Open Access Article
Open Access ArticleHelicobacter pylori and gastric cancer: current insights and nanoparticle-based interventions
Syed Ali Raza Shah*a,
Maria Mumtaza,
Sumaira Sharifa,
Imtiaz Mustafaa and
Iffat Nayila b
b
aInstitute of Molecular Biology and Biotechnology (IMBB), The University of Lahore, Lahore, Pakistan. E-mail: ali.raza@imbb.uol.edu.pk
bDepartment of Pharmacy, The University of Lahore, Sargodha Campus, Sargodha, Pakistan
First published on 18th February 2025
Abstract
Background: H. pylori is recognized as one of the main causes of gastric cancer, and this type of cancer is considered as one of the leading diseases causing cancer deaths all over the world. Knowledge on the interactions between H. pylori and gastric carcinogenesis is important for designing preventive measures. Objective: the objective of this review is to summarize the available literature on H. pylori and gastric cancer, specifically regarding the molecular mechanisms, nanoparticle-based therapy and clinical developments. Methods: the databases including PubMed, Google Scholar and web of science were searched as well as papers from 2010 to 2024 were considered for review. Research literature on H. pylori, gastric cancer, nanoparticles, nanomedicine, and therapeutic interventions was summarized for current findings and possible treatments. Results: the presence of H. pylori in gastric mucosa causes chronic inflammation and several molecular alterations such as DNA alteration, epigenetic changes and activation of oncogenic signaling pathways which causes gastric carcinogenesis. Conventional antibiotic treatments have some issues because of the constantly rising levels of antibiotic resistance. Lipid based nanoformulations, polymeric and metallic nanoparticles have been delivered in treatment of H. pylori to improve drug delivery and alter immunological responses. Conclusion: nanoparticle based interventions have been widely explored as drug delivery systems by improving the treatment strategies against H. pylori induced gastric cancer. Further studies and clinical trials are required to bring these findings into a clinical setting in order to possibly alter the management of H. pylori related gastric malignancies.
1. Introduction
Gastric cancer is still a strong public health issue and is ranked among the most common causes of cancer deaths across the globe.1 Even though diagnostic and therapeutic strategies have been improving, the prognosis of gastric cancer patients is still relatively grim mainly due to the advanced stage at diagnosis and lack of effective treatments.2 According to estimates, there were 1.09 million new cases of gastric cancer and 0.77 million gastric cancer-related deaths reported worldwide in 2020. Globally, the annual incidence rates for males and women were 15.6 to 18.1 and 6.7 to 7.8 per 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, respectively.3 Many cross-sectional studies have established that H. pylori positive people are at higher risk of developing gastric cancer in central Asia and Africa.4 For example, Helicobacter and cancer collaborative group meta-analysis published in 2001 assert that the risk of developing gastric cancer among patients with H. pylori infection is 2–3-fold that of the non-infected patients.5 The most significant risk factor for the formation of gastric cancer is the chronic infection of the stomach lining by the Gram-negative bacterium called Helicobacter pylori (H. pylori). H. pylori infection is very common, with 50% of the world population being infected, and the prevalence is even higher in the developing nations.6 H. pylori is a group 1 carcinogen having highest risk category for cancer development as described by International Agency for Research on Cancer (IARC) and is recognized as playing a substantial role in gastric carcinogenesis. Cytotoxin associated gene A (CagA) and Vacuolating cytotoxin A (VacA) are the main virulence factors of the bacterium that play a significant role in the development of gastric adenocarcinoma.7 These factors compromise the gastric epithelial barrier and induce chronic inflammation, which in addition to genetic and epigenetic changes, create a tumorigenic milieu.8 The progress from H. pylori infection to gastric cancer is a process of chronic gastritis, atrophic gastritis, intestinal metaplasia, dysplasia, and adenocarcinoma.9 Contemporary management of H. pylori infection is based on the use of antibiotics in the combination with proton pump inhibitors (PPIs). Treatment of H. pylori often involves triple therapy that includes clarithromycin, amoxicillin, or metronidazole.10 But antibiotic resistance has become a major concern and it has greatly reduced the effectiveness of these treatments making it important to look for other treatment options.11 Also, conventional treatments are inadequate to eliminate the infection or to hinder the occurrence of gastric cancer in those with a high risk. Nanotechnology has now been defined as a unique field in the discovery of various therapeutic interventions.12 Nanoparticles as a result of their physicochemical characteristic possess several advantages over the conventional therapy such as site-specific release, improved absorption, and minimal side effects.13 Antimicrobial medications, specifically antibiotics, had to be encapsulated for targeted drug delivery to combat H. pylori. Different categories of drug induced nanoparticles including zinc oxide nanoparticles, silver nanoparticles, chitosan based nanoparticles and liposomes are at the experimental stage to enhance the treatment outcomes of H. pylori and to prevent the oncogenic action of this bacterium.14 The antimicrobial effects of antibiotic mediated nanoparticles on H. pylori are mediated by medication transport, biofilm destruction, immunological modulation, and possible probiotic usage. The capacity of these nanoparticles to overcome challenges including poor medication absorption and antibiotic resistance makes them an effective weapon to fight against H. pylori infection.15
000, respectively.3 Many cross-sectional studies have established that H. pylori positive people are at higher risk of developing gastric cancer in central Asia and Africa.4 For example, Helicobacter and cancer collaborative group meta-analysis published in 2001 assert that the risk of developing gastric cancer among patients with H. pylori infection is 2–3-fold that of the non-infected patients.5 The most significant risk factor for the formation of gastric cancer is the chronic infection of the stomach lining by the Gram-negative bacterium called Helicobacter pylori (H. pylori). H. pylori infection is very common, with 50% of the world population being infected, and the prevalence is even higher in the developing nations.6 H. pylori is a group 1 carcinogen having highest risk category for cancer development as described by International Agency for Research on Cancer (IARC) and is recognized as playing a substantial role in gastric carcinogenesis. Cytotoxin associated gene A (CagA) and Vacuolating cytotoxin A (VacA) are the main virulence factors of the bacterium that play a significant role in the development of gastric adenocarcinoma.7 These factors compromise the gastric epithelial barrier and induce chronic inflammation, which in addition to genetic and epigenetic changes, create a tumorigenic milieu.8 The progress from H. pylori infection to gastric cancer is a process of chronic gastritis, atrophic gastritis, intestinal metaplasia, dysplasia, and adenocarcinoma.9 Contemporary management of H. pylori infection is based on the use of antibiotics in the combination with proton pump inhibitors (PPIs). Treatment of H. pylori often involves triple therapy that includes clarithromycin, amoxicillin, or metronidazole.10 But antibiotic resistance has become a major concern and it has greatly reduced the effectiveness of these treatments making it important to look for other treatment options.11 Also, conventional treatments are inadequate to eliminate the infection or to hinder the occurrence of gastric cancer in those with a high risk. Nanotechnology has now been defined as a unique field in the discovery of various therapeutic interventions.12 Nanoparticles as a result of their physicochemical characteristic possess several advantages over the conventional therapy such as site-specific release, improved absorption, and minimal side effects.13 Antimicrobial medications, specifically antibiotics, had to be encapsulated for targeted drug delivery to combat H. pylori. Different categories of drug induced nanoparticles including zinc oxide nanoparticles, silver nanoparticles, chitosan based nanoparticles and liposomes are at the experimental stage to enhance the treatment outcomes of H. pylori and to prevent the oncogenic action of this bacterium.14 The antimicrobial effects of antibiotic mediated nanoparticles on H. pylori are mediated by medication transport, biofilm destruction, immunological modulation, and possible probiotic usage. The capacity of these nanoparticles to overcome challenges including poor medication absorption and antibiotic resistance makes them an effective weapon to fight against H. pylori infection.15
In this review, our main objectives are to present the updated knowledge about the epidemiology and molecular pathways of H. pylori-related gastric cancer, to describe the modern treatment strategies and their drawbacks in the context of H. pylori infection treatment, and to introduce the recent developments and perspectives of the liposomes, polymeric, and metallic nanoparticles use for the treatment and diagnosis of H. pylori and gastric cancer.16 This review particularly focuses on the kinds of nanoparticles that have shown effectiveness against H. pylori, as well as their modes of action and their benefits over traditional antibiotic treatments. It was also evaluated the difficulties, and potential paths forward in creating nanoparticle-based treatments for the successful elimination of H. pylori, tackling problems such as antibiotic resistance, bioavailability, and targeted delivery. Thus, this review highlights the significance of adopting innovative approaches in the H. pylori associated gastric cancer treatment and patient care based on the integration of modern data and new therapeutic approaches.
