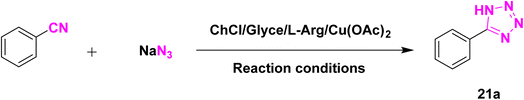Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Introduction and characterization of a novel Cu(II)-based quaternary deep eutectic solvent and its application in the efficient synthesis of triazoles and tetrazoles under mild conditions as an inexpensive, reusable, benign, and dual solvent/catalyst medium†
Laleh Golestanifar and
Ali Reza Sardarian *
*
Chemistry Department, College of Science, Shiraz University, Shiraz 71946-84795, Iran. E-mail: sardarian@shirazu.ac.ir; Fax: +98-36460788; Tel: +98-71-36137107
First published on 3rd February 2025
Abstract
Deep eutectic solvents consist of hydrogen bond donor and acceptor components. They are a new type of ionic liquids, and they have attracted the attention of many chemists in recent years. In this work, a quaternary deep eutectic solvent (QDES) was prepared using choline chloride, glycerol, L-arginine, and copper acetate. Its physicochemical properties were determined by Fourier transform infrared spectroscopy (FT-IR), thermal analysis (TGA), differential scanning calorimetry (DSC), hydrogen potential (pH), cyclic voltammetry (CV), viscosity, density, refractive index, ionic conductivity and spectrophotometer ultraviolet-visible (UV-Vis). Further, as a novel benign solvent/catalyst for the synthesis of 1,4-disubstituted-1,2,3-triazole, 4-substituted-1H-1,2,3-triazole, and 5-substituted-1H-tetrazole derivatives were used in a click reaction strategy. The special features of this method include mild conditions, a non-toxic environment, short reaction time, easy operation, biodegradability, deep eutectic solvent/catalyst recovery, access to cheaper raw materials, and environmental compatibility.
1. Introduction
Deep eutectic solvents refer to liquids close to the eutectic composition of mixtures (the molar ratio of components that produces the lowest melting point). They are a new alternative to ionic liquid (IL) analogs, which are now widely used and known as green counterparts of toxic organic solvents in organic transformations.1 In general, DESs, in contrast to ILs that are formed via a multistep strategy, can be prepared in one step with complete atom economy by simple mixing and heating of their components.1 Therefore, their preparation is consistent with green chemistry rules, environmentally and economically.2 In recent years, attention to green chemistry and using natural solvents and catalysts, have increased to preserve the environment. Heterocyclic chemicals have been evaluated as a lead constituent in medicinal chemistry. Because they are also copious in biopolymers such as enzymes, natural products, vitamins, and biologically active chemicals. Among heterocyclic materials, triazoles and tetrazoles have occupied a unique place. 1,2,3-Triazoles have many applications in industry, agriculture, and medicinal chemistry due to their stability in oxidation-reduction and acid-base hydrolysis. The large dipole moment of 1,2,3-triazoles causes these compounds to act as hydrogen bond acceptors that are useful for binding to biological targets and improving solubility.3,4 The medicinal properties of these compounds include anticancer, anti-allergy, anti-tuberculosis, anti-virus, anti-malaria, anti-AIDS, and antibiotics, and some of these compounds are now available in the market as drugs or are in clinical trials.5,6 The conventional methods for the synthesis of 1,2,3-triazoles are carried out using click reactions, which were reported by Sharpless and co-workers in 2001 to accelerate the synthesis of quasi-pharmaceutical molecules via azide–alkyne [1, 3]-dipolar cycloaddition.7 One of the click reactions is the copper-catalyzed azide–alkyne cycloaddition (CuAAC) reaction using various raw materials such as phenols, carboxylic acids, amines, amides, acetophenones to provide active acetylene substrates that produce 1,2,3-triazoles.8–13 Also, tetrazole derivatives are used in various pharmaceutical fields such as anti-arrhythmic, anti-diabetic, anti-fungal, anti-allergic, anti-AIDS and neurological diseases, and have been used in such a way that they are also present in the structure of the famous drug Losartan.14,15 The synthesis of tetrazoles is done using click reactions, through cyclization reaction [3 + 2] between an azide and a nitrile.16–18 The reported syntheses of 1,2,3-triazoles and 1H-tetrazoles have been are associated with pitfalls, such as the use of expensive and toxic metals, strong Lewis acids, and the in situ production of hydrazoic acid with a toxic and explosive nature.14,19–21 To avoid the use of toxic organic solvents and also expensive and harmful catalysts required in multi-step synthesis and purification for the formation of 1,2,3-triazoles and 1H-tetrazoles, herein, a novel QDES is designed to be prepared by employing safe, available, and inexpensive compounds such as choline chloride, glycerol, L-arginine and copper(II) acetate and used in the green production of the desired triazoles and tetrazoles.2. Experimental section
2.1. Materials and instrumentation
All pure chemical materials were purchased from Sigma-Aldrich and Merck. The progress of the reactions was monitored using thin-layer chromatography (TLC) on silica gel Polygram SILG/UV-254 plates. Physical and spectral information such as melting point, FT-IR, and NMR data were analyzed, along with available literature data, to characterize the obtained products. Bucci B-545 B.V.CHI apparatus was used to measure the melting point of the products. Fourier transform infrared (FT-IR) spectra were performed using a Shimadzu FT-IR 8300 spectrophotometer. The 1H and 13C NMR spectra were recorded using a Bruker Avance 400 (400 MHz) in DMSO-d6 and CDCl3. A typical sample was used to investigate the electrochemical behavior of ChCl/Glyce/L-Arg/Cu(II) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1
0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03 mmol). A silver wire and a platinum electrode were utilized as the reference electrode and the working and counter electrode, respectively, along with a computer-controlled AUTOLAB (model AUT84490) for electrochemical analysis. Thermogravimetric analysis was performed using a TGA Q600 instrument under an argon atmosphere with a heating rate of 10 °C min−1. Differential scanning calorimeter (DSC Q600) was used to study the phase transition of the mixture ChCl/Glyce/L-Arg/Cu(II) (1
0.03 mmol). A silver wire and a platinum electrode were utilized as the reference electrode and the working and counter electrode, respectively, along with a computer-controlled AUTOLAB (model AUT84490) for electrochemical analysis. Thermogravimetric analysis was performed using a TGA Q600 instrument under an argon atmosphere with a heating rate of 10 °C min−1. Differential scanning calorimeter (DSC Q600) was used to study the phase transition of the mixture ChCl/Glyce/L-Arg/Cu(II) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1
0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03 mmol) at a heating rate of 10 °C min−1 under an argon atmosphere. The conductivity of this DES was measured by Metrohm (SWISS MADE, model 644 conductivity meter). The viscosity was measured with a rheometric viscometer (Anton Paar, model MCR302), and the density was measured with a digital tube densitometer (Anton Paar, model DMA4000). Refractive index values were determined using equipment from A. Kruss Optronic GmbH. The UV-Vis absorption spectrum was recorded using an ultraviolet-visible spectrophotometer (UV-Vis PerkinElmer, Lambda 25). The pH of the DES was measured with a pH meter (Metrohm, model 780).
