Open Access Article
Open Access ArticleDesign and development of an isatin-1,2,3-triazole hybrid analogue as a potent anti-inflammatory agent with enhanced efficacy and gene expression modulation†
A. Niranjana Kumara,
Smruti Ranjan Dasb,
J. Kotesh Kumar *a,
K. V. N. S. Srinivas*a and
Sarada D. Tetalib
*a,
K. V. N. S. Srinivas*a and
Sarada D. Tetalib
aPhytochemistry Division, CSIR-Central Institute of Medicinal and Aromatic Plants, Research Centre, Boduppal, Hyderabad-500092, India. E-mail: koteshkumarj@cimap.res.in; kvn.satyasrinivas@cimap.res.in; Fax: +91-40-27202602
bDepartment of Plant Sciences, School of Life Sciences, University of Hyderabad, Hyderabad, India
First published on 22nd January 2025
Abstract
Isatin (1H-indole-2,3-dione) and its derivatives have been found to exhibit various biological activities, including anticancer and antidiabetic properties. In this study, a series of nine isatin-1,2,3-triazole conjugates were synthesized and evaluated for their anti-inflammatory potential via in vitro experiments. Their synthesis involved the propargylation of isatin 1 with propargyl bromide to obtain N-propargyl isatin 2, which was subjected to click reactions with different aromatic azides to yield isatin-N-1,2,3-triazoles (3a–i). The structures of all the compounds were confirmed via NMR and HR-MS. The final isatin analogues were tested for their ability to attenuate the production of proinflammatory cytokines in the lipopolysaccharide (LPS)-induced human leukemia monocytic THP-1 cells. Importantly, none of the compounds had any negative effect on THP-1 cell viability at the tested concentrations of 4 mM and 8 mM. LPS induced the production of the cytokines: Tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and monocyte chemoattractant protein-1 (MCP-1) by 351.4, 7.9 and 14.3 fold, respectively, in THP-1 cells. However, treatment with compound 3e markedly attenuated the levels of TNF-α (by 6.6 fold and 1.5 fold), IL-6 (by 1.03 fold and 1.41 fold) and MCP-1 (by 3.3 fold and 1.7 fold) by several fold at concentrations of 4 mM and 8 mM, respectively. Furthermore, in the gene expression modulation studies, 3e was found to downregulate the genes responsible for the production of TNF-α (24 and 25 fold), IL-6 (148 and 502 fold) and MCP-1 (50 and 25 fold) at the two tested concentrations compared with their expression in the LPS-induced THP-1 cells (135 fold, 6612 fold, and 68.8 fold, respectively). Thus, 3e markedly attenuated the secretion of TNF-α, IL-6 and MCP-1 from LPS-treated THP-1 cells, and also the expression of the concerned genes. At the lowest dose tested, i.e., 4 mM, 3e had the greatest effect on both gene expression and marker secretion.
1 Introduction
Isatin (1H-indole-2,3-dione) and substituted isatins are heterocyclic molecules found in plants such as Isatis spp,1 Calanthe discolor,2 Couroupita guianensis,3 and Melochia tomentosa,4 bacteria such as Streptomyces albus5 and fungi such as Chaetomium globosum.6 Isatin is a versatile substrate that can be used to prepare a variety of heterocyclic compounds, such as indoles and quinolones.7 Furthermore, Schiff bases and Mannich bases of isatin are known to possess a wide range of pharmacological properties including antibacterial I,8 anticonvulsant,9 anti-HIV II,10 antifungal III,11 antiviral IV12 and anti-TB13 activities. The bis-imine of isatin has shown antimicrobial properties14 and affected cell viability15 (Fig. 1). Isatin is used as an intermediate in the synthesis of drugs such as diclofenac and pirquinozol.The construction of molecular hybrids of potential bioactives using differently substituted nitrogen-rich heterocyclic pharmacophores such as 1,2,3-triazoles has enabled the production of new and biologically promising drugs.16–18 Chemically, 1,2,3-triazole is stable against acidic/basic hydrolysis and oxidative/reductive conditions, thus reflecting high aromatic stabilization and relative resistance to metabolic degradation.19–21 1,2,3-Triazole is one of the key structural units found in a large variety of bioactive molecules such as antifungal,22 antibacterial,23,24 antiallergic,25 anti-HIV,26,27 antitubercular28,29 and anti-inflammatory30 agents. For example, triazole nucleoside derivative V31 inhibited Mycobacterium tuberculosis, which disrupts siderophore biosynthesis. Carboxyamidotriazole VI is an angiogenesis inhibitor useful in cancer therapy,32 and 1,2,3-triazole-containing compound VII is a potential dual-action HIV-1 protease and nonnucleoside reverse transcriptase inhibitor.33 The cephalosporin analogue cefatrizine VIII is also an antibiotic used to treat bacterial infections of the urinary tract, liver and gallbladder, etc.34,35 (Fig. 2).
Our earlier research on the synthesis of novel hybrid analogues such as piperonal-thiazolidinedione-triazoles,36 chromanochalcone-triazoles,37 andrographolide-triazoles38 resulted in the production of potential anticancer, hypolipidemic and antidiabetic agents. In continuation, herein, we discuss the synthesis of isatin-1,2,3-triazole hybrid molecules via click chemistry and their anti-inflammatory potential in LPS-induced THP-1 cells.
