 Open Access Article
Open Access ArticleEnhanced platinum and palladium recovery from aqueous solutions: a comparative study of acylthiourea and amine-modified silica gel adsorbents†
Malehlogonolo R. R. Mphahlele a,
Alseno K. Mosaib,
Hlanganani Tutua and
Izak A. Kotzé
a,
Alseno K. Mosaib,
Hlanganani Tutua and
Izak A. Kotzé *a
*a
aMolecular Sciences Institute, School of Chemistry, University of the Witwatersrand, Private Bag X3, WITS, Johannesburg, 2050, South Africa. E-mail: Izak.Kotze@wits.ac.za
bDepartment of Chemistry, Faculty of Natural and Agricultural Sciences, University of Pretoria, Lynnwood Road, Pretoria 0002, South Africa
First published on 11th February 2025
Abstract
The recovery of precious metals from secondary sources is becoming increasingly important due to their natural scarcity and rising industrial demand. This study introduces a novel adsorbent, N-triethoxysilylpropyl-N′-benzoylthiourea-modified silica gel (TESP-BT-SG), developed for the selective recovery of platinum (Pt) and palladium (Pd) from aqueous solutions that simulate refinery wastewater. The extraction capabilities of TESP-BT-SG were compared with those of an amine-bearing adsorbent, (3-aminopropyl)triethoxy-silane-modified silica gel (APTES-SG), previously recognized for Pt and Pd recovery. Under optimal conditions, TESP-BT-SG achieved extraction efficiencies of 97% for Pt and 99% for Pd. Both adsorbents demonstrated rapid adsorption kinetics for Pd relative to Pt, reaching equilibrium within 3 hours for Pd and within 6 hours (TESP-BT-SG) and 24 hours (APTES-SG) for Pt. In solutions with elevated competing ion concentrations (5–100 mg L−1), both adsorbents retained high selectivity (>97%) for Pt and Pd. Adsorption isotherms and kinetic models were applied to elucidate the adsorption mechanisms, with the Langmuir isotherm and pseudo-second-order models providing the best fits, indicating monolayer coverage and chemisorption, respectively. Notably, the APTES-SG sorbent demonstrated enhanced performance with an increased loading capacity of 2.45 mmol g−1 compared to the previously reported 1.15 mmol g−1, achieved through our improved synthesis method. This modified APTES-SG showed significantly higher affinity for Pd (98%), Pt (97%), and iridium (Ir) (89%) compared to previous values of 8%, 33%, and 42%, respectively. The exceptional efficiency and selectivity of these silica-anchored adsorbents underscore their potential as cost-effective solutions for industries seeking to recover precious metals.
1. Introduction
Platinum group metals (PGMs), particularly platinum (Pt) and palladium (Pd), are essential components in various industries, including catalytic converters, electronics, pharmaceuticals, and, more recently, the hydrogen fuel cell sector.1–3 These diverse applications arise from their unique properties, such as corrosion resistance, high melting points, and remarkable catalytic activities.1–3 Despite their high cost, demand for PGMs continues to grow due to their irreplaceable roles in key industrial processes.4 Combined with their low natural abundance in the Earth's crust (0.0063 ppm), the rising demand may lead to potential shortages.3,4 Consequently, there is growing interest in exploring the recovery of these metals from secondary sources, such as refinery holding ponds and industrial waste, which may contain recoverable amounts of Pt and Pd (27–120 mg kg−1).2–5 In addition to PGMs, metals such as magnesium, nickel, manganese, copper, calcium, and iron (Mg, Ni, Mn, Cu, Ca, and Fe) can also enter the environment through industrial wastewater.6,7 Prolonged exposure and accumulation of these metals in the environment can lead to detrimental effects on mammals, plants, and humans.6–8 Recovering these metals from secondary sources with efficient separation techniques is thus both economically and environmentally advantageous.6,7Conventional recovery techniques used for removing PGMs from aqueous solutions include chemical precipitation, solvent extraction, and ion exchange.2,8,9 However, these methods often suffer from low efficiency, poor selectivity, and the generation of secondary waste.4,6,9 To overcome these limitations, there has been a growing focus on developing alternative methods, such as adsorption, which offer higher efficiency, selectivity, and ease of operation.4,8,9 Adsorption utilises solid supports, such as activated carbon, membranes, polymer resins, mesoporous silica, zeolite, and graphene oxide.4 These supports can be tailored to improve selectivity towards target ions, thus enhancing overall efficiency.4
In PGM separation studies, selectivity is a crucial parameter, as these metals are typically found alongside other transition metals.9–12 Adsorption has proven superior to traditional separation techniques, which are limited in their ability to selectively recover individual PGMs without co-extracting other ions.11–14 PGM-targeted adsorbents typically contain nitrogen (N), oxygen (O), and sulphur (S) bearing ligands.4,9,11 According to the hard–soft acid–base (HSAB) theory, Pt and Pd are classified as soft metals, thus having a high affinity for soft bases (S > N > O).4,15,16 Therefore, using ligands that contain soft donor atoms can be advantageous for the selective recovery of Pt and Pd from solutions containing a wide array of metal ions.4,13–16
In our previous work, we developed a novel acylthiourea-modified silica gel adsorbent, N,N-di(trimethoxysilylpropyl)-N′-benzoylthiourea-modified silica gel (DTMSP-BT-SG) (Fig. 1a), for recovering Pt and Pd from aqueous solutions.17 The acylthiourea ligand class was chosen for its multiple advantages, including:
(1) Absence of toxicity, sensitivity, and odour,
(2) Facile two-step one-pot synthesis,
(3) Multiple donor atoms (N, O, S), and
(4) High affinity for PGMs.10,17
We demonstrated the simplicity of synthesising this ligand (74% yield) and its successful immobilisation onto silica gel.17 The DTMSP-BT-SG adsorbent achieved Pt and Pd removal efficiencies of >98%, with adsorption capacities of 48.52 mg g−1 (Pt) and 29.68 mg g−1 (Pd).17 Moreover, the adsorbent maintained enhanced selectivity for Pt and Pd (>80%) even in the presence of high concentrations of competing ions. It also outperformed a silica-anchored amine adsorbent, bis(3-(trimethoxysilyl)propyl)amine-modified silica gel (BTMSPA-SG) (Fig. 1b), which was also used in the recovery studies.17 These findings established silica-anchored acylthioureas as promising adsorbents for Pt and Pd recovery from refinery wastewater.
Herein, we expand upon our previous work by exploring the synthesis of a second silica-anchored acylthiourea adsorbent (TESP-BT-SG, Fig. 1c) featuring one pendent chain to immobilise the ligand onto the solid support. This design aims to make the nitrogen donor atom more accessible by reducing steric hindrance. Additionally, we significantly enhanced the synthesis of another amine variant (APTES-SG, Fig. 1d) for Pt and Pd recovery from aqueous solutions (Schemes S1 and S2†). Our modified methodology achieved a ligand loading of 2.45 mmol g−1 on the solid support, compared to the previously reported value of 1.15 mmol g−1.11,12 Several parameters, including dosage, pH, concentration, and competing ions, were varied to simulate refinery wastewaters. Furthermore, pKa studies and extensive characterisation were conducted to gain insights into the properties of the adsorbents and their relationship to extraction efficiencies.