1.1 H. pylori and gastric carcinogenesis
H. pylori is a Gram-negative bacterium that infects the human stomach, and it is considered as a potent carcinogen for gastric cancer especially that of the non-cardia gastric adenocarcinoma.17 The infection begins by colonization of the columnar glandular epithelium of the stomach by the bacterium H. pylori mostly in the pyloric area. Its action is to secrete virulence factors that results into an inflammatory process.18 This condition can result to gastritis as well as ulcers. If the inflammation is chronic, there will be changes such as metaplasia in which the stomach cells alter their structure.19 These metaplastic cells are capable of mutations, which results to neoplasia, the abnormal and uncontrolled growth of cells. There are two main subtypes of adenocarcinoma (the most common type of gastric cancer, accounting for about 90% of cases): intestinal which is also called well-differentiated and diffuse which is also called undifferentiated. The diffuse type is characterized by mutation in the CDH1 gene and loss of E-CADHERIN function that results in cell disorganized division and cancer.20 The (Fig. 1;21) shows the sequence of events that occur in relation to the development of Helicobacter pylori infection to gastric cancer.1.2 Molecular mechanisms of H. pylori and role of inflammatory mediators
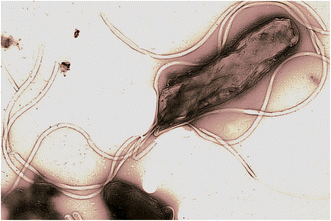
H. pylori through several molecular pathways such as inflammation, DNA damage and epigenetic changes contributes to gastric carcinogenesis.22 The important factors of virulence include cytotoxin-associated gene A (CagA) and vacuolating cytotoxin A (VacA) which affect the gastric epithelial cells and stimulate the oncogenic pathways.23 Electron microscopic image of H. pylori, in (Fig. 2;23) clearly signifies the spiraling nature of the bacteria. The bacterium is mobile and has several flagella that enables it to swim in the mucus layer of the stomach lining and causes chronic infections that can culminate into serious illnesses such as gastric cancer.24,25One of the critical pathways of carcinogenesis in the stomach is the chronic inflammation caused by H. pylori.26,27 It activates a continuous inflammatory response that results to the synthesis of various cytokines like interleukin-1 beta, interleukin-6, and tumor necrosis factor-alpha, as well as reactive oxygen species. These inflammatory mediators are pro genotoxic, pro carcinogenic and pro angiogenic, therefore they induce DNA damage, predispose to genetic instability and create a tumorigenic stroma.28,29 H. pylori infection directly caused DNA damage by producing ROS and indirectly by chronic inflammation. Also, the bacterium causes epigenetic modifications that involve DNA methylation and histone modification, leads to the down-regulation of tumor suppressor genes and up-regulation of oncogenes.30 For instance, the gene CDH1 that codes for E-cadherin, is commonly hypermethylated in H. pylori infected gastric mucosa and the risk of cancer is significantly higher in such cases.31 H. pylori infection is also known to cause epithelial–mesenchymal transition (EMT) which is known to increase the invasiveness and metastasizing abilities of the gastric epithelial cells.32 The H. pylori virulence factors are known to activate several signaling pathways that are involved in this process such as Wnt/β-catenin, TGF-β, and NF-κB signaling pathways.33,34
1.3 Current therapeutic approaches
1.3.3.1 Advantages of nanoparticles. Nanoparticles are considered to be advantageous over the conventional therapies for drug delivery as these are effective in targeted delivery, possess better bioavailability and possess low level of systemic toxicity.42 They afford the opportunity to encapsulate and deliver a large amount of therapeutic agents to the site of infection enhancing the efficiency of treatment with minimal side effects.43
1.3.3.2 Types of nanoparticles. (1) Lipid-based nanoparticles: liposomes and solid lipid nanoparticles (SLNs) can encapsulate antibiotics and deliver them directly to the gastric mucosa. Studies have shown that liposomal formulations of clarithromycin significantly enhance its antibacterial activity against H. pylori.44
(2) Polymeric nanoparticles: biodegradable polymers, such as PLGA and chitosan, are used to create nanoparticles that release antibiotics in a controlled manner. For instance, there is evidence based on PLGA nanoparticles containing amoxicillin that provides prolonged antibacterial effect and higher rates of eradication in animal trial.45
(3) Metallic nanoparticles: the antibacterial activity of silver, gold and zinc oxide nanoparticles is high against H. pylori. Due to their physicochemical properties, they can compromise the bacterial membranes and affect various metabolic activities. For instance, the synthesized silver nanoparticles proved to give a good result in elimination of H. pylori in the stomach.46,47
The polymeric and metallic nanoparticles cause damage to the bacterial cell membrane, denatured bacterial proteins, and heat shock proteins are produced (Fig. 3).1 They also suppress enzymatic activity, break down nucleic acids and produce a reactive oxygen species which leads to oxidative stress in bacteria. These individual actions cause the bacteria to enter a lethal stress response, which in turn leads to their death. This particular strategy focuses on one of the possible uses for nanoparticles and their capability to eliminate H. pylori, which is a factor that can lead to gastric cancer.48
1.4 Mechanisms of action
Nanoparticles can combat H. pylori through multiple mechanisms, including:• Direct antibacterial activity: metallic nanoparticles produce ROS resulting in oxidative stress and bacterial cell death. Also, nanoparticles can weaken the bacterial membranes and cause the release of cellular contents.49
• Enhanced drug delivery: one can use nanoparticles that can encapsulate the antibiotics and then release the drug directly to the stomach lining thus solving problems that are associated with conventional drug delivery systems.50 This targeted delivery increases the drug concentration at the site of infection and decreases the concentration in the whole body.51
• Modulation of immune response: nanoparticles can be engineered to modulate the host immune response, promoting anti-inflammatory effects and reducing gastric mucosal damage.52
1.5 Clinical applications and future directions
| Study | Year | Population | Relative risk (RR) | Conclusion | References |