Inflammation is an immunological response of the body to several factors including dietary lipids, mechanical injury, burns, allergens and noxious stimuli.39 Usually, inflammation is a defence mechanism of the body; however, chronic inflammation may damage normal functioning, especially in the context of autoimmune diseases, atherosclerosis, arthritis, etc. The inflammatory response involves various immune cells, blood vessels, and molecular mediators. Key players include white blood cells (such as neutrophils, macrophages, and lymphocytes), cytokines, chemokines, and other signalling molecules. The synthesized isatin-1,2,3-triazoles 3a–i were treated with LPS-induced human monocytic THP-1 cells in vitro to assess their ability to attenuate the production of three proinflammatory cytokines i.e., TNF-α, IL-6 and MCP-1, and investigate the nuclear factor character of the most potent analogue in modulating the expression of concerned genes in the tested monocytic cells.
2 Results and discussion
2.1 Chemistry
The synthetic strategy of novel isatin N-substituted 1,2,3-triazoles is outlined in Scheme 1. Isatin 1 was N-propargylated with propargyl bromide (1.5 eq.) in the presence of anhydrous K2CO3 (2 eq.) in 10 mL of DMF at room temperature for 4 h, resulting in N-propargyl-isatin 2. Compound 2 was reacted with substituted phenyl azides a and b and benzyl azides c–i (prepared in situ, as previously reported40 under click chemistry reaction conditions (Cu-catalyzed 1,3-dipolar cycloaddition)) in the presence of copper(I) iodide as the catalyst in dry THF at room temperature for 12–14 h, resulting in the production of novel substituted 1-((1-phenyl-1H-1,2,3-triazol-4-yl) methyl) indoline-2,3-diones 3a and b (yield: 90–92%) and 1-((1-benzyl-1H-1,2,3-triazol-4-yl)methyl) indoline-2,3-diones 3c–i (yield: 85–93%), respectively. | ||
| Scheme 1 General scheme for the synthesis of isatin-1,2,3-triazol derivatives (reaction conditions: step-1: propargyl bromide (1.0 mL), K2CO3, DMF, RT 4 h; step-2: CuI, THF, RT, 14 h). | ||
All the synthesized isatin-triazole derivatives 3a–i were characterized by 1H NMR, 13C NMR, IR and HR-MS spectra. In the IR spectra, compounds 3a–i presented IR absorption bands ranging from 1020–1250, 1724–1740 and 3000–3134 cm−1 for C–N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and aromatic C–H stretching, respectively. In the 1H NMR spectra, the characteristic protons of compounds 3a and 3e were assigned as follows. In compound 3a, one singlet at δ 5.57 (2H) confirmed the presence of an N–CH2 linkage. The characteristic triazole CH proton appeared at δ 8.28 (1H) and seven aromatic protons (four aromatic protons of the isatin ring plus three aromatic protons of the phenyl ring) resonated in the range of δ 7.39–8.27. Furthermore, the structure of compound 3a was confirmed by ESI-MS, and a molecular ion peak at m/z 386 (M + 23) was observed for the molecular formula C18H13N5O4 (M = 363). In the 1H NMR spectrum of compound 3e, the presence of two characteristic singlets at δ 5.6 (2H) to 4.9 (2H) confirmed the N–CH2 and Ar–CH2 linkages, respectively. A characteristic singlet peak was observed at δ 8.19 (1H) for the CH proton of the triazole ring. Furthermore, in the 13C-NMR spectrum of compound 3e, three characteristic peaks were observed for N–CH2, Ar–CH2 and CH of the triazole ring at δ 51.01, 35.36 and 123.1 respectively. In the ESI-mass spectrum, a molecular ion peak at m/z 409 (M + 23) was also observed for the molecular formula C18H12Cl2N4O2 (M = 386); thus, the structure of 3e was confirmed.
O and aromatic C–H stretching, respectively. In the 1H NMR spectra, the characteristic protons of compounds 3a and 3e were assigned as follows. In compound 3a, one singlet at δ 5.57 (2H) confirmed the presence of an N–CH2 linkage. The characteristic triazole CH proton appeared at δ 8.28 (1H) and seven aromatic protons (four aromatic protons of the isatin ring plus three aromatic protons of the phenyl ring) resonated in the range of δ 7.39–8.27. Furthermore, the structure of compound 3a was confirmed by ESI-MS, and a molecular ion peak at m/z 386 (M + 23) was observed for the molecular formula C18H13N5O4 (M = 363). In the 1H NMR spectrum of compound 3e, the presence of two characteristic singlets at δ 5.6 (2H) to 4.9 (2H) confirmed the N–CH2 and Ar–CH2 linkages, respectively. A characteristic singlet peak was observed at δ 8.19 (1H) for the CH proton of the triazole ring. Furthermore, in the 13C-NMR spectrum of compound 3e, three characteristic peaks were observed for N–CH2, Ar–CH2 and CH of the triazole ring at δ 51.01, 35.36 and 123.1 respectively. In the ESI-mass spectrum, a molecular ion peak at m/z 409 (M + 23) was also observed for the molecular formula C18H12Cl2N4O2 (M = 386); thus, the structure of 3e was confirmed.