These findings establish silica-anchored acylthioureas as promising adsorbents for the recovery of Pt and Pd from refinery wastewater.
2. Materials and methods
2.1. Reagents
The synthetic reagents (benzoyl chloride, potassium thiocyanate (KSCN), and (3-aminopropyl)triethoxysilane (APTES)) and analytical grade standards (platinum (Pt) and palladium (Pd) standard solutions (1000 mg L−1 in 5% w/w of c(HCl))) were purchased from Sigma Aldrich, South Africa, and used without further purification. To prepare the stock solutions, the Pt and Pd standard solutions (1000 mg L−1 in 5% w/w of c(HCl)) were diluted with deionised water to obtain the desired concentrations. The solutions were preserved by adding drops of HCl and stored in the refrigerator at 4 °C. To obtain the desired pH of the solutions, 0.01 mol L−1 of HCl and NaOH were used together with a pH meter (Mettler Toledo, Switzerland).2.2. Characterization techniques
The one- and two-dimensional nuclear magnetic resonance (NMR) spectra were obtained using a Bruker Avance III 500 MHz (Germany) to confirm the molecular structure of the ligand. Mass spectrometry was performed using a high-resolution Bruker Compact Q-TOF spectrometer (Germany) to determine the mass-to-charge ratio (m/z) of the ligand. The functional groups of the ligand and adsorbents were determined using Fourier transform infrared spectroscopy (FT-IR) (Tensor 27, Bruker, Germany). Scanning electron microscopy (SEM) (TESCAN Vega, Czech Republic) was used to observe the surface morphology of the adsorbents. X-ray fluorescence (XRF) (PAnalytical, Netherlands) and powder X-ray diffraction (PXRD) (D2 Phaser, Bruker, Germany) were used to study the chemical compositions and mineralogy of the adsorbents, respectively. The surface area and pore characteristics were determined with Brunauer–Emmet–Teller (BET) N2 adsorption–desorption isotherms (TriStar 3000 V6.05 A, Micrometrics, USA). The thermal stability of the adsorbents was assessed using thermogravimetric analysis (TGA) and differential thermal analysis (DTA) (PerkinElmer Pyris 1 TGA, Massachusetts, USA). Elemental analysis was carried out using varioELcube V4.0.13, Elementar (Germany) for determination of the C, H, N, and S elemental compositions present on the silica gel. Inductively coupled plasma optical emission spectroscopy (ICP-OES) (Spectro Genesis, Germany) was used to determine the concentrations of the metal ions.2.3. Synthesis of N-triethoxysilylpropyl-N′-benzoylthiourea (TESP-BT)
The synthesis of TESP-BT (Scheme S1, ESI†) was carried out by first dissolving potassium thiocyanate (1.0 g/10 mmol) in anhydrous acetonitrile (20 mL).10,17 Benzoyl chloride (1.2 mL/10 mmol) in acetonitrile (15 mL) was added dropwise to the solution and it was heated under reflux for 1 h. The reaction mixture was cooled to room temperature and then placed under reduced pressure for 2 h to remove the acetonitrile. To the remaining residue, anhydrous dichloromethane (DCM) (20 mL) was added, followed by a dropwise addition of (3-aminopropyl)triethoxysilane (APTES) (2.2 mL/9.5 mmol) in DCM (20 mL). The mixture was left to stir overnight at 40 °C to yield the product as a yellow liquid containing white particles. The reaction mixture was filtered, and the filtrate was placed under reduced pressure to remove the solvent. A yellow oil was obtained as the final product. A yield of 79% was obtained and the product was characterized by NMR and FT-IR spectroscopy, as well as mass spectrometry. 1H NMR (500 MHz, CDCl3), δ(ppm): 10.74 (s, 1H: N–H), 9.10 (s, 1H: N–H), 7.78–7.80 (d, J = 8.0, 8.0 Hz, 2H, Hh, Hl), 7.57 (t, J = 8 Hz, 1H, Hj), 7.45 (t, J = 8.0, 8.0 Hz, 2H, Hi, Hk), 3.79 (q, J = 6.67 Hz, 6H, Hb), 3.66 (q, J = 6.67 Hz, 2H, He), 1.80 (t, J = 8.0 Hz, 2H, Hd), 1.20 (t, J = 8.0 Hz, 9H, Ha), 0.67 (t, J = 8.0 Hz, 2H, Hc). 13C NMR (400 MHz) (CDCl3), δ (ppm): 179.39 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S), 166.57 (C
S), 166.57 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 133.07 (C9), 131.49 (C6) 128.67 (C8, C10), 127.13 (C7, C11), 55.09 (C2), 47.86 (C5), 21.44 (C4), 17.94 (C1), 7.44 (C3). FT-IR: v (cm−1): 3246 (N–H), 2974, 2928, 2886 (C–H), 1671 (C
O), 133.07 (C9), 131.49 (C6) 128.67 (C8, C10), 127.13 (C7, C11), 55.09 (C2), 47.86 (C5), 21.44 (C4), 17.94 (C1), 7.44 (C3). FT-IR: v (cm−1): 3246 (N–H), 2974, 2928, 2886 (C–H), 1671 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1080 (Si–O–Si). Chemical formula: C17H28N2O4SSi, ESI-MS (m/z): calc. 385.15 [M], exp. 385.1 [M + H]+.
O), 1080 (Si–O–Si). Chemical formula: C17H28N2O4SSi, ESI-MS (m/z): calc. 385.15 [M], exp. 385.1 [M + H]+.
2.4. Immobilization of TESP-BT and APTES onto silica gel
The procedure (Scheme S2†) reported by Erdem et al., was used to immobilize TESP-BT and APTES onto the silica gel (Davisil Grade 710, pore size 50–76 Å).18,19 This method involved pre-activation of the silica gel (0.50 g) by first stirring it in 0.01 M acetic acid (20 mL) for 10 min. The activated silica was filtered on a suction pump and washed several times with deionized water. The silica gel was added to a flask containing toluene (20 mL) and TESP-BT (0.30 mL), and thereafter stirred under reflux (110 °C) for 20 h. The reaction mixture was cooled to room temperature, filtered, and washed with toluene. The obtained functionalised silica particles were left to dry in the oven at 80 °C for 8 h. The immobilisation of APTES onto the silica gel was carried out in a similar manner (Scheme S2b†).2.5. Batch adsorption studies
The adsorption experiments (Fig. S1†) were conducted by pre-weighing the required mass of adsorbent into 50 mL centrifuge tubes, along with 20 mL of the relevant metal standard solution. The tubes were placed on an elliptical benchtop shaker to agitate at 150 rpm at room temperature. After mixing, the samples were centrifuged at 30 rpm for 5 min, to separate the metal-loaded adsorbent from the liquid. Subsequently, the samples were filtered, and the metal concentrations of the filtrates were measured using ICP-OES. The effects of adsorbent dosage (5–50 mg), pH (2–9), initial concentration (0.5–50 mg L−1), contact time (0–1440 min), and competing ions (Rh, Ir, Ru, Fe, Ca, Mg, K, Co, Ni, Zn) were studied. These parameters were investigated to simulate conditions of refinery wastewater and to determine the optimal conditions for the adsorption of Pt and Pd by TESP-BT-SG and APTES-SG. The adsorption capacity, qe (mg g−1) and adsorption efficiency (%) were calculated using equations eqn (1) and (2) respectively.