|---|---|---|---|---|---|
| Exploring the interactions between Helicobacter pylori (Hp) infection and other risk factors of gastric cancer. A pooled analysis in the stomach cancer pooling (StoP) project | 2021 | Global | 2.5 | H. pylori infection significantly increases gastric cancer risk | 59 |
| Helicobacter pylori and its role in gastric cancer | 2023 | Global | 3.8 | H. pylori-positive patients had a higher incidence of gastric cancer than H. pylori-negative patients | 60 |
| Nanoparticle type | Composition | Mechanism of action | Key studies | References |
|---|---|---|---|---|
| Lipid-based | Liposomes, SLNs | Encapsulation of antibiotics, targeted delivery | Liposomal clarithromycin, PLGA-amoxicillin | 61 |
| Polymeric | PLGA, chitosan | Controlled drug release, sustained antimicrobial activity | PLGA nanoparticles, chitosan nanoparticles | 62 |
| Metallic | Silver, gold, zinc oxide | ROS generation, membrane disruption | Silver nanoparticles, gold nanoparticles | 63 |
1.6 Immunotherapy approaches
• Immune boosters: nanoparticles can encapsulate immune-modulating agents that would boost the body's immune response against the H. pylori. For instance, nanoparticles can have cytokines that enhance the immune response against the particular infection incorporated in them.66,67
• Checkpoint inhibitors: the co-employment of immune checkpoint inhibitors and nanoparticles can assist in eradicating immune resistance hence effectively respond to H. pylori infected cells.68,69
1.7 Improving adherence to nanoparticle therapies
• Convenient drug delivery: create nanoparticle formulations that can be administered by increasing the patient's compliance with the prescribed schedules.70• Patient education: ensure that patient education is given to the patients on the need to adhere to their treatment schedule and how to use the new nanoparticle-based drugs.71
• Taste and comfort: design the oral nanoparticle drugs to be easily swallowed and with little discomfort in a bid to enhance patient's adherence to the medication.72
• Support systems: use the follow-up and reminder systems that can assist the patients to adhere to their prescribed treatments.73
1.7.1.1 Effectiveness. In general, the nanoparticle-based treatments have been found to be effective in increasing the therapeutic efficiency of H. pylori against the conventional antibiotics and the new treatment modalities that include the probiotics and the bismuth containing preparations.74 Research studies shown that nanoparticles enhance the solubility and stability of the antibiotics, decrease the frequency of administration, and decrease the propensity for the development of antibiotic resistance, thus, suggesting that they may be more effective than conventional methods.75
1.7.1.2 Safety. It is necessary to contrast nanoparticle treatments with standard therapies and their side effects and adverse reactions. This will also minimize the effects of the drugs on the rest of the body, hence reducing on systemic toxicity.76 Comparing the results with other types of particles is required to find out whether there are any specific threats of nanoparticles, including long-term toxicity or other improper behaviors in biological environments.77
1.7.1.3 Practicality. The reality of using nanoparticles in practice is therefore measured by how affordable it is to use nanoparticles, how easy it is to access nanoparticles and how easy it is to apply nanoparticles.78 Attributes like processes of making them, the storage conditions and the methods of administration are also gain much importance. Furthermore, the evidence evaluating patient's compliance with treatment regimens, which depends on dosing frequency and intolerance, is essential for practical application.79
1.8 Long-term follow-up data
1.9 Economic analysis
1.10 Personalized medicine implications
1.11 Regulatory and ethical considerations
1.12 Global health implications
| Study year | Intervention | Comparison | Outcome | Reference |
|---|---|---|---|---|
| 2024 | Nanoparticle-based therapy | Traditional antibiotics | Higher efficacy, lower resistance rates | 98 |
| 2024 | Silver nanoparticles with probiotics | Probiotics alone | Synergistic effect, enhanced antibacterial activity | 98 |
| 2023 | PLGA-amoxicillin nanoparticles | Amoxicillin | Sustained antimicrobial activity | 1 |
| 2023 | Nanoparticle-based vaccine | Traditional vaccine | Longer-lasting immunity, fewer doses needed | 99 |
| 2022 | Gold nanoparticles with antibiotics | Silver nanoparticles | Higher bioactivity, reduced colonization | 61 |
| 2022 | Zinc oxide nanoparticles | Conventional therapy | Effective against resistant strains | 48 |
| 2022 | Nanoparticle immunotherapy combination | Conventional treatment | Improved immune response, reduced side effects | 48 |
| 2021 | Liposomal clarithromycin | Standard clarithromycin | Enhanced antibacterial activity | 59 |
| 2021 | Polymeric nanoparticles with cytokines | Antibiotics alone | Enhanced immune response, better outcomes | 63 |
| 2020 | Chitosan nanoparticles | Standard antibiotic therapy | Improved drug delivery, lower toxicity | 100 |
2. Methodology
2.1 Literature search
A comprehensive literature search was conducted using PubMed, Google Scholar, and other relevant databases to gather data on the relationship between H. pylori and gastric cancer, and recent advancements in nanoparticle-based therapeutic interventions. Keywords used included “H. pylori”, “gastric cancer”, “nanoparticles”, “nanomedicine”, and “therapeutic interventions”. The search focused on articles published between 2010 and 2024.1022.2 Inclusion and exclusion criteria
• Inclusion criteria: scientific papers including published articles, clinical trials, review papers and meta-analysis regarding the correlation between H. pylori and gastric cancer, nanoparticle-based treatment for H. pylori.103• Exclusion criteria: review articles, editorials and letters, articles unrelated to H. pylori or gastric cancer, articles not available in English, and articles before 2010.104
2.3 Data extraction
Relevant data were extracted from the selected studies, including:• H. pylori infection and gastric cancer risk, an epidemiological study.
• The biological relationship between H. pylori and the development of gastric carcinoma.
• Current therapeutic strategies and their problems.
• Types of nanoparticles and their application in the management of H. pylori infection as well as the working mechanisms.
• Forced outputs from preclinical and clinical trials on nanoparticle-based theranostics.