0.03 mmol) at a heating rate of 10 °C min−1 under an argon atmosphere. The conductivity of this DES was measured by Metrohm (SWISS MADE, model 644 conductivity meter). The viscosity was measured with a rheometric viscometer (Anton Paar, model MCR302), and the density was measured with a digital tube densitometer (Anton Paar, model DMA4000). Refractive index values were determined using equipment from A. Kruss Optronic GmbH. The UV-Vis absorption spectrum was recorded using an ultraviolet-visible spectrophotometer (UV-Vis PerkinElmer, Lambda 25). The pH of the DES was measured with a pH meter (Metrohm, model 780).
2.2. General procedure
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 was used to extract the product into the contents of the flask. The merged organic phase was dried over Mg2SO4 and filtered. The concentration of filtrate under reduced pressure provides the corresponding pure acetylene product (Scheme 2).
3 was used to extract the product into the contents of the flask. The merged organic phase was dried over Mg2SO4 and filtered. The concentration of filtrate under reduced pressure provides the corresponding pure acetylene product (Scheme 2).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and the organic layer was filtered through anhydrous Mg2SO4 and dried under reduced pressure to obtain the crude triazole product. Finally, the desired pure triazole product was obtained through recrystallization in n-hexane/acetone.
2, and the organic layer was filtered through anhydrous Mg2SO4 and dried under reduced pressure to obtain the crude triazole product. Finally, the desired pure triazole product was obtained through recrystallization in n-hexane/acetone.3. Results and discussions
3.1. [ChCl][Glyce]2[L-Arg]0.1[Cu(OAc)2]0.03 characterizations
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N (guanidinium) and asymmetrical stretching COO− bands are seen in 1678 cm−1 and 1620 cm−1, respectively. The related peaks of scissoring NH2 (amino) and CH2 groups are seen at 1557 cm−1 and 1474 cm−1. The absorbance peak at 1421 cm−1 is related to the symmetrical stretching vibration COO−, and the out-of-plane bending absorption peak of N–H occurred at 770 cm−1.22 For [ChCl][Glyce]2[L-Arg]0.1[Cu(OAc)2]0.03 (Fig. 1d), the stretching vibrations of NH2 (amino, guanidinium) groups appeared at 3379 cm−1. Absorption in the regions of 2932 cm−1 and 2883 cm−1 was attributed to the stretching vibrations of CH2 and C–H groups in the mixture, and the asymmetric stretching vibration of the COO− group appeared in the region of 1648 cm−1. The scissor bending absorption of CH2 was observed in the region of 11
N (guanidinium) and asymmetrical stretching COO− bands are seen in 1678 cm−1 and 1620 cm−1, respectively. The related peaks of scissoring NH2 (amino) and CH2 groups are seen at 1557 cm−1 and 1474 cm−1. The absorbance peak at 1421 cm−1 is related to the symmetrical stretching vibration COO−, and the out-of-plane bending absorption peak of N–H occurred at 770 cm−1.22 For [ChCl][Glyce]2[L-Arg]0.1[Cu(OAc)2]0.03 (Fig. 1d), the stretching vibrations of NH2 (amino, guanidinium) groups appeared at 3379 cm−1. Absorption in the regions of 2932 cm−1 and 2883 cm−1 was attributed to the stretching vibrations of CH2 and C–H groups in the mixture, and the asymmetric stretching vibration of the COO− group appeared in the region of 1648 cm−1. The scissor bending absorption of CH2 was observed in the region of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 478 cm−1, and the absorption that occurred in the region of 1413 cm−1 is related to the symmetric stretching vibration of COO−. Also, symmetrical and asymmetrical stretching vibrations of Cu–N should be monitored at 430 cm−1 and 444 cm−1, but these areas could not be shown in the used device.22
478 cm−1, and the absorption that occurred in the region of 1413 cm−1 is related to the symmetric stretching vibration of COO−. Also, symmetrical and asymmetrical stretching vibrations of Cu–N should be monitored at 430 cm−1 and 444 cm−1, but these areas could not be shown in the used device.22
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mmol, 225 °C) and ChCl/Glyce/L-Arg (1
2 mmol, 225 °C) and ChCl/Glyce/L-Arg (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
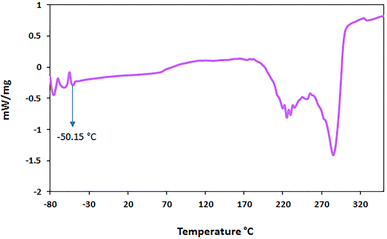
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 mmol, 160 °C), for the solvent/catalyst [ChCl][Glyce]2[L-Arg]0.1[Cu (OAc)2]0.03, thermal gravimetric analysis and differential thermal analysis were performed at 30–400 °C (Fig. 4). The weight loss occurred in the temperature range of 130–250 °C was assigned to the complete decomposition of DES and its related components. With the increase in the chain length of the [ChCl][Glyce]2[L-Arg]0.1[Cu (OAc)2]0.03 DES, followed by the reduction of ionic interactions and the creation of more hydrogen bonds between the components, its stability decreases. As a result, it starts to degrade at a lower temperature.23,24
0.1 mmol, 160 °C), for the solvent/catalyst [ChCl][Glyce]2[L-Arg]0.1[Cu (OAc)2]0.03, thermal gravimetric analysis and differential thermal analysis were performed at 30–400 °C (Fig. 4). The weight loss occurred in the temperature range of 130–250 °C was assigned to the complete decomposition of DES and its related components. With the increase in the chain length of the [ChCl][Glyce]2[L-Arg]0.1[Cu (OAc)2]0.03 DES, followed by the reduction of ionic interactions and the creation of more hydrogen bonds between the components, its stability decreases. As a result, it starts to degrade at a lower temperature.23,24
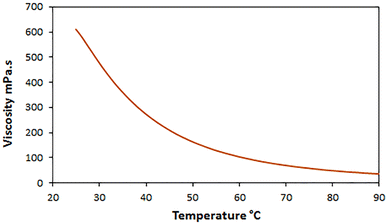
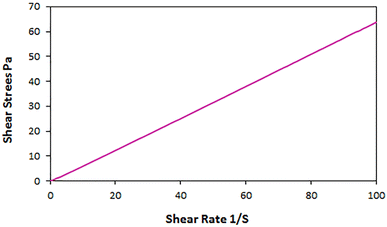
Also, to determine whether the ChCl/Glyce/L-Arg/Cu(OAc)2 fluid is Newtonian or non-Newtonian at a constant temperature (25 °C), its shear stress was measured based on the shear rate. The linear graph shows that the desired solvent/catalyst system is a Newtonian fluid (Fig. 6).
