection of exosomal MicroRNA, Talanta, 2020, 207, 120298 CrossRef CAS PubMed.
- H. Xie, K. Di, R. Huang, A. Khan, Y. Xia, H. Xu, C. Liu, T. Tanc, X. Tian, H. Shen, N. He and Z. Li, Extracellular vesicles based electrochemical biosensors for detection of cancer cells: A Review, Chin. Chem. Lett., 2020, 1–9 CrossRef CAS.
- M. Ç. Canbaz, Ç. S. Şimşek and M. K. Sezgintürk, Electrochemical biosensor based on self-assembled monolayers modified with gold nanoparticles for detection of HER-3, Anal. Chim. Acta, 2014, 814, 31–38 CrossRef CAS PubMed.
- M. Freitas, M. M. P. S. Neves, H. P. A. Nouws and C. Delerue-Matos, Quantum dots as nanolabels for breast cancer biomarker HER2-ECD analysis in human serum, Talanta, 2020, 208, 120430 CrossRef CAS PubMed.
- R. Heidari, J. Rashidiani, M. Abkar, R. A. Taheri, M. M. Moghaddam, S. A. Mirhosseini, R. Seidmoradi, M. R. Nourani, M. Mahboobi, A. H. Keihan and H. Kooshki, CdS Nanocrystals/Graphene Oxide-AuNPs based electrochemiluminescence immunosensor in sensitive quantification of a cancer biomarker: p53, Biosens. Bioelectron., 2019, 126, 7–14 CrossRef CAS PubMed.
- M. Mattarozzi, M. Giannetto and M. Careri, Electrochemical immunomagnetic assay as biosensing strategy for determination of ovarian cancer antigen HE4 in human serum, Talanta, 2020, 217, 120991 CrossRef CAS PubMed.
- J. G. Pacheco, M. S. V. Silva, M. Freitas, H. P. A. Nouws and C. Delerue-Matos, Molecularly imprinted electrochemical sensor for the point-of-care detection of a breast cancer biomarker (CA 15-3), Sens. Actuators, B, 2018, 256, 905–912 CrossRef CAS.
- K. S. Prasad, X. Cao, N. Gao, Q. Jin, S. T. Sanjay, G. Henao-Pabon and X. J. Li, A low-cost nanomaterial-based electrochemical immunosensor on paper for high-sensitivity early detection of pancreatic cancer, Sens. Actuators, B, 2020, 305, 127516 CrossRef CAS PubMed.
- C. Muñoz-San Martín, M. Gamella, M. Pedrero, A. Montero-Calle, R. Barderas, S. Campuzano and J. M. Pingarrón, Magnetic beads-based electrochemical immunosensing of HIF-1α, a biomarker of tumoral hypoxia, Sens. Actuators, B, 2020, 307, 127623 CrossRef.
- A. Barhoum, Z. Altintas, K. S. Devi and R. J. Forster, Electrochemiluminescence biosensors for detection of cancer biomarkers in biofluids: Principles, opportunities, and challenges, Nano Today, 2023, 50, 101874 CrossRef.
- P. Ranjan, A. Singhal, M. A. Sadique, S. Yadav, A. Parihar and R. Khan, Scope of biosensors, commercial aspects, and miniaturized devices for point-of-care testing from lab to clinics applications, Biosensor Based Advanced Cancer Diagnostics, Academic Press, 2022, pp. 395–410 Search PubMed.
- I. S. Kucherenko, O. O. Soldatkin, S. V. Dzyadevych and A. P. Soldatkin, Electrochemical biosensors based on multienzyme systems: main groups, advantages and limitations–a review, Anal. Chim. Acta, 2020, 1111, 114–131 CrossRef CAS PubMed.
- Q. Ali, H. Zheng, M. J. Rao, M. Ali, A. Hussain, M. H. Saleem and Y. Nehela, et al., Advances, limitations, and prospects of biosensing technology for detecting phytopathogenic bacteria, Chemosphere, 2022, 296, 133773 CrossRef CAS PubMed.
- Y. Ye, J. Ji, Z. Sun, P. Shen and X. Sun, Recent advances in electrochemical biosensors for antioxidant analysis in foodstuff, TrAC, Trends Anal. Chem., 2020, 122, 115718 CrossRef CAS.