2.4 Analysis
The data extracted were analyzed to give a brief on the existing knowledge of H. pylori in gastric carcinogenesis and the possibility of using nanoparticles for treatment.1053. Results
The present review study has also shown that H. pylori infection increases the risk of developing gastric cancer particularly non-cardia gastric adenocarcinoma. From the epidemiological perspective, it was demonstrated that patients with H. pylori infection have 2–3-fold increased risk of gastric cancer compared to the population without this infection. The role of H. pylori in gastric carcinogenesis is well established and it occurs by altering the molecular pathways related to chronic inflammation, DNA damage and epigenetic modifications. The pathogen factors including CagA and VacA weaken the gastric epithelial cells, which triggers oncogenic pathways. Inflammation due to H. pylori leads to the secretion of pro-inflammatory cytokines and ROS causing DNA damage, genetic instability and thus tumorigenicity. In addition to that, H. pylori also results in changes in methylation pattern and histone modification through which tumor suppressor genes are deactivated and oncogenes overexpressed. Standard medications used to treat H. pylori using antibiotics have been made ineffective by increasing resistance to antibiotics. Such difficulties have led to the emergence of new approaches to the problem, one of which is nanoparticle-based treatments. The use of nanoparticles has multiple advantages like high selectivity for the drug delivery, enhanced bioavailability and minimal systemic side effects. Lipid based nanoparticles, polymeric and metallic nanoparticles have been found to be effective in preclinical studies. These nanoparticles can improve the antibiotics uptake, redesign immune reactions and demonstrate the bactericidal effect on H. pylori. However, these therapies are still in the experimental stage and in clinical trial, therefore, their safety and effectiveness for use in humans has not been ascertained. Nanoparticle based therapies have the potential in the enhancement of the treatment of gastric malignancies associated with H. pylori.4. Discussion
The H. pylori infection is positively associated with the risk of gastric cancer. Subsequent meta-analyses and large cohorts have further affirmed the causal association between H. pylori and GC and in particular, the risk of developing non-cardia gastric adenocarcinoma.106 This explains why management of H. pylori infection is important in order to reduce the occurrences of gastric cancer. Evaluation of the roles of H. pylori and other factors showed that chronic inflammation being caused by H. pylori is a major step in the development of gastric cancer.107 CagA and VacA of the bacterium impair the epithelial cell function, cause DNA damage, and bring about epigenetic modifications. The following processes play a role in the initiation of gastric cancer as well as the development of the disease. Knowledge of these molecular processes forms a framework of how to reduce oncogenic properties of H. pylori through specific therapeutic interventions.108 Current H. pylori antibiotics have many limitations because of the increased resistance to antibiotics in the world. This has diminished the efficacy of the traditional treatments and made people seek for the other method of treating the diseases.109A study described that ano-amoxicillin, nano-azithromycin, and nano-metronidazole were the three nano-antibiotics with the corresponding optical density (OD) values of 0.386, 0.258, and 0.167. Each of the nano-antibiotics showed a higher percentage of inhibition than the micro-antibiotics. They were also discovered to be all non-oxidants and to be safe to employ as effective antioxidants in medical treatments. The novel antibiotic effectiveness was proportionate to its concentration, and all of the antibiotics in the nanomedicine had antibacterial properties.110 To eradicate H. pylori, antimicrobial medications, specifically antibiotics, had to be encapsulated in most cases. These nanoparticles (NP) were superior to non-targeted drugs because they prevented the bacteria from adhering to gastric cells and made it possible to administer medications inside the bacterium.111 Nanoparticles are an efficient targeted drug delivery technique for treating H. pylori, according to the cellular uptake mechanism. They can also be employed as potential nano-carriers for the oral delivery of other therapeutic medications that target H. pylori.112 New strategies include the use of probiotics and bismuth containing regimens that however need further confirmation and fine tuning. Therefore, nanoparticles can be considered as a new and effective strategy of H. pylori eradication. This increases therapeutic effectiveness and decreases the general toxicity of the antimicrobial agents employed in the treatment of bacterial infections. In the preclinical studies, different types of nanoparticles such as polymeric based and metallic based nanoparticles have shown promising results. The administrations of these nanoparticles can deliver antibiotics and have direct antibacterial actions including production of ROS or disrupting bacterial membrane.113 In vitro and in vivo database have revealed that nanoparticle mediated formulations of the existing antibiotics can improve the efficacy of drugs, decrease gastric irritation and even delay the carcinogenic transformation in the stomach. These nanoparticle-based therapies are only in the phase I trials for determining the safety, effectiveness, and metabolism in the human body. The outcomes of these trials will define the clinical potential of nanomedicine for the H. pylori related diseases.114 However, there are specific obstacles that need to be overcome in the case of nanoparticle-based therapies. These are the concerns that touch on scalability, regulatory approval, and stability over the extended period. However, more studies are required to fine-tune the nanoparticle formulations and to understand their biological behavior and response in various patients' groups. Future development in nanotechnology along with the enhanced understanding of the pathogenesis of H. pylori will provide the key to overcome these issues and to bring these advancements to clinics.115
5. Conclusion
H. pylori is one of the leading causes of gastric cancer in the global population, thus there is need to develop efficient therapeutic interventions to address this health burden. Nanoparticles have been considered as a therapeutic option against H. pylori infection and its carcinogenic consequences. These new approaches use the specific characteristics of nanoparticles and promote drug delivery more effectively, as well as have lesser side effects. Further scientific work and clinical studies will be required to implement these findings for clinical uses, this could transform the approach to managing H. pylori related gastric malignancies. The proposed approach has the potential to contribute to enhancement of outcomes in patients with gastric cancer and in general decrease the global morbidity rate of this disease.6. Future prospectives
Nanoparticles are a promising path for future research and application in the battle against H. pylori because of their ability to address global health issues including antibiotic resistance and their integration with personalized medicine approaches. Ultimately, nanoparticle-based treatment for H. pylori infections and gastric cancer offer encouraging benefits like increased effectiveness, and decreased resistance, there are still important issues that must be resolved. Clinical trials, economic evaluations, and regulatory frameworks must be given top priority in future research in order to get nanoparticle-based medicines from the lab to the clinic.Ethical statement
Our study requires no ethical approval as it was a review based study and all data collected from ethically approved sources of publicly available free databases and articles of PubMed, springer, BMC, nature.Consent to participate
All authors are equally participated in writing, data collection and preparation of this article.Consent to publish
All authors gave their consent to publish this article.Data availability
No data generated during this review article study.Author contributions
Conceptualization, writing original draft; Syed Ali Raza Shah, Maria Mumtaz; resources; Syed Ali Raza Shah, Sumaira Sharif; data curation, review and editing, Syed Ali Raza Shah, Iffat Nayila, and Sumaira Sharif; supervision; Syed Ali Raza Shah, Sumaira Sharif and Imtiaz Mustafa. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.References
- X. Yin, Y. Lai, Y. Du, T. Zhang, J. Gao and Z. Li, Metal-Based Nanoparticles: A Prospective Strategy for Helicobacter pylori Treatment, Int. J. Nanomed., 2023, 2413–2429 CrossRef CAS PubMed.
- S. Khan, M. Sharaf, I. Ahmed, T. U. Khan, S. Shabana and M. Arif, et al., Potential utility of nano-based treatment approaches to address the risk of Helicobacter pylori, Expert Rev. Anti-Infect. Ther., 2022, 20(3), 407–424 CrossRef CAS PubMed.
- M. Shirani, R. Pakzad, M. H. Haddadi, S. Akrami, A. Asadi and H. Kazemian, et al., The global prevalence of gastric cancer in Helicobacter pylori-infected individuals: a systematic review and meta-analysis, BMC Infect. Dis., 2023, 23(1), 543 CrossRef PubMed.
- R. S. Hendriksen, V. Bortolaia, H. Tate, G. H. Tyson, F. M. Aarestrup and P. F. McDermott, Using genomics to track global antimicrobial resistance, Front. Public Health, 2019, 7, 242 CrossRef PubMed.
- L. Yang, J. Zhang, J. Xu, X. Wei, J. Yang and Y. Liu, et al., Helicobacter pylori infection aggravates dysbiosis of gut microbiome in children with gastritis, Front. Cell. Infect. Microbiol., 2019, 9, 375 CrossRef CAS PubMed.
- T. A. Addissouky, Y. Wang, I. E. T. El Sayed, A. E. Baz, M. M. A. Ali and A. A. Khalil, Recent trends in Helicobacter pylori management: harnessing the power of AI and other advanced approaches, Beni-Suef Univ. J. Basic Appl. Sci., 2023, 12(1), 80 CrossRef.
- J. E. Francis, The Investigation of Adjuvanted Solid Lipid Nanoparticles as a Novel DNA Vaccine Delivery System against Helicobacter Pylori, RMIT University, 2021 Search PubMed.
- W. Song, A. C. Anselmo and L. Huang, Nanotechnology intervention of the microbiome for cancer therapy, Nat. Nanotechnol., 2019, 14(12), 1093–1103 CrossRef CAS PubMed.
- Y. Moodley, B. Linz, R. P. Bond, M. Nieuwoudt, H. Soodyall and C. M. Schlebusch, et al., Age of the association between Helicobacter pylori and man, PLoS Pathog., 2012, 8(5), e1002693 CrossRef CAS PubMed.
- K. M. Fock, D. Y. Graham and P. Malfertheiner, Helicobacter pylori research: historical insights and future directions, Nat. Rev. Gastroenterol. Hepatol., 2013, 10(8), 495–500 CrossRef PubMed.