 | ||
| Fig. 7 UV-visible absorption spectra for ChCl/Glyce/L-Arg/Cu(I), ChCl/Glyce/L-Arg/Cu (OAc)2, Cu(OAc)2 and CuCl in H2O. | ||
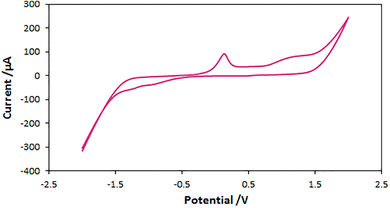
Based on UV-vis absorptions for copper(I) chloride (290 nm) and copper(II) acetate (280 nm),27,28 UV-Vis analysis for aqueous solution CuCl, Cu(OAc)2, NaN3/Cu(OAc)2 and NaN3/CuCl were performed and showed a broad absorption in the region of 300–400 nm which disclose the reduction of Cu(II) to Cu(I) (Fig. 8).
3.2. Evaluation of the catalytic activity of the ChCl/Glyce/L-Arg/Cu(OAc)2 in the preparation of 1H-1,2,3-triazole derivatives (1–20a)
To evaluate and optimize the ChCl/Glyce/L-Arg/Cu(OAc)2 as a green and new solvent/catalyst medium for the preparation of 1H-1,2,3-triazole derivatives, the reaction between phenylacetylene and sodium azide was taken as the model reaction. Initially, the reaction was taken in 3 mL of the prepared DES, and the expected triazole was formed in 80% yield after 3 h stirring at ambient temperature (Table 1, entry 1). Regarding dependency of the efficiency reaction to temperature, confirmed by TLC monitoring, repetition of the model reaction at 60 °C generated the desired triazole in 95% yield in 1 h (Table 1, entry 2). The efficiency of the model reaction was brought down when 1 and 2 mL of the DES were applied, so the yield of the title product reduced to 70% and 80% in the longer reaction times, 3 h and 1.5 h, separately (Table 1, entries 3 and 4). Enhancement of the molar ratio of Cu(OAc)2 in the DES structure, from 0.03 to 0.04 and 0.05, did not display any improvement in the efficiency of the reaction, and 4-phenyl-1H-triazole was formed in both cases, in excellent yield (95%) (Table 1, entries 5 and 6). When the reaction was performed in the absence of the ChCl/Glyce/L-Arg/Cu (OAc)2 DES, on the basis of TLC checking, all of the starting material remained intact, which is an indication of the critical role DES as a catalyst (Table 1, entry 7). For considering the effect of Cu(OAc)2 and [ChCl][Glyce]2[L-Arg]0.1 as the components of the desired DES, the reaction phenylacetylene with sodium azide was taken in the presence of these precursors separable. It should be noted that although the model reaction proceeded by Cu(OAc)2 in 20% yield at 60 °C after 6 h (Table 1, entry 8), most of the starting material remained unconsumed when the reaction was performed in [ChCl][Glyce]2[L-Arg]0.1 (Table 1, entry 9). These findings proved the synergic effect of these two precursors when they were combined and also the solvent/catalyst dual role [ChCl][Glyce]2[L-Arg]0.1[Cu(OAc)2]0.03. Based on the information gathered in Table 1, using 1 mmol phenylacetylene and 1.17 mmol sodium azide in 3 mL of [ChCl][Glyce]2[L-Arg]0.1[Cu(OAc)2]0.03 at 60 °C was selected as the optimized conditions for the providing of 1H-1,2,3-triazoles.| Entry | Reaction media | Molar ratio | Catalyst amount (mL) | Temperature (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: phenylacetylene (1 mmol) and sodium azide (1.17 mmol).b Yield refers to the net products isolated. | ||||||
| 1 | ChCl/Glyce/L-Arg/Cu(OAc)2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03 0.03 |
3 | R.T | 3 | 80 |
| 2 | ChCl/Glyce/L-Arg/Cu(OAc)2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03 0.03 |
3 | 60 | 1 | 95 |
| 3 | ChCl/Glyce/L-Arg/Cu(OAc)2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03 0.03 |
1 | 60 | 3 | 70 |
| 4 | ChCl/Glyce/L-Arg/Cu(OAc)2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03 0.03 |
2 | 60 | 1.5 | 83 |
| 5 | ChCl/Glyce/L-Arg/Cu(OAc)2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.04 0.04 |
3 | 60 | 1 | 95 |
| 6 | ChCl/Glyce/L-Arg/Cu(OAc)2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05 0.05 |
3 | 60 | 1 | 95 |
| 7 | — | — | — | 60 | 10 | — |
| 8 | Cu(OAc)2 | 0.03 | — | 60 | 6 | 20 |
| 9 | ChCl/Glyce/L-Arg | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1 |
3 | 60 | 8 | <5 |
After optimizing the reaction conditions, the click reaction strategy between phenylacetylene and the phenylacetylenes prepared from carboxylic acids and phenols with sodium azide was elected to investigate the efficiency and effectiveness of the ChCl/Glyce/L-Arg/Cu (OAc)2 DES as a green solvent/catalyst medium in the synthesis of 4-substituted-1H-1,2,3-triazole derivatives. The results are collected in Table 2. As can be seen, all acetylenes with electron-rich and electron-deficient substitutions underwent the [3 + 2] cycloaddition reaction and provided the title products in good to excellent yields (Table 2, entries 1–9). To find out the potential of the present DES in the making of 1,4-disubstituted-1,2,3-triazoles, the one-pot three-component reaction among the acetylenes prepared from carboxylic acids and phenols, sodium azide, and benzyl bromide or allyl bromide was tested. Based on the data achieved, the related products, except in cases of entries, were obtained in good to excellent yields under mild and optimized conditions (Table 2, entries 12–20). The products were often purified through recrystallization by n-hexane/acetone mixture after recovery of the DES.