- A. M. Akim, N. Safdar, A. Yasmin, Y. Y. Sung and T. S. T. Muhammad, Cancer and disease diagnosis-biosensor as potential diagnostic tool for biomarker detection, J. Adv. Pharm. Technol. Res., 2022, 13(4), 243–247 CrossRef PubMed.
- H. F. El-Sharif, S. Patel, E. N. Ndunda and S. M. Reddy, Electrochemical detection of dioctyl phthalate using molecularly imprinted polymer modified screen-printed electrodes, Anal. Chim. Acta, 2022, 1196, 339547 CrossRef CAS PubMed.
- M. Xiao, F. Tian, X. Liu, Q. Zhou, J. Pan, Z. Luo, M. Yang and C. Yi, Virus detection: from state-of-the-art laboratories to smartphone-based point-of-care testing, Adv. Sci., 2022, 9(17), 2105904 CrossRef CAS PubMed.
- A. A. Smith, R. Li and Z. T. H. Tse, Reshaping healthcare with wearable biosensors, Sci. Rep., 2023, 13(1), 4998 CrossRef CAS PubMed.
- M. Bolognesi, M. Prosa, M. Toerker, L. Lopez Sanchez, M. Wieczorek and C. Giacomelli, et al., A Fully Integrated Miniaturized Optical Biosensor for Fast and Multiplexing Plasmonic Detection of High-and Low-Molecular-Weight Analytes, Adv. Mater., 2023, 35(26), 2208719 CrossRef CAS PubMed.
- C. Wang, Y. Zhang, C. Liu, S. Gou, S. Hu and W. Guo, A portable colorimetric point-of-care testing platform for MicroRNA detection based on programmable entropy-driven dynamic DNA network modulated DNA-gold nanoparticle hybrid hydrogel film, Biosens. Bioelectron., 2023, 225, 115073 CrossRef CAS PubMed.
- G. F. Giordano, L. F. Ferreira, Í. Bezerra, J. A. Barbosa, J. N. Y. Costa, G. J. C. Pimentel and R. S. Lima, Machine learning toward high-performance electrochemical sensors, Anal. Bioanal. Chem., 2023, 415(18), 3683–3692 CrossRef CAS PubMed.
- V. Chugh, A. Basu, A. Kaushik and A. K. Basu, Progression in quantum sensing/bio-sensing technologies for healthcare, ECS Sens. Plus, 2023, 2(1), 015001 CrossRef CAS.
- S. W. Loo and T. S. Pui, Cytokine and cancer biomarkers detection: the dawn of electrochemical paper-based biosensor, Sensors, 2020, 20(7), 1854 CrossRef CAS PubMed.
- A. M. Chiorcea-Paquim, Advances in electrochemical biosensor technologies for the detection of nucleic acid breast cancer biomarkers, Sensors, 2023, 23(8), 4128 CrossRef CAS PubMed.
- A. Mohammadpour-Haratbar, S. B. A. Boraei, Y. Zare, K. Y. Rhee and S. J. Park, Graphene-based electrochemical biosensors for breast cancer detection, Biosensors, 2023, 13(1), 80 CrossRef CAS PubMed.
- H. Kamali, S. Golmohammadzadeh, H. Zare, R. Nosrati, M. Fereidouni and H. Safarpour, The recent advancements in the early detection of cancer biomarkers by DNAzyme-assisted aptasensors, J. Nanobiotechnol., 2022, 20(1), 438 CrossRef CAS PubMed.
- M. Sharafeldin, K. Kadimisetty, K. S. Bhalerao, T. Chen and J. F. Rusling, 3D-printed Immunosensor arrays for cancer diagnostics, Sensors, 2020, 20(16), 4514 CrossRef CAS PubMed.
- https://clinicaltrials.gov/study/NCT06211010.
- https://clinicaltrials.gov/study/NCT02659358.
- https://clinicaltrials.gov/study/NCT02957370.
- https://clinicaltrials.gov/study/NCT00813878.
- https://clinicaltrials.gov/study/NCT06033248.
- https://clinicaltrials.gov/study/NCT04549064.
- https://clinicaltrials.gov/study/NCT05940311.
Footnote |
| † These authors have equal contributions. |
| This journal is © The Royal Society of Chemistry 2025 |