- Y. Wang, S. lou Wang, J. yi Zhang, X. ning Song, Z. yong Zhang and J. feng Li, et al., Anti-ulcer and anti-Helicobacter pylori potentials of the ethyl acetate fraction of Physalis alkekengi L. var. franchetii (Solanaceae) in rodent, J. Ethnopharmacol., 2018, 211, 197–206 CrossRef CAS PubMed.
- B. D. Kocic, M. V. Dimitrijević, L. C. Miladinović, M. S. Marković, G. Ž. Ranković and D. L. Miladinović, In vitro anti-Helicobacter pylori activity of berberine and barberry extracts: A preliminary report, Nat. Prod. Commun., 2019, 14(6), 1934578X19857905 CrossRef CAS.
- N. H. Ibrahim, A. S. Awaad, R. A. Alnafisah, S. I. Alqasoumi, R. M. El-Meligy and A. Z. Mahmoud, In–vitro activity of Desmostachya bipinnata (L.) Stapf successive extracts against Helicobacter pylori clinical isolates, Saudi Pharm. J., 2018, 26(4), 535–540 CrossRef PubMed.
- V. Egas, G. Salazar-Cervantes, I. Romero, C. A. Méndez-Cuesta, J. L. Rodríguez-Chávez and G. Delgado, Anti-Helicobacter pylori metabolites from Heterotheca inuloides (Mexican arnica), Fitoterapia, 2018, 127, 314–321 CrossRef CAS PubMed.
- Y. Zou, X. Qian, X. Liu, Y. Song, C. Song and S. Wu, et al., The effect of antibiotic resistance on Helicobacter pylori eradication efficacy: A systematic review and meta-analysis, Helicobacter, 2020, 25(4), e12714 CrossRef CAS PubMed.
- E. Tshibangu-Kabamba and Y. Yamaoka, Helicobacter pylori infection and antibiotic resistance—from biology to clinical implications, Nat. Rev. Gastroenterol. Hepatol., 2021, 18(9), 613–629 CrossRef PubMed.
- S. A. R. Shah, H. Rahman, M. Qasim, M. S. Akram, Y. Saygideger and N. Puspita, et al., Differential Proteomics of Helicobacter pylori Isolates from Gastritis, Ulcer, and Cancer Patients: First Study from Northwest Pakistan, Medicina, 2022, 58(9), 1168 CrossRef PubMed.
- A. Ali and K. I. AlHussaini, Helicobacter pylori: A Contemporary Perspective on Pathogenesis, Diagnosis and Treatment Strategies, Microorganisms, 2024, 12(1), 222 CrossRef CAS PubMed.
- M. Mohammadi, B. Attaran, R. Malekzadeh and D. Y. Graham, Furazolidone, an underutilized drug for H. pylori eradication: lessons from Iran, Dig. Dis. Sci., 2017, 62, 1890–1896 CrossRef CAS PubMed.
- J. Baj, I. Korona-Głowniak, A. Forma, A. Maani, E. Sitarz and M. Rahnama-Hezavah, et al., Mechanisms of the epithelial–mesenchymal transition and tumor microenvironment in Helicobacter pylori-induced gastric cancer, Cells, 2020, 9(4), 1055 CrossRef CAS PubMed.
- R. Ivyna de Araújo Rêgo, G. F. Guedes Silvestre, D. Ferreira de Melo, S. L. Albino, M. M. Pimentel and S. B. Silva Costa Cruz, et al., Flavonoids-rich plant extracts against Helicobacter pylori infection as prevention to gastric cancer, Front. Pharmacol., 2022, 13, 951125 CrossRef PubMed.
- L. Wu, Z. Wang, G. Sun, L. Peng, Z. Lu and B. Yan, et al., Effects of anti-H. pylori triple therapy and a probiotic complex on intestinal microbiota in duodenal ulcer, Sci. Rep., 2019, 9(1), 12874 CrossRef PubMed.
- K. R. Jones, J. M. Whitmire and D. S. Merrell, A tale of two toxins: Helicobacter pylori CagA and VacA modulate host pathways that impact disease, Folia Microbiol., 2010, 1, 115 CAS.
- S. Y. Reigh and E. Lauga, Two-fluid model for locomotion under self-confinement, Phys Rev Fluids, 2017, 2(9), 93101 CrossRef.
- D. I. Andersson, H. Nicoloff and K. Hjort, Mechanisms and clinical relevance of bacterial heteroresistance, Nat. Rev. Microbiol., 2019, 17(8), 479–496 CrossRef CAS PubMed.
- N. Farzi, C. Behzad, Z. Hasani, M. Alebouyeh, H. Zojaji and M. R. Zali, Characterization of clarithromycin heteroresistance among Helicobacter pylori strains isolated from the antrum and corpus of the stomach, Folia Microbiol., 2019, 64, 143–151 CrossRef CAS PubMed.
- M. Sarem and R. Corti, Role of Helicobacter pylori coccoid forms in infection and recrudescence, Gastroenterol. Hepatol., 2016, 39(1), 28–35 CrossRef PubMed.
- A. Fishbein, B. D. Hammock, C. N. Serhan and D. Panigrahy, Carcinogenesis: Failure of resolution of inflammation?, Pharmacol. Ther., 2021, 218, 107670 CrossRef CAS PubMed.
- S. Kadkhodaei, F. Siavoshi and K. Akbari Noghabi, Mucoid and coccoid Helicobacter pylori with fast growth and antibiotic resistance, Helicobacter, 2020, 25(2), e12678 CrossRef PubMed.
- L. Zhuge, Y. Wang, S. Wu, R. Zhao, Z. Li and Y. Xie, Furazolidone treatment for Helicobacter Pylori infection: A systematic review and meta-analysis, Helicobacter, 2018, 23(2), e12468 CrossRef PubMed.
- S. Lee, G. T. Sneed and J. N. Brown, Treatment of Helicobacter pylori with nitazoxanide-containing regimens: a systematic review, Infect. Dis., 2020, 52(6), 381–390 CrossRef CAS PubMed.
- T. M. J. Eddin, S. M. O. Nasr, I. Gupta, H. Zayed and A. E. Al Moustafa, Helicobacter pylori and epithelial mesenchymal transition in human gastric cancers: An update of the literature, Heliyon, 2023, 9(8), e18945 CrossRef PubMed.
- J. Baj, A. Forma, M. Sitarz, P. Portincasa, G. Garruti and D. Krasowska, et al., Helicobacter pylori virulence factors—mechanisms of bacterial pathogenicity in the gastric microenvironment, Cells, 2020, 10(1), 27 CrossRef PubMed.
- C. Tiffon, J. Giraud, S. E. Molina-Castro, S. Peru, L. Seeneevassen and E. Sifré, et al., TAZ controls Helicobacter pylori-induced epithelial–mesenchymal transition and cancer stem cell-like invasive and tumorigenic properties, Cells, 2020, 9(6), 1462 CrossRef CAS PubMed.
- S. D. Lee, H. Jeong, B. R. Hwang, B. M. Yu, Y. Cho and K. T. Nam, et al., Helicobacter pylori promotes epithelial-to-mesenchymal transition by downregulating CK2β in gastric cancer cells, Biochim. Biophys. Acta, Mol. Basis Dis., 2023, 1869(1), 166588 CrossRef CAS PubMed.
- S. Courtois, M. Haykal, C. Bodineau, E. Sifre, L. Azzi-Martin and A. Ménard, et al., Autophagy induced by Helicobacter pylori infection is necessary for gastric cancer stem cell emergence, Gastric Cancer, 2021, 24, 133–144 CrossRef CAS PubMed.