| Entry | R | R1 | Product | TON | TOFc (h−1) |
|---|---|---|---|---|---|
| a Reaction conditions: acetylene derivative (1 mmol), organic halide (1 mmol), sodium azide (1.17 mmol) in ChCl/Glyce/L-Arg/Cu(OAc)2.b Yield refers to the net products isolated.c Defined as moles of product per mole of catalyst per h. | |||||
| 1 |  |
— |  |
54.29 | 54.29 |
| 2 |  |
— |  |
42.86 | 10.71 |
| 3 |  |
— |  |
44.57 | 11.14 |
| 4 |  |
— |  |
41.71 | 11.92 |
| 5 |  |
— |  |
38.86 | 7.77 |
| 6 | 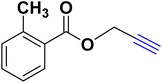 |
— | 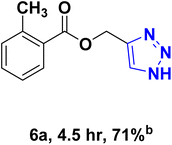 |
40.57 | 9.02 |
| 7 |  |
— |  |
39.43 | 7.89 |
| 8 |  |
— |  |
42.86 | 12.24 |
| 9 |  |
— | 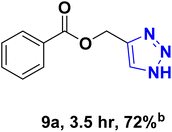 |
41.14 | 11.76 |
| 10 |  |
Allyl- |  |
30.20 | 6.04 |
| 11 |  |
PhCH2- |  |
33.56 | 6.71 |
| 12 |  |
Allyl- |  |
59.06 | 19.69 |
| 13 |  |
PhCH2- |  |
60.40 | 20.13 |
| 14 |  |
Allyl- |  |
53.69 | 15.34 |
| 15 |  |
PhCH2- |  |
55.70 | 18.57 |
| 16 |  |
Allyl- |  |
52.35 | 13.09 |
| 17 |  |
PhCH2- |  |
57.05 | 14.26 |
| 18 | 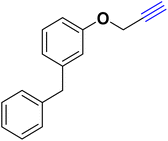 |
Allyl- |  |
55.70 | 13.93 |
| 19 |  |
PhCH2- |  |
61.74 | 20.58 |
| 20 |  |
Allyl- |  |
60.40 | 20.13 |
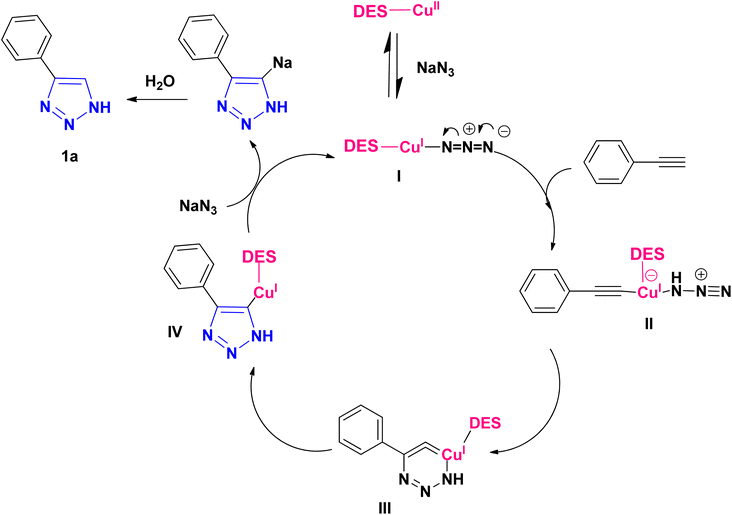
According to the methods presented in the references, the Cu(I) ion can catalyze the formation of 1H-1,2,3-triazoles.6,11,13 For this reason, in some protocols for the conversion of Cu(II) to copper(I), reducing agents such as sodium ascorbate and sodium azide have been used.28 Therefore, it seems reasonable to start the mechanism in the first step with the reduction of Cu(II) to Cu(I) to provide I without using additional reducing agents, and based on this, the proposed mechanism for the synthesis of 1H-1,2,3-triazole derivatives using phenylacetylene and sodium azide as the reactant models are presented as follows (Scheme 3). The copper II is reduced to copper I with sodium azide in the first step to give I. In the next step, the intermediate I react with the alkyne to form II, which is converted to III after an intramolecular cyclization. Then, the intermediate III rearranges and the isomer IV is created and transformed to triazole (1a) within the reaction environment via the protonolysis with water.