2.2 Anti-inflammatory activity
| Sample name | % of cell viability at different concentrations | ||
| Dexamethasone (μM) | |||
| 12 | 60 | 120 | |
| Dex | 92.18% ± 0.44% | 100% ± 1% | 100% ± 1.02% |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Isatin (mM) | |||
| 5 | 10 | ||
| Isatin | 100% ± 0.21% | 100% ± 0.28% | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Isatin derivatives (mM) | |||
| 4 | 8 | ||
| 3a | 99.41349% ± 8% | 99.40299% ± 4% | |
| 3b | 99.29078% ± 10% | 98.80478% ± 7% | |
| 3c | 98.97% ± 5% | 91% ± 13% | |
| 3d | 100% ± 2% | 98.69% ± 5% | |
| 3e | 99.68% ± 4% | 99.38% ± 5% | |
| 3f | 100% ± 2% | 99.30% ± 3% | |
| 3g | 99.66% ± 3% | 99.07% ± 8% | |
| 3h | 100% ± 9% | 99.33% ± 2% | |
| 3i | 100% ± 6% | 99.65% ± 7% | |
| TNF-α release (fold change) | |||
|---|---|---|---|
| Cells (control) | 1 ± 0.00177 | ||
| Cells + LPS (3 h) | 351.46 ± 0.0248 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Pretreatment with dexamethasone (μM) for 12 h | |||
| 12 | 60 | 120 | |
| Cells + Dex + LPS | 331.6891 ± 0.05 | 38.21044 ± 9.97 | 123.3 ± 29.83 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Isatin (mM) | |||
| 5 | 10 | ||
| Cells + isatin + LPS | 418.10 ± 0.06 | 324.2066 ± 0.09 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Isatin derivatives (mM) | |||
| 4 | 8 | ||
| Cells + 3a + LPS | 332.72 ± 0.01 | 336.55 ± 0.09 | |
| Cells + 3b + LPS | 318.06 ± 0.24 | 303.58 ± 0.07 | |
| Cells + 3c + LPS | 342.84 ± 0.06 | 341.04 ± 0.02 | |
| Cells + 3d + LPS | 306.9061 ± 0.08 | 313.6059 ± 0.14 | |
| Cells + 3e + LPS | 6.65 ± 0.01 | 1.50 ± 0.004 | |
| Cells + 3f + LPS | 328.15 ± 0.12 | 338.54 ± 0.05 | |
| Cells + 3g + LPS | 300.87 ± 0.06 | 152.19 ± 0.11 | |
| Cells + 3h + LPS | 335.6804 ± 0.11 | 276.31 ± 0.18 | |
| Cells + 3i + LPS | 353.60 ± 0.07 | 334.40 ± 0.08 | |
| IL-6 release (fold change) | |||
|---|---|---|---|
| Cells (control) | 1 ± 0.0062 | ||
| Cells + LPS (3 h) | 7.95 ± 1.67 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Pretreatment with dexamethasone (μM) for 12 h | |||
| 12 | 60 | 120 | |
| Cells + Dex + LPS | 5.74 ± 0.1 | 6.11 ± 0.36 | 5.53 ± 0.82 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Isatin (mM) | |||
| 5 | 10 | ||
| Cells + isatin + LPS | 4.08 ± 0.01 | 4.86 ± 0.15 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| 3e (mM) | |||
| 4 | 8 | ||
| Cells + 3e + LPS | 1.03 ± 0.20 | 1.41 ± 0.53 | |
| MCP-1 release (fold change) | |||
|---|---|---|---|
| Cells (control) | 1 ± 0.04 | ||
| Cells + LPS (3 h) | 14.37 ± 0.12 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Pretreatment with dexamethasone (μM) for 12 h | |||
| 12 | 60 | 120 | |
| Cells + Dex + LPS | 4.93 ± 0.08 | 7.75 ± 1.23 | 7.71 ± 1.1 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Isatin (mM) | |||
| 5 | 10 | ||
| Cells + isatin + LPS | 10.07 ± 0.15 | 10.95 ± 0.097 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| 3e (mM) | |||
| 4 | 8 | ||
| Cells + 3e + LPS | 3.32 ± 0.11 | 1.75 ± 0.08 | |
| Genes | Cells (control) | Cells + LPS | Cells + isatin + LPS | Cells + 3e + LPS | ||
|---|---|---|---|---|---|---|
| 5 mM | 10 mM | 4 mM | 8 mM | |||
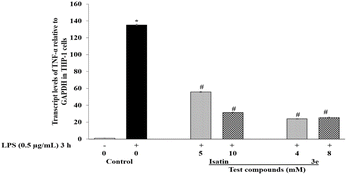
| TNF-α | 1 ± 0.31 | 135.2 ± 0.81 | 55.9 ± 0.50 | 31.4 ± 0.39 | 23.9 ± 0.41 | 25.3 ± 0.59 |
| IL-6 | 1 ± 0.18 | 6612.6 ± 0.21 | 1145.8 ± 1.01 | 596.8 ± 0.83 | 148.5 ± 0.53 | 502.5 ± 2.2 |
| MCP-1 | 1 ± 0.19 | 68.8 ± 0.70 | 6.21 ± 0.67 | 1.73 ± 0.54 | 49.9 ± 0.21 | 24.9 ± 0.49 |
3 Experimental
3.1 Chemistry
The melting points of all the compounds were recorded on a Casia-Siamia (VMP-AM) melting point apparatus and uncorrected. IR spectra were recorded on a PerkinElmer FT-IR 240 C spectrometer using KBr optics. NMR spectra were recorded on a Bruker Avance 400/500 MHz in DMSO-d6 using TMS as an internal standard. All reactions were monitored on silica gel percolated TLC plates from Merck, and the spots were visualized with UV light. The silica gel (100–200 mesh) used for column chromatography was procured from Merck.3.1.1.1 1-(Prop-2-ynyl)indoline-2,3-dione (2). Orange solid, yield: 98%, m.p.156–157 °C, 1H-NMR (DMSO-d6, 400 MHz) δ (ppm): 6.74–6.79 (t, 1H), 6.64–6.66 (d, J = 7.4 Hz, 1H), 6.22–6.30 (m, 2H), 3.62 (d, J = 2.4 Hz, 2H), 2.24 (t, 1H). ESI-MS found MNa+, 208 calculated for C11H7NO2: M, 185.1820.
3.1.2.1 1-((1-(2-Methyl-3-nitrophenyl)-1H-1,2,3-triazol-4-yl)methyl)indoline-2,3-dione (3a). Orange solid, yield: 92%, m.p. 198–199 °C, IR (KBr) 3145, 3093, 1739, 1612, 1527, 1467, 1296, 1175, 1097 cm−1; 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 8.26–8.28 (dd, J = 8.1, 1.3 Hz, 1H), 8.12 (s, 1H), 7.85–7.90 (m, 2H), 7.79–7.81 (dd, J = 7.9, 1.3 Hz, 1H), 7.73–7.76 (t, J = 8.0 Hz, 1H), 7.61 (t, J = 7.62 Hz, 1H), 7.50 (s, 1H), 7.36–7.43 (m, 1H), 5.37 (s, 2H), 1.84 (s, 3H). HR-MS found MH+, 364.1036. Calculated for C18H14N5O4: MH, 364.1046 [m/z: 364.10 (100.0%), 386.08.10 (30.2%), 146.06 (15%), 308.10 (12%), 158.06 (7%)].
3.1.2.2 1-((1-(4-Acetylphenyl)-1H-1,2,3-triazol-4-yl)methyl)indoline-2,3-dione (3b). Orange solid, yield: 90%, m.p. 252–253 °C, IR (KBr) 3153, 2959, 2924, 1737, 1687, 1608, 1468, 1263, 1158, 1098 cm−1; 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 8.05 (s, 1H), 7.19–7.22 (d, J = 8.7 Hz, 2H), 7.07–7.10 (d, J = 8.7 Hz, 2H), 6.65–6.71 (m, 2H), 6.21–6.28 (m, 2H), 4.15 (s, 2H), 1.68 (s, 3H). HR-MS found: MH+, 347.1154. Calculated for C19H15N4O3: MH, 347.1164 [m/z: 347.11 (100.0%), 348.11 (20%), 349.12 (4%), 345.13 (2%)].
3.1.2.3 1-((1-(4-Nitrobenzyl)-1H-1,2,3-triazol-4-yl)methyl)indoline-2,3-dione (3c). Orange solid, yield: 86%, m.p. 232–234 °C, IR (KBr) 3126, 2954, 1788, 1731, 1610, 1519, 1467, 1269, 1184, 1033 cm−1; 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 7.33 (s, 1H), 7.25–7.27 (d, J = 8.7 Hz, 2H), 6.66–6.71 (t, J = 7.8 Hz, 1H), 6.60–6.62 (d, J = 7.2 Hz, 1H), 6.53–6.56 (d, J = 8.6 Hz, 2H), 6.16–6.22 (m, 2H), 4.79 (s, 2H), 4.03 (s, 2H). ESI-MS found: MNa+, 386. Calculated for C15H14N5O4: MH, 364.3330 [m/z: 386 (100.0%), 235 (22.6%), 118 (10%), 166 (8%), 402 (6%)].
3.1.2.4 1-((1-(3-Chlorobenzyl)-1H-1,2,3-triazol-4-yl)methyl)indoline-2,3-dione (3d). Orange solid, yield: 89%, m.p. 145–146 °C, IR (KBr) 3130, 3069, 1732, 1609, 1468, 1322, 1217, 1124, 1049 cm−1; 1H-NMR (DMSO-d6, 400 MHz) δ (ppm): 9.01 (s, 1H), 8.03–8.15 (m, 4H), 7.60–7.64 (d, J = 15.8 Hz, 2H), 7.14–7.22 (m, 2H), 5.09 (s, 2H), 3.35 (s, 3H). 13C-NMR (CDCl3, 75 Hz) δ (ppm): 196.89, 182.97, 149.98, 143.20, 139.38, 138.09, 136.34, 130.06, 124.48, 123.40, 121.91, 119.56, 117.68, 111.07, 34.99, 26.81. HR-MS found: MH+, 353.0813. Calculated for C18H14ClN4O2: MH, 353.0823 [m/z: 353.08 (100.0%), 355.07 (31.2%), 356.08 (8%), 357.08 (1.8%)].
3.1.2.5 1-((1-(2,4-Dichlorobenzyl)-1H-1,2,3-triazol-4-yl)methyl)indoline-2,3-dione (3e). Orange solid, yield: 85%, m.p. 175–176 °C, IR (KBr) 3123, 3080, 2924, 2855, 1786, 1731, 1610, 1559, 1432, 1229, 1120, 1031 cm−1; 1H-NMR (DMSO-d6, 400 MHz) δ (ppm): 4.970 (s, 2H), 5.657 (s, 2H), 7.108–7.147 (m, 2H), 7.236–7.257 (d, 1H, J = 8.533 Hz), 7.552–7.575 (dd, 1H, J = 1.506 Hz), 7.608–7.651 (m, 1H), 7.688–7.694 (d, 1H, J = 2.259 Hz), 8.199 (s, 1H). 13C-NMR (CDCl3, 75 Hz) δ (ppm): 183.02, 157.92, 150.18, 142.12, 138.62, 135.88, 134.38, 131.42, 130.53, 129.93, 128.02, 125.34, 124.06, 123.17, 117.51, 111.50, 51.01, 35.36. HR-MS found: MH+, 387.0415. Found for C18H13C12N4O2: MH, 387.0425 [m/z: 387.04 (100.0%), 389.03 (63.5%), 359.04 (7%), 331.04 (4.2%)].
3.1.2.6 1-((1-(4-Fluorobenzyl)-1H-1,2,3-triazol-4-yl)methyl)indoline-2,3-dione (3f). Orange solid, yield: 93%, m.p. 186–187 °C, IR (KBr) 3150, 2924, 1786, 1726, 1607, 1509, 1466, 1354, 1266, 1051 cm−1; 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 7.27 (s, 1H), 6.61–6.68 (m, 2H), 6.39–6.43 (dd, J = 8.6, 5.6 Hz, 2H), 6.19–6.27 (dd, J = 17.5, 8.4 Hz, 4H), 4.61 (s, 2H), 4.01 (s, 2H). HR-MS found: MH+, 337.1114. Calculated for C18H14FN4O2: MH, 337.1124 [m/z: 337.11 (100.0%), 338.11 (21%), 281.11 (11.1%), 309.11 (8.6%), 282.11 (1.9%)].
3.1.2.7 1-((1-(2-Fluorobenzyl)-1H-1,2,3-triazol-4-yl)methyl)indoline-2,3-dione (3g). Orange solid, yield: 90%, m.p. 137–138 °C, IR (KBr) 3126, 3080, 2936, 1740, 1611, 1466, 1326, 1236, 1145, 1039 cm−1; 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 7.25 (s, 1H), 6.60–6.68 (dd, J = 19.1, 3.9 Hz, 2H), 6.25–6.45 (m, 4H), 6.18–6.20 (d, J = 7.8 Hz, 3H), 4.67 (s, 2H), 4.01 (s, 2H). HR-MS found: MH+, 337.1115. Calculated for C18H14FN4O2: MH, 337.1125 [m/z: 337.11 (100.0%), 338.11 (21%), 309.11 (18%), 310.11 (4%)].
3.1.2.8 2-((4-((2,3-Dioxoindolin-1-yl)methyl)-1H-1,2,3-triazol-1-yl)methyl)benzonitrile (3h). Orange solid, yield: 86%, m.p. 166–167 °C, IR (KBr) 3081, 3032, 2960, 2230, 1862, 1724, 1608, 1467, 1327, 1120, 1024 cm−1; 1H-NMR (DMSO-d6, 500 MHz) δ (ppm): 7.31 (s, 1H), 6.93–6.96 (dd, J = 7.7, 0.9 Hz, 1H), 6.6.-6.76 (m, 4H), 6.38–6.41 (d, J = 7.8 Hz, 1H), 6.18–6.22 (dd, J = 7.8, 1.6 Hz, 2H), 4.83 (s, 2H), 4.03 (s, 2H). HR-MS found: MH+, 344.1190. Calculated for C19H14N5O2: MH, 344.1200 [m/z: 344.11 (100.0%), 366.09 (60%), 345.11 (38%), 288.11 (37%), 316.11 (7.1%), 289.11 (7.6%)].
3.1.2.9 4′-((4-((2,3-Dioxoindolin-1-yl)methyl)-1H-1,2,3-triazol-1-yl)methyl)biphenyl-2-carbonitrile (3i). Orange solid, yield: 85%, m.p. 170–171 °C, IR (KBr) 3143, 2950, 2221, 1854, 1738, 1612, 1469, 1327, 1122, 1098 cm−1; 1H-NMR (DMSO-d6, 400 MHz) δ (ppm): δ 7.37 (s, 1H), 7.00–7.03 (d, J = 7.7 Hz, 1H), 6.83–6.86 (d, J = 6.8 Hz, 1H), 6.63–6.71 (dd, J = 15.5, 7.6 Hz, 6H), 6.47–6.50 (d, J = 8.1 Hz, 2H), 6.20–6.25 (m, 2H), 4.73 (s, 2H), 4.05 (s, 2H). HR-MS found: MH+, 420.1482. Calculated for C25H18N5O2: MH, 420.1492. [m/z: 420.14 (100.0%), 421.14 (18%), 442.12 (45%), 443.13 (8%)]
3.2 Anti-inflammatory activity
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Colourless and blue cells were recorded based on morphology through an inverted microscope while being watched with a haemocytometer.44
1 ratio. Colourless and blue cells were recorded based on morphology through an inverted microscope while being watched with a haemocytometer.44| % viability = (O.D. of treated cells//O.D. of untreated cells) × 100. |
4 Conclusion
The synthesized isatin-1,2,3-triazole hybrid analogues demonstrated significant anti-inflammatory activity, with compound 3e showing the most potent effects. Specifically, compound 3e significantly attenuated the secretion of TNF-α by 6.65 fold and 1.50 fold; that of IL-6 by 1.03 and 1.41 fold; and that of MCP-1 by 3.32 and 1.75 fold at concentrations of 4 mM and 8 mM, respectively. The gene expression analysis further revealed that 3e downregulated TNF-α by 24 fold and 25 fold; IL-6 by 148 fold and 502 fold; and MCP-1 by 50 fold and 25 fold at the same concentrations. Importantly, none of the synthesized analogues, including 3e, exhibited cytotoxicity toward THP-1 cells at the tested concentrations, confirming their safety. These findings highlight the potential of isatin-1,2,3-triazole hybrids, particularly compound 3e, as nontoxic and effective anti-inflammatory agents.Abbreviations
| C | Control |
| V-ctrl | Vehicle control |
| LPS | Lipopolysaccharides |
| TNF-α | Tumor necrosis factor-alpha |
| IL-6 | Interleukin-6 |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrezolium bromide |
| Dex | Dexamethasone |
| TC | Test compound |
Data availability
The data supporting the findings of this study are available from the corresponding author JKK upon reasonable request.Author contributions
A. N. K. performed the synthetic protocol and wrote the first draft of the manuscript; J. K. K. and K. V. N. S. S. edited the manuscript and formatted the last version; S. R. D. and S. D. T. performed the anti-inflammatory activity.Conflicts of interest
Authors declare no conflict of interest.Acknowledgements
The authors thank Director, CSIR-CIMAP, Lucknow, India, and Scientist in-charge, CSIR-CIMAP-RC, Hyderabad, India for their constant encouragement and support. Author Smruti expresses gratitude to CSIR for granting SRF and acknowledges UoH-IoE for their financial support towards consumables. CIMAP Publication Communication Number: CIMAP/PUB/2024/136.References
- Y. Guo and F. Chen, TLC-UV-spectrophotometric and TLC-scanning determination of isatin in leaf of Isatis, Zhongcaoyao, 1986, 17, 8–11 CAS
.
- M. Yoshikawa, T. Murakami, A. Kishi, T. Sakurama, H. Matsuda, M. Nomura, H. Matsuda and M. Kubo, Novel indole S,O-bisdesmoside, calanthoside, the precursor glycoside of tryptanthrin, indirubin, and isatin, with increasing skin blood flow promoting effects, from two Calanthe species (Orchidaceae), Chem. Pharm. Bull., 1998, 46, 886–888, DOI:10.1248/cpb.46.886
.
- J. Bergman, J. O. Lindström and U. L. F. Tilstam, The structure and properties of some indolic constituents in Couroupita guianensis aubl, Tetrahedron, 1985, 41(14), 2879–2881, DOI:10.1016/S0040-4020(01)96609-8
.
- G. J. Kapadia, B. K. Shukla, S. P. Chowdhury, H. M. Basak and E. A. Falesm, Phenylpelisatins: a novel class of alkaloids from Melochia tomentosa, J. Chem. Soc., Chem. Commun., 1997, 535–536, 10.1039/C39770000535
.
- U. D. O. Grafe and L. Rasics, Isolation and structure elucidation of 6-(3′-methylbuten-2′-yl) isatin, an unusual metabolite from Streptomyces albus, J. Antibiot., 1986, 39(1), 162–163, DOI:10.7164/antibiotics.39.162
.
- J. Breinholt, H. Demuth, M. Heide, G. W. Jensen, I. L. Moeller, R. I. Nielsen, C. E. Olsen and C. N. Rosendahl, Prenisatin (5-(3-Methyl-2-butenyl)-indole-2,3-dione): An antifungal isatin derivative from Chaetomium globosum, Acta Chem. Scand., 1996, 50(5), 443–445, DOI:10.3891/acta.chem.scand.50-0443
.
- J. F. M. Da Silva, S. J. Garden and A. C. Pinto, The Chemistry of Isatins: a Review from 1975 to 1999, J. Braz. Chem. Soc., 2001, 12(3), 273–324 CrossRef CAS
. https://ssrn.com/abstract=2969403.
- S. N. Pandeya and D. Sriram, Synthesis and screening for antibacterial activity of Schiff's and Mannich bases of Isatin and its derivatives, Acta. Pharm. Turc., 1998, 40, 33–38 CAS
.
- M. Verma, S. N. Pandeya, K. N. Singh and J. P. Stables, Anticonvulsant Activity of Schiff Bases of Isatin Derivatives, Acta Pharm., 2004, 54, 49–56 CAS
.
- S. N. Pandeya, D. Sriram, G. Nath and E. De Clercq, Synthesis, antibacterial, antifungal and anti-HIV evaluation of Schiff and Mannich bases of isatin and its derivatives with triazole, Arzneim.-Forsch., Drug Res., 2000, 50(2), 55–59, DOI:10.1055/s-0031-1300164
.
- S. N. Pandeya, D. Sriram, G. Nath and E. De Clercq, Synthesis, antibacterial, antifungal and anti-HIV activities of Schiff and Mannich bases derived from isatin derivatives and N-[4-(4′-chlorophenyl)thiazol-2-yl] thiosemicarbazide, Eur. J. Pharm. Sci., 1999, 9(1), 25–31, DOI:10.1016/s0928-0987(99)00038-x
.
- S. P. Singh, S. K. Shukla and L. P. Awasthi, Synthesis of some 3-(4′-nitrobenzoylhydrazono)-2-indolinones as a potential antiviral agents, Curr. Sci., 1983, 52, 766–769 CAS
.
- N. Karah, N. Terzioglu and A. Gursoy, Synthesis and Structure-Activity Relationships of 3-Hydrazono-1H-2-indolinones with Antituberculosis Activity, Arzneim.-Forsch., Drug Res., 1998, 48(7), 758–763, DOI:10.1002/chin.199844123
.
- A. Bacchi, M. Carcelli, P. Pelagatti, G. Pelizzi, M. C. Rodriguez-Arguelles, D. Rogolino, C. Solinas and F. Zani, Antimicrobial and mutagenic properties of organotin(IV) complexes with isatin and N-alkylisatin bisthiocarbonohydrazones, J. Inorg. Biochem., 2005, 99(2), 397–408, DOI:10.1016/j.jinorgbio.2004.10.008
.
- G. Cerchiaro, K. Aquilano, G. Filomeni, G. Rotilio, M. R. Ciriolo and A. M. D. C. Ferriera, Isatin-Schiff base copper(II) complexes and their influence on cellular viability, J. Inorg. Biochem., 2005, 99(7), 1433–1440, DOI:10.1016/j.jinorgbio.2005.03.013
.
- N. G. Aher, V. S. Pore, N. N. Mishra, A. Kumar, P. K. Shukla, A. Sharma and M. K. Bhat, Synthesis and antifungal activity of 1,2,3-triazole containing fluconazole analogues, Bioorg. Med. Chem. Lett., 2009, 19(3), 759–763, DOI:10.1016/j.bmcl.2008.12.026
.
- J. A. Demaray, J. E. Thuener, M. N. Dawson and S. J. Sucheck, Synthesis of triazole-oxazolidinones via a one-pot reaction and evaluation of their antimicrobial activity, Bioorg. Med. Chem. Lett., 2008, 18(17), 4868–4871, DOI:10.1016/j.bmcl.2008.07.087
.
- X. L. Wang, K. Wan and C. H. Zhou, Synthesis of novel sulfanilamide-derived 1,2,3-triazoles and their evaluation for antibacterial and antifungal activities, Eur. J. Med. Chem., 2010, 45(10), 4631–4639, DOI:10.1016/j.ejmech.2010.07.031
.
- S. B. Ferreira, A. C. Sodero, M. F. Cardoso, E. S. Lima, C. R. Kaiser, F. P. Silva and V. F. Ferreira, Synthesis, biological activity, and molecular modelling studies of 1H-1,2,3-triazole derivatives of carbohydrates as alpha-glucosidases inhibitors, J. Med. Chem., 2010, 53(6), 2364–2375, DOI:10.1021/jm901265h
.
- Y. Bourne, H. C. Kolb, Z. Radic, K. B. Sharpless, P. Taylor and P. Marchot, Freeze-frame inhibitor captures acetylcholinesterase in a unique conformation, Proc. Natl. Acad. Sci. U. S. A., 2004, 101(6), 1449–1454, DOI:10.1073/pnas.0308206100
.
- M. Whiting, J. Muldoon, Y. C. Lin, S. M. Silverman, W. Lindstron, A. J. Olson, H. C. Kolb, M. G. Finn, K. B. Sharpless, J. H. Elder and V. V. Fokin, Inhibitors of HIV-1 protease by using in situ click chemistry, Angew. Chem., Int. Ed., 2006, 45(9), 1435–1439, DOI:10.1002/anie.200502161
.
- M. S. Costa, N. Boechat, E. A. Rangel, F. D. C. D. Silva, A. M. T. D. Souza, C. R. Rodrigues, H. C. Castro, I. N. Junior, M. C. S. Lourenc, S. M. S. V. Wardell and V. F. Ferreirab, Synthesis, tuberculosis inhibitory activity, and SAR study of N-substituted-phenyl-1,2,3-triazole derivatives, Bioorg. Med. Chem., 2006, 14(24), 8644–8653, DOI:10.1016/j.bmc.2006.08.019
.
- R. Patpi, L. Pulipati, P. Yogeeswari, D. Sriram, N. Jain, B. Sridhar, R. Murthy, D. T. Anjana, S. V. Kalivendi and S. Kantevar, Design, Synthesis, and Structure–Activity Correlations of Novel Dibenzo[b,d]furan, Dibenzo[b,d]thiophene, and N-Methylcarbazole Clubbed 1,2,3-Triazoles as Potent Inhibitors of Mycobacterium tuberculosis, J. Med. Chem., 2012, 55, 3911–3922, DOI:10.1021/jm300125e
.
- R. D. Simone, M. G. Chini, I. Bruno, R. Riccio, D. Mueller, O. Werz and G. Bifulco, Structure-Based Discovery of Inhibitors of Microsomal Prostaglandin E2 Synthase−1,5-Lipoxygenase and 5-Lipoxygenase-Activating Protein: Promising Hits for the Development of New Anti-inflammatory Agents, J. Med. Chem., 2011, 54(6), 1565–1575 CrossRef PubMed
. doi.org/10.1021/jm101238d.
- I. S. Bennet, G. Brooks, N. J. P. Broom, S. H. Calvert, K. Coleman and I. Francois, 6-(Substituted methylene)penems, potent broad spectrum inhibitors of bacterial beta-lactamase. V. Chiral 1,2,3-triazolyl derivatives, J. Antibiot., 1991, 44(9), 969–978, DOI:10.7164/antibiotics.44.969
.
- M. J. Soltis, H. J. Yeh, K. A. Cole, N. Whittaker, R. P. Wersto and E. C. Kohn, Identification and characterization of human metabolites of CAI [5-amino-1-1(4′-chlorobenzoyl-3,5-dichlorobenzyl)-1,2,3-triazole- 4-carboxamide], Drug Metab. Dispos., 1996, 24(7), 799–806 CAS
.
- M. J. Fray, D. J. Bull, C. L. Carr, E. C. L. Gautier, C. E. Mowbray and A. Stobie, Structure−Activity Relationships of 1,4-Dihydro-(1H,4H)-quinoxaline-2,3-diones as N-Methyl-d-aspartate (Glycine Site) Receptor Antagonists. 1. Heterocyclic Substituted 5-Alkyl Derivatives, J. Med. Chem., 2001, 44(12), 1951–1962, DOI:10.1021/jm001124p
.
- D. Imperio, T. Pirali, U. Galli, F. Pagliai, L. Cafici, P. L. Canonico, G. Sorba, A. A. Genazzani and G. C. Tron, Replacement of the lactone moiety on podophyllotoxin and steganacin analogues with a 1,5-disubstituted 1,2,3-triazole via ruthenium-catalyzed click chemistry, Bioorg. Med. Chem., 2007, 15(21), 6748–6757, DOI:10.1016/j.bmc.2007.08.020
.
- L. S. K. Q. Lu, W. Chen, T. Tomaszek, G. Yang, D. Tew, T. D. Meek, G. A. Hofmann, C. K. S. Pritchard, W. W. Smith, T. F. Ho, P. W. Fisher, M. R. Mattern, R. K. Johnson, M. J. Hansbury, J. D. Winkler, K. W. Ward, D. F. Veber and S. K. Thompson, 4-Aryl-1,2,3-triazole: a novel template for a reversible methionine aminopeptidase 2 inhibitor, optimized to inhibit angiogenesis in vivo, J. Med. Chem., 2005, 48(18), 5644–5647, DOI:10.1021/jm050408c
.
- F. Pagliai, T. Pirali, E. D. Grosso, R. D. Brisco, G. C. Tron, G. Sorba and A. A. Genazzani, Rapid synthesis of triazole-modified resveratrol analogues via click chemistry, J. Med. Chem., 2006, 49(2), 467–470, DOI:10.1021/jm051118z
.
- R. V. Somu, H. Boshoff, C. Qiao, E. M. Bennett, C. E. Barry and C. C. Aldrich, Rationally designed nucleoside antibiotics that inhibit siderophore biosynthesis of Mycobacterium tuberculosis, J. Med. Chem., 2006, 49(1), 31–34, DOI:10.1021/jm051060o
.
- J. K. Luzzi, J. H. Varghese, C. I. MacDonald, E. E. Schmidt, C. Kohn, L. V. Morris, E. K. Marshall, F. A. Chamber and C. A. Groom, Inhibition of angiogenesis in liver metastases by carboxyamidotriazole (CAI), Angiogenesis, 1998, 2, 373–379, DOI:10.1023/a:1009259521092
.
- T. O. Olomola, R. Klein, K. A. Lobb, Y. Sayed and P. T. Kaye, Towards the synthesis of coumarin derivatives as potential dual-action HIV1 protease and reverse transcriptase inhibitors, Tetrahedron Lett., 2010, 51(48), 6325–6328, DOI:10.1016/j.tetlet.2010.09.121
.
- M. Kume, T. Kubota, Y. Kimura, K. Nakashimizu, M. Motokawa and M. Nakano, Synthesis and structure-activity relationships of new 7β-[(z)-2-(2-aminothiazol-4-yl)-2-hydroxyiminoacetamido]-cephalosporins with 1,2,3-triazole in c-3 side chain, J. Antibiot., 1993, 46, 177–192, DOI:10.7164/antibiotics.46.177
.
- C. H. Neu and P. K. Fu, Cefatrizine Activity Compared with That of Other Cephalosporins, Antimicrob. Agents Chemother., 1979, 15, 209–212, DOI:10.1128/aac.15.2.209
.
- Ch. Yakaiah, D. A. Kumar, A. Sarfaraz, S. P. Singh, A. N. Kumar, N. Gupta, K. V. N. S. Srinivas, J. K. Kumar, F. Khan, A. K. Tiwari and P. Grover, Synthesis, biological evaluation and molecular modelling studies of some novel thiazolidi-nediones with triazole ring, Eur. J. Med. Chem., 2013, 70, 308–314, DOI:10.1016/j.ejmech.2013.10.005
.
- Ch. Yakaiah, T. Sneha, T. Shalini, S. Chinde, D. A. Kumar, A. N. Kumar, K. V. N. S. Srinivas, S. Alam, J. K. Kumar, F. Khan, A. Tiwari and P. Grover, Synthesis, docking and ADMET studies of novel chalcone triazoles for anticancer and anti-diabetic activity, Eur. J. Med. Chem., 2015, 93, 564–573, DOI:10.1016/j.ejmech.2015.02.027
.
- Ch. Yakaiah, K. Manjulatha, P. Sharma, K. V. N. S. Srinivas, J. K. Kumar, A. N. Kumar, F. Khan and O. H. Setty, Synthesis and Cytotoxicity Evaluation of Novel Andrographolide-1,2,3-Triazole Derivatives, J. Heterocycl. Chem., 2016, 53(6), 1902–1910, DOI:10.1002/jhet.2505
.
- S. D. Silab, B. Kiskan, M. Antonietti and Y. Yagci, Mesoporous graphitic carbon nitride as a heterogeneous catalyst for photoinduced copper(I)-catalyzed azide–alkyne cycloaddition, RSC Adv., 2014, 4, 52170–52173, 10.1039/c4ra09954k
.
- H. Bosshart and M. Heinzelmann, THP-1 cells as a model for human monocytes, Ann. Transl. Med., 2016, 4, 438, DOI:10.21037/atm.2016.08.53
.
- S. S. Choudhury, L. Bashyam, N. Manthapuram, P. Bitla, P. Kollipara and S. D. Tetali, Ocimum sanctum leaf extracts attenuate human monocytic (THP-1) cell activation, J. Ethnopharmacol., 2014, 154(1), 148–155, DOI:10.1016/j.jep.2014.03.049
.
- K. V. Reddy, G. Bhattacharjee, G. Schabbauer, A. Hollis, K. Kempf, M. Tencati and N. Mackman, Dexamethasone enhances LPS induction of tissue factor expression in human monocytic cells by increasing tissue factor mRNA stability, J. Leukocyte Biol., 2004, 76(1), 145–151 CrossRef CAS PubMed
.
- K. K. Reddi and S. D. Tetali, Dry leaf extracts of Tinospora cordifolia (Willd.) Miers attenuate oxidative stress and inflammatory condition in human monocytic (THP-1) cells, Phytomedicine, 2019, 61, 152831–185, DOI:10.1016/j.phymed.2019.152831
.
- R. K. Kumar, M. Heuchel, K. Kratz, A. Lendlein, J. Jankowski and S. D. Tetali, Effects of extracts prepared from modified porous poly (ether imide) microparticulate absorbers on cytotoxicity, macrophage differentiation and proinflammatory behavior of human monocytic (THP-1) cells, Clin. Hemorheol. Microcirc., 2018, 69(1–2), 175–185, DOI:10.3233/CH-189112
.
- P. K. Kokkiripati, R. V. Kamsala, L. Bashyam, N. Manthapuram, P. Bitla, V. Peddada, A. S. Rafgavendra and S. D. Tetali, Stem-bark of Terminalia arjuna attenuates human monocytic (THP-1) and aortic endothelial cell activation, J. Ethnopharmacol., 2013, 146(2), 456–464, DOI:10.1016/j.jep.2012.12.050
.
Footnote |
| † Electronic supplementary information (ESI) available: The supplementary material includes experimental data and spectroscopy data of all the synthesized compounds such as IR, NMR and mass spectra. See DOI: https://doi.org/10.1039/d4ra07294d |
| This journal is © The Royal Society of Chemistry 2025 |