- P. K. Godavarthy and C. Puli, From antibiotic resistance to antibiotic renaissance: a new era in helicobacter pylori treatment, Cureus, 2023, 15(3), e36041 Search PubMed.
- D. G. Lee, H. S. Kim, Y. S. Lee, S. Kim, S. Y. Cha and I. Ota, et al., Helicobacter pylori CagA promotes Snail-mediated epithelial–mesenchymal transition by reducing GSK-3 activity, Nat. Commun., 2014, 5(1), 4423 CrossRef CAS PubMed.
- G. Krzysiek-Maczka, A. Targosz, U. Szczyrk, M. Strzałka, Z. Sliwowski and T. Brzozowski, et al., Role of Helicobacter pylori infection in cancer-associated fibroblast-induced epithelial-mesenchymal transition in vitro, Helicobacter, 2018, 23(6), e12538 CrossRef PubMed.
- S. Ansari and Y. Yamaoka, Helicobacter pylori virulence factor cytotoxin-associated gene A (CagA)-mediated gastric pathogenicity, Int. J. Mol. Sci., 2020, 21(19), 7430 CrossRef CAS PubMed.
- M. Chmiela and J. Kupcinskas, Pathogenesis of Helicobacter pylori infection, Helicobacter, 2019, 24, e12638 CrossRef PubMed.
- H. Yu, J. Zeng, X. Liang, W. Wang, Y. Zhou and Y. Sun, et al., Helicobacter pylori promotes epithelial–mesenchymal transition in gastric cancer by downregulating programmed cell death protein 4 (PDCD4), PLoS One, 2014, 9(8), e105306 CrossRef PubMed.
- Y. Shi, Z. Yang, T. Zhang, L. Shen, Y. Li and S. Ding, SIRT1-targeted miR-543 autophagy inhibition and epithelial–mesenchymal transition promotion in Helicobacter pylori CagA-associated gastric cancer, Cell Death Dis., 2019, 10(9), 625 CrossRef PubMed.
- C. M. B. Pinilla, N. A. Lopes and A. Brandelli, Lipid-based nanostructures for the delivery of natural antimicrobials, Molecules, 2021, 26(12), 3587 CrossRef CAS PubMed.
- Y. C. Yeh, T. H. Huang, S. C. Yang, C. C. Chen and J. Y. Fang, Nano-based drug delivery or targeting to eradicate bacteria for infection mitigation: a review of recent advances, Front. Chem., 2020, 8, 286 CrossRef CAS PubMed.
- A. M. Matei, C. Caruntu, M. Tampa, S. R. Georgescu, C. Matei and M. M. Constantin, et al., Applications of nanosized-lipid-based drug delivery systems in wound care, Appl. Sci., 2021, 11(11), 4915 CrossRef CAS.
- T. T. L. Nguyen and V. A. Duong, Solid lipid nanoparticles, Encyclopedia, 2022, 2(2), 952–973 CrossRef.
- S. Asgari, N. Nikkam and P. Saniee, Metallic nanoparticles as promising tools to eradicate H. pylori: a comprehensive review on recent advancements, Talanta Open, 2022, 6, 100129 CrossRef.
- A. Girma, Alternative mechanisms of action of metallic nanoparticles to mitigate the global spread of antibiotic-resistant bacteria, Cell Surf., 2023, 10, 100112 CrossRef CAS PubMed . Available from: https://www.sciencedirect.com/science/article/pii/S2468233023000191.
- F. Aflakian, F. Mirzavi, H. T. Aiyelabegan, A. Soleimani, J. G. Navashenaq and I. Karimi-Sani, et al., Nanoparticles-based therapeutics for the management of bacterial infections: a special emphasis on FDA approved products and clinical trials, Eur. J. Pharm. Sci., 2023, 106515 CrossRef CAS PubMed.
- N. Mammari, E. Lamouroux, A. Boudier and R. E. Duval, Current knowledge on the oxidative-stress-mediated antimicrobial properties of metal-based nanoparticles, Microorganisms, 2022, 10(2), 437 CrossRef CAS PubMed.
- S. Zhang, L. Lin, X. Huang, Y. G. Lu, D. L. Zheng and Y. Feng, Antimicrobial properties of metal nanoparticles and their oxide materials and their applications in oral biology, J. Nanomater., 2022, 2022(1), 2063265 CrossRef.
- Y. N. Slavin, J. Asnis, U. O. Hńfeli and H. Bach, Metal nanoparticles: understanding the mechanisms behind antibacterial activity, J. Nanobiotechnol., 2017, 15, 1–20 CrossRef PubMed.
- T. Liu, S. Chai, M. Li, X. Chen, Y. Xie and Z. Zhao, et al., A nanoparticle-based sonodynamic therapy reduces Helicobacter pylori infection in mouse without disrupting gut microbiota, Nat. Commun., 2024, 15(1), 844 CrossRef CAS PubMed.
- X. Zhu, T. Su, S. Wang, H. Zhou and W. Shi, New advances in nano-drug delivery systems: Helicobacter pylori and gastric cancer, Front. Oncol., 2022, 12, 834934 CrossRef CAS PubMed.
- S. Alkarri, H. Bin Saad and M. Soliman, On antimicrobial polymers: development, mechanism of action, international testing procedures, and applications, Polymers, 2024, 16(6), 771 CrossRef CAS PubMed.
- R. Kaur, K. Kaur, M. H. Alyami, D. K. Lang, B. Saini and M. F. Bayan, et al., Combating microbial infections using metal-based nanoparticles as potential therapeutic alternatives, Antibiotics, 2023, 12(5), 909 CrossRef CAS PubMed.
- Y. Zou, S. Tao, J. Li, M. Wu and X. Zhou, Applications of nanomaterials as treatments and diagnostic biosensors in microbial infections, MedComm: Biomater. Appl., 2023, 2(4), e62 CAS.
- G. Collatuzzo, C. Pelucchi, E. Negri, L. López-Carrillo, S. Tsugane and A. Hidaka, et al., Exploring the interactions between Helicobacter pylori (Hp) infection and other risk factors of gastric cancer: A pooled analysis in the Stomach cancer Pooling (StoP) Project, Int. J. Cancer, 2021, 149(6), 1228–1238 CrossRef CAS PubMed.
- V. E. Reyes, Helicobacter pylori and its role in gastric cancer, Microorganisms, 2023, 11(5), 1312 CrossRef CAS PubMed.
- R. Pop, A. F. Tăbăran, A. P. Ungur, A. Negoescu and C. Cătoi, Helicobacter Pylori-induced gastric infections: from pathogenesis to novel therapeutic approaches using silver nanoparticles, Pharmaceutics, 2022, 14(7), 1463 CrossRef CAS PubMed.
- R. Chitas, D. R. Fonseca, P. Parreira and M. C. L. Martins, Targeted nanotherapeutics for the treatment of Helicobacter pylori infection, J. Biomed. Sci., 2024, 31(1), 78, DOI:10.1186/s12929-024-01068-9.
- M. P. C. de Souza, B. A. F. de Camargo, L. Sposito, G. C. Fortunato, G. C. Carvalho and G. D. Marena, et al., Highlighting the use of micro and nanoparticles based-drug delivery systems for the treatment of Helicobacter pylori infections, Crit. Rev. Microbiol., 2021, 47(4), 435–460 CrossRef CAS PubMed.
- M. A. H. Abusalah, H. Chopra, A. Sharma, S. A. Mustafa, O. P. Choudhary and M. Sharma, et al., Nanovaccines: a game changing approach in the fight against infectious diseases, Biomed. Pharmacother., 2023, 167, 115597 CrossRef PubMed.