A comparison of the effect of the ChCl/Glyce/L-Arg/Cu(OAc)2 as a green and new solvent/catalyst medium in the formation of 1H-1,2,3-triazole derivatives with some other reported catalysts is collected in Table 3. As can be observed, in contrast to application of the quaternary ChCl/Glyce/L-Arg/Cu(OAc)2 DES, which can act as solvent/catalyst system and is obtained via quantitative atom economy with just by mixing of the constructive available components, the most of these published catalysts are magnetic and on a nanoscale, which their multi-step synthesis requires a lot of cost, time, production of waste, and the use of organic solvents.
| Entry | Catalyst | Conditions | Time (h) | Yield a(%) | Ref. |
|---|---|---|---|---|---|
| a Yield refers to the net products isolated. | |||||
| 1 | Cu(I)-AMPS | H2O/RT | 1 | 82 | 29 |
| 2 | Fe3O4@LDH@cysteine–Cu(I) | Choline azide/70 °C | 0.4 | 95 | 30 |
| 3 | Fe3O4@SiO2-pAMBA-CS-Cu2O | H2O/70 °C | 0.3 | 93 | 31 |
| 4 | Cu2O@Peanut shell | EtOH: H2O/50 °C | 1.5 | 93 | 32 |
| 5 | CuFe2O4@SiO2@L-arginine@Cu(I) | EtOH: H2O/60 °C | 0.6 | 89 | 33 |
| 6 | Fe3O4-DOPA-CuNPs | H2O/120 °C | 0.2 | 96 | 34 |
| 7 | Sulfated tungstate | DMF/60 °C | 1 | 95 | 35 |
| 8 | ChCl/Glyce/L-Arg/Cu(OAc)2 | 60 °C | 1 | 95 | This work |
Regarding the successful application of the biocompatible and biodegradable DES, [ChCl][Glyce]2[L-Arg]0.1[Cu(OAc)2]0.03, in the synthesis of the 1,2,3-triazoles, we encouraged to check its ability as a solvent/catalyst system in the providing 5-substituted tetrazoles. Therefore, we chose the model reaction of benzonitrile and sodium azide to optimize the reaction factors. The results were registered in Table 4. We ran the model reaction under ambient temperature, 60 °C, and 90 °C generated 5-phenyl tetrazole in 60%, 80%, and 90% yield in 2 mL of the DES, respectively (Table 4, entries 1–3). The best result was achieved when 2 mL of the ChCl/Glyce/L-Arg/Cu (OAc)2 DES was used, and the mixture was blended for 1.5 h at 110 °C (Table 4, entry 4). Performing the model reaction in a lower volume (1 mL) of the present DES reduced its efficiency and afforded the tetrazole product to 90% (Table 4, entry 5), and using 3 mL of the DES did not lead to any improvement in the efficiency and forming 97% yield of the title compound at 110 °C after 1.5 h (Table 4, entry 6). No significant change was observed in the efficiency of the reaction when a higher molar ratio of Cu (OAc)2 was utilized in the DES structure and a higher volume of DES was used (Table 4, entries 7 and 8). The reaction did not proceed without the ChCl/Glyce/L-Arg/Cu (OAc)2 DES, which proves the DES's crucial role as a solvent/catalyst media (Table 4, entry 9). When the reaction was accomplished under solvent-free conditions in the presence of Cu (OAc)2 as one of the DES precursors, only 40% of 4-phenyl-1H-1,2,3-tetrazole was produced after 6 h at 110 °C (Table 4, entry 10). The effect of the ChCl/Glyce/L-Arg DES, as the other precursor, was also not high in offering the desired product and resulted in a yield of about 30% (Table 4, entry 11). These two last facts, along with the result achieved in entry 2, exhibited the occurrence of synergic effect phenomena between Cu(OAc)2 and the ChCl/Glyce/L-Arg when they were blended to form the ChCl/Glyce/L-Arg/Cu(OAc)2 DES. As specified by the information in Table 4, using 1 mmol benzonitrile and 1.4 mmol sodium azide in 2 mL of [ChCl][Glyce]2[L-Arg]0.1[Cu (OAc)2]0.03 at 110 °C can be used as the optimized reaction conditions for the production of 5-substituted-1H-tetrazoles in follows.
| Entry | Reaction media | Molar ratio | Catalyst amount (mL) | Temp. (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: benzonitrile (1 mmol) and sodium azide (1.4 mmol).b Yield refers to the net products isolated. | ||||||
| 1 | ChCl/Glyce/L-Arg/Cu(OAc)2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03 0.03 |
2 | RT | 12 | 60 |
| 2 | ChCl/Glyce/L-Arg/Cu(OAc)2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03 0.03 |
2 | 60 | 6 | 80 |
| 3 | ChCl/Glyce/L-Arg/Cu(OAc)2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03 0.03 |
2 | 90 | 4 | 90 |
| 4 | ChCl/Glyce/L-Arg/Cu(OAc)2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03 0.03 |
2 | 110 | 1.5 | 97 |
| 5 | ChCl/Glyce/L-Arg/Cu(OAc)2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03 0.03 |
1 | 110 | 2 | 90 |
| 6 | ChCl/Glyce/L-Arg/Cu(OAc)2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03 0.03 |
3 | 110 | 1.15 | 97 |
| 7 | ChCl/Glyce/L-Arg/Cu(OAc)2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.04 0.04 |
2 | 110 | 1.5 | 97 |
| 8 | ChCl/Glyce/L-Arg/Cu(OAc)2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05 0.05 |
2 | 110 | 1.5 | 97 |
| 9 | — | — | — | 90 | 12 | — |
| 10 | Cu(OAc)2 | 0.03 | — | 110 | 6 | 40 |
| 11 | ChCl/Glyce/L-Arg | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1 |
2 | 110 | 10 | 30 |
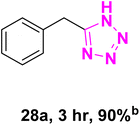
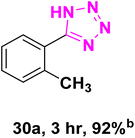
After optimizing the reaction conditions to check versatility and determine the reaction scope, varieties of the nitriles were treated with sodium azide in the ChCl/Glyce/L-Arg/Cu (OAc)2 DES (Table 5). The benzonitriles with electron-withdrawing and the electron-releasing substitutions undertook the reaction under the optimized conditions and offered the related tetrazoles in excellent yields (Table 5, entries 1–6, 9 and 10). Also, 4-cyanopyridine (as a heteroaromatic nitrile) and benzyl cyanide (as an aliphatic nitrile) provided the desired tetrazole in 92% and 90% yield indefinitely when reacted with sodium azide in the title DES.