 | (1) |
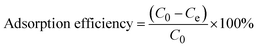 | (2) |
2.6. pKa determination experiments
To determine the pKa's of the adsorbents, titration experiments were performed with a potentiometric titrator (Metrohm, Switzerland), and a pH meter. The experiments were carried out by adding 50 mg of adsorbent and 40 mL of deionized water into a beaker. The contents were stirred until the pH of the solution stabilized, and the initial pH of the adsorbent was recorded. To titrate the solutions, 0.01 M HCl and NaOH were used to titrate the bases and acids respectively. pH values and volumes of titrant added were recorded until the endpoints were reached. The obtained readings were used to plot a curve of pH versus volume. To determine the pKa's, the Henderson–Hasselbach equation eqn (3) was used, and the alternative basic equation eqn (4) was used to determine the pKb's.20–26
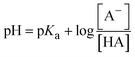 | (3) |
 | (4) |
2.7. Statistical analysis
All the experiments were conducted in duplicate (n = 2), and the mean values were used for data analysis. Data processing was performed using Microsoft Excel. The relative standard deviation (RSD) and relative standard error (RSE) of the measurements were calculated, with RSD values below 4% and RSE values below 3%.3. Results and discussion
3.1. Characterization of N-triethoxysilylpropyl-N′-benzoylthiourea (TESP-BT)
The molecular structure of TESP-BT was confirmed by one- and two-dimensional NMR spectroscopy, FT-IR spectroscopy, and mass spectrometry. The 1H NMR spectrum (Fig. S2†) displayed two singlets at 10.74 and 9.10 ppm corresponding to the amidic protons (NHf and NHg). Hf coupled to the quartet (He) at 3.66 ppm on COSY (Fig. S3†) thus, distinguishing it from Hg. In the aromatic region (7.45–7.80 ppm), the deshielded doublet at 7.80 ppm was assigned to Hh and Hl, which both experience the electron-withdrawing effect of the carbonyl substituent. The COSY spectrum was used to assign Hi/Hk and Hj at 7.45 and 7.57 ppm respectively. In the aliphatic region, Hb (3.79 ppm) and He (3.66 ppm) were found to be the most deshielded due to the adjacent electronegative oxygen and nitrogen atoms respectively. Hd, Ha and Hc were found upfield from 1.80–0.67 ppm.In the 13C NMR spectrum (Fig. S4†), the carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and thiocarbonyl (C
O) and thiocarbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S) carbons were assigned to the deshielded signals at 179.39 and 166.57 ppm respectively. HMBC (Fig. S5†), revealed that C
S) carbons were assigned to the deshielded signals at 179.39 and 166.57 ppm respectively. HMBC (Fig. S5†), revealed that C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S coupled to He through long-range coupling hence it was distinguished from C
S coupled to He through long-range coupling hence it was distinguished from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. The assignment of the aromatic (C7, C8, C9, C10, C11) and aliphatic carbons (C5, C4, C3, C2, C1) were achieved with HSQC (Fig. S6†) for C–H correlation, together with 13C DEPT (Fig. S7†). The signal at 131.49 ppm corresponding to a quaternary carbon was assigned to C6.
O. The assignment of the aromatic (C7, C8, C9, C10, C11) and aliphatic carbons (C5, C4, C3, C2, C1) were achieved with HSQC (Fig. S6†) for C–H correlation, together with 13C DEPT (Fig. S7†). The signal at 131.49 ppm corresponding to a quaternary carbon was assigned to C6.
TESP-BT was further characterized by FT-IR spectroscopy (Fig. S8†), which indicated the weak N–H stretch at 3246 cm−1. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch was found at 1671 cm−1 whilst the C
O stretch was found at 1671 cm−1 whilst the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S stretch can be assumed to be in the regions 800–1500 cm−1, which is characteristic of acylthioureas.10,27,28 The strong Si–O–Si stretch at 1080 cm−1 further confirmed the ligand structure.
S stretch can be assumed to be in the regions 800–1500 cm−1, which is characteristic of acylthioureas.10,27,28 The strong Si–O–Si stretch at 1080 cm−1 further confirmed the ligand structure.
Mass spectrometry (Fig. S9†) also confirmed the identity of TESP-BT as the predicted isotopologue pattern agreed with the experimentally obtained spectrum. The ESI-MS [M + H]+ of 385.15 m/z was observed and it correlated with the calculated mass of 385.15 m/z.