- Y. M. Park, S. J. Lee, Y. S. Kim, M. H. Lee, G. S. Cha and I. D. Jung, et al., Nanoparticle-based vaccine delivery for cancer immunotherapy, Immune Netw., 2013, 13(5), 177–183 CrossRef PubMed.
- R. Bezbaruah, V. P. Chavda, L. Nongrang, S. Alom, K. Deka and T. Kalita, et al., Nanoparticle-based delivery systems for vaccines, Vaccines, 2022, 10(11), 1946 CrossRef CAS PubMed.
- N. P. Koyande, R. Srivastava, A. Padmakumar and A. K. Rengan, Advances in nanotechnology for cancer immunoprevention and immunotherapy: a review, Vaccines, 2022, 10(10), 1727 CrossRef CAS PubMed.
- Y. Fan and J. J. Moon, Nanoparticle drug delivery systems designed to improve cancer vaccines and immunotherapy, Vaccines, 2015, 3(3), 662–685 CrossRef CAS PubMed.
- R. Pati, M. Shevtsov and A. Sonawane, Nanoparticle vaccines against infectious diseases, Front. Immunol., 2018, 9, 2224 CrossRef PubMed.
- C. Baruah, P. Das, P. Devi, P. M. Saikia and B. Deka, The emergence of nanovaccines as a new paradigm in virological vaccinology: a review, Explor. Immunol., 2023, 3(4), 361–383 CAS.
- Z. Sun, H. Zhao, L. Ma, Y. Shi, M. Ji and X. Sun, et al., The quest for nanoparticle-powered vaccines in cancer immunotherapy, J. Nanobiotechnol., 2024, 22(1), 61 CrossRef PubMed.
- Q. Li, Y. Liu, Z. Huang, Y. Guo and Q. Li, Triggering immune system with nanomaterials for cancer immunotherapy, Front. Bioeng. Biotechnol., 2022, 10, 878524 CrossRef PubMed.
- J. Varadé, S. Magadán and Á. González-Fernández, Human immunology and immunotherapy: main achievements and challenges, Cell. Mol. Immunol., 2021, 18(4), 805–828 CrossRef PubMed.
- M. A. Beach, U. Nayanathara, Y. Gao, C. Zhang, Y. Xiong and Y. Wang, et al., Polymeric Nanoparticles for Drug Delivery, Chem. Rev., 2024, 124(9), 5505–5616 CrossRef CAS PubMed.
- M. Chehelgerdi, M. Chehelgerdi, O. Q. B. Allela, R. D. C. Pecho, N. Jayasankar and D. P. Rao, et al., Progressing nanotechnology to improve targeted cancer treatment: overcoming hurdles in its clinical implementation, Mol. Cancer, 2023, 22(1), 169 CrossRef PubMed.
- X. Huang, T. He, X. Liang, Z. Xiang, C. Liu and S. Zhou, et al., Advances and applications of nanoparticles in cancer therapy, MedComm: Oncol., 2024, 3(1), e67, DOI:10.1002/mog2.67.
- B. K. Kashyap, V. V. Singh, M. K. Solanki, A. Kumar, J. Ruokolainen and K. K. Kesari, Smart Nanomaterials in Cancer Theranostics: Challenges and Opportunities, ACS Omega, 2023, 8(16), 14290–14320, DOI:10.1021/acsomega.2c07840.
- F. Raza, H. Zafar, S. Zhang, Z. Kamal, J. Su and W. E. Yuan, et al., Recent Advances in Cell Membrane-Derived Biomimetic Nanotechnology for Cancer Immunotherapy, Adv. Healthcare Mater., 2021, 10(6), 2002081, DOI:10.1002/adhm.202002081.
- Y. K. Katare, A. K. Panda, K. Lalwani, I. U. Haque and M. M. Ali, Potentiation of Immune Response from Polymer-Entrapped Antigen: Toward Development of Single Dose Tetanus Toxoid Vaccine, Drug Delivery, 2003, 10(4), 231–238, DOI:10.1080/drd_10_4_231.
- X. Ma, Y. Tian, R. Yang, H. Wang, L. W. Allahou and J. Chang, et al., Nanotechnology in healthcare, and its safety and environmental risks, J. Nanobiotechnol., 2024, 22(1), 715, DOI:10.1186/s12951-024-02901-x.
- M. Liu, H. Gao, J. Miao, Z. Zhang, L. Zheng and F. Li, et al., Helicobacter pylori infection in humans and phytotherapy, probiotics, and emerging therapeutic interventions: a review, Front. Microbiol., 2024, 14, 1330029 CrossRef PubMed.
- R. Nosrati, B. Golichenari, A. Nezami, S. M. Taghdisi, B. Karimi and M. Ramezani, et al., Helicobacter pylori point-of-care diagnosis: Nano-scale biosensors and microfluidic systems, TrAC, Trends Anal. Chem., 2017, 97, 428–444 CrossRef CAS . Available from: https://www.sciencedirect.com/science/article/pii/S0165993617303370.
- Y. Yang, W. J. Meng and Z. Q. Wang, The origin of gastric cancer stem cells and their effects on gastric cancer: Novel therapeutic targets for gastric cancer, Front. Oncol., 2022, 12, 960539 CrossRef CAS PubMed.
- A. Cano, M. Ettcheto, M. Espina, A. López-Machado, Y. Cajal and F. Rabanal, et al., State-of-the-art polymeric nanoparticles as promising therapeutic tools against human bacterial infections, J. Nanobiotechnol., 2020, 18(1), 156, DOI:10.1186/s12951-020-00714-2.
- A. H. Miri, M. Kamankesh, M. Rad-Malekshahi, A. Yadegar, M. Banar and M. R. Hamblin, et al., Factors associated with treatment failure, and possible applications of probiotic bacteria in the arsenal against Helicobacter pylori, Expert Rev. Anti-Infect. Ther., 2023, 21(6), 617–639, DOI:10.1080/14787210.2023.2203382.
- K. A. Adedokun, S. O. Imodoye, Z. S. Yahaya, I. T. Oyeyemi, I. O. Bello, M. T. Adeyemo-Imodoye, et al., Nanodelivery of Polyphenols as Nutraceuticals in Anticancer Interventions, in Polyphenols, 2023, pp. 188–224, DOI:10.1002/9781394188864.ch10.
- C. Chen, A. Beloqui and Y. Xu, Oral nanomedicine biointeractions in the gastrointestinal tract in health and disease, Adv. Drug Delivery Rev., 2023, 203, 115117 CrossRef CAS PubMed , Available from: https://www.sciencedirect.com/science/article/pii/S0169409X23004325.
- B. K. Das, A. Sarma and A. K. Goswami. Chapter 18 - Gut-health pharmacology: Integrating microbiota insights with natural product based therapies, in Drug Discovery Update, ed. Rudrapal M., Egbuna C., Cho W. C. B. T. B., Elsevier, 2024, pp. 377–99, Available from: https://www.sciencedirect.com/science/article/pii/B978044316013400018X Search PubMed.
- K. Nasiri, S. M. Masoumi, S. Amini, M. Goudarzi, S. M. Tafreshi and A. Bagheri, et al., Recent advances in metal nanoparticles to treat periodontitis, J. Nanobiotechnol., 2023, 21(1), 283, DOI:10.1186/s12951-023-02042-7.
- Z. Yang, D. Wang, C. Zhang, H. Liu, M. Hao and S. Kan, et al., The Applications of Gold Nanoparticles in the Diagnosis and Treatment of Gastrointestinal Cancer, Front. Oncol., 2022, 11, 819329 CrossRef PubMed.