| Entry | A | Product | TON | TOFc (h−1) |
|---|---|---|---|---|
| a Reaction conditions: different benzonitriles (1 mmol) and sodium azide (1.4 mmol) in ChCl/Glyce/L-Arg/Cu(OAc)2.b Yield refers to the net products isolated.c Defined as moles of product per mole of catalyst per h. | ||||
| 1 |  |
 |
53.59 | 35.72 |
| 2 | 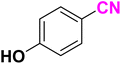 |
 |
50.83 | 25.41 |
| 3 |  |
 |
53.59 | 35.72 |
| 4 | 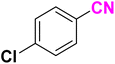 |
 |
51.93 | 34.62 |
| 5 |  |
 |
50.83 | 33.89 |
| 6 |  |
 |
53.04 | 35.36 |
| 7 |  |
 |
50.83 | 20.33 |
| 8 |  |
 |
49.72 | 16.57 |
| 9 |  |
 |
51.93 | 34.62 |
| 10 |  |
 |
50.83 | 16.94 |
An acceptable mechanism for preparing 5-substituted-1H-tetrazoles using sodium azide and benzonitrile as the prototype reaction is shown in Scheme 4. Initially, Cu(II) in the DES is reduced to DES-Cu(I)–N3, which activates the nitrile group in benzonitrile by coordination to provide the transient species I. The intramolecular cyclization reaction in I through attacking the terminal nitrogen atom of azide on the nitrile functional group to generates the particle II. The reaction of sodium azide with II is led to regenerate DES-Cu(I)–N3 to initiate a new reaction cycle and produce the sodium salt of tetrazole III, which is transformed to the tetrazole 21a by acidic hydrolysis in work up step.
 | ||
| Fig. 9 (a) Evaluation of the reusability of ChCl/Glyce/L-Arg/Cu(OAc)2 DES in the synthesis of 4-phenyl-1H-1,2,3-triazoles and (b) 5-phenyl-1H-tetrazoles. | ||
In addition, the efficiency evaluation of this method was performed in the synthesis of 5-phenyl-1H-tetrazole through click reaction using benzonitrile and sodium azide against some other catalysts available in the recent scientific sources, and the related data were registered in Table 6. As can be seen, the current DES, as a safe and biocompatible environment with the dual role of solvent and catalyst, can be formed by simply mixing the related biocompatible components with a complete atom economy, providing the title product with excellent efficiency at one of the relatively short times with avoiding application of toxic solvents, also toxic and expensive metal.
| Entry | Catalyst | Conditions | Time (h) | Yielda (%) | TOFb (h−1) | Ref. |
|---|---|---|---|---|---|---|
| a Yield refers to the net products isolated.b Defined as moles of product per mole of catalyst per h. | ||||||
| 1 | Boehmite@SiO2@Tris-Cu(I) | PEG/120 °C | 2 | 95 | 1.39 | 36 |
| 2 | Fe3O4@L-lysine-Pd(0) | H2O/100 °C | 1 | 99 | 330 | 17 |
| 3 | Cu(II)-DCC-CMK-3 | PEG/120 °C | 1.5 | 97 | 2.65 | 37 |
| 4 | MCM-41-SO3H | DMF/80 °C | 2 | 90 | 0.97 | 38 |
| 5 | Choline chloride/ZnCl2 | 140 °C | 0.5 | 90 | 50.42 | 39 |
| 6 | Choline chloride/glycerol | 140 °C | 2 | 70 | 9.46 | 39 |
| 7 | [BiPy](HSO3)2Cl2 | Ethylene glycol/100 °C | 1.5 | 99 | 2.2 | 18 |
| 8 | ChCl/Glyce/L-Arg/Cu(OAc)2 | 110 °C | 1.5 | 97 | 35.72 | This work |
4. Conclusion
We have prepared a novel, biocompatible and cost-effective Cu(II)-based quaternary deep eutectic solvent, [ChCl][Glyce]2[L-Arg]0.1[Cu (OAc)2]0.03, with a simple mixing of the non-toxic and available components using a quantitative atom economy way. After characterization, its efficiency and capability as a dual solvent/catalyst were explored for the synthesis of 1,4-disubstituted-1,2,3-triazoles, 4-substituted-1H-1,2,3-triazole, and 5-substituted-1H-tetrazole by click reaction strategy. This study disclosed the high to excellent efficiency of the QDES for the synthesis of the desired tetrazoles and triazoles under mild reaction conditions as a reusable, biodegradable, inexpensive, and non-toxic solvent/catalyst media.Data availability
The data that support the findings of this study are available in the ESI file.†Author contributions
L. G.: resources, validation, data curation, visualization. Methodology, formal analysis, investigation, writing – original draft. A. R. S.: resources, conceptualization, supervision, investigation, writing – review & editing.Conflicts of interest
The authors have no competing interests to declare that are relevant to the content of this article.Acknowledgements
The authors thank Shiraz University for the facilities the Department of Chemistry provides and partial financial support.References
- Q. Zhang, K. D. O. Vigier, S. Royer and F. Jérôme, Deep eutectic solvents: syntheses, properties and applications, Chem. Soc. Rev., 2012, 41, 7108–7146, 10.1039/c2cs35178a.
- M. Deetlefs and K. R. Seddon, Assessing the greenness of some typical laboratory ionic liquid preparations, Green Chem., 2010, 12, 17–30, 10.1039/B915049H.
- M. Whiting, J. Muldoon, Y. C. Lin, S. M. Silverman, W. Lindstrom, A. J. Olson and V. V. Fokin, Inhibitors of HIV-1 protease by using in situ click chemistry, Angew. Chem., Int. Ed., 2006, 45, 1435–1439, DOI:10.1002/anie.200502161.
- J. S. Lv, X. M. Peng, B. Kishore and C. H. Zhou, 1, 2, 3-Triazole-derived naphthalimides as a novel type of potential antimicrobial agents: Synthesis, antimicrobial activity, interaction with calf thymus DNA and human serum albumin, Bioorg. Med. Chem. Lett, 2014, 24, 308–313, DOI:10.1016/j.bmcl.2013.11.013.
- S. Zhang, Z. Xu, C. Gao, Q. C. Ren, L. Chang, Z. S. Lv and L. S. Feng, Triazole derivatives and their anti-tubercular activity, Eur. J. Med. Chem., 2017, 138, 501–513, DOI:10.1016/j.ejmech.2017.06.051.
- D. P. Vala, R. M. Vala and H. M. Patel, Versatile synthetic platform for 1, 2, 3-triazole chemistry, ACS Omega, 2022, 7, 36945–36987, DOI:10.1021/acsomega.2c04883.
- K. B. Sharpless, H. C. Kolb and M. G. Finn, Click chemistry: diverse chemical function from a few good reactions, Angew. Chem., Int. Ed., 2001, 40, 2004–2021, DOI:10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5.
- A. A. Saikia, R. N. Rao, S. Das, S. Jena, S. Rej, B. Maiti and K. Chanda, Sequencing [3+ 2]-cycloaddition and multicomponent reactions: A regioselective microwave-assisted synthesis of 1, 4-disubstituted 1, 2, 3-triazoles using ionic liquid supported Cu (II) precatalysts in methanol, Tetrahedron Lett., 2020, 61, 152273, DOI:10.1016/j.tetlet.2020.152273.
- F. S. D. Santos, R. P. D. Freitas, C. S. D. Freitas, D. V. C. Mendonça, D. P. Lage, G. D. S. V. Tavares and R. R. Teixeira, Synthesis of 1, 2, 3-Triazole-Containing Methoxylated Cinnamides and Their Antileishmanial Activity against the Leishmania braziliensis Species, Pharmaceuticals, 2023, 16, 1113, DOI:10.3390/ph16081113.
- A. Yadav, P. Patil, N. Birajdar, A. Gophane, K. C. Gunturu, S. Hangirgekar and S. Sankpal, An expeditious synthesis and histo–toxicological study of 1, 2, 3-triazoles catalyzed by a novel Fe3O4@ SiO2-Pr-Thiosemicarbazide-Cu (II) as a magnetically separable catalyst, Appl. Organomet. Chem., 2023, 37, e7181, DOI:10.1002/aoc.7181.
- E. Haldón, M. C. Nicasio and P. J. Pérez, Copper-catalysed azide-alkyne cycloadditions (CuAAC): an update, Org. Biomol. Chem., 2015, 13, 9528–9550, 10.1039/C5OB01457C.
- M. Kaur, D. Bharti, V. Kumar, P. K. Verma and R. Kumar, Decarboxylative click cycloaddition: an emerging strategy towards substituted 1, 2, 3-triazole derivatives, Mol. Divers., 2024, 1–17, DOI:10.1007/s11030-024-11014-4.
- L. Liang and D. Astruc, The copper (I)-catalyzed alkyne-azide cycloaddition (CuAAC)“click” reaction and its applications. An overview, Coord. Chem. Rev., 2011, 255, 2933–2945, DOI:10.1016/j.ccr.2011.06.028.
- S. Wu, A. Fluxe, J. Sheffer, J. M. Janusz, B. E. Blass, R. White and L. Djandjighian, Discovery and in vitro/in vivo studies of tetrazole derivatives as Kv1. 5 blockers, Bioorg. Med. Chem. Lett, 2006, 16, 6213–6218, DOI:10.1016/j.bmcl.2006.09.021.
- X. Xiong, C. Yi, X. Liao and S. Lai, A practical multigram-scale method for the green synthesis of 5-substituted-1H-tetrazoles in deep eutectic solvent, Tetrahedron Lett., 2019, 60, 402–406, DOI:10.1016/j.tetlet.2018.12.037.
- C. Tao, B. Wang, L. Sun, J. Yi, D. Shi, J. Wang and W. Liu, Cu (NO3) 2-catalyzed Direct Synthesis of 5-substituted 1 H-tetrazoles from Alcohols or Aldehydes, J. Chem. Res., 2017, 41, 25–29, DOI:10.3184/174751917X14815427219248.
- M. A. Ashraf, Z. Liu, C. Li and D. Zhang, Fe3O4@ L-lysine-Pd (0) organic-inorganic hybrid: As a novel heterogeneous magnetic nanocatalyst for chemo and homoselective [2+ 3] cycloaddition synthesis of 5-substituted 1H-tetrazoles, Appl. Organomet. Chem., 2021, 35, e6133, DOI:10.1002/aoc.6133.
- E. Aali, M. Gholizadeh and N. Noroozi-Shad, 1-Disulfo-[2, 2-bipyridine]-1, 1-diium chloride ionic liquid as an efficient catalyst for the green synthesis of 5-substituted 1H-tetrazoles, J. Mol. Struct., 2022, 1247, 131289, DOI:10.1016/j.molstruc.2021.131289.
- M. L. Kantam, K. S. Kumar and K. P. Raja, An efficient synthesis of 5-substituted-1H-tetrazoles using Zn/Al hydrotalcite catalyst, J. Mol. Catal. A:Chem., 2006, 247, 186–188, DOI:10.1016/j.molcata.2005.11.046.
- V. V. Sureshbabu, R. Venkataramanarao, S. A. Naik and G. Chennakrishnareddy, Synthesis of tetrazole analogs of amino acids using Fmoc chemistry: isolation of amino free tetrazoles and their incorporation into peptides, Tetrahedron Lett., 2007, 48, 7038–7041, DOI:10.1016/j.tetlet.2007.07.129.
- M. Nasrollahzadeh, Y. Bayat, D. Habibi and S. Moshaee, FeCl3–SiO2 as a reusable heterogeneous catalyst for the synthesis of 5-substituted 1H-tetrazoles via [2+ 3] cycloaddition of nitriles and sodium azide, Tetrahedron Lett., 2009, 50, 4435–4438, DOI:10.1016/j.tetlet.2009.05.048.
- K. Musioł, J. Janczak, K. Helios, M. Witwicki, M. Fitta, R. Pełka and A. Wojciechowska, Copper (II) coordination polymer based on l-arginine as a supramolecular hybrid inorganic-organic material: synthesis, structural, spectroscopic and magnetic properties, Res. Chem. Intermed., 2023, 49, 3563–3587, DOI:10.1007/s11164-023-04957-0.
- F. Chemat, H. Anjum, A. M. Shariff, P. Kumar and T. Murugesan, Thermal and physical properties of (Choline chloride+ urea+ l-arginine) deep eutectic solvents, J. Mol. Liq., 2016, 218, 301–308, DOI:10.1016/j.molliq.2016.02.062.
- F. Chemat, H. J. You, K. Muthukumar and T. Murugesan, Effect of l-arginine on the physical properties of choline chloride and glycerol based deep eutectic solvents, J. Mol. Liq., 2015, 212, 605–611, DOI:10.1016/j.molliq.2015.10.016.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Deep eutectic solvents (DESs) and their applications, Chem. Rev., 2014, 114, 11060–11082, DOI:10.1021/cr300162p.
- H. R. Ong, M. M. R. Khan, R. Ramli, Y. Du, S. Xi and R. M. Yunus, Facile synthesis of copper nanoparticles in glycerol at room temperature: Formation mechanism, RSC Adv., 2015, 5, 24544–24549, 10.1039/C4RA16919K.
- A. R. Hajipour, E. Boostani and F. Mohammadsaleh, Copper (I) catalyzed Sonogashira reactions promoted by monobenzyl nicotinium chloride, a N-donor quaternary ammonium salt, RSC Adv., 2015, 5, 94369–94374, 10.1039/C5RA19028B.
- A. R. Sardarian, F. Mohammadi and M. Esmaeilpour, Dendrimer-encapsulated copper (II) immobilized on Fe 3 O 4@ SiO 2 NPs: a robust recoverable catalyst for click synthesis of 1, 2, 3-triazole derivatives in water under mild conditions, Res. Chem. Intermed., 2019, 45, 1437–1456, DOI:10.1007/s11164-018-3672-x.
- L. Bahsis, H. Ben El Ayouchia, H. Anane, A. Pascual-Álvarez, G. De Munno, M. Julve and S. E. Stiriba, A reusable polymer-supported copper (I) catalyst for triazole click reaction on water: An experimental and computational study, Appl. Organomet. Chem., 2019, 33, e4669, DOI:10.1002/aoc.4669.
- F. Pazoki, A. Salamatmanesh, S. Bagheri and A. Heydari, Synthesis and Characterization of Copper (I)-Cysteine Complex Supported on Magnetic Layered Double Hydroxide as an Efficient and Recyclable Catalyst System for Click Chemistry Using Choline Azide as Reagent and Reaction Medium, Catal. Lett., 2020, 150, 1186–1195, DOI:10.1007/s10562-019-03011-2.
- F. Jafarzadeh, Z. Dolatkhah, S. Molaei and S. Javanshir, CS@ Cu2O and magnetic Fe3O4@ SiO2-pAMBA-CS-Cu2O as heterogeneous catalysts for CuAAC click reaction, Arab. J. Chem., 2022, 15, 103838, DOI:10.1016/j.arabjc.2022.103838.
- Z. Dolatkhah, A. Mohammadkhani, S. Javanshir and A. Bazgir, Peanut shell as a green biomolecule support for anchoring Cu 2 O: a biocatalyst for green synthesis of 1, 2, 3-triazoles under ultrasonic irradiation, BMC Chem., 2019, 13, 1–10, DOI:10.1186/s13065-019-0612-9.
- F. Salehzadeh, M. Esmkhani, M. Zallaghi, S. Javanshir and M. G. Dekamin, CuFe2O4@SiO2@L-arginine@Cu(I) as a new magnetically retrievable heterogeneous nanocatalyst with high efficiency for 1,4-disubstituted 1,2,3-triazoles synthesis, Sci. Rep., 2023, 13, 8675, DOI:10.1038/s41598-023-36012-8.
- M. Kumari, Y. Jain, P. Yadav, H. Laddha and R. Gupta, Synthesis of Fe 3 O 4-DOPA-Cu magnetically separable nanocatalyst: a versatile and robust catalyst for an array of sustainable multicomponent reactions under microwave irradiation, Catal. Lett., 2019, 149, 2180–2194, DOI:10.1007/s10562-019-02794-8.
- S. B. Autade and K. G. Akamanchi, Sulfated tungstate a heterogeneous acid catalyst for synthesis of 4-aryl-NH-1, 2, 3-triazoles by 1, 3-dipolar cycloaddition of nitroolefins with NaN3, Synth. Commun., 2019, 49, 1947–1956, DOI:10.1080/00397911.2019.1612919.
- A. Ghorbani-Choghamarani, H. Aghavandi and M. Mohammadi, Boehmite@ SiO2@ Tris (hydroxymethyl) aminomethane-Cu (I): a novel, highly efficient and reusable nanocatalyst for the C-C bond formation and the synthesis of 5-substituted 1H-tetrazoles in green media, Appl. Organomet. Chem., 2020, 34, e5804, DOI:10.1002/aoc.5804.
- R. Ghafouri-Nejad and M. Hajjami, Synthesis and characterization of Cu–N, N′-Dicyclohexylcarbodiimide supported on CMK-3 as a novel, efficient and recoverable nanocatalyst for synthesis of tetrazole, polyhydroquinoline, and sulfoxide derivatives, React. Kinet. Mech. Catal., 2020, 129, 371–389, DOI:10.1007/s11144-019-01695-6.
- F. Matloubi Moghaddam, M. Eslami and N. Ghadirian, MCM-41-SO3H as an efficient, reusable nano-ordered heterogeneous catalyst for the synthesis of divers 1-& 5-substituted 1H-tetrazoles, Sci. Iran., 2019, 26, 1463–1473, DOI:10.24200/sci.2018.21088.
- S. A. Padvi and D. S. Dalal, Choline chloride–ZnCl2: Recyclable and efficient deep eutectic solvent for the [2+ 3] cycloaddition reaction of organic nitriles with sodium azide, Synth. Commun., 2017, 47, 779–787, DOI:10.1080/00397911.2017.1285033.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra08090d |
| This journal is © The Royal Society of Chemistry 2025 |