3.2. Characterization of the adsorbents
The FT-IR spectra of the silica gel, TESP-BT ligand, and TESP-BT-SG adsorbent are illustrated in Fig. 2a. The silica gel is usually characterized by strong bands which are found in the low-frequency region (1000–500 cm−1).29,30These bands correspond to the Si–O–Si asymmetric and Si–O symmetric stretches at 1085 and 490 cm−1 respectively.29,30 The additional silica bands observed at 1649, and 3500 cm−1 are usually attributed to the bending vibrations of physically adsorbed H2O molecules and OH vibrations respectively and these are caused by the hydrogen-bonded surface silanol groups.29–31
Upon functionalization of TESP-BT onto the silica gel, there was an appearance of new peaks at 3245, 2900 and 1688 cm−1, which are respectively due to the N–H, C–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups. For the APTES-SG adsorbent (Fig S10†), two bands were observed at 3200 and 2928, and 2582 cm−1 which corresponded to the NH and CH2 groups of APTES, thus confirming that the desired adsorbent was obtained.29
O groups. For the APTES-SG adsorbent (Fig S10†), two bands were observed at 3200 and 2928, and 2582 cm−1 which corresponded to the NH and CH2 groups of APTES, thus confirming that the desired adsorbent was obtained.29
13C Solid-state NMR (Fig. 2b) further confirmed the functionalization of TESP-BT and APTES onto the silica gel. The aliphatic CH2 carbons (C3, C4 and C5) of both APTES-SG and
TESP-BT-SG were assigned to the peaks at 8.4, 16.5, and 43.2 ppm respectively. In the aromatic region of TESP-BT-SG, additional peaks were observed at 121.8, 161.2 and 174.9 ppm and these respectively corresponded to the aromatic, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S carbons of the TESP-BT ligand. Interestingly, these peaks were significantly sharper than the C
S carbons of the TESP-BT ligand. Interestingly, these peaks were significantly sharper than the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S signals observed for the DTMSP-BT-SG reported previously.17
S signals observed for the DTMSP-BT-SG reported previously.17
The PXRD patterns (Fig. 3a) of the adsorbents were all characterized by a dominant peak at 2θ = 21.9°, which is known to be indicative of the amorphous nature of the materials.31,32 The BET N2 isotherms (Fig. 3b) obtained are classified as type IV isotherms, which denote adsorption taking place in two stages.33,34 The initial stage involves filling of the micropores, whilst the second stage is due to filling of the mesopores.33,34 Fig. 3b also indicates that after the immobilization process, there was a reduction in the amount of N2 adsorbed, which is evidence that both TESP-BT and APTES were incorporated into the silica gel and as a result blocked access of more N2 gas molecules.29,34 Furthermore, the decrease in surface area and pore volumes (Table S1†) after the ligand immobilization proves that the silica surface and channels were covered and filled with the functional groups.29,34
The SEM images (Fig. 4) indicated that all the adsorbents exhibited a non-uniform surface containing particles of various sizes and shapes.32 The reduction in particle size observed in Fig. 4c can be attributed to mechanical abrasion which occurs during stirring in the adsorbent synthesis.33
The oxide chemical compositions of the adsorbents were determined by XRF (Table S2†). The unmodified silica gel was dominantly composed of an SiO2 content of 89.2%, which decreased to 78.81% (TESP-BT-SG) and 79.41% (APTES-SG), after the immobilization process. Perez et al. reported that the lower SiO2 content observed for TESP-BT-SG and APTES-SG indicates that the acylthiourea and amine groups are bound to the silica gel.29
Table 1 lists the elemental compositions of the unmodified silica gel and the adsorbents. The increased content of C, H, N and S after the immobilization process, further confirmed the introduction of the functional groups onto the silica gel to form TESP-BT-SG and APTES-SG.29,34 Additionally, the reported C and N elemental compositions of the adsorbents were used to calculate the ligand concentrations, which aided in calculating the theoretical loading capacities of the adsorbents in the extraction experiments.
| Adsorbents | C | H | N | S | ||
|---|---|---|---|---|---|---|
| % | mmol g−1 | % | % | mmol g−1 | % | |
| Silica gel | 0.0 | 0.86 | 0.0 | 0.0 | ||
| TESP-BT-SG | 8.32 | 0.630 | 1.26 | 1.55 | 0.553 | 1.26 |
| APTES-SG | 9.84 | 2.731 | 2.44 | 3.43 | 2.448 | 0.0 |
The thermal stability of the adsorbents was also determined and the TGA and DTA graphs of the silica gel, TESP-BT-SG and APTES-SG are presented in Fig. S11.† There was a two-stage weight loss trend observed on the graphs.30,31,35 The first stage between 25–100 °C, measured a 2–5% weight loss which is due to dehydration of adsorbed water molecules from adsorbents.30,31,35,36 In the second stage (100–800 °C), a further 4% weight loss was observed for the silica gel and a 15–18% loss was recorded for TESP-BT-SG and APTES-SG. The high quantity of weight loss observed for TESP-BT-SG and APTES-SG relative to the silica gel has been reported to be due to the loss of the functional groups anchored from the silica surface.35,36
The determined pKa and pKb plots of the adsorbents are tabulated below (Table 2), and the plots are reported in the ESI (Fig. S12†). The initial pH results of the adsorbents indicated that the acylthioureas were weak acids (pH 3–4), whilst the amines were weak bases (pH 6–8), hence the solutions were titrated with strong base (NaOH) and strong acid (HCl) respectively. When determining the pKa of weak acids, the volume added at half the equivalence point is considered because half-neutralization occurs here and the concentrations of the weak acid and conjugate base are at this point, hence the Hasselbalch equation eqn (3), becomes pH = pKa (Fig. S12a and b†).18–23 Similarly, when determining the pKb of weak bases, the midpoint which is located halfway between the initial point and equivalence point is considered (Fig. S12c and d†). At the midpoint, the number of moles of strong acid added, is equal to half the number of moles of the base that were initially in the flask. Thus, at the midpoint, the basic form of Henderson–Hasselbalch equation eqn (4) becomes pOH = pKb.18,21–26
| Acylthiourea adsorbents | pKa | Amine adsorbents | pKb |
|---|---|---|---|
| DTMSP-BT-SG | 5.65 | BTMSPA-SG | 11.84 |
| TESP-BT-SG | 5.87 | APTES-SG | 11.92 |
3.3. Adsorption studies
 | ||
| Fig. 5 Effect of (a) mass and (b) pH on the recovery of Pt and Pd (mass = 10 mg, pH = 2, conc. = 5 mg L−1, time = 4 h, temp. = 25 °C, vol. = 20 mL, n = 2, RSD < 4%). | ||
Compared to our previous results, the silica-anchored acylthioureas (TESP-BT-SG and DTMSP-BT-SG) displayed similar trends of Pt and Pd recovery, which can be attributed to the similarity in their active sites.17 The obtained maximum experimental adsorption capacities (qmax) and calculated adsorbent-to-metal ratios (Table S3†) of both adsorbents were also quite comparable, thus validating the similarity in their Pt and Pd extraction efficiencies. An increase in Pt and Pd adsorption was observed for the current silica-anchored amine (APTES-SG), whilst previously BTMSPA-SG recorded a low Pt (40%) and Pd (90%) uptake.17 The enhanced recovery displayed by APTES-SG can be attributed to its higher ligand concentration (2.448 mmol g−1) and superior adsorption capacities (25.06 mg g−1 (Pt) and 32.73 mg g−1 (Pd)) that surpassed those of BTMSPA-SG, which had a ligand concentration of 1.68 mmol g−1 and adsorption capacities of 6.63 mg g−1 (Pt) and 12.53 mg g−1 (Pd)).14,15,17,38
Nikoloski et al., reported that the active sites formed by the surface functional groups of the adsorbents are among one of the most dominant factors that can influence the extent of adsorption, alongside the solution's acidity and the presence of competing ions.15,38 Thus, the presence of one donor atom (N) on the amines versus three atoms (S, N, O) on the acylthioureas, accounts for their superiority in adsorption. From the results above, it can also be confirmed that the high adsorption exhibited by APTES-SG over BTMSPA-SG is a result of its elevated ligand concentration, which offers more active sites to capture the metals. The optimum dosage of adsorption was 0.5 and 2.5 g L−1 for Pd and Pt respectively, for both adsorbents.
This occurs because the concentration of hydronium ions (H+) is high at very low pH values and therefore, act to protonate the adsorbent surface functional groups.39 The primary amine group of APTES-SG will form NH3+ upon protonation.39 Whilst for TESP-BT-SG, a kinetic study done by Congdon and Edward, indicated that the sulphur atom of the N′-benzoylthiourea group gets protonated first, followed by a second protonation of the amidic moiety with increasing acid concentration.28,40 The protonation of these groups will contribute to positively charged adsorbents' surfaces, thus inducing adsorption through the ion-pairing mechanism where the most dominant and stable Pt and Pd chloro-complexes ([PtCl6]2− and [PdCl4]2−) will get captured.39,41,42 Many studies have reported that whilst the ion-pairing mechanism is more pronounced in the acidic regions, adsorption is not exclusive to only electrostatic attractions, but it can simultaneously take place with other mechanisms including ion exchange, chelation, and metal reduction.42–45
At pH > 2, the Pt removal efficiency decreased, especially for APTES-SG, whilst for Pd, adsorption remained high at these conditions. The adsorption of Pt and Pd has been reported to decrease at higher pH regions due to the low concentration of Cl− ions that normally form the stable chloro-complexes and as a result, hydrolysis occurs, forming aqua species which are known to be less adsorbable.44,45 Chassary et al., reported that Pt(IV) is more sensitive to hydrolysis relative to Pd(II), thus it readily forms these diverse aqua species ([PtCl5(H2O)]− and [PtCl4(H2O)2]) which aren't prone to adsorption, hence the observed decrease in Pt recovery.42 The high Pd recovery indicates that the present Pd(II) aqua species ([PdCl2(H2O)2], [PdCl3(OH)]2−, [PdCl(OH)3]2−) are still favourable for adsorption by both adsorbents.11,46 The difference in the adsorbents' efficiencies towards the two metals has also frequently been attributed to the varying ionic radius of their anionic complexes ([PdCl4]2− and [PtCl6]2−), as functional groups on the adsorbent surfaces will tend to approach the Pd complexes, which have a smaller radius.42,43
Additionally, the lack of hydronium ions in these basic regions, means that the dominant mechanism of adsorption will likely be through chelation of the metals to the acylthiourea, and amine groups of the adsorbents.39,41,42
The Pd adsorption behaviour by TESP-BT-SG was fairly similar to DTMSP-BT-SG in our previous work however, for Pt, the recovery decreased in the basic regions, as previously a >98% efficiency was observed at pH 8, whilst currently it dropped to 77%.17 For the silica-anchored amines, APTES-SG currently performed better than the previous BTMSPA-SG adsorbent in the Pd adsorption as a low recovery of 70% was observed for the latter, whilst presently its >97%.17 The Pt recovery efficiencies by both the silica-anchored amines were comparable.17 The pH results proved both acylthioureas (TESP-BT-SG and DTMSP-BT-SG) to be better adsorbents than the amines (BTMSPA-SG and APTES-SG) as they sustained great adsorption capability in the acidic and basic regions.
 | ||
| Fig. 6 Effect of (a) concentration (b) contact time on the recovery of Pt and Pd (mass = 10 mg, pH = 2, time = 4 h, conc. = 5 mg L−1, temp. = 25 °C, vol. = 20 mL, n = 2, RSD < 4%). | ||
An improvement in adsorption was noted for APTES-SG, which achieved maximum Pt and Pd recoveries of 91% and 98%, respectively, compared to the previously reported BTMSPA-SG with maximum adsorptions of 45% (Pt) and 94% (Pd). The silica-anchored acylthioureas recorded similar removal efficiencies under these conditions. Optimal Pt and Pd concentrations for adsorption were observed at 5 mg L−1 for TESP-BT-SG. For APTES-SG, the optimal concentration was 5 mg L−1 for Pd and 10 mg L−1 for Pt.
3.4 Adsorption isotherms
The Langmuir, Freundlich, and Dubinin–Radushkevich (D–R) isotherms are among the most generally used models to describe the equilibrium adsorption relationship between the absorbents and the metals.15,38,39 The Langmuir isotherm model (eqn (5)) describes the adsorption sites on the adsorbent as structurally homogenous, and energetically equivalent.6,39 This model also assumes a finite number of sites or capacity on the adsorbent, therefore if no further adsorption takes place, then the saturation value has been reached.6,39 Moreover, the Langmuir model describes adsorption to take place in a monolayer fashion.6
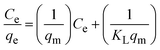 | (5) |
The Freundlich isotherm (eqn (6)), denotes adsorption taking place onto a heterogenous (multiple layers) surface where the active sites are assumed to have different energies.42,43
 | (6) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe vs. ln
qe vs. ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce and, the intercept respectively.36,37 The n factor can also be used to indicate whether the adsorption process is chemical (n > 1) or physical (n < 1).36,37
Ce and, the intercept respectively.36,37 The n factor can also be used to indicate whether the adsorption process is chemical (n > 1) or physical (n < 1).36,37
The Dubinin–Radushkevich (D–R) (eqn (7)) isotherm describes the adsorption of metals onto a heterogenous adsorbent surface with Gaussian energy distribution.36
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = ln qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Xm − Kadε2 Xm − Kadε2
| (7) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe vs. ε2. Xm (mg g−1) is the D–R theoretical saturation capacity (maximum adsorption capacity) that can be determined from the slope-intercept. ε is the Polanyi constant, expressed as eqn (8):
qe vs. ε2. Xm (mg g−1) is the D–R theoretical saturation capacity (maximum adsorption capacity) that can be determined from the slope-intercept. ε is the Polanyi constant, expressed as eqn (8):
 | (8) |
The D–R isotherm constant can also be used to estimate the mean free energy (Es (kJ mol−1)) (eqn (9)), which can be used to validate whether adsorption takes place via physical adsorption (1 < Es < 8), ion-exchange (8 < Es < 16), or chemical adsorption (Es > 20).8,13,37
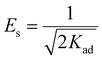 | (9) |
The data presented in Table 3 indicated that the adsorption process conformed to the Langmuir isotherm model, as the correlation coefficients (R2) of were closest to 1.13,14 This suggests that Pt and Pd adsorbed onto both adsorbents in a monolayer fashion.39
| Isotherm models | Parameters | TESP-BT-SG | APTES-SG | ||
|---|---|---|---|---|---|
| Pt | Pd | Pt | Pd | ||
| Langmuir | qm (mg g−1) | 29.01 | 28.66 | 25.06 | 32.73 |
| KL (L mol−1) | 2.13 | 71.18 | 0.61 | 73.00 | |
| R2 | 0.99 | 0.99 | 0.98 | 0.99 | |
| Freundlich | n | 4.44 | 6.85 | 2.63 | 4.127 |
| KF ((mg g−1) (mol−1 L−1)1/n) | 15.10 | 21.81 | 8.59 | 20.97 | |
| R2 | 0.60 | 0.37 | 0.50 | 0.73 | |
| (D–R) | Es (kJ mol−1) | 4450.96 | 9983.60 | 1196.33 | 6094.52 |
| Xm (mg g−1) | 27.39 | 27.42 | 30.60 | 30.91 | |
| R2 | 0.86 | 0.30 | 0.86 | 0.95 | |
The KL values revealed that both adsorbents had a higher selectivity for Pd, which explains its higher recovery relative to Pt. For Pt, the KL value was highest for TESP-BT-SG, suggesting a stronger affinity for this metal compared to APTES-SG, which aligns with the experimentally obtained data.15,38,39
From the Freundlich and D–R isotherms, the values of n and Es were found to be greater than 1 and 20 respectively, suggesting that chemical adsorption of Pt and Pd onto both adsorbents.13,37 These isotherm trends were consistent with data from our previous study, indicating similar equilibrium adsorption behaviour of the metals on the adsorbents.
The maximum experimental adsorption capacities (qm), indicating the adsorbents' efficiency for metal recovery, are also listed in Table 3. In comparison to other adsorbents in the literature (Table S4†), the loading capacities of Pt and Pd on both adsorbents were moderate. Additionally, qm values were combined with ligand concentrations to calculate the adsorbent-to-metal ratios. Table S3† shows that these ratios were smaller for TESP-BT-SG (Pt: 3.72; Pd: 2.05) than for APTES-SG (Pt: 19.06; Pd: 7.96), suggesting that a smaller amount of TESP-BT-SG would be needed to remove Pt and Pd from solution.38,42 Furthermore, these ratios were lower than those reported in our previous study, especially for APTES-SG, highlighting its higher efficiency over the previous amine-based adsorbent (BTMSPA-SG).
While both adsorbents showed similar behaviour in Pd recovery, TESP-BT-SG outperformed APTES-SG in Pt adsorption, making it a more efficient adsorbent as it effectively recovered both metals in a shorter time.
3.5 Adsorption kinetics
The mechanism by which adsorption of Pt and Pd occurred onto the adsorbents was determined by correlating the contact time experimental data with the pseudo-first and second-order kinetic order models.14,39 The rate of adsorption which is also known to be a good indicator for evaluating an adsorbent's performance was also determined from the kinetic model data.14,39 The pseudo-first-order model (eqn (10)) describes adsorption that takes place through physisorption.39 The pseudo-second-order model (eqn (11)) is indicative of chemical adsorption which involves covalency forces through the sharing of electrons between the metals and adsorbents.39
 | (10) |
 | (11) |
The obtained data in Table 4, indicated that the pseudo-second-order model best described the experimental data as the R2 values were closer to 1 and the qe(calc) values were also consistent with the qe(exp) of this model.13,14
| Kinetic models | Parameters | TESP-BT-SG | APTES-SG | ||
|---|---|---|---|---|---|
| Pt | Pd | Pt | Pd | ||
| Pseudo-first-order | qe(exp) (mg g−1) | 9.89 | 9.99 | 9.42 | 9.91 |
| qe(cal) (mg g−1) | 2.55 | 2.13 × 10−4 | 0.60 | 0.12 | |
| k1 (min−1) | −4.63 × 10−5 | −5.00 × 10−7 | −8.25 × 10−6 | −2.17 × 10−5 | |
| R2 | 0.90 | 0.034 | 0.67 | 0.36 | |
| Pseudo-second-order | qe(cal) (mg g−1) | 10.00 | 9.99 | 9.68 | 9.93 |
| k2 (mg g−1 min−1) | 0.0216 | 52.1365 | 0.0420 | 0.1992 | |
| R2 | 0.99 | 1.00 | 0.99 | 0.99 | |
These results, therefore, suggest that chemical adsorption had taken place through a chemical reaction between the functional groups of the adsorbents and the Pt and Pd anionic metal complexes.15,38,39 The rate of adsorption (k2) was also higher for the pseudo-second-order model, thus further validating that the experimental data conformed to this model. Additionally, Pd exhibited higher k2 values than Pt for both TESP-BT-SG and APTES-SG, thus confirming its fast kinetics of adsorption.38
Results in Fig. 7 show that competing ions did not affect Pt and Pd adsorption, with both metals being significantly recovered. Low recovery of competing ions at pH 2 likely arises from their prevalent cationic forms, while Pt and Pd exist as anionic complexes ([PtCl6]2− and [PdCl4]2−).44–47 The protonated adsorbent surface repels cations but attracts anionic Pt and Pd, facilitating selective adsorption.43–47 Koch et al. also found acylthioureas exhibit high selectivity for PGMs in highly acidic solutions.48 Similarly, Kramer et al. reported that amine ligands form stable complexes with PGMs in acidic environments, while other metals do not exhibit this tendency.11
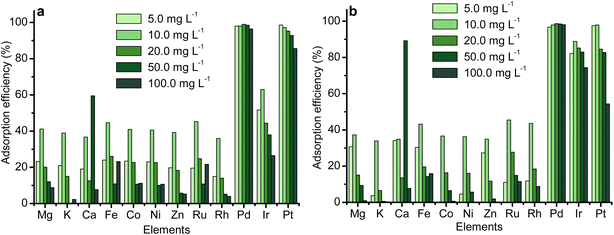 | ||
| Fig. 7 Effect of competing ions on the recovery of Pt and Pd by (a) TESP-BT-SG and (b) APTES-SG (mass = 10 mg, pH = 2, conc. = 5 mg L−1, temp. = 25 °C, time = 4 h, vol. = 20 mL, n = 2, RSD < 4%). | ||
Pd recovery exceeded 98% for both adsorbents at all ion concentrations. Pt removal efficiency with TESP-BT-SG dropped from 97% to 87% (5–100 mg L−1), with an even greater decrease observed with APTES-SG (97% to 54%).
Chassary et al., attributed the preferential Pd recovery to differences in the chemistries of Pt and Pd, specifically in their chloro-complex formation and hydrolysis behaviours.42 Pt(IV), more susceptible to hydrolysis, shows altered species distribution when Pd(II) is present, as Pd complexes diminish chloride ions needed to form Pt chloro-anions, thereby reducing Pt adsorption.43,44
TESP-BT-SG showed comparable selectivity to the previous acylthiourea adsorbent (DTMSP-BT-SG) for both metals, though with a lower ligand loading of 0.553 mmol g−1, compared to 1.03 mmol g−1 for DTMSP-BT-SG. This lower loading yielded a higher ligand-to-metal adsorption ratio of 3.72 (Pt) and 2.05 (Pd) versus 4.15 (Pt) and 3.69 (Pd) for DTMSP-BT-SG (Table S3†). The reduced loading likely results from intramolecular hydrogen bonding, typical for mono-alkyl acylthioureas, which results in a bulkier conformation.48 This is consistent with sharper C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S peaks in the 13C CP MAS NMR spectrum of TESP-BT-SG and a shifted IR carbonyl stretch (1671 cm−1 for TESP-BT-SG versus 1685 cm−1 for DTMSP-BT-SG). This conformation would be significantly bulkier and loading density is expected to be lower (Fig S13†).48
S peaks in the 13C CP MAS NMR spectrum of TESP-BT-SG and a shifted IR carbonyl stretch (1671 cm−1 for TESP-BT-SG versus 1685 cm−1 for DTMSP-BT-SG). This conformation would be significantly bulkier and loading density is expected to be lower (Fig S13†).48
APTES-SG showed improved selectivity for Pt and Pd (>97%) compared to the previous amine adsorbent, BTMSPA-SG (Pt < 50% and Pd 82%), likely due to APTES-SG's higher adsorption capacity. These results underscore the strong selectivity of both adsorbents for Pt and Pd, especially under the conditions tested (pH 2, dosage 0.5 g L−1).
As the pKb values for both adsorbents were similar, additional factors such as surface area, steric effects, and rate constants were examined. Jal et al. noted that bulkier ligand groups reduce pore volume on solid supports (such as silica gel) by occupying more space, which in turn decreases surface area and limits the availability of active sites.52 This effect is less evident with smaller molecules, which can provide more active sites by covering a larger surface area.52,53 Our BET results supported this, showing that BTMSPA-SG had a lower surface area (130.06 m2 g−1) than APTES-SG (180.19 m2 g−1).17
The conformation of amine groups may also influence adsorption kinetics. When the primary amine (APTES) is anchored to the silica surface, it retains flexibility, whereas the secondary amine (BTMSPA) adopts a more rigid structure.52 This flexibility may enhance APTES's activity and hinder BTMSPA's, a trend that aligned with our kinetic data.54
Moreover, we achieved a significantly higher amine loading on APTES-SG (2.45 mmol g−1) compared to the 1.15 mmol g−1 reported by Kramer and colleagues, as well as to BTMSPA-SG (1.68 mmol g−1), indicating that surface area and nitrogen content were critical factors in determining adsorption capacity.11,12,17,52,53 The high amine loading may enable multiple protonated amines to interact with an anionic species, which would make adsorption even more favourable due to entropy considerations.
4. Conclusion
This study successfully synthesised the novel TESP-BT ligand and immobilised both TESP-BT and APTES onto silica gel. Structural analyses through NMR, FT-IR, SEM, TGA/DTA, PXRD, and XRF confirmed the structures of the ligand and adsorbents. Under optimal recovery conditions (pH 2, metal concentration of 5 mg L−1, dosage of 0.5 g L−1 for Pd and 2.5 g L−1 for Pt), TESP-BT-SG efficiently recovered over 97% of both Pt and Pd. At these conditions, APTES-SG exhibited higher recovery for Pd (>97%) than for Pt (>83%). In solutions with increasing concentrations of competing ions (5–100 mg L−1), both adsorbents showed strong selectivity for Pd (>98%), while Pt recovery declined with rising ion concentration, from 97–87% for TESP-BT-SG and 97–54% for APTES-SG. These results highlight TESP-BT-SG's superior adsorption efficiency for both metals over APTES-SG, which showed a stronger recovery for Pd over Pt.Compared to our previous findings, the adsorption efficiency order was: DTMSP-BT-SG ≈ TESP-BT-SG > APTES-SG > BTMSPA-SG, with both acylthioureas displaying comparable performance. Among the amines, APTES-SG outperformed BTMSPA-SG. This difference in adsorption capacity was attributed to their exposed surface areas and ligand loading on the silica support. APTES-SG achieved a significantly higher ligand concentration (2.45 mmol g−1) compared to BTMSPA-SG (1.68 mmol g−1) and more than double the previously reported concentration (1.15 mmol g−1), thereby providing more active sites for Pt and Pd capture.
In conclusion, the silica-anchored acylthioureas demonstrated excellent adsorption efficiency and selectivity for both Pt and Pd under the tested conditions, achieving equilibrium adsorption faster than the silica-anchored amines. This positions silica-anchored acylthioureas as promising and cost-effective extracting agents for Pt and Pd recovery from secondary sources, while the new, highly modified APTES-SG shows potential as a viable, cost-effective alternative with additional capacity for Ir extraction from solution.
To further improve on this work, the selectivity of the adsorbents can be evaluated in competing ions solutions containing noble metals such as Ag and Au, to provide deeper insights into their preferential adsorption behaviour toward Pt and Pd. Additionally, investigating the adsorption performance of different oxidation states of Pt (II vs. IV) and Pd (II vs. IV) is recommended to better understand the impact of speciation on adsorption efficiency and mechanism.
Data availability
The data supporting the findings of this study are available within the article and the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the National Research Foundation (NRF (149354)) for financial support and the University of the Witwatersrand for the research facilities.References
- O. Grad, M. Ciopec, A. Negrea, N. Duţeanu, G. Vlase, P. Negrea, C. Dumitrescu, T. Vlase and R. Vodă, Sci. Rep., 2021, 11, 2016 CrossRef PubMed.
- I. Chidunchi, M. Kulikov, R. Safarov and E. Kopishev, Heliyon, 2024, 10, e25283 CrossRef PubMed.
- L. Chen, K. Wu, M. Zhang, N. Liu, C. Li, J. Qin, Q. Zhao and Z. Ye, Chem. Eng. J., 2023, 466, 43082 Search PubMed.
- D. Zhang, L. MacDonald, P. Raj and A. Karamalidis, Chem. Eng. J., 2024, 494, 152948 CrossRef.
- L. MacDonald, D. Zhang and A. Karamalidis, Resour. Conserv. Recycl., 2024, 205, 107590 CrossRef.
- M. R. Awual, M. A. Khaleque, Y. Ratna and H. Znad, J. Ind. Eng. Chem., 2015, 21, 405–413 CrossRef.
- K. Liu and X. Gao, Environ. Pollut., 2019, 255, 113192 CrossRef.
- M. R. Awual, M. M. Hasan, M. Naushad, H. Shiwaku and T. Yaita, Sens. Actuators, B, 2015, 209, 790–797 CrossRef.
- J. Lee, H. Hong, K. Chung and S. Kim, Sep. Purif. Technol., 2020, 246, 116896 CrossRef.
- S. Swaminathan, P. Jerome, R. Deepak, R. Karvembu and T. Oh, Coord. Chem. Rev., 2024, 503, 215620 CrossRef.
- J. Kramer, W. L. Driessen, K. R. Koch and J. Reedijk, Hydrometallurgy, 2002, 64, 59–68 CrossRef.
- J. Kramer, N. E. Dhladhla and K. R. Koch, Sep. Purif. Technol., 2006, 49, 181–185 CrossRef.
- M. Li, S. Tang, Z. Zhao, X. Meng, F. Gao, S. Jiang, Y. Chen, J. Feng and C. Feng, Chem. Eng. J., 2020, 386, 123947 CrossRef.
- A. Mavhungu, R. K. Mbaya and M. L. Moropeng, Int. J. Chem. Eng. Appl., 2013, 4, 354–358 Search PubMed.
- A. N. Nikoloski, K. L. Ang and D. Li, Hydrometallurgy, 2015, 152, 20–32 CrossRef CAS.
- R. G. Pearson, J. Am. Chem. Soc., 1963, 85, 3533–3539 CrossRef CAS.
- M. R. Mphahlele, A. K. Mosai, H. Tutu and I. A. Kotzé, RSC Adv., 2024, 14, 7507–7516 RSC.
- A. Erdem, T. Shahwan, A. Ağır and A. E. Eroğlu, Chem. Eng. J., 2011, 174, 76–85 CrossRef CAS.
- A. Purwanto, Y. Yusmaniar, F. Ferdiani and R. Damayanti, AIP Conf. Proc., 2017, 1891, 030018 Search PubMed.
- J. Reijenga, A. Van Hoof, A. Van Loon and B. Teunissen, Anal. Chem. Insights, 2013, 8 DOI:10.4137/ACI.S12304.
- H. N. Po and N. M. Senozan, J. Chem. Educ., 2001, 78, 1499 CrossRef.
- G. Khaira and A. Kot, Chemistry LibreTexts, https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Acids_and_Bases/Buffers/Henderson-Hasselbalch_Approximation, accessed May 5, 2024 Search PubMed.
- P. Thaplyal and P. C. Bevilacqua, Methods Enzymol., 2014, 549, 189–219 Search PubMed.
- L. Zaharani and N. G. Khaligh, Chem. Teach. Int., 2023, 5, 427–437 CrossRef.
- H. Sohn and J. Thornton, Chemistry LibreTexts, https://chem.libretexts.org/Ancillary_Materials/Demos_Techniques_and_Experiments/General_Lab_Techniques/Titration/Titration_of_a_Weak_Acid_with_a_Strong_Base accessed May 5, 2024 Search PubMed.
- K. Cox and C. Chang, Chemistry LibreTexts, https://chem.libretexts.org/Ancillary_Materials/Demos_Techniques_and_Experiments/General_Lab_Techniques/Titration/Titration_of_a_Weak_Base_with_a_Strong_Acid, accessed May 6, 2024 Search PubMed.
- A. Saeed, U. Flörke and M. F. Erben, J. Sulfur Chem., 2013, 35, 318–355 CrossRef.
- A. N. Mautjana, J. D. Miller, A. Gie, S. A. Bourne and K. R. Koch, Dalton Trans., 2003, 10, 1952–1960 RSC.
- G. Perez, E. Erkizia, J. J. Gaitero, I. Kaltzakorta, I. Jiménez and A. Guerrero, Mater. Chem. Phys., 2015, 165, 39–48 CrossRef.
- P. E. Imoisili, E. C. Nwanna and T.-C. Jen, Processes, 2022, 10, 2440 CrossRef.
- Z. A. Alothman and A. W. Apblett, J. Hazard. Mater., 2010, 182, 581–590 CrossRef PubMed.
- R. K. Sharma, M. Mishra, S. Sharma and S. Dutta, J. Coord. Chem., 2016, 69, 1152–1165 CrossRef.
- V. Zeleňák, M. Skříňská, A. Zukal and J. Čejka, Chem. Eng. J., 2018, 348, 327–337 CrossRef.
- S. Silviana, A. A. Janitra, A. N. Sa’adah and F. Dalanta, Ind. Eng. Chem. Res., 2022, 61, 9283–9299 Search PubMed.
- S. Jodeh, G. Hanbali, I. Warad, H. Lgaz, D. Jodeh, S. Radi and S. Tighadouini, Appl. J. Environ. Eng. Sci., 2015, 1, 66–84 Search PubMed.
- A. K. Mosai, L. Chimuka, E. M. Cukrowska, I. A. Kotzé and H. Tutu, Chemosphere, 2020, 239, 124768 CrossRef CAS PubMed.
- A. K. Mosai, L. Chimuka, E. M. Cukrowska, I. A. Kotzé and H. Tutu, Environ. Dev. Sustain., 2020, 23, 7041–7062 CrossRef.
- A. N. Nikoloski and K. L. Ang, Miner. Process. Extr. Metall. Rev., 2013, 35, 369–389 Search PubMed.
- A. K. Mosai, Miner. Eng., 2021, 163, 106770 CrossRef CAS.
- W. I. Congdon and J. T. Edward, J. Am. Chem. Soc., 1972, 94, 6099–6104 Search PubMed.
- K. Khunathai, K. Inoue, K. Ohto, H. Kawakita, M. Kurata, K. Atsumi, H. Fukuda and S. Alam, Solvent Extr. Ion Exch., 2013, 31, 320–334 CrossRef CAS.
- P. Chassary, T. Vincent, J. Sanchez Marcano, L. E. Macaskie and E. Guibal, Hydrometallurgy, 2005, 76, 131–147 Search PubMed.
- T. G. Asere, S. Mincke, K. Folens, F. Vanden Bussche, L. Lapeire, K. Verbeken, P. Van Der Voort, D. A. Tessema, G. Du Laing and C. V. Stevens, React. Funct. Polym., 2019, 141, 145–154 CrossRef.
- H. Sharififard, M. Soleimani and F. Z. Ashtiani, J. Taiwan Inst. Chem. Eng., 2012, 43, 696–703 CrossRef.
- Z. Hubicki and A. Wołowicz, J. Hazard. Mater., 2009, 164, 1414–1419 CrossRef.
- S. Lin, W. Wei, X. Wu, T. Zhou, J. Mao and Y. S. Yun, J. Hazard. Mater., 2015, 299, 10–17 CrossRef PubMed.
- W. Wang, X. Liu, X. Wang, L. Zong, Y. Kang and A. Wang, Front. Chem., 2021, 9, 662482 CrossRef.
- K. R. Koch, Coord. Chem. Rev., 2001, 216–217, 473–488 CrossRef.
- A. Cieszynska and D. Wieczorek, Hydrometallurgy, 2018, 175, 359–366 CrossRef.
- T. H. Nguyen, C. H. Sonu and M. S. Lee, J. Ind. Eng. Chem., 2015, 32, 238–245 CrossRef.
- B. Swain, C. Mishra and K. K. Sahu, Hydrometallurgy, 2010, 104, 1–7 CrossRef.
- P. Jal, S. Patel and B. K. Mishra, Talanta, 2004, 62, 1005–1028 CrossRef PubMed.
- O. E. Fayemi, J. A. Adekoya, E. E. Ebenso and A. S. Adekunle, Sep. Sci. Technol., 2014, 50, 1497–1506 CrossRef.
- C. Huang, J. Tian, J. Yan, M. Li and X. Li, Materials, 2020, 13, 2958 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra07935c |
| This journal is © The Royal Society of Chemistry 2025 |