- Y. P. Hung, Y. F. Chen, P. J. Tsai, I. H. Huang, W. C. Ko and J. S. Jan, Advances in the Application of Nanomaterials as Treatments for Bacterial Infectious Diseases, Pharmaceutics, 2021, 13(11), 1913 CrossRef CAS PubMed.
- M. Leja and A. Linē, Early detection of gastric cancer beyond endoscopy - new methods, Best Pract. Res., Clin. Gastroenterol., 2021, 50–51, 101731 CrossRef PubMed . Available from: https://www.sciencedirect.com/science/article/pii/S152169182100007X.
- L. Zhang, X. Li, G. Yue, L. Guo, Y. Hu and Q. Cui, et al., Nanodrugs systems for therapy and diagnosis of esophageal cancer, Front. Bioeng. Biotechnol., 2023, 11, 1233476 CrossRef PubMed.
- M. E. Taghavizadeh Yazdi, M. Qayoomian, S. Beigoli and M. H. Boskabady, Recent advances in nanoparticle applications in respiratory disorders: a review, Front. Pharmacol., 2023, 14, 1059343 CrossRef PubMed.
- M. Pereira-Silva, G. Chauhan, M. D. Shin, C. Hoskins, M. J. Madou and S. O. Martinez-Chapa, et al., Unleashing the potential of cell membrane-based nanoparticles for COVID-19 treatment and vaccination, Expert Opin. Drug Delivery, 2021, 18(10), 1395–1414, DOI:10.1080/17425247.2021.1922387.
- G. Yang, S. Chen and J. Zhang, Bioinspired and Biomimetic Nanotherapies for the Treatment of Infectious Diseases, Front. Pharmacol., 2019, 10, 751 CrossRef CAS PubMed.
- M. Leja, W. You, M. C. Camargo and H. Saito, Implementation of gastric cancer screening – The global experience, Best Pract. Res., Clin. Gastroenterol., 2014, 28(6), 1093–1106 CrossRef PubMed . Available from: https://www.sciencedirect.com/science/article/pii/S1521691814001486.
- L. Hochvaldova, G. Posselt, S. Wessler, L. Kvítek and A. Panáček, Implications of silver nanoparticles for H. pylori infection: modulation of CagA function and signaling, Front. Cell. Infect. Microbiol., 2024, 14, 1419568 CrossRef PubMed.
- J. A. Paes Dutra, S. Gonçalves Carvalho, A. Soares de Oliveira, J. R. Borges Monteiro, J. Rodrigues Pereira de Oliveira Borlot and M. Tavares Luiz, et al., Microparticles and nanoparticles-based approaches to improve oral treatment of Helicobacter pylori infection, Crit. Rev. Microbiol., 2024, 50(5), 728–749, DOI:10.1080/1040841X.2023.2274835.
- B. Almeida Furquim de Camargo, D. E. Soares Silva, A. Noronha da Silva, D. L. Campos, T. R. Machado Ribeiro and M. J. Mieli, et al., New Silver(I) Coordination Compound Loaded into Polymeric Nanoparticles as a Strategy to Improve In Vitro Anti-Helicobacter pylori Activity, Mol. Pharmaceutics, 2020, 17(7), 2287–2298, DOI:10.1021/acs.molpharmaceut.9b01264.
- M. Au, T. I. Emeto, J. Power, V. N. Vangaveti and H. C. Lai, Emerging Therapeutic Potential of Nanoparticles in Pancreatic Cancer: A Systematic Review of Clinical Trials, Biomedicines, 2016, 4(3), 20 CrossRef PubMed.
- M. Piscione, M. Mazzone, M. C. Di Marcantonio, R. Muraro and G. Mincione, Eradication of Helicobacter pylori and Gastric Cancer: A Controversial Relationship, Front. Microbiol., 2021, 12, 630852 CrossRef PubMed.
- A. Elbehiry, E. Marzouk, M. Aldubaib, A. Abalkhail, S. Anagreyyah and N. Anajirih, et al., Helicobacter pylori Infection: Current Status and Future Prospects on Diagnostic, Therapeutic and Control Challenges, Antibiotics, 2023, 12(2), 191 CrossRef CAS PubMed.
- C. Shu, Z. Xu, C. He, X. Xu, Y. Zhou and B. Cai, et al., Application of biomaterials in the eradication of Helicobacter pylori: A bibliometric analysis and overview, Front. Microbiol., 2023, 14, 1081271 CrossRef PubMed.
- L. X. Ling, Y. Ouyang and Y. Hu, Research trends on nanomaterials in gastric cancer: a bibliometric analysis from 2004 to 2023, J. Nanobiotechnol., 2023, 21(1), 248, DOI:10.1186/s12951-023-02033-8.
- S. H. Loosen, A. Mertens, I. Klein, C. Leyh, S. Krieg and J. Kandler, et al., Association between Helicobacter pylori and its eradication and the development of cancer, BMJ Open Gastroenterol., 2024, 11(1), e001377 CrossRef PubMed.
- G. D. Eslick, Helicobacter pylori infection causes gastric cancer A? review of the epidemiological, meta-analytic, and experimental evidence, World J. Gastroenterol., 2006, 12(19), 2991 CrossRef PubMed.
- C. Zhang, Y. Chen, Y. Long, H. Zheng, J. Jing and W. Pan, Helicobacter pylori and Gastrointestinal Cancers: Recent Advances and Controversies, Clin. Med. Insights:Oncol., 2024, 18, 11795549241234636 Search PubMed.
- W. L. E, M. PR and T. WK, Helicobacter pylori and Gastric Cancer: Factors That Modulate Disease Risk, Clin. Microbiol. Rev., 2010, 23(4), 713–739, DOI:10.1128/cmr.00011-10.
- S. Abbas Hussein Al-Saeed, R. M. Mizher and H. Z. Numan, Preparation of Nano-Medicine to Eliminate Helicobacter pylori Infection, Arch. Razi Inst., 2023, 78(4), 1313–1323 CAS.
- J. Prieložná, V. Mikušová and P. Mikuš, Advances in the delivery of anticancer drugs by nanoparticles and chitosan-based nanoparticles, Int. J. Pharm.:X, 2024, 8, 100281 Search PubMed , https://www.sciencedirect.com/science/article/pii/S2590156724000537.
- M. Moosazadeh Moghaddam, S. Bolouri, R. Golmohammadi, M. Fasihi-Ramandi, M. Heiat and R. Mirnejad, Targeted delivery of a short antimicrobial peptide (CM11) against Helicobacter pylori gastric infection using concanavalin A-coated chitosan nanoparticles, J. Mater. Sci.:Mater. Med., 2023, 34(9), 44, DOI:10.1007/s10856-023-06748-w.
- L. Wang, C. Hu and L. Shao, The antimicrobial activity of nanoparticles: present situation and prospects for the future, Int. J. Nanomed., 2017, 1227–1249 CrossRef PubMed.
- T. Safarov, B. Kiran, M. Bagirova, A. M. Allahverdiyev and E. S. Abamor, An overview of nanotechnology-based treatment approaches against Helicobacter Pylori, Expert Rev. Anti-Infect. Ther., 2019, 17(10), 829–840, DOI:10.1080/14787210.2019.1677464.
- E. Kouroumalis, I. Tsomidis and A. Voumvouraki, Helicobacter pylori and gastric cancer: a critical approach to who really needs eradication, Explor. Dig. Dis., 2024, 3(2), 107–142 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |